Published online Jun 28, 2020. doi: 10.3748/wjg.v26.i24.3432
Peer-review started: March 4, 2020
First decision: April 12, 2020
Revised: April 24, 2020
Accepted: May 30, 2020
Article in press: May 30, 2020
Published online: June 28, 2020
Processing time: 115 Days and 23.1 Hours
Alcoholic liver disease (ALD) is a worldwide health problem, and natural products have been shown to improve ALD due to their antioxidant activities. Some parts of Hovenia dulcis (H. dulcis), such as roots, peduncles, and stems, provide health benefits. Nevertheless, the effects and mechanisms of H. dulcis seeds on ALD have not yet been fully elucidated.
To determine H. dulcis antioxidant activity, evaluate its effects against ALD, and investigate the related mechanisms via network pharmacology.
The antioxidant activity of H. dulcis seed was determined by both ferric-reducing antioxidant power and trolox equivalent antioxidant capacity assays. The total phenolic and flavonoid contents were determined by Folin–Ciocalteu method and aluminum chloride colorimetry, respectively, and polysaccharide was determined by phenol-sulfuric acid method. The effects of H. dulcis seeds against alcoholic liver injury were investigated in mice with water extract pretreatment for 7 days followed by alcohol administration. Moreover, the mechanisms of action were explored with network pharmacology.
The results showed that H. dulcis seeds possessed strong antioxidant activity (245.11 ± 10.17 μmol Fe2+/g by ferric-reducing antioxidant power and 284.35 ± 23.57 μmol TE/g by trolox equivalent antioxidant capacity) and contained remarkable phenols and flavonoids, as well as a few polysaccharides. H. dulcis seeds attenuated alcohol-induced oxidative liver injury, showing reduced serum alanine and aspartate aminotransferases, alkaline phosphatase, and triglyceride, elevated hepatic glutathione, increased activities of superoxide dismutase and catalase, and reduced malondialdehyde and hepatic triglyceride. The results of network pharmacology analysis indicated that kaempferol, stigmasterol, and naringenin were the main bioactive compounds in H. dulcis seeds and that modulation of oxidative stress, inflammation, gut-derived products, and apoptosis were underlying mechanisms of the protective effects of H. dulcis seeds on ALD.
The results of this study demonstrate that H. dulcis seeds could be a good natural antioxidant source with protective effects on oxidative diseases such as ALD.
Core tip: Although some parts of Hovenia dulcis (H. dulcis) provide health impacts, the effects and mechanisms of H. dulcis seeds on alcoholic liver disease (ALD) have not been fully elucidated. In this study, we found that H. dulcis seeds possessed strong antioxidant activity and contained considerable phenols and flavonoids. H. dulcis seeds attenuated ALD and improved hepatic antioxidant capacity. Furthermore, results of network pharmacology analyses indicated that kaempferol, stigmasterol, and naringenin were the main bioactive compounds in H. dulcis seeds and that modulating oxidative stress, inflammation, gut-derived products, and apoptosis were involved in the mechanisms underlying the protective effects of H. dulcis seeds on ALD.
- Citation: Meng X, Tang GY, Zhao CN, Liu Q, Xu XY, Cao SY. Hepatoprotective effects of Hovenia dulcis seeds against alcoholic liver injury and related mechanisms investigated via network pharmacology. World J Gastroenterol 2020; 26(24): 3432-3446
- URL: https://www.wjgnet.com/1007-9327/full/v26/i24/3432.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i24.3432
Alcohol abuse often results in liver disease, and alcoholic liver disease (ALD) is a worldwide health problem that causes major health and economic burdens on both individuals and the society[1,2]. ALD can start with alcoholic liver injury, develop into alcohol-induced hepatitis and/or steatosis, and progress to fibrosis and/or cirrhosis; ALD can even lead to liver cancer[1,3]. Given the poor prognosis and the limited efficacy of current treatments for ALD, it would be more effective to prevent liver disease progression at the very beginning rather than to treat advanced conditions[4].
Natural products are economical sources of promising medicines and worth exploring more broadly and deeply. Numerous natural products have exhibited strong antioxidant activities, and their bioactive components, such as polyphenols, flavonoids, and polysaccharides, have been demonstrated to provide various health benefits, thus revealing their potential for being developed into effective therapeutics[5-7]. Hovenia dulcis (H. dulcis) has been used as a folk medicine for several centuries, especially in Japan, China, and Korea. Certain parts of H. dulcis exhibit various health effects. Namely, the peduncles of H. dulcis showed antioxidant and immunostimulatory effects, its fruit or stem showed antidiabetic effects through the AMP-activated protein kinase pathway, and the root could prevent proliferation of hepatic stellate cell-T6 cells[8-10]. It was also demonstrated that H. dulcis seeds, the edible seeds, could speed up alcohol degradation, leading to reduced alcohol concentration in the blood[11]. However, the effects and/or mechanisms of action of H. dulcis seeds on ALD have not been fully illustrated.
Network pharmacology is a cost-friendly and highly efficient method for conducting a comprehensive analysis based on the widely existing databases of bioinformatics, systems biology, genetic informatics, and systems pharmacology, and it could reveal the major bioactive ingredients of drugs, uncover the interactions between targets (proteins and/or genes) important to drugs and disease, and unveil possible disease mechanisms. Thus, network pharmacology could provide a theoretical foundation to help generate defined directions for further research in new drug development[12,13].
In this study, the antioxidant activities of H. dulcis seeds was determined, and its total phenol content (TPC), total flavonoid content (TFC), and polysaccharide content (PSC) were also assessed. Moreover, the effects of H. dulcis seeds on ALD were evaluated in mice, and network pharmacology was used to identify the main bioactive ingredients, the core targets, and possible mechanisms. The results of this study provide valid evidence for further application of the seeds of edible medicinal plant H. dulcis as nutraceuticals and pharmaceuticals to manage ALD.
Ethanol, acetic acid, and sodium chloride were obtained from Damao Chemical Reagent Factory (Tianjin, China). Polyformaldehyde was purchased from Wuhan Good Biotechnology Co., Ltd. (Wuhan, China). 2,4,6-Tripyridyl-S-triazine (TPTZ), Folin and Ciocalteu’s phenol, Trolox, and 2,20-azino-bis (3-ethylbenothiazoline-6-sulphonic acid) diammonium salt (ABTS) were obtained from Sigma-Aldrich (Saint Louis, MO, United States). All chemicals were of analytical grade, and the water used in this study was double distilled water. Detection kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China), including triglyceride (TG), catalase (CAT), superoxide dismutase (SOD), reduced glutathione (GSH), malondialdehyde (MDA), and total protein assay kits.
H. dulcis seeds were purchased from Beijing Tong Ren Tang Chinese Medicine Co., Ltd. (Guangzhou, China). The H. dulcis seed water extract (HWE) was prepared according to the literature with minor modifications[14]. Briefly, 10.00 g dried weight (DW) of the finely ground sample was soaked in 100 mL of water for 30 min at room temperature. Then, the decoction of mixture was placed in a water bath (98°C, 30 min). After being cooled down, the decoction was centrifuged (4200 × g, 10 min), and the supernatant was obtained. This extraction process was conducted twice, and then the supernatants were combined. Freeze drying process of water extract was conducted with a FreeZone Freeze Dryer (Labconco FreeZone®, Kansas City, MO, United Sates). The dried crude extract was dissolved in water at a concentration of 15 g/100 mL. Thus, when gavaged with 10 mL/kg HWE, each animal received a dose of 1.5 g DW/kg, equivalent to the desired human dose recommended by the National Administration of Traditional Chinese Medicine[15-17].
The ferric-reducing antioxidant power (FRAP) assay is a common method to determine the antioxidant activities of plants and was used in this study with slight modification[18]. The FRAP reagent was freshly prepared based on the volume ratio of 10:1:1 with the three solutions: (1) 300 mmol/L sodium acetate buffer (pH 3.6); (2) 10 mmol/L TPTZ solution (solvent: 40 mmol/L HCl); and (3) 20 mmol/L ferric chloride solution, which was kept in a water bath at 37 °C for further use. The 100 μL of HWE was mixed with 3 mL of FRAP reagent and incubated for 4 min at room temperature. Then, the absorbance of the mixture was taken at 593 nm with ferrous sulfate as a standard, and the results are stated as μmol Fe2+/g DW.
The trolox equivalent antioxidant capacity (TEAC) assay was used to test the free radical-scavenging activity of HWE based on a previously published approach that was slightly modified[19]. ABTS free radical (ABTS+) solution was made by mixing 7 mmol/L ABTS stock solution and 2.45 mmol/L potassium persulfate (1:1, v/v). Afterwards, it was kept in the dark for incubation at room temperature for over 16 h with a 2-d validity. The ABTS+ solution was diluted until its absorbance reached 0.710 ± 0.050 at 734 nm. Then, 3.8 mL of diluted solution and 100 μL of sample were mixed and allowed to react for 6 min. Thereafter, the absorbance was recorded, and the results are presented in terms of μmol trolox equivalent per g (μmol TE/g) DW.
