Published online May 14, 2020. doi: 10.3748/wjg.v26.i18.2138
Peer-review started: January 29, 2020
First decision: March 18, 2020
Revised: March 26, 2020
Accepted: April 29, 2020
Article in press: April 29, 2020
Published online: May 14, 2020
Processing time: 105 Days and 14.9 Hours
Liver transplantation represents the only curative option for patients with end-stage liver disease, fulminant hepatitis and advanced hepatocellular carcinoma. Even though major advances in transplantation in the last decades have achieved excellent survival rates in the early post-transplantation period, long-term survival is hampered by the lack of improvement in survival in the late post transplantation period (over 5 years after transplantation). The main etiologies for late mortality are malignancies and cardiovascular complications. The latter are increasingly prevalent in liver transplant recipients due to the development or worsening of metabolic syndrome and all its components (arterial hypertension, dyslipidemia, obesity, renal injury, etc.). These comorbidities result from a combination of pre-liver transplant features, immunosuppressive agent side-effects, changes in metabolism and hemodynamics after liver transplantation and the adoption of a sedentary lifestyle. In this review we describe the most prevalent metabolic and cardiovascular complications present after liver transplantation, as well as proposing management strategies.
Core tip: Recently there has been an increasing interest in extra hepatic-related complications after liver transplantation because they widely affect late morbidity and mortality. Metabolic and cardiovascular diseases and de novo neoplasia are considered to be among the main complications affecting long- and mid-term prognosis after liver transplantation. In this review, we will assess the prevalence of metabolic and cardiovascular complications after liver transplantation, their impact on post-transplant morbidity and mortality, and the optimal medical management currently available.
- Citation: Becchetti C, Dirchwolf M, Banz V, Dufour JF. Medical management of metabolic and cardiovascular complications after liver transplantation. World J Gastroenterol 2020; 26(18): 2138-2154
- URL: https://www.wjgnet.com/1007-9327/full/v26/i18/2138.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i18.2138
Liver transplantation represents the only curative treatment option for end-stage liver disease, chosen cases of acute liver failure and selected patients with hepatocellular carcinoma, providing patients with a complete recovery of their liver function with excellent survival and quality of life[1,2]. Advances in surgical techniques and post-operative medical management have resulted in very good early post-transplant survival rates in the last decades; however, late mortality has remained unchanged[3]. In Europe current reported survival rates are 83%, 71%, 61% and 51% at 1, 5, 10 and 15 years, respectively, with rates increasing up to 86% at 1-year and 74% at 5-year if the period from 2010 to 2014 is considered[4]. Similarly, data from the United States indicates 85%, 68% and 50% 1-year, 5-year and 10-year survival rates after liver transplantation, respectively, with significant differences according to the etiology of the underlying liver disease[5]. These excellent survival rates in the early post-transplantation period underline the importance of understanding the causes and risk factors for late post-transplant mortality, in order to improve overall survival.
Late mortality is traditionally defined as death occurring 5 years or more after liver transplantation[6]. Late mortality is predominantly not related to the liver graft (63%), with high rates of cardiovascular causes and malignancies[7] . Although these findings are in keeping with the main causes of mortality of the general population, patients who underwent liver transplantation show higher risk for developing metabolic, cardiovascular and neoplastic complications[8]. This is partially explained by the need for chronic immunosuppressive drugs, the majority of which are associated with the worsening or development of new-onset hypertension, dyslipidemia and diabetes[9,10]. However, the massive adoption of the Western-world lifestyle and diet have dramatically affected metabolic changes, predisposing and increasing the development of cardiovascular diseases[11]. Therefore, the unmet goal in the management of the post-liver transplantation follow-up is the prevention of these long-term complications. In this review, we aim to review the prevalence of these late-onset complications, their impact on post-transplant morbidity and mortality, and the optimal management currently available.
Metabolic syndrome is defined as a cluster of interrelated risk factors of metabolic origin, involving insulin resistance and inflammation, which directly promote the development of atherosclerotic cardiovascular disease and type 2 diabetes mellitus[12]. There are different definitions, but most of them consider hypertension, obesity, dyslipidemia, and diabetes mellitus as the main components of metabolic syndrome. Its initial defining criteria, known as the World Health Organization criteria, have not been consistently used because of the need to measure serum insulin and urinary microalbumin to allow for the diagnosis, two expensive analyses[13]. Later on, the Third Report of the National Cholesterol Education Program and the Adult Treatment Panel III[14], proposed a more practical classification (initially described in 2001 and successively revised in 2006[12]), that was widely accepted by the scientific community. According to the National Cholesterol Education Program and the Adult Treatment Panel III modified classification, metabolic syndrome is diagnosed when at least ≥ 3 of the following criteria are met: (1) Impaired glucose tolerance: Fasting plasma glucose ≥ 100 mg/dL (5.6 mmol/L); (2) Abdominal obesity: Waist circumference > 102 cm (40 in) in men, > 88 cm (35 in) in women; (3) Hypertriglyceridemia: ≥ 150 mg/dL (1.7 mmol/L) or drug treatment for high triglycerides; (4) Low levels of high-density lipoproteins (HDL): < 40 mg/dL (1 mmol/L) in men, < 50 mg/dL (1.3 mmol/L) in women or drug treatment for low HDL; and (5) High blood pressure: ≥ 130/85 mmHg or drug treatment for hypertension.
Although originally these considerations on metabolic syndrome were described for the general population, they are currently also adopted in transplanted patients. Another attempt to classify this syndrome was made in 2005 by the International Diabetes Federation criteria, establishing specific national cut-offs, in order to make the classification uniform all over the world[15].
The prevalence of metabolic syndrome is higher in liver transplant recipients when compared to the general population. Retrospective studies assessing the presence of metabolic syndrome post liver transplantation detected this problem in 43%-58% of recipients[16], compared to 30% of non-transplanted patients, with slight variations according to different geographical areas[17]. A recent meta-analysis evaluated eight original publications on metabolic syndrome after liver transplantation, underlining some modifiable and non-modifiable risk factors[16]. Male gender, and components present prior to transplantation such as high BMI[18], type 2 diabetes mellitus[19] and hypertension were all related to the development of de novo metabolic syndrome. In particular, patients suffering from diabetes mellitus before transplantation had a six-fold higher risk for developing de novo metabolic syndrome[19]. When considering the etiology of the underlying liver disease resulting in the indication for liver transplantation, patients affected by hepatitis C, cryptogenic cirrhosis [group that possibly could include patients with misdiagnosed non-alcoholic steatohepatitis (NASH)] and alcohol related cirrhosis were at higher risk of developing metabolic syndrome after liver transplantation[16,19].
Although the data are not completely conclusive on the effect of immu-nosuppressive therapy on metabolic syndrome, the metabolic effects of these drugs are well established. Prolonged exposure to these drugs may increase the risk of metabolic complications and/or affect the reversibility of comorbidities present before transplantation. Corticosteroids, usually used in the early post-transplant phase, can act directly on pancreas beta cells increasing insulin resistance, while calcineurin inhibitors can affect the development both of diabetes mellitus (particularly for tacrolimus) and of hypertension (mainly true for cyclosporine). Dyslipidemia is often related to the use of mammalian target of rapamycin (mTOR) inhibitors, whereas the use of anti-metabolites such as mycophenolate have fewer detrimental effects on metabolic syndrome related comorbidities[20]. Considering that all these metabolic side effects are related to immunosuppression, it is reasonable to think that these agents may be the cause of metabolic syndrome. Nevertheless, there is no robust data to support this relationship[21]. Minimizing the effective dose of immunosuppression and supporting a healthy lifestyle are all measures recommended in order to prevent and reduce the development of metabolic syndrome and its related comorbidities.
In the general population, metabolic syndrome is recognized as an independent risk factor for cardiovascular morbidity and mortality. Regardless of the single components of metabolic syndrome, which represent themselves cardiovascular risk factors, metabolic syndrome is a cluster of metabolic dysfunctions that play a multiplicative impact on cardiovascular prognosis[22]. In keeping with these findings, metabolic syndrome has been extensively studied in the setting of liver transplantation. In the aforementioned meta-analysis by Li et al[16], liver transplant recipients patients with metabolic syndrome exhibited a higher rate of cardiovascular events, but not poorer survival rates. Patients who are at high risk of developing metabolic syndrome after liver transplantation should undergo regular surveillance in order to achieve an earlier diagnosis and treatment. An early diagnosis of metabolic syndrome will limit possible comorbidities, thereby reducing the risk of cardiovascular events. Additionally, patients who develop metabolic syndrome after liver transplantation are at a higher risk of developing graft steatosis, leading to an increase in the recurrence or in the development of de novo non-alcoholic fatty liver disease (NAFLD). NAFLD de novo rates range from 20% to 40%[23], but they can increase to 78% when we consider patients transplanted for NASH[24]. This wide variability depends on the methodology used for liver steatosis diagnosis[25]. Nevertheless, in the majority of cases, the recurrence of NAFLD and NASH are harmless, without an evolution towards cirrhosis[26]. Notably, patients with recurrent NAFLD/NASH are more prone to develop cardiovascular comorbidities, type 2 diabetes mellitus and suffer from increased infection-related morbidity and mortality[27]. Interestingly, recipient genetic predisposition might play a role in the recurrence of NAFLD and NASH. The presence of the rs738409-G allele of the patatin-like phospholipase in liver transplant recipients represents an independent risk factor for post-procedure development of obesity and steatosis[28].
New-onset diabetes mellitus type II after-liver transplantation is increasingly recognized as a complication of solid organ transplantation. It is defined by a hemoglobin A1c (HbA1c) level ≥ 6.5% in the transplanted populations[29]. Data on the prevalence of type 2 diabetes in patients after liver transplantation are still controversial. This is due to the heterogeneity of the criteria used for the diagnosis of diabetes mellitus and to the variability in the follow-up time points in the different studies. Nevertheless, the prevalence of type 2 diabetes mellitus ranges from 31% to 38% in post-liver transplantation patients, while the incidence of new onset type 2 diabetes ranges from 13% to 28% in the first three years following transplantation[29,30]. Diabetes mellitus has been demonstrated to have significant consequences in both the early and late post-liver transplantation periods. When present, it was associated with a higher 10-year mortality, compared to non-diabetic liver transplant patients[31].
Patients with diabetes mellitus are more prone to experience complications with an increased risk of cardiovascular events, nephropathy, infections and death[32]. In addition, they experience a higher number of acute rejection episodes compared with non-diabetic patients, with higher rates of graft lost[33]. There are several well-established risk factors associated with the development of diabetes after liver transplantation. Male gender[34], ethnicity, family history[35], hepatitis C[36], citomegalovirus infections[10], and immunosuppressive drugs significantly contribute to the development of new-onset diabetes mellitus or worsening of pre-existing diabetes.
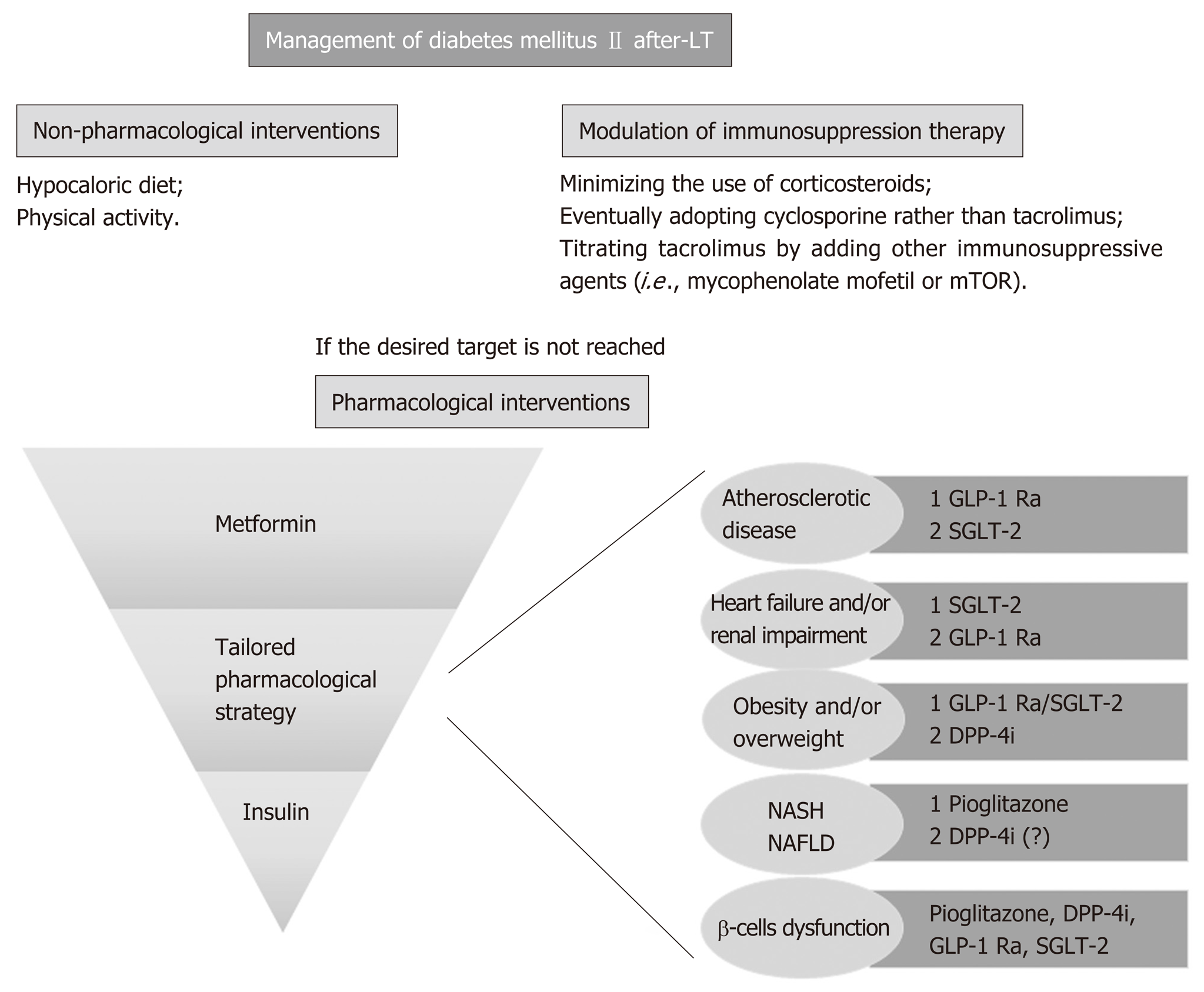
Among the available immunosuppressive drugs, corticosteroids are undisputedly known to increase the risk of new-onset diabetes in a length and dose-dependent manner[37]. This diabetogenic effect represents one of the most worrisome side-effects of glucocorticoids, justifying a strategy of rapid steroid withdrawal. Calcineurin-inhibitors also have a known diabetogenic effect, by directly damaging pancreatic islets cells. Although tacrolimus and cyclosporine share this mechanism of damage, the risk of developing or worsening of diabetes is significantly higher with tacrolimus than with cyclosporine (16.6% vs 9.8%, respectively), valid findings for all solid organ recipients[38]. There is convincing evidence from the non-liver transplant population that target glycemic levels significantly reduces morbidity and mortality in patients with type 2 diabetes mellitus[39]. Although this approach has not been specifically proven in the liver transplant population, and little information exists on the use of anti-diabetic drugs in this subset of patients, it is reasonable to assume that euglycemic status is a goal to achieve in post-liver transplantation management. Expert consensus and guideline recommendations suggest screening transplanted patients with basal glycaemia at weekly intervals during the first month following transplantation and subsequently at 3, 6, and 12 mo, with additional annual screening of diabetic complications[35,40]. The oral glucose test remains the best available test to definitely assess new-onset diabetes mellitus[41]. It should be noted that diagnosis of new-onset diabetes is not feasible in the first two months after liver transplantation[41], since in the immediate post-transplant period, insulin requirement is usually increased, being the safest and most effective therapy to treat hyperglycemia. However, once patients have returned to a regular eating pattern and stable immunosuppression, hyperglycemia may either disappear or, in the case of new onset diabetes mellitus, persist. In concordance, use of HbA1c test is recommended 3 mo post-liver transplantation due to possible peri-transplantation transfusions that render the test invalid[40]. The goal for transplanted patients with established type 2 diabetes mellitus should be an HbA1c level of less than 6.5%-7%[35]. An HbA1c level < 6.5% is recommended for patients with a shorter disease duration, younger age and fewer comorbidities. In older patients with multiple comorbidities and a high risk of hypoglycemia, an HbA1c of < 8.0% is considered a safer goal[42]. At present, there is insufficient data to recommend a specific algorithm of anti-diabetic agents in post-transplant diabetes mellitus, as studies addressing this specific population are lacking. However, if current guidelines for the treatment of type 2 diabetes in the general population are extrapolated to liver transplant patients, the choice of the anti-hyperglycemic agent should be tailored to patients’ preference and clinical characteristics[43]. Lifestyle changes represent the first line treatment for glycemic control, starting with a balanced diet low in calories and simple carbohydrates accompanied by moderate exercise, although this is often difficult in this patient population with general frailty persisting many years post-liver transplantation. When these measures are not sufficiently effective, pharmacological therapy with hypoglycemic agents and/or insulin needs to be considered. As the majority of oral diabetic medications are metabolized in the liver, they should be used with caution in patients in whom graft function is reduced[44]. Rosiglitazone, pioglitazone and sulfonylureas have been studied in the post-transplant population showing a possible minimization in insulin requirement[45,46]. Pioglitazone might also be considered in patients at risk of developing de novo or recurrent NAFLD after liver tr-ansplantation[47]. Metformin has not been extensively studied in the post-liver transplant setting despite its common use as the first-line therapy choice in type 2 diabetes. A single retrospective analysis of 24 renal transplant recipients treated with metformin reported a high rate of drug discontinuation due to gastrointestinal complaints or an increase in serum creatinine. However, no serious adverse events or severe alteration in immunosuppression drug levels were recorded[48]. Interestingly, in vitro analysis revealed that metformin optimally reverts diabetogenic genes that are dysregulated in the context of immunosuppression, which is something to take into account when evaluating the choice of therapy[49]. More recently, glucagon-like peptide-1 receptor agonist (GLP-1 RAs, i.e., liraglutide) and inhibitors of dipeptidyl peptidase 4 (DPP-4I or gliptins) were introduced as part of the current antidiabetic therapy. According to recent guidelines, GLP-1 RAs are recommended in the presence of established atherosclerotic cardiovascular disease and might be considered for their additive weight-loss properties. In this scenario, DPP-4I may also be useful because they do not affect body weight[50]. Sitagliptin or vildagliptin use after liver transplantation are not thought to have any significant effect on calcineurin inhibitor or mTOR inhibitor availability. A possible exception includes use of sitagliptin and cyclosporine as well as tacrolimus and vildagliptin, drug combinations that warrants further investigation[51,52]. Finally, there is next to no experience with sodium-glucose cotransporter type 2 (SGLT-2) inhibitors (i.e., canagliflozin, dapagliflozin and empagliflozin) in the setting of liver transplantation. All these drugs work by increasing urinary glucose excretion and are considered highly safe. Nevertheless, we have to consider that SGLT-2 inhibitors are associated with an increased risk of genital and urinary (the latter only for high doses of dapagliflozin) tract infections[53], leading to some controversy in a possible use in liver transplant patients. Moreover, drug elimination mainly occurs through hepatic and biliary excretion, making difficult the use of this medication when liver enzymes alterations are present[50]. The use of SGLT-2 inhibitors may have a positive effect in the setting of heart failure or renal impairment, with a specific dose adjustment needed according to renal function[50]. However, the only direct assessment of the potential interaction with immunosuppressive drugs to date was described in a study including healthy volunteers. Co-administration of cyclosporine resulted in a 23% increase in the mean canagliflozin area under the operating curve (AUC)[54]. The same mechanisms may result in an increased exposure to calcineurin inhibitors and mTOR inhibitors, although further studies are warranted to clarify this possible interaction. In addition, as impaired insulin secretion is a major determinant for liver transplantation, when hepatogenous diabetes[55,56] is suspected, drugs capable to improve β-cell function such as incretins or SGLT-2 inhibitors might be considered.
If therapeutic goals are not met with lifestyle changes and oral anti-diabetic medication, or if the patient becomes metabolically decompensated (symptomatic hyperglycemia with ketosis), insulin must be administered[57]. Ideally, glucagon-like peptide-1 receptor agonists should be used, in combination with basal insulin, in order to reduce the insulin requirement.
In summary, in addition to pharmacological treatment of diabetes mellitus, the adjustment of immunosuppressive regimens can aid in reducing the risk of post-liver transplantation diabetes and improve glycemic control. The possible strategy to adopt consists in minimizing the use of corticosteroids or adopting cyclosporine rather than the more diabetogenic tacrolimus, as well as titrating tacrolimus by adding other immunosuppressive agents (i.e., mycophenolate mofetil or mTOR inhibitors). These possible combinations may help improve glycemic control[57]. Careful attention also needs to be taken into account with regard to other cofactors, such as the occurrence of graft rejection, the concomitant presence of renal failure, etc. As mentioned, all immunosuppressive regimen adjustments should be combined with lifestyle modifications, and a carefully selected antidiabetic therapy (Figure 1).
Obesity is defined by the World Health Organization as a body mass index (BMI) > 30 kg/m2 and morbid obesity as a BMI of > 35 kg/m2[58]. In the past decade, obesity as a whole has progressively become a worldwide epidemic and a relevant comorbidity in the pre-liver transplant setting, with an overt adverse impact on the post-transplant outcome. Although dry weight is not always measurable in patients with decompensated cirrhosis, it is estimated that 15% to 30% of pre-transplant patients meet the criteria for obesity[59]. Following liver transplantation, weight gain tends to increase progressively over time. At one-year post-surgery, 33.7% of patients meet criteria for obesity, and by 5 years, 40.3% of patients are obese[60]. It is frequent that patients with a history of obesity prior to liver transplantation maintain this tendency after the surgical intervention. But additionally, one-third of patients with normal weight prior to transplantation become obese following the procedure[59]. Weight gain in the early post-transplantation period is related to several factors, such as an increased appetite as a result of the resolution of cirrhosis and thus, absence of a catabolic state, and the orexigenic effect of steroids. Risk factors for post-liver transplantation obesity include age greater than 50 years, obesity or type 2 diabetes prior to transplant and NASH as indication to liver transplantation[61]. Patients should be advised to achieve a healthy body weight prior to liver transplantation, as obesity is associated with numerous negative postoperative outcomes such as development of fatty deposits in the graft, development of diabetes, higher cardiovascular and oncogenic risk. Furthermore, patients should be made aware of the tendency to gain weight after transplantation and the problems associated herewith in order to adopt early preventive measures. Modulating immune suppression, such as adopting a regimen with a rapid withdrawal of steroids, is one of the possible strategies in patients at risk. However, it should be noted that steroids are needed not only in the early post-liver transplantation period, but also in the long-term follow-up as treatment of severe cellular rejection, where its use outweighs its associated risks. While corticosteroids are a well-known cause of weight increase, the effect of the exposure to the other available immunosuppressant drugs is not completely defined. Compared with tacrolimus, cyclosporine is associated with more weight gain in the first year following transplantation, whereas this difference is mitigated 2 years post-liver transplantation[60]. Recently, in a randomized trial by Charlton et al[62], the authors found that an early introduction of everolimus with reduced-exposure to tacrolimus at 1 month post liver transplantation reduced weight gain assessed at 1 and 2 years post-liver transplantation. When this reduced-exposure to tacrolimus arm was compared with the absent-exposure to tacrolimus arm, the weight gain was even lower, suggesting a beneficial effect of isolated everolimus therapy. In addition to selecting the best immunosuppression therapy, the fundamental approach to treat and prevent weight gain are lifestyle modifications. Supervised physical activity is considered safe and effective in stable liver transplant recipients[63]. It is proven that increasing aerobic capacity and muscle strength has a favorable impact on glucose homeostasis[64]. There are studies that describe a modified behavior in food intake before and after liver transplantation, with a positive energy balance in the first year after the surgery[65]. Therefore, ongoing physical exercise and the adoption of a healthy low-calorie diet are essential for the management of obesity in the post-transplant setting. Similar to the goals in the pre-transplant setting, the objectives of treating obesity after liver transplantation are to prevent obesity-related comorbidities and mortality, as well as to reduce the incidence of de novo NAFLD. When diet and exercise fail to achieve the proposed goal, pharmacologic therapies and/or bariatric surgery should be considered. Orlistat was tested in the post-liver transplant setting and was considered well-tolerated, safe, with no need for a close supervision of immunosuppressant drug levels, and dietary adherence. However, there was no significant evidence regarding its efficacy[66]. Liraglutide was recently approved for the treatment of obesity in non-diabetic patients[67], but to date there is no experience available in liver transplant recipients. Bariatric surgery is considered feasible, when indicated, although some issues remain. Potential problems include the presence of extensive adhesions, rendering surgery technically difficult, as well as the increased risk of wound complications in the setting of steroids or mTOR inhibitors[68]. Whenever possible, steroids should be tapered and stopped and mTOR inhibitors switched to other immunosuppressive agents to reduce the risks of wound healing problems. In the published literature, only case series are available describing the implementation of this therapy in liver transplant recipients (Table 1). Despite weight loss being observed in all reported series (range 21%-75%), high complication rates (30-40% of complications > grade III of the classification of Clavien-Dindo)[69] were documented for all types of procedures, particularly for sleeve gastrectomy[70]. Regarding mortality, there were no reports for sleeve gastrectomy, whereas gastric by-pass showed a mortality rate of 20%[71]. Regarding the pharmacokinetic of immunosuppressant drugs, studies have shown that the kinetics of tacrolimus and mycophenolate mofetil was not impacted by the performance of a sleeve gastrectomy[72]. On the other hand, patients with a gastric by-pass had significantly modified immunosuppressive drugs serum levels[73]. With currently limited data available on the effect of both bariatric surgery and pharmacological treatment on major outcomes such as survival in the post-liver transplant setting, diet and exercise are still considered the cornerstone treatment option for tackling and preventing weight gain.
| Ref. | Year | Number of patients | Follow-up, mean (range) | Type of bariatric surgery | Improvement of comorbidities | Complications, n (%) | Mortality, n |
| Duchini et al[119], United States | 2001 | 2 | 27 (18-36) | RYGB | Yes | 0 | 0 |
| Tichansky et al[120], United States | 2005 | 1 | 4 | RYGB | Yes | 0 | 0 |
| Butte et al[121], Chile | 2007 | 1 | 6 | SG | NE | 0 | 0 |
| Gentileschi et al[122], Italy | 2009 | 1 | 9 | BPD | NE | 0 | 1 (myocardial infarction) |
| Elli et al[123], United States | 2013 | 1 | 3 | SG | NE | 0 | 0 |
| Lin et al[124], United States | 2013 | 9 | 5 (3-12) | SG | Yes | 3 (33.3) (1 incisional hernia, 1 bile leakage, 1 dysphagia) | 0 |
| Al-Nowayalati et al[71], United States | 2013 | 7 | 59 (6-103) | RYGB | Yes | 4 (57.1) (2 incisional hernia, 2 wound infections) | 2 (1 septic shock at 6 mo after, 1 esophageal carcinoma at 9 mo after) |
| Pajecki et al[125], Brazil | 2014 | 1 | 5 | SG | Yes | 0 | 0 |
| Elli et al[126], United States | 2016 | 2 | 2 | SG | NE | 0 | 0 |
| Khoraki et al[127], United States | 2016 | 5 | 33.7 (13-79) | SG | Yes | 1 (20) (gastrointestinal bleeding) | 0 |
| Osseis et al[128], France | 2017 | 6 | 41 (12-94.4) | SG | Yes | 2 (33.3) (1 gastric fistula, 1 parietal mesh infection) | 1 (multi-organ failure at 19 mo after) |
| Tsamalaidze et al[129], Mexico | 2018 | 12 | 24 | SG | Yes | 4 (33.3) (2 dysphagia, 1 late drainage removal, 1 gastrostomy) | 0 |
High blood lipid levels are unusual in the pre-liver transplant population, due to the impaired hepatic synthetic function in end-stage liver disease. On the other hand, dyslipidemia is a very common finding in the post-liver transplant setting. The definition of hyperlipidemia varies widely among different studies in the post-liver transplant era and only a few consider the standard NCEP-ATP III criteria. In view of this lack of standardization, dyslipidemia is reported as present in 45% to 71% of liver transplant recipients[74]. In most cases, hypertriglyceridemia occurs in the first six months after transplantation, maintaining its prevalence throughout the first year, while it decreases in subsequent years. On the other hand, hypercholesterolemia and low levels of high-density lipoprotein concentration appear later, with an increasing prevalence that affects about 30% of patients at the end of the first post-transplant year[74].
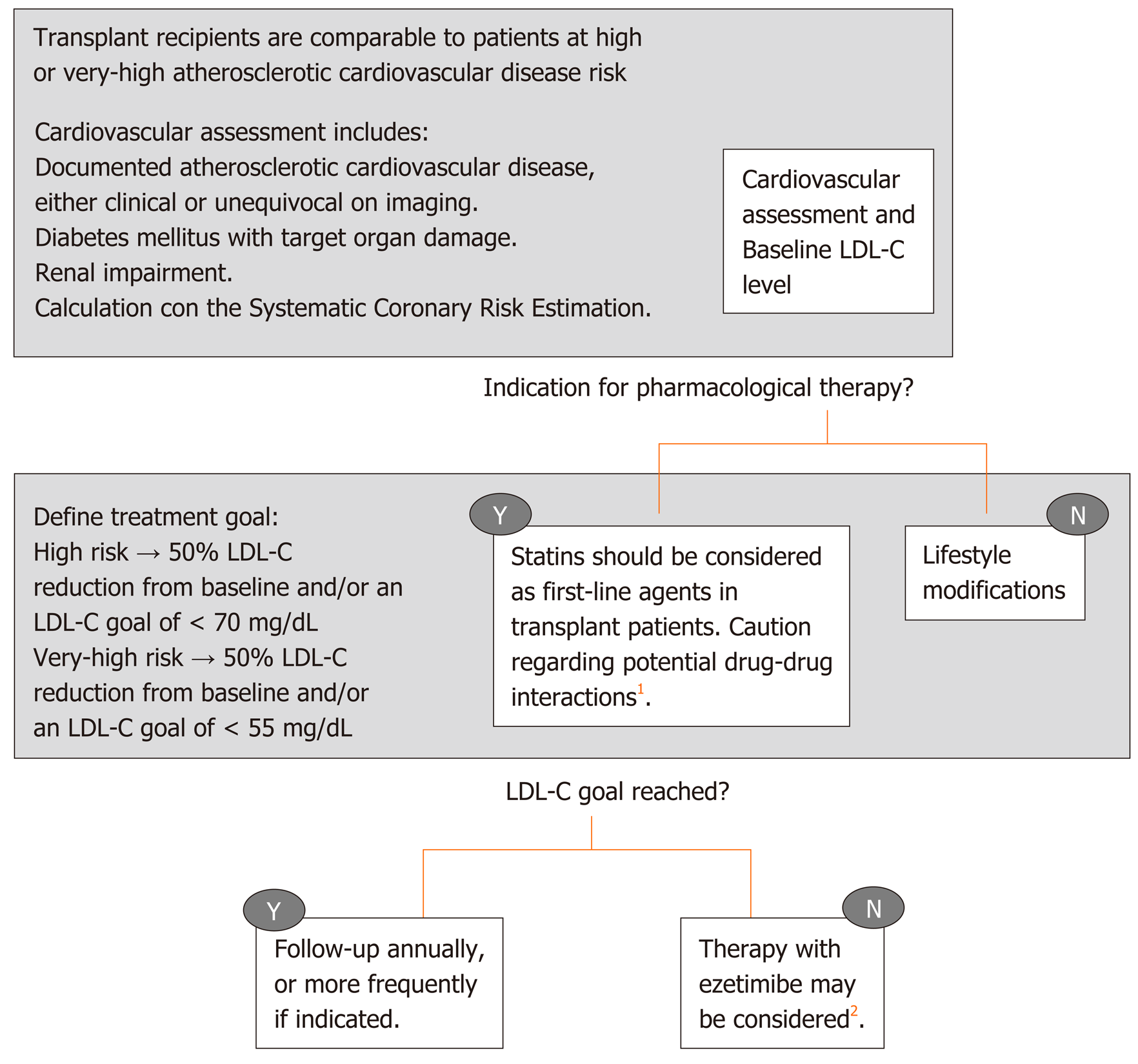
Dyslipidemia has multiple causes after liver transplantation that have been previously discussed in this review: Frequent body weight increase, poor glycemic control, genetic predisposition, donor related factors, early post-liver transplantation renal dysfunction[75] and the effect of immunosuppressant medication[76]. With regard to the latter, long-term corticosteroid use can contribute to hyperlipidemia[77]. Cyclosporine is more frequently associated with hyperlipidemia (14% vs 5%) and hypertriglyceridemia (49% vs 17%) when compared to tacrolimus, with a dose-dependent relationship[78]. The possible explanation for this cyclosporine effect is related to the inhibition of bile salt synthesis. In the case of tacrolimus, since this drug is known to cause hyperinsulinemia, this effect theoretically may lead to the development of hypertriglyceridemia. mTOR inhibitors are also known to increase the risk of hyperlipidemia (especially hypertriglyceridemia), through changes in insulin signaling pathways resulting in an excess of triglycerides production and secretion[79]. The combination therapy of mTOR inhibitors and tacrolimus results in lower rates of dyslipidemia[80], something also observed when switching from cyclosporine to tacrolimus[81]. Post-transplant dyslipidemia is generally resistant to dietary interventions, but it responds to traditional lipid-lowering agents. The most recent guidelines of the European Society of Cardiology[82] recommended the use of low-density lipoprotein concentrations (LDL-C) as the primary target for treatment of dyslipidemia, while for patients with elevated triglycerides, HDL-C is recommended as a secondary goal. In primary and secondary prevention for patients catalogued as having very-high risk for cardiovascular events, an LDL-C reduction of ≥ 50% from baseline and an LDL-C goal of < 1.4 mmol/L (< 55 mg/dL) are recommended, whereas in patients at high risk an LDL-C goal of < 1.8 mmol/L (< 70 mg/dL) is sufficient. This last version of the European Society of Cardiology guidelines dedicated a specific session on the management of dyslipidemia in solid organ recipients, although the recommendations are mostly based on studies on kidney recipients. They conclude that the management of dyslipidemia in transplant recipients should be comparable to that recommended for high or very-high risk patients, with an additional caution for possible drug-drug interactions (Figure 2). Statins are unanimously considered as a first line therapy for dyslipidemia in liver transplant patients, preferably pravastatin and fluvastatin because of the lack of interaction with cytochrome P450 and calcineurin inhibitors metabolization[21]. Generally, cyclosporine increases the blood levels of all statins, even more so than tacrolimus. Nevertheless, statins, with particular reference to pravastatin, have been established to be safe, efficacious and well tolerated in solid organ transplant recipients[83]. The concomitant use of other drugs metabolized by the cytochrome CYP3A4 should be carefully used in patients receiving both calcineurin inhibitors and statins[82], because a perturbation in the cytochrome P450 metabolic pathway can increase immunosuppressive drugs toxicity[84]. Ezetimibe may be considered in recipients who do not tolerate statins, although the experience is scant[85]. Concomitant use of calcineurin inhibitors may result in increased statin levels in the blood. Isolated hypertriglyceridemia can also be present post- liver transplantation and it generally responds well to fish oil. Omega 3 has less drug-drug interactions with im-munosuppressive therapy. In addition, omega 3 oil has other pleiotropic effects, such as anti-inflammatory and anti-proliferative properties, which can improve hepatic steatosis[86]. With regard to other lipid-lowering drugs, such as fibric acid derivatives, they are usually well tolerated, although there is scarce data available on their use in liver transplant patients. Importantly, the combination of fibrates with statin therapy increases the risk of myopathies and is thus not recommended. Patients on both these medications should be counseled regarding myalgia as a potential early symptom of rhabdomyolysis[87].
Arterial hypertension, which is defined as a systolic blood pressure greater than 140 mmHg and/or a diastolic blood pressure greater than 90 mmHg[88], occurs in 30%-50% of patients after liver transplantation, increasing up to 70% when evaluating patients in the long term[89,90]. Features such as high cardiac output, low systemic vascular resistance and low mean arterial pressure characterize end-stage liver disease. After patients are transplanted, this hemodynamic situation changes, leading to an increase in systemic blood pressure. Nevertheless, the etiology of post-liver transplantation hypertension is multifactorial and includes not only this change in hemodynamics, but also the use of immunosuppressive medications. These drugs are a well-known risk factor for hypertension, in particular calcineurin inhibitors. Although there are numerous pathogenetic mechanisms related to the development of hypertension in such patients, vasoconstriction seems to be the main causal factor. Vasoconstriction is caused by the excessive secretion of endothelin-1 and thromboxane and decreased production of prostacyclin, leading to an increase in sympathetic nervous system activity. In addition to these mechanisms, cyclosporine and tacrolimus act on sodium retention, resulting in an increase in effective-volume[90]. Nevertheless, cyclosporine seems to have a more deleterious effect compared to tacrolimus, showing a higher prevalence rate of arterial hypertension (73% vs 63%, respectively)[91]. Glu-cocorticosteroids are also a known cofactor for the development of arterial hypertension. They increase blood pressure through the renin-angiotensin-aldosterone system, causing a reduction in prostacyclin and nitric oxide production, and an increase in the quantity of angiotensin II receptors[92]. However, considering the usually short time of exposure to steroids in these patients, calcineurin inhibitors are the main agent responsible for the long-term development of arterial hypertension. The main concern about arterial hypertension is related to the direct damage on organs and its established association to increased risk for cardiovascular events[93]. Elevated blood pressure may lead to endothelial damage, atherosclerosis, kidney damage and left ventricle remodeling. Hypertensive control is essential to the improvement of long-term survival of both the graft and the recipient, related to the non-negligible risk of developing major cardiovascular events. The withdrawal of steroid therapy, the down-titration of calcineurin inhibitors (when adding mycophenolate mofetil or mTOR inhibitors) are possible strategies to reduce the increase in blood pressure values. Lifestyle modifications (i.e., low-sodium diet, cessation of smoking, avoidance of alcohol, and weight loss) should always be clearly explained to the patient. Nevertheless, when these measures are unsuccessful, medical therapy is mandatory. A blood pressure goal lower than 130/80 mmHg should be targeted to minimize cardiovascular risk[94]. Dihydropyridine calcium channel blockers are the preferred first-line agents in patients who do not exhibit proteinuria, in order to directly counteract the vasoconstriction associated with calcineurin inhibitors[95]. If proteinuria is present, liver transplant recipients benefit from angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blockers as a first line choice[95]. If a single-agent therapy is ineffective, a combination therapy should be evaluated, taking into account that the addition of beta-blockers is particularly indicated when a cardiac complication is well established[96]. Furthermore, angiotensin-converting enzyme inhibitors and angiotensin-2 blockers may magnify the collateral effects of calcineurin inhibitors such as hyperkalemia and metabolic acidosis, so it is advisable to use them during the long-term follow-up after liver transplantation, when the activation of the renin-angiotensin system becomes more evident. Diuretic therapy is typically avoided, due to the fact that volume contraction in the face of renal vasoconstriction may lead to further impairment in renal function[97].
It is important to keep in mind that drug-drug interactions are frequent in this subgroup of patients. Certain antihypertensive medications, namely beta-blockers, can significantly impact levels of immunosuppressive medication, thus they should be monitored during the introduction of any new drug. Independently from the chosen approach for blood pressure control, the end goal is to find a balance between antihypertensive therapy and immunosuppressive therapy modulation.
One of the most frequent medium- and long-term medical complications after liver transplantation is the development of nephrotoxicity, which is estimated to be 8%, 13.9% and 18.1% at 12, 36 and 60 mo post-transplant respectively[98]. Major causes of renal injury include the diagnosis of renal failure and/or hepatorenal syndrome prior to liver transplantation, critical intraoperative variables such as the need for vasopressors[99], donor-related variables such as donation after circulatory death, cold ischemia time, and graft steatosis[100,101]. All of these features are well established predictors of renal insufficiency after liver transplantation, particularly in the early post-surgical phase. However, in the majority of the cases, renal impairment is strongly associated with the direct side effects of immunosuppressive drugs in a dose dependent manner, such as calcineurin inhibitors. Nevertheless, it can also occur with combination regimens that aim to achieve low serum levels of tacrolimus. These findings have suggested the existence of different types of kidney damage based on different, partially reversible, mechanisms. The first type of damage is early and reversible, characterized by vasoconstriction of the kidney afferent arteriole, with a consensual reduction in glomerular filtration rate. A second and often irreversible damage is characterized by hyaline degeneration of renal arterioles, leading to glomerulosclerosis[102]. In this setting, the withdrawal of cyclosporine or tacrolimus is not effective for the recovery of kidney damage. Thus, early detection of renal impairment after liver transplantation is essential in order to implement different strategies to reduce calcineurin inhibitors levels. The introduction of immuno-suppressive combination therapies such as adding mTOR inhibitors to low doses of calcineurin inhibitors represents a possibility to minimize the exposure to nephrotoxic agents, sparing kidney function, without an increase in graft rejection rates[103]. Recently, new findings regarding the relationship between cardiovascular events after liver transplantation and renal impairment have been documented, underlying that even mild renal disease at the time of liver transplantation is a risk factor for post-transplant all-cause and cardiovascular mortality[104]. In a retrospective study on 202 transplant candidates pre-transplant renal impairment was found to be an independent predictor of post-transplant cardiac events (HR = 2.19, 95%CI: 1.25-3.85) and reduced cardiac event-free survival (HR = 2.27, 95%CI: 1.31-3.94)[105]. In addition, the velocity of the decline of glomerular filtrate rate after liver transplantation strongly correlated with the risk of adverse cardiovascular outcomes, highlighting the need to preserve early renal function in order to reduce these complications[104].
Cardiovascular diseases affect long-term prognosis after solid organ transplantation. Nevertheless, this risk is substantially different for liver transplant recipients compared to other solid organ recipients. This is partially related to hemodynamic and metabolic changes associated with chronic liver disease[106]. The marked peripheral vasodilatation present in patients with decompensated end-stage cirrhosis makes difficult the detection of a latent myocardial dysfunction with cardiac abnormalities, such as an attenuation in the systolic and diastolic contractile responses leading to the so-called cirrhotic cardiomyopathy. These changes, combined with reduced serum cholesterol, can mask pre-liver transplant cardiovascular risk factors, increasing the challenge to identify those patients at highest risk for cardiovascular diseases[97]. The relevance is notable when analyzing mortality after liver transplantation: It is estimated that 12%-16% of deaths one year after liver transplantation in the USA is due to cardiovascular disease[7]. In Europe, the median estimated 10-year risk of fatal cardiovascular disease is 1% (range: 0%-9%) and 10% of the affected patients have a high risk for these events[107]. A detailed cardiovascular assessment during pre-liver transplant evaluation is thus mandatory to not only assess the perioperative risk but also to allow for an early intervention, if needed, to ensure a good long-term outcome. Despite no guidelines being available in the pre-liver transplant assessment for cardiovascular disease, every transplant center adopts different routines for cardiovascular assessment, in order to stratify the population risk.
Standard evaluation before liver transplantation normally includes a full history and clinical examination, peripheral artery oxygen saturation, 12 lead electrocardiogram and a complete transthoracic ultrasound with assessment of left ventricular, right ventricular and valvular function (with an estimation of systolic pulmonary artery pressure). Further investigations, such as stress echocardiography, cardiac computerized tomographic scan, cardiac magnetic resonance or angiography are solicited based on medical history, cardiology indication and findings from the initial screening tests[108]. It should be noted that second line tests such as dobutamine stress echocardiography have shown a poor predictive value for coronary artery disease screening (sensitivity: 28%), although with high specificity (specificity: 82%), compared with the gold standard coronary angiography[109]. When using a protocol angiography in the pre-transplant cardiac evaluation, 36% of patients showed coronary artery disease in a recently published study, with NASH and hepatitis C being independent risk factors[110]. However, another study showed that the incidence of major cardiovascular events and overall survival after liver transplantation are similar between patients with and without coronary evaluation[111]. In another study where a control group without cardiovascular risk factors was matched with a group with coronary artery disease showed that the severity or extent of coronary artery disease does not affect post liver transplantation survival, if appropriately re-vascularized. However, early postoperative cardiac events could be associated with lower survival rate, irrespectively of underlying coronary artery disease[112]. Hence, it is unclear how many pre-liver transplant asymptomatic cardiovascular abnormalities could influence long-term outcome after transplantation. On the other hand, it seems that cardiac complications are significantly more frequent in patients with a pre-liver transplant known heart disease compared with those without pre-existing cardiovascular disease[113]. Since liver transplantation is a significantly stressful procedure from a cardiovascular standpoint, cardiovascular mortality is of the utmost importance, particularly when cirrhotic cardiomyopathy is unknown or un-derestimated[114]. Following liver transplantation, peripheral vascular resistance and blood pressure rapidly increase; these changes may cause an overt cardiac failure leading to pulmonary edema in predisposed patients. Other possible cardiovascular complications include the development of post-operative atrial fibrillation, defined as a new-onset atrial fibrillation during liver transplantation surgery or within 30 d after this procedure in a patient without previous episodes. This phenomenon can drive to hemodynamic and thromboembolic events, significantly affecting the prognosis of the patients in the early post-liver transplantation[115]. Although the impact of these early events on the long-term cardiovascular prognosis has not been explored in detail, a recent retrospective study in over 1000 liver transplant patients found that the development of postoperative atrial fibrillation is an independent risk factor for post-liver transplant mortality (OR = 2.0; 95%CI: 1.3-3.0; P < 0.01)[116]. Furthermore, as might be expected, NASH as an indication for liver transplantation had a significantly higher risk of a cardiovascular event 1 and 3 years after liver transplant. Even with a relatively low prevalence, major cardiac events are significantly associated with a lower 5-year survival rate, thus stressing the importance of identifying and stratifying high-risk patients prior to liver transplantation and offering targeted postoperative interventions. A study of Patel et al[117] has recently shown that despite that the presence and severity of pre-transplantation coronary artery disease may not affect post transplantation survival, the use of statins in the post-transplantation period might confer a survival benefit (HR = 0.25; 95%CI: 0.12-0.49; P < 0.001). This is independent of the use of aspirin, which did not show an effect on mortality. Nonetheless, the study highlighted that statin therapy is still very much underused, with only 46% of patients with proven coronary artery disease benefitting from this therapy. The medication was well tolerated (only 12% of recipients needed to stop the therapy due to side effects)[117], suggesting a promising role of statins in improving the outcomes after liver transplantation.
The proper identification of liver transplant recipients at risk of metabolic and cardiovascular morbidity and mortality after liver transplantation is still far from being satisfactory. Literature devoted to this topic is scarce and often of low quality, making it difficult to provide recommendations or to develop appropriate guidelines. Moreover, the efficacy and safety of the current treatment strategies for these metabolic complications needs to be validated in this specific population, as well as finding adequate surrogates which can be considered suitable targets to impact on the prognosis of liver transplant recipients.
However, as survival rate after liver transplantation increases, it seems clear that the management of metabolic complications and cardiovascular disease requires heightened attention. These comorbidities have a major impact on the morbidity, mortality and quality of life of liver transplant recipients in the late postoperative period. Early identification and proper management of these metabolic alterations, initially acting on lifestyle modifications, immunosuppression titration, and tailored medical therapy remain crucial to improve the outcome of liver transplant patients.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Switzerland
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ciccone MM, Cimen SG S-Editor: Yang Y L-Editor: A E-Editor: Zhang YL
| 1. | Merion RM, Schaubel DE, Dykstra DM, Freeman RB, Port FK, Wolfe RA. The survival benefit of liver transplantation. Am J Transplant. 2005;5:307-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 626] [Cited by in RCA: 617] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 2. | Merion RM. Current status and future of liver transplantation. Semin Liver Dis. 2010;30:411-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 3. | Durand F. How to improve long-term outcome after liver transplantation? Liver Int. 2018;38 Suppl 1:134-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 4. | Adam R, Karam V, Cailliez V, O Grady JG, Mirza D, Cherqui D, Klempnauer J, Salizzoni M, Pratschke J, Jamieson N, Hidalgo E, Paul A, Andujar RL, Lerut J, Fisher L, Boudjema K, Fondevila C, Soubrane O, Bachellier P, Pinna AD, Berlakovich G, Bennet W, Pinzani M, Schemmer P, Zieniewicz K, Romero CJ, De Simone P, Ericzon BG, Schneeberger S, Wigmore SJ, Prous JF, Colledan M, Porte RJ, Yilmaz S, Azoulay D, Pirenne J, Line PD, Trunecka P, Navarro F, Lopez AV, De Carlis L, Pena SR, Kochs E, Duvoux C; all the other 126 contributing centers (www. eltr.org) and the European Liver and Intestine Transplant Association (ELITA). 2018 Annual Report of the European Liver Transplant Registry (ELTR) - 50-year evolution of liver transplantation. Transpl Int. 2018;31:1293-1317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 331] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 5. | Kim WR, Lake JR, Smith JM, Schladt DP, Skeans MA, Harper AM, Wainright JL, Snyder JJ, Israni AK, Kasiske BL. OPTN/SRTR 2016 Annual Data Report: Liver. Am J Transplant. 2018;18 Suppl 1:172-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 301] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 6. | Rabkin JM, de La Melena V, Orloff SL, Corless CL, Rosen HR, Olyaei AJ. Late mortality after orthotopic liver transplantation. Am J Surg. 2001;181:475-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 84] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Watt KD, Pedersen RA, Kremers WK, Heimbach JK, Charlton MR. Evolution of causes and risk factors for mortality post-liver transplant: results of the NIDDK long-term follow-up study. Am J Transplant. 2010;10:1420-1427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 617] [Cited by in RCA: 586] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 8. | Aberg F, Jula A, Höckerstedt K, Isoniemi H. Cardiovascular risk profile of patients with acute liver failure after liver transplantation when compared with the general population. Transplantation. 2010;89:61-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 9. | Di Stefano C, Vanni E, Mirabella S, Younes R, Boano V, Mosso E, Nada E, Milazzo V, Maule S, Romagnoli R, Salizzoni M, Veglio F, Milan A. Risk factors for arterial hypertension after liver transplantation. J Am Soc Hypertens. 2018;12:220-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Prokai A, Fekete A, Pasti K, Rusai K, Banki NF, Reusz G, Szabo AJ. The importance of different immunosuppressive regimens in the development of posttransplant diabetes mellitus. Pediatr Diabetes. 2012;13:81-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Li Y, Pan A, Wang DD, Liu X, Dhana K, Franco OH, Kaptoge S, Di Angelantonio E, Stampfer M, Willett WC, Hu FB. Impact of Healthy Lifestyle Factors on Life Expectancies in the US Population. Circulation. 2018;138:345-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 500] [Cited by in RCA: 505] [Article Influence: 72.1] [Reference Citation Analysis (0)] |
| 12. | Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC Jr, Spertus JA, Fernando Costa. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement: Executive Summary. Crit Pathw Cardiol. 2005;4:198-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 374] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 13. | Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 115] [Reference Citation Analysis (0)] |
| 14. | Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20476] [Cited by in RCA: 20683] [Article Influence: 861.8] [Reference Citation Analysis (2)] |
| 15. | Zimmet P, M M Alberti KG, Serrano Ríos M. [A new international diabetes federation worldwide definition of the metabolic syndrome: the rationale and the results]. Rev Esp Cardiol. 2005;58:1371-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Thoefner LB, Rostved AA, Pommergaard HC, Rasmussen A. Risk factors for metabolic syndrome after liver transplantation: A systematic review and meta-analysis. Transplant Rev (Orlando). 2018;32:69-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 17. | McLaughlin T, Allison G, Abbasi F, Lamendola C, Reaven G. Prevalence of insulin resistance and associated cardiovascular disease risk factors among normal weight, overweight, and obese individuals. Metabolism. 2004;53:495-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 177] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 18. | Bianchi G, Marchesini G, Marzocchi R, Pinna AD, Zoli M. Metabolic syndrome in liver transplantation: relation to etiology and immunosuppression. Liver Transpl. 2008;14:1648-1654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 189] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 19. | Laish I, Braun M, Mor E, Sulkes J, Harif Y, Ben Ari Z. Metabolic syndrome in liver transplant recipients: prevalence, risk factors, and association with cardiovascular events. Liver Transpl. 2011;17:15-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 186] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 20. | Watt KD. Metabolic syndrome: is immunosuppression to blame? Liver Transpl. 2011;17 Suppl 3:S38-S42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 21. | Watt KD, Charlton MR. Metabolic syndrome and liver transplantation: a review and guide to management. J Hepatol. 2010;53:199-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 164] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 22. | Li J, Flammer AJ, Lennon RJ, Nelson RE, Gulati R, Friedman PA, Thomas RJ, Sandhu NP, Hua Q, Lerman LO, Lerman A. Comparison of the effect of the metabolic syndrome and multiple traditional cardiovascular risk factors on vascular function. Mayo Clin Proc. 2012;87:968-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Dumortier J, Giostra E, Belbouab S, Morard I, Guillaud O, Spahr L, Boillot O, Rubbia-Brandt L, Scoazec JY, Hadengue A. Non-alcoholic fatty liver disease in liver transplant recipients: another story of "seed and soil". Am J Gastroenterol. 2010;105:613-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 163] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 24. | Narayanan P, Mara K, Izzy M, Dierkhising R, Heimbach J, Allen AM, Watt KD. Recurrent or De Novo Allograft Steatosis and Long-term Outcomes After Liver Transplantation. Transplantation. 2019;103:e14-e21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 25. | Germani G, Becchetti C. Liver transplantation for non-alcoholic fatty liver disease. Minerva Gastroenterol Dietol. 2018;64:138-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Dureja P, Mellinger J, Agni R, Chang F, Avey G, Lucey M, Said A. NAFLD recurrence in liver transplant recipients. Transplantation. 2011;91:684-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 127] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 27. | Malik SM, Devera ME, Fontes P, Shaikh O, Sasatomi E, Ahmad J. Recurrent disease following liver transplantation for nonalcoholic steatohepatitis cirrhosis. Liver Transpl. 2009;15:1843-1851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 28. | Watt KD, Dierkhising R, Fan C, Heimbach JK, Tillman H, Goldstein D, Thompson A, Krishnan A, Charlton MR. Investigation of PNPLA3 and IL28B genotypes on diabetes and obesity after liver transplantation: insight into mechanisms of disease. Am J Transplant. 2013;13:2450-2457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 29. | Baid S, Cosimi AB, Farrell ML, Schoenfeld DA, Feng S, Chung RT, Tolkoff-Rubin N, Pascual M. Posttransplant diabetes mellitus in liver transplant recipients: risk factors, temporal relationship with hepatitis C virus allograft hepatitis, and impact on mortality. Transplantation. 2001;72:1066-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 215] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 30. | Ling Q, Xu X, Xie H, Wang K, Xiang P, Zhuang R, Shen T, Wu J, Wang W, Zheng S. New-onset diabetes after liver transplantation: a national report from China Liver Transplant Registry. Liver Int. 2016;36:705-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 31. | Ramos-Prol A, Hervás-Marín D, García-Castell A, Merino-Torres JF. Outcomes in patients with diabetes 10 years after liver transplantation. J Diabetes. 2017;9:1033-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 32. | Bhat V, Tazari M, Watt KD, Bhat M. New-Onset Diabetes and Preexisting Diabetes Are Associated With Comparable Reduction in Long-Term Survival After Liver Transplant: A Machine Learning Approach. Mayo Clin Proc. 2018;93:1794-1802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 33. | Bodziak KA, Hricik DE. New-onset diabetes mellitus after solid organ transplantation. Transpl Int. 2009;22:519-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 34. | Saab S, Shpaner A, Zhao Y, Brito I, Durazo F, Han S, Farmer DG, Ghobrial RM, Yersiz H, Goldstein LI, Tong MJ, Busuttil RW. Prevalence and risk factors for diabetes mellitus in moderate term survivors of liver transplantation. Am J Transplant. 2006;6:1890-1895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 35. | Davidson JA, Wilkinson A; International Expert Panel on New-Onset Diabetes after Transplantation. New-Onset Diabetes After Transplantation 2003 International Consensus Guidelines: an endocrinologist's view. Diabetes Care. 2004;27:805-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 126] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 36. | Delgado-Borrego A, Casson D, Schoenfeld D, Somsouk M, Terella A, Jordan SH, Bhan A, Baid S, Cosimi AB, Pascual M, Chung RT. Hepatitis C virus is independently associated with increased insulin resistance after liver transplantation. Transplantation. 2004;77:703-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 98] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 37. | Hjelmesaeth J, Hartmann A, Kofstad J, Stenstrøm J, Leivestad T, Egeland T, Fauchald P. Glucose intolerance after renal transplantation depends upon prednisolone dose and recipient age. Transplantation. 1997;64:979-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 229] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 38. | Diabetes Control and Complications Trial Research Group; Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, Rand L, Siebert C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17510] [Cited by in RCA: 16273] [Article Influence: 508.5] [Reference Citation Analysis (3)] |
| 39. | Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14327] [Cited by in RCA: 12665] [Article Influence: 469.1] [Reference Citation Analysis (0)] |
| 40. | Wilkinson A, Davidson J, Dotta F, Home PD, Keown P, Kiberd B, Jardine A, Levitt N, Marchetti P, Markell M, Naicker S, O'Connell P, Schnitzler M, Standl E, Torregosa JV, Uchida K, Valantine H, Villamil F, Vincenti F, Wissing M. Guidelines for the treatment and management of new-onset diabetes after transplantation. Clin Transplant. 2005;19:291-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 168] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 41. | Sharif A, Hecking M, de Vries AP, Porrini E, Hornum M, Rasoul-Rockenschaub S, Berlakovich G, Krebs M, Kautzky-Willer A, Schernthaner G, Marchetti P, Pacini G, Ojo A, Takahara S, Larsen JL, Budde K, Eller K, Pascual J, Jardine A, Bakker SJ, Valderhaug TG, Jenssen TG, Cohney S, Säemann MD. Proceedings from an international consensus meeting on posttransplantation diabetes mellitus: recommendations and future directions. Am J Transplant. 2014;14:1992-2000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 392] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 42. | Peláez-Jaramillo MJ, Cárdenas-Mojica AA, Gaete PV, Mendivil CO. Post-Liver Transplantation Diabetes Mellitus: A Review of Relevance and Approach to Treatment. Diabetes Ther. 2018;9:521-543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 43. | Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC); European Association for the Study of Diabetes (EASD); Rydén L, Grant PJ, Anker SD, Berne C, Cosentino F, Danchin N, Deaton C, Escaned J, Hammes HP, Huikuri H, Marre M, Marx N, Mellbin L, Ostergren J, Patrono C, Seferovic P, Uva MS, Taskinen MR, Tendera M, Tuomilehto J, Valensi P, Zamorano JL. ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD - summary. Diab Vasc Dis Res. 2014;11:133-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 145] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 44. | Marchetti P. New-onset diabetes after liver transplantation: from pathogenesis to management. Liver Transpl. 2005;11:612-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 45. | Villanueva G, Baldwin D. Rosiglitazone therapy of posttransplant diabetes mellitus. Transplantation. 2005;80:1402-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 46. | Luther P, Baldwin D. Pioglitazone in the management of diabetes mellitus after transplantation. Am J Transplant. 2004;4:2135-2138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 47. | Cusi K, Orsak B, Bril F, Lomonaco R, Hecht J, Ortiz-Lopez C, Tio F, Hardies J, Darland C, Musi N, Webb A, Portillo-Sanchez P. Long-Term Pioglitazone Treatment for Patients With Nonalcoholic Steatohepatitis and Prediabetes or Type 2 Diabetes Mellitus: A Randomized Trial. Ann Intern Med. 2016;165:305-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 736] [Article Influence: 81.8] [Reference Citation Analysis (0)] |
| 48. | Vanhove T, Remijsen Q, Kuypers D, Gillard P. Drug-drug interactions between immunosuppressants and antidiabetic drugs in the treatment of post-transplant diabetes mellitus. Transplant Rev (Orlando). 2017;31:69-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 49. | Bhat M, Pasini E, Das A, Baciu C, Angeli M, Humar A, Watt KD, Allard J. Diabetogenic Effects of Immunosuppression: An Integrative Analysis. Transplantation. 2020;104:211-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 50. | Grancini V, Resi V, Palmieri E, Pugliese G, Orsi E. Management of diabetes mellitus in patients undergoing liver transplantation. Pharmacol Res. 2019;141:556-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 51. | Bae J, Lee MJ, Choe EY, Jung CH, Wang HJ, Kim MS, Kim YS, Park JY, Kang ES. Effects of Dipeptidyl Peptidase-4 Inhibitors on Hyperglycemia and Blood Cyclosporine Levels in Renal Transplant Patients with Diabetes: A Pilot Study. Endocrinol Metab (Seoul). 2016;31:161-167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 52. | Sanyal D, Gupta S, Das P. A retrospective study evaluating efficacy and safety of linagliptin in treatment of NODAT (in renal transplant recipients) in a real world setting. Indian J Endocrinol Metab. 2013;17:S203-S205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 53. | Puckrin R, Saltiel MP, Reynier P, Azoulay L, Yu OHY, Filion KB. SGLT-2 inhibitors and the risk of infections: a systematic review and meta-analysis of randomized controlled trials. Acta Diabetol. 2018;55:503-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 162] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 54. | Devineni D, Vaccaro N, Murphy J, Curtin C, Mamidi RN, Weiner S, Wang SS, Ariyawansa J, Stieltjes H, Wajs E, Di Prospero NA, Rothenberg P. Effects of rifampin, cyclosporine A, and probenecid on the pharmacokinetic profile of canagliflozin, a sodium glucose co-transporter 2 inhibitor, in healthy participants. Int J Clin Pharmacol Ther. 2015;53:115-128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 55. | Perseghin G, Mazzaferro V, Sereni LP, Regalia E, Benedini S, Bazzigaluppi E, Pulvirenti A, Leão AA, Calori G, Romito R, Baratti D, Luzi L. Contribution of reduced insulin sensitivity and secretion to the pathogenesis of hepatogenous diabetes: effect of liver transplantation. Hepatology. 2000;31:694-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 102] [Article Influence: 4.1] [Reference Citation Analysis (2)] |
| 56. | Grancini V, Trombetta M, Lunati ME, Boselli ML, Gatti S, Donato MF, Palmieri E, Resi V, Pugliese G, Bonadonna RC, Orsi E. Central role of the β-cell in driving regression of diabetes after liver transplantation in cirrhotic patients. J Hepatol. 2019;70:954-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 57. | Davidson J, Wilkinson A, Dantal J, Dotta F, Haller H, Hernández D, Kasiske BL, Kiberd B, Krentz A, Legendre C, Marchetti P, Markell M, van der Woude FJ, Wheeler DC; International Expert Panel. New-onset diabetes after transplantation: 2003 International consensus guidelines. Proceedings of an international expert panel meeting. Barcelona, Spain, 19 February 2003. Transplantation. 2003;75:SS3-S24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 357] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 58. | Weir CB, Jan A. BMI Classification Percentile And Cut Off Points. StatPearls. Treasure Island: StatPearls Publishing 2020; . [PubMed] |
| 59. | Nair S, Verma S, Thuluvath PJ. Obesity and its effect on survival in patients undergoing orthotopic liver transplantation in the United States. Hepatology. 2002;35:105-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 289] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 60. | Richards J, Gunson B, Johnson J, Neuberger J. Weight gain and obesity after liver transplantation. Transpl Int. 2005;18:461-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 223] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 61. | Everhart JE, Lombardero M, Lake JR, Wiesner RH, Zetterman RK, Hoofnagle JH. Weight change and obesity after liver transplantation: incidence and risk factors. Liver Transpl Surg. 1998;4:285-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 142] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 62. | Charlton M, Rinella M, Patel D, McCague K, Heimbach J, Watt K. Everolimus Is Associated With Less Weight Gain Than Tacrolimus 2 Years After Liver Transplantation: Results of a Randomized Multicenter Study. Transplantation. 2017;101:2873-2882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 63. | Neuberger J, Armstrong MJ, Fisher J, Mark P, Schmidtke K, Sharif A, Vlaev I. Sport and Exercise in Improving Outcomes After Solid Organ Transplantation: Overview From a UK Meeting. Transplantation. 2019;103:S1-S11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 64. | Totti V, Tamè M, Burra P, Mosconi G, Roi GS, Sella G, Ermolao A, Ferrarese A, Sgarzi S, Savino G, Parodi G, Poggioli G, Ricchiuti A, Di Michele R, Trerotola M, Nanni Costa A. Physical Condition, Glycemia, Liver Function, and Quality of Life in Liver Transplant Recipients After a 12-Month Supervised Exercise Program. Transplant Proc. 2019;51:2952-2957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 65. | Anastácio LR, Ferreira SC. Nutrition, dietary intake, and eating behavior after liver transplantation. Curr Opin Clin Nutr Metab Care. 2018;21:381-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 66. | Cassiman D, Roelants M, Vandenplas G, Van der Merwe SW, Mertens A, Libbrecht L, Verslype C, Fevery J, Aerts R, Pirenne J, Muls E, Nevens F. Orlistat treatment is safe in overweight and obese liver transplant recipients: a prospective, open label trial. Transpl Int. 2006;19:1000-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 67. | Nuffer WA, Trujillo JM. Liraglutide: A New Option for the Treatment of Obesity. Pharmacotherapy. 2015;35:926-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 97] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 68. | Suraweera D, Dutson E, Saab S. Liver Transplantation and Bariatric Surgery: Best Approach. Clin Liver Dis. 2017;21:215-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 69. | Goitein D, Raziel A, Szold A, Sakran N. Assessment of perioperative complications following primary bariatric surgery according to the Clavien-Dindo classification: comparison of sleeve gastrectomy and Roux-Y gastric bypass. Surg Endosc. 2016;30:273-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 70. | Diwan TS, Rice TC, Heimbach JK, Schauer DP. Liver Transplantation and Bariatric Surgery: Timing and Outcomes. Liver Transpl. 2018;24:1280-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 71. | Al-Nowaylati AR, Al-Haddad BJ, Dorman RB, Alsaied OA, Lake JR, Chinnakotla S, Slusarek BM, Sampson BK, Ikramuddin S, Buchwald H, Leslie DB. Gastric bypass after liver transplantation. Liver Transpl. 2013;19:1324-1329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 72. | Diwan TS, Lichvar AB, Leino AD, Vinks AA, Christians U, Shields AR, Cardi MA, Fukuda T, Mizuno T, Kaiser T, Woodle ES, Alloway RR. Pharmacokinetic and pharmacogenetic analysis of immunosuppressive agents after laparoscopic sleeve gastrectomy. Clin Transplant. 2017;31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 73. | Rogers CC, Alloway RR, Alexander JW, Cardi M, Trofe J, Vinks AA. Pharmacokinetics of mycophenolic acid, tacrolimus and sirolimus after gastric bypass surgery in end-stage renal disease and transplant patients: a pilot study. Clin Transplant. 2008;22:281-291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 153] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 74. | Sheiner PA, Magliocca JF, Bodian CA, Kim-Schluger L, Altaca G, Guarrera JV, Emre S, Fishbein TM, Guy SR, Schwartz ME, Miller CM. Long-term medical complications in patients surviving > or = 5 years after liver transplant. Transplantation. 2000;69:781-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 194] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 75. | Ling Q, Wang K, Lu D, Guo HJ, Jiang WS, He XX, Xu X, Zheng SS. Major influence of renal function on hyperlipidemia after living donor liver transplantation. World J Gastroenterol. 2012;18:7033-7039. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 76. | Singh S, Watt KD. Long-term medical management of the liver transplant recipient: what the primary care physician needs to know. Mayo Clin Proc. 2012;87:779-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 77. | Stegall MD, Everson GT, Schroter G, Karrer F, Bilir B, Sternberg T, Shrestha R, Wachs M, Kam I. Prednisone withdrawal late after adult liver transplantation reduces diabetes, hypertension, and hypercholesterolemia without causing graft loss. Hepatology. 1997;25:173-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 106] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 78. | Clark W. Tacrolimus: immunosuppression following liver and kidney transplant. J Clin Pharm Ther. 1996;21:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 79. | Trotter JF, Wachs ME, Trouillot TE, Bak T, Kugelmas M, Kam I, Everson G. Dyslipidemia during sirolimus therapy in liver transplant recipients occurs with concomitant cyclosporine but not tacrolimus. Liver Transpl. 2001;7:401-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 66] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 80. | Morrisett JD, Abdel-Fattah G, Hoogeveen R, Mitchell E, Ballantyne CM, Pownall HJ, Opekun AR, Jaffe JS, Oppermann S, Kahan BD. Effects of sirolimus on plasma lipids, lipoprotein levels, and fatty acid metabolism in renal transplant patients. J Lipid Res. 2002;43:1170-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 215] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 81. | Neal DA, Gimson AE, Gibbs P, Alexander GJ. Beneficial effects of converting liver transplant recipients from cyclosporine to tacrolimus on blood pressure, serum lipids, and weight. Liver Transpl. 2001;7:533-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 82. | Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, Graham IM, Halliday A, Landmesser U, Mihaylova B, Pedersen TR, Riccardi G, Richter DJ, Sabatine MS, Taskinen MR, Tokgozoglu L, Wiklund O; ESC Scientific Document Group. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6024] [Cited by in RCA: 5318] [Article Influence: 1063.6] [Reference Citation Analysis (0)] |
| 83. | Martin JE, Cavanaugh TM, Trumbull L, Bass M, Weber F, Aranda-Michel J, Hanaway M, Rudich S. Incidence of adverse events with HMG-CoA reductase inhibitors in liver transplant patients. Clin Transplant. 2008;22:113-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 84. | Albekairy A, Alkatheri A, Fujita S, Hemming A, Howard R, Reed A, Karlix J. Cytochrome P450 3A4FNx011B as pharmacogenomic predictor of tacrolimus pharmacokinetics and clinical outcome in the liver transplant recipients. Saudi J Gastroenterol. 2013;19:89-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 85. | Almutairi F, Peterson TC, Molinari M, Walsh MJ, Alwayn I, Peltekian KM. Safety and effectiveness of ezetimibe in liver transplant recipients with hypercholesterolemia. Liver Transpl. 2009;15:504-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 86. | Lee S, Gura KM, Puder M. Omega-3 fatty acids and liver disease. Hepatology. 2007;45:841-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 87. | Barnard A, Konyn P, Saab S. Medical Management of Metabolic Complications of Liver Transplant Recipients. Gastroenterol Hepatol (N Y). 2016;12:601-608. [PubMed] |
| 88. | Fryar CD, Ostchega Y, Hales CM, Zhang G, Kruszon-Moran D. Hypertension Prevalence and Control Among Adults: United States, 2015-2016. NCHS Data Brief. 2017;1-8. [PubMed] |
| 89. | Parekh J, Corley DA, Feng S. Diabetes, hypertension and hyperlipidemia: prevalence over time and impact on long-term survival after liver transplantation. Am J Transplant. 2012;12:2181-2187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 90. | Gojowy D, Adamczak M, Dudzicz S, Gazda M, Karkoszka H, Wiecek A. High Frequency of Arterial Hypertension in Patients After Liver Transplantation. Transplant Proc. 2016;48:1721-1724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 91. | Hernández D, Alvarez A, Torres A, Oppenheimer F, Cobo M, González-Posada J, Jiménez A, Lorenzo V, Torregrosa V. Cardiovascular risk profile in nondiabetic renal transplant patients: cyclosporine versus tacrolimus. Transplant Proc. 2003;35:1727-1729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 92. | Saruta T. Mechanism of glucocorticoid-induced hypertension. Hypertens Res. 1996;19:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 91] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 93. | Fernández-Miranda C, Sanz M, dela Calle A, Loinaz C, Gómez R, Jiménez C, García I, Gómez de la Cámara A, Moreno E. Cardiovascular risk factors in 116 patients 5 years or more after liver transplantation. Transpl Int. 2002;15:556-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 94. | Issa DH, Alkhouri N. Long-term management of liver transplant recipients: A review for the internist. Cleve Clin J Med. 2015;82:361-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 95. | Guckelberger O. Long-term medical comorbidities and their management: hypertension/cardiovascular disease. Liver Transpl. 2009;15 Suppl 2:S75-S78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 96. | Galioto A, Semplicini A, Zanus G, Fasolato S, Sticca A, Boccagni P, Frigo AC, Cillo U, Gatta A, Angeli P. Nifedipine versus carvedilol in the treatment of de novo arterial hypertension after liver transplantation: results of a controlled clinical trial. Liver Transpl. 2008;14:1020-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 97. | Fussner LA, Heimbach JK, Fan C, Dierkhising R, Coss E, Leise MD, Watt KD. Cardiovascular disease after liver transplantation: When, What, and Who Is at Risk. Liver Transpl. 2015;21:889-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 148] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 98. | Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, Arndorfer J, Christensen L, Merion RM. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349:931-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1703] [Cited by in RCA: 1637] [Article Influence: 74.4] [Reference Citation Analysis (0)] |
| 99. | Rymarz A, Serwacki M, Rutkowski M, Pakosiński K, Grodzicki M, Patkowski W, Kacka A, Ołdakowska-Jedynak U, Krawczyk M. Prevalence and predictors of acute renal injury in liver transplant recipients. Transplant Proc. 2009;41:3123-3125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 100. | Umbro I, Tinti F, Scalera I, Evison F, Gunson B, Sharif A, Ferguson J, Muiesan P, Mitterhofer AP. Acute kidney injury and post-reperfusion syndrome in liver transplantation. World J Gastroenterol. 2016;22:9314-9323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 101. | Leithead JA, Rajoriya N, Gunson BK, Muiesan P, Ferguson JW. The evolving use of higher risk grafts is associated with an increased incidence of acute kidney injury after liver transplantation. J Hepatol. 2014;60:1180-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 102. | Hošková L, Málek I, Kopkan L, Kautzner J. Pathophysiological mechanisms of calcineurin inhibitor-induced nephrotoxicity and arterial hypertension. Physiol Res. 2017;66:167-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 97] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 103. | Lin M, Mittal S, Sahebjam F, Rana A, Sood GK. Everolimus with early withdrawal or reduced-dose calcineurin inhibitors improves renal function in liver transplant recipients: A systematic review and meta-analysis. Clin Transplant. 2017;31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 104. | VanWagner LB, Montag S, Zhao L, Allen NB, Lloyd-Jones DM, Das A, Skaro AI, Hohmann S, Friedewald JJ, Levitsky J. Cardiovascular Disease Outcomes Related to Early Stage Renal Impairment After Liver Transplantation. Transplantation. 2018;102:1096-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 105. | Josefsson A, Fu M, Björnsson E, Castedal M, Kalaitzakis E. Pre-transplant renal impairment predicts posttransplant cardiac events in patients with liver cirrhosis. Transplantation. 2014;98:107-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 106. | Carey WD, Dumot JA, Pimentel RR, Barnes DS, Hobbs RE, Henderson JM, Vogt DP, Mayes JT, Westveer MK, Easley KA. The prevalence of coronary artery disease in liver transplant candidates over age 50. Transplantation. 1995;59:859-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 183] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 107. | Guillaud O, Boillot O, Sebbag L, Walter T, Bouffard Y, Dumortier J. Cardiovascular risk 10 years after liver transplant. Exp Clin Transplant. 2014;12:55-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 108. | Raval Z, Harinstein ME, Skaro AI, Erdogan A, DeWolf AM, Shah SJ, Fix OK, Kay N, Abecassis MI, Gheorghiade M, Flaherty JD. Cardiovascular risk assessment of the liver transplant candidate. J Am Coll Cardiol. 2011;58:223-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 178] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 109. | Soldera J, Camazzola F, Rodríguez S, Brandão A. Cardiac stress testing and coronary artery disease in liver transplantation candidates: Meta-analysis. World J Hepatol. 2018;10:877-886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (2)] |
| 110. | Patel SS, Nabi E, Guzman L, Abbate A, Bhati C, Stravitz RT, Reichman T, Matherly SC, Driscoll C, Lee H, Luketic VA, Sterling RK, Sanyal AJ, Patel V, Levy M, Siddiqui MS. Coronary artery disease in decompensated patients undergoing liver transplantation evaluation. Liver Transpl. 2018;24:333-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 111. | Romero-Cristóbal M, Mombiela T, Caballero A, Clemente A, Fernández-Yunquera A, Diaz-Fontenla F, Rincón D, Ripoll C, Bermejo J, Catalina MV, Matilla AM, Ibáñez-Samaniego L, Pérez-Peña J, López-Baena JÁ, Díaz-Zorita B, Fernández-Avilés F, Salcedo MM, Bañares R. Clinical Utility of a Risk-Adapted Protocol for the Evaluation of Coronary Artery Disease in Liver Transplant Recipients. Liver Transpl. 2019;25:1177-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 112. | Satapathy SK, Vanatta JM, Helmick RA, Flowers A, Kedia SK, Jiang Y, Ali B, Eason J, Nair SP, Ibebuogu UN. Outcome of Liver Transplant Recipients With Revascularized Coronary Artery Disease: A Comparative Analysis With and Without Cardiovascular Risk Factors. Transplantation. 2017;101:793-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 113. | Dec GW, Kondo N, Farrell ML, Dienstag J, Cosimi AB, Semigran MJ. Cardiovascular complications following liver transplantation. Clin Transplant. 1995;9:463-471. [PubMed] |
| 114. | Liu H, Jayakumar S, Traboulsi M, Lee SS. Cirrhotic cardiomyopathy: Implications for liver transplantation. Liver Transpl. 2017;23:826-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 94] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 115. | Xia VW, Worapot A, Huang S, Dhillon A, Gudzenko V, Backon A, Agopian VG, Aksoy O, Vorobiof G, Busuttil RW, Steadman RH. Postoperative atrial fibrillation in liver transplantation. Am J Transplant. 2015;15:687-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 116. | Rachwan RJ, Kutkut I, Hathaway TJ, Timsina LR, Kubal CA, Lacerda MA, Ghabril MS, Bourdillon PD, Mangus RS. Postoperative Atrial Fibrillation and Flutter in Liver Transplantation: An Important Predictor of Early and Late Morbidity and Mortality. Liver Transpl. 2020;26:34-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 117. | Patel SS, Rodriguez VA, Siddiqui MB, Faridnia M, Lin FP, Chandrakumaran A, Laurenzano J, Clinton J, Kowlgi GN, Kirkman D, Sima AP, Liptrap E, Bhati C, Siddiqui MS. The Impact of Coronary Artery Disease and Statins on Survival After Liver Transplantation. Liver Transpl. 2019;25:1514-1523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 118. | Authors/Task Force Members; ESC Committee for Practice Guidelines (CPG); ESC National Cardiac Societies. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Atherosclerosis. 2019;290:140-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 729] [Cited by in RCA: 684] [Article Influence: 114.0] [Reference Citation Analysis (0)] |
| 119. | Duchini A, Brunson ME. Roux-en-Y gastric bypass for recurrent nonalcoholic steatohepatitis in liver transplant recipients with morbid obesity. Transplantation. 2001;72:156-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 62] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 120. | Tichansky DS, Madan AK. Laparoscopic Roux-en-Y gastric bypass is safe and feasible after orthotopic liver transplantation. Obes Surg. 2005;15:1481-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 121. | Butte JM, Devaud N, Jarufe NP, Boza C, Pérez G, Torres J, Pérez-Ayuso RM, Arrese M, Martínez J. Sleeve gastrectomy as treatment for severe obesity after orthotopic liver transplantation. Obes Surg. 2007;17:1517-1519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 122. | Gentileschi P, Venza M, Benavoli D, Lirosi F, Camperchioli I, D'Eletto M, Lazzaro A, Stolfi VM, Anselmo A, Di Lorenzo N, Tisone G, Gaspari AL. Intragastric balloon followed by biliopancreatic diversion in a liver transplant recipient: a case report. Obes Surg. 2009;19:1460-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 123. | Elli EF, Masrur MA, Giulianotti PC. Robotic sleeve gastrectomy after liver transplantation. Surg Obes Relat Dis. 2013;9:e20-e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 124. | Lin MY, Tavakol MM, Sarin A, Amirkiai SM, Rogers SJ, Carter JT, Posselt AM. Safety and feasibility of sleeve gastrectomy in morbidly obese patients following liver transplantation. Surg Endosc. 2013;27:81-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 125. | Pajecki D, Cesconetto DM, Macacari R, Joaquim H, Andraus W, de Cleva R, Santo MA, D'Albuquerque LA, Cecconello I. Bariatric surgery (sleeve gastrectomy) after liver transplantation: case report. Arq Bras Cir Dig. 2014;27 Suppl 1:81-83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 126. | Elli EF, Gonzalez-Heredia R, Sanchez-Johnsen L, Patel N, Garcia-Roca R, Oberholzer J. Sleeve gastrectomy surgery in obese patients post-organ transplantation. Surg Obes Relat Dis. 2016;12:528-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 127. | Khoraki J, Katz MG, Funk LM, Greenberg JA, Fernandez LA, Campos GM. Feasibility and outcomes of laparoscopic sleeve gastrectomy after solid organ transplantation. Surg Obes Relat Dis. 2016;12:75-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 128. | Osseis M, Lazzati A, Salloum C, Gavara CG, Compagnon P, Feray C, Lim C, Azoulay D. Sleeve Gastrectomy After Liver Transplantation: Feasibility and Outcomes. Obes Surg. 2018;28:242-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 129. | Tsamalaidze L, Stauffer JA, Arasi LC, Villacreses DE, Franco JSS, Bowers S, Elli EF. Laparoscopic Sleeve Gastrectomy for Morbid Obesity in Patients After Orthotopic Liver Transplant: a Matched Case-Control Study. Obes Surg. 2018;28:444-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |