STRUCTURE AND FUNCTION OF THE LIVER
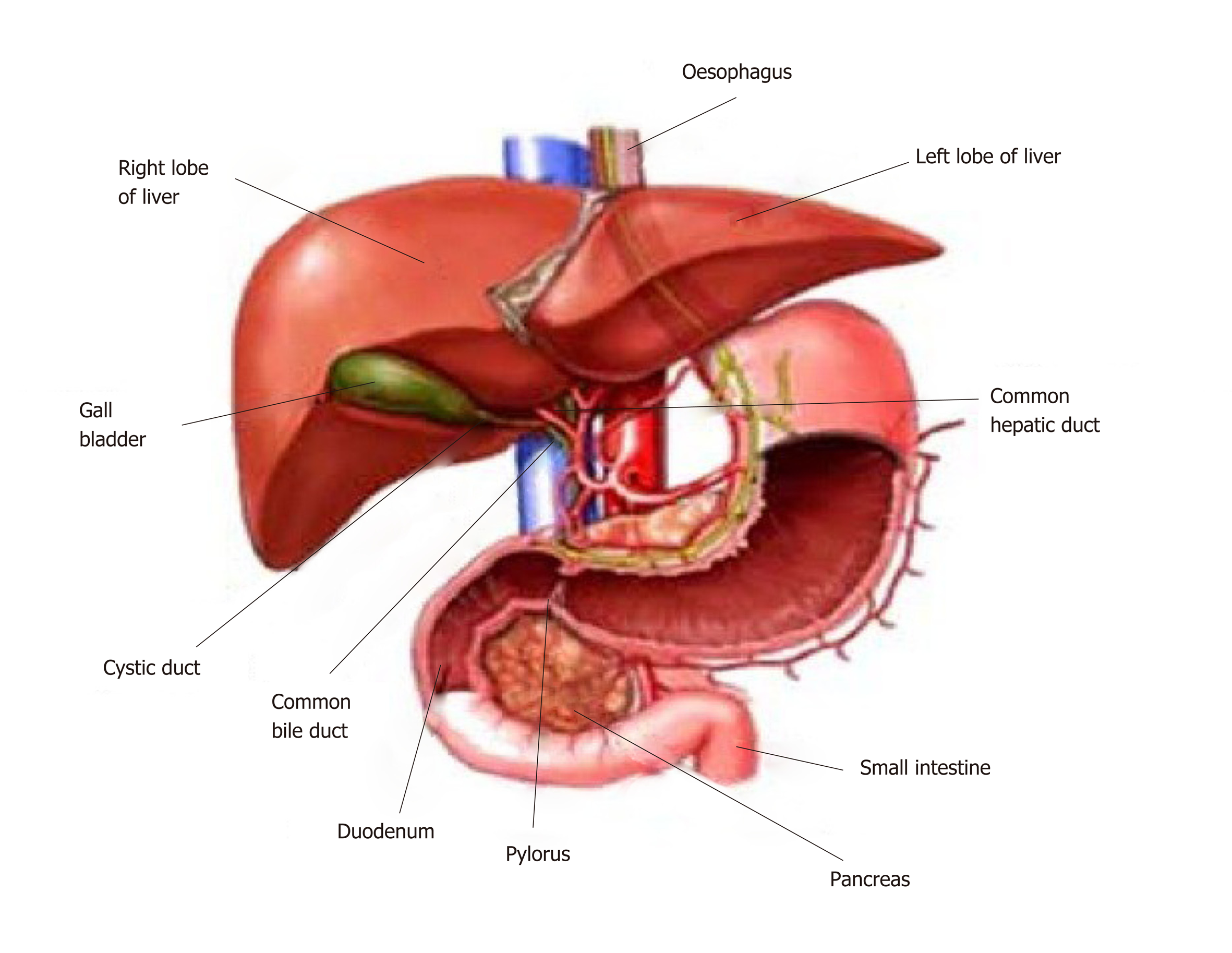
The liver is the largest solid organ in the body and has a median weight of 1.6 kg and 1.4 kg in adult males and females respectively[1]. The liver receives 75% of its blood supply via the portal vein and 25% via the hepatic arterial system. The portal vein carries blood from the entire capillary system of the digestive tract, spleen, pancreas and gallbladder. The hepatic artery is the second major branch of the celiac axis. The venous drainage of the liver is via the hepatic veins which open into the superior vena cava (Figure 1). The liver can be divided into eight functional segments or “lobules” based upon blood supply and biliary drainage. Hepatic lobules are comprised of a central hepatic vein and peripheral portal tracts that contain the final tributaries of the bile ducts (bile ductule), portal vein (portal venule) and hepatic vein (hepatic venule). Blood is drained from the portal tracts to the central vein by specialised capillaries known as the hepatic sinusoids[2].
Figure 1 Anatomy of the liver and its macroscopic relationship to the intestinal tract and vasculature.
Reproduced with permission of Creative Commons Attribution License from Ebaid et al[164].
The sinusoids are lined by a fenestrated endothelial layer containing numerous microvilli[2]. This structural organisation facilitates exchange of solutes between the portal tracts and the hepatocytes through the space of Disse. Endothelial cells, Kupffer cells and hepatic stellate cells (HSC) lie in juxtaposition with the hepatic sinusoid (Figures 2 and 3). Kupffer cells are the resident macrophages of the liver and their major functions include the clearance of particles, immune complexes, senescent red blood cells and endotoxins. In addition, Kupffer cells have a role in the innate immune response and produce pro-inflammatory cytokines including interleukin 1 and 6, tumour necrosis factor-α (TNF-α) and interferons. HSCs are distributed throughout the liver and form the main perisinusoidal cell type with a diverse range of functions.
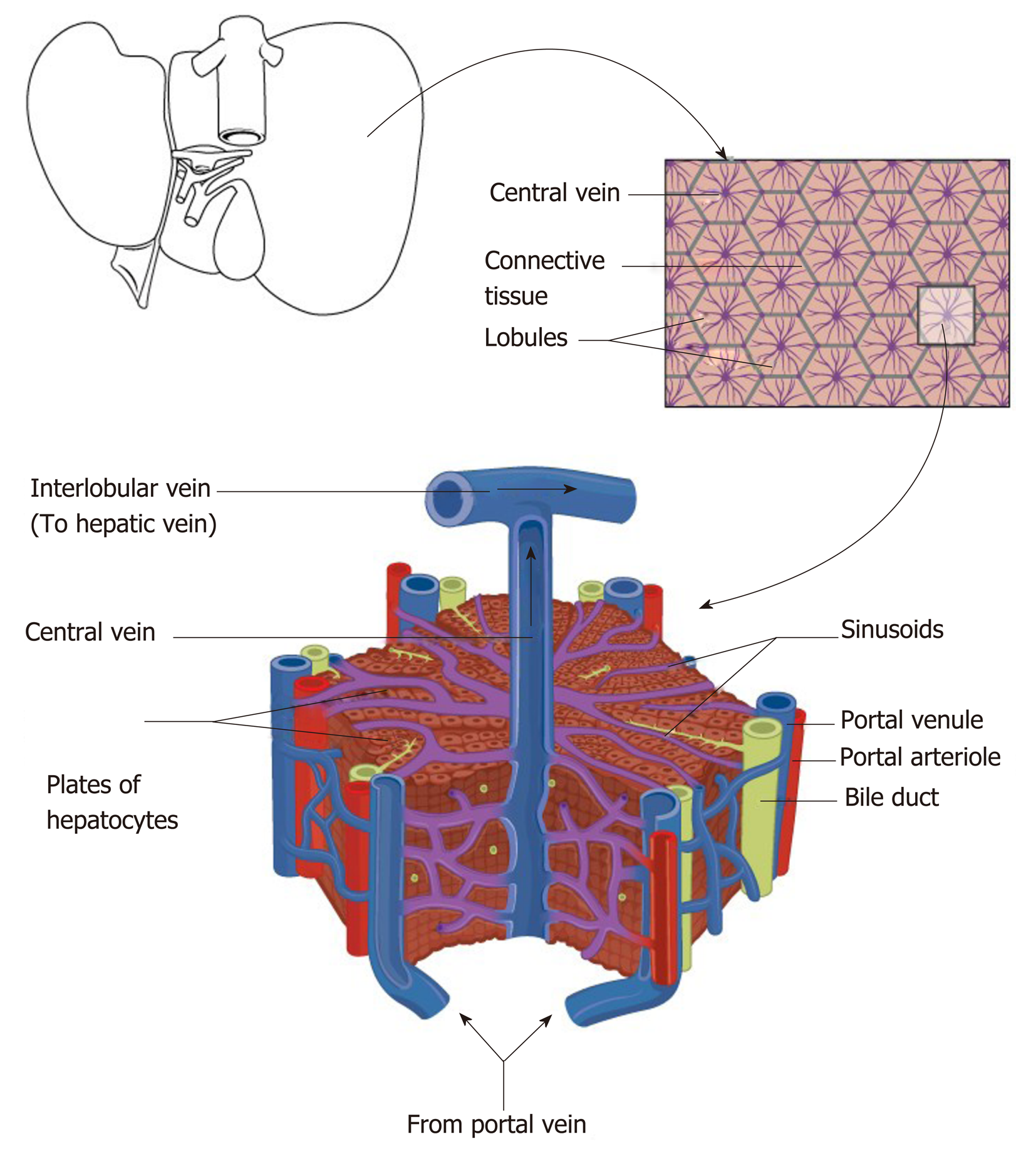
Figure 2 Schematic diagram representing the relationship of the macroscopic structure of the liver with the functional hepatic lobule with hepatic venules (blue), hepatic arteriole (red), bile ductules (yellow).
Reproduced with permission of Creative Commons Attribution License from Anatomy & Physiology textbook[165].
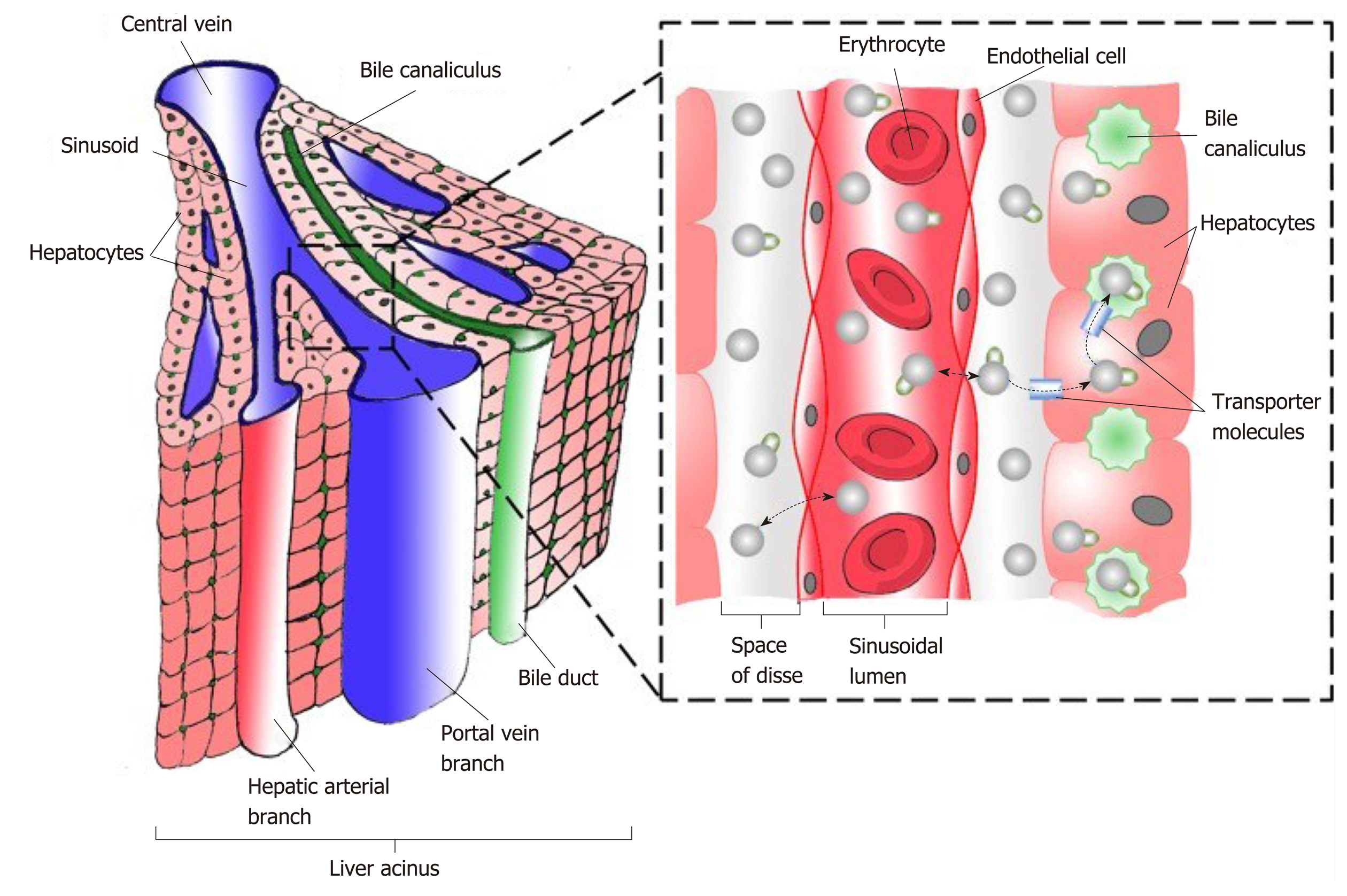
Figure 3 Schematic diagram representing functional hepatic acinus with hepatic venules (blue), hepatic arteriole (red), bile ductules (green) together with the relationship to the Space of Disse and the sinusoidal lumen.
Reproduced with permission of Creative Commons Attribution License from Chouhan et al[166].
HEPATIC EXTRACELLULAR MATRIX
The extracellular matrix (ECM) is the array of macromolecules that forms the liver “scaffolding”[3]. In the normal liver, ECM contributes to approximately 0.5% of the total weight of the liver, comprising less than 3% of the area on cross sectional imaging[3]. Normal ECM is composed of collagens (types I, III, IV, V, VI, XIV and XVIII), elastin, structural glycoproteins (laminin, fibronectin, nidogen/enactin, tenascin, osteopontin, various acidic proteins), proteoglycans (heparan sulfate, syndecan, biglycan and decorin), and hyaluronic acid (a glycosaminoglycan)[4]. All 3 of the cell types (hepatocytes, endothelial cells, HSC) that surround the space of Disse produce matrix components.
FUNCTION OF THE LIVER
The liver has a number functions which in broad terms can be defined as “the regulation of the concentrations of solutes in the blood that affect the function of other organs”[1]. Through the uptake, metabolism and secretion of solutes, the liver performs an integral role in the metabolism of amino acids (for example transamination), carbohydrates (for example gluconeogenesis), lipids (for example lipid production), haemoglobin, bile salts, iron, copper, vitamins, ammonia and drugs. The liver is the major synthetic organ producing albumin, serum binding proteins (for example haptoglobin) and clotting factors. Furthermore, the liver is an important immunological site with functions such as cytokine signalling, antigen surveillance and immune tolerance.
HEPATIC INFLAMMATION IN RESPONSE TO CHRONIC INJURY
Whereas immune responses in CLD may contribute to the restoration of tissue function they may also lead to tissue injury. An overactive or exaggerated immune response, for example in non-alcoholic steatohepatitis (NASH) or chronic hepatitis C (CHC), can result in organ dysfunction by the replacement of hepatic parenchyma by scar tissue and by vascular architectural distortion. These immune responses are the subject of active investigation to not only extend understanding of the pathology of liver injury but to also identify diagnostic and therapeutic targets.
INNATE IMMUNE RESPONSE
Pattern recognition receptors (PRRs) are an essential part of the innate immune system that facilitate the detection of pathogens. The liver is enriched with both parenchymal liver cells and non-parenchymal liver cells that express PRRs. Hepatocytes express PRRs and are the main liver parenchymal cells. Non-parenchymal cells that express PRRs include liver sinusoidal endothelial cells (LSEC), HSC and bone marrow derived immune cells (Kupffer cells, and dendritic cells)[5]. PRR can be divided into 4 classes: Toll-like receptors (TLR), Nucleotide oligomerisation receptors (NLR), C-like lectin receptors and RIG-1 like receptors. PRR recognise conserved molecular structures called pathogen-associated molecular patterns that are pathogen specific. Of the 4 types of PRR, TLRs are the key sensor of the innate immune system for the recognition of pathogens[6] including viruses[7]. For example, TLR-3 is able to recognise viral dsRNA leading to the production of type 1 Interferons (IFN α or β)[8]. Three main pathways are activated by TLRs: Mitogen activated protein kinase pathway (ERK, p38 and JNK), nuclear factor κB pathway and interferon regulatory transcription factor pathway. Pro-inflammatory and anti-inflammatory cytokines can be induced following TLR stimulation. Inflammation is characterized by both the activation of innate immune cells and the production of pro-inflammatory cytokines such as IL-1α, IL-1β, and TNFα.
INNATE IMMUNE CELL RECRUITMENT TO SITES OF HEPATIC INFLAMMATION
Neutrophils (also known as neutrophilic granulocytes or polymorphonuclear leukocytes) infiltrate the site of injury within minutes in response to the injury itself following the release of damage-associated molecular patterns (DAMPS)[9] from damaged cells. Neutrophil recruitment peaks within hours[10]. Neutrophils are key effectors of the innate immune system with high phagocytic potential and possess a vast number of antimicrobial molecules. In addition to clearing pathogens, neutrophils may also exacerbate macrophage cytotoxicity and help promote a chronic inflammatory state[11].
Monocyte infiltration following tissue injury peaks between 24-48 h[12]. Recruited monocytes demonstrate a variety of functions including production of inflammatory mediators, clearance of neutrophils, ECM production and angiogenesis. In humans, 3 types of monocyte subsets have been identified: Classic (CD14++CD16-), intermediate (CD14++CD16+), and non-classic (CD14++CD16++)[13]. Furthermore, as derived from murine studies, monocytes can also be categorised into inflammatory (CCR2hi, CX3CR1lo) and anti-inflammatory (CX3CR1hi, CCR2lo) subtypes. Pro-inflammatory monocytes produce inflammatory cytokines and chemokines, produce proteases and facilitate clearance. Anti-inflammatory monocytes produce anti-inflammatory cytokines (IL-10), transforming growth factor-β (TGF-β), and vascular endothelial growth factor (VEGF) that promote resolution and restitution[14].
Kupffer cells are the resident macrophages of the liver[15]. In addition to detecting tissue injury, Kupffer cells can recruit inflammatory cells, promote tissue repair and remodelling. In addition, new populations of macrophages can be recruited following acute inflammation (emergency repopulation). Macrophages may be pro-inflammatory (classic M1 type) or anti-inflammatory (alternative M2 type) and their relative balance contributes to either injury or repair[16].
Platelets are metabolically and synthetically active cells that can produce many inflammatory cytokines and chemokines thus attracting neutrophils to sites of inflammation[17].
Natural killer T cells and natural killer (NK) cells (granular lymphocytes) can be thought of as being part of the innate immune system as they can kill target cells without priming[18]. NK cells can contribute to HSC clearance in a TNF-related apoptosis inducing ligand dependent manner[19].
ADAPTIVE IMMUNE RESPONSE TO HEPATIC INJURY
Both T (thymus) and B (bone marrow derived) lymphocytes mediate the adaptive immune response. Whereas T cells are involved in cell-mediated immunity, B cells are primarily responsible for humoral immunity. Regardless, the function of both T and B cells is to recognise “non self” antigens by generating specific responses to eliminate specific pathogens or cells expressing “non self” antigens[20].
T helper (CD4+) cells can activate other immune cells (including B cells), switch antibody classes, activate cytotoxic (CD8+) T cells and enhance macrophage phagocytosis. In response to cytokine stimulation, T helper cells can either assume a proinflammatory phenotype (Th1) or an anti-inflammatory phenotype (Th2). Th1 T cells secrete the inflammatory cytokines interferon-γ and TGF-β. Th2 T cells are characterised by the secretion of IL-4, IL-5 and IL-10. Proinflammatory M1 macrophages are induced by the release of interferon-γ by Th1 T cells.
In addition to Th1 and Th2 cells, Il-17 producing T helper cells (Th17) and regulatory T cells (Tregs) also function as effector T cells that are heavily involved in inflammation[21] and the development of liver fibrosis[22]. After activation Th17 cells secrete IL-17A, IL-17F, IL-21, IL-22, and TNFα, which promote tissue inflammation by induction of other proinflammatory cytokines and recruitment of leukocytes[23]. Treg cells are a unique subset of T-helper cells that control effector T-cell responses to prevent autoimmune reactions. Activated Treg suppress the development of functional immune reactions by the production of anti-inflammatory cytokines including IL-10 and TGFβ[24]. The transcription factors Foxp3, STAT5 and CD25 are expressed on the surface of Treg cells[25].
B cells secrete antibodies which provides humoral immunity within the adaptive immune system. In addition, B cells secrete cytokines and function as antigen presenting cells. All B cells express B cell receptors on their cell membrane. B cell receptors allow B cells to bind specific antigens which is followed by the production of antibodies. When naïve or memory B cells are activated by antigen, they proliferate and differentiate into effector B cells with the formation of large plasma cell representing the end stage of their maturation pathway.
CELL DEATH AND ONGOING INFLAMMATORY RESPONSE
Cell death can be categorised in several ways including non-inflammatory cell death (apoptosis) and inflammatory cell death (necrosis, pyroptosis and necroptosis). Regardless, the nature of the accompanying immune response is dependent on the signals liberated from dead cells. Apoptosis fails to generate an immune response as apoptotic cells retain membrane integrity. In addition, apoptotic cells also release factors that inhibit the recruitment and activation of neutrophils. By contrast, membrane integrity is disrupted during necrosis which results in the release of DAMPS[9]. Broadly, DAMPS are molecules that can activate inflammation. Necrosis also produces inflammatory signals by modifying extracellular matrix components including hyaluronic acid[26]. Moreover, necrosis can also result from programmed cell death pathways: Pyroptosis and necroptosis. Pyroptosis is initiated by inflammasome mediated activation of capsase-1 which results in lytic cell death and the production of the interleukins (IL) IL-1β and IL-18[27]. Necroptosis is activated by the presentation of “external death signals” to cells such as TNF, interferon receptor and selected TLR pathways[28]. Following activation by these signals, necroptosis involves inhibition of caspase-8 (promotes apoptosis), mixed-lineage kinase domain-like protein and RIP kinase family members 1 and RIP kinase family members 3.
INFLAMMASOME
Inflammasomes are an intracellular multiprotein scaffolding that are expressed in both parenchymal and non-parenchymal cells of the liver. Functionally, inflammasomes are both sensors and receptors of the innate immune system that can induce inflammation in response to pathogens and molecules derived from host proteins (DAMPs). In response to these “cellular danger signals” inflammasomes activate caspase-1 and release both IL-1β and IL-18[29].
PATHOPHYSIOLOGY OF LIVER FIBROSIS
Whereas the development of liver fibrosis itself is often a prerequisite for the morbidity associated with CLD it is important to highlight that fibrogenesis is also a part of the normal wound healing process in response to noxious stimuli.
Liver fibrosis is a structural change defined by an accumulation of ECM proteins such as collagen often triggered by chronic, sustained inflammation. The pattern of fibrosis deposition is dependent on the aetiology of liver injury. Pericellular and perisinusoidal fibrosis deposition in the centrilobular areas are characteristic of fibrosis related to NASH and alcohol related liver disease. By contrast, periportal fibrosis deposition is characteristic of autoimmune and viral liver diseases.
Regardless, it is important to recognise that maintenance of the liver matrix is a dynamic process in which deposition and resorption of matrix are balanced. In a steady physiologic state, both ongoing ECM deposition and removal are equal resulting in an unchanged amount of ECM[30]. At a cellular level, the HSC is recognised to be the most important cell lineage in the development of liver fibrosis. In its quiescent or resting state, the HSC is a lipid storing cell representing the body’s major location of Vitamin A. However, on activation the HSC undergoes transformation to become a myofibroblast capable of regulating matrix deposition and resorption whilst also possessing contractile properties. In addition to those derived from HSC, profibrogenic myofibroblasts can also be derived from portal fibroblasts, recruited bone marrow cells and epithelial cells (for example hepatocytes and cholangiocytes) that have undergone epithelial to mesenchymal transition[31]. HSC reside in the space of Disse and are the resident non-paranchymal cell type. Their embryonic origin is likely to be mesenchymal given that they produce α-smooth muscle actin when activated in addition to vimentin and desmin[32]. HSC themselves represent approximately 10% of the total liver cell number and 1.5% of the total liver cell volume[33]. The location of HSC within the space of Disse allows for direct (within 140 µm) contact of the HSC with their other cell types including hepatocytes, endothelial cells and Kupfer cells[33] thus facilitating the intercellular transport of soluble mediators and cytokines. In addition to this intimate location within the space of Disse, intercellular communication between HSC and the neighbouring cells are also facilitated by their prominent dendritic cytoplasmic processes. Their contractility may contribute to the regulation of portal pressure[34]. When quiescent, the HSC serve as a reservoir for retinol (a precursor for vitamin A) and other lipid soluble compounds. Indeed, the presence of Vitamin A esters in cytoplasmic perinuclear lipid droplets is the characteristic microscopic feature of quiescent HSC. HSCs represent resting profibrogenic myofibroblasts which are a major constituent of the extracellular matrix in both the healthy and diseased liver. The phenotypic transformation of HSCs into profibrogenic myofibroblasts is associated with the acquisition of α-smooth muscle reactivity. The activation of HSC is comprised of 2 stages: Initiation and perpetuation.
HSC ACTIVATION
In response to liver injury, lipid peroxides and apoptotic bodies that accumulate in damaged hepatocytes initiate HSC activation in a process mediated by Fas and TNF-related apoptosis inducing ligand[35]. This activation process is initiated by profibrogenic cytokines (for example TGF-β), fibronectin, platelet derived growth factor (PDGF), reactive oxygen species (ROS) and apoptotic bodies derived from neighbouring cells, immune cells and platelets (Figures 4 and 5)[30]. Thereafter, HSC undergo characteristic phenotypic changes and resemble myofibroblasts[31].
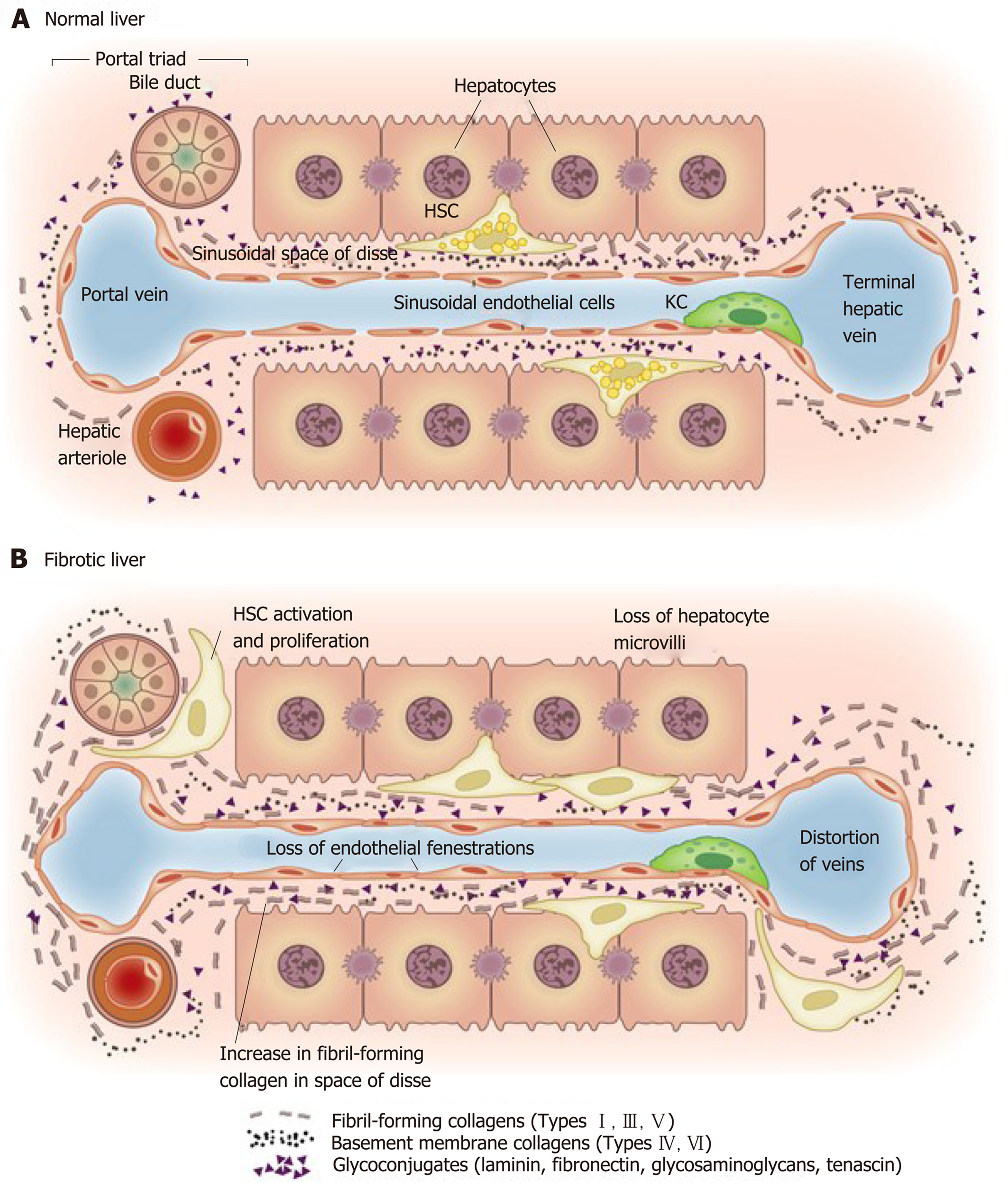
Figure 4 Matrix and cellular alteration in hepatic fibrosis.
Normal liver parenchyma contains epithelial cells (hepatocytes) and nonparenchymal cells: fenestrated sinusoidal endothelium, hepatic stellate cells, and Kupffer cells. A: After injury, the stellate cells become activated and secrete large amounts of extracellular matrix (ECM); B: Deposition of ECM in the space of Disse leads to the loss of both endothelial fenestrations and hepatocyte microvilli. Reproduced with permission from Hernandez-Gea and Friedman[37]. HSC: Hepatic stellate cells; KC: Kupffer cells.
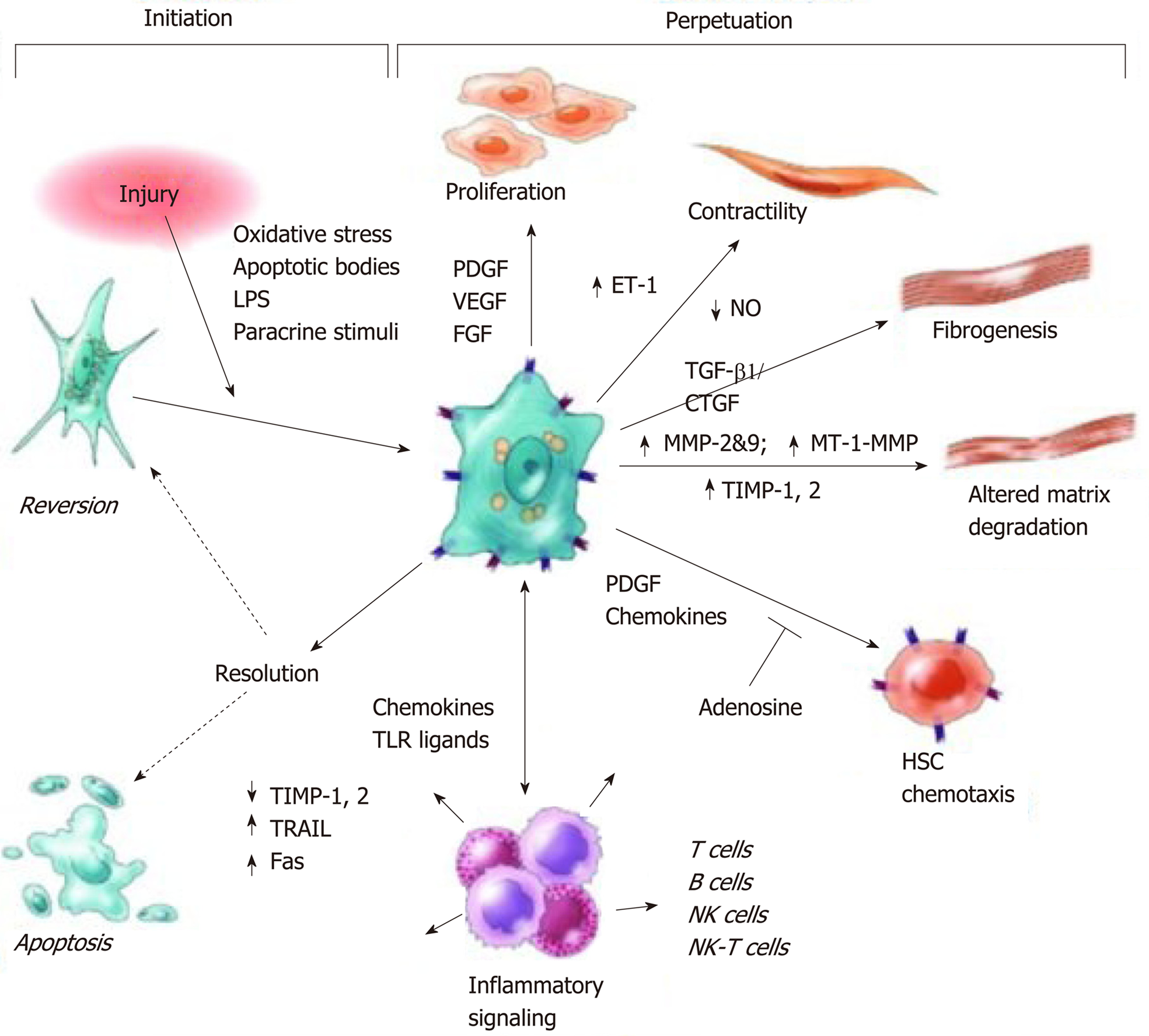
Figure 5 Pathways of hepatic stellate cell activation including those contributing to initiation and perpetuation.
Initiation is provoked by soluble stimuli that include oxidant stress signals, apoptotic bodies, lipopolysaccharide, and cytokine stimuli from neighbouring cells. Perpetuation is characterised by specific phenotypic changes including proliferation, contractility, fibrogenesis, altered matrix degradation, chemotaxis, and cytokine signalling. Reproduced with permission from Friedman[30]. CTGF: Connective tissue growth factor; ET: Endothelin; FGF: Fibroblast growth factor; LPS: Lipopolysaccharide; MMP: Matrix metalloproteinase; MT-1: membrane type-1, NK: Natural killer, NO: Nitrous oxide; PDGF: Platelet derived growth factor; TIMP: Tissue inhibitor of metalloproteases; TGF: Transforming growth factor; TLR: Toll like receptor; TRAIL: Tumour necrosis factor-related apoptosis-inducing ligand; VEGF: Vascular endothelial growth factor.
HSC PERPETUATION
Following on from activation, the activated HSC enters the phase of perpetuation in which ECM is accumulated resulting in scar tissue formation (Figures 4 and 5). Tissue hypoxia, apoptosis and cell matrix interactions maintain ongoing HSC activation. HSC perpetuation is comprised of number of functional responses including proliferation, fibrogenesis, chemotaxis, contractility, matrix degradation, retinoid loss and cytokine expression.
Proliferation
HSC proliferate rapidly and gain a profibrogenic phenotype; this occurs primarily in response to both an increase in PDGF and PDGF responsivity[36,37]. Other mediators involved in HSC proliferation include TNFα, VEGF, thrombin and epidermal growth factor (EGF)[30].
Fibrogenesis
An accumulation of ECM (particularly collagen type I) occurs follows on from increased synthesis by activated myofibroblasts and decreased degradation. TGF-β1 is the main driver for ECM production by activated HSCs. Connective tissue growth factor (CTGF) also acts as a profibrogenic cytokine towards HSC.
Chemotaxis
HSCs migrate towards chemokines allowing cells to organise within regions of injury. Examples of chemokines toward which HSCs migrate to include PDGF, VEGF, Ang-1, TGF-β1, EGF, b-FGF, CCL2, CXR3 and CXR4[38]. In addition, tissue hypoxia enhances HSC migration with ROS activating extracellular signal-regulated kinase 1/2 (ERK) and JNK1/2 pathways.
Contraction
Hepatic sinusoidal remodelling occurs following HSC activation which is mediated by collagen matrix deposition, loss of fenestration and an increase in the number of contractile HSCs[39]. These events contribute in an increase in sinusoidal resistance; in the context of advanced fibrosis this can contribute to portal hypertension. Other factors that stimulate HSC contraction include nitrous oxide deficiency, ET-1, angiotensinogen II, eicosanoids, atrial naturetic peptide and somatostatin.
Retinoid loss
HSC activation is characterised by the loss of perinuclear retinoid droplets[40]. As described earlier HSC are the largest reserve of retinoids in the body and conversion of retinol into retinyl ester is a characteristic feature of HSC activation.
Matrix degradation
Matrix metalloproteinases (MMPs) are the are the main enzymes responsible for ECM degradation; the MMP activity is in turn regulated by tissue inhibitors of metalloproteinases (TIMPs). Both MMPs and TIMPs are produced by several liver cell populations including Kupffer cells, myofibroblasts and hepatocytes[41]. However, TIMPs are predominantly expressed by activated HSCs. The A Disintegrin and Metallopoteinase-domain proteins form another stimulus for stellate cell collagen production via TGF-β activation[42].
Cytokine expression
HSCs express a vast array of chemokines that have been identified to recruit neutrophils, macrophages, NK/Natural killer T cells, dendritic cells and T cells. As a result, HSCs play an important role in immune cell infiltration.
PROGRESSION OF FIBROSIS IN CHRONIC LIVER DISEASES TO CIRRHOSIS
Cirrhosis can be defined as “the histological development of regenerative nodules surrounded by fibrous bands in response to chronic liver injury that leads to portal hypertension and end stage liver disease”[43]. Cirrhosis is a consequence of long standing excessive fibrogenesis resulting in encapsulation and/or replacement of injured liver parenchyma by a collagenous scar. Histologically, cirrhosis is characterized by fibrotic septa that link portal tracts with each other and central vein. This produces liver parenchyma that is composed of hepatocyte islands that are surrounded by fibrotic septa and devoid of a central vein. These changes result in an increase in intravascular resistance within the portal venous system and decreased hepatic perfusion. In addition, the development of cirrhosis confers a significant increase in the risk of developing hepatocellular carcinoma (incidence up to 30% over a 5 year period)[43]. Progression of fibrosis to cirrhosis is variable and is dependent on the cause of liver disease, environmental and host factors[43]. Cirrhosis is frequently asymptomatic and unsuspected until complications of liver disease present with include variceal bleeding, ascites, spontaneous bacterial peritonitis, encephalopathy and death.
EXPLORING THE PATHOGENESIS OF INFLAMMATION AND FIBROSIS IN NAFLD
The pathogenesis of NASH is complex involving hepatic parenchymal and non-parenchymal cells together with immune cells. With regard to pathogenesis, a “two hit” hypothesis was first proposed in 1998[44]; this has subsequently been modified into “three hit”[45] and “multiple hit” hypotheses[46]. In the original “two hit” hypothesis, the development of insulin resistance results in excessive lipid accumulation within hepatocytes (the first hit). The first hit is followed by lipotoxic metabolite-induced mitochondrial dysfunction, oxidative stress and endoplasmic reticular (ER) stress which leads to hepatocyte death (the second hit). Under normal physiological circumstances, existing hepatocyte replication results in the replacement of dead hepatocytes. In NASH however, it is believed that progenitor cell replication is enhanced as hepatocyte replication is impaired. Although progenitor cell proliferation results in the replacement of dead hepatocytes it also results in hepatic stellate cell activation and fibrogenesis (the third hit). The “multiple hit” hypothesis describes further additional insults derived from other sites such as the GI tract (such as gut-derived endotoxins due to impaired gut permeability and stasis) and adipose tissue (adipokines). Moreover, the “multiple hit” hypothesis allows one to understand that the development of NASH is the result of a complex interplay between factors such as the genetic variation in immune balance and the influence of additional aetiologies such as alcohol consumption and obesity. Whereas several pathways (including direct lipotoxicity, inflammasome activation, toll-like receptor signalling, hedgehog signalling) have all been implicated in the pathogenesis of cellular inflammation in NASH, the final pathway appears to be the development of a profibrotic state.
EPIGENETIC AND GENETIC REGULATION IN NASH
Recent genome-wide association studies have identified several genes that confer an increased risk of NASH amongst individuals with NAFLD[47]. These include patatin-like phospholipase domain containing 3 (PNPLA3), transmembrane 6 superfamily member 2 (TM6SF2), farnesyl diphosphate farnesyl transferase 1 (FDFT1), EF-hand calcium binding domain 4B and glucokinase receptor. Of these identified genes, the most widely studied is the PNPLA3 gene located on chromosome 22. PNPLA3 gene encodes a 481 amino acid protein that mediates triacylglycerol hydrolysis. In studies of Huh7 hepatoma cell line cells, PNPLA3 variant p.I148M (rs738409 substitution of cytosine to guanine) was associated with reduced enzymatic activity of emulsified triglycerides following hydrolysis. Within clinical studies, the PNPLA3 variant p.I148M has also been found to be associated with a high risk of NASH in adult[48], paediatric[49] and also lean patients[50].
In addition, genome-wide association studies have identified that epigenetics (a reversible phenomenon affecting gene expression) also appears to contribute to the pathogenesis of NASH. A study of rat liver tissues and high fat emulsion induced fatty liver identified that the expression of genes involved in apoptosis, biosynthesis and inflammation increase in NASH[51]. By contrast, these studies have revealed down-regulation of expression of genes involved in DNA damage response signal transduction, cholesterol biosynthesis and carbohydrate metabolism.
LIPOTOXIC HEPATOCYTE INJURY
The accumulation of excess free fatty acids (FFA) in hepatocytes results in the generation of toxic lipid metabolites. This process, lipotoxicity, is thought to be fundamental in the development of NASH. Following exposure to these toxic metabolites, injured hepatocytes appear enlarged and swollen in a process termed ballooning. In addition to steatosis and lobular inflammation, the presence of ballooning is an essential diagnostic criterion for the presence of histologic NASH[52].
The source of excess FFA is multifactorial and excess dietary intake is certainly a contributing factor. In addition, an increase in intrahepatic FFA can occur because of de novo lipogenesis, adipose lipolysis and impaired FFA oxidation. Under normal circumstances, hepatocytes store FFA as triglycerides and it is postulated that the conversion of FFA into triglycerides may be protective against lipotoxicity[53]. However, in the context of FFA excess, the conversion to tryglycerides becomes saturated and alternative highly toxic lipid metabolites are formed: Ceramides, diacylglycerols and lysophosphatidylcholine and oxidised cholesterol metabolites[54]. These toxic metabolites cause liver injury through the overproduction of ROS.
Two major mechanisms of oxidative stress in NASH have been identified. The first, direct cell injury, occurs when there is an imbalance between pro-oxidants and antioxidants. The second, indirect cell injury, occurs when damaging cellular pathways are activated such as those involving NF-κB. Increased production of ROS induces activation of NF-κB which regulates the production of pro-inflammatory cytokines such as interleukin-1β (IL-1β), TNFα and interleukin-6 (IL-6).
ER stress is activated by FFA induced oxidative stress and appears to be an important mechanism in the development of NASH[55]. Prolonged and severe ER stress can lead to cell death. Studies have identified that ER stress markers are elevated in NASH[56], and that ER stress can activate inflammatory pathways such as Jun-(N)-terminal Kinase (JNK) and NF-κB[57].
INFLAMMATORY AND IMMUNE MEDIATORS IN NAFLD
Analogous to other liver diseases, a variety of inflammatory and immunologic mechanisms contribute to NASH and NAFLD progression. These include innate immunity (neutrophils, macrophages, NK cells and NK T cells), adaptive immunity (T and B cells), inflammasome activation and the gut-liver axis[58].
GUT MICROBIOTA AND MACROPHAGES IN THE PATHOGENESIS OF NASH
As described above, the liver is exposed to low level endotoxaemia and antigenaemia via the portal vein. Thereafter, Kupffer cells are the principle cell type responsible for antigen and endotoxin clearance thus maintaining immune tolerance and homeostasis. This balance can, however, be impaired in the context of changes to gut flora, gut permeability and Kupffer cell responsivity. Studies have identified evidence for gut dysbiosis as a potential factor in the aetiology of NAFLD. Lower levels of Bateriodetes together with higher levels of Prevotella and Porphoyromonas have been identified in patients with NAFLD as compared to healthy controls[59]. Moreover, in mouse models, hepatic steatosis and inflammation driven by inflammasome mediated dysbiosis were associated with enhanced hepatic TNF-α expression[60]. In another study examining differences in gut microbiota between patients health subjects, obese patients and those with NASH, marked differences were identified in the composition of gut flora in either obese (without NASH) or obese (with NASH) patients as compared with healthy subjects[61]. Whereas the gut composition of patients with obese and NASH patients were more similar, there were significant differences in the concentrations of Proteobacteria, Enterobacteriaceae, and Escherichia species. Interestingly, whilst similar blood-ethanol concentrations were observed between healthy subjects and obese non-NASH patients, obese NASH patients exhibited significantly elevated blood ethanol levels. It was therefore hypothesised that elevated ethanol blood levels in patients with NASH may be the result of alcohol producing gut bacteria.
Macrophages may be proinflammatory (classic M1 type) or anti-inflammatory (alternative M2 type) and their relative balance contributes to either injury or repair[16]. In both mouse models and human subjects, an increase in M2 Kupffer cells was found to promote M1 Kupffer cell apoptosis which in turn was found to inhibit progression of NAFLD[62]. Toll-like receptor activation (particularly TLR4) is associated with macrophage mediated inflammation in NASH[63] and is associated with the release of IL-1β, TNFα and IL-6[64]. Studies have identified that both TLR4 inhibition and macrophage depletion reduces NAFLD progression in both animal and human liver biopsy studies[65,66]. In addition, liver macrophages transform into “foam cells” in NASH by internalising oxidised low-density lipoprotein (ox-LDL) resulting in storage of cholesterol and cholesterol crystals in enlarged lysosomes[67]. Studies in mice have revealed that inhibition of ox-LDL recognition and ox-LDL uptake into foam cells is associated with reduced hepatic histologic progression[68].
NEUTROPHILS IN THE PATHOGENESIS OF NASH
A number have studies have identified a possible role of neutrophils in the progression of NAFLD and NASH. Neutrophil infiltration is a common histologic finding in patients with NASH with neutrophils frequently surrounded steatotic hepatocytes, resembling the crown-like structures found in obese adipose tissue[69]. Moreover, patients with NASH and also advanced fibrosis related to NASH have been found to have a higher neutrophil-to-lymphocyte ratio than patients without either NASH or advanced fibrosis[70]. Human neutrophil peptides are proteins produced by neutrophils that induce cytokine and chemokine production under inflammatory conditions. Mouse studies have identified that human neutrophil peptides can enhance hepatic fibrosis in fatty liver by inducing hepatic stellate cell proliferation[71]. In addition, the deletion of elastase (a protease secreted by neutrophils) in high fat diet induced obese mice was found to improve hepatic histologic inflammation with reduced of neutrophil and macrophage infiltration[72].
T AND B LYMPHOCYTES IN THE PATHOGENESIS OF NASH
Recent studies have confirmed that the innate immune system appears to play an important role in the pathogenesis of NASH. Analysis of liver histology obtained from patients with NASH has identified that the inflammatory infiltrate in NASH is heavily enriched with both lymphocytes and macrophages[73]. Moreover, the inflammatory infiltrate at the portal tracts is composed predominantly of CD8+ lymphocytes[74]. Regulatory T cells play a critical role in regulating inflammatory processes in NASH. Th17 T cells functionally oppose regulatory T cells and produce IL-17. Elevated levels of IL-17 have been identified in patients with obesity[75]. In mouse studies, the neutralisation of IL-17 was found to reduce the severity of NASH, implicating IL-17 as a mediator of NASH and identifying this as a potential therapeutic target[76,77].
More recently, B cells have emerged as additional protagonists in the development of NASH[78]. Studies have identified that NASH is associated with the presence of circulating antibodies targeting neoantigens formed from the interaction between lipid peroxidation products and cellular proteins[79]. These IgG antibodies against malondialdehyde-derived products may be present in up to 60% of patients with NASH[80]. Furthermore, raised anti-malondialdehyde IgG titres have been identified in patients with severe histologic NASH and advanced fibrosis[38]. In both serum and adipose tissue, B cell levels have been found to increase markedly in mice fed a high fat diet[81]. Interestingly, whilst B cell deficient mice display exhibit reduced insulin resistance, the transfer of B cells or specific IgG (isolated from wild type mice fed a high fat diet) is able to induce insulin resistance.
IL-17 has also been identified as an important mediator in the development of atherosclerosis in NAFLD[82]. In a 2014 study, the severity of atherosclerosis was assessed in patients with ultrasound evidence of NAFLD and normal liver function tests using carotid intima-media thickness. Multiple regression identified that a raised intima-media thickness was predicted both by the IL-17-related chemokine eotaxin, intima-media thickness and the amount of visceral fat. These results support the hypothesis that visceral adipose tissue releases IL-17 which in turn induces smooth muscle eotaxin secretion in atherosclerotic lesions.
INTERFERON ALPHA AND GAMMA IN THE PATHOGE-NESIS OF NAFLD
The association between intramuscular triglycerides–as assessed by ultrasonography of skeletal muscles - and interferons in NAFLD was explored in a recent study[83]. As compared to healthy subjects, patients with NAFLD were found to have higher levels of interferon alpha 2 and lower levels of interferon gamma. Moreover, the severity of intramuscular triglyceride was found to be inversely proportional to interferon alpha 2 levels whereas interferon gamma levels were not associated with intramuscular triglycerides severity. These results suggest the interplay between these cytokines may be instrumental in the development of NAFLD.
INFLAMMASOME ACTIVATION IN THE PATHOGENESIS OF NASH
Several studies have identified that inflammasome activation contributes to the development of NASH and fibrosis in NAFLD. In both mouse and human studies, both pro-IL-1β and pro-IL-18 have been found to be markedly increased in NASH[84,85]. In addition, mice deficient of either NLRP3, IL1-α or IL-1β have been found to be from both from diet induced hepatic inflammation and fibrosis[85,86]. In a further mouse study, inhibition of caspase-1 was also able to prevent the development of diet induced NASH and fibrosis[87].
HSC ACTIVATION IN THE PATHOGENESIS OF NASH
Prior to the development of NASH, HSC are hypothesised to be in a quiescent state. Thereafter, the development of both lipotoxicity and steatohepatitis is followed by HSC activation which results in ongoing fibrogenesis. Hitherto, ongoing studies have attempted to elucidate the mechanism for HSC activation in NASH: Studies of human hepatocytes have identified that lipid metabolite accumulation increases TGF-β and impairs adiponectin-mediated induction of activin A[88].
The Notch single-pass transmembrane receptor signalling pathway has been implicated in HSC activation. In a recent study, Notch pathway components were found to be upregulated in TGF-β activated HSC and in fibrotic livers with inhibition of Notch signalling decreasing HSC activation[89].
The stress-activated protein kinases, c-Jun N-terminal kinase (JNK) and p38 have been identified to have distinct and opposed roles in rat HSC[90]. Whereas JNK was identified to promote HSC proliferation, P38 was identified to inhibit HSC proliferation.
Hedgehog pathway activation (sonic hedgehog) has been identified to corollate with the severity of histologic injury and fibrosis in human NAFLD biopsy samples[91].
EXPLORING THE PATHOGENESIS OF INFLAMMATION AND FIBROSIS IN CHC
HCV structure and replication
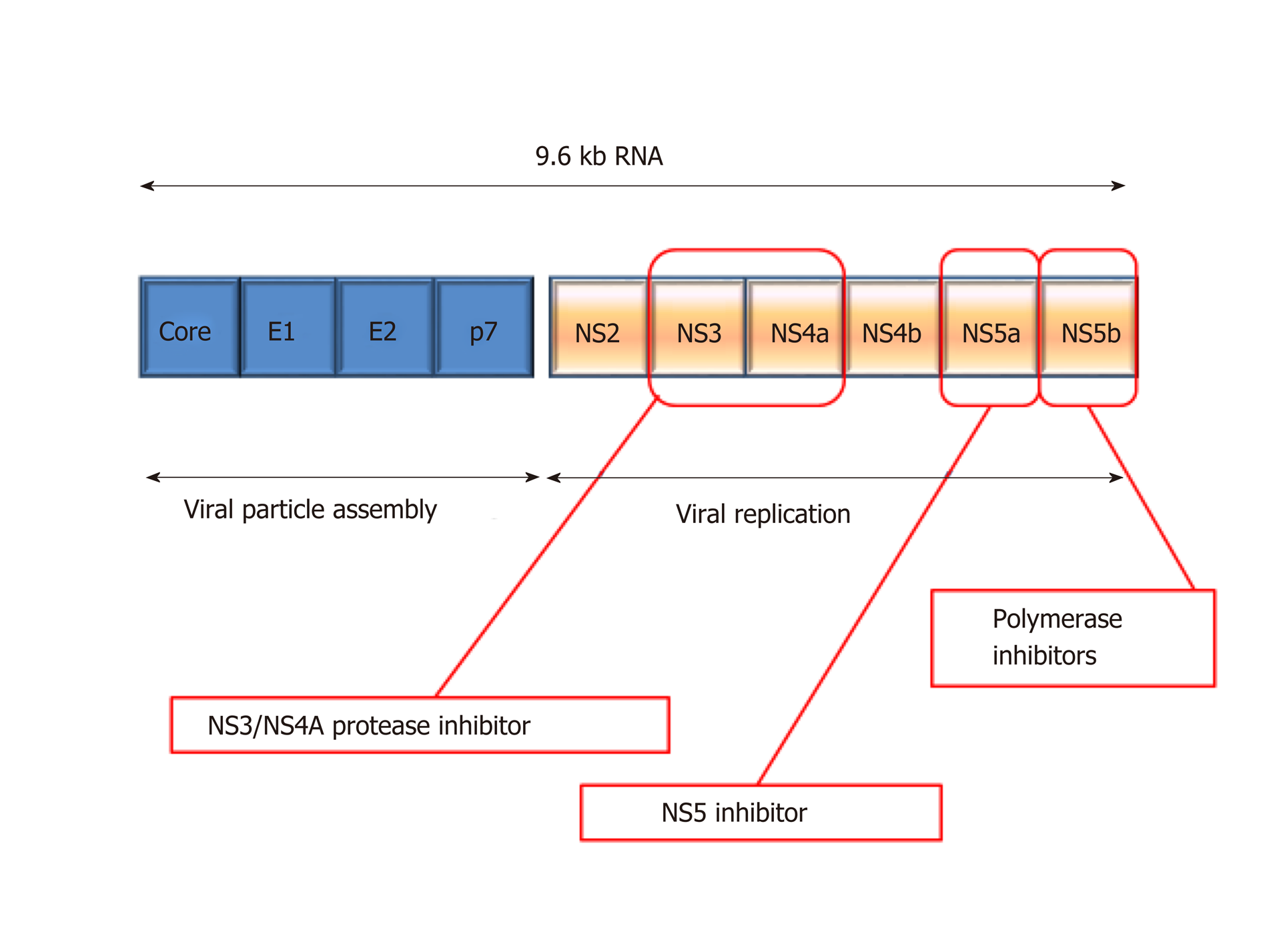
HCV is an enveloped, single stranded RNA molecule that is approximately 9600 nucleotides in length and encodes a polyprotein of approximately 3000 amino acids[92]. During HCV replication, the HCV polyprotein is cleaved by proteolytic enzymes into four structural and six non-structural proteins (Figure 6)[93]. New viral particles are assembled by the four structural proteins whilst the six non-structural proteins support viral replication. The post-translational processing of the non-structural proteins from the polypeptide is catalysed by the NS3 serine protease and cofactor NS4A. NS3/NS4A together complete the post translational processing of the NS proteins at the NS3/NS4A, NS4A/NS4B, NS4B/NS5A and NS5A/NS5B junctions[94]. These non-structural replicative products are then released and form a complex responsible for forming viral RNA.
Figure 6 Depiction of hepatitis C virus genome structure and drug targets.
NS: Non-structural.
The lack of a proof-reading capacity in the HCV RNA polymerase contributes to the extensive genetic variation within HCV isolates. Six major genotypes of HCV are recognised with numerous sub-types. Within an infected individual an HCV may exist as multiple quasi-species or minor genetic variants within a sub-type. The geographic distribution of HCV genotypes varies at an epidemiological level so that specific genotypes and sub-types are more common in certain countries.
Fifty five to 85% of individuals who develop acute HCV infection will develop CHC which is associated with progressive liver fibrosis and cirrhosis, portal hypertension, liver failure and hepatocellular carcinoma[95].
The primary goal of HCV treatment is to cure infection: Successfully treated patients may attain a sustain virologic response (SVR) which is considered tantamount to a “virological cure” (defined as the absence of HCV RNA 12-24 wk after the discontinuation of antiviral therapy). The attainment of SVR is typically associated with normalisation of liver function tests and either significant improvement or resolution of hepatic necroinflammation. In addition, this may also be associated with stabilisation or regression of liver fibrosis.
More recently, direct-acting antiviral (DAA) agents for the treatment of HCV have been developed that specifically target HCV viral replication. The development of DAA therapies is a consequence of the development of in-vitro HCV replication models[96] including the HCV replicon system. DAA agents were discovered by screening for their ability to inhibit viral replication[97]. In addition, the replicon systems were used to select and characterise resistant mutations to specific DAAs and also assess replication fitness[98].
The introduction of DAA agents has led to HCV therapy in treatment regimens that are highly efficacious (SVR 95-100%), well tolerated and abbreviated even in difficult to treat populations such as those with advanced liver disease[99].
Despite these remarkable advances in the treatment of established infection, a vaccine for HCV is still in its infancy. Regardless, it is likely that both prophylactic and therapeutic vaccines will be developed over the next decade through a greater understanding of host immunological factors, cell culture systems and animal models.
TLRs and HCV
As described earlier, TLRs are an important component of virus-mediated innate immune responses; this has also been confirmed in HCV. TLR-3 has been found to mediate antiviral responses against HCV in hepatoma cells[100]. In addition to inducing the production of type I interferons, TLR-3 activation also induces type III interferon (IFN-λ/IL-28) production[101]. A recent study also identified that a pro-fibrogenic HSC phenotype is mediated by TLR-2 signalling pathway following HCV core and NS3 protein exposure[102].
Direct activation of fibrogenesis by HCV
The interaction between HSCs and HCV is now well established. Hepatocytes infected with HCV have been found to release several pro-fibrotic factors including TGF-β that modify the expression of several profibrogenic genes in HSCs[103]. HSC have also been identified to engulf apoptotic bodies derived from HCV infection-induced hepatocyte apoptosis; this process has been found to elicit a profibrogenic response[104]. There is also evidence that HSC and HCV directly communicate with each other[105,106]; there is evidence that this may be mediated through both the core and non-structural proteins directly. Studies have identified that the core protein can active pro-mitogenic intracellular pathways with HSC with the NS3 and NS5 proteins stimulating pro-inflammatory pathways via NF-κB and c-JNK[106]. In addition, the E2 structural protein has also been identified as profibrotic with MMP-2 activation occurring after binding to CD81 of HSC[105]. In HCV replicon models studies using rat and human HSCs, HCV replication has been followed by the activation of TGFβ1 and other profibrotic cytokines[103,107].
Indirect activation of fibrogenesis by HCV
Analogous to other liver diseases, the ongoing immune response to HCV infection results in ongoing fibrogenesis via the activation of HSC. The mediators involved in the activation and proliferation of HSC include PDGF, TGFα, VEGF and activation of EGF receptor. Thereafter, several factors promote ECM production via TGF-β1 (stimulating collagen production via Smad signalling). Importantly, connective tissue growth factor (CTGF) and leptin also activate fibrogenesis via TGFβ1 signalling. Leptin also acts by suppressing proliferator-activated receptor γ (PPARγ). As described earlier, NF-κB signalling promotes a variety of chemotactic and mitogenic HSC effects.
REVERSIBILITY OF LIVER FIBROSIS
Fibrosis regression in CHC
Hitherto, treatment for chronic liver disease has been primarily aetiology specific in which the causative agent or factor has been eliminated or modified. The treatment of CHC is an excellent example of this in which successful antiviral therapy results in SVR which is tantamount to a virologic cure. The attainment of SVR abolishes ongoing liver injury and studies have confirmed that not only is fibrogenesis arrested but fibrosis reversal can also manifest[108-110].
Clinical regression of fibrosis in cirrhosis
Studies have identified that the potential for liver fibrosis regression may also be dependent on the duration of fibrosis and on several scar factors including scar composition, cellularity and spatial distribution[111]. Areas that may not be completely degraded include those that are acellular, rich in elastin and heavily cross-linked. Both animal and human studies have also identified that the regression of cirrhosis results in the transformation of cirrhotic micronodules into larger macronodules[111,112]. Furthermore, due to the significant vascular distortion, shunting and angiogenesis that is associated with cirrhosis, significant fibrosis regression in a previously cirrhotic liver may not result in portal pressure reduction[113].
Mechanisms for fibrosis regression
An understanding of the mechanisms that underlie fibrosis regression have led to the identification of antifibrotic targets and the developments of antifibrotic agents[114].
Studies have confirmed that rather than being a fixed entity, the hepatic scar is in a state of continuous dynamic flux with respect to its cellular and matrix composition. In keeping with fibrosis progression, both myofibroblasts and hepatic macrophages are key protagonists in the regression of fibrosis. MMPs are a group of proteolytic enzymes that are responsible for the degradation of extracellular matrix components. The MMP proteolytic activity is regulated by their potent specific inhibitors tissue inhibitors TIMPs as expressed by activated HSCs[115]. Moreover, the apoptosis of activated HSC may be inhibited by TIMPs.
In both animal models and human studies documenting fibrosis regression with scar degradation, TIMP levels decrease dramatically and MMP levels rise[111,115]. Thereafter, profibrogenic myofibroblasts are removed from the receding hepatic scar by apoptosis. In addition, recent studies have identified that hepatic macrophages are important regulators of ECM remodelling[116] highlighting the importance of specific macrophage subsets in either fibrogenesis or fibrosis regression (Ly-6Clo)[117].
HSCS AND FIBROSIS REGRESSION
As described earlier, HSCs play an important contribution to ECM remodelling in fibrosis evolution. Three mechanisms have been described through which HSCs can contribute to fibrosis regression: Apoptosis, senescence or quiescence.
HSC apoptosis
HSC apoptosis leads to a decrease in the number of activated HSCs and has been observed during fibrosis regression[118]. This results in a decrease in TIMP-1 expression and thus inhibiting MMP activity. Apoptosis can be initiated in HSCs via CD95L (Fas ligand), Bcl-2 and p53[119]. Moreover, apoptosis can be initiated by hepatocytes (via NGF)[120], NK cells (via TNF-related apoptosis inducing ligand and NKG2D)[19] and Kupffer cells (via capsase-9)[121].
HSC senescence
There is evidence that HSCs can enter a senescent phase adopting a more inflammatory but less fibrogenic phenotype[122]. Both in culture and in vivo there is evidence that HSC senescence is mediated by p53.
HSC quiescence
Studies have demonstrated that a large proportion (approximately 50%) of activated HSCs can revert to a quiescent phenotype[123,124]. What has also been demonstrated, however, is that quiescent HSCs have a lower threshold to enter an activated state than naïve HSCs. HSC quiescence is thought to be mediated by PPAR-γ.
TARGETS OF HSC MEDIATED ANTIFIBROTIC THERAPY
Reduction of the inflammatory/immune response or inhibition hepatocyte apoptosis/injury to avoid HSC activation.
Promotion of fibrosis regression by inhibiting scar formation; promotion of matrix degradation; inhibition HSC activation or stimulate HSC apoptosis/sene-scence/quiescence.
Inhibition of signalling pathways (extracellular and intracellular) that initiate and perpetuate HSCs
CLASSES OF ANTIFIBROTIC THERAPY
Treatments for liver fibrosis can be categorised into several ways. Antifibrotic treatment may target the underlying aetiology of liver disease specifically (for example in CHC). Conversely, antifibrotic treatments may act with a direct anti-fibrotic action in the absence of any effect on the underlying aetiology of liver disease. These may be established drugs licenced for other indications and rediscovered to investigate their potential antifibrotic effect (drug repositioning).
Type 1 interferons
In CHC, type 1 interferons were found to exhibit an antifibrotic effect in animal models[125,126] with a reduction in portal pressure[127] and significant histological improvement even in the absence of achieving SVR[128]. These observations were translated into the three large clinical trials that addressed the use of long term interferon in patients with advanced fibrosis[129-131]. All 3 trials identified that low maintenance dose of PEG-IFN did not improve outcomes in patients with compensated cirrhosis and CHC after a lead-in phase of PEG-IFN.
Activated myofibroblasts
Activated myofibroblasts can contribute to fibrosis regression by the release of proteolytic enzymes (mainly MMPs) that degrade ECM when subjected to favourable stimuli integrin receptor-mediated) in a process called “stress relaxation”. Myofibroblast stress relaxation resulting in a reduction in fibrogenesis and portal hyper tension has been demonstrated in cirrhotic rats by inhibiting Rho kinase[132]. Myofibroblasts have also been targeted by liposomes loaded with siRNA-Loaded Cationic Nanohydrogel Particles[133].
Damaged hepatocytes
Myofibroblasts can become activated by the phagocytosis of apoptotic hepatocytes. Biliary fibrosis has been found to be ameliorated in mice by the inhibition of hepatocyte apoptosis using a pan-cases inhibitor or an antagonist of cathespin B (a lysosomal trigger of apoptosis)[134,135].
Biliary progenitors
The “ductular reaction” is classically seen in biliary fibrosis where biliary progenitor cells proliferate and secrete cytokines that promote HSC activation. In advanced fibrosis of “non-biliary” liver diseases, liver progenitor cell proliferation is also marked after the development of portal fibrosis. Several drugs targeting biliary progenitor cells have been used as antifibrotics including those inhibiting integrin αvβ6 (a receptor for fibronectin and tenascin-C)[136] and the hedgehog pathway[137,138].
LSECs
During perisinusoidal fibrosis, activated LSECs produce ECM and secrete fibrogenic cytokines. LSECs themselves are activated by angiogenic factors produced by myofibroblasts including VEGF and angiopoetin-1[139]. Polykinase inhibitors such as sunitinib and sorafenib are antiangiogenic and have been identified to ameliorate experimental liver fibrosis[39,140].
Monocytes
Monocytes are the precursors of myofibroblasts, macrophages and dendritic cells playing an important role in inflammation and fibrosis. The chemokine CXCL9 has been identified to inhibit fibrogenesis by activating the monocyte receptor CX3CR[141,142].
Lysyl oxidase (LOXL2)
The enzyme LOXL2 mediates ECM crosslinking of matrix proteins including collagen. Antifibrotic activity has been demonstrated using LOXL an experiment models of liver fibrosis[143]. Simtuzumab is a monoclonal antibody targeting LOX2 and is currently being evaluated in phase II trials in patients with NAFLD with and without cirrhosis.
TLRs
TLR activation promotes a proinflammatory environment and TLR3 and TLR4 are being investigated for their antifibrotic properties.
TREATMENTS AND THERAPIES FOR NASH
Numerous medications are currently being studied for the treatment of NASH and fibrosis related to NAFLD. Whereas many of these medications have been shown to be effective in improving liver histology, the magnitude of the treatment effect is at best modest and there may also be associated adverse events.
Diet and physical activity
Sustained weight loss of more than 10% has been identified to be effective at improving liver histology (inflammation and fibrosis)[144-146].
PPAR agonists
Perioxisome proliferator-activator receptors (PPARs) are nuclear receptors that are expressed in a variety of organs including the liver. There are 3 types of PPAR receptor: α, β/δ and γ. PPARs regular metabolic processes including oxidation and lipid transport. PPARα agonists (for example fibrates) have not shown significant histologic benefit in NAFLD. In a phase IIb study, the dual PPARα/δ agonist was found to increase resolution of histologic NASH[147]; phase III studies are currently being conducted. Thiazolidinediones (for example pioglitazone) are PPARγ agonists that commonly used to treat diabetes and act as insulin sensitisers. In patients with NAFLD, treatment with pioglitazone has been shown to improve liver histology[148-150]. There is however, a risk of weight gain and congestive heart failure with pioglitazone treatment.
Farnesoid X receptor bile acid axis
Farnesoid X receptor (FXR) is a bile acid intracellular receptor that both regulates bile acid synthesis and decreases hepatic gluconeogenesis[151]. Obeticholic acid is a synthetic bile acid derivative and an FXR agonist. In phase II studies treatment with Obeticholic acid significantly improved liver histology (NASH and fibrosis) in patients with NAFLD[152]. Side effects of Obeticholic acid include reversible pruritus and worsening lipid profile. Phase III trials of Obeticholic acid are currently being conducted. Other agents work via the FXR-bile acid are also under investigation (FGF-19 and NGM-282)
Lipid-altering agents
Aramchol is Stearoyl-CoA desaturase inhibitor that is currently being investigated in phase III studies for NASH. In a small phase II study Aramchol was associated with a decrease in hepatic fat content[153]. HMG-CoA reductase inhibitors (statins) are used extensively in both primary and secondary care for the treatment of hyperlipidaemia. There is evidence that statin use may improve liver histology (steatosis, steatohepatitis and fibrosis) in patients with NAFLD[154].
Incretin based therapies
Glucagon-like peptide 1 agonists such as liraglutide and exenatide are primarily used for the treatment of diabetes. Glucagon-like peptide 1 belongs to the incretin family of proteins and acts on the pancreas to cause beta-cell proliferation and enhance insulin biosynthesis. A meta-analysis of several studies identified that treatment with Glucagon-like peptide 1 agonist was associated with improved hepatic steatosis and fibrosis[155].
Agents targeting inflammation, cell injury and inflammation
Vitamin E is an antioxidant that has been shown in both paediatric and adult NAFLD studies to improve hepatic steatosis, inflammation and NASH but not hepatic fibrosis[156,157]. Pentoxyphilline is a methylxanthine derivative that modulates several cytokines including inhibiting TNFα. In a small study of 55 patients, as compared to placebo, patients with biopsy proven NASH treated with pentoxyphilline were found to have improved NAS scores and fibrosis scores[158]. Larger studies will be needed to confirm these findings.
CONCLUSION
As CLD often develops insidiously most CLD is diagnosed at the stage of cirrhosis when interventions are ineffective, resulting in considerable morbidity and mortality[159,160]. Standard “liver function tests” have poor sensitivity and specificity for the detection of liver fibrosis[161,162]. The reference standard for staging liver fibrosis remains the histological staging of a liver biopsy specimen. Liver biopsy itself, even in experienced centres, is associated with complications such as pain (20%), serious morbidity (0.6%) and even death (0.01%)[163]. Moreover, the accuracy and reliability liver biopsy is limited due to sampling error and both inter- and intra-observer variability. As such the reference standard for assessing liver disease is not practicable for the detection of liver injury amongst individuals at risk of CLD and instead a non-invasive approach is desirable.
Non-invasive alternatives to liver biopsy include imaging modalities and serum markers. Imaging modalities such as transient elastography have been developed to detect liver fibrosis by measuring the degree of liver stiffness. Whereas serum markers of fibrosis may not be entirely liver specific, liver stiffness itself is importantly an intrinsic physical property of liver tissue. On an individual patient basis however, it can be more time consuming to assess liver fibrosis using an imaging modality than by using a serum marker. Although scanning itself may take only a few minutes the procedure time may be prolonged due to factors such as patient positioning and patient compliance. By contrast, serum markers of fibrosis can be measured at the time of routine blood tests. Liver stiffness values can be influenced by a variety of factors including concurrent inflammation and recent food ingestion. Whereas imaging modalities are reliable clinical discriminators (for example advanced versus non-advanced fibrosis) they have a limited role in furthering our understanding of hepatic pathophysiology.
Only by having a comprehensive understanding of the pathophysiology of chronic liver disease can appropriate and meaningful diagnostic targets for research studies be identified. Regardless, for clinicians who are not engaged in laboratory research this also requires a revision of basic anatomy, physiology and immunology. In this review article we have attempted to go “back to basics” to enable liver specific pathophysiology to be contextualised.
Through an understanding of hepatic inflammation and matrix biology, candidate direct biomarkers have been successfully identified and validated for use in clinical practice with these studies complementing the indirect candidate biomarker approach that have also proved successful. Indeed, these approaches have been combined and there are now numerous examples of hybrid biomarker panels.
Regardless, clinically meaningful diagnostic targets can change dramatically in response to advances in therapy. This point is highlighted by our focus on both CHC and NAFLD. Whereas the treatment of NAFLD is still in its infancy, treatment for CHC has evolved from toxic interferon containing regimens to the abbreviated highly efficacious regimens of today. As such, whereas fibrosis stratification and CHC related fibrosis are no longer the priorities that they were perhaps a decade ago, the lessons learnt from these studies remain invaluable. Moreover, these studies continue to foster the development of antifibrotic therapies through both drug repositioning and the development of novel agents. Even after the successful eradication of CHC, therapies to accelerate fibrosis regression are needed for patients with cirrhosis as ongoing fibrotic reversal is slow and in some cases liver fibrosis can continue to progress.
By contrast to the treatment of CHC, therapies for NAFLD are still very much in their infancy. Notwithstanding, the development of novel agents targeting hepatic inflammation and fibrosis is now progressing at a rapid pace. Furthermore, we hope that we will soon embrace an era of personalised treatment of liver fibrosis and inflammation that can be accurately monitored by non-invasive biomarkers.