Published online Aug 28, 2020. doi: 10.3748/wjg.v26.i32.4857
Peer-review started: May 23, 2020
First decision: June 12, 2020
Revised: June 18, 2020
Accepted: July 30, 2020
Article in press: July 30, 2020
Published online: August 28, 2020
Processing time: 96 Days and 18.1 Hours
The diagnosis of bacterial infection is difficult in patients with acute-on-chronic liver failure (ACLF).
To evaluate the diagnostic accuracy of widely used parameters for bacterial infection in ACLF and to develop a simple scoring system to improve diagnostic efficiency.
This was a retrospective study. Procalcitonin (PCT), white blood cells (WBC), proportion of neutrophils (N%), and C-reactive protein (CRP) were examined. Logistic regression was used to select variables for the scoring models and receiver operating characteristic curve (ROC) analysis was used to evaluate the diagnostic value of different indices.
This study included 386 patients with ACLF, 169 (43.78%) of whom had bacterial infection on admission. The area under the ROC (AUROC) of PCT, CRP, WBC and N% for the diagnosis of bacterial infection ranged from 0.637 to 0.692, with no significant difference between them. Logistic regression showed that only N%, PCT, and CRP could independently predict infection. A novel scoring system (infection score) comprised of N%, PCT and CRP was developed. The AUROC of the infection score was 0.740, which was significantly higher than that for the other four indices (infection score vs N%, PCT, CRP, and WBC, P = 0.0056, 0.0001, 0.0483 and 0.0008, respectively). The best cutoff point for the infection score was 4 points, with a sensitivity of 78.05%, a specificity of 55.29%, a positive predictive value of 57.91% and a negative predictive value of 76.16%.
The infection score is a simple and useful tool for discriminating bacterial infection in ACLF.
Core tip: This is a retrospective study evaluating the diagnostic value of widely used biomarkers for infection, including procalcitonin (PCT), white blood cells, proportion of neutrophils (N%), and C-reactive protein (CRP) for bacterial infection in ACLF. The results showed that all four parameters did not perform well in ACLF, with no significant difference found among them. A novel scoring system was developed comprised of N%, PCT and CRP which demonstrated higher accuracy for bacterial infection in ACLF than the indicators used alone. Further validation of this scoring system is required in prospective studies.
- Citation: Lin S, Yan YY, Wu YL, Wang MF, Zhu YY, Wang XZ. Development of a novel score for the diagnosis of bacterial infection in patients with acute-on-chronic liver failure. World J Gastroenterol 2020; 26(32): 4857-4865
- URL: https://www.wjgnet.com/1007-9327/full/v26/i32/4857.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i32.4857
Acute-on-chronic liver failure (ACLF) is a severe syndrome characterized by the loss of hepatocyte function and consequent multiple organ failure[1,2]. Patients with ACLF are usually vulnerable to infection[3,4]. On the other hand, bacterial infection can lead to deterioration of liver function or even death in such populations[5-8]. Thus, early detection of bacterial infection and timely treatment are crucial in the management of ACLF. However, compared with the general population or cases with liver cirrhosis, patients with ACLF demonstrate different clinico-pathophysiological features[9]. For example, significant systemic inflammation is commonly observed in ACLF[10-13], which makes the diagnosis of infection more difficult in the ACLF population than in other populations.
Routine blood testing is the preferred method for detecting infection in the general population. However, its value is not satisfactory in ACLF as leucopenia resulting from hypersplenism is common in patients with chronic liver disease. Serum C-reactive protein (CRP) and serum procalcitonin (PCT) level are widely used as diagnostic indicators for bacterial infection[14]. Previous studies have shown that CRP level decreases with the severity of liver failure[15,16]; therefore, the diagnostic capacity of CRP is interfered by hepatocyte dysfunction[17]. We previously demonstrated that the threshold of PCT should be elevated in ACLF when it is used for the diagnosis of bacterial infection[18], and this view was supported by several other studies[19-22]. Therefore, it is important to identify a new biomarker or to develop a model to improve diagnostic efficiency. This retrospective study aimed to develop a novel scoring system containing common biomarkers for the identification of bacterial infection in ACLF.
This study included ACLF patients who were hospitalized in the First Affiliated Hospital of Fujian Medical University from January 2014 to March 2019. The First Affiliated Hospital of Fujian Medical University is a teaching hospital that receives patients referred from other hospitals. Many of these patients may have previously been treated for some time in other hospitals and have developed nosocomial infection; therefore, the infection rate on admission in this cohort is relatively higher than reported. We excluded patients without a PCT test on admission and those with severe trauma, acute pancreatitis, malignancy, fungal infection, and those who had undergone major surgery.
ACLF was diagnosed based on the guideline of the Asian Pacific Association for the Study of the Liver (2014)[23], which are the most widely used criteria in Asia[24]. ACLF was confirmed in patients with chronic liver disease who had a total serum bilirubin ≥ 5 mg/dL or an international normalized ratio of ≥ 1.5 within 4 wk, complicated by encephalopathy and/or ascites[23].
The following data were collected from all patients on admission: Sex, age, the etiology of liver diseases, etiologies, infection source, PCT, CRP, white blood cell count (WBC), proportion of neutrophils (N%), liver and kidney function tests. The normal ranges of the four parameters were as follows: WBC 3.5-9.5 × 109/L, N% 40%-75%, CRP 0-8 mg/L, and PCT 0-0.05 ng/mL.
Continuous variables are expressed as the mean ± standard deviation and were compared using the Student’s t test in the case of normal distribution or the Mann-Whitney test in the remaining cases. Categorical variables are expressed as counts (percentages) and were compared by the Chi-squared test or Fisher’s exact test[25]. The diagnostic accuracy of PCT and the other parameters were examined by the receiver operating characteristic curve (ROC curve). The best cut-off value of each indicator was chosen based on Youden’s index. The sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV) were calculated based on the cut-off point. Statistical analyses were performed using SPSS software, version 18.0 (SPSS, Chicago, IL, United States). The ROC curves were compared using MedCalc software version15.2.2 (MedCalc Software, Mariakerke, Belgium). A P value < 0.05 was considered statistically significant.
This study was in compliance with the Declaration of Helsinki. All patients signed a written consent form for use of their clinical data. The study was approved by the Institutional Ethics Committee of the First Affiliated Hospital of Fujian Medical University.
This study included 386 patients with ACLF, 169 (43.78%) of whom had bacterial infection on admission and 217 (56.22%) did not (Figure 1). The baseline characteristics of these patients are shown in Table 1. Hepatitis B virus-related ACLF was the predominant etiology of liver disease in this population (75%). Patients with infection were older (52.18 ± 14.70 years vs 45.96 ± 13.94 years, P < 0.001) and had significantly higher MELD scores (22.24 ± 7.36 vs 18.94 ± 4.39, P < 0.001) than non-infected cases. Sex and etiologies were similar between the two groups.
| Non-infected (n = 217) | Infected (n = 169) | P value | |
| Age (yr) | 45.96 ± 13.94 | 52.18±14.70 | < 0.001 |
| Male, n (%) | 177 (81.6) | 126 (74.6) | 0.096 |
| Etiology, n (%) | 0.051 | ||
| HBV infection | 173 (79.7) | 114 (67.5) | |
| Alcohol | 17 (7.8) | 23 (13.6) | |
| HBV and alcohol | 8 (3.7) | 8 (4.7) | |
| Others | 19 (8.8) | 24 (14.2) | |
| MELD score | 18.94 ± 4.39 | 22.24 ± 7.36 | < 0.001 |
| PT (s) | 21.97 ± 6.52 | 26.22 ± 9.90 | < 0.001 |
| INR | 1.90 ± 0.56 | 2.35 ± 1.16 | < 0.001 |
| Total bilirubin (μmol/L) | 272.56 ± 127.41 | 317.70 ± 153.10 | 0.001 |
| Albumin (g/L) | 31.34 ± 4.76 | 28.81 ± 5.36 | < 0.001 |
| ALT (U/L) | 663.30 ± 694.59 | 524.97 ± 800.98 | 0.070 |
| AST (U/L) | 463.18 ± 494.97 | 477.25 ± 662.96 | 0.811 |
| GGT (U/L) | 159.74 ± 160.13 | 141.85 ± 143.03 | 0265 |
| Creatinine (μmol/L) | 61.67 ± 19.37 | 81.71 ± 56.61 | < 0.001 |
| PCT (ng/mL) | 0.75 ± 0.60 | 1.46 ± 1.81 | < 0.001 |
| CRP (mg/L) | 15.86 ± 9.61 | 25.59 ± 17.50 | < 0.001 |
| WBC (× 109/L) | 6.67 ± 3.11 | 9.01 ± 5.62 | < 0.001 |
| N% | 66.80 ± 10.57 | 73.03 ± 11.56 | < 0.001 |
The most common infection in this ACLF cohort was pneumonia, accounting for 55.62%. The proportions of spontaneous bacterial peritonitis (SBP) (13.60%) and both pneumonia and SBP (12.43%) were similar. The remaining infections were from an unidentified source (7.69%), biliary tract infection (7.10%), urinary tract infection (5.92%) and blood stream infection (1.78%).
The PCT levels were increased in patients with ACLF even in the absence of bacterial infection (0.75 ± 0.60 ng/mL), which was demonstrated in our previous study[18]. This increase was nearly 15 times higher than the normal range (0.05 ng/mL). However, the PCT levels in the infected group were still significantly higher than those in the non-infected group (1.46 ± 1.81 ng/mL, P < 0.05, Table 1). In the group with infection, CRP levels differed significantly with infection site, while PCT, WBC and N% levels showed no significant differences regarding the infection site (Table 2).
| PCT (ng/mL) | CRP (mg/L) | WBC (× 109/L) | N% | |
| Overall (n = 169) | 1.46 ± 1.81 | 25.59 ± 17.50 | 9.01 ± 5.62 | 73.03 ± 11.56 |
| Pneumonia (n = 94) | 1.35 ± 1.77 | 25.26 ± 17.80 | 8.87 ± 5.98 | 71.77 ± 11.23 |
| SBP (n = 23) | 1.29 ± 0.83 | 18.55 ± 9.19 | 8.46 ± 3.88 | 72.05 ± 14.06 |
| Pneumonia and SBP (n = 21) | 2.30 ± 2.83 | 37.71 ± 24.00 | 11.30 ± 6.72 | 77.26 ± 13.85 |
| Infection without identified source (n = 13) | 2.12 ± 2.04 | 29.26 ± 11.98 | 9.35 ± 4.81 | 75.57 ± 10.87 |
| Biliary tract (n = 12) | 0.59 ± 0.37 | 14.61 ± 8.12 | 7.92 ± 4.66 | 73.42 ± 10.25 |
| Urinary (n = 10) | 0.74 ± 0.54 | 19.72 ± 5.90 | 5.38 ± 2.43 | 67.43 ± 9.25 |
| Bloodstream (n = 3) | 1.02 ± 0.42 | 32.65 ± 17.90 | 8.21 ± 1.13 | 74.67 ± 5.80 |
| P value | 0.084 | 0.001 | 0.203 | 0.406 |
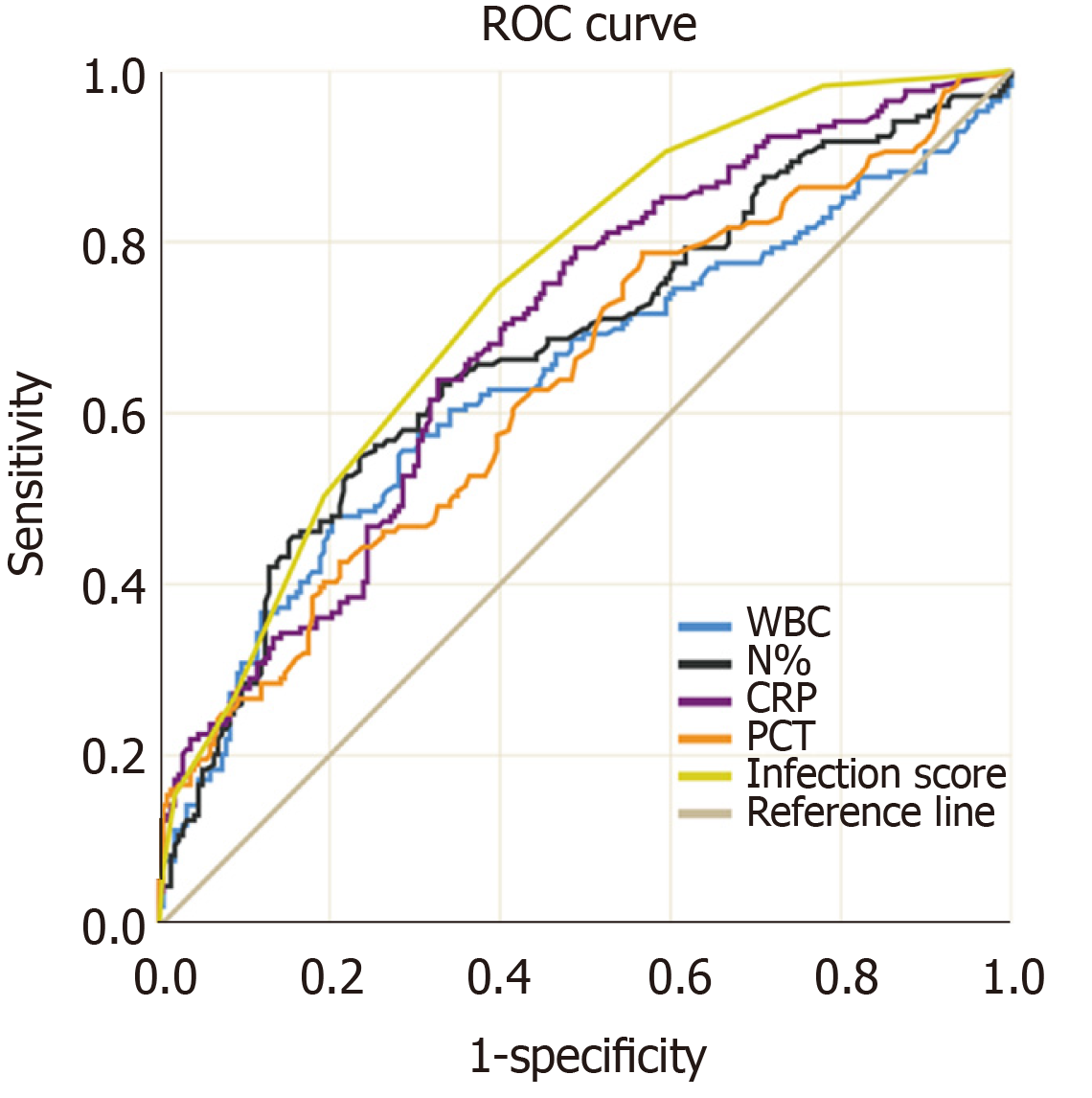
Figure 2 shows the ROC curves of PCT, CRP, WBC, and N% for the diagnosis of bacterial infection. Table 3 shows the best cutoff points and the area under the ROC (AUROC) of these parameters. There were no significant differences in multiple comparisons of the AUROCs (all P values > 0.05).
| Cut offs | Sensitivity (%) | Specificity (%) | PLR | NLR | PPV (%) | NPV (%) | P value | AUROC | |
| PCT | ≥ 1.01 | 42.60 | 78.80 | 2.01 | 0.73 | 61.01 | 63.81 | < 0.0001 | 0.637 |
| CRP | ≥ 17.5 | 63.91 | 67.28 | 1.95 | 0.54 | 60.33 | 70.54 | < 0.0001 | 0.692 |
| WBC | ≥ 7.90 | 47.93 | 79.72 | 2.36 | 2.36 | 64.79 | 66.28 | < 0.0001 | 0.638 |
| N% | ≥ 73.8 | 55.03 | 76.50 | 2.34 | 0.59 | 64.58 | 68.60 | < 0.0001 | 0.674 |
The results of logistic regression showed that PCT, CRP and N% could independently predict infection, with an odds ratio (OR) of 1.595 (95%CI: 1.202-2.116), 1.047 (95%CI: 1.025-1.069) and 1.030 (95%CI: 1.005-1.055), respectively, while the WBC was not an independent indicator for infection (OR = 1.063, 95%CI: 0.993-1.137).
According to the variables selected for logistic regression analysis and the cut-offs defined by ROC analysis, we developed a scoring system that contained three components, N%, PCT, and CRP (Table 4). For example, the OR of PCT for infection was 1.595, which meant that each additional unit (1 ng/mL) increment in PCT increased the risk of infection by 59%. The baseline level of PCT in the non-infected group was approximately 0.75 ng/mL and the optimal cutoff point of PCT for discriminating infection and non-infection was 1 ng/mL. After combining the above information and making the scoring system more user-friendly, PCR < 0.5 ng/mL was assigned 0 point, 1 > PCT ≥ 0.5 was assigned 1 point, and 2 > PCT ≥ 1 was assigned 2 points. The AUROC of this infection score for the diagnosis of bacterial infection in patients with ACLF was 0.740, which was significantly higher than the other four biomarkers (infection score vs N%, PCT, CRP, and WBC, P = 0.0056, 0.0001, 0.0483 and 0.0008, respectively). The best cutoff value of the infection score was 4 points, with a sensitivity of 78.05%, specificity of 55.29%, PPV of 57.91%, and NPV of 76.16%. Cases with an infection score of 0-2 points were not likely to have a bacterial infection (NPV was 94.10%). On the other hand, cases with an infection score of 8 points and greater were largely considered infected and empiric antibiotics were strongly recommended (PPV was 91.68%) (Table 5).
| Items | Definition | Score |
| N% | < 60 | 0 |
| 70 > N ≥ 60 | 1 | |
| 80 > N ≥ 70 | 2 | |
| ≥ 80 | 3 | |
| PCT | < 0.5 | 0 |
| 1 > PCT ≥ 0.5 | 1 | |
| 2 > PCT ≥ 1 | 2 | |
| ≥ 2 | 3 | |
| CRP | < 10 | 0 |
| 20 >N ≥ 10 | 1 | |
| ≥ 20 | 2 |
| Score | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | PLR | NLR |
| ≥ 2 | 98.22 | 22.12 | 49.55 | 94.10 | 1.26 | 0.08 |
| ≥ 4 | 78.05 | 55.29 | 57.91 | 76.16 | 1.75 | 0.40 |
| ≥ 6 | 26.63 | 91.24 | 70.30 | 61.49 | 3.04 | 0.80 |
| ≥ 8 | 6.51 | 99.54 | 91.68 | 57.76 | 14.12 | 0.94 |
The early diagnosis of bacterial infection is crucial for the management of liver failure. This study firstly demonstrated that common indicators of infection, including WBC, N%, CRP, and PCT, did not perform well in ACLF as all the AUROCs were less than 0.7 and no differences were found between these indicators. A novel scoring system was developed in this study and the results showed that this infection score had better accuracy than those four parameters alone for the diagnosis of bacterial infection in ACLF.
This infection score comprised three commonly used indicators, the N%, PCT and CRP. Neutrophils are known to be the first immune cells in the response to infection[26]. The N% is independent of the WBC, which means that hypersplenism might not significantly influence the N%. In this cohort, the N% alone was not an excellent biomarker of bacterial infection as the AUROC was only 0.674 and its best cutoff value was still within the normal range. However, when combined with other biomarkers, the diagnostic value was greatly improved.
PCT has been questioned as an index of bacterial infection in patients with liver diseases as several studies have demonstrated elevated PCT levels in the absence of bacterial infection in such patients[18,27,28]. We previously showed that PCT was not the best parameter of infection in cirrhotic patients following the onset of sepsis[29]. Mallet et al[21] showed that the diagnostic value of PCT for infections was related to the etiology of liver failure. PCT has also been shown to be a better biomarker for liver cell injury than a biomarker for bacterial infection in patients with acute liver failure[20]. A study from China suggested adjusting the threshold of PCT according to liver function[19]. The results of this study showed that the diagnostic value of PCT alone was similar to that of WBC, N% and CRP even though the threshold for PCT was increased to 20 times greater than the upper limit of the normal range. This result was supported by the aforementioned studies.
Similar to PCT and N%, the diagnostic value of CRP alone was not satisfactory. Inflammation and bacterial translocation in liver cirrhosis may lead to an increase in the synthesis of CRP[30]. On the other hand, a previous study showed that CRP levels will remain high even when bacterial infections are resolved[31]. The CRP might partially reflect systemic inflammation in ACLF in addition to bacterial infection.
With the combination of these three widely used parameters, the performance of the infection score was better in diagnosing bacterial infection. The AUROC values were significantly higher than any of the other indicators used alone. When the infection score was equal to or less than 2 points, the NPV was 90%, suggesting that infection can be ruled out and antibiotics should not be administered. When the score was 8 points, the PPV was 90%, suggesting that anti-infective therapy was required. The variables in this model are widely used in clinical practice and the score is easy to compute. This novel scoring system might be a useful tool for early detection of infection in patients with ACLF and help to improve the outcome of this population.
The limitation of this research is that it is a retrospective study performed in a single medical center. As ACLF is an uncommon disease, we were unable to include sufficient cases to validate this model. Studies regarding the diagnosis of infection in liver failure are still limited, thus studies with a large sample size are needed to validate this scoring system.
In conclusion, a novel scoring system comprised of N%, CRP and PCT is useful for the diagnosis of bacterial infection in ACLF.
Patients with acute-on-chronic liver failure (ACLF) are prone to have bacterial infection. However, the diagnosis of infection is difficult in the ACLF population due to their specific clinico-pathophysiological features.
Early detection of bacterial infection and timely treatment are crucial in the management of ACLF. Therefore, it is important to identify a new biomarker or to develop a model to improve diagnostic efficiency.
This retrospective study aimed to develop a novel scoring system containing common biomarkers for the identification of bacterial infection in ACLF.
This was a retrospective study. Procalcitonin (PCT), white blood cells (WBC), proportion of neutrophils (N%), and C-reactive protein (CRP) were examined. Logistic regression was used to select variables for the scoring models and receiver operating characteristic curve (ROC) analysis was used to evaluate the diagnostic value of different indices.
This study included 386 patients with ACLF, 169 (43.78%) of whom had bacterial infection on admission. The area under the ROC (AUROC) of PCT, CRP, WBC and N% for the diagnosis of bacterial infection ranged from 0.637 to 0.692, with no significant difference between them. Logistic regression showed that only N%, PCT, and CRP could independently predict infection. A novel scoring system (infection score) comprised of N%, PCT and CRP was developed. The AUROC of the infection score was 0.740, which was significantly higher than that for the other four indices (infection score vs N%, PCT, CRP, and WBC, P = 0.0056, 0.0001, 0.0483 and 0.0008, respectively). The best cutoff point for the infection score was 4 points, with a sensitivity of 78.05%, a specificity of 55.29%, a positive predictive value of 57.91% and a negative predictive value of 76.16%.
The common indicators of infection, including WBC, N%, CRP, and PCT, did not perform well in ACLF as all the AUROCs were less than 0.7 and no differences were found between these indicators. A novel scoring system comprised of N%, PCT and CRP demonstrated higher accuracy for bacterial infection in ACLF than the indicators used alone.
Further validation of this scoring system is required in prospective studies.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: El-Shabrawi MHF S-Editor: Yan JP L-Editor: Webster JR P-Editor: Ma YJ
| 1. | Lange CM, Bechstein WO, Berg T, Engelmann C, Bruns T, Canbay A, Moreau R, Trebicka J. Acute-on-Chronic Liver Failure. Visc Med. 2018;34:296-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 2. | Chen T, Yang Z, Choudhury AK, Al Mahtab M, Li J, Chen Y, Tan SS, Han T, Hu J, Hamid SS, Huei LG, Ghazinian H, Nan Y, Chawla YK, Yuen MF, Devarbhavi H, Shukla A, Abbas Z, Sahu M, Dokmeci AK, Lesmana LA, Lesmana CRA, Xin S, Duan Z, Guo W, Ma K, Zhang Z, Cheng Q, Jia J, Sharma BC, Sarin SK, Ning Q. Complications constitute a major risk factor for mortality in hepatitis B virus-related acute-on-chronic liver failure patients: a multi-national study from the Asia-Pacific region. Hepatol Int. 2019;13:695-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 3. | Fernández J, Acevedo J, Wiest R, Gustot T, Amoros A, Deulofeu C, Reverter E, Martínez J, Saliba F, Jalan R, Welzel T, Pavesi M, Hernández-Tejero M, Ginès P, Arroyo V; European Foundation for the Study of Chronic Liver Failure. Bacterial and fungal infections in acute-on-chronic liver failure: prevalence, characteristics and impact on prognosis. Gut. 2018;67:1870-1880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 400] [Article Influence: 57.1] [Reference Citation Analysis (2)] |
| 4. | Engelmann C, Berg T. Management of Infectious Complications Associated with Acute-on-Chronic Liver Failure. Visc Med. 2018;34:261-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Grgurevic I, Trkulja V, Bozin T, Madir A, Miletic M, Marusic S, Skrlin J, Sestan Crnek S, Dobrovic K. Infection as a predictor of mortality in decompensated liver cirrhosis: exploring the relationship to severity of liver failure. Eur J Gastroenterol Hepatol. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Li B, Gao Y, Wang X, Qian Z, Meng Z, Huang Y, Deng G, Lu X, Liu F, Zheng X, Li H, Chen J. Clinical features and outcomes of bacterascites in cirrhotic patients: A retrospective, multicentre study. Liver Int. 2020;40:1447-1456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Cao Z, Liu Y, Wang S, Lu X, Yin S, Jiang S, Chen L, Cai M, Zeng B, Yao Y, Tang W, Zhao G, Xiang X, Wang H, Cai W, Zhu C, Li H, Xie Q. The impact of HBV flare on the outcome of HBV-related decompensated cirrhosis patients with bacterial infection. Liver Int. 2019;39:1943-1953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Mücke MM, Rumyantseva T, Mücke VT, Schwarzkopf K, Joshi S, Kempf VAJ, Welsch C, Zeuzem S, Lange CM. Bacterial infection-triggered acute-on-chronic liver failure is associated with increased mortality. Liver Int. 2018;38:645-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 9. | Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M, Gerbes A, Wendon J, Alessandria C, Laleman W, Zeuzem S, Trebicka J, Bernardi M, Arroyo V; CANONIC Study Investigators of the EASL–CLIF Consortium. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426-1437, 1437.e1-1437.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1720] [Cited by in RCA: 2172] [Article Influence: 181.0] [Reference Citation Analysis (5)] |
| 10. | Hensley MK, Deng JC. Acute on Chronic Liver Failure and Immune Dysfunction: A Mimic of Sepsis. Semin Respir Crit Care Med. 2018;39:588-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Clària J, Stauber RE, Coenraad MJ, Moreau R, Jalan R, Pavesi M, Amorós À, Titos E, Alcaraz-Quiles J, Oettl K, Morales-Ruiz M, Angeli P, Domenicali M, Alessandria C, Gerbes A, Wendon J, Nevens F, Trebicka J, Laleman W, Saliba F, Welzel TM, Albillos A, Gustot T, Benten D, Durand F, Ginès P, Bernardi M, Arroyo V; CANONIC Study Investigators of the EASL-CLIF Consortium and the European Foundation for the Study of Chronic Liver Failure (EF-CLIF). Systemic inflammation in decompensated cirrhosis: Characterization and role in acute-on-chronic liver failure. Hepatology. 2016;64:1249-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 560] [Article Influence: 62.2] [Reference Citation Analysis (0)] |
| 12. | Yang D, Xie Y, Pan H, Huang Y, Dai Y, Tong Y, Chen M. Clinical characteristics and prognostic factors of liver cirrhosis patients with systemic inflammatory response syndrome. Hepatol Res. 2017;47:1174-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Laleman W, Claria J, Van der Merwe S, Moreau R, Trebicka J. Systemic Inflammation and Acute-on-Chronic Liver Failure: Too Much, Not Enough. Can J Gastroenterol Hepatol. 2018;2018:1027152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 14. | Pfäfflin A, Schleicher E. Inflammation markers in point-of-care testing (POCT). Anal Bioanal Chem. 2009;393:1473-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 112] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 15. | Park WB, Lee KD, Lee CS, Jang HC, Kim HB, Lee HS, Oh MD, Choe KW. Production of C-reactive protein in Escherichia coli-infected patients with liver dysfunction due to liver cirrhosis. Diagn Microbiol Infect Dis. 2005;51:227-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Mackenzie I, Woodhouse J. C-reactive protein concentrations during bacteraemia: A comparison between patients with and without liver dysfunction. Intensive Care Med. 2006;32:1344-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Fernández J, Gustot T. Management of bacterial infections in cirrhosis. J Hepatol. 2012;56 Suppl 1:S1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 250] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 18. | Lin S, Yan Y, Wu Y, Van Poucke S, Wang M, Zhu Y, Wang X. Procalcitonin as a biomarker for diagnose of bacterial infection in patients with acute-on-chronic liver failure. Clin Res Hepatol Gastroenterol. 2020;44:e32-e34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Qu J, Feng P, Luo Y, Lü X. Impact of hepatic function on serum procalcitonin for the diagnosis of bacterial infections in patients with chronic liver disease: A retrospective analysis of 324 cases. Medicine (Baltimore). 2016;95:e4270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Rule JA, Hynan LS, Attar N, Sanders C, Korzun WJ, Lee WM; Acute Liver Failure Study Group. Procalcitonin Identifies Cell Injury, Not Bacterial Infection, in Acute Liver Failure. PLoS One. 2015;10:e0138566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | Mallet M, Haq M, Tripon S, Bernard M, Benosman H, Thabut D, Rudler M. Elevated procalcitonin is associated with bacterial infection during acute liver failure only when unrelated to acetaminophen intoxication. Eur J Gastroenterol Hepatol. 2017;29:811-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Sugihara T, Koda M, Okamoto T, Miyoshi K, Matono T, Oyama K, Hosho K, Okano JI, Isomoto H. Serum Procalcitonin in Patients with Acute Liver Failure. Yonago Acta Med. 2017;60:40-46. [PubMed] |
| 23. | Sarin SK, Kedarisetty CK, Abbas Z, Amarapurkar D, Bihari C, Chan AC, Chawla YK, Dokmeci AK, Garg H, Ghazinyan H, Hamid S, Kim DJ, Komolmit P, Lata S, Lee GH, Lesmana LA, Mahtab M, Maiwall R, Moreau R, Ning Q, Pamecha V, Payawal DA, Rastogi A, Rahman S, Rela M, Saraya A, Samuel D, Saraswat V, Shah S, Shiha G, Sharma BC, Sharma MK, Sharma K, Butt AS, Tan SS, Vashishtha C, Wani ZA, Yuen MF, Yokosuka O; APASL ACLF Working Party. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL) 2014. Hepatol Int. 2014;8:453-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 541] [Cited by in RCA: 500] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 24. | Lin S, Zhang K, Zhang J, Wang M, Velani B, Zhu Y. Long-term outcomes of patients with hepatitis B virus-related acute on chronic liver failure: An observational cohort study. Liver Int. 2019;39:854-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Zhang Z. Univariate description and bivariate statistical inference: the first step delving into data. Ann Transl Med. 2016;4:91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 144] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 26. | Lawrence SM, Corriden R, Nizet V. The Ontogeny of a Neutrophil: Mechanisms of Granulopoiesis and Homeostasis. Microbiol Mol Biol Rev. 2018;82:e00057-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 148] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 27. | Woźnica E, Łysenko L. Procalcitonin in liver dysfunction - Dr Jekyll or Mr Hyde? Anaesthesiol Intensive Ther. 2018;50:226-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 28. | Dong R, Wan B, Lin S, Wang M, Huang J, Wu Y, Wu Y, Zhang N, Zhu Y. Procalcitonin and Liver Disease: A Literature Review. J Clin Transl Hepatol. 2019;7:51-55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 29. | Lin S, Huang Z, Wang M, Weng Z, Zeng D, Zhang Y, Zhu Y, Jiang J. Interleukin-6 as an early diagnostic marker for bacterial sepsis in patients with liver cirrhosis. J Crit Care. 2015;30:732-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 30. | Jalan R, Fernandez J, Wiest R, Schnabl B, Moreau R, Angeli P, Stadlbauer V, Gustot T, Bernardi M, Canton R, Albillos A, Lammert F, Wilmer A, Mookerjee R, Vila J, Garcia-Martinez R, Wendon J, Such J, Cordoba J, Sanyal A, Garcia-Tsao G, Arroyo V, Burroughs A, Ginès P. Bacterial infections in cirrhosis: a position statement based on the EASL Special Conference 2013. J Hepatol. 2014;60:1310-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 644] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 31. | Cervoni JP, Thévenot T, Weil D, Muel E, Barbot O, Sheppard F, Monnet E, Di Martino V. C-reactive protein predicts short-term mortality in patients with cirrhosis. J Hepatol. 2012;56:1299-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 136] [Article Influence: 10.5] [Reference Citation Analysis (0)] |