Published online Sep 21, 2020. doi: 10.3748/wjg.v26.i35.5354
Peer-review started: May 7, 2020
First decision: May 15, 2020
Revised: May 17, 2020
Accepted: September 2, 2020
Article in press: September 2, 2020
Published online: September 21, 2020
Processing time: 132 Days and 22.9 Hours
We have previously reported that Helicobacter pylori (H. pylori)-associated nodular gastritis could occur in both the antrum and the cardia. Cardiac nodularity-like appearance (hereafter, called as cardiac nodularity) had a high predictive accuracy for the diagnosis of H. pylori infection. In the previous study, we included only the patients who were evaluated for H. pylori infection for the first time, and excluded patients with a history of eradication. Therefore, the prevalence and clinical features of cardiac nodularity remains unknown.
To perform this cross-sectional study to explore the characteristics of cardiac nodularity.
Consecutive patients who underwent esophagogastroduodenoscopy between May, 2017 and August, 2019 in the Toyoshima Endoscopy Clinic were enrolled in this study. We included H. pylori-negative, H. pylori-positive, and H. pylori-eradicated patients, and excluded patients with unclear H. pylori status and eradication failure. H. pylori infection was diagnosed according to serum anti-H. pylori antibody and the urea breath test or histology. Cardiac nodularity was defined as a miliary nodular appearance or the presence of scattered whitish circular small colorations within 2 cm of the esophagogastric junction. Nodularity was visualized as whitish in the narrow-band imaging mode. We collected data on the patients’ baseline characteristics.
A total of 1078 patients were finally included. Among H. pylori-negative patients, cardiac nodularity and antral nodularity were recognized in 0.14% each. Among H. pylori-positive patients, cardiac nodularity and antral nodularity were recognized in 54.5% and 29.5%, respectively. Among H. pylori-eradicated patients, cardiac nodularity and antral nodularity were recognized in 4.5% and 0.6%, respectively. The frequency of cardiac nodularity was significantly higher than that of antral nodularity in H. pylori-positive and -eradicated patients. The frequencies of cardiac nodularity and antral nodularity in H. pylori-eradicated patients were significantly lower than those in H. pylori-positive patients (P < 0.001). The patients with cardiac nodularity were significantly younger than those without cardiac nodularity (P = 0.0013). Intestinal metaplasia score of the patients with cardiac nodularity were significantly lower than those without cardiac nodularity (P = 0.0216). Among H. pylori-eradicated patients, the patients with cardiac nodularity underwent eradication significantly more recently compared with those without cardiac nodularity (P < 0.0001).
This report outlines the prevalence and clinical features of cardiac nodularity, and confirm its close association with active H. pylori infection.
Core Tip: The prevalence of cardiac and antral nodularity in Helicobacter pylori (H. pylori)-negative, -positive, and -eradicated patients were 0.14% and 0.14%, 54.5% and 29.5%, and 4.5% and 0.6%, respectively. Cardiac nodularity was more frequent than antral nodularity in H. pylori-positive and -eradicated patients. Cardiac nodularity was often found in younger patients and patients with less intestinal metaplasia. Cardiac nodularity decreased after eradication, especially in patients who underwent eradication a long time ago.
- Citation: Nishizawa T, Sakitani K, Suzuki H, Yoshida S, Kataoka Y, Nakai Y, Ebinuma H, Kanai T, Toyoshima O, Koike K. Clinical features of cardiac nodularity-like appearance induced by Helicobacter pylori infection. World J Gastroenterol 2020; 26(35): 5354-5361
- URL: https://www.wjgnet.com/1007-9327/full/v26/i35/5354.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i35.5354
Helicobacter pylori (H. pylori) infection leads to the development of gastric atrophy, peptic ulcer, and gastric cancer[1-5]. Eradication of H. pylori infection has been reported as an effective strategy for treating atrophic gastritis and peptic ulcer, and preventing gastric cancer[6-9]. Therefore, it is important to evaluate H. pylori infection status[10-12].
Nodular gastritis is a form of chronic gastritis that has a unique miliary pattern on endoscopy, with “gooseflesh-like” appearance. Many studies have shown a strong association between nodular gastritis and H. pylori infection[13-15]. Children and young women are reported to be predisposed to nodular gastritis. Nodular gastritis improves gradually with age[16]. Several reports have suggested an association between nodular gastritis and diffuse type gastric cancer[13,15,17].
We have previously reported that nodularity could occur in both the antrum and the cardia[18]. Cardiac nodularity-like appearance is found more frequently than antral nodularity. Cardiac nodularity-like appearance (hereafter, called as cardiac nodularity) had a high predictive accuracy for the diagnosis of H. pylori infection. Our previous report also showed excellent interobserver agreement on cardiac nodularity. Furthermore, histological examination of cardiac nodularity often revealed lymphoid follicles displaying lymphocyte infiltration in the cardiac gland[18].
However, the prevalence and clinical features of cardiac nodularity remains unknown. Therefore, we performed this cross-sectional study to explore the characteristics of cardiac nodularity.
This study was approved by the ethical review committee of Hattori Clinic on September 6, 2019 (approval no. S1909-U06)[12,19]. All clinical investigations were conducted according to the ethical guidelines of the Declaration of Helsinki.
Consecutive patients who underwent esophagogastroduodenoscopy (EGD) between May, 2017 and August, 2019 in the Toyoshima Endoscopy Clinic were enrolled in this study. Inclusion criteria included defined H pylori status (H pylori-negative patients, H pylori-positive patients, and H pylori-eradicated patients). The patients with unclear H pylori status and eradication failure were excluded from the study. EGD was conducted for the examination of symptoms and screening. We collected data on the patients’ baseline characteristics, including age and sex, and period since eradication for eradicated patients.
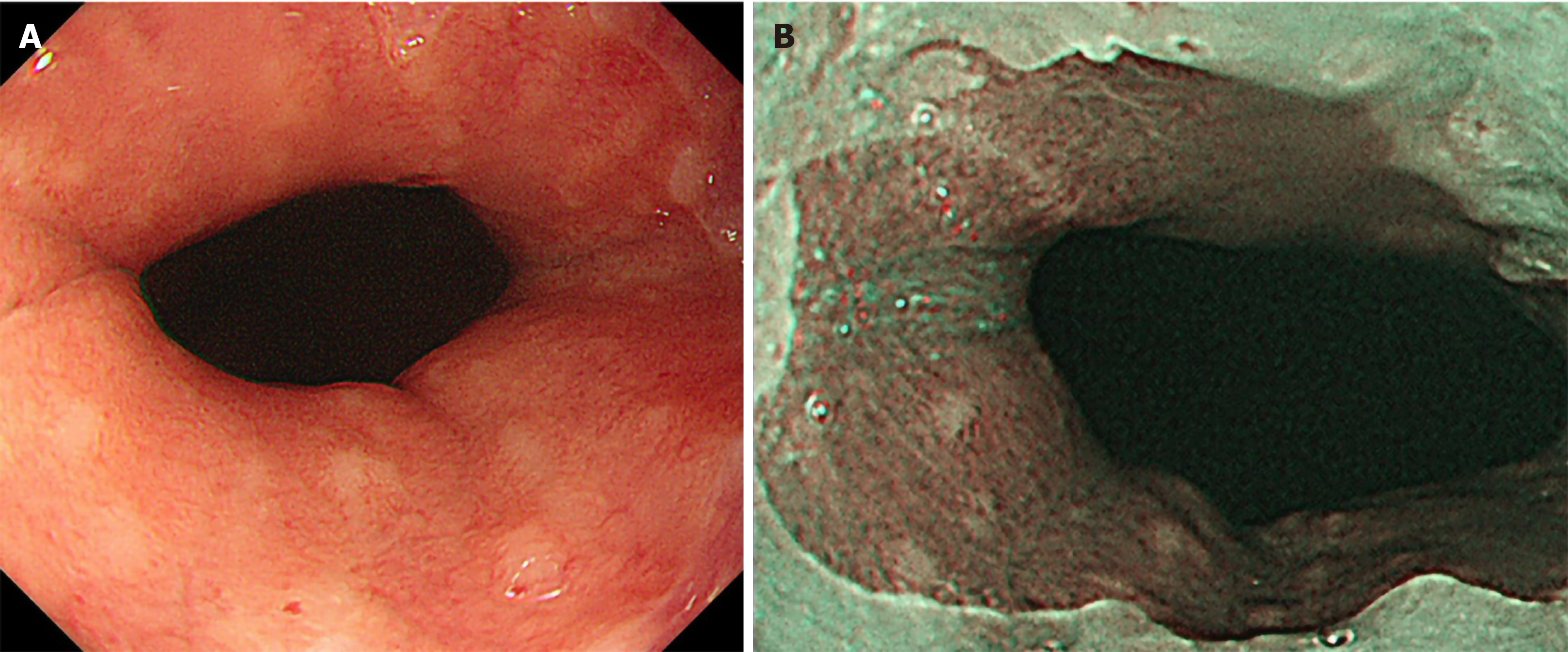
EGD was performed using the Olympus Evis Lucera Elite system with a GIF-H290Z or GIF-HQ290 endoscope (Olympus Corporation, Tokyo, Japan)[20]. An expert physician (Toyoshima O) performed endoscopic procedures. Furthermore, EGD images were retrospectively reviewed by other expert physicians. Discrepancies in diagnosis between the two sets of physicians were resolved through discussion. Sedation with midazolam and/or pethidine was performed at the patient’s discretion[21-23]. Antral nodularity was defined as a miliary nodular appearance consisting of whitish circular micronodules measuring ≤ 1 mm in both height and diameter. Cardiac nodularity was defined as a miliary nodular appearance or the presence of scattered whitish circular small colorations within 2 cm of the esophagogastric junction. Nodularity was visualized as whitish in the narrow-band imaging (NBI) mode. The representative endoscopic images are shown in Figure 1.
We scored atrophy, intestinal metaplasia, diffuse redness, and enlarged folds, according to the Kyoto classification[24].
Endoscopic atrophy was diagnosed based on the Kimura and Takemoto classification[25]. Non-atrophy and C1 were scored as Atrophy score 0, C2, and C3 as Atrophy score 1, and O1 to O3 as Atrophy score 2.
The absence of intestinal metaplasia was scored as Intestinal metaplasia score 0, the presence of intestinal metaplasia within the antrum as Intestinal metaplasia score 1, and intestinal metaplasia extending into the corpus as Intestinal metaplasia score 2. The Intestinal metaplasia score was diagnosed using white light imaging.
The absence of diffuse redness was scored as Diffuse redness score 0, mild diffuse redness or diffuse redness with regular arrangement of collecting venules (RAC) as Diffuse redness score 1, and severe diffuse redness or diffuse redness without RAC as Diffuse redness score 2.
The absence and presence of enlarged folds was scored as Enlarged folds score 0 and 1, respectively.
Serum anti-H. pylori antibody was measured on the day of EGD. The antibody titer was measured using an enzyme immunoassay kit with antigens derived from Japanese individuals (E-plate Eiken H. pylori antibody II; Eiken Chemical, Tokyo, Japan). An antibody titer ≥ 10 U/mL (the cut-off value recommended by the manufacturer) was considered positive for H. pylori[26]. When the serum anti-H. pylori antibody titer was 3.0-9.9 U/mL, the findings of urea breath test or histological assessment was added. If either the urea breath test or histology was positive, patients were considered positive for H. pylori[27,28]. An antibody titer < 3.0 U/mL or negative urea breath test was considered to indicate H. pylori negativity. Eradication was confirmed by urea breath test.
Categorical data were compared using the chi-square test. Continuous data were compared using Student’s or Welch’s t-test, as appropriate. A two-sided P value of < 0.05 was considered as statistically significant. Calculations were carried out by the Stat Mate IV software (ATOMS, Tokyo, Japan).
The endoscopist performed 1215 EGDs during the study period. We excluded 137 patients (135 patients with unclear H. pylori infection status and two with eradication failure). A total of 1078 patients were finally included.
The characteristics of the participants in the present study are shown in Table 1. Among H. pylori-negative patients, cardiac nodularity and antral nodularity were recognized in one patient each (0.14% each). Among H. pylori-positive patients, cardiac nodularity and antral nodularity were recognized in 24 (54.5%) and 13 (29.5%) patients, respectively. The frequency of cardiac nodularity was significantly higher than that of antral nodularity (P < 0.05). Among H. pylori-eradicated patients, cardiac nodularity and antral nodularity were recognized in 15 (4.5%) and 2 (0.6%) patients, respectively. The frequency of cardiac nodularity was significantly higher than that of antral nodularity (P < 0.01). The frequencies of cardiac nodularity and antral nodularity in H. pylori-eradicated patients were significantly lower than those in H. pylori-positive patients (P < 0.001).
Clinical characteristics of cardiac nodularity in H. pylori-positive patients are shown in Table 2. The patients with cardiac nodularity were significantly younger than those without cardiac nodularity (P = 0.0013). Intestinal metaplasia score of the patients with cardiac nodularity were significantly lower than those without cardiac nodularity (P = 0.0216).
| Cardiac nodularity (+) | Cardiac nodularity (-) | P value | |
| Patient number | 24 | 20 | |
| Mean age (standard deviation) | 44.9 ± 7.8 | 58.3 ± 15.1 | 0.0013 |
| Male:female | 9:15 | 10:10 | 0.598 |
| Atrophy score | 1.46 ± 0.59 | 1.55 ± 0.51 | 0.588 |
| Intestinal metaplasia score | 0.21 ± 0.59 | 0.80 ± 0.95 | 0.0216 |
| Enlarged fold score | 0.54 ± 0.51 | 0.60 ± 0.50 | 0.705 |
| Diffuse redness score | 1.75 ± 0.55 | 1.67 ± 0.64 | 0.648 |
Among H. pylori-eradicated patients, the patients with cardiac nodularity were also significantly younger than those without cardiac nodularity (P = 0.0003, Table 3). Furthermore, the patients with cardiac nodularity underwent eradication significantly more recently compared with those without cardiac nodularity (P < 0.0001).
| Cardiac nodularity (+) | Cardiac nodularity (-) | P value | |
| Patient number | 15 | 315 | |
| Mean age (standard deviation) | 49.2 ± 12.3 | 60.8 ± 12.1 | 0.0003 |
| Male:female | 5:10 | 180:135 | 0.121 |
| Months after eradication | 41.5 ± 30.1 | 91.6 ± 100.0 | < 0.0001 |
The prevalence of cardiac nodularity was 0.14%, 54.5%, and 4.5% in H. pylori-negative, -positive, and -eradicated patients, respectively. Cardiac nodularity was more frequent than antral nodularity in H. pylori-positive and -eradicated patients. Cardiac nodularity was often found in younger patients and patients with less intestinal metaplasia. Cardiac nodularity decreased after eradication, especially in patients who underwent eradication a long time ago.
Our previous study showed excellent prediction accuracy of cardiac nodularity due to H. pylori infection, with 0.928 of accuracy, 0.996 of specificity, 0.571 of sensitivity, 0.960 of positive predictive value, and 0.925 of negative predictive value[18]. In our previous study, we included only the patients who were evaluated for H pylori infection for the first time, and excluded patients with a history of eradication. However, the present cross-sectional study included the patients with a history of eradication also. The frequency of cardiac nodularity in H. pylori-positive patients was remarkably higher than that in H. pylori-negative patients and H. pylori-eradicated patients. Cardiac nodularity may serve as one of the predictive markers for active H. pylori infection.
Nodular gastritis is more frequent in children than in adults[29]. The prevalence of nodular gastritis has been reported to be 32.9%-85% in H. pylori-positive children[30-34]. The prevalence of nodular gastritis gradually decreased with age[13]. Our study also showed that the patients with cardiac nodularity were significantly younger than those without cardiac nodularity. Age dependence of cardiac nodularity is in line with that of antral nodularity.
Miyamoto et al[13] demonstrated that atrophy scores were lower in patients with nodular gastritis than in H. pylori-positive controls. Nakashima et al[35] also reported that atrophy and intestinal metaplasia were rare in nodular gastritis. Our study also showed that compared with patients without cardiac nodularity, Intestinal metaplasia score of the patients with cardiac nodularity was significantly lower. Cardiac nodularity seemed to disappear with the progression of intestinal metaplasia.
Dwivedi et al[36] reported that 87.5% of nodular gastritis patients showed complete normalization of the gastric mucosa after H. pylori eradication therapy. Our study also showed significantly low prevalence of cardiac nodularity in H. pylori-eradicated patients, especially in patients who underwent eradication a long time ago. Cardiac nodularity seemed to disappear with improvement in gastric inflammation after H. pylori eradication.
This study had some limitations. First, this study employed only a single experienced endoscopist. Second, the study was a retrospective review at a single institution. Our results should be validated in diverse settings for generalizability.
This report outlined the prevalence and clinical features of cardiac nodularity, and confirmed its close association with active H. pylori infection.
Helicobacter pylori (H. pylori)-associated nodular gastritis could occur in both the antrum and the cardia. Cardiac nodularity-like appearance is found more frequently than antral nodularity.
Previous study included only the patients who were evaluated for H. pylori infection for the first time. There still remains a lack of the prevalence and clinical features of cardiac nodularity-like appearance.
We aimed to evaluate the characteristics of cardiac nodularity-like appearance.
We enrolled consecutive patients who underwent esophagogastroduodenoscopy between May, 2017 and August, 2019 in the Toyoshima Endoscopy Clinic. We included H. pylori-negative, H. pylori-positive, and H. pylori-eradicated patients, and excluded patients with unclear H. pylori status and eradication failure. Cardiac nodularity was defined as a miliary nodular appearance or the presence of scattered whitish circular small colorations within 2 cm of the esophagogastric junction.
A total of 1078 patients were finally included. The prevalence of cardiac and antral nodularity in H. pylori-negative, -positive, and -eradicated patients were 0.14% and 0.14%, 54.5% and 29.5%, and 4.5% and 0.6%, respectively. Cardiac nodularity-like appearance was more frequent than antral nodularity in H. pylori-positive and -eradicated patients. Cardiac nodularity-like appearance was often found in younger patients and patients with less intestinal metaplasia. Cardiac nodularity-like appearance decreased after eradication, especially in patients who underwent eradication a long time ago.
This report outlines the prevalence and clinical features of cardiac nodularity-like appearance, and confirm its close association with active H. pylori infection.
Our results should be validated in diverse settings for generalizability.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ahmadi Hedayati M, Dinç T S-Editor: Gong ZM L-Editor: A P-Editor: Ma YJ
| 1. | Suzuki H, Nishizawa T, Tsugawa H, Mogami S, Hibi T. Roles of oxidative stress in stomach disorders. J Clin Biochem Nutr. 2012;50:35-39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 134] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 2. | Šterbenc A, Jarc E, Poljak M, Homan M. Helicobacter pylori virulence genes. World J Gastroenterol. 2019;25:4870-4884. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 72] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (2)] |
| 3. | Kubosawa Y, Mori H, Kinoshita S, Nakazato Y, Fujimoto A, Kikuchi M, Nishizawa T, Suzuki M, Suzuki H. Changes of gastric ulcer bleeding in the metropolitan area of Japan. World J Gastroenterol. 2019;25:6342-6353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Toyoshima O, Tanikawa C, Yamamoto R, Watanabe H, Yamashita H, Sakitani K, Yoshida S, Kubo M, Matsuo K, Ito H, Koike K, Seto Y, Matsuda K. Decrease in PSCA expression caused by Helicobacter pylori infection may promote progression to severe gastritis. Oncotarget. 2018;9:3936-3945. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Toyoshima O, Yamaji Y, Yoshida S, Matsumoto S, Yamashita H, Kanazawa T, Hata K. Endoscopic gastric atrophy is strongly associated with gastric cancer development after Helicobacter pylori eradication. Surg Endosc. 2017;31:2140-2148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 6. | Sugimoto M, Murata M, Yamaoka Y. Chemoprevention of gastric cancer development after Helicobacter pylori eradication therapy in an East Asian population: Meta-analysis. World J Gastroenterol. 2020;26:1820-1840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (1)] |
| 7. | Mori H, Suzuki H. Update on quinolone-containing rescue therapies for Helicobacter pylori infection. World J Gastroenterol. 2020;26:1733-1744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 8. | Nishizawa T, Suzuki H, Maekawa T, Harada N, Toyokawa T, Kuwai T, Ohara M, Suzuki T, Kawanishi M, Noguchi K, Yoshio T, Katsushima S, Tsuruta H, Masuda E, Tanaka M, Katayama S, Kawamura N, Nishizawa Y, Hibi T, Takahashi M. Dual therapy for third-line Helicobacter pylori eradication and urea breath test prediction. World J Gastroenterol. 2012;18:2735-2738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Nishizawa T, Maekawa T, Watanabe N, Harada N, Hosoda Y, Yoshinaga M, Yoshio T, Ohta H, Inoue S, Toyokawa T, Yamashita H, Saito H, Kuwai T, Katayama S, Masuda E, Miyabayashi H, Kimura T, Nishizawa Y, Takahashi M, Suzuki H. Clarithromycin Versus Metronidazole as First-line Helicobacter pylori Eradication: A Multicenter, Prospective, Randomized Controlled Study in Japan. J Clin Gastroenterol. 2015;49:468-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | de Brito BB, da Silva FAF, Soares AS, Pereira VA, Santos MLC, Sampaio MM, Neves PHM, de Melo FF. Pathogenesis and clinical management of Helicobacter pylori gastric infection. World J Gastroenterol. 2019;25:5578-5589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 198] [Cited by in RCA: 167] [Article Influence: 27.8] [Reference Citation Analysis (15)] |
| 11. | Suzuki H, Nishizawa T, Tsugawa H, Hibi T. Molecular approaches and modern clinical strategies for the management of Helicobacter pylori infection in Japan. Keio J Med. 2012;61:109-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Sakitani K, Nishizawa T, Arita M, Yoshida S, Kataoka Y, Ohki D, Yamashita H, Isomura Y, Toyoshima A, Watanabe H, Iizuka T, Saito Y, Fujisaki J, Yahagi N, Koike K, Toyoshima O. Early detection of gastric cancer after Helicobacter pylori eradication due to endoscopic surveillance. Helicobacter. 2018;23:e12503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Miyamoto M, Haruma K, Yoshihara M, Hiyama T, Sumioka M, Nishisaka T, Tanaka S, Chayama K. Nodular gastritis in adults is caused by Helicobacter pylori infection. Dig Dis Sci. 2003;48:968-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 82] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Hayashi S, Imamura J, Kimura K, Saeki S, Hishima T. Endoscopic features of lymphoid follicles in Helicobacter pylori-associated chronic gastritis. Dig Endosc. 2015;27:53-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Nishikawa I, Kato J, Terasoma S, Matsutani H, Tamaki H, Tamaki T, Kuwashima F, Nakata H, Tomeki T, Matsunaka H, Ibata Y, Yamashita Y, Maekita T, Higashi K, Ichinose M. Nodular gastritis in association with gastric cancer development before and after Helicobacter pylori eradication. JGH Open. 2018;2:80-86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | Zerbib F, Vialette G, Cayla R, Rudelli A, Sauvet P, Bechade D, Seurat PL, Lamouliatte H. [Follicular gastritis in adults. Relations with Helicobacter pylori, histological and endoscopic aspects]. Gastroenterol Clin Biol. 1993;17:529-534. [PubMed] |
| 17. | Kitamura S, Yasuda M, Muguruma N, Okamoto K, Takeuchi H, Bando Y, Miyamoto H, Okahisa T, Yano M, Torisu R, Takayama T. Prevalence and characteristics of nodular gastritis in Japanese elderly. J Gastroenterol Hepatol. 2013;28:1154-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Toyoshima O, Nishizawa T, Sakitani K, Yamakawa T, Watanabe H, Yoshida S, Nakai Y, Hata K, Ebinuma H, Suzuki H, Koike K. Nodularity-like appearance in the cardia: novel endoscopic findings for Helicobacter pylori infection. Endosc Int Open. 2020;8:E770-E774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Nishizawa T, Suzuki H, Arano T, Yoshida S, Yamashita H, Hata K, Kanai T, Yahagi N, Toyoshima O. Characteristics of gastric cancer detected within 1 year after successful eradication of Helicobacter pylori. J Clin Biochem Nutr. 2016;59:226-230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Nishizawa T, Sakitani K, Suzuki H, Yamakawa T, Takahashi Y, Yoshida S, Nakai Y, Hata K, Ebinuma H, Koike K, Toyoshima O. Small-caliber endoscopes are more fragile than conventional endoscopes. Endosc Int Open. 2019;7:E1729-E1732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Nishizawa T, Suzuki H, Arita M, Kataoka Y, Fukagawa K, Ohki D, Hata K, Uraoka T, Kanai T, Yahagi N, Toyoshima O. Pethidine dose and female sex as risk factors for nausea after esophagogastroduodenoscopy. J Clin Biochem Nutr. 2018;63:230-232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Toyoshima O, Yoshida S, Nishizawa T, Yamakawa T, Sakitani K, Hata K, Takahashi Y, Fujishiro M, Watanabe H, Koike K. CF290 for pancolonic chromoendoscopy improved sessile serrated polyp detection and procedure time: a propensity score-matching study. Endosc Int Open. 2019;7:E987-E993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Nishizawa T, Sakitani K, Suzuki H, Takeuchi M, Takahashi Y, Takeuchi K, Yamakawa T, Yoshida S, Hata K, Ebinuma H, Koike K, Toyoshima O. Adverse events associated with bidirectional endoscopy with midazolam and pethidine. J Clin Biochem Nutr. 2020;66:78-81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Toyoshima O, Nishizawa T, Koike K. Endoscopic Kyoto classification of Helicobacter pylori infection and gastric cancer risk diagnosis. World J Gastroenterol. 2020;26:466-477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 101] [Cited by in RCA: 79] [Article Influence: 15.8] [Reference Citation Analysis (2)] |
| 25. | Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy. 1969;3:87-97. [RCA] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 743] [Article Influence: 43.7] [Reference Citation Analysis (3)] |
| 26. | Toyoshima O, Nishizawa T, Sakitani K, Yamakawa T, Takahashi Y, Yamamichi N, Hata K, Seto Y, Koike K, Watanabe H, Suzuki H. Serum anti-Helicobacter pylori antibody titer and its association with gastric nodularity, atrophy, and age: A cross-sectional study. World J Gastroenterol. 2018;24:4061-4068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 27. | Toyoshima O, Nishizawa T, Arita M, Kataoka Y, Sakitani K, Yoshida S, Yamashita H, Hata K, Watanabe H, Suzuki H. Helicobacter pylori infection in subjects negative for high titer serum antibody. World J Gastroenterol. 2018;24:1419-1428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (1)] |
| 28. | Nishizawa T, Sakitani K, Suzuki H, Yamakawa T, Takahashi Y, Yamamichi N, Watanabe H, Seto Y, Koike K, Toyoshima O. A combination of serum anti-Helicobacter pylori antibody titer and Kyoto classification score could provide a more accurate diagnosis of H pylori. United European Gastroenterol J. 2019;7:343-348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | Al-Enezi SA, Alsurayei SA, Aly NY, Ismail AE, Ismail WA, Al-Brahim N, El-Dousari A. Endoscopic nodular gastritis in dyspeptic adults: prevalence and association with Helicobacter pylori infection. Med Princ Pract. 2010;19:40-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Bujanover Y, Konikoff F, Baratz M. Nodular gastritis and Helicobacter pylori. J Pediatr Gastroenterol Nutr. 1990;11:41-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 68] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 31. | Mitchell HM, Bohane TD, Tobias V, Bullpitt P, Daskalopoulos G, Carrick J, Mitchell JD, Lee A. Helicobacter pylori infection in children: potential clues to pathogenesis. J Pediatr Gastroenterol Nutr. 1993;16:120-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 86] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Shiotani A, Kamada T, Kumamoto M, Nakae Y, Nakamura Y, Kakudo K, Haruma K. Nodular gastritis in Japanese young adults: endoscopic and histological observations. J Gastroenterol. 2007;42:610-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 33. | Prieto G, Polanco I, Larrauri J, Rota L, Lama R, Carrasco S. Helicobacter pylori infection in children: clinical, endoscopic, and histologic correlations. J Pediatr Gastroenterol Nutr. 1992;14:420-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 76] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Luzza F, Pensabene L, Imeneo M, Mancuso M, Giancotti L, La Vecchia AM, Costa MC, Strisciuglio P, Pallone F. Antral nodularity and positive CagA serology are distinct and relevant markers of severe gastric inflammation in children with Helicobacter pylori infection. Helicobacter. 2002;7:46-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 35. | Nakashima R, Nagata N, Watanabe K, Kobayakawa M, Sakurai T, Akiyama J, Hoshimoto K, Shimbo T, Uemura N. Histological features of nodular gastritis and its endoscopic classification. J Dig Dis. 2011;12:436-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Dwivedi M, Misra SP, Misra V. Nodular gastritis in adults: clinical features, endoscopic appearance, histopathological features, and response to therapy. J Gastroenterol Hepatol. 2008;23:943-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |