Published online Oct 7, 2020. doi: 10.3748/wjg.v26.i37.5673
Peer-review started: June 15, 2020
First decision: July 25, 2020
Revised: August 8, 2020
Accepted: September 12, 2020
Article in press: September 12, 2020
Published online: October 7, 2020
Processing time: 104 Days and 22.9 Hours
The management strategies for recurrent ampullary adenoma after endoscopic papillectomy are still controversial. Patients with the recurrent papillary lesions need to receive repetitive endoscopic interventions due to the limitations of conventional endoscopic techniques.
To assess the feasibility, efficacy, and safety of hybrid endoscopic submucosal dissection (ESD) by duodenoscope for recurrent, laterally spreading papillary lesions.
We enrolled two patients with recurrent, laterally spreading, duodenal papillary adenomas with no intraductal extension confirmed by follow-up between March 2017 and September 2018. After marking the resection borders of the lesion using a dual knife, a submucosal cushion was created by injecting a mixture of saline solution, methylene blue, and adrenaline. A total circumferential incision and submucosal excision was performed by dual knife combined with insulated-tip diathermic knife, and then the lesion was ligated and resected using an electric snare. Endoscopic hemostasis was applied during the endoscopic procedures. Moreover, the endoscopic retrograde cholangiopancreatography (ERCP) procedures, including selective cannulation and stent implantation of biliary and pancreatic ducts, were performed. Additionally, we performed endoclip closure for mucosal defect after ESD.
Hybrid ESD using a duodenoscope and biliary and pancreatic stent placement were performed successfully in two patients. The endoscopic size of recurrent papillary lesions was no more than 2 cm. Generally, the average total procedure time was 95.5 min, and the procedure time of ESD and ERCP was 38.5 min and 15.5 min, respectively. No serious complications occurred during the intraoperative and postoperative periods. The histopathological examination revealed tubulovillous adenoma negative for neoplastic extension at the cut margin in both patients. The duodenoscopic follow-up and histopathology of biopsy specimens at 3 mo after ESD showed no residual or recurrent lesions in ampullary areas in both cases. Both cases have been followed up with no recurrence to June 2020.
Hybrid ESD by duodenoscope is technically challenging, and may be curative for recurrent, laterally spreading papillary adenomas < 2 cm. It should be performed cautiously in selected patients by experienced endoscopists.
Core Tip: The management strategies for recurrent ampullary adenomas after endoscopic papillectomy are still controversial. Our preliminary experience showed that hybrid endoscopic submucosal dissection by duodenoscope could be feasible for recurrent, laterally spreading ampullary adenomas. Follow-up after hybrid endoscopic submucosal dissection showed no residual or recurrent lesions in ampullary areas. However, it should be performed with caution by experienced endoscopists in selected patients, and the effectiveness should be verified by large-scale studies.
- Citation: Wang ZK, Liu F, Wang Y, Wang XD, Tang P, Li W. Preliminary experience of hybrid endoscopic submucosal dissection by duodenoscope for recurrent laterally spreading papillary lesions. World J Gastroenterol 2020; 26(37): 5673-5681
- URL: https://www.wjgnet.com/1007-9327/full/v26/i37/5673.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i37.5673
The clinical practice or consensus for endoscopic management of duodenal papillary lesions is not fully established[1]. Several endoscopic resection techniques such as snare polypectomy, endoscopic mucosal resection (EMR) and argon plasma coagulation (APC) ablation are available for papillary lesions[1-3]. Endoscopic resection of the laterally spreading ampullary lesions is difficult technically and usually involves surgery. Moreover, the management strategies for recurrent duodenal papillary adenomas after endoscopic papillectomy are still controversial[2]. Patients with recurrent papillary adenomas need to receive repetitive endoscopic interventions due to the limitations of conventional endoscopic techniques, such as incomplete resection. At present, endoscopic submucosal dissection (ESD) has become common and has gradually replaced most surgical procedures for management of early gastrointestinal neoplasms[4], which could provide a higher en bloc resection rate than conventional endoscopic resection techniques. Until now, ESD has rarely been used for the treatment of duodenal papillary lesions by duodenoscope due to the complex anatomy of the duodenal papilla and technical difficulties.
In this paper, we report our preliminary experience of hybrid ESD by duodenoscope combined with biliary and pancreatic stent placement for the treatment of recurrent, laterally spreading, duodenal papillary lesions.
Ten patients with recurrent duodenal papillary adenomatous lesions after endoscopic snare papillectomy were admitted to the Department of Gastroenterology and Hepatology, The First Medical Center of Chinese PLA General Hospital between March 2017 and September 2018. We excluded patients who received endoscopic management using an electric snare or APC ablation for small, locally recurrent adenomas < 1 cm without intraductal growth, and those who underwent pancreatoduodenectomy and/or chemoradiotherapy due to cancerization and ductal infiltration. Finally, we enrolled two patients with recurrent, laterally spreading papillary adenomas confirmed by duodenoscopic and biopsy examination. The patients had undergone hybrid ESD by duodenoscope and subsequent endoscopic retrograde cholangiopancreatography (ERCP). The clinical symptoms and physical and laboratory examination were evaluated. Computed tomography (CT), magnetic resonance imaging, magnetic resonance cholangiopancreatography and abdominal ultrasound were performed preoperatively, and the imaging examinations confirmed no infiltration of tumor into biliary and pancreatic ducts. Moreover, these two patients refused surgical management and gave informed consent.
Hybird ESD was performed using a standard duodenoscope (TJF240 or TJF260V; Olympus, Tokyo, Japan). The equipment included a dual knife (KD-650L; Olympus), insulated-tip diathermic (IT) knife II (KD-611L; Olympus), injection needle (NM-200L-0525), hemostatic forceps (FD-410LR; Olympus), electric snare (Cook Medical, Bloomington, IL, United States), endoclips (Olympus), SureClips (Micro-Tech, Nanjing, China), intraductal ultrasound (IDUS) (UM-DG20-31R; Olympus), cannulating sphincterotome (Dreamtome RX, Boston, MA, United States), bile duct stent (10 Fr in diameter, 3-8 cm in length; Cook Medical), pancreatic duct stent (5-7 Fr in diameter, 7-8 cm in length; Cook Medical), and a high-frequency electrosurgical generator (VIO 200D; ERBE, Tubingen, Germany). An endoscopic CO2 insufflator (UCR; Olympus) was used during the endoscopic procedures. Saline solution with diluted methylene blue, sodium hyaluronate and adrenaline was used for submucosal injection, and 1:10000 diluted epinephrine submucosal injection was used for hemostasis.
All endoscopic procedures were performed by an experienced endoscopist who had > 20 years’ experience in advanced endoscopic techniques and had performed > 2000 ESD and ERCP procedures. Patients were placed in the prone position and underwent intravenous anesthesia. Before ESD, the duodenal papillary lesions were evaluated by white light and narrow band imaging. Hybrid ESD with snaring was performed using a standard duodenoscope. After marking the resection borders of the lesion using a dual knife, a submucosal cushion was created by injecting a mixture of saline solution, methylene blue and adrenaline. A total circumferential incision and submucosal excision were performed by Dual knife combined with IT knife, and the lesion was ligated and resected using an electric snare. The electric coagulation, injection of saline solution with epinephrine and endoclips placement were applied for hemostasis during the endoscopic procedures. After hybrid ESD, ERCP, including selective cannulation of biliary and pancreatic ducts, and IDUS were performed to identify whether there was tumor ductal infiltration. Moreover, stent implantation of the biliary and pancreatic ducts was performed to prevent postoperative complications. Endoclip closure for mucosal defect after hybrid ESD was performed. During the postoperative period, intravenous proton pump inhibitors, somatostatin and antibiotics were given to prevent infection and post-ERCP pancreatitis. Meanwhile, diet was gradually restored if no complications occurred after postoperative fasting for 2-3 d.
The primary outcome measures included clinical symptoms; physical and laboratory examination; imaging characteristics, especially endoscopic and histopathological characteristics; detailed procedure-related outcome data, including the time of hybrid ESD and ERCP; en bloc resection; procedure-related complications; and hospital stay. Endoscopic and histopathological follow-up was performed to assess the presence of duodenal papillary lesions after hybrid ESD procedures.
This study included two patients with recurrent, laterally spreading, duodenal papillary adenomas. The previous characteristics of recurrent cases are listed in Table 1, and the duodenoscopic follow-up confirmed recurrent adenomas located in the therapeutic scar tissues. The main characteristics of all patients are shown in Table 2. Preoperative imaging confirmed no infiltration of tumor into biliary and pancreatic ducts, and the endoscopic findings showed a clear border with no evidence of malignancy, such as ulceration and spontaneous bleeding.
| Case | Pathological characteristics of initial resected specimen | En bloc resection | Complete resection | The first recurrent time (mo) | Time of follow-up (mo) | Previous endoscopic managements |
| Case 1 | Tubulovillous adenoma with local HIN | Yes | Yes | 31 | 96 | Endoscopic snare papillectomy, and multiple APC ablation for adenoma recurrence and three ERCP procedures for biliary stones and acute cholangitis |
| Case 2 | Tubulovillous adenoma with local LIN | Yes | Yes | 15 | 15 | Endoscopic snare papillectomy |
| Characteristics | Case 1 | Case 2 |
| Age (yr) /sex | 54/male | 54/female |
| Clinical symptoms | Negative | Negative |
| Physical and laboratory examinations | Normal | Normal |
| Recent endoscopic characteristics | Laterally spreading adenomatous lesion with a diameter of 1.5 cm on the resected scar | A red and protuberant laterally spreading lesion with a diameter of 1 cm on the resected scar |
| Total procedure time (min) | 107 | 84 |
| Hybrid ESD procedure time (min) | 57 | 30 |
| Bleeding and wound control time (min) | 24 | 22 |
| ERCP procedure time (min) | 16 | 15 |
| ERCP characteristics | No intraductal growth of lesion, but biliary stones with dilated biliary and pancreatic ducts; bile duct stent (10 Fr in diameter, 8 cm in length) and pancreatic stent (7 Fr in diameter, 8 cm in length) placement | No intraductal growth of lesion and no dilatation of biliary and pancreatic ducts; biliary stent (10 Fr in diameter, 3 cm in length) and pancreatic stent (5 Fr in diameter, 7 cm in length) placement |
| IDUS characteristics | Clear layer of the biliary and pancreatic ducts without intraductal extension; bile duct stones with dilated biliary and pancreatic ducts | Clear layer of the biliary and pancreatic ducts without intraductal extension; no dilatation of biliary and pancreatic ducts |
| No. of endoscopic clips | 2 | 5 |
| Size of resected specimen (cm) | 1.4 × 1.0 | 2.0 × 1.5 |
| Histology of resected specimen | Tubulovillous adenoma | Tubulovillous adenoma |
| En blot resection | Yes | Yes |
| R0 resection | Yes | Yes |
| Complications | None | Postoperative transient hyperamylasemia |
| Postoperative hospital stay (d) | 4 | 4 |
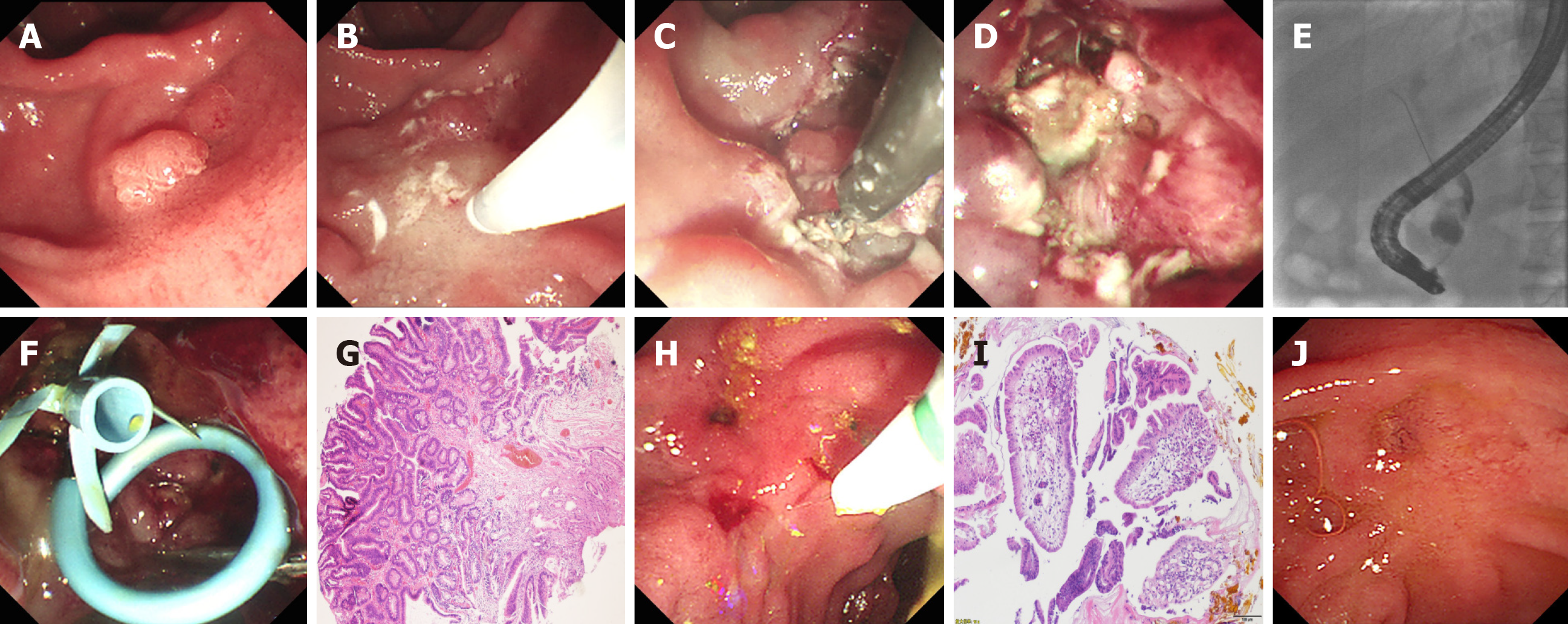
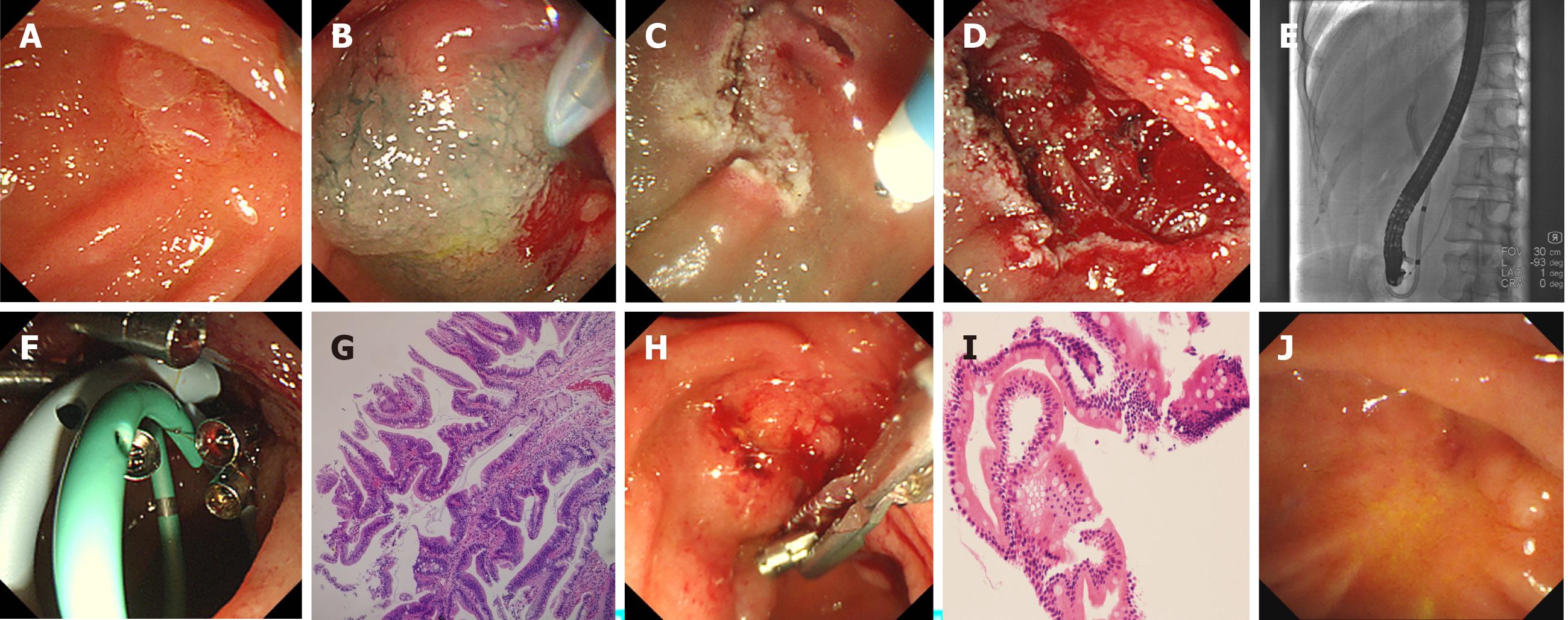
Hybrid ESD combined with ERCP was performed successfully using duodenoscope. The detailed endoscopic procedures are shown in Figures 1 and 2. The average total procedure time was 95.5 min, and the procedure time of hybrid ESD and ERCP was 38.5 min and 15.5 min, respectively. After submucosal injection, the surrounding mucosa of the lesion was lifted, except the central scar tissues. When the circumferential incision was completed, the IT knife was used to dissect the fibrotic area for prevention of perforation. The lesion was ligated and resected en bloc using a polypectomy snare. After hybrid ESD, ERCP and IDUS procedures were performed. Selective cannulation, angiography, and IDUS revealed a clear layer of the biliary and pancreatic ducts and no intraductal growth of the lesions in these two patients. Meanwhile, cholangiopancreatography and IDUS revealed common bile duct stones with obvious dilated biliary and pancreatic ducts in case 1, but the stones were not extracted simultaneously, considering the increasing risk of complications. Subsequently, the biliary and pancreatic stents were placed successfully in both cases (Figures 1 and 2, and Table 2). Furthermore, endoscopic clips were deployed for closure of mucosal defects. In both cases, no serious complications occurred during the intraoperative and postoperative periods. Postoperative laboratory tests showed normal routine blood and biochemical examinations, except for transient hyperamylasemia in case 2. Histopathological examination revealed tubulovillous adenoma negative for neoplastic extension at the horizontal and vertical margins in both cases (Figures 1 and 2).
Duodenoscopic and histopathological follow-up was undertaken at 3 mo after hybrid ESD. Endoscopic examination showed no residual or recurrent lesions in ampullary areas in both cases. ERCP showed dilated common bile duct and pancreatic ducts, and the common bile duct stones were extracted successfully in case 1, and the biliary stent was removed in case 2 (Figures 1 and 2). Histopathology of biopsy specimens showed chronic and acute inflammation of small intestinal mucosa with no adenomatous tissues in both cases (Figures 1 and 2). Both cases have been followed up with no recurrence up to June 2020.
Duodenal papillary neoplasms are uncommon and occur sporadically with a prevalence of 0.1%-0.2%[5]. The majority of papillary adenomas undergo the adenoma–carcinoma sequence, and complete removal is mandatory for curative therapy due to the malignant potential[6]. Traditionally, recommended surgical management has included pancreaticoduodenectomy and local surgical excision for complete removal of duodenal papillary tumors[7], but surgery often results in extensive resection with significant morbidity and mortality[1]. This is considered overtreatment for some benign duodenal papillary lesions or early noninvasive tumors of the papilla without intraductal growth. Endoscopic papillectomy by snare polypectomy or EMR has the advantages of being less invasiveness, which represents an alternative to surgical resection[1]. At present, it is not definitive which size or endoscopic morphology of ampullary lesions should not be treated by endoscopic resection[2]. Most studies have not recommended endoscopic resection for the duodenal papillary lesions ≥ 3-4 cm or those with extrapapillary extension[8]. Endoscopic snare polypectomy or EMR for ampullary lesions often requires repetitive interventions due to incomplete resection, residual lesion and tumor recurrence. The recurrent rate of ampullary adenomas after endoscopic papillectomy is 7%-33%[9-12]. Our team reported that 13 of 110 patients who underwent endoscopic papillectomy experienced recurrence during a mean follow-up period of 16.3 mo, and the predictive factors related to recurrence were complete resection and final pathological findings[13]. Although 75% of recurrences can be cleared endoscopically[12], surgery is usually the last choice. In our experience, there is still the possibility of multiple recurrence after endoscopic snare polypectomy or APC ablation for recurrent ampullary lesions. This indicates the technical difficulty and limitations of the conventional endoscopic papillectomy for recurrent duodenal papillary lesions.
Preoperative evaluation of ampullary lesions is important for the subsequent management strategy. In this study, preoperative endoscopic findings showed a clear border with no evidence of malignancy, such as ulceration or spontaneous bleeding, and the appropriate ampullary lesion size (≤ 2 cm). Meanwhile, preoperative histopathological examination of biopsy sample revealed papillary adenoma for both cases. Furthermore, endoscopic ultrasonography (EUS) and IDUS are useful diagnostic tools for selecting endoscopic or surgical treatment for ampullary adenomas[14,15]. These methods may be more efficient than CT for preoperative evaluation of local T staging, regional lymph node metastasis, ductal infiltration, and major vascular invasion in patients with ampullary lesions. It is reported that the accuracy of EUS in diagnosing extension into the bile duct and pancreatic duct is as high as 86%-90% and 77%-92%, respectively. IDUS can be more accurate in visualizing mucosal layers than conventional EUS, with a high accuracy of 90%-95% and 88%-100% in diagnosing extension into the bile duct and pancreatic duct, respectively[16]. In our study, both patients received careful preoperative evaluation, and several imaging examinations indicated no intraductal involvement of ampullary lesions. EUS for the estimation of tumor staging and ductal infiltration was not performed routinely in this study, but subsequent ERCP and IDUS confirmed no intraductal extension. Whatever, accurate preoperative evaluation could reduce the technical difficulties and the risk of severe complications[17].
At present, ESD has been widely accepted as standard treatment for early gastrointestinal neoplasms and large laterally spreading lesions[4], and as an appropriate resection technique for locally remnant and recurrent lesions in patients receiving endoscopic resection[18,19]. Several studies have reported successful ESD for duodenal lesions[20-22] using therapeutic gastroscopy, but ESD is rarely used for treatment of recurrent, laterally spreading ampullary lesions by duodenoscope. This is mainly due to the special physiology and complex anatomical features of duodenal papillary lesions (e.g., lesion location, lesion size, thin muscle layer, rich blood supply, secretion of biliary and pancreatic juice, and fibrosis of recurrent lesions), and technical difficulties (e.g., limited operating space and operational difficulties to achieve good endoscopic control and optimal visual fields based on forward-viewing endoscopy)[2]. Meanwhile, ESD for recurrent, laterally spreading ampullary adenomas raises concerns about a high risk of procedure-related complications. Therefore, ESD should be performed by experienced endoscopists with both ERCP and ESD techniques to reduce the risk of severe complications. In our study, all endoscopic procedures were performed by an experienced endoscopist who had > 20 years’ experience in advanced endoscopic techniques and had performed > 2000 ESD and ERCP procedures. Carefully attention was focused on endoscopic management for procedure-related complications. Submucosal fibrosis after endoscopic papillectomy for recurrent cases is a significant risk factor for adverse events. Therefore, we used a hybrid ESD technique for complete resection with a polypectomy snare to remove the lesions after dissecting the submucosal layer. Additionally, strict hemostasis including injection, electrocoagulation and endoclip placement during hybrid ESD were compulsory. The resection wound after ampullary hybrid ESD is exposed directly to biliary and pancreatic juices, which is believed to cause postoperative complications, so we suggest that after biliary and pancreatic stent placement, the resection wound should be closed by endoclips as much as possible. In this study, hybrid ESD with snaring using duodenoscope was technically successful, and en bloc resection of the therapeutic scar tissue was achieved. The average procedure time of hybrid ESD was 38.5 min, which was acceptable. No severe adverse events such as bleeding and perforation occurred in the recurrent cases.
ERCP as an important procedure and should be performed after ampullary ESD treatment. Generally, it is easy to perform selective cannulation due to the exposure of biliary and pancreatic duct after papillectomy. Limited endoscopic resection of ampullary lesions may have a risk of leaving residual tissues; therefore, ERCP and IDUS are useful for further evaluation of the condition of the biliary and pancreatic ducts. For these cases, selective cannulation for biliary and pancreatic ducts was successfully performed, and ERCP and IDUS demonstrated no biliary or pancreatic extension and no residual lesions after ESD. Prophylactic stent placement in the pancreatic duct after endoscopic removal is necessary to minimize the risk of post-ERCP pancreatitis[23]. In this study, we implanted both biliary and pancreatic stents to avoid early complications and late adverse events. There are four purposes of deploying biliary and pancreatic stenting after ESD: to prevent post-ERCP pancreatitis and cholangitis; to drain bile and pancreatic juice far from the wound surface to reduce the risk of perforation and bleeding; to avoid closure of the pancreaticobiliary opening when the hemostatic clip closes the wound; and to prevent biliary and/or pancreatic stenosis.
In conclusion, hybrid ESD by duodenoscope is technically challenging, and may be curative for recurrent, laterally spreading ampullary adenomas < 2 cm in diameter. It should be performed with caution by experienced endoscopists in selected patients to avoid severe complications.
Management of recurrent ampullary adenomas after endoscopic papillectomy is still controversial. Some patients have to receive repetitive endoscopic interventions due to the limitations of conventional endoscopic techniques.
Endoscopic submucosal dissection (ESD) has become a standard treatment for early gastrointestinal neoplasms, as well as an appropriate technique for resection locally remnant and recurrent lesions. At present, ESD is rarely used in the treatment of duodenal papillary lesions by duodenoscope due to the complex anatomy of duodenal papilla and technical difficulties.
In this retrospective study, we report our preliminary experience of hybrid ESD by duodenoscope combined with biliary and pancreatic stent placement for recurrent, laterally spreading, duodenal papillary lesions.
Two patients with recurrent, laterally spreading, papillary adenomas underwent hybrid ESD by duodenoscope and endoscopic retrograde cholangiopancreatography (ERCP). Outcomes, including endoscopic and histopathological characteristics, time of hybrid ESD and ERCP procedures, en bloc resection, procedure-related complications, and hospital stay were recorded.
Hybrid ESD using duodenoscope and subsequent biliary and pancreatic stent placement was performed successfully for both patients. The endoscopic size of recurrent papillary lesions was no more than 2 cm. No serious complications occurred during the intraoperative and postoperative periods. Histopathological examination revealed tubulovillous adenoma negative for neoplastic extension at the cut margin in both patients. No recurrence were observed during follow-up.
Hybrid ESD by duodenoscope is technically challenging, and may be curative for recurrent, laterally spreading papillary adenomas < 2 cm.
Hybrid ESD by duodenoscope should be performed cautiously in selected patients by experienced endoscopists. A prospective study should be conducted to compare hybrid ESD with conventional endoscopic techniques to gain more evidence.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kishida Y, Weiss H S-Editor: Gao CC L-Editor: Filipodia P-Editor: Li JH
| 1. | El Hajj II, Coté GA. Endoscopic diagnosis and management of ampullary lesions. Gastrointest Endosc Clin N Am. 2013;23:95-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 2. | ASGE Standards of Practice Committee, Chathadi KV, Khashab MA, Acosta RD, Chandrasekhara V, Eloubeidi MA, Faulx AL, Fonkalsrud L, Lightdale JR, Salztman JR, Shaukat A, Wang A, Cash BD, DeWitt JM. The role of endoscopy in ampullary and duodenal adenomas. Gastrointest Endosc. 2015;82:773-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 134] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 3. | Napoleon B, Gincul R, Ponchon T, Berthiller J, Escourrou J, Canard JM, Boyer J, Barthet M, Ponsot P, Laugier R, Helbert T, Coumaros D, Scoazec JY, Mion F, Saurin JC; Sociéte Française d’Endoscopie Digestive (SFED; French Society of Digestive Endoscopy). Endoscopic papillectomy for early ampullary tumors: long-term results from a large multicenter prospective study. Endoscopy. 2014;46:127-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 4. | Tanaka S, Kashida H, Saito Y, Yahagi N, Yamano H, Saito S, Hisabe T, Yao T, Watanabe M, Yoshida M, Kudo SE, Tsuruta O, Sugihara KI, Watanabe T, Saitoh Y, Igarashi M, Toyonaga T, Ajioka Y, Ichinose M, Matsui T, Sugita A, Sugano K, Fujimoto K, Tajiri H. JGES guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc. 2015;27:417-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 436] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 5. | Martin JA, Haber GB. Ampullary adenoma: clinical manifestations, diagnosis, and treatment. Gastrointest Endosc Clin N Am. 2003;13:649-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Fischer HP, Zhou H. Pathogenesis of carcinoma of the papilla of Vater. J Hepatobiliary Pancreat Surg. 2004;11:301-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 132] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 7. | de Castro SM, van Heek NT, Kuhlmann KF, Busch OR, Offerhaus GJ, van Gulik TM, Obertop H, Gouma DJ. Surgical management of neoplasms of the ampulla of Vater: local resection or pancreatoduodenectomy and prognostic factors for survival. Surgery. 2004;136:994-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 83] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 8. | Cheng CL, Sherman S, Fogel EL, McHenry L, Watkins JL, Fukushima T, Howard TJ, Lazzell-Pannell L, Lehman GA. Endoscopic snare papillectomy for tumors of the duodenal papillae. Gastrointest Endosc. 2004;60:757-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 151] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 9. | Sahar N, Krishnamoorthi R, Kozarek RA, Gluck M, Larsen M, Ross AS, Irani S. Long-Term Outcomes of Endoscopic Papillectomy for Ampullary Adenomas. Dig Dis Sci. 2020;65:260-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 10. | Tringali A, Valerii G, Boškoski I, Familiari P, Landi R, Perri V, Costamagna G. Endoscopic snare papillectomy for adenoma of the ampulla of vater: Long-term results in 135 consecutive patients. Dig Liver Dis. 2020;52:1033-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Lü S, Jiang M, Liu F, Tang H, Yang Y, Zhang W, Zhang M, Jin Z, Li Z. Endoscopic papillectomy of benign papillary tumors: A single-center experience. Medicine (Baltimore). 2020;99:e20414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Lee R, Huelsen A, Gupta S, Hourigan LF. Endoscopic ampullectomy for non-invasive ampullary lesions: a single-center 10-year retrospective cohort study. Surg Endosc. 2020;:. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Li S, Wang Z, Cai F, Linghu E, Sun G, Wang X, Meng J, Du H, Yang Y, Li W. New experience of endoscopic papillectomy for ampullary neoplasms. Surg Endosc. 2019;33:612-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 14. | Peng CY, Lv Y, Shen SS, Wang L, Ding XW, Zou XP. The impact of endoscopic ultrasound in preoperative evaluation for ampullary adenomas. J Dig Dis. 2019;20:248-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Okano N, Igarashi Y, Hara S, Takuma K, Kamata I, Kishimoto Y, Mimura T, Ito K, Sumino Y. Endosonographic preoperative evaluation for tumors of the ampulla of vater using endoscopic ultrasonography and intraductal ultrasonography. Clin Endosc. 2014;47:174-177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Yamamoto K, Iwasaki E, Itoi T. Insights and updates on endoscopic papillectomy. Expert Rev Gastroenterol Hepatol. 2020;14:435-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Catalano MF, Linder JD, Chak A, Sivak MV Jr, Raijman I, Geenen JE, Howell DA. Endoscopic management of adenoma of the major duodenal papilla. Gastrointest Endosc. 2004;59:225-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 217] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 18. | Rahmi G, Tanaka S, Ohara Y, Ishida T, Yoshizaki T, Morita Y, Toyonaga T, Azuma T. Efficacy of endoscopic submucosal dissection for residual or recurrent superficial colorectal tumors after endoscopic mucosal resection. J Dig Dis. 2015;16:14-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Oka S, Tanaka S, Kaneko I, Mouri R, Hirata M, Kanao H, Kawamura T, Yoshida S, Yoshihara M, Chayama K. Endoscopic submucosal dissection for residual/Local recurrence of early gastric cancer after endoscopic mucosal resection. Endoscopy. 2006;38:996-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 94] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 20. | Yamamoto Y, Yoshizawa N, Tomida H, Fujisaki J, Igarashi M. Therapeutic outcomes of endoscopic resection for superficial non-ampullary duodenal tumor. Dig Endosc. 2014;26 Suppl 2:50-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 21. | Marques J, Baldaque-Silva F, Pereira P, Arnelo U, Yahagi N, Macedo G. Endoscopic mucosal resection and endoscopic submucosal dissection in the treatment of sporadic nonampullary duodenal adenomatous polyps. World J Gastrointest Endosc. 2015;7:720-727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Jung JH, Choi KD, Ahn JY, Lee JH, Jung HY, Choi KS, Lee GH, Song HJ, Kim DH, Kim MY, Bae SE, Kim JH. Endoscopic submucosal dissection for sessile, nonampullary duodenal adenomas. Endoscopy. 2013;45:133-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 23. | Yamao T, Isomoto H, Kohno S, Mizuta Y, Yamakawa M, Nakao K, Irie J. Endoscopic snare papillectomy with biliary and pancreatic stent placement for tumors of the major duodenal papilla. Surg Endosc. 2010;24:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |