Published online Aug 14, 2020. doi: 10.3748/wjg.v26.i30.4489
Peer-review started: March 30, 2020
First decision: May 29, 2020
Revised: June 10, 2020
Accepted: July 14, 2020
Article in press: July 14, 2020
Published online: August 14, 2020
Processing time: 137 Days and 5.2 Hours
Sequential transarterial chemoembolization (TACE) and portal vein embolization (PVE) are associated with long time interval that can allow tumor growth and nullify treatments' benefits.
To evaluate the effect of simultaneous TACE and PVE for patients with large hepatocellular carcinoma (HCC) prior to elective major hepatectomy.
Fifty-one patients with large HCC who underwent PVE combined with or without TACE prior to hepatectomy were included in this study, with 13 patients in the simultaneous TACE + PVE group, 17 patients in the sequential TACE + PVE group, and 21 patients in the PVE-only group. The outcomes of the procedures were compared and analyzed.
All patients underwent embolization. The mean interval from embolization to surgery, the kinetic growth rate of the future liver remnant (FLR), the degree of tumor size reduction, and complete tumor necrosis were significantly better in the simultaneous TACE + PVE group than in the other groups. Although the patients in the simultaneous TACE + PVE group had a higher transaminase levels after PVE and TACE, they recovered to comparable levels with the other two groups before surgery. The intraoperative course and the complication and mortality rates were similar among the three groups. The overall survival and disease-free survival were higher in the simultaneous TACE + PVE group than in the other two groups.
Simultaneous TACE and PVE is a safe and effective approach to increase FLR volume for patients with large HCC before major hepatectomy.
Core tip: Simultaneous transarterial chemoembolization (TACE) and portal vein embolization (PVE) is a safe and effective approach to increase the future liver remnant volume quickly in a short time, and it has achieved a longer median overall survival and disease-free survival time compared with sequential TACE and PVE or PVE-only for patients with large hepatocellular carcinoma before major hepatectomy.
- Citation: Zhang CW, Dou CW, Zhang XL, Liu XQ, Huang DS, Hu ZM, Liu J. Simultaneous transcatheter arterial chemoembolization and portal vein embolization for patients with large hepatocellular carcinoma before major hepatectomy. World J Gastroenterol 2020; 26(30): 4489-4500
- URL: https://www.wjgnet.com/1007-9327/full/v26/i30/4489.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i30.4489
Hepatocellular carcinoma (HCC) is a highly lethal invasive carcinoma arising in the liver[1,2]. The most important risk factors for HCC are infection with hepatitis B or hepatitis C and/or preexisting liver cirrhosis[3,4]. The incidence of HCC varies across the world and associated with the geographical distribution of hepatitis B and hepatitis C[5-7]. The worldwide age-standardized annual mortality rates of HCC are 12.7 per 100000 in men and 4.6 per 100000 in women[2,8].
Major hepatectomy is increasingly performed for patients with large HCC to achieve curative resection and provide the only opportunity for long-term survival[9,10]. Nevertheless, major hepatectomy is frequently contraindicated in many patients with HCC due to insufficient future liver remnant (FLR) along with the increased possibility of postoperative liver failure, especially in patients with chronic liver disease or cirrhosis.
Preoperative portal vein embolization (PVE) aimed to induce atrophy of the embolized segments and compensatory hypertrophy of contralateral segments has been widely accepted as the standard method to reduce the risk of postoperative liver failure and convert patients with large HCC from an unresectable to a resectable status[11,12]. The fastest liver hypertrophy was reported to appear 2 wk after PVE and could be sustained for about 8 wk[13]. Nevertheless, the buffering increase of the hepatic arterial flow resulting from PVE might lead to rapid ipsilateral tumor growth as well as insufficient contralateral liver hypertrophy. To prevent this detrimental effect of preoperative PVE and to further facilitate FLR regeneration, transarterial chemoembolization (TACE) is recommended before PVE. Recent researches have demonstrated that sequential TACE and PVE before major hepatectomy is safe and effective for patients with large HCC and can improve their clinical outcomes and survival[14-16]. Ogata et al[17] reported that the mean increase in percentage FLR volume, the rate of complete tumor necrosis, and the 5-year disease-free survival (DFS) in the TACE+PVE group were significantly higher compared with those in the PVE alone group. Nevertheless, one obvious drawback of sequential TACE and PVE is the long wait time between TACE and PVE (3-4 wk) and before surgery (4-6 wk), which may result in tumor progression and thus nullify the obtained efficacy.
In view of the potential risk of tumor progression during the long wait time before surgery, simultaneous TACE and PVE before hepatectomy should theoretically have a stronger anticancer effect through the double-obstruction of the tumor feeding vessels and should reduce the wait time before surgery, and have been reported to be safe and effective[18]. Nevertheless, this attempt was only made in very few patients with small HCC confined to superficial liver areas. More importantly, no study has ever compared the results of simultaneous TACE and PVE vs sequential TACE and PVE or vs PVE alone. Therefore, this is the first study to evaluate the effect of simultaneous TACE and PVE before major hepatectomy in patients with large HCC and to compare their clinical outcome with sequential TACE+PVE or PVE only.
Between January 2010 and December 2018, 51 patients with large HCC and insufficient FLR were enrolled in this study. The inclusion criteria were: (1) 18-70 years of age; (2) Diagnosed with HCC based on the EASL-EORTC Recommendations[19]; (3) Tumor size ≥ 5 cm based on computed tomography (CT) or magnetic resonance imaging (MRI); (4) Chest/abdominal-pelvic CT or positron emission tomography (PET)-CT were performed to exclude extrahepatic metastasis; (5) Major hepatectomy was deemed to be required in patients with threshold preoperative FLR < 40% and indocyanine green retention rate at 15 min (ICGR15) < 10%; (6) No tumor thrombus in portal vein and branches of the retained lobe; (7) Absence of severe esophageal and gastric varices; and (8) Eastern Cooperative Oncology Group (ECOG) performance status of 0-1. The exclusion criteria were: (1) Child-Pugh C; (2) More than four lesions; (3) Tumors distributed in the two lobes; or (4) Other malignant tumors. Written informed consent was obtained from all enrolled patients. The study was approved by the Ethics Committee of Zhejiang Provincial People’s Hospital and followed the principle of declaration of Helsinki.
TACE procedures were performed under local anesthesia as selectively as possible, depending on tumor distribution. A conventional or drug-eluting beads TACE was used, with the latter being used in more recent patients. TACE procedures were performed by a team of experienced interventional radiologists. Conventional TACE included an intra-arterial injection of a mixture of chemotherapeutic agents (150 mg doxorubicin; adriamycin; Pharmacia Upjohn, Kalamazoo, MI, United States), emulsified in iodized oil (Lipiodol, Gerbet, Aulnay-sous-Bois, France). Embolization was achieved by injection of gelatin sponge (Gelitaspon, Gelita Medical B.V., Amsterdam, Netherlands) or polyvinyl alcohol particles (Bead Block, Biocompatibles, Farnham, United Kingdom). The drug-eluting beads included 100-300 mm and/or 300-500 mm sized particles (Biocompatibles, Farnham, United Kingdom), as described elsewhere[20]. Bead loading was performed at an intended dose of 150 mg/patient.
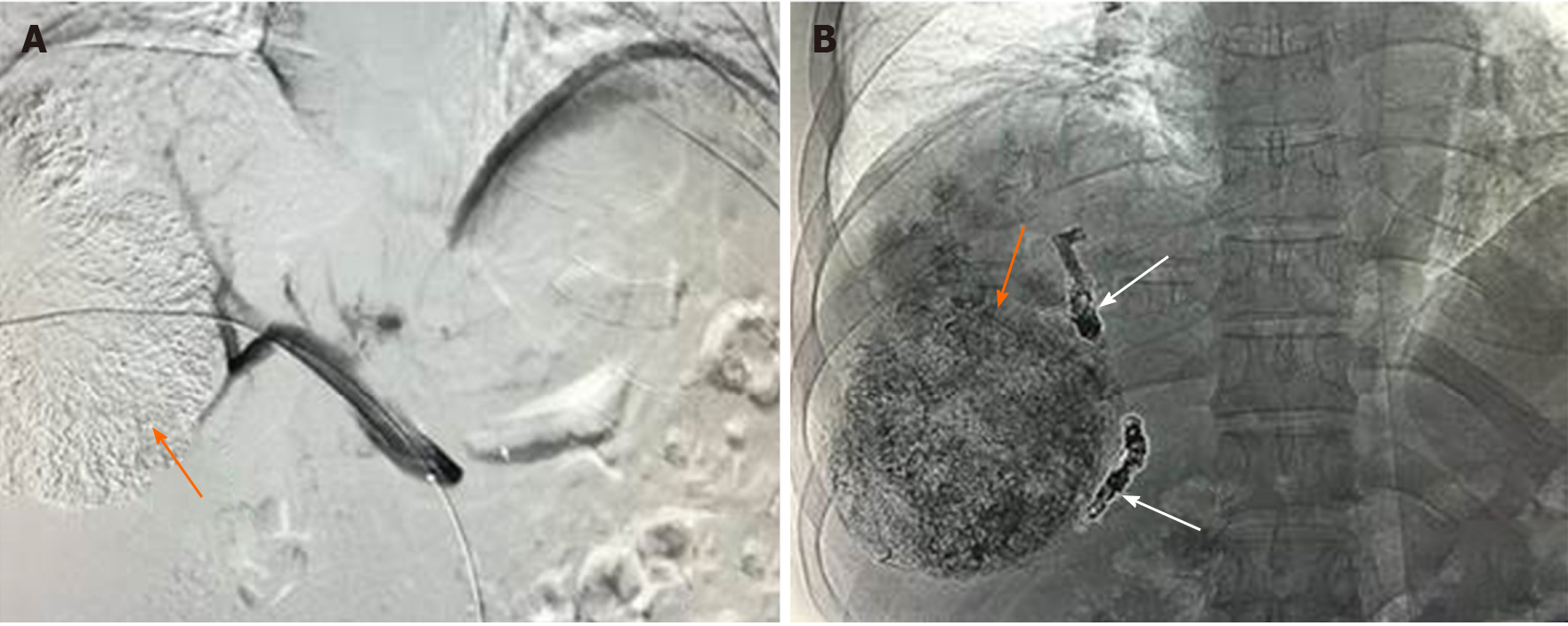
PVE procedures were performed 2-4 wk after TACE in the sequential TACE + PVE (seTP) group or concurrently in the simultaneous TACE + PVE (siTP) group by the same team of interventional radiologists. Procedures were performed under general anesthesia. The technical aspects of PVE have been described elsewhere[21]. The percutaneous approach was the technique of choice. After catheterizing the main portal trunk, a portography was performed, and a mixture of N-butyl-2-cyanoacrylate and iodized oil (Lipiodol, Guerbet, Aulnay-sous-Bois, France) was used for embolization. Embolization was completed with 0.018-inch coils (MicroNester 0.018, Cook, Bloomington, United States), and polyvinyl alcohol particles (Beadblock, Biocompatibles, Farnham, United Kingdom), when necessary (Figure 1).
Hepatectomy was considered feasible after achieving substantial hypertrophy of the FLR. Hepatectomy was not performed if the FLR was still < 40% or distant metastasis was found. Laparoscopic or open hepatectomy was performed in all patients depending on the size of the tumor and judgment of the chief surgeon. Intraoperative ultrasound was used to verify the location of the tumor and its relationship with major vascular structures as well as to detect satellite nodules. Selective hemihepatic blood flow occlusion or intermittent Glisson pedicle clamping was routinely performed.
Baseline patient and tumor characteristics were obtained from the prospective institutional database. All patients underwent volumetric helical computed tomographic estimation of liver volume before PVE with or without TACE and before surgery. Those with a 10% decrease before surgery compared to the baseline size were defined as with tumor size reduction. As the most accurate indicator to assess the ability of liver hypertrophy and to estimate the possibility of postoperative hepatic failure, the kinetic growth rate (KGR)[22], which reflects the average hypertrophy of liver volume each week, was calculated for all patients. For patients who finally underwent hepatectomy, the ratio of residual tumor cells (RT) was quantitatively assessed by pathologists as the volume of the RT compared to the total surface volume of the lesion. Lesions were classified into three groups: major pathological response (MPR, RT < 10%), partial pathologic response (PPR, RT from 10% to 50%), and no pathologic response (RT > 50%)[23]. In addition, patients who did not undergo resection because of tumor progression, insufficient FLR hypertrophy, or liver failure after PVE with or without TACE were also recorded. Other data, including liver function tests (LFTs) before and after TACE and/or PVE, perioperative results, and long-term outcomes were collected.
Follow-up information included clinical examination, liver function tests, measurement of serum alpha-fetoprotein (AFP), and abdominal MRI every month during the first half-year after liver resection and every 3 months thereafter for resected patients, but close observation every month was necessary to unresectable patients.
Statistical analyses were performed using SPSS 20.0 (IBM, Armonk, NY, United States). Continuous data were presented as mean ± SD or median (range), and compared with one-way analysis of variance (ANOVA) or the Kruskal-Wallis test among groups. Categorical variables were analyzed by Pearson’s χ2 or Fisher’s exact test, as appropriate. Kaplan-Meier curve and the log-rank test were used to compare the survival among groups. A P value < 0.05 was considered statistically significant.
A total of 51 patients who met the eligibility criteria underwent TACE/PVE during the study period, with 13 patients in the siTP group, 17 patients in the seTP group and 21 patients in the PVE-only group. The baseline patient and tumor characteristics in the three groups are summarized in Table 1. No significant differences were found among these three groups regarding the baseline features.
| Characteristics | siTP group (n = 13) | seTP group (n = 17) | PVE-only group (n = 21) | P value |
| Male, n (%) | 10 (77) | 13 (76) | 17 (81) | 0.935 |
| Age (yr) | 51.5 ± 10.0 | 54.0 ± 11.2 | 59.2 ± 8.3 | 0.068 |
| Etiology, n (%) | 0.928 | |||
| HBV | 9 (69) | 12 (70) | 15 (71) | |
| Alcoholic | 1 (8) | 2 (12) | 2 (10) | |
| NASH | 3 (23) | 3 (18) | 3 (14) | |
| Schistosomiasis | 0 | 0 | 1 (5) | |
| ECOG performance status, n (%) | 0.725 | |||
| 0 | 10 (77) | 13 (76) | 18 (86) | |
| 1 | 3 (23) | 4 (24) | 3 (14) | |
| Tumor multiplicity, n (%) | 0.809 | |||
| Single | 11 (85) | 14 (82) | 15 (71) | |
| Multiple | 2 (15) | 3 (18) | 6 (29) | |
| Tumor size (cm) | 8.4 ± 2.7 | 8.3 ± 2.3 | 7.7 ± 1.9 | 0.630 |
| ICGR15 (%) | 5.7 ± 3.0 | 5.0 ± 2.6 | 6.0 ± 2.7 | 0.511 |
| Baseline FLR (%) | 31.2 ± 3.7 | 31.8 ± 4.5 | 33.2 ± 3.5 | 0.150 |
| AFP (ng/mL) | 377 (11-3419) | 487 (16-12000) | 551 (9-10344) | 0.640 |
| ALT (U/mL) | 30.2 ± 13.8 | 40.5 ± 26.0 | 40.4 ± 24.0 | 0.380 |
| AST (U/mL) | 39.5 ± 16.2 | 58.2 ± 40.0 | 49.5 ± 20.8 | 0.212 |
| TBIL (μg/mL) | 19.0 ± 5.1 | 17.4 ± 5.2 | 21.2 ± 6.9 | 0.147 |
All patients underwent PVE with or without TACE. Common and minor complications included abdominal pain, fever, nausea, and transient LFTs elevation. All these complications were resolved by themselves or after symptomatic treatment. In the seTP group, one male patient suffered from acute pulmonary infarction after TACE because of an intrahepatic arteriovenous fistula. This case recovered after respiration support and PVE.
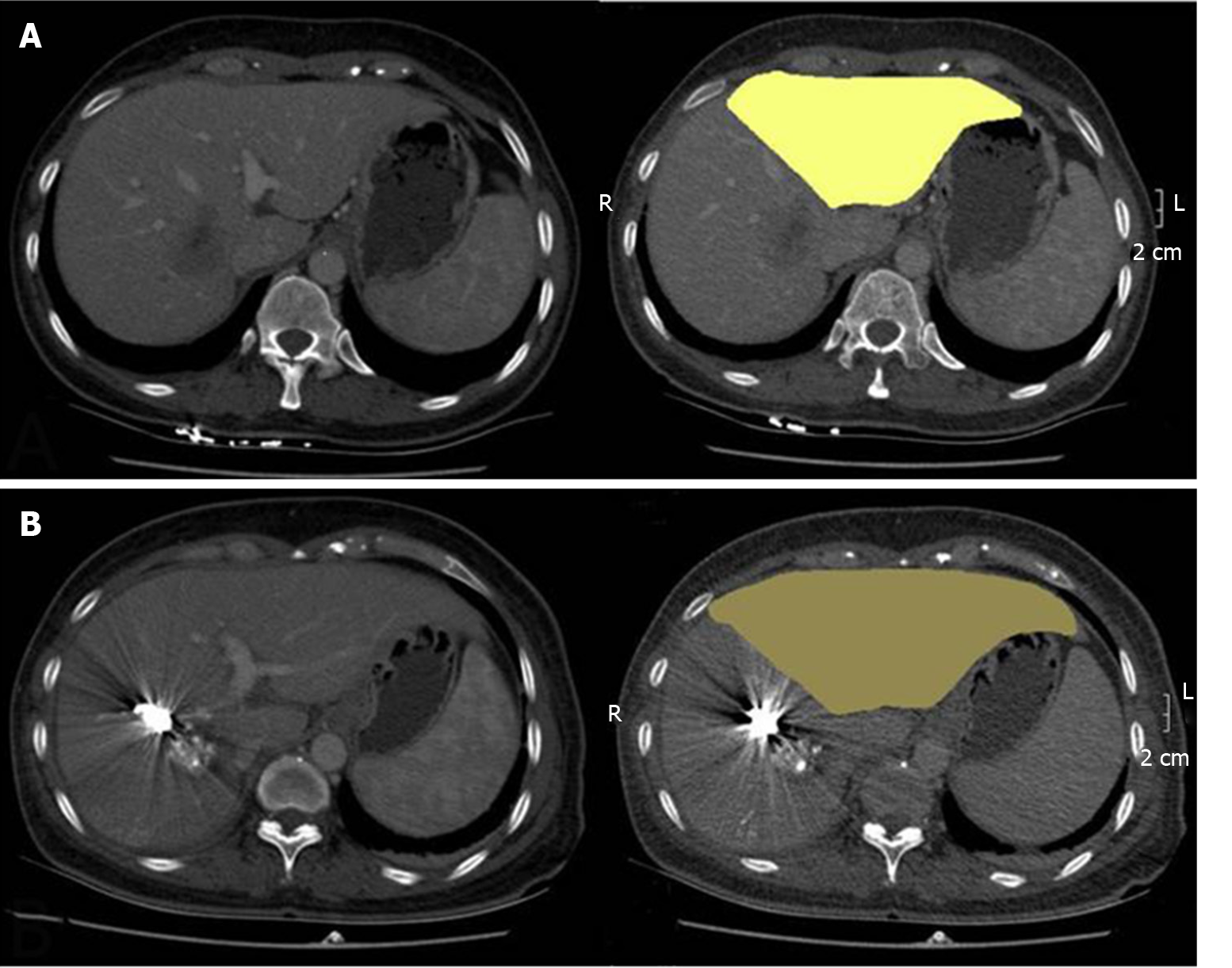
The mean ratio of FLR volume was similar in all groups before surgery (45.9% ± 4.2% vs 43.3% ± 6.6% vs 43.0% ± 4.7%, P = 0.262). The mean interval from TACE/PVE to surgery was significantly lower in the siTP group (16.2 ± 2.7 d vs 37.9 ± 6.5 vs 35.4 ± 10.6 d, respectively, in the siTP, seTP, and PVE-only groups, P < 0.001), and the KGR of FLR was significantly higher in the siTP group than in the other groups (21.1% ± 5.9% vs 7.6% ± 2.9% vs 6.8% ± 3.6%, P < 0.001) (Figure 2). The rate of tumor size reduction was 100% (13/13) in the siTP group, was 76% (13/17) in the seTP group, and was 10% (2/21) in the PVE-only group (P < 0.001) (Table 2).
| Variable | siTP group (n = 13) | seTP group (n = 17) | PVE-only group (n = 21) | P value |
| FLR (%) (before hepatectomy) | 45.9 ± 4.2 | 43.3 ± 6.6 | 43.0 ± 4.7 | 0.262 |
| Interval from TACE/PVE to hepatectomy | 16.2 ± 2.7 | 37.9 ± 6.5 | 35.4 ± 10.6 | < 0.001 |
| KGR (%) | 21.1 ± 5.9 | 7.6 ± 2.9 | 6.8 ± 3.6 | < 0.001 |
| Tumor size reduction, n (%) | 13 (100) | 13 (76) | 2 (10) | < 0.001 |
| Complications, n (%) | 0.875 | |||
| Abdominal pain | 6 (46) | 9 (53) | 3 (14) | |
| Fever | 7 (54) | 8 (47) | 3 (14) | |
| Nausea | 2 (15) | 4 (24) | 2 (10) | |
| Acute pulmonary infarction | 0 | 1 (6) | 0 |
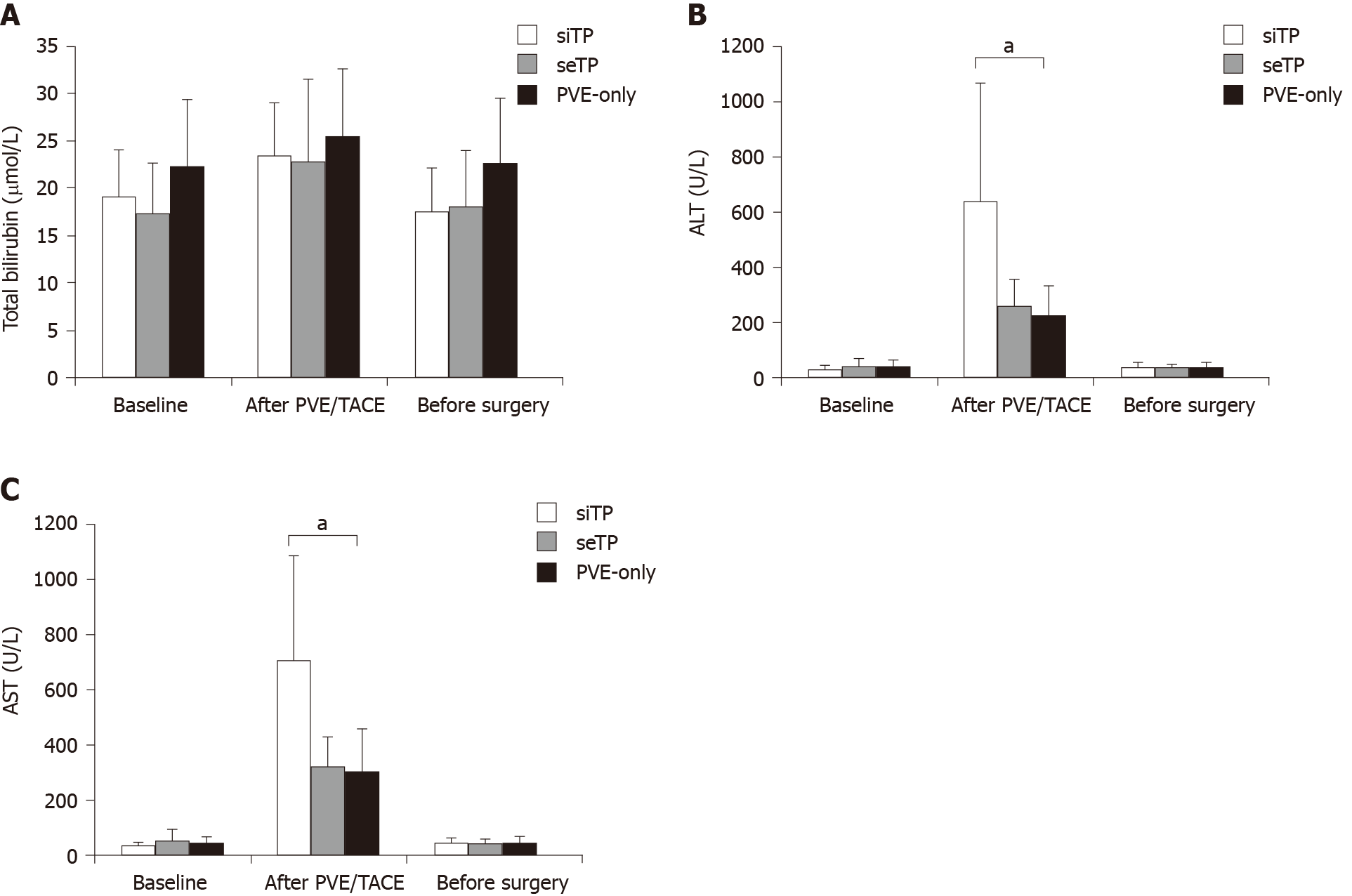
The results of LFTs after PVE and before surgery in the three groups are shown in Figure 1. The total bilirubin (TBIL) levels were similar among the three groups before or after PVE/TACE intervention. The patients in the three groups showed comparable alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels before TACE/PVE intervention. Compared with those in the seTP group and PVE-only group, the patients in the siTP group experienced the most prominent elevation of AST (706.7 ± 376.8 U/L vs 321.3 ± 112.2 U/L vs 310.1 ± 153.0 U/L, P < 0.001) and ALT (641.2 ± 429.8 U/L vs 261.5 ± 95.7 U/L vs 227.4 ± 107.3 U/L, P < 0.001) after PVE and TACE (Figure 3). The transient elevation of AST and ALT levels in the siTP group was recovered to comparable levels with the other two groups before surgery.
Ten patients failed to undergo major hepatectomy due to inadequate remnant liver hypertrophy (n = 6) or extrahepatic metastasis (n = 4). The resection rate was 100% in the siTP group (13/13), which was higher than that in the seTP group (82%, 14/17) and the PVE-only group (67%, 14/21), but the difference was not significant (P = 0.057). Intraoperative course including operation time, blood loss, transfusion, and the incidence of complications were similar among the three groups, as shown in Table 3. One patient in the PVE-only group died of liver failure 7 days after surgery, despite medical management, but no significant difference in the mortality was observed among the groups.
| Variables | siTP group (n = 13) | seTP group (n = 17) | PVE-only group (n = 21) | P value |
| Patients with major hepatectomy, n (%) | 13 (100) | 14 (82) | 14 (67) | 0.057 |
| Surgical approach, n (%) | 0.868 | |||
| Right hemihepatectomy | 9 (69) | 12 (71) | 9 (43) | |
| Right hepatic trisegmentectomy | 2 (15) | 1 (6) | 3 (14) | |
| Left hemihepatectomy | 1 (8) | 0 | 1 (5) | |
| Left hepatic trisegmentectomy | 1 (8) | 1 (6) | 1 (5) | |
| Operation time (min) | 261.4 ± 51.8 | 210.7 ± 53.7 | 246.1 ± 66.4 | 0.080 |
| Intraoperative blood loss (mL) | 285.0 ± 138.7 | 265.7 ± 153.6 | 343.9 ± 136.7 | 0.266 |
| Transfusion, n (%) | 4 (31) | 4 (29) | 2 (10) | 0.550 |
| Hospital stay after surgery (d) | 10.9 ± 2.1 | 11.9 ± 3.9 | 12.3 ± 3.4 | 0.503 |
| Complications, n (%) | 0.842 | |||
| Liver failure | 1 (8) | 0 | 2 (10) | |
| Bilirary fistula | 3 (23) | 4 (24) | 2 (10) | |
| Hydroperitonium | 1 (8) | 2 (12) | 0 | |
| Hydrothorax | 2 (15) | 1 (6) | 1 (5) | |
| Abdominal infection | 2 (15) | 1 (6) | 1 (5) | |
| Perioperative death, n (%) | 0 | 0 | 1 (5) | 0.372 |
| Pathologic response, n (%) | < 0.001 | |||
| MPR (RT < 10%) | 6 (46) | 2 (12) | 0 | |
| PPR (RT from 10% to 50%) | 3 (23) | 2 (12) | 0 |
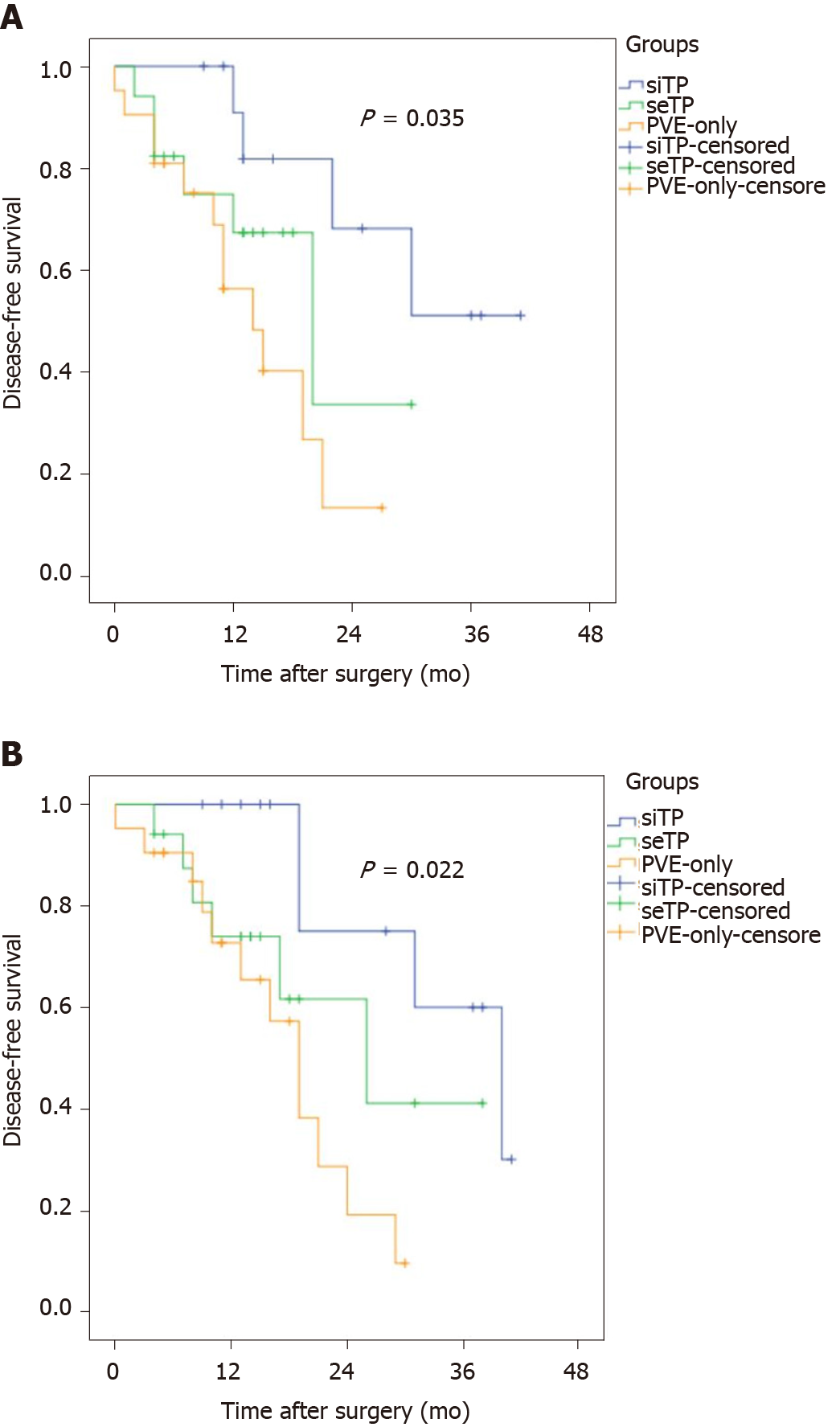
The pathological examination of the resected specimens showed that MPR and PPR of tumor-induced by simultaneous TACE + PVE occurred in 9 of 13 patients, 4 of 17 in the seTP group and none in the PVE-only group (P < 0.001). Compared with those in the seTP or PVE-only group, the patients in the siTP group showed significantly longer median DFS (P = 0.035) and overall survival (OS) (P = 0.022) after major hepatectomy (Figure 4).
Sequential TACE and PVE are associated with long time interval that can allow tumor growth and nullify their benefits. Therefore, this study aimed to evaluate the effect of simultaneous TACE and PVE for patients with large HCC prior to elective major hepatectomy. The results suggest that simultaneous TACE and PVE is a safe and effective approach to increase FLR volume for patients with large HCC before major hepatectomy.
The risk of postoperative liver failure is increasing with more and more major hepatectomy was performed, as 70% of patients with HCC have liver cirrhosis[4]. Therefore, the protocol of liver resection in the treatment of large liver tumors has been evaluated recently. As the most commonly used technique for increasing FLR volume, PVE is performed in many patients with HCC and has a low incidence of complications[11]. Nevertheless, one drawback of PVE is inadequate liver hypertrophy, especially in patients with cirrhosis. More importantly, the possibility of tumor progression caused by the long interval between PVE and surgery and compensatory increase of arterial blood flow after cessation of the portal supply is unavoidable.
Sequential TACE and PVE, which was firstly reported by Makuuchi et al[24], has been accepted as an effective preoperative treatment for patients with insufficient FLR and favorable hepatic reserve function. This approach has three main purposes: (1) To prevent tumor progression during the weeks between PVE and surgery; (2) To strengthen the effects of PVE by embolizing possible arterio-portal shunts (frequently found in HCC) whose blood contribution to the tumor may attenuate the effect of PVE; and (3) To improve the FLR volumetric increase through accelerating hepatocytes proliferation that may be induced by the increased inflammatory cytokine production as well as the massive HCC necrosis following the obstruction of both arterial and portal flow[25]. Animal experiments showed that liver injury caused by TACE+PVE was mild and recoverable. TACE + PVE showed a stronger tumor-inhibiting effect than TACE or PVE alone and induced a higher level of tumor cell apoptosis[26]. Sequential TACE and PVE before surgery is a safe and effective method to increase the rate of hypertrophy of the FLR and lead to longer OS and recurrence-free survival (RFS) in patients with large HCC. Indeed, Yoo et al[14] reported the mean increase in percentage FLR volume, OS and RFS were significantly higher in the TACE +PVE group than in the PVE only group.
Nevertheless, the possibility of tumor progression caused by incomplete tumor necrosis after TACE and during the interval time between TACE and PVE remains an inevitable problem. Therefore, simultaneous TACE and PVE was proposed and performed in several liver cancer patients with small tumors located near the surface of the liver as a preoperative treatment and obtained satisfactory efficacy[18]. One of the major concerns regarding simultaneous TACE and PVE is the risk of severe liver parenchymal necrosis resulting from the simultaneous occlusion of arterial and portal blood supply, which may influence the prognosis of patients. Therefore, the safety and efficacy of simultaneous TACE+PVE require urgent investigation in large numbers of patients, especially in those with large HCC.
In the present study, 13 patients with large HCC received simultaneous TACE + PVE before liver resection, and no liver failure occurred. TBIL level showed a minor and transient elevation in all three groups, which was consistent with previous data[18]. Although the mean liver transaminases levels, including ALT and AST, peaked temporarily after simultaneous TACE + PVE and were significantly higher than in the seTP and PVE-only groups, they almost returned to normal before surgery. Therefore, we proposed that the transient transaminases elevation after simultaneous PVE and TACE result from tumor necrosis rather than from noncancerous liver parenchymal necrosis. This perspective is also supported by the data of higher mean RT of the pathological specimens in the siTP group compared to that in the seTP and PVE-only groups, and mild liver parenchymal damage. It should be noted that all eligible patients had good liver function (ICGR15 < 10%) at baseline, which might be the reason why no liver failure occurred after TACE and/or PVE in all the three groups.
Although the mean FLR volume before surgery was similar in the three groups, the average interval time before surgery in the siTP group when the liver reached the minimum requirements of FLR volume was shorter than in the other two groups. More importantly, the KGR was significantly higher in the siTP group than in the seTP and PVE-only groups, indicating that simultaneous TACE + PVE is more efficient for increasing FLR volume than sequential TACE + PVE and PVE alone in patients with large HCC. We speculate that compared with sequential TACE + PVE or PVE alone, simultaneous PVE + TACE can lead to more thorough tumor necrosis, and thus more dramatic increase of inflammatory cytokines due to more obvious increase of blood flow of contralateral liver parenchyma. Remarkably, no significant differences were observed among the three groups regarding the operation time, blood loss, transfusion, and hospital stay after surgery, indicating that simultaneous TACE and PVE did not increase the difficulty of liver resection. In addition, no significant difference was found regarding the complications and perioperative mortality among the three groups, suggesting that simultaneous TACE and PVE did not increase postoperative complications.
We compared the survival among the three groups. The median OS and DFS were longer in the siTP group than in seTP and PVE-only groups. This may be explained with following reasons: (1) Simultaneous TACE and PVE can completely block the hematogenous metastasis, which is a fundamental determinant of patients’ survival; (2) It can induce more prominent tumor necrosis by the simultaneous occlusion of the double blood supply; and (3) It can shorten the wait time before surgery, which further reduces the chance of tumor progression.
This study has limitations. Data of portal pressure were not available to evaluate the effect of the major hepatic resections because portal pressure was not routinely measured in our center. The sample size was too small to conduct a multivariable analysis of the outcomes. Therefore, the conclusions can only be used as preliminary evidence that should be further confirmed in larger groups of patients and with longer follow-up.
In conclusion, our study demonstrates that simultaneous TACE and PVE is a safe and effective approach to increase the FLR volume quickly in a short time for patients with large HCC before major hepatectomy.
Sequential transarterial chemoembolization (TACE) and portal vein embolization (PVE) can improve the clinical outcomes and survival of patients with large hepatocellular carcinoma (HCC). However, the sequential treatment needs long wait time that can allow tumor growth and nullify treatments' benefits.
No study has ever compared the results of simultaneous TACE and PVE vs. sequential TACE and PVE or vs. PVE alone.
To evaluate the effect of simultaneous TACE and PVE before major hepatectomy in patients with large HCC and to compare their clinical outcome with sequential TACE+PVE or PVE only.
Fifty-one patients with large HCC who underwent PVE combined with or without TACE prior to major hepatectomy were included in this study, with 13 patients in the simultaneous TACE + PVE group, 17 patients in the sequential TACE + PVE group, and 21 patients in the PVE-only group. The outcomes of the procedures were compared and analyzed.
All patients underwent embolization. The mean interval from embolization to surgery, the kinetic growth rate of the future liver remnant (FLR), the degree of tumor size reduction, and complete tumor necrosis were significantly better in the simultaneous TACE + PVE group than in the other two groups. Although the patients in the simultaneous TACE + PVE group had a higher transaminase levels after PVE and TACE, they recovered to comparable levels with the other two groups before surgery. The intraoperative course and the complication and mortality rates were similar among the three groups. The overall survival and disease-free survival were higher in the simultaneous TACE + PVE group than in the other two groups.
Simultaneous TACE and PVE is a safe and effective approach to increase FLR volume for patients with large HCC who need major hepatectomy.
Although the data were extracted from a prospective database, portal pressure data were not available because portal pressure was not routinely measured in our center. Prospective studies are needed to verify the present preliminary evidence, with available data of portal pressure. Multicenter, randomized controlled trials with large sample size and long-term follow-up should be conducted to confirm our results.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aoki T S-Editor: Gong ZM L-Editor: MedE-Ma JY P-Editor: Ma YJ
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12667] [Cited by in RCA: 15299] [Article Influence: 3059.8] [Reference Citation Analysis (4)] |
| 2. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55773] [Article Influence: 7967.6] [Reference Citation Analysis (132)] |
| 3. | Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6569] [Article Influence: 469.2] [Reference Citation Analysis (1)] |
| 4. | NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Hepatobiliary Cancers. Version 4.2019. Washington: National Comprehensive Cancert Network, 2019. |
| 5. | Verslype C, Rosmorduc O, Rougier P; ESMO Guidelines Working Group. Hepatocellular carcinoma: ESMO-ESDO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23 Suppl 7:vii41-vii48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 282] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 6. | Zheng R, Qu C, Zhang S, Zeng H, Sun K, Gu X, Xia C, Yang Z, Li H, Wei W, Chen W, He J. Liver cancer incidence and mortality in China: Temporal trends and projections to 2030. Chin J Cancer Res. 2018;30:571-579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 241] [Article Influence: 34.4] [Reference Citation Analysis (1)] |
| 7. | Chen JG, Zhang SW. Liver cancer epidemic in China: past, present and future. Semin Cancer Biol. 2011;21:59-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 188] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 8. | Wong MC, Jiang JY, Goggins WB, Liang M, Fang Y, Fung FD, Leung C, Wang HH, Wong GL, Wong VW, Chan HL. International incidence and mortality trends of liver cancer: a global profile. Sci Rep. 2017;7:45846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 180] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 9. | Palavecino M, Chun YS, Madoff DC, Zorzi D, Kishi Y, Kaseb AO, Curley SA, Abdalla EK, Vauthey JN. Major hepatic resection for hepatocellular carcinoma with or without portal vein embolization: Perioperative outcome and survival. Surgery. 2009;145:399-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 10. | Ribero D, Abdalla EK, Madoff DC, Donadon M, Loyer EM, Vauthey JN. Portal vein embolization before major hepatectomy and its effects on regeneration, resectability and outcome. Br J Surg. 2007;94:1386-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 342] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 11. | Abulkhir A, Limongelli P, Healey AJ, Damrah O, Tait P, Jackson J, Habib N, Jiao LR. Preoperative portal vein embolization for major liver resection: a meta-analysis. Ann Surg. 2008;247:49-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 473] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 12. | Shindoh J, D Tzeng CW, Vauthey JN. Portal vein embolization for hepatocellular carcinoma. Liver Cancer. 2012;1:159-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Glantzounis GK, Tokidis E, Basourakos SP, Ntzani EE, Lianos GD, Pentheroudakis G. The role of portal vein embolization in the surgical management of primary hepatobiliary cancers. A systematic review. Eur J Surg Oncol. 2017;43:32-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 14. | Yoo H, Kim JH, Ko GY, Kim KW, Gwon DI, Lee SG, Hwang S. Sequential transcatheter arterial chemoembolization and portal vein embolization versus portal vein embolization only before major hepatectomy for patients with hepatocellular carcinoma. Ann Surg Oncol. 2011;18:1251-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 15. | Xu C, Lv PH, Huang XE, Wang SX, Sun L, Wang FA, Wang LF. Safety and efficacy of sequential transcatheter arterial chemoembolization and portal vein embolization prior to major hepatectomy for patients with HCC. Asian Pac J Cancer Prev. 2014;15:703-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Goumard C, Komatsu S, Brustia R, Fartoux L, Soubrane O, Scatton O. Technical feasibility and safety of laparoscopic right hepatectomy for hepatocellular carcinoma following sequential TACE-PVE: a comparative study. Surg Endosc. 2017;31:2340-2349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Ogata S, Belghiti J, Farges O, Varma D, Sibert A, Vilgrain V. Sequential arterial and portal vein embolizations before right hepatectomy in patients with cirrhosis and hepatocellular carcinoma. Br J Surg. 2006;93:1091-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 184] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 18. | Di Carlo I, Pulvirenti E, Toro A, Patanè D. Simultaneous transarterial and portal embolization for unresectable tumors of the liver. Hepatogastroenterology. 2010;57:140-145. [PubMed] |
| 19. | European Association for the Study of the Liver. European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4059] [Cited by in RCA: 4517] [Article Influence: 347.5] [Reference Citation Analysis (2)] |
| 20. | Wáng YX, De Baere T, Idée JM, Ballet S. Transcatheter embolization therapy in liver cancer: an update of clinical evidences. Chin J Cancer Res. 2015;27:96-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 64] [Reference Citation Analysis (0)] |
| 21. | Azoulay D, Castaing D, Krissat J, Smail A, Hargreaves GM, Lemoine A, Emile JF, Bismuth H. Percutaneous portal vein embolization increases the feasibility and safety of major liver resection for hepatocellular carcinoma in injured liver. Ann Surg. 2000;232:665-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 283] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 22. | Shindoh J, Truty MJ, Aloia TA, Curley SA, Zimmitti G, Huang SY, Mahvash A, Gupta S, Wallace MJ, Vauthey JN. Kinetic growth rate after portal vein embolization predicts posthepatectomy outcomes: toward zero liver-related mortality in patients with colorectal liver metastases and small future liver remnant. J Am Coll Surg. 2013;216:201-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 233] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 23. | Bryant MK, Dorn DP, Zarzour J, Smith JK, Redden DT, Saddekni S, Abdel Aal AK, Gray SH, Eckhoff DE, Dubay DA. Computed tomography predictors of hepatocellular carcinoma tumour necrosis after chemoembolization. HPB (Oxford). 2014;16:327-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Aoki T, Imamura H, Hasegawa K, Matsukura A, Sano K, Sugawara Y, Kokudo N, Makuuchi M. Sequential preoperative arterial and portal venous embolizations in patients with hepatocellular carcinoma. Arch Surg. 2004;139:766-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 137] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 25. | Veteläinen R, Dinant S, van Vliet A, van Gulik TM. Portal vein ligation is as effective as sequential portal vein and hepatic artery ligation in inducing contralateral liver hypertrophy in a rat model. J Vasc Interv Radiol. 2006;17:1181-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Song D, Hu M, Guo W. Liver Injury and Tumor-Inhibiting Effect of Sequential Transcatheter Arterial Chemoembolization and Portal Venous Embolization on Rabbit VX2 Liver Carcinoma. Med Sci Monit. 2017;23:1471-1476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |