Published online Jun 21, 2020. doi: 10.3748/wjg.v26.i23.3182
Peer-review started: December 30, 2019
First decision: January 16, 2020
Revised: April 1, 2020
Accepted: May 23, 2020
Article in press: May 23, 2020
Published online: June 21, 2020
Processing time: 174 Days and 11.3 Hours
Acupuncture has been used in China for thousands of years and has become more widely accepted by doctors and patients around the world. A large number of clinical studies and animal experiments have confirmed that acupuncture has a benign adjustment effect on gastrointestinal (GI) movement; however, the mechanism of this effect is unclear, especially in terms of neural mechanisms, and there are still many areas that require further exploration. This article reviews the recent data on the neural mechanism of acupuncture on GI movements. We summarize the neural mechanism of acupuncture on GI movement from four aspects: acupuncture signal transmission, the sympathetic and parasympathetic nervous system, the enteric nervous system, and the central nervous system.
Core tip: Acupuncture has been applied in the treatment of gastrointestinal (GI) dysmotility diseases worldwide for decades. However, its underlying neuromechanisms in regulating GI motility have not been fully established. The neural regulation of GI function depends on its endogenous and exogenous nervous system. This review discusses the mechanisms of acupuncture on GI motility from various perspectives including the afferent signals, autonomic nervous system, as well as central nervous system based on its physical/pathological neural control.
- Citation: Yu Z. Neuromechanism of acupuncture regulating gastrointestinal motility. World J Gastroenterol 2020; 26(23): 3182-3200
- URL: https://www.wjgnet.com/1007-9327/full/v26/i23/3182.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i23.3182
Acupuncture, as a complementary and alternative medical treatment, is used worldwide due to its advantages in terms of efficacy and safety. A literature analysis summarized the clinical diseases and symptoms that could be treated by acupuncture and moxibustion by collecting and analyzing published randomized controlled studies in the PubMed database. This analysis identified more than one hundred diseases and symptoms that were unanimously considered effectively treated, among which gastrointestinal (GI) diseases accounted for a quarter of the diseases[1-3]. GI motility disorder is a common pathological feature in many clinical diseases[4,5], including gastroparesis, irritable bowel syndrome (IBS), functional dyspepsia, pseudo-obstruction, and chronic constipation, causing premature satiety, nausea, vomiting, abdominal pain, bloating, loss of appetite, and other clinical symptoms[6-10]. Taking diabetic gastroparesis as an example, as many as 25%–55% of patients with type 1 diabetes have gastroparesis, with a slightly higher incidence rate in patients with type 2 diabetes, causing great suffering and poor quality of life in patients[11]. GI motility disorder is becoming a serious public health problem and a significant burden to society; however, current treatment options for gut dysmotility are limited because of serious side effects[12,13].
In previous decades, many studies confirmed the efficacy of acupuncture for functional GI disorders and more recent medical research has made some progress in determining its mechanisms. The nervous system plays a key role in the benign regulation of GI movement in acupuncture. GI motility is the result of the combined effects of the nervous system, humoral factors, and the myoelectric activity of the GI tract itself, in which the nervous system plays a main role. Neural regulation of GI function depends on the following three levels: The local enteric nervous system (ENS) (endogenous regulation), the autonomic nervous system (ANS), and the central nervous system (CNS) (exogenous regulation). The aim of this review is to discuss the possible neuromechanisms of acupuncture in GI motility, based on its afferent signals and the neural regulation of GI motility.
The effect of acupuncture is based on the somatic afferent signals induced by the insertion of needles into the body manually (manual acupuncture, MA) or electrically (electroacupuncture, EA). Somatic afferent nerve fibers are classified into several types according to their diameter, including A-α, A-β, A-δ, and C-fibers, and it is widely accepted that these fibers underlie the neural mechanism of acupuncture[14]. MA stimulation can activate all four groups of somatic afferents and deliver the signal to the CNS, while the type of fibers activated by EA stimulation depends on the intensity[15]. Nevertheless, only when the intensity of stimuli is over the threshold of Aδ and/or C-fibers can acupuncture obviously modulate GI motility[16].
A strong mechanical stimulus applied to the abdominal skin induces an inhibitory effect on jejunal motility, and this effect was proven to be produced by excitation of group IV (unmyelinated) cutaneous afferent nerve fibers in the T10 spinal nerve. Similar stimuli applied to the skin of the upper chest, neck, forepaws, or hind paws elicited a facilitative jejunal reflex that was evoked by excitation of mainly group III (A-δ) cutaneous afferent nerve fibers[17]. Another study indicated that duodenal motility was inhibited by EA at intensities of more than 5.0 mA (suprathreshold of group IV afferent excitation) at acupoints located in the abdominal area, and facilitated by EA at acupoints located in the hind paw with intensities of more than 2.0 mA (subthreshold of group IV, and suprathreshold for groups II + III afferent excitation)[18].
A variety of somatosensory receptors are present in the skin and muscles, including mechanoreceptors, thermoreceptors, and nociceptors. Among them, acid-sensing ion channel 3 (ASIC3) is mainly located in Aβ-fibers innervating the skin and muscle to respond to mechanical stimuli, and transient receptor potential vanilloid-1 (TRPV1) is co-expressed with C-fibers rather than with myelinated nociceptors as a heat and mechanical sensor[19,20]. EA at acupoints located in the abdominal area markedly inhibits gastric motility in an intensity-dependent manner, and this inhibitory effect is alleviated by capsazepine injection (an antagonist of the TRPV1 receptor) or TRPV1 gene knockout with no intensity dependence, indicating that TRPV1 is partially involved in the EA-mediated modulation of gastric motility[21,22]. A similar result for EA was observed on jejunal motility[23]. In Asic3 gene deleted mice, the effect of EA at ST36 and CV12 on gastric motility was slightly decreased, with no statistical significance compared with that in wild-type mice[24]. The mean temperature was around 43°C, referring to the thermal activation of Aδ- and C-fibers[25-27]. Nociceptive 43°C and 45°C heat stimuli at ST36 and CV12, rather than non-nociceptive 41°C, produced significant regulatory effects on gastric motility, and this effect was also decreased in Trpv1−/− mice, but not in Asic3−/− mice[28,29]. Results from morphological observations confirmed the role of TRPV1 and ASIC3. Western blotting and immunofluorescence results demonstrated an abundance of TRPV1, TRPV4, and ASIC3 in the anatomical layers of ST36[30]. Components of calcium wave propagation (CWP, the proposed downstream sensing pathway) were also co-expressed with TRPV1. However, only TRPV1 is regarded as a responding channel for acupuncture by sensing peripheral information and conducting signaling via the CWP and the excitatory phosphorylated glutamate ionotropic receptor NMDA type subunit 1-phosphorylated calcium/calmodulin dependent protein kinase II pathway, offering a comprehensive understanding of the physical stimulation by acupuncture of neurological signaling[31].
Neural networks to control GI motility are positioned at three levels: The parasympathetic and sympathetic nervous system (PNS, SNS), the ENS, and the CNS. Although the intestines are capable of functioning in the absence of extrinsic innervation, movements of the upper GI tract are much more dependent on extrinsic neural pathways, and the motility of the small and large intestines are mainly monitored by the ENS[32,33]. The PNS and SNS comprise one of the factors affecting extrinsic innervation of GI contractility. The SNS provides a predominantly inhibitory effect on GI motility, and in contrast, the PNS exerts both excitatory and inhibitory control over GI motility.
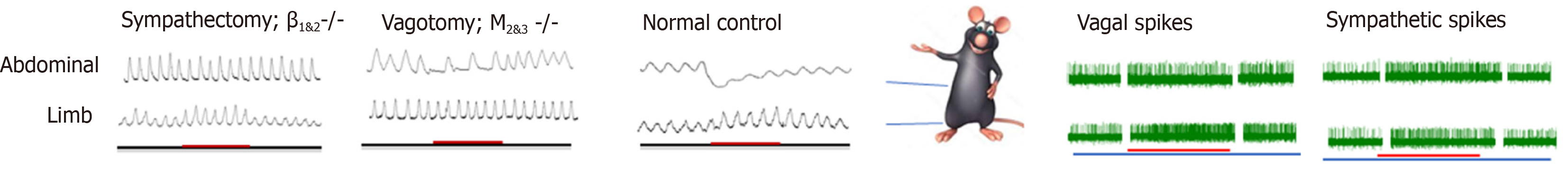
The role of the somatoautonomic reflex, which was demonstrated by Sato and his colleagues in the 1960s[34,35], is now considered the neurological basis of acupuncture in GI motility modulation[36,37], and this modulation exerts a regional-specific effect[38,39] (Figure 1). Stimulating acupoints in the abdominal area inhibits gastric, duodenal, and jejunal motility by increasing sympathetic efferent fiber activity, and stimulating acupoints in the limb, which, in contrast, facilitates the above-mentioned gut motility by exciting vagal efferent fiber activity[18,40-43]. The effect of acupuncture on GI motility could be attenuated by vagotomy or sympathectomy[16,44-46]. The β1 and β2 receptors are expressed in the GI tract and serve as the predominant subtypes in the inhibitory effects of the SNS on GI motility[47,48], while the M2 and M3 receptors serve as the predominant subtypes in the excitatory effects of the PNS on GI motility[49,50]. The effect of acupuncture on GI motility could also be reduced by the corresponding antagonist[51-54] and deletion of the gene encoding the β1&2 receptor or M2&3 receptor[55-57]. In addition to the excitement effect, lower extremity acupoints also suggest an inhibitory effect on GI movement, and the inhibitory rate of ST37/LI11 on gastric movement fluctuates between 6.67%–1.33%[58,59]. Moreover, the effect of ST36 on gastric movement is reversed to inhibition after gene knockout of M2 and M3 receptor subtypes[45], suggesting the involvement of other receptors. This might be caused by activation of the other vagus pathway, the inhibitory non-adrenergic, noncholinergic (NANC) pathway, whose transmitter is nitric oxide (NO) or vasoactive intestinal polypeptide (VIP), to cause smooth muscle relaxation[60]. Zhu and his team explained this type of region-specific effect using the spinal segmental innervation theory[46]. Stimulation at homotopic acupoints (with the same spinal segment from the innervated visceral organs) decreases GI pressure via the sympathetic pathways, while stimulation at heterotopic acupoints (with different spinal segments from the innervated visceral organs) facilitates GI motility via the parasympathetic pathways[41,56]. This effect applied not only to normal conditions, but also to rats with diarrhea and constipation. It should be noted that this region- specific effect seems to apply only to the regulation of GI movements. When it comes to the effects of acupuncture on modulating the cardiovascular system, stimulations of the limbs, chest, and abdomen all produce a similar effect in terms of heart rate and blood pressure, yet whether the effect is excited or suppressed depends on the intensity of the stimulus (or what type of fiber is activated) and the tissue structure (skin or muscle)[61-64].
The response time of acupoints at different sites might be another feature of the regional specificity of the somatoautonomic reflex; however, this feature is rarely noticed. According to a previous study[65], the abdominal acupoints for gastric motility could be effective within 30 s with a shorter response time, while the acupoint of the hindlimb was effective after 30 s with a longer response time. ST36 showed an excitatory effect in a fast onset manner in sympathectomy rats and β1&2 knockout mice, suggesting that sympathetic inhibition might be one of the key factors for the delayed onset of ST36 and β1 and β2 receptors may underlie its receptor mechanisms[44,45]. Undoubtedly, the sympathetic pathway is activated by acupoints in the lower limbs; evidence shows that EA at ST36/ST37 increased sympathetic activity in healthy volunteers using a heart rate variability analysis or microneurography evaluation of the left peroneal nerve[66,67]. This mechanism is fully elucidated in terms of the cardiovascular system and anti-inflammatory effects[68,69]; however, it is rarely mentioned in the regulation of GI function. Therefore, the role of sympathetic nerve pathways in regulating GI function by limbic acupoints is greatly underestimated and further evidence is required.
When it comes to colonic motility, the situation is a bit more complicated, because the effects of acupoints located in different areas on the proximal and distal colon are inconsistent. Acupuncture at the acupoints of the forelimb (LI1–LI6 and LI11), abdomen (ST25), and back (BL25) had no significant effects on the proximal colonic motility; and acupoints of the hind paw (ST37) increased proximal colonic pressure[70-72]. Stimulation of the limbs (LI11, ST37, and ST36), abdomen (ST25), and back (ST37) all produced an augmented effect on distal colonic motility regardless of normal, constipated, or diarrheic state[73]. The mechanism underlies the cholinergic pathway[74]. Apart from the segmental innervation theory, the regional neuro-feature between the proximal and distal colon might offer a partial explanation. Vagal innervation to the colon varies among species. In humans, the vagal efferent to the large intestine ends at the splenic flexure, and the remaining part receives parasympathetic input from the pelvic nerves[75-77]. However, studies in rodents suggest that the efferent fibers of the vagus distribute throughout the entire colon[78,79], which is supported by evidence from a retrograde neuronal tracing study[80]. Moreover, the distal colon is innervated via dual parasympathetic nerves, because the pelvic nerve projects from the rectum through the mid colon. In addition, there are regional differences in the innervation of the vagus nerve between the proximal and distal colon[81-84], i.e., the fiber density and distribution of neurotransmitters, such as acetylcholine, within the ganglions is different from each other. It is possible that the regional difference underlies the inconsistency of EA in regulating the motility of the proximal and distal colons. Besides, the regional complexity in the enteric neuron[85] and pelvic ganglion (contain both postganglionic sympathetic and parasympathetic neurons) innervated colon might also add to the complexity of acupuncture-mediated modulation of GI motility[86-88].
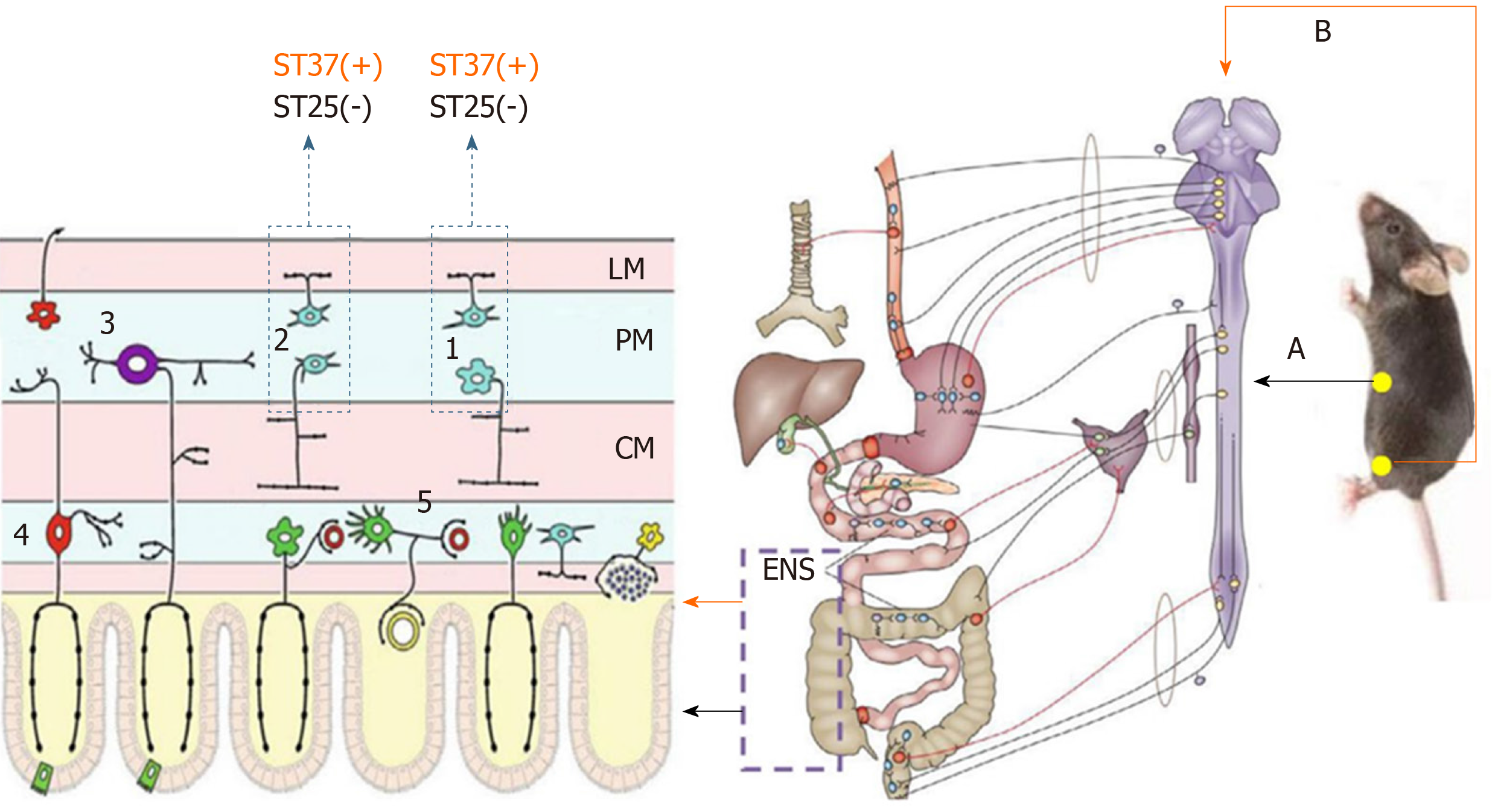
In contrast to the innervation of other peripheral organs, the GI tract has an extensive intrinsic nervous system (the ENS), which modulates its functions, including motility, secretion, blood flow, immunity, and maintenance of the integrity of the epithelial barrier. The major components of the ENS are myenteric plexuses and submucosal plexuses, which contain numerous enteric neurons and glial cells. The myenteric plexuses distribute from the upper esophagus to the internal anal sphincter, forming a continuous network to control GI motility. The submucosal plexuses are closer, allowing them to sense the lumen environment and regulate GI blood flow, as well as controlling epithelial cell functions and secretion[89]. Basically, enteric neurons can be classified into three types according to their function: Motor neurons (including inhibitory neurons and excitatory neurons), sensory neurons, and interneurons. Different neurons in the ENS neural network regulate GI movement by releasing different transmitters. These transmitters include excitatory transmitters, like acetylcholine, opioid peptides, and 5-HT; and inhibitory transmitters such as VIP and NO.
The importance of the ENS in the neuropathy of GI dysmotility is highlighted by a wide range of diseases that are grouped as congenital, acquired (such as slow transit constipation), and secondary disorders (such as diabetic gastroparesis)[32]. Wedel et al[90] found that the number of nerve cells decreased significantly within the myenteric plexus and external submucous plexus in patients with idiopathic slow-transit constipation by observing the neuronal marker Protein Gene Product 9.5 (PGP9.5), confirming the role of ENS in GI motility. PGP9.5-positive cells were also markedly decreased in the jejunum, ileum, and proximal colon myenteric plexus of mice with constipation[91]. EA has shown a benign regulatory effect in STC; however, much less is known about its mechanism with reference to the ENS, and a series of studies suggested that EA could restore GI motility by controlling excitatory and inhibitory neurons. ST37, an acupoint in the limb, improved the carbon propulsion rates and defecation time and increased the total PGP9.5 expression in the jejunum, ileum, and proximal colonic myenteric plexus[92]. Further results indicated the restoration of enteric neuron function by downregulating the level of nitric oxide synthase (nNOS, secreted by inhibitory neurons to induce relaxation) and upregulating the expression of choline acetyltransferase (ChAT, secreted by excitatory neurons to induce contraction). While ST25, located in the abdomen, could only downregulate the expression of nNOS; therefore, the regulatory effect of EA on enteric neurons seems to act in a region-specific manner[72] (Figure 2).
Loss of enteric neurons is one the most important neuropathies that contribute to GI dysmotility in diabetic rats, and this change may be mediated, in part, via a reduction of glial cell derived neurotrophic factor (GDNF) and its main downstream signaling pathway phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)-protein kinase B (AKT), which is a survival signal for enteric neurons[93,94]. Decreases in PGP9.5, nNOS, and ChAT neurons in the colon of diabetic rats led to disordered gastric emptying and intestinal transit[95,96]. After long-term intervention, EA at ST36 induced regeneration of lost enteric neurons[97]. Meanwhile, the mRNA and protein level of GDNF, an important neurotrophic factor of enteric neurons, and p-AKT in the colon were upregulated, suggesting the role of GDNF and the PI3K-AKT signal pathway in the mechanism of EA-induced regeneration of impaired enteric neurons.
Purinergic P2X receptors contribute to neurotransmission in subsets of enteric neurons of the GI tract and P2X3 receptor immunoreactivity was found in the myenteric neurons of the esophagus, stomach, and small and large intestines of rodents, of which the major types of neurons are excitatory, inhibitory muscle motor neurons, and interneurons[98,99]. However, the evidence for P2X3 receptor involvement in GI motility is limited. Studies have reported that P2X3 gene knockout mice exhibit a feature of impaired gut peristalsis[100,101]. P2X3 subunits are localized to after hyperpolarizing neurons[102], which are likely to be intrinsic intestinal sensory neurons. The impairment of peristalsis caused by P2X3 gene deletion was independent of the changes in the muscarinic receptor or nicotinic receptor function in the longitudinal muscle layer, suggesting that the P2X3 receptor underlies the sensory mechanisms in the myenteric plexus that mediate peristalsis, and plays an important role in mechanosensitivity and hypersensitivity[103]. The therapeutic effect of EA on IBS has been confirmed by a substantial body of literature from clinical data, and the mechanism involves alleviating mechanosensitivity and hypersensitivity[104], where P2X receptors might serve as novel targets[105]. Electroacupuncture has been suggested to regulate the P2X3 receptor located in the neurons of the colonic myenteric plexus of IBS rats[106]. Whether this has a crucial role in the other parts of the GI tract has not yet been reported, and the results of this study[104] provide clues for further research.
The ENS contains a large amount of enteric glial cells (EGCs) surrounding neurons to regulate neuronal activity and protect the gut in different ways, including maintaining the intestinal barrier function and the inflammatory process, which facilitate the control of GI motility[107]. Animals with genetic elimination of enteric glia develop jejuno-ileitis inflammation[108] and intestinal dysmotility in a sex-dependent manner[109], confirming the pivotal role of EGCs in GI motility disorders[110].
Hemorrhagic shock is frequently accompanied by bowel stasis following intestinal ischemia and reperfusion injury. The initiated inflammatory response, including the production of cytokines and recruitment of leukocytes within the intestinal muscularis, contributes to impaired muscle contractility and mucosal barrier function[111]. In addition to promoting gastric emptying, EA at ST36 was also reported to decrease intestinal injury and alleviate inflammation to prevent intra-abdominal adhesion, which serves as one of the main causes of intestinal obstruction[112,113]. This protective effect provided by EA acts partly via activation of EGCs to secrete substances that regulate the gut function via activation of the cholinergic anti-inflammatory pathway[114,115], because both blockage of the cholinergic α7nAChR subunit by α-bungarotoxin and vagotomy could weaken or eliminate the effects of EA at ST36.
Apart from the above-mentioned function, enteric glia are emerging as novel regulators of enteric reflex circuits that contribute to the regulation of GI motility[116]. Delvalle et al[117] provided the first evidence that EGCs have effects on gut motility through modulation of excitatory reflexes involved in GI motility, and activation of glial M3 and M5 subtypes of muscarinic receptors contributes to this kind of physiological regulation. Genetic ablation of M2 and M3 receptors diminished the enhancement of GI motility by EA at ST37[53]; however, whether the subtypes of muscarinic receptors of EGCs are involved requires more evidence.
As described previously, the extrinsic neural pathway participates in the modulation of GI functions. The CNS, as another important factor of extrinsic innervation, provides its extrinsic neural inputs to control GI motility in a more widespread and integrated manner, which involves the spinal cord, medulla, midbrain, and mesencephalon. The regulatory effect of acupuncture on GI movement also requires the participation of the CNS at multiple levels by changing the activities of the nuclei related to GI movement, including the dorsal motor nucleus (DMV), the solitary tract nucleus (NTS), the raphe nuclei, the lateral hypothalamic area (LHA), and the paraventricular nucleus of the hypothalamus (PVN), which have been confirmed by neuroanatomical tracing following the injection of a neuronal tracer into the stomach and ST36, respectively[118,119]. The role of acupuncture on the nuclei of the CNS is summarized in Table 1.
| The nuclei | Acupoint | Function | Ref. |
| Brain stem | |||
| DVC | ST36, ST37 | Activate neurons discharge | [123] |
| DVC | RN12, BL21 | Increase fos expression | [126] |
| DVC | RN12, BL21 | Motilin and gastrin | [190] |
| DVC | ST36 | Inhibited the release of SP | [133] |
| DVC | ST36 | Increased the expression of astrocytic and microglial | [138] |
| DMV | PC6 | Modulate vagovagal neurocircuits | [44] |
| DMV | ST36 | Modulate vagovagal neurocircuits | [45] |
| NTS and DMV | ST36, T25 | Increased the number of c-Fos immunopositive cells | [150] |
| The cerebellum | |||
| FN | ST36, LI11, BL21, and CV12 | Elevate the spontaneous discharge | [159] |
| MV | ST36, LI11, and BL21 | Modulated the activity of GD neurons | [176] |
| The hypothalamus | |||
| PVN | BL23, BL18, LR14, GB25, GB24, LR13, DU14 | Activate CRH-like neurons; modulate the expression of CRH and the GR | [182] |
| PVN | ST36 and PC6 | Decreased the numbers of double-labeled OT neurons and c-fos neurons | [187] |
| LHA | ST36 | Abolished the inhibitory reaction induced by GD | [170,199] |
| LHA | ST36, ST25 | Modulate the levels of NA, serotonin (5-HT), and the activity of ATPases | [200] |
| LHA | ST36 and ST25 | Alter the activity of the glucose-sensitive neurons | [201] |
| ARC | ST36, SP6 | Increased α-MSH and POMC | [218] |
| ARC | SP6, ST36 | Regulates the expression of neuropeptide Y and POMC neurons | [220] |
The dorsal vagal complex: A large volume of sensory information of the GI tract, both mechanical and chemical, is transmitted mainly to the NTS via the vagal afferent fibers. This information is integrated by NTS neurons and then conveyed to the motor DMV, using γ-aminobutyric acid (GABA), glutamate (Glu), and norepinephrine as the main neurotransmitters to modulate vagal efferent innervation and GI motility through the vagal ACh or NANC pathways[120-122]. The NTS and DMN constitute the dorsal vagal complex (DVC), the main neuroanatomical structure of vagovagal reflexes. The role of DVC in acupuncture-mediated regulation of GI function is supported by multiple pieces of evidence from morphological, neuroele-ctrophysiological, and neurochemical studies. While regulating the electro activity of the stomach, EA at ST36 and ST37 could modulate the firing of the neurons in the NTS and DMV, and increase their Fos expression[46,123-126]. Moreover, with the help of a lesion-making device, the DVC was damaged, which resulted in GI tract dysfunction[127], and EA lost its regulatory effect on gastric motility, confirming the important role of DVC in EA-mediated regulation of GI function[123,128].
Brain-gut peptides play an important role in GI function. There is a long list of GI peptides that are capable of regulating GI motility. They are secreted by endocrine cells of the gut as neurotransmitters and neuromodulators, and distribute to the CNS to regulate GI functions[129-131]. A fewer years later, another study demonstrated that EA at RN12 and BL21 enhanced the production of GI hormones, such as motilin and gastrin, in the DVC[126]. In addition, acupuncture stimulation at ST36 could modulate the concentration of VIP, motilin, ghrelin, and gastrin in serum[132]. Microinjection of substance P (SP), another candidate brain-gut peptide, into the DVC inhibited gastric motility via the NK-1 receptor, whereas EA at ST36 enhanced gastric myoelectric activity and simultaneously inhibited the release of SP in the DVC, suggesting that SP in the DVC is involved in the effect of EA on gastric myoelectric activity[133]. These data implied the role of neurons in the DVC in the effect of EA on GI movement. The activities of brain-gut peptides are complicated, and their mechanisms differ from each other. In the detection of the same brain-gut peptide in patients, different scholars may have drawn different conclusions, and have not mentioned their complex interconnections. An in-depth study of brain-gut peptides and their relationship with acupuncture would be conducive to a better understanding of the mechanism of acupuncture in GI motility.
Glia constitute almost half of the cells in the CNS. Given the emerging evidence, glial cells have been highlighted in many aspects of CNS function, rather than acting as static bystanders as was long believed. Continued elucidation of glial cell biology, and the dynamic interactions of neurons and glia, will enrich our understanding of nervous system health and diseases[134]. Research on acupuncture-mediated regulation of glial cells is increasing year by year, in such fields as analgesic processes, anti-inflammation, and GI function[135-137]. Studies suggest that EA at ST36 significantly increased the expression of astrocytic (glial fibrillary acidic protein) and microglial (OX-42) marker cells in the DVC and regulated gastric activity. Propentofylline (PPF, a blocker of both microglial cells and astrocytes) attenuated the activation by EA of gastric activity, suggesting that EA regulation of gastric activity might be related to the glia in the DVC[138]. Although the function of DVC glia in the effect of EA on GI motility has been suggested, the data remains very limited, and the hypothesis must be verified via further studies.
Vagovagal neurocircuits comprise specific nuclei, including the NTS and DMV. NTS neurons receive and converge the somatic and visceral sensory information[139-141], whereas the DMV is the origin of vagal efferent fibers, modulating gastric motility as well as other visceral functions (e.g., vagal neurocircuitry and its influence on gastric motility). GABA is the main neurotransmitter that transmits information from the NTS to the DMV. Injection of L-Glu into the DMV might lead to a change in gastric activity[142-144], and whether the effect is excited or suppressed depends on the injection site (rostrum or posterior)[145]. Microinjection of GABA into the DMV decreased gastric motility, whereas microinjection of bicuculline, a GABA antagonist, increased gastric motility[146,147]. EA at PC6 showed no marked change after the injection of L-Glu; however, gastric movement and the parasympathetic nerve firing after injection of GABA were enhanced, indicating that GABA transmission of the DMV underlies the facilitation of EA at PC6 on gastric motility[44]. EA at ST36[45] is capable of modulating gastric motility after the injection of L-Glu and GABA, suggesting that both Glu and the GABA receptor in the DMV participate in promoting the gastric motility effect of EA at ST36, i.e., EA at ST36 and PC6 modulate gastric motility via the vagovagal reflex.
The rostral ventrolateral medulla: The rostral ventrolateral medulla (RVLM), the final area for the integration of sympathetic nerve activity (SNA)[148], processes a large volume of pre-sympathetic (sympathoexcitatory) neurons that project directly into the intermediolateral (IML) column of the spinal cord, and modulates the sympathetic control of visceral functions[149]. Takahashi et al[150] reported that acupuncture at ST-36, but not at ST25, increased the number of c-Fos immunopositive cells at the NTS and DMV, while acupuncture at ST25 specifically increased the number of cells at the NTS and RVLM. This suggested that somatic afferents activated by acupuncture at ST36 are conveyed to the NTS and then stimulate the DMV neurons. In contrast, somatic afferents activated by acupuncture at ST25 are conveyed to the NTS and stimulate RVLM neurons. This deduction was supported by anatomical and functional evidence that demonstrated a robust excitatory input from NTS neurons to neurons within the RVLM[151]. In spinal functional magnetic resonance imaging research, multiple activations of grey matter (including the spinal dorsal horn and IML) in T8 and T9 vertebrae showed a response to stimulation on ST25[152]. Another study found that ST25, ST37, and LI11 could all change the firing rate of the IML; however, ST25 showed the strongest effect. It could be concluded that the NTS–RVLM–IML-sympathetic and NTS–DMV–parasympathetic pathways, respectively, underlie the neural anatomical evidence of regional specific effects of acupuncture on gastric motor function in rats. However, RN12, BL 21, and ST25 are located in the abdomen and back, and share similar segment innervation, yet c-fos expression in the NTS and DMV was statistically increased by EA stimulation at RN12 and BL21; therefore, this anatomical evidence of a regional specific effect requires further research for verification.
The fastigial nucleus: The cerebellum is traditionally viewed as a subcortical motor center; however, behavioral, anatomical, and neuroelectrophysiological studies have shown that the cerebellum also has an important regulatory role in the visceral activities of the body[153]. The fastigial nucleus (FN) is the oldest nucleus in the cerebellum and inhabits a key position in the regulation of nonsomatic functions, including the GI movement and feeding, among the nuclei of the cerebellum[154,155]. Electrical stimulation of the cerebellum can affect GI movement through the vagal and sympathetic nerve pathways[156-158]: Activation of adrenergic fibers to release catecholamines, and activation of cholinergic fibers to inhibit or facilitate gastric movement. In addition, studies have shown that electrical stimulation of the FN has a protective effect on the gastric mucosa by reducing gastric ischemia-reperfusion injury and apoptosis of gastric mucosal cells in rats. Needling at ST36, LI11, BL21, and CV12 could elevate the spontaneous discharge frequency of cerebellar FN neurons; however, CV12 seems to have an advantageous role in regulating the cerebellar FN, partly confirming the role of the FN in the central pathway of acupuncture-mediated regulation of GI function[159].
The direct bidirectional pathways between the cerebellum and the hypothalamus have been demonstrated in a series of neuroanatomical investigations, forming a part of the circuits underlying cerebellar autonomic modulation[160,161]. The FN is one of the originating nuclei of the cerebellar-hypothalamic projection, and is the main structure that modulates GI activities in the cerebellum. Recently, the FN-LHA loop has been investigated[162], and is likely to serve as the neural basis for regulating GI functions[163]. Gastric vagal and cerebellar fastigial nuclear signals converge on glycemia-sensitive neurons of the LHA in rats[164]. Electrical and chemical stimulation of the FN had neuroprotective effects on gastric mucosal stress injury and gastric ischemia-reperfusion damage in rats, and pre-damage of the FN or LHA could eliminate the protective effect of the FN[165-169]. Extracellular recording results suggested that the signal of gastric distention (GD) and acupuncture at ST36 converged in the LHA and FN, and acupuncture could modulate the activities of different types of GD-sensitive neurons in the LHA and FN, indicating that the LHA-FN circuit might participate in the central integration mechanism of acupuncture’s effects on gastric function[170]. However, whether the LHA-FN circuit, which underlies the effect of acupuncture on GI function, acts through the sympathetic nerve or parasympathetic nerve requires further research.
The vestibular nucleus: The vestibular nucleus (VN) is the initial structure of the central processing of vestibular orientation information and receives projections from the vestibular nerve, playing a special role in regulating GI function. The work of Gagliuso et al[171] and Ruggiero et al[172] demonstrated the neuroanatomy of the vestibulo-solitary pathway, providing an anatomical explanation for the autonomic changes observed, including GI responses, after peripheral vestibular activation[171,172]. The medial vestibular nucleus (MV) is the area with the largest volume and cell number in the vestibular nucleus. Direct fiber projection also exists in the MV and dorsal vagus nucleus[173,174]. The technique of retrograde axonal transport of horseradish peroxidase in the MV identified a group of neurons sending axons to the "stomach" region of a single tract nucleus, and local irritation of the neurons initiated relaxation of the stomach wall[175]. GD and acupuncture at different acupoints (ST36, LI11, and BL21) modulated the activity of the neurons within the MVN, suggesting that the MVN is partly involved in the central integration mechanism underlying acupuncture-mediated regulation of gastric functions[176].
The hypothalamus is located on the ventral thalamus and contains distinct groups of nuclei and bundles that regulate a variety of autonomic functions by interconnecting with other parts of the CNS. Among them, the PVN, the LHA, and the arcuate nucleus (ARC) are particularly important in the regulation of GI functions.
The paraventricular nucleus of the hypothalamus: The PVN is located near the periventricular zone of the medial hypothalamus, and emerges as one of the most important autonomic control centers via its secretion of a variety of peptides[177,178]. Among them, corticotrophin-releasing hormone (CRH) and oxytocin (OT), synthesized by magnocellular and parvocellular neurons within the PVN for release into the hypophysial portal system or projection to other central nuclei, are particularly important in the regulation of GI functions, especially stress-induced alterations of GI motility[179,180]. Studies have shown that corticotropin-releasing factor (CRF) activates DMV neurons and decreases gastric motility via activation of the vagal NANC pathway[181]. Few data are available focusing on acupuncture-mediated regulation of CRH function, and these data suggest that acupuncture could activate CRH-like neurons and modulate the expression of CRH and the glucocorticoid receptor (GR) in the PVN[182]. Rinaman et al[183] showed that the PVN is the sole source of OT-immunopositive fibers and terminals in the DVC. Microinjection of OT into the DMV or PVN resulted in inhibition of gastric motility; however, this effect was ameliorated by vagotomy or microinjection of an OT receptor antagonist into the PVN[184]. Llewellyn’ s work provided the anatomical basis for the functional pathways in GI motility regulation by OT: As a neurotransmitter, OT is transmitted from the PVN to the DVC, where it activates motor neurons of the brainstem vagus nerve via the NANC pathway, thereby inhibiting GI movement[185,186]. EA at the limbic acupoints ST36 and PC6 increased the intragastric pressure and decreased the numbers of double-labeled OT neurons and c-fos neurons within the PVN[187]. However, the effect of acupuncture was significantly decreased after dorsolateral funiculus or spinal cord transection, suggesting that the dorsolateral funiculus is the main conduction bundle responsible for acupuncture signal transduction into the PVN[188]. Knockout of the OT gene accelerated gastric emptying, and influenced the effect of acupuncture, further confirming the paraventricular nucleus OT neurons as the initiating device of autonomic nervous pathways, which might participate in EA-mediated regulation of gastric motility, in the form of neural and neuroendocrine stimuli[189]. Thus, acupuncture signals gather in the DVC and PVN. PVN lesions cause a decrease in gastric motility, and EA is unable to restore it, demonstrating that the PVN is another central target of acupuncture’s regulation of gastric motility[190]. Taken together, these data suggest that the acupuncture signal is transmitted to the solitary nucleus through the spinal cord, and after initial integration by the solitary nucleus, the ascending projection activates the PVN. The descending projection is performed by the PVN-DVC-vagal nerve pathway, through the nerve system and neuroendocrine system to regulate GI function.
The lateral hypothalamic area: The LHA is linked to celiac sympathetic and parasympathetic nerves, and regulates metabolic, behavioral, and autonomic functions, and serves as the feeding center[191]. Its influence on GI function and feeding behavior might be mediated by the DVC. As early as the 1980s, with the help of tracing techniques, an anatomical connection was found between the LHA and the dorsal DVC[192,193]. Moreover, this pathway involves multiple neurotransmitters and neuropeptides, including the projection of ghrelin neurons[194-196]. Ghrelin has central and peripheral actions in gut motility, and its receptors are regarded as targets for novel motility drugs[197,198]. Ghrelin fibers originating in the LHA project into the DVC. The electrical stimulation of the LHA promotes gastric motility and GD neurons in the DVC, and this excitatory effect is partially blocked by pretreatment with a ghrelin receptor antagonist and is diminished by an electrical lesion of the DVC. Cisplatin-treatment could weaken this excitatory effect of electrical stimulation in the LHA.
Somatic input from ST36 abolished the inhibitory reaction of the LHA induced by gastric distention[170,199]. Acupuncture could modulate the levels of noradrenaline, serotonin (5-HT), and the activity of ATPases in the LHA[200]. However, ST36 and ST25 are able to alter the activity of the glucose-sensitive neurons within the LHA, which is also the candidate target of ghrelin[201-203]. Increasing numbers of investigations have focused on the role of acupuncture in the peripheral actions of ghrelin in gut motility in the clinic[204,205]. The level of plasma ghrelin was measured in patients with GI cancer and obesity[206,207] and in mice. The results suggested that acupuncture has a benign effect on the modulation of ghrelin secretion. However, the literature on the central role of acupuncture on ghrelin secretion is limited. It has been confirmed that acupuncture can activate the TRPV1 receptor[21,23,30,31], which can also be activated by capsaicin during GI motility, i.e., acupuncture has a similar effect to capsaicin. Capsaicin regulates the central action of ghrelin[194-196]; therefore, we speculated that acupuncture also has a central regulatory effect on ghrelin, which requires further data to prove this.
Hypothalamic arcuate nucleus: The ARC is located at the base of the hypothalamus and adjacent to the third ventricle. It receives extensive afferent inputs and processes efferent fibers projecting to other central regions, underlying its role in the regulation of autonomic functions, including GI functions[208-210]. Stimulation of neurons in the ARC has been demonstrated to increase colonic motility[211] and inhibit gastric phasic contractions[212] as well as gastric acid secretion via vagal pathways[213]. Its effect on the colon could be blocked by microinjection of CRF antagonists into the PVN, suggesting that an Arc-PVN neurocircuit might be involved in the regulation of GI functions via vagal efferent fibers.
The role of ARC in the modulation of acupuncture has been demonstrated; however, most research has been confined to the area of analgesia[214]. Applying the tracing technique, the afferent pathways of CV4 were explored. After the injection of Pseudorabies virus (PRV) into the acupoint of CV4, nuclei such as ARC and PVN showed PRV-immunoreactive neurons in normal rats[215]. Other results from Fos-CreER-based genetic mapping indicated that acupuncture at PC6 activates a series of regions, including the ARC and PVN[216]. These studies confirmed that ARC could receive the somatic fibers of acupuncture and modulate its activity. Following ligation of the ARC, the effect of acupuncture on the electrogastrography of rabbits was attenuated[217], providing anatomical and physiological evidence for the ability of acupuncture to regulate GI motility via the ARC. EA treatment increased the peptide levels of the melanocyte-stimulating hormone receptor (α-MSH) and the mRNA levels of its precursor proopiomelanocortin (POMC) in the ARC of hypothalamic neurons of rats with diet-induced obesity[218]. In addition, acupuncture also regulates the expression of neuropeptide Y, a cocaine and amphetamine-regulated transcript peptide[219,220], together with POMC neurons in the ARC, playing an important role in feeding and gastric emptying, which is potentially in a pathological state[221,222]; therefore, feeding-related peptides in the ARC might underlie another mechanism by which acupuncture regulates GI motility.
Although marked progress has been made in revealing the neural mechanism underlying acupuncture’s effects on the GI tract, there is still a lot of work to be done to unravel the complex interaction between the peripheral and CNSs, as well as the distinct CNS areas related to the integration of GI homeostatic functions. Acupuncture is a physical stimulus. When the needle penetrates the body, the mechanical force generated directly or indirectly acts on the acupoint area. The mechanical stimulation translates into neurochemical signals, inducing a somatic afferent signal; however, only when the intensity of the stimulus is over the threshold of the Aδ and/or C-fibers can acupuncture obviously modulate GI motility, whose receptor mechanism mainly relies on TRPV1. In the process of acupuncture, a regional-specific effect is exhibited in the modulation of GI motility, the neurological basis of which is now considered to be the somatoautonomic reflex, and the spinal segmental innervation hypothesis could be borrowed to explain it: Stimulation at homotopic acupoints (with the same spinal segments from the innervated visceral organs) decrease the GI pressure via the sympathetic pathways (mainly involving β1 and β2 receptors), while stimulation at heterotopic acupoints (with different spinal segments from the innervated visceral organs) induces GI facilitation via parasympathetic pathways (both cholinergic and NANC vagal efferents are involved). Other than that, the ENS, including enteric neurons and glia cells, also adds to the effect of acupuncture on the modulation of GI motility. The CNS provides its extrinsic neural inputs to control GI motility in a more widespread and integrated manner. The regulatory effect of acupuncture on GI movements also requires the participation of the CNS at multiple levels. In this review, only some nuclei of the brain stem, cerebellum, and hypothalamus are discussed, because of the complexity of the CNS and the blurred relationship between distinct nuclei in the CNS and acupuncture. Those connections among the nuclei constitute the anatomical and functional basis controlling GI motility. The acupuncture signals over GI functions were also analyzed and integrated through this connection. Applying modern and constantly updated technology is conducive to gaining a better understanding of the mechanism of acupuncture’s effects on GI motility.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Adams JD, Inchauspe AA S-Editor: Wang JL L-Editor: Webster JR E-Editor: Ma YJ
| 1. | Du YH, Huang W, Xiong J, Li B, Shi L, Fan XN. [Preliminary study on disease menu of acupuncture and moxibustion abroad]. Zhongguo Zhen Jiu. 2009;29:53-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Li B, Du YH, Xiong J, Xu YL, Li Y, Wang X, Li ZH, Liu JL, Zhang YY. [Indications of acupuncture outpatient based on clinical investigation]. Zhongguo Zhen Jiu. 2011;31:733-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Tack J, Carbone F, Rotondo A. Gastroparesis. Curr Opin Gastroenterol. 2015;31:499-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Billiauws L, Corcos O, Joly F. Dysmotility disorders: a nutritional approach. Curr Opin Clin Nutr Metab Care. 2014;17:483-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Keller J, Bassotti G, Clarke J, Dinning P, Fox M, Grover M, Hellström PM, Ke M, Layer P, Malagelada C, Parkman HP, Scott SM, Tack J, Simren M, Törnblom H, Camilleri M; International Working Group for Disorders of Gastrointestinal Motility and Function. Expert consensus document: Advances in the diagnosis and classification of gastric and intestinal motility disorders. Nat Rev Gastroenterol Hepatol. 2018;15:291-308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 169] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 6. | Rychter J, Espín F, Gallego D, Vergara P, Jiménez M, Clavé P. Colonic smooth muscle cells and colonic motility patterns as a target for irritable bowel syndrome therapy: mechanisms of action of otilonium bromide. Therap Adv Gastroenterol. 2014;7:156-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Tanaka Y, Kanazawa M, Palsson OS, Tilburg MAV, Gangarosa LM, Fukudo S, Drossman DA, Whitehead WE. Increased Postprandial Colonic Motility and Autonomic Nervous System Activity in Patients With Irritable Bowel Syndrome: A Prospective Study. J Neurogastroenterol Motil. 2018;24:87-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | He Y, Yang C, Wang P, Yang L, Wu H, Liu H, Qi M, Guo Z, Li J, Shi H, Wu X, Hu Z. Child compound Endothelium corneum attenuates gastrointestinal dysmotility through regulating the homeostasis of brain-gut-microbiota axis in functional dyspepsia rats. J Ethnopharmacol. 2019;240:111953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Lindberg G. Pseudo-obstruction, enteric dysmotility and irritable bowel syndrome. Best Pract Res Clin Gastroenterol. 2019;40-41:101635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Rao SS, Rattanakovit K, Patcharatrakul T. Diagnosis and management of chronic constipation in adults. Nat Rev Gastroenterol Hepatol. 2016;13:295-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 222] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 11. | Moshiree B, Potter M, Talley NJ. Epidemiology and Pathophysiology of Gastroparesis. Gastrointest Endosc Clin N Am. 2019;29:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 12. | Everhart JE, Ruhl CE. Burden of digestive diseases in the United States part I: overall and upper gastrointestinal diseases. Gastroenterology. 2009;136:376-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 418] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 13. | Madisch A, Vinson BR, Abdel-Aziz H, Kelber O, Nieber K, Kraft K, Storr M. Modulation of gastrointestinal motility beyond metoclopramide and domperidone: Pharmacological and clinical evidence for phytotherapy in functional gastrointestinal disorders. Wien Med Wochenschr. 2017;167:160-168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Kagitani F, Uchida S, Hotta H. Afferent nerve fibers and acupuncture. Auton Neurosci. 2010;157:2-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Kagitani F, Uchida S, Hotta H, Aikawa Y. Manual acupuncture needle stimulation of the rat hindlimb activates groups I, II, III and IV single afferent nerve fibers in the dorsal spinal roots. Jpn J Physiol. 2005;55:149-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Li YQ, Zhu B, Rong PJ, Ben H, Li YH. Neural mechanism of acupuncture-modulated gastric motility. World J Gastroenterol. 2007;13:709-716. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 79] [Cited by in RCA: 87] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Koizumi K, Sato A, Terui N. Role of somatic afferents in autonomic system control of the intestinal motility. Brain Res. 1980;182:85-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Noguchi E, Ohsawa H, Tanaka H, Ikeda H, Aikawa Y. Electro-acupuncture stimulation effects on duodenal motility in anesthetized rats. Jpn J Physiol. 2003;53:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Li WG, Xu TL. ASIC3 channels in multimodal sensory perception. ACS Chem Neurosci. 2011;2:26-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2577] [Cited by in RCA: 2691] [Article Influence: 107.6] [Reference Citation Analysis (0)] |
| 21. | Yu Z, Cao X, Xia Y, Ren B, Feng H, Wang Y, Jiang J, Xu B. Electroacupuncture Stimulation at CV12 Inhibits Gastric Motility via TRPV1 Receptor. Evid Based Complement Alternat Med. 2013;2013:294789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Pang T, Lu C, Wang K, Liang C, Yu Z, Zhu B, Xu B. Electroacupuncture at ST25 Inhibits Cisapride-Induced Gastric Motility in an Intensity-Dependent Manner. Evid Based Complement Alternat Med. 2016;2016:3457025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Yu Z, Zhang N, Lu CX, Pang TT, Wang KY, Jiang JF, Zhu B, Xu B. Electroacupuncture at ST25 inhibits jejunal motility: Role of sympathetic pathways and TRPV1. World J Gastroenterol. 2016;22:1834-1843. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Su YS, He W, Wang C, Shi H, Zhao YF, Xin JJ, Wang XY, Shang HY, Hu L, Jing XH, Zhu B. "Intensity-response" effects of electroacupuncture on gastric motility and its underlying peripheral neural mechanism. Evid Based Complement Alternat Med. 2013;2013:535742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Andrew D, Greenspan JD. Mechanical and heat sensitization of cutaneous nociceptors after peripheral inflammation in the rat. J Neurophysiol. 1999;82:2649-2656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 162] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 26. | McMullan S, Lumb BM. Spinal dorsal horn neuronal responses to myelinated versus unmyelinated heat nociceptors and their modulation by activation of the periaqueductal grey in the rat. J Physiol. 2006;576:547-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Cain DM, Khasabov SG, Simone DA. Response properties of mechanoreceptors and nociceptors in mouse glabrous skin: an in vivo study. J Neurophysiol. 2001;85:1561-1574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 159] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 28. | Su YS, Xin JJ, Yang ZK, He W, Shi H, Wang XY, Hu L, Jing XH, Zhu B. Effects of Different Local Moxibustion-Like Stimuli at Zusanli (ST36) and Zhongwan (CV12) on Gastric Motility and Its Underlying Receptor Mechanism. Evid Based Complement Alternat Med. 2015;2015:486963. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Su YS, Yang ZK, Xin JJ, He W, Shi H, Wang XY, Hu L, Jing XH, Zhu B. Somatosensory Nerve Fibers Mediated Generation of De-qi in Manual Acupuncture and Local Moxibustion-Like Stimuli-Modulated Gastric Motility in Rats. Evid Based Complement Alternat Med. 2014;2014:673239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 30. | Wu SY, Chen WH, Hsieh CL, Lin YW. Abundant expression and functional participation of TRPV1 at Zusanli acupoint (ST36) in mice: mechanosensitive TRPV1 as an "acupuncture-responding channel". BMC Complement Altern Med. 2014;14:96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 31. | Chen HC, Chen MY, Hsieh CL, Wu SY, Hsu HC, Lin YW. TRPV1 is a Responding Channel for Acupuncture Manipulation in Mice Peripheral and Central Nerve System. Cell Physiol Biochem. 2018;49:1813-1824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 32. | Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol. 2012;9:286-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 856] [Cited by in RCA: 1066] [Article Influence: 82.0] [Reference Citation Analysis (0)] |
| 33. | Furness JB, Callaghan BP, Rivera LR, Cho HJ. The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv Exp Med Biol. 2014;817:39-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 532] [Article Influence: 48.4] [Reference Citation Analysis (1)] |
| 34. | Sato A, Schmidt RF. Muscle and cutaneous afferents evoking sympathetic reflexes. Brain Res. 1966;2:399-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 35. | Sato A, Kaufman A, Koizumi K, Brooks CM. Afferent nerve groups and sympathetic reflex pathways. Brain Res. 1969;14:575-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Uchida S, Kagitani F, Sato-Suzuki I. Somatoautonomic reflexes in acupuncture therapy: A review. Auton Neurosci. 2017;203:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 37. | Takahashi T. Effect and mechanism of acupuncture on gastrointestinal diseases. Int Rev Neurobiol. 2013;111:273-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 38. | Iwa M, Tateiwa M, Sakita M, Fujimiya M, Takahashi T. Anatomical evidence of regional specific effects of acupuncture on gastric motor function in rats. Auton Neurosci. 2007;137:67-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 39. | Kametani H, Sato A, Sato Y, Simpson A. Neural mechanisms of reflex facilitation and inhibition of gastric motility to stimulation of various skin areas in rats. J Physiol. 1979;294:407-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 69] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 40. | Li YQ, Zhu B, Rong PJ, Ben H, Li YH. Effective regularity in modulation on gastric motility induced by different acupoint stimulation. World J Gastroenterol. 2006;12:7642-7648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 41. | Qin QG, Gao XY, Liu K, Yu XC, Li L, Wang HP, Zhu B. Acupuncture at heterotopic acupoints enhances jejunal motility in constipated and diarrheic rats. World J Gastroenterol. 2014;20:18271-18283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 42. | Li H, He T, Xu Q, Li Z, Liu Y, Li F, Yang BF, Liu CZ. Acupuncture and regulation of gastrointestinal function. World J Gastroenterol. 2015;21:8304-8313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 114] [Cited by in RCA: 117] [Article Influence: 11.7] [Reference Citation Analysis (3)] |
| 43. | Hu X, Yuan M, Yin Y, Wang Y, Li Y, Zhang N, Sun X, Yu Z, Xu B. Electroacupuncture at LI11 promotes jejunal motility via the parasympathetic pathway. BMC Complement Altern Med. 2017;17:329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 44. | Lu M, Chen C, Li W, Yu Z, Xu B. EA at PC6 Promotes Gastric Motility: Role of Brainstem Vagovagal Neurocircuits. Evid Based Complement Alternat Med. 2019;2019:7457485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 45. | Lu MJ, Yu Z, He Y, Yin Y, Xu B. Electroacupuncture at ST36 modulates gastric motility via vagovagal and sympathetic reflexes in rats. World J Gastroenterol. 2019;25:2315-2326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (1)] |
| 46. | Tada H, Fujita M, Harris M, Tatewaki M, Nakagawa K, Yamamura T, Pappas TN, Takahashi T. Neural mechanism of acupuncture-induced gastric relaxations in rats. Dig Dis Sci. 2003;48:59-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 47. | Kaan SK, Mei QB, Cho CH. A mechanistic study of beta-adrenoceptor antagonists on ethanol-induced gastric damage. Eur J Pharmacol. 1996;317:115-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (1)] |
| 48. | Yu O, Ouyang A. Distribution of beta-adrenoceptor subtypes in gastrointestinal tract of nondiabetic and diabetic BB rats. A longitudinal study. Dig Dis Sci. 1997;42:1146-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 49. | Stengel PW, Gomeza J, Wess J, Cohen ML. M(2) and M(4) receptor knockout mice: muscarinic receptor function in cardiac and smooth muscle in vitro. J Pharmacol Exp Ther. 2000;292:877-885. [PubMed] |
| 50. | Matsui M, Motomura D, Karasawa H, Fujikawa T, Jiang J, Komiya Y, Takahashi S, Taketo MM. Multiple functional defects in peripheral autonomic organs in mice lacking muscarinic acetylcholine receptor gene for the M3 subtype. Proc Natl Acad Sci USA. 2000;97:9579-9584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 331] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 51. | Gao LL, Guo Y, Sha T, Liu YY, Tang JB, Yuan F, Zhou T, Hong SH, Ming D. Differential effects of variable frequencies of manual acupuncture at ST36 in rats with atropine-induced inhibition of gastric motility. Acupunct Med. 2016;34:33-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 52. | Li H, Wang YP. Effect of auricular acupuncture on gastrointestinal motility and its relationship with vagal activity. Acupunct Med. 2013;31:57-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 53. | Okada M, Itoh K, Kitakoji H, Imai K. Mechanism of Electroacupuncture on Postoperative Ileus Induced by Surgical Stress in Rats. Med Acupunct. 2019;31:109-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 54. | Iwa M, Sakita M. Effects of acupuncture and moxibustion on intestinal motility in mice. Am J Chin Med. 1994;22:119-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 55. | Gao X, Zhao Y, Su Y, Liu K, Yu X, Cui C, Yang Z, Shi H, Jing X, Zhu B. β1/2 or M2/3 Receptors Are Required for Different Gastrointestinal Motility Responses Induced by Acupuncture at Heterotopic or Homotopic Acupoints. PLoS One. 2016;11:e0168200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 56. | Gao X, Qin Q, Yu X, Liu K, Li L, Qiao H, Zhu B. Acupuncture at heterotopic acupoints facilitates distal colonic motility via activating M3 receptors and somatic afferent C-fibers in normal, constipated, or diarrhoeic rats. Neurogastroenterol Motil. 2015;27:1817-1830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 57. | Yuan M, Li Y, Wang Y, Zhang N, Hu X, Yin Y, Zhu B, Yu Z, Xu B. Electroacupuncture at ST37 Enhances Jejunal Motility via Excitation of the Parasympathetic System in Rats and Mice. Evid Based Complement Alternat Med. 2016;2016:3840230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 58. | Tatewaki M, Harris M, Uemura K, Ueno T, Hoshino E, Shiotani A, Pappas TN, Takahashi T. Dual effects of acupuncture on gastric motility in conscious rats. Am J Physiol Regul Integr Comp Physiol. 2003;285:R862-R872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 90] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 59. | Yu Z, Xia YB, Lu MX, Lin J, Yu WJ, Xu B. [Influence of electroacupuncture stimulation of "tianshu" (ST 25), "quchi" (LI 11) and "shangjuxu" (ST 37) and their pairs on gastric motility in the rat]. Zhen Ci Yan Jiu. 2013;38:40-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 60. | Browning KN, Travagli RA. Plasticity of vagal brainstem circuits in the control of gastrointestinal function. Auton Neurosci. 2011;161:6-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 61. | Uchida S, Kagitani F, Hotta H. Neural mechanisms of reflex inhibition of heart rate elicited by acupuncture-like stimulation in anesthetized rats. Auton Neurosci. 2010;157:18-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 62. | Uchida S, Shimura M, Ohsawa H, Suzuki A. Neural mechanism of bradycardiac responses elicited by acupuncture-like stimulation to a hind limb in anesthetized rats. J Physiol Sci. 2007;57:377-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 63. | Uchida S, Kagitani F, Hotta H. Mechanism of the reflex inhibition of heart rate elicited by acupuncture-like stimulation in anesthetized rats. Auton Neurosci. 2008;143:12-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 64. | Kimura A, Ohsawa H, Sato A, Sato Y. Somatocardiovascular reflexes in anesthetized rats with the central nervous system intact or acutely spinalized at the cervical level. Neurosci Res. 1995;22:297-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 69] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 65. | Chen JZ, Lu MJ, Han X, Xu B, Yu Z. [Influence of electroacupuncture of "Zhongwan" (CV12) and "Zusanli" (ST36) in different combinations of stimulating strength and sequence on intragastric pressure in normal rats]. Zhen Ci Yan Jiu. 2019;44:125-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 66. | Jia BA, Cheng CY, Lin YW, Li TC, Liu HJ, Hsieh CL. The 2 Hz and 15 Hz electroacupuncture induced reverse effect on autonomic function in healthy adult using a heart rate variability analysis. J Tradit Complement Med. 2011;1:51-56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 67. | Kimura K, Ishida K, Takahashi N, Toge Y, Tajima F. Effects of acupuncture at the ST-36 point on muscle sympathetic nerve activity and blood pressure in normal adults. Auton Neurosci. 2017;208:131-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 68. | Kim JB, Uk NN. Sympathetic Nervous Activity is Involved in the Anti-Inflammatory Effects by Electroacupuncture Stimulation. Korean J Acupunct. 2019;36:162-170. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 69. | Tracey KJ. The inflammatory reflex. Nature. 2002;420:853-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2390] [Cited by in RCA: 2487] [Article Influence: 108.1] [Reference Citation Analysis (0)] |
| 70. | Kim HY, Hahm DH, Pyun KH, Lee HJ, Nam TC, Shim I. Effect of traditional acupuncture on proximal colonic motility in conscious dogs. J Vet Med Sci. 2006;68:603-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 71. | Yuxue Z, Changxiang C, Qingguang Q, Hui B, Junhong G, Xiaochun Y, Bing Z. Effect of manual acupuncture on bowel motility in normal kunming mouse. J Tradit Chin Med. 2015;35:227-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 72. | Liang C, Wang KY, Gong MR, Li Q, Yu Z, Xu B. Electro-acupuncture at ST37 and ST25 induce different effects on colonic motility via the enteric nervous system by affecting excitatory and inhibitory neurons. Neurogastroenterol Motil. 2018;30:e13318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 73. | Iwa M, Matsushima M, Nakade Y, Pappas TN, Fujimiya M, Takahashi T. Electroacupuncture at ST-36 accelerates colonic motility and transit in freely moving conscious rats. Am J Physiol Gastrointest Liver Physiol. 2006;290:G285-G292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 99] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 74. | Luo D, Liu S, Xie X, Hou X. Electroacupuncture at acupoint ST-36 promotes contractility of distal colon via a cholinergic pathway in conscious rats. Dig Dis Sci. 2008;53:689-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 75. | Corman ML. Colon and Rectal Surgery. Philadelphia: Lippincott Williams & Wilkins, 2005: 1743. |
| 76. | Willard FH, Robert C, Ward RJH, John A, Jerome editors. Robert C, Ward RJH, John A, Jerome editors. Foundations for osteopathic medicine. Philadelphia: Lippincott Williams & Wilkins, 2002: 105-111. |
| 77. | Dorofeeva AA, Panteleev SS, Makarov FN. Involvement of the sacral parasympathetic nucleus in the innervation of the descending colon and rectum in cats. Neurosci Behav Physiol. 2009;39:207-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 78. | Altschuler SM, Escardo J, Lynn RB, Miselis RR. The central organization of the vagus nerve innervating the colon of the rat. Gastroenterology. 1993;104:502-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 129] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 79. | Tong WD, Ridolfi TJ, Kosinski L, Ludwig K, Takahashi T. Effects of autonomic nerve stimulation on colorectal motility in rats. Neurogastroenterol Motil. 2010;22:688-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 80. | Berthoud HR, Carlson NR, Powley TL. Topography of efferent vagal innervation of the rat gastrointestinal tract. Am J Physiol. 1991;260:R200–R207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 159] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 81. | Kwak JM, Babygirija R, Gribovskaja-Rupp I, Takahashi T, Yamato S, Ludwig K. Regional difference in colonic motility response to electrical field stimulation in Guinea pig. J Neurogastroenterol Motil. 2013;19:192-203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 82. | Gribovskaja-Rupp I, Babygirija R, Takahashi T, Ludwig K. Autonomic nerve regulation of colonic peristalsis in Guinea pigs. J Neurogastroenterol Motil. 2014;20:185-196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 83. | Takahashi T, Owyang C. Regional differences in the nitrergic innervation between the proximal and the distal colon in rats. Gastroenterology. 1998;115:1504-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 84. | Hasler WL, Kurosawa S, Chung OY. Regional cholinergic differences between distal and proximal colonic myenteric plexus. Am J Physiol. 1990;258:G404-G410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 85. | Li Z, Hao MM, Van den Haute C, Baekelandt V, Boesmans W, Vanden Berghe P. Regional complexity in enteric neuron wiring reflects diversity of motility patterns in the mouse large intestine. Elife. 2019;8:e42914. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 86. | Espinosa-Medina I, Saha O, Boismoreau F, Chettouh Z, Rossi F, Richardson WD, Brunet JF. The sacral autonomic outflow is sympathetic. Science. 2016;354:893-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 102] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 87. | Horn JP. The sacral autonomic outflow is parasympathetic: Langley got it right. Clin Auton Res. 2018;28:181-185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 88. | Espinosa-Medina I, Saha O, Boismoreau F, Brunet JF. The "sacral parasympathetic": ontogeny and anatomy of a myth. Clin Auton Res. 2018;28:13-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 89. | Nezami BG, Srinivasan S. Enteric nervous system in the small intestine: pathophysiology and clinical implications. Curr Gastroenterol Rep. 2010;12:358-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 90. | Wedel T, Roblick UJ, Ott V, Eggers R, Schiedeck TH, Krammer HJ, Bruch HP. Oligoneuronal hypoganglionosis in patients with idiopathic slow-transit constipation. Dis Colon Rectum. 2002;45:54-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 73] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 91. | Liang C, Wang KY, Yu Z, Xu B. Development of a novel mouse constipation model. World J Gastroenterol. 2016;22:2799-2810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 92. | Liang C, Wang K, Xu B, Yu Z. Electroacupuncture at acupoint ST 37(Shangjuxu) improves function of the enteric nervous system in a novel mouse constipation model. BMC Complement Altern Med. 2016;16:392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 93. | Yarandi SS, Srinivasan S. Diabetic gastrointestinal motility disorders and the role of enteric nervous system: current status and future directions. Neurogastroenterol Motil. 2014;26:611-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 144] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 94. | Du F, Wang L, Qian W, Liu S. Loss of enteric neurons accompanied by decreased expression of GDNF and PI3K/Akt pathway in diabetic rats. Neurogastroenterol Motil. 2009;21:1229-e114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 95. | Takahashi T. Pathophysiological significance of neuronal nitric oxide synthase in the gastrointestinal tract. J Gastroenterol. 2003;38:421-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 199] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 96. | Yoneda S, Kadowaki M, Kuramoto H, Fukui H, Takaki M. Enhanced colonic peristalsis by impairment of nitrergic enteric neurons in spontaneously diabetic rats. Auton Neurosci. 2001;92:65-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 97. | Du F, Liu S. Electroacupuncture with high frequency at acupoint ST-36 induces regeneration of lost enteric neurons in diabetic rats via GDNF and PI3K/AKT signal pathway. Am J Physiol Regul Integr Comp Physiol. 2015;309:R109-R118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 98. | Kestler C, Neuhuber WL, Raab M. Distribution of P2X(3) receptor immunoreactivity in myenteric ganglia of the mouse esophagus. Histochem Cell Biol. 2009;131:13-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 99. | Poole DP, Castelucci P, Robbins HL, Chiocchetti R, Furness JB. The distribution of P2X3 purine receptor subunits in the guinea pig enteric nervous system. Auton Neurosci. 2002;101:39-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 87] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 100. | Bian X, Ren J, DeVries M, Schnegelsberg B, Cockayne DA, Ford AP, Galligan JJ. Peristalsis is impaired in the small intestine of mice lacking the P2X3 subunit. J Physiol. 2003;551:309-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 85] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 101. | Devries MP, Vessalo M, Galligan JJ. Deletion of P2X2 and P2X3 receptor subunits does not alter motility of the mouse colon. Front Neurosci. 2010;4:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 102. | Kunze WA, Furness JB. The enteric nervous system and regulation of intestinal motility. Annu Rev Physiol. 1999;61:117-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 265] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 103. | Shinoda M, Feng B, Gebhart GF. Peripheral and central P2X receptor contributions to colon mechanosensitivity and hypersensitivity in the mouse. Gastroenterology. 2009;137:2096-2104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 104. | Wu IXY, Wong CHL, Ho RST, Cheung WKW, Ford AC, Wu JCY, Mak ADP, Cramer H, Chung VCH. Acupuncture and related therapies for treating irritable bowel syndrome: overview of systematic reviews and network meta-analysis. Therap Adv Gastroenterol. 2019;12:1756284818820438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 105. | Galligan JJ. Enteric P2X receptors as potential targets for drug treatment of the irritable bowel syndrome. Br J Pharmacol. 2004;141:1294-1302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 106. | Weng ZJ, Wu LY, Zhou CL, Dou CZ, Shi Y, Liu HR, Wu HG. Effect of electroacupuncture on P2X3 receptor regulation in the peripheral and central nervous systems of rats with visceral pain caused by irritable bowel syndrome. Purinergic Signal. 2015;11:321-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 107. | Arbogast LA, Ben-Jonathan N. Tyrosine hydroxylase in the stalk-median eminence and posterior pituitary is inactivated only during the plateau phase of the preovulatory prolactin surge. Endocrinology. 1989;125:667-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 139] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 108. | Bush TG, Savidge TC, Freeman TC, Cox HJ, Campbell EA, Mucke L, Johnson MH, Sofroniew MV. Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell. 1998;93:189-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 460] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 109. | Rao M, Rastelli D, Dong L, Chiu S, Setlik W, Gershon MD, Corfas G. Enteric Glia Regulate Gastrointestinal Motility but Are Not Required for Maintenance of the Epithelium in Mice. Gastroenterology. 2017;153:1068-1081.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 138] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 110. | Grubišić V, Verkhratsky A, Zorec R, Parpura V. Enteric glia regulate gut motility in health and disease. Brain Res Bull. 2018;136:109-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 111. | Hierholzer C, Kalff JC, Chakraborty A, Watkins SC, Billiar TR, Bauer AJ, Tweardy DJ. Impaired gut contractility following hemorrhagic shock is accompaied by IL-6 and G-CSF production and neutrophil infiltration. Dig Dis Sci. 2001;46:230-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 112. | Du MH, Luo HM, Tian YJ, Zhang LJ, Zhao ZK, Lv Y, Xu RJ, Hu S. Electroacupuncture ST36 prevents postoperative intra-abdominal adhesions formation. J Surg Res. 2015;195:89-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 113. | Du MH, Luo HM, Hu S, Lv Y, Lin ZL, Ma L. Electroacupuncture improves gut barrier dysfunction in prolonged hemorrhagic shock rats through vagus anti-inflammatory mechanism. World J Gastroenterol. 2013;19:5988-5999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 114. | Hu S, Zhao ZK, Liu R, Wang HB, Gu CY, Luo HM, Wang H, Du MH, Lv Y, Shi X. Electroacupuncture activates enteric glial cells and protects the gut barrier in hemorrhaged rats. World J Gastroenterol. 2015;21:1468-1478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 115. | Hu S, Du MH, Luo HM, Wang H, Lv Y, Ma L, Lin ZL, Shi X, Gaischek I, Wang L, Litscher G. Electroacupuncture at Zusanli (ST36) Prevents Intestinal Barrier and Remote Organ Dysfunction following Gut Ischemia through Activating the Cholinergic Anti-Inflammatory-Dependent Mechanism. Evid Based Complement Alternat Med. 2013;2013:592127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 116. | Gulbransen BD, Sharkey KA. Novel functional roles for enteric glia in the gastrointestinal tract. Nat Rev Gastroenterol Hepatol. 2012;9:625-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 286] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 117. | Delvalle NM, Fried DE, Rivera-Lopez G, Gaudette L, Gulbransen BD. Cholinergic activation of enteric glia is a physiological mechanism that contributes to the regulation of gastrointestinal motility. Am J Physiol Gastrointest Liver Physiol. 2018;315:G473-G483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (1)] |
| 118. | Lee CH, Jung HS, Lee TY, Lee SR, Yuk SW, Lee KG, Lee BH. Studies of the central neural pathways to the stomach and Zusanli (ST36). Am J Chin Med. 2001;29:211-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 119. | Wang X, Shi H, Shang H, He W, Chen S, Litscher G, Gaischek I, Jing X. Effect of Electroacupuncture at ST36 on Gastric-Related Neurons in Spinal Dorsal Horn and Nucleus Tractus Solitarius. Evid Based Complement Alternat Med. 2013;2013:912898. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 120. | Babic T, Browning KN, Travagli RA. Differential organization of excitatory and inhibitory synapses within the rat dorsal vagal complex. Am J Physiol Gastrointest Liver Physiol. 2011;300:G21-G32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 121. | Browning KN, Travagli RA. Short-term receptor trafficking in the dorsal vagal complex: an overview. Auton Neurosci. 2006;126-127:2-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 122. | Travagli RA, Anselmi L. Vagal neurocircuitry and its influence on gastric motility. Nat Rev Gastroenterol Hepatol. 2016;13:389-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 205] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 123. | Wang JJ, Ming Q, Liu XD, Huang YX, Chen LW, Qiu JY, Duan L, Cao R, Rao ZR. Electro-acupuncture of Foot YangMing regulates gastric activity possibly through mediation of the dorsal vagal complex. Am J Chin Med. 2007;35:455-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 124. | Wang H, Wang CY, Zhang JS, Sun L, Sun JP, Tian QH, Jin XL, Yin L. Acupuncture therapy for experimental stomach ulcer and c-Fos expression in rats. World J Gastroenterol. 2005;11:5517-5520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 125. | Liu JH, Li J, Yan J, Chang XR, Cui RF, He JF, Hu JM. Expression of c-fos in the nucleus of the solitary tract following electroacupuncture at facial acupoints and gastric distension in rats. Neurosci Lett. 2004;366:215-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 126. | Wang H, Shen GM, Liu WJ, Huang S, Zhang MT. The Neural Mechanism by Which the Dorsal Vagal Complex Mediates the Regulation of the Gastric Motility by Weishu (RN12) and Zhongwan (BL21) Stimulation. Evid Based Complement Alternat Med. 2013;2013:291764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 127. | Lim HD, Kim MH, Lee CY, Namgung U. Anti-Inflammatory Effects of Acupuncture Stimulation via the Vagus Nerve. PLoS One. 2016;11:e0151882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 128. | Wang JJ, Liu XD, Qin M, Chen LW, Qiu JY, Duan L, Cao R, Rao ZR, Huang YX. Electro-acupuncture of Tsusanli and Shangchuhsu regulates gastric activity possibly through mediation of the vagus-solotary complex. Hepatogastroenterology. 2007;54:1862-1867. [PubMed] |
| 129. | Furness JB. Integrated Neural and Endocrine Control of Gastrointestinal Function. Adv Exp Med Biol. 2016;891:159-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 130. | Aziz Q, Thompson DG. Brain-gut axis in health and disease. Gastroenterology. 1998;114:559-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 204] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 131. | Shen GM, Zhou MQ, Xu GS, Xu Y, Yin G. Role of vasoactive intestinal peptide and nitric oxide in the modulation of electroacupucture on gastric motility in stressed rats. World J Gastroenterol. 2006;12:6156-6160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 132. | Jang JH, Lee DJ, Bae CH, Ha KT, Kwon S, Park HJ, Hahm DH, Lee H, Kim S. Changes in small intestinal motility and related hormones by acupuncture stimulation at Zusanli (ST 36) in mice. Chin J Integr Med. 2017;23:215-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 133. | Liu JH, Yan J, Yi SX, Chang XR, Lin YP, Hu JM. Effects of electroacupuncture on gastric myoelectric activity and substance P in the dorsal vagal complex of rats. Neurosci Lett. 2004;356:99-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 134. | Allen NJ, Lyons DA. Glia as architects of central nervous system formation and function. Science. 2018;362:181-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 573] [Cited by in RCA: 592] [Article Influence: 84.6] [Reference Citation Analysis (0)] |
| 135. | Choi DC, Lee JY, Lim EJ, Baik HH, Oh TH, Yune TY. Inhibition of ROS-induced p38MAPK and ERK activation in microglia by acupuncture relieves neuropathic pain after spinal cord injury in rats. Exp Neurol. 2012;236:268-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 136. | Zhang F, Wu L, Zhao J, Lv T, Hu Z, Weng Z, Wang S, Wu H, Liu H. Neurobiological Mechanism of Acupuncture for Relieving Visceral Pain of Gastrointestinal Origin. Gastroenterol Res Pract. 2017;2017:5687496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 137. | Shan S, Qi-Liang MY, Hong C, Tingting L, Mei H, Haili P, Yan-Qing W, Zhi-Qi Z, Yu-Qiu Z. Is functional state of spinal microglia involved in the anti-allodynic and anti-hyperalgesic effects of electroacupuncture in rat model of monoarthritis? Neurobiol Dis. 2007;26:558-568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 79] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 138. | Wang SZ, Liu XD, Huang YX, Ma QJ, Wang JJ. Disruption of glial function regulates the effects of electro-acupuncture at Tsusanli on gastric activity in rats. Am J Chin Med. 2009;37:647-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 139. | He JF, Yan J, Li JS, Liu JH, Wang C, Chang XR, Qu YT. Neuron discharge and c-Fos expression in the nucleus of the solitary tract following electroacupuncture at acupoints of the Yangming Stomach Meridian of Foot. J Acupunct Meridian Stud. 2013;6:82-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 140. | Fang JF, Du JY, Shao XM, Fang JQ, Liu Z. Effect of Electroacupuncture on the NTS is modulated primarily by acupuncture point selection and stimulation frequency in normal rats. BMC Complement Altern Med. 2017;17:182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 141. | He J, Yan J, Chang X, Liu J, Li J, Yi S, Lin Y. Neurons in the NTS of rat response to gastric distention stimulation and acupuncture at body surface points. Am J Chin Med. 2006;34:427-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 142. | Sun HZ, Zhao SZ, Cui XY, Ai HB. Hindbrain Effects of L-Glutamate on Gastric Motility in Rats. Gastroenterology Res. 2009;2:43-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 143. | Krowicki ZK, Nathan NA, Hornby PJ. Opposing effects of vasoactive intestinal polypeptide on gastric motor function in the dorsal vagal complex and the nucleus raphe obscurus of the rat. J Pharmacol Exp Ther. 1997;282:14-22. [PubMed] |
| 144. | Rogers RC, Hermann GE, Travagli RA. Brainstem pathways responsible for oesophageal control of gastric motility and tone in the rat. J Physiol. 1999;514:369-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 113] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 145. | Cruz MT, Murphy EC, Sahibzada N, Verbalis JG, Gillis RA. A reevaluation of the effects of stimulation of the dorsal motor nucleus of the vagus on gastric motility in the rat. Am J Physiol Regul Integr Comp Physiol. 2007;292:R291-R307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 146. | Sivarao DV, Krowicki ZK, Hornby PJ. Role of GABAA receptors in rat hindbrain nuclei controlling gastric motor function. Neurogastroenterol Motil. 1998;10:305-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 91] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 147. | Washabau RJ, Fudge M, Price WJ, Barone FC. GABA receptors in the dorsal motor nucleus of the vagus influence feline lower esophageal sphincter and gastric function. Brain Res Bull. 1995;38:587-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 148. | Zanzinger J, Czachurski J, Seller H. Inhibition of basal and reflex-mediated sympathetic activity in the RVLM by nitric oxide. Am J Physiol. 1995;268:R958-R962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 42] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 149. | Oshima N, McMullan S, Goodchild AK, Pilowsky PM. A monosynaptic connection between baroinhibited neurons in the RVLM and IML in Sprague-Dawley rats. Brain Res. 2006;1089:153-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 150. | Takahashi T. Mechanism of acupuncture on neuromodulation in the gut--a review. Neuromodulation. 2011;14:8-12; discussion 12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 151. | Shafton AD, Ryan A, McGrath B, Badoer E. Volume expansion does not activate neuronal projections from the NTS or depressor VLM to the RVLM. Am J Physiol. 1999;277:R39-R46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 152. | Ma SM, Yan CQ, Wu RH, Shen ZW, Fan H, Li JL, Liu CZ. [Feasibility of lower spinal cord functional MRI in neural conduction study following electrical stimulation at Tianshu (ST25) acupoint and Zusanli (ST36) acupoint]. Shi Zhen Guo Yi Guo Yao. 2019;30:217-219. [DOI] [Full Text] |
| 153. | Li B, Zhu JN, Wang JJ. Histaminergic afferent system in the cerebellum: structure and function. Cerebellum Ataxias. 2014;1:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 154. | Zhang XY, Wang JJ, Zhu JN. Cerebellar fastigial nucleus: from anatomic construction to physiological functions. Cerebellum Ataxias. 2016;3:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 155. | Zhu JN, Zhang YP, Song YN, Wang JJ. Cerebellar interpositus nuclear and gastric vagal afferent inputs reach and converge onto glycemia-sensitive neurons of the ventromedial hypothalamic nucleus in rats. Neurosci Res. 2004;48:405-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 156. | Martner J. Influences on colonic and small intestinal motility by the cerebellar fastigial nucleus. Acta Physiol Scand. 1975;94:82-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 157. | Lisander B, Martner J. Effects on gastric motility from the cerebellar fastigial nucleus. Acta Physiol Scand. 1975;94:368-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 158. | Martner J. Cerebellar influences on autonomic mechanisms. An experimental study in the cat with special reference to the fastigial nucleus. Acta Physiol Scand Suppl. 1975;425:1-42. [PubMed] |
| 159. | Liang C, Wang Y, Xu B, Yu Z. [Effect of acupuncture at different acupoints on electric activities of rat cerebellar fastigial nuclear]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2015;35:476-480. [PubMed] |
| 160. | Haines DE, Dietrichs E, Mihailoff GA, McDonald EF. The cerebellar-hypothalamic axis: basic circuits and clinical observations. Int Rev Neurobiol. 1997;41:83-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 105] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 161. | Zhu JN, Yung WH, Kwok-Chong Chow B, Chan YS, Wang JJ. The cerebellar-hypothalamic circuits: potential pathways underlying cerebellar involvement in somatic-visceral integration. Brain Res Rev. 2006;52:93-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 151] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 162. | Li Y, Zhang X, Chen L, Yang B, Sui R. Cerebellar fastigial nucleus is involved in post-stroke depression through direct cerebellar-hypothalamic GABAergic and glutamatergic projections. Exp Ther Med. 2019;18:2885-2892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 163. | Zhu JZ, Fei SJ, Zhang JF, Zhu SP, Liu ZB, Li TT, Qiao X. Lateral hypothalamic area mediated the aggravated effect of microinjection of Baclofen into cerebellar fastigial nucleus on stress gastric mucosal damage in rats. Neurosci Lett. 2012;509:125-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 164. | Zhang YP, Ma C, Wen YQ, Wang JJ. Convergence of gastric vagal and cerebellar fastigial nuclear inputs on glycemia-sensitive neurons of lateral hypothalamic area in the rat. Neurosci Res. 2003;45:9-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 165. | Gao L, Fei S, Qiao W, Zhang J, Xing H, Du D. Protective effect of chemical stimulation of cerebellar fastigial nucleus on stress gastric mucosal injury in rats. Life Sci. 2011;88:871-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 166. | Zhu JZ, Fei SJ, Zhang JF, Zhu SP, Liu ZB, Li TT, Qiao X. Muscimol microinjection into cerebellar fastigial nucleus exacerbates stress-induced gastric mucosal damage in rats. Acta Pharmacol Sin. 2013;34:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 167. | Qiao X, Yang J, Fei SJ, Zhu JZ, Zhu SP, Liu ZB, Li TT, Zhang JF. Protective effect of histamine microinjected into cerebellar fastigial nucleus on stress gastric mucosal damage in rats. Brain Res. 2015;1629:351-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 168. | Zhen LL, Miao B, Chen YY, Su Z, Xu MQ, Fei S, Zhang J. Protective effect and mechanism of injection of glutamate into cerebellum fastigial nucleus on chronic visceral hypersensitivity in rats. Life Sci. 2018;203:184-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 169. | Gao L, Zhao H, Zhu T, Liu Y, Hu L, Liu Z, Huang H, Chen F, Deng Z, Chu D, Du D. The Regulatory Effects of Lateral Hypothalamus Area GABAB Receptor on Gastric Ischemia-Reperfusion Injury in Rats. Front Physiol. 2017;8:722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 170. | Yu Z, Wang Y, Liang C, Xu B. [Effects of acupuncture at "Zusanli" (ST 36) on sensitive neurons of gastric distention in LHA-FN circuit in rats]. Zhongguo Zhen Jiu. 2016;36:851-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 171. | Gagliuso AH, Chapman EK, Martinelli GP, Holstein GR. Vestibular neurons with direct projections to the solitary nucleus in the rat. J Neurophysiol. 2019;122:512-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 172. | Ruggiero DA, Mtui EP, Otake K, Anwar M. Vestibular afferents to the dorsal vagal complex: substrate for vestibular-autonomic interactions in the rat. Brain Res. 1996;743:294-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 173. | Balaban CD, Beryozkin G. Vestibular nucleus projections to nucleus tractus solitarius and the dorsal motor nucleus of the vagus nerve: potential substrates for vestibulo-autonomic interactions. Exp Brain Res. 1994;98:200-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 101] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 174. | Porter JD, Balaban CD. Connections between the vestibular nuclei and brain stem regions that mediate autonomic function in the rat. J Vestib Res. 1997;7:63-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 175. | Panteleev SS, Aleksandrov VG, Bagaev VA, Busygina II, Liubashina OA. [The neurons of the medial vestibular nucleus initiating the relaxation of the rat's stomach wall]. Aviakosm Ekolog Med. 1999;33:15-19. [PubMed] |
| 176. | Liang C, Wang Y, Xu B, Yu Z. Effect of acupuncture at three different acupoints on electrical activity of gastric distention-affected neurons in rat medial vestibular nucleus. J Tradit Chin Med. 2018;38:125-131. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 177. | Ferguson AV, Latchford KJ, Samson WK. The paraventricular nucleus of the hypothalamus - a potential target for integrative treatment of autonomic dysfunction. Expert Opin Ther Targets. 2008;12:717-727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 268] [Cited by in RCA: 236] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 178. | Rogers RC, Hermann GE. Oxytocin, oxytocin antagonist, TRH, and hypothalamic paraventricular nucleus stimulation effects on gastric motility. Peptides. 1987;8:505-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 134] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 179. | Jiang Y, Coleman FH, Kopenhaver Doheny K, Travagli RA. Stress Adaptation Upregulates Oxytocin within Hypothalamo-Vagal Neurocircuits. Neuroscience. 2018;390:198-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 180. | Taché Y, Bonaz B. Corticotropin-releasing factor receptors and stress-related alterations of gut motor function. J Clin Invest. 2007;117:33–40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 266] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 181. | Lewis MW, Hermann GE, Rogers RC, Travagli RA. In vitro and in vivo analysis of the effects of corticotropin releasing factor on rat dorsal vagal complex. J Physiol. 2002;543:135–146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 182. | Wang SJ, Zhang JJ, Yang HY, Wang F, Li ST. Acupoint specificity on acupuncture regulation of hypothalamic- pituitary-adrenal cortex axis function. BMC Complement Altern Med. 2015;15:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 183. | Rinaman L. Postnatal development of hypothalamic inputs to the dorsal vagal complex in rats. Physiol Behav. 2003;79:65-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 184. | Flanagan LM, Olson BR, Sved AF, Verbalis JG, Stricker EM. Gastric motility in conscious rats given oxytocin and an oxytocin antagonist centrally. Brain Res. 1992;578:256-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 185. | Llewellyn-Smith IJ, Kellett DO, Jordan D, Browning KN, Travagli RA. Oxytocin-immunoreactive innervation of identified neurons in the rat dorsal vagal complex. Neurogastroenterol Motil. 2012;24:e136-e146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 186. | Holmes GM, Browning KN, Babic T, Fortna SR, Coleman FH, Travagli RA. Vagal afferent fibres determine the oxytocin-induced modulation of gastric tone. J Physiol. 2013;591:3081-3100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 187. | Yong CY, Chen S, Chen H, Chu X, Zhang C, Tan C, Ye L, Li JS. Effects of needling acupoints at different nerve segments on oxytocin neurons in rat’s hypothalamic paraventricular nucleus and intragastric pressure. J Acupunct Tuina Sci. 2019;17:297-304. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 188. | Yong CY, Chen S, Chen H, Chu X, Zhang C, Tan C, Ye L, Li JS. Central neuromechanisms underlying control of intragastric pressure through acupuncture at Zusanli (ST36) in rats: the upper cervical cord is the key link between the ascending and descending pathways. Neural Regen Res. 2016;11:971-976. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 189. | Liu W, Zhang C, Zhang Y, Ning J, Li J. [Effects of electroacupuncture at acupoints with different nerve segments on gastric emptying in OT gene knockout mice]. Zhongguo Zhen Jiu. 2017;37:735-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 190. | Wang H, Liu WJ, Shen GM, Zhang MT, Huang S, He Y. Neural mechanism of gastric motility regulation by electroacupuncture at RN12 and BL21: A paraventricular hypothalamic nucleus-dorsal vagal complex-vagus nerve-gastric channel pathway. World J Gastroenterol. 2015;21:13480-13489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 191. | Jansen AS, Hoffman JL, Loewy AD. CNS sites involved in sympathetic and parasympathetic control of the pancreas: a viral tracing study. Brain Res. 1997;766:29-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 130] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 192. | Jiang C, Fogel R, Zhang X. Lateral hypothalamus modulates gut-sensitive neurons in the dorsal vagal complex. Brain Res. 2003;980:31-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 193. | Berk ML. Projections of the lateral hypothalamus and bed nucleus of the stria terminalis to the dorsal vagal complex in the pigeon. J Comp Neurol. 1987;260:140-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 55] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 194. | Gong Y, Liu Y, Guo Y, Su M, Zhong Y, Xu L, Guo F, Gao S. Ghrelin projection from the lateral hypothalamus area to the dorsal vagal complex and its regulation of gastric motility in cisplatin-treated rats. Neuropeptides. 2017;66:69-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 195. | Gong Y, Liu Y, Liu F, Wang S, Jin H, Guo F, Xu L. Ghrelin fibers from lateral hypothalamus project to nucleus tractus solitaries and are involved in gastric motility regulation in cisplatin-treated rats. Brain Res. 2017;1659:29-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 196. | Liu Y, Yan M, Guo Y, Niu Z, Sun R, Jin H, Gong Y. Ghrelin and electrical stimulating the lateral hypothalamus area regulated the discharges of gastric distention neurons via the dorsal vagal complex in cisplatin-treated rats. Gen Comp Endocrinol. 2019;279:174-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 197. | Sessenwein JL, Lomax AE. Ghrelin receptors as targets for novel motility drugs. Neurogastroenterol Motil. 2015;27:589-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 198. | Colldén G, Tschöp MH, Müller TD. Therapeutic Potential of Targeting the Ghrelin Pathway. Int J Mol Sci. 2017;18:798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 112] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 199. | Wu H, Chen X. [Effect of electro-acupuncture of zusanli on unit discharges in the lateral hypothalamic area induced by stomach distension]. Zhen Ci Yan Jiu. 1990;15:194-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 200. | Liu Z, Sun F, Han Y. [Effect of acupuncture on level of monoamines and activity of adenosine triphosphatase in lateral hypothalamic area of obese rats]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2000;20:521-523. [PubMed] |
| 201. | Yu Z, Xia Y, Ju C, Shao Q, Mao Z, Gu Y, Xu B. Electroacupuncture regulates glucose-inhibited neurons in treatment of simple obesity. Neural Regen Res. 2013;8:809-816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 202. | Wang WG, Chen X, Jiang H, Jiang ZY. Effects of ghrelin on glucose-sensing and gastric distension sensitive neurons in rat dorsal vagal complex. Regul Pept. 2008;146:169-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 203. | Alamri BN, Shin K, Chappe V, Anini Y. The role of ghrelin in the regulation of glucose homeostasis. Horm Mol Biol Clin Investig. 2016;26:3-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 204. | Hong SH, Ding SS, Wu F, Bi Y, Xu F, Wan YJ, Xuan LH. Efficacy and safety of manual acupuncture manipulations with different frequencies on epigastric pain syndrome (EPS) in functional dyspepsia (FD) patients: study protocol for a randomized controlled trial. Trials. 2017;18:102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 205. | Han G, Ko SJ, Park JW, Kim J, Yeo I, Lee H, Kim SY, Lee H. Acupuncture for functional dyspepsia: study protocol for a two-center, randomized controlled trial. Trials. 2014;15:89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 206. | Güçel F, Bahar B, Demirtas C, Mit S, Cevik C. Influence of acupuncture on leptin, ghrelin, insulin and cholecystokinin in obese women: a randomised, sham-controlled preliminary trial. Acupunct Med. 2012;30:203-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 207. | Grundmann O, Yoon SL, Williams JJ, Gordan L, George TJ Jr. Augmentation of Cancer Cachexia Components With Targeted Acupuncture in Patients With Gastrointestinal Cancers: A Randomized Controlled Pilot Study. Integr Cancer Ther. 2019;18:1534735418823269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 208. | Chronwall BM. Anatomy and physiology of the neuroendocrine arcuate nucleus. Peptides. 1985;6 Suppl 2:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 238] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 209. | Blevins JE, Baskin DG. Hypothalamic-brainstem circuits controlling eating. Forum Nutr. 2010;63:133-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 210. | DeFalco J, Tomishima M, Liu H, Zhao C, Cai X, Marth JD, Enquist L, Friedman JM. Virus-assisted mapping of neural inputs to a feeding center in the hypothalamus. Science. 2001;291:2608-2613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 332] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 211. | Tebbe JJ, Pasat IR, Mönnikes H, Ritter M, Kobelt P, Schäfer MK. Excitatory stimulation of neurons in the arcuate nucleus initiates central CRF-dependent stimulation of colonic propulsion in rats. Brain Res. 2005;1036:130-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 212. | Ma R, Zhang JX, Xu GR, Qian XJ. Effect of Stimulation of Hypothalamic of Arcuate Nucleus on Intragastric Pressure of Rat. Acta Universitis Medicinalis Anhui. 1989;1:6-8. [DOI] [Full Text] |
| 213. | Tebbe JJ, Dietze T, Grote C, Mönnikes H. Excitatory stimulation of neurons in the arcuate nucleus inhibits gastric acid secretion via vagal pathways in anesthetized rats. Brain Res. 2001;913:10-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 214. | Zhao ZQ. Neural mechanism underlying acupuncture analgesia. Prog Neurobiol. 2008;85:355-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 665] [Cited by in RCA: 727] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 215. | Zhang F, Feng Y, Zhou R, Zhang YQ, Chen BY. [Observation on the central afferent pathway of "Guanyuan" (CV 4) under normal and pathological states and the influence of electroacupuncture]. Zhen Ci Yan Jiu. 2008;33:147-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 216. | Guo Z, Lin X, Samaniego T, Isreb A, Cao S, Malik S, Holmes TC, Xu X. Fos-CreER-based genetic mapping of forebrain regions activated by acupuncture. J Comp Neurol. 2020;528:953-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 217. | Zhang XD, Zheng XZ. [The study of the relationship between the regulation of electroacupuncture on electrogastrography of rabbits and ARC]. Mudanjiang Yi Xue Yuan Xue Bao. 2001;22:5-7. [DOI] [Full Text] |
| 218. | Fei Wang, Tian DR, Tso P, Han JS. Arcuate nucleus of hypothalamus is involved in mediating the satiety effect of electroacupuncture in obese rats. Peptides. 2011;32:2394-2399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 219. | Lee JD, Jang MH, Kim EH, Kim CJ. Acupuncture decreases neuropeptide Y expression in the hypothalamus of rats with Streptozotocin-induced diabetes. Acupunct Electrother Res. 2004;29:73-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 220. | Tian DR, Li XD, Wang F, Niu DB, He QH, Li YS, Chang JK, Yang J, Han JS. Up-regulation of the expression of cocaine and amphetamine-regulated transcript peptide by electroacupuncture in the arcuate nucleus of diet-induced obese rats. Neurosci Lett. 2005;383:17-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 221. | Larsen PJ, Hunter RG. The role of CART in body weight homeostasis. Peptides. 2006;27:1981-1986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 222. | Guan X, Shi X, Li X, Chang B, Wang Y, Li D, Chan L. GLP-2 receptor in POMC neurons suppresses feeding behavior and gastric motility. Am J Physiol Endocrinol Metab. 2012;303:E853-E864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |