Published online May 21, 2020. doi: 10.3748/wjg.v26.i19.2294
Peer-review started: December 30, 2019
First decision: February 19, 2020
Revised: March 29, 2020
Accepted: May 1, 2020
Article in press: May 1, 2020
Published online: May 21, 2020
Processing time: 142 Days and 20.8 Hours
Hepatocellular adenomas (HCAs) represent rare, benign liver tumours occurring predominantly in females taking oral contraceptives. In children, HCAs comprise less than 5% of hepatic tumours and demonstrate association with various conditions. The contemporary classification of HCAs, based on their distinctive genotypes and clinical phenotypes, includes hepatocyte nuclear factor 1 homeobox alpha-inactivated HCAs, beta-catenin-mutated HCAs, inflammatory HCAs, combined beta-catenin-mutated and inflammatory HCAs, sonic hedgehog-activated HCAs, and unclassified HCAs. In children, there is a lack of literature on the characteristics and distribution of HCA subtypes. In this review, we summarized different HCA subtypes and the clinicopathologic spectrum of HCAs in the paediatric population.
Core tip: Hepatocellular adenomas (HCAs) are uncommon liver tumours with 2 major complications: bleeding and malignant transformation; these lesions are classified based on their distinctive genotypes and clinical phenotypes. HCAs in children may be identified in the setting of conditions such as glycogen storage disorder and familial adenomatous polyposis. However, the molecular subtypes do not always correlate with predisposing risk factors and syndromes. Herein, we will discuss the different subtypes of HCA and the clinicopathological characteristics in children.
- Citation: Hahn E, Putra J. Hepatocellular adenoma in the paediatric population: Molecular classification and clinical associations. World J Gastroenterol 2020; 26(19): 2294-2304
- URL: https://www.wjgnet.com/1007-9327/full/v26/i19/2294.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i19.2294
Hepatocellular adenomas (HCAs) are rare benign neoplasms arising from hepatocytes, occurring at a rate of 3-4 per 100000[1]. There is a female predominance with a strong association with oral contraceptive pill (OCP) use[2,3]. Other risk factors for the development of HCAs include androgen hormone imbalance, obesity, alcohol intake, liver vascular disease, chronic viral hepatitis, cirrhosis, previous malignancy, and germline genetic susceptibility[4-6]. Although HCAs are considered benign, these lesions have 2 major complications: severe bleeding and malignant transformation[4]. The current HCA classification provides considerable benefits in terms of management and prognostication. The literature of HCAs in the paediatric population is still limited. In children, HCAs have been associated with glycogen storage diseases (GSDs), galactosemia, Hurler syndrome (mucopolysaccharidosis type 1), familial adenomatous polyposis syndrome, and Fanconi anemia (FA), among others[7-10].
In this review, we will discuss the current molecular classification of HCAs, followed by select clinical associations in children.
HCAs were initially categorized into 4 subtypes based on the genotypes and clinical phenotypes: hepatocyte nuclear factor 1 homeobox alpha (HNF1A)-inactivated HCAs (HHCAs), inflammatory HCAs (IHCAs), beta-catenin-mutated HCAs (bHCAs), and unclassified HCAs (UHCAs)[11]. Further evaluation using gene expression profiling, RNA sequencing, whole-exome and -genome sequencing, resulted in an expanded classification which includes bHCAs involving exon 3 (bex3HCAs) and exon 7 or 8 (bex7,8HCAs), ICHAs with beta-catenin mutations (bex3IHCAs and bex7,8IHCAs), and a newly defined entity of sonic hedgehog HCAs (shHCAs)[4]. The clinical and pathological characteristics of these subtypes are summarized in Table 1.
| HCA subtype | Risk factors | Specific clinical features | Histologic features | IHCs |
| HHCA | HNF1A germline mutations, MODY type 3, microsatellite instability | Hepatic adenomatosis | Intralesional steatosis | LFABP (absent/decreased) |
| IHCA | Obesity, alcohol, glycogenosis | Inflammatory syndrome | Sinusoidal dilatation, inflammatory infiltrate | CRP, SAA |
| bex3HCA | Male, liver vascular disease, androgen therapy | Frequent malignant transformation | Pseudoacinar formation, mild nuclear atypia | beta-catenin (nuclear staining), GS (diffuse and strong) |
| bex7,8HCA | No specific risk factors | No specific clinical features | No specific features | GS (weak, heterogeneous) |
| shHCA | Obesity | Symptomatic bleeding | Intratumoural hemorrhage | Prostaglandin D2 synthase |
| UHCA | No specific risk factors | No specific clinical features | No specific features | None |
HNF1A is a gene located on chromosome 12 (12q24.31) that encodes the protein hepatocyte nuclear factor 1 (HNF1) which acts as a transcription factor, developmentally regulating gene expression through interactions with the promoters of genes expressed in the liver[12]. Zucman-Rossi et al[11] demonstrated that bi-allelic inactivating mutations of HNF1A constituted a homogenous, morphologically distinct subgroup of adenomas (HHCAs). These mutations are exclusive of mutations in genes associated with other subtypes of HCA (CTNNB1, IL6ST, JAK1, GNAS and STAT3)[13].
HHCAs most commonly affect female patients with an average age of 37 years at diagnosis in one series, with 8% of patients demonstrating germline HNF1A mutations[4]. Risk factors for the development of HHCAs include oral contraceptive use, which is especially potent due to the decreased estradiol detoxification in these tumours, and HNF1A germline mutations[4,14]. The familial form of hepatic adenomatosis (multiple HHCAs) secondary to germline mutations of HNF1A has been identified in patients with maturity-onset diabetes of the young type 3[15-19]. Additionally, HNF1A contains a poly-cytosine C8-microsatellite, making it susceptible to microsatellite instability; this phenomenon has been observed to result in HHCA development in 3 unrelated children with bi-allelic mutations of MLH1 and PMS2[20].
Histologically, HHCAs are characterized by intralesional steatosis, along with a lack of inflammation and cytologic atypia[21]. This phenomenon (intratumoural steatosis) is due to increased lipogenesis secondary to HNF1A inactivating mutations, occurring via down-regulation of liver fatty acid binding protein (LFABP)[22]. It is important to note that the degree of steatosis in each lesion varies, and steatosis is not exclusively seen in this subtype of HCA. The diagnosis of HHCA can be confirmed by decreased or absent LFABP immunostaining in the lesional cells[21]. Although rare, malignant transformation has been associated with this subtype of HCA[19,23,24].
IHCAs are one of the most common subtypes (30%-50% of HCAs) which are characterized by IL6ST mutations[4,25]. The gene is located on chromosome 5 (5q11.2) and encodes glycoprotein 130 (gp130), a signal transducer for the JAK/STAT pathway[26]. Mutations in gp130 lead to sustained activation of the pathway, resulting in hepatocellular proliferation and HCA development[25,27]. GNAS also plays a role in activating this pathway, with somatic mutations leading to the development of HCA and HCC[25].
IHCAs demonstrate a female predominance, with an average age of diagnosis of 40 years[4]. Clinically, IHCA patients may present with fever, leukocytosis, and elevated C-reactive protein (CRP), gamma-glutamyl transferase, alkaline phosphatase, and amyloid-associated proteins[4]. In general, this subtype has been associated with high body mass index, alcohol consumption, GSD type I, and primary sclerosing cholangitis[21]. IHCAs carry an increased risk of bleeding due to their highly vascularized morphology[25].
Microscopically, IHCAs are characterized by display inflammatory infiltrates (predominantly lymphocytes and histiocytes, admixed with plasma cells and neutrophils), sinusoidal dilatation, dystrophic arteries, and variable ductular reaction in the periphery of the lesions[4,21]. By immunohistochemistry, the tumours are positive for CRP and serum amyloid A[4]. Malignant transformation occurs in 5-10% of IHCAs, with coexisting beta-catenin mutations implicated in the pathogenesis[28].
CTNNB1 (catenin, beta-1) is a gene located on chromosome 3 (3p22.1) that encodes the protein beta-catenin, an adherens junction protein[29]. This protein anchors the actin cytoskeleton between epithelial cells, communicating a contact inhibition signal, regulating normal cell growth and behaviour[29]. The Wnt/beta-catenin pathway regulates hepatocellular development, growth, and regeneration[30]. Mutations in CTNNB1 may result in uncontrolled hepatocyte proliferation. These mutations can occur in exon 3, 7, or 8, giving rise to HCAs and HCC[4]. bex3HCAs has the highest malignant transformation potential[4]. In one series, approximately half of all bHCAs co-demonstrated inflammatory phenotypes with mutations affecting genes implicated in IHCAs (6% of all HCAs being classified as bex3IHCAs and 4% as bex7,8IHCAs)[4].
bHCAs occur in younger patients than the other subtypes, with an average age of 27.5-28.5 years at diagnosis, and a female predominance, although a higher proportion of males are affected than in other subtypes[4]. An association with androgen therapy is well-described.
The characteristic morphological features include mild cytologic atypia and pseudoacinar formation in addition to typical HCA findings. In bex3HCAs, the lesional cells demonstrate diffuse and strong immunohistochemical expression of glutamine synthetase (GS) and aberrant, nuclear positivity for beta-catenin[4]. Meanwhile, bex7,8HCAs are characterized by perivenular and heterogeneous staining of GS without nuclear beta-catenin expression.
A subset of HCAs demonstrates small deletions of INHBE (inhibin, beta-E) which lead to INHBE−GLI1 fusions[4]. INHBE is a gene located on chromosome 12 (12q13.3) that encodes a protein which plays a role in pancreatic exocrine growth and proliferation[31]. GLI1 is a gene located on chromosome 12 (12q13.3) as well; it is involved in signal transduction in the sonic hedgehog signaling pathway, and activates transcription of target genes[32]. In the liver, the sonic hedgehog pathway leads to growth of progenitor hepatocyte populations, thereby promoting regeneration, with accompanying compensatory reparative changes, including inflammation, fibrosis and vascular remodeling[33]. These changes are classically associated with cirrhosis but can play a role in the pathogenesis of shHCAs, HCC, and cholangiocarcinoma[33]. GLI1 fusions have been observed in other benign neoplasms, as have other mutations affecting the sonic hedgehog pathway[34-36]. In one series, shHCAs accounted for 4% of previously unclassified HCAs[4].
shHCAs have a strong female predominance, with an average age of diagnosis of 43 years[4]. This subtype shows intratumoural hemorrhage on microscopic examination[4]. By immunohistochemistry, the lesional cells are positive for prostaglandin D2 synthase, while argininosuccinate synthetase 1, albeit molecularly enhanced in shHCAs, shows non-specific staining in this subtype as well as others[4,37]. Currently, the malignant potential of shHCAs is unknown[4].
HCAs represent < 5% of all paediatric hepatic tumours[8]. In addition to sex hormone disturbances as seen in adults, HCAs in children may arise in the background of FA, GSDs type I, III, and IV, galactosemia, immunodeficiency, congenital portosystemic shunts (CPSS), cardiac hepatopathy status-post Fontan procedure, Hurler syndrome, familial adenomatous polyposis, germline HNF1A mutations and maturity-onset diabetes of the young type 3, among others[7-10,38]. HCAs may also occur spontaneously in the paediatric setting. In one series, up to 30% of HCAs developed without risk factors[9]. Table 2 highlights different conditions which have been associated with HCAs in the paediatric population.
| Sex hormone dysregulation |
| Oral contraceptive use |
| Obesity |
| Klinefelter’s syndrome |
| Polycystic ovary syndrome |
| Sex hormone producing tumours (e.g., ertoli-Leydig cell tumours) |
| Androgen therapy (Turner’s syndrome, Fanconi anemia, Glanzmann's thrombasthenia) |
| Antiepileptic therapies with sodium ion channel modulation |
| Metabolic disorders |
| Glycogen storage diseases type I, III, and IV |
| Galactosemia |
| Hurler syndrome (mucopolysaccharidosis type 1) |
| Fanconi Anemia (with or without androgen therapy) |
| Diabetes mellitus type II |
| Immunodeficiency |
| Congenital portosystemic shunts |
| Cardiac hepatopathy (status-post Fontan procedure) |
| Other syndromes |
| Alagille syndrome |
| Familial adenomatous polyposis syndrome |
| Maturity-onset diabetes of the young type 3 |
| McCune-Albright syndrome |
| Noonan syndrome with multiple lentigines |
| Prader Willi syndrome |
| Wolf-Hirschhorn syndrome |
The average age of HCA presentation in children is 14 years, although HCAs may be detected as early as prenatally[10,39,40]. The lesions most commonly present in the right lobe of female patients[7]. Clinically, patients present with HCAs found incidentally on imaging or with abdominal pain, which can be related to bleeding and rupture which occur in 27.2% and 17.5% of patients, respectively[9,41].
Similar to adults, HCAs predominantly manifest as solitary lesions, while multiple lesions are more frequently observed in children with predisposition, such as GSD and Hurler syndrome[8,42]. Currently, there are no published recommendations about screening protocols for HCA in patients with predisposing factors except for children with GSD[10]. In children with GSD type I, liver imaging is routinely performed every 12-24 mo[43]. Computed tomography or magnetic resonance imaging with contrast should be considered in older children to look for evidence of increasing lesion size, poorly defined margins, or hemorrhage[43].
Histologic evaluation of the tumor should be considered in sporadic cases with no known predisposing factor for diagnostic confirmation and evaluation of the background liver[10]. The molecular classification is currently the same as in adults. The main differential diagnoses of HCAs in the paediatric population include focal nodular hyperplasia, hemangiomas, fibrolamellar carcinoma, and HCCs; the detailed clinicopathological features of these entities are beyond the scope of this review. Selected entities associated with paediatric HCAs are discussed below.
Sex hormone dysregulation is a shared pathway for development of HCAs, across all subtypes and age groups. Besides OCP, sex hormone dysregulation in the paediatric population can occur with obesity, polycystic ovarian syndrome (PCOS), Klinefelter’s syndrome, sex hormone producing tumours, such as Sertoli-Leydig cell tumours, and in the treatment of other diseases, such as hormone therapy for Turner’s syndrome, steroid therapy for FA and Glanzmann's thrombasthenia, and oxcarbazepine therapy for seizures[10,44-51]. Oxcarbazepine and other sodium ion channel modulating antiepileptic drugs have been found to cause reproductive endocrine dysfunction, and this is the proposed pathogenesis of HCAs in these cases[51].
The molecular subtype of these tumours is not well-described. There is one report of a 13 year old girl, with obesity, PCOS, and diabetes mellitus type II who had a HCA that demonstrated a variant of unknown significance in HNF1A with accompanying characteristic prominent lesional steatosis, along with acinar growth and conspicuous nucleoli[48]. Conversely, there are reports of bHCAs (including coexisting inflammatory phenotype) arising in obese adolescents, a large UHCA (GS positive, beta-catenin negative) in an 8-year-old girl without predisposing risk factors, and an IHCA in a 30-year-old woman with Turner’s syndrome[45,52-54].
FA is a rare autosomal recessive disorder (1 in 90000), which is characterized by pancytopenia and dysmorphic features, and treated with anabolic steroids[55,56]. Patients affected by FA have increased development of liver tumours, including HCAs and HCC[57]. In a study that reviewed 32 patients with FA and associated hepatic lesions, 32% of neoplasms were determined to be HCAs[58]. Additionally, androgen therapy and iron overload increase the risk of HCA development in these patients[49,59]. HCC in FA patients may develop as a malignant transformation of HCA[49,59,60].
There is a strong link between GSDs and HCAs, occurring in GSD types I, III, and IV[61]. Additionally, Roshcer et al[62] reported a likely hepatic adenoma detected on ultrasound in a patient with GSD type VI. HCAs are seen in approximately 16%-75% of patients with GSD type I, and are usually detectable by age 15[63]. GSD-associated HCAs are frequently multiple, and, in contrast to the hormone-related etiologies, these occur without female predominance and with metabolic control leading to regression of lesional size and burden[64].
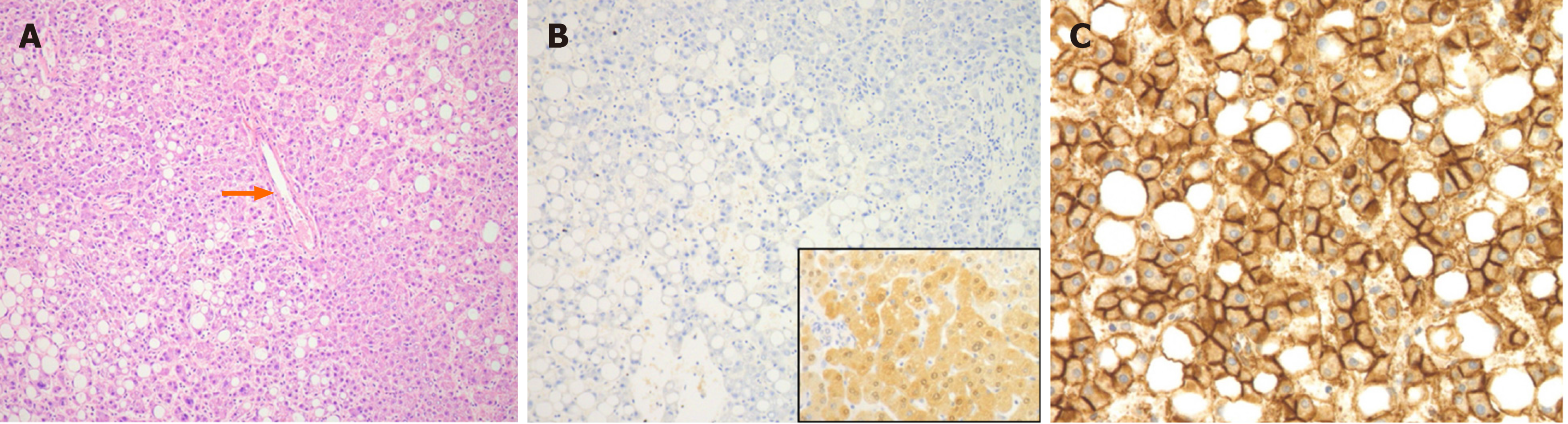
In a large series of GSD-related HCAs, the majority (52%) were classified as IHCAs, harbouring IL6ST or GNAS mutations, with the remainder classified as bHCAs (28%, with 57% bex3HCA and 43% bex7,8HCA) or UHCAs (20%)[65]. At the Hospital of Sick Children, we encountered an adolescent with GSD type IA who developed a HHCA (Figure 1) despite no previous reports of this subtype in GSD patients.
Chromosomal aberrations affecting chromosome 6 (gain of 6p and loss of 6q) have been observed in 60% of GSD I-related HCAs[66]. The high frequency of bHCAs, in particular bex3HCAs, in this population, as well as shared abnormalities of chromosome 6 with HCC, correlates with our understanding of the behaviour of these neoplasms and the increased frequency of malignant transformation of GSD I-related HCAs, which occurs through the adenoma-carcinoma sequence[66,67].
HCAs are seen in 4%-25% of patients with GSD type III[68,69]. Compared to GSD I-related HCAs, malignant transformation is less frequently observed in GSD type III, and almost exclusively in the setting of cirrhosis[68]. GSD type IV has documented association with HCAs and HCC development, however the pathologic progression is also not well understood[70].
Alagille syndrome is an autosomal dominant condition caused by mutations in JAG1 (94% of cases) and NOTCH2 (1.5% of cases)[71]. Alagille syndrome is pathologically characterized by a paucity of intrahepatic bile ducts, with other syndromic sequelae, including cardiac malformations, vascular malformations, vertebral abnormalities, and abnormal facies[72]. The association with HCA development is tenuous. In one series of 20 patients with AS who received imaging, 6 were found to have nodular hepatic masses, and of the 5 that underwent pathological evaluation, none met the criteria for diagnosis as HCAs[73]. However, there is a reported case of a 9 year old boy with AS, with a proven mutation in NOTCH2, who was incidentally found to have a HCA on abdominal ultrasound for portal hypertension, which was consistent with a HHCA upon histologic evaluation[74].
Congenital portosystemic shunts (CPSS) are rare vascular malformations, affecting approximately 1 in 30000 children[75]. These shunts can be evident on prenatal ultrasounds, and are classified as intrahepatic or extrahepatic. Patients with CPSS are at risk for the development of HCAs and HCCs, in addition to other complications, such as cholestasis, hepatopulmonary syndrome, and encephalopathy[75,76]. The CPSS-related hepatic lesions generally respond well to shunt correction[76]. CPSS-associated HCAs can occur in the presence of other hereditary syndromes, such as Noonan syndrome with multiple lentigines (LEOPARD syndrome) and other undiagnosed multisystem syndromes[76]. The patients included in one series displayed a variety of dysmorphic features in addition to CPSS-related HCAs, which may indicate that multiple genetic signaling pathways are involved in HCA development in these patients, in addition to hepatic and systemic blood flow abnormalities[76].
Terracciano et al[77] reported a child with a family history of Carney complex who underwent enucleation of a HCA at the age of 9. She re-presented at the age of 14 with fibrolamellar carcinoma, which has not been well-documented to develop from HCAs[77]. An association between IHCAs and McCune-Albright syndrome has been described in adults, both driven by GNAS mutations[25,78]. Additionally, HCAs have been described in Wolf-Hirschhorn syndrome, a rare contiguous gene deletion syndrome involving the short arm of chromosome 4[79].
In a large meta-analysis, 4.2% of HCAs were found to undergo malignant transformation, with 4.5% of resected HCAs containing focal malignancy[80]. The highest risk of malignant transformation is seen in bex3HCAs, although the phenomenon has been identified in other subtypes as well[4,81]. In bex3HCAs, the initial CTNNB1 mutation is sufficient for development of benign HCAs, with accompanying telomerase reverse transcriptase (TERT) mutations required for malignant transformation[82-84]. Other risk factors for malignant transformation include large size (> 5 cm), male sex, high alcohol intake, diabetes mellitus type II (DMII), fibrosis of the background liver, and acquired TP53 mutations[4,85]. Malignant transformation of HCAs is a rare phenomenon in the paediatric population; it has been described in association with GSD type I, FA, CPSS, and Wolff-Hirschhorn syndrome[49,67,76,79].
Rare cases of malignant transformation from HCA into hepatoblastoma have also been reported. Louie et al[86] reported hepatoblastoma arising in a pigmented bHCA of a 4-year-old male patient, while Gupta et al[87] reported 3 children with familial adenomatous polyposis syndrome who developed hepatoblastoma in the background of hepatic adenomatosis[86,87].
Determining malignant transformation is often challenging pathologically, as the distinction between HCA and well-differentiated HCC is not always straight forward. Current pathological features that are helpful in this distinction include assessment for architectural distortion, HCC with a rim of residual HCA, cytologic atypia, loss of reticulin staining, and increased immunohistochemical staining for CD34[85,88]. Even after workup this distinction may still be difficult, prompting the suggestion of a separate diagnostic category of atypical hepatocellular neoplasm or hepatocellular neoplasm of uncertain malignant potential[89,90]. Some have recommended chromosomal analysis of adenomas with atypical features for abnormalities shared by HCC, namely those affecting chromosomes 1, 8, and 6, in an attempt to elucidate their potential behaviour[91,92].
The assessment of malignant transformation in children should be based on the histopathology and its molecular subtyping, similar to the diagnostic approach in adults.
The current molecular classification of HCAs demonstrates a reliable correlation to risk factors and prognosis. When it comes to the paediatric population, the molecular subtypes are identifiable, however, often do not correlate with predisposing risk factors and syndromes. Our understanding of the molecular pathways involved in liver tumourigenesis, paediatric HCC, and neoplasia in general, will play a large role in our approach to patients with liver lesions, predisposing risk factors, and seemingly unrelated syndromes with molecular aberrations in associated genes. Better documentation of HCA subtypes in this age group and further study of these lesions, patients, and tumours will continue to illuminate pathogenesis. HCAs remain an area of future study and a clinical entity best managed with a multidisciplinary approach.
We would like to thank Dr. Iram Siddiqui (the Hospital for Sick Children) for providing us with the case included in this manuscript.
Manuscript source: Invited Manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Canada
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Govindarajan GK, Kim JH, Misdraji J S-Editor: Wang YQ L-Editor: A E-Editor: Ma YJ
| 1. | Rooks JB, Ory HW, Ishak KG, Strauss LT, Greenspan JR, Hill AP, Tyler CW. Epidemiology of hepatocellular adenoma. The role of oral contraceptive use. JAMA. 1979;242:644-648. [PubMed] |
| 2. | Edmondson HA, Henderson B, Benton B. Liver-cell adenomas associated with use of oral contraceptives. N Engl J Med. 1976;294:470-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 335] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 3. | Svrcek M, Jeannot E, Arrivé L, Poupon R, Fromont G, Fléjou JF, Zucman-Rossi J, Bouchard P, Wendum D. Regressive liver adenomatosis following androgenic progestin therapy withdrawal: a case report with a 10-year follow-up and a molecular analysis. Eur J Endocrinol. 2007;156:617-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Nault JC, Couchy G, Balabaud C, Morcrette G, Caruso S, Blanc JF, Bacq Y, Calderaro J, Paradis V, Ramos J, Scoazec JY, Gnemmi V, Sturm N, Guettier C, Fabre M, Savier E, Chiche L, Labrune P, Selves J, Wendum D, Pilati C, Laurent A, De Muret A, Le Bail B, Rebouissou S, Imbeaud S; GENTHEP Investigators, Bioulac-Sage P, Letouzé E, Zucman-Rossi J. Molecular Classification of Hepatocellular Adenoma Associates With Risk Factors, Bleeding, and Malignant Transformation. Gastroenterology. 2017;152:880-894.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 269] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 5. | Seo JM, Lee SJ, Kim SH, Park CK, Ha SY. Hepatocellular carcinoma arising from hepatocellular adenoma in a hepatitis B virus-associated cirrhotic liver. Clin Radiol. 2012;67:329-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Tonorezos ES, Barnea D, Abou-Alfa GK, Bromberg J, D'Angelica M, Sklar CA, Shia J, Oeffinger KC. Hepatocellular adenoma among adult survivors of childhood and young adult cancer. Pediatr Blood Cancer. 2017;64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Wheeler DA, Edmondson HA, Reynolds TB. Spontaneous liver cell adenoma in children. Am J Clin Pathol. 1986;85:6-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Resnick MB, Kozakewich HP, Perez-Atayde AR. Hepatic adenoma in the pediatric age group. Clinicopathological observations and assessment of cell proliferative activity. Am J Surg Pathol. 1995;19:1181-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Vaithianathan R, Philipchandran, Selvambigai G, Jayaganesh P. Spontaneous hepatocellular adenoma in paediatric age group - case report. J Clin Diagn Res. 2013;7:2962-2963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Franchi-Abella S, Branchereau S. Benign hepatocellular tumors in children: focal nodular hyperplasia and hepatocellular adenoma. Int J Hepatol. 2013;2013:215064. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Zucman-Rossi J, Jeannot E, Nhieu JT, Scoazec JY, Guettier C, Rebouissou S, Bacq Y, Leteurtre E, Paradis V, Michalak S, Wendum D, Chiche L, Fabre M, Mellottee L, Laurent C, Partensky C, Castaing D, Zafrani ES, Laurent-Puig P, Balabaud C, Bioulac-Sage P. Genotype-phenotype correlation in hepatocellular adenoma: new classification and relationship with HCC. Hepatology. 2006;43:515-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 606] [Cited by in RCA: 535] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 12. | Courtois G, Morgan JG, Campbell LA, Fourel G, Crabtree GR. Interaction of a liver-specific nuclear factor with the fibrinogen and alpha 1-antitrypsin promoters. Science. 1987;238:688-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 411] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 13. | Védie AL, Sutter O, Ziol M, Nault JC. Molecular classification of hepatocellular adenomas: impact on clinical practice. Hepat Oncol. 2018;5:HEP04. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Jeannot E, Poussin K, Chiche L, Bacq Y, Sturm N, Scoazec JY, Buffet C, Van Nhieu JT, Bellanné-Chantelot C, de Toma C, Laurent-Puig P, Bioulac-Sage P, Zucman-Rossi J. Association of CYP1B1 germ line mutations with hepatocyte nuclear factor 1alpha-mutated hepatocellular adenoma. Cancer Res. 2007;67:2611-2616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Yamagata K, Oda N, Kaisaki PJ, Menzel S, Furuta H, Vaxillaire M, Southam L, Cox RD, Lathrop GM, Boriraj VV, Chen X, Cox NJ, Oda Y, Yano H, Le Beau MM, Yamada S, Nishigori H, Takeda J, Fajans SS, Hattersley AT, Iwasaki N, Hansen T, Pedersen O, Polonsky KS, Bell GI. Mutations in the hepatocyte nuclear factor-1alpha gene in maturity-onset diabetes of the young (MODY3). Nature. 1996;384:455-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 824] [Cited by in RCA: 787] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 16. | Bacq Y, Jacquemin E, Balabaud C, Jeannot E, Scotto B, Branchereau S, Laurent C, Bourlier P, Pariente D, de Muret A, Fabre M, Bioulac-Sage P, Zucman-Rossi J. Familial liver adenomatosis associated with hepatocyte nuclear factor 1alpha inactivation. Gastroenterology. 2003;125:1470-1475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 117] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 17. | Reznik Y, Dao T, Coutant R, Chiche L, Jeannot E, Clauin S, Rousselot P, Fabre M, Oberti F, Fatome A, Zucman-Rossi J, Bellanne-Chantelot C. Hepatocyte nuclear factor-1 alpha gene inactivation: cosegregation between liver adenomatosis and diabetes phenotypes in two maturity-onset diabetes of the young (MODY)3 families. J Clin Endocrinol Metab. 2004;89:1476-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 89] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Willson JS, Godwin TD, Wiggins GA, Guilford PJ, McCall JL. Primary hepatocellular neoplasms in a MODY3 family with a novel HNF1A germline mutation. J Hepatol. 2013;59:904-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Stueck AE, Qu Z, Huang MA, Campreciós G, Ferrell LD, Thung SN. Hepatocellular Carcinoma Arising in an HNF-1α-Mutated Adenoma in a 23-Year-Old Woman with Maturity-Onset Diabetes of the Young: A Case Report. Semin Liver Dis. 2015;35:444-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Holter S, Pollett A, Zogopoulos G, Kim H, Schwenter F, Asai K, Gallinger S, Clendenning M, Steinbach G, Jacobson A, Boycott KM. Hepatic adenomas caused by somatic HNF1A mutations in children with biallelic mismatch repair gene mutations. Gastroenterology. 2011;140:735-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Dhingra S, Fiel MI. Update on the new classification of hepatic adenomas: clinical, molecular, and pathologic characteristics. Arch Pathol Lab Med. 2014;138:1090-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 22. | Rebouissou S, Imbeaud S, Balabaud C, Boulanger V, Bertrand-Michel J, Tercé F, Auffray C, Bioulac-Sage P, Zucman-Rossi J. HNF1alpha inactivation promotes lipogenesis in human hepatocellular adenoma independently of SREBP-1 and carbohydrate-response element-binding protein (ChREBP) activation. J Biol Chem. 2007;282:14437-14446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 100] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 23. | Nault JC, Bioulac-Sage P, Zucman-Rossi J. Hepatocellular benign tumors-from molecular classification to personalized clinical care. Gastroenterology. 2013;144:888-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 193] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 24. | Putra J, Ferrell LD, Gouw ASH, Paradis V, Rishi A, Sempoux C, Balabaud C, Thung SN, Bioulac-Sage P. Malignant transformation of liver fatty acid binding protein-deficient hepatocellular adenomas: histopathologic spectrum of a rare phenomenon. Mod Pathol. 2020;33:665-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 25. | Nault JC, Fabre M, Couchy G, Pilati C, Jeannot E, Tran Van Nhieu J, Saint-Paul MC, De Muret A, Redon MJ, Buffet C, Salenave S, Balabaud C, Prevot S, Labrune P, Bioulac-Sage P, Scoazec JY, Chanson P, Zucman-Rossi J. GNAS-activating mutations define a rare subgroup of inflammatory liver tumors characterized by STAT3 activation. J Hepatol. 2012;56:184-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 26. | Carbia-Nagashima A, Arzt E. Intracellular proteins and mechanisms involved in the control of gp130/JAK/STAT cytokine signaling. IUBMB Life. 2004;56:83-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Pilati C, Amessou M, Bihl MP, Balabaud C, Nhieu JT, Paradis V, Nault JC, Izard T, Bioulac-Sage P, Couchy G, Poussin K, Zucman-Rossi J. Somatic mutations activating STAT3 in human inflammatory hepatocellular adenomas. J Exp Med. 2011;208:1359-1366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 188] [Cited by in RCA: 201] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 28. | Dokmak S, Paradis V, Vilgrain V, Sauvanet A, Farges O, Valla D, Bedossa P, Belghiti J. A single-center surgical experience of 122 patients with single and multiple hepatocellular adenomas. Gastroenterology. 2009;137:1698-1705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 259] [Article Influence: 16.2] [Reference Citation Analysis (1)] |
| 29. | Peifer M. Cancer, catenins, and cuticle pattern: a complex connection. Science. 1993;262:1667-1668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Thompson MD, Monga SP. WNT/beta-catenin signaling in liver health and disease. Hepatology. 2007;45:1298-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 394] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 31. | Hashimoto O, Ushiro Y, Sekiyama K, Yamaguchi O, Yoshioka K, Mutoh K, Hasegawa Y. Impaired growth of pancreatic exocrine cells in transgenic mice expressing human activin betaE subunit. Biochem Biophys Res Commun. 2006;341:416-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Motoyama J, Liu J, Mo R, Ding Q, Post M, Hui CC. Essential function of Gli2 and Gli3 in the formation of lung, trachea and oesophagus. Nat Genet. 1998;20:54-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 408] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 33. | Omenetti A, Choi S, Michelotti G, Diehl AM. Hedgehog signaling in the liver. J Hepatol. 2011;54:366-373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 232] [Cited by in RCA: 220] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 34. | Dahlén A, Fletcher CD, Mertens F, Fletcher JA, Perez-Atayde AR, Hicks MJ, Debiec-Rychter M, Sciot R, Wejde J, Wedin R, Mandahl N, Panagopoulos I. Activation of the GLI oncogene through fusion with the beta-actin gene (ACTB) in a group of distinctive pericytic neoplasms: pericytoma with t(7;12). Am J Pathol. 2004;164:1645-1653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 117] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 35. | Spans L, Fletcher CD, Antonescu CR, Rouquette A, Coindre JM, Sciot R, Debiec-Rychter M. Recurrent MALAT1-GLI1 oncogenic fusion and GLI1 up-regulation define a subset of plexiform fibromyxoma. J Pathol. 2016;239:335-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 102] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 36. | Amakye D, Jagani Z, Dorsch M. Unraveling the therapeutic potential of the Hedgehog pathway in cancer. Nat Med. 2013;19:1410-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 449] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 37. | Nault JC, Couchy G, Caruso S, Meunier L, Caruana L, Letouzé E, Rebouissou S, Paradis V, Calderaro J, Zucman-Rossi J. Argininosuccinate synthase 1 and periportal gene expression in sonic hedgehog hepatocellular adenomas. Hepatology. 2018;68:964-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 38. | Babaoglu K, Binnetoglu FK, Aydoğan A, Altun G, Gürbüz Y, Inan N, Corapçioğlu F. Hepatic adenomatosis in a 7-year-old child treated earlier with a Fontan procedure. Pediatr Cardiol. 2010;31:861-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 39. | Gold JH, Guzman IJ, Rosai J. Benign tumors of the liver. Pathologic examination of 45 cases. Am J Clin Pathol. 1978;70:6-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 29] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 40. | Applegate KE, Ghei M, Perez-Atayde AR. Prenatal detection of a solitary liver adenoma. Pediatr Radiol. 1999;29:92-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 41. | van Aalten SM, de Man RA, IJzermans JN, Terkivatan T. Systematic review of haemorrhage and rupture of hepatocellular adenomas. Br J Surg. 2012;99:911-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 42. | Chiorean L, Cui XW, Tannapfel A, Franke D, Stenzel M, Kosiak W, Schreiber-Dietrich D, Jüngert J, Chang JM, Dietrich CF. Benign liver tumors in pediatric patients - Review with emphasis on imaging features. World J Gastroenterol. 2015;21:8541-8561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 91] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (2)] |
| 43. | Kishnani PS, Austin SL, Abdenur JE, Arn P, Bali DS, Boney A, Chung WK, Dagli AI, Dale D, Koeberl D, Somers MJ, Wechsler SB, Weinstein DA, Wolfsdorf JI, Watson MS; American College of Medical Genetics and Genomics. Diagnosis and management of glycogen storage disease type I: a practice guideline of the American College of Medical Genetics and Genomics. Genet Med. 2014;16:e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 298] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 44. | Beuers U, Richter WO, Ritter MM, Wiebecke B, Schwandt P. Klinefelter's syndrome and liver adenoma. J Clin Gastroenterol. 1991;13:214-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 45. | Espat J, Chamberlain RS, Sklar C, Blumgart LH. Hepatic adenoma associated with recombinant human growth hormone therapy in a patient with Turner's syndrome. Dig Surg. 2000;17:640-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 46. | Nemoto S, Ariizumi SI, Kotera Y, Omori A, Yamashita S, Kato TA, Aoyama S, Egawa H, Yamamoto M. Inflammatory hepatocellular adenoma in a patient with Turner's syndrome: A case report. Int J Surg Case Rep. 2019;56:5-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 47. | Lautz TB, Finegold MJ, Chin AC, Superina RA. Giant hepatic adenoma with atypical features in a patient on oxcarbazepine therapy. J Pediatr Surg. 2008;43:751-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 48. | Triantafyllopoulou M, Whitington PF, Melin-Aldana H, Benya EC, Brickman W. Hepatic adenoma in an adolescent with elevated androgen levels. J Pediatr Gastroenterol Nutr. 2007;44:640-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 49. | Velazquez I, Alter BP. Androgens and liver tumors: Fanconi's anemia and non-Fanconi's conditions. Am J Hematol. 2004;77:257-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 143] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 50. | Rossor T, Quaglia A, Zacharoulis S, Strautnieks S, Davenport M, Hadžić N. Hepatic adenoma mimicking a Leydig-sertoli cell tumor metastasis. Cent Eur J Paediatr. 2013;9:201-204. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 51. | Löfgren E, Tapanainen JS, Koivunen R, Pakarinen A, Isojärvi JI. Effects of carbamazepine and oxcarbazepine on the reproductive endocrine function in women with epilepsy. Epilepsia. 2006;47:1441-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 52. | Rosencrantz RA, Wu Y, Sonke PY, Yusuf Y. Giant hepatocellular adenoma in a previously obese thirteen-year-old boy. Ann Hepatol. 2015;14:559-563. [PubMed] |
| 53. | Yadav R, Mallick S, Mittal D, Madhusudan KS, Jana M, Bajpai M, Gupta SD, Das P. Giant hepatocellular adenoma with peliosis hepatis in a child: A diagnostic dilemma. Trop Gastroenterol. 2015;36:200-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 54. | Oliveira S, Samba AK, Towbin AJ, Gupta A, Geller JI, Nathan JD, Kohli R. Incidental inflammatory adenoma with β-catenin activation in the setting of paediatric NASH. Pediatr Obes. 2018;13:70-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 55. | Lobitz S, Velleuer E. Guido Fanconi (1892-1979): a jack of all trades. Nat Rev Cancer. 2006;6:893-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 56. | Nalepa G, Clapp DW. Fanconi anaemia and cancer: an intricate relationship. Nat Rev Cancer. 2018;18:168-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 282] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 57. | Mulvihill JJ, Ridolfi RL, Schultz FR, Borzy MS, Haughton PB. Hepatic adenoma in Fanconi anemia treated with oxymetholone. J Pediatr. 1975;87:122-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 50] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 58. | Touraine RL, Bertrand Y, Foray P, Gilly J, Philippe N. Hepatic tumours during androgen therapy in Fanconi anaemia. Eur J Pediatr. 1993;152:691-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 59. | Ozenne V, Paradis V, Vullierme MP, Vilgrain V, Leblanc T, Belghiti J, Imbert A, Valla DC, Degos F. Liver tumours in patients with Fanconi anaemia: a report of three cases. Eur J Gastroenterol Hepatol. 2008;20:1036-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 60. | Colle I, Laureys G, Raevens S, Libbrecht L, Leroy JG, Reyntjens K, Geerts A, Rogiers X, Troisi RI, Hoehn H, Schindler D, Hanenberg H, De Wilde V, Van Vlierberghe H. Living related liver transplantation in an adult patient with hepatocellular adenoma and carcinoma 13 years after bone marrow transplantation for Fanconi anemia: A case report. Hepatol Res. 2013;43:991-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 61. | Schady DA, Roy A, Finegold MJ. Liver tumors in children with metabolic disorders. Transl Pediatr. 2015;4:290-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 62. | Roscher A, Patel J, Hewson S, Nagy L, Feigenbaum A, Kronick J, Raiman J, Schulze A, Siriwardena K, Mercimek-Mahmutoglu S. The natural history of glycogen storage disease types VI and IX: Long-term outcome from the largest metabolic center in Canada. Mol Genet Metab. 2014;113:171-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 63. | Rake JP, Visser G, Labrune P, Leonard JV, Ullrich K, Smit GP. Glycogen storage disease type I: diagnosis, management, clinical course and outcome. Results of the European Study on Glycogen Storage Disease Type I (ESGSD I). Eur J Pediatr. 2002;161 Suppl 1:S20-S34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 145] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 64. | Khanna R, Verma SK. Pediatric hepatocellular carcinoma. World J Gastroenterol. 2018;24:3980-3999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 81] [Cited by in RCA: 98] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 65. | Calderaro J, Labrune P, Morcrette G, Rebouissou S, Franco D, Prévot S, Quaglia A, Bedossa P, Libbrecht L, Terracciano L, Smit GP, Bioulac-Sage P, Zucman-Rossi J. Molecular characterization of hepatocellular adenomas developed in patients with glycogen storage disease type I. J Hepatol. 2013;58:350-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 120] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 66. | Kishnani PS, Chuang TP, Bali D, Koeberl D, Austin S, Weinstein DA, Murphy E, Chen YT, Boyette K, Liu CH, Chen YT, Li LH. Chromosomal and genetic alterations in human hepatocellular adenomas associated with type Ia glycogen storage disease. Hum Mol Genet. 2009;18:4781-4790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 67. | Bianchi L. Glycogen storage disease I and hepatocellular tumours. Eur J Pediatr. 1993;152 Suppl 1:S63-S70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 106] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 68. | Demo E, Frush D, Gottfried M, Koepke J, Boney A, Bali D, Chen YT, Kishnani PS. Glycogen storage disease type III-hepatocellular carcinoma a long-term complication? J Hepatol. 2007;46:492-498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 69. | Labrune P, Trioche P, Duvaltier I, Chevalier P, Odièvre M. Hepatocellular adenomas in glycogen storage disease type I and III: a series of 43 patients and review of the literature. J Pediatr Gastroenterol Nutr. 1997;24:276-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 180] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 70. | Alshak NS, Cocjin J, Podesta L, van de Velde R, Makowka L, Rosenthal P, Geller SA. Hepatocellular adenoma in glycogen storage disease type IV. Arch Pathol Lab Med. 1994;118:88-91. [PubMed] |
| 71. | Leonard LD, Chao G, Baker A, Loomes K, Spinner NB. Clinical utility gene card for: Alagille Syndrome (ALGS). Eur J Hum Genet. 2014;22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 72. | Alagille D, Odièvre M, Gautier M, Dommergues JP. Hepatic ductular hypoplasia associated with characteristic facies, vertebral malformations, retarded physical, mental, and sexual development, and cardiac murmur. J Pediatr. 1975;86:63-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 414] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 73. | Rapp JB, Bellah RD, Maya C, Pawel BR, Anupindi SA. Giant hepatic regenerative nodules in Alagille syndrome. Pediatr Radiol. 2017;47:197-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 74. | Pacheco MC, Monroe EJ, Horslen SP. Hepatic Adenoma Arising in a Patient With Alagille Syndrome: A Case Report. Pediatr Dev Pathol. 2018;21:585-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 75. | Bernard O, Franchi-Abella S, Branchereau S, Pariente D, Gauthier F, Jacquemin E. Congenital portosystemic shunts in children: recognition, evaluation, and management. Semin Liver Dis. 2012;32:273-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 177] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 76. | Franchi-Abella S, Branchereau S, Lambert V, Fabre M, Steimberg C, Losay J, Riou JY, Pariente D, Gauthier F, Jacquemin E, Bernard O. Complications of congenital portosystemic shunts in children: therapeutic options and outcomes. J Pediatr Gastroenterol Nutr. 2010;51:322-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 181] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 77. | Terracciano LM, Tornillo L, Avoledo P, Von Schweinitz D, Kühne T, Bruder E. Fibrolamellar hepatocellular carcinoma occurring 5 years after hepatocellular adenoma in a 14-year-old girl: a case report with comparative genomic hybridization analysis. Arch Pathol Lab Med. 2004;128:222-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 78. | Gaujoux S, Salenave S, Ronot M, Rangheard AS, Cros J, Belghiti J, Sauvanet A, Ruszniewski P, Chanson P. Hepatobiliary and Pancreatic neoplasms in patients with McCune-Albright syndrome. J Clin Endocrinol Metab. 2014;99:E97-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 79. | Battaglia A, Calhoun ARUL, Lortz A, Carey JC. Risk of hepatic neoplasms in Wolf-Hirschhorn syndrome (4p-): Four new cases and review of the literature. Am J Med Genet A. 2018;176:2389-2394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 80. | Stoot JH, Coelen RJ, De Jong MC, Dejong CH. Malignant transformation of hepatocellular adenomas into hepatocellular carcinomas: a systematic review including more than 1600 adenoma cases. HPB (Oxford). 2010;12:509-522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 275] [Cited by in RCA: 242] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 81. | Micchelli ST, Vivekanandan P, Boitnott JK, Pawlik TM, Choti MA, Torbenson M. Malignant transformation of hepatic adenomas. Mod Pathol. 2008;21:491-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 82. | Pilati C, Letouzé E, Nault JC, Imbeaud S, Boulai A, Calderaro J, Poussin K, Franconi A, Couchy G, Morcrette G, Mallet M, Taouji S, Balabaud C, Terris B, Canal F, Paradis V, Scoazec JY, de Muret A, Guettier C, Bioulac-Sage P, Chevet E, Calvo F, Zucman-Rossi J. Genomic profiling of hepatocellular adenomas reveals recurrent FRK-activating mutations and the mechanisms of malignant transformation. Cancer Cell. 2014;25:428-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 199] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 83. | Nault JC, Mallet M, Pilati C, Calderaro J, Bioulac-Sage P, Laurent C, Laurent A, Cherqui D, Balabaud C, Zucman-Rossi J. High frequency of telomerase reverse-transcriptase promoter somatic mutations in hepatocellular carcinoma and preneoplastic lesions. Nat Commun. 2013;4:2218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 414] [Cited by in RCA: 511] [Article Influence: 46.5] [Reference Citation Analysis (1)] |
| 84. | Nault JC, Calderaro J, Di Tommaso L, Balabaud C, Zafrani ES, Bioulac-Sage P, Roncalli M, Zucman-Rossi J. Telomerase reverse transcriptase promoter mutation is an early somatic genetic alteration in the transformation of premalignant nodules in hepatocellular carcinoma on cirrhosis. Hepatology. 2014;60:1983-1992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 258] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 85. | Farges O, Ferreira N, Dokmak S, Belghiti J, Bedossa P, Paradis V. Changing trends in malignant transformation of hepatocellular adenoma. Gut. 2011;60:85-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 204] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 86. | Louie CY, Concepcion W, Park JK, Rangaswami A, Finegold MJ, Hazard FK. Hepatoblastoma Arising in a Pigmented β-catenin-activated Hepatocellular Adenoma: Case Report and Review of the Literature. Am J Surg Pathol. 2016;40:998-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 87. | Gupta A, Sheridan RM, Towbin A, Geller JI, Tiao G, Bove KE. Multifocal hepatic neoplasia in 3 children with APC gene mutation. Am J Surg Pathol. 2013;37:1058-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 88. | Sempoux C, Balabaud C, Bioulac-Sage P. Malignant transformation of hepatocellular adenoma. Hepat Oncol. 2014;1:421-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 89. | Evason KJ, Grenert JP, Ferrell LD, Kakar S. Atypical hepatocellular adenoma-like neoplasms with β-catenin activation show cytogenetic alterations similar to well-differentiated hepatocellular carcinomas. Hum Pathol. 2013;44:750-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 90. | Bedossa P, Burt AD, Brunt EM, Callea F, Clouston AD, Dienes HP, Goodman ZD, Gouw AS, Hubscher SG, Roberts EA, Roskams T, Terracciano L, Tiniakos DG, Torbenson MS, Wanless IR. Well-differentiated hepatocellular neoplasm of uncertain malignant potential: proposal for a new diagnostic category. Hum Pathol. 2014;45:658-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 91. | Kakar S, Chen X, Ho C, Burgart LJ, Adeyi O, Jain D, Sahai V, Ferrell LD. Chromosomal abnormalities determined by comparative genomic hybridization are helpful in the diagnosis of atypical hepatocellular neoplasms. Histopathology. 2009;55:197-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 92. | Kakar S, Grenert JP, Paradis V, Pote N, Jakate S, Ferrell LD. Hepatocellular carcinoma arising in adenoma: similar immunohistochemical and cytogenetic features in adenoma and hepatocellular carcinoma portions of the tumor. Mod Pathol. 2014;27:1499-1509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |