Published online May 28, 2020. doi: 10.3748/wjg.v26.i20.2472
Peer-review started: December 23, 2019
First decision: March 27, 2020
Revised: April 13, 2020
Accepted: May 13, 2020
Article in press: May 13, 2020
Published online: May 28, 2020
Processing time: 156 Days and 21.1 Hours
The regulation of food intake is a complex mechanism, and the hypothalamus is the main central structure implicated. In particular, the arcuate nucleus appears to be the most critical area in the integration of multiple peripheral signals. Among these signals, those originating from the white adipose tissue and the gastrointestinal tract are known to be involved in the regulation of food intake. The present paper focuses on adiponectin, an adipokine secreted by white adipose tissue, which is reported to have a role in the control of feeding by acting centrally. The recent observation that adiponectin is also able to influence gastric motility raises the question of whether this action represents an additional peripheral mechanism that concurs with the central effects of the hormone on food intake. This possibility, which represents an emerging aspect correlating the central and peripheral effects of adiponectin in the hunger-satiety cycle, is discussed in the present paper.
Core tip: Central structures involved in the regulation of food intake receive and integrate signals from peripheral organs, including white adipose tissue. This paper summarizes the main findings on the influence of adiponectin, a pleiotropic hormone secreted by adipocytes, on food intake. In addition to its central actions, adiponectin has been recently reported to cause gastric motor changes known to be peripheral signals implicated in the hunger-satiety cycle. In this light, we discuss the potential role of adiponectin as a peripheral signal in the signaling network underlying hunger and satiety.
- Citation: Idrizaj E, Garella R, Squecco R, Baccari MC. Can adiponectin have an additional effect on the regulation of food intake by inducing gastric motor changes? World J Gastroenterol 2020; 26(20): 2472-2478
- URL: https://www.wjgnet.com/1007-9327/full/v26/i20/2472.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i20.2472
The regulation of food intake consists of complex signals that allow the maintenance of energy and nutrient balance. Several neurotransmitters that act at the central level are involved in energy homeostasis by regulating food intake and/or energy expenditure. A number of pathological conditions may lead to a loss of this control. This can be observed in obesity, various metabolic diseases or eating disorders, where the signaling systems that underlie appetite control are dysregulated and not yet fully clarified. Therefore, it is important to elucidate the role of any single actor in this extremely complex mechanism that could largely benefit the pathophysiology and treatment of obesity and eating disorders. However, due to the complexity of the physiological mechanisms underlying the regulation of food intake, we attempt to make a simplified synthesis of the main central structures and some of the main peripheral signals involved in the regulation of food intake to provide the reader with a better understanding of the topic discussed.
In brief, central nervous system structures, the hypothalamus in particular, are able to integrate signals of different nature through afferent and efferent pathways to and from the brainstem as well as peripheral organs[1]. The arcuate hypothalamic nucleus appears to be the most critical area in the integration of such signals, some of which are prevalently involved in the control of energy homeostasis and others related to the short-term regulation of food intake[2]. The arcuate hypothalamic nucleus contains neurons whose activation influences food intake by exerting anorexigenic or orexigenic effects. Particularly, the activation of neurons that express pro-opiomelanocortin (POMC), a precursor for many peptides, leads to a decrease in food intake. In contrast, the stimulation of adjacent neurons, which synthesize neuropeptide Y (NPY) and agouti-related peptide, is known to cause an increase in food intake. These two systems influence each other in opposite ways. The arcuate hypothalamic nucleus represents the main integrative center of signals coming either from other hypothalamic areas involved in feeding behavior (such as the paraventricular nucleus, the dorsomedial nucleus and the lateral nucleus) or from extra hypothalamic areas[3], such as the nucleus tractus solitaris. The latter receives signals from the periphery and transmits them to the hypothalamus.
There are many signals of both neural and hormonal nature that originate from the periphery and are able to modulate feeding. In particular, the gastrointestinal tract is a source of signals able to influence food intake either through the release of gut hormones or through gastrointestinal motor changes[4]. The latter could modify the activity of the vagal afferent fibers, which send signals to the hypothalamic regions through the interposition of the nucleus tractus solitaris. Gut hormones, such as cholecystokinin, glucagon-like peptide-1, ghrelin and peptide tyrosine tyrosine, can reach the brain by entering the systemic circulation and may also send signals to the central structures by stimulating receptors present on vagal afferent neurons of the gut mucosa[5].
Gastrointestinal motor changes, particularly those related to the stomach, are recognized as key mediators of the hunger-satiety cycle. Gastric accommodation as well as gastric emptying indeed play a significant role in the regulation of stomach distension[6]. The latter has been shown to trigger stretch and tension, thus stimulating mechanosensitive receptors. These, in turn, transmit their information via the afferent nerves[7,8] to several brain areas[9]. It is known that the tone of the gastric proximal portion decreases during food intake. This process of active relaxation is mediated by different parasympathetic reflex pathways, which lead to a decrease in contractile cholinergic input and an increase in nitric oxide release[10]. Several studies reported that the delay of gastric emptying, physiologically or artificially induced, and hence the dilation of the gastric wall are related to an increase in satiety feelings and fullness, leading to food intake termination[11-13]. Interestingly, obese people showed enhanced gastric emptying, whereas a subgroup of patients with anorexia nervosa had significantly delayed gastric emptying[14,15]. Moreover, several studies combining ultrasound and scintigraphy experiments found an inverse correlation between satiety and gastric distension/emptying[16-18].
Evidence exists that gastric distension-induced satiety can also be regulated by some anorexigenic gut hormones (e.g., cholecystokinin and glucagon-like peptide-1), which induce gastric distension and inhibit gastric emptying (and at the same time increase gastric compliance). Such effects on gastric motility can contribute to their effects on inducing satiety[19].
In this regard, several hormones, not only from the gastrointestinal tract but also from other organs, such as white adipose tissue (WAT), have been reported to modulate appetite in humans[20]. WAT secretes adipokines and bioactive signaling molecules[21-23]; thus, it is a part of the endocrine signaling system. This is the reason why WAT cannot be thought of as an inactive organ but rather an endocrine organ. Adipose tissue has complex interactions with the brain and peripheral organs and plays an active role in energy homeostasis and several other processes[24,25]. For instance, it controls the hunger-satiety cycle by providing hormonal signals about energy stores to the brain via adipokines. Among them, in addition to leptin, whose effects have been extensively investigated, adiponectin has also been reported to have a role in regulating feeding behavior by sending signals to the hypothalamus[26].
Among adipokines, adiponectin has recently attracted much attention because of its multiple peripheral actions: It seems to be implicated in several physiological conditions and to have a significant role in the maintenance of whole-body homeostasis[27]. Moreover, adiponectin has been reported to exert numerous beneficial effects as an antidiabetic, anti-atherosclerotic, antiapoptotic and anti-inflammatory agent in both animals and humans[28,29]. The hormone has also been shown to have a role in suppressing lipogenesis and gluconeogenesis and to increase fatty acid oxidation and energy consumption[30].
Adiponectin is one of the most concentrated hormones in plasma. However, despite its abundance, circulating adiponectin plasma concentrations change significantly[27] in a number of health and pathological conditions. In fact, a peculiar feature of adiponectin is the inverse correlation of its circulating concentrations with weight, waist circumference, body mass index and obesity[31,32]. In this regard, a significant increase in adiponectin concentration in adipose tissue and plasma has been described after caloric restriction or bariatric surgery in obese subjects[33,34]. However, the mechanisms underlying obesity-associated reductions in plasma adiponectin concentrations have not yet been elucidated in detail.
In addition to peripheral actions, adiponectin has also been reported to exert central actions in the regulation of food intake, but they are still controversial. Nonetheless, the presence of adiponectin receptors has been proven in different regions of the human brain[35,36], including the hypothalamic arcuate and lateral nuclei[37].
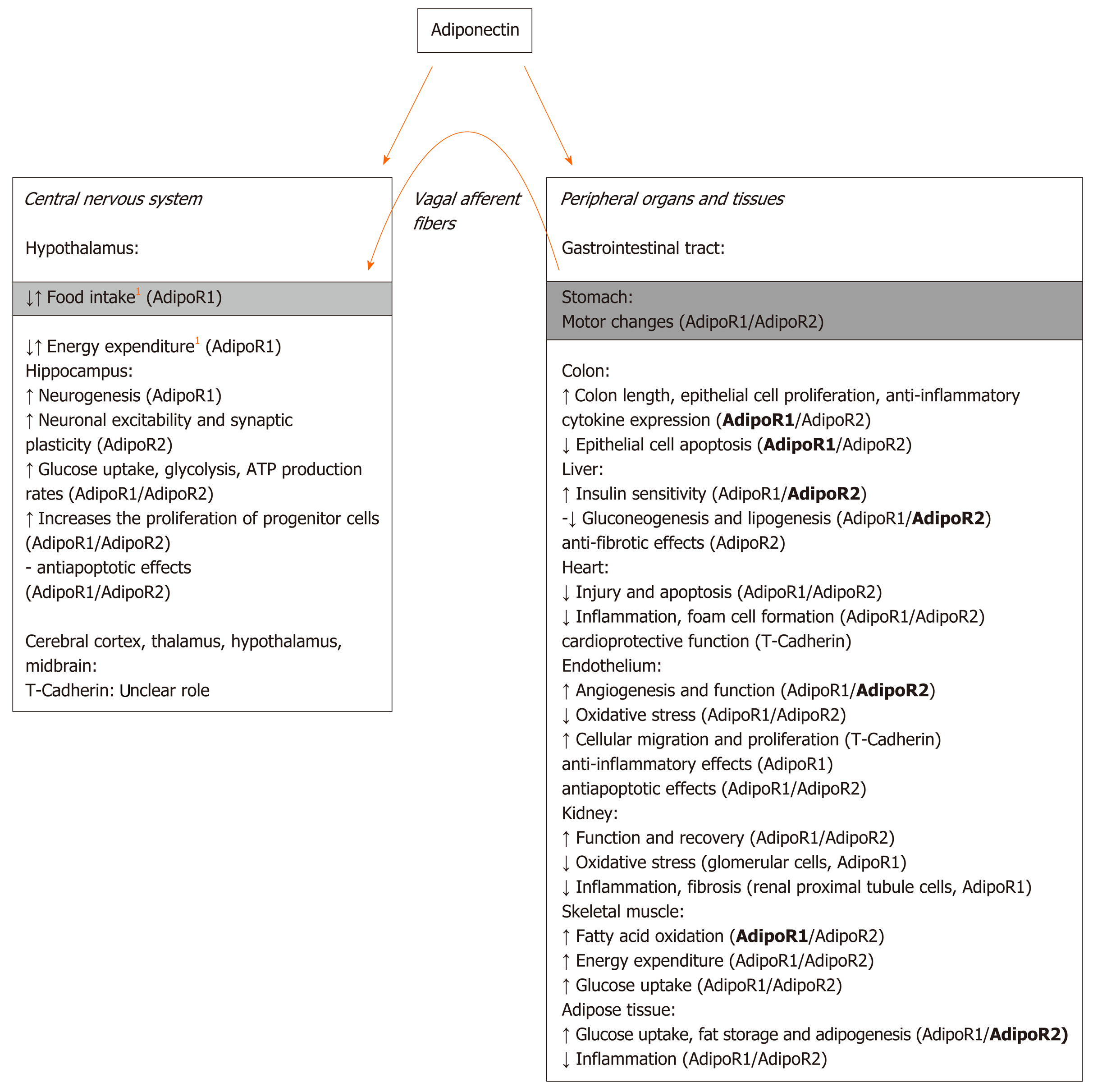
Adiponectin receptors (AdipoR1, AdipoR2, and T-cadherin) are indeed expressed in the brain as well as in the peripheral tissues and show different affinities for specific adiponectin isoforms, i.e., trimeric, hexameric, and high-molecular-weight multimeric isoforms[38]. Posttranslational modifications are important for the determination of adiponectin functionality since different isoforms of adiponectin exhibit different biological activities. In particular, trimeric and hexameric forms are reported to be those mainly involved in the central regulation of food intake. Hexamers and high-molecular-weight adiponectin oligomers have been proposed to bind to the T-cadherin receptor in the brain, even if their interaction remains unclear[38,39]. Although AdipoR1 and AdipoR2 are both highly expressed in various brain areas, only hypothalamic AdipoR1 has been suggested to mediate adiponectin regulation of food intake and energy expenditure in mice[35]. A simplified scheme illustrating the main central and peripheral effects of adiponectin and the related receptors involved is reported in Figure 1.
Whether different isoforms of adiponectin enter the brain has long been a debated issue, but recent literature has reported that numerous adipokines are indeed able to pass through the blood-brain barrier and act right on the brain[40]. Specifically, adiponectin can cross the blood-brain barrier and can also be secreted locally in the brain tissue[41], likely leading to different biological effects[38,40]. In particular, some adiponectin isoforms (trimeric and hexameric) are indeed able to reach the brain and hypothalamic centers[40] to interact with the areas that control hunger and satiety. Although this is a very exciting finding, many other aspects remain to be solved, primarily whether adiponectin exerts anorexigenic or orexigenic effects.
Notwithstanding an early observation of Qi and coworkers[42], who reported no central effects of adiponectin on food intake in mice, numerous subsequent studies indeed refer to its ability to influence feeding by acting on hypothalamic nuclei. In this view, Kubota et al[35] observed that full-length adiponectin, intravenously injected in mice, enhanced adenosine monophosphate-activated protein kinase activity in the arcuate hypothalamus[43] via AdipoR1, thus promoting food intake during fasting. Contrasting results have been reported by Coope et al[26], who demonstrated that direct intracerebroventricular injection of adiponectin in fasted rats induced a dose-related reduction in food intake. This effect was abolished following the inhibition of AdipoR1 but not AdipoR2. Coope et al[26] proposed that the discrepancy between their results and those obtained by Kubota et al[35] could be related to the different protocols employed. In fact, the perfusion of the hormone through the jugular vein[35] did not take into account that adiponectin may not be able to easily pass through the blood-brain barrier according to its different isoforms[5]. As a matter of fact, only the trimer and low-molecular-weight hexamer have been detected in the human cerebrospinal fluid, with the adiponectin trimer being the predominant oligomer[38]. This also gives a possible explanation for the lack of effects observed by Qi et al[42]. Indeed, a reduction in food intake has been recently observed following intracerebroventricular injection of adiponectin in rats[44], in agreement with the above-reported results by Coope and coworkers[26].
The effects of adiponectin on food intake and hypothalamic neuronal activity appear to also be related to different nutritional states and glucose plasma concentrations. In this view, electrophysiological experiments highlight that adiponectin inhibits NPY neurons and activates POMC neurons in a pho-sphoinositide-3-kinase-dependent and activated protein kinase-independent manner at various physiological glucose levels[45]. Two other recent studies[46,47] showed that intracerebroventricular injection of adiponectin in mice influences feeding and activates POMC neurons in relation to low or high glucose concentrations, whereas the inhibitory action on NPY neurons is glucose-independent. These results proposed an exciting new role for adiponectin as an attenuator of the effect of changes in the glucose level on POMC neuron activity and feeding behavior.
Moreover, many substances that act centrally to regulate food intake are also able to exert their effects at the gastrointestinal smooth muscle level. Among these, leptin is one of the best characterized[48]. However, notwithstanding the above-reported effects of adiponectin in the hunger-satiety cycle at the central level, no data were present in the literature about its possible peripheral effects on gastrointestinal motility until recently. The ability of the hormone to influence the mechanical responses in strips from the mouse gastric fundus has been recently reported, and the expression of both AdipoR1 and AdipoR2 receptors in these preparations has been revealed[49]. Particularly, a neuromodulatory action for the hormone has been suggested due to its ability to reduce the amplitude of the neurally induced contractile responses and to enhance that of the relaxant ones (likely through the involvement of the nitric oxide pathway). Interestingly, in addition to its neuromodulatory action, adiponectin is capable of inducing gastric motor changes through a direct relaxant effect on smooth muscle[49]. Likewise, electrophysiological investigations[50] indicate the ability of the hormone to hyperpolarize the resting membrane potential, suggesting a reduction of gastric smooth muscle cell excitability and thus an inhibitory action on excitation-contraction coupling.
All the above considerations indicate that adiponectin may have an additional effect on the regulation of food intake by inducing gastric motor changes. The depressant actions on the gastric muscle exerted by adiponectin may indeed be directed to facilitate relaxation, which may lead to gastric wall distension, known to be a peripheral satiety signal. Thus, the most attractive hypothesis is certainly that such adiponectin effects may be truly regarded as a reinforcing peripheral mechanism engaged by the hormone itself to concur with its central anorexigenic effects. Going a step further, we can speculate that the depressant actions of the hormone on gastric motility may also cause delayed gastric emptying, thus concurring with an increase in satiety feelings and fullness, leading to food intake termination. With this in mind, we can hypothesize the existence of a link between the depressant peripheral effects exerted by adiponectin at the gastric level and its central anorexigenic effects.
However, it must be remembered that most of the studies have been carried out in animals. Both central and peripheral effects of adiponectin in the regulation of food intake certainly deserve to be further investigated from a translational perspective. Dysfunction in generating signals or in the interpretation of these signals by the brain may indeed lead to obesity and/or eating disorders.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Cure E, Khattab MA, Perse M S-Editor: Wang J L-Editor: A E-Editor: Zhang YL
| 1. | Abdalla MM. Central and peripheral control of food intake. Endocr Regul. 2017;51:52-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 2. | Elmquist JK, Coppari R, Balthasar N, Ichinose M, Lowell BB. Identifying hypothalamic pathways controlling food intake, body weight, and glucose homeostasis. J Comp Neurol. 2005;493:63-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 299] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 3. | Duca FA, Covasa M. Current and emerging concepts on the role of peripheral signals in the control of food intake and development of obesity. Br J Nutr. 2012;108:778-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Neary NM, Goldstone AP, Bloom SR. Appetite regulation: from the gut to the hypothalamus. Clin Endocrinol (Oxf). 2004;60:153-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 101] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Murphy KG, Bloom SR. Gut hormones and the regulation of energy homeostasis. Nature. 2006;444:854-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 532] [Cited by in RCA: 531] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 6. | Janssen P, Vanden Berghe P, Verschueren S, Lehmann A, Depoortere I, Tack J. Review article: the role of gastric motility in the control of food intake. Aliment Pharmacol Ther. 2011;33:880-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 228] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 7. | Phillips RJ, Powley TL. Tension and stretch receptors in gastrointestinal smooth muscle: re-evaluating vagal mechanoreceptor electrophysiology. Brain Res Brain Res Rev. 2000;34:1-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 177] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 8. | Sengupta JN, Gebhart GF. Gastrointestinal afferents fibers and sensation. In: Johnson LR, ed. Physiology of the Gastrointestinal Tract. New York: Raven, 1994: 483-519. |
| 9. | Van Oudenhove L, Dupont P, Vandenberghe J, Geeraerts B, van Laere K, Bormans G, Demyttenaere K, Tack J. The role of somatosensory cortical regions in the processing of painful gastric fundic distension: an update of brain imaging findings. Neurogastroenterol Motil. 2008;20:479-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Tack J, Demedts I, Meulemans A, Schuurkes J, Janssens J. Role of nitric oxide in the gastric accommodation reflex and in meal induced satiety in humans. Gut. 2002;51:219-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 158] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 11. | Wisén O, Hellström PM. Gastrointestinal motility in obesity. J Intern Med. 1995;237:411-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Hunt JN. A possible relation between the regulation of gastric emptying and food intake. Am J Physiol. 1980;239:G1-G4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Di Lorenzo C, Williams CM, Hajnal F, Valenzuela JE. Pectin delays gastric emptying and increases satiety in obese subjects. Gastroenterology. 1988;95:1211-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 96] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Johansson C, Ekelund K. Relation between body weight and the gastric and intestinal handling of an oral caloric load. Gut. 1976;17:456-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Dubois A, Gross HA, Ebert MH, Castell DO. Altered gastric emptying and secretion in primary anorexia nervosa. Gastroenterology. 1979;77:319-323. [PubMed] |
| 16. | Sturm K, Parker B, Wishart J, Feinle-Bisset C, Jones KL, Chapman I, Horowitz M. Energy intake and appetite are related to antral area in healthy young and older subjects. Am J Clin Nutr. 2004;80:656-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 127] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Hveem K, Jones KL, Chatterton BE, Horowitz M. Scintigraphic measurement of gastric emptying and ultrasonographic assessment of antral area: relation to appetite. Gut. 1996;38:816-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 132] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 18. | Jones KL, Doran SM, Hveem K, Bartholomeusz FD, Morley JE, Sun WM, Chatterton BE, Horowitz M. Relation between postprandial satiation and antral area in normal subjects. Am J Clin Nutr. 1997;66:127-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 140] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J Clin Invest. 2007;117:13-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 793] [Cited by in RCA: 791] [Article Influence: 43.9] [Reference Citation Analysis (0)] |
| 20. | Stanley S, Wynne K, McGowan B, Bloom S. Hormonal regulation of food intake. Physiol Rev. 2005;85:1131-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 247] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 21. | Ahima RS. Adipose tissue as an endocrine organ. Obesity (Silver Spring). 2006;14 Suppl 5:242S-249S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 446] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 22. | Idrizaj E, Garella R, Squecco R, Baccari MC. Adipocytes-released Peptides Involved in the Control of Gastrointestinal Motility. Curr Protein Pept Sci. 2019;20:614-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Harwood HJ. The adipocyte as an endocrine organ in the regulation of metabolic homeostasis. Neuropharmacology. 2012;63:57-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 186] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 24. | Ahima RS. Metabolic actions of adipocyte hormones: focus on adiponectin. Obesity (Silver Spring). 2006;14 Suppl 1:9S-15S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 111] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 25. | Flier JS. Obesity wars: molecular progress confronts an expanding epidemic. Cell. 2004;116:337-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 837] [Cited by in RCA: 798] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 26. | Coope A, Milanski M, Araújo EP, Tambascia M, Saad MJ, Geloneze B, Velloso LA. AdipoR1 mediates the anorexigenic and insulin/leptin-like actions of adiponectin in the hypothalamus. FEBS Lett. 2008;582:1471-1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 117] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 27. | Maeda N, Funahashi T, Matsuzawa Y, Shimomura I. Adiponectin, a unique adipocyte-derived factor beyond hormones. Atherosclerosis. 2020;292:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 28. | Kadowaki T, Yamauchi T, Kubota N. The physiological and pathophysiological role of adiponectin and adiponectin receptors in the peripheral tissues and CNS. FEBS Lett. 2008;582:74-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 196] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 29. | van Andel M, Heijboer AC, Drent ML. Adiponectin and Its Isoforms in Pathophysiology. Adv Clin Chem. 2018;85:115-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 30. | Yamauchi T, Kadowaki T. Adiponectin receptor as a key player in healthy longevity and obesity-related diseases. Cell Metab. 2013;17:185-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 320] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 31. | Mahdi BM. Significant of adiponectin in gastropathy: Case-controlled study. Ann Med Surg (Lond). 2019;47:16-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 32. | Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, Tataranni PA. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930-1935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1862] [Cited by in RCA: 1959] [Article Influence: 81.6] [Reference Citation Analysis (0)] |
| 33. | Tiaka EK, Manolakis AC, Kapsoritakis AN, Potamianos SP. The implication of adiponectin and resistin in gastrointestinal diseases. Cytokine Growth Factor Rev. 2011;22:109-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | Linscheid P, Christ-Crain M, Stoeckli R, Reusch CE, Lutz TA, Müller B, Keller U. Increase in high molecular weight adiponectin by bariatric surgery-induced weight loss. Diabetes Obes Metab. 2008;10:1266-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Kubota N, Yano W, Kubota T, Yamauchi T, Itoh S, Kumagai H, Kozono H, Takamoto I, Okamoto S, Shiuchi T, Suzuki R, Satoh H, Tsuchida A, Moroi M, Sugi K, Noda T, Ebinuma H, Ueta Y, Kondo T, Araki E, Ezaki O, Nagai R, Tobe K, Terauchi Y, Ueki K, Minokoshi Y, Kadowaki T. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab. 2007;6:55-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 584] [Cited by in RCA: 603] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 36. | Guillod-Maximin E, Roy AF, Vacher CM, Aubourg A, Bailleux V, Lorsignol A, Pénicaud L, Parquet M, Taouis M. Adiponectin receptors are expressed in hypothalamus and colocalized with proopiomelanocortin and neuropeptide Y in rodent arcuate neurons. J Endocrinol. 2009;200:93-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 117] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 37. | Kos K, Harte AL, da Silva NF, Tonchev A, Chaldakov G, James S, Snead DR, Hoggart B, O'Hare JP, McTernan PG, Kumar S. Adiponectin and resistin in human cerebrospinal fluid and expression of adiponectin receptors in the human hypothalamus. J Clin Endocrinol Metab. 2007;92:1129-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 162] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 38. | Bloemer J, Pinky PD, Govindarajulu M, Hong H, Judd R, Amin RH, Moore T, Dhanasekaran M, Reed MN, Suppiramaniam V. Role of Adiponectin in Central Nervous System Disorders. Neural Plast. 2018;2018:4593530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 119] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 39. | Rizzo MR, Fasano R, Paolisso G. Adiponectin and Cognitive Decline. Int J Mol Sci. 2020;21:2010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 40. | Lee TH, Cheng KK, Hoo RL, Siu PM, Yau SY. The Novel Perspectives of Adipokines on Brain Health. Int J Mol Sci. 2019;20:5638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 41. | Li HY, Hong X, Cao QQ, So KF. Adiponectin, exercise and eye diseases. Int Rev Neurobiol. 2019;147:281-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 42. | Qi Y, Takahashi N, Hileman SM, Patel HR, Berg AH, Pajvani UB, Scherer PE, Ahima RS. Adiponectin acts in the brain to decrease body weight. Nat Med. 2004;10:524-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 596] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 43. | Wang B, Cheng KK. Hypothalamic AMPK as a Mediator of Hormonal Regulation of Energy Balance. Int J Mol Sci. 2018;19:3552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 44. | Halah MP, Marangon PB, Antunes-Rodrigues J, Elias LLK. Neonatal nutritional programming impairs adiponectin effects on energy homeostasis in adult life of male rats. Am J Physiol Endocrinol Metab. 2018;315:E29-E37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 45. | Sun J, Gao Y, Yao T, Huang Y, He Z, Kong X, Yu KJ, Wang RT, Guo H, Yan J, Chang Y, Chen H, Scherer PE, Liu T, Williams KW. Adiponectin potentiates the acute effects of leptin in arcuate Pomc neurons. Mol Metab. 2016;5:882-891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 46. | Suyama S, Maekawa F, Maejima Y, Kubota N, Kadowaki T, Yada T. Glucose level determines excitatory or inhibitory effects of adiponectin on arcuate POMC neuron activity and feeding. Sci Rep. 2016;6:30796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 47. | Suyama S, Lei W, Kubota N, Kadowaki T, Yada T. Adiponectin at physiological level glucose-independently enhances inhibitory postsynaptic current onto NPY neurons in the hypothalamic arcuate nucleus. Neuropeptides. 2017;65:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 48. | Yarandi SS, Hebbar G, Sauer CG, Cole CR, Ziegler TR. Diverse roles of leptin in the gastrointestinal tract: modulation of motility, absorption, growth, and inflammation. Nutrition. 2011;27:269-275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 49. | Idrizaj E, Garella R, Castellini G, Mohr H, Pellegata NS, Francini F, Ricca V, Squecco R, Baccari MC. Adiponectin affects the mechanical responses in strips from the mouse gastric fundus. World J Gastroenterol. 2018;24:4028-4035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 50. | Idrizaj E, Garella R, Castellini G, Francini F, Ricca V, Baccari MC, Squecco R. Adiponectin Decreases Gastric Smooth Muscle Cell Excitability in Mice. Front Physiol. 2019;10:1000. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |