Published online Mar 28, 2020. doi: 10.3748/wjg.v26.i12.1286
Peer-review started: November 5, 2019
First decision: December 5, 2019
Revised: January 8, 2020
Accepted: February 28, 2020
Article in press: February 28, 2020
Published online: March 28, 2020
Processing time: 144 Days and 6.1 Hours
Tamarix chinensis Lour (TCL) is a shrub that usually grows in arid or semiarid desert areas and saline-alkali fields. It is a traditional Chinese herbal medicine with hepatoprotective, antioxidant, antibacterial, and antitumor activities.
To investigate the possible protective effects of TCL against liver injury induced by chronic ethanol intake.
C57BL/6J male mice were fed a Lieber-DeCarli lipid diet containing alcohol and received (by gavage) a water-alcohol extract (80%) of TCL (100 and 200 mg/kg BW) or distilled water for 4 wk. After euthanasia, liver tissues were observed histologically with hematoxylin and eosin staining and Oil red O staining, and the levels of alanine aminotransferase, aspartate transaminase, hepatic lipids, reactive oxygen species, malondialdehyde, and superoxide dismutase were measured. In addition, expression of the NOD-like receptor family, pyrin domain-containing 3 (NLRP3) inflammasome and downstream proinflammatory cytokines were determined.
Compared with the ethanol group, mice in the TCL-treated group (200 mg/kg) had significantly lower serum levels of alanine aminotransferase (mean, 34.1 IU/L vs 45.3 IU/L, P < 0.01) and aspartate transaminase (mean, 89.6 IU/L vs 115.7 IU/L, P < 0.01), as well as marked reduction of hepatic tissue reactive oxygen species (decreased by 27.5%, P < 0.01) and malondialdehyde (decreased by 76.6%, P < 0.01) levels, with a significant increase of superoxide dismutase (Increased by 73.2%, P < 0.01). Expression of the NLRP3 inflammasome and its downstream cytokines [interleukin (IL)-1β, tumor necrosis factor-α, and IL-6], and recruitment of natural killer T cells to the liver, were reduced in the TCL-treated incubation with a Lieber-DeCaril ethanol lipid diet group.
These findings suggest that a TCL extract (200 mg/kg) protects against chronic ethanol-induced liver injury, probably by inhibiting the NLRP3-caspase-1-IL-1β signaling pathway and suppressing oxidative stress.
Core tip: Administration of alcoholic extract of Tamarix chinensis Lour (TCL) inhibits the upregulation of NOD-like receptor family, pyrin domain containing 3 inflammasome and its downstream proinflammatory cytokines (i.e., interleukin-1β, tumor necrosis factor-α, interleukin-6) induced by chronic ethanol exposure. Alcoholic extract of TCL inhibits the recruitment of natural killer T cells in the liver induced by chronic ethanol exposure. Administration of alcoholic extract of TCL ameliorates the oxidative stress induced by chronic ethanol exposure.
- Citation: Wang ZD, Zhang Y, Dai YD, Ren K, Han C, Wang HX, Yi SQ. Tamarix chinensis Lour inhibits chronic ethanol-induced liver injury in mice. World J Gastroenterol 2020; 26(12): 1286-1297
- URL: https://www.wjgnet.com/1007-9327/full/v26/i12/1286.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i12.1286
Consumption of alcohol is customary in most countries and alcohol abuse is common worldwide. Chronic liver disease caused by long-term alcohol abuse is called alcoholic liver disease (ALD). ALD represents a spectrum of clinical and morphological changes that range initially from simple steatosis to steatohepatitis, fibrosis, and cirrhosis[1], and is a primary cause of mortality among people who abuse alcohol[2]. The pathogenesis of ALD is complex and poorly understood. Several factors are involved in the pathogenesis of ALD, including oxidative stress and inflammatory mediators. Activation of innate immunity and inflammation are also pivotal in the progression of ALD[3]. Increasing evidence indicates that inflammasome-mediated release of inflammatory cytokines is a critical contributor to the development and progression of ALD[4]. Inflammasomes are multiprotein cytoplasmic complexes, among which the NOD-like receptor family, pyrin domain containing 3 (NLRP3) inflammasome has been most extensively studied. The NLRP3 inflammasome is comprised of a receptor (NLRP3), an adapter protein (apoptosis-associated speck-like protein [ASC]), and an effector protein (pro-caspase-1, the precursor of cysteinyl aspartate specific proteinase-1). When the NLRP3 inflammasome is activated, the inactive pro-caspase-1 is cleaved to form active caspase-1[5], which subsequently cleaves pro-interleukin (IL)-1β to release mature IL-1β that contributes to hepatic steatosis, apoptosis, inflammation, and fibrosis/cirrhosis[6]. Alcohol intake can activate NLRP3 inflammasomes in cells such as Kupffer cells in the liver, and activated NLRP3 inflammasomes can cleave immature pre-IL-1β into mature IL-1β through caspase-1, thus triggering liver injury[7,8]. Thus, ALD causes serious liver damage, but there is still no therapy that is effective for preventing the onset and progression of ALD with few side effects, except abstinence from alcohol[9]. In the last few years, an increasing number of studies on the role of herbal medicines in the prevention and treatment of ALD have been published, because of their multiple actions and few side effects[10,11].
Tamarix chinensis Lour (TCL) is a tree that is used for afforestation and sand fixation in arid areas with secondary salinization. Its leaf is a material used in traditional Chinese herbal medicines, which is recorded in the Compendium of Materia Medica and other ancient Chinese medical works, with the functions alleviating rashes, dispelling wind and dehumidification, being mainly used for measles and rheumatism. Pharmacology studies have shown that the chemical constituents of TCL are mainly flavonoids, triterpenes, organic acids, and volatile oils[12], which have various biological activities such as hepatoprotective, anti-inflammatory, antioxidant, bacteriostastic, and antitumor effects[13,14]. TCL has certain protective effects against liver injury induced by carbon tetrachloride and acetaminophen, and can reduce the severity of liver damage[13]. However, the regulatory effect of TCL on alcohol-induced activation of NLRP3 inflammasomes is still unclear. The Lieber-DeCaril ethanol lipid diet has been widely used to reproduce chronic alcoholic hepatic injury in animals[15,16]. Feeding animals the Lieber-DeCarli diet containing alcohol for 4 wk is a classical approach to reproduce chronic liver injury due to alcohol that has been employed in many laboratories[17,18].
Therefore, in this study, mice were fed the Lieber-DeCaril ethanol lipid diet to establish a chronic ethanol-induced liver injury model, and to investigate the protective effect of a TCL extract (200 mg/kg BW) against liver injury due to chronic alcohol intake and the possible mechanisms involved.
TCL was provided from trees grown in the Yellow River Delta region (Wudi, China), and was authenticated by Dr. Dexi Liu from the Shandong Province Forestry Academy of Science of China (Figure 1). TCL contains a variety of chemical components such as flavonoids, triterpenes, steroids, phenylpropanoids, organic acids, and phenolic acids[12]. Based on the literature[19], extraction was performed as follows: The dried branches and leaves of TCL (100 g) were minced and sifted using an 80-mesh sieve, and then extracted by maceration in an 80% aqueous ethanol solution (1000 mL). The resulting extract was filtered to remove bacteria, concentrated (to 31 g) in a rotary vacuum evaporator, and stored in tightly sealed sample tubes.
Eighty C57BL/6J male mice (8-10 wk old) were purchased from Beijing HFK Bioscience Co. Ltd. (Beijing, China). The mice were housed 2 to 3 per cage and maintained in pathogen-free microisolator cages with controlled temperature (22.5 °C ± 0.5 °C) and humidity (50% ± 5%) and a 12-h light/dark cycle. The liquid diets were provided ad libitum. First, all mice were acclimatized to the liquid diet for 1 wk. Then the mice were randomly assigned to the following five groups: Control (Ctrl) group, ethanol (EtOH) group, reduced glutathione (GSH) group as the positive Ctrl (EtOH + GSH at 86 mg/kg BW), and two TCL groups (EtOH + TCL-L at 100 mg/kg BW and EtOH + TCL-H at 200 mg/kg BW). GSH exists in the normal liver and reportedly improves clinical signs and hepatic dysfunction induced by alcohol[20]. Therefore, GSH was used as a positive Ctrl when assessing the effects of TCL. The doses of 100 mg/kg and 200 mg/kg TCL extracts used here are based on a previous study[21], and refer to the dose of TCL decoction in traditional Chinese medicine. All groups, except for the Ctrl group, were fed a modified Lieber-DeCarli alcohol liquid diet, with the following energy composition: 11% carbohydrate, 18% protein, 36% ethanol, and 35% fat. The Ctrl group was fed an isocaloric Ctrl Lieber-DeCarli diet without ethanol (Trophic Animal Feed High-Tech Co., Ltd., China). The two kinds of liquid diet were prepared every 2 d according to the manufacturer's protocol. Administration of reduced GSH (YaoPharma Co., Ltd. Chongqing, China) and TCL extract was done by gavage. The dose of reduced GSH used in this study was based on the clinical dosage. After 4 wk on the liquid diet, all mice were euthanized and blood samples were collected. Liver tissue was harvested and stored in a freezer at -80 °C. All animal experiments were performed in strict accordance with the guide for the Care and Use of Laboratory Animals of the Institute of Basic Medicine, Shandong Academy of Medical Sciences. The experimental protocols were approved by the animal ethics committee of the Institute of Basic Medicine, Shandong Academy of Medical Sciences (07/2016). All efforts were made to minimize animal suffering.
Histological examination of the liver was done after staining sections with hematoxylin and eosin (H&E). After fixation in 10% neutral formaldehyde, liver specimens were dehydrated, wrapped, cut into sections, and stained with H&E solution. Lipid deposition in the liver was assessed by staining with Oil Red O. Briefly, cryostat sections (8 μm) of the liver were fixed, stained with Oil Red O (Sigma-Aldrich, St. Louis, MO, United states) solution, and then counterstained with hematoxylin (Sigma-Aldrich). The sections were examined under the Leica DM4000B light microscope.
Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) are commonly used as biochemical indicators of liver injury. Serum levels of ALT and AST were measured by using commercial spectrophotometric kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instructions.
After liver tissue was homogenized in RIPA lysis buffer and centrifuged at 13000 g for 15 min, the protein concentration of the lysates was measured by the Nanodrop 1000 spectrophotometer. Then proteins were separated by 10% or 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride membrane by electroblotting. Subsequently, the membrane was probed overnight at 4 °C with primary antibodies directed against NLRP3, ASC, caspase-1, IL-1β, and β-actin diluted in primary antibody dilution buffer (Shanghai Biyuntian Biological Technology Co. Ltd., Shanghai, China), followed by incubation for 1 h at room temperature with horseradish peroxidase (HRP)-labeled secondary antibodies. Protein bands were visualized with Millipore ECL chemical luminescence HRP substrate (Millipore Corporation, Billerica, MA, United States) and the ImageQuant LAS 4000 mini biomolecular imager (GE Healthcare Life Science, Uppsala, Sweden). The levels of target proteins were subsequently quantitated by densitometry using ImageJ software, version 1.47v, with normalization for β-actin.
Hepatic expression of NLRP3, ASC, caspase-1, and IL-1β mRNA was also measured by quantitative PCR (qPCR). First, total RNA was extracted from liver tissue using TRIzol reagent (Invitrogen, Carlsbad, CA, United States), and first-strand cDNA was synthesized by reverse transcription with the FastQuant RT Kit (Tiangen Biotech Co., Ltd., Beijing, China) according to the manufacturer’s instructions. The cDNA thus obtained was amplified using SYBR Green PCR Master Mix (Tiangen Biotech Co., Ltd., Beijing, China) and a LightCycler® 480 Real-Time PCR System (F. Hoffmann-La Roche Ltd., Basel, Switzerland). Specific primers for NLRP3, ASC, caspase-1, IL- 1β, IL-6, tumor necrosis factor (TNF)-α and β-actin were purchased from BioSune Biotechnology Co., Ltd. (Shanghai, China). Expression of the target mRNAs was normalized to that of β-actin. The primer sequences were as follows: NLRP3 (forward: 5’-ATTACCCGCCCGAGAAAGG-3’, reverse: 5’-TCGCAGCAAAGATCCACACAG-3’); ASC (forward: 5’-CTTGTCAGGGGATGAACTCAAAA-3’, reverse: 5’-GCC ATACGACTCCAGATAGTAGC-3’); caspase-1 (forward: 5’-AATACAACCACT CGTACACGTC-3’, reverse: 5’-AGCTCCAACCCTCGGAGAAA-3’); IL-1β (forward: 5’-GCCACCTTTTGACAGTGATGA-3’, reverse: 5’-ATGTGCTGCTGCGAGATTTG-3’); IL-6 (forward: 5’-ACAAAGCCAGAGTCCTTCAGAG-3’, reverse: 5’-TGT GACTCCAGCTTATCTCTTGG-3’); TNF-α (forward: 5’-ACCCTCACACTC ACAAACCAC-3’, reverse: 5’-ACAAGGTACAACCCATCGGC-3’); β-actin (forward: 5’-GGCTGTATTCCCCTCCATCG-3’, reverse: 5’-CCAGTTGGTAACAATGCCATGT-3’). In the PCR reaction mixture for each target mRNA (20 μL), the quantity of cDNA and the concentration of the specific forward/reverse primer were 100 ng and 0.3 μmol/L, respectively. After denaturation at 95 °C for 10 s, PCR was done with 40 cycles of 95 °C for 10 s, 60 °C for 32 s, and 72 °C for 32 s, followed by a final cycle of 95 °C for 15 s, 60 °C for 1 min, and 95 °C for 15 s. Data were analyzed by using the 2−ΔΔCt method and fold induction of target mRNA expression was normalized by that of GAPDH.
Hepatic lymphocytes were isolated by Discontinuous Percoll Gradient Centrifugation and immunostained with fluorescent antibodies (FITC-CD3 and PE-CY7-NK1.1). Fluorescence was detected by using the BD FACS caliber flow cytometer, and data were analyzed with FLOW JO 7.6.1software.
Liver homogenate was prepared and hepatic tissue levels of reactive oxygen species (ROS), malondialdehyde (MDA), and superoxide dismutase (SOD) were determined by using commercial assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer's protocols. Protein concentrations were measures using the Nanodrop 1000 spectrophotometer, and all values were normalized to the hepatic total protein content.
Statistical analyses were performed with SPSS 22.0 software. Values are expressed as the mean ± standard deviation. Between-group comparisons were evaluated by one-way analysis of variance (with Dunnett's test and Bonferroni's multiple comparison test). Statistical significance was established at P < 0.05. All experiments were performed at least in triplicate.
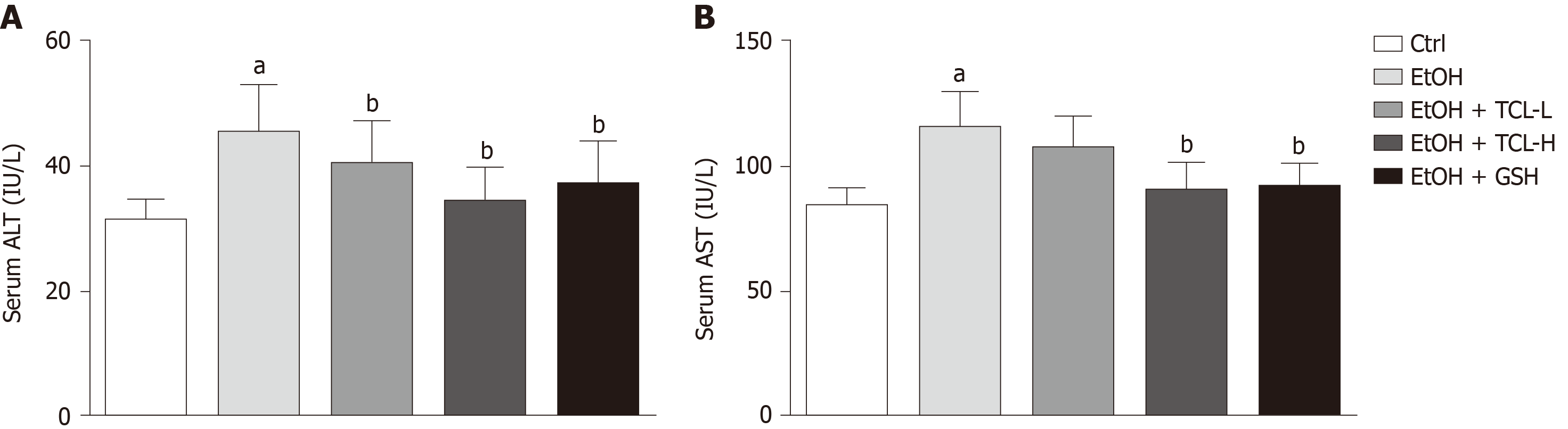
We examined the serum levels of ALT and AST to explore the effect of TCL extract on chronic ethanol-induced hepatoxicity in mice. Compared with Ctrl mice, mice in the alcohol group displayed severe hepatotoxicity with high serum levels of ALT (mean, 45.3 IU/L vs 31.4 IU/L, P < 0.01) and AST (mean, 115.7 IU/L vs 84.1 IU/L, P < 0.01). The increase of these enzymes was reversed by treatment with either TCL or GSH, especially the higher dose of TCL (200 mg/kg BW) (ALT mean, 34.1 IU/L vs 45.3 IU/L, P < 0.01; AST mean, 89.6 IU/L vs 115.7 IU/L, P < 0.01 ) (Figure 2).
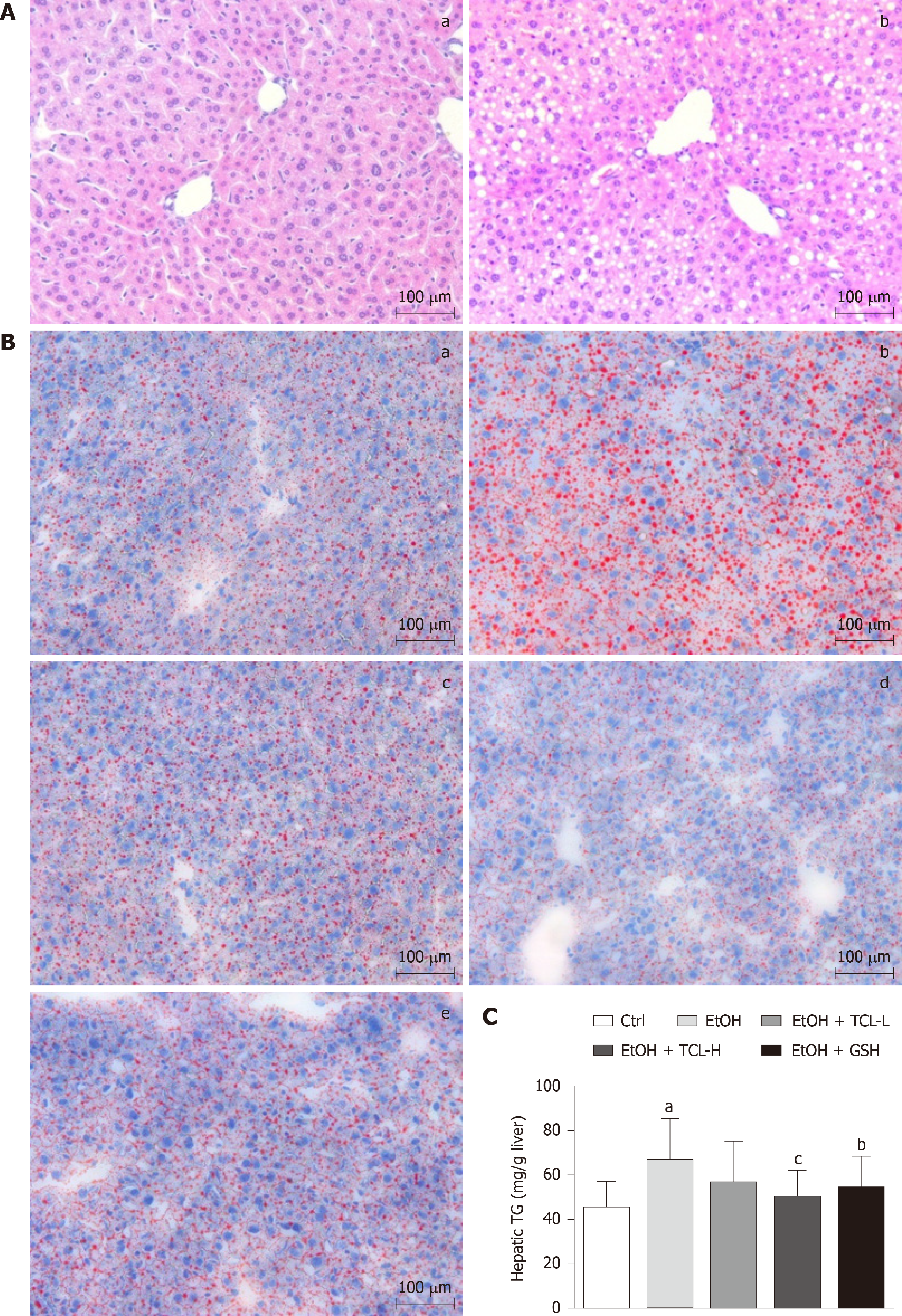
Morphological changes of the liver were observed by light microscopy after H&E staining, revealing pan-lobular steatosis and focal necrosis in the ethanol group (Figure 3A). To clarify the extent of lipid accumulation, we used Oil Red O staining and detection of triglycerides for qualitative and quantitative analysis of the hepatic lipid content. As shown in Figure 3B, numerous red lipid droplets were found in the hepatocytes of mice in the ethanol group, consistent with the results of the H&E staining, while there were fewer and smaller lipid droplets in the GSH and TCL groups than in the ethanol group. Quantitation of triglycerides (TGs) confirmed the results of Oil Red O staining, demonstrating that elevation of the hepatic TG content induced by ethanol was significantly reversed by treatment with either TCL or GSH (mean, EtOH+TCL-H 49.2 mg/g vs EtOH 66.8 mg/g, P < 0.01) (Figure 3C).
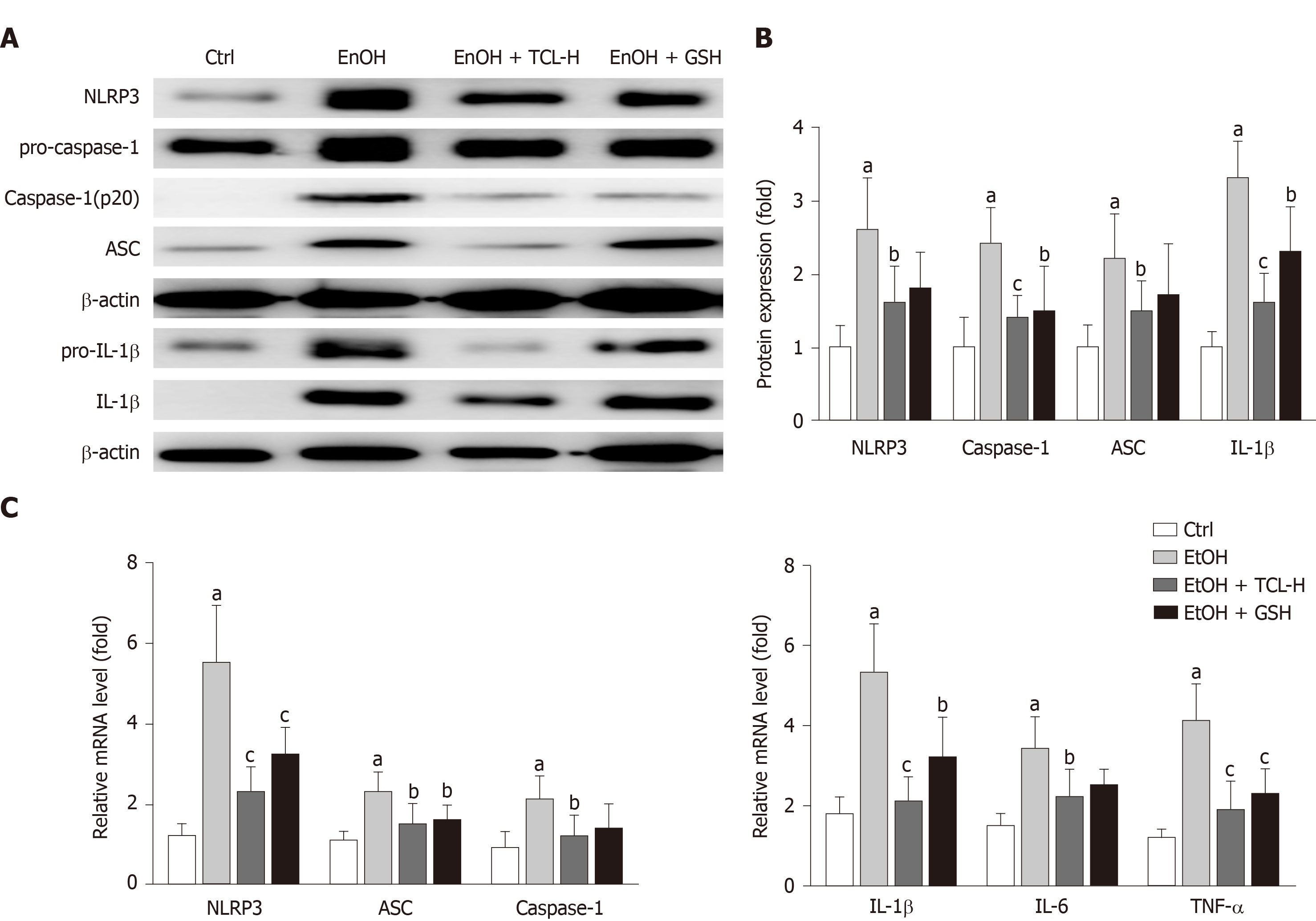
To explore whether the protective effect of TCL on chronic ethanol-induced liver injury in mice was related to inhibition of NLRP3 inflammasomes, we evaluated the hepatic tissue expression of proteins that are components of this inflammasome (NLRP3, ASC, and caspase-1) and downstream cytokines (IL-1β, IL-6, and TNF-α) by Western blot analysis. We also quantified mRNA expression by qPCR. Compared with the Ctrl group, both hepatic tissue protein and mRNA levels were significantly elevated for NLRP3, ASC, caspase-1, IL-1β, IL-6, and TNF-α in the liver tissues of the ethanol group. These changes were markedly inhibited by treatment with either TCL or GSH (Figure 4).
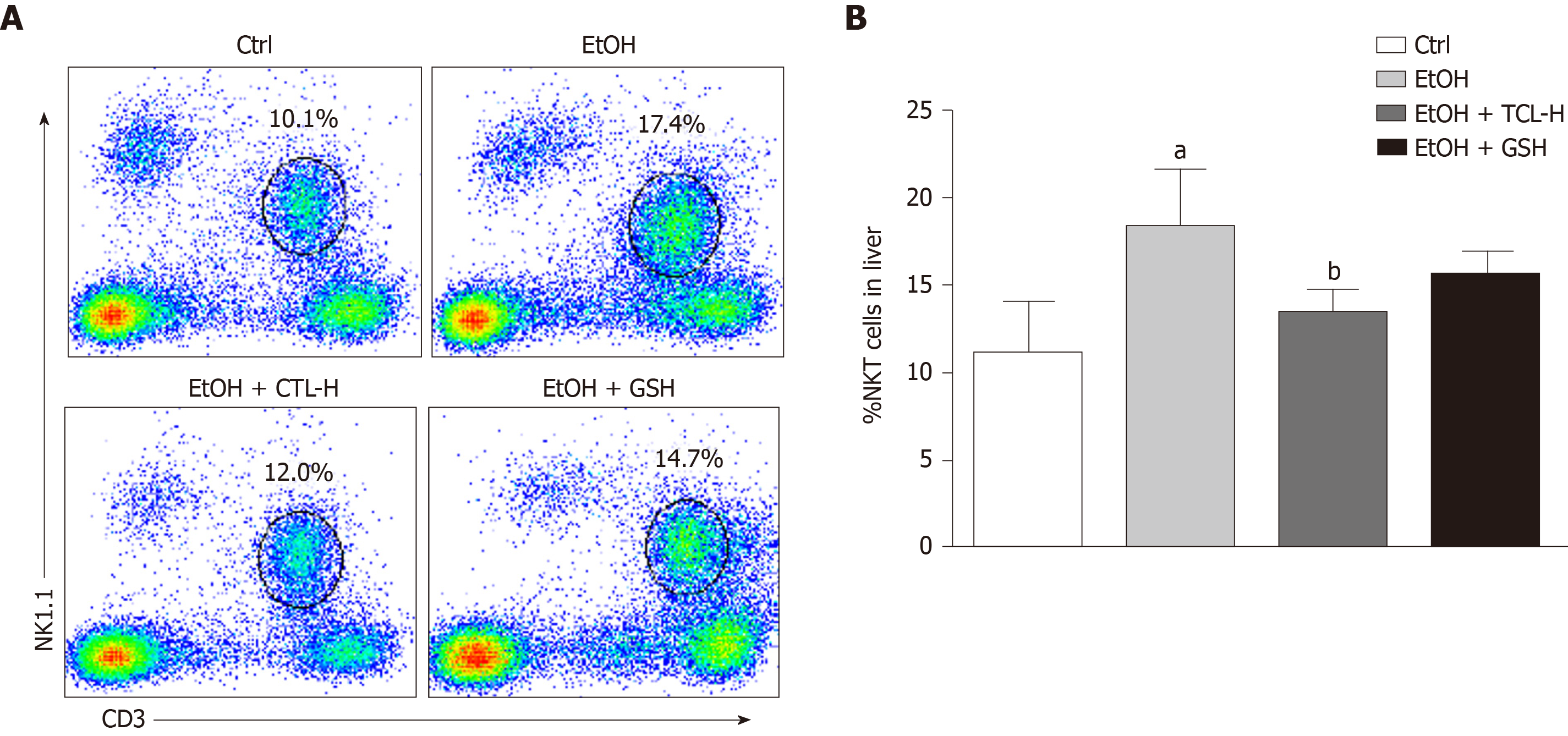
To explore the effect of TCL on natural killer T (NKT) cells in the liver, we investigated the proportion of NKT cells among total lymphocytes. Chronic ethanol exposure increased the proportion of NK1.1+CD3+ cells in the liver compared to the Ctrl group (Figure 5A), while treatment with TCL reduced it by 4.9% compared to the ethanol group (Figure 5B). The GSH group also showed a decrease of NKT cells compared to the ethanol group, but there was no significant difference.
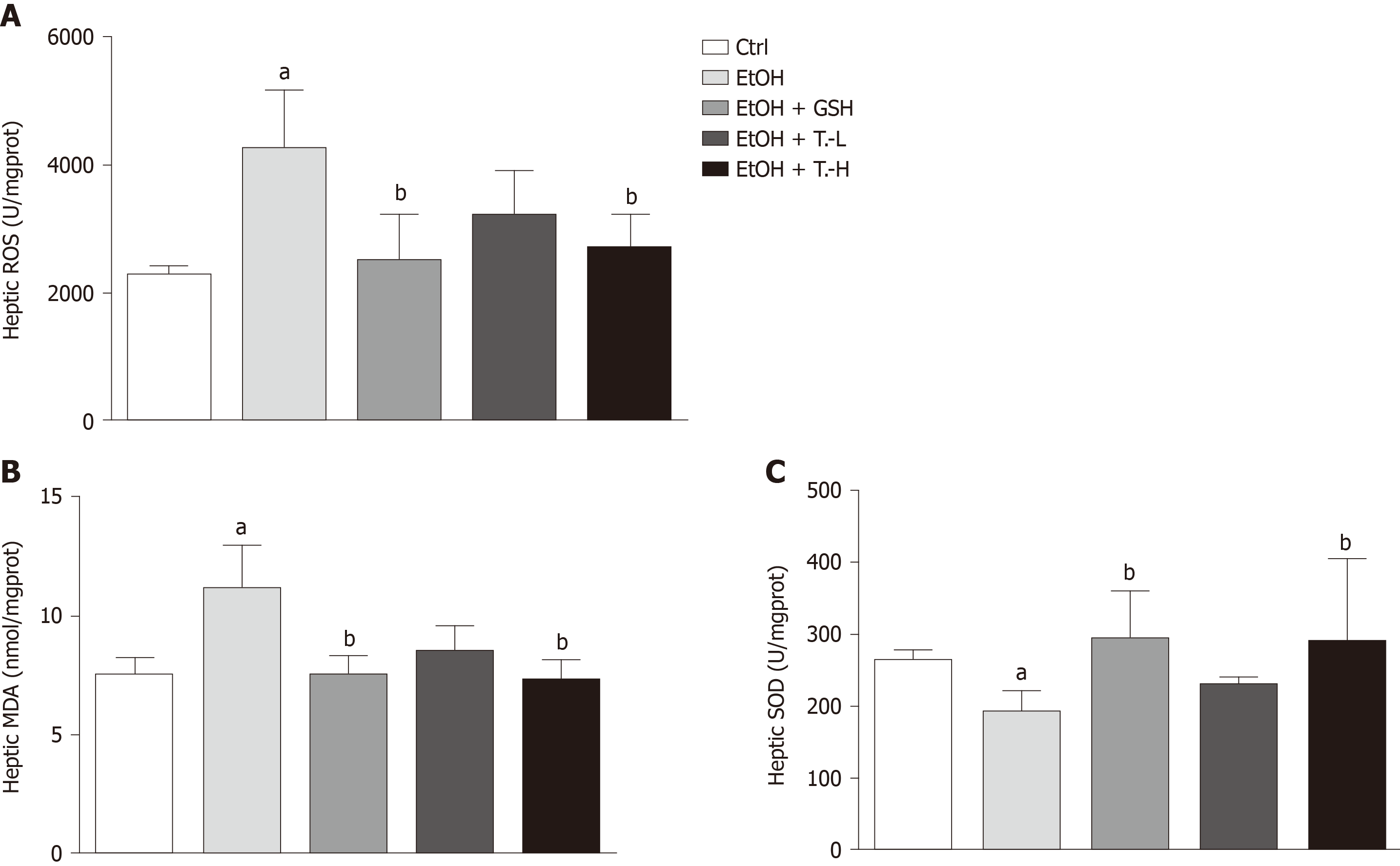
We measured the hepatic tissue levels of ROS, MDA, and SOD to examine the effect of TCL on ethanol-induced oxidative stress. Chronic ethanol exposure significantly elevated ROS and MDA levels in liver tissue, while reducing SOD. These changes were largely prevented by treatment with TCL (200 mg/kg BW) or GSH. Briefly, compared with the ethanol group, ROS production was decreased by 27.5% (P < 0.01) and 32.6% (P < 0.01) in the TCL group and the GSH group, respectively. Similarly, hepatic MDA production was reduced to 76.6% (P < 0.01) and 73.0% (P < 0.01) in TCL group and the GSH group, respectively, compared with the ethanol group. In addition, the hepatic SOD content was elevated to 73.2% (P < 0.01) and 85.2% (P < 0.01) of the Ctrl level in the TCL group and GSH group, respectively (Figure 6).
ALD is a spectrum of liver abnormalities induced by chronic alcohol consumption that results in more than 2 million deaths annually worldwide[22]. Recently, a growing number of studies have shown that some Chinese herbal medicines have benefits for prevention and treatment of ALD[22]. The purpose of the present study was to verify whether TCL protects against liver injury induced by alcohol and explore the possible mechanisms involved.
After receiving the Lieber-DeCarli ethanol liquid diet for 4 wk, mice in the ethanol group developed liver damage, demonstrated by marked increase of serum AST and ALT, hepatic steatosis, expression of NLRP3 inflammasome components and proinflammatory factors, increased ROS and MDA production, accumulation of NKT cells, and a significant decrease of SOD activity. GSH exists in the normal liver and reportedly improves clinical signs and hepatic dysfunction induced by alcohol[20]. Therefore, GSH was used as a positive Ctrl when assessing the effects of TCL. This study showed that treatment with TCL could successfully prevent liver injury induced by chronic alcohol consumption.
The pathogenic mechanisms involved in ALD are complicated and still not well understood. However, based on the available evidence, the “2-hit theory” of pathogenesis has been proposed[23]. The first hit is alcohol-induced disruption of lipid metabolism leading to hepatic steatosis, which makes hepatocytes vulnerable to liver injury by a second hit, resulting in inflammation and necrosis. Thus, prevention of fat accumulation can probably inhibit the progression of ALD. This study indicated that treatment with TLC significantly reduced lipid accumulation induced by chronic alcohol consumption, as shown by H&E staining, Oil red O staining, and quantification of the hepatic triglyceride content. Thus, TCL relieves hepatic steatosis by exerting a protective effect against liver injury induced by chronic alcohol consumption.
Studies have shown that inflammatory mediators and pro-inflammatory cytokines are crucially involved in liver injury induced by alcohol consumption. Alcohol intake activates the NLRP3 inflammasome, which is present in hepatic immune cells, and the activated NLRP3 inflammasome triggers liver damage by cleaving pro-IL-1β to release mature IL-1β[24]. IL-1β induces triglyceride accumulation in hepatocytes and triggers inflammation by its synergistic action with Toll-like receptor signaling that markedly amplifies inflammation via lipopolysaccharide-inducible inflammatory cytokines[25,26]. In this study, the ethanol group showed a significant increase of NLRP3 inflammasome components and IL-1β, while these increases were significantly attenuated by treatment with TCL. These data illustrate that TCL can suppress NLRP3 inflammasome activation and IL-1β release induced by chronic alcohol consumption. Recently, Cui et al[4] found that IL-1β promoted alcoholic liver injury by recruiting NKT cells. To investigate whether TCL has a protective effect against alcoholic liver injury by inhibiting the NLRP3-IL-1β signaling pathway, we investigated NKT cells in the liver. Consistent with our expectations, the results showed that TCL significantly reversed the accumulation of NKT cells induced by chronic alcohol consumption. Taken together, these data suggest that the protective effect of TCL against alcoholic liver injury might be due to reduction of inflammation via inhibition of the NLRP3-IL-1β signaling pathway. At the same time, it was also found that GSH significantly inhibited the activation of NLRP3 inflammasomes induced by alcohol.
Chronic ethanol consumption also increases ROS production, and decreases the levels of antioxidants that normally scavenge excess ROS[27,28]. This leads to an imbalance between pro-oxidants and antioxidants, which is called oxidative stress, and is thought to represent a major pathogenetic pathway for alcoholic liver injury. ROS cause liver damage in various ways, including permanent changes to DNA, lipid peroxidation that damages biological membranes[29], and inhibition of mitochondrial and peroxisomal β-oxidation enzymes, leading to hepatic steatosis[30]. ROS are also important in mediating activation of the NLRP3 inflammasome[31,32], and there is a ROS-thioredoxin-interacting protein signal axis during the alcohol-induced hit process. Through this signal axis, alcohol and its metabolites can activate the NLRP3 inflammasome domain, causing the activation of downstream cytokines of NLRP3[33]. We evaluated whether ROS were reduced when NLRP3 activation was inhibited, and we showed that TCL decreased ROS production induced by chronic alcohol consumption, which might suppress NLRP3 inflammasome activation. MDA, a product of ethanol, is frequently used as an indicator of oxidative stress. SOD is an endogenous antioxidant enzyme[2]. SOD overexpression in liver cells mediated by infection with an adenovirus vector was shown to diminish free radicals and reduce alcohol-induced liver injury[34]. To determine whether TCL reduces oxidative stress, the MDA level and SOD activity were also examined, and we confirmed that treatment with TCL alleviates oxidative stress induced by chronic alcohol consumption.
In conclusion, this study demonstrated that treatment with TCL (200 mg/kg BW) effectively alleviates chronic ethanol-induced liver injury. The protective effect of TCL might be associated with inhibition of the NLRP3-IL-1β signaling pathway and reduction of oxidative stress. Our findings provide information that TCL may be a potential candidate for prevention and treatment of ethanol-induced liver damage.
Tamarix chinensis Lour (TCL) is a shrub that usually grows in arid or semiarid desert areas and saline-alkali fields. It is a traditional Chinese herbal medicine with hepatoprotective, antioxidant, antibacterial, and antitumor activities.
To investigate the possible protective effects of TCL against liver injury induced by chronic ethanol intake.
Eighty C57BL/6J male mice (8-10 wk old) were purchased from Beijing HFK Bioscience Co. Ltd. (Beijing, China).
The mice were randomly assigned to the following 5 groups: Control (Ctrl) group, ethanol (EtOH) group, reduced glutathione (GSH) group as the positive Ctrl (EtOH + GSH at 86 mg/kg BW), and two TCL groups (EtOH + TCL-L at 100 mg/kg BW and EtOH+TCL-H at 200 mg/kg BW). Histological examination of the liver was done after staining sections with hematoxylin-eosin. Alanine aminotransferase and aspartate aminotransferase are commonly used as biochemical indicators of liver injury. TCL inhibits hepatic expression of NOD-like receptor family, pyrin domain containing 3 (NLRP3), apoptosis-associated speck-like protein (ASC), caspase-1, and interleukin (IL)-1β at the transcriptional and protein levels by Western blotting. Hepatic expression of NLRP3, ASC, caspase-1 and IL-1β mRNA was also measured by quantitative PCR. Hepatic lymphocytes were isolated by Discontinuous Percoll Gradient Centrifugation and immunostained with fluorescent antibodies (FITC-CD3 and PE-CY7-NK1.1). Fluorescence was detected by using a BD FACS caliber flow cytometry and data were analyzed with FLOW JO 7.6.1software. Liver homogenate was prepared and hepatic tissue levels of reactive oxygen species, malondialdehyde, and superoxide dismutase were determined by using commercial assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Statistical analyses were performed with SPSS 22.0 software. Values are expressed as the mean ± standard deviation. Between-group comparisons were evaluated by one-way analysis of variance (with Dunnett's test and Bonferroni's multiple comparison test). Statistical significance was established at P < 0.05. All experiments were performed at least in triplicate.
Compared with the ethanol group, mice in the TCL-treated group (200 mg/kg) had significantly lower serum levels of alanine aminotransferase (mean, 34.1 IU/L vs 45.3 IU/L, P < 0.01) and aspartate aminotransferase (mean, 89.6 IU/L vs 115.7 IU/L, P < 0.01), as well as marked reduction of hepatic tissue reactive oxygen species (decreased by 27.5%, P < 0.01) and malondialdehyde (decreased by 76.6%, P < 0.01) levels, with a significant increase of superoxide dismutase (Increased by 73.2%, P < 0.01). Expression of the NLRP3 inflammasome and its downstream cytokines (IL-1β, tumor necrosis factor-α, and IL-6), and recruitment of natural killer T cells to the liver, were reduced in the TCL-treated incubation with a Lieber-DeCaril ethanol lipid diet group.
These findings suggest that a TCL extract (200 mg/kg) protects against chronic ethanol-induced liver injury, probably by inhibiting the NLRP3-caspase-1-IL-1β signaling pathway and suppressing oxidative stress.
Our findings provide information that TCL may be a potential candidate for prevention and treatment of ethanol-induced liver damage.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aureliano M, Zhang H S-Editor: Wang YQ L-Editor: Filipodia E-Editor: Qi LL
| 1. | Torruellas C, French SW, Medici V. Diagnosis of alcoholic liver disease. World J Gastroenterol. 2014;20:11684-11699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 95] [Cited by in RCA: 123] [Article Influence: 11.2] [Reference Citation Analysis (8)] |
| 2. | Carvalho AF, Heilig M, Perez A, Probst C, Rehm J. Alcohol use disorders. Lancet. 2019;394:781-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 413] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 3. | Mandrekar P, Szabo G. Signalling pathways in alcohol-induced liver inflammation. J Hepatol. 2009;50:1258-1266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 396] [Cited by in RCA: 393] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 4. | Cui K, Yan G, Xu C, Chen Y, Wang J, Zhou R, Bai L, Lian Z, Wei H, Sun R, Tian Z. Invariant NKT cells promote alcohol-induced steatohepatitis through interleukin-1β in mice. J Hepatol. 2015;62:1311-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 124] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 5. | Ozaki E, Campbell M, Doyle SL. Targeting the NLRP3 inflammasome in chronic inflammatory diseases: current perspectives. J Inflamm Res. 2015;8:15-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 201] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 6. | Lage SL, Longo C, Branco LM, da Costa TB, Buzzo Cde L, Bortoluci KR. Emerging Concepts about NAIP/NLRC4 Inflammasomes. Front Immunol. 2014;5:309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Petrasek J, Bala S, Csak T, Lippai D, Kodys K, Menashy V, Barrieau M, Min SY, Kurt-Jones EA, Szabo G. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J Clin Invest. 2012;122:3476-3489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 587] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 8. | Xiao J, Zhu Y, Liu Y, Tipoe GL, Xing F, So KF. Lycium barbarum polysaccharide attenuates alcoholic cellular injury through TXNIP-NLRP3 inflammasome pathway. Int J Biol Macromol. 2014;69:73-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 9. | Kaiser JP, Beier JI, Zhang J, David Hoetker J, von Montfort C, Guo L, Zheng Y, Monia BP, Bhatnagar A, Arteel GE. PKCepsilon plays a causal role in acute ethanol-induced steatosis. Arch Biochem Biophys. 2009;482:104-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Guan MJ, Zhao N, Xie KQ, Zeng T. Hepatoprotective effects of garlic against ethanol-induced liver injury: A mini-review. Food Chem Toxicol. 2018;111:467-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 11. | Del Prete A, Scalera A, Iadevaia MD, Miranda A, Zulli C, Gaeta L, Tuccillo C, Federico A, Loguercio C. Herbal products: benefits, limits, and applications in chronic liver disease. Evid Based Complement Alternat Med. 2012;2012:837939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Sultanova N, Makhmoor T, Yasin A, Abilov ZA, Omurkamzinova VB, Atta-ur-Rahman, Choudhary MI. Isotamarixen - a new antioxidant and prolyl endopeptidase-inhibiting triterpenoid from Tamarix hispida. Planta Med. 2004;70:65-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Abouzid S, Sleem A. Hepatoprotective and antioxidant activities of Tamarix nilotica flowers. Pharm Biol. 2011;49:392-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Orabi MA, Taniguchi S, Sakagami H, Yoshimura M, Amakura Y, Hatano T. Hydrolyzable Tannins of Tamaricaceous Plants. 7.1 Structures and Cytotoxic Properties of Oligomeric Ellagitannins from Leaves of Tamarix nilotica and Cultured Tissues of Tamarix tetrandra. J Nat Prod. 2016;79:984-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | McCullough RL, McMullen MR, Sheehan MM, Poulsen KL, Roychowdhury S, Chiang DJ, Pritchard MT, Caballeria J, Nagy LE. Complement Factor D protects mice from ethanol-induced inflammation and liver injury. Am J Physiol Gastrointest Liver Physiol. 2018;315:G66-G79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 16. | Mandrekar P, Ambade A, Lim A, Szabo G, Catalano D. An essential role for monocyte chemoattractant protein-1 in alcoholic liver injury: regulation of proinflammatory cytokines and hepatic steatosis in mice. Hepatology. 2011;54:2185-2197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 245] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 17. | Liangpunsakul S, Rahmini Y, Ross RA, Zhao Z, Xu Y, Crabb DW. Imipramine blocks ethanol-induced ASMase activation, ceramide generation, and PP2A activation, and ameliorates hepatic steatosis in ethanol-fed mice. Am J Physiol Gastrointest Liver Physiol. 2012;302:G515-G523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 18. | Cao YW, Jiang Y, Zhang DY, Wang M, Chen WS, Su H, Wang YT, Wan JB. Protective effects of Penthorum chinense Pursh against chronic ethanol-induced liver injury in mice. J Ethnopharmacol. 2015;161:92-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 19. | Ksouri R, Falleh H, Megdiche W, Trabelsi N, Mhamdi B, Chaieb K, Bakrouf A, Magné C, Abdelly C. Antioxidant and antimicrobial activities of the edible medicinal halophyte Tamarix gallica L. and related polyphenolic constituents. Food Chem Toxicol. 2009;47:2083-2091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 152] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 20. | Li XX, Jiang ZH, Zhou B, Chen C, Zhang XY. Hepatoprotective effect of gastrodin against alcohol-induced liver injury in mice. J Physiol Biochem. 2019;75:29-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 21. | Urfi MK, Mujahid M, Rahman MA, Rahman MA. The Role of Tamarix gallica Leaves Extract in Liver Injury Induced by Rifampicin Plus Isoniazid in Sprague Dawley Rats. J Diet Suppl. 2018;15:24-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med. 2009;360:2758-2769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 693] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 23. | Day CP, James OF. Steatohepatitis: a tale of two "hits"? Gastroenterology. 1998;114:842-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2953] [Cited by in RCA: 3130] [Article Influence: 115.9] [Reference Citation Analysis (36)] |
| 24. | Shulga N, Pastorino JG. Hexokinase II binding to mitochondria is necessary for Kupffer cell activation and is potentiated by ethanol exposure. J Biol Chem. 2014;289:26213-26225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Yin H, Guo Q, Li X, Tang T, Li C, Wang H, Sun Y, Feng Q, Ma C, Gao C, Yi F, Peng J. Curcumin Suppresses IL-1β Secretion and Prevents Inflammation through Inhibition of the NLRP3 Inflammasome. J Immunol. 2018;200:2835-2846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 151] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 26. | Petrasek J, Dolganiuc A, Csak T, Kurt-Jones EA, Szabo G. Type I interferons protect from Toll-like receptor 9-associated liver injury and regulate IL-1 receptor antagonist in mice. Gastroenterology. 2011;140:697-708.e4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 27. | Zhu H, Jia Z, Misra H, Li YR. Oxidative stress and redox signaling mechanisms of alcoholic liver disease: updated experimental and clinical evidence. J Dig Dis. 2012;13:133-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 125] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 28. | Tilg H, Moschen AR. IL-1 cytokine family members and NAFLD: neglected in metabolic liver inflammation. J Hepatol. 2011;55:960-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 29. | Smathers RL, Galligan JJ, Stewart BJ, Petersen DR. Overview of lipid peroxidation products and hepatic protein modification in alcoholic liver disease. Chem Biol Interact. 2011;192:107-112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 303] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 30. | Baes M, Van Veldhoven PP. Hepatic dysfunction in peroxisomal disorders. Biochim Biophys Acta. 2016;1863:956-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 31. | Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11:136-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1681] [Cited by in RCA: 2111] [Article Influence: 131.9] [Reference Citation Analysis (0)] |
| 32. | Harijith A, Ebenezer DL, Natarajan V. Reactive oxygen species at the crossroads of inflammasome and inflammation. Front Physiol. 2014;5:352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 341] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 33. | Zhang X, Zhang JH, Chen XY, Hu QH, Wang MX, Jin R, Zhang QY, Wang W, Wang R, Kang LL, Li JS, Li M, Pan Y, Huang JJ, Kong LD. Reactive oxygen species-induced TXNIP drives fructose-mediated hepatic inflammation and lipid accumulation through NLRP3 inflammasome activation. Antioxid Redox Signal. 2015;22:848-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 203] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 34. | Wheeler MD, Kono H, Yin M, Rusyn I, Froh M, Connor HD, Mason RP, Samulski RJ, Thurman RG. Delivery of the Cu/Zn-superoxide dismutase gene with adenovirus reduces early alcohol-induced liver injury in rats. Gastroenterology. 2001;120:1241-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 92] [Article Influence: 3.8] [Reference Citation Analysis (0)] |