Published online Jul 21, 2020. doi: 10.3748/wjg.v26.i27.3989
Peer-review started: March 31, 2020
First decision: April 29, 2020
Revised: June 9, 2020
Accepted: June 23, 2020
Article in press: June 23, 2020
Published online: July 21, 2020
Processing time: 112 Days and 7.8 Hours
The incidence of intestinal NK/T cell lymphoma (NKTCL) is extremely low, and the clinical symptoms are atypical, which makes it difficult to distinguish this disorder from Crohn's disease (CD), T lymphocyte proliferative disease, and other immune disorders. The misdiagnosis rate is high, and the patient's prognosis is poor.
In this case, the patient had repeated high fever, colonoscopy revealed multiple ulcers, and the initial diagnosis was CD. The patient’s condition did not improve after treatment with hormones and infliximab, and she eventually died. Positron emission tomographic-computed tomographic and B-ultrasound were performed in our hospital and showed that multiple lymph nodes were enlarged. Immunohistochemi-stry showed that CD3 and Epstein-Barr virus encoded RNA expression was positive. Colonoscopy, tissue biopsy, and histopathology showed intestinal focal mucosal infiltration of heterotypic lymphocytes with an abnormal immune phenotype. On the basis of the patient’s medical history, auxiliary examination, and pathological findings, digestive physicians and pathologists gave the diagnosis of NKTCL.
Clinicians need to improve their comprehensive knowledge of NKTCL, and combination of clinical symptoms, histological characteristics, as well as colonoscopy biopsies should be considered to improve the diagnosis and thereby reduce misdiagnosis.
Core tip: The incidence of intestinal NK/T cell lymphoma (NKTCL) is extremely low, and the clinical symptoms are atypical. The misdiagnosis rate is high, and the patient's prognosis is poor. It is commonly caused by Epstein-Barr virus infection. At present, the etiology of this disease is unclear. The patient in this case was eventually diagnosed with intestinal NKTCL. The patient’s condition did not improve after treatment with hormones and infliximab, and she eventually died. This case reminds us that more potential molecular markers need to be explored. The molecular regulatory network needs to be supplemented to help with the differential diagnosis. Clinicians and pathologists need to improve their awareness of this disease and reduce the rate of misdiagnosis.
- Citation: Li H, Lyu W. Intestinal NK/T cell lymphoma: A case report. World J Gastroenterol 2020; 26(27): 3989-3997
- URL: https://www.wjgnet.com/1007-9327/full/v26/i27/3989.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i27.3989
In recent years, there have been reports in the literature of NK/T cell lymphoma (NKTCL) originating from the gastrointestinal tract[1]. NKTCL has a low incidence and lacks typical clinical manifestations. The inner lining of the gastrointestinal tract exhibits multiple intestinal mucosa ulcers of different sizes, which are related to Crohn's disease (CD) and indolent T lymphocyte proliferative disease (ITLPD)[2]. It is difficult to identify proliferative diseases, and the tumor cells show extensive variation under microscope. When the infection is complicated, the microscopic findings are often confused with inflammatory infiltration, which results in a high misdiagnosis rate and a difficult diagnosis. We here report a case of an adult female with recurrent fever and diarrhea. CD was diagnosed multiple times by colonoscopy and pathology. The patient was treated with immunosuppressive agents, biological agents, and antibiotics. She had recurrent high fever with diarrhea and weight loss, which became more severe, and she eventually died. Based on consultation of gastroenterologists and pathologists, the diagnosis in this case was considered to be intestinal NKTCL.
A 40-year-old female patient was admitted to the hospital with recurrent fever and loose stools for more than 3 mo and aggravation for 3 d.
The patient had developed fever with loose stools 3 mo ago. She went to a local hospital, and diagnosed with acute gastroenteritis and was discharged after anti-infection, fluid replacement and other symptomatic supportive treatments. Subsequently, the patient repeatedly developed fever and loose stools, which resembled a dilute water sample. The stools were yellow with no blood, and the patient was monitored for signs of emergency. She visited the local hospital many times for treatment, and showed no obvious improvement in her symptoms.
The patient reported no remarkable history of past illness.
Physical examination showed pale mucosa and tenderness of upper abdomen.
The auxiliary examination after admission showed that the white blood cells were 9.9 × 109/L, neutral ratio was 75.7%, hemoglobin was 87 g/L, C-reactive protein was 106 mg/L, and procalcitonin was 3.92 ng/mL. Calprotectin and ANCA IgG were positive. Tests were negative for autoantibody, immunization, blood culture, stool culture, Widal reaction, CMV DNA, and inflammatory bowel disease-associated antibodies.
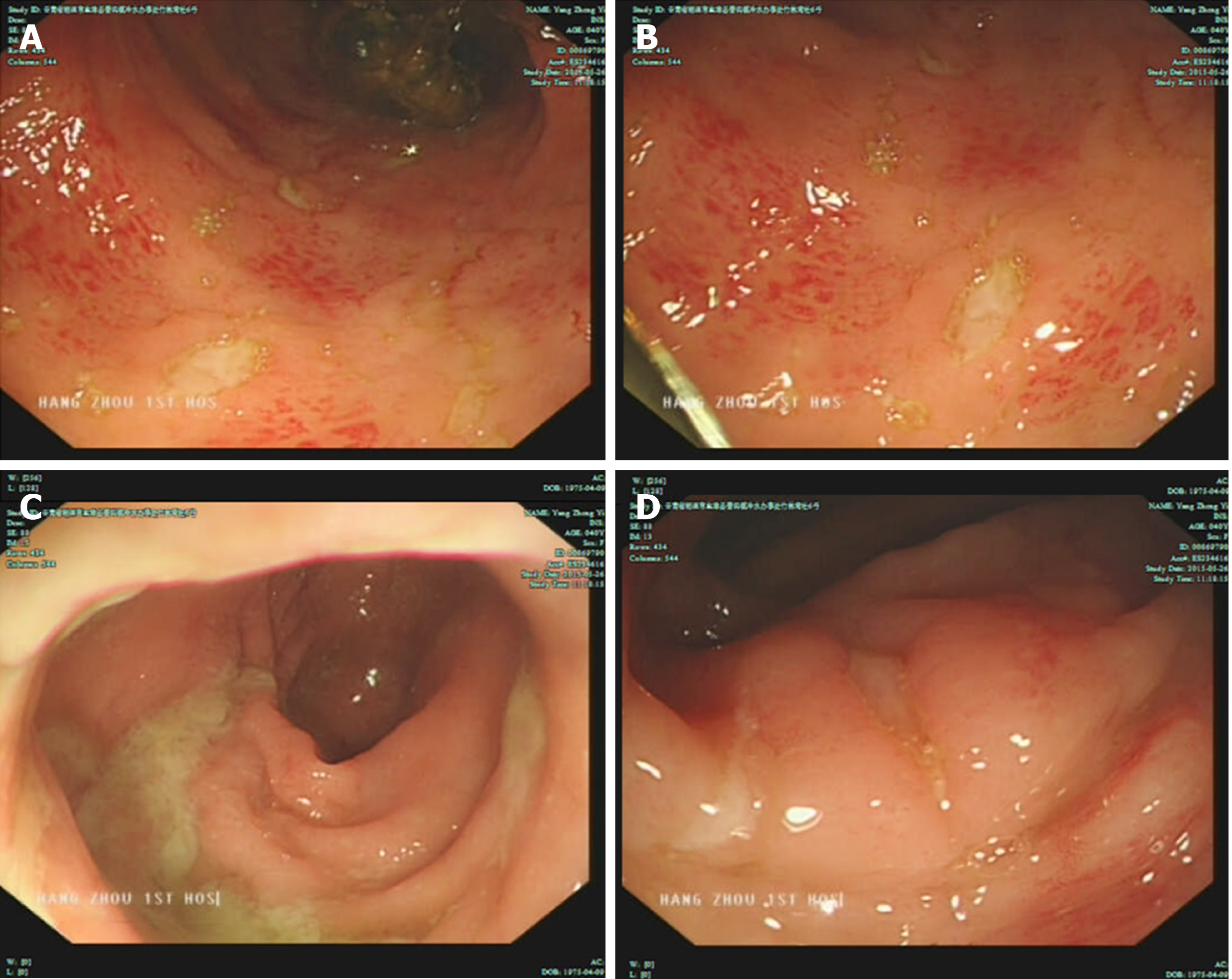
B-ultrasound revealed that multiple lymph nodes in the neck were accessible, including lymph nodes near the root of the neck; and multiple mesenteric nodes were accessible. Colonoscopy revealed multiple colonic ulcers on May 26, 2015 (Figure 1). The colonoscopic biopsy pathology showed chronic mucosal inflammation, interstitial lymphocytes, plasma cells, and infiltration of eosinophils and neutrophils. The patient was administered metronidazole for anti-infection and salford enema fluid combined with Pattersian. The colonoscopic biopsy pathology showed interstitial lymphocyte hyperplasia with erosion and granulation tissue formation, and immunohisto-chemistry showed chronic inflammation of the sigmoid mucosa on May 29, 2015. The lymphocyte results mainly indicated T lymphocyte hyperplasia. The patient underwent positron emission tomographic-computed tomographic (PET-CT) on June 10, 2015, which showed that 18F-fluorodeoxyglucose metabolism in the colon and rectum was increased, the intestinal wall of some segments of the colon was slightly thickened, prompting inflammatory bowel disease, and there were multiple low-metabolized lymph nodes in the mesenteric root of the abdominal cavity.
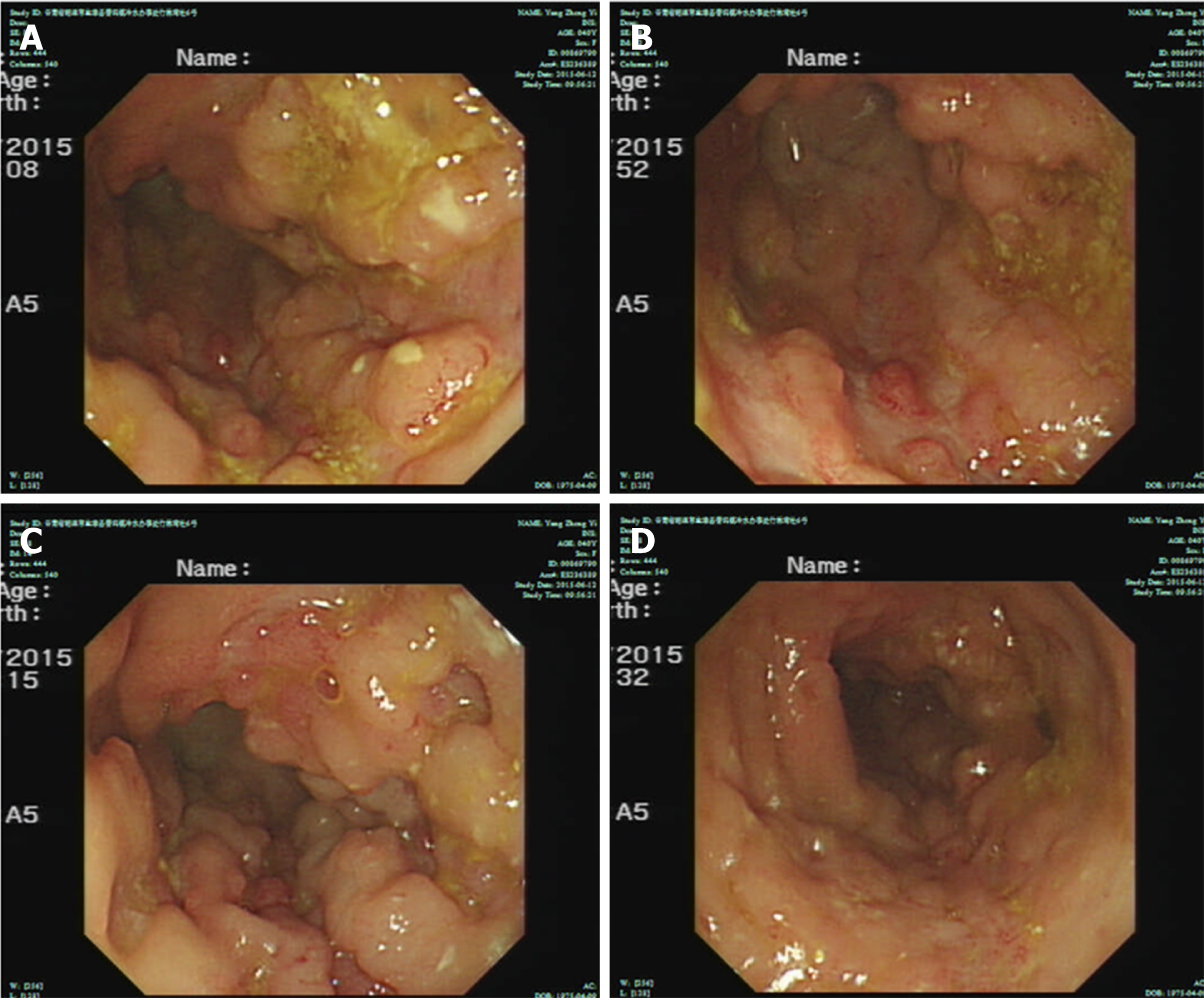
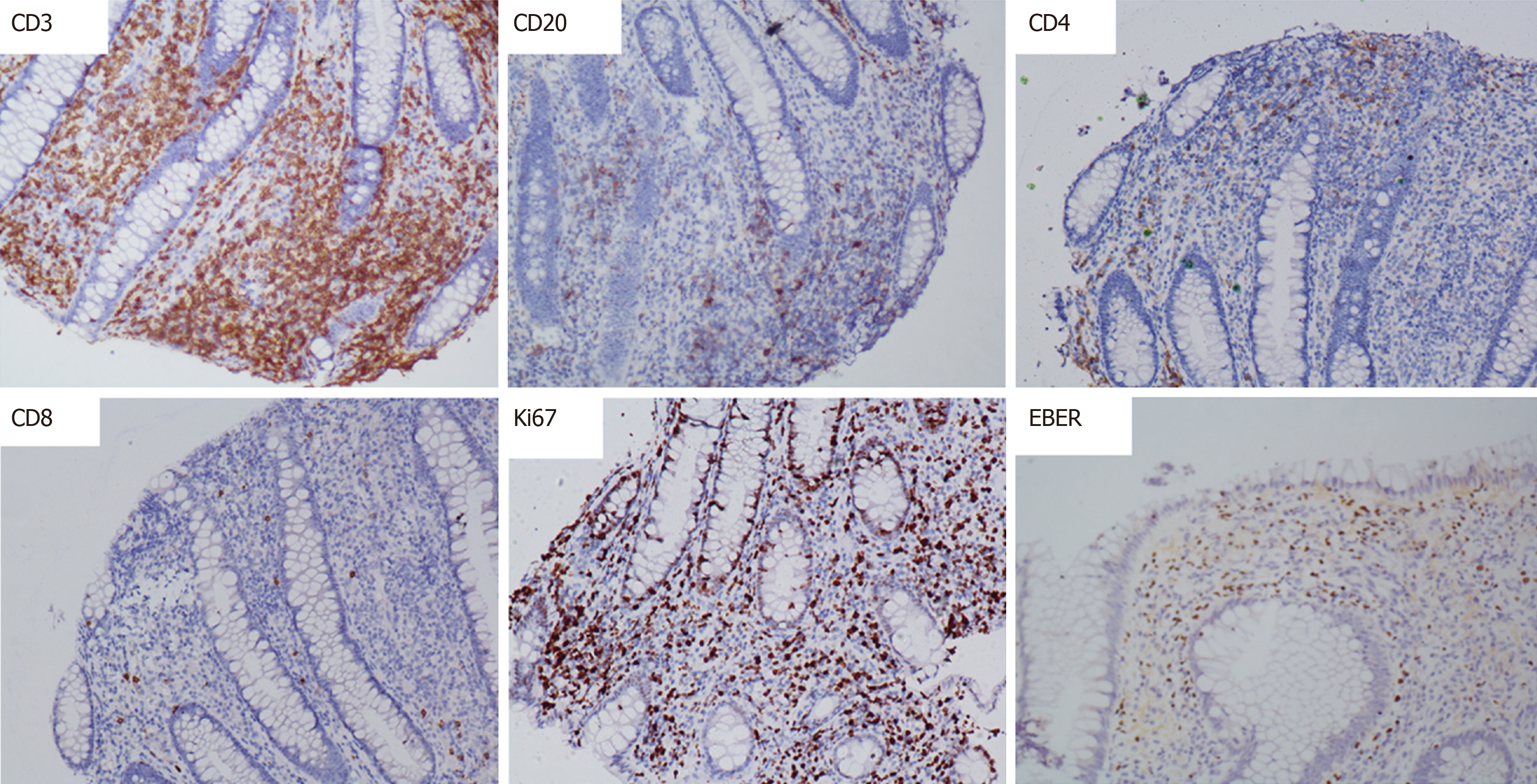
The patient had a high fever; the highest body temperature was 40.3 ºC, the white blood cells were 17.5 × 109/L, and the neutrophil ratio was 91.8%, and meropenem anti-infective treatment was administered. The patient subsequently experienced recurrent high fevers. A pathologist of Sir Run Run Shaw Hospital Affiliated with Zhejiang University was consulted. The expression of CD3, Epstein-Barr virus encoded RNA (EBER), and T-cell intracellular antigen (TIA) was positive; CD20, CD5, CD4, and CD8 were negative; CD56 was suspected to be positive; and Ki-67 was approximately 40%. Pathological diagnosis showed intestinal mucosal focal heterotypic lymphocyte infiltration with an abnormal immune phenotype, and T cell lymphoma could not be ruled out on June 28, 2015. Enteroscopy showed hyperemia and edema of the sigmoid colon mucosa, multiple longitudinal ulcers and proliferative changes of the surrounding mucosa on June 12, 2015 (Figure 2). Bone marrow aspiration showed lymphocytes (accounting for 3.5%, mainly mature) on June 17, 2015. Immunohistochemical analysis on the bone marrow was performed and revealed that CD3, CD2, CD5, CD20, CD79α, myeloperoxidase, and CD61 and CD34 megakaryocytes, were positive, and terminal deoxynucleotidyl transferase was negative on June 18, 2015. Blood tumor immunotyping showed that the proportion of granulocytes increased, which showed abnormal differentiation on the CD15-CD11b and CD16-CD13 dot plot on June 18, 2015. The Ig gene rearrangement test did not detect a monoclonal rearrangement of the gene on June 23, 2015. The hematologist consultation did not consider lymphoma.
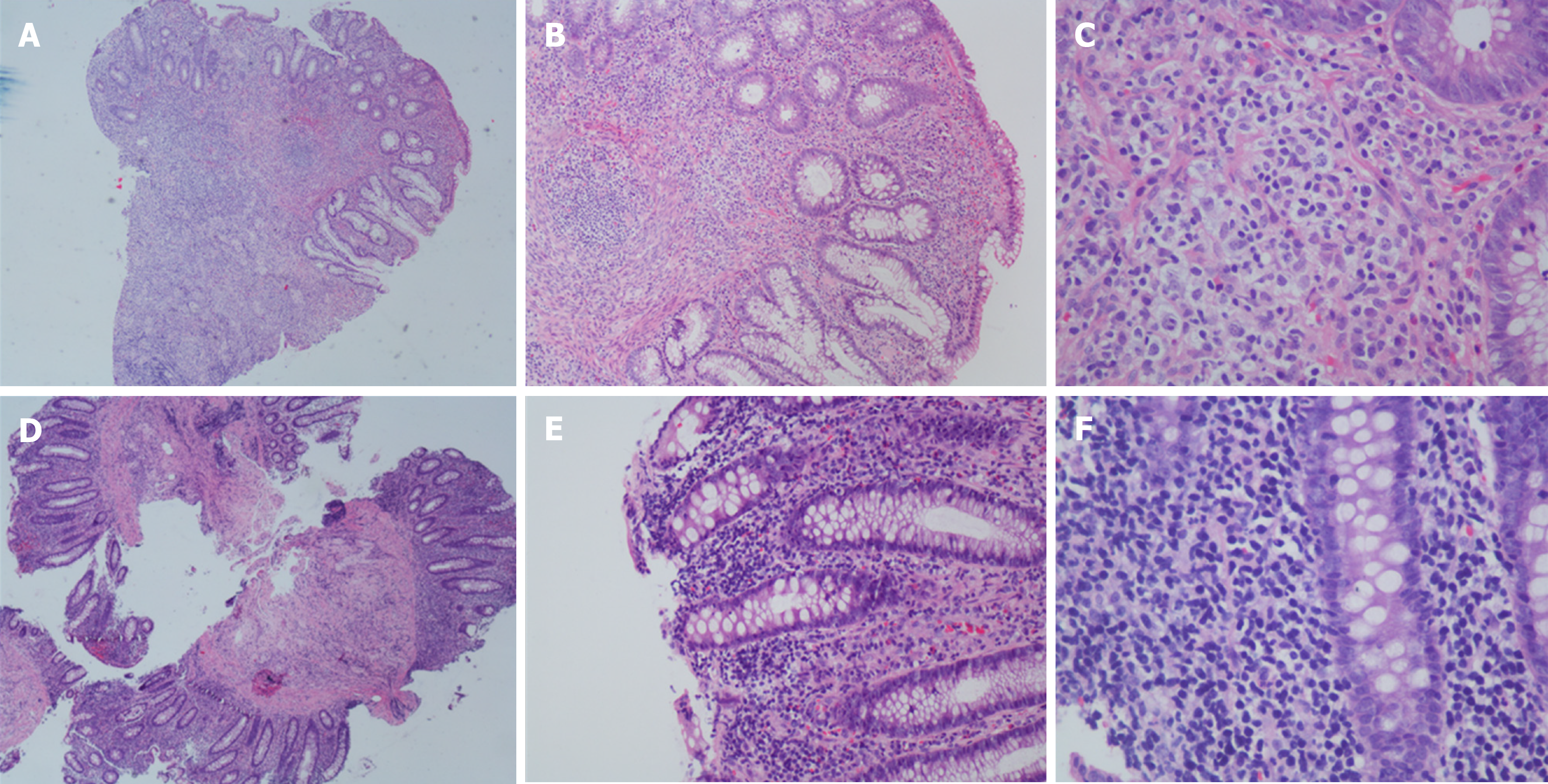
Colonoscopy showed multiple superficial ulcers. Biopsy showed heterotypic lymphocyte clusters, CD3, and EBER were positively expressed (Figures 3 and 4). Consultation of gastroenterologists and pathologists resulted in a final diagnosis of intestinal NKTCL.
The patient was administered metronidazole for anti-infection and salford enema fluid combined with Pattersian. The patient had a high fever; the highest body temperature was 40.3 ºC, which was managed by meropenem anti-infective treatment. The patient had a recurrent high-grade fever and was diagnosed with severe CD; lymphoma was also considered. And she was given an intravenous injection of methylprednisolone 40 mg for 3 d in combination with Podexan orally and a salford enema on June 6, 2015, which was shifted to metzo at 40 mg orally 3 d later. The patient's temperature gradually stabilized, her diarrhea improved, and the dose of hormone was reduced according to the guidelines.
After discharge, the patient's temperature gradually became normal, her diarrhea improved, and the hormone dose was reduced to 20 mg orally each day. Subsequently, the patient developed a high-grade fever with significant weight loss. She was diagnosed with “severe CD” in another hospital and was treated with infliximab. The patient achieved a short-term clinical remission. Eventually, the patient relapsed and died a few months later.
NKTCL is a rare type of lymphoma with a high degree of malignancy. It is commonly caused by Epstein-Barr virus (EBV) infection, and it occurs more frequently in the middle aged individuals[3]. The median age is 37-47 years. The extra-articular NKTCL occurs most commonly in the gastrointestinal tract, accounting for approximately 30%-50% of all extra-articular NKTCL. The lesion most commonly involves the colon in the intestinal tract, which also involves part of the small intestine[4]. It is estimated that approximately 57.4% of NKTCL cases occur in the colon. Intestinal lesions of adult systemic EBV-positive NKTCL are reported in domestic and foreign literature to have primary clinical features of chronic diarrhea, accompanied by high fever and emaciation; in the early stage of the concurrent blood syndrome or progression to highly malignant lymphoma, the common symptoms of malignant NKTCL are fever, skin rashes, lymph node hyperplasia and hepatosplenomegaly. The adult-onset prognosis is poor, and infection and multiple organ failure are the main causes of death. Symptoms usually last from weeks to months. Endoscopic manifestations are nonspecific, and superficial/erosive, ulcerative/ulcerative infiltrating lesions of the intestinal mucosa are common, and the endoscopic manifestations are variable, which also brings difficulties in diagnosis[5]. EBER was positive by immunohistochemistry, with most patients expressing CD3, CD56, TIA-1, and occasionally CD4 (0%-11%) or CD8 (0%-20%)[4,6,7]. TR clonal rearrangements occur in 17%-40% of NKTCL patients[8-10]. Gene array in lymphoma revealed that the most common chromosomal region deletions were 6q, 8p, 11q, 13q, and 17p. The most common chromosomal regions were 1q, 17q, and 20q, and the smallest chromosomal deletion region was 6q, which was seen in 40%-50% of cases. This region contained tumor suppressor genes such as prdm1, foxo3, and hace1[11-15]. Second-generation sequencing of nasopharyngeal ENKTCL tissues detected mutant genes (jak3, stat3, stat5b), epigenetic regulators (bcor and mll2), and DNA damage repair genes (tp53, ddx3x) in the JAK-STAT pathway[3,16]. NKTCL has no typical clinical features and no effective treatment is available. Cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) chemotherapy is currently recommended, but patients can only achieve short-term clinical remission and prognosis is poor, with a median survival of 7 mo. EBV infection also predicts a worse prognosis[3,8].
ITLPD is a disease with indolent behavior. This lymphocytic proliferative disease has a rare clinical onset, and due to atypical clinical symptoms, pathological diagnosis is difficult, and the misdiagnosis rate is high. ITLPD is classified as a type of lymphoma in the fourth edition of the WHO classification, and its occurrence is rare. At present, the etiology of ITLPD is not clear, and some patients have a history of inflammatory bowel disease (IBD) and autoimmune diseases[17,18]. The clinical symptoms of ITLPD occurring in the gastrointestinal tract are nonspecific, including abdominal pain, nausea and vomiting, diarrhea, and gastrointestinal bleeding, which are often misdiagnosed as CD. The onset age of CD ranges from 15 to 77 years (the median age is 51 years). Ten patients diagnosed with ITLPD have been reported. The ten patients were young and middle-aged. The patients’ main clinical features were abdominal pain and diarrhea. Six of the patients had endoscopic lesions involving the large intestine, and some patients had lesions involving the whole digestive tract, characterized by shallow mucosal ulcers or multiple small polyps that could be involved in colonic lesions. It is difficult for colonoscopy to identify IBD. Colonoscopy usually shows intestinal mucosal ulcers, nodular hyperplasia, polyps, and mucosal erosion. Microscopically, small-to medium-sized mature lymphocytes in the mucosal lamina propria can be seen. In some cases, lymphocytes can be seen with mild atypia. Clusters of lymphocytes (lymph cytopathic lesions) are seen in the crypt epithelium. Biopsy molecular expression characteristics showed that CD3 was positively expressed in all 10 patients, and eight patients were CD4-, CD8+, and TIA+; one patient was CD4-/CD8-; and one patient was CD4-/CD8-. Another study reported a case of CD4-/CD8- ITLPD, which was characterized by abdominal pain and diarrhea. The patient had a history of CD and died within 1 year after receiving 5 courses of CHOP chemotherapy. ITLPD with CD4-/CD8- cells is rare, there is no effective treatment, and the average survival of patients is short. ITLPD can affect the whole digestive tract. The histological diagnosis is that the mucosa or (and) lamina propria infiltrates small and single lymphocytes. In some cases, lymphocytes may infiltrate into submucosal tissue, usually not covering the entire tissue layer nor forming lumps. Lesions may be continuous or multifocal, with variable immunophenotypes.
NKTCL may be misdiagnosed as CD. CD is a chronic intestinal mucosal inflammatory change and dysfunction of the intestinal epithelium and is a comprehensive disease. The etiology of CD is unclear, and it can be induced by genetic susceptibility, immune abnormalities, the environment, and changes in the intestinal microenvironment. The typical clinical features of CD are chronic diarrhea, abdominal pain, emaciation, and malnutrition[19]. Patients with CD concurrent with septicemia may have high fever and it is difficult to identify NKTCL. Typical laboratory tests of CD include thrombocytosis, anemia, and hypersensitive C-reactive protein. Fecal calprotectin is an important CD screening and activity evaluation index. Endoscopy is still an important examination method. Endoscopic changes of typical CD include segmental intestinal inflammation, longitudinal ulcers, and mucosal changes in visible pebble samples. The pathological biopsy showed no caseating granuloma change and no atypical lymphocyte infiltration. Noncaseous granuloma formation is one of the diagnostic criteria for Crohn's disease,with only a 15% positive rate of endoscopic biopsy, and a positive rate of 70% in surgical biopsy specimens. Digestive endoscopy combined with pathological biopsy is an important basis for the diagnosis of CD. In addition, capsule endoscopy is also a safe and noninvasive method for intestinal examination, which can observe early intestinal mucosal lesions. The prognosis of CD is relatively good, but there is still a lack of effective radical treatment at present[20]. Drug therapy is mostly used to control disease activity and maintain disease remission, and prevent complications and intestinal damage. After remission using biological agents, maintenance therapy can be continued or immunosuppressant maintenance therapy can be used[21]. If drug therapy fails or complete intestinal obstruction, acute perforation, uncontrolled massive bleeding and other serious complications occur, surgical treatment can be performed[22].
The relationship between NKTCL, CD, and ITLPD is unclear[17,23,24]. It is usually difficult to distinguish them based on clinical symptoms. The common symptoms are similar. The general characteristics, such as age of onset, can also overlap, and the endoscopic features are usually nonspecific. Some NKTCL and ITLPD patients have a history of CD, and some ITLPD patients may have lymphoid follicular hyperplasia and granulomas. Histopathology of gastrointestinal tract derived ITLPD may show inflammatory changes, and most cases are initially misdiagnosed as inflammatory diseases. Significant lymphocytic infiltration or single lymphocytic infiltration in the lamina propria helps to distinguish ITLPD from CD. ITLPD is an inert disease with slow disease development and usually with mild clinical symptoms. In addition to typical cell and tissue morphological differences, the lack of epithelial cell characteristics, rare mitosis, and low cell proliferation activity can be diagnosed as ITLPD. NKTCL has a relatively high degree of malignancy. NKTCL is invasive and severe and usually has a shorter median survival than ITLPD and CD[6,25]. NKTCL seen under the microscope is easily confused with CD and ITLPD with respect to histological phenotypes. Hormone and pedestrian therapy is effective for CD, while hormone therapy can also clinically relieve NKTCL and ITLPD in the short term, which increases the difficulty of diagnosis. The high degree of overlap among these three diseases, including clinical symptoms, endoscopy, and pathology, is the main reason for the difficulty in diagnosis. Pathological diagnosis is still the gold standard. It requires that multiple end-point biopsies be taken during the endoscopic biopsy, and the diseased tissue is excavated if necessary. At the same time, it is necessary to closely integrate clinical symptoms and pathological characteristics and perform a comprehensive evaluation with gene sequencing if necessary, so as to establish a correct diagnosis.
The clinical manifestations of the present patient were characterized by chronic diarrhea and repeated fever. B-ultrasound and PET-CT showed multiple lymph node enlargement. Several analyses of the endoscopic biopsy pathology suggested T lymphocyte hyperplasia, and heterotypic lymphocytes were visible. Immunohistochemistry showed positive CD3 and EBER expression. Digestive physicians and pathologists diagnosed NKTCL after several discussions. This disease is rare in adults, the prognosis of patients is poor, and the mortality rate is high. At present, the disease is not well understood, so early diagnosis and treatment are particularly important. Due to the lack of typical clinical symptoms and colonoscopic manifestations, the biopsy tissue under colonoscopy is small and shallow, and early lymphocyte aberration is not obvious, which often makes it difficult to distinguish this disorder from other inflammatory bowel diseases and intestinal lymphoproliferative diseases. At present, there is no unified treatment of NKTCL. Some patients receiving chemotherapy can achieve short-term remission, but cannot reduce the viral load in the body. Antiviral and immunosuppressive treatments may have short-term effects. In this case, hormone therapy was given several times, and short-term clinical remission was obtained, but long-term clinical remission was unsuccessful.
We reported a case of malignant intestinal NKTCL, which is a rare type of lymphoma and is hard to distinguish from CD, ITLPD, and other immune disorders. The development of medicine, biology and computer technology has enhanced our understanding of these diseases, including a more detailed and comprehensive classification of this type of immune disorder. However, the etiology and origin of this disease are still unclear. More potential molecular markers need to be explored. The molecular regulatory network needs to be supplemented to help with the differential diagnosis. Individualized treatment plans need to be formulated to improve the overall survival of patients. Clinicians and pathologists need to improve their awareness of this disease and reduce the rate of misdiagnosis in order to achieve better clinical management of patients with this disease.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Barberio B S-Editor: Liu M L-Editor: MedE-Ma JY E-Editor: Ma YJ
| 1. | Kim SJ, Choi CW, Mun YC, Oh SY, Kang HJ, Lee SI, Won JH, Kim MK, Kwon JH, Kim JS, Kwak JY, Kwon JM, Hwang IG, Kim HJ, Lee JH, Oh S, Park KW, Suh C, Kim WS. Multicenter retrospective analysis of 581 patients with primary intestinal non-hodgkin lymphoma from the Consortium for Improving Survival of Lymphoma (CISL). BMC Cancer. 2011;11:321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 2. | Wan Ahmad Kammal WS, Mohd Rose I, Md Zin RR, Raja Ali RA, Masir N. Extranodal NK/T-cell lymphoma mimicking Crohn's colitis. Malays J Pathol. 2019;41:195-199. [PubMed] |
| 3. | Tse E, Kwong YL. NK/T-cell lymphomas. Best Pract Res Clin Haematol. 2019;32:253-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 4. | Jiang M, Chen X, Yi Z, Zhang X, Zhang B, Luo F, Jiang Y, Zou L. Prognostic characteristics of gastrointestinal tract NK/T-cell lymphoma: an analysis of 47 patients in China. J Clin Gastroenterol. 2013;47:e74-e79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Kim JH, Lee JH, Lee J, Oh SO, Chang DK, Rhee PL, Kim JJ, Rhee JC, Lee J, Kim WS, Ko YH. Primary NK-/T-cell lymphoma of the gastrointestinal tract: clinical characteristics and endoscopic findings. Endoscopy. 2007;39:156-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | van Vliet C, Spagnolo DV. T- and NK-cell lymphoproliferative disorders of the gastrointestinal tract: review and update. Pathology. 2020;52:128-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 7. | Yu BH, Shui RH, Sheng WQ, Wang CF, Lu HF, Zhou XY, Zhu XZ, Li XQ. Primary Intestinal Extranodal Natural Killer/T-Cell Lymphoma, Nasal Type: A Comprehensive Clinicopathological Analysis of 55 Cases. PLoS One. 2016;11:e0161831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Chuang SS, Chang ST, Chuang WY, Huang WT, Hsieh PP, Tsou MH, Liao YL, Lin SH, Hsieh YC, Lu CL, Sheu MJ, Liu H. NK-cell lineage predicts poor survival in primary intestinal NK-cell and T-cell lymphomas. Am J Surg Pathol. 2009;33:1230-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Pongpruttipan T, Sukpanichnant S, Assanasen T, Wannakrairot P, Boonsakan P, Kanoksil W, Kayasut K, Mitarnun W, Khuhapinant A, Bunworasate U, Puavilai T, Bedavanija A, Garcia-Herrera A, Campo E, Cook JR, Choi J, Swerdlow SH. Extranodal NK/T-cell lymphoma, nasal type, includes cases of natural killer cell and αβ, γδ, and αβ/γδ T-cell origin: a comprehensive clinicopathologic and phenotypic study. Am J Surg Pathol. 2012;36:481-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 166] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 10. | Sun J, Lu Z, Yang D, Chen J. Primary intestinal T-cell and NK-cell lymphomas: a clinicopathological and molecular study from China focused on type II enteropathy-associated T-cell lymphoma and primary intestinal NK-cell lymphoma. Mod Pathol. 2011;24:983-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Siu LL, Wong KF, Chan JK, Kwong YL. Comparative genomic hybridization analysis of natural killer cell lymphoma/leukemia. Recognition of consistent patterns of genetic alterations. Am J Pathol. 1999;155:1419-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 182] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 12. | Siu LL, Chan V, Chan JK, Wong KF, Liang R, Kwong YL. Consistent patterns of allelic loss in natural killer cell lymphoma. Am J Pathol. 2000;157:1803-1809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 81] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Nakashima Y, Tagawa H, Suzuki R, Karnan S, Karube K, Ohshima K, Muta K, Nawata H, Morishima Y, Nakamura S, Seto M. Genome-wide array-based comparative genomic hybridization of natural killer cell lymphoma/leukemia: different genomic alteration patterns of aggressive NK-cell leukemia and extranodal Nk/T-cell lymphoma, nasal type. Genes Chromosomes Cancer. 2005;44:247-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 203] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 14. | Huang Y, de Reyniès A, de Leval L, Ghazi B, Martin-Garcia N, Travert M, Bosq J, Brière J, Petit B, Thomas E, Coppo P, Marafioti T, Emile JF, Delfau-Larue MH, Schmitt C, Gaulard P. Gene expression profiling identifies emerging oncogenic pathways operating in extranodal NK/T-cell lymphoma, nasal type. Blood. 2010;115:1226-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 242] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 15. | Karube K, Nakagawa M, Tsuzuki S, Takeuchi I, Honma K, Nakashima Y, Shimizu N, Ko YH, Morishima Y, Ohshima K, Nakamura S, Seto M. Identification of FOXO3 and PRDM1 as tumor-suppressor gene candidates in NK-cell neoplasms by genomic and functional analyses. Blood. 2011;118:3195-3204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 135] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 16. | Song TL, Nairismägi ML, Laurensia Y, Lim JQ, Tan J, Li ZM, Pang WL, Kizhakeyil A, Wijaya GC, Huang DC, Nagarajan S, Chia BK, Cheah D, Liu YH, Zhang F, Rao HL, Tang T, Wong EK, Bei JX, Iqbal J, Grigoropoulos NF, Ng SB, Chng WJ, Teh BT, Tan SY, Verma NK, Fan H, Lim ST, Ong CK. Oncogenic activation of the STAT3 pathway drives PD-L1 expression in natural killer/T-cell lymphoma. Blood. 2018;132:1146-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 236] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 17. | Perry AM, Warnke RA, Hu Q, Gaulard P, Copie-Bergman C, Alkan S, Wang HY, Cheng JX, Bacon CM, Delabie J, Ranheim E, Kucuk C, Hu X, Weisenburger DD, Jaffe ES, Chan WC. Indolent T-cell lymphoproliferative disease of the gastrointestinal tract. Blood. 2013;122:3599-3606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 139] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 18. | Malamut G, Meresse B, Kaltenbach S, Derrieux C, Verkarre V, Macintyre E, Ruskone-Fourmestraux A, Fabiani B, Radford-Weiss I, Brousse N, Hermine O, Cerf-Bensussan N, Cellier C. Small intestinal CD4+ T-cell lymphoma is a heterogenous entity with common pathology features. Clin Gastroenterol Hepatol. 2014;12:599-608.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Baumgart DC, Sandborn WJ. Crohn's disease. Lancet. 2012;380:1590-1605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1347] [Cited by in RCA: 1529] [Article Influence: 117.6] [Reference Citation Analysis (0)] |
| 20. | Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn's disease. Lancet. 2017;389:1741-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1121] [Cited by in RCA: 1797] [Article Influence: 224.6] [Reference Citation Analysis (111)] |
| 21. | Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295:2275-2285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1816] [Cited by in RCA: 1813] [Article Influence: 95.4] [Reference Citation Analysis (0)] |
| 22. | Larson DW, Pemberton JH. Current concepts and controversies in surgery for IBD. Gastroenterology. 2004;126:1611-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 72] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Carbonnel F, d'Almagne H, Lavergne A, Matuchansky C, Brouet JC, Sigaux F, Beaugerie L, Nemeth J, Coffin B, Cosnes J, Gendre JP, Rambaud JC. The clinicopathological features of extensive small intestinal CD4 T cell infiltration. Gut. 1999;45:662-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Matnani R, Ganapathi KA, Lewis SK, Green PH, Alobeid B, Bhagat G. Indolent T- and NK-cell lymphoproliferative disorders of the gastrointestinal tract: a review and update. Hematol Oncol. 2017;35:3-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 25. | Ko YH, Cho EY, Kim JE, Lee SS, Huh JR, Chang HK, Yang WI, Kim CW, Kim SW, Ree HJ. NK and NK-like T-cell lymphoma in extranasal sites: a comparative clinicopathological study according to site and EBV status. Histopathology. 2004;44:480-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 168] [Article Influence: 8.0] [Reference Citation Analysis (0)] |