Published online Aug 21, 2020. doi: 10.3748/wjg.v26.i31.4639
Peer-review started: February 20, 2020
First decision: March 24, 2020
Revised: April 9, 2020
Accepted: July 18, 2020
Article in press: July 18, 2020
Published online: August 21, 2020
Processing time: 182 Days and 20.1 Hours
Colorectal cancer is the third most common malignancy worldwide. Therefore, it is critically important to identify new useful markers that can be easily obtained in routine practice. Inflammation is a crucial issue in the pathogenesis and development of cancer.
To evaluate the prognostic value of absolute monocyte count, monocyte to lymphocyte ratio (MLR), the combination of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio (NLR-PLR), and combined platelet and neutrophil-to-lymphocyte ratio (PLT-NLR) in peripheral blood samples of patients with colorectal cancer undergoing surgery.
We conducted a retrospective study of 160 patients with colorectal cancer who underwent surgery, and 42 healthy controls. The status of absolute monocyte count, MLR, NLR-PLR and PLT-NLR was calculated on the basis of blood samples obtained before and after surgery. Haematologic factors were examined in correlation with the type of tumour growth, tumour size, histological type, percentage of mucinous component, grade of malignancy, Tumour-Node-Metastasis stage, venous, lymphatic and perineural invasion of cancer cells, status of lymph node invasion and the presence of cancer cell deposits. The Kaplan-Meier method and the long-rank test were used to compare survival curves. To determine independent prognostic factors, univariate and multivariate Cox proportional hazards regression models were applied.
The PLT-NLR status was correlated with tumour size and the presence of perineural invasion (P = 0.015; P = -0.174, P = 0.037). Moreover, high NLR-PLR and PLR-NLR ratios in the blood samples obtained after surgery were positively associated with histological type of cancer and percentage of the mucinous component (NLR-PLR: P = 0.002; P = 0.009; PLR-NLR status: P = 0.002; P = 0.007). The analysis of 5-year disease-free survival showed that the MLR of whole blood obtained after surgery [HR = 2.903, 95%CI: (1.368-6.158), P = 0.005] and the status of lymph node metastasis [HR = 0.813, 95%CI: (0.653-1.013), P = 0.050] were independent prognostic factors in colorectal cancer patients.
The postoperative MLR in whole blood samples can be used as an independent prognostic factor in patients diagnosed with colorectal cancer.
Core tip: This is a retrospective study evaluating the monocyte-to-lymphocyte ratio in peripheral whole blood samples of colorectal cancer patients. Haematologic parameters can be useful in the diagnosis and prognosis of colorectal cancer. The monocyte-to-lymphocyte ratio of whole blood obtained after surgery was an independent factor in the 5-year disease-free survival time of colorectal cancer patients.
- Citation: Jakubowska K, Koda M, Grudzińska M, Kańczuga-Koda L, Famulski W. Monocyte-to-lymphocyte ratio as a prognostic factor in peripheral whole blood samples of colorectal cancer patients. World J Gastroenterol 2020; 26(31): 4639-4655
- URL: https://www.wjgnet.com/1007-9327/full/v26/i31/4639.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i31.4639
According to the latest data, colorectal cancer (CRC) is the third most common malignancy worldwide. It is estimated to be the third leading cause of death due to cancer in both males and females[1]. The appearance of regional lymph-node metastasis in CRC is a relevant risk factor for the development not only of regional recurrences but also distant metastatic lesions[2]. Distant metastases are the main cause of death in CRC patients. It is assessed that roughly one-fifth of CRC patients will have liver metastases. A significant number of patients die within 5 years[3]. Surgery is the most frequent and primary treatment in non-metastatic CRC. In addition, patients with stage II and III undergo chemotherapy based on 5fluorouracil[4]. Early identification of patients with a poor prognosis could optimize treatment. It is currently believed that the characteristics of a tumour mass are insufficient to determine prognosis and treatment.
A systemic inflammatory response is related to tumour progression[5]. In addition, inflammatory cells are key players in the tumour microenvironment[6]. Their count can be measured by simple laboratory tests. The available data on peripheral blood cells in cancer patients show that a rise in monocyte count in the peripheral blood is a predictive factor of a poor prognosis in patients with hepatocarcinoma or stomach cancer[7,8]. In another study, monocyte count was an independent factor correlated with survival in patients with metastasis from CRC[9]. Another important parameter is the monocyte/lymphocyte ratio (MLR). In recent times, the MLR has been investigated in patients with solid tumours. The monocyte level divided by the lymphocyte level may be an efficient prognostic indicator in tumours[10,11]. The neutrophil/lymphocyte ratio (NLR) has been described as a prognostic factor dependent on CRC stage. The NLR ratio was found to be higher in cases with more advanced disease[12]. Another inflammation-based marker is the combination of platelet count (PLT) and NLR (PLT–NLR status). This indicator was examined to predict survival in patients with gastric cancer[13]. Identifying novel prognostic markers of CRC may help identify patients at high risk and choose appropriate treatment in selected cases.
Therefore, the aim of the present study was to investigate the correlation of monocyte count, MLR, neutrophil-to-monocyte ratio (NMR), NLR-PLR status and PLT-NLR status with clinical outcome in CRC patients.
We retrospectively reviewed the medical records of 160 patients diagnosed with CRC (96 men and 64 women) who underwent surgery in the Department of Oncological Surgery, Comprehensive Cancer Centre (Bialystok, Poland) between April 2014 and December 2016. The mean age of the patients was 67.5 years, including 40 patients < 60 years old and 120 patients ≥ 60 years old. The majority presented similar symptoms, including abdominal pain, anaemia, rectal bleeding, constipation, diarrhoea, vomiting and anorexia. In most cases, patients additionally received treatment for hypertension, type II diabetes, osteoarthritis and coronary heart disease. However, none of the patients had received anti-inflammatory therapy. All patients underwent routine diagnostic tests, including basic diagnostic laboratory tests (morphological tests and lipid profiles), electrocardiography, spirometry, arterial blood gas analysis, X-ray and chest computed tomography. The clinical stage of CRC was evaluated according to the Tumour-Node-Metastasis (TNM) classification[14]. Prior to surgery, patients with tumours identified in other sites had not received anti-inflammatory or immu-nosuppressive therapy.
Patients diagnosed with neoplasms in the rectum received preoperative therapy (n = 53). They received radiotherapy (n = 39), chemotherapy (n = 7) and radio-chemotherapy (n = 7), and received a dose of 25 Gy in fractions of 5 Gy during one week in the pelvic area. The response to preoperative therapy was estimated according to the Response Evaluation Criteria in Solid Tumours[15]. Stable disease was observed in 26 patients, while 27 patients had a partial response.
The inclusion criteria were as follows: (1) Pathologically confirmed CRC; (2) Treatment with radical resection; and (3) No previous anti-inflammatory therapy. The exclusion criteria were: (1) Incomplete clinicopathological and follow-up data; and (2) Presence of haematological disorders.
Tissues obtained from surgery were fixed in 4% buffered formalin for 24 to 72 h at room temperature. Small sections of tissue were embedded in paraffin. Sections (4 µm-thick) were cut from paraffin blocks and stained with haematoxylin and eosin (HE) at room temperature for 4 min (cat. no. 468802128; POCH S.A.; Avantor Performance Materials Poland, Gliwice, Poland) according to the manufacturer's protocol. The slides were deparaffinised in an oven at 60°C for 5 min. The slides were subsequently rehydrated in xylene (three washes, 10 min each) and graded ethanol (100%, 95%, 85% and 75%, 1 min at each concentration). The type of tumour growth, tumour size, histological type, percentage of mucinous component, grade of malignancy and TNM stage were determined by pathologists. Venous, lymphatic and perineural invasions of cancer cells were also analysed. Characteristic features of lymph node invasion were examined, including the number of resected and invaded lymph nodes, the presence of micro- and macro-metastases, invasion of the pouch lymph node, the presence of distant metastases and the size of metastases. The presence, number and size of cancer cell deposits were also assessed[16].
Blood samples were obtained within 3 d before and after surgical treatment. Venous blood samples were also obtained from 42 healthy controls (female: 21, male: 21; mean age: 45 years old; min-max: 25-65 years old). The differential white blood cell count was determined using an XN-1000 automated haematology analyser (Sysmex Co., Kobe, Japan).
We analysed monocyte count and the MLR in whole blood before and after surgery of patients with CRC. The MLR was defined as the absolute monocyte count divided by the absolute lymphocyte count. Receiver operating characteristic (ROC) curve analysis was used to investigate cut-off values of the pre- and postoperative monocyte count (preMONO/postMONO) and MLR (preMLR/postMLR). The cut-off of pre- and postoperative monocyte count were 0.39 and 0.68 with sensitivity (preMONO-75.8%; postMONO-27.89%) and specificity (preMONO-16.67%; postMONO-85.71%) The area under the ROC curve for pre and post monocyte count had weak prognostic value in CRC patients (0.505 and 0.532). Moreover, the cut-off of pre- and postoperative MLR was 1.46 in both cases. The sensitivity and specificity of analysis in preoperative blood samples were 94.27% and 73.81% and were similar to those in postoperative samples (90.41% and 73.81%). The area under the ROC curve for pre and postNMR showed that the parameter exhibits strong diagnostic power (1.000). Pre and post MLR had moderate diagnostic power of 0.751 and 0.746. Patients were divided into the high and low groups according to the cut-off values in pre- and postoperative blood samples.
The NLR-PLR was calculated according to Hirahara et al[17]. The NLR-PLR status consists of two parameters: NLR (the absolute neutrophil count divided by the absolute lymphocyte count) and PLR (the platelet count divided by the absolute lymphocyte count). The cut-off values were 2.5 and 267.3, respectively. Patients with low values of both parameters (NLR < 2.5, PLR < 267.3) were scored as group 0. Score 1 consisted of one high parameter: NLR (> 2.5) or PLR (> 267.3). Score 2 was generated by combining both high parameters (NLR > 2.5, PLR > 267.3). The PLT-NLR status was generated by PLT with the NLR score. The cut-off values were 300 and 2.5 based on the ROC curve. Score 0 was calculated as PLT (< 300) and NLR (< 2.5). Patients with either elevated NLR or PLR were allocated to score 1. Patients with both increased NLR (> 2.5) and PLR (> 300) were in group 2. For disease-free survival (DFS) analysis, we divided the study group into two subgroups with score 0 and score 1 + 2.
Patients were followed up for 2-5.0 years. They were monitored by the measurement of carcinoembryonic antigen and CA19-9 levels, physical examination, colonoscopy or/and radiological imaging including computed tomography of the chest, abdomen and pelvis, bone scan, and positron emission tomography scans. Local and distant recurrences were defined as pathologic evidence of the spread of tumours in the region of anastomosis (local recurrence) or/and present outside the primary tumour at other sites such as liver, lungs, bones, brain (distant recurrence) and confirmed by the techniques mentioned above.
Statistical analysis was performed using the STATISTICA 13.0 program. Comparisons between groups were analysed using the independent-samples t-test, and comparisons within groups were analysed using the paired t-test. Enumeration data were analysed using the χ2 test. Comparisons among multiple groups were analysed using one-way ANOVA. DFS time was calculated as the duration between the date of diagnosis and the date of disease progression, including local or distant relapse. The DFS rate was estimated using the Kaplan Meier estimator method and the survival curves were compared using log-rank tests. Prognostic factors were assessed using univariate and multivariate analyses (Cox proportional hazard regression model). A P value < 0.05 was considered statistically significant.
The study group was heterogeneous in terms of sex (40% female, 60% male) and age (25% < 60 years old, 75% > 60 years old). Tumour locations were the right-side of the colon in 20 patients (12.5%), transverse colon in 14 patients (8.75%), left-side in 15 patients (9.37%), sigmoid colon in 29 patients (18.12%) and rectum in 82 patients (51.25%). There were 42 (26.25%) patients with TNM stage I, 31 (19.37%) with stage II, 69 (43.12%) with stage III and 18 (11.25%) with stage IV. In more than 92% of patients the grade of malignancy was determined as 2. Tumour size was between 2.5 and 5.0 cm in 106 patients (66.25%). Among 160 evaluable patients, only 17 had distant metastasis. Lymph-node metastasis was found in almost half of patients in the study group (49.37%). The presence of venous invasion and lymphatic invasion was determined in 46 and 38 patients, respectively. The complete characteristics of the study group are shown in Table 1.
| Parameter | n | Percentage (%) | |
| Age (yr) | < 60 | 40 | 25.0 |
| ≥ 60 | 120 | 75.0 | |
| Sex | Female | 64 | 40.0 |
| Male | 96 | 60.0 | |
| Location | Right-side | 20 | 12.5 |
| Transverse | 14 | 8.75 | |
| Left-side | 15 | 9.375 | |
| Sigmoid | 29 | 18.125 | |
| Rectum | 82 | 51.25 | |
| Tumour growth | Expanding | 133 | 83.125 |
| Infiltrate | 27 | 16.875 | |
| Tumour size | < 2.5 cm | 27 | 16.875 |
| 2.5-5.0 cm | 106 | 66.25 | |
| 5.0 cm | 27 | 16.875 | |
| TNM stage | 1 | 42 | 26.25 |
| 2 | 31 | 19.375 | |
| 3 | 69 | 43.125 | |
| 4 | 18 | 11.25 | |
| Grade of malignancy | 2 | 148 | 92.5 |
| 3 | 12 | 7.5 | |
| pT stage | 1-2 | 65 | 40.625 |
| 3-4 | 95 | 59.375 | |
| Venous invasion | Absent | 113 | 70.625 |
| Present | 46 | 28.75 | |
| Lymphatic invasion | Absent | 121 | 75.625 |
| Present | 38 | 23.75 | |
| Lymph-node metastasis | Absent | 81 | 50.625 |
| Present | 79 | 49.375 | |
| Distant metastasis | Absent | 143 | 89.375 |
| Present | 17 | 10.625 | |
| Tumour deposits | Absent | 133 | 83.125 |
| Present | 27 | 16.875 | |
| CEA levels (ng/mL) | < 3.5 | 124 | 77.5 |
| > 3.5 | 36 | 22.5 | |
| CA19-9 levels (U/mL) | < 27 | 150 | 93.75 |
| > 27 | 10 | 6.25 | |
| Monocyte count | Low group | 152 | 95.0 |
| High group | 8 | 5.0 | |
| MLR | Low group | 112 | 70.0 |
| High group | 48 | 30.0 | |
| PLT-NLR status | Score 0 | 46 | 28.75 |
| Score 1 | 82 | 51.25 | |
| Score 2 | 32 | 20.0 | |
| NLR-PLR status | Score 0 | 16 | 10.0 |
| Score 1 | 21 | 13.125 | |
| Score 2 | 123 | 76.875 |
The correlations between monocyte count and MLR and anatomoclinical variables were published previously. The PLT-NLR status calculated based on the preoperative blood samples was correlated with tumour size and the presence of perineural invasion (P = 0.015; P = 0.037). Moreover, high values of NLR-PLR and PLT-NLR in the blood samples obtained after surgery were positively associated with histological type of the cancer and the percentage of mucinous component (NLR-PLR: P = 0.002; P = 0.009; PLT-NLR status: P = 0.002; P = 0.007). We found no correlation between NLR-PLR and PLT-NLR and any other variables such as age, sex, TNM stage, lymph node status, the presence of distant metastasis and cancer cell deposits. These results are shown in Table 2.
| Parameter | n | NLR-PLR status | PLT-NLR status | |||
| 160 | Pre- P value | Post- P value | Pre- P value | Post- P value | ||
| Age (yr) | < 60 | 40 | 0.326 | 0.120 | 0.427 | 0.155 |
| > 60 | 120 | |||||
| Sex | Female | 64 | 0.729 | 0.370 | 0.258 | 0.435 |
| Male | 96 | |||||
| Location | Right-side | 20 | 0.963 | 0.167 | 0.475 | 0.183 |
| Transverse | 14 | |||||
| Left-side | 15 | |||||
| Sigmoid | 29 | |||||
| Rectum | 82 | |||||
| Tumour growth | Expanding | 133 | 0.671 | 0.776 | 0.349 | 0.728 |
| Infiltrate | 27 | |||||
| Tumour size | < 2.5 cm | 27 | 0.359 | 0.401 | 0.015a | 0.554 |
| 2.5-5.0 cm | 106 | |||||
| > 5.0 cm | 27 | |||||
| Histological type | Mucinous | 30 | 0.226 | 0.002a | 0.895 | 0.002a |
| Adenocarcinoma | 130 | |||||
| Percentage of mucinous component | 10%-30% | 15 | 0.730 | 0.009a | 0.810 | 0.007a |
| 30%-50% | 15 | |||||
| TNM stage | 1 | 42 | 0.722 | 0.583 | 0.266 | 0.703 |
| 2 | 31 | |||||
| 3 | 69 | |||||
| 4 | 18 | |||||
| Grade of malignancy | 2 | 148 | 0.687 | 0.883 | 0.464 | 0.864 |
| 3 | 12 | |||||
| pT stage | 1 | 3 | 0.590 | 0.196 | 0.853 | 0.253 |
| 2 | 62 | |||||
| 3 | 91 | |||||
| 4 | 4 | |||||
| Venous invasion | Absent | 113 | 0.437 | 0.993 | 0.618 | 0.917 |
| Present | 46 | |||||
| Lymphatic invasion | Absent | 121 | 0.917 | 0.994 | 0.414 | 0.940 |
| Present | 38 | |||||
| Perineural invasion | Absent | 143 | 0.714 | 0.343 | 0.037a | 0.368 |
| Present | 17 | |||||
| Lymph node metastasis | Absent | 81 | 0.292 | 0.913 | 0.070 | 0.995 |
| Present | 79 | |||||
| Distant metastasis | Absent | 143 | 0.152 | 0.354 | 0.157 | 0.378 |
| Present | 17 | |||||
| Tumour deposits | Absent | 133 | 0.910 | 0.320 | 0.670 | 0.293 |
| Present | 27 | |||||
| Tumour budding | Absent | 94 | 0.771 | 0.575 | 0.302 | 0.692 |
| Present | 66 | |||||
| Necrosis | Absent | 45 | 0.599 | 0.521 | 0.273 | 0.449 |
| Focal | 61 | |||||
| Moderate | 36 | |||||
| Extensive | 18 | |||||
| Fibrosis | Absent | 11 | 0.258 | 0.390 | 0.400 | 0.282 |
| Focal | 72 | |||||
| Moderate | 43 | |||||
| Extensive | 34 | |||||
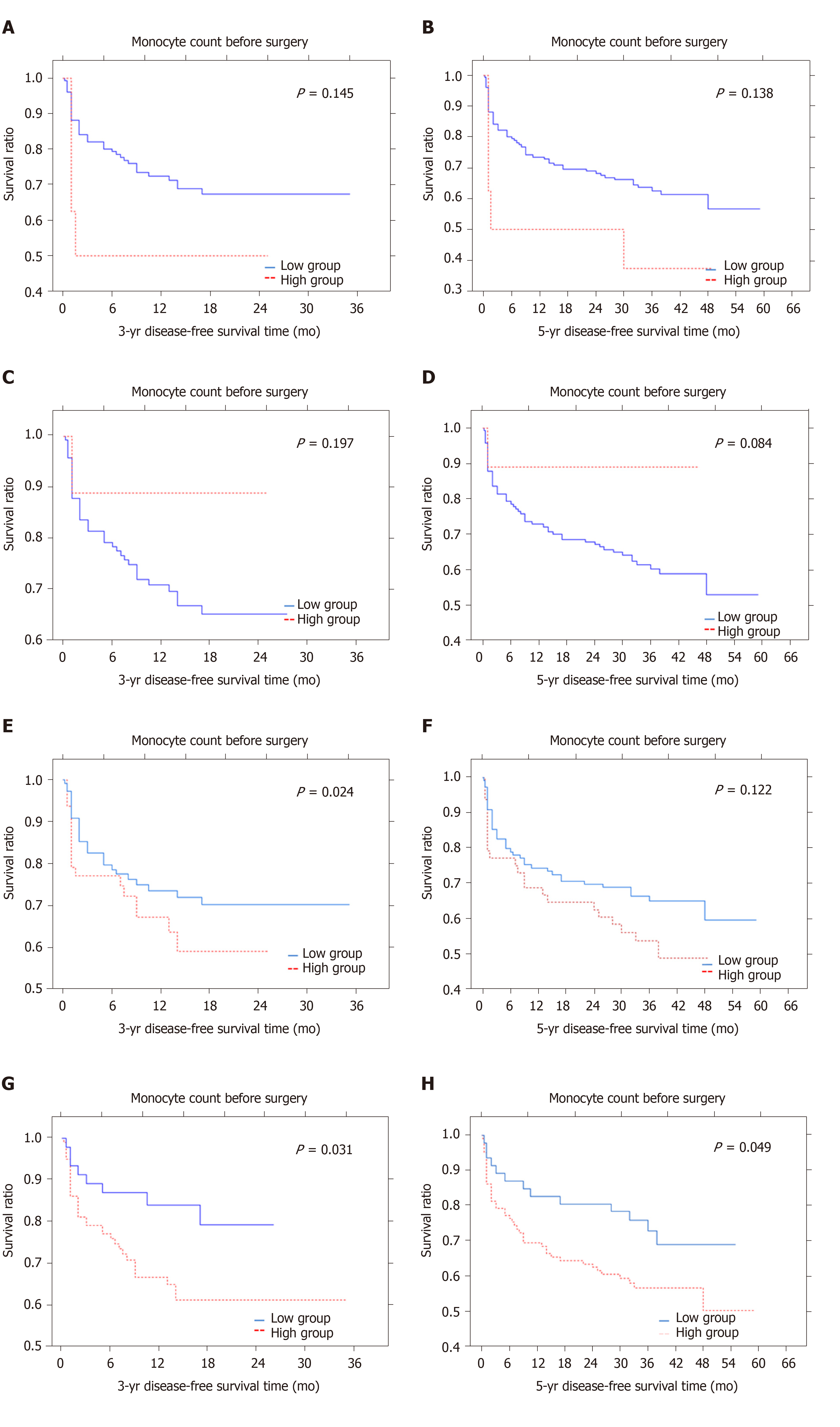
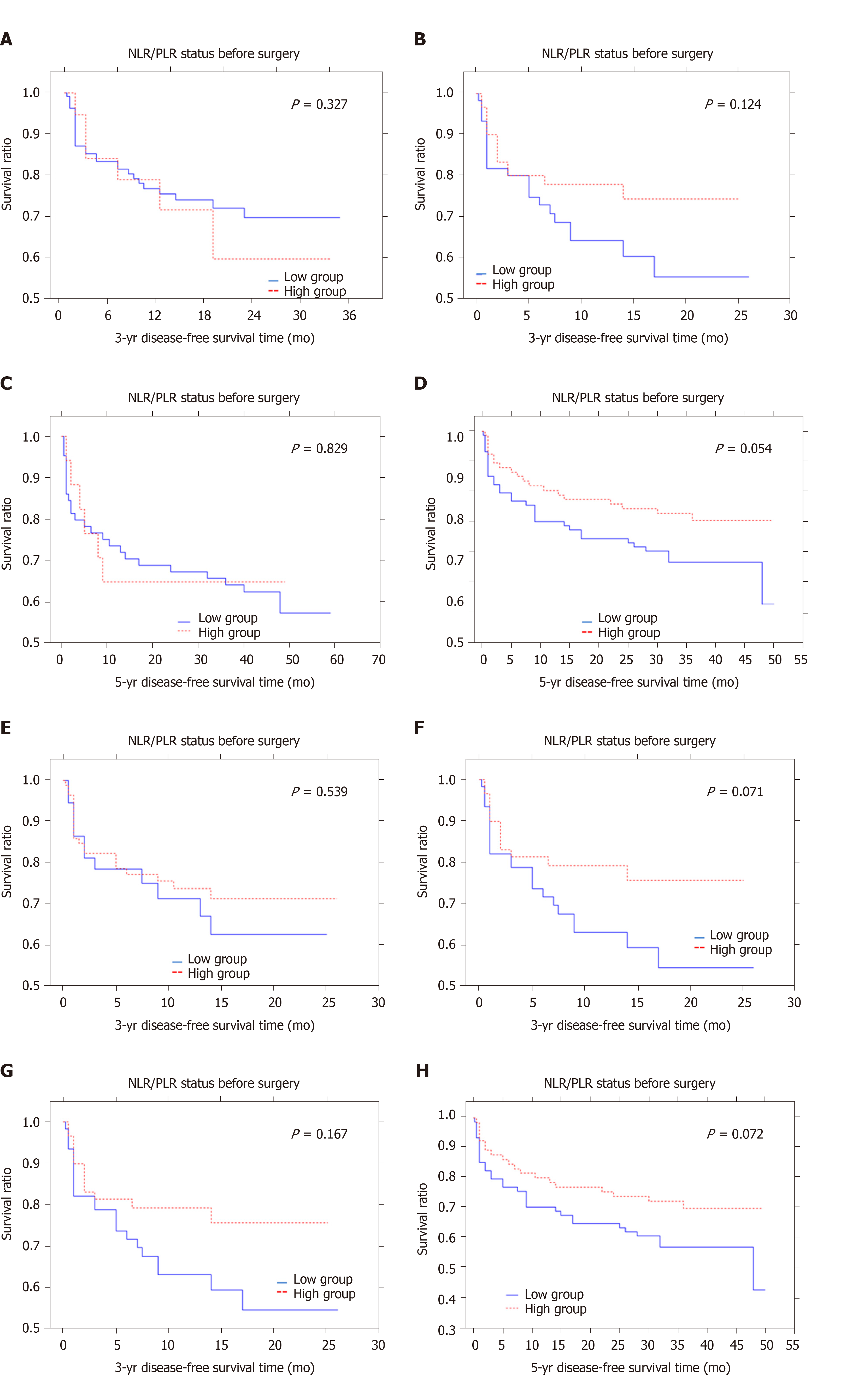
The median 3- and 5-year DFS time was 11.6 and 27.6 mo, respectively. Patients with a low monocyte count in preoperative blood samples lived approximately 12.3 mo (3-year DFS survival) and 28.3 mo (5-year DFS survival) compared to 8.25 mo (3-year DFS time) and 20.3 mo (5-year DFS time) in those with a high monocyte count. These values for monocyte count differed from each other but they were not statistically significant (P = 0.145; P = 0.138) (Figure 1A and B). In the whole blood samples obtained after surgery, the monocyte count tended to differ significantly for the 5-year DFS time which was 0.084. The mean 3-year and 5-year DFS were 15.7 mo and 27.6 mo in the high group and 11.6 mo and 37.1 mo in the low group (Figure 1C and D). Patients with a higher preMLR value tended to have shorter 3- and 5-year DFS time (P = 0.248, P = 0.122) (Figure 1E and F). Patients with low postMLR had longer 3- and 5-year DFS time (P = 0.031, P = 0.049) (Figure 1G and H). Analysis of the NLR-PLR status measured after surgery showed only a tendency for shorter 5-year DFS in the low group (P = 0.054) (Figure 2A-D). Moreover, the results of the PLT-NLR status obtained after surgery showed a tendency for shorter 3- and 5-year DFS in the low group as compared to the high group (P = 0.071; P = 0.072) (Figure 2E-H).
Factors found to be predictive of 3-year DFS in univariate Cox regression analysis included: The number of lymph nodes removed (HR = 0.415, 95%CI: 0.193-0.890, P = 0.023), invasion of the lymph node pouch (HR = 14.191, 95%CI: 1.458-138.08, P = 0.022), inflammatory cell infiltration in the invasive front (HR = 1.590, 95%CI: 1.011-2.525, P = 0.044) and MLR obtained before surgery (HR = 2.000, 95%CI: 0.814-5.063, P = 0.015). According to the multivariate Cox proportional model, the number of lymph nodes removed (P = 0.020) and inflammatory cell infiltration in the invasive front (P = 0.015) were independent factors of 3-year DFS in CRC. Moreover, the analysis of 5-year DFS showed that the MLR in whole blood obtained after surgery (HR = 2.903, 95%CI: 1.368-6.158, P = 0.005) and the status of lymph node metastasis (HR = 0.813, 95%CI: 0.653-1.013, P = 0.050) were prognostic factors in patients diagnosed with CRC. Using the multivariate Cox proportional model, we demonstrated that only the MLR calculated from whole blood after surgical treatment was an independent factor of 5-year DFS in CRC patients. These results are shown in Tables 3 and 4.
| Variables | Univariate P value | Multivariate P value | HR (95%CI) |
| Age ( ≤ 60 vs ≥ 60 yr) | 0.554 | - | 1.020 (0.953-1.093) |
| Sex (female vs male) | 0.971 | - | 1.015 (0.426-2.421) |
| Tumour growth (expanding vs infiltrate) | 0.902 | - | 0.925 (0.265-3.223) |
| Tumour size, < 2.5 cm vs 2.5-5 cm vs > 5 cm | 0.313 | - | 1.472 (0.694-3.123) |
| TNM stage (I-IV) | 0.328 | - | 1.011 (0.988-1.033) |
| Adenocarcinoma type (nonmuc vs partim mucin) | 0.182 | - | 0.026 (0.000-5.528) |
| Grade of malignancy (2 vs 3) | 0.196 | - | 0.288 (0.043-1.893) |
| Preoperative treatment (yes vs no) | 0.097 | - | 2.153 (0.869-5.335) |
| pT stage (1-4) | 0.234 | - | 1.723 (0.702-4.226) |
| Venous invasion (yes vs no) | 0.379 | - | 0.358 (0.003-3.535) |
| Lymphatic invasion (yes vs no) | 0.194 | - | 4.737 (0.451-49.770) |
| Perineural invasion (yes vs no) | 0.100 | - | 0.134 (0.012-1.474) |
| Number of lymph nodes removed (< 5 vs 5-10 vs < 10) | 0.023a | 0.020a | 0.415 (0.193-0.890) |
| Lymph node metastasis (yes vs no) | 0.145 | - | 0.091 (0.003-2.286) |
| Type of lymph node metastasis (micro vs macro) | 0.256 | - | 0.480 (0.135-1.703) |
| Number of metastatic lymph nodes (< 5 vs > 5) | 0.060 | - | 1.768 (0.974-3.201) |
| Lymph node pouch invasion (yes vs no) | 0.022a | 0.385 | 14.191 (1.458-138.08) |
| Distant metastasis (yes vs no) | 0.847 | - | 1.186 (0.209-6.729) |
| Distant metastasis size (< 10 mm vs > 10 mm) | 0.333 | - | 0.829 (0.568-1.211) |
| Tumour deposits (yes vs no) | 0.074 | - | 1.996 (0.933-4.270) |
| Inflammatory cell infiltration in the invasive front (present vs absent) | 0.044a | 0.015a | 1.590 (1.011-2.525) |
| Monocyte-to-lymphocyte ratio (MLR) before surgery (low vs high) | 0.015a | 0.214 | 2.000 (0.814-5.063) |
| Monocyte-to-lymphocyte ratio (MLR) after surgery (low vs high) | 0.265 | - | 1.000 (0.265-4.302) |
| Variables | Univariate P value | Multivariate P value | HR (95%CI) |
| Age, ≤ 60 vs ≥ 60 yr | 0.207 | - | 0.665 (0.325-1.253) |
| Sex (female vs male) | 0.466 | - | 0.982 (0.937-1.030) |
| Tumour growth (expanding vs infiltrate) | 0.815 | - | 1.103 (0.484- 2.514) |
| Tumour size (< 2.5 cm vs 2.5-5 cm vs > 5 cm) | 0.082 | - | 0.613 (0.354-1.064) |
| TNM stage (I-IV) | 0.487 | - | 0.919 (0.726-1.164) |
| Adenocarcinoma type (nonmucin vs partim mucin) | 0.701 | - | 1.326 (0.312-5.627) |
| Grade of malignancy (2 vs 3) | 0.489 | - | 0.575 (0.119-2.762) |
| Preoperative treatment (yes vs no) | - | ||
| pT stage (1-4) | 0.074 | - | 1.895 (0.937-3.832) |
| Venous invasion (yes vs no) | 0.315 | - | 0.453 (0.096-2.121) |
| Lymphatic invasion (yes vs no) | 0.633 | - | 1.486 (0.290-7.599) |
| Perineural invasion (yes vs no) | 0.677 | - | 0.776 (0.236-2.552) |
| Number of lymph nodes removed (< 5 vs 5-10 vs < 10) | 0.140 | - | 1.036 (0.988-1.087) |
| Lymph node metastasis (yes vs no) | 0.050a | 0.105 | 0.813 (0.653-1.013) |
| Type of lymph node metastasis (micro vs macro) | 0.369 | - | 0.747 (0.369-1.411) |
| Number of metastatic lymph nodes (< 5 vs > 5) | 0.806 | - | 1.188 (0.290-4.741) |
| Lymph node pouch invasion (yes vs no) | 0.529 | - | 0.719 (0.257-2.006) |
| Distant metastasis (yes vs no) | 0.933 | - | 0.954 (0.324-2.812) |
| Distant metastasis size (< 10 mm vs > 10 mm) | 0.371 | - | 1.109 (0.883-1.394) |
| Tumour deposits (yes vs no) | 0.536 | - | 1.242 (0.623-2.475) |
| Inflammatory cell infiltration in the invasive front (present vs absent) | 0.991 | - | 0.997 (0.642-1.549) |
| Monocyte-to-lymphocyte ratio before surgery (low vs high) | 0.996 | - | 1.001 (0.548-1.826) |
| Monocyte-to-lymphocyte ratio after surgery (low vs high) | 0.005a | 0.034a | 2.903 (1.368-6.158) |
Systemic inflammation is closely associated with carcinogenesis. The tumour microenvironment consists of tumour cells and inflammatory cells which release various cytokines and chemokines. Literature data have shown that monocytes appear to be recruited as inflammatory cells with cellular machinery to directly kill malignant cells. They are involved in TRAIL-mediated apoptosis via the production of interferon alfa[18]. Monocytes are also able to stimulate cancer cell death through Ab-dependent cytolysis and phagocytosis[19]. However, cancer cells appear to protect themselves from phagocytosis in the circulation via chemokine CD47 overexpression. Despite high expression of the ligand for CD47 on the surface of circulating monocytes, they are unable to stop cancer cell invasion through phagocytosis[20]. Multiple reports have documented that monocytes can be responsible for tumour progression, including the early stages of tumour growth and the formation of distant metastases[21]. Both human and mouse models of colorectal and breast cancer have shown that monocytes can be recruited to primary tumours and pulmonary metastases in a CCL2 Monocyte chemoattractant protein-1 (MCP-1/CCL2)-dependent manner[22,23]. In our study we showed that patients with CRC and a low preoperative absolute monocyte count had a tendency for longer 3-year and 5-year DFS. Our results are in contrast with those of Li et al[24] who demonstrated shorter one, three-, five-year overall survival rates in CRC patients with a low absolute monocyte count. Also, Hu et al[25] proved that preoperative monocyte counts in peripheral blood samples were associated with 5-year overall survival and appear to be an independent risk factor for liver metastasis of CRC. Sasaki et al[9] and Haruki et al[26] observed that an increase in preoperative peripheral blood monocyte count can be an independent risk factor for overall and cancer-related survival of patients with CRC metastasis after hepatic resection. Our results suggest that preoperative circulating monocytes in the blood of patients with CRC take part in tumour progression and can be responsible for reduced patient survival. Moreover, we also showed that the peripheral absolute monocyte count in postoperative blood samples had a reverse tendency for survival time. Additional work on the evaluation of monocyte count in postoperative blood samples in a larger group is needed to better understand the observed correlation.
Monocytes are able to interact with adaptive immunity by directing the recruitment and function of lymphocytes within the tumour microenvironment. Circulating monocytes take part in paracrine signalling and produce higher levels of many inflammatory cytokines and chemokines, including tumour necrosis factor alfa, interleukin 1 (IL-1η), interleukin 6 (IL-6), and chemokine ligand 3[27]. They can be recruited into tumours and determine the reduction of cytotoxic CD8+T cell infiltration. Literature data have shown that the inhibition of CCR2-dependent monocyte-derived cells leads to intensive infiltration of CD8+T cells and a reduction in tumour growth in various tumours, including malignant melanoma, pancreatic and liver tumours[28,29]. Monocyte-derived cells can also secrete chemokine ligand 5 (CCL5) that recruits immune suppressive regulatory T cells into the tumour micro-environment[30]. In addition, nonclassical monocytes are important for the recruitment of natural killer (NK) cells to metastatic sites of malignant tumours[31]. Monocytes can overexpress chemoattractants for NK cells, including chemokine ligand 3, CCL4, and CCL5, and may be able to attract NK cells to lung metastases[32]. The close relation between monocytes and lymphocytes prompted us to determine a simple, haematologic MLR. Literature data have demonstrated that the MLR is an independent prognostic factor for patients with advanced gastric cancer and hepatocellular carcinoma undergoing neoadjuvant treatment[33,34]. Most of the research was focused on the preoperative MLR. The significant role of the MLR as a prognostic factor has been confirmed in various malignant neoplasms, including lung cancer, urothelial carcinoma, ovarian carcinoma and gastrointestinal stromal tumours[35-37]. In the present study, CRC patients with a low MLR in peripheral blood obtained after surgery had longer 5-year DFS. Moreover, the multivariate Cox regression model showed that the MLR in peripheral blood obtained after surgery is an independent prognostic factor for 5-year DFS. Our results are in accordance with those of Huang et al[38] who found that CRC patients with a high MLR had a poor prognosis. More studies are required to determine the significance of the MLR in CRC patients.
Moreover, we also examined the neutrophil to lymphocyte and platelet to lymphocyte ratios in pre- and postoperative blood samples from CRC patients. In the current study, the NLR-PLR status in postoperative peripheral blood was found to correlate with histological type and percentage of the mucinous component. The preoperative NLR-PLR status has been reported to be a useful prognostic factor in patients with various gastrointestinal malignancies[39-41]. Recio-Boiles et al[39] showed that NLR-PLR status in perioperative blood samples from patients with resectable pancreatic ductal adenocarcinoma was associated with invasion of the resected margins and lymph nodes. The NLR-PLR score was higher in patients with progressive advanced gastric cancer[40]. Moreover, Kabir et al[41] confirmed that the elevated NLR-PLR value in preoperative blood samples from hepatocellular carcinoma patients predicts both overall survival and recurrence-free survival. In multivariate analysis, we failed to confirm these relationships in patients with CRC. We only found a tendency for shorter 3- and 5-year DFS in postoperative samples. Our observation was in contrast to Li et al[42] who demonstrated an inverse correlation between NLR-PLR status and survival time.
Patients with CRC may suffer from thrombocythemia and have a poor prognosis[43]. It is well known that platelets are closely related to cancer cells. Cancer cells can modify platelet behaviour which includes tumour-platelet aggregates, triggering platelet granules and extracellular vesicle release and altering platelet phenotype. Platelets can secrete angiogenic factors and enhance tumour growth by promoting proliferation and antiapoptotic signals. Moreover, they promote tumour invasion and sustain metastasis. Furthermore, platelets assist tumours in evading immune destruction[44]. Therefore, they appear to be important in the immune response against cancer in correlation with neutrophils and lymphocytes. We analysed the platelet count and NLR in pre- and postoperative peripheral blood samples from CRC patients. The present study showed that high PLT-NLR status was found to positively correlate with tumour size in preoperative samples and with histological type and the percentage of mucinous component in samples obtained after surgery. Moreover, the analysis showed that patients with high PLT-NLR status had a longer 3-year and 5-year DFS. Our observations suggest that the PLT-NLR status may be a useful haematologic marker of CRC. To our knowledge, this is the first research on PLT-NLR status in CRC and should be expanded in a larger group of CRC patients in the near future.
In conclusion, the postoperative MLR in whole blood samples can be used as an independent prognostic factor in patients diagnosed with CRC undergoing surgery. In addition, invasion of the lymph node pouch, the number of lymph nodes removed and inflammatory cell infiltration in the invasive front can be considered directly related to DFS in patients with CRC.
Colorectal cancer (CRC) is the third most common malignancy worldwide. Therefore, it is critically important to identify new useful markers that can be easily obtained in routine practice. Recent studies showed that inflammation is a crucial issue in the pathogenesis and development of cancer, especially in tumour progression. However, little is known about the prognostic value of the absolute monocyte count, monocyte to lymphocyte ratio (MLR), the combination of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio (NLR-PLR), and combined platelet and neutrophil-to-lymphocyte ratio (PLT-NLR) in patients diagnosed with CRC.
Effective research on haematologic markers that can improve the diagnosis of colorectal cancer and establish patient prognosis.
The aim of the study was to evaluate the prognostic value of absolute monocyte count, MLR, the combination of NLR-PLR, and PLT-NLR in peripheral blood samples of patients with colorectal cancer undergoing surgery.
We respectively enrolled CRC patients who undergone surgery between April 2014 and December 2016 in the Department of Oncological Surgery, Comprehensive Cancer Centre (Bialystok, Poland). The status of absolute monocyte count, MLR, NLR-PLR and PLT-NLR was calculated on the basis of blood samples obtained before and after surgery and were examined in correlation with various pathomorphological and clinical factors. Receiver operating characteristic curve analysis was used to investigate cut-off values for the pre- and postoperative haematologic factors examined. The Kaplan-Meier method and the long-rank test were used to compare survival curves. To determine independent prognostic factors, univariate and multivariate Cox proportional hazards regression models were applied.
The analysis showed that PLT-NLR status was correlated with tumour size and the presence of perineural invasion (P = 0.015; P = -0.174, P = 0.037). Moreover, high NLR-PLR and PLR-NLR in the blood samples obtained after surgery were positively associated with the histological type of cancer and percentage of the mucinous component (NLR-PLR: P = 0.002; P = 0.009; PLR-NLR status: P = 0.002; P = 0.007). The analysis of 5-year disease-free survival showed that the MLR in whole blood obtained after surgery (HR = 2.903, 95%CI: 1.368-6.158, P = 0.005) and the status of lymph node metastasis (HR = 0.813, 95%CI: 0.653-1.013, P = 0.050) were independent prognostic factors in colorectal cancer patients.
The present study demonstrated that the postoperative MLR in whole blood samples can be used as an independent prognostic factor in patients diagnosed with colorectal cancer.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Poland
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Fei XM, Ji G, Perse M S-Editor: Zhang L L-Editor: Webster JR P-Editor: Ma YJ
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11573] [Cited by in RCA: 13155] [Article Influence: 1879.3] [Reference Citation Analysis (4)] |
| 2. | Saha S, Nora D, Wong JH, Weise D. Sentinel lymph node mapping in colorectal cancer--a review. Surg Clin North Am. 2000;80:1811-1819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | McArdle CS, Hole DJ. Outcome following surgery for colorectal cancer: analysis by hospital after adjustment for case-mix and deprivation. Br J Cancer. 2002;86:331-335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Schmoll HJ, Van Cutsem E, Stein A, Valentini V, Glimelius B, Haustermans K, Nordlinger B, van de Velde CJ, Balmana J, Regula J, Nagtegaal ID, Beets-Tan RG, Arnold D, Ciardiello F, Hoff P, Kerr D, Köhne CH, Labianca R, Price T, Scheithauer W, Sobrero A, Tabernero J, Aderka D, Barroso S, Bodoky G, Douillard JY, El Ghazaly H, Gallardo J, Garin A, Glynne-Jones R, Jordan K, Meshcheryakov A, Papamichail D, Pfeiffer P, Souglakos I, Turhal S, Cervantes A. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. A personalized approach to clinical decision making. Ann Oncol. 2012;23:2479-2516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1035] [Cited by in RCA: 1106] [Article Influence: 85.1] [Reference Citation Analysis (1)] |
| 5. | Yamashita H, Katai H. Systemic inflammatory response in gastric cancer. World J Surg. 2010;34:2399-2400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Ribatti D. Inflammatory Cells in Tumor Microenvironment. In: The Role of Microenvironment in the Control of Tumor Angiogenesis, 2016: 1-86. |
| 7. | Bruckner HW, Lavin PT, Plaxe SC, Storch JA, Livstone EM. Absolute granulocyte, lymphocyte, and moncyte counts. Useful determinants of prognosis for patients with metastatic cancer of the stomach. JAMA. 1982;247:1004-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 34] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Sasaki A, Iwashita Y, Shibata K, Matsumoto T, Ohta M, Kitano S. Prognostic value of preoperative peripheral blood monocyte count in patients with hepatocellular carcinoma. Surgery. 2006;139:755-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 9. | Sasaki A, Kai S, Endo Y, Iwaki K, Uchida H, Tominaga M, Okunaga R, Shibata K, Ohta M, Kitano S. Prognostic value of preoperative peripheral blood monocyte count in patients with colorectal liver metastasis after liver resection. J Gastrointest Surg. 2007;11:596-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Xiang J, Zhou L, Li X, Bao W, Chen T, Xi X, He Y, Wan X. Preoperative Monocyte-to-Lymphocyte Ratio in Peripheral Blood Predicts Stages, Metastasis, and Histological Grades in Patients with Ovarian Cancer. Transl Oncol. 2017;10:33-39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 11. | Zhou D, Zhang Y, Xu L, Zhou Z, Huang J, Chen M. A monocyte/granulocyte to lymphocyte ratio predicts survival in patients with hepatocellular carcinoma. Sci Rep. 2015;5:15263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol. 2005;91:181-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 717] [Cited by in RCA: 843] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 13. | Sun X, Liu X, Liu J, Chen S, Xu D, Li W, Zhan Y, Li Y, Chen Y, Zhou Z. Preoperative neutrophil-to-lymphocyte ratio plus platelet-to-lymphocyte ratio in predicting survival for patients with stage I-II gastric cancer. Chin J Cancer. 2016;35:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Benson AB, Venook AP, Al-Hawary MM, Cederquist L, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Engstrom PF, Garrido-Laguna I, Grem JL, Grothey A, Hochster HS, Hoffe S, Hunt S, Kamel A, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Murphy JD, Nurkin S, Saltz L, Sharma S, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Wuthrick E, Gregory KM, Freedman-Cass DA. NCCN Guidelines Insights: Colon Cancer, Version 2.2018. J Natl Compr Canc Netw. 2018;16:359-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 635] [Cited by in RCA: 684] [Article Influence: 97.7] [Reference Citation Analysis (1)] |
| 15. | Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15860] [Cited by in RCA: 21581] [Article Influence: 1348.8] [Reference Citation Analysis (1)] |
| 16. | Lin Q, Wei Y, Ren L, Zhong Y, Qin C, Zheng P, Xu P, Zhu D, Ji M, Xu J. Tumor deposit is a poor prognostic indicator in patients who underwent simultaneous resection for synchronous colorectal liver metastases. Onco Targets Ther. 2015;8:233-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Hirahara T, Arigami T, Yanagita S, Matsushita D, Uchikado Y, Kita Y, Mori S, Sasaki K, Omoto I, Kurahara H, Maemura K, Okubo K, Uenosono Y, Ishigami S, Natsugoe S. Combined neutrophil-lymphocyte ratio and platelet-lymphocyte ratio predicts chemotherapy response and prognosis in patients with advanced gastric cancer. BMC Cancer. 2019;19:672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 153] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 18. | Griffith TS, Wiley SR, Kubin MZ, Sedger LM, Maliszewski CR, Fanger NA. Monocyte-mediated tumoricidal activity via the tumor necrosis factor-related cytokine, TRAIL. J Exp Med. 1999;189:1343-1354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 362] [Cited by in RCA: 373] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 19. | Yeap WH, Wong KL, Shimasaki N, Teo EC, Quek JK, Yong HX, Diong CP, Bertoletti A, Linn YC, Wong SC. CD16 is indispensable for antibody-dependent cellular cytotoxicity by human monocytes. Sci Rep. 2016;6:34310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 165] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 20. | Jaiswal S, Jamieson CH, Pang WW, Park CY, Chao MP, Majeti R, Traver D, van Rooijen N, Weissman IL. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138:271-285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1313] [Cited by in RCA: 1234] [Article Influence: 77.1] [Reference Citation Analysis (0)] |
| 21. | Kitamura T, Doughty-Shenton D, Cassetta L, Fragkogianni S, Brownlie D, Kato Y, Carragher N, Pollard JW. Monocytes Differentiate to Immune Suppressive Precursors of Metastasis-Associated Macrophages in Mouse Models of Metastatic Breast Cancer. Front Immunol. 2017;8:2004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 121] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 22. | Chun E, Lavoie S, Michaud M, Gallini CA, Kim J, Soucy G, Odze R, Glickman JN, Garrett WS. CCL2 Promotes Colorectal Carcinogenesis by Enhancing Polymorphonuclear Myeloid-Derived Suppressor Cell Population and Function. Cell Rep. 2015;12:244-257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 212] [Cited by in RCA: 299] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 23. | Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222-225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2327] [Cited by in RCA: 2221] [Article Influence: 158.6] [Reference Citation Analysis (0)] |
| 24. | Li Z, Xu Z, Huang Y, Zhao R, Cui Y, Zhou Y, Wu X. The predictive value and the correlation of peripheral absolute monocyte count, tumor-associated macrophage and microvessel density in patients with colon cancer. Medicine (Baltimore). 2018;97:e10759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Hu S, Zou Z, Li H, Zou G, Li Z, Xu J, Wang L, Du X. The Preoperative Peripheral Blood Monocyte Count Is Associated with Liver Metastasis and Overall Survival in Colorectal Cancer Patients. PLoS One. 2016;11:e0157486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Haruki K, Shiba H, Fujiwara Y, Furukawa K, Wakiyama S, Ogawa M, Ishida Y, Misawa T, Yanaga K. Perioperative change in peripheral blood monocyte count may predict prognosis in patients with colorectal liver metastasis after hepatic resection. J Surg Oncol. 2012;106:31-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Chittezhath M, Dhillon MK, Lim JY, Laoui D, Shalova IN, Teo YL, Chen J, Kamaraj R, Raman L, Lum J, Thamboo TP, Chiong E, Zolezzi F, Yang H, Van Ginderachter JA, Poidinger M, Wong AS, Biswas SK. Molecular profiling reveals a tumor-promoting phenotype of monocytes and macrophages in human cancer progression. Immunity. 2014;41:815-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 224] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 28. | Mitchem JB, Brennan DJ, Knolhoff BL, Belt BA, Zhu Y, Sanford DE, Belaygorod L, Carpenter D, Collins L, Piwnica-Worms D, Hewitt S, Udupi GM, Gallagher WM, Wegner C, West BL, Wang-Gillam A, Goedegebuure P, Linehan DC, DeNardo DG. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 2013;73:1128-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 774] [Article Influence: 59.5] [Reference Citation Analysis (0)] |
| 29. | Li X, Yao W, Yuan Y, Chen P, Li B, Li J, Chu R, Song H, Xie D, Jiang X, Wang H. Targeting of tumour-infiltrating macrophages via CCL2/CCR2 signalling as a therapeutic strategy against hepatocellular carcinoma. Gut. 2017;66:157-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 542] [Article Influence: 67.8] [Reference Citation Analysis (0)] |
| 30. | Schlecker E, Stojanovic A, Eisen C, Quack C, Falk CS, Umansky V, Cerwenka A. Tumor-infiltrating monocytic myeloid-derived suppressor cells mediate CCR5-dependent recruitment of regulatory T cells favoring tumor growth. J Immunol. 2012;189:5602-5611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 323] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 31. | Kubo H, Mensurado S, Gonçalves-Sousa N, Serre K, Silva-Santos B. Primary Tumors Limit Metastasis Formation through Induction of IL15-Mediated Cross-Talk between Patrolling Monocytes and NK Cells. Cancer Immunol Res. 2017;5:812-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 32. | Hanna RN, Cekic C, Sag D, Tacke R, Thomas GD, Nowyhed H, Herrley E, Rasquinha N, McArdle S, Wu R, Peluso E, Metzger D, Ichinose H, Shaked I, Chodaczek G, Biswas SK, Hedrick CC. Patrolling monocytes control tumor metastasis to the lung. Science. 2015;350:985-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 350] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 33. | Zhu Z, Xu L, Zhuang L, Ning Z, Zhang C, Yan X, Lin J, Shen Y, Wang P, Meng Z. Role of monocyte-to-lymphocyte ratio in predicting sorafenib response in patients with advanced hepatocellular carcinoma. Onco Targets Ther. 2018;11:6731-6740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 34. | Chen L, Hao Y, Zhu L, Li S, Zuo Y, Zhang Y, Song H, Xue Y. Monocyte to lymphocyte ratio predicts survival in patients with advanced gastric cancer undergoing neoadjuvant chemotherapy. Onco Targets Ther. 2017;10:4007-4016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 35. | Chen X, Wu J, Zhang F, Ying L, Chen Y. Prognostic Significance of Pre-Operative Monocyte-to-Lymphocyte Ratio in Lung Cancer Patients Undergoing Radical Surgery. Lab Med. 2018;49:e29-e39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 36. | Zhu X, Wu SQ, Xu R, Wang YH, Zhong ZH, Zhang L, Zhao XK. The evaluation of monocyte lymphocyte ratio as a preoperative predictor in urothelial malignancies: a pooled analysis based on comparative studies. Sci Rep. 2019;9:6280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Cananzi FCM, Minerva EM, Samà L, Ruspi L, Sicoli F, Conti L, Fumagalli Romario U, Quagliuolo VL. Preoperative monocyte-to-lymphocyte ratio predicts recurrence in gastrointestinal stromal tumors. J Surg Oncol. 2019;119:12-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 38. | Huang L, Fang J, Wu J, Zhou X, Wei H. [Prognostic value of combining preoperative serum tumor markers and peripheral blood routine indexes in patients with colorectal cancer]. Zhonghua Wei Chang Wai Ke Za Zhi. 2018;21:1421-1426. [PubMed] |
| 39. | Recio-Boiles A, Nallagangula A, Veeravelli S, Vondrak J, Saboda K, Roe D, Elquza E, McBride A, Babiker HM. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios inversely correlate to clinical and pathologic stage in patients with resectable pancreatic ductal adenocarcinoma. Ann Pancreat Cancer. 2019;2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 40. | Zhu GS, Tian SB, Wang H, Ma MG, Liu Y, Du HS, Long YP. Preoperative Neutrophil Lymphocyte Ratio and Platelet Lymphocyte Ratio Cannot Predict Lymph Node Metastasis and Prognosis in Patients with Early Gastric Cancer: a Single Institution Investigation in China. Curr Med Sci. 2018;38:78-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 41. | Kabir T, Ye M, Mohd Noor NA, Woon W, Junnarkar SP, Shelat VG. Preoperative Neutrophil-to-Lymphocyte Ratio Plus Platelet-to-Lymphocyte Ratio Predicts the Outcomes after Curative Resection for Hepatocellular Carcinoma. Int J Hepatol. 2019;2019:4239463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 42. | Li C, Wen TF, Yan LN, Li B, Wang WT, Yang JY, Xu MQ. Postoperative neutrophil-to-lymphocyte ratio plus platelet-to-lymphocyte ratio predicts the outcomes of hepatocellular carcinoma. J Surg Res. 2015;198:73-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 43. | Rao XD, Zhang H, Xu ZS, Cheng H, Shen W, Wang XP. Poor prognostic role of the pretreatment platelet counts in colorectal cancer: A meta-analysis. Medicine (Baltimore). 2018;97:e10831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 44. | Xu XR, Yousef GM, Ni H. Cancer and platelet crosstalk: opportunities and challenges for aspirin and other antiplatelet agents. Blood. 2018;131:1777-1789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 228] [Article Influence: 32.6] [Reference Citation Analysis (0)] |