Published online Aug 21, 2020. doi: 10.3748/wjg.v26.i31.4718
Peer-review started: January 20, 2020
First decision: February 27, 2020
Revised: May 15, 2020
Accepted: August 1, 2020
Article in press: August 1, 2020
Published online: August 21, 2020
Processing time: 213 Days and 19.7 Hours
Congenital intrahepatic bile duct dilatation without fibrosis is called Caroli disease (CD), and is called Caroli syndrome (CS) when it has fibrotic and cirrhotic liver morphology. The development of intrahepatic carcinoma is described in both conditions, but the reported incidence varies extensively. Potential risk factors for the malignant transformation were not described. Furthermore, conservative or surgical treatment is performed depending on the extent of cystic malformation, hepatic dysfunction and structural hepatic changes, but little is known about which treatment should be offered to patients with CD or CS and cancer.
To further investigate the malignant transformation in these conditions.
A systematic review of the current literature until January 2019 was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement. A search using Medline (PubMed) was performed using a combination of Medical Subject Headings terms “caroli disease”, “caroli syndrome”, “tumor”, “malignant”, and “cholangiocarcinoma”. Only human studies published in English were used for this systematic review. The following parameters were extracted from each article: year of publication, type of study, number of patients, incidence of malignant tumor, duration of symptoms, age, sex, diagnostics, identification of tumor, surgical therapy, survival and tumor recurrence.
Twelve retrospective studies reporting the courses of 561 patients (53% females) were included in this systematic review. With a mean age of 41.6 years old (range 23 to 56 years old), patients were younger than other populations undergoing liver surgery. Depending on the size of the study population the incidence of cholangiocarcinoma varied from 2.7% to 37.5% with an overall incidence of 6.6%. There were only few detailed reports about preoperative diagnostic work-up, but a multimodal work-up including ultrasound of the liver, computed tomography, magnetic resonance imaging and endoscopic retrograde cholangiopancreatography was used in most studies. Disease duration was variable with up to several years. Most patients had episodes of cholangitis, sepsis, fever or abdominal pain. Tumor detection was an incidental finding of the surgical specimen in most cases because it is currently often impossible to detect tumor manifestation during preoperative diagnostics. Liver resection or liver transplantation was performed depending on the extent of the biliary pathology and additional alterations of the liver structure or function. No postoperative adjuvant chemotherapy was reported, but chemotherapy was administered in selected cases of tumor recurrence. Overall survival rates after one year were low at 36% and a high recurrence rate of up to 75% during the observation period.
Only few retrospective studies reported a low tumor incidence. Despite the high rate of mortality and tumor recurrence, definite surgical treatment should be offered as soon as possible.
Core tip: Congenital intrahepatic bile duct dilatation with or without fibrosis is called Caroli syndrome or Caroli disease, respectively. We performed a systematic review of the current literature to investigate the incidence of malignant transformation, risk factors, and surgical treatment options. There were only 12 retrospective cohort studies reporting 561 patients, including 37 patients with malignancy, so an exact preoperative diagnostic work-up, risk factors and preferred surgical treatment were impossible to conclude due to lack of data. Overall survival rates after one year were low (36%), and recurrence rate was high (75%) during the observation period.
- Citation: Fahrner R, Dennler SG, Inderbitzin D. Risk of malignancy in Caroli disease and syndrome: A systematic review. World J Gastroenterol 2020; 26(31): 4718-4728
- URL: https://www.wjgnet.com/1007-9327/full/v26/i31/4718.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i31.4718
Vachell first described Caroli disease (CD) in 1906[1]. The segmental dilatation of the intrahepatic biliary ducts in combination with renal cystic disease and liver fibrosis is named Caroli syndrome (CS)[2,3]. Due to focal inflammation, likely initiated by an intrauterine malformation of the bile ductal plate, the biliary tract is continuously destroyed over decades[4,5]. Consequently, the further progression of this destructive inflammatory state leads to recurrent episodes of cholangitis, the development of biliary stones and malignant transformation in some cases, and cholangiocarcinoma is described, but is rare overall with reports up to 14%[5-7]. However, the risk of developing intrahepatic cholangiocellular carcinoma in CD or CS is increased 100-fold compared to healthy individuals[8]. The association between intrahepatic cystic lesions in CS or CD and cholangiocarcinoma was first published in 1968[9]. The distinct etiology of this association and malformation is not clear[8], but several theories are postulated: Carcinogenic component of the bile with prolonged effect during biliary stasis[8]; permanent stone irritation and formation of carcinogens[10]; and cellular predisposition for neoplastic change of the congenital cystic malformation of the biliary tract[8]. Unfortunately, the preoperative diagnosis of malignant transformation is difficult, and final diagnosis of the cholangiocarcinoma is typically made during pathological examination of the surgical specimen and less common in preoperative liver biopsies[6,11,12]. Furthermore, malignancy in CS or CD is suggested to be more likely in older patients[11], insufficient bile drainage[8] and long disease duration[8,10,12]. Therefore, the time span between the onset of first symptoms and definitive treatment is up to 60 mo in many cases[13,14]. The complaint symptoms may be unspecific and vary from pain, pruritus, fever, sepsis, recurrent cholangitis, abscesses, pancreatitis, weight loss, and adynamia[3,5,15-22]. Besides biliary drainage operations or interventions[7,22-24], liver resection[7,12,25] or orthotopic liver transplantation (OLT)[7] are performed. The decision to perform a specific intervention depends on the extent of biliary pathology and hepatic fibrosis and portal hypertension consecutively.
The present systematic review (1) analyzed the existing literature on the current incidence of malignancy in CS or CD; (2) the potential risk factors for the development of malignancy in patients with CS or CD; (3) the postoperative outcome of CS or CD after liver resection or OLT; and (4) the best treatment options for patients with CS or CD and suspected malignancy.
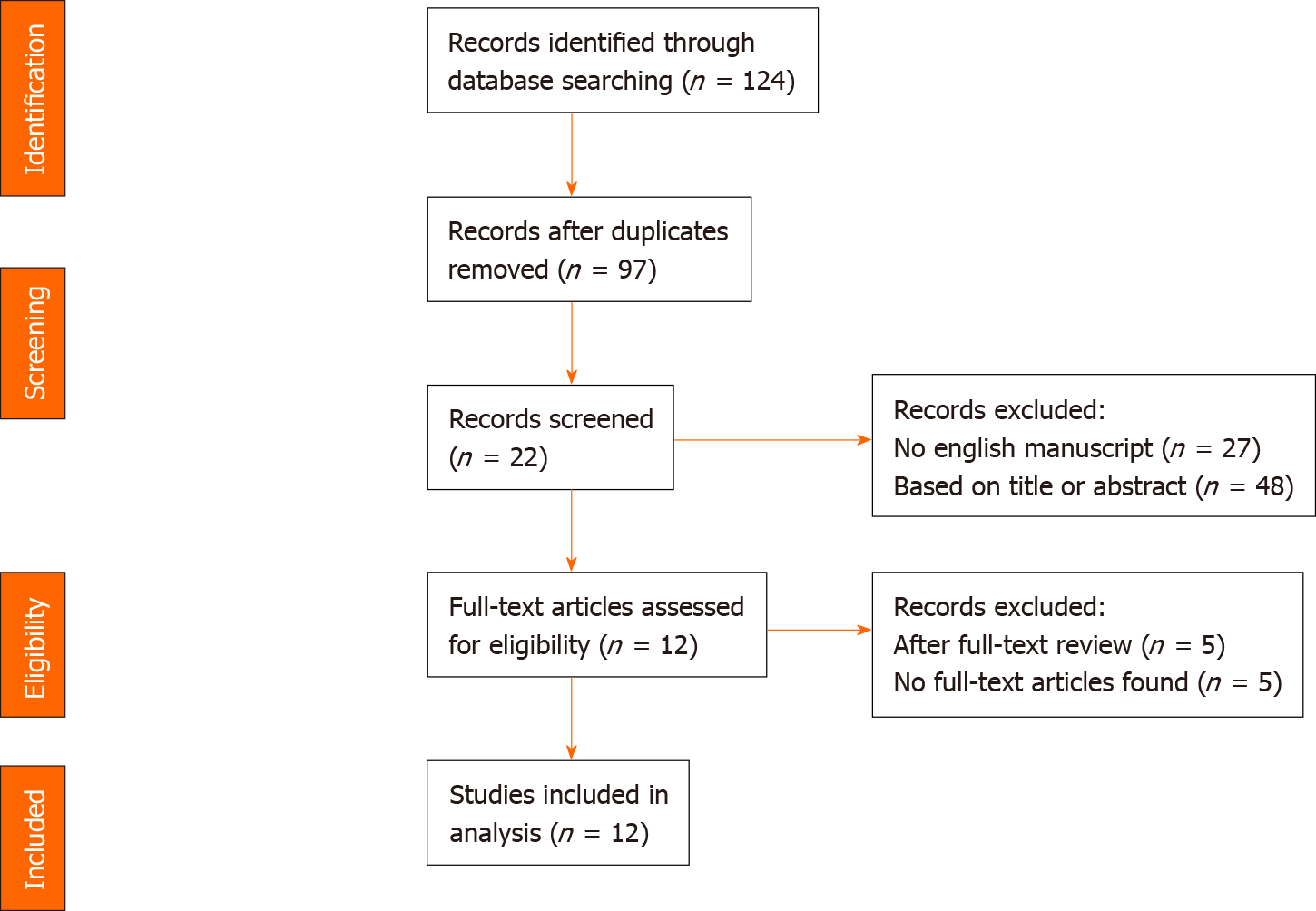
A systematic review of the literature until January 2019 was performed according the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement[26] to identify studies reporting the incidence of tumors in patients with CS and CD underwent resection or liver transplantation. Therefore, a literature search was performed using Medline (PubMed) from January 1993 to January 2019. During screening process, non-English articles were excluded. Reports with less than five patients and review articles were excluded. The search was performed using a combination of Medical Subject Headings terms “caroli disease”, “caroli syndrome”, “tumor”, “malignant”, and “cholangiocarcinoma”. Only human studies were used for this systematic review. The titles and abstracts of the articles were screened first for scientific relevance of this review. The full article was reviewed for potential eligibility and reported incidence of malignancy in the next step. The flow-chart show the process of identification, screening and final inclusion of articles in Figure 1.
The following parameters were extracted from each article: Year of publication, type of study, number of patients with CD or CS, incidence of malignant tumor, duration of symptoms (months), age in years, sex (females vs males), used diagnostics (ultrasound, computed tomography (CT), magnetic resonance imaging (MRI), endoscopic retrograde cholangiopancreatography (ERCP), and percutaneous cholangiography), identification of tumor (preoperative biopsy or incidental finding), surgical therapy (liver resection, OLT), survival and tumor recurrence.
Twelve manuscripts were included in this systematic review (Table 1). All studies were of a retrospective nature. Most studies were single-center studies (n = 8), and four studies included data of an official registry (n = 2) or multicenter (n = 2) analysis. The study populations of each study were small, with only three studies including more than 100 patients and 75% of the studies included less than 45 patients, with a total of 561 patients. The reported incidence of cholangiocarcinoma in patients treated for CS or CD varied from 2.7% to 37.5%, depending on the size of the study population. The overall incidence of cholangiocarcinoma in all included patients was 6.6% (n = 37).
| Ref. | Country | Year of publication | Type of study | n of reported patients | n of cancer (%) |
| Ammori et al[27] | United Kingdom | 2002 | Single-center, retrospective | 8 | 3 (37.5) |
| De Kerckhove et al[19] | Belgium | 2005 | ELTR registry, retrospective | 113 | 3 (2.7) |
| Kassahun et al[21] | Germany | 2005 | Single-center, retrospective | 33 | 3 (9) |
| Habib et al[17] | United States | 2006 | Single-center, retrospective | 33 | 1 (3) |
| Bockhorn et al[30] | Germany | 2006 | Single-center, retrospective | 12 | 3 (25) |
| Millwala et al[5] | United States | 2008 | UNOS registry, retrospective | 104 | 3 (2.9) |
| Ulrich et al[3] | Germany | 2008 | Single-center, retrospective | 44 | 4 (9) |
| Hori et al[24] | Japan | 2010 | Single-center, retrospective | 5 | 1 (20) |
| Lendoire et al[13] | Argentina | 2011 | Multicenter, retrospective | 24 | 1 (4.2) |
| Mabrut et al[20] | France | 2013 | Multicenter, retrospective | 155 | 8 (5) |
| Moslim et al[12] | United States | 2015 | Single-center, retrospective | 9 | 3 (33.3) |
| Fahrner et al[25] | Germany | 2018 | Single-center, retrospective | 21 | 4 (19) |
Only few studies reported a detailed preoperative diagnostic work-up for patients with CS and CD in general, and no study presented a work-up to identify cholangiocarcinoma within CS and CD (Table 2). Ultrasound of the liver, CT, MRI, and ERCP revealed the standard of preoperative diagnostics in most of the studies. Three studies reported percutaneous cholangiography to estimate the extent of biliary pathology.
| Ref. | CT | MRI | ERCP | Ultrasound | Others |
| Ammori et al[27] | Yes | Yes | 100% | Yes | No data |
| De Kerckhove et al[19] | Yes | Yes | Yes | Yes | Percutaneous cholangiography |
| Kassahun et al[21] | 100% | 16.2% | 83.9% | 100% | No data |
| Habib et al[17] | No data | No data | No data | No data | No data |
| Bockhorn et al[30] | 100% | No data | 92% | 100% | No data |
| Millwala et al[5] | No data | No data | No data | No data | No data |
| Ulrich et al[3] | Yes | Yes | Yes | Yes | Percutaneous cholangiography |
| Hori et al[24] | No data | No data | No data | No data | No data |
| Lendoire et al[13] | Yes | Yes | Yes | Yes | Percutaneous cholangiography |
| Mabrut et al[20] | No data | 74.7% | 27.7% | No data | No data |
| Moslim et al[12] | No data | No data | No data | No data | No data |
| Fahrner et al[25] | No data | No data | No data | No data | No data |
There was a slight predominance of female sex in all patients (53% females, Table 3). Patients suffering from CS or CD are younger than other populations undergoing liver resection, for e.g., liver cancer, the mean patient age ranged from 23 to 56 years old, with a total mean of 41.6 years old at the time point of surgery. Only five studies reported data on the duration of disease or symptoms. Patients reported at least 7 mo of mean duration of symptoms, but the most patients suffered from cholangitis, fever or abdominal pain for several years (Table 3). In most cases (89%), tumor identification was finally made in definitive histological assessment as an incidental finding. The diagnosis was suspected prior surgery or proven by preoperative biopsy only in less than five patients (14%).
| Ref. | Age (yr) | Female sex (%) | Duration of disease | Tumor identification |
| Ammori et al[27] | 35 | 75 | No data | No data |
| De Kerckhove et al[19] | 39.7 | 52 | No data | No data |
| Kassahun et al[21] | 51.5 | 55 | < 1 yr 51%, 1-5 yr 13%, > 5 yr 35% | No data |
| Habib et al[17] | 26 | 50 | No data | No data |
| Bockhorn et al[30] | 39 | 42 | No data | 67% incidental finding |
| Millwala et al[5] | 35 | 55 | No data | No data |
| Ulrich et al[3] | 49 | 55 | < 1 yr 55%, > 5 yr 2.5% | 100% incidental finding |
| Hori et al[24] | 23.74 | No data | 6.4 yr | 100% puncture of nodule prior surgery |
| Lendoire et al[13] | 48.7 | 70 | 2.6 yr | 100% incidental finding |
| Mabrut et al[20] | 55.7 | 43 | 7 mo | No data |
| Moslim et al[12] | 40 | 22 | No data | 100% incidental finding |
| Fahrner et al[25] | 56 | 74 | No data | 75% incidental finding 25% suspicious nodules in diagnostics |
Patients with CS or CD were treated via liver resection or OLT (Table 4). The indication for the onco-surgical treatment (liver resection or OLT) was primarly based on the extent of the biliary pathology and an additional alteration of the liver structure or function. Most of the tumors were incidental findings, and no exact proposal of liver resection or liver transplantation was possible on the basis of the data, but the proposed treatment of cholangiocarcinomas without CS or CD is likely applicable in these patients. In cases of tumor identification in histological assessment of the surgical specimen, no postoperative adjuvant chemotherapy was reported. Chemotherapy was administered in selected cases of tumor recurrence. Unfortunately, few data were available on the tumor-node-metastasis (TNM) classification or grading of the tumors. Most tumors were localized within in the liver (pT1 or pT2), but local lymph node involvement or peritoneal seeding was described (Table 4). However, overall survival rates after one year were low at 36% in total. It was impossible to distinguish the effect of liver resection or liver transplantation due to the retrospective reports of both different surgical treatments. The reported recurrence rate during the observation period in 7 studies was high, at up to 75%.
| Ref. | Surgery for all patients (LR vs OLT)1 | TNM | 1-yr survival cancer patients | Recurrence |
| Ammori et al[27] | 50% vs 50% | No data | 33% | 0% |
| De Kerckhove et al[19] | 0% vs 100% | No data | 66% | 33% |
| Kassahun et al[21] | 87% vs 0% | No data | 0% | 33% |
| Habib et al[17] | 0% vs 100% | No data | 0% | No data |
| Bockhorn et al[30] | 83% vs 17% | n = 1 pT1, n = 1 pT2 | 66% | 0% |
| Millwala et al[5] | 0% vs 100% | No data | 0% | No data |
| Ulrich et al[3] | 90% vs 10% | No data | 0% | No data |
| Hori et al[24] | 0% vs 100% | No data | No data | No data |
| Lendoire et al[13] | 91.6% vs 0% | No data | 100% | No data |
| Mabrut et al[20] | 75% vs 18.9% | n = 6 R0, n = 1 peritoneal seeding | 33% | 50% |
| Moslim et al[12] | 22% vs 78% | No data | 0% | 33%, 66% tumor progression |
| Fahrner et al[25] | 90% vs 10% | n = 1 pT1N1, n = 2 pT2, n = 1 pT3 | 100% | 75% |
Only a few reports investigated CS and CD and the development of cancer, and there was no suitable explanation for this fact in most of the publications. The present review analyzed the data of 561 patients included in 12 studies who were treated either with liver resection or OLT. Unfortunately, most of the studies were reports of less than 45 patients, and retrospective single or multicenter experiences were reported over several years in all cases. In addition, various case reports were not included in this systematic review.
The total incidence of cancerous transformation or simultaneous cancer in CS and CD is approximately 7%, but it ranged from approximately 3% to almost 40% in the beginning of the 2000s depending on the cohort[19,27]. Over time this incidence did not change, and the latest publications during the last six years reported incidences between 5% to 33%[12,20,25]. There were no new aspects reported on the development of cancerous transformation, and biliary stasis[8], stone irritation[10], a cellular predisposition of the cystic congenital malformation[8] and a combination of these factors are possible pathways[28]. Most cases of cancer are cholangiocarcinoma or adenocarcinoma, and the biliary epithelium is propagated as the origin of the cancerous transformation[6]. As one trigger for the malignant transformation, chronic inflammation of the biliary epithelium during cholangitis with consecutive dysplasia and carcinogenesis is postulated[29]. Unfortunately, due to little data, the hypothesis of an increased incidence for malignant transformation in large bile ducts rather than in small peripheral ducts could not be proven in this review[14].
There are only few data on the structured preoperative work-up of patients with CS and CD regarding the used method. Ultrasound was used in most studies to identify cystic lesions within the liver and potential altered liver structure, such as liver cirrhosis[21,30]. To further evaluate the biliary tract, ERCP or percutaneous cholan-giography was used in selected cases in some of the studies[3,13,19,27]. However, the use of invasive biliary diagnostics should be avoided and performed only in selected patients because they are often associated with an increased risk for infections[19]. If reported, CT or MRI scans were used to investigate the nature of the cysts, identify possible malignant transformation, and plan and assess the surgical resectability[20,21,27]. The tumor markers Ca-19-9 is often elevated in cases of benign biliary obstruction or cholangitis, and higher levels may mislead the correct diagnosis[7]. Overall, the diagnosis of malignancy in CS and CD is difficult, and the tumor identification was an incidental finding in most of the reports in this review, which was made during the final histological assessment of the surgical specimen, because the preoperative identification of a tumor within a dilated cystic bile tract is almost impossible[3,8,11,13,25]. Therefore, the value of screening patients with CS and CD for malignancy is quite inaccurate, and definite treatment should be sought, because delayed diagnosis and widespread tumor are associated with a poor prognosis[18]. In the future, a more aggressive diagnostic work-up including endoscopic ultrasound, puncture of suspected segments within the biliary tract, cholangioscopy (percutaneously) should be used, with the goal to detect more frequently patients with malignant trans-formation and therefore avoiding delay of definite surgical treatment.
The initial treatment of CS and CD is often nonsurgical and supportive therapy using analgesia, antibiotics in case of biliary infections and ursodeoxycholic acid[31,32]. Surgical or interventional drainage procedures may be performed to remove biliary tract obstructions and improve biliary passage to decrease the risk and burden of cholangitis[33,34]. Beside this conservative treatment, the final diagnosis of CS or CD often takes months or years until the patient is referred for definite surgical treatment. During that time, patients suffer recurrent infections, sepsis, hepatic abscesses, portal hypertension, and the risk of malignant transformation without solving the problem of the dilated and cystic biliary tract[30,35-38]. There are reports that malignant transformation does not depend on the disease duration, because cancer occurred in patients with symptomatic CS or CD of two months to two decades. Therefore, it has been postulated that there are likely more aggressive subgroups in CS or CD with increased potential for malignancy[30]. Due to a lack of data, there is no explanation or postulated pathway for this fact.
As a conclusion for this observation, surgical treatment with liver resection or OLT should be offered to patients with CS or CD as soon as possible to avoid recurrent sepsis and primarily malignant transformation[3,25,30]. In cases of conservative treatment, even asymptomatic patients should be regularly screened clinically and radiologically for malignancy[30]. To obtain the best treatment and surveillance, an interdisciplinary management team, including surgeons, gastroenterologists, hepatologists, radiologists, pediatrician and specialists in infectious diseases, is needed. Therefore, these patients are often treated in transplant centers. Overall, a high suspicion for potential malignant transformation is needed, and early treatment should be intended.
After one year, the survival rate of all tumor patients was 35%, which is low and much lower than in hepatocellular carcinoma patients[39-41] or selected surgical treated cholangiocarcinoma patients[42-45]. At least 32% had tumor recurrence during the observation period, but five studies did not report any data about recurrence. Therefore, the incidence may be even higher, and some patients likely died before the detection of any tumor recurrence probably due to the high mortality rate. In some studies, a high morbidity and in-house mortality was reported after OLT due to infectious complications and therapy-resistant sepsis[17,21]. The general treatment suggestion is to perform liver resection in defined and localized cystic degeneration and evaluate OLT in diffuse cystic affection with structural hepatic changes[3,25,27,30].
On the basis of the data, no adjuvant chemotherapy was administered in cases of cholangiocarcinoma or adenocarcinoma. There were only a few reports of chemotherapy in cases of recurrence when surgery was no longer an option. This result is comparable to patients with cholangiocarcinoma without CS or CD, where chemotherapy is used only in nonresectable and extended tumors or recurrence[46].
The studies were not comparable. For example, TNM staging was available only in a few studies. The extent of cystic degeneration and underlying structural liver changes greatly influences the choice of treatment as mentioned previously. In cases of cancer in CS patients treated with OLT, the long-term survival rate was 0%[17,27], and recurrence rates were high, which are comparable to studies of OLT and cholangiocarcinoma without CS or CD[47]. Patients with localized and small cholangiocarcinoma likely benefit from OLT, although little current data exist[7].
The following limitations of this systematic review must be addressed. Overall, there were predominantly small, retrospective cohort studies with nonhomogeneous patient collectives treated with liver resection or OLT. Therefore, it was not possible to identify specific risk factors indicating the risk for malignant transformation in CS or CD, because these two entities were not reported separately. Another aspect was that most of the tumors were incidental findings, so a specific work-up algorithm is likely not possible to define. Further prospective and multicenter studies or databases with the specific goal to identify risk factors for malignant transformation are needed.
In conclusion, there were only few retrospective studies of patients treated with surgery in cases of CS and CD. Therefore, it was not possible to identify valuable risk factors for the malignant transformation in these patients. On the other hand, the previously postulated factors for the development of malignancy could neither be proven nor rebutted. Overall, the incidence of simultaneous malignancy in CS or CD is low, but high tumor rates to almost 40% were reported depending on the investigated cohort. Because a high proportion of malignant tumors were incidental findings, the definite surgical treatment should be offered as soon as possible to avoid septic complications and the development of carcinoma. Surgical treatment should include liver resection in localized affection (primarily CD) and OLT in diffuse affection and structural liver changes, such as liver fibrosis (CS). In proven malignant transformation, small tumors may be treated with OLT successfully, but extensive tumors are mostly associated with an inferior outcome, e.g., due to tumor recurrence. Overall, the postoperative results are associated with a high rate of mortality and tumor recurrence. Therefore, patient selection and the timing of surgery are of high importance. On the basis of the current literature, there are no optimal screening methods described to identify patients with malignant transformation early and safely. Therefore, a high suspicion of malignancy is needed to find the exact time point for surgery and avoid delay in definite surgical treatment. Finally, new screening tools are needed to increase the rate of tumor identification during long-term surveillance of patients with CS or CD.
Congenital intrahepatic bile duct dilatation without fibrosis is called Caroli disease (CD), and is called Caroli syndrome (CS) when it has fibrotic and cirrhotic liver morphology. The development of intrahepatic carcinoma is described in both conditions, but the reported incidence varies extensively. Furthermore, there are no valid guidelines how to treat and diagnose malignant transformation in these patients.
So far no valid potential risk factors for the malignant transformation were described. Furthermore, conservative or surgical treatment is performed depending on the extent of cystic malformation, hepatic dysfunction and structural hepatic changes, but little is known about which treatment should be offered to patients with CD or CS and especially cancer.
We performed a systematic review of the current literature to investigate specific conditions for malignant transformation in patients with CD and CS and to identify potential risk factors and recommendations regarding diagnosis and surgical treatment.
A systematic review of the current literature until January 2019 was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement. During screening process, non-English articles and reports with less than five patients were excluded. The search was performed using a combination of Medical Subject Headings terms “caroli disease”, “caroli syndrome”, “tumor”, “malignant”, and “cholangiocarcinoma”. Only human studies were used for this systematic review.
Twelve retrospective studies reporting the courses of 561 patients (53% females) were included in this systematic review. The patients’ mean age was 41.6 years old (range 23 to 56 years old), therefore patients were younger than other populations undergoing liver surgery. The incidence of cholangiocarcinoma varied from 2.7% to 37.5% with an overall incidence of 6.6%. There were only few detailed reports about preoperative diagnostic work-up. Disease duration was variable with up to several years. In most cases tumor detection was an incidental finding of the surgical specimen as tumor detection in preoperative diagnostics was often impossible. Surgical treatment depended on the extent of the biliary pathology and additional alterations of the liver structure or function. No adjuvant chemotherapy was reported. Overall survival rates after one year were low at 36% and a high recurrence rate of up to 75% during the observation period was seen.
Only few retrospective studies are available and reported a low tumor incidence. On the basis of these studies distinct suggestions regarding diagnosis and treatment are impossible to define. Despite the high rate of mortality and tumor recurrence, definite surgical treatment should be offered as soon as possible.
Further prospective multicenter investigations regarding patient surveillance, diagnostic tools, time point of surgery and risk factors of malignant transformation are needed.
Manuscript source: Invited manuscript
Specialty type: Surgery
Country/Territory of origin: Switzerland
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bugaj AM S-Editor: Zhang H L-Editor: A P-Editor: Ma YJ
| 1. | Vachell HR, Stevens WM. CASE OF INTRAHEPATIC CALCULI. Br Med J. 1906;1:434-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 2. | Caroli J, Soupault R, Kossakowski J, Plocker L, Paradowska. [Congenital polycystic dilation of the intrahepatic bile ducts; attempt at classification]. Sem Hop. 1958;34:488-95/SP. [PubMed] |
| 3. | Ulrich F, Pratschke J, Pascher A, Neumann UP, Lopez-Hänninen E, Jonas S, Neuhaus P. Long-term outcome of liver resection and transplantation for Caroli disease and syndrome. Ann Surg. 2008;247:357-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Desmet VJ. Congenital diseases of intrahepatic bile ducts: variations on the theme "ductal plate malformation". Hepatology. 1992;16:1069-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 340] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 5. | Millwala F, Segev DL, Thuluvath PJ. Caroli's disease and outcomes after liver transplantation. Liver Transpl. 2008;14:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Balsells J, Margarit C, Murio E, Lazaro JL, Charco R, Vidal MT, Bonnin J. Adenocarcinoma in Caroli's disease treated by liver transplantation. HPB Surg. 1993;7:81-6; discussion 86-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Lai Q, Lerut J. Proposal for an algorithm for liver transplantation in Caroli's disease and syndrome: putting an uncommon effort into a common task. Clin Transplant. 2016;30:3-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Dayton MT, Longmire WP, Tompkins RK. Caroli's Disease: a premalignant condition? Am J Surg. 1983;145:41-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 99] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Schiewe R, Baudisch E, Ehrhardt G. [Congenital intrahepatic bile duct cysts with calcinosis and malignant degenerations]. Bruns Beitr Klin Chir (1971). 1968;216:264-271. [PubMed] |
| 10. | Flanigan DP. Biliary carcinoma associated with biliary cysts. Cancer. 1977;40:880-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 11. | Vlachogiannakos J, Potamianos S, Triantos C, Makri I, Imvrios G, Patsiaoura K, Avgerinos A. Monolobar Caroli's disease complicated by cholangiocarcinoma in a 70-year-old man, previously asymptomatic. Gastrointest Endosc. 2004;60:297-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Moslim MA, Gunasekaran G, Vogt D, Cruise M, Morris-Stiff G. Surgical Management of Caroli's Disease: Single Center Experience and Review of the Literature. J Gastrointest Surg. 2015;19:2019-2027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Lendoire JC, Raffin G, Grondona J, Bracco R, Russi R, Ardiles V, Gondolesi G, Defelitto J, de Santibañes E, Imventarza O. Caroli's disease: report of surgical options and long-term outcome of patients treated in Argentina. Multicenter study. J Gastrointest Surg. 2011;15:1814-1819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Mabrut JY, Partensky C, Jaeck D, Oussoultzoglou E, Baulieux J, Boillot O, Lerut J, de Ville de Goyet J, Hubert C, Otte JB, Audet M, Ducerf C, Gigot JF. Congenital intrahepatic bile duct dilatation is a potentially curable disease: long-term results of a multi-institutional study. Ann Surg. 2007;246:236-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Clemente G, Giuliante F, De Rose AM, Ardito F, Giovannini I, Nuzzo G. Liver resection for intrahepatic stones in congenital bile duct dilatation. J Visc Surg. 2010;147:e175-e180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Taylor AC, Palmer KR. Caroli's disease. Eur J Gastroenterol Hepatol. 1998;10:105-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 60] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Habib S, Shakil O, Couto OF, Demetris AJ, Fung JJ, Marcos A, Chopra K. Caroli's disease and orthotopic liver transplantation. Liver Transpl. 2006;12:416-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Abdalla EK, Forsmark CE, Lauwers GY, Vauthey JN. Monolobar Caroli's Disease and cholangiocarcinoma. HPB Surg. 1999;11:271-6; discussion 276-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | De Kerckhove L, De Meyer M, Verbaandert C, Mourad M, Sokal E, Goffette P, Geubel A, Karam V, Adam R, Lerut J. The place of liver transplantation in Caroli's disease and syndrome. Transpl Int. 2006;19:381-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Mabrut JY, Kianmanesh R, Nuzzo G, Castaing D, Boudjema K, Létoublon C, Adham M, Ducerf C, Pruvot FR, Meurisse N, Cherqui D, Azoulay D, Capussotti L, Lerut J, Reding R, Mentha G, Roux A, Gigot JF. Surgical management of congenital intrahepatic bile duct dilatation, Caroli's disease and syndrome: long-term results of the French Association of Surgery Multicenter Study. Ann Surg. 2013;258:713-21; discussion 721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Kassahun WT, Kahn T, Wittekind C, Mössner J, Caca K, Hauss J, Lamesch P. Caroli's disease: liver resection and liver transplantation. Experience in 33 patients. Surgery. 2005;138:888-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Jarufe N, Figueroa E, Muñoz C, Moisan F, Varas J, Valbuena JR, Bambs C, Martínez J, Pimentel F. Anatomic hepatectomy as a definitive treatment for hepatolithiasis: a cohort study. HPB (Oxford). 2012;14:604-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Otani K, Shimizu S, Chijiiwa K, Ogawa T, Morisaki T, Sugitani A, Yamaguchi K, Tanaka M. Comparison of treatments for hepatolithiasis: hepatic resection versus cholangioscopic lithotomy. J Am Coll Surg. 1999;189:177-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 80] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Hori T, Oike F, Ogura Y, Ogawa K, Hata K, Yonekawa Y, Ueda M, Sakamoto S, Kasahara M, Egawa H, Takada Y, Kaido T, Hatano E, Nguyen JH, Chen F, Baine AM, Uemoto S. Liver transplantation for congenital biliary dilatation: a single-center experience. Dig Surg. 2010;27:492-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Fahrner R, Dennler SGC, Dondorf F, Ardelt M, Rauchfuss F, Settmacher U. Liver resection and transplantation in Caroli disease and syndrome. J Visc Surg. 2019;156:91-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 47171] [Article Influence: 2948.2] [Reference Citation Analysis (0)] |
| 27. | Ammori BJ, Jenkins BL, Lim PC, Prasad KR, Pollard SG, Lodge JP. Surgical strategy for cystic diseases of the liver in a western hepatobiliary center. World J Surg. 2002;26:462-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 28. | Phinney PR, Austin GE, Kadell BM. Cholangiocarcinoma arising in Caroli's disease. Arch Pathol Lab Med. 1981;105:194-197. [PubMed] |
| 29. | Ulrich F, Steinmüller T, Settmacher U, Müller AR, Jonas S, Tullius SG, Neuhaus P. Therapy of Caroli's disease by orthotopic liver transplantation. Transplant Proc. 2002;34:2279-2280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Bockhorn M, Malagó M, Lang H, Nadalin S, Paul A, Saner F, Frilling A, Broelsch CE. The role of surgery in Caroli's disease. J Am Coll Surg. 2006;202:928-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Ros E, Navarro S, Bru C, Gilabert R, Bianchi L, Bruguera M. Ursodeoxycholic acid treatment of primary hepatolithiasis in Caroli's syndrome. Lancet. 1993;342:404-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 54] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Yonem O, Bayraktar Y. Clinical characteristics of Caroli's disease. World J Gastroenterol. 2007;13:1930-1933. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 65] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 33. | Yonem O, Bayraktar Y. Clinical characteristics of Caroli's syndrome. World J Gastroenterol. 2007;13:1934-1937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 66] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 34. | Moreno González E, Gómez Sanz R, Hidalgo Pascual M, Garcia Garcia I, Rico Selas P, Calle Santiuste A, Loinaz Segurola C, Bercedo Martinez J. Surgical treatment of congenital dilatation of the biliary system. Hepatogastroenterology. 1993;40:134-138. [PubMed] |
| 35. | Tsuchida Y, Sato T, Sanjo K, Etoh T, Hata K, Terawaki K, Suzuki I, Kawarasaki H, Idezuki Y, Nakagome Y. Evaluation of long-term results of Caroli's disease: 21 years' observation of a family with autosomal "dominant" inheritance, and review of the literature. Hepatogastroenterology. 1995;42:175-181. [PubMed] |
| 36. | Sun WB, Han BL, Cai JX. The surgical treatment of isolated left-sided hepatolithiasis: a 22-year experience. Surgery. 2000;127:493-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 37. | Totkas S, Hohenberger P. Cholangiocellular carcinoma associated with segmental Caroli's disease. Eur J Surg Oncol. 2000;26:520-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 38. | Shimonishi T, Sasaki M, Nakanuma Y. Precancerous lesions of intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 2000;7:542-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 80] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 39. | Han JH, Kim DG, Na GH, Kim EY, Lee SH, Hong TH, You YK. Evaluation of prognostic factors on recurrence after curative resections for hepatocellular carcinoma. World J Gastroenterol. 2014;20:17132-17140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 40. | Wang JH, Wang CC, Hung CH, Chen CL, Lu SN. Survival comparison between surgical resection and radiofrequency ablation for patients in BCLC very early/early stage hepatocellular carcinoma. J Hepatol. 2012;56:412-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 272] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 41. | Kluger MD, Salceda JA, Laurent A, Tayar C, Duvoux C, Decaens T, Luciani A, Van Nhieu JT, Azoulay D, Cherqui D. Liver resection for hepatocellular carcinoma in 313 Western patients: tumor biology and underlying liver rather than tumor size drive prognosis. J Hepatol. 2015;62:1131-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 97] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 42. | Dondorf F, Uteβ F, Fahrner R, Felgendreff P, Ardelt M, Tautenhahn HM, Settmacher U, Rauchfuβ F. Liver Transplant for Perihilar Cholangiocarcinoma (Klatskin Tumor): The Essential Role of Patient Selection. Exp Clin Transplant. 2019;17:363-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 43. | Laurent S, Verhelst X, Geerts A, Geboes K, De Man M, Troisi R, Vanlander A, Rogiers X, Berrevoet F, Van Vlierberghe H. Update on liver transplantation for cholangiocarcinoma : a review of the recent literature. Acta Gastroenterol Belg. 2019;82:417-420. [PubMed] |
| 44. | Yoh T, Cauchy F, Le Roy B, Seo S, Taura K, Hobeika C, Dokmak S, Farges O, Gelli M, Sa Cunha A, Adam R, Uemoto S, Soubrane O. Prognostic value of lymphadenectomy for long-term outcomes in node-negative intrahepatic cholangiocarcinoma: A multicenter study. Surgery. 2019;166:975-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 45. | Bagante F, Spolverato G, Merath K, Weiss M, Alexandrescu S, Marques HP, Aldrighetti L, Maithel SK, Pulitano C, Bauer TW, Shen F, Poultsides GA, Soubrane O, Martel G, Koerkamp BG, Guglielmi A, Endo I, Pawlik TM. Intrahepatic cholangiocarcinoma tumor burden: A classification and regression tree model to define prognostic groups after resection. Surgery. 2019;166:983-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 46. | Lowery MA, Goff LW, Keenan BP, Jordan E, Wang R, Bocobo AG, Chou JF, O'Reilly EM, Harding JJ, Kemeny N, Capanu M, Griffin AC, McGuire J, Venook AP, Abou-Alfa GK, Kelley RK. Second-line chemotherapy in advanced biliary cancers: A retrospective, multicenter analysis of outcomes. Cancer. 2019;125:4426-4434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 47. | Meyer CG, Penn I, James L. Liver transplantation for cholangiocarcinoma: results in 207 patients. Transplantation. 2000;69:1633-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 348] [Article Influence: 13.9] [Reference Citation Analysis (0)] |