The TPC was determined by the Folin–Ciocalteu method[20]. Specifically, 0.5 mL of properly diluted sample and 2.5 mL of the Folin–Ciocalteu reagent (0.2 mol/L) were mixed. After 4 min, 2 mL of saturated sodium carbonate solution (75 g/L) was added into the mixture, followed by a 2 h incubation at room temperature. Then, the absorbance of the mixture was taken at 760 nm. The results are expressed as mg gallic acid equivalent per g (mg GAE/g) DW.
The TFC was determined by aluminum chloride colorimetry with slight modifications[21]. Briefly, 0.5 mL of diluted sample was mixed with 1.5 mL of ethanol (95%, v/v), 0.1 mL of aluminum chloride (10%, w/v), 0.1 mL of potassium acetate (1 M), and 2.8 mL of water. The mixture was incubated for 30 min at room temperature, and the absorbance was recorded at 415 nm. The results were stated in the pattern of mg quercetin equivalent per g (mg QE/g) DW.
The PSC was determined by the phenol-sulfuric acid method[22]. Specifically, 2 mL of properly diluted sample and 8 mL of absolute ethanol were mixed together and then centrifuged (4200 × g) for 10 min. The precipitate was collected and washed with 10 mL of ethanol (80%, v/v) prior to vortexing and centrifuging (4200 × g) again for 5 min. The supernatant was removed, and the described washing process was performed twice. The final precipitate was dissolved in 4 mL of water, and 1 mL of the resulting solution was mixed with 1 mL of phenol (6%, m/m) and then 5 mL of sulfuric acid (98%); the reaction was allowed to proceed for 10 min. The absorbance was then measured at 490 nm, and the results are expressed as mg glucose equivalent per g (mg GE/g) DW.
Male Kunming mice weighing 18-22 g were obtained from the Laboratory Animal Center, Sun Yat-Sen University. The animals were housed with 40%-60% relative humidity at 22 ± 1°C; they were provided a 12/12 h light-dark cycle and free access to water and rodent chow access to food and water for 2 wk prior to experimentation. The animal protocol was designed to minimize pain or discomfort to the animals. All experimental procedures were performed based on the approval of Animal Ethics Committee of School of Public Health, Sun Yat-Sen University (No. 2017-011).
Mice were randomly divided into a control group, a model group, and a treatment group, with each group consisting of eight mice. Each morning on days 1-7, HWE (10 mL/kg) was administered orally to the mice in the treatment group, while mice in the other two groups were gavaged with an equivalent amount of water. On day 7, 1 h after the regular administration, alcohol (10 mL/kg, 52%, v/v) was given intragastrically to the model group and the treatment group, and equivalent amount of water was given to the control group. After 9 h, all of the mice were euthanized by barbiturate overdose (intravenous injection, 150 mg/kg pentobarbital sodium), and their blood samples were obtained. Finally, liver samples were weighed, and two portions were collected for biomarker measurements and histopathological examination, respectively.
Serum samples were obtained by centrifuging the blood samples at 800 × g for 15 min and then analyzed by a Beckman Coulter Chemistry Analyzer (AU5821, Tokyo, Japan). The tested biomarkers in the serum included aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin (TBIL), and TG.
The liver weight was measured to calculate the liver coefficient, i.e., liver weight/body weight in terms of percentage. One slice of liver sampled from the middle of the left lobe was fixed and then stained by hematoxylin and eosin for histopathologic observation using a NIKON Biological Microscope Eclipse Ci-S (Tokyo, Japan). A second slice from the same lobe was applied for the hepatic biomarker tests. Liver homogenate was made by mixing liver tissue (0.2 g) and ice-cold physiological saline (1.8 mL), which was centrifuged twice at 3700 × g for 10 min each time. The supernatant was collected to assess the hepatic biomarkers, including GSH, SOD, CAT, MDA, and TG, with the corresponding commercial detection kits.
Selecting potential bioactive components of H. dulcis seeds and their related putative targets: H. dulcis seeds as a folk medicine has a long history in Asian countries; therefore, its bioactive components were searched in the pharmacological databases of traditional Chinese Medicine Systems Pharmacology (TCMSP) database and the TCM Database@Taiwan; PubChem was also used to verify the compounds and remove the duplicates with synonyms[23,24]. The potential bioactive compounds were selected based on their pharmacokinetic characteristics, i.e., absorption, distribution, metabolism, and excretion (ADME). The ingredients obtained from TCMSP were screened by oral bioavailability ≥ 30% and drug-likeness ≥ 0.18, while for those from TCM Database@Taiwan, the eligible ingredients were selected by Lipinski's rule: (1) No more than five hydrogen bond donors; (2) No more than 10 hydrogen bond acceptors; (3) A molecular mass less than 500 Daltons; and (4) An octanol-water partition coefficient log P not greater than 5[25,26].
Two bioinformatics and cheminformatics platforms, the TCMSP database and the DrugBank database, were consulted for comprehensive and detailed target information regarding H. dulcis seeds[24,27]. Additionally, gene symbol identification and annotation of the target proteins were achieved by UniProt Knowledgebase[28].
Predicting known therapeutic targets of ALD: Following the ALD definition by the medical subject headings, alcoholic liver disease refers to liver disease in association with alcoholism and commonly at least two subentities (alcoholic fatty liver, alcoholic hepatitis, and alcoholic cirrhosis) coexist[29]. Two databases, the Online Mendelian Inheritance in Man (OMIM) databases and GeneCards, were used to identify the known therapeutic targets of ALD[30,31]. The OMIM, which is authored and edited at Johns Hopkins University School of Medicine, provides information on more than 15000 genes and all known Mendelian disorders[30]. Additionally, based on 168 integrated databases, GeneCards provides comprehensive and detailed information regarding all human genes that have been annotated and predicted[31]. The available data from these two databases were combined after removing the duplicates.
The 173 common targets, which were associated both with eligible ingredients of H. dulcis seeds and ALD, were obtained by overlapping the two abovementioned groups (Supplementary Figure 1).
Protein–protein interaction construction: A functional protein–protein interaction (PPI) network was constructed with the common target proteins by STRING (medium confidence, 0.40; species, Mus musculus)[29,31,32] .
H. dulcis seed-ingredient-target interaction network construction: Cytoscape software (https://cytoscape.org/, Version 3.7.1) was used to construct and visualize a network of H. dulcis seed-ingredient-target interaction with the obtained common targets[33].
Enrichment analysis: The possible mechanisms of H. dulcis seeds on ALD were explored by gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses[34,35]. A bioconductor was used to provide effective analysis of genomic data, and ClusterProfiler as well as Pathview were applied to statistically analyze, integrate, and visualize gene functional profiles[36]. A false discovery rate (FDR) < 0.05 was considered as statistically significant for the GO terms and KEGG pathways[37].
The results were reported in terms of means ± standard deviation. Statistical analysis was performed with IBM SPSS Statistics 20.0 (SPSS Inc., Armonk, NY, United States). One-way analysis of variance and the least significant difference test were applied for the evaluation of the differences between groups. P < 0.05 and P < 0.01 were regarded as two statistically significant levels, respectively. The statistical methods of this study were reviewed by Chan-Juan Zhao from the Department of Bio-statistics, School of Public Health, Hainan Medical University, Hainan Province, China.
The antioxidant activity of H. dulcis seeds was assessed with two assays in this study, and the results showed an antioxidant capacity of 245.11 ± 10.17 μmol Fe2+/g by the FRAP assay and 284.35 ± 23.57 μmol TE/g by the TEAC assay (Table 1). The following values were also determined in this study: The TPC values were 6.29 ± 0.56 mg GAE/g DW, TFC, 2.25 ± 0.17 mg QE/g DW, and PSC, 0.03 ± 0.00 mg GE/g DW.
| FRAP value, μmol Fe2+/g DW | TEAC value, μmol TE/g DW | TPC value, mg GAE/g DW | TFC value, mg QE/g DW | PSC value, mg GE/g DW |
| 245.11 ± 10.17 | 284.35 ± 23.57 | 6.29 ± 0.56 | 2.25 ± 0.17 | 0.03 ± 0.00 |
Significant increases of serum biomarkers, including ALT, AST, TBIL, and TG, were observed in the model group after alcohol administration, indicating the occurrence of alcoholic liver injury (Table 2). In this study, significant reductions in ALT and AST were observed in the treatment group (P < 0.01). Moreover, TBIL and TG were also significantly reduced in the group treated with HWE compared with the model group. The results provided evidence that H. dulcis seeds could attenuate ALD.
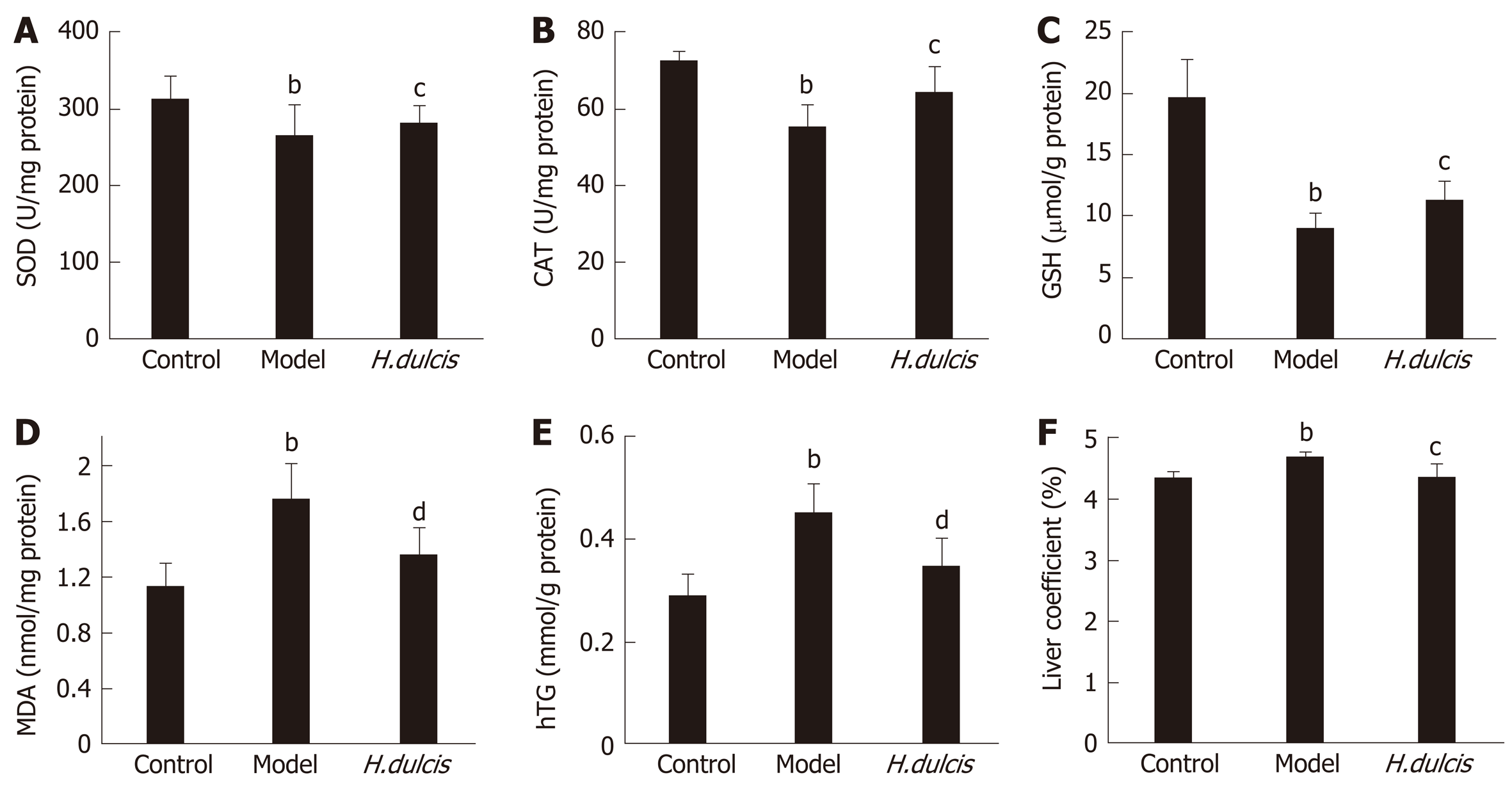
In addition to the serum biomarkers, hepatic biomarkers were also altered by alcohol intake, which induced significant decreases of SOD and CAT activities as well as GSH levels, together with increases in MDA, TG, and the liver coefficient (Figure 1). All these alterations were largely reversed in the group with HWE treatments, i.e. SOD, CAT, and GSH were increased (P < 0.05), and on the other hand, MDA and hepatic TG was reduced (P < 0.01), together with decreased liver coefficient (P < 0.05). Such results indicated that H. dulcis seeds improved the in vivo antioxidant activity.
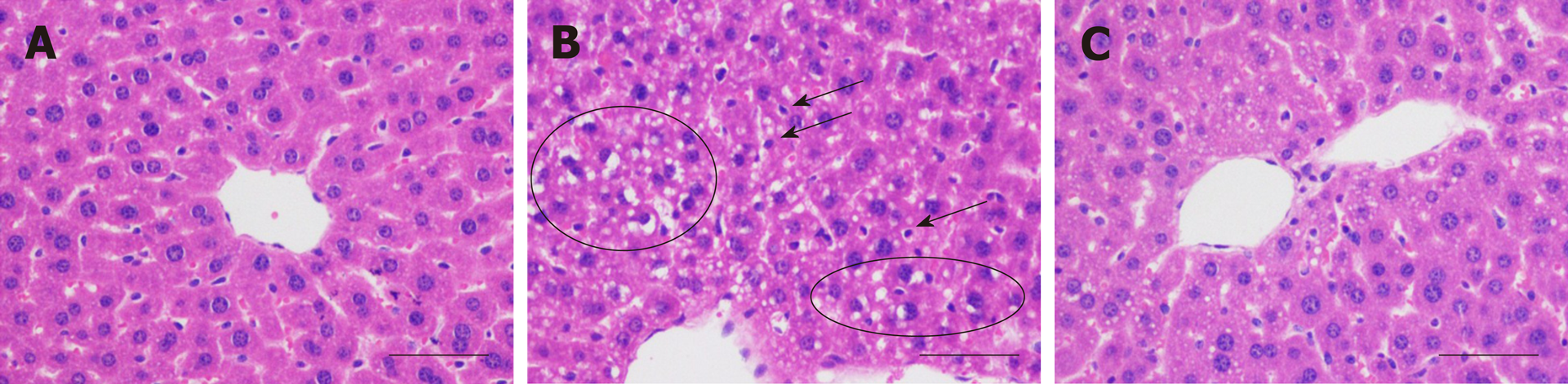
The histopathological examinations indicated that in the model group, the involved hepatocytes were swollen and in disarray, with uneven-sized lipid droplets having accumulated (Figure 2B), and inflammatory cell infiltration was also found. These observations showed that alcoholic hepatitis and steatosis coexisted. On the other hand, the histopathological changes in the treatment group were noticeably alleviated by H. dulcis seeds (Figure 2C).
Identification of targets related to both H. dulcis seeds and ALD: A total of 44 bioactive components of H. dulcis seeds were obtained, with 39 from TCMSP and five from TCM Database@Taiwan. According to the criteria regarding ADME, eight bioactive components remained as the eligible ingredients without duplicates, i.e. perlolyrine, β-sitosterol, kaempferol, naringenin, stigmasterol, ceanothic acid, quercetin and (2R,3R)-2-(3,5-dihydroxyphenyl)-3,5,7-trihydroxychroman-4-one (Supplementary Table 1)[25,26]. A total of 582 putative targets were found based on all ingredients of H. dulcis seeds from TCMSP and DrugBank, from which 182 were related to the eight eligible ingredients. Meanwhile, a total of 6087 known therapeutic targets regarding ALD were obtained from two sources, GeneCards and OMIM. Consequently, 173 common targets in association with both the eligible ingredients of H. dulcis seeds and ALD were obtained by overlapping the two groups (182 and 6087 targets, respectively) (Supplementary Figure 1).
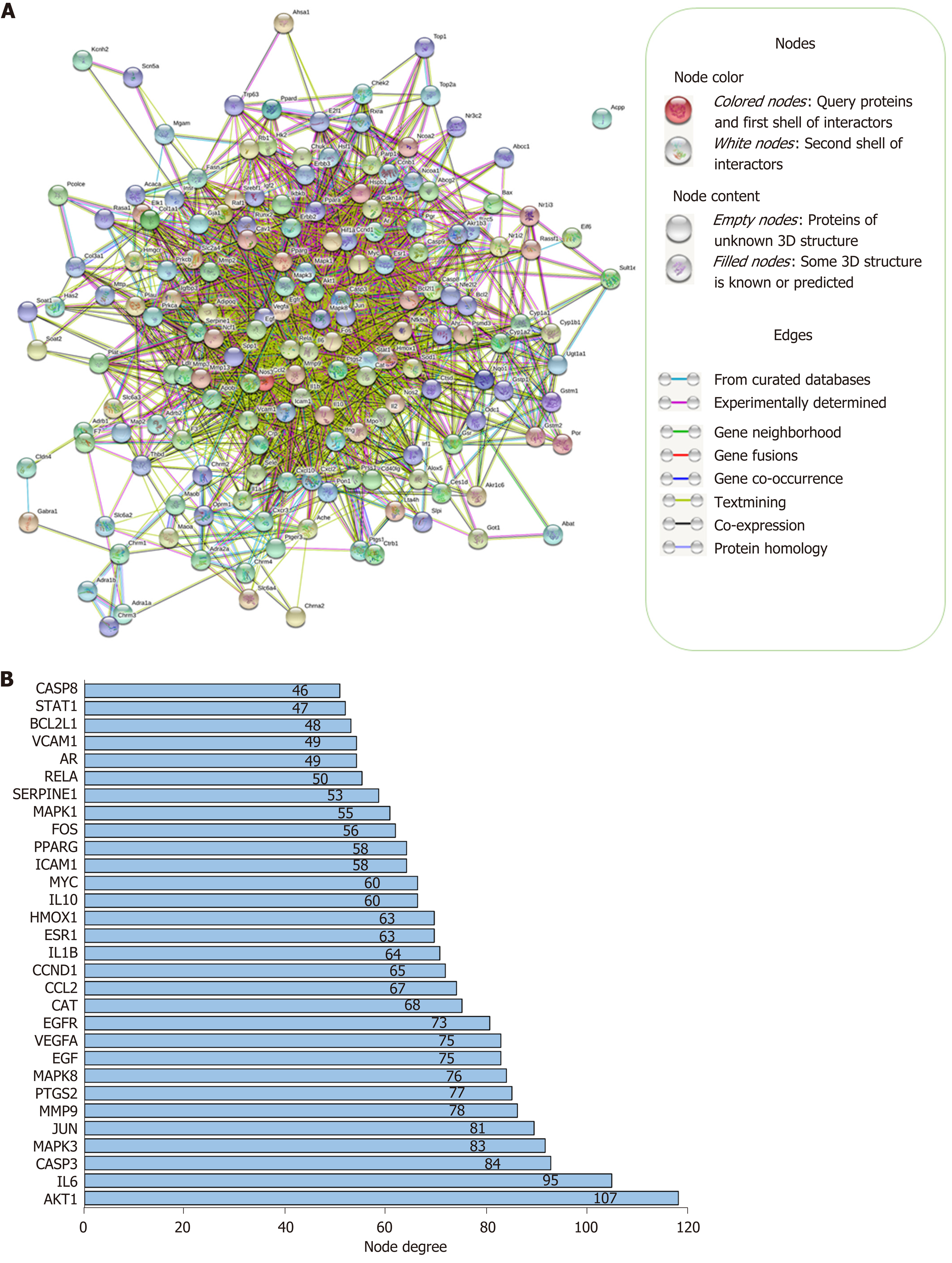
Network construction: A PPI network of 173 targets was constructed and visualized by STRING, as shown in Figure 3A. There were 169 target proteins (nodes) and 2249 interactions (edges) in the PPI network, with average node degree of 26.6 and 610 expected edges. The PPI enrichment P value was less than 1.0e-16. The 30 targets with the highest node degrees are shown in Figure 3B (node degrees of all targets were listed in Supplementary Table 2).
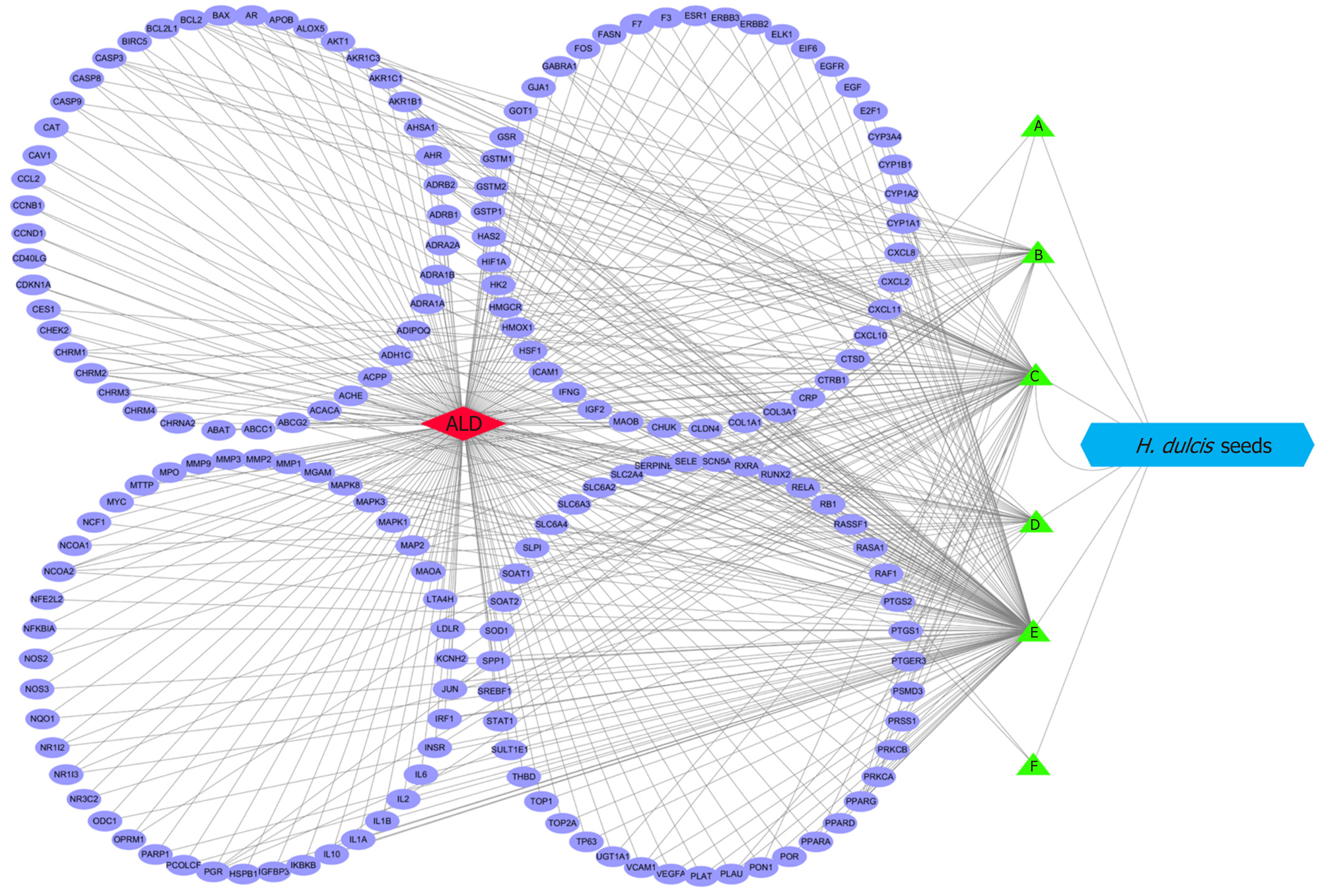
The interaction network was constructed by Cytoscape to represent the relationships between the eligible ingredients of H. dulcis seeds, the targets, and ALD (Figure 4). The network consisted of one node for ALD (red diamond in Figure 4), one node for H. dulcis seeds (blue hexogen), six nodes for ingredients (green triangles), 173 nodes for the targets (purple ellipses), and 443 edges for the interactions. The two ingredients with the highest node degrees were stigmasterol and kaempferol (degree = 130 and 80, respectively), which could be regarded as the key bioactive ingredients, while the others’ degrees were below 30. The targets with top three degrees were prostaglandin-endoperoxide synthase (PTGS) 2, PTGS1, and peroxisome proliferator-activated receptor γ, with degrees of 8, 7, and 6, respectively, and the degrees of the other targets were 5 or less.
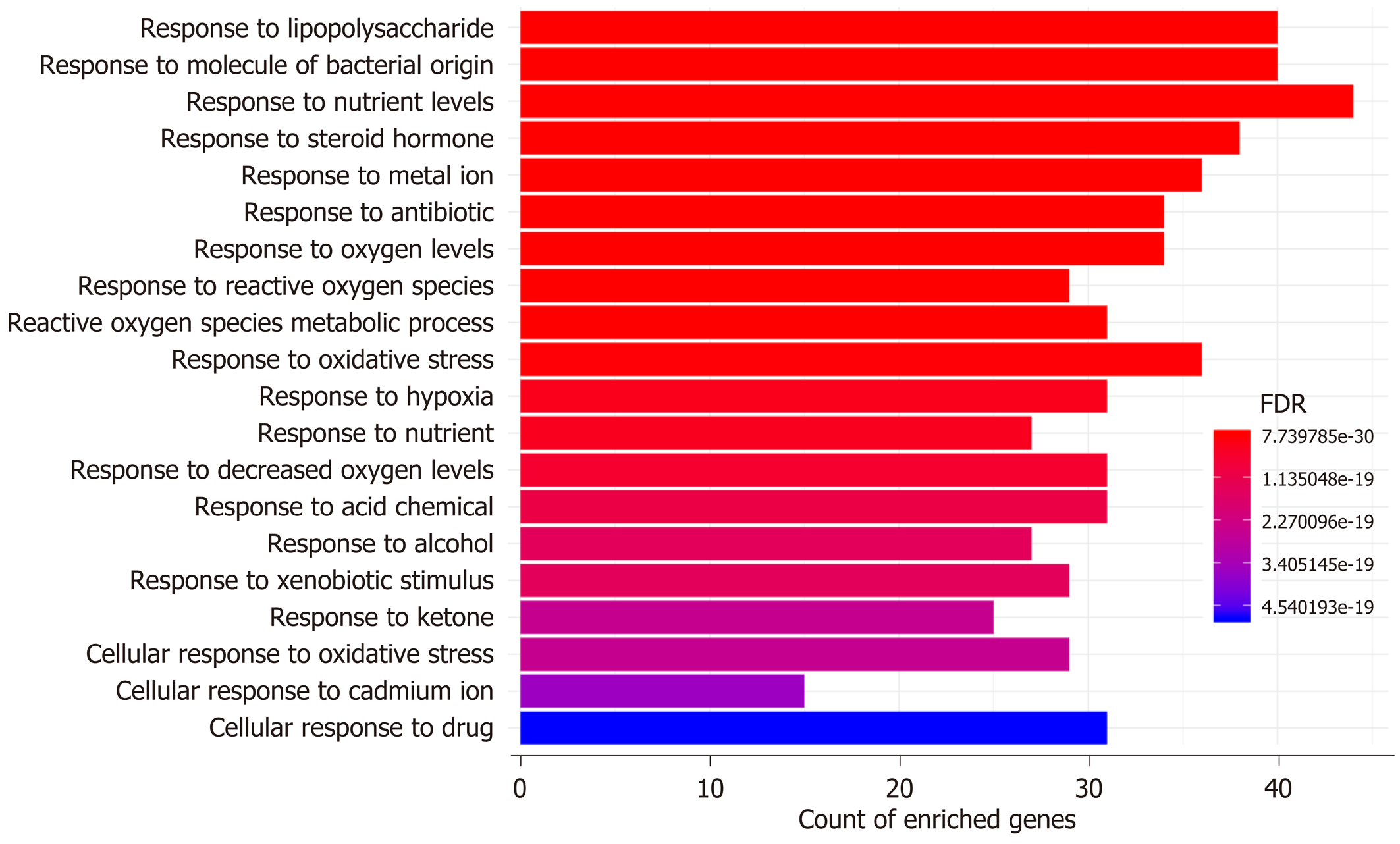
GO and KEGG enrichment analyses: The possible mechanisms of action involved in the prevention of H. dulcis seeds against ALD were explored by means of GO and KEGG enrichment analyses. GO enrichment analysis was performed from three ontologies, i.e. biological process (BP), molecular function (MF), and cellular component (CC). In a total of 4956 GO terms, 2369 were of statistical significance (FDR < 0.05), including 2101 BP, 180 MF, and 88 CC (Supplementary Table 3). The 20 most enriched BPs are illustrated in Figure 5, mainly including four biological processes that were directly associated with reactive oxygen species (ROS)/oxidative stress and hypoxia, two related to molecule of bacterial origin/lipopolysaccharide, two associated with nutrient levels, and one directly linked to alcohol. In addition, major enriched MF also covered those in association with oxidoreductase activity and antioxidant activity, cytokine activity, transcription factor activity, and nuclear receptor activity, indicating that the regulation might occur at transcriptional levels (Supplementary Table 3).
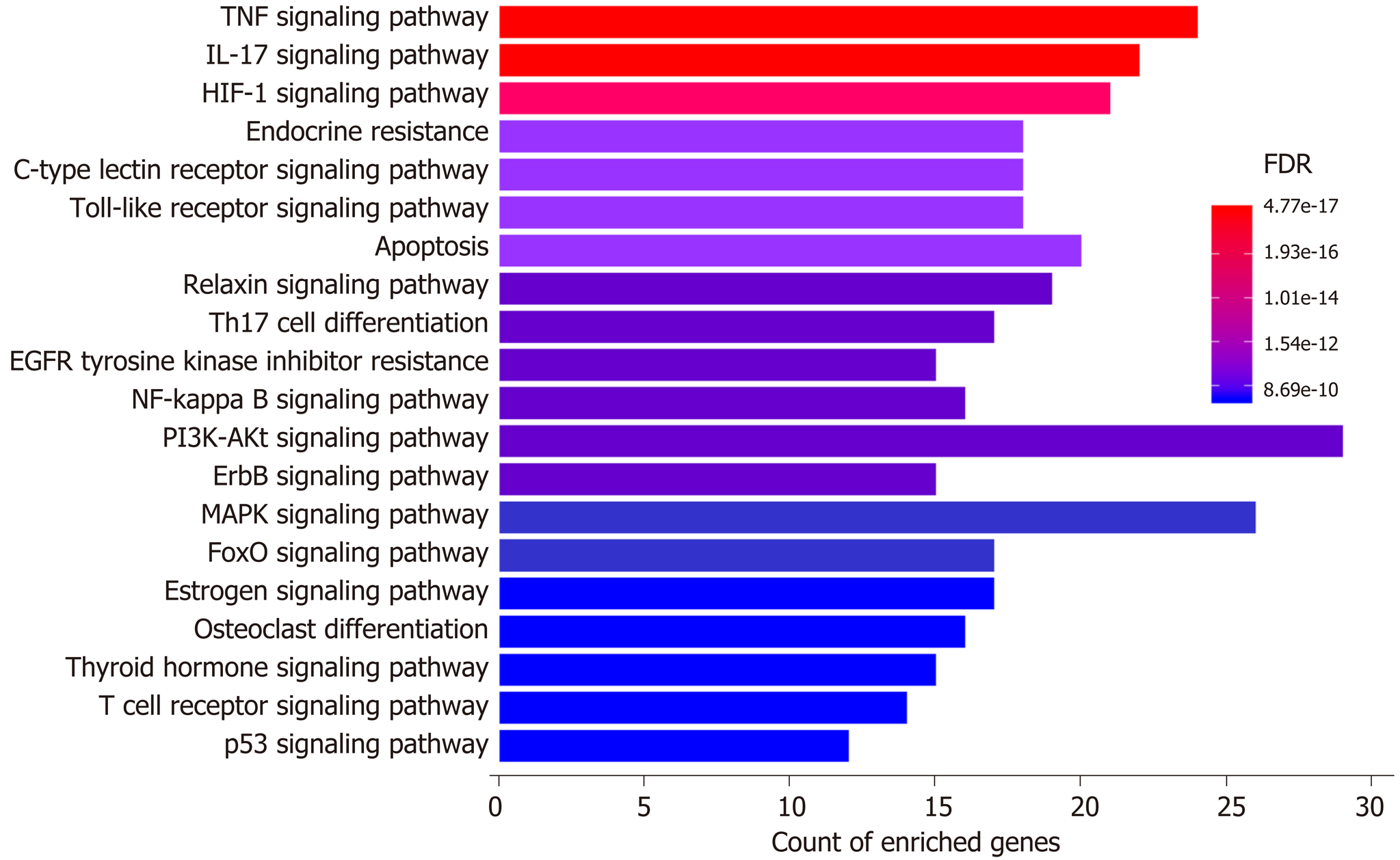
Moreover, a total of 253 pathways were available from KEGG enrichment analysis; 158 of these pathways were statistically significant (FDR < 0.05), and 98 were not related to other diseases (Supplementary Table 4). The top 10 most enriched nondisease associated pathways included “Tumor necrosis factor (TNF) signaling pathway” (24 gene enriched), “Interleukin 17 (IL-17) signaling pathway” (22 gene enriched), “Hypoxia-inducible factor 1 (HIF-1) signaling pathway” (21 gene enriched), “Endocrine resistance” (18 gene enriched), “Toll-like receptor (TLR) signaling pathway” (18 gene enriched), “C-type lectin receptor (CLR) signaling pathway” (18 gene enriched), “Apoptosis” (20 gene enriched), “Relaxin signaling pathway” (19 gene enriched), “Th17 cell differentiation” (17 gene enriched), and “Epidermal growth factor receptor tyrosine kinase inhibitor resistance” (15 gene enriched). The results from GO and KEGG enrichment analyses suggested that oxidative stress, inflammation, hypoxia, bacteria/bacterial product translocation, and apoptosis were all involved in the mechanisms underlying the protective effects of H. dulcis seeds on ALD.
Increasing evidence has demonstrated that natural products provide various health benefits, and antioxidant activity is one of the major contributors[5,6]. In this study, the antioxidant activity of H. dulcis seeds was determined by the FRAP assay, depending on the capacity of the sample to reduce [Fe (TPTZ)2]3+ into [Fe (TPTZ)2]2+[18,38]. Additionally, due to the complex composition and multifunctional features of most natural antioxidants that may influence antioxidant activity, using more than one method would provide a more comprehensive and reliable antioxidant activity evaluation[39]. Therefore, the TEAC assay was also applied in this study, which is a common method to determine the activity of antioxidant scavenging ABTS+[19,38]. The results from these two assays revealed that H. dulcis seeds exhibited much higher antioxidant activities than those of many fruits (e.g., kiwi fruit and blueberries), vegetables (e.g., broccoli and garlic), herbs (e.g., Pueraria lohata root, Taraxacum mongolicum, and seeds of Perilla frutescens and Ginkgo biloba), which are commonly considered to provide health benefits due to their strong antioxidant properties[14,40,41]. In addition, polyphenols, flavonoids, and polysaccharides have been reported as major contributors to the antioxidant activity of natural products[41,42]. In this study, H. dulcis seeds were found to contain much higher TPC than various fruits (e.g., cherries and blueberries), certain edible macrofungi (e.g., Agaricus bisporus, Auricularia auricular and Hericium erinaceus), and many medicinal plants, including Angelica biserrata, Millettia dielsiana Harms, Pinellia ternata, and Pueraria lohata root[14,40,41]. In addition, the TFC of H. dulcis seeds was higher than that of edible beans, such as mung bean, and even higher than that of Strobilanthes crispus leaves, a famous folk medicine in Malaysia[43,44]. Overall, H. dulcis seeds showed strong antioxidant activity and contained relatively high total phenolic content, considerable flavonoids, and a few polysaccharides, indicating that it could be a good source of natural antioxidants.
Alcohol consumption can lead to elevated transaminases, ALT and AST, the two common indicators of liver damage[45]. In this study, H. dulcis seeds showed hepatoprotective effects against alcohol by significantly decreasing these two transaminases (Table 2). In addition, liver dysfunction often results in bile and lipid mis-metabolism, and serum TBIL and TG may increase as a result[46]. Significantly reduced levels of TBIL and TG were found in the treatment group compared with the model group, thus showing that treatments of H. dulcis seeds could effectively protect liver function.
Alcohol is mainly metabolized in the liver, where it is oxidized into acetaldehyde and then into acetate; this biotransformation process can cause oxidative stress and lipid peroxidation[1]. In this study, alcohol administration induced depletion of GSH, one of the endogenous antioxidants, and decreased the activities of SOD and CAT, the antioxidant enzymes in the liver, all of which play a crucial role in scavenging ROS and reducing oxidative stress[47-49]. The reversal of these alterations was observed in mice treated with HWE (Figure 1). Moreover, hepatic MDA and TG were also markedly lower in the treatment group than those in the model group, indicating lipid peroxidation and dysmetabolism were less serious with the intervention of H. dulcis seeds. In addition, the liver coefficient was significantly decreased in the treatment group compared to the model group, indicating lower severity of liver damage due to the HWE.
To identify the major bioactive ingredients and obtain a comprehensive investigation of the mechanisms of H. dulcis seeds on ALD, network pharmacology was employed in this study as a highly efficient and cost-friendly tool[12,13]. Eight out of 44 bioactive compounds were screened due to eligibility of their pharmacokinetic features, and 173 corresponding known therapeutic targets, which were also associated with ALD, were selected (Supplementary Figure 1). The STRING-constructed PPI network of these common targets consisted of 169 nodes and 2249 edges (Figure 3A). As shown in Figure 3B, the targets with the top three node degrees were protein kinase B (AKT1), IL-6, and caspase-3, indicating they might be the pivotal modulatory targets contributing to the interactions between H. dulcis seeds and ALD. In addition, given the 610 expected edges and an enrichment P value < 1.0e-16, it could be indicated that the network had significantly more interactions than expected, showing the complex interactions between the targets.
The relations between the eligible ingredients of H. dulcis seeds, the targets, and ALD are represented by the Cytoscape-constructed interaction network (Figure 4). As shown, the ingredients with the top two node degrees (degree = 130 and 80, respectively) were stigmasterol and kaempferol, which could be considered as the key bioactive ingredients. Further, PTGS2, PTGS1, and peroxisome proliferator-activated receptor γ, the targets with the top three degrees (degree = 8, 7 and 6, respectively), could be regarded as the core targets in the interactions of H. dulcis seeds and ALD. Such predictions could be backed up by some previous studies[50-52]. For instance, PTGS, also known as cyclooxygenase (COX), plays a key role in prostaglandin biosynthesis. Alcohol may cause downregulation of COX-1 in Kupffer cells and induce COX-2 expression via pro-inflammatory factors, such as cytokines and endotoxin, consequently leading to advanced ALD[50,51]. Moreover, the PPAR-γ signaling pathway is deeply involved in fatty acid storage and glucose metabolism, and the activation of this pathway could not only reduce alcohol intake and preference but also attenuate inflammation by inhibiting activity of the nuclear factor-κB[52]. In addition, there were 443 edges in the network, presenting the mutually regulatory interactions between the targets and the ingredients of H. dulcis seeds.
GO enrichment analysis indicated that the top two enriched biological processes were “response to lipopolysaccharide” and “response to molecule of bacterial origin” (Figure 5), which not only demonstrated that the translocated bacterial products were involved in the actions of H. dulcis seeds on ALD but also verified the antibacterial impacts of H. dulcis seeds, as reported in the literature[53]. There were four biological processes that were directly associated with ROS/oxidative stress (“response to reactive oxygen species”, “reactive oxygen species metabolic process”, “response to oxidative stress”, and “cellular response to oxidative stress”), and three processes were related to hypoxia (“response to oxygen levels”, “response to hypoxia”, and “response to decreased oxygen levels”) and were also associated with oxidative stress in ALD[54]. Therefore, reducing oxidative stress might be one of the main mechanisms in the protective effects of H. dulcis seeds against ALD. Since malnutrition and ALD were the mutual causalities and given the two nutrient-linked biological processes (“response to nutrient levels” and “response to nutrient”), it could be speculated that H. dulcis seeds might improve ALD by modulating nutrient metabolism[55]. Notably, the enriched biological process of “response to alcohol” was also found, showing that H. dulcis seeds could directly react with alcohol, which might be attributable to its capability of speeding up alcohol degradation and consequently shortening the action time of alcohol in the liver[11].
KEGG enrichment analysis illustrated the enriched pathways involved in the hepatoprotective effects of H. dulcis seeds against alcohol (Figure 6). These pathways have been shown to interweave with each other, and such an interplay could influence the pathogenesis of ALD[56-59]. It has been reported that HIF-1α could be a major and complex factor in ALD[56]. On the one hand, alcohol consumption could cause oxidative stress and hypoxia, resulting in HIF-1α upregulation; this process was adaptive and protective, which led to the inhibition of excessive lipid accumulation[60]. On the other hand, HIF-1α could be a determinant in the development of steatosis and hypertriglyceridemia as well as a mediator involved in pro-inflammation and upregulation of vascular endothelial growth factor[56,61]. In short, as a transcription regulator, changing HIF-1α activity might lead to various and complex consequences that could be influenced by other concurrent signals received by the hepatocytes[56]. Additionally, alcohol could increase the Th17 cell population and result in increased IL-17 secretion, which could promote the production of other cytokines, such as TNF-α and IL-6, and promote liver inflammation[57,58]. Moreover, increased permeability of the gut barrier caused by alcohol allows more bacteria/bacterial products to translocate into the liver and bind to the TLRs in hepatic stellate cells and Kupffer cells, thus inducing nuclear factor-κB activation, cytokine production, and immune activation and promoting the development of advanced ALD[62]. Furthermore, the activation of CLR, such as macrophage-inducible C-type lectin produced by Kupffer cells, could exacerbate ALD by increasing IL-1β production, thus leading to the increased infiltration of inflammatory immune cells[59]. In addition, apoptosis was also involved in the top 10 enriched nondisease related pathways. Taken together, by integrating the results from PPI networks as well as GO and KEGG enrichment analyses, oxidative stress, inflammation, translocated bacteria and related products, nutrient levels, and apoptosis could be involved in the mechanisms of H. dulcis seeds against ALD, and it could be predicted that this effect occurs most likely through TNF/IL-17/HIF-1/TLR/CLR/apoptosis signaling pathways.
In conclusion, our results showed that H. dulcis seeds possessed strong antioxidant activity and contained remarkable levels of polyphenols and flavonoids as well as a few polysaccharides. Moreover, H. dulcis seeds were demonstrated to protect against ALD by reducing oxidative stress and preventing lipid dysmetabolism in vivo. Network pharmacology analysis indicated that kaempferol, stigmasterol, and naringenin were the main bioactive compounds in H. dulcis seeds and that modulating oxidative stress, inflammation, gut-derived products, nutrient levels, and apoptosis were involved in the ALD-preventing mechanisms of H. dulcis seeds. The results of this study demonstrated that H. dulcis seeds could be a good natural antioxidant source with protective effects on oxidative diseases such as ALD.
Alcoholic liver disease (ALD) is a worldwide health problem with poor prognosis and limited efficacy of current treatments, resulting in major health and economic burdens on both individuals and the society. Many natural products have been shown to improve ALD due to their antioxidant activities.
Some parts of Hovenia dulcis (H. dulcis) provide health benefits. Nevertheless, the effects and mechanisms of H. dulcis seeds on ALD have not yet been fully elucidated.
The present study aimed to determine H. dulcis antioxidant activity, evaluate its effects against ALD, and investigate the related mechanisms via network pharmacology.
The antioxidant activity of H. dulcis seed was determined by both ferric-reducing antioxidant power (FRAP) and trolox equivalent antioxidant capacity (TEAC) assays. The total phenolic and flavonoid contents were determined by Folin–Ciocalteu method and aluminum chloride colorimetry, respectively, as well as polysaccharide by phenol-sulfuric acid method. The effects of H. dulcis seeds against alcoholic liver injury were investigated in mice with water extract pretreatment for 7 d followed by alcohol administration. Moreover, the mechanisms of action were explored with network pharmacology.
The results showed that H. dulcis seeds possessed strong antioxidant activity (245.11 ± 10.17 μmol Fe2+/g by FRAP, and 284.35 ± 23.57 μmol TE/g by TEAC, respectively), and contained remarkable phenols and flavonoids, as well as a few polysaccharides. H. dulcis seeds attenuated alcohol-induced oxidative liver injury, showing reduced serum alanine and aspartate aminotransferases, alkaline phosphatase and triglyceride, elevated hepatic glutathione, increased activities of superoxide dismutase and catalase, and reduced malondialdehyde as well as hepatic triglyceride. The results of network pharmacology analysis indicated that kaempferol, stigmasterol, and naringenin were the main bioactive compounds in H. dulcis seeds, and that modulating oxidative stress, inflammation, gut-derived products, and apoptosis were involved in the mechanisms of H. dulcis seeds’ protective effects on ALD.
The results of this study demonstrated that H. dulcis seeds could be a good natural antioxidant source with protective effects on oxidative diseases such as ALD.
The results of this study provide valid evidence for further application of the seeds of edible medicinal plant H. dulcis as nutraceuticals and pharmaceuticals to manage ALD.
The authors would like to acknowledge Sha Li (Hong Kong, China, PhD) for skillful technical assistance.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Alberts SR, Lee MK, Vollmers HP S-Editor: Wang JL L-Editor: Filipodia E-Editor: Zhang YL
| 1. | Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1244] [Cited by in RCA: 1496] [Article Influence: 106.9] [Reference Citation Analysis (0)] |
| 2. | World Health Organization. Global status report on alcohol and health 2018. Available from: https://apps.who.int/iris/bitstream/handle/10665/274603/9789241565639-eng.pdf. |
| 3. | Louvet A, Mathurin P. Alcoholic liver disease: mechanisms of injury and targeted treatment. Nat Rev Gastroenterol Hepatol. 2015;12:231-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 646] [Article Influence: 64.6] [Reference Citation Analysis (0)] |
| 4. | Beier JI, McClain CJ. Mechanisms and cell signaling in alcoholic liver disease. Biol Chem. 2010;391:1249-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 5. | Xu XY, Meng X, Li S, Gan RY, Li Y, Li HB. Bioactivity, Health Benefits, and Related Molecular Mechanisms of Curcumin: Current Progress, Challenges, and Perspectives. Nutrients. 2018;10:1553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 209] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 6. | Meng X, Li Y, Li S, Zhou Y, Gan RY, Xu DP, Li HB. Dietary Sources and Bioactivities of Melatonin. Nutrients. 2017;9:367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 206] [Cited by in RCA: 216] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 7. | Maruthur NM, Tseng E, Hutfless S, Wilson LM, Suarez-Cuervo C, Berger Z, Chu Y, Iyoha E, Segal JB, Bolen S. Diabetes Medications as Monotherapy or Metformin-Based Combination Therapy for Type 2 Diabetes: A Systematic Review and Meta-analysis. Ann Intern Med. 2016;164:740-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 544] [Cited by in RCA: 468] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 8. | Kim HL, Sim JE, Choi HM, Choi IY, Jeong MY, Park J, Jung Y, Youn DH, Cho JH, Kim JH, Hwang MW, Jin JS, Hong SH, Cho HW, Um JY. The AMPK pathway mediates an anti-adipogenic effect of fruits of Hovenia dulcis Thunb. Food Funct. 2014;5:2961-2968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Kang KB, Jun JB, Kim JW, Kim HW, Sung SH. Ceanothane- and lupane-type triterpene esters from the roots of Hovenia dulcis and their antiproliferative activity on HSC-T6 cells. Phytochemistry. 2017;142:60-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Wang M, Jiang C, Ma L, Zhang Z, Cao L, Liu J, Zeng X. Preparation, preliminary characterization and immunostimulatory activity of polysaccharide fractions from the peduncles of Hovenia dulcis. Food Chem. 2013;138:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 11. | Kim H, Kim YJ, Jeong HY, Kim JY, Choi EK, Chae SW, Kwon O. A standardized extract of the fruit of Hovenia dulcis alleviated alcohol-induced hangover in healthy subjects with heterozygous ALDH2: A randomized, controlled, crossover trial. J Ethnopharmacol. 2017;209:167-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 12. | Hopkins AL. Network pharmacology: the next paradigm in drug discovery. Nat Chem Biol. 2008;4:682-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2254] [Cited by in RCA: 2811] [Article Influence: 165.4] [Reference Citation Analysis (0)] |
| 13. | Calvert S, Tacutu R, Sharifi S, Teixeira R, Ghosh P, de Magalhães JP. A network pharmacology approach reveals new candidate caloric restriction mimetics in C. elegans. Aging Cell. 2016;15:256-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 14. | Li S, Li SK, Gan RY, Song FL, Kuang L, Li HB. Antioxidant capacities and total phenolic contents of infusions from 223 medicinal plants. Ind CroP Prod. 2013;51:289-298. [DOI] [Full Text] |
| 15. | U.S. Food and Drug Administration. Guidance for Industry: Estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers (2005). Available from: https://www.fda.gov/media/72309/download. |
| 16. | China National Administration of Traditional Chinese Medicine. Hovenia dulcis seeds (2015). Available from: https://baike.baidu.com/item/%E6%9E%B3%E6%A4%87%E5%AD%90/758262?fr=aladdin. |
| 17. | Walpole SC, Prieto-Merino D, Edwards P, Cleland J, Stevens G, Roberts I. The weight of nations: an estimation of adult human biomass. BMC Public Health. 2012;12:439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 441] [Cited by in RCA: 445] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 18. | Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of "antioxidant power": the FRAP assay. Anal Biochem. 1996;239:70-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12606] [Cited by in RCA: 12172] [Article Influence: 419.7] [Reference Citation Analysis (0)] |
| 19. | Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14802] [Cited by in RCA: 13630] [Article Influence: 524.2] [Reference Citation Analysis (0)] |
| 20. | Singleton VL, Orthofer R, Lamuela-Raventós RM, Packer L. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Packer L. In: Packer L, editor. Oxidants and Antioxidants Part A. San Diego: Elsevier Academic Press Inc., 1999: 152-178. |
| 21. | Gouveia S, Castilho PC. Antioxidant potential of Artemisia argentea L'Hér alcoholic extract and its relation with the phenolic composition. Food Res Int. 2011;44:1620-1631. [RCA] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 22. | Guo YJ, Deng GF, Xu XR, Wu S, Li S, Xia EQ, Li F, Chen F, Ling WH, Li HB. Antioxidant capacities, phenolic compounds and polysaccharide contents of 49 edible macro-fungi. Food Funct. 2012;3:1195-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 101] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 23. | Ru J, Li P, Wang J, Zhou W, Li B, Huang C, Li P, Guo Z, Tao W, Yang Y, Xu X, Li Y, Wang Y, Yang L. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminform. 2014;6:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1623] [Cited by in RCA: 3148] [Article Influence: 286.2] [Reference Citation Analysis (0)] |
| 24. | Chen CY. TCM Database@Taiwan: the world's largest traditional Chinese medicine database for drug screening in silico. PLoS One. 2011;6:e15939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 503] [Cited by in RCA: 572] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 25. | Xu X, Zhang W, Huang C, Li Y, Yu H, Wang Y, Duan J, Ling Y. A novel chemometric method for the prediction of human oral bioavailability. Int J Mol Sci. 2012;13:6964-6982. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 564] [Cited by in RCA: 622] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 26. | Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46:3-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7448] [Cited by in RCA: 9390] [Article Influence: 391.3] [Reference Citation Analysis (0)] |
| 27. | Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, Assempour N, Iynkkaran I, Liu Y, Maciejewski A, Gale N, Wilson A, Chin L, Cummings R, Le D, Pon A, Knox C, Wilson M. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2018;46:D1074-D1082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3266] [Cited by in RCA: 5291] [Article Influence: 881.8] [Reference Citation Analysis (0)] |
| 28. | UniProt Consortium. UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res. 2019;47:D506-D515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4450] [Cited by in RCA: 5250] [Article Influence: 1050.0] [Reference Citation Analysis (0)] |
| 29. | Medical Subject Headings. Liver Diseases, Alcoholic, 1979. Available from: https://www.ncbi.nlm.nih.gov/mesh/68008108. |
| 30. | Hamosh A, Scott AF, Amberger JS, Bocchini CA, McKusick VA. Online Mendelian Inheritance in Man (OMIM), a knowledgebase of human genes and genetic disorders. Nucleic Acids Res. 2005;33:D514-D517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1574] [Cited by in RCA: 1903] [Article Influence: 95.2] [Reference Citation Analysis (0)] |
| 31. | Stelzer G, Rosen N, Plaschkes I, Zimmerman S, Twik M, Fishilevich S, Stein TI, Nudel R, Lieder I, Mazor Y, Kaplan S, Dahary D, Warshawsky D, Guan-Golan Y, Kohn A, Rappaport N, Safran M, Lancet D. The GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. Curr Protoc Bioinformatics. 2016;54:1.30.1-1.30.33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1289] [Cited by in RCA: 2902] [Article Influence: 322.4] [Reference Citation Analysis (0)] |
| 32. | Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos A, Tsafou KP, Kuhn M, Bork P, Jensen LJ, von Mering C. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43:D447-D452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6477] [Cited by in RCA: 7628] [Article Influence: 693.5] [Reference Citation Analysis (0)] |
| 33. | Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498-2504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24663] [Cited by in RCA: 33595] [Article Influence: 1599.8] [Reference Citation Analysis (0)] |
| 34. | Carbon S, Ireland A, Mungall CJ, Shu S, Marshall B, Lewis S; AmiGO Hub; Web Presence Working Group. AmiGO: online access to ontology and annotation data. Bioinformatics. 2009;25:288-289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1243] [Cited by in RCA: 1378] [Article Influence: 81.1] [Reference Citation Analysis (0)] |
| 35. | Kanehisa M, Furumichi M, Tanabe M, Sato Y, Morishima K. KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017;45:D353-D361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4407] [Cited by in RCA: 5834] [Article Influence: 648.2] [Reference Citation Analysis (0)] |
| 36. | Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, Hornik K, Hothorn T, Huber W, Iacus S, Irizarry R, Leisch F, Li C, Maechler M, Rossini AJ, Sawitzki G, Smith C, Smyth G, Tierney L, Yang JY, Zhang J. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 2004;5:R80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9318] [Cited by in RCA: 9601] [Article Influence: 457.2] [Reference Citation Analysis (0)] |
| 37. | Chen J, Li C, Zhu Y, Sun L, Sun H, Liu Y, Zhang Z, Wang C. Integrating GO and KEGG terms to characterize and predict acute myeloid leukemia-related genes. Hematology. 2015;20:336-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 38. | Alam MN, Bristi NJ, Rafiquzzaman M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm J. 2013;21:143-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 794] [Cited by in RCA: 796] [Article Influence: 61.2] [Reference Citation Analysis (0)] |
| 39. | Huang D, Ou B, Prior RL. The chemistry behind antioxidant capacity assays. J Agric Food Chem. 2005;53:1841-1856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3740] [Cited by in RCA: 3229] [Article Influence: 161.5] [Reference Citation Analysis (0)] |
| 40. | Deng GF, Lin X, Xu XR, Gao LL, Xie JF, Li HB. Antioxidant capacities and total phenolic contents of 56 vegetables. J Funct Foods. 2013;5:260-266. [RCA] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 182] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 41. | Fu L, Xu BT, Xu XR, Gan RY, Zhang Y, Xia EQ, Li HB. Antioxidant capacities and total phenolic contents of 62 fruits. Food Chem. 2011;129:345-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 371] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 42. | Wang H, Liu YM, Qi ZM, Wang SY, Liu SX, Li X, Wang HJ, Xia XC. An overview on natural polysaccharides with antioxidant properties. Curr Med Chem. 2013;20:2899-2913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 43. | Ghasemzadeh A, Jaafar HZ, Rahmat A. Phytochemical constituents and biological activities of different extracts of Strobilanthes crispus (L.) Bremek leaves grown in different locations of Malaysia. BMC Complement Altern Med. 2015;15:422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 44. | Singh B, Singh N, Thakur S, Kaur A. Ultrasound assisted extraction of polyphenols and their distribution in whole mung bean, hull and cotyledon. J Food Sci Technol. 2017;54:921-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 45. | Giboney PT. Mildly elevated liver transaminase levels in the asymptomatic patient. Am Fam Physician. 2005;71:1105-1110. [PubMed] |
| 46. | Zhao CN, Tang GY, Liu Q, Xu XY, Cao SY, Gan RY, Zhang KY, Meng SL, Li HB. Five-Golden-Flowers Tea: Green Extraction and Hepatoprotective Effect against Oxidative Damage. Molecules. 2018;23:2216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 47. | Pompella A, Visvikis A, Paolicchi A, De Tata V, Casini AF. The changing faces of glutathione, a cellular protagonist. Biochem Pharmacol. 2003;66:1499-1503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 868] [Cited by in RCA: 877] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 48. | Hayyan M, Hashim MA, AlNashef IM. Superoxide Ion: Generation and Chemical Implications. Chem Rev. 2016;116:3029-3085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1053] [Cited by in RCA: 1188] [Article Influence: 132.0] [Reference Citation Analysis (0)] |
| 49. | Chelikani P, Fita I, Loewen PC. Diversity of structures and properties among catalases. Cell Mol Life Sci. 2004;61:192-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1123] [Cited by in RCA: 1003] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 50. | Zhou JY, Jiang ZA, Zhao CY, Zhen Z, Wang W, Nanji AA. Long-term binge and escalating ethanol exposure causes necroinflammation and fibrosis in rat liver. Alcohol Clin Exp Res. 2013;37:213-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 51. | Nanji AA, Miao L, Thomas P, Rahemtulla A, Khwaja S, Zhao S, Peters D, Tahan SR, Dannenberg AJ. Enhanced cyclooxygenase-2 gene expression in alcoholic liver disease in the rat. Gastroenterology. 1997;112:943-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 130] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 52. | Blednov YA, Benavidez JM, Black M, Ferguson LB, Schoenhard GL, Goate AM, Edenberg HJ, Wetherill L, Hesselbrock V, Foroud T, Harris RA. Peroxisome proliferator-activated receptors α and γ are linked with alcohol consumption in mice and withdrawal and dependence in humans. Alcohol Clin Exp Res. 2015;39:136-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 53. | Diao WR, Zhang LL, Feng SS, Xu JG. Chemical composition, antibacterial activity, and mechanism of action of the essential oil from Amomum kravanh. J Food Prot. 2014;77:1740-1746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 54. | Wang X, Wu D, Yang L, Gan L, Cederbaum AI. Cytochrome P450 2E1 potentiates ethanol induction of hypoxia and HIF-1α in vivo. Free Radic Biol Med. 2013;63:175-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 55. | Barve S, Chen SY, Kirpich I, Watson WH, Mcclain C. Development, Prevention, and Treatment of Alcohol-Induced Organ Injury: The Role of Nutrition. Alcohol Res. 2017;38:289-302. [PubMed] |
| 56. | Mehal WZ. HIF-1α is a major and complex player in alcohol induced liver diseases. J Hepatol. 2012;56:311-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 57. | Cho ML, Kang JW, Moon YM, Nam HJ, Jhun JY, Heo SB, Jin HT, Min SY, Ju JH, Park KS, Cho YG, Yoon CH, Park SH, Sung YC, Kim HY. STAT3 and NF-kappaB signal pathway is required for IL-23-mediated IL-17 production in spontaneous arthritis animal model IL-1 receptor antagonist-deficient mice. J Immunol. 2006;176:5652-5661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 255] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 58. | Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel F, Lecron JC, Kastelein RA, Cua DJ, McClanahan TK, Bowman EP, de Waal Malefyt R. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1490] [Cited by in RCA: 1566] [Article Influence: 87.0] [Reference Citation Analysis (0)] |
| 59. | Kim JW, Roh YS, Jeong H, Yi HK, Lee MH, Lim CW, Kim B. Spliceosome-Associated Protein 130 Exacerbates Alcohol-Induced Liver Injury by Inducing NLRP3 Inflammasome-Mediated IL-1β in Mice. Am J Pathol. 2018;188:967-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 60. | Nishiyama Y, Goda N, Kanai M, Niwa D, Osanai K, Yamamoto Y, Senoo-Matsuda N, Johnson RS, Miura S, Kabe Y, Suematsu M. HIF-1α induction suppresses excessive lipid accumulation in alcoholic fatty liver in mice. J Hepatol. 2012;56:441-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 61. | Nath B, Levin I, Csak T, Petrasek J, Mueller C, Kodys K, Catalano D, Mandrekar P, Szabo G. Hepatocyte-specific hypoxia-inducible factor-1α is a determinant of lipid accumulation and liver injury in alcohol-induced steatosis in mice. Hepatology. 2011;53:1526-1537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 164] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 62. | Meng X, Li S, Li Y, Gan RY, Li HB. Gut Microbiota's Relationship with Liver Disease and Role in Hepatoprotection by Dietary Natural Products and Probiotics. Nutrients. 2018;10:1457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |