Published online May 21, 2020. doi: 10.3748/wjg.v26.i19.2323
Peer-review started: April 10, 2020
First decision: April 18, 2020
Revised: April 25, 2020
Accepted: May 16, 2020
Article in press: May 16, 2020
Published online: May 21, 2020
Processing time: 41 Days and 9.3 Hours
The severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) that causes coronavirus disease-2019 (COVID-19) is a global pandemic, manifested by an infectious pneumonia. Although patients primarily present with fever, cough and dyspnea, some patients also develop gastrointestinal (GI) and hepatic manifestations. The most common GI symptoms reported are diarrhea, nausea, vomiting, and abdominal discomfort. Liver chemistry abnormalities are common and include elevation of aspartate transferase, alanine transferase, and total bilirubin. Studies have shown that SARS-CoV-2 infects the GI tract via its viral receptor angiotensin converting enzyme II, which is expressed on enterocytes of the ileum and colon. Viral RNA has also been isolated from stool specimens of COVID-19 patients, which raised the concern for fecal-oral transmission in addition to droplet transmission. Although indirect evidence has suggested possible fecal-oral transmission of SARS-CoV-2, more effort is needed to establish the role of the fecal-oral transmission route. Further research will help elucidate the association between patients with underlying GI diseases, such as chronic liver disease and inflammatory bowel disease, and severity of COVID-19. In this review, we summarize the data on GI involvement to date, as well as the impact of COVID-19 on underlying GI diseases.
Core tip: The coronavirus disease-2019 (COVID-19) pandemic, caused by the novel coronavirus termed severe acute respiratory syndrome coronavirus 2, is rapidly gripping the world. COVID-19 can cause a wide spectrum of disease, ranging from mild to severe symptoms and even death. Despite initial reports of the disease primarily presenting with respiratory symptoms, a surge in patients presenting with gastrointestinal symptoms is being reported. This review provides a detailed knowledge on gastrointestinal and hepatic manifestations of COVID-19.
- Citation: Cha MH, Regueiro M, Sandhu DS. Gastrointestinal and hepatic manifestations of COVID-19: A comprehensive review. World J Gastroenterol 2020; 26(19): 2323-2332
- URL: https://www.wjgnet.com/1007-9327/full/v26/i19/2323.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i19.2323
The severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2), a novel coronavirus, was isolated and named by the International Committee on Taxonomy of Viruses in early January 2020, approximately 1 mo after it caused an outbreak in the city of Wuhan, Hubei Province of China[1,2]. Since the preceding outbreak, in December 2019, the pneumonia disease, which was named coronavirus disease-2019 (COVID-19) by the World Health Organization, has evolved into a pandemic that involves every continent, except Antarctica. As of the time of writing of this review, the number of global confirmed cases of COVID-19 has surpassed one and a half million, threatening to cause long-term effects on the global economy and societal structures[3].
The clinical presentation of COVID-19 is predominantly respiratory in nature, with cough and dyspnea being the most prominent features. Other prevalent clinical symptoms in patients with COVID-19 include fever and fatigue[4-7]. The information on extra-pulmonary manifestations, however, has been scarce. Despite being frequently overlooked, involvement of the gastrointestinal (GI) tract and the hepatic system is now being increasingly reported[8]. Indeed, the first case of COVID-19 in the United States presented with nausea and vomiting in addition to systemic and respiratory symptoms, and later developed abdominal discomfort and diarrhea[9]. In this article, we will review the GI and hepatic manifestations and the related pathophysiology (however limited) in patients with COVID-19 infection. Lastly, we will discuss the effect of COVID-19 in patients with underlying chronic GI diseases.
A detailed search of all published and in-press articles using search terms “COVID-19”, “SARS-CoV-2”, “gastrointestinal”, “liver”, “biliary”, “diarrhea”, “abdominal pain”, “nausea”, “vomiting”, “anosmia”, “dysgeusia”, “anorexia”, and “jaundice” was performed on PubMed and Google to extract data for this review.
GI symptoms are common in COVID-19 and can be present in up to 26% of patients in some populations[10]. The most common GI presentation in patients with COVID-19 is diarrhea (3.8%-34%), followed by nausea and/or vomiting (3.9%-10.1%) and abdominal pain (1.1%-2.2%)[5-7,11-21]. Other common GI symptoms reported in patients with COVID-19 are anorexia, anosmia, and dysgeusia[22]. Cheung et al[23] in their meta-analysis of 60 studies involving 4243 COVID-19 patients from six countries found GI symptoms in 17.6% of the patients with anorexia (26.8%), diarrhea (12.5%), nausea/vomiting (10.2%), and abdominal pain/discomfort (9.2%). Most notably, the latest and most comprehensive meta-analysis to date was produced by Borges and colleagues[6], involving 59254 patients from 11 countries. The results of the meta-analysis showed that 9% of all included patients displayed GI symptoms. These findings are not unexpected, as previous studies of SARS (2002-2003), which was caused by SARS-CoV, also revealed concurrent GI manifestations, similar to the current pandemic.
In the cases of SARS infection, these clinical presentations can be explained by the presence of active viral replication in the intestinal tract of infected patients, which was demonstrated via both electronic microscopy and viral culture[24]. Despite recent genetic sequencing showing significant genetic divergence between SARS-CoV and SARS-CoV-2, structural analysis suggests that both coronaviruses may have used angiotensin converting enzyme II (ACE2) as an entry receptor to establish infection[25-27]. These findings indicate that SARS-CoV-2 has the potential to infect multiple organs, since ACE2 is expressed by various tissues, including epithelial cells of the GI tract[28,29].
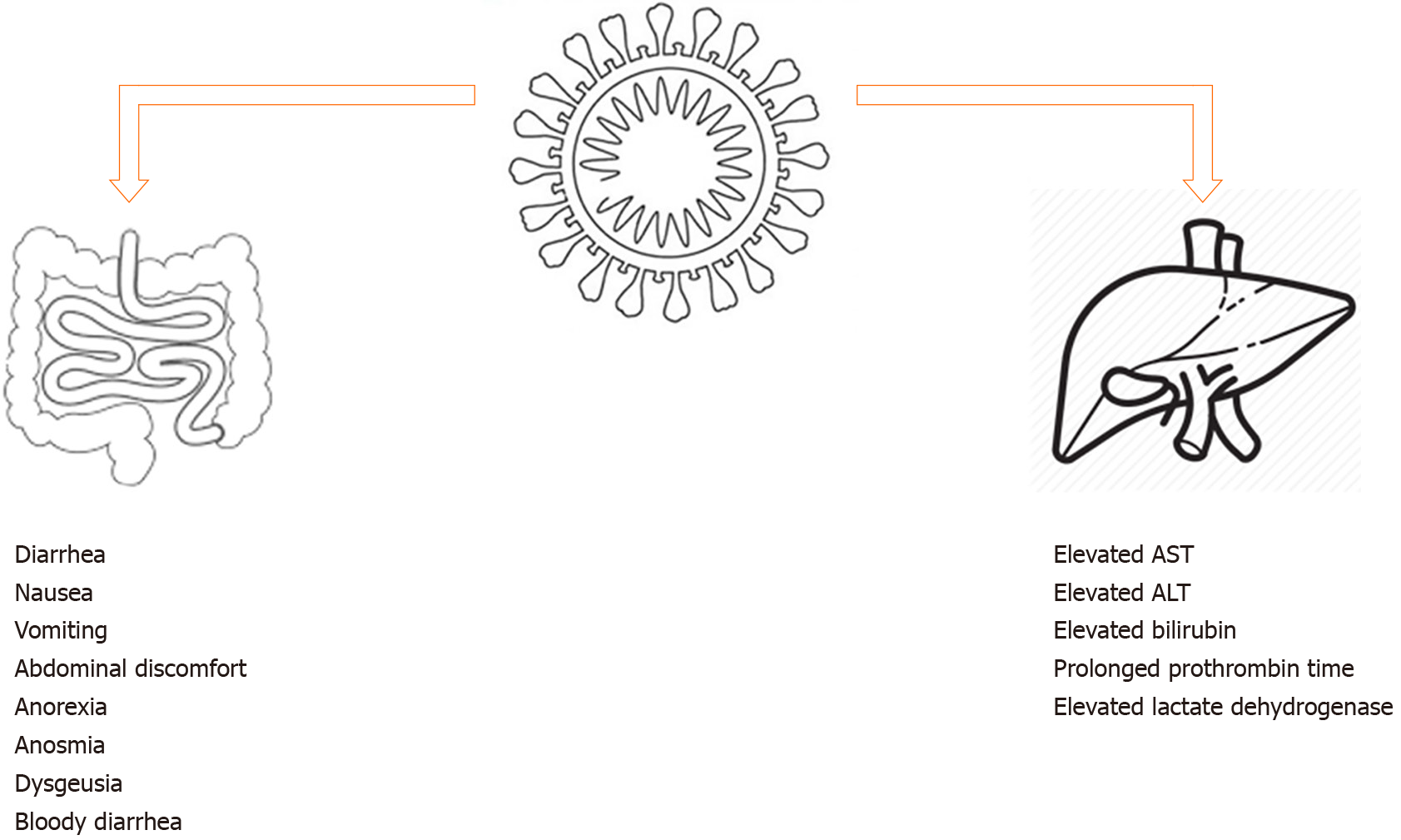
Evidence of GI infection by SARS-CoV-2 has also been provided by isolation of viral RNA from GI epithelial cells and intracellular staining of viral nucleocapsid protein in the same[30]. Building on existing knowledge, it is hypothesized that SARS-CoV-2 infection of intestinal enterocytes causes dysfunction of the ileum and colon, subsequently leading to the various GI manifestations seen in COVID-19 patients. Such GI manifestations are summarized in Figure 1.
To date, the studies looking at the association between severity of COVID-19 in patients with concurrent GI symptoms has yielded mixed results. A meta-analysis by Cheung et al[23] suggested GI symptoms are more common in severe disease (17.1%). These results, however, were not duplicated in other descriptive studies, likely due to low number of subjects[16,19,21]. A case series study focusing on GI manifestations, conducted by Pan et al[31], found that most cases of diarrhea are mild and non-dehydrating, typically up to three times daily. Both adult and pediatric populations can present with GI symptoms, although vomiting may be more prominent in the pediatric population[32].
Interestingly, few COVID-19 case studies and case series have been reporting cases with GI symptoms preceding respiratory symptoms, with some patients only presenting with digestive symptoms in the absence of respiratory symptoms[33-35]. One particularly unusual presentation was reported for a 71-year-old female, who, after a trip to Egypt, presented with hemorrhagic colitis without any respiratory symptoms. She tested positive on nasopharyngeal polymerase chain reaction for SARS-CoV-2. The cause of her lower GI bleed was determined to be the SARS-CoV-2 infection, after extensive tests for other etiologies of hemorrhagic colitis returned negative[36]. Another case report of 41-year-old woman from Michigan (United States), who presented with voluminous diarrhea and electrolyte abnormalities before onset of pulmonary symptoms, highlights that COVID-19 can have variable GI presentation[37]. Prior to this, only low volume and non-dehydrating diarrhea had been reported.
Although uncommon, these presentations can potentially lead to delay in diagnosis if clinicians are unfamiliar with GI presentation of COVID-19[38]. It is, however, currently uncertain how this unusual presentation would affect clinical course and prognosis.
The elevation of liver chemistries, including aspartate transferase, alanine transferase, and total bilirubin, has been reported since early observational studies[4-7,11,13,15,21]. Unlike the GI signs and symptoms discussed above, abnormal levels of liver chemistries have consistently shown to be more prevalent in severe disease[5,11,15,39]. Four potential causes of liver injury have been proposed. First is direct assault of SARS-CoV-2 on hepatocytes, leading to abnormal liver enzyme levels. Although this hypothesis is the most direct explanation, hepatocytes have not been shown to express high-level ACE2, making the liver an unlikely target for infection[28]. Although a preliminary study revealed a high level of ACE2 expression in cholangiocytes (suggesting an indirect cause of elevated liver enzymes as cholangiocyte dysfunction[40]), alkaline phosphatase has not been consistently shown to be high in COVID-19 patients, in support of this hypothesis. In addition, the pathological report of 3 COVID-19 cases published by Yao et al[41] did not provide evidence of SARS-CoV-2 infection of hepatocytes, suggesting this as an unlikely cause of liver injury. The second proposal suggests drug hepatotoxicity as the cause of abnormal liver function tests. This is possible, as acetaminophen is commonly used to control fever, which is a common presentation in COVID-19 infections. The use of acetaminophen within recommended dose, however, is unlikely to cause liver injuries as seen in the reported case series. It is also possible that systemic inflammatory response syndrome and multiorgan dysfunction contributed to the development of a cytokine storm and subsequently liver impairment, which may explain the higher prevalence of abnormal liver chemistries in patients with severe COVID-19 infection. Lastly, the occurrence of SARS can lead to hypoxic injury, which then leads to liver dysfunction[39]. Liver chemistries’ abnormalities are summarized in Figure 1.
Although abnormal liver enzymes are associated with higher risk of severe disease, daily check of liver function test may not be warranted. Most patients only have mild elevation of liver enzymes’ levels, which resolves as the patient improves clinically[42,43]. The most common cause of death in COVID-19 infection is respiratory failure and sepsis. To date, only 1 case of COVID-19 has been reported with presentation of acute liver failure[44]. As there are no effective hepatoprotective treatments currently, it is important for clinicians to not be distracted by minimally elevated liver enzymes’ level and to instead focus on general management and supportive care[42].
The concern of COVID-19 transmission through a fecal-oral route, in addition to respiratory droplets, has been raised by several authors[32,45]. It is noted that RNA of SARS-CoV-2 was detected in the stool specimen of the first COVD-19 patient in the United States, raising the concern for fecal-oral transmission of the virus[9]. The viral components found in the stool sample likely originated from infected enterocytes of the patient’s ileum and colon. As previously mentioned, the expression of ACE2 on intestinal enterocytes makes both the small and large intestines susceptible to SARS-CoV-2 infection[28-30]. Evidence of GI infection by SARS-CoV-2 has also been provided by the isolation of viral RNA from GI epithelial cells and intracellular staining of viral nucleocapsid protein in the same[30]. Therefore, it is physiologically possible for SARS-CoV-2 to be transmittable through an oral-fecal route. A study of COVID-19 infection in a familial cluster in China identified 2 young adults from the same family who presented with diarrhea, suggesting that fecal-oral transmission may play a vital role in disease transmission[46]. Interestingly, patients with SARS-CoV-2 detected in stool have longer delay before viral clearance[38]. Of note, viral RNA are also reported to be detectable in stool samples of patients without GI symptoms, such as diarrhea[47].
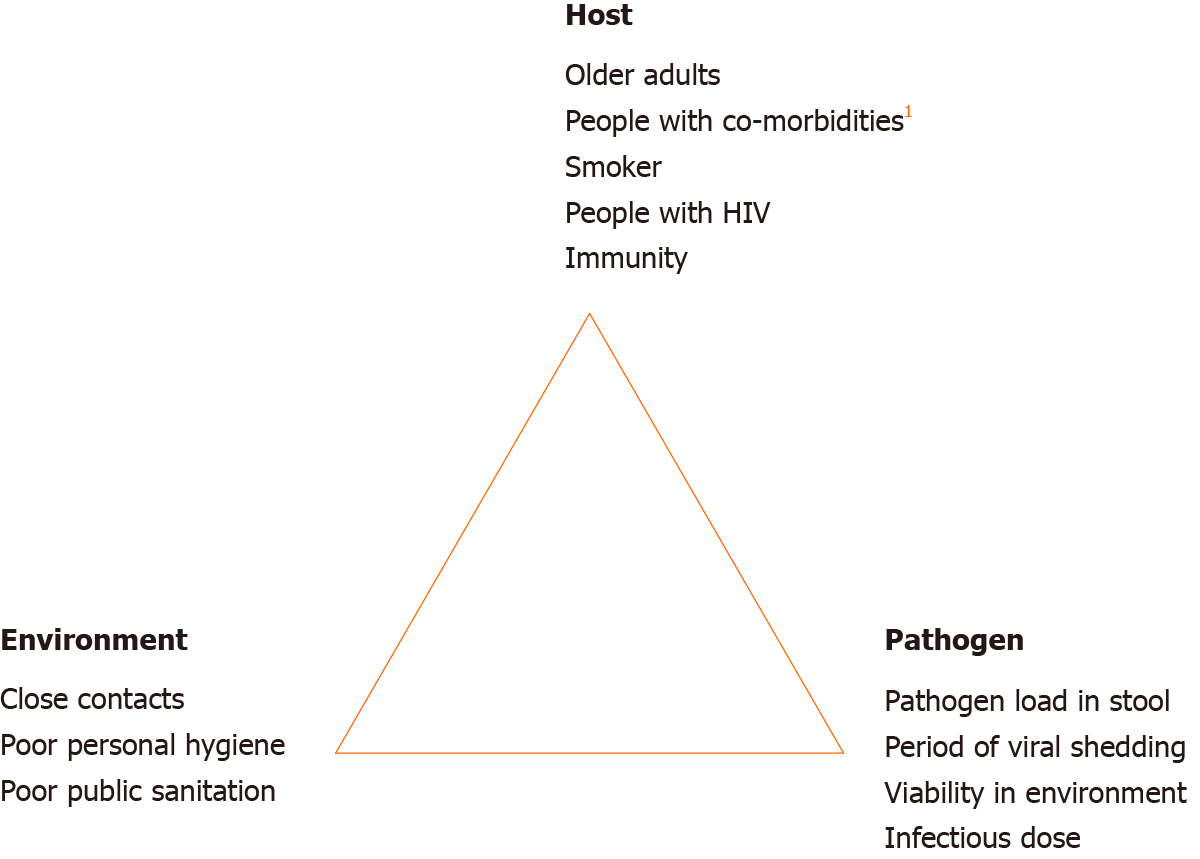
Test of SARS-CoV-2 virus in stool was found to be positive even after a negative throat swab test in 42.9%–81.8% of cases[13,48,49]. In addition, stool samples can remain positive for up to day-16 of illness[48]. These findings raise the question regarding the need for longer isolation and stool viral testing after clinical recovery. This is especially concerning in relation to a recent study that demonstrated SARS-CoV-2 virus isolated from stool is infectious (manuscript in preparation)[30]. Pathogen, host and environment factors influencing the fecal-oral transmissibility of SARS-CoV-2 are illustrated in Figure 2. It is important to note here that presence of COVID-19 nucleic acid in stool has been reported as not correlated with severity of pneumonia or GI manifestation[50]. On the other hand, shedding of viral RNA in stool has provided an opportunity to develop stool-based diagnostic test for SARS-CoV-2 infection. A study by Zhang and colleagues[50] demonstrated equal accuracy in COVID-19 detection by using stool specimens compared to pharyngeal swab specimens. This finding can potentially lead to development of a less invasive COVID-19 diagnostic test.
The potential for fecal-oral transmission also leads to the possibility of transmission to healthcare providers during endoscopic interventions. On March 15th, 2020, a guideline produced by joint gastroenterology societies in the United States recommended its members to delay all elective procedures and use N95 (or N99 or powered air-purifying respirator) masks instead of surgical masks for all endoscopy procedures as part of personal protective equipment[51]. The guidelines also recommend use of double gloves and of negative pressure rooms over regular endoscopy rooms, when available. Healthcare providers, however, are still at risk for infection during urgent and emergent interventions. A guidance composed by Rashid and colleagues[52] laid out various strategies to minimize risks of nosocomial infection. Briefly, the document recommends identification of potential and confirmed COVID-19 cases, appropriate personal protective equipment and proper disinfection of scopes and working space.
Similarly, the fecal microbiota transplant procedure also faces various challenges in ensuring stool samples obtained from donors are not infected with SARS-CoV-2 virus. A group of experts has recommended to screen high-risk donors for SARS-CoV-2 infection, including donors with typical COVID-19 symptoms and donors with recent (within 30 d) high-risk travel to affected regions or SARS-CoV-2-positive contact. Alternatively, donors’ stools could be isolated before use and then only released if the donor remains asymptomatic after 30 d. Lastly, stool banks can also retrospectively check the health status of a donor prior to using the donor’s stool for fecal microbiota transplant[53]. This approach, however, may miss asymptomatic carriers and thus it is recommended to screen all stool donors, regardless of risk factors[54].
Patients with chronic liver disease and cirrhosis may be at higher risk of COVID-19 infection, due to systemic immunodeficiency[55]. Current published description studies, however, found that only a small number of COVID-19 patients have underlying chronic liver disease and no statistically significant association has been established between underlying chronic liver disease and severity of COVID-19 or outcomes[12,15,16,20]. A case series from the Lombardy region in Italy that described the characteristics COVID-19 patients admitted to Intensive Care Units reported patients with chronic liver disease accounting for only 3%. Another study from China that described a population of COVID-19 patients with 2.1% of patients having underlying hepatitis B found that it is associated with more severe COVID-19 disease[5].
Acknowledging the lack of relevant data, the Surveillance Epidemiology of Coronavirus (COVID-19) Under Research Exclusion (SECURE-Cirrhosis) and COVID-HEP registries have been initiated. These are international voluntary registries of COVID-19 patients with underlying liver disease (with or without cirrhosis) or transplantation[56,57]. At the time of writing of this review article, SECURE-Cirrhosis has recorded a total of 29 cases of COVID-19 with underlying chronic liver disease and post-liver transplant in the Americas, China, Japan and Korea, which includes 4 (13.8%) reported deaths. The counterpart COVID-HEP registry, which is composed of cases from all other countries, has 52 cases of COVID-19 with 19 (36.5%) deaths among patients with chronic liver disease and liver transplant. In the weekly report concluded on April 4th, 2020 by COVID-HEP, 10 out of 12 patients who died had underlying cirrhosis. Those patients primarily expired due to respiratory failure, followed by cardiogenic shock. Data of COVID-19 patients with underlying chronic liver disease or liver transplant specific to the United States is not available as of the time of this writing.
Similarly, patients taking biologics or immunosuppressive medications for various conditions, including inflammatory bowel diseases (IBDs), also raised concerns to clinicians. According to the Chinese IBD Elite Union, which includes the seven of the largest IBD referral centers in China, no patients with IBD were reported to be infected with SARS-CoV-2[55]. The lack of data may be due to lower prevalence of disease in China compared to the prevalence of IBD in the United States which is more than 5 times higher than in most Asian countries[58,59].
Since then, SECURE-IBD database started, which is an open access online reporting of COVID-19 infection in patients with IBD[60]. At the time of writing this review, a total of 326 cases of COVID-19 is reported in patients with IBD from 32 countries, with maximum of 108 cases reported from the United States. Out of the reported cases, 189 are with Crohn’s disease and 135 with ulcerative colitis/unspecified. Overall, 95 (61%) were hospitalized, 11 (7%) placed on ventilator, and 13 (9%) patients died from COVID-19. The International Organization For the Study of Inflammatory Bowel Disease has issued detailed guidance with regard to IBD and COVID-19[61]. It is important to note that these recommendations are the summation of expert opinions and need interpretation in the context of the individual patient. Additionally, the recommendations may change as the situation evolves. As per the International Organization For the Study of Inflammatory Bowel Disease, the risk of SARS-CoV-2 in patients with IBD is the same as in the general population.
It is uncertain if active inflammation from IBD increases the risk of getting SARS-CoV-2. Patients on 5-aminosalicyclic acid therapy should continue with their therapy, even if they test positive for SARS-CoV-2 or have COVID-19. Patients on budesonide therapy should not decrease their dose or discontinue treatment to prevent infection from SARS-CoV-2; however, it is uncertain if budesonide therapy should be stopped if they test positive for SARS-CoV-2 or develop COVID-19. Patients on anti-tumor necrosis factor should stop therapy if they develop COVID-19, although it is uncertain if they should stop if they test positive for SARS-CoV-2. It is uncertain if patients on vedolizumab should stop therapy if they test positive for SARS-CoV-2 or develop COVID-19. Patients on ustekinumab, however, should stop therapy if they develop COVID-19[61]. It is currently recommended that patients on prednisone ≥ 20 g/d should stop (taper as appropriate) if they test positive for SARS-CoV-2 or develop COVID-19, and also to prevent infection since it increases both the risk of SARS-CoV-2 infection and COVID-19. Similarly, thiopurines (6-mercaptopurine, azathioprine), methotrexate and tofacitinib also tend to inhibit the body’s immune response to viral infections and they should be stopped if the patient tests positive for the SARS-CoV-2 or develops COVID-19.
In an IBD patient who develops COVID-19 and their IBD medications were stopped, the IBD medications can be restarted after resolution of COVID-19 symptoms and after two nasopharyngeal polymerase chain reaction tests are negative. As the situation is evolving, it is very important for other countries to continue collecting descriptive data in order to determine if patients with underlying chronic GI diseases are more vulnerable to the new SARS-CoV-2 virus.
Since its beginning in Wuhan City, China in December 2019, the COVID-19 pandemic continues to cause more infections and mortalities globally. A significant number of research studies have been conducted to understand the nature of the viral infection and ways to mitigate the harms caused. Although many researchers have described the possibility of fecal-oral transmission of the virus, no case report or clinical research has been able to confirm the hypothesis. The demonstration of confirmed fecal-oral transmission is difficult, as it requires researchers to control for respiratory droplet exposure, which may be difficult in a clinical setting. It also poses an ethical challenge by purposefully exposing human subjects to a potentially deadly viral infection. One plausible way to circumvent the challenges would be experimentation with animal models. Currently, various research laboratories have utilized the ACE2 transgenic mouse model and Macaca Rhesus model to conduct COVID-19 related researches. It is important for the scientific community to understand the potential role of fecal-oral transmission in the current pandemic, as it may affect isolation recommendations and prevent new infection foci.
In summary, COVID-19 is a new coronavirus infection that can lead to acute respiratory infection, with a high rate of morbidity and mortality. In addition to the respiratory manifestations, patients with COVID-19 also frequently presents with GI symptoms. The role of fecal viral shedding in the current pandemic and the impact of COVID-19 patients with underlying GI disease is still unknown. Until a vaccine is developed, the global impact of COVID-19 will continue. Future studies will help us to better understand the GI involvement of SARS-CoV-2 infection.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ricciuto A, Takemura N, Zhang C S-Editor: Wang JL L-Editor: A E-Editor: Li X
| 1. | Phelan AL, Katz R, Gostin LO. The Novel Coronavirus Originating in Wuhan, China: Challenges for Global Health Governance. JAMA. 2020;323:709-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 774] [Cited by in RCA: 744] [Article Influence: 148.8] [Reference Citation Analysis (6)] |
| 2. | Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536-544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5202] [Cited by in RCA: 4632] [Article Influence: 926.4] [Reference Citation Analysis (0)] |
| 3. | World Health Organization. Coronavirus Disease (COVID-19) Pandemic, 2020. Available from: https://www.who.int/health-topics/coronavirus#tab=tab_1. |
| 4. | Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, Ji R, Wang H, Wang Y, Zhou Y. Prevalence of comorbidities and its effects in coronavirus disease 2019 patients: A systematic review and meta-analysis. Int J Infect Dis. 2020;94:91-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2672] [Cited by in RCA: 2509] [Article Influence: 501.8] [Reference Citation Analysis (2)] |
| 5. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19202] [Cited by in RCA: 18876] [Article Influence: 3775.2] [Reference Citation Analysis (7)] |
| 6. | Borges do Nascimento IJ, Cacic N, Abdulazeem HM, von Groote TC, Jayarajah U, Weerasekara I, Esfahani MA, Civile VT, Marusic A, Jeroncic A, Carvas Junior N, Pericic TP, Zakarija-Grkovic I, Meirelles Guimarães SM, Luigi Bragazzi N, Bjorklund M, Sofi-Mahmudi A, Altujjar M, Tian M, Arcani DMC, O'Mathúna DP, Marcolino MS. Novel Coronavirus Infection (COVID-19) in Humans: A Scoping Review and Meta-Analysis. J Clin Med. 2020;9:E941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 367] [Cited by in RCA: 330] [Article Influence: 66.0] [Reference Citation Analysis (2)] |
| 7. | Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, Cereda D, Coluccello A, Foti G, Fumagalli R, Iotti G, Latronico N, Lorini L, Merler S, Natalini G, Piatti A, Ranieri MV, Scandroglio AM, Storti E, Cecconi M, Pesenti A; COVID-19 Lombardy ICU Network, Nailescu A, Corona A, Zangrillo A, Protti A, Albertin A, Forastieri Molinari A, Lombardo A, Pezzi A, Benini A, Scandroglio AM, Malara A, Castelli A, Coluccello A, Micucci A, Pesenti A, Sala A, Alborghetti A, Antonini B, Capra C, Troiano C, Roscitano C, Radrizzani D, Chiumello D, Coppini D, Guzzon D, Costantini E, Malpetti E, Zoia E, Catena E, Agosteo E, Barbara E, Beretta E, Boselli E, Storti E, Harizay F, Della Mura F, Lorini FL, Donato Sigurtà F, Marino F, Mojoli F, Rasulo F, Grasselli G, Casella G, De Filippi G, Castelli G, Aldegheri G, Gallioli G, Lotti G, Albano G, Landoni G, Marino G, Vitale G, Battista Perego G, Evasi G, Citerio G, Foti G, Natalini G, Merli G, Sforzini I, Bianciardi L, Carnevale L, Grazioli L, Cabrini L, Guatteri L, Salvi L, Dei Poli M, Galletti M, Gemma M, Ranucci M, Riccio M, Borelli M, Zambon M, Subert M, Cecconi M, Mazzoni MG, Raimondi M, Panigada M, Belliato M, Bronzini N, Latronico N, Petrucci N, Belgiorno N, Tagliabue P, Cortellazzi P, Gnesin P, Grosso P, Gritti P, Perazzo P, Severgnini P, Ruggeri P, Sebastiano P, Covello RD, Fernandez-Olmos R, Fumagalli R, Keim R, Rona R, Valsecchi R, Cattaneo S, Colombo S, Cirri S, Bonazzi S, Greco S, Muttini S, Langer T, Alaimo V, Viola U. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574-1581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3537] [Cited by in RCA: 3826] [Article Influence: 765.2] [Reference Citation Analysis (0)] |
| 8. | Sommer P, Lukovic E, Fagley E, Long D, Sobol J, Heller K, Moitra V, Pauldine R, O'Connor M, Shahul S, Nunnally M, Tung A. Initial Clinical Impressions of the Critical Care of COVID-19 Patients in Seattle, New York City, and Chicago. Anesth Analg. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 9. | Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, Spitters C, Ericson K, Wilkerson S, Tural A, Diaz G, Cohn A, Fox L, Patel A, Gerber SI, Kim L, Tong S, Lu X, Lindstrom S, Pallansch MA, Weldon WC, Biggs HM, Uyeki TM, Pillai SK; Washington State 2019-nCoV Case Investigation Team. First Case of 2019 Novel Coronavirus in the United States. N Engl J Med. 2020;382:929-936. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4155] [Cited by in RCA: 3821] [Article Influence: 764.2] [Reference Citation Analysis (1)] |
| 10. | Zhou Z, Zhao N, Shu Y, Han S, Chen B, Shu X. Effect of Gastrointestinal Symptoms on Patients Infected With Coronavirus Disease 2019. Gastroenterology. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 167] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 11. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30114] [Article Influence: 6022.8] [Reference Citation Analysis (3)] |
| 12. | Chen L, Liu HG, Liu W, Liu J, Liu K, Shang J, Deng Y, Wei S. [Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia]. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:E005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 108] [Reference Citation Analysis (0)] |
| 13. | Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14869] [Cited by in RCA: 12975] [Article Influence: 2595.0] [Reference Citation Analysis (1)] |
| 14. | Wang Y, Wang Y, Chen Y, Qin Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J Med Virol. 2020;92:568-576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 972] [Cited by in RCA: 851] [Article Influence: 170.2] [Reference Citation Analysis (0)] |
| 15. | Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, Fan Y, Zheng C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425-434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2493] [Cited by in RCA: 2310] [Article Influence: 462.0] [Reference Citation Analysis (0)] |
| 16. | Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, Akdis CA, Gao YD. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2139] [Cited by in RCA: 2342] [Article Influence: 468.4] [Reference Citation Analysis (0)] |
| 17. | Liu K, Fang YY, Deng Y, Liu W, Wang MF, Ma JP, Xiao W, Wang YN, Zhong MH, Li CH, Li GC, Liu HG. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl). 2020;133:1025-1031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 728] [Cited by in RCA: 856] [Article Influence: 171.2] [Reference Citation Analysis (0)] |
| 18. | Cheng JL, Huang C, Zhang GJ, Liu DW, Li P, Lu CY, Li J. [Epidemiological characteristics of novel coronavirus pneumonia in Henan]. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:327-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 19. | Young BE, Ong SWX, Kalimuddin S, Low JG, Tan SY, Loh J, Ng OT, Marimuthu K, Ang LW, Mak TM, Lau SK, Anderson DE, Chan KS, Tan TY, Ng TY, Cui L, Said Z, Kurupatham L, Chen MI, Chan M, Vasoo S, Wang LF, Tan BH, Lin RTP, Lee VJM, Leo YS, Lye DC; Singapore 2019 Novel Coronavirus Outbreak Research Team. Epidemiologic Features and Clinical Course of Patients Infected With SARS-CoV-2 in Singapore. JAMA. 2020;323:1488-1494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1346] [Cited by in RCA: 1366] [Article Influence: 273.2] [Reference Citation Analysis (0)] |
| 20. | Arentz M, Yim E, Klaff L, Lokhandwala S, Riedo FX, Chong M, Lee M. Characteristics and Outcomes of 21 Critically Ill Patients With COVID-19 in Washington State. JAMA. 2020;323:1612-1614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1511] [Cited by in RCA: 1624] [Article Influence: 324.8] [Reference Citation Analysis (0)] |
| 21. | Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14113] [Cited by in RCA: 14767] [Article Influence: 2953.4] [Reference Citation Analysis (0)] |
| 22. | Giacomelli A, Pezzati L, Conti F, Bernacchia D, Siano M, Oreni L, Rusconi S, Gervasoni C, Ridolfo AL, Rizzardini G, Antinori S, Galli M. Self-reported olfactory and taste disorders in SARS-CoV-2 patients: a cross-sectional study. Clin Infect Dis. 2020;ciaa330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 881] [Cited by in RCA: 864] [Article Influence: 172.8] [Reference Citation Analysis (0)] |
| 23. | Cheung KS, Hung IF, Chan PP, Lung KC, Tso E, Liu R, Ng YY, Chu MY, Chung TW, Tam AR, Yip CC, Leung KH, Yim-Fong Fung A, Zhang RR, Lin Y, Cheng HM, Zhang AJ, To KK, Chan KH, Yuen KY, Leung WK. Gastrointestinal Manifestations of SARS-CoV-2 Infection and Virus Load in Fecal Samples from the Hong Kong Cohort and Systematic Review and Meta-analysis. Gastroenterology. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1113] [Cited by in RCA: 1132] [Article Influence: 226.4] [Reference Citation Analysis (1)] |
| 24. | Leung WK, To KF, Chan PK, Chan HL, Wu AK, Lee N, Yuen KY, Sung JJ. Enteric involvement of severe acute respiratory syndrome-associated coronavirus infection. Gastroenterology. 2003;125:1011-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 295] [Article Influence: 13.4] [Reference Citation Analysis (1)] |
| 25. | Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, Bi Y, Ma X, Zhan F, Wang L, Hu T, Zhou H, Hu Z, Zhou W, Zhao L, Chen J, Meng Y, Wang J, Lin Y, Yuan J, Xie Z, Ma J, Liu WJ, Wang D, Xu W, Holmes EC, Gao GF, Wu G, Chen W, Shi W, Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8473] [Cited by in RCA: 7601] [Article Influence: 1520.2] [Reference Citation Analysis (0)] |
| 26. | Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271-280.e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11946] [Cited by in RCA: 14271] [Article Influence: 2854.2] [Reference Citation Analysis (0)] |
| 27. | Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367:1444-1448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3274] [Cited by in RCA: 3676] [Article Influence: 735.2] [Reference Citation Analysis (0)] |
| 28. | Du M, Cai G, Chen F, Christiani DC, Zhang Z, Wang M. Multi-omics Evaluation of Gastrointestinal and Other Clinical Characteristics of SARS-CoV-2 and COVID-19. Gastroenterology. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 112] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 29. | Zhang H, Kang Z, Gong H, Xu D, Wang J, Li Z, Cui X, Xiao J, Meng T, Zhou W, Liu J, Xu H. The digestive system is a potential route of 2019-nCov infection: a bioinformatics analysis based on single-cell transcriptomes. 2020 Preprint. bioRxiv:2020.01.30.927806. [DOI] [Full Text] |
| 30. | Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology. 2020;158:1831-1833.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1985] [Cited by in RCA: 1995] [Article Influence: 399.0] [Reference Citation Analysis (1)] |
| 31. | Pan L, Mu M, Yang P, Sun Y, Wang R, Yan J, Li P, Hu B, Wang J, Hu C, Jin Y, Niu X, Ping R, Du Y, Li T, Xu G, Hu Q, Tu L. Clinical Characteristics of COVID-19 Patients With Digestive Symptoms in Hubei, China: A Descriptive, Cross-Sectional, Multicenter Study. Am J Gastroenterol. 2020;115:766-773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1160] [Cited by in RCA: 1205] [Article Influence: 241.0] [Reference Citation Analysis (0)] |
| 32. | Tian Y, Rong L, Nian W, He Y. Review article: gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment Pharmacol Ther. 2020;51:843-851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 594] [Cited by in RCA: 583] [Article Influence: 116.6] [Reference Citation Analysis (0)] |
| 33. | Tran J, Glavis-Bloom J, Bryan T, Harding KT, Chahine C, Houshyar R. COVID-19 patient presenting with initial gastrointestinal symptoms. Eurorad. 2020;Case 16654. |
| 34. | Wang S, Guo L, Chen L, Liu W, Cao Y, Zhang J, Feng L. A case report of neonatal COVID-19 infection in China. Clin Infect Dis. 2020;ciaa225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 237] [Cited by in RCA: 304] [Article Influence: 60.8] [Reference Citation Analysis (0)] |
| 35. | An P, Chen H, Jiang X, Su J, Xiao Y, Ding Y, Ren H, Ji M, Chen Y, Chen W, Lv X, Shen L, Chen M, Li J, Yin A, Kang J, Liu S, Tan W, Wu L, Dong W, Cao J, Zhou Z, Tan S, Chen G, Zhou J, Yang Y, Yu H. Clinical Features of 2019 Novel Coronavirus Pneumonia Presented Gastrointestinal Symptoms But Without Fever Onset. 2020 Preprint. Available from: https://ssrn.com/abstract=3532530. [RCA] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Reference Citation Analysis (2)] |
| 36. | Carvalho A, Alqusairi R, Adams A, Paul M, Kothari N, Peters S, DeBenedet AT. SARS-CoV-2 Gastrointestinal Infection Causing Hemorrhagic Colitis: Implications for Detection and Transmission of COVID-19 Disease. Am J Gastroenterol. 2020;PreProof. |
| 37. | Cappell M. Severe Diarrhea and Impaired Renal Function in COVID-19 Disease. Am J Gastroenterol. 2020;PreProof. |
| 38. | Han C, Duan C, Zhang S, Spiegel B, Shi H, Wang W, Zhang L, Lin R, Liu J, Ding Z, Hou X. Digestive Symptoms in COVID-19 Patients with Mild Disease Severity: Clinical Presentation, Stool Viral RNA Testing, and Outcomes. Am J Gastroenterol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 413] [Cited by in RCA: 383] [Article Influence: 76.6] [Reference Citation Analysis (0)] |
| 39. | Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428-430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1348] [Cited by in RCA: 1295] [Article Influence: 259.0] [Reference Citation Analysis (4)] |
| 40. | Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A, Zhou J, Shi G, Fang N, Fan J, Cai J, Fan J, Lan F. Specific ACE2 Expression in Cholangiocytes May Cause Liver Damage After 2019-nCoV Infection. 2020 Preprint. Available from: bioRxiv:2020.02.03.931766. [DOI] [Full Text] |
| 41. | Yao XH, Li TY, He ZC, Ping YF, Liu HW, Yu SC, Mou HM, Wang LH, Zhang HR, Fu WJ, Luo T, Liu F, Chen C, Xiao HL, Guo HT, Lin S, Xiang DF, Shi Y, Li QR, Huang X, Cui Y, Li XZ, Tang W, Pan PF, Huang XQ, Ding YQ, Bian XW. [A pathological report of three COVID-19 cases by minimally invasive autopsies]. Zhonghua Bing Li Xue Za Zhi. 2020;49:E009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 461] [Reference Citation Analysis (0)] |
| 42. | Bangash MN, Patel J, Parekh D. COVID-19 and the liver: little cause for concern. Lancet Gastroenterol Hepatol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 324] [Cited by in RCA: 357] [Article Influence: 71.4] [Reference Citation Analysis (0)] |
| 43. | Zhang Y, Zheng L, Liu L, Zhao M, Xiao J, Zhao Q. Liver impairment in COVID-19 patients: A retrospective analysis of 115 cases from a single centre in Wuhan city, China. Liver Int. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 310] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 44. | Wander P, Epstein M, Bernstein D. COVID-19 Presenting as Acute Hepatitis. Am J Gastroenterol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 125] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 45. | Gu J, Han B, Wang J. COVID-19: Gastrointestinal Manifestations and Potential Fecal-Oral Transmission. Gastroenterology. 2020;158:1518-1519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 954] [Cited by in RCA: 949] [Article Influence: 189.8] [Reference Citation Analysis (1)] |
| 46. | Chan JF, Yuan S, Kok KH, To KK, Chu H, Yang J, Xing F, Liu J, Yip CC, Poon RW, Tsoi HW, Lo SK, Chan KH, Poon VK, Chan WM, Ip JD, Cai JP, Cheng VC, Chen H, Hui CK, Yuen KY. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514-523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6483] [Cited by in RCA: 5422] [Article Influence: 1084.4] [Reference Citation Analysis (0)] |
| 47. | Ong J, Young BE, Ong S. COVID-19 in gastroenterology: a clinical perspective. Gut. 2020;69:1144-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 48. | Ling Y, Xu SB, Lin YX, Tian D, Zhu ZQ, Dai FH, Wu F, Song ZG, Huang W, Chen J, Hu BJ, Wang S, Mao EQ, Zhu L, Zhang WH, Lu HZ. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin Med J (Engl). 2020;133:1039-1043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 641] [Cited by in RCA: 577] [Article Influence: 115.4] [Reference Citation Analysis (0)] |
| 49. | Yang Z, Li G, Dai X, Liu G, Li G, Jie Y. [Three cases of novel coronavirus pneumonia with viral nucleic acids still positive in stool after throat swab detection turned negative]. Zhonghua Xiaohua Zazhi. 2020;. [DOI] [Full Text] |
| 50. | Zhang J, Wang S, Xue Y. Fecal specimen diagnosis 2019 novel coronavirus-infected pneumonia. J Med Virol. 2020;92:680-682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 278] [Cited by in RCA: 301] [Article Influence: 60.2] [Reference Citation Analysis (0)] |
| 51. | Bezerra J, Pochapin M, El-Serag HB, Vargo JJ. COVID-19 Clinical Insights for Our Community of Gastroenterologists and Gastroenterology Care Providers. American College of Gastroenterology, 2020. Available from: https://www.gastro.org/press-release/joint-gi-society-message-covid-19-clinical-insights-for-our-community-of-gastroenterologists-and-gastroenterology-care-providers. |
| 52. | Lui RN, Wong SH, Sánchez-Luna SA, Pellino G, Bollipo S, Wong MY, Chiu PWY, Sung JJY. Overview of guidance for endoscopy during the coronavirus disease 2019 pandemic. J Gastroenterol Hepatol. 2020;35:749-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 172] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 53. | Ianiro G, Mullish BH, Kelly CR, Sokol H, Kassam Z, Ng S, Fischer M, Allegretti JR, Masucci L, Zhang F, Keller J, Sanguinetti M, Costello SP, Tilg H, Gasbarrini A, Cammarota G. Screening of faecal microbiota transplant donors during the COVID-19 outbreak: suggestions for urgent updates from an international expert panel. Lancet Gastroenterol Hepatol. 2020;5:430-432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 97] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 54. | Green CA, Quraishi MN, Shabir S, Sharma N, Hansen R, Gaya DR, Hart AL, Loman NJ, Iqbal TH. Screening faecal microbiota transplant donors for SARS-CoV-2 by molecular testing of stool is the safest way forward. Lancet Gastroenterol Hepatol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 55. | Mao R, Liang J, Shen J, Ghosh S, Zhu LR, Yang H, Wu KC, Chen MH; Chinese Society of IBD, Chinese Elite IBD Union; Chinese IBD Quality Care Evaluation Center Committee. Implications of COVID-19 for patients with pre-existing digestive diseases. Lancet Gastroenterol Hepatol. 2020;5:426-428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 218] [Cited by in RCA: 241] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 56. | Surveillance Epidemiology of Coronavirus (COViD-19) Under Research Exclusion. SECURE-Cirrhosis, 2020. Available from: https://covidcirrhosis.web.unc.edu/. |
| 57. | COVID-Hep. COVID-Hep.net: Coronavirus (COVID-19) in liver disease reporting registry, 2020. Available from: https://www.covid-hep.net/. |
| 58. | Ng WK, Wong SH, Ng SC. Changing epidemiological trends of inflammatory bowel disease in Asia. Intest Res. 2016;14:111-119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 240] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 59. | Loftus EV. Update on the Incidence and Prevalence of Inflammatory Bowel Disease in the United States. Gastroenterol Hepatol (N Y). 2016;12:704-707. [PubMed] |
| 60. | Surveillance Epidemiology of Coronavirus (COVID-19) Under Research Exclusion. SECURE-IBD, 2020. Available from: https://covidibd.org/. |
| 61. | International Organization For the Study Of Inflammatory Bowel Disease. IOIBD Update on COVID19 for Patients with Crohn’s Disease and Ulcerative Colitis, 2020. Available from: https://www.ioibd.org/. |