Published online May 21, 2020. doi: 10.3748/wjg.v26.i19.2388
Peer-review started: December 30, 2019
First decision: February 16, 2020
Revised: March 27, 2020
Accepted: April 21, 2020
Article in press: April 21, 2020
Published online: May 21, 2020
Processing time: 142 Days and 23.2 Hours
Neoadjuvant chemotherapy is currently recommended as preoperative treatment for locally advanced rectal cancer (LARC); however, evaluation of treatment response to neoadjuvant chemotherapy is still challenging.
To create a multi-modal radiomics model to assess therapeutic response after neoadjuvant chemotherapy for LARC.
This retrospective study consecutively included 118 patients with LARC who underwent both computed tomography (CT) and magnetic resonance imaging (MRI) before neoadjuvant chemotherapy between October 2016 and June 2019. Histopathological findings were used as the reference standard for pathological response. Patients were randomly divided into a training set (n = 70) and a validation set (n = 48). The performance of different models based on CT and MRI, including apparent diffusion coefficient (ADC), dynamic contrast enhanced T1 images (DCE-T1), high resolution T2-weighted imaging (HR-T2WI), and imaging features, was assessed by using the receiver operating characteristic curve analysis. This was demonstrated as area under the curve (AUC) and accuracy (ACC). Calibration plots with Hosmer-Lemeshow tests were used to investigate the agreement and performance characteristics of the nomogram.
Eighty out of 118 patients (68%) achieved a pathological response. For an individual radiomics model, HR-T2WI performed better (AUC = 0.859, ACC = 0.896) than CT (AUC = 0.766, ACC = 0.792), DCE-T1 (AUC = 0.812, ACC = 0.854), and ADC (AUC = 0.828, ACC = 0.833) in the validation set. The imaging performance for extramural venous invasion detection was relatively low in both the training (AUC = 0.73, ACC = 0.714) and validation (AUC = 0.578, ACC = 0.583) sets. The multi-modal radiomics model reached an AUC of 0.925 and ACC of 0.886 in the training set, and an AUC of 0.93 and ACC of 0.875 in the validation set. For the clinical radiomics nomogram, good agreement was found between the nomogram prediction and actual observation.
A multi-modal nomogram using traditional imaging features and radiomics of preoperative CT and MRI adds accuracy to the prediction of treatment outcome, and thus contributes to the personalized selection of neoadjuvant chemotherapy for LARC.
Core tip: Our study developed and validated a radiomics model that incorporated computed tomography and magnetic resonance imaging radiomics features for noninvasive and individualized prediction of clinical response to neoadjuvant chemotherapy in patients with locally advanced rectal cancer. The combination of computed tomography and magnetic resonance imaging radiomics features was associated with better performance than any individual sequence. In contrast, the clinical model based on extramural venous invasion achieved relatively low diagnostic performance. The multi-modal nomogram facilitated the easy and noninvasive estimation of clinical response to neoadjuvant chemotherapy. The proposed radiomics model performs well, thereby guiding clinical decision-making and preoperative assessment of neoadjuvant chemotherapy for locally advanced rectal cancer.
- Citation: Li ZY, Wang XD, Li M, Liu XJ, Ye Z, Song B, Yuan F, Yuan Y, Xia CC, Zhang X, Li Q. Multi-modal radiomics model to predict treatment response to neoadjuvant chemotherapy for locally advanced rectal cancer. World J Gastroenterol 2020; 26(19): 2388-2402
- URL: https://www.wjgnet.com/1007-9327/full/v26/i19/2388.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i19.2388
With the improvement in inspection technology and implementation of the concept of individualized treatment, the early detection rate of rectal cancer has markedly increased[1]. However, the postoperative recurrence rate of locally advanced rectal cancer (LARC) is high, leading to poor prognosis, and neoadjuvant chemo-radiotherapy (CRT) followed by total mesorectal excision (TME) is recommended for LARC[2,3]. As previously reported[2,4-6], neoadjuvant CRT can downstage the rate of LARC by 50%-60%, and achieve a pathological complete response (PCR) rate of 15%-27%.
However, several studies[7-10] have reported that there were still 7%-37% of LARC patients who do not respond to neoadjuvant CRT, which may not only increase CRT-related side effects and economic burden, but also delay surgery time. Furthermore, non-responders were associated with lower recurrence-free survival rate, distant metastasis, and local recurrence rate compared with good responders[11]. Therefore, it is necessary to identify which patients can benefit from neoadjuvant CRT treatment.
Enhanced computed tomography (CT) and magnetic resonance imaging (MRI) are recommended and commonly used for LARC[3] to noninvasively evaluate the therapeutic responses to neoadjuvant CRT. Different imaging techniques have been reported to identify the response to neoadjuvant CRT, including fluorodeoxyglucose positron emission tomography (FDG PET), T2-weighted MRI, dynamic contrast-enhanced MRI, and diffusion-weighted imaging (DWI); however, their performance is varied and limited[12-15]. Thus, there is an increasing need to identify a more reliable method for evaluating therapeutic response.
Radiomics, which involves computer-based extraction of a large number of quantitative imaging features, are analyzed with a specific clinical question in mind to help clinical decision-making[16]. There are several reports of the successful use of radiomics analysis for the classification of benign and malignant tissue[17], adding information about tumor aggressiveness[18-20], and predicting responsiveness to neoadjuvant CRT prior to initiation[21,22]. We hypothesize that CT and MRI-based radiomics may add value in the evaluation of therapeutic responses to neoadjuvant chemotherapy in patients with LARC; thus, improving qualitative assessment will help differentiate patients with a clinical response from those with no response after neoadjuvant chemotherapy.
The aim of this retrospective study was to create a multi-modal radiomics model derived from CT and MRI, and to investigate the added value for predicting clinical response in patients with LARC after neoadjuvant chemotherapy.
This study was approved by the Ethics Committee of West China Hospital of Sichuan University (No. 2019-140). Patient approval or informed consent for the review of medical images was not required.
LARC was defined as the primary tumor invading the muscularized layer of the intestinal wall (T3-4), with or without peripheral lymph node metastasis (N0-2), and without distant metastasis, as detected by imaging or pathological examination[23]. We retrospectively included patients with LARC who underwent total TME after neoadjuvant chemotherapy in the Gastrointestinal Surgery Department of our hospital from October 2016 to June 2019. Inclusion criteria were: (1) Rectal MRI and abdominal enhanced CT scan were both performed before neoadjuvant chemotherapy; and (2) All patients received neoadjuvant chemotherapy before TME. Exclusion criteria were: (1) Familial polyposis; (2) History of neoadjuvant CRT for other malignant tumors; (3) CT and MRI revealed incomplete images prior to neoadjuvant chemotherapy. Image quality was poor, and artifacts were obvious, which could not be used for image segmentation and radiomic feature extraction and analysis; and (4) Clinical, laboratory, and pathology data were incomplete. The patient selection process is summarized in Figure 1.
The first chemotherapy course adopted the CapeOx plan (oxaliplatin 30 mg/m², day 1 and capecitabine 850-1000 mg/m², bid, day 1-14). With no break time after the first course, the second to fourth courses adopted the CapeOx plan with sequential oral apatinib 250 mg qd for 10 consecutive days. There were breaks of 3 wk for the second to the fourth course; and 3 wk after the fourth course of neoadjuvant chemotherapy, TME surgery was performed.
Enhanced CT was performed using a 128-MDCT scanner (Somatom Definition AS+, Siemens Healthcare Sector, Forchheim, Germany) and a dual-source CT system (Somatom Definition Flash, Siemens Healthcare Sector, Forchheim, Germany). Both CT models had the same tube voltage (120 kV), tube current (200-210 mAs), and slice thickness (2 mm). Intravenous nonionic contrast material (1.2 mL/kg; omnipaque 300 mg/mL, GE Healthcare) was administered via the antecubital vein, using a power injector at a rate of 3 mL/s. The area of interest was located in the center of the abdominal aorta at the level of the abdominal trunk. With a trigger threshold of the aorta reaching 170 HU, the arterial phase (at the trigger) and the portal vein phase (30 s after the trigger) images were obtained.
MRI was performed using a 3.0-T magnet (Magnetom Skyra, Siemens Healthcare, Erlangen, Germany) with an 18-channel matrix coil. All patients underwent bowel preparation with antispasmodic medications before imaging. A routine clinical imaging protocol was performed including axial HR-T2WI and axial DWI MRI with apparent diffusion coefficient (ADC). Dynamic contrast-enhanced (DCE) images were obtained using a fat-suppressed 3D gradient-echo T1 weighted sequence (volumetric interpolated breath-hold examination, known as ‘VIBE’). Dynamic contrast-enhanced images were obtained using 3D T1-VIBE with a volumetric acquisition of the entire rectum that began simultaneously with the intravenous administration of gadolinium (0.5 mmol/mL; Omniscan, GE Healthcare, Cork, Ireland) followed by a 30 mL saline flush (3 mL/s). The entire volume was acquired in one second, and the acquisition was repeated over a one-minute scan time to acquire an exact evaluation of the medium contrast kinetics in the tumor tissue of all vascular phases. The MRI parameters at our institution are summarized in Supplementary Table 1.
| Variable | Training cohort, n = 70 | Validation cohort, n = 48 | ||||
| Response, n = 48 | Non-response, n = 22 | P value | Response, n = 32 | Non-response, n = 16 | P value | |
| T3 | 9 (18.8) | 0 (0) | 0.002 | 9 (28.1) | 0 (0) | 0.03 |
| T4a | 31 (64.6) | 10 (45.5) | 16 (50) | 8 (50) | ||
| T4b | 8 (16.7) | 12 (54.6) | 7 (21.9) | 8 (50) | ||
| N0 | 8 (16.7) | 1 (4.6) | 0.4 | 6 (18.8) | 2 (12.5) | 0.6 |
| N1 | 26 (54.2) | 13 (59.1) | 13 (40.6) | 9 (56.3) | ||
| N2 | 14 (29.2) | 8 (36.4) | 13 (40.6) | 5 (31.3) | ||
| Site: Ultralow | 2 (4.2) | 1 (4.55) | 0.2 | 4 (12.5) | 1 (6.3) | 0.01 |
| Site: Low | 34 (70.8) | 12 (54.6) | 21 (65.6) | 5 (31.3) | ||
| Site: High | 12 (25) | 9 (40.9) | 7 (21.9) | 10 (62.5) | ||
| EMVI positive | 33 (68.8) | 5 (22.7) | < 0.001 | 24 (75) | 2 (12.5) | < 0.001 |
| EMVI negative | 15 (31.3) | 17 (77.3) | 8 (25) | 14 (87.5) | ||
| Female | 14 (29.2) | 10 (45.5) | 0.2 | 9 (28.1) | 6 (37.5) | 0.5 |
| Male | 34 (70.8) | 12 (54.6) | 23 (71.9) | 10 (62.5) | ||
| CEA ≤ 3.4 | 32 (66.7) | 14 (63.6) | 0.8 | 21 (65.6) | 12 (75) | 0.5 |
| CEA > 3.4 | 16 (33.3) | 8 (36.4) | 11 (34.4) | 4 (25) | ||
| CA199 ≤ 22 | 39 (81.3) | 19 (86.4) | 0.6 | 29 (90.6) | 14 (87.5) | 0.7 |
| CA199 > 22 | 9 (18.8) | 3 (13.6) | 3 (9.4) | 2 (12.50) | ||
| Age in yr | 59.2 ± 9.7 | 54.8 ± 10.5 | 0.09 | 60.8 ± 9.6 | 55.3 ± 11.1 | 0.08 |
| BMI in kg/m2 | 22.9 ± 3.2 | 23.1 ± 3.2 | 0.8 | 22.8 ± 3.4 | 23.3 ± 2.9 | 0.6 |
| Hb in g/L | 134.8 ± 20.5 | 127.9 ± 19.5 | 0.2 | 131.4 ± 19.5 | 127.1 ± 22.2 | 0.5 |
The features of extramural venous invasion (EMVI) and tumor location were evaluated by two radiologists (with 8-12 years of experience in rectal cancer imaging) who were blinded to pathological results using a scoring system from 0 to 4[24]. EMVI scoring from 0 to 2 was defined as negativity, and EMVI scoring from 3 to 4 was defined as positivity. Upon disagreement, they would reach a consensus through negotiation.
The open source software ITK-SNAP (3.6.0, open source, http://www.itksnap.org) was used for image segmentation. Pre-treatment enhanced CT and MRI findings were analyzed by a radiologist (with 8 years of experience in rectal cancer imaging), and validated by a senior radiologist (with 12 years of experience in rectal cancer imaging) within 1-2 wk to calculate intraclass correlation coefficients (ICCs). Both radiologists were blinded to the histopathology results. The regions of interest (ROIs) were created manually using the enhanced CT, HR-T2WI, and last phase (60 s after contrast injection) images from DCE-T1 (DCE-T1-60s) and ADC data, including the whole tumor and excluding the intestinal lumen. ROIs of rectal tumors were manually drawn on each slice. In order to decrease data variability and make it easier to evaluate quantitative radiomic features, intensity normalization was performed to transform original CT and MR images into a similar intensity distribution[25].
The 118 patients were randomly divided into a training set (n = 70) and a validation set (n = 48). A total of 396 radiomic features of each sequence were extracted from all CT and MR images using the in-house Artificial Intelligence Kit software v.3.0.0. Normalization of extracted features was performed in the first step before feature selection. We replaced outliers with the median of the particular variance vector when the values reached beyond the range of the mean and standard deviation. In addition, we standardized the data in a specific interval. The standardized formula was as follows: (fi-u)/std, where fi represents a single characteristic datum, u is the average value of the data column, and std is the standard deviation of the data column.
Adding a prior feature ranking procedure may be helpful for improving final performance. Therefore, after elimination of redundant features and features with low reproducibility, we used a multivariate ranking method [minimum redundancy maximum relevance (mRMR)] to identify the most important features on the basis of a heuristic scoring criterion, and only the top ranked features were retained[26-28]. The least absolute shrinkage and selection operator (LASSO) was then used for selection bias of the features from the 20 top ranked features. λ was the regularization parameter of LASSO regression and was selected when the ten-fold-cross-validation error was minimal.
Considering that the non-responders group contained fewer patients than the responders group, this might have an adverse impact on classifier performance; thus, the synthetic minority oversampling technique was applied with the joint weighting of features in the optimal subset to generate samples from the minority group to balance the size of the majority group[29-31]. The advantage of this method is to obtain synthetic samples that have similar attribution values to existing samples and are “not merely replications”, thus enhancing the representation of the minority group while retaining the original structure of the samples.
Finally, the most significant features were determined to construct the radiomics model on the basis of logistic regression. We first created five different models based on individual enhanced CT, MRI (DCE-T1, HR-T2WI, ADC), and EMVI, and then compared the performance of the models based on individual sequences and their combination. The best of these models was constructed into the multi-modal radiomics nomogram. Clinical risk factors were compared via univariate analysis, and variables with P < 0.05 were included in the clinical model. Models were trained using the repeated 10-fold-cross-validation method in the training set, and estimation performance was evaluated in the validation set.
The reference standard was the histopathological results generated by assessing the basic histopathology of the tumor specimens after TME. Histopathological analysis was performed by specialized gastrointestinal histopathologists with more than 10 years of experience who was blinded to imaging findings. Pathological grading of primary tumor regression was defined according to the four-tier American Joint Committee on Cancer tumor regression grade (TRG) system[32]. TRG 0-2 was defined as a response to neoadjuvant chemotherapy, and TRG 3 was defined as non-response.
Statistical analyses were performed with R software v. 3.4.3 (R Core Team, Vienna, Austria). Performance of the different models was assessed using the receiver operating characteristic curve (ROC) analysis, and demonstrated as area under the curve (AUC) and accuracy (ACC). Calibration plots were used to graphically investigate the performance characteristics of the nomogram. The DeLong test was used for statistical comparison of the ROC curves. The t test or Mann-Whitney U test was performed to compare continuous variables, while a χ2 or Fisher’s exact test was used for classifying variables between groups. All statistical tests were two-sided, and a Bonferroni-corrected P value was used to identify the significance of the feature by multiple comparisons.
In this study, we enrolled 118 LARC patients who underwent TME after neoadjuvant chemotherapy, including 38 (32.2%) non-responders (22 males, 16 females) and 80 (67.8%) responders (57 males, 23 females). The patients were randomly divided into the training set (n = 70) and the validation set (n = 48). Demographic and clinicopathological characteristics of the cohort are presented in Table 1. Univariate factor analysis showed statistically significant differences in EMVI between the two groups (P < 0.001). There were no differences in gender, age, BMI, TNM stage[3] (T, tumor; N, Lymph Node; M, Metastasis), carcinoembryonic antigen (CEA) level, Carbohydrate antigen 199 (CA199) level, and lesion region between the response and non-response groups.
A total of 396 radiomic features of each sequence were extracted from all CT and MR images (42 first-order histogram features, 334 second-order texture features, 9 morphological features, and 11 gray-level zone size matrix features). After elimination of redundant features and features with low reproducibility, 65 radiomic features, including 18 features from enhanced CT, 14 from HR-T2WI, 17 from ADC, and 16 from DCE-T1 (Supplementary Table 2) were used to build individual radiomics models. For the combined model, we used a multivariate ranking method (mRMR) to identify the most important features, and only the top ranked features were retained. The most significant features were then investigated to construct the radiomics model on the basis of logistic regression. Finally, 13 radiomic features with non-zero coefficients (two features from enhanced CT, one from HR-T2WI, five from ADC, and five from DCE-T1) were selected to calculate the radiomics score. The names of the selected features can be found in Supplementary Table 2. The agreement between the two radiologists on selected radiomic features was considered excellent (ICC range: 0.654 to 0.923). The Lasso process is shown in Figure 3.
| Characteristic | AUC | 95%CI | Cut-off | ACC | Specificity | Sensitivity |
| EMVI | ||||||
| Training | 0.73 | 0.619-0.842 | 0.331 | 0.714 | 0.688 | 0.773 |
| Validation | 0.578 | 0.426-0.731 | 0.583 | 0.594 | 0.562 | |
| CT | ||||||
| Training | 0.809 | 0.745-0.872 | 0.5 | 0.818 | 0.875 | 0.742 |
| Validation | 0.766 | 0.632-0.899 | 0.792 | 0.844 | 0.688 | |
| DCE-T1 | ||||||
| Training | 0.848 | 0.79-0.907 | 0.5 | 0.818 | 0.875 | 0.742 |
| Validation | 0.812 | 0.688-0.937 | 0.854 | 0.938 | 0.688 | |
| HR-T2WI | ||||||
| Training | 0.845 | 0.786-0.903 | 0.5 | 0.857 | 0.932 | 0.758 |
| Validation | 0.859 | 0.746-0.973 | 0.896 | 0.969 | 0.75 | |
| ADC | ||||||
| Training | 0.847 | 0.789-0.904 | 0.5 | 0.844 | 0.83 | 0.864 |
| Validation | 0.828 | 0.71-0.946 | 0.833 | 0.844 | 0.812 | |
| CRM | ||||||
| Training | 0.921 | 0.842-1 | 0.318 | 0.886 | 0.854 | 0.955 |
| Validation | 0.908 | 0.823-0.994 | 0.812 | 0.812 | 0.812 | |
| MRM | ||||||
| Training | 0.925 | 0.845-1 | 0.447 | 0.886 | 0.896 | 0.864 |
| Validation | 0.93 | 0.86-1 | 0.875 | 0.875 | 0.875 | |
As shown in Table 2, for an individual sequence, the HR-T2WI model performed better (AUC = 0.859, ACC = 0.896) than the CT (AUC = 0.766, ACC = 0.792), DCE-T1 (AUC = 0.812, ACC = 0.854), and ADC (AUC = 0.828, ACC = 0.833) models in the validation set. The combined radiomics model had a significantly better performance than CT (P = 0.03), while no significant differences were found when compared with DCE-T1, HR-T2WI, and ADC in the training set (Figure 4A). In the validation set, the combined radiomics model (AUC = 0.908, ACC = 0.812) had a better performance than the individual DCE-T1, HR-T2WI, and ADC models, but the differences were not significant. The EMVI model achieved a relatively low performance in both the training (AUC = 0.73, ACC = 0.714) and validation (AUC = 0.578, ACC = 0.583) sets. When combined with radiomic features, the multi-modal radiomics model performed better, and reached an AUC of 0.925 and ACC of 0.886 in the training set (Figure 4B), and an AUC of 0.93 and ACC of 0.875 in the validation set. The comparisons of different radiomic feature model performances were presented in Supplementary Table 3.
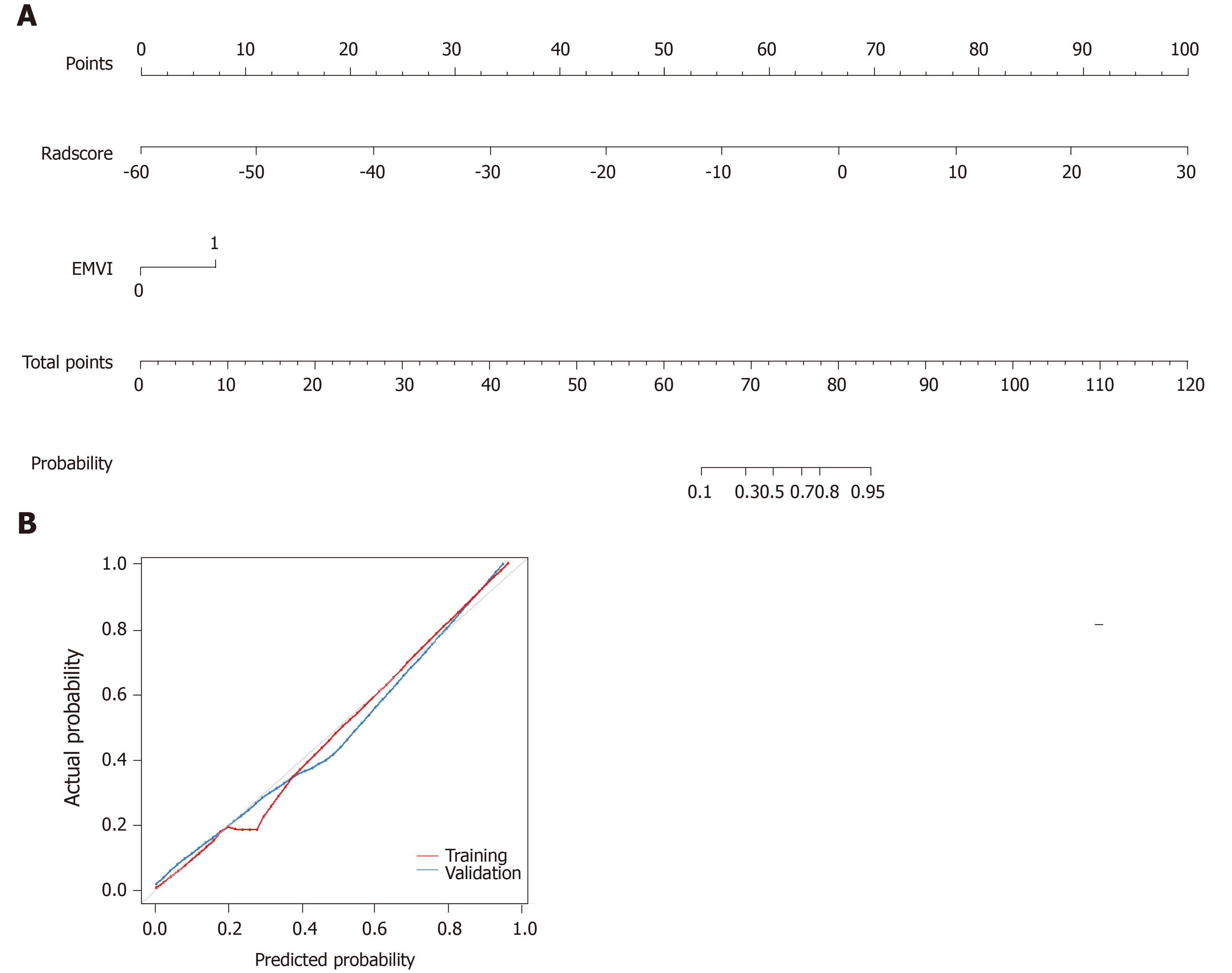
For the clinical radiomics nomogram, EMVI features and the radiomics score were identified by univariate analysis (P < 0.05). In multivariable logistic regression analysis, the radiomics score [odds ratio (OR) =1.34, P < 0.001] and EMVI (OR = 6.72, P = 0.01) significantly predicted the clinical response of neoadjuvant chemotherapy for LARC. Therefore, the radiomics nomogram was constructed with the radiomics score and EMVI, and good agreement was found between the nomogram prediction and actual observation in the calibration plots (Hosmer-Lemeshow test; P = 0.17) (Figure 5).
Our study developed and validated a radiomics model that incorporated CT and MRI features for the noninvasive and individualized prediction of clinical response to neoadjuvant chemotherapy in patients with LARC. The combination of CT and MRI (DCE-T1, HR-T2WI, and ADC) features was associated with better performance than any individual sequence. In contrast, the clinical model based on EMVI showed relatively low performance. A multi-modal nomogram facilitated an easy and noninvasive estimation of clinical response to neoadjuvant chemotherapy. The proposed radiomics model performed well, thereby adding accuracy to decision-making and the clinical assessment of neoadjuvant chemotherapy for LARC.
Although neoadjuvant CRT has been considered the standard treatment option for LARC, the main target of radiotherapy is local control, and its effects on distant metastasis or overall survival are still controversial, especially in patients undergoing TME surgery[33-35]. Moreover, the short- and long-term adverse effects of radiotherapy, such as urogenital anal dysfunction and occurrence of secondary cancer, are not negligible[36-38]. Due to progress in new effective chemotherapy strategies, the concept of neoadjuvant chemotherapy without radiotherapy has emerged. Recently, several studies[39-41] have evaluated the feasibility and efficacy of neoadjuvant chemotherapy for LARC, and the results were promising; therefore, neoadjuvant chemotherapy without radiotherapy was performed in our study.
Imaging examination plays a key role in the evaluation of response to neoadjuvant chemotherapy for LARC. Our study showed that MRI features of EMVI were significantly positive in the responder group compared with the non-responder group, which is consistent with the literature[42,43]. However, there were still some limitations[44,45] in the evaluation of EMVI using MRI: First, the limitation of MRI resolution ratio and scan slice thickness resulted in a poor display of small vessels, and the examination results of EMVI may be false-negatives. Second, some local advanced cancers are accompanied by high vein invasion or extensive destruction of the vascular wall and cell structure with no normal vascular structure. Furthermore, MRI may indicate positive EMVI findings, which might increase the false-negative rate of pathological diagnosis. Therefore, a more accurate model is needed to predict the efficacy of neoadjuvant chemotherapy for LARC.
Recently, several studies have reported that radiomic features based on CT and MRI showed a good relationship with tumor biological characteristics and heterogeneities, which was helpful in predicting the curative effect of neoadjuvant CRT for LARC[21,22,46-48]. Most of the above studies aimed to predict patients with pathologic complete response (PCR) by radiomics analysis, so as to omit surgery and over-treatment. Under neoadjuvant therapy, the rate of PCR was low, ranging from 15-27%[21,22,46-48], which indicated that approximately 80% of patients did not achieve PCR and possibly received surgical intervention. However, even without PCR, the patients achieved partial responsiveness and benefited from neoadjuvant CRT by shrinking the tumor and descending the tumor stage, which could improve resectability rate[8,49]. According to the TRG, the response rate of neoadjuvant chemotherapy was up to 67.8%, which means that 32.2% of patients did not benefit from the therapeutic strategy, and suffered from side effects and pain due to systemic chemotherapy. Therefore, the purpose of our study was to differentiate clinical response (including complete and partial) from non-responding LARC patients receiving neoadjuvant chemotherapy.
Multiparametric MRI radiomic features were reported to hold potential in predicting non-responsiveness to neoadjuvant therapy in patients with LARC, which achieved an area under the ROC curve of 0.82-0.83[9,10]. In the present study, the HR-T2WI model performed better (AUC 0.859, ACC 0.896) than the above. However, most studies adopted a single examination method, and did not integrate multimodal imaging examination methods. Our study combined enhanced CT with multiparameter MRI to construct a multi-modal model with higher prediction efficiency than a single radiomics model.
In the era of precision medicine, a single feature or model can no longer meet the requirements of individualized treatment. Only the comprehensive analysis of all potentially useful information can improve the accuracy of prediction and diagnosis. A recent study[50] has shown that combining clinical variables with the radiomics model can improve predictive performance of neoadjuvant CRT for LARC. However, no differences were found in clinical variables of gender, age, BMI, TNM stage, CEA, CA199, and lesion region between responder and non-responder groups in our study. Thus, we created the multi-parameter radiomics model only combined with EMVI to build a comprehensive prediction model, which had the highest prediction value in the training (ROC 0.925) and validation (ROC 0.93) group, respectively. In addition, this prediction model was made into a visual nomogram, which calculated the specific probability of each patient's curative effect based on the sum of the scores of each risk factor, making it easier for clinicians to judge the specific situation of each patient, and provide personalized treatments for patients.
There are several studies[22,46,51-53] reporting the use of joint models to construct nomograms, which have achieved a considerable prediction of neoadjuvant CRT for LARC. However, most studies only used a single imaging method, such as CT, MRI, or ultrasound. In contrast, the present study created a prediction model combined with CT and MRI, which has been the routine imaging examination method recommended by guidelines. Therefore, our multi-modal radiomics prediction model is economical and easy for clinical practice. Besides, some studies evaluated F18-FDG PET/CT and/or MRI radiomic features, and reported a high predictive value for curative effect[51,54]; however, F18-FDG PET examination is expensive and difficult to popularize.
Our research has several limitations. First, due to its retrospective design, there might be selection bias, although the patients were continuously enrolled. Second, the sample size was small as the neoadjuvant chemotherapy protocol is new in our hospital, and has not yet been widely used. Therefore, the results of the present study remain to be proven using different protocols of neoadjuvant chemotherapy or CRT. Third, some biological characteristics such as overexpression of human epidermal growth factor receptor 2 and Ki-67 were reported to have good prediction of response to neoadjuvant chemotherapy[55-57]; however, in our study, the above biological markers were not available in all included patients. Thus, the multi-modal nomogram combined with biological characteristics is desirable in the future.
In conclusion, the findings of this study showed that the multi-modal nomogram established by radiomics of preoperative CT and MRI adds accuracy to prediction and could contribute to the personalized selection of neoadjuvant chemotherapy for LARC.
Neoadjuvant chemotherapy is currently recommended as preoperative treatment for locally advanced rectal cancer (LARC); however, evaluation of treatment response to neoadjuvant chemotherapy is still challenging.
Several studies have reported that there were still 7-37% of LARC patients who do not respond to neoadjuvant CRT, which may not only increase CRT-related side effects and economic burden, but also delay surgery time. Therefore, it is necessary to identify which patients can benefit from neoadjuvant CRT treatment.
To create a multi-modal radiomics model to assess therapeutic response after neoadjuvant chemotherapy for LARC.
This retrospective study consecutively included 118 patients with LARC who underwent both computed tomography (CT) and magnetic resonance imaging (MRI) before neoadjuvant chemotherapy between October 2016 and June 2019. Histopathological findings were used as the reference standard for pathological response. Patients were randomly divided into a training set (n = 70) and a validation set (n = 48). The performance of different models based on CT and MRI, including apparent diffusion coefficient (ADC), dynamic contrast enhanced T1 images (DCE-T1), high resolution T2-weighted imaging (HR-T2WI), and imaging features, was assessed by using the receiver operating characteristic curve (ROC) analysis and was demonstrated as area under the curve (AUC) and accuracy (ACC). Calibration plots with Hosmer-Lemeshow tests were used to investigate the agreement and performance characteristics of the nomogram.
Eighty of 118 patients (68%) achieved a pathological response. For an individual radiomics model, HR-T2WI performed better (AUC 0.859, ACC 0.896) than CT (AUC = 0.766, ACC = 0.792), DCE-T1 (AUC = 0.812, ACC = 0.854), and ADC (AUC = 0.828, ACC = 0.833) in the validation set. The imaging performance for extramural venous invasion (EMVI) detection was relatively low in both the training (AUC = 0.73, ACC = 0.714) and validation (AUC = 0.578, ACC = 0.583) sets. The multi-modal radiomics model reached an AUC of 0.925 and ACC of 0.886 in the training set, and an AUC of 0.93 and ACC of 0.875 in the validation set. For the clinical radiomics nomogram, good agreement was found between the nomogram prediction and actual observation.
A multi-modal nomogram using traditional imaging features and radiomics of preoperative CT and MRI adds accuracy to the prediction of treatment outcome, and thus contributes to the personalized selection of neoadjuvant chemotherapy for LARC.
Some biological characteristics such as overexpression of human epidermal growth factor receptor 2 and Ki-67 were reported to have good prediction of response to neoadjuvant chemotherapy; however, in our study, the above biological markers were not available in all included patients. Thus, the multi-modal nomogram combined with biological characteristics is desirable in the future.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Morelli L S-Editor: Wang YQ L-Editor: Filipodia E-Editor: Liu MY
| 1. | Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, Jemal A, Schymura MJ, Lansdorp-Vogelaar I, Seeff LC, van Ballegooijen M, Goede SL, Ries LA. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1390] [Cited by in RCA: 1504] [Article Influence: 100.3] [Reference Citation Analysis (1)] |
| 2. | Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, Karstens JH, Liersch T, Schmidberger H, Raab R; German Rectal Cancer Study Group. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4342] [Cited by in RCA: 4464] [Article Influence: 212.6] [Reference Citation Analysis (1)] |
| 3. | Benson AB, Venook AP, Al-Hawary MM, Cederquist L, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Engstrom PF, Grem JL, Grothey A, Hochster HS, Hoffe S, Hunt S, Kamel A, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Mulcahy MF, Murphy JD, Nurkin S, Saltz L, Sharma S, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Wuthrick E, Gregory KM, Gurski L, Freedman-Cass DA. Rectal Cancer, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16:874-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 682] [Article Influence: 113.7] [Reference Citation Analysis (0)] |
| 4. | Maas M, Nelemans PJ, Valentini V, Das P, Rödel C, Kuo LJ, Calvo FA, García-Aguilar J, Glynne-Jones R, Haustermans K, Mohiuddin M, Pucciarelli S, Small W, Suárez J, Theodoropoulos G, Biondo S, Beets-Tan RG, Beets GL. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11:835-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1189] [Cited by in RCA: 1460] [Article Influence: 97.3] [Reference Citation Analysis (0)] |
| 5. | Habr-Gama A, Perez RO, Nadalin W, Sabbaga J, Ribeiro U, Silva e Sousa AH, Campos FG, Kiss DR, Gama-Rodrigues J. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. 2004;240:711-7; discussion 717-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1120] [Cited by in RCA: 1360] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 6. | Påhlman L, Bohe M, Cedermark B, Dahlberg M, Lindmark G, Sjödahl R, Ojerskog B, Damber L, Johansson R. The Swedish rectal cancer registry. Br J Surg. 2007;94:1285-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 281] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 7. | Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, Daban A, Bardet E, Beny A, Ollier JC, EORTC Radiotherapy Group Trial 22921. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1993] [Cited by in RCA: 2041] [Article Influence: 107.4] [Reference Citation Analysis (0)] |
| 8. | Rödel C, Liersch T, Becker H, Fietkau R, Hohenberger W, Hothorn T, Graeven U, Arnold D, Lang-Welzenbach M, Raab HR, Sülberg H, Wittekind C, Potapov S, Staib L, Hess C, Weigang-Köhler K, Grabenbauer GG, Hoffmanns H, Lindemann F, Schlenska-Lange A, Folprecht G, Sauer R; German Rectal Cancer Study Group. Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: initial results of the German CAO/ARO/AIO-04 randomised phase 3 trial. Lancet Oncol. 2012;13:679-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 506] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 9. | Yang C, Jiang ZK, Liu LH, Zeng MS. Pre-treatment ADC image-based random forest classifier for identifying resistant rectal adenocarcinoma to neoadjuvant chemoradiotherapy. Int J Colorectal Dis. 2020;35:101-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 10. | Zhou X, Yi Y, Liu Z, Cao W, Lai B, Sun K, Li L, Zhou Z, Feng Y, Tian J. Radiomics-Based Pretherapeutic Prediction of Non-response to Neoadjuvant Therapy in Locally Advanced Rectal Cancer. Ann Surg Oncol. 2019;26:1676-1684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 11. | Park IJ, You YN, Agarwal A, Skibber JM, Rodriguez-Bigas MA, Eng C, Feig BW, Das P, Krishnan S, Crane CH, Hu CY, Chang GJ. Neoadjuvant treatment response as an early response indicator for patients with rectal cancer. J Clin Oncol. 2012;30:1770-1776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 382] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 12. | Janssen MH, Ollers MC, Riedl RG, van den Bogaard J, Buijsen J, van Stiphout RG, Aerts HJ, Lambin P, Lammering G. Accurate prediction of pathological rectal tumor response after two weeks of preoperative radiochemotherapy using (18)F-fluorodeoxyglucose-positron emission tomography-computed tomography imaging. Int J Radiat Oncol Biol Phys. 2010;77:392-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Engelen SM, Beets-Tan RG, Lahaye MJ, Lammering G, Jansen RL, van Dam RM, Konsten J, Leijtens JW, van de Velde CJ, Beets GL. MRI after chemoradiotherapy of rectal cancer: a useful tool to select patients for local excision. Dis Colon Rectum. 2010;53:979-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Intven M, Reerink O, Philippens ME. Dynamic contrast enhanced MR imaging for rectal cancer response assessment after neo-adjuvant chemoradiation. J Magn Reson Imaging. 2015;41:1646-1653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 15. | De Felice F, Magnante AL, Musio D, Ciolina M, De Cecco CN, Rengo M, Laghi A, Tombolini V. Diffusion-weighted magnetic resonance imaging in locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy. Eur J Surg Oncol. 2017;43:1324-1329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Gillies RJ, Kinahan PE, Hricak H. Radiomics: Images Are More than Pictures, They Are Data. Radiology. 2016;278:563-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4541] [Cited by in RCA: 5550] [Article Influence: 616.7] [Reference Citation Analysis (3)] |
| 17. | Beig N, Khorrami M, Alilou M, Prasanna P, Braman N, Orooji M, Rakshit S, Bera K, Rajiah P, Ginsberg J, Donatelli C, Thawani R, Yang M, Jacono F, Tiwari P, Velcheti V, Gilkeson R, Linden P, Madabhushi A. Perinodular and Intranodular Radiomic Features on Lung CT Images Distinguish Adenocarcinomas from Granulomas. Radiology. 2019;290:783-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 239] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 18. | Sidhu HS, Benigno S, Ganeshan B, Dikaios N, Johnston EW, Allen C, Kirkham A, Groves AM, Ahmed HU, Emberton M, Taylor SA, Halligan S, Punwani S. "Textural analysis of multiparametric MRI detects transition zone prostate cancer". Eur Radiol. 2017;27:2348-2358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 19. | Ueno Y, Forghani B, Forghani R, Dohan A, Zeng XZ, Chamming's F, Arseneau J, Fu L, Gilbert L, Gallix B, Reinhold C. Endometrial Carcinoma: MR Imaging-based Texture Model for Preoperative Risk Stratification-A Preliminary Analysis. Radiology. 2017;284:748-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 138] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 20. | Lakhman Y, Veeraraghavan H, Chaim J, Feier D, Goldman DA, Moskowitz CS, Nougaret S, Sosa RE, Vargas HA, Soslow RA, Abu-Rustum NR, Hricak H, Sala E. Differentiation of Uterine Leiomyosarcoma from Atypical Leiomyoma: Diagnostic Accuracy of Qualitative MR Imaging Features and Feasibility of Texture Analysis. Eur Radiol. 2017;27:2903-2915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 122] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 21. | De Cecco CN, Ciolina M, Caruso D, Rengo M, Ganeshan B, Meinel FG, Musio D, De Felice F, Tombolini V, Laghi A. Performance of diffusion-weighted imaging, perfusion imaging, and texture analysis in predicting tumoral response to neoadjuvant chemoradiotherapy in rectal cancer patients studied with 3T MR: initial experience. Abdom Radiol (NY). 2016;41:1728-1735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 22. | Liu Z, Zhang XY, Shi YJ, Wang L, Zhu HT, Tang Z, Wang S, Li XT, Tian J, Sun YS. Radiomics Analysis for Evaluation of Pathological Complete Response to Neoadjuvant Chemoradiotherapy in Locally Advanced Rectal Cancer. Clin Cancer Res. 2017;23:7253-7262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 406] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 23. | Provenzale D, Gupta S, Ahnen DJ, Markowitz AJ, Chung DC, Mayer RJ, Regenbogen SE, Blanco AM, Bray T, Cooper G, Early DS, Ford JM, Giardiello FM, Grady W, Hall MJ, Halverson AL, Hamilton SR, Hampel H, Klapman JB, Larson DW, Lazenby AJ, Llor X, Lynch PM, Mikkelson J, Ness RM, Slavin TP, Sugandha S, Weiss JM, Dwyer MA, Ogba N. NCCN Guidelines Insights: Colorectal Cancer Screening, Version 1.2018. J Natl Compr Canc Netw. 2018;16:939-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 106] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 24. | Smith NJ, Barbachano Y, Norman AR, Swift RI, Abulafi AM, Brown G. Prognostic significance of magnetic resonance imaging-detected extramural vascular invasion in rectal cancer. Br J Surg. 2008;95:229-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 328] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 25. | Kickingereder P, Götz M, Muschelli J, Wick A, Neuberger U, Shinohara RT, Sill M, Nowosielski M, Schlemmer HP, Radbruch A, Wick W, Bendszus M, Maier-Hein KH, Bonekamp D. Large-scale Radiomic Profiling of Recurrent Glioblastoma Identifies an Imaging Predictor for Stratifying Anti-Angiogenic Treatment Response. Clin Cancer Res. 2016;22:5765-5771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 203] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 26. | Parmar C, Grossmann P, Bussink J, Lambin P, Aerts HJWL. Machine Learning methods for Quantitative Radiomic Biomarkers. Sci Rep. 2015;5:13087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 696] [Cited by in RCA: 700] [Article Influence: 70.0] [Reference Citation Analysis (0)] |
| 27. | Parmar C, Grossmann P, Rietveld D, Rietbergen MM, Lambin P, Aerts HJ. Radiomic Machine-Learning Classifiers for Prognostic Biomarkers of Head and Neck Cancer. Front Oncol. 2015;5:272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 232] [Cited by in RCA: 269] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 28. | Peng H, Long F, Ding C. Feature selection based on mutual information: criteria of max-dependency, max-relevance, and min-redundancy. IEEE Trans Pattern Anal Mach Intell. 2005;27:1226-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6560] [Cited by in RCA: 3434] [Article Influence: 171.7] [Reference Citation Analysis (0)] |
| 29. | Feng Z, Rong P, Cao P, Zhou Q, Zhu W, Yan Z, Liu Q, Wang W. Machine learning-based quantitative texture analysis of CT images of small renal masses: Differentiation of angiomyolipoma without visible fat from renal cell carcinoma. Eur Radiol. 2018;28:1625-1633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 162] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 30. | Xu X, Liu Y, Zhang X, Tian Q, Wu Y, Zhang G, Meng J, Yang Z, Lu H. Preoperative prediction of muscular invasiveness of bladder cancer with radiomic features on conventional MRI and its high-order derivative maps. Abdom Radiol (NY). 2017;42:1896-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 31. | Xu X, Zhang X, Tian Q, Zhang G, Liu Y, Cui G, Meng J, Wu Y, Liu T, Yang Z, Lu H. Three-dimensional texture features from intensity and high-order derivative maps for the discrimination between bladder tumors and wall tissues via MRI. Int J Comput Assist Radiol Surg. 2017;12:645-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 32. | Trakarnsanga A, Gönen M, Shia J, Nash GM, Temple LK, Guillem JG, Paty PB, Goodman KA, Wu A, Gollub M, Segal N, Saltz L, Garcia-Aguilar J, Weiser MR. Comparison of tumor regression grade systems for locally advanced rectal cancer after multimodality treatment. J Natl Cancer Inst. 2014;106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 194] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 33. | Peeters KC, Marijnen CA, Nagtegaal ID, Kranenbarg EK, Putter H, Wiggers T, Rutten H, Pahlman L, Glimelius B, Leer JW, van de Velde CJ; Dutch Colorectal Cancer Group. The TME trial after a median follow-up of 6 years: increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann Surg. 2007;246:693-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 853] [Cited by in RCA: 875] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 34. | Abraha I, Aristei C, Palumbo I, Lupattelli M, Trastulli S, Cirocchi R, De Florio R, Valentini V. Preoperative radiotherapy and curative surgery for the management of localised rectal carcinoma. Cochrane Database Syst Rev. 2018;10:CD002102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 35. | De Caluwé L, Van Nieuwenhove Y, Ceelen WP. Preoperative chemoradiation versus radiation alone for stage II and III resectable rectal cancer. Cochrane Database Syst Rev. 2013;CD006041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 36. | Birgisson H, Påhlman L, Gunnarsson U, Glimelius B. Occurrence of second cancers in patients treated with radiotherapy for rectal cancer. J Clin Oncol. 2005;23:6126-6131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 195] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 37. | Peeters KC, van de Velde CJ, Leer JW, Martijn H, Junggeburt JM, Kranenbarg EK, Steup WH, Wiggers T, Rutten HJ, Marijnen CA. Late side effects of short-course preoperative radiotherapy combined with total mesorectal excision for rectal cancer: increased bowel dysfunction in irradiated patients--a Dutch colorectal cancer group study. J Clin Oncol. 2005;23:6199-6206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 667] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 38. | Parc Y, Zutshi M, Zalinski S, Ruppert R, Fürst A, Fazio VW. Preoperative radiotherapy is associated with worse functional results after coloanal anastomosis for rectal cancer. Dis Colon Rectum. 2009;52:2004-2014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 39. | Hasegawa S, Goto S, Matsumoto T, Hida K, Kawada K, Matsusue R, Yamaguchi T, Nishitai R, Manaka D, Kato S, Kadokawa Y, Yamanokuchi S, Kawamura J, Zaima M, Kyogoku T, Kanazawa A, Mori Y, Kanai M, Matsumoto S, Sakai Y. A Multicenter Phase 2 Study on the Feasibility and Efficacy of Neoadjuvant Chemotherapy Without Radiotherapy for Locally Advanced Rectal Cancer. Ann Surg Oncol. 2017;24:3587-3595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 40. | Koike J, Funahashi K, Yoshimatsu K, Yokomizo H, Kan H, Yamada T, Ishida H, Ishibashi K, Saida Y, Enomoto T, Katsumata K, Hisada M, Hasegawa H, Koda K, Ochiai T, Sakamoto K, Shiokawa H, Ogawa S, Itabashi M, Kameoka S. Efficacy and safety of neoadjuvant chemotherapy with oxaliplatin, 5-fluorouracil, and levofolinate for T3 or T4 stage II/III rectal cancer: the FACT trial. Cancer Chemother Pharmacol. 2017;79:519-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 41. | Patel UB, Brown G, Machado I, Santos-Cores J, Pericay C, Ballesteros E, Salud A, Isabel-Gil M, Montagut C, Maurel J, Ramón-Ayuso J, Martin N, Estevan R, Fernandez-Martos C. MRI assessment and outcomes in patients receiving neoadjuvant chemotherapy only for primary rectal cancer: long-term results from the GEMCAD 0801 trial. Ann Oncol. 2017;28:344-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 42. | Liang P, Nakada I, Hong JW, Tabuchi T, Motohashi G, Takemura A, Nakachi T, Kasuga T, Tabuchi T. Prognostic significance of immunohistochemically detected blood and lymphatic vessel invasion in colorectal carcinoma: its impact on prognosis. Ann Surg Oncol. 2007;14:470-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 43. | Suzuki T, Sadahiro S, Tanaka A, Okada K, Saito G, Kamijo A, Akiba T, Kawada S. Relationship between histologic response and the degree of tumor shrinkage after chemoradiotherapy in patients with locally advanced rectal cancer. J Surg Oncol. 2014;109:659-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 44. | Siddiqui MRS, Simillis C, Hunter C, Chand M, Bhoday J, Garant A, Vuong T, Artho G, Rasheed S, Tekkis P, Abulafi AM, Brown G. A meta-analysis comparing the risk of metastases in patients with rectal cancer and MRI-detected extramural vascular invasion (mrEMVI) vs mrEMVI-negative cases. Br J Cancer. 2017;116:1513-1519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 110] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 45. | Sohn B, Lim JS, Kim H, Myoung S, Choi J, Kim NK, Kim MJ. MRI-detected extramural vascular invasion is an independent prognostic factor for synchronous metastasis in patients with rectal cancer. Eur Radiol. 2015;25:1347-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 106] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 46. | Chee CG, Kim YH, Lee KH, Lee YJ, Park JH, Lee HS, Ahn S, Kim B. CT texture analysis in patients with locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy: A potential imaging biomarker for treatment response and prognosis. PLoS One. 2017;12:e0182883. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 47. | Nie K, Shi L, Chen Q, Hu X, Jabbour SK, Yue N, Niu T, Sun X. Rectal Cancer: Assessment of Neoadjuvant Chemoradiation Outcome based on Radiomics of Multiparametric MRI. Clin Cancer Res. 2016;22:5256-5264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 307] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 48. | Horvat N, Veeraraghavan H, Khan M, Blazic I, Zheng J, Capanu M, Sala E, Garcia-Aguilar J, Gollub MJ, Petkovska I. MR Imaging of Rectal Cancer: Radiomics Analysis to Assess Treatment Response after Neoadjuvant Therapy. Radiology. 2018;287:833-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 260] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 49. | Aschele C, Cionini L, Lonardi S, Pinto C, Cordio S, Rosati G, Artale S, Tagliagambe A, Ambrosini G, Rosetti P, Bonetti A, Negru ME, Tronconi MC, Luppi G, Silvano G, Corsi DC, Bochicchio AM, Chiaulon G, Gallo M, Boni L. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the STAR-01 randomized phase III trial. J Clin Oncol. 2011;29:2773-2780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 544] [Cited by in RCA: 581] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 50. | Dinapoli N, Barbaro B, Gatta R, Chiloiro G, Casà C, Masciocchi C, Damiani A, Boldrini L, Gambacorta MA, Dezio M, Mattiucci GC, Balducci M, van Soest J, Dekker A, Lambin P, Fiorino C, Sini C, De Cobelli F, Di Muzio N, Gumina C, Passoni P, Manfredi R, Valentini V. Magnetic Resonance, Vendor-independent, Intensity Histogram Analysis Predicting Pathologic Complete Response After Radiochemotherapy of Rectal Cancer. Int J Radiat Oncol Biol Phys. 2018;102:765-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 51. | Giannini V, Mazzetti S, Bertotto I, Chiarenza C, Cauda S, Delmastro E, Bracco C, Di Dia A, Leone F, Medico E, Pisacane A, Ribero D, Stasi M, Regge D. Predicting locally advanced rectal cancer response to neoadjuvant therapy with 18F-FDG PET and MRI radiomics features. Eur J Nucl Med Mol Imaging. 2019;46:878-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 104] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 52. | Zhao RS, Wang H, Zhou ZY, Zhou Q, Mulholland MW. Restaging of locally advanced rectal cancer with magnetic resonance imaging and endoluminal ultrasound after preoperative chemoradiotherapy: a systemic review and meta-analysis. Dis Colon Rectum. 2014;57:388-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 53. | Tsai C, Hague C, Xiong W, Raval M, Karimuddin A, Brown C, Phang PT. Evaluation of endorectal ultrasound (ERUS) and MRI for prediction of circumferential resection margin (CRM) for rectal cancer. Am J Surg. 2017;213:936-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 54. | Lovinfosse P, Polus M, Van Daele D, Martinive P, Daenen F, Hatt M, Visvikis D, Koopmansch B, Lambert F, Coimbra C, Seidel L, Albert A, Delvenne P, Hustinx R. FDG PET/CT radiomics for predicting the outcome of locally advanced rectal cancer. Eur J Nucl Med Mol Imaging. 2018;45:365-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 123] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 55. | Meng X, Xia W, Xie P, Zhang R, Li W, Wang M, Xiong F, Liu Y, Fan X, Xie Y, Wan X, Zhu K, Shan H, Wang L, Gao X. Preoperative radiomic signature based on multiparametric magnetic resonance imaging for noninvasive evaluation of biological characteristics in rectal cancer. Eur Radiol. 2019;29:3200-3209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 113] [Article Influence: 16.1] [Reference Citation Analysis (2)] |
| 56. | Martin V, Landi L, Molinari F, Fountzilas G, Geva R, Riva A, Saletti P, De Dosso S, Spitale A, Tejpar S, Kalogeras KT, Mazzucchelli L, Frattini M, Cappuzzo F. HER2 gene copy number status may influence clinical efficacy to anti-EGFR monoclonal antibodies in metastatic colorectal cancer patients. Br J Cancer. 2013;108:668-675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 126] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 57. | Imaizumi K, Suzuki T, Kojima M, Shimomura M, Sakuyama N, Tsukada Y, Sasaki T, Nishizawa Y, Taketomi A, Ito M, Nakatsura T. Ki67 expression and localization of T cells after neoadjuvant therapies as reliable predictive markers in rectal cancer. Cancer Sci. 2020;111:23-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |