Published online May 21, 2020. doi: 10.3748/wjg.v26.i19.2305
Peer-review started: January 1, 2020
First decision: January 16, 2020
Revised: March 27, 2020
Accepted: April 28, 2020
Article in press: April 28, 2020
Published online: May 21, 2020
Processing time: 141 Days and 7.2 Hours
Pancreatic neuroendocrine tumors (pNETs) are a heterogeneous group of tumors with complicated treatment options that depend on pathological grading, clinical staging, and presence of symptoms related to hormonal secretion. With regard to diagnosis, remarkable advances have been made: Chromogranin A is recommended as a general marker for pNETs. But other new biomarker modalities, like circulating tumor cells, multiple transcript analysis, microRNA profile, and cytokines, should be clarified in future investigations before clinical application. Therefore, the currently available serum biomarkers are insufficient for diagnosis, but reasonably acceptable in evaluating the prognosis of and response to treatments during follow-up of pNETs. Surgical resection is still the only curative therapeutic option for localized pNETs. However, a debulking operation has also been proven to be effective for controlling the disease. As for drug therapy, steroids and somatostatin analogues are the first-line therapy for those with positive expression of somatostatin receptor, while everolimus and sunitinib represent important progress for the treatment of patients with advanced pNETs. Great progress has been achieved in the combination of systematic therapy with local control treatments. The optimal timing of local control intervention, planning of sequential therapies, and implementation of multidisciplinary care remain pending.
Core tip: Pancreatic neuroendocrine tumors are a heterogeneous group of tumors with complicated treatment. There are several highlights of our manuscript. First, we summarize conventional and new advances in serum biomarkers, like circulating tumor cells, multiple transcript analysis, microRNA profile, and cytokines. Then we review the changes of each guidelines of grading and staging systems and the clinical evidence behind them. Lastly, surgical resection is still the only curative therapeutic option for localized pancreatic neuroendocrine tumors. Great progress has been achieved in drug therapy and the combination with local control treatments. We summarize new advances in detail and provide potential strategies for the management of neuroendocrine tumor associated with liver metastases.
- Citation: Ma ZY, Gong YF, Zhuang HK, Zhou ZX, Huang SZ, Zou YP, Huang BW, Sun ZH, Zhang CZ, Tang YQ, Hou BH. Pancreatic neuroendocrine tumors: A review of serum biomarkers, staging, and management. World J Gastroenterol 2020; 26(19): 2305-2322
- URL: https://www.wjgnet.com/1007-9327/full/v26/i19/2305.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i19.2305
Neuroendocrine neoplasms (NENs), a group of tumors that originate from neuroendocrine cells, are found in all organs, especially in the lung, the digestive tract, and the pancreas[1]. Pancreatic neuroendocrine tumors (pNETs), which were first described in 1869, are a subgroup of NENs that have relatively distinct biological behavior and clinical management compared with pancreatic adenocarcinoma. Although the incidence of pNETs is less than or equal to one case per 100000 individuals per year and they only comprise 1% to 2% of pancreatic neoplasms, their incidence is increasing[2]. pNETs are clinically classified as functioning or nonfunctioning depending on whether they release hormones that produce symptoms: 60% to 90% of pNETs are nonfunctioning and largely asymptomatic[3]. In contrast, functioning pNETs are much more uncommon and associated with symptoms related to their capacity to produce different hormones, including insulin, gastrin, vasoactive intestinal peptide (VIP), glucagon, somatostatin, and serotonin. Since the late 2000s, basic and clinical research of pNETs has notably progressed and therapy has trended toward comprehensive and minimal invasion treatment[4]. Despite these advances, pNETs, as a distinct clinical entity, remain largely unexplored. Reliable knowledge of the histologic characteristics, biological mechanism, and definition criteria of pNETs is a prerequisite for diagnosis, staging, treatment, and prognosis. Therefore, the aim of this review is to provide an overview of the serum biomarkers and controversial pathologic grading and clinical staging criteria and give an updated review of the comprehensive treatment of pNETs.
Chromogranin A: Chromogranin A (CgA), a glycoprotein secreted by neurons and neuroendocrine cells, is considered one of the best-described clinical biomarkers of NETs with a sensitivity of 66%, specificity of 95%, and overall accuracy of 71% in pNETs[5]. Several guidelines, including those by both the North American Neuroendocrine Tumor Society (NANETS) and the European Neuroendocrine Tumor Society (ENETS), recommend serum CgA as a marker during diagnosis and follow-up in nonfunctioning-pNETs[6,7]. However, CgA is not specified as a diagnostic biomarker because it is false-positively elevated in other tumor- and non-tumor-related situations, such as inflammatory bowel disease, chronic gastritis, renal or liver failure, prostate and thyroid cancer, and pancreatitis. Therapy with proton pump inhibitors (PPIs), steroids, and somatostatin analogues (SSAs) also increases serum CgA levels[8,9]. Nevertheless, CgA could be an independent prognosis factor of progression-free survival (PFS) and overall survival (OS) in patients with pNETs. Pulvirenti et al[5] analyzed a cohort of 65 patients with pNETs and found a CgA baseline value of > 15 ng/mL as a significant predictor of OS. Giusti et al[9] reported that preoperative plasma CgA in patients with postoperative recurrence was significantly higher than that in patients without recurrence. Several studies showed that both response to treatment and the presence of metastases, particularly in the liver, are correlated with serum CgA levels[10]. Therefore, the measurement of CgA levels may serve as a reliable marker for clinical management in follow-up rather than for diagnosis.
Neuron-specific enolase: Neuron-specific enolase (NSE) is a glycolytic enzyme expressed in the neurons and neuroendocrine and paraneuronal cells. NSE is not commonly used alone in clinical practice due to its diagnostic sensitivity of 31%[11]. However, NSE levels are associated with poor differentiation and shorter PFS, even if CgA levels are normal[12]. Yao et al[13] assessed the prognostic value of CgA combined with NSE and found that elevated baseline CgA/NSE provided prognostic information on PFS and survival; early CgA/NSE responses are potential prognostic markers for treatment outcomes in patients with advanced pNET.
Progastrin-releasing peptide: Progastrin-releasing peptide (ProGRP) is a precursor of a neuropeptide hormone called GRP, which is frequently used as a marker for diagnosing and monitoring small-cell lung cancer[14,15]. ProGRP is a biologically active protein that stimulates tumor cell proliferation. Thus, proGRP may be associated with more aggressive tumor behavior and poor prognosis. Korse et al[16] found that the serum ProGRP level is associated with tumor grade in NENs. A combination of tumor markers – CgA, NSE, proGRP, and cytokeratin fragments - provided more diagnostic and prognostic information than each marker alone.
Pancreatic polypeptide: Pancreatic polypeptide (PP) is produced by pancreatic islet cells that are located in the head and uncinate of the pancreas. PP is generally considered a secondary pNETs marker due to its limited sensitivity and specificity. A study of 323 patients with pNET reported an elevated serum level of PP in 45% of patients[17]. Other studies reported a diagnostic sensitivity for PP ranging from 41% to 68% for pNETs[18]. A higher serum concentration of PP can also be detected in several physiological conditions, including physical exercise, hypoglycemia, and food intake. PP false-negatively identify some CgA cases, and a combination of PP with CgA could improve the sensitivity by 25% to a total of 93% in the group of nonfunctioning-pNETs (NF-pNETs)[19]. Walter et al[20] found that during the follow-up period, PP had a high specificity (84%) for predicting the disease control rate (DCR), and an increase > 50% in PP serum level was correlated with tumor progression on imaging.
Insulin: Insulin-secreting pNETs, also known as insulinomas, are almost specific to the pancreas and the most common functioning pNET. Patients with insulinoma show increased serum insulin levels and other clinical symptoms associated with hypoglycemia. Some studies have reported the 72 h fasting test as an effective gold standard for diagnosing insulinoma with a nearly 100% sensitivity and specificity[21]. Developing the classic symptoms of hypoglycemia requires 12 h of fasting and in the first 48 h most patients suffer from Whipple’s triad: Symptoms and signs of hypoglycaemia, low plasma level of glucose, and resolution of symptoms after correction of the hypoglycaemia[22].
Glucagon: Glucagon, a hormone produced by pancreatic islet α cells, plays an opposite role to insulin in glycometabolism. Elevated levels of glucagon above 500 pg/mL can be detected in glucagon-secreting pNETs, also termed glucagonomas[12]. Glucagonomas, accompanied as part of multiple endocrine neoplasia 1 syndrome[23], has malignancy potential. Glucagonoma syndrome includes symptoms of necrotic migratory erythema, weight loss, hypoalbuminemia, and diabetes mellitus or impaired glucose tolerance. However, glucagon levels, the only specific indicator, is also elevated in other conditions, such as cirrhosis, diabetes mellitus, sepsis, and burns. Therefore, hyperglucagonemia must be considered together with other typical symptoms of glucagonoma syndrome for diagnosis.
VIP: VIP, a 28 amino acid peptide hormone produced by the brain, gut, and pancreas, plays an important role in gastrointestinal contraction and pancreatic exocrine secretion. Thus, it naturally links VIP-secreting tumors of the pancreas (also known as VIPomas), which represent an infrequent subtype of pancreatic islet cell tumors, with a characteristic clinical presentation (Verner–Morrison syndrome), involving watery diarrhea, hypokalemia, and achlorhydria. The rate of metastasis (commonly in regional lymph nodes and the liver) of VIPomas has been reported to be 50%-89% at the initial diagnosis[24,25]. In a large cohort of 1000 patients with various etiology factors of diarrhea, elevated plasma VIP levels were found to be 100% specific for the presence of VIPoma in 39 patients[25].
Gastrin: Gastrinomas, localized in the pancreas (10%-40%) or duodenum (60%-80%), often cause the oversecretion of gastrin, which generally functions as a factor promoting the release of gastric acid[26]. Increased serum gastrin may be indicative of the presence of a gastrinoma and the diagnosis of Zollinger–Ellison syndrome (gastroesophageal reflux and complicated peptic ulcer disease). Because serum gastrin can be elevated in patients with atrophic gastritis and during the treatment of PPIs, it is suggested to stop taking PPIs or seek an alternative to histamine type 2 receptor (H2) blockers for at least 7 d[27]. A more than 1000-fold increase in the serum level of gastrin can be diagnosed as gastrinoma[28], whereas for intermediately elevated gastrin levels, a secretin test is needed. Secretin (2 U/kg weight) is administered by intravenous bolus and the serum gastrin is measured. An increase in gastrin by ≥ 120 pg/mL over baseline is considered positive with a diagnostic sensitivity of 94% and specificity of 100%[29]. An imaging test is required to confirm the localization of the tumor prior to seeking surgical treatment.
Somatostatin: Somatostatin-producing NETs (SSoma) mainly originate from the pancreas, the duodenum close to the ampulla, and the peri-ampullary area. Because somatostatin inhibits the endocrine secretion and the motility of the stomach and gallbladder, somatostatin-producing NETs always cause a classical triad of syndromes: Hyperglycemia, cholelithiasis, and maldigestion of food[30]. Serum somatostatin levels can be elevated with regard to various extra-pancreatic NETs, and a prevalence of 1 in 40 million in the morbidity of pancreatic somatostatin-producing NETs makes drawing conclusions difficult based on both typical clinical symptoms and laboratory assessments.
Ectopic hormones: In other cases, identification of the elevated serum level of a specific hormone is also useful for diagnosis. The ectopic secretion of adr-enocorticotropic hormone (ACTH) can be observed in pNET, which can lead to the manifestation of Cushing’s syndrome. The primary differential diagnosis is Cushing’s disease, which can be excluded by an enhanced pituitary magnetic resonance imaging (MRI)[31]. Growth hormone (GRH), another hormone released by the pituitary, can also be elevated in NETs. The measurement of GRH and insulin like growth factor-1, and a GRH suppression test are needed for diagnosis[32]. To date, only Melmed et al[33] have reported a single case of ectopic GRH in pNET. Other specific serum biomarkers of functioning-pNETs are listed in Table 1.
| Tumor/syndrome | Location | Incidence (million per year) | Biomarker | Main symptoms |
| Insulinoma | Pancreas | 1-32 | Insulin | Hypoglycaemia |
| Gastrinoma | Pancreas, duodenum | 0.5-21.5 | Gastrin | Zollinger-Ellison syndrome: Gastroesophageal reflux and complicated peptic ulcer disease |
| VIPoma | Pancreas | 0.05-0.2 | Vasoactive intestinal peptide | Verner-Morrison syndrome: Watery diarrhea, hypokalemia, and achlorhydria |
| Glucagonoma | Pancreas | 0.01-0.1 | Glucagon | Necrotic migratory erythema, weight loss, hypoalbuminemia, and diabetes mellitus or impaired glucose tolerance |
| SSoma | Pancreas, duodenum, jejunum | Rare | Somatostatin | Hyperglycemia, cholelithiasis, and maldigestion of food |
| ACTHoma | Pancreas | Rare | ACTH | Cushing syndrome |
| GRHoma | Pancreas, lung, jejunum | Rare | GRH | Acromegaly |
| Carcinoid syndrome caused by pNET | Pancreas | Rare | Serotonin, 5-hydroxyindoleacitic acid | Skin flushing, diarrhoea, bronchospasm, and cardiac valve fibrosis |
| Hypercalcemia caused by pNET | Pancreas | Rare | Parathyroid hormone-related peptide | Hypercalcaemia and abdominal pain |
Circulating tumor cells: The tumor cells in the peripheral blood are termed circulating tumor cells (CTCs), which are supposed to be useful biomarkers for providing diagnostic and prognostic information. The identified characteristics of CTCs from circulation include the size of tumor cells and the expression of epithelial cell adhesion molecule (EpCAM)[34]. In a retrospective study, CTCs were detected in 36% of patients with pNETs through the use of the Food and Drug Administration-approved Cell Search platform. The presence of CTCs appears to be associated with a higher tumor grade, tumor burden, an increased circulating CgA concentration, and a higher Ki67 index[35]. Several large studies of other tumors (colorectal cancer and prostate cancer) reported CTCs as an independent factor for predicting PFS and OS[36,37]. For NETs, Khan et al[35] reported that CTCs are also independently associated with PFS and OS, indicating a 3.3-fold increased risk of progression and 3.7-fold increased risk of death in patients with the presence of one or more CTCs. For the pancreatic cohort, a similar but nonsignificant trend was observed, possibly due to the smaller number in this subgroup. Changes in CTCs after treatments were strongly associated with OS, with the best prognostic group being patients with 0 CTCs after therapy, followed by those with a ≥ 50% reduction in CTCs, with those with a < 50% reduction or increase in CTCs having the worst outcome. Current CTC analyses may not be sensitive and specific enough as diagnostic biomarkers to detect all NET types or to distinguish pNETs from different types of NETs. Although its concept and technology have attractive value, CTCs cannot be used in their current form as an effective biomarker for pNETs[10].
NETest: A novel multianalyte biomarker, multiple transcript analysis PCR-based test (NETest) using blood-based quantitative real-time PCR to measure 51 different NET-related transcripts presented promising results in both the diagnosis and prognosis of NETs[38]. Captured by gene co-expression networks from tissue and blood transcriptome databases, these 51 relevant transcripts include a series of genes that are associated with neoplastic behavior and the proliferation, signaling, and secretion of NETs[39]. The first prospective study included 206 patients with gastroenteropancreatic NETs (GEP-NETs), in which NETest had areas under the curve of 0.95 and 0.98 for diagnosis in two different validation sets. The sensitivity and specificity of the test applied to pNETs are 80% and 94%, respectively[40]. NETests can also be used in the prediction of progression and response to treatment in follow-up. Pavel et al[40] and Ćwikła et al[41] demonstrated that NETest is significantly associated with disease progression. The NETest was more informative than CgA changes in predicting disease alterations. The NETest had an earlier time point change than imaging. A study assessing the predictive value of the NETest on the therapeutic response to SSAs found that NETest (P = 0.002) and tumor grading (P = 0.054) were the only factors associated with treatment response in a prospective group of 35 GEP-NETs (9 for pNETs)[41]. These outcomes are encouraging, but concerns exist about the technical availability and cost-effectiveness of this biomarker in clinical practice.
MicroRNAs: MicroRNAs (miRNAs) are a series of small non-coding RNAs with the capability to regulate gene expression at the post-transcriptional level in biological processes, including carcinogenesis[42]. In contrast with several studies that described miRNAs as biomarkers in GEP-NET tissues, little is known about serum miRNA levels and only a few oncogenic and suppressor serum miRNAs were identified in pNETs. Upregulation of serum miR-193b and plasma miR-21 levels was noted in patients with pNETs[43,44].
In a separate study, down-regulation of serum miR-1290 was found to discriminate pNET from pancreatic adenocarcinomas (area under the curve of 0.80). Other significantly down-regulated serum miRNAs in pNETs include miR-584, miR-1285, miR-550-002410, and miR-1825[45]. Although the clinical application of miRNAs in the diagnosis of pNETs remains an attractive research interest, further studies are needed to understand their biological mechanism in the development of pNETs, and to form a measurement standard or to develop a diagnostic reagent kit[46].
Cytokines: The vascular endothelial growth factor (VEGF) signaling pathway plays a pivotal role in regulating tumor angiogenesis and has been proven to be related to cell survival, growth, and metastasis. VEGF, as a therapeutic target, has been validated in various types of cancers; GEP-NETs also express high levels of VEGF and its transmembrane receptors (VEFGR-1, VEFGR-2, and VEFGR-3), which can be detected in peripheral blood[47]. Relationships between VEGFR and prognosis have been described. High baseline levels of VEGFR-2 are associated with decreased OS in pNETs[48]. Interleukin-8 (IL-8) plays a vital part in proangiogenesis, mitogenesis, and mitogenesis through interaction with two receptors, IL-8RA and IL-8RB (also known as CXCR1 and CXCR2, respectively)[49]. In addition to IL-8, its receptor IL-8RB is elevated in patients with pNETs[50,51]. In patients with carcinoids, low pre-treatment IL-8 levels predicted longer PFS, longer OS, and better response to sunitinib, indicating that IL-8 is a candidate marker of prognosis and sunitinib treatment benefits this subset of patients[50]. Similar to IL-8, stromal cell-derived factor-1α is also an important regulatory factor of cell migration, proliferation, and angiogenesis. Stromal cell-derived factor-1α levels are significantly higher in pNETs compared to other NETs and are inversely correlated with disease-free survival[48].
Overall, various types of cytokines produced promising results in diagnosis and prognosis of pNETs, but large-sample and well controlled trials are still required to validate and qualify the results.
To guide clinical practice, of the two most common staging systems for pNETs, one was constructed by ENETS and the other by the American Joint Committee on Cancer (AJCC).
The sixth edition of the AJCC Cancer Staging Manual, published in 2002, excluded pNETs when staging pancreatic tumors[52]. pNETs were first isolated from pancreatic adenocarcinoma in the seventh edition of the AJCC staging system, published in 2010; however, the same staging classification criteria in pancreatic adenocarcinoma were directly applied to pNETs in this edition[53]. The biological behaviors and prognosis are absolutely different between pNETs and pancreatic adenocarcinoma, so it seems inappropriate to apply the pancreatic adenocarcinoma staging system to pNETs without any adjustments. Two large cohort studies found that the proportion of patients diagnosed with stage III disease according to the seventh AJCC edition was relatively small. Rindi et al[54] reported a poor discrimination of survival between patients diagnosed with stages II and III disease[54,55]. All these findings support the need for revising the staging system for pNETs.
As a result, the newly revised eighth edition did not follow the seventh edition and introduced another classification criterion asserted by ENETS. Several changes were made: First, the eighth edition staging system only applied to well differentiated G1, G2, and G3 tumors (World Health Organization Classification, 2017 edition), whereas the remaining poorly differentiated G3 tumors [also known as neuroendocrine carcinoma (NEC)] followed the pancreatic cancer staging system. Second, the T category was more detailed in emphasizing the classification of tumor size. The presence of invasion around the pancreas was excluded. Third, adjacent structures, including major vascular invasion, were categorized as belonging to the T4 category. Finally, the M1 category was specified according to metastatic sites. These significant changes reveal the biological behavior of pNETs and allow the accurate assessment of prognosis[56,57]. However, some controversies remain in this staging system. A similar OS was found between patients diagnosed with stage I and stage IIA disease. Patients with stage IIIB disease (TxN1M0) had a lower hazard ratio for death than patients with stage IIIA disease (T4N0M0)[58]. Given these findings, a modified ENETS (mENETS) staging classification was proposed by maintaining the ENETS T, N, and M definitions but adopting the seventh AJCC edition’s staging definitions[59]. Confirmed by two large pNET cohorts, the mENETS staging classification is more suitable for pNETs than the AJCC or ENETS system and may be adopted in the next AJCC edition. The definitions and groups of several important staging editions for pNETs are shown in Tables 2 and 3.
| AJCC 7th staging classification | AJCC 8th and ENETS staging classification | ||
| T1 | Limited to the pancreas, ≤ 2 cm in greatest dimension | T1 | Tumor limited to the pancreas, ≤ 2 cm |
| T2 | Limited to the pancreas, > 2 cm in greatest dimension | T2 | Tumor limited to the pancreas, 2-4 cm |
| T3 | Beyond the pancreas but without involvement of the superior mesenteric artery | T3 | Tumor limited to the pancreas, > 4 cm, or invading the duodenum or common bile duct |
| T4 | Involvement of the celiac axis or superior mesenteric artery (unresectable tumor) | T4 | Tumor invades adjacent structures |
| N0 | No regional lymph node metastasis | N0 | No regional lymph node metastasis |
| N1 | Regional lymph node metastasis | N1 | Regional lymph node metastasis |
| M0 | No distant metastasis | M0 | No distant metastasis |
| M1 | Distant metastasis | M1 | Distant metastasis |
| M1a | Metastasis confined to liver | ||
| M1b | Metastasis in at least one extrahepatic site | ||
| M1c | Both hepatic and extrahepatic metastases |
| AJCC 7th staging classification | AJCC 8th and ENETS staging classification | mENETS | |||||||
| Stage | T | N | M | T | N | M | T | N | M |
| IA | T1 | N0 | M0 | T1 | N0 | M0 | T1 | N0 | M0 |
| IB | T2 | N0 | M0 | T2 | N0 | M0 | T2 | N0 | M0 |
| IIA | T3 | N0 | M0 | T3 | N0 | M0 | T3 | N0 | M0 |
| IIB | T1-3 | N1 | M0 | T4 | N0 | M0 | T1-3 | N1 | M0 |
| III | T4 | Any N | M0 | Any T | N1 | M0 | T4 | Any N | M0 |
| IV | Any T | Any N | M1 | Any T | Any N | M1 | Any T | Any N | M1 |
Several common consensuses exist for the pathological classification and diagnosis of pNENs: The World Health Organization (WHO) pathological classification, the ENETS guideline, NANETS consensus, and the National Comprehensive Cancer Network (NCCN) guideline, as well as the Chinese Society of Clinical Oncology expert consensus. The most general and commonly used consensus is the WHO pathological classification (Table 4).
| Edition | Grading standards | |||
| 2000/2004 | G1: Well-differentiated NET; ≤ 2 cm in size, Ki-67 ≤ 2% | G2: Well-differentiated NEC; > 2 cm in size, Ki-67 3%-20% or angioinvasive | G3: Poorly differentiated NEC; Ki-67 > 20% | |
| 2010 | NET-G1: Well-differentiated, mitotic count < 2/2 mm2, Ki-67 ≤ 2% | NET-G2: Well-differentiated, mitotic count 2-20/2 mm2, Ki-67 3%-20% | NEC-G3: Poorly differentiated, mitotic count > 20/2 mm2, Ki-67 > 20% | |
| 2017/2019 | NET-G1: Well-differentiated, mitotic count < 2/2 mm2, Ki-67 ≤ 2% | NET-G2: Well-differentiated, mitotic count 2-20/2 mm2, Ki-67 3%-20% | NET-G3: Well-differentiated, mitotic count > 20/2 mm2, Ki-67 > 20% | NEC-G3: Poorly differentiated, mitotic count > 20/2 mm2, Ki-67 > 20% |
In the first NENs WHO classification published in 1980, the term carcinoid was applied to most of the NETs[60]. However, both pathologists and clinicians struggle to apply this term due to misleading neuroendocrine features and carcinoid syndrome. Therefore, it was finally termed NET and NEC in the WHO 2000 classification of GEP-NETs and the WHO 2004 classification of pNETs[58,61]. Based on tumor size, morphology, and the presence of invasion or metastasis, a distinction was formed between well differentiated NETs (G1), which show benign or uncertain malignant potential behavior; well differentiated NECs (G2), characterized by low-grade malignancy; and poorly differentiated NECs (G3) of high-grade malignancy. This version of the classification acknowledged the existence of benign NENs, which is contrary to the clinical practice of patients with a small and indiscoverable primary focus but a clear liver metastasis.
As all GEP-NENs have metastatic potential, the 2010 WHO classification considered them malignant tumors for the first time and adjusted the grading[62]. Under the auspices of ENETS proposals, a grading tool was devised mainly based on mitotic count and Ki-67. Low–intermediate grade (G1-G2), well differentiated tumors were defined as NETs; high-grade (G3), poorly differentiated tumors were defined as NEC[63]. The 2010 WHO classification was proven to be practicable and effective for predicting the survival of patients with pNENs[55]. However, a subset of neuroendocrine cancers, especially originating from the pancreas, was observed to be actually well differentiated according to standard morphology but classified into high grade (G3) according to the 2010 WHO classification grading tool[64,65]. In 2014, La Rosa et al[66] proposed this type of cancer as a separate group named NET-G3, and NEC can be defined as poorly differentiated small-cell carcinoma and large-cell NEC. In another study, a series of 136 patients diagnosed with NEC-G3 were classified into three groups according to the degree of morphologic differentiation (well vs poorly differentiated) and Ki-67 index (< 55% vs ≥ 55%). Patients with well differentiated neoplasms and a low Ki-67 index have a better OS than other groups[67].
Based on the studies mentioned above, the 2017 WHO classification was devised for NENs of the pancreas alone[68]. The WHO 2010 principles were endorsed in this edition, but a new definition of NET G3 was introduced for neoplasms that are well differentiated in morphology but have a Ki-67 index in the G3 range. The WHO 2017 classification highlighted the association of morphologic differentiation with the definition of NET and NEC. A new concept of mixed neuroendocrine-non-NEN was used to define that mixed neoplasms may occasionally include different types and grades of neuroendocrine components and non-neuroendocrine components (e.g., adeno or squamous). The newest WHO 2019 classification followed the previous version in 2017.
Surgical principle and indication: In most cases, surgical resection remains the only potentially curative treatment for patients with pNETs. All the guidelines mentioned above (including NCCN, ENETS, Chinese Society of Clinical Oncology, and NANETS) recommend surgical resection for all functioning pNETs and localized NF-PNETs (without widespread metastasis), and the surgical options include simple enucleation, central pancreatectomy, distal pancreatectomy with or without splenectomy, and pancreatoduodenectomy (Whipple’s operation), depending on tumor location[69]. Tumor size has traditionally been thought to be directly related to malignant potential; therefore, all tumors larger than 2 cm that are always locally invasive or have positive lymph node involvement should include regional lymph node dissection. However, whether surgical resection or conservative observation of “watch-and-wait” (first presented in ENETS 2012) is more suitable in patients with NF-pNETs smaller than 2 cm remains controversial[70]. Several studies demonstrated a distinct correlation between tumor size and lower malignancy potential, so this observation is acceptable for patients with pNETs smaller than 2 cm[71,72]. Conversely, Sharpe and Finkelstein observed that patients with localized pNETs ≤ 2 cm had an OS advantage with resection compared with observation[73,74].
A novel strategy to manage these small NF-pNETs is obtaining histopathologic grading through endoscopic ultrasound and fine-needle aspiration, so the final decision making is based on histopathologic diagnosis[75].
Studies have reported that small WHO Grade 3 NF-pNETs should be treated surgically due to their malignancy potential, which is inconclusive for those WHO Grade 2 tumors[76,77]. For the rest of small NF-pNETs with low WHO grading, the regimen of conservative observation involves repeat axial imaging and detection of serum biomarker levels (CgA or PP) every 3 mo following diagnosis and then at 6-mo intervals for one year and yearly thereafter. Due to the better biological behavior of pNETs compared with pancreatic adenocarcinoma, more aggressive surgical approaches should be adopted for border resectable and locally advanced pNETs. Some studies have shown that aggressive surgical strategies may also prolong survival as long as an R0 or even R1 resection is conducted[78,79].
A systematic review investigating the role of primary tumor resection with unresectable metastatic disease at the time of diagnosis showed that primary tumor resection without liver debulking surgery remained associated with a decreased risk of death at five years compared with patients who did not have the primary tumor resected. Surgical morbidity, surgical timing, and predictive response to adjuvant treatment should be considered before primary tumor resection with unresectable metastatic disease[80-82].
Lymph nodes dissection: Lymph node metastasis is an independent risk factor of disease-free survival and OS for patients with pNETs; hence, a certain range of lymph node dissection is needed for radical tumor resection[83,84]. However, determining whether the patient has lymph node metastasis before or during operation is difficult. Lopez-Aguiar et al[83] analyzed 695 patients with NF-pNETs and considered a tumor diameter > 2 cm as the predictive factor for lymph node metastasis[85]. Several other studies on NF-pNETs concluded similarly, but this is contrary to the report by Jutric et al[86,87]: Nearly 24% of patients with grade 1 tumors that are less than 1 cm in size undergoing resection have lymph node metastases. Summarizing the current guidelines and studies, lymph nodes dissection is recommended for NF-pNETs > 2 cm in diameter and all functioning pNETs, except insulinoma. This is also applicable for NF-pNETs 1-2 cm in diameter. No lymphadenectomy is allowed in patients with NF-pNETs < 1 cm in diameter but without any high-risk factors.
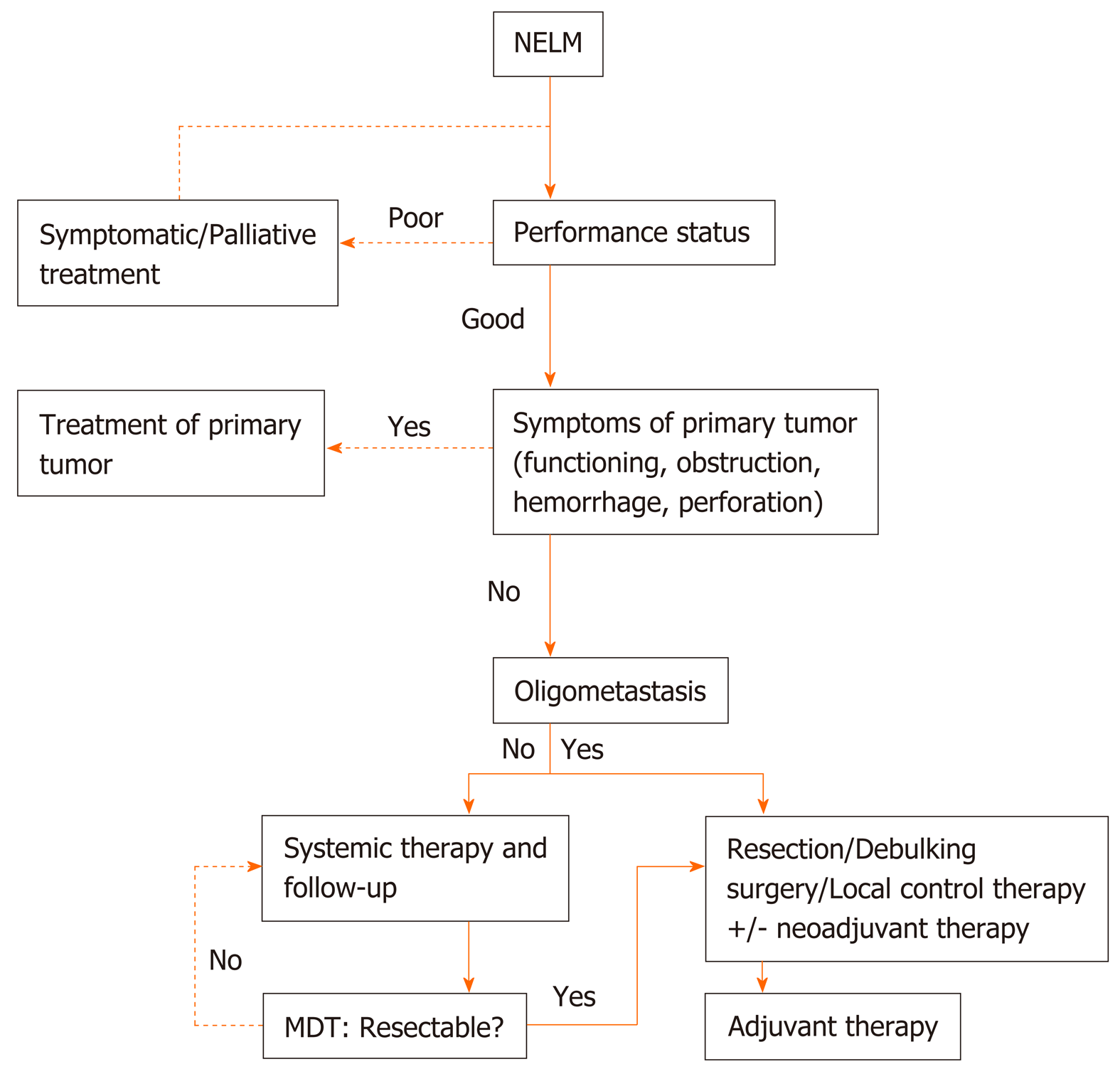
Distant metastasis: About 65%-95% of patients are initially diagnosed with distant metastases, especially liver metastases. Three-to-five-year survival is 13%-54% compared with 75%-99% for patients without hepatic metastases[86,87]. Surgical resection is the only curative treatment for pNETs with liver metastases. A recent systematic review analyzed 1542 pNET patients with liver metastasis and found that the 1-, 3-, and 5-year survival rates of patients undergoing hepatectomy were significantly higher than those of patients without hepatectomy[88]. However, clinical practice recommendations are limited. Complete resection of liver metastases has been associated with better long-term survival in previous studies, survival rates of 60%-80% at five years may be achieved in the resection group, while this decreases to approximately 30% when liver metastases are not resected. Radical resection is suggested in patients with type I and part of type II liver metastases with an anticipated liver remnant of at least 30%, and other indications include well differentiated (G1-G2) tumors, absence of extra-abdominal metastases, and expecting R0/R1 resection[89]. For some complicated type II liver metastases, a two-step surgery, which includes a resection of left metastases associated with right portal vein ligation followed by right hepatectomy, may be proposed[89,90]. Palliative hepatectomy is not recommended in patients with WHO Grade 3 or type III liver metastases. A multi-institutional analysis that identified 612 patients who underwent liver-directed therapy showed that the 5-year survival is higher among patients who underwent an R0/R1 resection compared with those who underwent a debulking (≥ 80%) operation. Among patients with ≥ 50% liver involvement, the 5-year survival rate following debulking was 40.6%. Hepatic debulking for liver metastases is a reasonable therapeutic option for patients with grossly unresectable disease[91]. Potential strategies for management of NET associated with liver metastases (NELM) are shown in Figure 1.
Postoperative follow-up: Follow-up consultation with cross-sectional imaging [triple-phase computed tomography (CT) of the abdomen] or MRI should be performed every year for the first three years after surgical resection, then every one to two years for a total of 10 years[92]. The routine uses of serum biomarkers (including general markers and relevant hormone) for surveillance of recurrence are mentioned above. Patients with a Ki-67 index greater than 5% or any positive lymph nodes are considered at a sufficiently high risk of recurrence to warrant increased frequency of follow-up[92].
Biological therapy: According to NCCN and ENETS guidelines, SSAs are the first choice for pNETs with a low proliferation index (Ki-67 < 10%) and positive expression of somatostatin receptor (SSRT)[40,93]. SSAs show an antiproliferative effect and mainly consist of two therapeutic agents: Lanreotide and octreotide long-acting release (LAR). CLARINET Research (a phase III trial) showed a significant association between the treatment of lanreotide and prolonged PFS among patients with advanced pNETs[94]. The use of octreotide LAR was also proven to be correlated to a high DCR and long time to tumor progression[95]. Although new generation SSAs (pasireotide LAR) can be combined with more SSRTs simultaneously and produce a more extensive antiproliferation effect, up to 79% of patients treated with pasireotide LAR had hyperglycemia, so currently, it is not recommended as the drug of choice[96]. SSAs also remain the treatment of choice for the hormone-excess state in pNETs prior to surgery or if resection cannot be performed.
Interferonα-2b can be used instead or in combination when the curative effect of SSAs is poor in patients with refractory carcinoid syndrome[97]. Other drugs can relieve symptoms related to specific functioning-pNETs: PPIs for oversecretion of gastric acid by gastrinoma and metyrapone for oversecretion of ACTH by ACTHoma.
Targeted therapy: Among the numerous targeted agents investigated in GEP-NETs, the mammalian target of rapamycin inhibitors, everolimus, and of tyrosine kinase inhibitors, sunitinib, are the only two agents approved presently by the Food and Drug Administration for the treatment of pNETs. These two targeted agents are generally recommended as second-line treatment after SSAS treatment in patients with tumor-positive expression of SSTR, and as the first-line treatment in patients with tumor-negative expression of SSTR[40]. Prospective randomized controlled studies have showed that everolimus and sunitinib could improve the PFS of advanced pNETs with a median PFS of 11.0 and 11.4 mo compared with placebo, respectively[98,99]. The objective response rate (ORR) of sunitinib in advanced panNET ranged from 9% to 33.3%, higher than that of everolimus (5%-9.5%)[100]. Apart from sunitinib, the clinical trials of other tyrosine kinase inhibitors as targeting agents for pNETs including cabozamtinib, sulfatinib, and lenvatinib are also being conducted continuously. Cabozamtinib inhibits the expression of c-MET and a phase II clinical trial has shown a high DCR (90%, 18/20) for pNETs[101]. Preclinical research showed that sulfatinib inhibits VEGFRs, fibroblast growth factor receptor, and colony stimulating factor 1 receptor simultaneously, and a phase II trial in pNETs showed a high DCR of 92.9%[102]. Lenvatinib was studied in a phase II clinical study and achieved a 40% ORR in patients with pNETs and a median PFS of 15.8 mo even after treatment with everolimus and sunitinib[103]. Currently, numerous drugs aiming at other new targets in pNETs have been researched and developed; for instance, palbociclib targets CDK4/6 and patients treated with palbociclib were observed to be evaluable for ORR with a median follow-up period of 10 mo[104,105].
Chemotherapy: Chemotherapy is beneficial in patients with advanced pNETs (i.e., progressive, with high tumor burden or high Ki67 index), or in a neoadjuvant setting to obtain tumor shrinkage for secondary tumor resection. At present, three kinds of chemotherapy schemes are recommended for pNETs: Temozolomide-based and streptozotocin-based chemotherapies (streptozocin mono- or plus 5-fluroracil) are mainly used for tumors with good differentiation and relatively fast growth, whereas the platinum-based scheme (cisplatin plus etoposide) is used for pNEC but not well differentiated NET[40]. A prospective randomized controlled study compared the curative effect of temozolomide monotherapy with temozolomide combined with capecitabine (CAPTEM Scheme) and found that the CAPTEM scheme prolonged PFS significantly to 22.7 mo with an ORR of 33.3%, which shows considerable promise for combined chemotherapy for pNETs. The ORR of streptozotocin-based chemotherapy ranges from 21.6% to 42.7%. The ORR among G3 NEC to platinum-based regimen was reasonably high, up to 61.3%[106]. Rb loss and KRAS mutation showed additional benefits than those without (Rb loss, 80% vs normal Rb, 24%; mutated KRAS, 77% vs wild type, 23%)[107].
Immunotherapy: Immunotherapy for NENs is still in the early stage of clinical trials and the efficacy of anti-programmed cell death protein 1 (PD-1) immunotherapy for GEP-NETs is lower, with an ORR < 10%[108]. The expression of some potential immune-related biomarkers in pNETs has been preliminarily investigated. Expression of PD-L1 in pNET is rare, at 7.4%. Microsatellite instability was observed in 12.5% of patients with pNET[109,110]. Stable microsatellite, low PD-L1 expression, and tumor mutation burden are associated with a poor response to immunotherapy in NENs[111]. PD-L1 expression, high tumor mutation burden, and microsatellite instability are more pronounced in poorly differentiated NENs. Thus, avelumab, an anti-PD-L1 antibody, was approved for the treatment of Merkel cell carcinoma (MCC), a high-grade cutaneous NEC[112].
Peptide receptor radionuclide therapy (PRRT) is applied in patients with advanced NETs through injecting radiolabeled SSAs. A radioisotope, such as 90Yttrium or 177Lutetium (177Lu), binds to an SSA via a chelator and delivers targeted radiation precisely to tumors. The b-emission can effectively produce toxic effects when the radiolabeled SSA binds to the surface of the tumor cells with high expression of SSTR[113]. Several retrospective studies have demonstrated the role of PRRT in the treatment of advanced pNETs. The ORRs ranged from 16.5% to 61.3%. The pivotal phase 3 Lu-DOTATATE PRRT trial in NETs was restricted to patients with midgut NETs with an ORR of 18%[114]. No phase 3 trial data are currently available for pNET patients. The largest study, which included 610 patients with bronchial and GEP-NETs treated with 177Lu infusion, achieved an ORR of 39% in all the sites. pNETs showed the best response compared with NETs from other sites. The ORR was 58%, among which F-pNET had additional benefits with an ORR of 62%[115]. Therefore, the current guidelines suggest that PRRT can be attempted in patients with high expression of SSTR.
Although surgical resection is the primary treatment for liver metastases, postoperative complications due to remnant liver volume being insufficient, ischemia-reperfusion injury, postoperative hemorrhage, and infection delay the systematic treatment schedule. This technique includes radiofrequency ablation (RFA), microwave ablation, and cryotherapy, which can be completed via ultrasound/CT guided, or laparoscopic approaches. For lesions smaller than 3 cm, local ablation has the same safety and effectiveness as surgical resection[116,117], whereas for lesions with a diameter of 3-5 cm, the curative effect remains controversial[118]. An improved technique, real-time ultrasonography/CT-MRI image fusion-guided RFA, has been increasingly widely used. A pilot study showed that ultrasonography/CT-MRI image fusion improved tumor visibility and the technical feasibility of RFA. Fusion imaging guided RFA using multiple electrodes demonstrated a highly effective ablation rate for lesions up to 5 cm, and a low local tumor progression rate during a two-year follow-up period. The debulking (≥ 80%) operation has been proven to be effective in selected patients with liver metastases[93]. Local ablation, expecting an R0/R1 or debulking ablation, would be an acceptable option combined with systematic therapy for liver metastases.
Liver-directed transarterial embolization (TAE), transarterial chemoembolization (TACE), and selective internal radiation therapy are widely used effective treatment modalities for liver metastases. TACE combines intra-arterial injection of cytotoxic agents with particulate embolization, achieving a relatively higher intra-tumor chemotherapy concentration and prolonged dwell time of the agent within the tumor compared with systemic administration[119]. TAE and TACE are both effective in NET patients with liver metastases[120]. TAE would be preferred due to slightly better toxicity profile. Aiming at NELM, partial or complete symptom relief has been reported in 60%-85% of patients treated with TACE. A significant biological response is achieved in 45%-75% of cases. A better tumor response and prolonged OS were observed in pNETs in comparison with small-intestine NET[121,122]. Several modalities were discovered to predict the OS in NELMs treated with TACE. Extremely high levels of pancreastatin before TACE could predict a poor prognosis, whereas significant drops in pancreastatin after TACE are correlated with an improved survival. A rebound in the level after the initial decrease might predict progressive liver disease, requiring repeated TACE[123]. A semi-quantitative visual assessment of hepatic tumor burden on multiparametric MRI could accurately, reproducibly, and efficiently predict the OS[124].
Disappointingly, in recent decades, patients with NET experienced long delays (5-7 years) before diagnosis, and most lacked access to the multidisciplinary care necessary for management of these complex tumors[125]. Differently from the traditional referral model, multidisciplinary care is ideal for the management of patients with complex conditions. A multidisciplinary team of NET usually consists of physicians from both the medical and surgical oncology departments, pathology, endocrinology, diagnostic, and interventional radiology teams, as well as a professional nursing team, to integrate the opinions of various aspects of diagnosis and treatment quickly[126]. Tamagno et al[127] compared the changes in diagnosis and treatment of GEP-NET patients before and after the establishment of the multidisciplinary team and identified a lack of consistency in the biochemical, imaging, and pathological findings. These inconsistencies have been reduced by the systematic multidisciplinary approach and the therapeutic management of GEP-NET patients has been altered and became more consistent with recommended guidelines. A more striking finding is that disease imaging staging and grading were modified in 30.7% and 17.9% of patients after a multidisciplinary approach. A change in therapeutic management was proposed in 50.3% of patients[128].
PNETs are a heterogeneous group of tumors with complicated treatment options that depend on pathological grading, clinical staging, and the presence of symptoms related to hormonal secretion. With regard to diagnosis, remarkable advances have been made: CgA is recommended as a general marker for pNETs, whereas specific hormones have been suggested to be analyzed in relation to clinical symptoms. However, other new biomarker modalities, like CTCs, NETest, miRNA profile, and cytokines, should be clarified in future investigations before clinical application. Therefore, the currently available serum biomarkers are insufficient for diagnosis, but reasonably acceptable in evaluating the prognosis of and response to treatments during follow-up of pNETs. Morphology, immunohistochemical staining, pathological grading, and clinical staging remain the gold standards for diagnosis. Surgical resection is still the only curative therapeutic option for localized pNETs. However, a debulking operation has also been proven to be effective for the control of the disease. As for drug therapy, SSAs are the first-line therapy for those with positive expression of SSRT, whereas everolimus and sunitinib represent important progress in the target therapy of patients with advanced pNETs. The best strategy for adjuvant or neoadjuvant chemotherapy is controversial. However, progress has been achieved in the combination of systematic therapy with local control treatments. The optimal timing of local control intervention, planning of sequential therapies, and implementation of multidisciplinary care remain pending. With a clearer understanding of the genetic and molecular pathogenesis of pNETs, the next decade of studies will provide new insight into early diagnosis, precise grading and staging systems, novel drug therapy, and optimal combination with local control therapies.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Perrotti S, Qayed E, Ramsay MA S-Editor: Dou Y L-Editor: Wang TQ E-Editor: Zhang YL
| 1. | Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, Shih T, Yao JC. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017;3:1335-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1510] [Cited by in RCA: 2464] [Article Influence: 308.0] [Reference Citation Analysis (4)] |
| 2. | Hallet J, Law CH, Cukier M, Saskin R, Liu N, Singh S. Exploring the rising incidence of neuroendocrine tumors: a population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer. 2015;121:589-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 610] [Article Influence: 55.5] [Reference Citation Analysis (1)] |
| 3. | Metz DC, Jensen RT. Gastrointestinal neuroendocrine tumors: pancreatic endocrine tumors. Gastroenterology. 2008;135:1469-1492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 637] [Cited by in RCA: 542] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 4. | Chabot J. Editorial: Pancreatic neuroendocrine tumors: Primum non nocere. Surgery. 2016;159:348-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Pulvirenti A, Rao D, Mcintyre CA, Gonen M, Tang LH, Klimstra DS, Fleisher M, Ramanathan LV, Reidy-Lagunes D, Allen PJ. Limited role of Chromogranin A as clinical biomarker for pancreatic neuroendocrine tumors. HPB (Oxford). 2019;21:612-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 6. | Kulke MH, Anthony LB, Bushnell DL, de Herder WW, Goldsmith SJ, Klimstra DS, Marx SJ, Pasieka JL, Pommier RF, Yao JC, Jensen RT; North American Neuroendocrine Tumor Society (NANETS). NANETS treatment guidelines: well-differentiated neuroendocrine tumors of the stomach and pancreas. Pancreas. 2010;39:735-752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 467] [Cited by in RCA: 397] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 7. | Salazar R, Wiedenmann B, Rindi G, Ruszniewski P. ENETS 2011 Consensus Guidelines for the Management of Patients with Digestive Neuroendocrine Tumors: an update. Neuroendocrinology. 2012;95:71-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | Massironi S, Conte D, Sciola V, Spampatti MP, Ciafardini C, Valenti L, Rossi RE, Peracchi M. Plasma chromogranin A response to octreotide test: prognostic value for clinical outcome in endocrine digestive tumors. Am J Gastroenterol. 2010;105:2072-2078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Giusti M, Sidoti M, Augeri C, Rabitti C, Minuto F. Effect of short-term treatment with low dosages of the proton-pump inhibitor omeprazole on serum chromogranin A levels in man. Eur J Endocrinol. 2004;150:299-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 86] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 10. | Modlin IM, Bodei L, Kidd M. Neuroendocrine tumor biomarkers: From monoanalytes to transcripts and algorithms. Best Pract Res Clin Endocrinol Metab. 2016;30:59-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 11. | Nobels FR, Kwekkeboom DJ, Coopmans W, Schoenmakers CH, Lindemans J, De Herder WW, Krenning EP, Bouillon R, Lamberts SW. Chromogranin A as serum marker for neuroendocrine neoplasia: comparison with neuron-specific enolase and the alpha-subunit of glycoprotein hormones. J Clin Endocrinol Metab. 1997;82:2622-2628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 82] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Appetecchia M, Lauretta R, Rota F, Carlini M, Massimo C. Massimo C. Neuroendocrine Tumors Biomarkers. In: Massimo C. Abdominal Neuroendocrine Tumors. Springer: Milano, 2018: 65-78. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Yao JC, Pavel M, Phan AT, Kulke MH, Hoosen S, St Peter J, Cherfi A, Öberg KE. Chromogranin A and neuron-specific enolase as prognostic markers in patients with advanced pNET treated with everolimus. J Clin Endocrinol Metab. 2011;96:3741-3749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 160] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 14. | Wu XY, Hu YB, Li HJ, Wan B, Zhang CX, Zhang B, Hu H, Zhang Q, Lv TF, Zhan P, Song Y. Diagnostic and therapeutic value of progastrin-releasing peptide on small-cell lung cancer: A Single-Center Experience in China. J Cell Mol Med. 2018;22:4328-4334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Oh HJ, Park HY, Kim KH, Park CK, Shin HJ, Lim JH, Kwon YS, Oh IJ, Kim YI, Lim SC, Kim YC, Kim SH, Shin MG. Progastrin-releasing peptide as a diagnostic and therapeutic biomarker of small cell lung cancer. J Thorac Dis. 2016;8:2530-2537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Korse CM, Taal BG, Vincent A, van Velthuysen ML, Baas P, Buning-Kager JC, Linders TC, Bonfrer JM. Choice of tumour markers in patients with neuroendocrine tumours is dependent on the histological grade. A marker study of Chromogranin A, Neuron specific enolase, Progastrin-releasing peptide and cytokeratin fragments. Eur J Cancer. 2012;48:662-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 17. | Adrian TE, Uttenthal LO, Williams SJ, Bloom SR. Secretion of pancreatic polypeptide in patients with pancreatic endocrine tumors. N Engl J Med. 1986;315:287-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Hofland J, Zandee WT, de Herder WW. Role of biomarker tests for diagnosis of neuroendocrine tumours. Nat Rev Endocrinol. 2018;14:656-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 82] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 19. | Panzuto F, Severi C, Cannizzaro R, Falconi M, Angeletti S, Pasquali A, Corleto VD, Annibale B, Buonadonna A, Pederzoli P, Delle Fave G. Utility of combined use of plasma levels of chromogranin A and pancreatic polypeptide in the diagnosis of gastrointestinal and pancreatic endocrine tumors. J Endocrinol Invest. 2004;27:6-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Walter T, Chardon L, Chopin-laly X, Raverot V, Caffin AG, Chayvialle JA, Scoazec JY, Lombard-Bohas C. Is the combination of chromogranin A and pancreatic polypeptide serum determinations of interest in the diagnosis and follow-up of gastro-entero-pancreatic neuroendocrine tumours? Eur J Cancer. 2012;48:1766-1773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 21. | Dizon AM, Kowalyk S, Hoogwerf BJ. Neuroglycopenic and other symptoms in patients with insulinomas. Am J Med. 1999;106:307-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 114] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 22. | Batcher E, Madaj P, Gianoukakis AG. Pancreatic neuroendocrine tumors. Endocr Res. 2011;36:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Soga J, Yakuwa Y. Glucagonomas/diabetico-dermatogenic syndrome (DDS): a statistical evaluation of 407 reported cases. J Hepatobiliary Pancreat Surg. 1998;5:312-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 68] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Solcia E, Capella C, Riva C, Rindi G, Polak JM. The morphology and neuroendocrine profile of pancreatic epithelial VIPomas and extrapancreatic, VIP-producing, neurogenic tumors. Ann N Y Acad Sci. 1988;527:508-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Bloom SR. Vasoactive intestinal peptide, the major mediator of the WDHA (pancreatic cholera) syndrome: value of measurement in diagnosis and treatment. Am J Dig Dis. 1978;23:373-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 49] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Ellison EC, Johnson JA. The Zollinger-Ellison syndrome: a comprehensive review of historical, scientific, and clinical considerations. Curr Probl Surg. 2009;46:13-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 27. | Ito T, Cadiot G, Jensen RT. Diagnosis of Zollinger-Ellison syndrome: increasingly difficult. World J Gastroenterol. 2012;18:5495-5503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 79] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 28. | Berna MJ, Hoffmann KM, Serrano J, Gibril F, Jensen RT. Serum gastrin in Zollinger-Ellison syndrome: I. Prospective study of fasting serum gastrin in 309 patients from the National Institutes of Health and comparison with 2229 cases from the literature. Medicine (Baltimore). 2006;85:295-330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 146] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 29. | Berna MJ, Hoffmann KM, Long SH, Serrano J, Gibril F, Jensen RT. Serum gastrin in Zollinger-Ellison syndrome: II. Prospective study of gastrin provocative testing in 293 patients from the National Institutes of Health and comparison with 537 cases from the literature. evaluation of diagnostic criteria, proposal of new criteria, and correlations with clinical and tumoral features. Medicine (Baltimore). 2006;85:331-364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 129] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 30. | Tanaka S, Yamasaki S, Matsushita H, Ozawa Y, Kurosaki A, Takeuchi K, Hoshihara Y, Doi T, Watanabe G, Kawaminami K. Duodenal somatostatinoma: a case report and review of 31 cases with special reference to the relationship between tumor size and metastasis. Pathol Int. 2000;50:146-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 54] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Lacroix A, Feelders RA, Stratakis CA, Nieman LK. Cushing's syndrome. Lancet. 2015;386:913-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 602] [Article Influence: 60.2] [Reference Citation Analysis (0)] |
| 32. | Stelmachowska-Banaś M, Głogowski M, Vasiljevic A, Raverot V, Raverot G, Zgliczyński W. Ectopic acromegaly due to growth hormone-releasing hormone secretion from bronchial carcinoid causing somatotroph hyperplasia and partial pituitary insufficiency. Pol Arch Intern Med. 2019;129:208-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Melmed S, Ezrin C, Kovacs K, Goodman RS, Frohman LA. Acromegaly due to secretion of growth hormone by an ectopic pancreatic islet-cell tumor. N Engl J Med. 1985;312:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 73] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | Ehlers M, Allelein S, Haase M, Willenberg HS, Knoefel WT, Schott M. Circulating tumor cells in patients with neuroendocrine neoplasms. Horm Metab Res. 2014;46:744-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 35. | Khan MS, Kirkwood A, Tsigani T, Garcia-Hernandez J, Hartley JA, Caplin ME, Meyer T. Circulating tumor cells as prognostic markers in neuroendocrine tumors. J Clin Oncol. 2013;31:365-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 134] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 36. | Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, Picus J, Morse M, Mitchell E, Miller MC, Doyle GV, Tissing H, Terstappen LW, Meropol NJ. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:3213-3221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1354] [Cited by in RCA: 1409] [Article Influence: 82.9] [Reference Citation Analysis (0)] |
| 37. | Resel Folkersma L, San José Manso L, Galante Romo I, Moreno Sierra J, Olivier Gómez C. Prognostic significance of circulating tumor cell count in patients with metastatic hormone-sensitive prostate cancer. Urology. 2012;80:1328-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 38. | Modlin IM, Drozdov I, Kidd M. The identification of gut neuroendocrine tumor disease by multiple synchronous transcript analysis in blood. PLoS One. 2013;8:e63364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 136] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 39. | Kidd M, Drozdov I, Modlin I. Blood and tissue neuroendocrine tumor gene cluster analysis correlate, define hallmarks and predict disease status. Endocr Relat Cancer. 2015;22:561-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 40. | Pavel M, Jann H, Prasad V, Drozdov I, Modlin IM, Kidd M. NET Blood Transcript Analysis Defines the Crossing of the Clinical Rubicon: When Stable Disease Becomes Progressive. Neuroendocrinology. 2017;104:170-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 41. | Ćwikła JB, Bodei L, Kolasinska-Ćwikła A, Sankowski A, Modlin IM, Kidd M. Circulating Transcript Analysis (NETest) in GEP-NETs Treated With Somatostatin Analogs Defines Therapy. J Clin Endocrinol Metab. 2015;100:E1437-E1445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 42. | Garzon R, Fabbri M, Cimmino A, Calin GA, Croce CM. MicroRNA expression and function in cancer. Trends Mol Med. 2006;12:580-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 550] [Cited by in RCA: 582] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 43. | Thorns C, Schurmann C, Gebauer N, Wallaschofski H, Kümpers C, Bernard V, Feller AC, Keck T, Habermann JK, Begum N, Lehnert H, Brabant G. Global microRNA profiling of pancreatic neuroendocrine neoplasias. Anticancer Res. 2014;34:2249-2254. [PubMed] |
| 44. | Vicentini C, Fassan M, D'Angelo E, Corbo V, Silvestris N, Nuovo GJ, Scarpa A. Clinical application of microRNA testing in neuroendocrine tumors of the gastrointestinal tract. Molecules. 2014;19:2458-2468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 45. | Li A, Yu J, Kim H, Wolfgang CL, Canto MI, Hruban RH, Goggins M. MicroRNA array analysis finds elevated serum miR-1290 accurately distinguishes patients with low-stage pancreatic cancer from healthy and disease controls. Clin Cancer Res. 2013;19:3600-3610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 241] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 46. | Malczewska A, Kidd M, Matar S, Kos-Kudla B, Modlin IM. A Comprehensive Assessment of the Role of miRNAs as Biomarkers in Gastroenteropancreatic Neuroendocrine Tumors. Neuroendocrinology. 2018;107:73-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 47. | Pavel ME, Hassler G, Baum U, Hahn EG, Lohmann T, Schuppan D. Circulating levels of angiogenic cytokines can predict tumour progression and prognosis in neuroendocrine carcinomas. Clin Endocrinol (Oxf). 2005;62:434-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 48. | Zurita AJ, Khajavi M, Wu HK, Tye L, Huang X, Kulke MH, Lenz HJ, Meropol NJ, Carley W, DePrimo SE, Lin E, Wang X, Harmon CS, Heymach JV. Circulating cytokines and monocyte subpopulations as biomarkers of outcome and biological activity in sunitinib-treated patients with advanced neuroendocrine tumours. Br J Cancer. 2015;112:1199-1205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 49. | Mateo J, Heymach JV, Zurita AJ. Circulating biomarkers of response to sunitinib in gastroenteropancreatic neuroendocrine tumors: current data and clinical outlook. Mol Diagn Ther. 2012;16:151-161. [PubMed] |
| 50. | Hussain F, Wang J, Ahmed R, Guest SK, Lam EW, Stamp G, El-Bahrawy M. The expression of IL-8 and IL-8 receptors in pancreatic adenocarcinomas and pancreatic neuroendocrine tumours. Cytokine. 2010;49:134-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 51. | Tecimer T, Dlott J, Chuntharapai A, Martin AW, Peiper SC. Expression of the chemokine receptor CXCR2 in normal and neoplastic neuroendocrine cells. Arch Pathol Lab Med. 2000;124:520-525. [PubMed] |
| 52. | Frederick LG, David LP, Irvin DF, April GF, Charles MB, Daniel GH, Monica M: American Joint Committee on Cancer. AJCC cancer staging manual. New York: Springer, 2002. |
| 53. | Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5537] [Cited by in RCA: 6449] [Article Influence: 429.9] [Reference Citation Analysis (0)] |
| 54. | Rindi G, Falconi M, Klersy C, Albarello L, Boninsegna L, Buchler MW, Capella C, Caplin M, Couvelard A, Doglioni C, Delle Fave G, Fischer L, Fusai G, de Herder WW, Jann H, Komminoth P, de Krijger RR, La Rosa S, Luong TV, Pape U, Perren A, Ruszniewski P, Scarpa A, Schmitt A, Solcia E, Wiedenmann B. TNM staging of neoplasms of the endocrine pancreas: results from a large international cohort study. J Natl Cancer Inst. 2012;104:764-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 335] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 55. | Strosberg JR, Cheema A, Weber J, Han G, Coppola D, Kvols LK. Prognostic validity of a novel American Joint Committee on Cancer Staging Classification for pancreatic neuroendocrine tumors. J Clin Oncol. 2011;29:3044-3049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 171] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 56. | You Y, Jang JY, Kim SC, Yoon YS, Park JS, Cho CK, Park SJ, Yang JD, Lee WJ, Hong TH, Ahn KS, Jeong CY, Lee HK, Lee SE, Roh YH, Kim HJ, Kim H, Han IW. Validation of the 8th AJCC Cancer Staging System for Pancreas Neuroendocrine Tumors Using Korean Nationwide Surgery Database. Cancer Res Treat. 2019;51:1639-1652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 57. | Yang M, Zhang Y, Zeng L, Ke NW, Tan CL, Tian BL, Xiang B, Liu XB. Prognostic Validity of the American Joint Committee on Cancer Eighth Edition TNM Staging System for Surgically Treated and Well-Differentiated Pancreatic Neuroendocrine Tumors: A Comprehensive Analysis of 254 Consecutive Patients From a Large Chinese Institution. Pancreas. 2019;48:613-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 58. | Solcia E, Klöppel G, Sobin LH. Histological typing of endocrine tumours. 2nd ed. New York: Springer-Verlag, 2000. [DOI] [Full Text] |
| 59. | Luo G, Javed A, Strosberg JR, Jin K, Zhang Y, Liu C, Xu J, Soares K, Weiss MJ, Zheng L, Wolfgang CL, Cives M, Wong J, Wang W, Sun J, Shao C, Wang W, Tan H, Li J, Ni Q, Shen L, Chen M, He J, Chen J, Yu X. Modified Staging Classification for Pancreatic Neuroendocrine Tumors on the Basis of the American Joint Committee on Cancer and European Neuroendocrine Tumor Society Systems. J Clin Oncol. 2017;35:274-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 101] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 60. | Klöppel G, Perren A, Heitz PU. The gastroenteropancreatic neuroendocrine cell system and its tumors: the WHO classification. Ann NY Acad Sci. 2004;1014:13-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 538] [Cited by in RCA: 519] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 61. | DeLellis RA, Lloyd RV, Heitz PU, Eng C. Pathology and genetics of tumours of endocrine organs, WHO classification of tumours, vol. 8. 3rd ed. Lyon: IARC Press, 2004. |
| 62. | Bosman F, Carneiro F, Hruban RH, Theise ND. WHO Classification of Tumours of the Digestive System, WHO Classification of Tumours, vol. 3. 4th ed. Lyon: IARC Press, 2010. |
| 63. | Inzani F, Petrone G, Rindi G. The New World Health Organization Classification for Pancreatic Neuroendocrine Neoplasia. Endocrinol Metab Clin North Am. 2018;47:463-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 150] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 64. | Scoazec J, Couvelard A, Monges G, Leteurtre E, Belleannee G, Guyetant S, Duvillard P, Danjoux M, Parot X, Lepage C. Well-differentiated grade 3 digestive neuroendocrine tumors: Myth or reality? The PRONET study group. J Clin Oncol. 2012;30:15. [RCA] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 65. | Tang LH, Untch BR, Reidy DL, O'Reilly E, Dhall D, Jih L, Basturk O, Allen PJ, Klimstra DS. Well-Differentiated Neuroendocrine Tumors with a Morphologically Apparent High-Grade Component: A Pathway Distinct from Poorly Differentiated Neuroendocrine Carcinomas. Clin Cancer Res. 2016;22:1011-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 248] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 66. | La Rosa S, Sessa F. High-grade poorly differentiated neuroendocrine carcinomas of the gastroenteropancreatic system: from morphology to proliferation and back. Endocr Pathol. 2014;25:193-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 67. | Milione M, Maisonneuve P, Spada F, Pellegrinelli A, Spaggiari P, Albarello L, Pisa E, Barberis M, Vanoli A, Buzzoni R, Pusceddu S, Concas L, Sessa F, Solcia E, Capella C, Fazio N, La Rosa S. The Clinicopathologic Heterogeneity of Grade 3 Gastroenteropancreatic Neuroendocrine Neoplasms: Morphological Differentiation and Proliferation Identify Different Prognostic Categories. Neuroendocrinology. 2017;104:85-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 175] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 68. | Lloyd RV, Osamura R, Kloppel G, Rosai J. WHO classification of tumours of endocrine organs, WHO Classification of Tumours, vol. 10. 4th ed. Lyon: IARC Press, 2017. |
| 69. | Bar-Moshe Y, Mazeh H, Grozinsky-Glasberg S. Non-functioning pancreatic neuroendocrine tumors: Surgery or observation? World J Gastrointest Endosc. 2017;9:153-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 70. | Falconi M, Bartsch DK, Eriksson B, Klöppel G, Lopes JM, O'Connor JM, Salazar R, Taal BG, Vullierme MP, O'Toole D; Barcelona Consensus Conference participants. ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms of the digestive system: well-differentiated pancreatic non-functioning tumors. Neuroendocrinology. 2012;95:120-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 358] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 71. | Massironi S, Rossi RE, Zilli A, Casazza G, Ciafardini C, Conte D. A wait-and-watch approach to small pancreatic neuroendocrine tumors: prognosis and survival. Oncotarget. 2016;7:18978-18983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 72. | Sallinen V, Le Large TY, Galeev S, Kovalenko Z, Tieftrunk E, Araujo R, Ceyhan GO, Gaujoux S. Surveillance strategy for small asymptomatic non-functional pancreatic neuroendocrine tumors - a systematic review and meta-analysis. HPB (Oxford). 2017;19:310-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 73. | Sharpe SM, In H, Winchester DJ, Talamonti MS, Baker MS. Surgical resection provides an overall survival benefit for patients with small pancreatic neuroendocrine tumors. J Gastrointest Surg. 2015;19:117-123; discussion 123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 74. | Finkelstein P, Sharma R, Picado O, Gadde R, Stuart H, Ripat C, Livingstone AS, Sleeman D, Merchant N, Yakoub D. Pancreatic Neuroendocrine Tumors (panNETs): Analysis of Overall Survival of Nonsurgical Management Versus Surgical Resection. J Gastrointest Surg. 2017;21:855-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 75. | Rustagi T, Farrell JJ. Endoscopic diagnosis and treatment of pancreatic neuroendocrine tumors. J Clin Gastroenterol. 2014;48:837-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 76. | Weynand B, Borbath I, Bernard V, Sempoux C, Gigot JF, Hubert C, Lannoy V, Deprez PH, Jouret-Mourin A. Pancreatic neuroendocrine tumour grading on endoscopic ultrasound-guided fine needle aspiration: high reproducibility and inter-observer agreement of the Ki-67 labelling index. Cytopathology. 2014;25:389-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 77. | Liu JB, Baker MS. Surgical Management of Pancreatic Neuroendocrine Tumors. Surg Clin North Am. 2016;96:1447-1468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 78. | Haugvik SP, Labori KJ, Waage A, Line PD, Mathisen Ø, Gladhaug IP. Pancreatic surgery with vascular reconstruction in patients with locally advanced pancreatic neuroendocrine tumors. J Gastrointest Surg. 2013;17:1224-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 79. | Thiels CA, Bergquist JR, Laan DV, Croome KP, Smoot RL, Nagorney DM, Thompson GB, Kendrick ML, Farnell MB, Truty MJ. Outcomes of Pancreaticoduodenectomy for Pancreatic Neuroendocrine Tumors: Are Combined Procedures Justified? J Gastrointest Surg. 2016;20:891-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 80. | Tsilimigras DI, Ntanasis-Stathopoulos I, Kostakis ID, Moris D, Schizas D, Cloyd JM, Pawlik TM. Is Resection of Primary Midgut Neuroendocrine Tumors in Patients with Unresectable Metastatic Liver Disease Justified? A Systematic Review and Meta-Analysis. J Gastrointest Surg. 2019;23:1044-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 81. | Curran T, Pockaj BA, Gray RJ, Halfdanarson TR, Wasif N. Importance of lymph node involvement in pancreatic neuroendocrine tumors: impact on survival and implications for surgical resection. J Gastrointest Surg. 2015;19:152-160; discussion 160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 82. | Toste PA, Kadera BE, Tatishchev SF, Dawson DW, Clerkin BM, Muthusamy R, Watson R, Tomlinson JS, Hines OJ, Reber HA, Donahue TR. Nonfunctional pancreatic neuroendocrine tumors <2 cm on preoperative imaging are associated with a low incidence of nodal metastasis and an excellent overall survival. J Gastrointest Surg. 2013;17:2105-2113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 83. | Lopez-Aguiar AG, Zaidi MY, Beal EW, Dillhoff M, Cannon JGD, Poultsides GA, Kanji ZS, Rocha FG, Marincola Smith P, Idrees K, Beems M, Cho CS, Fisher AV, Weber SM, Krasnick BA, Fields RC, Cardona K, Maithel SK. Defining the Role of Lymphadenectomy for Pancreatic Neuroendocrine Tumors: An Eight-Institution Study of 695 Patients from the US Neuroendocrine Tumor Study Group. Ann Surg Oncol. 2019;26:2517-2524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 84. | Jilesen AP, van Eijck CH, Busch OR, van Gulik TM, Gouma DJ, van Dijkum EJ. Postoperative Outcomes of Enucleation and Standard Resections in Patients with a Pancreatic Neuroendocrine Tumor. World J Surg. 2016;40:715-728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 85. | Jutric Z, Grendar J, Hoen HM, Cho SW, Cassera MA, Newell PH, Hammill CW, Hansen PD, Wolf RF. Regional Metastatic Behavior of Nonfunctional Pancreatic Neuroendocrine Tumors: Impact of Lymph Node Positivity on Survival. Pancreas. 2017;46:898-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 86. | Frilling A, Modlin IM, Kidd M, Russell C, Breitenstein S, Salem R, Kwekkeboom D, Lau WY, Klersy C, Vilgrain V, Davidson B, Siegler M, Caplin M, Solcia E, Schilsky R; Working Group on Neuroendocrine Liver Metastases. Recommendations for management of patients with neuroendocrine liver metastases. Lancet Oncol. 2014;15:e8-e21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 367] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 87. | Fairweather M, Swanson R, Wang J, Brais LK, Dutton T, Kulke MH, Clancy TE. Management of Neuroendocrine Tumor Liver Metastases: Long-Term Outcomes and Prognostic Factors from a Large Prospective Database. Ann Surg Oncol. 2017;24:2319-2325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 88. | Yu X, Gu J, Wu H, Fu D, Li J, Jin C. Resection of Liver Metastases: A Treatment Provides a Long-Term Survival Benefit for Patients with Advanced Pancreatic Neuroendocrine Tumors: A Systematic Review and Meta-Analysis. J Oncol. 2018;2018:6273947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 89. | Partelli S, Bartsch DK, Capdevila J, Chen J, Knigge U, Niederle B, Nieveen van Dijkum EJM, Pape UF, Pascher A, Ramage J, Reed N, Ruszniewski P, Scoazec JY, Toumpanakis C, Kianmanesh R, Falconi M; Antibes Consensus Conference participants. ENETS Consensus Guidelines for Standard of Care in Neuroendocrine Tumours: Surgery for Small Intestinal and Pancreatic Neuroendocrine Tumours. Neuroendocrinology. 2017;105:255-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 218] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 90. | Kianmanesh R, Sauvanet A, Hentic O, Couvelard A, Lévy P, Vilgrain V, Ruszniewski P, Belghiti J. Two-step surgery for synchronous bilobar liver metastases from digestive endocrine tumors: a safe approach for radical resection. Ann Surg. 2008;247:659-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 91. | Ejaz A, Reames BN, Maithel S, Poultsides GA, Bauer TW, Fields RC, Weiss MJ, Marques HP, Aldrighetti L, Pawlik TM. Cytoreductive debulking surgery among patients with neuroendocrine liver metastasis: a multi-institutional analysis. HPB (Oxford). 2018;20:277-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 92. | Singh S, Moody L, Chan DL, Metz DC, Strosberg J, Asmis T, Bailey DL, Bergsland E, Brendtro K, Carroll R, Cleary S, Kim M, Kong G, Law C, Lawrence B, McEwan A, McGregor C, Michael M, Pasieka J, Pavlakis N, Pommier R, Soulen M, Wyld D, Segelov E; Commonwealth Neuroendocrine Tumour Collaboration (CommNETS) Follow-up Working Group. Follow-up Recommendations for Completely Resected Gastroenteropancreatic Neuroendocrine Tumors. JAMA Oncol. 2018;4:1597-1604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 93. | Clark OH, Benson AB, Berlin JD, Choti MA, Doherty GM, Engstrom PF, Gibbs JF, Heslin MJ, Kessinger A, Kulke MH, Kvols L, Salem R, Saltz L, Shah MH, Shibata S, Strosberg JR, Yao JC; NCCN Neuroendocrine Tumors Panel Members. NCCN Clinical Practice Guidelines in Oncology: neuroendocrine tumors. J Natl Compr Canc Netw. 2009;7:712-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 124] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 94. | Caplin ME, Pavel M, Ruszniewski P. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371:1556-1557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 95. | Jann H, Denecke T, Koch M, Pape UF, Wiedenmann B, Pavel M. Impact of octreotide long-acting release on tumour growth control as a first-line treatment in neuroendocrine tumours of pancreatic origin. Neuroendocrinology. 2013;98:137-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 96. | Wolin EM, Jarzab B, Eriksson B, Walter T, Toumpanakis C, Morse MA, Tomassetti P, Weber MM, Fogelman DR, Ramage J, Poon D, Gadbaw B, Li J, Pasieka JL, Mahamat A, Swahn F, Newell-Price J, Mansoor W, Öberg K. Phase III study of pasireotide long-acting release in patients with metastatic neuroendocrine tumors and carcinoid symptoms refractory to available somatostatin analogues. Drug Des Devel Ther. 2015;9:5075-5086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 147] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 97. | Falconi M, Eriksson B, Kaltsas G, Bartsch DK, Capdevila J, Caplin M, Kos-Kudla B, Kwekkeboom D, Rindi G, Klöppel G, Reed N, Kianmanesh R, Jensen RT; Vienna Consensus Conference participants. ENETS Consensus Guidelines Update for the Management of Patients with Functional Pancreatic Neuroendocrine Tumors and Non-Functional Pancreatic Neuroendocrine Tumors. Neuroendocrinology. 2016;103:153-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1097] [Cited by in RCA: 985] [Article Influence: 109.4] [Reference Citation Analysis (1)] |
| 98. | Yao JC, Pavel M, Lombard-Bohas C, Van Cutsem E, Voi M, Brandt U, He W, Chen D, Capdevila J, de Vries EGE, Tomassetti P, Hobday T, Pommier R, Öberg K. Everolimus for the Treatment of Advanced Pancreatic Neuroendocrine Tumors: Overall Survival and Circulating Biomarkers From the Randomized, Phase III RADIANT-3 Study. J Clin Oncol. 2016;34:3906-3913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 213] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 99. | Faivre S, Niccoli P, Castellano D, Valle JW, Hammel P, Raoul JL, Vinik A, Van Cutsem E, Bang YJ, Lee SH, Borbath I, Lombard-Bohas C, Metrakos P, Smith D, Chen JS, Ruszniewski P, Seitz JF, Patyna S, Lu DR, Ishak KJ, Raymond E. Sunitinib in pancreatic neuroendocrine tumors: updated progression-free survival and final overall survival from a phase III randomized study. Ann Oncol. 2017;28:339-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 139] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 100. | Hauser H, Gerson DS, Reidy-Lagunes D, Raj N. Systemic Therapies for Metastatic Pancreatic Neuroendocrine Tumors. Curr Treat Options Oncol. 2019;20:87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 101. | Chan JA, Faris JE, Murphy JE, Blaszkowsky LS, Kwak EL, McCleary NJ, Fuchs CS, Meyerhardt JA, Ng K, Zhu AX, Abrams TA, Wolpin BM, Zhang S, Reardon A, Fitzpatrick B, Kulke MH, Ryan DP. Phase II trial of cabozantinib in patients with carcinoid and pancreatic neuroendocrine tumors (pNET). J Clin Oncol. 2017;35 Suppl_4:228-228. [RCA] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 102. | Xu J, Li J, Bai C, Xu N, Zhou Z, Li Z, Zhou C, Jia R, Lu M, Cheng Y, Mao C, Wang W, Cheng K, Su C, Hua Y, Qi C, Li J, Wang W, Li K, Sun Q, Ren Y, Su W. Surufatinib in Advanced Well-Differentiated Neuroendocrine Tumors: A Multicenter, Single-Arm, Open-Label, Phase Ib/II Trial. Clin Cancer Res. 2019;25:3486-3494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 103. | Capdevila J, Fazio N, Lopez C, Teule A, Valle JW, Tafuto S, Custodio A, Reed N, Raderer M, Grande E, Garcia-Carbonero R, Jimenez Fonseca P, Alonso V, Antonuzzo L, Spallanzani A, Berruti A, Sevilla Garcia I, La Casta A, Hernando J, Ibrahim T. 1307OEfficacy of lenvatinib in patients with advanced pancreatic (panNETs) and gastrointestinal (giNETs) grade 1/2 (G1/G2) neuroendocrine tumors: Results of the international phase II TALENT trial (GETNE 1509). Ann Oncol. 2018;29 Suppl_8:467-478. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 104. | Tang LH, Contractor T, Clausen R, Klimstra DS, Du YC, Allen PJ, Brennan MF, Levine AJ, Harris CR. Attenuation of the retinoblastoma pathway in pancreatic neuroendocrine tumors due to increased cdk4/cdk6. Clin Cancer Res. 2012;18:4612-4620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 105. | Grande E, Teulé A, Alonso-Gordoa T, Jiménez-Fonseca P, Benavent M, Capdevila J, Custodio A, Vera R, Munarriz J, La Casta A, Díez JJ, Gajate P, Molina-Cerrillo J, Matos I, Cristóbal EM, Ruffinelli JC, Palacios J, García-Carbonero R. The PALBONET Trial: A Phase II Study of Palbociclib in Metastatic Grade 1 and 2 Pancreatic Neuroendocrine Tumors (GETNE-1407). Oncologist. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 106. | Vijayvergia N, Dasari A, Deng M, Litwin S, Al-Toubah T, Alpaugh RK, Dotan E, Hall MJ, Ross NM, Runyen MM, Denlinger CS, Halperin DM, Cohen SJ, Engstrom PF, Strosberg JR. Pembrolizumab monotherapy in patients with previously treated metastatic high-grade neuroendocrine neoplasms: joint analysis of two prospective, non-randomised trials. Br J Cancer. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 85] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 107. | Hijioka S, Hosoda W, Matsuo K, Ueno M, Furukawa M, Yoshitomi H, Kobayashi N, Ikeda M, Ito T, Nakamori S, Ishii H, Kodama Y, Morizane C, Okusaka T, Yanagimoto H, Notohara K, Taguchi H, Kitano M, Yane K, Maguchi H, Tsuchiya Y, Komoto I, Tanaka H, Tsuji A, Hashigo S, Kawaguchi Y, Mine T, Kanno A, Murohisa G, Miyabe K, Takagi T, Matayoshi N, Yoshida T, Hara K, Imamura M, Furuse J, Yatabe Y, Mizuno N. Rb Loss and KRAS Mutation Are Predictors of the Response to Platinum-Based Chemotherapy in Pancreatic Neuroendocrine Neoplasm with Grade 3: A Japanese Multicenter Pancreatic NEN-G3 Study. Clin Cancer Res. 2017;23:4625-4632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 148] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 108. | Sampedro-Núñez M, Serrano-Somavilla A, Adrados M, Cameselle-Teijeiro JM, Blanco-Carrera C, Cabezas-Agricola JM, Martínez-Hernández R, Martín-Pérez E, Muñoz de Nova JL, Díaz JÁ, García-Centeno R, Caneiro-Gómez J, Abdulkader I, González-Amaro R, Marazuela M. Analysis of expression of the PD-1/PD-L1 immune checkpoint system and its prognostic impact in gastroenteropancreatic neuroendocrine tumors. Sci Rep. 2018;8:17812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 109. | da Silva A, Bowden M, Zhang S, Masugi Y, Thorner AR, Herbert ZT, Zhou CW, Brais L, Chan JA, Hodi FS, Rodig S, Ogino S, Kulke MH. Characterization of the Neuroendocrine Tumor Immune Microenvironment. Pancreas. 2018;47:1123-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 110. | Salem ME, Puccini A, Grothey A, Raghavan D, Goldberg RM, Xiu J, Korn WM, Weinberg BA, Hwang JJ, Shields AF, Marshall JL, Philip PA, Lenz HJ. Landscape of Tumor Mutation Load, Mismatch Repair Deficiency, and PD-L1 Expression in a Large Patient Cohort of Gastrointestinal Cancers. Mol Cancer Res. 2018;16:805-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 167] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 111. | Bösch F, Brüwer K, Altendorf-Hofmann A, Auernhammer CJ, Spitzweg C, Westphalen CB, Boeck S, Schubert-Fritschle G, Werner J, Heinemann V, Kirchner T, Angele M, Knösel T. Immune checkpoint markers in gastroenteropancreatic neuroendocrine neoplasia. Endocr Relat Cancer. 2019;26:293-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 112. | Lanitis T, Proskorovsky I, Ambavane A, Hunger M, Zheng Y, Bharmal M, Phatak H. Survival Analysis in Patients with Metastatic Merkel Cell Carcinoma Treated with Avelumab. Adv Ther. 2019;36:2327-2341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 113. | Cives M, Strosberg J. Radionuclide Therapy for Neuroendocrine Tumors. Curr Oncol Rep. 2017;19:9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 105] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 114. | Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, Mittra E, Kunz PL, Kulke MH, Jacene H, Bushnell D, O'Dorisio TM, Baum RP, Kulkarni HR, Caplin M, Lebtahi R, Hobday T, Delpassand E, Van Cutsem E, Benson A, Srirajaskanthan R, Pavel M, Mora J, Berlin J, Grande E, Reed N, Seregni E, Öberg K, Lopera Sierra M, Santoro P, Thevenet T, Erion JL, Ruszniewski P, Kwekkeboom D, Krenning E; NETTER-1 Trial Investigators. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med. 2017;376:125-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1702] [Cited by in RCA: 2230] [Article Influence: 278.8] [Reference Citation Analysis (0)] |
| 115. | Brabander T, van der Zwan WA, Teunissen JJM, Kam BLR, Feelders RA, de Herder WW, van Eijck CHJ, Franssen GJH, Krenning EP, Kwekkeboom DJ. Long-Term Efficacy, Survival, and Safety of [177Lu-DOTA0,Tyr3]octreotate in Patients with Gastroenteropancreatic and Bronchial Neuroendocrine Tumors. Clin Cancer Res. 2017;23:4617-4624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 411] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 116. | Fang Y, Chen W, Liang X, Li D, Lou H, Chen R, Wang K, Pan H. Comparison of long-term effectiveness and complications of radiofrequency ablation with hepatectomy for small hepatocellular carcinoma. J Gastroenterol Hepatol. 2014;29:193-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 137] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 117. | Zhang L, Ge NL, Chen Y, Xie XY, Yin X, Gan YH, Zhang BH, Zhang JB, Chen RX, Wang YH, Ye SL, Ren ZG. Long-term outcomes and prognostic analysis of radiofrequency ablation for small hepatocellular carcinoma: 10-year follow-up in Chinese patients. Med Oncol. 2015;32:77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 118. | Kutlu OC, Chan JA, Aloia TA, Chun YS, Kaseb AO, Passot G, Yamashita S, Vauthey JN, Conrad C. Comparative effectiveness of first-line radiofrequency ablation versus surgical resection and transplantation for patients with early hepatocellular carcinoma. Cancer. 2017;123:1817-1827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 119. | de Mestier L, Zappa M, Hentic O, Vilgrain V, Ruszniewski P. Liver transarterial embolizations in metastatic neuroendocrine tumors. Rev Endocr Metab Disord. 2017;18:459-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 120. | Fiore F, Del Prete M, Franco R, Marotta V, Ramundo V, Marciello F, Di Sarno A, Carratù AC, de Luca di Roseto C, Colao A, Faggiano A. Transarterial embolization (TAE) is equally effective and slightly safer than transarterial chemoembolization (TACE) to manage liver metastases in neuroendocrine tumors. Endocrine. 2014;47:177-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 121. | Gupta S, Johnson MM, Murthy R, Ahrar K, Wallace MJ, Madoff DC, McRae SE, Hicks ME, Rao S, Vauthey JN, Ajani JA, Yao JC. Hepatic arterial embolization and chemoembolization for the treatment of patients with metastatic neuroendocrine tumors: variables affecting response rates and survival. Cancer. 2005;104:1590-1602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 301] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 122. | Marrache F, Vullierme MP, Roy C, El Assoued Y, Couvelard A, O'Toole D, Mitry E, Hentic O, Hammel P, Lévy P, Ravaud P, Rougier P, Ruszniewski P. Arterial phase enhancement and body mass index are predictors of response to chemoembolisation for liver metastases of endocrine tumours. Br J Cancer. 2007;96:49-55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 123. | Strosberg D, Schneider EB, Onesti J, Saunders N, Konda B, Shah M, Dillhoff M, Schmidt CR, Shirley LA. Prognostic Impact of Serum Pancreastatin Following Chemoembolization for Neuroendocrine Tumors. Ann Surg Oncol. 2018;25:3613-3620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 124. | Luo Y, Ameli S, Pandey A, Khoshpouri P, Ghasabeh MA, Pandey P, Li Z, Hu D, Kamel IR. Semi-quantitative visual assessment of hepatic tumor burden can reliably predict survival in neuroendocrine liver metastases treated with transarterial chemoembolization. Eur Radiol. 2019;29:5804-5812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 125. | Modlin IM, Oberg K, Chung DC, Jensen RT, de Herder WW, Thakker RV, Caplin M, Delle Fave G, Kaltsas GA, Krenning EP, Moss SF, Nilsson O, Rindi G, Salazar R, Ruszniewski P, Sundin A. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008;9:61-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1268] [Cited by in RCA: 1180] [Article Influence: 69.4] [Reference Citation Analysis (0)] |
| 126. | Singh S, Law C. Multidisciplinary reference centers: the care of neuroendocrine tumors. J Oncol Pract. 2010;6:e11-e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 127. | Tamagno G, Sheahan K, Skehan SJ, Geoghegan JG, Fennelly D, Collins CD, Maguire D, Traynor O, Brophy DP, Cantwell C, Swan N, McGowan L, O'Toole D, O'Shea D. Initial impact of a systematic multidisciplinary approach on the management of patients with gastroenteropancreatic neuroendocrine tumor. Endocrine. 2013;44:504-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 128. | Magi L, Mazzuca F, Rinzivillo M, Arrivi G, Pilozzi E, Prosperi D, Iannicelli E, Mercantini P, Rossi M, Pizzichini P, Laghi A, Signore A, Marchetti P, Annibale B, Panzuto F. Multidisciplinary Management of Neuroendocrine Neoplasia: A Real-World Experience from a Referral Center. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |