Published online Sep 7, 2020. doi: 10.3748/wjg.v26.i33.4960
Peer-review started: May 19, 2020
First decision: July 29, 2020
Revised: August 3, 2020
Accepted: August 26, 2020
Article in press: August 26, 2020
Published online: September 7, 2020
Processing time: 108 Days and 3 Hours
Effective treatment of osteoporosis is essential for improving morbidity and health-related quality of life in chronic liver disease (CLD) patients. Denosumab has been shown to increase bone mineral density (BMD) and decrease the risk of osteoporotic fracture in the general population. However, there are few reports evaluating the efficacy of denosumab in CLD patients.
To investigated the effects and safety of denosumab in CLD patients with osteoporosis.
Sixty CLD patients with osteoporosis were subcutaneously administered denosumab once every 6 mo. The study period for evaluating efficacy and safety was 12 mo. Changes from baseline in BMD at the lumbar spine, femoral neck, and total hip were evaluated at 12 mo of denosumab treatment. Bone turnover and quality were assessed by measuring serum tartrate-resistant acid phosphatase-5b (bone resorption marker), serum total procollagen type I N-terminal propeptide (bone formation maker), and plasma pentosidine (bone quality marker).
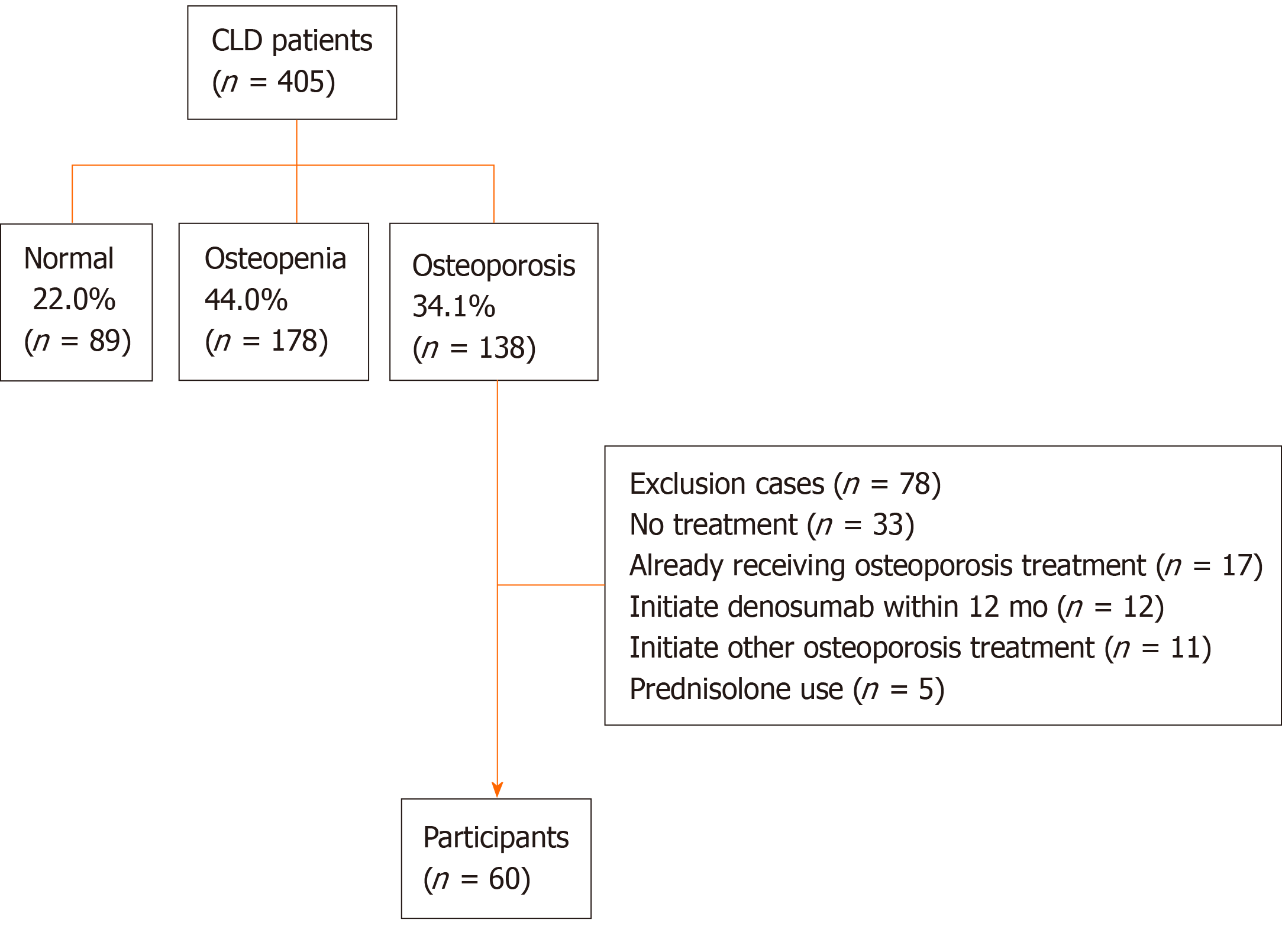
Among the 405 CLD patients, 138 (34.1%) patients were diagnosed with osteoporosis; among these, 78 patients met the exclusion criteria and thus 60 patients were finally included in the present study. The median percentage changes from baseline to 12 mo of denosumab treatment in BMD at the lumbar spine, femoral neck, and total hip were +4.44%, +3.71%, and +4.03%, respectively. Denosumab significantly improved BMD, regardless of sex, patient age, and presence of liver cirrhosis. Serum tartrate-resistant acid phosphatase-5b and procollagen type I N-terminal propeptide levels constantly and significantly declined after denosumab treatment (P < 0.001). Plasma pentosidine levels were also significantly lower at 12 mo of treatment (P = 0.010). No patients experienced fractures and moderate-to-severe adverse events, except for transient hypocalcemia.
Denosumab treatment was safe and increased BMD, suppressed bone turnover, and improved bone quality marker levels in CLD patients with osteoporosis, irrespective of differences in baseline characteristics.
Core tip: Osteoporosis is a common complication that causes fragility fractures and reduces health-related quality of life in patients with chronic liver disease. Denosumab treatment significantly increased bone mineral density at the lumbar spine, femoral neck, and total hip; suppressed bone turnover markers; and improved a bone quality marker at 12 mo, regardless of differences in baseline characteristics. No patients experienced fractures and adverse events, except for transient hypocalcemia. Denosumab treatment is safe and beneficial treatment option for osteoporosis in chronic liver disease patients.
- Citation: Saeki C, Saito M, Oikawa T, Nakano M, Torisu Y, Saruta M, Tsubota A. Effects of denosumab treatment in chronic liver disease patients with osteoporosis. World J Gastroenterol 2020; 26(33): 4960-4971
- URL: https://www.wjgnet.com/1007-9327/full/v26/i33/4960.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i33.4960
Osteoporosis is a frequent complication in patients with chronic liver disease (CLD), particularly in those with liver cirrhosis (LC) and primary biliary cholangitis[1-4]. Osteoporosis can cause fragility fractures, thereby increasing morbidity and decreasing health-related quality of life in CLD patients[5]. Therefore, early diagnosis and effective treatment of osteoporosis are crucial in preventing fragility fracture and maintaining health-related quality of life.
In terms of tissue material properties, bone mineral density (BMD) and bone quality are essential for determining bone strength[6-8]. Bone mineralization, which is regulated mainly by bone remodeling, provides stiffness to the bones, whereas collagen fibers influence the tensile strength, ductility, and toughness of the bones[7,8]. Collagen cross-link formation, a post-translational modification of collagen, plays an important role in the material level of bone quality[7,8]. The formations of collagen cross-links are classified into two types: Enzymatic and non-enzymatic cross-links. Enzymatic cross-links induced in an enzyme-dependent manner and produced by osteoblasts promote ductility and strength as well as bone mineralization. In contrast, non-enzymatic cross-links represented by advanced glycation end products (AGEs), which are induced by glycation and oxidant stress, impair the function of osteoblasts and bone’s mechanical properties. Therefore, impaired enzymatic cross-links and/or accelerated non-enzymatic cross-links in bone collagen deteriorate ductility and increase bone fragility[7,8]. Pentosidine is a biomarker for AGEs that accumulate in bone with increasing age and enhance the bone resorption activities of osteoclasts[9,10]. Urinary and serum pentosidine levels were reported to be positively and independently correlated to fractures in postmenopausal women and diabetic patients[11-14] and are now being used to estimate fracture risk in patients with osteoporosis and diabetes.
Receptor activator of nuclear factor kappa-B ligand (RANKL), also known as tumor necrosis factor-related activation-induced cytokine and osteoprotegerin ligand, is expressed on various cells including osteoblasts, binds to the receptor RANK on osteoclasts and precursors surfaces, and promotes differentiation and activation of osteoclasts, which are involved in bone resorption[15,16]. Osteoprotegerin (OPG) is expressed on several cells including osteoblasts, serves as a soluble decoy receptor for RANKL, and interrupts the interaction between RANK and RANKL by competing with RANK, suppressing osteoclastogenesis and bone resorption[15]. Hence, the RANK/RANKL/OPG signaling pathway plays a critical role in regulating bone resorption by osteoclasts and bone formation by osteoblasts.
Denosumab is a human monoclonal antibody with high affinity and specificity for RANKL and mimics the endogenous effect of OPG, thereby inhibiting bone resorption and remodeling[17,18]. Reportedly, denosumab increased BMD and decreased the levels of bone turnover markers, leading to a reduction in the osteoporotic fracture risk in postmenopausal women[19-24]. Furthermore, long-term treatment with denosumab was proved safe and associated with low bone remodeling rates and a constant increase in BMD without reaching a plateau[24]. Therefore, denosumab has attracted attention as an effective osteoporosis treatment that improves health-related quality of life[25]. However, there are few reports evaluating the efficacy of denosumab in CLD patients.
The aim of this study was to clarify the effects of denosumab treatment on BMD, bone turnover, and bone quality in CLD patients with osteoporosis.
This was a retrospective study of denosumab treatment for osteoporosis, which was conducted at the Jikei University School of Medicine (Tokyo, Japan) and Fuji City General Hospital (Shizuoka, Japan). A total of 60 CLD patients met the inclusion criteria and initiated denosumab treatment between 2017 and 2019. The inclusion criteria were as follows: (1) Presence of CLD with any etiology (hepatitis B or C, alcoholic liver disease, autoimmune hepatitis, primary biliary cholangitis, or nonalcoholic steatohepatitis); (2) Presence of osteoporosis diagnosed according to the World Health Organization criteria (T score ≤ -2.5); (3) No history of osteoporosis treatment; and (4) Subjects receiving denosumab treatment over 12 mo. The exclusion criteria were as follows: (1) Hypocalcemia (defined as serum calcium concentration < 8.5 mg/dL); (2) Subjects with glucocorticoid use; and (3) Subjects constantly receiving dental treatment. Denosumab was subcutaneously administered at a dose of 60 mg once every 6 months in combination with daily oral supplements of 610 mg of calcium and vitamin D [400 IU of cholecalciferol (native vitamin D) for patients with an estimated glomerular filtration rate ≥ 35 mL/min/1.73 m2 or 0.75 µg of eldecalcitol (active vitamin D) for those with estimated glomerular filtration rate < 35 mL/min/1.73 m2]. Although the study period was 12 mo, denosumab treatment is ongoing.
LC was diagnosed based on laboratory tests, morphological assessment with imaging (ultrasonography, computed tomography, and/or magnetic resonance), and/or the presence of esophageal/gastric varices confirmed using upper gastrointestinal endoscopy. BMD at the lumbar spine (L2-L4), femoral neck, and total hip was evaluated at 0 and 12 mo of treatment. Serum tartrate-resistant acid phosphatase-5b (TRACP-5b; bone resorption marker) and total procollagen type I N-terminal propeptide (P1NP; bone formation maker) were measured at 0, 1, 3, 6, and 12 mo. Plasma pentosidine (bone quality marker) was measured at 0 and 12 mo. Hypocalcemia was graded according to the Common Terminology Criteria for Adverse Events version 5.0. This study was conducted in accordance with the Declaration of Helsinki and was approved by the ethics committee of the Jikei University School of Medicine (approval No. 28-194) and Fuji City General Hospital (approval No. 162). Written informed consent was obtained from all patients.
The primary end point included changes from baseline to 12 mo of treatment in BMD at the lumbar spine, femoral neck, and total hip. The secondary end point included changes from baseline to 12 mo of treatment in bone turnover (serum TRACP-5b and P1NP) and quality (plasma pentosidine) markers.
BMD was assessed at the lumbar spine (L2-L4), femoral neck, and total hip using dual-energy X-ray absorptiometry (PRODIGY; GE Healthcare, Madison, WI, United States). Osteoporosis was diagnosed according to the World Health Organization criteria (T-score ≤ -2.5 for osteoporosis, between -2.5 and -1.0 for osteopenia, and > -1.0 for normality)[26]. Vertebral fractures were evaluated using spinal lateral X-rays at baseline and 12 mo of treatment.
A blood sample was obtained from each patient after overnight fasting. Serum aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, gamma-glutamyltransferase, albumin, and total bilirubin were measured using routine laboratory methods. Serum TRACP-5b, serum P1NP, and plasma pentosidine were measured using enzyme immunoassay (SB Bioscience, Tokyo, Japan), electrochemiluminescence immunoassay (Roche Diagnostics, Tokyo), and enzyme-linked immunosorbent assay (FUSHIMI Pharmaceutical, Kagawa, Japan), respectively.
Continuous variables are represented as medians and interquartile ranges. The Mann-Whitney U test was used to compare continuous variables between the two groups. The Wilcoxon signed rank test and Friedman test were used to compare changes in treatment-related biomarkers over time. Categorical variables are represented as the number of patients and percentages. Statistical analyses were performed using SPSS version 26 software (IBM, Armonk, NY, United States). A P value of < 0.05 was considered statistically significant.
Among the 405 CLD patients who underwent the assessment of BMD, 138 patients were diagnosed with osteoporosis; among these, 78 patients met the exclusion criteria and thus were excluded from this study. Therefore, 60 patients were finally included in the analysis (Figure 1).
The baseline clinical characteristics of the 60 patients enrolled in this study are shown in Table 1. The median age of the patients was 74.0 (68.5–79.8) years. The study included 47 female patients (78.3%). Twenty-five patients (41.7%) had LC. The number of patients with history of osteoporotic fracture was 25 (41.7%). The median BMD values at the lumber spine, femoral neck, and total hip were 0.84 (0.76–0.94) g/cm2, 0.61 (0.56–0.66) g/cm2, and 0.67 (0.59–0.71) g/cm2, respectively. Only one patient was treated with denosumab in combination with oral eldecalcitol due to chronic kidney disease.
| Variable | All patients (n = 60) |
| Age (yr) | 74.0 (68.5–79.8) |
| Female | 47 (78.3) |
| Menopause | 47 (100) |
| BMI (kg/m2) | 21.0 (19.2–23.6) |
| Etiology | |
| HBV/HCV/PBC/Others | 10/25/19/6 |
| Liver cirrhosis | 25 (41.7) |
| Child-Pugh A/B | 21/4 |
| Child-Pugh score | 5 (5–6) |
| MELD score | 3.0 (0.5–6.0) |
| Osteoporotic fracture | 25 (41.7) |
| AST (U/L) | 26 (20–28) |
| ALT (U/L) | 15 (12–24) |
| ALP (U/L) | 283 (235–332) |
| GGT (U/L) | 24 (17–40) |
| Total bilirubin (mg/dL) | 0.7 (0.5–0.9) |
| Platelet (× 104/μL) | 16.6 (12.6–22.7) |
| Prothrombin time | 98 (86–100) |
| Albumin (g/dL) | 4.1 (3.9–4.4) |
| Creatinine (mg/dL) | 0.7 (0.6–0.9) |
| eGFR (mL/min/1.73 m2) | 64 (53–75) |
| Ca (mg/dL) | 9.4 (9.1–9.7) |
| Pentosidine (μg/mL) | 0.0584 (0.0475–0.0761) |
| TRACP-5b (mU/dL) | 533 (358–661) |
| Total P1NP (ng/mL) | 57 (44-80) |
| Lumbar spine BMD (g/cm2) | 0.84 (0.76–0.94) |
| Lumbar spine T score | -2.41 (-1.28)–(-2.94) |
| Femoral neck BMD (g/cm2) | 0.61 (0.56–0.66) |
| Femoral neck T score | -2.94 (-2.64)–(-3.32) |
| Total hip BMD (g/cm2) | 0.67 (0.59–0.71) |
| Total hip T score | -2.48 (-2.07)–(-2.95) |
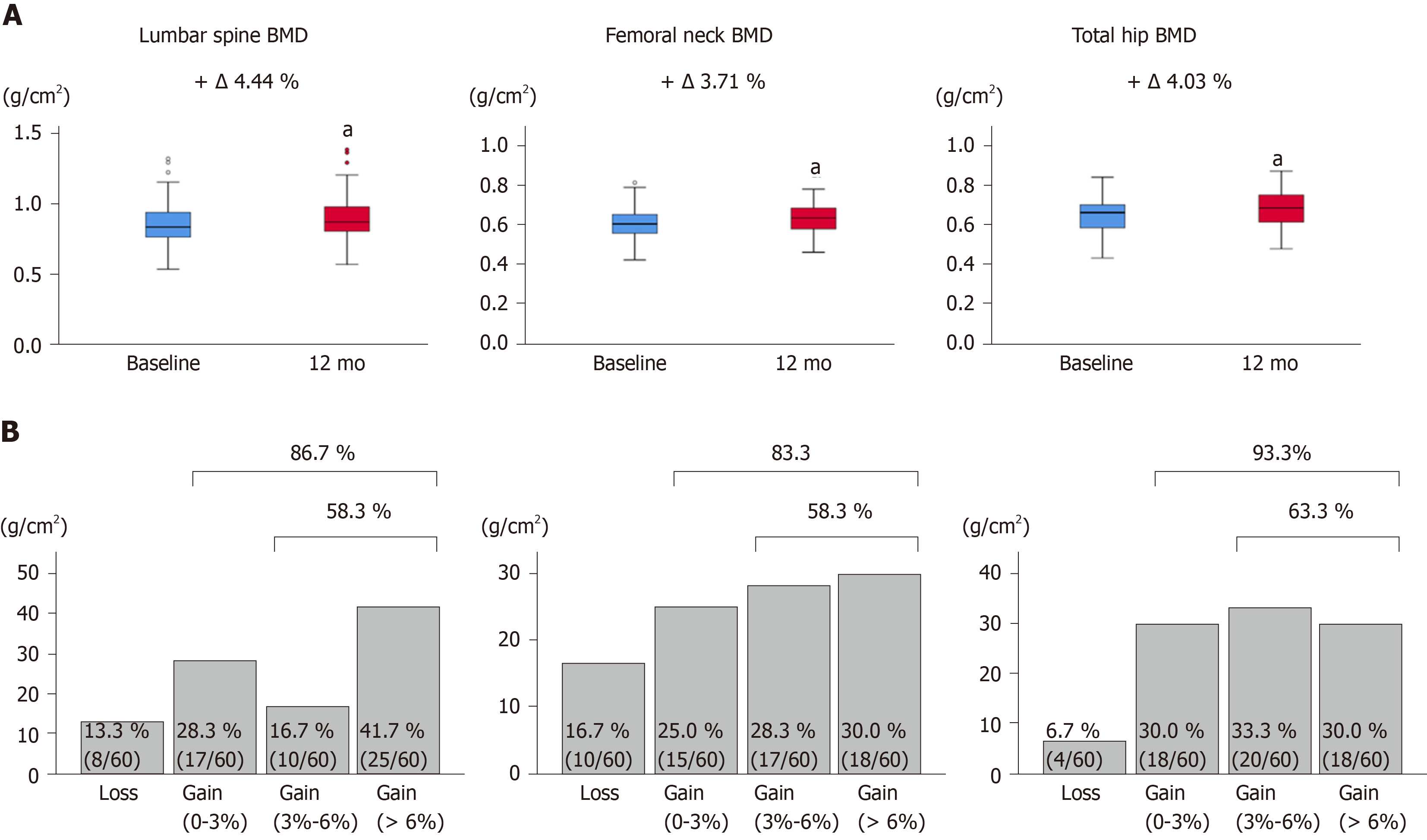
The changes from baseline to 12 mo of treatment in BMD at the lumbar spine, femoral neck, and total hip are shown in Figure 2A and Table S1. All BMD values were significantly improved at 12 mo of treatment (P < 0.001 for all). The median percentage changes were +4.44% for the lumbar spine, +3.71% for the femoral neck, and +4.03 for the total hip. The proportion of BMD gains at 12 mo is shown in Figure 2B. The percentages of patients with increased BMD were 86.7% (52/60) for the lumbar spine, 83.3% (50/60) for the femoral neck, and 93.3% (56/60) for the total hip. When a putative least significant change was defined as a gain of > 3%[27,28], percentages of patients with increased BMD were 58.3% (35/60), 58.3% (35/60), and 63.3% (38/60), respectively. Taken together, 12-mo denosumab treatment increased BMD in most CLD patients, although there were individual differences in BMD gains.
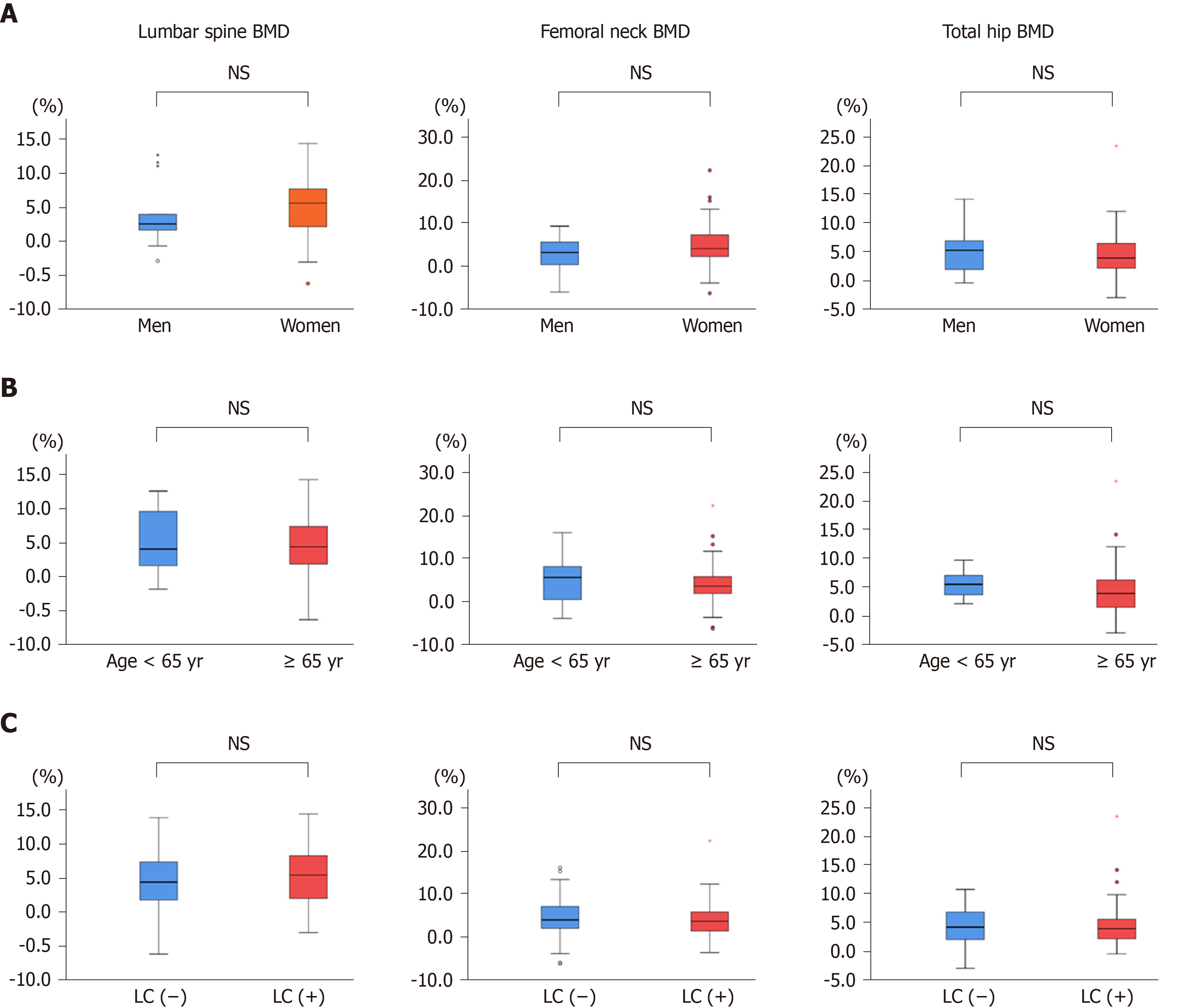
Next, we compared the changes from baseline in BMD between men and women, between patients aged < 65 years and ≥ 65 years, and between LC and non-LC groups (Figure 3A-C, Table S2-S4). The BMD values in all groups except for femoral neck BMD in men were significantly improved at 12 mo of treatment (Table S2-S4). However, the percentage changes were not significantly different between any two groups (Figure 3A-C). These findings indicate that denosumab treatment significantly improved BMD, irrespective of gender, patient age, and presence/absence of LC.
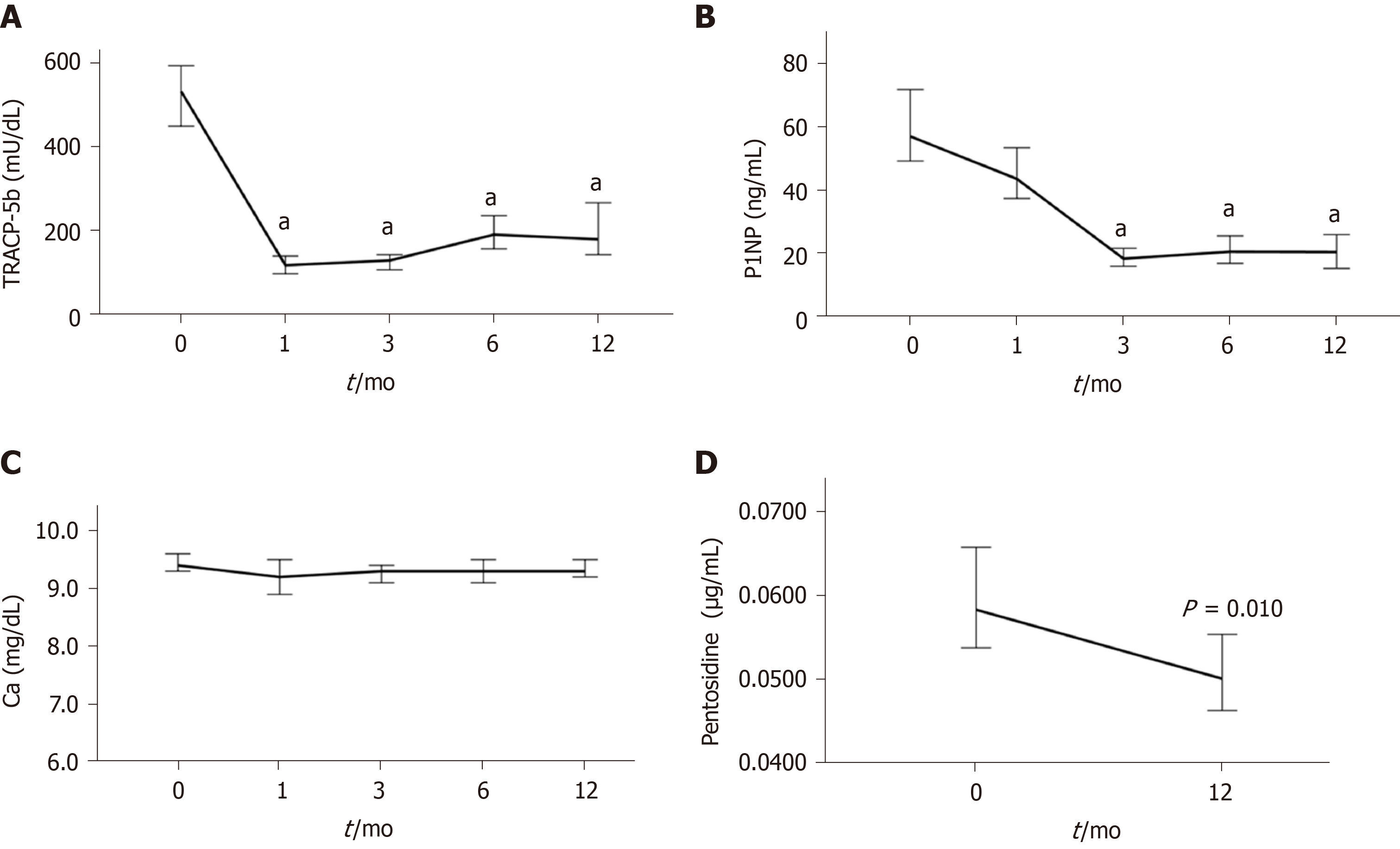
The changes in bone turnover and quality markers during denosumab treatment are shown in Figure 4 and Table S5. The median serum TRACP-5b level was 533 mU/dL at baseline and rapidly decreased by 77.7% at 1 mo of treatment and remained at a similar level thereafter (Figure 4A, Table S5). The median serum P1NP level was 57 ng/mL at baseline and gradually decreased by 22.8% at 1 mo and 68.4% at 3 mo, and remained at a similar level thereafter (Figure 4B, Table S5). There was little or no change in the serum calcium concentration during denosumab treatment (Figure 4C, Table S5). The median plasma pentosidine level was 0.0584 μg/mL at baseline and significantly decreased at 12 months (0.0501 μg/mL; P = 0.010) (Figure 4D, Table S5).
The incidence of hypocalcemia (< 8.5 mg/dL) was 11.7 % (7 of the 60 patients): 4 incidents occurred at 1 mo, 2 at 3 mo, and 1 at 12 mo of treatment. Two of the hypocalcemic patients were non-compliant with calcium and vitamin D supplementation. All cases were asymptomatic and classified as grade 1 hypocalcemia, with levels returning to the normal range without additional treatment. No patients experienced any fractures or other moderate-to-severe adverse events during the 12-mo study period.
Osteoporosis, resulting in frequent fractures and leading to significant morbidity, is a common complication in CLD[1-5]. Therefore, an appropriate treatment for osteoporosis is essential for improving health-related quality of life in CLD patients. In the present study, we demonstrated that denosumab significantly improved BMD in CLD patients, regardless of gender, patient age, and presence/absence of liver cirrhosis. The median percentage changes from baseline to 12 mo of treatment in BMD at the lumbar spine, femoral neck, and total hip were +4.44%, +3.71%, and +4.03%, respectively, in the present study; they were +5%–6%, +2%–3%, and +2%–3.5%, respectively, in previous reports for men and postmenopausal women with primary osteoporosis[28-30]. In a large phase 3 trial for primary osteoporosis, 10-year denosumab treatment increased BMD by +21.7%, +9.2%, and +9.0%, respectively[24]. A recent pilot study for a small number of patients with autoimmune liver disease showed that 3-year denosumab treatment significantly improved BMD without any adverse events[31]. These findings suggest that denosumab treatment is effective for osteoporosis in CLD patients. The present study is the first to highlight the efficacy of denosumab in treating osteoporosis in CLD patients.
According to the definition of osteoporosis, bone strength is determined by both BMD and bone quality[7,8]. BMD depends mainly on bone remodeling, whose status is reflected by bone turnover markers. Bone quality is determined by enzymatic and non-enzymatic collagen cross-links. Increased AGE cross-links (e.g., pentosidine as its surrogate biomarker) have been proposed as a major cause of bone fragility[7,8]. Reportedly, pentosidine levels in the cortical and cancellous bones of patients with femoral neck fracture were higher than those in age-matched controls[32,33]. Higher serum and urine pentosidine levels were associated with increased fracture risk and incidence of vertebral fractures in diabetic patients[12,14]. Furthermore, serum and bone pentosidine levels were higher in patients with hip fractures than in those with osteoarthritis; additionally, a significant, positive correlation was found between serum and bone pentosidine levels in the fracture patients[34]. These results suggest that pentosidine could be a useful biomarker for estimating bone quality.
In the present study, TRACP-5b declined sharply at 1 mo of denosumab treatment and was sustained thereafter, whereas P1NP declined gradually and reached the nadir at 3 mo of treatment. These pharmacokinetic profiles are consistent with those reported in previous studies[20,35]. Meanwhile, the present study is the first to demonstrate that denosumab significantly reduced plasma pentosidine levels in CLD patients, given that only few studies have investigated the effect of denosumab on bone quality markers. Similar findings were observed in denosumab treatment for prostate cancer patients receiving androgen deprivation therapy[36]. Taken together, denosumab treatment could not only efficiently modify bone remodeling in the early phase of the treatment but also improve bone quality in terms of tissue material properties.
Long-term denosumab treatment constantly increased BMD without reaching a plateau, despite persistently reduced bone turnover markers, and seemed to be more effective than bisphosphonate (BP)[24,37]. In the ovariectomized cynomolgus monkeys study, denosumab induced continuous modeling-based bone formation in the cortical bone, which indicates the increase of the content of enzymatic immature and mature cross-links, and continuously increased BMD, despite the reductions of bone resorption and formation biomarkers[38]. Similarly, administration of parathyroid hormone (1-34) (teriparatide), which stimulates bone formation, increased the content of enzymatic cross-links, bone volume, and trabecular thickness, and decreased pentosidine (non-enzymatic AGE cross-links), leading to improvement of bone strength, in ovariectomized monkeys[39]. In contrast, BP treatment increases BMD with reaching a plateau[37], and long-term suppression of bone remodeling (bone resorption and formation) increased pentosidine levels in dogs and humans[40,41]. Taken together, denosumab treatment may not impede the bone formation and increases the content of enzymatic cross-links, which could contribute to the reduction of non-enzymatic AGE cross-links (such as pentosidine) and improvement of bone quality and strength.
Adherence to osteoporosis medication is a critical issue, given that low compliance is associated with a higher risk of osteoporotic fractures[42]. Indeed, approximately 50% of patients treated with oral BP discontinued their prescribed treatment regimen within one year[42,43]. However, denosumab users had better 1-year and 2-year adherence than weekly oral BP users[43]. Hence, denosumab treatment is a recommended therapeutic option for CLD patients with osteoporosis.
During the 12-mo study period, no patients experienced any fractures or moderate-to-severe adverse events. Hypocalcemia was observed in 11.7% (7 of the 60 patients) and mainly developed in the early phase after the first dosing, which was similar to results in a previous report[44]; however, it was asymptomatic, transient, and mild (grade 1). Thus, denosumab treatment was safe in CLD patients.
This study had some limitations. First, the sample size was not large enough to evaluate the efficacy of denosumab in each subgroup. Second, given that the study period of 12 mo was short, we could not clarify the long-term treatment outcomes (such as fragility fractures and health-related quality of life) and adverse events associated with long-term administration. Third, given that this was not a randomized control study to evaluate the efficacy of denosumab versus BP, we could not demonstrate the real differences in CLD patients with osteoporosis.
In conclusion, denosumab increased BMD, suppressed bone turnover, and improved bone quality marker levels in CLD patients with osteoporosis, irrespective of gender, patient age, and presence/absence of liver cirrhosis. Given that denosumab treatment is effective and safe, it is a beneficial treatment option for osteoporosis in CLD patients. A large-scale, long-term, randomized controlled study is needed to confirm these findings.
Chronic liver disease (CLD) patients are frequently complicated by osteoporosis, which causes fragility fractures and reduces health-related quality of life. Denosumab, a fully human anti-receptor activator of nuclear factor kappa-B ligand antibody, has been shown to be effective for osteoporosis and reduce the risk of osteoporotic fracture in the general population. However, the efficacy and safety of denosumab in CLD patients remains unknown.
Adherence to osteoporosis medication is a crucial problem, given that we frequently encounter poor adherence to prescribed osteoporosis medications in a real-world clinical setting, which can result in an increased risk of osteoporotic fractures. Denosumab treatment, subcutaneously administered once every 6 mo, is expected to improve medication adherence compared to weekly oral drugs. Recently, denosumab treatment is suggested to improve health-related quality of life in patients with osteoporosis. Therefore, denosumab has been the focus of public attention as an attractive treatment for osteoporosis.
The primary objective was to investigate the effect of denosumab on bone mineral density (BMD) and its safety in CLD patients with osteoporosis. The secondary objective was to assess the effect of denosumab on bone quality.
We screened 405 CLD patients for osteoporosis and diagnosed osteoporosis in 138 (34.1%) patients. Among these patients with osteoporosis, 60 were finally included in the present study. Denosumab was administered once every 6 mo. Changes from baseline in BMD at the lumbar spine, femoral neck, and total hip were evaluated at 12 mo of denosumab treatment. Changes in bone turnover and quality were assessed by measurement of serum tartrate-resistant acid phosphatase-5b (bone resorption marker), serum total procollagen type I N-terminal propeptide (bone formation maker), and plasma pentosidine (bone quality marker).
BMD values at the lumbar spine (+4.44%), femoral neck (+3.71%), and total hip (+4.03%) were significantly improved at 12 mo of treatment, regardless of differences in baseline characteristics. Denosumab treatment significantly suppressed bone turnover markers and improved a bone quality marker at 12 mo. No patients experienced fractures and adverse events, except for transient hypocalcemia.
Denosumab treatment is effective and safe even in CLD patients with osteoporosis. Thus, denosumab is a beneficial treatment option for osteoporosis in CLD patients.
This study opened up new possibilities for osteoporosis treatment in CLD patients. Specifically, it is noteworthy that denosumab treatment improved a bone quality marker along with BMD. A large-scale, randomized controlled study is needed to confirm the long-term effects of denosumab.
We thank the medical staff at Jikei University School of Medicine and Fuji City General Hospital for data collection.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Feretis M, Li YH, Macias-Rodríguez RU, Rodrigues AT S-Editor: Zhang L L-Editor: A P-Editor: Ma YJ
| 1. | Jeong HM, Kim DJ. Bone Diseases in Patients with Chronic Liver Disease. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 2. | Nakchbandi IA, van der Merwe SW. Current understanding of osteoporosis associated with liver disease. Nat Rev Gastroenterol Hepatol. 2009;6:660-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Saeki C, Takano K, Oikawa T, Aoki Y, Kanai T, Takakura K, Nakano M, Torisu Y, Sasaki N, Abo M, Matsuura T, Tsubota A, Saruta M. Comparative assessment of sarcopenia using the JSH, AWGS, and EWGSOP2 criteria and the relationship between sarcopenia, osteoporosis, and osteosarcopenia in patients with liver cirrhosis. BMC Musculoskelet Disord. 2019;20:615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 4. | Guañabens N, Parés A, Ros I, Caballería L, Pons F, Vidal S, Monegal A, Peris P, Rodés J. Severity of cholestasis and advanced histological stage but not menopausal status are the major risk factors for osteoporosis in primary biliary cirrhosis. J Hepatol. 2005;42:573-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 124] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 5. | Santos LA, Romeiro FG. Diagnosis and Management of Cirrhosis-Related Osteoporosis. Biomed Res Int. 2016;2016:1423462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Seeman E, Delmas PD. Bone quality--the material and structural basis of bone strength and fragility. N Engl J Med. 2006;354:2250-2261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1422] [Cited by in RCA: 1365] [Article Influence: 71.8] [Reference Citation Analysis (0)] |
| 7. | Saito M, Marumo K. Collagen cross-links as a determinant of bone quality: a possible explanation for bone fragility in aging, osteoporosis, and diabetes mellitus. Osteoporos Int. 2010;21:195-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 617] [Cited by in RCA: 651] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 8. | Saito M, Marumo K. Effects of Collagen Crosslinking on Bone Material Properties in Health and Disease. Calcif Tissue Int. 2015;97:242-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 130] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 9. | Karim L, Tang SY, Sroga GE, Vashishth D. Differences in non-enzymatic glycation and collagen cross-links between human cortical and cancellous bone. Osteoporos Int. 2013;24:2441-2447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 10. | Dong XN, Qin A, Xu J, Wang X. In situ accumulation of advanced glycation endproducts (AGEs) in bone matrix and its correlation with osteoclastic bone resorption. Bone. 2011;49:174-183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Shiraki M, Kuroda T, Tanaka S, Saito M, Fukunaga M, Nakamura T. Nonenzymatic collagen cross-links induced by glycoxidation (pentosidine) predicts vertebral fractures. J Bone Miner Metab. 2008;26:93-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 115] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 12. | Schwartz AV, Garnero P, Hillier TA, Sellmeyer DE, Strotmeyer ES, Feingold KR, Resnick HE, Tylavsky FA, Black DM, Cummings SR, Harris TB, Bauer DC; Health, Aging, and Body Composition Study. Pentosidine and increased fracture risk in older adults with type 2 diabetes. J Clin Endocrinol Metab. 2009;94:2380-2386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 228] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 13. | Shiraki M, Kashiwabara S, Imai T, Tanaka S, Saito M. The association of urinary pentosidine levels with the prevalence of osteoporotic fractures in postmenopausal women. J Bone Miner Metab. 2019;37:1067-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Yamamoto M, Yamaguchi T, Yamauchi M, Yano S, Sugimoto T. Serum pentosidine levels are positively associated with the presence of vertebral fractures in postmenopausal women with type 2 diabetes. J Clin Endocrinol Metab. 2008;93:1013-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 174] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 15. | Tanaka S, Nakamura K, Takahasi N, Suda T. Role of RANKL in physiological and pathological bone resorption and therapeutics targeting the RANKL-RANK signaling system. Immunol Rev. 2005;208:30-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 246] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 16. | Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA. 1998;95:3597-3602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3136] [Cited by in RCA: 3052] [Article Influence: 113.0] [Reference Citation Analysis (0)] |
| 17. | Chandran T, Venkatachalam I. Efficacy and safety of denosumab compared to bisphosphonates in improving bone strength in postmenopausal osteoporosis: a systematic review. Singapore Med J. 2019;60:364-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 18. | Dahiya N, Khadka A, Sharma AK, Gupta AK, Singh N, Brashier DB. Denosumab: A bone antiresorptive drug. Med J Armed Forces India. 2015;71:71-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | McClung MR, Lewiecki EM, Cohen SB, Bolognese MA, Woodson GC, Moffett AH, Peacock M, Miller PD, Lederman SN, Chesnut CH, Lain D, Kivitz AJ, Holloway DL, Zhang C, Peterson MC, Bekker PJ; AMG 162 Bone Loss Study Group. Denosumab in postmenopausal women with low bone mineral density. N Engl J Med. 2006;354:821-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 889] [Cited by in RCA: 827] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 20. | Bone HG, Bolognese MA, Yuen CK, Kendler DL, Wang H, Liu Y, San Martin J. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women. J Clin Endocrinol Metab. 2008;93:2149-2157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 271] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 21. | Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, Delmas P, Zoog HB, Austin M, Wang A, Kutilek S, Adami S, Zanchetta J, Libanati C, Siddhanti S, Christiansen C; FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2264] [Cited by in RCA: 2278] [Article Influence: 142.4] [Reference Citation Analysis (0)] |
| 22. | Kendler DL, Chines A, Brandi ML, Papapoulos S, Lewiecki EM, Reginster JY, Muñoz Torres M, Wang A, Bone HG. The risk of subsequent osteoporotic fractures is decreased in subjects experiencing fracture while on denosumab: results from the FREEDOM and FREEDOM Extension studies. Osteoporos Int. 2019;30:71-78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Bilezikian JP, Lin CJF, Brown JP, Wang AT, Yin X, Ebeling PR, Fahrleitner-Pammer A, Franek E, Gilchrist N, Miller PD, Simon JA, Valter I, Zerbini CAF, Libanati C, Chines A. Long-term denosumab treatment restores cortical bone loss and reduces fracture risk at the forearm and humerus: analyses from the FREEDOM Extension cross-over group. Osteoporos Int. 2019;30:1855-1864. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | Bone HG, Wagman RB, Brandi ML, Brown JP, Chapurlat R, Cummings SR, Czerwiński E, Fahrleitner-Pammer A, Kendler DL, Lippuner K, Reginster JY, Roux C, Malouf J, Bradley MN, Daizadeh NS, Wang A, Dakin P, Pannacciulli N, Dempster DW, Papapoulos S. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017;5:513-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 709] [Cited by in RCA: 619] [Article Influence: 77.4] [Reference Citation Analysis (2)] |
| 25. | Hayashi S, Fukuda K, Maeda T, Chinzei N, Kihara S, Miura Y, Sakai Y, Hashimoto S, Matsumoto T, Takayama K, Niikura T, Kuroda R. Denosumab Treatment Improved Health-Related Quality of Life in Osteoporosis: A Prospective Cohort Study. JBMR Plus. 2019;3:e10191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Kanis JA. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group. Osteoporos Int. 1994;4:368-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1393] [Cited by in RCA: 1574] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 27. | Iwamoto N, Okamoto M, Tsuji S, Endo Y, Takatani A, Shimizu T, Umeda M, Fukui S, Sumiyoshi R, Igawa T, Koga T, Kawashiri SY, Aramaki T, Ichinose K, Tamai M, Nakamura H, Origuchi T, Eguchi K, Ueki Y, Kawakami A. Denosumab is effective toward glucocorticoid-induced osteoporosis patients complicated with rheumatic diseases regardless of prior anti-osteoporotic drugs. J Bone Miner Metab. 2019;37:554-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Bolognese MA, Teglbjærg CS, Zanchetta JR, Lippuner K, McClung MR, Brandi ML, Høiseth A, Lakatos P, Moffett AH, Lorenc RS, Wang A, Libanati C. Denosumab significantly increases DXA BMD at both trabecular and cortical sites: results from the FREEDOM study. J Clin Densitom. 2013;16:147-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 29. | Tsai JN, Uihlein AV, Lee H, Kumbhani R, Siwila-Sackman E, McKay EA, Burnett-Bowie SA, Neer RM, Leder BZ. Teriparatide and denosumab, alone or combined, in women with postmenopausal osteoporosis: the DATA study randomised trial. Lancet. 2013;382:50-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 320] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 30. | Orwoll E, Teglbjærg CS, Langdahl BL, Chapurlat R, Czerwinski E, Kendler DL, Reginster JY, Kivitz A, Lewiecki EM, Miller PD, Bolognese MA, McClung MR, Bone HG, Ljunggren Ö, Abrahamsen B, Gruntmanis U, Yang YC, Wagman RB, Siddhanti S, Grauer A, Hall JW, Boonen S. A randomized, placebo-controlled study of the effects of denosumab for the treatment of men with low bone mineral density. J Clin Endocrinol Metab. 2012;97:3161-3169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 144] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 31. | Arase Y, Tsuruya K, Hirose S, Ogiwara N, Yokota M, Anzai K, Deguchi R, Shiraishi K, Shirai T, Kagawa T. Efficacy and Safety of 3-Year Denosumab Therapy for Osteoporosis in Patients With Autoimmune Liver Diseases. Hepatology. 2020;71:757-759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | Saito M, Fujii K, Soshi S, Tanaka T. Reductions in degree of mineralization and enzymatic collagen cross-links and increases in glycation-induced pentosidine in the femoral neck cortex in cases of femoral neck fracture. Osteoporos Int. 2006;17:986-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 161] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 33. | Saito M, Fujii K, Marumo K. Degree of mineralization-related collagen crosslinking in the femoral neck cancellous bone in cases of hip fracture and controls. Calcif Tissue Int. 2006;79:160-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 181] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 34. | Vaculík J, Braun M, Dungl P, Pavelka K, Stepan JJ. Serum and bone pentosidine in patients with low impact hip fractures and in patients with advanced osteoarthritis. BMC Musculoskelet Disord. 2016;17:308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 35. | Sugimoto T, Matsumoto T, Hosoi T, Miki T, Gorai I, Yoshikawa H, Tanaka Y, Tanaka S, Fukunaga M, Sone T, Nakano T, Ito M, Matsui S, Yoneda T, Takami H, Watanabe K, Osakabe T, Okubo N, Shiraki M, Nakamura T. Three-year denosumab treatment in postmenopausal Japanese women and men with osteoporosis: results from a 1-year open-label extension of the Denosumab Fracture Intervention Randomized Placebo Controlled Trial (DIRECT). Osteoporos Int. 2015;26:765-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 36. | Miyazawa Y, Sekine Y, Syuto T, Nomura M, Koike H, Matsui H, Shibata Y, Ito K, Suzuki K. Evaluation of Bone Turnover / Quality Markers and Bone Mineral Density in Prostate Cancer Patients Receiving Androgen Deprivation Therapy with or without Denosumab. Anticancer Res. 2017;37:3667-3671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 37. | Reid IR. Short-term and long-term effects of osteoporosis therapies. Nat Rev Endocrinol. 2015;11:418-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 137] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 38. | Ominsky MS, Libanati C, Niu QT, Boyce RW, Kostenuik PJ, Wagman RB, Baron R, Dempster DW. Sustained Modeling-Based Bone Formation During Adulthood in Cynomolgus Monkeys May Contribute to Continuous BMD Gains With Denosumab. J Bone Miner Res. 2015;30:1280-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 39. | Saito M, Marumo K, Kida Y, Ushiku C, Kato S, Takao-Kawabata R, Kuroda T. Changes in the contents of enzymatic immature, mature, and non-enzymatic senescent cross-links of collagen after once-weekly treatment with human parathyroid hormone (1-34) for 18 months contribute to improvement of bone strength in ovariectomized monkeys. Osteoporos Int. 2011;22:2373-2383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 40. | Saito M, Mori S, Mashiba T, Komatsubara S, Marumo K. Collagen maturity, glycation induced-pentosidine, and mineralization are increased following 3-year treatment with incadronate in dogs. Osteoporos Int. 2008;19:1343-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 96] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 41. | Uchiyama S, Ikegami S, Kamimura M, Mukaiyama K, Nakamura Y, Nonaka K, Kato H. The skeletal muscle cross sectional area in long-term bisphosphonate users is smaller than that of bone mineral density-matched controls with increased serum pentosidine concentrations. Bone. 2015;75:84-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 42. | Gallagher AM, Rietbrock S, Olson M, van Staa TP. Fracture outcomes related to persistence and compliance with oral bisphosphonates. J Bone Miner Res. 2008;23:1569-1575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 111] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 43. | Reyes C, Tebe C, Martinez-Laguna D, Ali MS, Soria-Castro A, Carbonell C, Prieto-Alhambra D. One and two-year persistence with different anti-osteoporosis medications: a retrospective cohort study. Osteoporos Int. 2017;28:2997-3004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 44. | Imatoh T, Sai K, Takeyama M, Segawa K, Yamashita T, Nakashima N, Kataoka Y, Yokoi H, Hiramatsu T, Ohe K, Kimura M, Hori K, Kawakami J, Saito Y. Evaluating the impact of regulatory action on denosumab-induced hypocalcaemia in Japan. J Clin Pharm Ther. 2019;44:788-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |