INTRODUCTION
Barrett’s esophagus (BE) is a condition in which the esophageal mucosa extending at least one centimeter above the gastroesophageal junction converts from normal esophageal squamous epithelium to a specialized intestinal columnar type epithelium. BE is a premalignant condition in which intestinal metaplasia (IM) may progress to dysplasia and eventually esophageal adenocarcinoma. While the annual malignant conversion risk of IM is only 0.3%, the risk increases with low-grade dysplasia (LGD) to 0.5%, and with high-grade dysplasia to 6%[1].
While non-dysplastic BE can be adequately conservatively managed with chemoprevention via proton-pump inhibitor therapy and endoscopic surveillance programs[2,3], dysplastic BE requires more aggressive intervention to mitigate the risk of development of esophageal adenocarcinoma. Historically, esophagectomy was considered the only option for dysplastic and early neoplastic BE, however, over the past twenty years, minimally invasive and highly efficacious endoscopic interventions with low risk profiles have been developed that have replaced esophagectomy as the mainstay of therapy[4-7].
These endoscopic interventions have collectively been termed “endoscopic eradication therapy” (EET) and, to date, consist of both endoscopic resection and ablative techniques. For any visible raised or suspicious lesion, endoscopic mucosal resection (EMR), via band ligation or a cap-assisted technique[8], or endoscopic submucosal dissection (ESD), are recommended as the first step in standard of care therapy. EMR and ESD have comparable rates of remission of dysplasia at 3-mo follow-up, however ESD is technically more difficult and has a higher adverse-event rate[9], making EMR the more feasible option for treatment of visible lesions. In patients with dysplastic BE, resection of visible lesions is not sufficient and should be followed by ablative therapy in order to eradicate flat lesions and invisible dysplasia[3,10]. Additionally, ablation of all intestinal metaplasia has been shown to reduce the recurrence of dysplasia and thus is the standard of care[11,12].
Radiofrequency ablation (RFA) has been the most widely used and studied ablative technique and is consider the primary ablation therapy for BE[11-14]. RFA involves the delivery of radiofrequency energy through a circumferential balloon or focal ablation catheter directly to the flat Barrett’s mucosa with the goal of thermal destruction of dysplastic tissue and subsequent promotion of regrowth of normal squamous esophageal epithelium. RFA can be performed for any length of BE and on average, requires 3-4 sessions for complete eradication. RFA was first developed in 1999 and since then, has shown to be highly effective in eradication of BE. A landmark trial in 2009, entitled the AIM dysplasia trial, demonstrated that 90% of subjects with high-grade dysplasia and 81% of subjects with LGD achieved complete eradication of dysplasia as compared to < 5% in the sham procedure arm. The patients who underwent RFA also achieved a significantly higher rate of complete eradication of intestinal metaplasia and of disease progression[11]. The usefulness of RFA specifically for patients with LGD was re-emphasized in the SURF study in 2014[12], and its overall efficacy was repeatedly highlighted in a growing body of literature[13,15]. Furthermore, RFA is safe, tolerable, and has been shown to have a low adverse event rate[16]. A 2016 meta-analysis showed that the overall adverse event rate was 8.8%, with the most common event being stricture formation (5.6%). Post-procedure pain occurred in 3.7% of patients[17]. Taking all of this level 1 evidence into account, RFA has been deemed the first-line therapy for ablation of BE[3].
Despite RFA’s great success, however, there is a subset of patients in whom complete eradication of intestinal metaplasia (CE-IM) cannot be achieved. A meta-analysis of 18 studies showed the pooled CE-IM rate to be 78%[13]. Several factors have been implicated in the failure of RFA to eradicate Barrett’s dysplasia and metaplasia. A multi-center prospective trial in 2013 identified active reflux esophagitis, endoscopic resection scar regeneration with Barrett’s epithelium, narrow pre-RFA esophageal diameters, and longer years of dysplasia presence to be independent predictors for poor response to RFA[18]. Additionally, the presence of a hiatal hernia, advanced patient age, longer segments of BE, and incomplete mucosal healing on subsequent endoscopy were found to also contribute to incomplete eradication of dysplasia and metaplasia after RFA[19,20]. Finally, procedure volume for the endoscopist performing RFA was positively correlated with complete eradication of intestinal metaplasia rates[21,22].
This review will focus on the management of patients with dysplastic Barrett’s esophagus refractory to RFA therapy. Management strategies discussed in this review include optimizing the RFA procedure, optimizing acid suppression (with medical, endoscopic, and surgical management), cryotherapy, hybrid argon plasma coagulation, and EndoRotor resection.
OPTIMIZATION OF MODIFIABLE FACTORS IN RADIOFREQUENCY ABLATION
Pasricha et al[22] found that there was indeed a significant learning curve effect of case volume on successful rates of complete eradication of dysplasia and metaplasia. However, the curve started to flatten at 30 procedures, suggesting that this could be considered the threshold, or minimum standard, for which better outcomes could be expected. There was no difference between recurrence rates at community hospitals or academic centers. Thus, referral to an endoscopist who performs a high volume of RFA for re-treatment of refractory patients could be considered. Additionally, as suggested by Eluri and Shaheen[23], attendance at society-sponsored RFA-specific training courses and improving training in fellowships may improve the endoscopist’s individual outcomes.
ANTI-REFLUX MEDICAL THERAPY
Acid exposure has been shown to cause changes in the esophageal epithelium that lead to BE[24,25]. Furthermore, patients with BE have the highest risk for esophageal acid reflux of any patient population[26]. The 2016 guidelines recommend that all patients with BE should receive once-daily proton-pump inhibitor (PPI) therapy, which should be increased to twice daily for the treatment of uncontrolled acid reflux[3]. Despite this, it has been shown that approximately one quarter of patients with BE on twice-daily PPI therapy have abnormal pH-impedance studies, suggesting a significant proportion of BE patients have physiologically significant esophageal acid exposure despite adherence with recommended medical therapy[27]. Additionally, studies have suggested that uncontrolled reflux was associated with persistence and recurrence of intestinal metaplasia and dysplasia after RFA[28].
It is important to note that many patients with BE and acid reflux are asymptomatic, which makes the diagnosis of uncontrolled acid reflux challenging. Komanduri et al[29] demonstrated that a structured reflux management protocol, in addition to EET, significantly decreased the rate of recurrent or persistent IM or dysplasia after EET. This protocol consisted of early patient counseling about the important of reflux management in the treatment of BE, administration of a twice-daily PPI regimen taken 30 min before breakfast and dinner, medication reconciliation and remediation at each subsequent visit, and objective on-treatment pH testing in the cases of poor symptom control, reflux esophagitis, or inability to achieve complete eradication of IM after completion of EET. This study revealed that medication compliance was poor initially (only 59% were adherent) and that a reduction in dose or frequency of PPI use was the only independent factor associated with incomplete eradication of metaplasia. In the patients with incomplete eradication, optimization of their PPI regimen back to twice daily pre-meal dosing with, on average, one additional session of RFA, subsequently resulted in almost 90% of the patient achieving complete eradication. Thus, this highlights the importance of optimizing patient adherence with a twice-daily PPI regimen in patients with dysplastic BE that fails initial EET with RFA.
ANTI-REFLUX SURGERY
Sometimes, despite optimal PPI therapy, acid reflux persists and incomplete eradication of metaplasia and dysplasia results that is refractory to EET. In the same Komanduri et al[29] study, these patients were referred for surgical fundoplication, with resultant complete eradication of intestinal metaplasia. Skrobić et al[30] specifically evaluated patients who had recurrence of metaplasia after RFA after either staying on PPI therapy or those who underwent Nissen fundoplication[30]. They found recurrence in 20% of patients on PPI, whereas recurrence occurred only in 9% of patients who had undergone fundoplication. This difference became significant in the cohort of patients with long-segment BE. Furthermore, Johnson et al[31] suggested that Nissen fundoplication after RFA treatment of BE might be more effective than PPI therapy at preventing further progression of disease or the development of cancer[31].
Another minimally invasive surgical anti-reflux option includes Magnetic Sphincter Augmentation, which was approved by the FDA in 2012 and involves the laparoscopic placement of magnetic titanium beads on a stainless steel cable at the level of the gastroesophageal junction. These beads reinforce and close the lower esophageal sphincter, and have been shown to be similarly effective at 1-year follow-up in terms of acid reflux control, and with less side effects, than to laparoscopic Nissen fundoplication[32,33].
These data suggest that patients with BE who fail to achieve complete eradication with RFA therapy due to persistent uncontrolled esophageal acid reflux may benefit from subsequent fundoplication or sphincter augmentation for better reflux control.
ENDOSCOPIC ANTI-REFLUX THERAPY
There will remain a cohort of patients with persistent dysplasia and metaplasia in whom optimized PPI therapy is not sufficient for reflux control, but who also are not candidates for or do not want to undergo surgical anti-reflux therapy. For these patients, endoscopic anti-reflux procedures may be a possibility. Transoral incisionless fundoplication (TIF) is an endoscopic suturing procedure that repairs the anti-reflux barrier by creating a 2-4 cm flap valve and a 270 degree fundoplication with the deployment of endoscopic fasteners. Multiple studies have shown that long term reflux control through a 10 year follow-up period after the procedure are better with TIF than with treatment with PPI therapy alone[34-39]. Further randomized trials are needed to directly compare TIF with Nissen fundoplication, however, in patients who failed RFA and optimal PPI therapy, and who are unable to undergo surgery, endoscopic TIF should be considered as a feasible option to better treat uncontrolled reflux.
CRYOTHERAPY
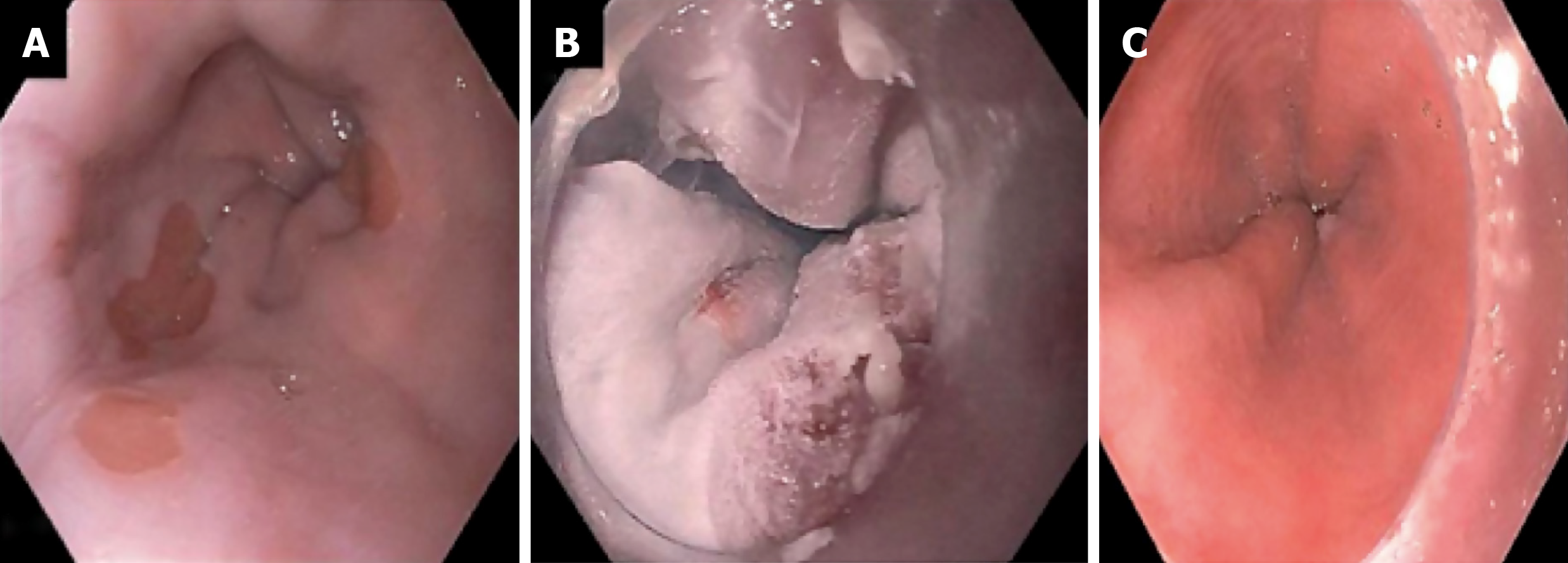
Cryotherapy is perhaps the main modality for treatment of RFA refractory BE. The mechanism of action is that application of a cryogen to the target tissue induces cell necrosis. Two cryotherapy platforms are commercially available in the United States. The first is liquid nitrogen spray cryotherapy (truFreeze, CSA Medical, Lexington, MA, United States). In this platform, a catheter is passed down the channel of 2.8 mm channel adult gastroscope, after a decompression tube is placed in the stomach over a wire. The catheter is attached to a liquid nitrogen compressor and tank. A foot-pedal activates the flow of liquid nitrogen through the catheter and freezes the target tissue to -196 degrees Celsius. Excess gas is absorbed through the decompression tube while an assistant is ensuring the stomach is not distended. Generally 2-3 freeze-thaw cycles are performed over the target tissue. Figure 1 shows endoscopy images of a patient with RFA refractory Barrett’s after multiple sessions of RFA. Full details on the set up and equipment can be found on a recent American Society of Gastrointestinal Endoscopy Technical review on cryotherapy[40].
Figure 1 Endoscopy images of a patient with radiofrequency ablation refractory Barrett’s after multiple sessions of radiofrequency ablation.
A: A patient with dysplastic Barrett’s refractory to radiofrequency ablation; B: Who was treated with liquid nitrogen spray cryotherapy; C: Achieved complete eradication of intestinal metaplasia.
There are three retrospective studies examining the efficacy of liquid nitrogen cryotherapy in BE refractory to RFA. The first study evaluated 46 patients who did not achieve complete eradication of dysplasia (CE-D) with RFA and subsequently underwent cryotherapy[41]. Of the 46 patients, 38 (83%) achieved CE-D, and 21 (46%) achieved CE-IM. The second study, from our center, evaluated 18 patients who were RFA refractory[42]. Of the 18, 13 (72%) achieved CE-D, and 9 (50%) achieved CE-IM. The third study evaluated 16 patients who were RFA refractory[43]. Of the 16, 12 (75%) achieved CE-D, and 5 (31%) achieved CE-IM. Overall these studies show that in this difficult to treat cohort, liquid nitrogen cryotherapy allows for excellent CE-D rates and acceptable CE-IM rates considering the patient population.
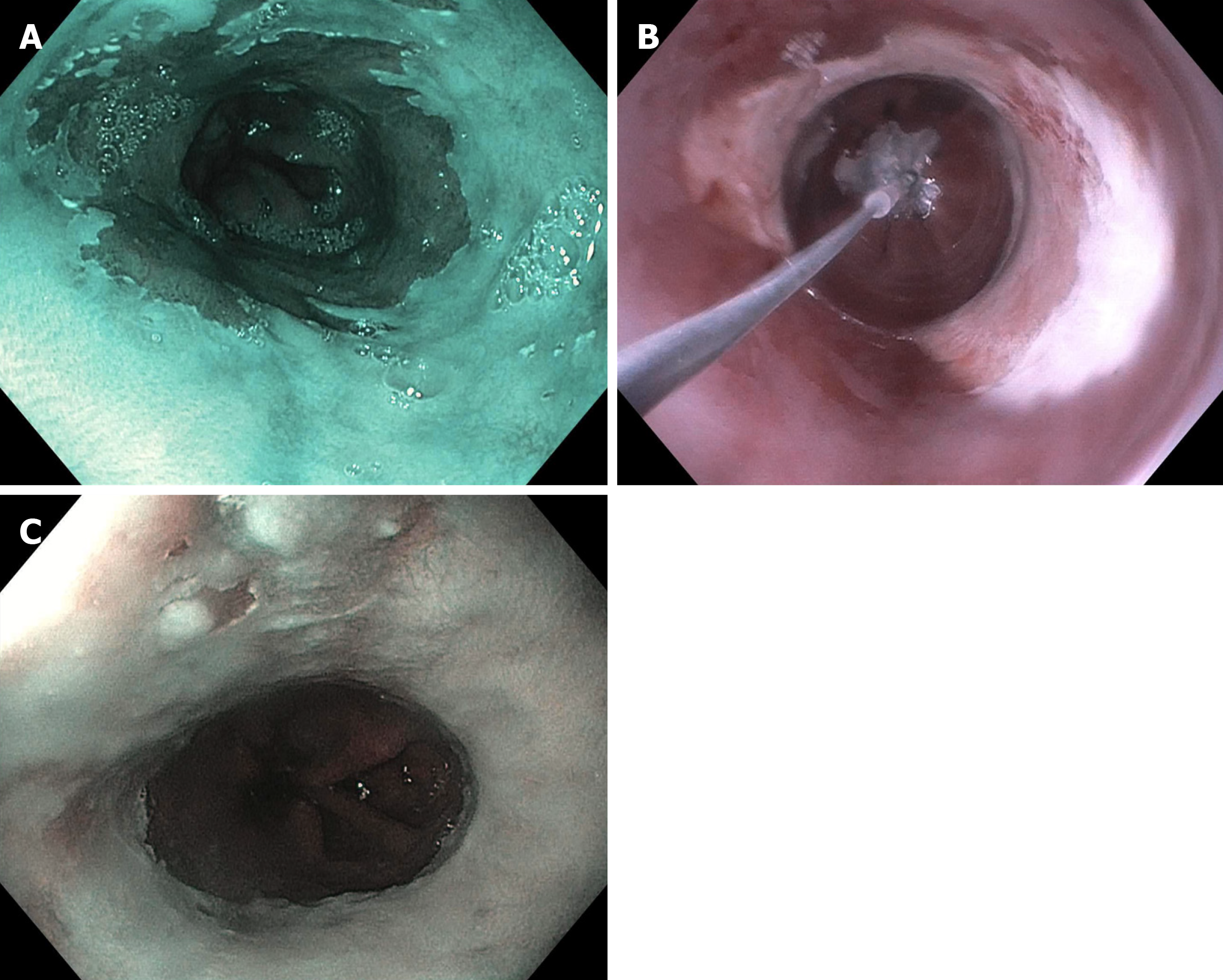
The second cryotherapy platform is a balloon-based system (C2 CryoBalloon, Pentax Medical, Redwood City, CA, United States) that has recently been developed. A catheter is passed through the channel of a therapeutic gastroscope (3.7 mm channel). The catheter is attached to battery powered handle that contains cartridges of liquid nitrous oxide. The balloon is inflated once it is positioned in the target area to be ablated and directly apposes the esophageal tissue to be ablated. Cryogen in the form of nitrous oxide is then sprayed from a diffuser, centered within the balloon, against the balloon apposing the esophageal tissue. Through contact, the esophageal tissue freezes to -78 degrees Celsius. The diffuser can be turned to spray a different quadrant of the esophagus by having an assistant turn the handle. Full details on the set up and equipment can be found on a recent ASGE Technical review on cryotherapy[40,44]. Recently the second-generation system has been developed that allows for the diffuser to be controlled (both up/down and rotational movements) by a foot pedal; and thus provides more control to the endoscopist. Given the recent release of this product, data is emerging on the use of CryoBalloon in treatment naïve patients[45]. To our knowledge, there is no study evaluating cryotherapy in RFA refractory disease published in full manuscript form. However there is currently a multi-center prospective trial evaluating this for RFA refractory disease (NCT03554356). In our limited experience, CryoBalloon cryotherapy works well for RFA refractory disease. Figure 2 shows a patient from our practice who underwent five RFA sessions, who then underwent salvage CryoBalloon cryotherapy. He was able to achieve CE-IM with two sessions of cryotherapy.
Figure 2 A patient from our practice who underwent five radiofrequency ablation sessions, who then underwent salvage CryoBalloon cryotherapy.
A: A patient with dysplastic Barrett’s refractory to radiofrequency ablation (note endoscopy image is in narrow band imaging mode); B: Who was treated with CryoBalloon cryotherapy; C: Achieved complete eradication of intestinal metaplasia (note endoscopy image is in narrow band imaging mode).
HYBRID ARGON PLASMA COAGULATION
The latest ablation modality in BE is hybrid Argon Plasma Coagulation (APC). Prior to argon plasma coagulation, a submucosal injection is performed of saline using a water jet system (ErbeJet 2; Erbe United States, Marietta, GA, United States) at high PSI settings (typically Effect 25/360 PSI-Effect 50/725 PSI in our unit). After an adequate submucosal injection is created using the lowest PSI setting, APC is performed typically at higher wattage than with standard APC (Erbe VIO D device, pulsed APC, effect 2, 60 Watts).
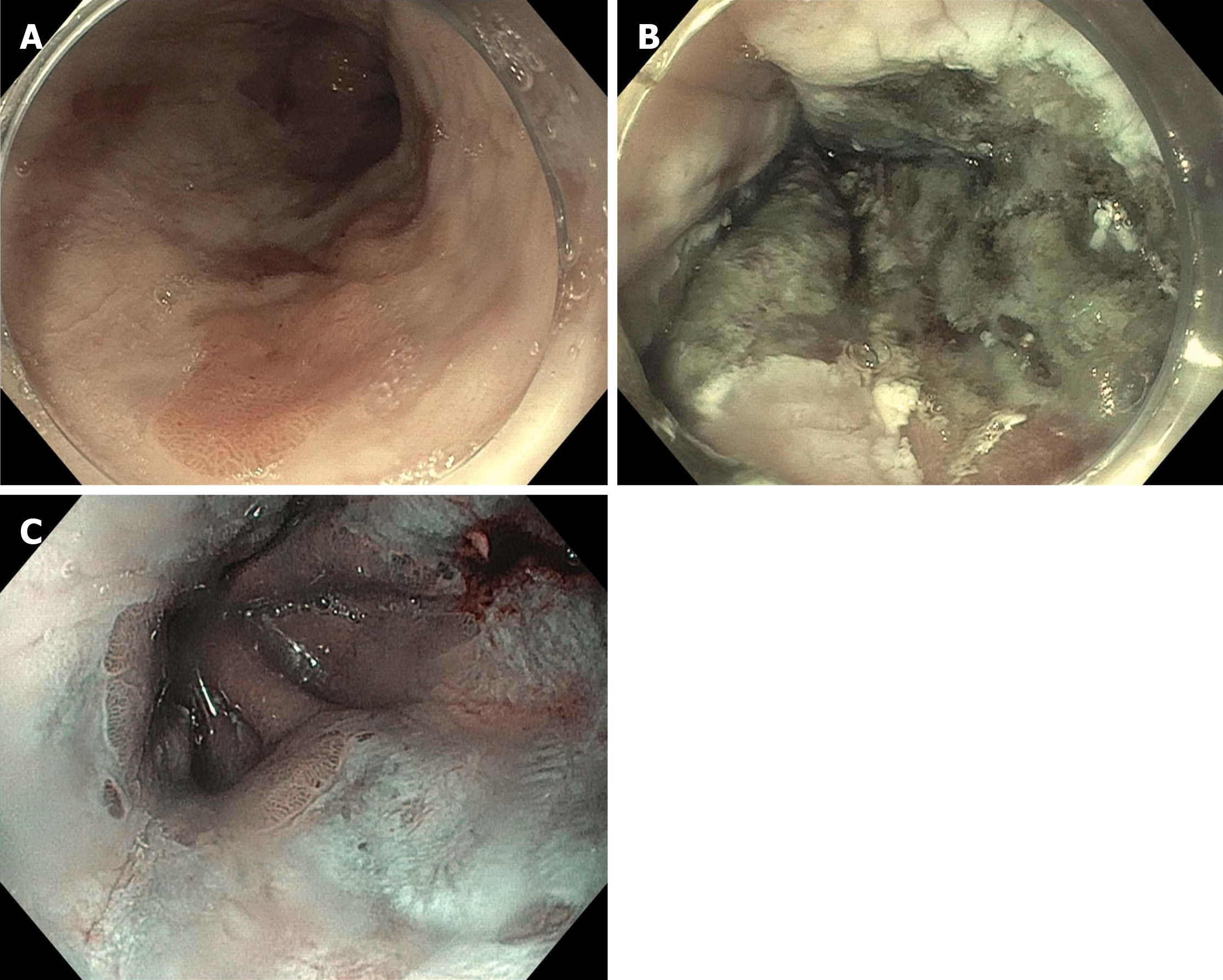
Given the novelty of this device, most data only exists for patients with treatment naïve Barrett’s after endoscopic resection of raised lesions[46]. In a prospective study of 50 treatment naïve patients, 48 (96%) were able to achieve CE-IM. In our practice, we use Hybrid APC for RFA refractory disease with excellent results. Our group published a case series of five BE patients with dysplasia who were refractory to RFA +/- cryotherapy[47]. These patients were able to achieve CE-IM with hybrid APC. Figure 3 shows a patient who underwent multiple sessions of RFA with refractory dysplasia. CE-IM was achieved after two sessions with hybrid APC.
Figure 3 A patient who underwent multiple sessions of radiofrequency ablation with refractory dysplasia.
A: A patient with dysplastic Barrett’s refractory to radiofrequency ablation; B: Who was treated with hybrid argon plasma coagulation; C: Achieved complete eradication of intestinal metaplasia (note endoscopy image is in narrow band imaging mode).
ENDOROTOR ENDOSCOPIC RESECTION
Another device that is promising for use in refractory Barrett’s esophagus is the EndoRotor® Endoscopic Resection System (Interscope Medical, Inc, Whitinsville, MA, United States). It is an automated mechanical non-thermal endoscopic resection system for use in the gastrointestinal tract for benign or pre-malignant tissue removal. It is FDA-cleared for removal of residual tissue from peripheral margins at the time of endoscopic mucosal resection in the colon. The EndoRotor system is comprised of the catheter with the cutting tool, a console (that houses the motor drive, peristaltic pump, and vacuum regulation), a foot pedal for activation of the device, and a specimen collection trap.
The 3.1 mm diameter disposable catheter is compatible with therapeutic endoscopes with a 3.2 mm or larger working channel. The foot pedal-activated console motor rotates the cutting tool in the catheter. Simultaneously, irrigation fluid is delivered between the inner wall of the braided sheath and the cutting tool, and suction is applied via the hollow lumen of the inner cutting tool, which aspirates the resected tissue onto a micron filter within the specimen trap. The catheter may be rotated to alter the orientation of the cutting window.
There has been one feasibility study evaluating the use of EndoRotor in treatment naïve neoplastic Barrett’s[48]. Fourteen patients with BE underwent EMR of T1a or T1b lesions and the subsequently underwent EndoRotor ablation of the residual Barrett’s. The study showed that the device is feasible to remove Barrett’s mucosa, with follow up endoscopy showing neosquamous re-epithelialization on follow up. However adverse events were not insignificant with six patients with intra-procedural bleeding events and nine with post-procedural pain.
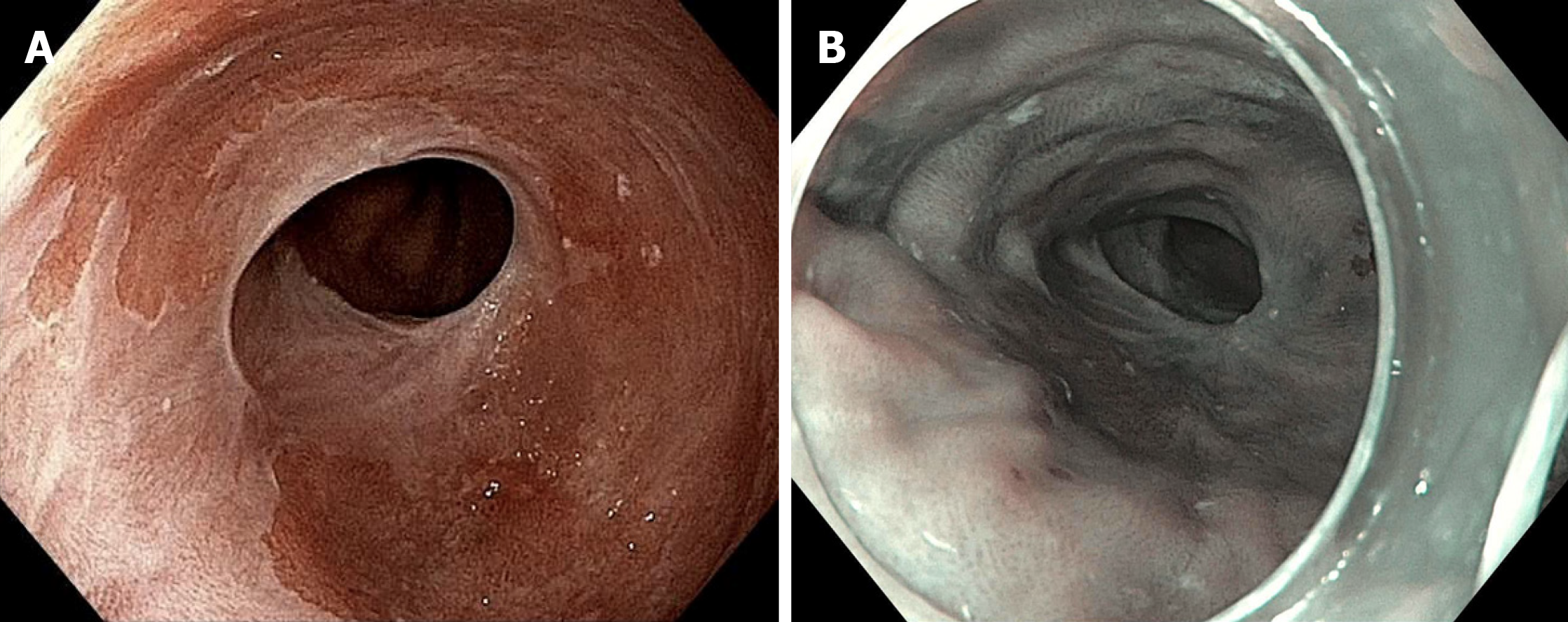
There is currently a prospective randomized study evaluating EndoRotor versus continued ablation for refractory Barrett’s (NCT03364114). In our practice we find EndoRotor useful for eradication of Barrett’s within a narrowed or strictured lumen in which ablation therapy with RFA or cryotherapy would be difficult. Figure 4 shows such a case of Barrett’s esophagus refractory to multiple sessions of RFA and cryotherapy in long segment Barrett’s (Prague C8M12). Ablation therapy was able to eradicate BE mucosa in the entire segment except for within a narrowed segment of the esophagus (patient asymptomatic) that returned high-grade dysplasia. EndoRotor therapy was applied to this narrowed area and eradication of this tissue was achieved.
Figure 4 A case of Barrett’s esophagus refractory to multiple sessions of radiofrequency ablation and cryotherapy in long segment Barrett’s.
A: A patient with dysplastic Barrett’s refractory to radiofrequency ablation and had a narrowing in the esophagus with residual dysplastic Barrett’s who was treated with the EndoRotor ablation system; B: Achieved complete eradication of intestinal metaplasia (note endoscopy image is in narrow band imaging mode).
CONCLUSION
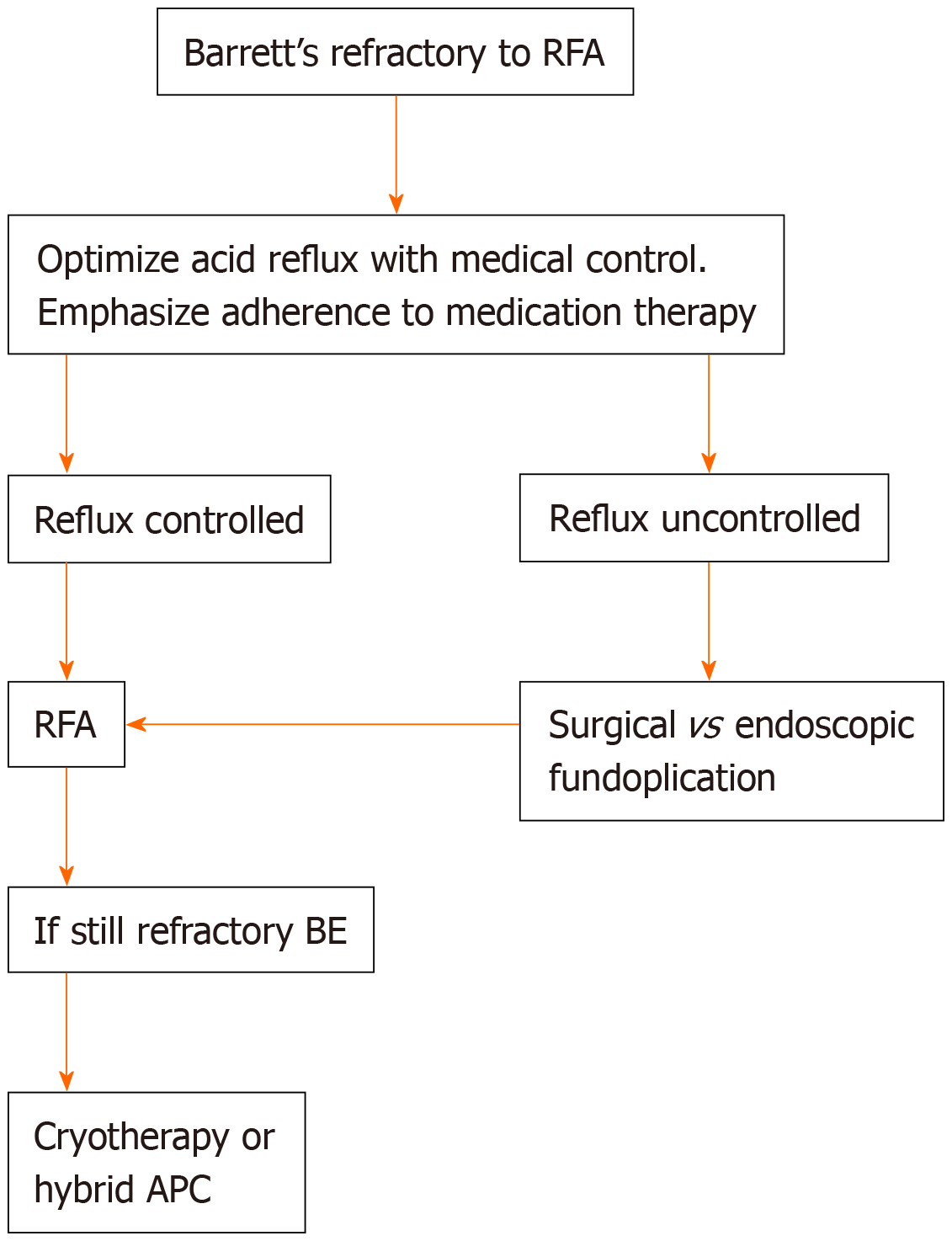
This review discusses the management of dysplastic BE that is RFA refractory. Our algorithm is to optimize acid control via medical, endoscopic, or surgical therapy depending on patient characteristics (Figure 5). If the patient is optimized on medical management then we usually opt for surgical hernia repair and fundoplication as many of these patients have large hiatal hernias precluding endoscopic fundoplication. Large areas of residual Barrett’s are usually treated with cryotherapy. Smaller areas of residual Barrett’s can be treated with cryotherapy or hybrid APC. As mentioned, EndoRotor resection is a useful tool for resection of BE mucosa in narrowed segments that may not be amenable to the ablation platforms discussed.
Figure 5 Proposed algorithm for management of Barrett’s refractory to radiofrequency ablation.
RFA: Radiofrequency ablation; BE: Barrett’s esophagus; APC: Argon plasma coagulation.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mastracci L S-Editor: Zhang L L-Editor: A E-Editor: Qi LL