Published online Sep 21, 2020. doi: 10.3748/wjg.v26.i35.5302
Peer-review started: March 24, 2020
First decision: April 25, 2020
Revised: June 23, 2020
Accepted: August 29, 2020
Article in press: August 29, 2020
Published online: September 21, 2020
Processing time: 176 Days and 15.6 Hours
The incidence of peptic ulcer disease has decreased during the last few decades, but the incidence of reported peptic ulcer complications has not decreased. Perforating peptic ulcer (PPU) is a severe form of the disease.
To assess trends in the incidence, presentation, and outcome of PPU over a period of 40 years.
This was a single-centre, retrospective, cohort study of all patients admitted to Levanger Hospital, Norway, with PPU from 1978 to 2017. The patients were identified in the Patient Administrative System of the hospital using International Classification of Diseases (ICD), revision 8, ICD-9, and ICD-10 codes for perforated gastric and duodenal ulcers. We reviewed the medical records of the patients to retrieve data. Vital statistics were available for all patients. The incidence of PPU was analysed using Poisson regression with perforated ulcer as the dependent variable, and sex, age, and calendar year from 1978 to 2017 as covariates. Relative survival analysis was performed to compare long-term survival over the four decades.
Two hundred and nine patients were evaluated, including 113 (54%) men. Forty-six (22%) patients were older than 80 years. Median age increased from the first to the last decade (from 63 to 72 years). The incidence rate increased with increasing age, but we measured a decline in recent decades for both sexes. A significant increase in the use of acetylsalicylic acid, from 5% (2/38) to 18% (8/45), was observed during the study period. Comorbidity increased significantly over the 40 years of the study, with 22% (10/45) of the patients having an American Society of Anaesthesiologists (ASA) score 4-5 in the last decade, compared to 5% (2/38) in the first decade. Thirty-nine percent (81/209) of the patients had one or more postoperative complications. Both 100-day mortality and long-term survival were associated with ASA score, without significant variations between the decades.
Declining incidence rates occurred in recent years, but the patients were older and had more comorbidity. The ASA score was associated with both short-term mortality and long-term survival.
Core Tip: We sought to review the epidemiology of perforated peptic ulcer in a stable population at a primary hospital over a period of 40 years. The incidence rate has declined in recent decades for both sexes, though median age and comorbidity have both increased. Complications occurred more frequently and were more serious in recent decades, in older patients, in patients with comorbidities, and in patients with higher American Society of Anaesthesiologists (ASA) scores. Both short- and long-term survival were associated with ASA score, without significant variation between the decades.
- Citation: Dadfar A, Edna TH. Epidemiology of perforating peptic ulcer: A population-based retrospective study over 40 years. World J Gastroenterol 2020; 26(35): 5302-5313
- URL: https://www.wjgnet.com/1007-9327/full/v26/i35/5302.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i35.5302
The incidence of peptic ulcer disease (PUD), either gastric or duodenal, has decreased during the last few decades with the discovery of the role of Helicobacter pylori (H. pylori)[1-5]. However, the incidence of peptic ulcer complications has not decreased in the same manner[6,7]. Bleeding and perforation are the most severe complications of PUD[8]. Due to progress in endoscopic and interventional radiological techniques, bleeding is mostly considered a medical emergency and outcomes have improved[9]. Although bleeding is far more common than perforated peptic ulcer (PPU), perforation accounts for most deaths associated with PUD[6,10,11] and PPU remains a surgical emergency, with high short-term mortality of 10%-30%[12-14].
Surgical repair with closure of the perforation, with or without an omental pedicle, is the preferred treatment for PPU[11,15,16]. This repair can be achieved through either open repair or laparoscopy[15,17]. Previous studies have shown a change in the demography of PPU over the last few decades, with an increasing age at diagnosis in recent years[15,18]. Less is known about the implications of increased age in patients with PPU in regards to treatment, complications, and mortality[12,13,15,19].
Thus, the aim of this study was to investigate changes in demography and the effect on treatment, complications, and short- and long-term mortality in patients admitted to our hospital with PPU over four decades.
Levanger Hospital is located in Middle Norway, with a catchment area of 85000 at the start of the study period and 100000 in recent years. This retrospective study included all patients diagnosed with benign PPU between January 1978 and December 2017. The patients were identified in the Patient Administrative System using International Classification of Diseases (ICD), ICD-8 codes (531.00-531.09, 532.00, 533.00, 534.00), ICD-9 codes (531.1-531.2, 531.5-531.6, 532- 533 with same decimals as for 531), and ICD-10 codes (K25.1-K25.2, K25.5-K25.6, K26-K28 with same decimals as for K25). Additional searches were done for the surgical codes for gastroraphy and duodenoraphy. Demographic and clinical data were collected from the hospital records.
The American Society of Anaesthesiologists (ASA) score was used to compare preoperative comorbidity[20], which was further classified using the Charlson Comorbidity Index[21]. Complications were classified according to the Clavien-Dindo classification[22,23]: Grade I, any small deviation from the normal postoperative course treated at bedside or with certain drugs (e.g., antiemetics); grade II, complications treated with transfusion or medicines other than allowed for grade 1 (e.g., antibiotics); grade III, complications requiring endoscopic, radiological, or surgical intervention; grade IV, life-threatening complications; grade V, complications leading to death of the patient.
The incidence of PPU was defined as the number of new cases of PPU in the defined population within 1 year. The incidence rate (IR) was defined as the incidence divided by the total person-time at risk during the same year. The incidence rate ratio (IRR) was defined as the ratio between two incidence rates.
Ulcer localisation was considered gastric when present anywhere in the stomach, including pyloric ulcers. Localisation distal to the pylorus was categorised as duodenal.
The study was approved by the Regional Ethics Committee (REK Midt # 2018/1510). We also performed a data protection impact assessment in accordance with the European General Data Protection Regulation before collecting data[24].
The medians of two samples, such as age in men compared to women, were compared by the Wilcoxon rank sum test. The Cochran-Armitage test was used to test for trends in proportions. The Joncheere-Terpstra test was used to test for the distribution of age as a dependent variable across decade groups as an independent variable. Ordinal logistic regression was used to test associations in doubly ordered r × c tables, such as for the Charlson Comorbidity Index and ASA score by decades. Logistic regression analysis was used to test for an association between 100-day mortality as a dependent variable and sex, age, year of admittance, Charlson Comorbidity Index, and ASA score as independent variables.
The incidence of PPU was analysed using Poisson regression with perforated ulcer as the dependent variable and sex, age in 5-year intervals (20-24, 25-29, up to 90-94, 95-99), and calendar year from 1978 to 2017 as covariates. Non-linear relationships were explored using fractional polynomials[25]. Fractional polynomials are a method for checking if the effect of an explanatory variable is linear, as in the basic Poisson regression model.
The age and sex distribution for the 10 municipalities around Levanger Hospital for every year from 1980 to 2016 was obtained from Statistics Norway. To study long-term survival in this patient population over 40 years, we performed the relative survival analysis using the Ederer II method[26,27]. Multivariable analyses were performed using the full likelihood approach. Survival probabilities by sex and age for the Norwegian population for every year from 1978 were downloaded from the Human Mortality Database[28].
Two-sided P values < 0.05 were considered significant. Medians were reported with the range (minimum to maximum) and standard deviation (SD), as well as 95% confidence intervals (CIs), as appropriate. Analyses were carried out in Stata 16 (Stata Corp LP, College Station, Texas, United States), IBM SPSS Statistics 25 (SPSS Inc., Chicago, IL, United States), and StatXact 9 (1050 Winter St, Waltham, MA, United States).
Over 40 years, 209 patients with PPU were treated, including 113 (54%) men and 46 (22%) patients older than 80 years. In the first two decades of the observation period, PPU occurred more frequently among men than women (ratio 3:2). In the last two decades, this has evened out to nearly 1:1. Eighty-five percent of the patients presented within 24 h after the onset of pain. Only 7 patients (3.3%) were admitted with a systolic blood pressure < 90 mmHg.
Trends in patient characteristics according to decade of treatment are shown in Table 1. The median age increased from 63 to 72 years from the first 10 years to the last 10 years of study (P = 0.018). The mean time from debut of symptoms until hospital admission increased from 7 (SD 9) h in the first 10 years to 13 (SD 14) h in the last 10 years (P = 0.019).
| n = 209 | 1978–1987 (n = 38) | 1988–1997 (n = 64) | 1998–2007 (n = 62) | 2008-2017 (n = 45) | P value |
| Sex | |||||
| Women | 14 (36.8) | 26 (40.6) | 31 (50.0) | 25 (55.6) | 0.049a |
| Men | 24 (63.2) | 38 (59.4) | 31 (50.0) | 20 (44.4) | |
| Incidence (No./100000) | |||||
| Women | 3.3 (1.8 to 5.5) | 6.0 (3.9 to 8.8) | 7.0 (4.8 to 9.9) | 5.3 (3.4 to 7.8) | 0.14b |
| Men | 5.5 (3.6 to 8.3) | 8.7 (6.2 to 12.0) | 7.0 (4.8 to 10.0) | 4.2 (2.6 to 6.5) | 0.32b |
| Age, mean ± SD, years | 62 ± 17 | 64 ± 16 | 67 ± 16 | 69 ± 17 | 0.018c |
| Hours from symptom debut until admission, mean ± SD) | 7 ± 9 | 8 ± 12 | 16 ± 29 | 13 ± 14 | 0.019c |
| ASA class | |||||
| II | 30 (78.9) | 46 (71.9) | 33 (53.2) | 24 (53.3) | 0.001d |
| III | 6 (15.8) | 13 (20.3) | 19 (30.6) | 11 (24.2) | |
| IV | 2 (5.3) | 5 (7.8) | 10 (16.1) | 9 (20.0) | |
| V | 0 | 0 | 0 | 1 (2.2) | |
| Ulcer localisation | |||||
| Gastric | 25 (65.8) | 27 (42.2) | 26 (41.9) | 19 (42.2) | 0.059a |
| Duodenal | 13 (34.2) | 37 (57.8) | 36 (58.1) | 26 (57.8) | |
| Past ulcer history | 5 (13.2) | 25 (39.1) | 16 (26.2) | 0 | 0.022a |
| Smoker at present | 19 (57.6) | 40 (64.5) | 27 (49.1) | 26 (59.1) | 0.89a |
| NSAID use | 4 (10.5) | 11 (17.2) | 19 (31.1) | 8 (17.8) | 0.18a |
| Steroid use | 2 (5.3) | 2 (3.1) | 7 (11.5) | 3 (6.7) | 0.42a |
| Salicylate use | 2 (5.3) | 4 (6.3) | 10 (16.4) | 8 (17.8) | 0.025a |
| Charlson Comorbidity index | |||||
| 0 | 26 (68.4) | 37 (57.8) | 29 (46.8) | 18 (40.0) | 0.003d |
| 1 | 5 (13.2) | 22 (34.4) | 24 (38.7) | 13 (28.9) | |
| 2+ | 7 (18.4) | 5 (7.8) | 9 (14.5) | 14 (31.1) |
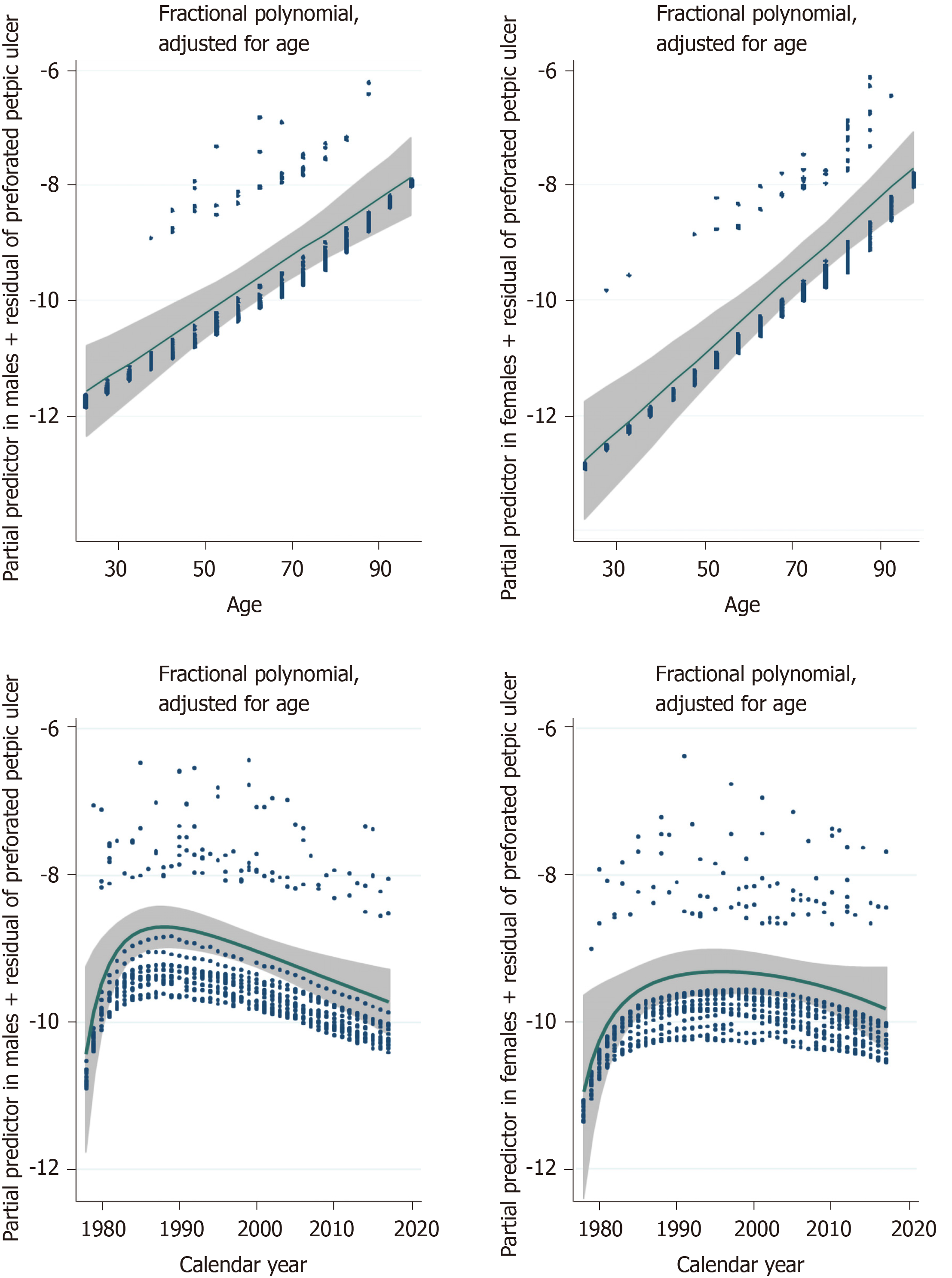
The IR varied between 3.3-5.3 and 4.2-8.7 per 100000/year for women and men, respectively, throughout the observation period. The IR increased with increasing age, without an upper limit (see Figure 1), until the second decade of the study period in men and to the third decade of the study period in women. Recent years have shown a declining tendency in both sexes (see Figure 1). Adjusted IRRs obtained from Poisson regression with calendar year and age as covariates are shown in Table 2. The IR increased significantly with age for both gastric and duodenal PPUs.
| Male | P value | Female | P value | |
| IRR (CI) | IRR (CI) | |||
| Total peptic ulcer perforation | ||||
| Calendar year | 0.986 (0.970 to 1.001) | 0.074 | 1.005 (0.988 to 1.023) | 0.55 |
| Age (per 5 yr) | 1.040 (1.029 to 1.051) | < 0.001 | 1.060 (1.047 to 1.073) | < 0.001 |
| Gastric ulcer perforation | ||||
| Calendar year | 0.979 (0.956 to 1.001) | 0.063 | 0.998 (0.973 to 1.024) | 0.90 |
| Age (per 5 yr) | 1.037 (1.022 to 1.053) | < 0.001 | 1.056 (1.038 to 1.075) | < 0.001 |
| Duodenal ulcer perforation | ||||
| Calendar year | 0.992 (0.971 to 1.014) | 0.49 | 1.011 (0.988 to 1.035) | 0.36 |
| Age (per 5 yr) | 1.043 (1.028 to 1.058) | < 0.001 | 1.063 (1.045 to 1.080) | < 0.001 |
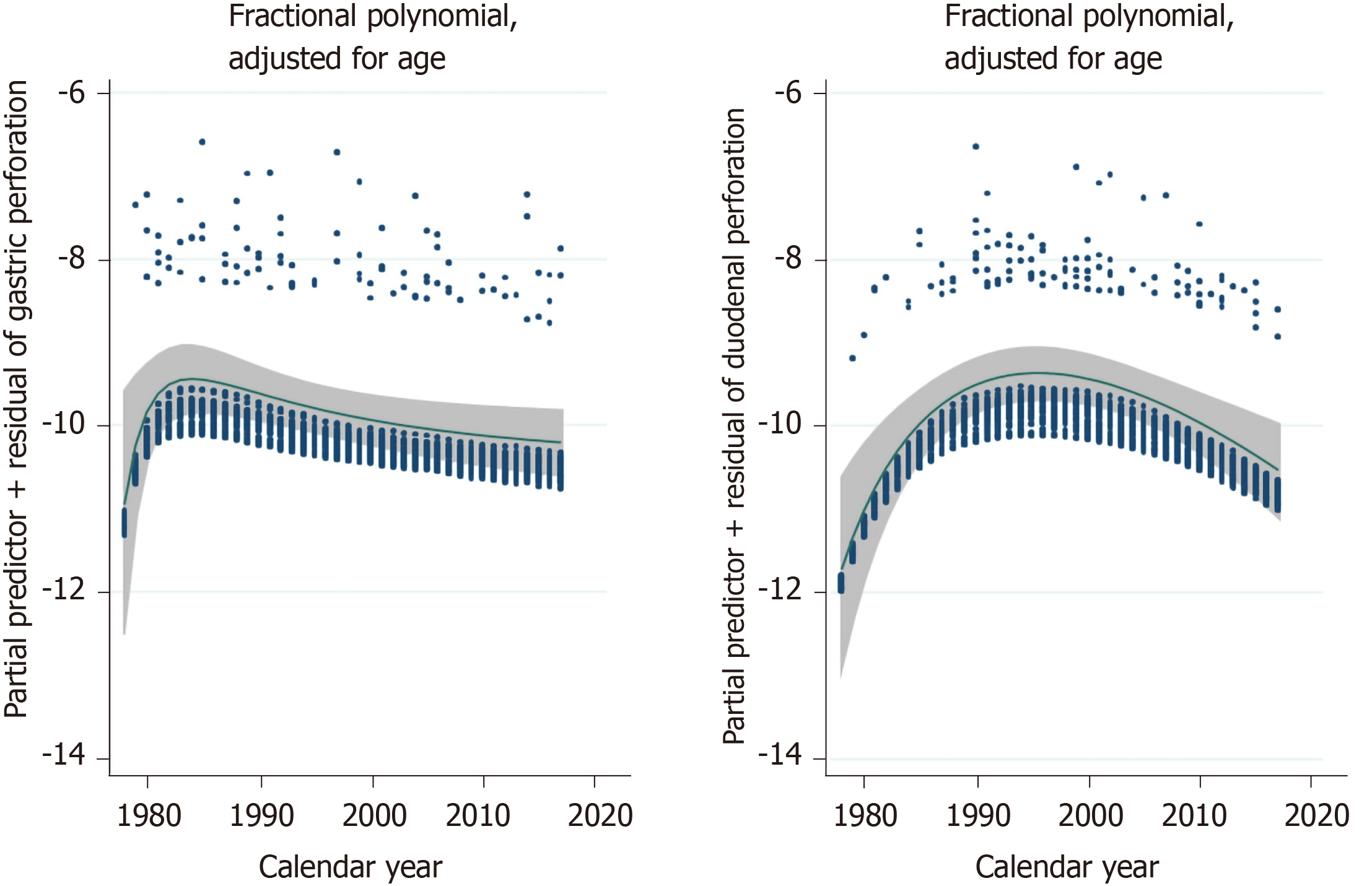
Figure 2 shows that the incidence of gastric ulcer perforations peaked around 1984, whereas duodenal ulcer perforations peaked approximately 15 years later.
The use of acetylsalicylic acid increased significantly over 40 years, from 5% (2/38) the first 10 years, to 18% (8/45) the last 10 years (Table 1). The proportion of patients with PPU who used non-steroidal anti-inflammatory drugs (NSAIDs) or smoked did not change significantly throughout the course of the study.
The Charlson Comorbidity Index and ASA score increased significantly in patients with PPU over 40 years (Table 1). In 1978-1987, 5% (2/38) of the patients had an ASA score 4-5, increasing to 22% (10/45) during 2008-2017.
Two hundred and six patients had open surgery; 201 with suture and omental patch, 5 with resections. Three patients were not operated on; one was 90 years old and about to die at admission. Another 90-year-old was deemed too sick to tolerate narcosis and operation. The third patient was 70 years old, multimorbid, and had previously undergone difficult operations involving the upper abdomen. He had localised peritonitis and was treated conservatively with nasogastric suction, intravenous drip, antibiotics, and close clinical supervision. He survived. All three were admitted in the last decade.
The median time from debut of symptoms to operation increased significantly (P = 0.004), from 8 h in the first decade to 17 h in the last decade. The median time from admittance to hospital to operation and the duration of the operation and hospital stay were stable through all four decades (see Table 3).
| n = 209 | 1978–1987 (n = 38) | 1988–1997 (n = 64) | 1998–2007 (n = 62) | 2008-2017 (n = 45) | P value |
| Treatment | |||||
| Simple closure with orwithout omentopexy | 37 (97) | 64 (100) | 62 (100) | 38 (84) | |
| Gastric resection | 1 (3) | 0 | 0 | 4 (9) | |
| No operation | 0 | 0 | 0 | 3 (7) | |
| Hours from admission to operation, mean ± SD | 7 ± 9 | 8 ± 12 | 16 ± 29 | 13 ± 14 | 0.019a |
| Duration of operation, mean ± SD, min | 72 ± 29 | 78 ± 35 | 61 ± 24 | 78 ± 40 | 0.15a |
| Re-operation | 1 (3) | 4 (6) | 6 (10) | 4 (9) | 0.18b |
| Clavien-Dindo classification of complications | |||||
| 0 | 28 (74) | 45 (70) | 38 (61) | 17 (38) | 0.001c |
| 1-2 | 6 (16) | 8 (13) | 9 (15) | 13 (29) | |
| 3 | 1 (3) | 1 (29) | 4 (7) | 4 (9) | |
| 4 | 0 | 4 (6) | 3 (5) | 3 (7) | |
| 5 | 3 (8) | 6 (9) | 8 (13) | 8 (18) | |
| 100-day mortality | 7 (18) | 11 (17) | 16 (26) | 9 (20) | 0.56b |
| Estimated 10-yr relative survival in patients surviving 100 d, (95%CI) | 97 (70-114) | 71 (52-87) | 86 (64-103) | 86 (51-108) | 0.44d |
One or more complications occurred in 39% of patients (81/209). The two most common complications were pneumonia (n = 18, 8.6%) and wound infections (n = 12, 5.7%). Reoperation was performed in 14 (6.8%) patients for wound dehiscence (n = 7, 3.4%), postoperative leak (n = 4, 1.9%), intestinal obstruction (n = 1), severe bleeding from duodenal ulcer (n = 1), and drainage of subphrenic and pelvic abscesses (n = 1). The Clavien-Dindo classification of complications is shown in Table 3. Complications occurred more frequently and were more serious in recent decades, in older patients, and in patients with comorbidities and higher ASA scores.
The 100-day mortality was 20.6% (43/209) without significant variations between decades. Based on ASA score, the 100-day mortality was 6% in patients with ASA score 2, 39% with ASA score 3, and 59% with ASA score 4-5. We performed a multivariable, logistic regression analysis of 100-day mortality as a dependent variable and sex, age, year of admittance, Charlson Comorbidity Index, and ASA score as independent variables. Only ASA score was significantly associated with 100-day mortality [odds ratio (OR) = 12.5; 95%CI: 3.5-41.8 for ASA score 3 and OR = 31.2 (7.4-132) for ASA score 4-5].
The overall estimated 5-year relative survival was 95% (95%CI: 86-101) with ASA score 2, 56% (95%CI: 37-74) with ASA score 3, and 12% (95%CI: 2-35) with ASA score 4-5.
In those who survived the first 100 days, the estimated 5-year relative survival was 98% (95%CI: 89-104) for ASA score 2, 84% (95%CI: 58-102) for ASA score 3, and 26% (95%CI: 3-63) for ASA score 4 (see Figure 3).
This study demonstrated a trend of increasing age and comorbidity in patients admitted for PPU over 40 years. Complications were more common in recent decades. However, we found no significant variations between short-term mortality between the decennia. Comorbidity measured bythrough ASA score was a good prognostic factor regarding short-term mortality and long-term survival.
The median age of patients with PPU increased with each decade. In the last decade, more than half of the patients with PPU were > 70 years old. Women constituted a greater share of the patients with PPU with time, surpassing men in the last decade.
The incidence rates for PPU in our population were similar to previous studies, which reported an incidence of 4-15 per 100000/year[4,7,12]. The IR tended to decline during the last half of the observation period, and this tendency occurred almost one decade earlier for men than women. Over the same period, the IR was similar between the sexes. The Poisson regression with fractional polynomials indicated an increase in IR with increasing age for both sexes.
These epidemiological findings are in agreement with existing data showing a declining trend in PPU, equal gender distribution, and more frequent occurrence among the elderly[4,15,29,30]. These changes in the epidemiology of PPU may have numerous explanations. The identification and treatment of H. pylori as a cause of PUD is considered the main cause of reduced PPU incidence, especially in younger age groups[1,15]. The introduction of proton pump inhibitors is also postulated to be related to a reduced IR for PPU[4]. A shift in the occurrence of predisposing factors may also have contributed to these changes.
In addition to H. pylori infection, use of NSAIDs, corticosteroids, smoking, and previous history of PUD are known risk factors for PPU[31,32]. In our study, we found no significant change in trends regarding the use of NSAIDs, corticosteroids, or smoking habits.
Acetylsalicylic acid is also technically considered an NSAID due to similar mechanisms of action[32,33]. It also has a similar profile regarding adverse events and is associated with an increased risk of PPU[32]. However, acetylsalicylic acid has a completely different area of use than other NSAIDs, mostly in secondary prevention of thrombotic cardiovascular events[33]. The significant increase in the use of acetylsalicylic acid through the study period may contribute to the increased IR with increasing age and the elderly being more prone to adverse effects[15,34].
The patients admitted with PPU had increasing comorbidity in recent decades according to the Charlson Comorbidity Index and ASA class. Increased comorbidity is associated with increased age[35]. Previous studies have also shown an association between comorbidity, complications, and mortality after PPU[14]. This is thought to be related to delayed admittance, diagnosis, and treatment[15].
The surgical treatment for PPU in our cohort barely changed over four decades, with simple closure with or without omentopexy being the procedure of choice in 98% (201/206) of patients. Only one of the patients were treated with a laparoscopic technique. The length of the operation was stable over all decades, and this could be explained with little variation in surgical access and method.
Three patients in the cohort were treated without an operation, all in the last decade, two of whom died within a short timeframe. This could reflect more frequent “failure-to-rescue” in recent years, especially in elderly patients with severe comorbidity[29,36].
The increase in time from the debut of symptoms to hospital admission in recent decades may be related to the previously described epidemiological shift in the age of patients with PPU[4,14,30]. Elderly patients with comorbidity are less likely to present with peritonitis[29].
Post-operative complications occurred more frequently in recent decades. Increasing age and greater comorbidity in the patients treated for PPU could explain the increase in serious complications (Clavien-Dindo grade 4 and 5)[37]. We found no significant change in the frequency of reoperation. We also found a substantial increase in the frequency of grade 1-2 complications. This does not necessarily reflect an actual increase in low-grade complications, but may be related to a change in doctors’ habits towards more frequent and detailed descriptions in documentation[38]. The accuracy of the collected data is limited to the amount of detail in the patient’s journal. This represents a limitation of the retrospective study design.
Short-term mortality measured 100 d post-operatively was stable through all four decades and in concordance with existing data. The short-term mortality was associated with ASA score, supporting the understanding that patient comorbidity affects mortality. A similar association with degree of comorbidity expressed through ASA score was seen regarding long-term survival, measured as 5-year relative survival. This supports previous data suggesting that ASA score can be used as a prognostic factor regarding both short- and long-term survival[39-41].
The study had some limitations. The retrospective design has weaknesses. The quality of the database was dependent on the quality of the different patient records. Grade 1 Clavien-Dindo complications were often not documented in the patient records. More severe complications were regularly documented, and we expect very few missing grade II to V complications in the database.
In conclusion, this study confirmed that the IRR of PPU increased with increasing age, without an upper limit. The IRR increased until the second decade of the study period in men and the third decade in women. Recent years indicate a declining tendency in both sexes. In recent decades, patients were older and had more comorbidity. Post-operative complications increased over the 40 years of the study. ASA score was associated with both short-term mortality and long-term survival.
The results of this study would have external validity for populations similar to the Norwegian population.
The incidence of peptic ulcer disease (PUD) has decreased during the last few decades. However, complicated PUD has not decreased likewise. Perforation is the complication that accounts for most deaths associated with PUD, and it remains a surgical emergency. Perforated peptic ulcer (PPU) has a high short-term mortality.
With the discovery of the role of Helicobacter pylori in PUD, it is important to investigate trends and changes in demography in patients with PPU. This will provide more precise characteristics regarding these patients, which in turn might contribute towards more rapid diagnostics and treatment.
The aim of this study was to investigate changes in demography and the effect on treatment, complications, and short- and long-term mortality in patients admitted to our hospital with PPU over four decades.
All patients who were admitted to our hospital with PPU from 1978-2017 were retrospectively identified and included. We retrieved their medical records and reviewed them to obtain data concerning patient characteristics, treatment and complications.
The median age increased from 63 to 72 years from the first to the last decade. The incidence rate increased with increasing age, although we observed a decline in incidence rate in recent decades. Comorbidity increased significantly over the 40 years of the study. The median time from debut of symptoms to operation increased from 8 to 17 h from the first to the last decade. One or more complications occurred in 39 %. Both short- and long-term mortality were associated with American Society of Anaesthesiologists (ASA) score.
Declining incidence rates occurred in recent years, but the patients were older and had more comorbidity. The ASA score was associated with both short-term mortality and long-term survival.
This study has shown a demographic shift among patients with PPU. Future research should assess a better understanding of the association of increasing age, comorbidity and other risk factors with PPU. Clinical trials might serve to reduce the high number of complications in these patients.
We thank Stian Lydersen, Professor of Medical Statistics at NTNU, for advice on statistical methods and comments on the manuscript. We want to thank the clinicians and other employees at Nord-Trøndelag Hospital Trust for their support and for contributing to the data collection in this research project.
Manuscript source: Unsolicited manuscript
Specialty type: Surgery
Country/Territory of origin: Norway
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zhu Y S-Editor: Liu M L-Editor: A P-Editor: Ma YJ
| 1. | Oderda G, Forni M, Dell'Olio D, Ansaldi N. Cure of peptic ulcer associated with eradication of Helicobacter pylori. Lancet. 1990;335:1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Eisner F, Hermann D, Bajaeifer K, Glatzle J, Königsrainer A, Küper MA. Gastric Ulcer Complications after the Introduction of Proton Pump Inhibitors into Clinical Routine: 20-Year Experience. Visc Med. 2017;33:221-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (36)] |
| 3. | Dutta AK, Chacko A, Balekuduru A, Sahu MK, Gangadharan SK. Time trends in epidemiology of peptic ulcer disease in India over two decades. Indian J Gastroenterol. 2012;31:111-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Hermansson M, Ekedahl A, Ranstam J, Zilling T. Decreasing incidence of peptic ulcer complications after the introduction of the proton pump inhibitors, a study of the Swedish population from 1974-2002. BMC Gastroenterol. 2009;9:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Lassen A, Hallas J, Schaffalitzky de Muckadell OB. Complicated and uncomplicated peptic ulcers in a Danish county 1993-2002: a population-based cohort study. Am J Gastroenterol. 2006;101:945-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 137] [Article Influence: 7.2] [Reference Citation Analysis (1)] |
| 6. | Lau JY, Sung J, Hill C, Henderson C, Howden CW, Metz DC. Systematic review of the epidemiology of complicated peptic ulcer disease: incidence, recurrence, risk factors and mortality. Digestion. 2011;84:102-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 313] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 7. | Thorsen K, Søreide JA, Kvaløy JT, Glomsaker T, Søreide K. Epidemiology of perforated peptic ulcer: age- and gender-adjusted analysis of incidence and mortality. World J Gastroenterol. 2013;19:347-354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 139] [Cited by in RCA: 134] [Article Influence: 11.2] [Reference Citation Analysis (4)] |
| 8. | Malfertheiner P, Chan FK, McColl KE. Peptic ulcer disease. Lancet. 2009;374:1449-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 618] [Cited by in RCA: 534] [Article Influence: 33.4] [Reference Citation Analysis (1)] |
| 9. | Lu Y, Loffroy R, Lau JY, Barkun A. Multidisciplinary management strategies for acute non-variceal upper gastrointestinal bleeding. Br J Surg. 2014;101:e34-e50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Søreide K, Thorsen K, Søreide JA. Strategies to improve the outcome of emergency surgery for perforated peptic ulcer. Br J Surg. 2014;101:e51-e64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 106] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 11. | Tarasconi A, Coccolini F, Biffl WL, Tomasoni M, Ansaloni L, Picetti E, Molfino S, Shelat V, Cimbanassi S, Weber DG, Abu-Zidan FM, Campanile FC, Di Saverio S, Baiocchi GL, Casella C, Kelly MD, Kirkpatrick AW, Leppaniemi A, Moore EE, Peitzman A, Fraga GP, Ceresoli M, Maier RV, Wani I, Pattonieri V, Perrone G, Velmahos G, Sugrue M, Sartelli M, Kluger Y, Catena F. Perforated and bleeding peptic ulcer: WSES guidelines. World J Emerg Surg. 2020;15:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 154] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 12. | Svanes C. Trends in perforated peptic ulcer: incidence, etiology, treatment, and prognosis. World J Surg. 2000;24:277-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 153] [Article Influence: 6.1] [Reference Citation Analysis (1)] |
| 13. | Møller MH, Vester-Andersen M, Thomsen RW. Long-term mortality following peptic ulcer perforation in the PULP trial. A nationwide follow-up study. Scand J Gastroenterol. 2013;48:168-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Thorsen K, Glomsaker TB, von Meer A, Søreide K, Søreide JA. Trends in diagnosis and surgical management of patients with perforated peptic ulcer. J Gastrointest Surg. 2011;15:1329-1335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 15. | Søreide K, Thorsen K, Harrison EM, Bingener J, Møller MH, Ohene-Yeboah M, Søreide JA. Perforated peptic ulcer. Lancet. 2015;386:1288-1298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 196] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 16. | Byrne BE, Bassett M, Rogers CA, Anderson ID, Beckingham I, Blazeby JM; Association of Upper Gastrointestinal Surgeons for the National Emergency Laparotomy Project Team. Short-term outcomes after emergency surgery for complicated peptic ulcer disease from the UK National Emergency Laparotomy Audit: a cohort study. BMJ Open. 2018;8:e023721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Tan S, Wu G, Zhuang Q, Xi Q, Meng Q, Jiang Y, Han Y, Yu C, Yu Z, Li N. Laparoscopic versus open repair for perforated peptic ulcer: A meta analysis of randomized controlled trials. Int J Surg. 2016;33 Pt A:124-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 18. | Sarosi GA, Jaiswal KR, Nwariaku FE, Asolati M, Fleming JB, Anthony T. Surgical therapy of peptic ulcers in the 21st century: more common than you think. Am J Surg. 2005;190:775-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Thorsen K, Søreide JA, Søreide K. Long-Term Mortality in Patients Operated for Perforated Peptic Ulcer: Factors Limiting Longevity are Dominated by Older Age, Comorbidity Burden and Severe Postoperative Complications. World J Surg. 2017;41:410-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | ASA House of Delegates/Executive Committee. ASA Physical Status Classification System. 2019. [accessed 2020, June 22]. Available from: https://www.asahq.org/standards-and-guidelines/asa-physical-status-classification-system. |
| 21. | Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32099] [Cited by in RCA: 38075] [Article Influence: 1002.0] [Reference Citation Analysis (0)] |
| 22. | Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6210] [Cited by in RCA: 8533] [Article Influence: 533.3] [Reference Citation Analysis (0)] |
| 23. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 24657] [Article Influence: 1174.1] [Reference Citation Analysis (0)] |
| 24. | The European Parliament and the Council of the European Union. Regulation (EU) 2016/679 of the European Parliament and the Council of 27 April 2016 on the protection of natural persons with regard to the processing of personal data and on the free movement of such data, and repealing Directive 95/46/EC (General Data Protection Regulation). European Union: The Official Journal of the European Union, 2016. [accessed 2020, June 22]. Available from: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32016R0679&from=EN. |
| 25. | Royston PA, Altman DG. Regression Using Fractional Polynomials of Continuous Covariates: Parsimonious Parametric Modelling. Appl Statist. 1994;43:429-453. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1016] [Cited by in RCA: 1073] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 26. | Dickman PW, Sloggett A, Hills M, Hakulinen T. Regression models for relative survival. Stat Med. 2004;23:51-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 599] [Cited by in RCA: 629] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 27. | Dickman PW, Coviello E. Estimating and modeling relative survival. Stata Journal. 2015;186-215. [DOI] [Full Text] |
| 28. | Database HM. Human Mortality Database; 2018. [accessed 2020, March 20]. Available from: http://www.mortality.org. |
| 29. | Søreide K, Thorsen K, Søreide JA. Clinical patterns of presentation and attenuated inflammatory response in octo- and nonagenarians with perforated gastroduodenal ulcers. Surgery. 2016;160:341-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Bashinskaya B, Nahed BV, Redjal N, Kahle KT, Walcott BP. Trends in Peptic Ulcer Disease and the Identification of Helicobacter Pylori as a Causative Organism: Population-based Estimates from the US Nationwide Inpatient Sample. J Glob Infect Dis. 2011;3:366-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Chung KT, Shelat VG. Perforated peptic ulcer - an update. World J Gastrointest Surg. 2017;9:1-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 183] [Cited by in RCA: 147] [Article Influence: 18.4] [Reference Citation Analysis (5)] |
| 32. | Lanas A, Chan FKL. Peptic ulcer disease. Lancet. 2017;390:613-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 555] [Article Influence: 69.4] [Reference Citation Analysis (37)] |
| 33. | Meek IL, Van de Laar MA, E Vonkeman H. Non-Steroidal Anti-Inflammatory Drugs: An Overview of Cardiovascular Risks. Pharmaceuticals (Basel). 2010;3:2146-2162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 134] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 34. | Lanas A, Serrano P, Bajador E, Esteva F, Benito R, Sáinz R. Evidence of aspirin use in both upper and lower gastrointestinal perforation. Gastroenterology. 1997;112:683-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 125] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 35. | Divo MJ, Martinez CH, Mannino DM. Ageing and the epidemiology of multimorbidity. Eur Respir J. 2014;44:1055-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 425] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 36. | Sheetz KH, Waits SA, Krell RW, Campbell DA, Englesbe MJ, Ghaferi AA. Improving mortality following emergent surgery in older patients requires focus on complication rescue. Ann Surg. 2013;258:614-7; discussion 617-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 37. | Møller MH, Adamsen S, Thomsen RW, Møller AM. Preoperative prognostic factors for mortality in peptic ulcer perforation: a systematic review. Scand J Gastroenterol. 2010;45:785-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 38. | Kuhn T, Basch P, Barr M, Yackel T; Medical Informatics Committee of the American College of Physicians. Clinical documentation in the 21st century: executive summary of a policy position paper from the American College of Physicians. Ann Intern Med. 2015;162:301-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 140] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 39. | Thorsen K, Søreide JA, Søreide K. What is the best predictor of mortality in perforated peptic ulcer disease? A population-based, multivariable regression analysis including three clinical scoring systems. J Gastrointest Surg. 2014;18:1261-1268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 40. | Thorsen K, Søreide JA, Søreide K. Scoring systems for outcome prediction in patients with perforated peptic ulcer. Scand J Trauma Resusc Emerg Med. 2013;21:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 41. | Søreide K, Thorsen K, Søreide JA. Predicting outcomes in patients with perforated gastroduodenal ulcers: artificial neural network modelling indicates a highly complex disease. Eur J Trauma Emerg Surg. 2015;41:91-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |