Published online Mar 21, 2020. doi: 10.3748/wjg.v26.i11.1128
Peer-review started: December 6, 2019
First decision: January 16, 2020
Revised: February 21, 2020
Accepted: March 1, 2020
Article in press: March 1, 2020
Published online: March 21, 2020
Processing time: 106 Days and 1.1 Hours
Accurate diagnosis of Pancreatic cysts (PC) is key in the management. The knowledge of indications for surgery, the role of endoscopic ultrasound-guided fine needle aspiration, cyst fluid analysis, imaging, and surveillance of PC are all important in the diagnosis and management of PC. Currently, there are many guidelines for the management of PC. The optimal use of these guidelines with a patient-centered approach helps diagnose early cancer and prevent the spread of cancer.
Core tip: The differentiation of mucinous and non-mucinous cysts is key in the effective management of pancreatic cysts. Thorough understanding of the absolute indications for surgery, the role of endoscopic ultrasound-guided fine needle aspiration, cyst fluid analysis, imaging, and the guidelines for surveillance are important in the diagnosis and treatment of pancreatic cysts. Patient-centered approach with a multidisciplinary team involving the surgeon, radiologist, pathologist, oncologist, and advanced endoscopist improves the management of pancreatic cysts.
- Citation: Lanke G, Lee JH. Similarities and differences in guidelines for the management of pancreatic cysts. World J Gastroenterol 2020; 26(11): 1128-1141
- URL: https://www.wjgnet.com/1007-9327/full/v26/i11/1128.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i11.1128
Pancreatic cysts (PC) are diagnosed more frequently with the widespread use of cross-sectional imaging, and most of them are found incidentally. The prevalence of PC varies with age and race and they are found approximately 3%-14% on routine computed tomography (CT) and Magnetic resonance imaging (MRI) for unrelated reasons[1,2]. They can be benign or neoplastic. Accurate determination of cyst categorization is key in the management. With the introduction of endoscopic ultrasound (EUS) and fine-needle aspiration (FNA), the accuracy of pancreatic cyst classification is improved. This review article focuses on the management of PC using different guidelines.
The pathological classification of PC (Table 1) includes inflammatory fluid collections (IFCs), non-neoplastic PC, and pancreatic cystic neoplasms (PCNs). PC can also be associated with an underlying disorder such as Von Hippel-Lindau or polycystic kidney disease[3,4]. IFCs are usually as a result of a complication of acute pancreatitis. IFCs are categorized according to the revised Atlanta classification into acute peripancreatic fluid collections, pseudocysts, acute necrotic collections, and walled-off pancreatic necrosis[5].
| Inflammatory fluid collections |
| Acute peripancreatic fluid collections |
| Pseudocysts |
| Acute necrotic collections |
| Walled-off pancreatic necrosis |
| Non-neoplastic |
| True cysts |
| Mucinous non-neoplastic cysts |
| Lymphoepithelial cysts |
| Pancreatic cystic neoplasms |
| Serous cystic neoplasms |
| Mucinous cystic neoplasm |
| Intrapapillary mucinous neoplasm |
| Solid papillary neoplasm |
Non-neoplastic or benign PC include true cysts, retention cysts, mucinous non-neoplastic cysts, and lymphoepithelial cysts. They are typically seen after surgical resection of a pancreatic lesion that is suspected to be a pancreatic cystic neoplasm (PCN) preoperatively. According to World Health Organization histological classification, PCNs are classified into serous cystic tumors, mucinous cystic neoplasms (MCNs), intraductal papillary mucinous neoplasms (IPMNs) and solid pseudopapillary neoplasms (SPN).
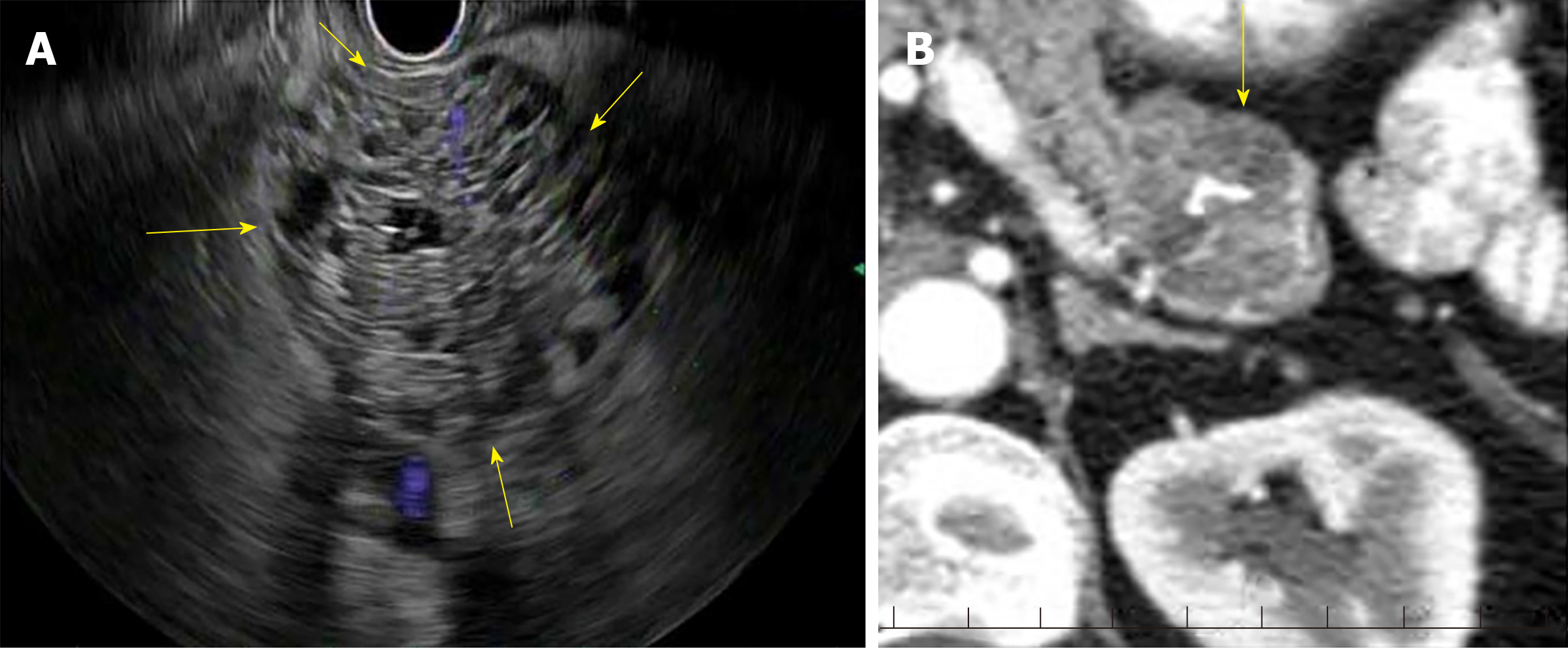
Serous cystic tumors are usually serous cystadenomas commonly seen in women over the age of 60 years and can arise anywhere in the pancreas[6]. Serous cystadenomas are classified into microcystic (composed of multiple small cystic spaces) and oligocystic (composed of fewer larger cystic spaces)[7,8]. Most of them are benign and malignant potential is low[9]. Serous cystadenomas are often found incidentally on imaging. Sometimes it is difficult to distinguish serous cystic neoplasms from mucinous neoplasms on imaging. EUS finding of honeycomb appearance with central calcification can be diagnostic (Figure 1)[10]. EUS-FNA with low carcinoembryonic antigen (CEA) in cyst fluid can help distinguish mucinous and serous cystic tumors[11]. Histologically, the cysts are lined by cuboidal epithelial cells with clear cytoplasm filled with glycogen[12].
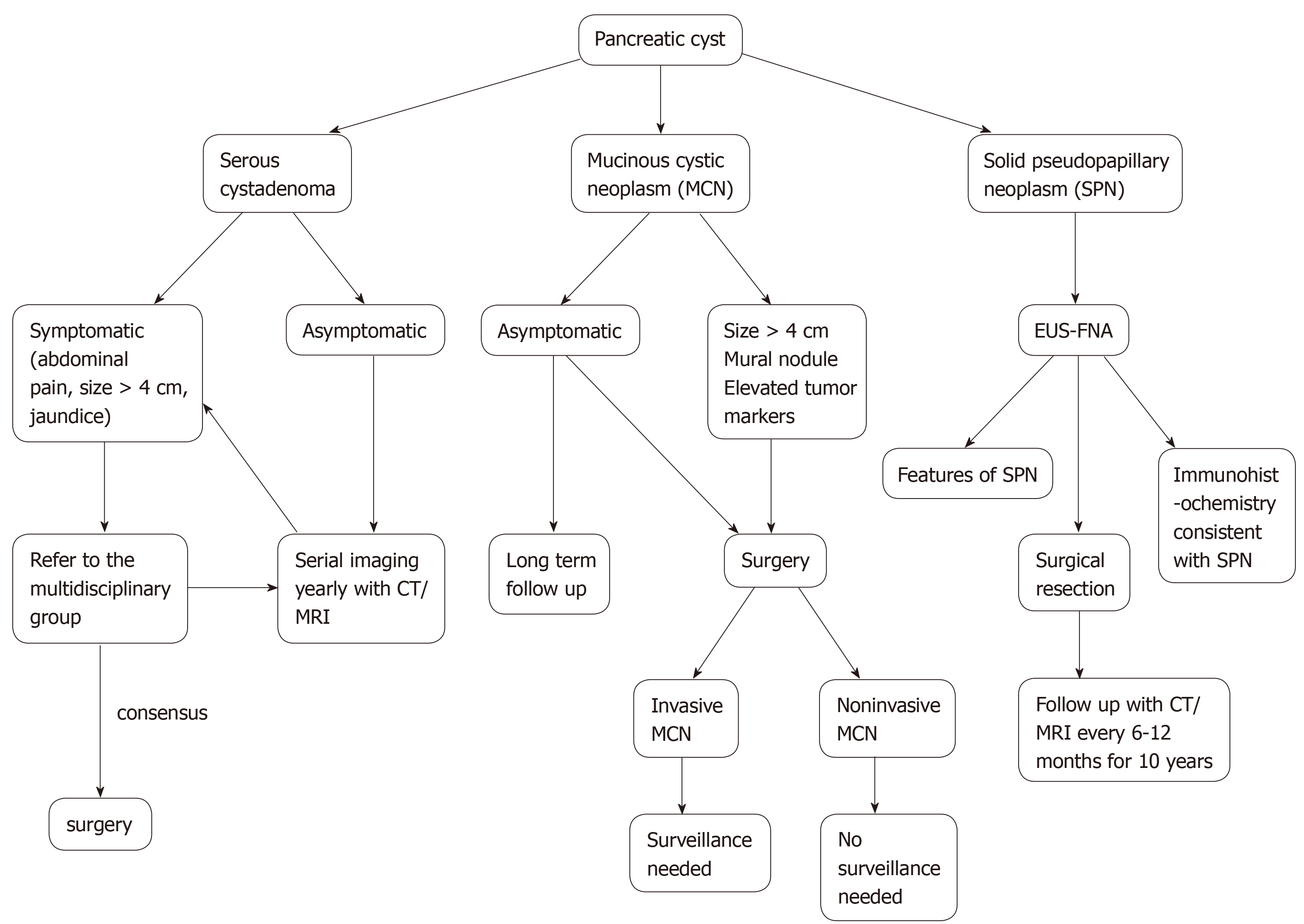
The risk of malignant transformation to cystadenocarcinoma is approximately 0.2-3%[12,13]. Surgery should be reserved for symptomatic (jaundice, extrinsic organ compression) patients and when in doubt close follow up with multidisciplinary team approach is advocated[14,15]. Size > 4 cm alone should not be an indication for surgery, although some authors advocate it[16,17]. There is no consensus on guidelines for follow up in terms of imaging. Many authors recommend yearly CT/MRI although currently, it is uncertain about how long the patient needs follow up. Overall, conservative management is recommended for serous cystic tumors and the algorithm for management of serous cystic tumors is shown in Figure 2.
MCNs are found commonly in women over the age of 40 years and can occur in the body or tail of the pancreas (Figure 3)[8]. They secrete mucin, demonstrate ovarian like stroma, exhibit cellular atypia and do not communicate with the main pancreatic duct[8]. MCNs have the risk of malignant potential. MCNs are classified according to the grade of dysplasia into low, intermediate, high grade, or invasive carcinoma[18]. The prevalence of invasive carcinoma in MCNs is approximately 12% and most patients are young at presentation, which would require long term surveillance[19]. The current treatment recommendation for MCNs is surgical resection[20]. Also, MCNs do not require surveillance after surgical resection unless there is invasive cancer[21]. Some authors do not recommend surgery for MCNs < 3 cm without mural nodules or elevated tumor markers[22]. The algorithm for the management of MCNs is shown in Figure 2.
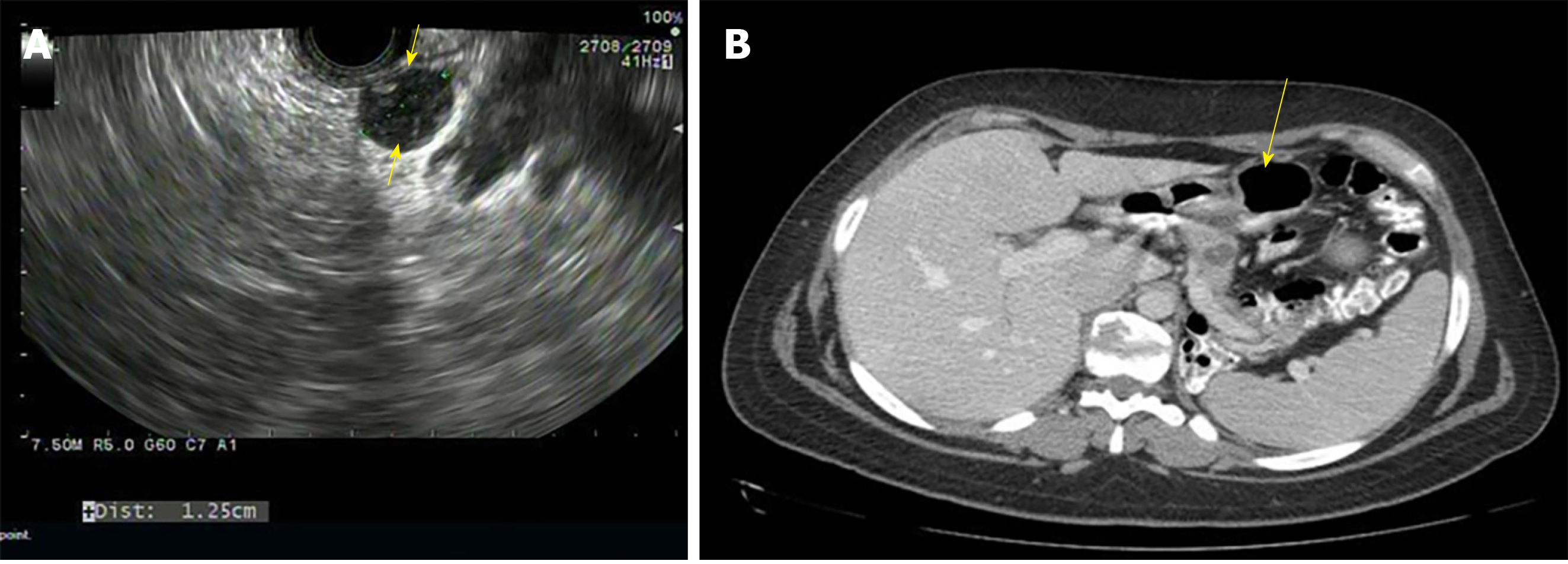
SPNs predominantly affect young females and are generally located in the tail of the pancreas (Figure 4A)[23]. They appear as solid and cystic components with areas of hemorrhage, calcification, and a rim of the fibrous capsule[24]. They have malignant potential. Surgery is the treatment of choice and R0 resection is curative[25]. The algorithm for the management of SPNs is shown in Figure 2.
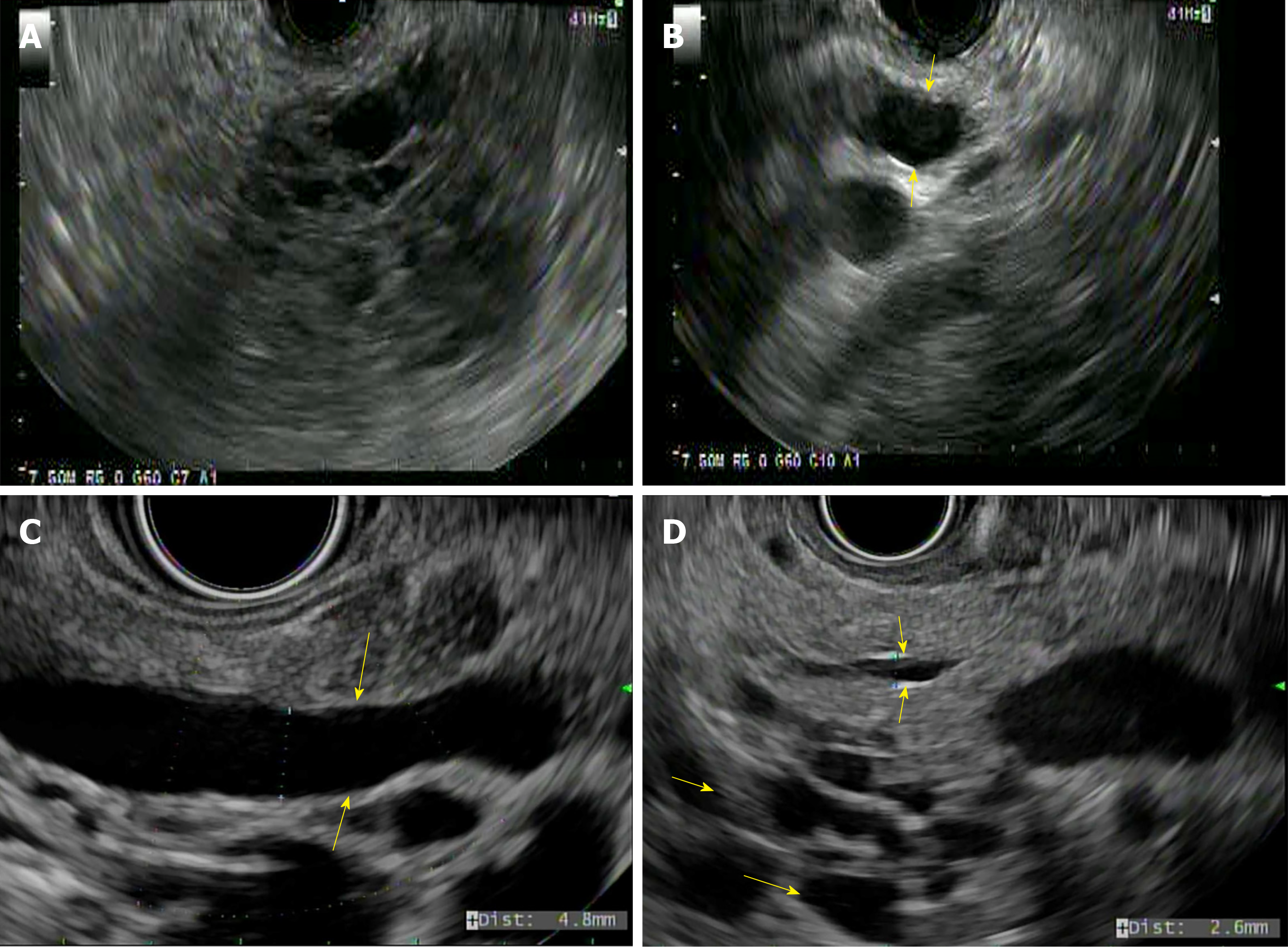
IPMNs are mucin-producing papillary neoplasms of the pancreatic duct that exhibit variable cellular atypia with dilation of the pancreatic ducts and are more common over the age of 60 years[26]. Based on the involvement of the pancreatic duct, they are classified into branch duct, main duct (MD-IPMN), or mixed type of IPMN (Figure 4B-D) respectively[27]. IPMNs have malignant potential and according to the grade of dysplasia they are classified into mild dysplasia, moderate dysplasia, high-grade dysplasia, or invasive carcinoma[27].
MCNs size < 4 cm without symptoms or mural nodules can undergo surveillance every 6 mo during the 1st year using EUS/MRI or both[21,29]. They can be followed annually if no interval change in cyst size. Lifelong surveillance is advocated if they are fit for surgery. IPMNs cyst size < 4 cm or low-grade dysplasia can be followed with serum CA 19-9 level, EUS/MRI or both every 6 mo during the 1st year. Followed by every year if no interval change in cyst size until no longer fit for surgery[28]. After surgical resection of high-grade dysplasia or MD-IPMN, EUS/MRI is recommended every 6 mo for the 1st two years and yearly follow-up afterward. Low-grade dysplasia or remnant IPMN after surgical resection should be followed in the same manner as non-resected IPMN. Lifelong follow up after surgical resection is recommended if the patient is fit and willing to undergo surgery.
The surveillance of IPMN/MCN is based on cyst size. Cyst size < 1 cm, MRI every 2 years for 4 years is recommended and lengthen the interval if cyst size is stable. Cyst size 1-2 cm, MRI every year for 3 years, followed by every 2 years for 4 years and lengthen the interval if cyst size is stable. Cyst size 2-3 cm, MRI/EUS every 6-12 mo for 3 years, followed by every year for 4 years and lengthen the interval once stable in size. Cyst size > 3 cm, MRI alternating with EUS every 6 mo for 3 years, followed by MRI alternating with EUS every year for 4 years and lengthen the interval once stable in size. Consider EUS-FNA if any increase in cyst size during follow up.
The risk of recurrence of IPMN after surgery varies based on the degree of dysplasia. EUS/MRI every 6 mo after surgical resection of IPMN-HGD is recommended. With low to intermediate grade dysplasia in the absence of PC in the remnant pancreas after surgical resection, MRI every 2 years is recommended. However, if IPMN or PC are present in the remnant pancreas after surgical resection, surveillance should be according to cyst size. Stop surveillance after surgical resection of MCN if no invasive cancer is present or no longer a surgical candidate.
In asymptomatic pancreatic neoplastic cysts < 3 cm without a solid component or PD dilation, MRI is recommended in 1 year and every 2 years for 5 years. American Gastroenterology Association (AGA) recommends stopping surveillance when no longer fit for surgery or no change in cyst characteristics after 5 years of follow up.
In revised IAP 2017 guidelines, for cyst size 1-2 cm, CT/MRI every 6 mo for a year, followed by every year for 2 years and lengthen the interval if stable. For cyst size 2-3 cm, EUS in 3-6 mo for 1 year. Increase the interval to 1 year with EUS/MRI as appropriate. For cyst size > 3 cm, close surveillance alternating MRI with EUS every 3-6 mo. In surgically resected IPMN, surveillance is recommended with cross-sectional imaging twice a year for patients with a family history of pancreatic ductal adenocarcinoma, surgical margin positive for HGD, and non-intestinal sub-type of IPMN. For all others, every 6-12 mo of cross-sectional imaging is recommended.
ACR guidelines are for the management of PC found incidentally on CT/MRI. These guidelines are based on the age of the patient and the size of the cyst. Cyst size < 1.5 cm and age < 65 years, CT/MRI every year for 5 years, followed by every 2 years for 4 years. Stop surveillance if stable over 9 years. Cyst size < 1.5 cm and age 65-79 years, CT/MRI every 2 years for a total of 10 years. Stop surveillance if the cyst is stable for 10 years. If there is interval change and cyst size < 1.5 cm, consider CT/MRI every year or EUS-FNA. If EUS-FNA shows a mucinous cyst or indeterminate cyst, CT/MRI every 6 mo for 2 years, followed by every year for 2 years and every 2 years for 6 years. Stop surveillance if the cyst is stable after 10 years.
Cyst size 1.5-1.9 cm with MPD communication, CT/MRI every year for 5 years, followed by every 2 years for 4 years. Stop surveillance if cyst size stable for over 9 years. Cyst size 2-2.5 cm with MPD communication, CT/MRI every 6 mo for 2 years, followed by every year for 2 years and subsequently every 2 years for 6 years. Stop surveillance if cyst size is stable for 10 years. If there is interval change and cyst size ≤ 2.5 cm, CT/MRI every 6 mo for 2 years, followed by every year for 2 years and subsequently every 2 years. If cyst size > 2.5 cm, consider EUS-FNA. If EUS-FNA shows a mucinous cyst or indeterminate cyst, CT/MRI every 6 mo for 2 years, followed by every year for 2 years and every 2 years for 6 years.
Cyst size 1.5-2.5 cm without MPD communication or cannot be determined, CT or MRI every 6 mo for 2 years, followed by every year for 2 years and subsequently every 2 years for 6 years. Stop surveillance if cyst size is stable after 10 years. If there is interval change and cyst size ≤ 2.5 cm, consider CT/MRI every 6 mo for 1 year, followed by every year for 5 years and subsequently every 2 years. If cyst size is > 2.5 cm, consider EUS-FNA.
Cyst size > 2.5 cm and low risk by imaging, consider CT/MRI every 6 mo for 2 years. If stable after 2 years, CT/MRI every year for 2 years and subsequently every 2 years for 6 years. Stop surveillance if stable in cyst size. Any interval changes in cyst size, consider EUS-FNA. Age ≥ 80 years with cyst size ≤ 2.5 cm, CT/MRI every 2 years for 4 years. Stop surveillance if the cyst is stable in size. If there is interval change and cyst size ≤ 2.5 cm, consider CT/MRI every year. Stop surveillance if cyst size stable or not a surgical candidate. If there is interval change and cyst size > 2.5 cm, consider EUS-FNA. Age ≥ 80 years with cyst size > 2.5 cm and low risk by imaging, consider CT/MRI every 2 years for 4 years. Stop surveillance if cyst size is stable. If there is interval change in cyst size, consider EUS-FNA.
Surveillance of PC using different guidelines is illustrated in Table 2. Overall, there is no consensus on the surveillance of PC without high-risk stigmata or worrisome features. European guidelines recommend surveillance of MCN/IPMN cysts < 4 cm with EUS/MRI. American College of Gastroenterology (ACG) guidelines recommend surveillance of IPMN/MCN based on cyst size (< 1 cm, 1-2 cm, 2-3 cm, > 3 cm) with MRI and after surgical resection, follow up is recommended based on the degree of dysplasia. AGA recommends follow up with MRI if cyst size < 3 cm without solid component or MPD dilation. Lifelong surveillance is recommended if they are fit for surgery. Revised IAP or Fukuoka guidelines are based on cyst size (< 1 cm, 1-2 cm, 2-3 cm, and > 3 cm) but with increased surveillance using CT/MRI and EUS as needed. ACR guidelines are proposed for the management of asymptomatic incidental PC found on imaging and they are based on cyst size, age, low risk on imaging, and MPD communication. They are classified into age < 65 years with cyst size < 1.5 cm, age 65-79 years with cyst size < 1.5 cm, cyst size 1.5-1.9 cm with MPD communication, cyst size 1.5-2.5 cm without MPD communication, low risk by imaging with cyst size > 2.5 cm, age ≥ 80 years with cyst size ≤ 2.5 cm and age ≥ 80 years with low risk by imaging and cyst size > 2.5 cm. Surveillance is recommended using CT/MRI and EUS-FNA as needed.
| Surveillance of pancreatic cysts | |
| European guidelines[28] | Mucinous cystic neoplasm: Cyst size < 4 cm without symptoms or mural nodules should undergo surveillance every 6 mo for the 1st year using EUS/MRI or both[29]. Followed by annually, if no changes. Lifelong surveillance if they are fit for surgery |
| Intraductal papillary mucinous neoplasm (IPMN): Every 6 mo for cysts less than 4cm or low-grade dysplasia for the 1st year with CA 19-9, EUS/MRI or both. Followed by yearly, until no longer fit for surgery | |
| After surgical resection, HGD or MD-IPMN should have imaging every 6 mo for the 1st 2 yr. Followed by yearly surveillance. Lifelong surveillance if they are fit for surgery | |
| American College of Gastroenterology (ACG) guidelines[30] | Intraductal papillary mucinous neoplasm/Mucinous cystic neoplasm (IPMN/MCN): Cyst size < 1 cm: MRI every 2 yr × 4 yr. If stable in size, consider prolonging the time interval. Any increase in size, consider EUS-FNA in 6 mo and reevaluate |
| Cyst size 1-2 cm: MRI every 1 yr × 3 yr. If stable, consider MRI every 2 yr × 4 yr. Once stable, consider prolonging the interval | |
| Cyst size 2-3 cm: MRI/EUS every 6-12 mo for 3 yr. If stable, MRI every 1-year × 4 yr. Once stable, consider prolonging the interval. Any increase in cyst size should be referred to the multidisciplinary group and consider EUS-FNA | |
| Cyst size > 3 cm: Referral to the multidisciplinary team. MRI alternating with EUS every 6 mo for 3 yr. Once stable in size, MRI alternating with EUS every year for 4 yr. Once stable in size, consider prolonging the interval | |
| Stop surveillance when a patient is no longer a surgical candidate or after surgical resection of MCN if no invasive cancer | |
| The risk of recurrence of IPMN after surgery varies based on the degree of dysplasia | |
| EUS/MRI every 6 mo after surgical resection of IPMN with HGD | |
| MRI every 2 yr after surgical resection of IPMN with low to intermediate grade dysplasia in the absence of pancreatic cysts in the remnant pancreas. However, if IPMN or pancreatic cysts are present in the remnant pancreas, then surveillance should be based on cyst size | |
| American Gastroenterology Association (AGA) guidelines[31] | Cyst size < 3 cm without a solid component or PD dilation recommend MRI in 1 yr, followed by every 2 yr for 5 yr. |
| Recommend stopping surveillance if no change in cyst characteristics after 5 yr or not a surgical candidate | |
| Revised IAP 2017 or revised Fukuoka guidelines[32] | Branch duct-Intraductal papillary mucinous neoplasm (BD-IPMN): Cysts without high-risk stigmata should undergo CT/MRI every 3-6 mo to establish stability if prior imaging is not available. Subsequently, surveillance should be based on size stratification |
| For cyst size < 1 cm, CT/MRI every 2 yr | |
| For cyst size 1-2 cm, CT/MRI every 6 mo for a year, followed by every year for 2 yr and prolong the interval if stable | |
| For cyst size 2-3 cm, EUS in 3-6 mo for 1 year. Increase the interval to 1 yr with EUS/MRI as appropriate. Consider surgery in young patients with a need for prolonged surveillance | |
| For cyst size > 3 cm, close surveillance alternating MRI with EUS every 3-6 mo. Strongly recommend surgery in young patients | |
| In surgically resected IPMN, surveillance is recommended with cross-sectional imaging twice a year for patients with a family history of pancreatic ductal adenocarcinoma, surgical margin positive for HGD and non-intestinal sub-type of IPMN. For all others, every 6-12 mo of cross-sectional imaging is recommended | |
| American College of Radiology (ACR) guidelines[33] | Cyst size < 1.5 cm and age < 65 yr: CT/MRI every year for 5 yr, followed by every 2 yr for 4 yr. Stop surveillance if stable over 9 yr |
| Cyst size < 1.5 cm and age 65-79 yr: CT/MRI every 2 yr for a total of 10 yr. Stop surveillance if the cyst is stable for 10 yr | |
| If there is interval change and cyst size < 1.5 cm, consider CT/MRI every year or EUS-FNA. EUS-FNA shows a mucinous cyst or indeterminate cyst, CT/MRI every 6 mo for 2 yr, followed by every year for 2 yr and every 2 yr for 6 yr. Stop surveillance if the cyst is stable after 10 yr | |
| Any further interval growth of cyst should be referred to surgery for further evaluation | |
| Cyst size 1.5-1.9 cm with MPD communication: CT/MRI every year for 5 yr, followed by every 2 yr for 4 yr. Stop surveillance if cyst size stable for over 9 yr | |
| Cyst size 2-2.5 cm with MPD communication: CT/MRI every 6 mo for 2 yr, followed by every year for 2 yr and subsequently every 2 yr for 6 yr. Stop surveillance if cyst size is stable for 10 yr | |
| If there is interval change and cyst size ≤ 2.5 cm, CT/MRI every 6 mo for 2 yr, followed by every year for 2 yr and subsequently every 2 yr. If cyst size > 2.5 cm, consider EUS-FNA | |
| If EUS-FNA shows a mucinous cyst or indeterminate cyst, CT/MRI every 6 mo for 2 yr, followed by every year for 2 yr and every 2 yr for 6 yr | |
| EUS-FNA is recommended for any mural nodule, wall thickening, dilation of MPD ≥ 7 mm or extrahepatic biliary obstruction/Jaundice irrespective of cyst size | |
| Cyst size 1.5-2.5 cm without MPD communication or cannot be determined: CT or MRI every 6 mo for 2 yr, followed by every year for 2 yr and subsequently every 2 yr for 6 yr. Stop surveillance if cyst size is stable after 10 yr | |
| If there is interval change and cyst size < 2.5 cm, consider CT/MRI every 6 mo for 1 year, followed by every year for 5 yr and subsequently every 2 yr. If cyst size is > 2.5 cm, consider EUS-FNA | |
| Cyst size >2.5 cm: If a cyst is a low risk by imaging, consider CT/MRI every 6 mo for 2 yr. If stable after 2 yr, CT/MRI every yr for 2 yr and subsequently every 2 yr for 6 yr. Stop surveillance if stable in cyst size | |
| Any interval changes in cyst size, consider EUS-FNA. Any high-risk stigmata like jaundice, enhancing mural nodule, wall thickening and MPD ≥ 10 mm refer to surgery for evaluation | |
| Age ≥ 80 yr with cyst size ≤ 2.5 cm: CT/MRI every 2 yr for 4 yr. Stop surveillance if cyst size is stable in size: If there is interval change and cyst size ≤ 2.5 cm, consider CT/MRI every year. Stop surveillance if the cyst stabilizes or not a surgical candidate; If there is interval change and cyst size > 2.5 cm, consider EUS-FNA | |
| Age ≥ 80 yer with cyst size ≥ 2.5 cm: If low risk by imaging, consider CT/MRI every 2 yr for 4 yr. Stop surveillance if cyst size is stable; If there is interval change in cyst size, consider EUS-FNA | |
| High risk (mural nodule, wall thickening, dilation of MPD ≥ 7 mm or extrahepatic biliary obstruction/Jaundice) features by imaging should be referred to EUS-FNA | |
| High-risk stigmata (jaundice, enhancing mural nodule, wall thickening, and MPD ≥ 10 mm) by EUS or imaging refer to surgery for evaluation |
Serum cancer antigen (CA 19-9) can be considered when there is a concern for the malignant transformation of IPMN[34,35]. Guanine nucleotide-binding protein (GNAS) and Kirsten rat sarcoma viral oncogene homolog (KRAS) mutations using next-generation sequencing techniques can be used in identifying mucin-producing cysts when the diagnosis is not clear[36,37]. Cyst fluid carcinoembryonic antigen (CEA) > 192 ng/mL can help distinguish mucinous from non-mucinous cysts[38]. Cyst fluid amylase level can help identify pseudocysts but may not differentiate mucinous from non-mucinous cysts[11,39]. A combination of cytology, cyst fluid amylase, CEA and molecular markers can help differentiate mucinous from non-mucinous cysts.
Cyst fluid cytology can assess for HGD-IPMN or cancer when imaging features are insufficient to warrant surgery. Cyst fluid CEA (> 192 ng/mL) can help differentiate IPMNs and MCNs from other cyst types[40]. Molecular markers like KRAS and GNAS mutations can help identify IPMNs or MCNs when the diagnosis is not clear[41,42]. Cyst fluid amylase level < 250 IU/L can help exclude the diagnosis of pseudocyst[11].
Cyst fluid cytology is recommended for the evaluation of high-risk features on imaging and positive cytology increases the specificity for diagnosing malignancy. The role of cyst fluid molecular markers is not clear and further research is needed.
Cyst fluid CEA (> 192 ng/mL) can distinguish mucinous from non-mucinous but not benign from malignant cyst[38]. Cyst fluid cytology can be diagnostic but sometimes limited by scant cellularity[43,44]. Cyst fluid amylase can differentiate benign from malignant MCN and amylase levels are higher in pseudocysts than non-pseudocysts[45]. The role of molecular markers like KRAS and GNAS mutations is still evolving.
Cyst fluid CEA > 192 ng/mL can help identify a mucinous cyst[46]. Cyst fluid amylase >250 IU/L suggests pseudocyst[11]. KRAS and GNAS molecular markers can help differentiate mucinous from non-mucinous cysts[47]. Cyst cytology can identify dysplastic cells. The role of cystic fluid analysis in the diagnosis of PC using different guidelines is illustrated in Table 3. Overall, cyst fluid analysis compliments imaging and can help differentiate mucinous from non-mucinous cysts. Cytology can aid in distinguishing HGD-IPMN and cancer. Cyst fluid CEA > 192 ng/mL can differentiate mucinous from non-mucinous cysts. Cyst fluid amylase > 250 IU/L can accurately diagnose pseudocysts. The use of molecular markers is still evolving, and it is promising for the future.
| Cyst fluid analysis | |
| European guidelines[28] | Cyst fluid CEA with cytology, or KRAS/GNAS mutation analysis for differentiating IPMN or MCN from other pancreatic cysts |
| American College of Gastroenterology (ACG) guidelines[30] | Cyst fluid CEA to differentiate IPMNs and MCNs from other cyst types |
| Cyst fluid cytology to assess for HGD or pancreatic cancer when imaging features are alone insufficient for surgery | |
| Molecular markers like KRAS or GNAS mutations can help identify IPMNs or MCNs when the diagnosis is not clear | |
| American Gastroenterology Association (AGA) guidelines[31] | Cyst fluid cytology is recommended for the evaluation of high-risk features on imaging. The role of molecular markers is not clear and further research is needed |
| Revised IAP 2017 guidelines[32] | Cyst fluid CEA can distinguish mucinous from non-mucinous cysts. CEA level ≥ 192-200 ng/mL is 80% accurate for the diagnosis of mucinous cyst[38,45] |
| Cyst fluid cytology can be diagnostic but sometimes limited by scant cellularity[43,44] | |
| Cyst fluid amylase can differentiate benign from malignant MCN and amylase levels are higher in pseudocysts than non-pseudocysts[45]. The role of molecular markers like KRAS and GNAS mutations is still evolving | |
| American College of Radiology guidelines[33] | Cyst fluid CEA ≥ 192 ng/mL can help identify a mucinous cyst[46] |
| Cyst fluid amylase > 250 IU/L suggests pseudocyst[11] | |
| KRAS and GNAS molecular markers can help differentiate mucinous from non-mucinous cysts[47] | |
| Cyst cytology can identify dysplastic cells |
EUS-FNA can improve diagnostic accuracy with cyst fluid CEA, amylase, and cytology in differentiating mucinous vs non-mucinous cysts. Also, it distinguishes malignant vs benign cysts when CT or MRI is unclear. EUS-FNA should be performed only when results are expected to change clinical management.
EUS-FNA is indicated in IPMNs/MCNs with jaundice or acute pancreatitis secondary to the cyst, new-onset or worsening diabetes and increase in cyst size > 3 mm/year during surveillance, significantly elevated serum CA 19-9, mural nodule, solid component within cyst or pancreatic parenchyma, dilation of MPD > 5 mm, focal dilation of PD concerning for MD-IPMN or obstructing lesion, and mucin-producing cyst size ≥ 3 cm; it is also indicated when the diagnosis of cysts is unclear; results will likely alter management; and when cyst fluid CEA can differentiate IPMNs and MCNs from other cyst types.
EUS-FNA is indicated when a pancreatic cyst has at least 2 high-risk features such as cyst size ≥ 3 cm, dilated MPD, and solid component.
EUS-FNA is recommended with pancreatitis, cyst size ≥ 3 cm, thickened/enhancing cyst wall, the main duct size 5-9 mm, non-enhancing mural nodule, an abrupt change in caliber of the pancreatic duct with distal pancreatic atrophy, lymphadenopathy, increased serum level of CA19-9, and cyst growth rate ≥ 5 mm/2 years.
EUS-FNA is indicated for a mural nodule, wall thickening, dilation of MPD ≥ 7 mm, or extrahepatic biliary obstruction/jaundice. The indications of EUS-FNA for the diagnosis of PC using different guidelines are illustrated in Table 4. Overall, EUS-FNA is indicated when the results are likely to change the management. EUS-FNA is recommended for cyst ≥ 3 cm, mural nodule, thickened cyst wall, solid component in cyst, MPD > 5 mm, abrupt change in caliber of PD with distal pancreatic atrophy, lymphadenopathy, cyst growth ≥ 3-5 mm/year, acute pancreatitis, new-onset or worsening diabetes, jaundice and increased serum CA 19-9 level.
| Endoscopic ultrasound-Fine needle aspiration indications | |
| European guidelines[28] | Differentiating mucinous vs non-mucinous |
| Malignant vs benign | |
| CT or MRI unclear | |
| Only when results are expected to change clinical management | |
| American College of Gastroenterology guidelines[30] | Jaundice |
| Acute pancreatitis | |
| Significantly elevated serum CA 19-9 | |
| Mural nodule | |
| A solid component within cyst or pancreatic parenchyma | |
| Dilation of MPD ≥ 5 mm | |
| Focal dilation of PD | |
| Cyst size > 3 cm | |
| When the diagnosis of cysts is unclear or results will likely alter management | |
| Cyst fluid CEA to differentiate IPMNs and MCNs from other cyst types | |
| New onset or worsening diabetes | |
| Increase in cyst size > 3 mm/yr | |
| American Gastroenterology Association guidelines[31] | At least 2 high-risk features |
| Cyst size ≥ 3 cm | |
| Dilated MPD | |
| Solid component | |
| Revised IAP 2017 or revised Fukuoka guidelines[32] | Pancreatitis |
| Cyst ≥ 3 cm | |
| Enhancing mural nodule < 5 mm | |
| Thickened/enhancing cyst wall | |
| Main duct size 5-9 mm | |
| An abrupt change in caliber of the pancreatic duct with distal pancreatic atrophy | |
| Lymphadenopathy | |
| Increased serum level of CA19-9 | |
| Cyst growth rate ≥ 5 mm/2 yr | |
| American College of Radiology guidelines[33] | Mural nodule |
| Wall thickening | |
| Dilation of MPD ≥ 7 mm | |
| Extrahepatic biliary obstruction/Jaundice |
Absolute indications for surgery include positive cytology for malignant/ HGD-IPMN, solid mass, jaundice (tumor-related), enhancing mural nodule ≥ 5 mm, and MPD ≥ 10 mm. Relative indications for surgery include cyst growth rate ≥ 5mm/year, increased levels of serum CA 19-9 (≥ 37 U/mL), MPD dilation 5-9.9 mm, cyst diameter ≥ 40 mm, new-onset diabetes mellitus, acute pancreatitis caused by the cyst, or enhancing mural nodule < 5 mm.
Referral to multidisciplinary team is recommended for evaluation of surgery with jaundice or acute pancreatitis secondary to the cyst, significantly elevated serum CA 19-9 level, presence of a mural nodule or solid component within the cyst, MPD dilation > 5 mm, focal dilation of PD for MD-IPMN or an obstructing lesion, IPMNs or MCNs ≥ 3 cm and the presence of HGD-IPMN or pancreatic cancer on cytology.
Surgery is recommended for cysts with both a solid component and a dilated PD and/ or concerning features on EUS-FNA positive for HGD/cancer.
Absolute indications for surgery include obstructive jaundice in a patient with a cystic lesion of the head of the pancreas, enhancing mural nodule > 5 mm and MPD ≥ 10 mm. Relative indications for surgery include cyst ≥ 3 cm, enhancing mural nodule < 5 mm, thickened cyst wall, MPD 5-9 mm, an abrupt change in caliber of PD with distal pancreatic atrophy, lymphadenopathy, increased serum level of CA 19-9 and cyst growth rate ≥ 5 mm/2 years.
Absolute indications for surgery include obstructive jaundice with a cyst in the head of the pancreas, enhancing solid component within the cyst and MPD ≥ 10 mm in the absence of obstruction. Relative indications include cyst size ≥ 3 cm, thickened cyst wall, non-enhancing mural nodule, and MPD ≥ 7 mm. The indications of surgery for various PCNs using different guidelines are illustrated in Table 5. Overall, the absolute indications for surgery are consistent among all the guidelines and the cysts with relative indications for surgery can be closely followed with imaging and/EUS-FNA.
| Absolute indications of surgery | Relative indications of surgery | |
| European guidelines[28] | Intraductal papillary mucinous neoplasm: Cytology positive for malignancy/High-grade dysplasia; Solid mass; Jaundice; Mural nodule ≥ 5 mm; Main pancreatic duct dilation > 10 mm | Cyst growth rate > 5 mm/yr |
| Mucinous cystic neoplasm: Size ≥ 4 cm | Serum CA 19-9 > 37 U/mL | |
| Symptomatic Mural nodule | MPD dilation 5-9 mm | |
| Cyst diameter ≥ 40 mm | ||
| New-onset diabetes mellitus | ||
| Acute pancreatitis related to IPMN | ||
| Mural nodule < 5 mm | ||
| American College of Gastroenterology guidelines[30] | Intraductal papillary mucinous neoplasm or Mucinous cystic neoplasm: | N/A |
| Referral to EUS-FNA/Multidisciplinary; team: | ||
| Jaundice | ||
| Acute pancreatitis | ||
| Significantly elevated CA 19-9 | ||
| Mural nodule | ||
| A solid component in cyst/pancreatic parenchyma | ||
| MPD > 5 mm | ||
| Focal dilation of PD or MD-IPMN | ||
| HGD/Pancreatic cancer on cytology | ||
| American Gastroenterology Association guidelines[31] | Pancreatic cysts: | N/A |
| EUS-FNA cytology positive for - | ||
| HGD/cancer | ||
| Both solid component and dilated PD on MRI and EUS | ||
| Revised IAP 2017 or revised Fukuoka guidelines[32] | Obstructive jaundice with pancreatic head cyst | Pancreatitis |
| Enhancing mural nodule ≥ 5 mm | Enhancing mural nodule < 5 mm | |
| MPD ≥ 10 mm | Thickened/enhancing cyst wall | |
| Main duct size 5-9 mm | ||
| An abrupt change in caliber of the pancreatic duct with distal pancreatic atrophy | ||
| Lymphadenopathy | ||
| Increase in serum level of CA 19-9 | ||
| Cyst growth rate ≥ 5 mm/2 yr | ||
| American College of Radiology guideline[33] | Obstructive jaundice with a cyst in the head of the pancreas | Cyst ≥ 3 cm |
| Enhancing solid component within a cyst | Thickened/enhancing cyst wall | |
| MPD > 10 mm in the absence of obstruction | Non-enhancing mural nodule | |
| MPD ≥ 7 mm |
At MD Anderson cancer center, we get referrals from all over the country and abroad for evaluation of PC. By the time, patients come to us, they have already been seen two or three physicians with the recommendation for surgical resection. The majority of these patients do not need require resection but can be clinically followed with repeat imaging studies. Accurate characterization of the pancreatic cyst is the key in the management of PC. All patients with pancreatic cyst who are referred to MD Anderson cancer center get automatically enrolled in the pancreatic cyst database. There is a team of pancreatic surgeons, advanced endoscopists with expertise in EUS-FNA, oncologists, radiologists, and gastrointestinal pathologists who work closely with a concerted effort in accurately diagnosing and managing PC. Any high-risk features on imaging will be referred for EUS-FNA. If EUS-FNA shows HGD/cancer, the patients will be referred for surgical evaluation. Cyst fluid analysis can help distinguish mucinous from non-mucinous cysts. Surveillance is based on the type, size of the cyst, MPD dilation, and any high-risk features. We use both ACG and revised Fukuoka guidelines in the surveillance of PC.
With the increased incidence of asymptomatic PC on imaging, accurate diagnosis is the key in the management. A multidisciplinary team approach involving advanced endoscopist, pathologist, radiologist, and surgeon is paramount in the comprehensive management of PC. Surgical resection should be selectively offered considering absolute indications, high-risk features on imaging/EUS, and clinical setting of each patient. Surveillance using a cross-sectional imaging or EUS should be individualized based on the cyst type, size, involvement of the main duct, and/or presence of a mural nodule. Lastly, surgical resection should be performed at high volume centers to optimize the outcomes in morbidity and mortality.
Manuscript source: Invited Manuscript
Corresponding Author's Membership in Professional Societies: Texas Society of Gastroenterology and Endoscopy.
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kovacevic B S-Editor: Wang J L-Editor: A E-Editor: Ma YJ
| 1. | Laffan TA, Horton KM, Klein AP, Berlanstein B, Siegelman SS, Kawamoto S, Johnson PT, Fishman EK, Hruban RH. Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am J Roentgenol. 2008;191:802-807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 724] [Cited by in RCA: 658] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 2. | de Jong K, Nio CY, Hermans JJ, Dijkgraaf MG, Gouma DJ, van Eijck CH, van Heel E, Klass G, Fockens P, Bruno MJ. High prevalence of pancreatic cysts detected by screening magnetic resonance imaging examinations. Clin Gastroenterol Hepatol. 2010;8:806-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 376] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 3. | Hammel PR, Vilgrain V, Terris B, Penfornis A, Sauvanet A, Correas JM, Chauveau D, Balian A, Beigelman C, O'Toole D, Bernades P, Ruszniewski P, Richard S. Pancreatic involvement in von Hippel-Lindau disease. The Groupe Francophone d'Etude de la Maladie de von Hippel-Lindau. Gastroenterology. 2000;119:1087-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 237] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 4. | Kim JA, Blumenfeld JD, Chhabra S, Dutruel SP, Thimmappa ND, Bobb WO, Donahue S, Rennert HE, Tan AY, Giambrone AE, Prince MR. Pancreatic Cysts in Autosomal Dominant Polycystic Kidney Disease: Prevalence and Association with PKD2 Gene Mutations. Radiology. 2016;280:762-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4323] [Article Influence: 360.3] [Reference Citation Analysis (45)] |
| 6. | Pyke CM, van Heerden JA, Colby TV, Sarr MG, Weaver AL. The spectrum of serous cystadenoma of the pancreas. Clinical, pathologic, and surgical aspects. Ann Surg. 1992;215:132-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 144] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 7. | Lewandrowski K, Warshaw A, Compton C. Macrocystic serous cystadenoma of the pancreas: a morphologic variant differing from microcystic adenoma. Hum Pathol. 1992;23:871-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 101] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Brugge WR. Diagnosis and management of cystic lesions of the pancreas. J Gastrointest Oncol. 2015;6:375-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 42] [Reference Citation Analysis (0)] |
| 9. | Compagno J, Oertel JE. Microcystic adenomas of the pancreas (glycogen-rich cystadenomas): a clinicopathologic study of 34 cases. Am J Clin Pathol. 1978;69:289-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 311] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 10. | Kimura W, Moriya T, Hirai I, Hanada K, Abe H, Yanagisawa A, Fukushima N, Ohike N, Shimizu M, Hatori T, Fujita N, Maguchi H, Shimizu Y, Yamao K, Sasaki T, Naito Y, Tanno S, Tobita K, Tanaka M. Multicenter study of serous cystic neoplasm of the Japan pancreas society. Pancreas. 2012;41:380-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 11. | van der Waaij LA, van Dullemen HM, Porte RJ. Cyst fluid analysis in the differential diagnosis of pancreatic cystic lesions: a pooled analysis. Gastrointest Endosc. 2005;62:383-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 383] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 12. | Strobel O, Z'graggen K, Schmitz-Winnenthal FH, Friess H, Kappeler A, Zimmermann A, Uhl W, Büchler MW. Risk of malignancy in serous cystic neoplasms of the pancreas. Digestion. 2003;68:24-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 105] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Jais B, Rebours V, Malleo G, Salvia R, Fontana M, Maggino L, Bassi C, Manfredi R, Moran R, Lennon AM, Zaheer A, Wolfgang C, Hruban R, Marchegiani G, Fernández Del Castillo C, Brugge W, Ha Y, Kim MH, Oh D, Hirai I, Kimura W, Jang JY, Kim SW, Jung W, Kang H, Song SY, Kang CM, Lee WJ, Crippa S, Falconi M, Gomatos I, Neoptolemos J, Milanetto AC, Sperti C, Ricci C, Casadei R, Bissolati M, Balzano G, Frigerio I, Girelli R, Delhaye M, Bernier B, Wang H, Jang KT, Song DH, Huggett MT, Oppong KW, Pererva L, Kopchak KV, Del Chiaro M, Segersvard R, Lee LS, Conwell D, Osvaldt A, Campos V, Aguero Garcete G, Napoleon B, Matsumoto I, Shinzeki M, Bolado F, Fernandez JM, Keane MG, Pereira SP, Acuna IA, Vaquero EC, Angiolini MR, Zerbi A, Tang J, Leong RW, Faccinetto A, Morana G, Petrone MC, Arcidiacono PG, Moon JH, Choi HJ, Gill RS, Pavey D, Ouaïssi M, Sastre B, Spandre M, De Angelis CG, Rios-Vives MA, Concepcion-Martin M, Ikeura T, Okazaki K, Frulloni L, Messina O, Lévy P. Serous cystic neoplasm of the pancreas: a multinational study of 2622 patients under the auspices of the International Association of Pancreatology and European Pancreatic Club (European Study Group on Cystic Tumors of the Pancreas). Gut. 2016;65:305-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 213] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 14. | Galanis C, Zamani A, Cameron JL, Campbell KA, Lillemoe KD, Caparrelli D, Chang D, Hruban RH, Yeo CJ. Resected serous cystic neoplasms of the pancreas: a review of 158 patients with recommendations for treatment. J Gastrointest Surg. 2007;11:820-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 128] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 15. | Le Borgne J, de Calan L, Partensky C. Cystadenomas and cystadenocarcinomas of the pancreas: a multiinstitutional retrospective study of 398 cases. French Surgical Association. Ann Surg. 1999;230:152-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 317] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 16. | Malleo G, Bassi C, Rossini R, Manfredi R, Butturini G, Massignani M, Paini M, Pederzoli P, Salvia R. Growth pattern of serous cystic neoplasms of the pancreas: observational study with long-term magnetic resonance surveillance and recommendations for treatment. Gut. 2012;61:746-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 17. | Tseng JF, Warshaw AL, Sahani DV, Lauwers GY, Rattner DW, Fernandez-del Castillo C. Serous cystadenoma of the pancreas: tumor growth rates and recommendations for treatment. Ann Surg. 2005;242:413-419; discussion 419-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 231] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 18. | Sarr MG, Carpenter HA, Prabhakar LP, Orchard TF, Hughes S, van Heerden JA, DiMagno EP. Clinical and pathologic correlation of 84 mucinous cystic neoplasms of the pancreas: can one reliably differentiate benign from malignant (or premalignant) neoplasms? Ann Surg. 2000;231:205-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 232] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 19. | Crippa S, Salvia R, Warshaw AL, Domínguez I, Bassi C, Falconi M, Thayer SP, Zamboni G, Lauwers GY, Mino-Kenudson M, Capelli P, Pederzoli P, Castillo CF. Mucinous cystic neoplasm of the pancreas is not an aggressive entity: lessons from 163 resected patients. Ann Surg. 2008;247:571-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 274] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 20. | Farrell JJ. Pancreatic Cysts and Guidelines. Dig Dis Sci. 2017;62:1827-1839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 21. | Tanaka M, Fernández-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, Kimura W, Levy P, Pitman MB, Schmidt CM, Shimizu M, Wolfgang CL, Yamaguchi K, Yamao K; International Association of Pancreatology. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1714] [Cited by in RCA: 1614] [Article Influence: 124.2] [Reference Citation Analysis (0)] |
| 22. | Park JW, Jang JY, Kang MJ, Kwon W, Chang YR, Kim SW. Mucinous cystic neoplasm of the pancreas: is surgical resection recommended for all surgically fit patients? Pancreatology. 2014;14:131-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 23. | Lanke G, Ali FS, Lee JH. Clinical update on the management of pseudopapillary tumor of pancreas. World J Gastrointest Endosc. 2018;10:145-155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 48] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (1)] |
| 24. | Cai H, Zhou M, Hu Y, He H, Chen J, Tian W, Deng Y. Solid-pseudopapillary neoplasms of the pancreas: clinical and pathological features of 33 cases. Surg Today. 2013;43:148-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Romics L, Oláh A, Belágyi T, Hajdú N, Gyurus P, Ruszinkó V. Solid pseudopapillary neoplasm of the pancreas--proposed algorithms for diagnosis and surgical treatment. Langenbecks Arch Surg. 2010;395:747-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | D'Angelica M, Brennan MF, Suriawinata AA, Klimstra D, Conlon KC. Intraductal papillary mucinous neoplasms of the pancreas: an analysis of clinicopathologic features and outcome. Ann Surg. 2004;239:400-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 256] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 27. | Sohn TA, Yeo CJ, Cameron JL, Hruban RH, Fukushima N, Campbell KA, Lillemoe KD. Intraductal papillary mucinous neoplasms of the pancreas: an updated experience. Ann Surg. 2004;239:788-97; discussion 797-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 674] [Cited by in RCA: 623] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 28. | European Study Group on Cystic Tumours of the Pancreas. European evidence-based guidelines on pancreatic cystic neoplasms. Gut. 2018;67:789-804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1006] [Cited by in RCA: 890] [Article Influence: 127.1] [Reference Citation Analysis (1)] |
| 29. | Del Chiaro M, Verbeke C, Salvia R, Klöppel G, Werner J, McKay C, Friess H, Manfredi R, Van Cutsem E, Löhr M, Segersvärd R; European Study Group on Cystic Tumours of the Pancreas. European experts consensus statement on cystic tumours of the pancreas. Dig Liver Dis. 2013;45:703-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 334] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 30. | Elta GH, Enestvedt BK, Sauer BG, Lennon AM. ACG Clinical Guideline: Diagnosis and Management of Pancreatic Cysts. Am J Gastroenterol. 2018;113:464-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 422] [Article Influence: 60.3] [Reference Citation Analysis (1)] |
| 31. | Vege SS, Ziring B, Jain R, Moayyedi P; Clinical Guidelines Committee; American Gastroenterology Association. American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology. 2015;148:819-22; quize12-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 629] [Cited by in RCA: 757] [Article Influence: 75.7] [Reference Citation Analysis (1)] |
| 32. | Tanaka M, Fernández-Del Castillo C, Kamisawa T, Jang JY, Levy P, Ohtsuka T, Salvia R, Shimizu Y, Tada M, Wolfgang CL. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology. 2017;17:738-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 868] [Cited by in RCA: 1154] [Article Influence: 144.3] [Reference Citation Analysis (1)] |
| 33. | Megibow AJ, Baker ME, Morgan DE, Kamel IR, Sahani DV, Newman E, Brugge WR, Berland LL, Pandharipande PV. Management of Incidental Pancreatic Cysts: A White Paper of the ACR Incidental Findings Committee. J Am Coll Radiol. 2017;14:911-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 216] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 34. | Wang W, Zhang L, Chen L, Wei J, Sun Q, Xie Q, Zhou X, Zhou D, Huang P, Yang Q, Xie H, Zhou L, Zheng S. Serum carcinoembryonic antigen and carbohydrate antigen 19-9 for prediction of malignancy and invasiveness in intraductal papillary mucinous neoplasms of the pancreas: A meta-analysis. Biomed Rep. 2015;3:43-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 35. | Kim JR, Jang JY, Kang MJ, Park T, Lee SY, Jung W, Chang J, Shin Y, Han Y, Kim SW. Clinical implication of serum carcinoembryonic antigen and carbohydrate antigen 19-9 for the prediction of malignancy in intraductal papillary mucinous neoplasm of pancreas. J Hepatobiliary Pancreat Sci. 2015;22:699-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 36. | Singhi AD, Nikiforova MN, Fasanella KE, McGrath KM, Pai RK, Ohori NP, Bartholow TL, Brand RE, Chennat JS, Lu X, Papachristou GI, Slivka A, Zeh HJ, Zureikat AH, Lee KK, Tsung A, Mantha GS, Khalid A. Preoperative GNAS and KRAS testing in the diagnosis of pancreatic mucinous cysts. Clin Cancer Res. 2014;20:4381-4389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 148] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 37. | Springer S, Wang Y, Dal Molin M, Masica DL, Jiao Y, Kinde I, Blackford A, Raman SP, Wolfgang CL, Tomita T, Niknafs N, Douville C, Ptak J, Dobbyn L, Allen PJ, Klimstra DS, Schattner MA, Schmidt CM, Yip-Schneider M, Cummings OW, Brand RE, Zeh HJ, Singhi AD, Scarpa A, Salvia R, Malleo G, Zamboni G, Falconi M, Jang JY, Kim SW, Kwon W, Hong SM, Song KB, Kim SC, Swan N, Murphy J, Geoghegan J, Brugge W, Fernandez-Del Castillo C, Mino-Kenudson M, Schulick R, Edil BH, Adsay V, Paulino J, van Hooft J, Yachida S, Nara S, Hiraoka N, Yamao K, Hijioka S, van der Merwe S, Goggins M, Canto MI, Ahuja N, Hirose K, Makary M, Weiss MJ, Cameron J, Pittman M, Eshleman JR, Diaz LA, Papadopoulos N, Kinzler KW, Karchin R, Hruban RH, Vogelstein B, Lennon AM. A combination of molecular markers and clinical features improve the classification of pancreatic cysts. Gastroenterology. 2015;149:1501-1510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 326] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 38. | Cizginer S, Turner BG, Bilge AR, Karaca C, Pitman MB, Brugge WR. Cyst fluid carcinoembryonic antigen is an accurate diagnostic marker of pancreatic mucinous cysts. Pancreas. 2011;40:1024-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 210] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 39. | Al-Rashdan A, Schmidt CM, Al-Haddad M, McHenry L, Leblanc JK, Sherman S, Dewitt J. Fluid analysis prior to surgical resection of suspected mucinous pancreatic cysts. A single centre experience. J Gastrointest Oncol. 2011;2:208-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 40. | Thornton GD, McPhail MJ, Nayagam S, Hewitt MJ, Vlavianos P, Monahan KJ. Endoscopic ultrasound guided fine needle aspiration for the diagnosis of pancreatic cystic neoplasms: a meta-analysis. Pancreatology. 2013;13:48-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 191] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 41. | Rosenbaum MW, Jones M, Dudley JC, Le LP, Iafrate AJ, Pitman MB. Next-generation sequencing adds value to the preoperative diagnosis of pancreatic cysts. Cancer Cytopathol. 2017;125:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 42. | Jones M, Zheng Z, Wang J, Dudley J, Albanese E, Kadayifci A, Dias-Santagata D, Le L, Brugge WR, Fernandez-del Castillo C, Mino-Kenudson M, Iafrate AJ, Pitman MB. Impact of next-generation sequencing on the clinical diagnosis of pancreatic cysts. Gastrointest Endosc. 2016;83:140-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 108] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 43. | Pitman MB, Deshpande V. Endoscopic ultrasound-guided fine needle aspiration cytology of the pancreas: a morphological and multimodal approach to the diagnosis of solid and cystic mass lesions. Cytopathology. 2007;18:331-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 44. | Pitman MB, Genevay M, Yaeger K, Chebib I, Turner BG, Mino-Kenudson M, Brugge WR. High-grade atypical epithelial cells in pancreatic mucinous cysts are a more accurate predictor of malignancy than "positive" cytology. Cancer Cytopathol. 2010;118:434-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 45. | Park WG, Mascarenhas R, Palaez-Luna M, Smyrk TC, O'Kane D, Clain JE, Levy MJ, Pearson RK, Petersen BT, Topazian MD, Vege SS, Chari ST. Diagnostic performance of cyst fluid carcinoembryonic antigen and amylase in histologically confirmed pancreatic cysts. Pancreas. 2011;40:42-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 46. | Rockacy M, Khalid A. Update on pancreatic cyst fluid analysis. Ann Gastroenterol. 2013;26:122-127. [PubMed] |
| 47. | Thiruvengadam N, Park WG. Systematic Review of Pancreatic Cyst Fluid Biomarkers: The Path Forward. Clin Transl Gastroenterol. 2015;6:e88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (1)] |