Published online May 14, 2020. doi: 10.3748/wjg.v26.i18.2126
Peer-review started: December 31, 2019
First decision: March 18, 2020
Revised: April 8, 2020
Accepted: April 21, 2020
Article in press: April 21, 2020
Published online: May 14, 2020
Processing time: 134 Days and 12.2 Hours
Hepatocellular carcinoma (HCC) is the most common primary liver cancer with a dismal prognosis, especially when diagnosed at advanced stages. Annexin A2 (ANXA2), is found to promote cancer progression and therapeutic resistance. However, the underlining mechanisms of ANXA2 in immune escape of HCC remain poorly understood up to now. Herein, we summarized the molecular function of ANXA2 in HCC and its relationship with prognosis. Furthermore, we tentatively elucidated the underlying mechanism of ANXA2 immune escape of HCC by upregulating the proportion of regulatory T cells and the expression of several inhibitory molecules, and by downregulating the proportion of natural killer cells and dendritic cells and the expression of several inhibitory molecules or effector molecules. We expect a lot of in-depth studies to further reveal the underlying mechanism of ANXA2 in immune escape of HCC in the future.
Core tip: Annexin A2 (ANXA2) has been found to promote cancer progression and therapeutic resistance in patients with hepatocellular carcinoma. However, the mechanism by which annexin A2 facilitates the immune escape of hepatocellular carcinoma remains poorly understood. In this opinion review, we discuss in detail the latest findings on the role of annexin A2 in hepatocellular carcinoma immune escape.
- Citation: Qiu LW, Liu YF, Cao XQ, Wang Y, Cui XH, Ye X, Huang SW, Xie HJ, Zhang HJ. Annexin A2 promotion of hepatocellular carcinoma tumorigenesis via the immune microenvironment. World J Gastroenterol 2020; 26(18): 2126-2137
- URL: https://www.wjgnet.com/1007-9327/full/v26/i18/2126.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i18.2126
As an aggressive malignancy, liver cancer is the fifth leading cause of death from cancer globally[1]. Hepatocellular carcinoma (HCC), the most common type of primary liver cancer, has a poor prognosis, especially when diagnosed at the advanced stages[2]. One of the leading risk factors for HCC is infection with the hepatitis B virus, particularly in East Asia[3]. Although surgical treatment for HCC may be effective in the early stages, the 5-year overall survival (OS) rate is only 50%-70%[4,5]. According to a recent study based on proteomic and phosphoproteomic profiling, early-stage HCC can be further stratified into three subtypes with different clinical outcomes[6]. The third subtype, which is characterized by disrupted cholesterol homeostasis, is associated with the lowest postoperative OS rate and the greatest risk of a poor prognosis[7,8]. Despite global advancement of social development and implementation of the annual physical examination program to increase the diagnosis of patients with early-stage HCC, the proportion of patients with advanced HCC at first diagnosis remains high[9]. Although first-line drugs such as sorafenib and lovatinib were used in the treatment of advanced HCC, the landscape of advanced HCC management was not optimistic until the advent of immunotherapy and the knowledge gained about the molecular pathogenesis of the disease[2,10,11].
HCC is considered to be an immunogenic tumor resulting from diseases that lead to chronic inflammation of the liver[12]. Therefore, immunotherapeutic strategies may represent a key treatment direction for improving the clinical outcomes of patients with HCC[13,14]. In recent years, immune checkpoint inhibitors have emerged as potential drugs with promising therapeutic effects against advanced HCC[15-17]; examples include nivolumab[18-20] or pembrolizumab[21,22] for programmed cell death protein 1 (PD-1) blockade, atezolizumab (MPDL3280A) for programmed cell death-ligand 1 blockade[23,24], and ipilimumab for cytotoxic T-lymphocyte-associated protein 4 blockade[25]. Of course, the combination of different immunotherapies or of immunotherapies with conventional therapeutic approaches may provide synergistic effects and facilitate the development of personalized medicine[16,26]. However, the molecular mechanisms underlying HCC immune escape remain poorly un-derstood[27-29].
Annexin A2 (ANXA2, also termed annexin II, p36, calpactin 1 heavy chain, or lipocortin II) was originally extracted from human placenta as an inhibitor of phospholipase A2[30]. The human ANXA2 gene, which is located on chromosome 15q21, is 40 kb in length and has 13 exons. It can be cleaved by chymotrypsin into a 3 kDa amino-terminal domain and a 33 kDa carboxyl-terminal domain. The ANXA2 protein can exist as a monomer, heterodimer, or heterotetramer in vivo. The heterodimeric form consists of one subunit of ANXA2 in complex with a molecule of 3-phosphoglycerate kinase, whereas the heterotetrameric form consists of two ANXA2 subunits combined with an S100A10dimer[31].
The function of ANXA2 is closely related to the form in which it exists. It has been shown that the ANXA2 monomer is localized mainly in the cytoplasm but may transition to the intracellular membrane in response to signals such as changes in the Ca2+ concentration, pH, or membrane phospholipid composition. However, the specific biological roles it plays in the subsequent processes are still unclear[32]. The ANXA2 dimer is involved in the formation of intracellular vesicles through combination with multiple endosomes and mediation of membrane fusion[33]. Additionally, the dimer is required for the biogenesis of multivesicular bodies as well as being a constituent of exosomes that are frequently cited in proteomic studies[34]. The ANXA2 heterotetramers are the most well studied of the three forms, and it is now well established that they serve as an assembly site for plasminogen and tissue plasminogen activator on the endothelial cell surface, thereby promoting the generation of plasmin and allowing the clearance of fibrin formed on the blood vessel surface in response to more subtle forms of vascular injury[35,36].
In recent years, increasingly more studies have focused on the relationship between ANXA2 and immune-related diseases, such as lupus nephritis, rheumatoid arthritis, and cancer[37-39]. ANXA2 was found to promote various processes related to cancer progression, such as cancer proliferation, migration, epithelial–mesenchymal transition (EMT), invasion, and stem cell formation, as well as their resistance to radiotherapy, chemotherapy, and immunotherapy[40]. There is growing evidence that ANXA2 plays an important role in tumor immune escape[41].
A scientific literature search was conducted using the PubMed, Web of Science, and Google Scholar databases. The keywords used included “cancer,” “hepatocellular carcinoma,” “ANXA2,” “immune escape,” “immunotherapy,” “overall survival,” and combinations of the aforementioned terms.
A high level of ANXA2 is characteristic of malignant salivary gland tumors[42] and pulmonary invasive mucinous adenocarcinoma[43] and is associated with DNA repair as well as metabolic alteration in pancreatic ductal adenocarcinoma[44]. ANXA2 is highly expressed in gastric cancer tissues and is related to the tumor size, histological differentiation, tumor–node–metastasis stage, and lymph node metastasis[45]. The ANXA2–S100A10 heterotetramers are upregulated by the promyelocytic leu-kemia–retinoic acid receptor alpha fusion protein and promotes plasminogen-dependent fibrinolysis and matrix invasion in acute promyelocytic leukemia[46]. ANXA2 overexpression contributes to the aggressive phenotype of triple-negative breast cancer in the African American population[47]. The ANXA2 protein content harbored by extracellular vesicles represents a promising prognostic biomarker in endometrial cancer[48]. The ginsenoside compound K inhibits nuclear factor-kappa B by targeting ANXA2[49,50].
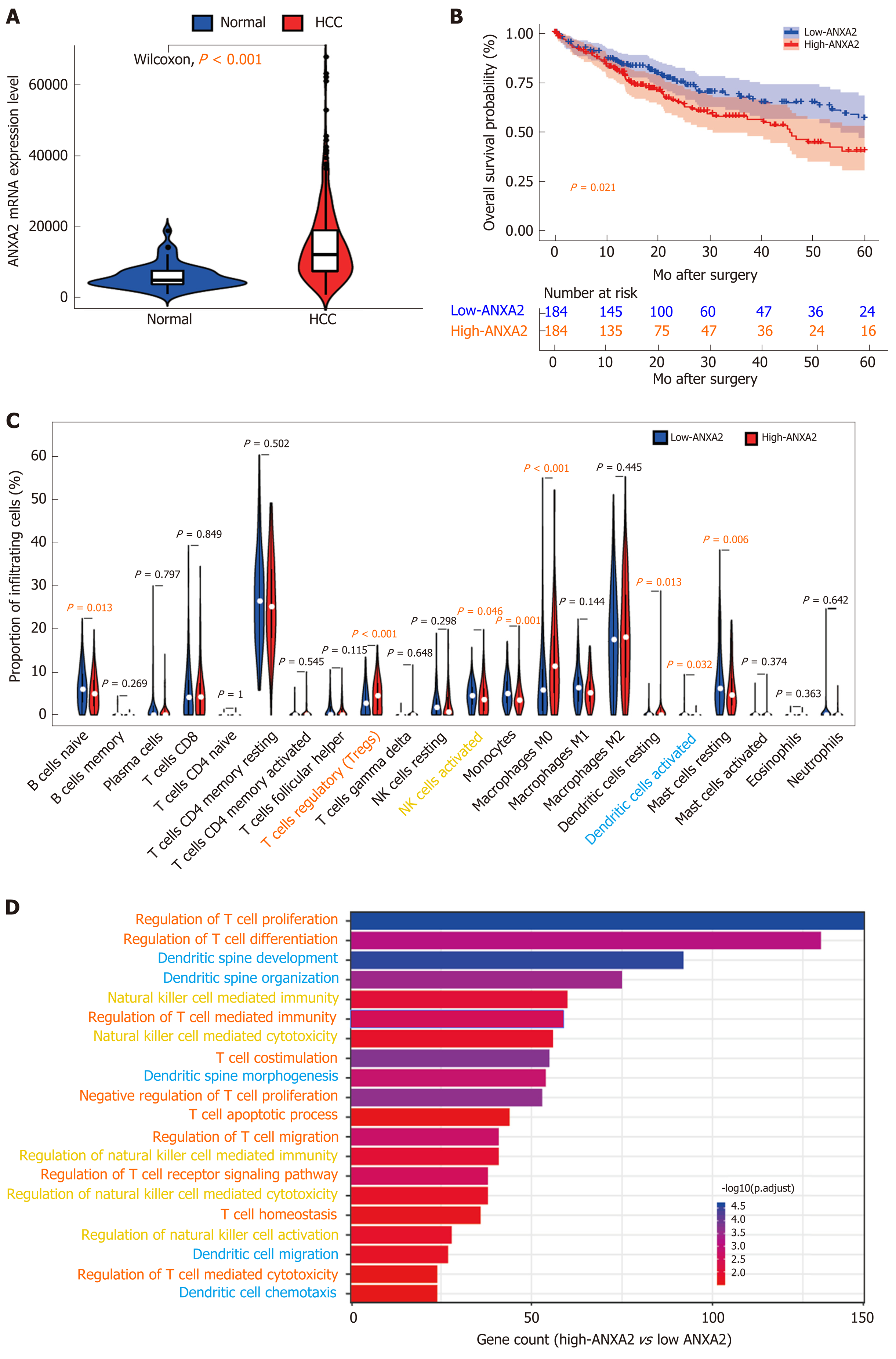
In a rat model of cirrhosis, after 30 wk of thioacetamide induction, the level of ANXA2 in the liver increased three times over the level before modeling with the dynamic increasing trend being positively correlated with immune factors, such as interleukin and transforming growth factor-beta, indicating the close relationship between ANXA2 and precancerous lesions of HCC[51]. Our previous study results suggested that the circulating levels of ANXA2 in patients with HCC were significantly higher than those in patients with other liver diseases[52]. ANXA2 was frequently found to be upregulated in HCC tissues compared with its levels in benign liver disease tissues and was significantly correlated with the degree of histological differentiation, intrahepatic metastasis, portal vein thrombosis, and tum-or–node–metastasis stage[53]. From our present search of The Cancer Genome Atlas database, we have further confirmed that ANXA2 is upregulated in HCC tissues compared with its level in normal tissues (Figure 1A, P < 0.001). In addition, ANXA2 is a critical differentially expressed gene in nonalcoholic fatty liver disease, where it is associated with the disease severity and modifiable lifestyle factors[54].
The interaction of human epididymis protein 4 with ANXA2 promotes the migration of various malignant cells[55]. ANXA2 enhances the progression of colorectal cancer and HCC via structural rearrangement of the cytoskeleton[56]. The protein also promotes glioma cell proliferation through the signal transducer and activator of transcription 3-cyclin D1 pathway[57]. ANXA2 has been shown to be a specific target of bleomycin, where its binding with the drug impeded the induction of pulmonary fibrosis mediated by the transcription factor EB-induced autophagic flux[58]. The miR155HG-miR-185-ANXA2 loop contributes to glioblastoma progression[59].The long noncoding (lnc) RNA lung cancer-associated transcript 1 promotes tumorigenesis by inhibiting ANXA2 phosphorylation in HCC[60]. The miR-23b-3p-ANXA2 axis inhibits the development and progression of pancreatic ductal adenocarcinoma[61]. ANXA2 was found to promote cancer progression and therapeutic resistance in nasopharyngeal carcinoma[40]. The lncRNA cytoskeleton regulator RNA induces the upregulation of ANXA2 by binding competitively to miR-613, leading to nasopharyngeal carcinoma metastasis[62]. Another lncRNA, colon cancer-associated transcript 1, interacts with ANXA2 to promote beta-catenin translocation to the nucleus where it then activates T-cell factor 4, leading to breast cancer progression[63,64]. The lncRNA small nucleolar RNA host gene 14 potentiates pancreatic cancer progression via ANXA2 expression upregulation by acting as a competing endogenous RNA for miR-613[65]. Our previous results have suggested that ANXA2 silencing inhibits the invasion, migration, and tumorigenic potential of hepatoma cells[66].
ANXA2 overexpression is associated with colorectal cancer invasiveness and TGFß-induced EMT through the Src-ANXA2-signal transducer and activator of transcription 3 axis[67]. Mesenchymal stem cells promote hepatocarcinogenesis via the interaction of ANXA2 with a novel lncRNA termed mesenchymal stem cell-upregulated factor[68]. ANXA2 inhibition suppresses ovarian cancer progression through the control of beta-catenin and hence EMT[69]. ANXA2 silencing inhibits the proliferation, invasion, and migration of gastric cancer cells[70] as well as non-small cell lung cancer proliferation and EMT through a p53-dependent pathway[71].
The phosphorylation of ANXA2 at its tyrosine residue promotes the invasion and metastasis of drug-resistant breast cancer cells[72]. Highly expressed phosphorylated ANXA2 (Tyr23) also promotes esophageal cancer progression by activating the MYC-hypoxia-inducible factor 1 alpha-vascular endothelial growth factor axis[73]. The tumor suppressor sirtuin 6, which is ubiquitylated and degraded by E3 ubiquitin ligase, contributes to liver tumorigenesis in an ANXA2-dependent manner[74]. Tripartite motif-containing, a novel marker of poor prognosis in ovarian cancer, promotes the malignant progression of the disease by inducing ANXA2 expression[75]. Likewise, tripartite motif-containing 65 supports the aggressiveness of bladder urothelial carcinoma cells by promoting ANXA2 ubiquitination and degradation[76].
ANXA2 is an independent prognostic biomarker for the malignant progression of laryngeal cancer[77]. The protein may also be a potential prognostic biomarker of liver cancer[78]. The high expression level of ANXA2 in stromal tissue is associated with a reduced OS rate in patients with epithelial ovarian cancer[79], and when highly expressed in cancer cell membranes is associated with poor prognosis in pancreatic cancer[80]. ANXA2 overexpression is predictive of decreased survival in patients with pancreatic cancer[81] and triple-negative breast cancer[82]. According to a quantitative phosphoproteomic analysis, the phosphorylation of ANXA2 Tyr23 is associated with poor prognosis in HCC[83]. Our previous research results confirmed that an increased level of ANXA2 was closely associated with a shortened OS rate in patients with HCC and was therefore identified as an independent prognostic factor of this disease[53]. Herein, working with data from the Cancer Genome Atlas database, we further confirmed that patients with HCC with high ANXA2 expression levels had a shorter OS (Figure 1B, P < 0.001).
A combined ANXA2-N-Myc downstream regulated 1-signal transducer and activator of transcription 1 gene signature predicts the response of patients with cervical cancer to chemoradiotherapy[84]. The interaction of P37 with ANXA2 is required for the mycoplasma-associated multidrug resistance of hepatocarcinoma cells[85]. ANXA2 contributes to cisplatin resistance in cells of non-small cell lung cancer by activating the c-Jun N-terminal kinase-p53 pathway[86] and enhances multidrug resistance in pediatric neuroblastoma by regulating the nuclear factor-kappa B signaling pathway[87]. MiR-101 alleviates the chemoresistance of gastric cancer cells by targeting ANXA2[88]. Chemotherapy combined with bevacizumab can effectively destroy advanced lung adenocarcinoma cells harboring epidermal growth factor receptor-ANXA2 mutations[89].
A study of the antitumor effect of a vaccine prepared from H22 hepatocarcinoma cells induced by cartilage polysaccharides found ANXA2 to be closely related to oncogenesis and cancer development, invasion, and metastasis. A major increase in ANXA2 mRNA was found in the cartilage polysaccharide-induced H22 cells. The data suggested that ANXA2, a specific antigen, may play a key role in the antitumor immune response of HCC and in activating the immune system[90]. ANXA2 was found to be a tumor-associated antigen in patients with lung cancer who had been exposed to asbestos[91]. It has also been implicated in the attachment and entry, genome replication and expression, assembly, and egress of viruses[92].
ANXA2 is essential for the trafficking and capsid disassembly of oncogenic human papillomavirus and protects the virions from lysosomal degradation[93]. The cell-surface translocation of ANXA2 contributes to bleomycin-induced pulmonary fibrosis through its mediation of the inflammatory response in mice[94]. Stromal cell-derived factor-1 alpha triggers the engulfment and cell motility 1 gene-dependent membrane translocation of ANXA2 for the regulation of HCC chemotaxis and metastasis[95].
Cancer-associated fibroblasts promote EMT and epidermal growth factor receptor–tyrosine kinase inhibitor resistance in non-small cell lung cancers via hepatocyte growth factor-insulin-like growth factor-1-ANXA2 signaling[96]. A study on the pathogenesis of immune-mediated liver fibrosis found that after modeling of the disease in rats by injection with pig serum, the ANXA2 concentration increased continually in the rat liver during the process of fibrosis. Similarly, the serum levels of ANXA2 in patients with liver fibrosis were upregulated by 1.4-fold compared with the levels in healthy individuals. When Huh7 cells were exposed to the hepatitis B virus in vitro, ANXA2 translocated from the nucleus and cytoplasm to the cytoplasmic membrane, which suggested that it was involved in the immune-mediated liver injury caused by the virus[97]. Dendritic cells (DCs) respond to nasopharyngeal carcinoma cells through an ANXA2-recognizing C-type lectin, named DC-specific intercellular adhesion molecule 3-grabbing nonintegrin[98].
In this present review, our analysis of Cell-type Identification By Estimating Relative Subsets Of RNA Transcripts data[99] revealed that elevated ANXA2 levels resulted in a higher proportion of Treg cells (P < 0.001) and lower proportions of activated natural killer (NK) cells (P = 0.046) and DCs (P = 0.032) than those found in the low-ANXA2 group and in some nonfunctional immune cells (Figure 1C). Furthermore, our Gene Ontology analysis of differentially expressed genes (false discovery rate < 0.05; fold change = 2) suggested that signatures of functional regulation in Treg, NK, and DC cells were enriched (Figure 1D) in patients with HCC.
In addition, ANXA2 plays a key role in nontumorous immunological diseases. Soluble ANXA2 activates human macrophages via mitogen-activated protein kinases and may be capable of acting as an inflammatory mediator[100]. ANXA2 expression was downregulated in myeloid cells that had been induced to differentiate through stimulation with all-trans retinoic acid[101]. An immune response mediated by ANXA2 autoantibodies resulted in high circulating levels of interleukin-6 in serum samples from patients with lung cancer[102].
A recent study has shown that T-cell activation, proliferation, and cluster formation are dependent on the proteases tissue plasminogen activator and plasmin[103]. The tissue plasminogen activator treatment of T cells increased the cleavage of ANXA2, which regulates the actin cytoskeleton. Live cell imaging of the activated T cells further indicated a negative role of the ANXA2-regulated actin cytoskeleton in T-cell clustering. This may be one of the mechanisms by which the upregulation of ANXA2 in tumors leads to decreased T-cell activation and an imbalance of the tumor microenvironment[103].The soluble ANXA2 released by tumor cells has novel immunosuppressive properties in patients with renal cell carcinoma[104]. Elevated serum levels of ANXA2 may be important for the suppression of the immune response[105]. A Listeria-based ANXA2-targeting immunotherapy in combination with anti-PD-1 antibodies demonstrated high efficacy against pancreatic tumors[39]. ANXA2 in the cancerous cell membrane was identified as the direct antigenic ligand of the Vγ8Vδ3 T-cell receptor of γδ T cells, which make up the first line of defense of stressed cells[41].
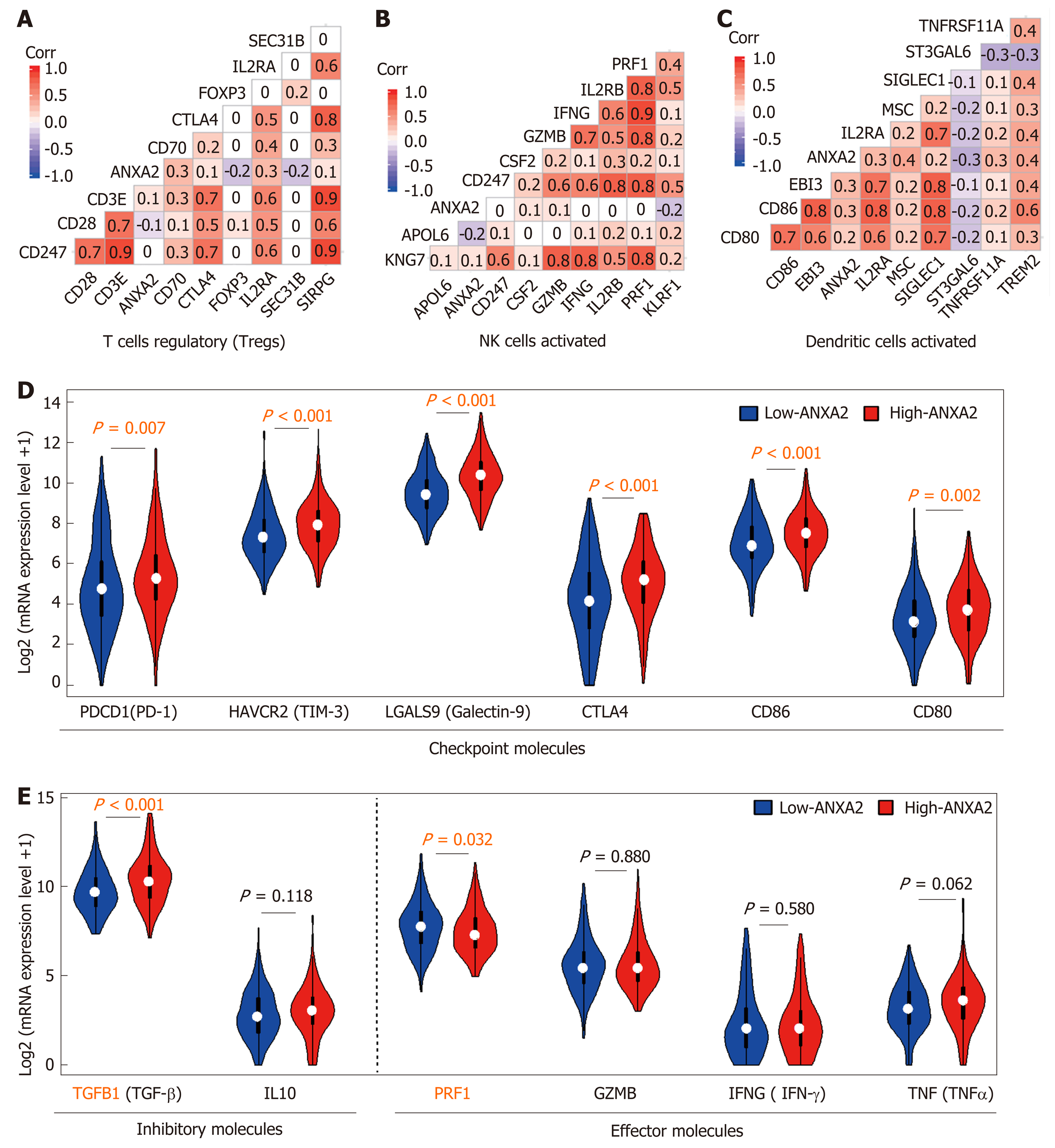
At present, there are limited published studies on the role of ANXA2 in immune escape. In this review, we analyzed the correlations of partially labeled genes of Treg cells (Figure 2A), activated NK cells (Figure 2B), and DCs (Figure 2C). We further confirmed that an elevated ANXA2 level results in the upregulation of several checkpoint molecules, such as PD-1, hepatitis A virus cellular receptor 2, gelectin-9, cytotoxic T-lymphocyte-associated protein 4, CD86, and CD80 (Figure 2D). Moreover, we also found that elevated ANXA2 levels result in the downregulation of several inhibitory molecules (e.g., TGFβ and interleukin-10), and effector molecules (e.g., perforin 1, granzyme B, interferon-gamma, and tumor necrosis factor-alpha; Figure 2E). These results suggest that elevated ANXA2 levels contribute to HCC immune escape.
ANXA2 is usually overexpressed in cancerous tissue and results in shorter OS and chemotherapy resistance in patients with HCC[106]. Furthermore, an elevated ANXA2 level results in the upregulation of both the proportion of Treg cells and the expression of several checkpoint molecules as well as the downregulation of both the proportions of activated NK cells and DCs and of several inhibitory molecules. Although there are few research studies to date on the role of ANXA2 in tumor immune escape, we expect a future increase in the number of in-depth studies being carried out to reveal the mechanism through which ANXA2 mediates the immune escape of HCC.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tamori A S-Editor: Wang J L-Editor: Filipodia E-Editor: Zhang YL
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13300] [Cited by in RCA: 15475] [Article Influence: 2579.2] [Reference Citation Analysis (2)] |
| 2. | Tella SH, Mahipal A, Kommalapati A, Jin Z. Evaluating the Safety and Efficacy of Nivolumab in Patients with Advanced Hepatocellular Carcinoma: Evidence to Date. Onco Targets Ther. 2019;12:10335-10342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Goh MJ, Sinn DH, Kim S, Woo SY, Cho H, Kang W, Gwak GY, Paik YH, Choi MS, Lee JH, Koh KC, Paik SW. Statin Use and the Risk of Hepatocellular Carcinoma in Patients With Chronic Hepatitis B. Hepatology. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 4. | Gao Q, Zhu H, Dong L, Shi W, Chen R, Song Z, Huang C, Li J, Dong X, Zhou Y, Liu Q, Ma L, Wang X, Zhou J, Liu Y, Boja E, Robles AI, Ma W, Wang P, Li Y, Ding L, Wen B, Zhang B, Rodriguez H, Gao D, Zhou H, Fan J. Integrated Proteogenomic Characterization of HBV-Related Hepatocellular Carcinoma. Cell. 2019;179:561-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 620] [Article Influence: 124.0] [Reference Citation Analysis (0)] |
| 5. | Su TH, Tseng TC, Kao JH. HCC risk in patients with HBV-related cirrhosis receiving nucleos(t)ide analogues therapy: Is HCC prevented or delayed? Hepatology. 2018;67:1634-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Jiang Y, Sun A, Zhao Y, Ying W, Sun H, Yang X, Xing B, Sun W, Ren L, Hu B, Li C, Zhang L, Qin G, Zhang M, Chen N, Zhang M, Huang Y, Zhou J, Zhao Y, Liu M, Zhu X, Qiu Y, Sun Y, Huang C, Yan M, Wang M, Liu W, Tian F, Xu H, Zhou J, Wu Z, Shi T, Zhu W, Qin J, Xie L, Fan J, Qian X, He F; Chinese Human Proteome Project (CNHPP) Consortium. Proteomics identifies new therapeutic targets of early-stage hepatocellular carcinoma. Nature. 2019;567:257-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 683] [Cited by in RCA: 623] [Article Influence: 103.8] [Reference Citation Analysis (0)] |
| 7. | Khatib SA, Wang XW. Proteomic heterogeneity reveals SOAT1 as a potential biomarker for hepatocellular carcinoma. Transl Gastroenterol Hepatol. 2019;4:37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Chan LK, Ng IO. Proteomic profiling in liver cancer: another new page. Transl Gastroenterol Hepatol. 2019;4:47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Colagrande S, Inghilesi AL, Aburas S, Taliani GG, Nardi C, Marra F. Challenges of advanced hepatocellular carcinoma. World J Gastroenterol. 2016;22:7645-7659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 128] [Cited by in RCA: 130] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 10. | Bouattour M, Mehta N, He AR, Cohen EI, Nault JC. Systemic Treatment for Advanced Hepatocellular Carcinoma. Liver Cancer. 2019;8:341-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 11. | Bangaru S, Marrero JA, Singal AG. Review article: new therapeutic interventions for advanced hepatocellular carcinoma. Aliment Pharmacol Ther. 2020;51:78-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 12. | Tagliamonte M, Mauriello A, Cavalluzzo B, Ragone C, Manolio C, Petrizzo A, Buonaguro L. Tackling hepatocellular carcinoma with individual or combinatorial immunotherapy approaches. Cancer Lett. 2020;473:25-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 13. | Dawkins J, Webster RM. The hepatocellular carcinoma market. Nat Rev Drug Discov. 2019;18:13-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 14. | Jones RL, Young LS, Adams DH. Immunotherapy in hepatocellular carcinoma. Lancet. 2000;356:784-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Ringelhan M, Pfister D, O'Connor T, Pikarsky E, Heikenwalder M. The immunology of hepatocellular carcinoma. Nat Immunol. 2018;19:222-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 736] [Article Influence: 105.1] [Reference Citation Analysis (0)] |
| 16. | Llovet JM, Montal R, Sia D, Finn RS. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol. 2018;15:599-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1458] [Cited by in RCA: 1384] [Article Influence: 197.7] [Reference Citation Analysis (0)] |
| 17. | Liu Z, Lin Y, Zhang J, Zhang Y, Li Y, Liu Z, Li Q, Luo M, Liang R, Ye J. Molecular targeted and immune checkpoint therapy for advanced hepatocellular carcinoma. J Exp Clin Cancer Res. 2019;38:447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 167] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 18. | Killock D. Immunotherapy: Nivolumab keeps HCC in check and opens avenues for checkmate. Nat Rev Clin Oncol. 2017;14:392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Yau T, Hsu C, Kim TY, Choo SP, Kang YK, Hou MM, Numata K, Yeo W, Chopra A, Ikeda M, Kuromatsu R, Moriguchi M, Chao Y, Zhao H, Anderson J, Cruz CD, Kudo M. Nivolumab in advanced hepatocellular carcinoma: Sorafenib-experienced Asian cohort analysis. J Hepatol. 2019;71:543-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 189] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 20. | Stotts MJ, Adjapong O, Kaplan DE. A Psoriasiform Drug Eruption Secondary to Nivolumab for Hepatocellular Carcinoma: A Case Report. Hepatology. 2019;70:1477-1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Rammohan A, Reddy MS, Farouk M, Vargese J, Rela M. Pembrolizumab for metastatic hepatocellular carcinoma following live donor liver transplantation: The silver bullet? Hepatology. 2018;67:1166-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 22. | Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, Breder V, Edeline J, Chao Y, Ogasawara S, Yau T, Garrido M, Chan SL, Knox J, Daniele B, Ebbinghaus SW, Chen E, Siegel AB, Zhu AX, Cheng AL; KEYNOTE-240 investigators. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J Clin Oncol. 2020;38:193-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1365] [Cited by in RCA: 1343] [Article Influence: 268.6] [Reference Citation Analysis (0)] |
| 23. | Deng S, Yang Q, Shu X, Lang J, Lu S. The Relative Risk of Immune-Related Liver Dysfunction of PD-1/PD-L1 Inhibitors Versus Chemotherapy in Solid Tumors: A Meta-Analysis of Randomized Controlled Trials. Front Pharmacol. 2019;10:1063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Reck M, Mok TSK, Nishio M, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu D, Moro-Sibilot D, Thomas CA, Barlesi F, Finley G, Lee A, Coleman S, Deng Y, Kowanetz M, Shankar G, Lin W, Socinski MA; IMpower150 Study Group. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med. 2019;7:387-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 714] [Article Influence: 119.0] [Reference Citation Analysis (0)] |
| 25. | Zen Y, Yeh MM. Checkpoint inhibitor-induced liver injury: A novel form of liver disease emerging in the era of cancer immunotherapy. Semin Diagn Pathol. 2019;36:434-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 26. | Feng Z, Rong P, Wang W. Meta-analysis of the efficacy and safety of PD-1/PD-L1 inhibitors administered alone or in combination with anti-VEGF agents in advanced hepatocellular carcinoma. Gut. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 27. | Li W, Wang H, Ma Z, Zhang J, Ou-Yang W, Qi Y, Liu J. Multi-omics Analysis of Microenvironment Characteristics and Immune Escape Mechanisms of Hepatocellular Carcinoma. Front Oncol. 2019;9:1019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 28. | Ruiz de Galarreta M, Bresnahan E, Molina-Sánchez P, Lindblad KE, Maier B, Sia D, Puigvehi M, Miguela V, Casanova-Acebes M, Dhainaut M, Villacorta-Martin C, Singhi AD, Moghe A, von Felden J, Tal Grinspan L, Wang S, Kamphorst AO, Monga SP, Brown BD, Villanueva A, Llovet JM, Merad M, Lujambio A. β-Catenin Activation Promotes Immune Escape and Resistance to Anti-PD-1 Therapy in Hepatocellular Carcinoma. Cancer Discov. 2019;9:1124-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 623] [Article Influence: 103.8] [Reference Citation Analysis (0)] |
| 29. | Han Q, Zhao H, Jiang Y, Yin C, Zhang J. HCC-Derived Exosomes: Critical Player and Target for Cancer Immune Escape. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 30. | Huang KS, Wallner BP, Mattaliano RJ, Tizard R, Burne C, Frey A, Hession C, McGray P, Sinclair LK, Chow EP. Two human 35 kd inhibitors of phospholipase A2 are related to substrates of pp60v-src and of the epidermal growth factor receptor/kinase. Cell. 1986;46:191-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 349] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 31. | Gerke V, Moss SE. Annexins: from structure to function. Physiol Rev. 2002;82:331-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1483] [Cited by in RCA: 1545] [Article Influence: 67.2] [Reference Citation Analysis (0)] |
| 32. | Hajjar KA. The Biology of Annexin A2: From Vascular Fibrinolysis to Innate Immunity. Trans Am Clin Climatol Assoc. 2015;126:144-155. [PubMed] |
| 33. | Emans N, Gorvel JP, Walter C, Gerke V, Kellner R, Griffiths G, Gruenberg J. Annexin II is a major component of fusogenic endosomal vesicles. J Cell Biol. 1993;120:1357-1369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 227] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 34. | Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics. 2010;73:1907-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1718] [Cited by in RCA: 1946] [Article Influence: 129.7] [Reference Citation Analysis (0)] |
| 35. | Luo M, Hajjar KA. Annexin A2 system in human biology: cell surface and beyond. Semin Thromb Hemost. 2013;39:338-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 36. | O'Connell PA, Surette AP, Liwski RS, Svenningsson P, Waisman DM. S100A10 regulates plasminogen-dependent macrophage invasion. Blood. 2010;116:1136-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 121] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 37. | Cheung KF, Yung S, Chau MK, Yap DY, Chan KW, Lee CK, Tang CS, Chan TM. Annexin II-binding immunoglobulins in patients with lupus nephritis and their correlation with disease manifestations. Clin Sci (Lond). 2017;131:653-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 38. | Haridas V, Shetty P, Sarathkumar E, Bargale A, Vishwanatha JK, Patil V, Dinesh US. Reciprocal regulation of pro-inflammatory Annexin A2 and anti-inflammatory Annexin A1 in the pathogenesis of rheumatoid arthritis. Mol Biol Rep. 2019;46:83-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 39. | Kim VM, Blair AB, Lauer P, Foley K, Che X, Soares K, Xia T, Muth ST, Kleponis J, Armstrong TD, Wolfgang CL, Jaffee EM, Brockstedt D, Zheng L. Anti-pancreatic tumor efficacy of a Listeria-based, Annexin A2-targeting immunotherapy in combination with anti-PD-1 antibodies. J Immunother Cancer. 2019;7:132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 40. | Chen CY, Lin YS, Chen CH, Chen YJ. Annexin A2-mediated cancer progression and therapeutic resistance in nasopharyngeal carcinoma. J Biomed Sci. 2018;25:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 41. | Marlin R, Pappalardo A, Kaminski H, Willcox CR, Pitard V, Netzer S, Khairallah C, Lomenech AM, Harly C, Bonneville M, Moreau JF, Scotet E, Willcox BE, Faustin B, Déchanet-Merville J. Sensing of cell stress by human γδ TCR-dependent recognition of annexin A2. Proc Natl Acad Sci USA. 2017;114:3163-3168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 113] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 42. | Cardoso CM, de Jesus SF, de Souza MG, Queiroz LDRP, Santos EM, Dos Santos EP, Oliveira LP, Cordeiro Santos CK, Santos SHS, de Paula AMB, Farias LC, Guimaraes ALS. High levels of ANXA2 are characteristic of malignant salivary gland tumors. J Oral Pathol Med. 2019;48:929-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 43. | Arai K, Hirose M. Annexin A2 Expression in Aerogenous Metastasis of Pulmonary Invasive Mucinous Adenocarcinoma: A Case Report including Immunohistochemical Analysis. Case Rep Oncol Med. 2019;2019:5064852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 44. | Takahashi H, Katsuta E, Yan L, Dasgupta S, Takabe K. High expression of Annexin A2 is associated with DNA repair, metabolic alteration, and worse survival in pancreatic ductal adenocarcinoma. Surgery. 2019;166:150-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 45. | Han F, Shrestha S, Huang H, Lv HY, Nie C, Lin L, Lu ML. Expression of annexin II in gastric carcinoma and its role in gastric cancer metastasis. World J Gastroenterol. 2017;23:7009-7015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 46. | Huang D, Yang Y, Sun J, Dong X, Wang J, Liu H, Lu C, Chen X, Shao J, Yan J. Annexin A2-S100A10 heterotetramer is upregulated by PML/RARα fusion protein and promotes plasminogen-dependent fibrinolysis and matrix invasion in acute promyelocytic leukemia. Front Med. 2017;11:410-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 47. | Gibbs LD, Chaudhary P, Mansheim K, Hare RJ, Mantsch RA, Vishwanatha JK. ANXA2 expression in African American triple-negative breast cancer patients. Breast Cancer Res Treat. 2019;174:113-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 48. | Herrero C, de la Fuente A, Casas-Arozamena C, Sebastian V, Prieto M, Arruebo M, Abalo A, Colás E, Moreno-Bueno G, Gil-Moreno A, Vilar A, Cueva J, Abal M, Muinelo-Romay L. Extracellular Vesicles-Based Biomarkers Represent a Promising Liquid Biopsy in Endometrial Cancer. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 49. | Wang YS, Zhu H, Li H, Li Y, Zhao B, Jin YH. Ginsenoside compound K inhibits nuclear factor-kappa B by targeting Annexin A2. J Ginseng Res. 2019;43:452-459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 50. | Wang YS, Li H, Li Y, Zhu H, Jin YH. Identification of natural compounds targeting Annexin A2 with an anti-cancer effect. Protein Cell. 2018;9:568-579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 51. | An JH, Seong J, Oh H, Kim W, Han KH, Paik YH. [Protein expression profiles in a rat cirrhotic model induced by thioacetamide]. Korean J Hepatol. 2006;12:93-102. [PubMed] |
| 52. | Zhang HJ, Yao DF, Yao M, Huang H, Wu W, Yan MJ, Yan XD, Chen J. Expression characteristics and diagnostic value of annexin A2 in hepatocellular carcinoma. World J Gastroenterol. 2012;18:5897-5904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 53. | Zhang H, Yao M, Wu W, Qiu L, Sai W, Yang J, Zheng W, Huang J, Yao D. Up-regulation of annexin A2 expression predicates advanced clinicopathological features and poor prognosis in hepatocellular carcinoma. Tumour Biol. 2015;36:9373-9383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 54. | Arendt BM, Teterina A, Pettinelli P, Comelli EM, Ma DWL, Fung SK, McGilvray ID, Fischer SE, Allard JP. Cancer-related gene expression is associated with disease severity and modifiable lifestyle factors in non-alcoholic fatty liver disease. Nutrition. 2019;62:100-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 55. | Wang J, Deng L, Zhuang H, Liu J, Liu D, Li X, Jin S, Zhu L, Wang H, Lin B. Interaction of HE4 and ANXA2 exists in various malignant cells-HE4-ANXA2-MMP2 protein complex promotes cell migration. Cancer Cell Int. 2019;19:161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 56. | He H, Xiao L, Cheng S, Yang Q, Li J, Hou Y, Song F, Su X, Jin H, Liu Z, Dong J, Zuo R, Song X, Wang Y, Zhang K, Duan W, Hou Y. Annexin A2 Enhances the Progression of Colorectal Cancer and Hepatocarcinoma via Cytoskeleton Structural Rearrangements. Microsc Microanal. 2019;25:950-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 57. | Chen L, Lin L, Xian N, Zheng Z. Annexin A2 regulates glioma cell proliferation through the STAT3‑cyclin D1 pathway. Oncol Rep. 2019;42:399-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 58. | Wang K, Zhang T, Lei Y, Li X, Jiang J, Lan J, Liu Y, Chen H, Gao W, Xie N, Chen Q, Zhu X, Liu X, Xie K, Peng Y, Nice EC, Wu M, Huang C, Wei Y. Identification of ANXA2 (annexin A2) as a specific bleomycin target to induce pulmonary fibrosis by impeding TFEB-mediated autophagic flux. Autophagy. 2018;14:269-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 113] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 59. | Wu W, Yu T, Wu Y, Tian W, Zhang J, Wang Y. The miR155HG/miR-185/ANXA2 loop contributes to glioblastoma growth and progression. J Exp Clin Cancer Res. 2019;38:133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 96] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 60. | Lou Y, Yu Y, Xu X, Zhou S, Shen H, Fan T, Wu D, Yin J, Li G. Long non-coding RNA LUCAT1 promotes tumourigenesis by inhibiting ANXA2 phosphorylation in hepatocellular carcinoma. J Cell Mol Med. 2019;23:1873-1884. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 61. | Wei DM, Dang YW, Feng ZB, Liang L, Zhang L, Tang RX, Chen ZM, Yu Q, Wei YC, Luo DZ, Chen G. Biological Effect and Mechanism of the miR-23b-3p/ANXA2 Axis in Pancreatic Ductal Adenocarcinoma. Cell Physiol Biochem. 2018;50:823-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 62. | Chen W, Du M, Hu X, Ma H, Zhang E, Wang T, Yin L, He X, Hu Z. Long noncoding RNA cytoskeleton regulator RNA promotes cell invasion and metastasis by titrating miR-613 to regulate ANXA2 in nasopharyngeal carcinoma. Cancer Med. 2020;9:1209-1219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 63. | Tang T, Guo C, Xia T, Zhang R, Zen K, Pan Y, Jin L. LncCCAT1 Promotes Breast Cancer Stem Cell Function through Activating WNT/β-catenin Signaling. Theranostics. 2019;9:7384-7402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 117] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 64. | Wang CCN, Li CY, Cai JH, Sheu PC, Tsai JJP, Wu MY, Li CJ, Hou MF. Identification of Prognostic Candidate Genes in Breast Cancer by Integrated Bioinformatic Analysis. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 65. | Deng PC, Chen WB, Cai HH, An Y, Wu XQ, Chen XM, Sun DL, Yang Y, Shi LQ, Yang Y. LncRNA SNHG14 potentiates pancreatic cancer progression via modulation of annexin A2 expression by acting as a competing endogenous RNA for miR-613. J Cell Mol Med. 2019;23:7222-7232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 66. | Zhang HJ, Yao DF, Yao M, Huang H, Wang L, Yan MJ, Yan XD, Gu X, Wu W, Lu SL. Annexin A2 silencing inhibits invasion, migration, and tumorigenic potential of hepatoma cells. World J Gastroenterol. 2013;19:3792-3801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 46] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 67. | Rocha MR, Barcellos-de-Souza P, Sousa-Squiavinato ACM, Fernandes PV, de Oliveira IM, Boroni M, Morgado-Diaz JA. Annexin A2 overexpression associates with colorectal cancer invasiveness and TGF-ß induced epithelial mesenchymal transition via Src/ANXA2/STAT3. Sci Rep. 2018;8:11285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 68. | Yan X, Zhang D, Wu W, Wu S, Qian J, Hao Y, Yan F, Zhu P, Wu J, Huang G, Huang Y, Luo J, Liu X, Liu B, Chen X, Du Y, Chen R, Fan Z. Mesenchymal Stem Cells Promote Hepatocarcinogenesis via lncRNA-MUF Interaction with ANXA2 and miR-34a. Cancer Res. 2017;77:6704-6716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 195] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 69. | Liu Y, Li H, Ban Z, Nai M, Yang L, Chen Y, Xu Y. Annexin A2 inhibition suppresses ovarian cancer progression via regulating β-catenin/EMT. Oncol Rep. 2017;37:3643-3650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 70. | Xie R, Liu J, Yu X, Li C, Wang Y, Yang W, Hu J, Liu P, Sui H, Liang P, Huang X, Wang L, Bai Y, Xue Y, Zhu J, Fang T. ANXA2 Silencing Inhibits Proliferation, Invasion, and Migration in Gastric Cancer Cells. J Oncol. 2019;2019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 71. | Wu M, Sun Y, Xu F, Liang Y, Liu H, Yi Y. Annexin A2 Silencing Inhibits Proliferation and Epithelial-to-mesenchymal Transition through p53-Dependent Pathway in NSCLCs. J Cancer. 2019;10:1077-1085. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 72. | Fan Y, Si W, Ji W, Wang Z, Gao Z, Tian R, Song W, Zhang H, Niu R, Zhang F. Rack1 mediates tyrosine phosphorylation of Anxa2 by Src and promotes invasion and metastasis in drug-resistant breast cancer cells. Breast Cancer Res. 2019;21:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 73. | Ma S, Lu CC, Yang LY, Wang JJ, Wang BS, Cai HQ, Hao JJ, Xu X, Cai Y, Zhang Y, Wang MR. ANXA2 promotes esophageal cancer progression by activating MYC-HIF1A-VEGF axis. J Exp Clin Cancer Res. 2018;37:183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 91] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 74. | Kohli S, Bhardwaj A, Kumari R, Das S. SIRT6 Is a Target of Regulation by UBE3A That Contributes to Liver Tumorigenesis in an ANXA2-Dependent Manner. Cancer Res. 2018;78:645-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 75. | Wang Y, Zhou Z, Wang X, Zhang X, Chen Y, Bai J, Di W. TRIM59 Is a Novel Marker of Poor Prognosis and Promotes Malignant Progression of Ovarian Cancer by Inducing Annexin A2 Expression. Int J Biol Sci. 2018;14:2073-2082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 76. | Wei WS, Chen X, Guo LY, Li XD, Deng MH, Yuan GJ, He LY, Li YH, Zhang ZL, Jiang LJ, Chen RX, Ma XD, Wei S, Ma NF, Liu ZW, Luo JH, Zhou FJ, Xie D. TRIM65 supports bladder urothelial carcinoma cell aggressiveness by promoting ANXA2 ubiquitination and degradation. Cancer Lett. 2018;435:10-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 77. | Luo S, Xie C, Wu P, He J, Tang Y, Xu J, Zhao S. Annexin A2 is an independent prognostic biomarker for evaluating the malignant progression of laryngeal cancer. Exp Ther Med. 2017;14:6113-6118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 78. | Zhuang C, Wang P, Sun T, Zheng L, Ming L. Expression levels and prognostic values of annexins in liver cancer. Oncol Lett. 2019;18:6657-6669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 79. | Christensen MV, Høgdall C, Jensen SG, Lokman N, Ricciardelli C, Christensen IJ, Christiansen P, Brask J, Karlsen MA, Nissen TK, Jochumsen KM, Høgdall E. Annexin A2 and S100A10 as Candidate Prognostic Markers in Epithelial Ovarian Cancer. Anticancer Res. 2019;39:2475-2482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 80. | Hagiwara K, Harimoto N, Yokobori T, Muranushi R, Hoshino K, Gantumur D, Yamanaka T, Ishii N, Tsukagoshi M, Igarashi T, Tanaka H, Watanabe A, Kubo N, Araki K, Hosouchi Y, Shirabe K. High Co-expression of Large Tenascin C Splice Variants in Stromal Tissue and Annexin A2 in Cancer Cell Membranes is Associated with Poor Prognosis in Pancreatic Cancer. Ann Surg Oncol. 2020;27:924-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 81. | Murphy AG, Foley K, Rucki AA, Xia T, Jaffee EM, Huang CY, Zheng L. Stromal Annexin A2 expression is predictive of decreased survival in pancreatic cancer. Oncotarget. 2017;8:106405-106414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 82. | Gibbs LD, Vishwanatha JK. Prognostic impact of AnxA1 and AnxA2 gene expression in triple-negative breast cancer. Oncotarget. 2018;9:2697-2704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 83. | Xing X, Yuan H, Sun Y, Ke K, Dong X, Chen H, Liu X, Zhao B, Huang A. ANXA2Tyr23 and FLNASer2152 phosphorylation associate with poor prognosis in hepatic carcinoma revealed by quantitative phosphoproteomics analysis. J Proteomics. 2019;200:111-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 84. | Buttarelli M, Babini G, Raspaglio G, Filippetti F, Battaglia A, Ciucci A, Ferrandina G, Petrillo M, Marino C, Mancuso M, Saran A, Villani ME, Desiderio A, D'Ambrosio C, Scaloni A, Scambia G, Gallo D. A combined ANXA2-NDRG1-STAT1 gene signature predicts response to chemoradiotherapy in cervical cancer. J Exp Clin Cancer Res. 2019;38:279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 85. | Liu D, Hu Y, Guo Y, Zhu Z, Lu B, Wang X, Huang Y. Mycoplasma-associated multidrug resistance of hepatocarcinoma cells requires the interaction of P37 and Annexin A2. PLoS One. 2017;12:e0184578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 86. | Feng X, Liu H, Zhang Z, Gu Y, Qiu H, He Z. Annexin A2 contributes to cisplatin resistance by activation of JNK-p53 pathway in non-small cell lung cancer cells. J Exp Clin Cancer Res. 2017;36:123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 87. | Wang Y, Chen K, Cai Y, Cai Y, Yuan X, Wang L, Wu Z, Wu Y. Annexin A2 could enhance multidrug resistance by regulating NF-κB signaling pathway in pediatric neuroblastoma. J Exp Clin Cancer Res. 2017;36:111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 88. | Bao J, Xu Y, Wang Q, Zhang J, Li Z, Li D, Li J. miR-101 alleviates chemoresistance of gastric cancer cells by targeting ANXA2. Biomed Pharmacother. 2017;92:1030-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 89. | Zhong R, Li H, Liu Y, Zhang S, Liu J, Huang Z, Cheng Y. Chemotherapy combined with bevacizumab for the treatment of advanced lung adenocarcinoma cancer harboring EGFR-ANXA2, EGFR-RAD51, ATR and BRCA2 mutations: A case report. Thorac Cancer. 2020;11:456-460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 90. | Liu A, Liu D, Zhao S, Zheng J, Cao D, Zhang H. Up regulation of annexin A2 on murine H22 hepatocarcinoma cells induced by cartilage polysaccharide. Cancer Epidemiol. 2011;35:490-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 91. | Yasuda M, Hanagiri T, Shigematsu Y, Onitsuka T, Kuroda K, Baba T, Mizukami M, Ichiki Y, Uramoto H, Takenoyama M, Yasumoto K. Identification of a tumour associated antigen in lung cancer patients with asbestos exposure. Anticancer Res. 2010;30:2631-2639. [PubMed] |
| 92. | Taylor JR, Skeate JG, Kast WM. Annexin A2 in Virus Infection. Front Microbiol. 2018;9:2954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 93. | Taylor JR, Fernandez DJ, Thornton SM, Skeate JG, Lühen KP, Da Silva DM, Langen R, Kast WM. Heterotetrameric annexin A2/S100A10 (A2t) is essential for oncogenic human papillomavirus trafficking and capsid disassembly, and protects virions from lysosomal degradation. Sci Rep. 2018;8:11642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 94. | Lei Y, Wang K, Li X, Li Y, Feng X, Zhou J, Zhang Z, Huang C, Zhang T. Cell-surface translocation of annexin A2 contributes to bleomycin-induced pulmonary fibrosis by mediating inflammatory response in mice. Clin Sci (Lond). 2019;133:789-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 95. | Li H, Wang Y, Lu Y, Li F. Annexin A2 interacting with ELMO1 regulates HCC chemotaxis and metastasis. Life Sci. 2019;222:168-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 96. | Yi Y, Zeng S, Wang Z, Wu M, Ma Y, Ye X, Zhang B, Liu H. Cancer-associated fibroblasts promote epithelial-mesenchymal transition and EGFR-TKI resistance of non-small cell lung cancers via HGF/IGF-1/ANXA2 signaling. Biochim Biophys Acta Mol Basis Dis. 2018;1864:793-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 97] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 97. | Zhang L, Peng X, Zhang Z, Feng Y, Jia X, Shi Y, Yang H, Zhang Z, Zhang X, Liu L, Yin L, Yuan Z. Subcellular proteome analysis unraveled annexin A2 related to immune liver fibrosis. J Cell Biochem. 2010;110:219-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 98. | Chao PZ, Hsieh MS, Cheng CW, Hsu TJ, Lin YT, Lai CH, Liao CC, Chen WY, Leung TK, Lee FP, Lin YF, Chen CH. Dendritic cells respond to nasopharygeal carcinoma cells through annexin A2-recognizing DC-SIGN. Oncotarget. 2015;6:159-170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 99. | Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, Hoang CD, Diehn M, Alizadeh AA. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12:453-457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4763] [Cited by in RCA: 8920] [Article Influence: 892.0] [Reference Citation Analysis (0)] |
| 100. | Swisher JF, Khatri U, Feldman GM. Annexin A2 is a soluble mediator of macrophage activation. J Leukoc Biol. 2007;82:1174-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 101. | Gilmore WS, Olwill S, McGlynn H, Alexander HD. Annexin A2 expression during cellular differentiation in myeloid cell lines. Biochem Soc Trans. 2004;32:1122-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 102. | Brichory FM, Misek DE, Yim AM, Krause MC, Giordano TJ, Beer DG, Hanash SM. An immune response manifested by the common occurrence of annexins I and II autoantibodies and high circulating levels of IL-6 in lung cancer. Proc Natl Acad Sci USA. 2001;98:9824-9829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 234] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 103. | Loef EJ, Brooks AES, Lorenz N, Birch NP, Dunbar PR. Neuroserpin regulates human T cell-T cell interactions and proliferation through inhibition of tissue plasminogen activator. J Leukoc Biol. 2020;107:145-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 104. | Aarli A, Skeie Jensen T, Kristoffersen EK, Bakke A, Ulvestad E. Inhibition of phytohaemagglutinin-induced lymphoproliferation by soluble annexin II in sera from patients with renal cell carcinoma. APMIS. 1997;105:699-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 105. | Ulvestad E, Kristoffersen EK, Jensen TS, Matre R. Identification of a soluble Fc gamma-binding molecule (annexin II) in human serum using a competitive ELISA. APMIS. 1994;102:667-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 106. | Sharma MC. Annexin A2: An emerging biomarker and potential therapeutic target for aggressive cancers. Int J Cancer. 2019;144:2074-2081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |