Published online Apr 14, 2020. doi: 10.3748/wjg.v26.i14.1638
Peer-review started: December 19, 2019
First decision: February 18, 2020
Revised: March 6, 2020
Accepted: March 14, 2020
Article in press: March 14, 2020
Published online: April 14, 2020
Processing time: 117 Days and 10.7 Hours
Biliary diseases are common digestive system disorders which may combine with biliary tract infection such as cholecystitis or cholangitis. Thus, rapid identification of the bacteria and their antibiotic susceptibility profiles are crucial for reducing the mortality of patients with biliary tract infection.
To identify bacterial species and antibiotic susceptibility for antibacterial therapy and analyze bile cultivation risk factors for increasing detection rates.
This retrospective study was conducted from July 2008 to July 2017. In total, 1339 bile samples which were collected during therapeutic endoscopic retrograde cholangiopan-creatography or percutaneous transhepatic cholangiodrainage or other biliary surgeries or biliary drainage were obtained to characterize pathogen spectra, antibiotic susceptibility, and clinical features. Clinical data including age, sex, comorbidities, clinical symptoms, protopathies, and history of biliary tract diseases and surgeries were collated from hospital medical records. Species identification and initial drug susceptibility were further identified by biochemical characterization using the VITEK 2 Compact test.
Positive microbiological findings were observed in 738 samples. The most frequently encountered strains were gram-negative bacteria (74.94%), including Escherichia coli (37.78%), Pseudomonas aeruginosa (8.96%), and Klebsiella pneumoniae (10.29%). Bile bacteria were largely sensitive to carbapenems, piperacillin/tazobactam, and gentamicin. Gram-negative strains had low susceptibility to ceftriaxone, quinolones and ampicillin. Almost the same micro-organisms were present in patients with malignant and benign diseases. The number of samples with Klebsiella pneumoniae in the bile culture were significantly different between patients with malignant and benign diseases (55 vs 30; P = 0.019). Age (P < 0.001), fever (P < 0.001), history of biliary tract diseases and surgeries (both P < 0.001), benign disease (P = 0.002), and the comorbidity chronic renal insufficiency (P = 0.007) affected the positive rates of the bile samples.
Gram-negative bacteria were the most commonly isolated biliary bacteria. We determined the major factors associated with positive detection rates. Microbiological analysis of bile samples allowed accurate antibiotic treatments.
Core tip: In this large ten year retrospective study, we analyzed bacterial species in bile and their antibiotic susceptibility for antibacterial therapy, and analyzed bile cultivation risk factors to increase detection rates. The most frequently encountered strains were gram-negative bacteria, which were largely sensitive to carbapenems, piperacillin/tazobactam, and gentamicin. Almost the same micro-organisms were present in patients with malignant and benign diseases. Major risk factors for positive detection rates were age, fever, history of biliary tract diseases and surgeries, benign diseases, and the comorbidity chronic renal insufficiency.
- Citation: Gu XX, Zhang MP, Zhao YF, Huang GM. Clinical and microbiological characteristics of patients with biliary disease. World J Gastroenterol 2020; 26(14): 1638-1646
- URL: https://www.wjgnet.com/1007-9327/full/v26/i14/1638.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i14.1638
Biliary diseases are common digestive system disorders and include gallstones, gallbladder polyps, gallbladder carcinoma, and cholangiocarcinoma. These diseases may be combined with biliary tract infection such as cholecystitis or cholangitis. Normally, bile is a lipid-rich sterile solution produced in the liver[1,2]. However, bacterial colonization of bile has been found in some healthy people, and due to the normal bile flow, such individuals have no clinical symptoms. Bacteria may proliferate through the retrograde intrusion path owing to obstruction of the normal excretion of bile by tumors, stones, or worms that increase the pressure within the biliary ducts. These bacteria may also invade through the blood and lymphatic system. Furthermore, certain interventions such as surgery or endoscopic manipulations may negatively influence human defense mechanisms. Thus, bacteria translocate into the circulation, causing infection, possibly leading to severe sepsis and septic shock or even multiple organ dysfunction syndrome and eventually death[3-5]. Thus, rapid identification of the bacteria and their antibiotic susceptibility profiles is crucial for reducing the mortality of patients with biliary tract infection[6]. However, bile culture requires time and has lower positive rates. Moreover, insufficient data are available regarding the microbiological flora of the biliary tract, and most studies were conducted decades ago[7,8]. In addition, microbes show both regional and temporal variations[9].
Furthermore, there is no agreement concerning an optimum initial antibiotic therapy[5,10], and few data are available regarding the antibiotic susceptibility profiles of bacteria isolated from bile[11]. In addition, the rapid development of multidrug-resistant bacteria complicates the choice of an appropriate empiric antimicrobial therapy even more.
Thus, the aim of this study was to identify bacterial species and their antibiotic susceptibility for early empiric antibacterial therapy and to analyze risk factors in order to increase the detection rates of bile cultivation in patients with biliary diseases.
In total, 1339 bile samples were collected between July 2008 and July 2017 from 1339 patients who underwent therapeutic endoscopic retrograde cholangiopan-creatography or percutaneous transhepatic cholangiodrainage or other biliary surgeries or biliary drainage at the Second Affiliated Hospital of Nanjing Medical University. For this retrospective study, clinical data were collected from medical records. The following variables from medical records were included in our research: Age, sex, comorbidities, clinical symptoms, protopathies, and history of biliary tract diseases and surgeries (Table 1). The study was conducted in accordance with the principles of the Declaration of Helsinki, and the study protocol was approved by the ethics committee of the Second Affiliated Hospital of Nanjing Medical University.
| Group 1 (n = 601) (%) | Group 2 (n = 738) (%) | Total (n = 1339) (%) | P value | |
| Age (mean in years) | 60.93 ± 14.99 | 63.62 ± 14.72 | 62.42 ± 14.90 | 0.000a |
| Sex (male) | 293 (48.75) | 375 (50.81) | 668 (49.89) | 0.453 |
| Clinical manifestations | ||||
| Fever | 62 (10.32) | 206 (27.91) | 268 (20.01) | 0.000a |
| Abdominal pain | 459 (76.37) | 606 (82.11) | 1065 (79.54) | 0.906 |
| Jaundice | 252 (41.93) | 327 (44.31) | 579 (43.24) | 0.382 |
| Benign diseases | 405 (67.39) | 554 (75.07) | 959 (71.62) | 0.002a |
| Malignant diseases | 196 (32.61) | 184 (24.93) | 380 (28.38) | |
| History of biliary tract diseases | 191 (31.78) | 369 (50.00) | 560 (41.82) | 0.000a |
| History of biliary tract surgery | 128 (21.30) | 329 (44.58) | 457 (34.13) | 0.000a |
| Comorbidities | ||||
| Diabetes | 77 (12.81) | 86 (11.65) | 163 (12.17) | 0.519 |
| Hypertension | 170 (28.29) | 182 (24.66) | 352 (26.29) | 0.134 |
| Brain infarction | 52 (8.65) | 56 (7.59) | 108 (8.07) | 0.477 |
| Coronary heart disease | 30 (4.99) | 33 (4.47) | 63 (4.71) | 0.655 |
| Chronic bronchitis | 14 (2.33) | 19 (2.57) | 33 (2.46) | 0.774 |
| Chronic renal insufficiency | 4 (0.67) | 19 (2.57) | 23 (1.72) | 0.007a |
Bacterial isolates were obtained from the Second Affiliated Hospital of Nanjing Medical University, a large hospital in Jiangsu Province, China, between July 2008 and July 2017. They were confirmed by classic microbiological methods including Gram stain and catalase. Species identification and initial drug susceptibility were further identified by biochemical characterization using the VITEK 2 Compact test (bioMérieux, Lyon, France). Escherichia coli (E. coli) ATCC25922, Pseudomonas aeruginosa (P. aeruginosa) ATCC 27853, and Staphylococcus aureus ATCC 29213 were used as the quality control strains. Imipenem, meropenem, ceftriaxone, ampicillin, piperacillin/tazobactam, quinolones, gentamicin, vancomycin, and linezolid were purchased from Oxoid Ltd (Basingstoke, United Kingdom). Minimum inhibitory concentrations of the antibiotics were determined using the broth dilution method, E test (bioMérieux), or disk diffusion methods according to Clinical Laboratory Standards Institute guidelines.
All statistical analyses were performed using the SPSS software (Chicago, IL, United States). Between-group analyses were conducted using t-test or χ2 test. A P value of < 0.05 was considered to indicate statistical significance.
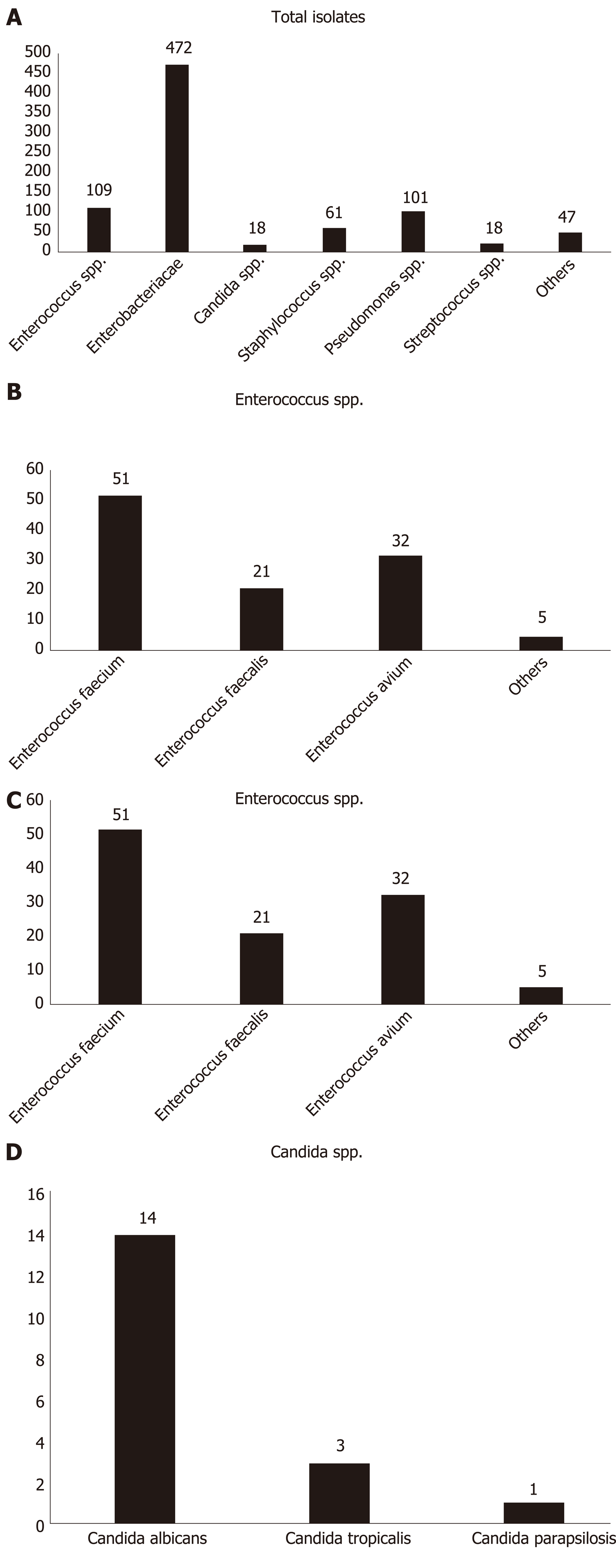
As shown in Figure 1, 738 of 1339 bile samples showed positive culture results (55.12%); 826 bacterial isolates were identified from 738 bile samples. Of these 738 samples, 652 contained single bacterial species, 84 had simultaneous growth of two different bacterial species, and two had simultaneous growth of three diverse bacterial species. In total, 619 strains were gram-negative bacteria (74.94%), 189 strains were gram-positive bacteria (22.88%), and 18 strains were fungi (2.18%). At the family level, the most frequently isolated pathogens were Enterobacteriaceae (472; 57.14%) and Enterococcaceae (109; 13.20%). The most common gram-negative bacterial strains at the species level were E. coli (312; 37.78%), Klebsiella pneumoniae (K. pneumoniae) (85; 10.29%), and P. aeruginosa (74; 8.96%). The most frequently detected gram-positive pathogenic bacteria were Enterococcus faecium (51; 6.17%), E. avium (32; 3.87%), and E. faecalis (21; 2.54%). Of the 18 fungal strains identified, 14 (77.78%) belonged to Candida albicans, and the remaining four represented C. tropicalis (16.67%) and C. parapsilosis (5.55%).
We analyzed the most common isolates in our study for susceptibility to antibiotics. Overall, for isolated E. coli, ceftriaxone and ampicillin resistance was observed in 251/312 cases (80.45%) and 277/312 isolates (88.78%), respectively. Ampicillin showed activity against 27.45% of the E. faecium isolates, and quinolones were active against 33.33% of the isolates. Furthermore, 78.38% of P. aeruginosa strains were resistant to ceftriaxone, and 100% of the isolates were resistant to ampicillin. Ceftriaxone and ampicillin resistance were observed in 71.76% and 95.29% of the K. pneumoniae isolates, respectively (Table 2).
| Antibiotic | Escherichia coli (n = 312) (%) | Pseudomonas aeruginosa (n = 74) (%) | Klebsiella pneumoniae (n = 85) (%) | Enterococcus faecium (n = 51) (%) |
| Ceftriaxone | 251 (80.45%) | 58 (78.38%) | 61 (71.76%) | NA |
| Ampicillin | 277 (88.78%) | 74 (100%) | 81 (95.29%) | 37 (72.55%) |
| Piperacillin/tazobactam | 37 (11.86%) | 9 (12.16%) | 18 (21.18%) | 28 (54.90%) |
| Quinolones | 204 (65.38%) | 11 (14.86%) | 34 (40.00%) | 34 (66.67%) |
| Carbapenems | 0 (0%) | 17 (22.97%) | 2 (2.35%) | NA |
| Vancomycin | NA | NA | NA | 0 (0%) |
| Gentamicin | 144 (46.15%) | 9 (12.16%) | 43 (50.59%) | 18 (35.29%) |
| Linezolid | NA | NA | NA | 0 (0%) |
The patients admitted to our hospital had a mean age of 62.42 years (SD, 14.90; range, 1-97 years) and were mostly men (n = 668) and aged > 65 years (n = 619). Of 1339 selected bile samples, 959 were collected from patients with benign diseases such as gallstones, cholecystitis, and gallbladder polyps. In addition, 380 bile samples were collected from patients with malignant diseases such as adenocarcinoma of the duodenal papilla, pancreatic cancer, gallbladder carcinoma, and cholangiocarcinoma. The most common clinical symptoms were abdominal pain (82.23%), fever (20.01%), and jaundice (43.24%). Major comorbidities were diabetes (163 cases), hypertension (352 cases), brain infarction (108 cases), coronary heart disease (63 cases), chronic bronchitis (33 cases), and chronic renal insufficiency (23 cases). Altogether, 560 patients had a history of biliary tract diseases, and 457 patients underwent biliary tract surgeries (Table 1).
We compared the main clinical differences between 738 cases with positive bile culture results (group 2) and 601 cases with negative bile culture results (group 1) (Table 1). We found that older patients (≤ 60 vs > 60) had high positive rates (P < 0.05). There was a statistically significant difference between the two groups with and without clinical manifestations such as fever (62 of 601, 10.32% vs 206 of 738, 27.91%; P < 0.001). The patients with benign diseases had higher positive rates that those with malignant diseases (405 of 601, 67.39% vs 554 of 738, 75.07%; P = 0.002). We also found statistically significant differences in patients with a history of biliary tract diseases and surgeries (191 of 601, 31.78% vs 369 of 738, 50% and 128 of 601, 21.30% vs 329 of 738, 44.58%; both P < 0.001). Four patients in group 1 and 19 patients in group 2 had chronic renal insufficiency (P = 0.007).
We found that the most common strains in patients (n = 554) with benign diseases were E. coli (231; 41.7%), P. aeruginosa (55; 9.93%), K. pneumoniae (55; 9.93%), and E. faecium (39; 7.04%). The predominant strains identified in patients (n = 184) with malignant diseases were E. coli (81; 44.02%), K. pneumoniae (30; 16.30%), P. aeruginosa (19; 10.33%), and E. faecium (12; 6.52%). Both for benign and malignant disease, the prevalence was almost the same. A significant difference was observed between patients with malignant and those with benign diseases with regard to K. pneumoniae bile cultures (55 vs 30; P = 0.019; Table 3).
| Bacteria | Benign diseases (n = 554) (%) | Malignant diseases (n = 184) (%) | Total (n = 738) (%) | P value |
| Escherichia coli | 231 (41.7) | 81 (44.02) | 312 (42.28) | 0.580 |
| Enterococcus faecium | 39 (7.04) | 12 (6.52) | 51 (6.91) | 0.810 |
| Klebsiella pneumoniae | 55 (9.93) | 30 (16.30) | 85 (11.52) | 0.019a |
| Pseudomonas aeruginosa | 55 (9.93) | 19 (10.33) | 74 (10.03) | 0.876 |
| Proteus mirabilis | 14 (2.53) | 7 (3.8) | 21 (2.85) | 0.367 |
| Staphylococcus | 40 (7.22) | 21 (11.41) | 61 (8.27) | 0.074 |
It has been reported that biliary pathogenic bacteria may be associated with intestinal flora distribution, such as E. coli, K. pneumoniae, and Enterococcus[12-14]. In the present study, we analyzed 1339 bile samples over ten years and established that biliary bacteria were mainly gram-negative bacteria, accounting for 74.94%, the rest included 22.88% of gram-positive bacteria and 2.18% of fungus. E. coli (37.78%) and K. pneumoniae (10.29%) were the most common gram-negative bacteria, and Enterococcus (13.20%) and Staphylococcus (7.38%) were the main gram-positive bacteria. Therefore, at the species level, our results, are consistent with previous results[12-14]. Normally, due to the protective effects of bile salts, flushing of bile, and phagocytosis by Kupffer cells[1,15], the numbers of bacteria in the biliary tract are low. In a previous study, gut microbes were established to shift to the bile ducts and liver via the duodenal papilla and portal system following obstruction of the biliary tract, which caused infection[16]. In our study, the mixed infection rate was lower than the individual bacterial infection rate (11.65% vs 88.35%), which was different to previous reports[17,18]. This may have been because we did not perform anaerobic bacteria cultivation and the wide application of antimicrobial agents.
In the past, the combination of ampicillin and an aminoglycoside was considered to be the first choice for treatment of biliary tract infection. However, due to increasing resistance to penicillin and kidney toxicity of aminoglycoside, empiric therapy was changed. Current guidelines now recommend treatment with third-generation cephalosporins or a penicillin/beta-lactamase inhibitor-based agent for empiric therapy of biliary bacteria by intravenous infusion[19]. In conclusion, bacterial resistance has changed. In our study, gram-negative strains had low susceptibility to ceftriaxone, quinolones and ampicillin, which is inconsistent with the guidelines. This high resistance may be related to common inappropriate use of these antibiotics, the selection of third-generation cephalosporins and no classification of quinolones. Thus, ceftriaxone and ampicillin were not recommended. On the other hand, they were reasonably susceptible to piperacillin/tazobactam and carbapenems. However, not all patients with biliary infections are treated with carbapenems and piperacillin/ tazobactam due to cost issues and emerging resistance. It was reported that univariate risk factors for biliary multidrug resistant bacteria were male sex, nosocomial acute cholangitis, prior antibiotic exposure and prior biliary stenting[13]. We analyzed the risk factors for this high ceftriaxone resistance rate with regard to E. coli (Supplementary Table 1). Unfortunately, we did not find any relevant risk factors. Further in-depth analysis is required in the future. In our study, the resistance rates of E. faecium were exceedingly high. In our series, gentamicin and piperacillin/tazobactam led to insignificant susceptibility rates, and only narrow-spectrum antibiotics such as vancomycin were effective. These findings must be considered during future empiric antibiotic treatments.
We suspected that microbiological profiles may be related to different diseases. Therefore, we analyzed the differences in microbiological profiles of patients with benign and malignant diseases. However, we found almost the same micro-organisms were positively cultured, and E. coli, Enterococcus, P. aeruginosa, and K. pneumoniae were the most common bacteria present in patients with malignant and benign diseases. We also attempted to demonstrate a possible association between bactibilia and the emergence of malignant diseases, such as Helicobacter pylori, which is associated with pancreatic cancer[20] and biliary tract cancer[21]. However, only a significant difference was observed between patients with benign diseases and those with malignant diseases with regard to K. pneumoniae (P = 0.019).
When the bile duct is obstructed, bacteria in the bile proliferate and inflammation occurs. Thus, choosing suitable antibiotics according to the profiles of the different bacteria identified is essential. However, in clinical practice, there is a certain time delay in obtaining bacterial culture and drug susceptibility results. At the same time, positive cultivation rates are low. Therefore, analyzing the factors that affect the positive cultivation rates of patients to increase detection rates is important. We found that older patients presented with high positive rates, possibly because they had comorbid diseases and had low immunity to resist bacteria. Moreover, weakened gastrointestinal motility, decreased secretion of gastric acid and bile, and lower gastric acid concentration and intestinal disorders in older people promoted the growth of bacteria. Abdominal pain, fever, and jaundice were the most frequently observed clinical manifestations in patients with biliary diseases[10]. However, in our study, we found that only patients with fever tended to present with high positive rates, possibly because fever had higher specificity than other symptoms.
Salvador et al[22] revealed that patients with benign diseases had higher positive rates than those with malignant diseases (P = 0.002), which is consistent with our findings, but is contrary to those of another study[14]. This may be due to other reasons. According to some investigators, sphincter of Oddi function in patients is normal before the onset of malignant disease. Normal function can adjust the flow of bile and pancreatic juices to maintain normal bile duct pressure. In addition, it can prevent reflux of duodenal contents. When sphincter of Oddi dysfunction occurs, it will lead to obstruction of the biliary tract and growth of bacteria. Furthermore, patients with benign diseases had higher rates of bile duct stones than those with malignant diseases in our study, which would also lead to obstruction of the biliary tract and the growth of biliary pathogenic bacteria.
A history of biliary tract diseases and surgeries was also another risk factor (P < 0.001). Previous research has shown that biliary tract diseases such as gallstones provide beneficial conditions for adhesion, growth, and propagation of pathogenic bacteria. In addition, the symptoms of these diseases cause disorders of bodily function and change the tissue structure, which decreases the ability to remove the bacteria. There is evidence of damage to the normal structure of the sphincter of Oddi[23] and influence on the function of bile ducts[24] due to previous surgeries such as endoscopic retrograde cholangiopancreatography. Similar surgical interventions can easily lead to duodenal–biliary reflux, mucosal hyperemia edema, and anastomosis of the biliary stricture, which creates beneficial conditions for rapid multiplication of bacteria.
We analyzed the different comorbidities of patients with high positive rates. In contrast to the findings of other studies, few of the observed comorbidities had a significant risk for positive rates[25]. We only found chronic renal insufficiency (P = 0.007) to be significant. The kidneys of patients with chronic renal insufficiency may have long-term serious injuries. As a result, their lymphocyte levels decrease, neutrophil function is damaged, and immune function is weakened.
In conclusion, Gram-negative bacteria were the most commonly isolated biliary bacteria. Risk factors such as age, fever, history of biliary tract diseases and surgeries, benign diseases, and comorbidities such as chronic renal insufficiency positively influenced the detection rates. Bile samples for microbiological analysis may enable a more accurate selection of antibiotic treatments.
Biliary diseases may combine with biliary tract infection such as cholecystitis or cholangitis which possibly lead to severe sepsis and septic shock or even multiple organ dysfunction syndrome and eventually death. However, bile culture requires more time and has lower positive rates. Most related studies were conducted decades ago and lack a large sample size.
Using a large sample size and ten years of study, we fully understand the bacterial species and antibiotic susceptibility for antibacterial therapy in patients with biliary diseases.
The identification of bacterial species and their antibiotic susceptibility for early empiric antibacterial therapy are crucial for reducing the mortality of patients with biliary tract infection.
Clinical data were collected from hospital medical records. Species identification and initial drug susceptibility were further identified by biochemical characterization using the VITEK 2 Compact test. All statistical analyses were performed using the SPSS software. Between-group analyses were conducted using the t-test or χ2 test.
The most frequently encountered strains were gram-negative bacteria (74.94%), including Escherichia coli (37.78%), Pseudomonas aeruginosa (8.96%), and Klebsiella pneumoniae (10.29%). Bile bacteria were largely sensitive to carbapenems, piperacillin/tazobactam, and gentamicin. We found almost the same micro-organisms present in patients with malignant and benign diseases. Age (P < 0.001), fever (P < 0.001), history of biliary tract diseases and surgeries (both P < 0.001), benign disease (P = 0.002), and the comorbidity chronic renal insufficiency (P = 0.007) affected the positive rates of the bile samples.
We found that gram-negative strains had low susceptibility to ceftriaxone, quinolones and ampicillin. In addition, some risk factors such as age, fever, history of biliary tract diseases and surgeries, benign diseases, and the comorbidity chronic renal insufficiency positively influenced the detection rates. Bile samples for microbiological analysis may enable a more accurate selection of antibiotic treatments.
The risk factors for antibiotic resistance rate and bacterial resistance genes should be analyzed.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ahboucha S, Khoury T S-Editor: Dou Y L-Editor: Webster JR E-Editor: Zhang YL
| 1. | D'Aldebert E, Biyeyeme Bi Mve MJ, Mergey M, Wendum D, Firrincieli D, Coilly A, Fouassier L, Corpechot C, Poupon R, Housset C, Chignard N. Bile salts control the antimicrobial peptide cathelicidin through nuclear receptors in the human biliary epithelium. Gastroenterology. 2009;136:1435-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 167] [Article Influence: 10.4] [Reference Citation Analysis (1)] |
| 2. | Csendes A, Fernandez M, Uribe P. Bacteriology of the gallbladder bile in normal subjects. Am J Surg. 1975;129:629-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 68] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Melzer M, Toner R, Lacey S, Bettany E, Rait G. Biliary tract infection and bacteraemia: presentation, structural abnormalities, causative organisms and clinical outcomes. Postgrad Med J. 2007;83:773-776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 101] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 4. | Takada T, Strasberg SM, Solomkin JS, Pitt HA, Gomi H, Yoshida M, Mayumi T, Miura F, Gouma DJ, Garden OJ, Büchler MW, Kiriyama S, Yokoe M, Kimura Y, Tsuyuguchi T, Itoi T, Gabata T, Higuchi R, Okamoto K, Hata J, Murata A, Kusachi S, Windsor JA, Supe AN, Lee S, Chen XP, Yamashita Y, Hirata K, Inui K, Sumiyama Y; Tokyo Guidelines Revision Committee. TG13: Updated Tokyo Guidelines for the management of acute cholangitis and cholecystitis. J Hepatobiliary Pancreat Sci. 2013;20:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 191] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 5. | Gomi H, Solomkin JS, Schlossberg D, Okamoto K, Takada T, Strasberg SM, Ukai T, Endo I, Iwashita Y, Hibi T, Pitt HA, Matsunaga N, Takamori Y, Umezawa A, Asai K, Suzuki K, Han HS, Hwang TL, Mori Y, Yoon YS, Huang WS, Belli G, Dervenis C, Yokoe M, Kiriyama S, Itoi T, Jagannath P, Garden OJ, Miura F, de Santibañes E, Shikata S, Noguchi Y, Wada K, Honda G, Supe AN, Yoshida M, Mayumi T, Gouma DJ, Deziel DJ, Liau KH, Chen MF, Liu KH, Su CH, Chan ACW, Yoon DS, Choi IS, Jonas E, Chen XP, Fan ST, Ker CG, Giménez ME, Kitano S, Inomata M, Mukai S, Higuchi R, Hirata K, Inui K, Sumiyama Y, Yamamoto M. Tokyo Guidelines 2018: antimicrobial therapy for acute cholangitis and cholecystitis. J Hepatobiliary Pancreat Sci. 2018;25:3-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 244] [Article Influence: 34.9] [Reference Citation Analysis (1)] |
| 6. | Jang DK, Kim J, Park WB, Yi SY, Lee JK, Yoon WJ. Increasing burden of biliary tract infection caused by extended-spectrum beta-lactamase-producing organisms in Korea: A nationwide population-based study. J Gastroenterol Hepatol. 2020;35:56-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Ehrenstein BP, Salamon L, Linde HJ, Messmann H, Schölmerich J, Glück T. Clinical determinants for the recovery of fungal and mezlocillin-resistant pathogens from bile specimens. Clin Infect Dis. 2002;34:902-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Lorenz R, Herrmann M, Kassem AM, Lehn N, Neuhaus H, Classen M. Microbiological examinations and in-vitro testing of different antibiotics in therapeutic endoscopy of the biliary system. Endoscopy. 1998;30:708-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Kwon JS, Han J, Kim TW, Oh JH, Kwon HH, Jung JT, Kwon JG, Kim EY, Kim HG. Changes in causative pathogens of acute cholangitis and their antimicrobial susceptibility over a period of 6 years. Korean J Gastroenterol. 2014;63:299-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Lee JG. Diagnosis and management of acute cholangitis. Nat Rev Gastroenterol Hepatol. 2009;6:533-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 99] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 11. | Nomura T, Shirai Y, Hatakeyama K. Enterococcal bactibilia in patients with malignant biliary obstruction. Dig Dis Sci. 2000;45:2183-2186. [PubMed] |
| 12. | Zhao J, Wang Q, Zhang J. Changes in Microbial Profiles and Antibiotic Resistance Patterns in Patients with Biliary Tract Infection over a Six-Year Period. Surg Infect (Larchmt). 2019;20:480-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 13. | Reuken PA, Torres D, Baier M, Löffler B, Lübbert C, Lippmann N, Stallmach A, Bruns T. Correction: Risk Factors for Multi-Drug Resistant Pathogens and Failure of Empiric First-Line Therapy in Acute Cholangitis. PLoS One. 2017;12:e0172373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Kaya M, Beştaş R, Bacalan F, Bacaksız F, Arslan EG, Kaplan MA. Microbial profile and antibiotic sensitivity pattern in bile cultures from endoscopic retrograde cholangiography patients. World J Gastroenterol. 2012;18:3585-3589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Schubert K, Olde Damink SWM, von Bergen M, Schaap FG. Interactions between bile salts, gut microbiota, and hepatic innate immunity. Immunol Rev. 2017;279:23-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 16. | Lee DW, Chung SC. Biliary infection. Baillieres Clin Gastroenterol. 1997;11:707-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Weber A, Huber W, Kamereck K, Winkle P, Voland P, Weidenbach H, Schmid RM, Prinz C. In vitro activity of moxifloxacin and piperacillin/sulbactam against pathogens of acute cholangitis. World J Gastroenterol. 2008;14:3174-3178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Alves JR, Silva Rdo C, Guerra SC, Freitas TT, Souza DL, Amico EC. MICROBIOLOGICAL ANALYSIS OF BILE IN PATIENTS WITH BENIGN AND MALIGNANT BILIOPANCREATIC DISEASES AND ITS CONSEQUENCES. Arq Gastroenterol. 2016;53:156-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Sun Z, Zhu Y, Zhu B, Xu G, Zhang N. Controversy and progress for treatment of acute cholangitis after Tokyo Guidelines (TG13). Biosci Trends. 2016;10:22-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Nilsson HO, Stenram U, Ihse I, Wadstrom T. Helicobacter species ribosomal DNA in the pancreas, stomach and duodenum of pancreatic cancer patients. World J Gastroenterol. 2006;12:3038-3043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 61] [Cited by in RCA: 81] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 21. | Zhou D, Wang JD, Weng MZ, Zhang Y, Wang XF, Gong W, Quan ZW. Infections of Helicobacter spp. in the biliary system are associated with biliary tract cancer: a meta-analysis. Eur J Gastroenterol Hepatol. 2013;25:447-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | Salvador VB, Lozada MC, Consunji RJ. Microbiology and antibiotic susceptibility of organisms in bile cultures from patients with and without cholangitis at an Asian academic medical center. Surg Infect (Larchmt). 2011;12:105-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 23. | Negm AA, Schott A, Vonberg RP, Weismueller TJ, Schneider AS, Kubicka S, Strassburg CP, Manns MP, Suerbaum S, Wedemeyer J, Lankisch TO. Routine bile collection for microbiological analysis during cholangiography and its impact on the management of cholangitis. Gastrointest Endosc. 2010;72:284-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (1)] |
| 24. | Barrett M, Asbun HJ, Chien HL, Brunt LM, Telem DA. Bile duct injury and morbidity following cholecystectomy: a need for improvement. Surg Endosc. 2018;32:1683-1688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 101] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 25. | Chao CM, Lai CC, Tang HJ, Ko WC, Hsueh PR. Biliary tract infections caused by Aeromonas species. Eur J Clin Microbiol Infect Dis. 2013;32:245-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |