Published online Apr 28, 2020. doi: 10.3748/wjg.v26.i16.1938
Peer-review started: December 30, 2019
First decision: January 19, 2020
Revised: March 30, 2020
Accepted: April 17, 2020
Article in press: April 17, 2020
Published online: April 28, 2020
Processing time: 119 Days and 19.1 Hours
Liver transplantation (LT) is the best treatment for patients with liver cancer or end stage cirrhosis, but it is still associated with a significant mortality. Therefore identifying factors associated with mortality could help improve patient management. The impact of iron metabolism, which could be a relevant therapeutic target, yield discrepant results in this setting. Previous studies suggest that increased serum ferritin is associated with higher mortality. Surprisingly iron deficiency which is a well described risk factor in critically ill patients has not been considered.
To assess the impact of pre-transplant iron metabolism parameters on post-transplant survival.
From 2001 to 2011, 553 patients who underwent LT with iron metabolism parameters available at LT evaluation were included. Data were prospectively recorded at the time of evaluation and at the time of LT regarding donor and recipient. Serum ferritin (SF) and transferrin saturation (TS) were studied as continuous and categorical variable. Cox regression analysis was used to determine mortality risks factors. Follow-up data were obtained from the local and national database regarding causes of death.
At the end of a 95-mo median follow-up, 196 patients were dead, 38 of them because of infections. In multivariate analysis, overall mortality was significantly associated with TS > 75% [HR: 1.73 (1.14; 2.63)], SF < 100 µg/L [HR: 1.62 (1.12; 2.35)], hepatocellular carcinoma [HR: 1.58 (1.15; 2.26)], estimated glomerular filtration rate (CKD EPI Cystatin C) [HR: 0.99 (0.98; 0.99)], and packed red blood cell transfusion [HR: 1.05 (1.03; 1.08)]. Kaplan Meier curves show that patients with low SF (< 100 µg/L) or high SF (> 400 µg/L) have lower survival rates at 36 mo than patients with normal SF (P = 0.008 and P = 0.016 respectively). Patients with TS higher than 75% had higher mortality at 12 mo (91.4% ± 1.4% vs 84.6% ± 3.1%, P = 0.039). TS > 75% was significantly associated with infection related death [HR: 3.06 (1.13; 8.23)].
Our results show that iron metabolism imbalance (either deficiency or overload) is associated with post-transplant overall and infectious mortality. Impact of iron supplementation or depletion should be assessed in prospective study.
Core tip: Iron is an essential element for many biological functions. Its deficiency or overload is associated with poor outcomes in many settings. Few data are available in patients undergoing liver transplantation, and more specifically on infection related deaths. Our study is the first to describe in a large number of patients, the impact or iron metabolism imbalance on mortality after liver transplantation. Our results show that both iron deficiency and overload are significantly associated with increased mortality. Further we show that transferrin saturation higher than 75% is associated with mortality.
- Citation: Fallet E, Rayar M, Landrieux A, Camus C, Houssel-Debry P, Jezequel C, Legros L, Uguen T, Ropert-Bouchet M, Boudjema K, Guyader D, Bardou-Jacquet E. Iron metabolism imbalance at the time of listing increases overall and infectious mortality after liver transplantation. World J Gastroenterol 2020; 26(16): 1938-1949
- URL: https://www.wjgnet.com/1007-9327/full/v26/i16/1938.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i16.1938
Liver transplantation (LT) is the most effective treatment for patients with end-stage liver disease. However, it is still associated with significant mortality thus promoting the search for associated factors whose optimization could improve survival. Since 2002 in France, liver graft allocation is based on the Model of End-Stage Liver Disease (MELD), which is an objective score improving 3-mo mortality prediction compared to the Child-Turcotte-Pugh score[1-3]. Other factors of mortality in addition to MELD have been studied to better manage patients waiting for LT like serum sodium[4-7], renal function[8], sarcopenia[9] and ferritin[10,11].
Serum ferritin (SF) concentration is a reproducible and routinely available biochemical marker. It is the major intracellular iron storage protein and although it correlates with body iron stores, serum level is also influenced by inflammation and cell lysis. Therefore SF increases in patients with elevated body iron stores or in many liver diseases as chronic hepatitis C, alcohol-related liver disease and non-alcoholic steatohepatitis[12] or in extra-hepatic causes like systemic inflammatory states, systemic immune-mediated diseases (rheumatoid arthritis, adult Still’s disease)[13,14], metabolic syndrome[15], in case of chronic kidney failure[16,17], or hematological disease[18,19].
The relevance of the iron burden in LT was first raised by studies suggesting that patients who undergo LT for cirrhosis related to hereditary hemochromatosis have higher morbidity and mortality[20,21], mainly due to an increased risk of infections and recurrence of hepatocellular carcinoma[22].
Results of studies investigating SF as a risk factor in LT are discordant. Increased SF was associated with an increased risk of death in patients on waiting list and following LT[10,11].
In contrast, Al-Freah et al[23] using different cut-off values and without considering serum transferrin saturation (TS), showed the lack of influence of SF on wait-list or post-transplant survival, but a significant negative impact of hepatic iron overload on 12 mo' survival. Conversely in a retrospective study addressing iron metabolism and liver iron content, Stuart et al[24] showed that increased hepatic iron concentration was a marker of the severity of the liver disease but was not associated with lower survival after LT.
Further, high SF and TS were found to be risk factors of mortality in other contexts than LT. High SF and TS are associated with increased mortality in acute-on-chronic liver failure[25] and allogeneic hematopoietic stem cell transplantation[26]. Similarly in the setting of intensive care, serum iron parameters at admission were correlated with short and long term mortality[27]. From a biochemical point of view, elevated TS is associated with the appearance of non-transferrin bound iron (NTBI) and labile plasma iron (LPI), which are toxic forms of iron which induce reactive oxygen radicals promoting oxidative stress, leading to cellular damage[28-30]. Depending on the underlying disease and the assay, NTBI can be detected starting from TS level of 50% to 75%, on the other hand, LPI is always present if TS is higher than 75%[28,31]. NTBI Imbalance of iron metabolism also fosters siderophilic bacterial infection[32,33], including pneumonia[34], with involvement of NTBI[35].
These discrepant results and the multiple factors influencing or influenced by iron metabolism suggest that more data are required to assess the relevance of iron metabolism imbalance in the management of patients with cirrhosis listed for LT. The aim of our study was to evaluate in a large retrospective cohort of liver transplanted patients the impact of pre-transplant iron metabolism imbalance on post-transplant survival.
All patients who underwent LT between January 2001 and December 2011 in our centre and with iron metabolism parameters available at the time of LT listing evaluation were included. Patients with previous history of LT, emergency LT or multiple-organ transplantation were excluded.
Following data were prospectively recorded at the time of evaluation: age, sex, body mass index (BMI) of donor and recipient, cause of cirrhosis, presence of hepatocellular carcinoma, Child-Pugh score, serum sodium, serum iron, TS, serum ferritin, cystatin C, warm and cold ischemia times, perioperative transfusion (red blood cell units, platelets, fresh frozen plasma units), duration of surgery, intensive care unit (ICU) and regular unit length of stay. MELD score was available for all patients after March 2007 and retrospectively calculated for others when INR was available.
Iron metabolism parameters (serum iron, SF, transferrin, TS) were routinely measured during pre-transplant evaluation. Serum ferritin was determined using che-miluminescence immunoassay from 2001 to 2009 (ACS 180 Bayer), then turbidimetric assay (Olympus AU 800) from 2009 to the end of the study, internal control and calibration proved consistent measurement and comparability of value between assay.
All patients had orthotopic liver transplantation with inferior vena cava preservation. The graft was harvested from a brain death donor in all cases. No organs from executed prisoners were used. After the procedure, patients were transferred to the ICU until graft function was satisfactory. Routine immunosuppression consisting on low-dose tacrolimus (TAC), mycophenolatemofetil and a short course of corticosteroids was initiated. TAC dosage was initiated at 0.1 mg/kg daily or at 0.06 mg/kg daily in case of concomitant administration of fluconazole and then adapted according to TAC blood through concentration with a target value between 6 and 10 ng/mL. No significant modification regarding the surgical procedure or the postoperative medical care was observed during the study period.
Follow-up data were obtained from the local database and the National Biomedicine Agency database in March 2017. The following complications are prospectively recorded during the follow-up: Anastomotic complications (arterial, venous, biliary), cardiovascular event, renal failure (dialysis), graft rejection.
Patients lost to follow up were considered to be dead. Causes of death were prospectively recorded and categorized as: Cancer (including recurrence of he-patocellular carcinoma), infection related [any causes of bacterial, viral (excluding chronic hepatitis) or fungal infection identified as the main cause leading to death], recurrence of the initial liver disease, graft failure, cardiovascular event, other causes, and undetermined.
This study protocol conformed to the declaration of Helsinki and was approved by the Rennes University Ethics Committee. According to French law, the corresponding database was declared to the “National Committee of Informatics and Freedom” (CNIL, n°96-025).
Quantitative variables are described as median [quartiles] and qualitative variables are presented as number and percentage (%).
As SF distribution was skewed and exhibited nonlinearity (according to martingale residuals) in the Cox regression model, it was studied as a categorical variable using tertiles (lower than 100 µg/L - between 100 and 400 µg/L - higher than 400 µg/L). TS was studied as a categorical variable with a cut-off value of 75% as at this level NTBI and LPI are always present[28].
Clinically relevant variables and variables associated with mortality in univariate analysis (Cox regression) with a P value < 0.2 were included in the multivariate analysis. Multivariate Cox regression analysis was performed with backward stepwise selection of variable according to likelihood ratio.
Survival curves were established using the Kaplan-Meier method and compared using the Log-rank test.
Data were analyzed using version 22.0 of SPSS (SPSS Inc., Chicago, IL, United States). P < 0.05 was considered significant with a two-tailed test.
A total of 553 patients underwent LT during the study period with iron metabolism parameters available at the time of evaluation. Their clinical and biochemical characteristics are described in Table 1.
| Variable | n = 553 |
| Age, yr | 55 (49-60.5) |
| Sex male/female | 414 (74.9)/139 (25.1) |
| BMI, kg/m² | 26.2 (23.3-29.7) |
| Iron metabolism | |
| Serum iron, µmol/L | 21.9 (1.6; 57.9) |
| Transferrin, g/L | 2.10 (1.5-2.5) |
| Transferrin saturation, % | 43.6 (25.1-74.5) |
| Transferrin saturation > 75% | 136 (24.6) |
| Serum ferritin, µg/L | 241 (75.5-593.5) |
| < 100 | 168 (30.4) |
| 100-400 | 185 (33.5) |
| > 400 | 200 (36.2) |
| Cirrhosis etiology | |
| Alcohol | 340 (61.5) |
| Viral hepatitis C | 89 (16.1) |
| Viral hepatitis B | 18 (3.3) |
| Primary sclerosing cholangitis | 20 (3.6) |
| Primary biliary cholangitis | 12 (2.2) |
| Auto-immune | 10 (1.8) |
| Hemochromatosis | 17 (3.0) |
| Others | 47 (8.4) |
| Hepatocellular carcinoma | 214 (38.7) |
| Child pugh score | |
| A | 206 (39.1) |
| B | 158 (30.0) |
| C | 163 (31.0) |
| MELD | 15.1 (10.6-20.7) |
| Donor age, yr | 49 (37-62) |
| Donor BMI, kg/m2 | 24.3 (21.9-27.6) |
| Cold ischemia, minutes | 592 (446-723) |
| Perioperative transfusion | |
| Packed red cell, n | 5 (2-8) |
| Fresh frozen plasma, n | 6 (2-9) |
| ICU length of stay, d | 4 (3-7) |
The median age at evaluation for LT was 55 (49-60.5) years. They were preferentially men (74.9%) with alcohol-related liver disease (61.5%). The median SF was 241 (75.5-593.5) µg/L, 168 patients had SF < 100 µg/L and 200 had SF > 400 µg/L. The median TS was 43.6% (25.1%-74.5%) with 24.6% of patients having a TS higher than 75%. MELD was available in 407 (73.6%) patients and median MELD was 15.1 (10.6-20.7). Median time between listing and LT was 4 (2-9) mo. Median follow-up time after LT was 95 (55-126) mo. At the end of the follow-up period, 5 patients were lost to follow up, and 196 patients died, 38 (6.9%) of died from infection. Of them 13 died of septicaemia, 12 of pulmonary bacterial infection, 5 of peritonitis, 3 of angiocholitis, 3 of severe viral infection and 2 of fungal infection. Patient outcomes and causes of death are summarized in Table 2.
| n (%) | |
| Dead | 196 |
| Lost to follow up | 5 |
| Causes of death | |
| Cancer | 41 (20.92) |
| Infection | 38 (19.39) |
| Recurrence of initial liver disease | 30 (15.31) |
| Graft failure | 12 (6.12) |
| Cardio vascular event | 28 (14.29) |
| Others | 31 (15.82) |
| Undetermined | 16 (8.16) |
In univariate analysis, variable associated (P < 0.2) with overall mortality were: fresh frozen plasma transfusion units (P < 0.001), packed red blood cell transfusion (P < 0.001), estimated glomerular filtration rate through the CKD Epi Cystatin C equation (P = 0.01), duration of surgery (P = 0.07), cirrhosis etiology (P = 0.07), SF (P = 0.08), TS > 75% (P = 0.08), ascites (P = 0.04), albumin (P = 0.10) and Cystatin C (P = 0.11).
Results from the multivariate analysis are reported in Table 3. In multivariate analysis, TS higher than 75% was significantly associated with mortality [HR: 1.73 (1.14; 2.63)]. Low SF was significantly associated with mortality [HR: 1.62 (1.12; 2.35)] whereas high SF was not significant.
| Parameters | HR (95%CI) | P value |
| Hepatocellular carcinoma (yes/no) | 1.58 (1.15; 2.16) | 0.004 |
| eGFR CKD EPI Cystatin C (mL/min) | 0.99 (0.98; 0.99) | 0.01 |
| Cirrhosis aetiology (HCV as reference) | 0.028 | |
| Alcohol | 0.57 (0.39; 0.83) | 0.003 |
| Hepatitis B virus | 0.50 (0.19; 1.28) | 0.14 |
| Other | 0.66 (0.41; 1.08) | 0.10 |
| Transferrin saturation > 75% | 1.73 (1.14; 2.63) | 0.01 |
| Ferritin (100-400 µg/L as reference) | 0.009 | |
| < 100 µg/L | 1.62 (1.12; 2.35) | 0.01 |
| > 400 µg/L | 0.90 (0.59; 1.37) | 0.63 |
| Packed red blood cell (per unit) | 1.05 (1.03; 1.08) | < 0.001 |
Because one could consider that abnormal SF or TS may only be markers of advanced liver disease, we performed an analysis entering the MELD score in the final model. MELD was not significantly associated with mortality [HR: 1.00 (0.96; 1.03), P = 0.87], this did not change significance of SF or TS, nor significantly changed their Hazard Ratio.
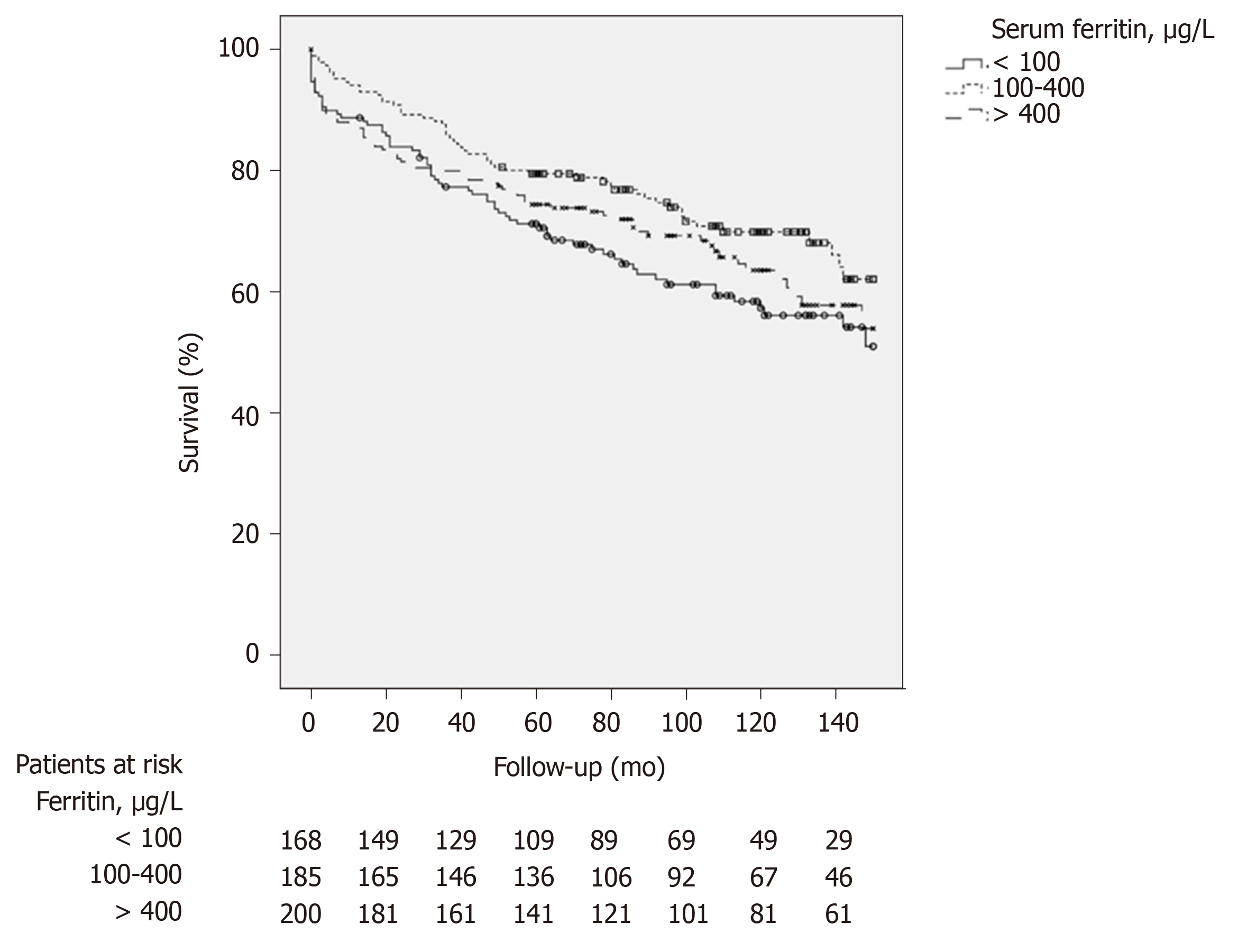
Kaplan Meier survival analysis was performed to explore the relationship between serum ferritin and overall survival. Kaplan Meier survival curves suggest that patients with low SF and high SF have a lower survival than patients with SF within the normal range (Figure 1). This difference was not statistically significant between the three groups over the whole study period (P = 0.07). But short-term survival analysis restricted to 6, 12 and 36 mo of follow-up showed a significant difference between the three groups (P = 0.016 at 6 mo, P = 0.026 at 12 mo and P = 0.032 at 36 mo). At 36 mo, patients with ferritin < 100 µg/L and patients with ferritin > 400 µg/L had lower survival than patients with ferritin 100-400 µg/L (P = 0,008 and P = 0,016 respectively).
Kaplan Meier survival curves show that overall survival was similar between the two groups of TS (lower or higher than 75%) (P = 0.08) (Figure 2). However short term survival analysis restricted to 6 and 12 mo of follow-up showed a significant difference with an increased mortality in patients with TS higher than 75% (92.6% ± 1.3 vs 86.0% ± 3.0, P = 0.012 at 6 mo and 91.4% ± 1.4% vs 84.6% ± 3.1%, P = 0.039 at 12 mo) suggesting an impact of TS on the early phase after LT.
Because iron overload and NTBI are thought to promote sepsis, we then addressed the impact of iron metabolism on infection related deaths and infections after LT.
In univariate analysis, factors associated (P < 0.2) with infection related deaths were: ICU length of stay (P < 0.001), estimated glomerular filtration rate through the CKD Epi Cystatin C equation (P = 0.03), recipient BMI (P = 0.07), packed red blood cell transfusion (P = 0.07), TS > 75% (P = 0.15), SF (P = 0.16), bilirubin (P = 0.17), and fresh frozen plasma transfusion (P = 0.17).
In multivariate analysis using Cox regression (Table 4), increased TS was significantly associated with the risk of infection related deaths [HR: 3.06 (1.13; 8.23)]. SF was associated with infection related deaths with high SF associated with lower infection related death [HR: 0.35 (0.12; 0.98)] whereas low SF was not significant.
| Parameters | HR (95%CI) | P value |
| Transferrin saturation > 75% | 3.06 (1.13; 8.23) | 0.02 |
| Ferritin (100-400 µg/L as reference) | 0.02 | |
| < 100 µg/L | 1.74 (0.77; 3.93) | 0.17 |
| > 400 µg/L | 0.35 (0.12; 0.98) | 0.48 |
| eGFR CKD EPI Cystatin C (mL/min) | 0.98 (0.97; 0.99) | 0.03 |
| ICU length of stay (d) | 1.02 (1.01; 1.03) | < 0.01 |
In our study, we examined the impact of iron metabolism at the time of evaluation for LT on post-transplant mortality. Our results show that TS > 75% and low SF were risk factors of overall mortality. This suggests that iron metabolism is a double-edged sword as both overload and deficiency are associated with mortality. Kaplan Meier survival curves show that increased mortality in patients with TS > 75% is seen at 6 and 12 mo after LT. Further our results show that TS > 75% was significantly associated with infection related deaths after LT.
The first interesting results of our study was the surprising fact that low SF was associated with mortality while high SF was not, contrary to other studies[10,11]. Because iron metabolism can be either in excess or deficiency situation, we think that both must be considered. Our results show that iron deficiency and overload were both risk factors of overall mortality. This U shape association is consistent with previous finding. Iron deficiency has already been demonstrated as a risk factor of mortality in ICU[36-38] and as a risk factor of infections because of immune depression induced by iron deficiency[39-44]. On the other hand, iron supplementation particularly by perfusion is associated with an increased risk of infections[45-47] and low SF is a protective host mechanism[48]. This two-way effect could explain the discrepant results regarding the impact of SF on mortality after LT[11,23].
Weismüller et al[11] showed that SF ≥ 365 µg/L in combination with TS < 55% before LT was an independent risk factor for mortality following LT, suggesting an impact of both iron overload and other factors (acute phase, immune response). However the cut-off level of < 55% for TS was chosen by receiver operating curve analysis in a subgroup of patients with SF > 365 µg/L with the goal to distinguish which patients survived. Although this may represent iron deficiency or inflammation, there is little information on the potential mechanisms by which low TS could be associated with increased mortality. Further, multivariate analysis was only performed using a binary variable distinguishing patients with TS < 55% and SF ≥ 365 µg/L to all others, without describing multivariate results regarding SF or TS separately.
We choose to assess TS from a physiological point of view, in agreement with the appearance and toxicity of NTBI and LPI we choose high TS > 75% as a cut-off[28]. This might be considered a better surrogate of functional iron overload, as it represents a high level of biologically available iron in the bloodstream. Our multivariate analysis taking into account TS > 75% shows that high serum ferritin is not associated with overall mortality. Further high serum ferritin is associated with a lower infection related mortality, while TS > 75% is associated with higher infection related mortality. This emphasizes the paradoxical situation between functional iron overload (TS > 75%) and high serum ferritin that may only reflect inflammation as ferritin is also an acute phase protein.
For Al-Freah et al[23] SF as a categorical variable (> or < 300 µg/L) is not associated with post LT survival, but patients with explant siderosis grade ≥ 2 had inferior 12-mo post-LT survival (P = 0.03). As the fast and efficient uptake of NTBI by liver parenchymal cell is well described[49], and is a major mechanism for liver siderosis, this could be in accordance with our result showing that high TS is a risk factor. However TS was not assessed in this study.
We think that a major limitation of these study is the lack of consideration for iron deficiency. Iron deficiency can affect a significant part of the population, even more in the setting of end-stage liver disease as chronic bleeding and frailty are common. Actually one third of our patient had a serum ferritin lower than 100 µg/L that can be considered as low in this setting. This may be a major cause of discrepancy between studies and this may also explain the non-linearity between SF and mortality that prevent its simple use as a continuous variable in Cox regression analysis.
The use of different SF cut-offs across studies is in part explained by the heterogeneity of biochemical methods used by centres and laboratories and the lack of international standards and reference materials. Moreover, SF may be affected by several factors besides iron metabolism, especially in the context of liver disease and inflammation. Therefore we think one should more consider an indication toward iron deficiency or overload rather than a definite cut-off that is hard to define in this setting.
Iron overload with high SF and TS have been shown to be risk factors of mortality in other situations than LT[25,27,50,51]. High SF has been demonstrated to be a risk factor of infections after kidney transplantation[52]. Chow et al[53] recently shown that increased levels of serum iron were independently associated with an increased risk for several types of infection and deaths. Recently, a second study of Chow et al[54] also showed a link between high SF, high Hepcidin and low serum iron, with infection after LT.
Our finding that TS higher than 75% is associated with increased overall and infection related mortality support our initial hypothesis of NTBI and LPI production, a toxic free iron present in case of TS > 75%[28,30]. Increasing iron availability with high TS and production of NTBI/LPI promotes development of bacterial infection via increased bacterial growth. Further our results suggest that these patients may be more prone to infection in the post-operative period.
Overall our results are in accordance with the fact that iron is an essential nutrient for all organisms but can paradoxically become toxic, inducing oxidative stress in case of iron overload[29,30]. Iron deficiency leads to immunosuppression, which in turn increases the risk of infections. Conversely, iron overload provokes production of free radicals and oxidative damage and influence pathogen growth.
In conclusion, our results show that imbalance in iron metabolism is significantly associated with sepsis-related and overall mortality in the post-transplant period. The role of iron should be prospectively studied in these patients to identify patients at risk and guide optimization of iron metabolism management to improve post-transplant survival.
Liver transplantation (LT) is the best treatment for patients with liver cancer or end stage cirrhosis, but it is still associated with a significant mortality. Therefore identifying factors associated with mortality could help improve patient management. The impact of iron metabolism, which could be a relevant therapeutic target, yield discrepant results in this setting. Previous studies suggest that increased serum ferritin is associated with higher mortality. Surprisingly iron deficiency which is a well described risk factor in critically ill patients has not been considered.
Iron metabolism could be easily corrected before liver transplantation, thus assessing its impact on mortality is crtitical before designing clinical trials in that purpose.
The main objectives, the objectives that were realized, and the significance of realizing these objectives for future research in this field should be described in detail.
Retrospective cohort analysis with Cox multivariate Regression. Survival was also estimated though Kaplan Meier analysis.
A large number of patients was studied (553) and followed for 95 mo. At the end of follow-up 196 patients were dead, 38 of them because of infections. In multivariate analysis, overall mortality was significantly associated with transferrin saturation (TS) higher than 75% [HR: 1.73 (1.14; 2.63)], serum ferritin lower than 100 µg/L [HR: 1.62 (1.12; 2.35)], hepatocellular carcinoma [HR: 1.58 (1.15; 2.26)], estimated glomerular filtration rate (CKD EPI Cystatin C) [HR: 0.99 (0.98; 0.99)], and packed red blood cell transfusion [HR: 1.05 (1.03; 1.08)]. Kaplan Meier curves show that patients with low serum ferritin (< 100 µg/L) or high serum ferritin (> 400 µg/L) have lower survival rates at 36 mo than patients with normal SF (P = 0,008 and P = 0,016 respectively). Patients with TS higher than 75% had higher mortality at 12 mo (91.4% ± 1.4% vs 84.6% ± 3.1%, P = 0.039). Moreover TS higher than 75% was significantly associated with infection related death [HR: 3.06 (1.13; 8.23)].
Our study is the first to describe the respective impact of iron deficiency in patient undergoing liver transplantation. This suggests that active management of iron deficiency with iron supplementation before liver transplantation could significantly improves outcome after liver transplantation. Further this is the first study showing that high TS is associated with higher short term mortality. This suggests that exposure to toxic forms of iron increase cellular damages in the perioperative period and that treating iron overload before liver transplantation could improves outcome.
A prospective randomized clinical trial is required to assess the beneficial effect of correction iron metabolism imbalance before liver transplantation.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: France
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aygun C, Mousa HA, Murotomi K S-Editor: Gong ZM L-Editor: A E-Editor: Ma YJ
| 1. | Ghobrial RM, Gornbein J, Steadman R, Danino N, Markmann JF, Holt C, Anselmo D, Amersi F, Chen P, Farmer DG, Han S, Derazo F, Saab S, Goldstein LI, McDiarmid SV, Busuttil RW. Pretransplant model to predict posttransplant survival in liver transplant patients. Ann Surg. 2002;236:315-322; discussion 322-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 123] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 2. | Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D'Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3462] [Cited by in RCA: 3671] [Article Influence: 153.0] [Reference Citation Analysis (0)] |
| 3. | Wiesner R, Edwards E, Freeman R, Harper A, Kim R, Kamath P, Kremers W, Lake J, Howard T, Merion RM, Wolfe RA, Krom R; United Network for Organ Sharing Liver Disease Severity Score Committee. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1825] [Cited by in RCA: 1863] [Article Influence: 84.7] [Reference Citation Analysis (0)] |
| 4. | Biggins SW, Rodriguez HJ, Bacchetti P, Bass NM, Roberts JP, Terrault NA. Serum sodium predicts mortality in patients listed for liver transplantation. Hepatology. 2005;41:32-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 292] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 5. | Ruf AE, Kremers WK, Chavez LL, Descalzi VI, Podesta LG, Villamil FG. Addition of serum sodium into the MELD score predicts waiting list mortality better than MELD alone. Liver Transpl. 2005;11:336-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 321] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 6. | Luca A, Angermayr B, Bertolini G, Koenig F, Vizzini G, Ploner M, Peck-Radosavljevic M, Gridelli B, Bosch J. An integrated MELD model including serum sodium and age improves the prediction of early mortality in patients with cirrhosis. Liver Transpl. 2007;13:1174-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 187] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 7. | Kim WR, Biggins SW, Kremers WK, Wiesner RH, Kamath PS, Benson JT, Edwards E, Therneau TM. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359:1018-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 935] [Cited by in RCA: 1046] [Article Influence: 61.5] [Reference Citation Analysis (0)] |
| 8. | Uguen T, Jezequel C, Ropert M, Houssel-Debry P, Latournerie M, Lainé F, Deugnier Y, Vigneau C, Boudjema K, Guyader D, Bardou-Jacquet E. Pretransplant renal function according to CKD-EPI cystatin C equation is a prognostic factor of death after liver transplantation. Liver Int. 2016;36:547-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Durand F, Buyse S, Francoz C, Laouénan C, Bruno O, Belghiti J, Moreau R, Vilgrain V, Valla D. Prognostic value of muscle atrophy in cirrhosis using psoas muscle thickness on computed tomography. J Hepatol. 2014;60:1151-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 285] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 10. | Walker NM, Stuart KA, Ryan RJ, Desai S, Saab S, Nicol JA, Fletcher LM, Crawford DH. Serum ferritin concentration predicts mortality in patients awaiting liver transplantation. Hepatology. 2010;51:1683-1691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Weismüller TJ, Kirchner GI, Scherer MN, Negm AA, Schnitzbauer AA, Lehner F, Klempnauer J, Schlitt HJ, Manns MP, Strassburg CP. Serum ferritin concentration and transferrin saturation before liver transplantation predict decreased long-term recipient survival. Hepatology. 2011;54:2114-2124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Bell H, Skinningsrud A, Raknerud N, Try K. Serum ferritin and transferrin saturation in patients with chronic alcoholic and non-alcoholic liver diseases. J Intern Med. 1994;236:315-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 83] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Zandman-Goddard G, Shoenfeld Y. Ferritin in autoimmune diseases. Autoimmun Rev. 2007;6:457-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 148] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 14. | Kong XD, Xu D, Zhang W, Zhao Y, Zeng X, Zhang F. Clinical features and prognosis in adult-onset Still's disease: a study of 104 cases. Clin Rheumatol. 2010;29:1015-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Abril-Ulloa V, Flores-Mateo G, Solà-Alberich R, Manuel-y-Keenoy B, Arija V. Ferritin levels and risk of metabolic syndrome: meta-analysis of observational studies. BMC Public Health. 2014;14:483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 16. | Hasuike Y, Nonoguchi H, Tokuyama M, Ohue M, Nagai T, Yahiro M, Nanami M, Otaki Y, Nakanishi T. Serum ferritin predicts prognosis in hemodialysis patients: the Nishinomiya study. Clin Exp Nephrol. 2010;14:349-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Kalantar-Zadeh K, Kalantar-Zadeh K, Lee GH. The fascinating but deceptive ferritin: to measure it or not to measure it in chronic kidney disease? Clin J Am Soc Nephrol. 2006;1 Suppl 1:S9-S18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 132] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 18. | Lim ZY, Fiaccadori V, Gandhi S, Hayden J, Kenyon M, Ireland R, Marsh J, Ho AY, Mufti GJ, Pagliuca A. Impact of pre-transplant serum ferritin on outcomes of patients with myelodysplastic syndromes or secondary acute myeloid leukaemia receiving reduced intensity conditioning allogeneic haematopoietic stem cell transplantation. Leuk Res. 2010;34:723-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Tunçcan OG, Yegin ZA, Ozkurt ZN, Erbaş G, Akı SZ, Senol E, Yağcı M, Sucak G. High ferritin levels are associated with hepatosplenic candidiasis in hematopoietic stem cell transplant candidates. Int J Infect Dis. 2010;14 Suppl 3:e104-e107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Crawford DH, Fletcher LM, Hubscher SG, Stuart KA, Gane E, Angus PW, Jeffrey GP, McCaughan GW, Kerlin P, Powell LW, Elias EE. Patient and graft survival after liver transplantation for hereditary hemochromatosis: Implications for pathogenesis. Hepatology. 2004;39:1655-1662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Kowdley KV, Brandhagen DJ, Gish RG, Bass NM, Weinstein J, Schilsky ML, Fontana RJ, McCashland T, Cotler SJ, Bacon BR, Keeffe EB, Gordon F, Polissar N; National Hemochromatosis Transplant Registry. Survival after liver transplantation in patients with hepatic iron overload: the national hemochromatosis transplant registry. Gastroenterology. 2005;129:494-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 22. | Dar FS, Faraj W, Zaman MB, Bartlett A, Bomford A, O'Sullivan A, O'Grady J, Heneghan M, Rela M, Heaton ND. Outcome of liver transplantation in hereditary hemochromatosis. Transpl Int. 2009;22:717-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Al-Freah MA, Kriese S, Foxton MR, Quaglia A, Bomford A, Heaton ND, O'Grady JG, Agarwal K, Wendon JA, Heneghan MA. The association of pretransplant ferritin level with waiting list and post-transplant survival. Does ferritin actually predict outcome? Transpl Int. 2013;26:1070-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Stuart KA, Fletcher LM, Clouston AD, Lynch SV, Purdie DM, Kerlin P, Crawford DH. Increased hepatic iron and cirrhosis: no evidence for an adverse effect on patient outcome following liver transplantation. Hepatology. 2000;32:1200-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Bruns T, Nuraldeen R, Mai M, Stengel S, Zimmermann HW, Yagmur E, Trautwein C, Stallmach A, Strnad P. Low serum transferrin correlates with acute-on-chronic organ failure and indicates short-term mortality in decompensated cirrhosis. Liver Int. 2017;37:232-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 26. | Koreth J, Antin JH. Iron overload in hematologic malignancies and outcome of allogeneic hematopoietic stem cell transplantation. Haematologica. 2010;95:364-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Tacke F, Nuraldeen R, Koch A, Strathmann K, Hutschenreuter G, Trautwein C, Strnad P. Iron Parameters Determine the Prognosis of Critically Ill Patients. Crit Care Med. 2016;44:1049-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 28. | Le Lan C, Loréal O, Cohen T, Ropert M, Glickstein H, Lainé F, Pouchard M, Deugnier Y, Le Treut A, Breuer W, Cabantchik ZI, Brissot P. Redox active plasma iron in C282Y/C282Y hemochromatosis. Blood. 2005;105:4527-4531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 114] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 29. | Koskenkorva-Frank TS, Weiss G, Koppenol WH, Burckhardt S. The complex interplay of iron metabolism, reactive oxygen species, and reactive nitrogen species: insights into the potential of various iron therapies to induce oxidative and nitrosative stress. Free Radic Biol Med. 2013;65:1174-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 313] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 30. | Brissot P, Ropert M, Le Lan C, Loréal O. Non-transferrin bound iron: a key role in iron overload and iron toxicity. Biochim Biophys Acta. 2012;1820:403-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 470] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 31. | de Swart L, Hendriks JC, van der Vorm LN, Cabantchik ZI, Evans PJ, Hod EA, Brittenham GM, Furman Y, Wojczyk B, Janssen MC, Porter JB, Mattijssen VE, Biemond BJ, MacKenzie MA, Origa R, Galanello R, Hider RC, Swinkels DW. Second international round robin for the quantification of serum non-transferrin-bound iron and labile plasma iron in patients with iron-overload disorders. Haematologica. 2016;101:38-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 32. | Willemetz A, Beatty S, Richer E, Rubio A, Auriac A, Milkereit RJ, Thibaudeau O, Vaulont S, Malo D, Canonne-Hergaux F. Iron- and Hepcidin-Independent Downregulation of the Iron Exporter Ferroportin in Macrophages during Salmonella Infection. Front Immunol. 2017;8:498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 33. | Ganz T. Systemic iron homeostasis. Physiol Rev. 2013;93:1721-1741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 623] [Cited by in RCA: 773] [Article Influence: 64.4] [Reference Citation Analysis (0)] |
| 34. | Michels KR, Zhang Z, Bettina AM, Cagnina RE, Stefanova D, Burdick MD, Vaulont S, Nemeth E, Ganz T, Mehrad B. Hepcidin-mediated iron sequestration protects against bacterial dissemination during pneumonia. JCI Insight. 2017;2:e92002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 35. | Stefanova D, Raychev A, Arezes J, Ruchala P, Gabayan V, Skurnik M, Dillon BJ, Horwitz MA, Ganz T, Bulut Y, Nemeth E. Endogenous hepcidin and its agonist mediate resistance to selected infections by clearing non-transferrin-bound iron. Blood. 2017;130:245-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 102] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 36. | Uscinska E, Sobkowicz B, Sawicki R, Kiluk I, Baranicz M, Stepek T, Dabrowska M, Szmitkowski M, Musial WJ, Tycinska AM. Parameters influencing in-hospital mortality in patients hospitalized in intensive cardiac care unit: is there an influence of anemia and iron deficiency? Intern Emerg Med. 2015;10:337-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 37. | Patteril MV, Davey-Quinn AP, Gedney JA, Murdoch SD, Bellamy MC. Functional iron deficiency, infection and systemic inflammatory response syndrome in critical illness. Anaesth Intensive Care. 2001;29:473-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 38. | Muñoz M, Romero A, Morales M, Campos A, García-Erce JA, Ramírez G. Iron metabolism, inflammation and anemia in critically ill patients. A cross-sectional study. Nutr Hosp. 2005;20:115-120. [PubMed] [DOI] [Full Text] |
| 39. | Wander K, Shell-Duncan B, McDade TW. Evaluation of iron deficiency as a nutritional adaptation to infectious disease: an evolutionary medicine perspective. Am J Hum Biol. 2009;21:172-179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 40. | Oppenheimer SJ. Iron and its relation to immunity and infectious disease. J Nutr. 2001;131:616S-633S; discussion 633S-635S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 410] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 41. | Ekiz C, Agaoglu L, Karakas Z, Gurel N, Yalcin I. The effect of iron deficiency anemia on the function of the immune system. Hematol J. 2005;5:579-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 162] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 42. | Kumar V, Choudhry VP. Iron deficiency and infection. Indian J Pediatr. 2010;77:789-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 43. | Tansarli GS, Karageorgopoulos DE, Kapaskelis A, Gkegkes I, Falagas ME. Iron deficiency and susceptibility to infections: evaluation of the clinical evidence. Eur J Clin Microbiol Infect Dis. 2013;32:1253-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 44. | de Pontual L. [Iron and susceptibility to infections]. Arch Pediatr. 2017;24:5S14-5S17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 45. | Hoen B, Renoult E, Jonon B, Kessler M. Septicemia due to Yersinia enterocolitica in a long-term hemodialysis patient after a single desferrioxamine administration. Nephron. 1988;50:378-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 46. | Fishbane S. Review of issues relating to iron and infection. Am J Kidney Dis. 1999;34:S47-S52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 47. | Patruta SI, Edlinger R, Sunder-Plassmann G, Hörl WH. Neutrophil impairment associated with iron therapy in hemodialysis patients with functional iron deficiency. J Am Soc Nephrol. 1998;9:655-663. [PubMed] |
| 48. | Jurado RL. Iron, infections, and anemia of inflammation. Clin Infect Dis. 1997;25:888-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 256] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 49. | Brissot P, Wright TL, Ma WL, Weisiger RA. Efficient clearance of non-transferrin-bound iron by rat liver. Implications for hepatic iron loading in iron overload states. J Clin Invest. 1985;76:1463-1470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 195] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 50. | Oikonomou T, Goulis I, Soulaidopoulos S, Karasmani A, Doumtsis P, Tsioni K, Mandala E, Akriviadis E, Cholongitas E. High serum ferritin is associated with worse outcome of patients with decompensated cirrhosis. Ann Gastroenterol. 2017;30:217-224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 51. | Anastasiou OE, Kälsch J, Hakmouni M, Kucukoglu O, Heider D, Korth J, Manka P, Sowa JP, Bechmann L, Saner FH, Paul A, Gerken G, Baba HA, Canbay A. Low transferrin and high ferritin concentrations are associated with worse outcome in acute liver failure. Liver Int. 2017;37:1032-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 52. | Fernández-Ruiz M, López-Medrano F, Andrés A, Morales JM, Lumbreras C, San-Juan R, Polanco N, González E, Aguado JM. Serum iron parameters in the early post-transplant period and infection risk in kidney transplant recipients. Transpl Infect Dis. 2013;15:600-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 53. | Chow JK, Werner BG, Ruthazer R, Snydman DR. Increased serum iron levels and infectious complications after liver transplantation. Clin Infect Dis. 2010;51:e16-e23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 54. | Chow JKL, Ganz T, Ruthazer R, Simpson MA, Pomfret EA, Gordon FD, Westerman ME, Snydman DR. Iron-related markers are associated with infection after liver transplantation. Liver Transpl. 2017;23:1541-1552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |