Published online Mar 14, 2020. doi: 10.3748/wjg.v26.i10.1088
Peer-review started: November 14, 2019
First decision: December 30, 2019
Revised: January 6, 2020
Accepted: January 19, 2020
Article in press: January 19, 2020
Published online: March 14, 2020
Processing time: 121 Days and 12 Hours
Hepatopulmonary syndrome (HPS) is an arterial oxygenation defect induced by intrapulmonary vascular dilatation (IPVD) in the setting of liver disease and/or portal hypertension. This syndrome occurs most often in cirrhotic patients (4%–32%) and has been shown to be detrimental to functional status, quality of life, and survival. The diagnosis of HPS in the setting of liver disease and/or portal hypertension requires the demonstration of IPVD (i.e., diffuse or localized abnormally dilated pulmonary capillaries and pulmonary and pleural arteriovenous communications) and arterial oxygenation defects, preferably by contrast-enhanced echocardiography and measurement of the alveolar-arterial oxygen gradient, respectively.
To compare brain and whole-body uptake of technetium for diagnosing HPS.
Sixty-nine patients with chronic liver disease and/or portal hypertension were prospectively included. Brain uptake and whole-body uptake were calculated using the geometric mean of technetium counts in the brain and lungs and in the entire body and lungs, respectively.
Thirty-two (46%) patients had IPVD as detected by contrast-enhanced echocardiography. The demographics and clinical characteristics of the patients with and without IPVD were not significantly different with the exception of the creatinine level (0.71 ± 0.18 mg/dL vs 0.83 ± 0.23 mg/dL; P = 0.041), alveolar-arterial oxygen gradient (23.2 ± 13.3 mmHg vs 16.4 ± 14.1 mmHg; P = 0.043), and arterial partial pressure of oxygen (81.0 ± 12.1 mmHg vs 90.1 ± 12.8 mmHg; P = 0.004). Whole-body uptake was significantly higher in patients with IPVD than in patients without IPVD (48.0% ± 6.1% vs 40.1% ± 8.1%; P = 0.001). The area under the curve of whole-body uptake for detecting IPVD was significantly higher than that of brain uptake (0.75 vs 0.54; P = 0.025). The optimal cut-off values of brain uptake and whole-body uptake for detecting IPVD were 5.7% and 42.5%, respectively, based on Youden’s index. The sensitivity, specificity, and accuracy of brain uptake > 5.7% and whole-body uptake > 42.5% for detecting IPVD were 23%, 89%, and 59% and 100%, 52%, and 74%, respectively.
Whole-body uptake is superior to brain uptake for diagnosing HPS.
Core tip: Hepatopulmonary syndrome is a common complication of liver disease that impairs the lungs’ ability to oxygenate blood, leading to debilitating symptoms, such as shortness of breath. Intrapulmonary vascular dilations, a hallmark of hepatopulmonary syndrome, can be detected using technetium-99m-labeled macroaggregated albumin lung perfusion scan; however, of the two most commonly used methods of result interpretation (i.e., brain uptake and whole-body uptake), it is unclear which is more accurate. In this study of 69 patients with liver cirrhosis, we found that whole-body uptake is more accurate than brain uptake for detecting intrapulmonary vascular dilations.
- Citation: Zhao H, Tsauo J, Zhang XW, Ma HY, Weng NN, Tang GS, Li X. Technetium-99m-labeled macroaggregated albumin lung perfusion scan for diagnosis of hepatopulmonary syndrome: A prospective study comparing brain uptake and whole-body uptake. World J Gastroenterol 2020; 26(10): 1088-1097
- URL: https://www.wjgnet.com/1007-9327/full/v26/i10/1088.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i10.1088
Hepatopulmonary syndrome (HPS) is an arterial oxygenation defect induced by intrapulmonary vascular dilatation (IPVD) in the setting of liver disease and/or portal hypertension[1,2]. This syndrome occurs most often in cirrhotic patients (4%–32%) and has been shown to be detrimental to functional status, quality of life, and survival[3,4]. The diagnosis of HPS in the setting of liver disease and/or portal hypertension requires the demonstration of IPVD (i.e., diffuse or localized abnormally dilated pulmonary capillaries and pulmonary and pleural arteriovenous communications) and arterial oxygenation defects, preferably by contrast-enhanced echocardiography (CEE) and measurement of the alveolar-arterial oxygen gradient (AaO2), respectively[1]. Although a technetium-99m (Tc-99m)-labeled macroaggregated albumin (MAA) lung perfusion scan is not the preferred method for detecting IPVD due to its low sensitivity (20%–96%) compared to CEE, it is an important complimentary tool to CEE for diagnosing HPS[1,5-8]. An MAA lung perfusion scan can determine the degree of IPVD via the shunt fraction, which can be used to assess the contribution of HPS to arterial oxygenation defects in patients with coexisting pulmonary disease and to determine the prognosis of HPS patients undergoing liver transplantation[9,10]. In addition, CEE is unfeasible in 5%–7% of patients due to inadequate echocardiographic window[3,11,12].
Both brain uptake and whole-body uptake have been used to determine the shunt fraction[8,13,14]. In the former, the shunt fraction is calculated based on the technetium uptake in the brain, which is assumed to receive 13% of the cardiac output, and the lungs, whereas the latter is based on the technetium uptake in the whole body and the lungs[7]. Although whole-body uptake has been more widely used in general, brain uptake is considered the standard technique for diagnosing HPS[1,14]. This is mainly because whole-body scans require a longer acquisition time than local scans; therefore, they may be associated with a theoretical risk of overestimating the shunt fraction due to the breakdown of MAA particles over time. However, the assumption that the brain receives 13% of the cardiac output may not be applicable to patients with liver disease and/or portal hypertension because this value is based on healthy subjects[15]. In cirrhotic patients, the brain may receive a lower percentage of the cardiac output than in healthy subjects due to cerebral vasoconstriction[16,17]. Therefore, brain uptake may be associated with a theoretical risk of underestimating the shunt fraction in cirrhotic patients. To the best of our knowledge, brain uptake and whole-body uptake have not been compared for the diagnosis of HPS. This question is of interest because its answer may allow for more accurate diagnosis of HPS in patients unfeasible to CEE and more accurate quantification of the degree of IPVD. The purpose of this study was to compare brain uptake and whole-body uptake for diagnosing HPS.
This prospective study was approved by the Institutional Review Board of West China Hospital (Identifier, 2014-234). All patients provided written informed consent. From December 2014 to October 2015, all patients with chronic liver disease and/or portal hypertension admitted to our institution for an elective interventional radiologic procedure (e.g., transjugular intrahepatic portosystemic shunt or balloon angioplasty of the obstructed hepatic veins and/or inferior vena cava) were eligible for inclusion. Chronic liver disease and portal hypertension were diagnosed by clinical, laboratory, imaging, and, when necessary, histologic findings. The exclusion criteria were age < 18 years, forced expiratory volume in one second (FEV1) < 70% predicted, forced vital capacity (FVC) < 70% predicted, FEV1/FVC < 70% predicted, pulmonary artery systolic pressure > 35 mmHg, QT interval > 50% of the RR interval, intracardiac shunting, active infection, pregnancy, or a previous transjugular intrahepatic or surgical portosystemic shunt.
CEE was used to detect IPVD. The technique used to perform CEE has been described in detail elsewhere[18]. Briefly, agitated saline produced by flushing 9.5 mL of saline with 0.5 mL of room air between two 10 mL Luer-Lock syringes was intravenously injected, and transthoracic two-dimensional echocardiography in the parasternal four-chamber view was used to detect the appearance of microbubbles. The result was regarded as positive if any microbubbles appeared in the left heart chambers three to six heartbeats after their initial appearance in the right heart chambers[1]. The appearance of any microbubbles in the left heart chambers less than three heartbeats after their initial appearance in the right heart chambers indicated the presence of an intracardiac shunt. The result was regarded as negative if no microbubbles appeared in the left heart chambers after three injections. All CEE results were interpreted by consensus of two blinded echocardiologists with more than 10 years of experience. The diagnosis of HPS was established by a positive CEE result and an elevated AaO2 [i.e., ≥ 15 mmHg (≥ 20 mmHg in patients aged ≥ 65 years)][19]. The severity of HPS was classified as very severe [arterial partial pressure of oxygen (PaO2) < 50 mmHg], severe (PaO2 50–59 mmHg), moderate (PaO2 60–79 mmHg), or mild (PaO2 ≥ 80 mmHg)[1].
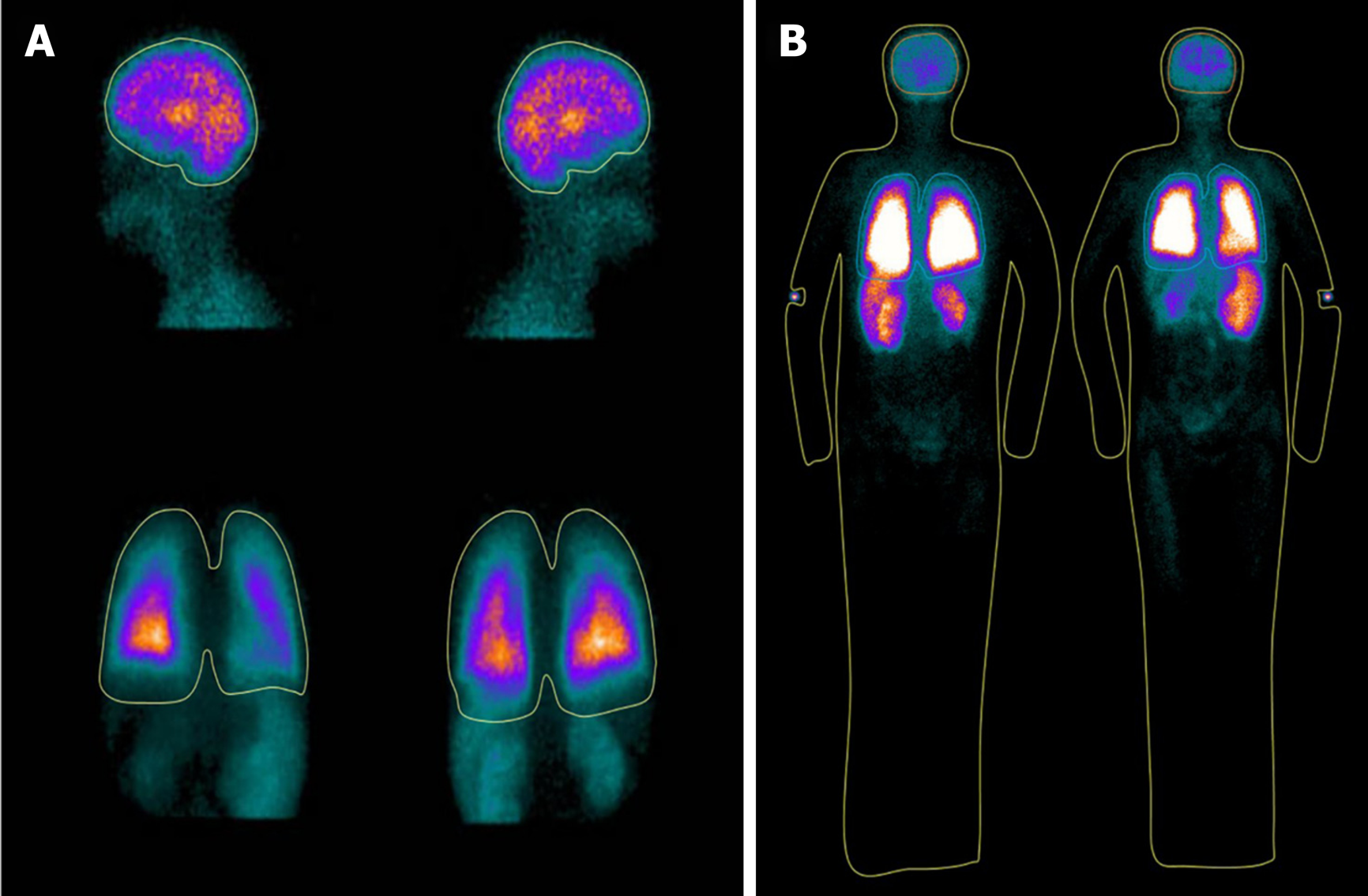
The MAA kit used in this study was purchased from by Jiangsu Institute of Nuclear Medicine (Jiangsu, China). A total of 10–30 mCi of Tc-99m in 5 mL of saline was injected into a 10-mL bottle containing 2 mg of aggregated albumin (particles size between 10–90 μm) and 0.15 mg stannous chloride. The bottle was rocked for 1 min and, occasionally, the radiochemical purity of the Tc-99m MAA was determined with paper chromatography (purity normally > 99%) 5 min later. With the patient in an upright position, 2 mCi of Tc-99m MAA particles was injected through an intravenous line placed in the upper extremity. Subsequently, local images (i.e., left and right lateral head and anterior and posterior chest) and whole-body images (i.e., anterior and posterior whole-body) were acquired sequentially using a single photon emission computed tomography system (Precedence; Philips Healthcare, Cleveland, OH, United States) immediately after injection of MAA particles. In all patients, the order of acquisition of images (i.e., local images followed by whole-body images or vice versa) was decided using computer-generated random numbers. The acquisition parameters were as follows: 64 × 64 matrix and 2 min of acquisition time per local image or 15–25 cm/min per whole-body image. Regions of interest were manually drawn using postprocessing software (Jetstream Workspace, Philips, the Netherlands) by consensus of two blinded experienced nuclear radiologists. Brain uptake was calculated using the geometric mean of the technetium (GMT) counts in the brain, which is assumed to receive 13% of the cardiac output, and the lungs as follows: (GMTbrain / 0.13) / (GMTbrain / 0.13 + GMTlungs). In contrast, the whole-body uptake was calculated using the GMT counts in the whole-body and the lungs as follows: (1 - GMTlungs/GMTwhole-body).
Variables were compared using Student’s t-test, the Mann-Whitney U test, the χ2 test, or Fisher’s exact test as appropriate. Pearson’s correlation or Spearman's rank correlation test were used to investigate relationships between the variables. Multivariable logistic regression with backward elimination was performed to identify variables that were independently associated with IPVD. Receiver operating characteristic curve analysis was conducted to evaluate the accuracy of brain uptake and whole-body uptake for detecting IPVD. A two-sided P value < 0.05 was considered to indicate a significant difference. All statistical analyses were conducted using MedCalc (version 15.2.2; MedCalc, Ostend, Belgium) or SPSS (version 21.0; SPSS, Armonk, NY, United States) statistical software.
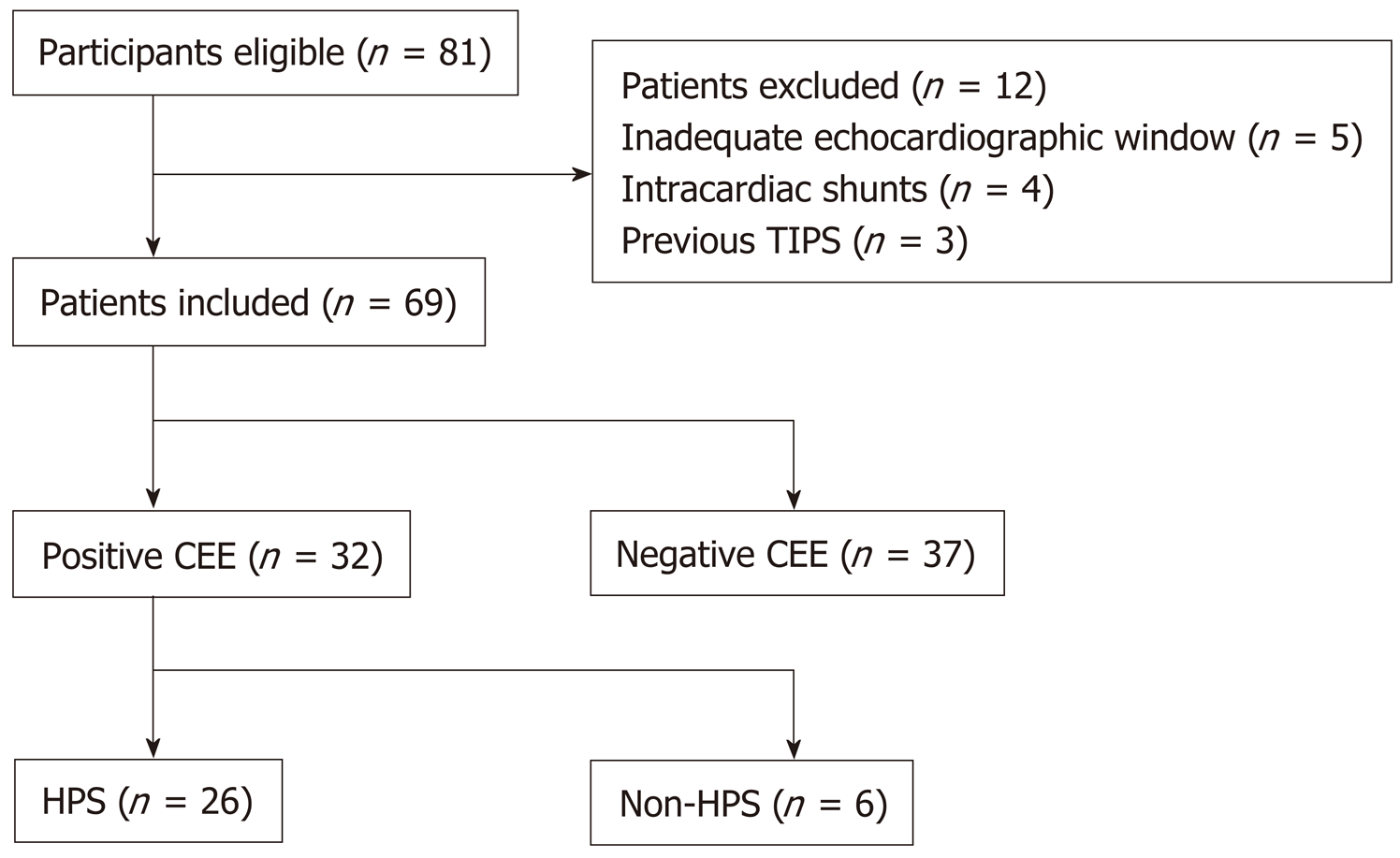
A total of 81 patients were considered eligible for inclusion in this study. Twelve patients were excluded: Five due to an inadequate echocardiographic window, four due to intracardiac shunt, and three due to a previous transjugular intrahepatic portosystemic shunt. The remaining 69 patients were included (Figure 1). Of these patients, 32 (46%) had a positive CEE result and were therefore considered to have IPVD. The demographics and clinical characteristics of the patients with and without IPVD were not significantly different, with the exception of the creatinine level (0.71 ± 0.18 mg/dL vs 0.83 ± 0.23 mg/dL; P = 0.041), AaO2 (23.2 ± 13.3 mmHg vs 16.4 ± 14.1 mmHg; P = 0.043), and PaO2 (81.0 ± 12.1 mmHg vs 90.1 ± 12.8 mmHg; P = 0.004) (Table 1). Among the 32 patients with IPVD, 26 (81%) were considered to have HPS, as they had an elevated AaO2. The remaining six (19%) patients were considered to have subclinical HPS. Of the 26 patients with HPS, one (4%) had very severe HPS, 11 (42%) had moderate HPS, and 14 (54%) had mild HPS.
| All patients, n = 69 | IPVD, n = 32 | Non-IPVD, n = 37 | P value | |
| Age in yr | 53.6 ± 12.2 | 54.1 ± 11.6 | 53.1 ± 12.8 | 0.739 |
| Sex, male/female | 46/23 (67/33) | 21/11 (66/34) | 25/12 (68/32) | 0.864 |
| Etiology | 0.460 | |||
| Hepatitis B virus infection | 16 (23) | 7 (22) | 9 (24) | |
| Budd-Chiari syndrome | 15 (22) | 6 (19) | 9 (24) | |
| Hepatocellular carcinoma | 15 (22) | 9 (28) | 6 (16) | |
| Alcoholic liver disease | 10 (14) | 5 (16) | 5 (14) | |
| Cryptogenic | 4 (6) | 3 (9) | 1 (3) | |
| Others | 9 (13) | 2 (6) | 7 (19) | |
| Child-Pugh class, A/B/C | 31/32/6 (45/46/9) | 11/18/3 (34/56/10) | 20/14/3 (54/38/8) | 0.262 |
| Child-Pugh score | 7.0 ± 1.6 | 7.2 ± 1.6 | 6.9 ± 1.6 | 0.457 |
| MELD score | 9.8 ± 2.9 | 9.8 ± 2.5 | 9.7 ± 3.2 | 0.913 |
| Bilirubin in mg/dL | 1.5 ± 0.8 | 1.7 ± 1.0 | 1.3 ± 0.5 | 0.198 |
| Albumin in g/L | 35.4 ± 5.2 | 35.2 ± 5.5 | 35.6 ± 5.1 | 0.720 |
| Creatinine in mg/dL | 0.77 ± 0.22 | 0.71 ± 0.18 | 0.83 ± 0.23 | 0.041 |
| International normalized ratio | 1.26 ± 0.18 | 1.26 ± 0.15 | 1.26 ± 0.20 | 0.963 |
| Variceal bleeding | 38 (55) | 17 (45) | 21 (55) | 0.762 |
| Ascites | 37 (54) | 19 (51) | 18 (49) | 0.373 |
| FEV1/FVC, % predicted | 85.3 ± 6.8 | 84.4 ± 6.2 | 86.2 ± 7.5 | 0.553 |
| Arterial blood gas | ||||
| PaO2 in mmHg | 85.8 ± 13.2 | 81.0 ± 12.1 | 90.1 ± 12.8 | 0.004 |
| PaCO2 in mmHg | 35.7 ± 5.1 | 36.5 ± 4.5 | 35.1 ± 5.6 | 0.255 |
| AaO2 in mmHg | 19.6 ± 14.0 | 23.2 ± 13.3 | 16.4 ± 14.1 | 0.043 |
| MAA shunt fractions derived from different formulas | ||||
| Brain-lung uptake | 4.4 ± 4.3 | 5.3 ± 6.0 | 3.6 ± 1.8 | 0.245 |
| Whole-body uptake | 43.8 ± 8.2 | 48.0 ± 6.1 | 40.1 ± 8.1 | 0.001 |
Thirty-three (48%) patients underwent acquisition of local images followed by whole-body images, whereas 36 (52%) underwent acquisition of whole-body images followed by local images. The demographics and clinical characteristics of the patients who underwent acquisition of local images followed by whole-body images were not significantly different from those of patients who underwent acquisition of whole-body images followed by local images (Table 2). The results of the MAA lung perfusion scan are presented in Table 1. No thyroid uptake was noted in any patient. Brain uptake was not significantly different between patients with and those without IPVD (5.3% ± 6.0% vs 3.6% ± 1.8%; P = 0.245) (Figure 2A). Conversely, whole-body uptake was significantly higher in patients with than in patients without IPVD (48.0% ± 6.1% vs 40.1% ± 8.1%; P = 0.001) (Figure 2B). Among the variables that were significantly different between patients with and those without IPVD, PaO2 and AaO2 were significantly correlated with one another (P < 0.001). Multivariable logistic regression showed that whole-body uptake was the only variable independently associated with IPVD [odds ratio, 1.29 (95% confidence interval (CI): 1.07–1.55); P = 0.008] (Table 3). The Hosmer-Lemeshow goodness-of-fit test showed that the logistic regression model used to identify variables independently associated with IPVD was well fit for the data (P = 0.551).
| Local images followed by whole-body images, n = 33 | Whole-body images followed by local images, n = 36 | P value | |
| Age in yr | 52.8 ± 12.0 | 54.4 ± 12.5 | 0.588 |
| Sex, male/female | 20/13 (61/39) | 26/10 (72/28) | 0.307 |
| Etiology | 0.161 | ||
| Hepatitis B virus infection | 12 (36) | 4 (11) | |
| Budd-Chiari syndrome | 7 (21) | 8 (22) | |
| Hepatocellular carcinoma | 4 (12) | 11 (31) | |
| Alcoholic liver disease | 4 (12) | 6 (17) | |
| Cryptogenic | 2 (6) | 2 (6) | |
| Others | 4 (12) | 5 (14) | |
| Child-Pugh class, A/B/C | 16/16/1 (48/48/3) | 15/16/5 (42/44/14) | 0.344 |
| Child-Pugh score | 6.9 ± 1.5 | 7.1 ± 1.7 | 0.458 |
| MELD score | 9.4 ± 2.4 | 10.0 ± 3.2 | 0.436 |
| Bilirubin in mg/dL | 1.5 ± 0.9 | 1.5 ± 0.7 | 0.958 |
| Albumin in g/L | 35.0 ± 5.0 | 35.8 ± 5.5 | 0.510 |
| Creatinine in mg/dL | 0.76 ± 0.21 | 0.78 ± 0.23 | 0.757 |
| International normalized ratio | 1.24 ± 0.14 | 1.28 ± 0.20 | 0.298 |
| Variceal bleeding | 22 (58) | 16 (42) | 0.064 |
| Ascites | 16 (43) | 21 (57) | 0.413 |
| FEV1/FVC, % predicted | 86.3 ± 7.5 | 84.3 ± 6.2 | 0.524 |
| Arterial blood gas | |||
| PaO2 in mmHg) | 87.6 ± 10.0 | 84.2 ± 15.6 | 0.283 |
| PaCO2 in mmHg | 35.9 ± 5.1 | 35.6 ± 5.2 | 0.785 |
| AaO2 in mmHg | 17.5 ± 11.4 | 21.6 ± 16.0 | 0.225 |
| IPVD | 13 (41) | 19 (59) | 0.265 |
| HPS | 12 (46) | 14 (54) | 0.829 |
| Brain-lung uptake | 3.5 ± 1.7 | 5.3 ± 5.6 | 0.158 |
| Whole-body uptake | 42.7 ± 10.0 | 44.7 ± 6.5 | 0.393 |
| Variable | β coefficients | SE | P value | OR | 95%CI |
| Age | 0.008 | 0.028 | 0.768 | 1.008 | 0.954–1.066 |
| Sex | 0.372 | 0.938 | 0.692 | 1.450 | 0.230–9.122 |
| Creatinine | -2.350 | 2.257 | 0.298 | 0.095 | 0.001–7.949 |
| Whole-body uptake | 0.254 | 0.095 | 0.008 | 1.289 | 1.069–1.554 |
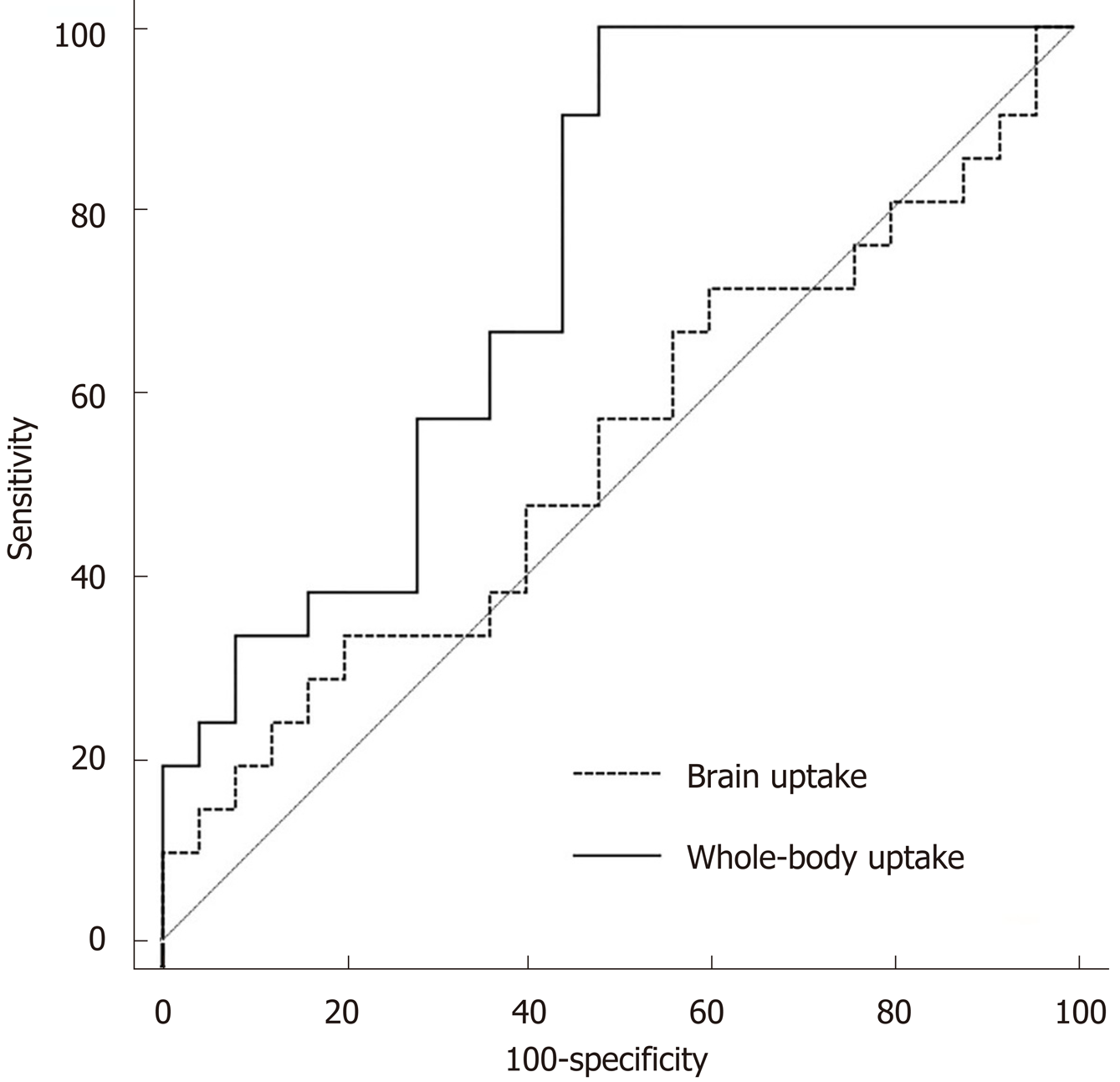
The area under the curve of whole-body uptake for detecting IPVD was significantly greater than that of brain uptake [0.75 (95%CI: 0.60–0.86) vs 0.54 (95%CI: 0.38–0.69); P = 0.025] (Figure 3). The optimal cut-off values of brain and whole-body uptake for detecting IPVD were 5.7% and 42.5%, respectively, based on Youden’s index. The sensitivity, specificity, and accuracy of CEE for detecting IPVD were 100%, 86%, and 91%, respectively (Table 4). Comparatively, the sensitivity, specificity, and accuracy of brain uptake > 5.7% and > 6% for detecting IPVD were only 23%, 89%, and 59% and 19%, 92%, and 59%, respectively. In contrast, the sensitivity, specificity, and accuracy of whole-body uptake > 42.5% for detecting IPVD were 100%, 52%, and 74%, respectively.
| Sensitivity, % | Specificity, % | PPV, % | NPV, % | Accuracy, % | |
| CEE | 100 | 86 | 81 | 100 | 91 |
| Brain-lung uptake > 6% | 19 | 92 | 67 | 58 | 59 |
| Brain-lung uptake > 5.7% | 23 | 89 | 64 | 58 | 59 |
| Whole-body uptake > 42.5% | 100 | 52 | 65 | 100 | 74 |
Brain uptake is calculated based on the assumption that the brain receives 13% of the cardiac output[7]. However, this assumption was derived from healthy subjects; therefore, it may not be applicable to patients with liver disease and/or portal hypertension. In fact, cirrhotic patients present a widespread reduction in intracranial blood flow, particularly in patients with ascites and more advanced cirrhosis[16]. In addition, brain uptake may also be affected by medications that affect cerebral circulation, such as propanol, vasopressin, and terlipressin[20,21]. Another concern with brain uptake is that the cut-off value of 6% was derived from only eight healthy subjects[6,17,22]. Despite these drawbacks, brain uptake is considered the standard technique for diagnosing HPS[1]. In contrast, as a more widely used technique in general, whole-body uptake is seldom used for diagnosing HPS, mainly because of the theoretical risk of overestimating the shunt fraction due to breakdown of MAA particles during whole-body scans, which require longer acquisition times than local scans. However, in this study, the order of acquisition of images did not significantly affect whole-body uptake and brain uptake, suggesting that the alterations in the shunt fraction caused by breakdown of MAA particles during these scans may be negligible.
In this study, we found: (1) Whole-body uptake was significantly greater in patients with than in patients without IPVD, whereas brain uptake was not significantly different between patients with and those without IPVD; (2) Whole-body uptake was the only independent predictor associated with the presence of IPVD; (3) The area under the curve of whole-body uptake for detecting IPVD was significantly higher than that of brain uptake; and (4) The sensitivity of whole-body uptake > 42.5% for detecting IPVD was 100%, whereas it was only 19% for brain uptake > 6%. Collectively, these results suggest that whole-body uptake is superior to brain uptake for diagnosing HPS. Underestimation of the shunt fraction due to inaccurate estimation of the distribution of the cardiac output to the brain may explain these results. In contrast, the suboptimal cut-off value (6%) was unlikely to be the reason for these results because the optimal cut-off value (5.7%) was comparable and produced similar results. It should be noted that the sensitivity of brain uptake in this study (19%) was lower than values reported in previous studies (20%–96%), most likely due to the high percentage of patients with mild or moderate HPS (96%), as the sensitivity of brain uptake is much lower in these patients than in patients with severe or very severe HPS[6-8,23].
Although whole-body uptake was more accurate than brain uptake for detecting IPVD, it remains to be less accurate than CEE, which suggests that CEE should remain the preferred method for diagnosing HPS. It should be noted, however, that MAA is an important complimentary tool to CEE in detecting HPS. This is because in approximately 5%–7% of patients, a conclusive result of IPVD cannot be obtained with CEE owing to inadequate echocardiographic windows[3,11,12]. Although transesophageal contrast-enhanced echocardiography could be an alternative in patients with poor image quality using CEE, it is more invasive than MAA lung perfusion scan[11]. In addition, CEE does not allow for quantifying the degree of IPVD, which is especially useful to estimate the degree of hypoxemia due to intrapulmonary shunting in patients with coexistent intrinsic cardiopulmonary disease and HPS. Therefore, in patients who were indicated for MAA lung perfusion scan to detect IPVD, whole-body uptake should be the preferred method over brain uptake, because it allows for more accurate diagnosis of HPS.
This study has several important limitations. First, the vast majority of the patients had only mild or moderate HPS; therefore, our results may not be applicable to patients with severe or very severe HPS. However, it should be noted that it is much more difficult to detect IPVD in patients with mild or moderate HPS than in patients with severe or very severe HPS[6,23]. Second, patients with different underlying liver diseases were included. Third, the order of acquisition of images was different between the two groups of patients, although our results did not show any statistically significant difference in uptake between the two groups.
In conclusion, whole-body uptake is superior to brain uptake for diagnosing HPS; therefore, it should be considered as the standard technique for calculating the shunt fraction. However, it should remain a complementary tool to CEE for diagnosing HPS due to its relatively lower sensitivity.
Hepatopulmonary syndrome (HPS) is a common complication of liver disease that impairs the lungs’ ability to oxygenate blood, leading to debilitating symptoms, such as shortness of breath. Intrapulmonary vascular dilations (IPVD), a hallmark of HPS, can be detected using technetium-99m-labeled macroaggregated albumin lung perfusion scan.
Of the two most commonly used methods of result interpretation (i.e., brain uptake and whole-body uptake) for macroaggregated albumin lung perfusion scan, it is unclear which is more accurate for detecting IPVD and diagnosing HPS.
This study aimed to compare brain and whole-body uptake of technetium for diagnosing HPS and establish the standard technique for calculating the shunt fraction for the future research.
Sixty-nine patients with chronic liver disease and/or portal hypertension were prospectively included. Brain uptake and whole-body uptake were calculated using the geometric mean of technetium counts in the brain and lungs and in the entire body and lungs, respectively.
Thirty-two (46%) patients had IPVD as detected by contrast-enhanced echocardiography. The area under the curve of whole-body uptake for detecting IPVD was significantly higher than that of brain uptake (0.75 vs 0.54; P = 0.025). The optimal cut-off values of brain uptake and whole-body uptake for detecting IPVD were 5.7% and 42.5%, respectively. The sensitivity, specificity, and accuracy of brain uptake > 5.7% and whole-body uptake > 42.5% for detecting IPVD were 23%, 89%, and 59% and 100%, 52%, and 74%, respectively.
Whole-body uptake is superior to brain uptake for diagnosing HPS.
Whole-body uptake is superior to brain uptake for diagnosing HPS. Further studies are needed to confirm the findings of the current study. For detecting IPVD and diagnosing HPS, whole-body uptake should be considered as the standard technique for calculating the shunt fraction from macroaggregated albumin lung perfusion scan.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Corresponding Author's Membership in Professional Societies: Chinese Society of Interventional Radiology (Vice President).
P-Reviewer: Auzinger G S-Editor: Zhang L L-Editor: Filipodia E-Editor: Zhang YL
| 1. | Machicao VI, Balakrishnan M, Fallon MB. Pulmonary complications in chronic liver disease. Hepatology. 2014;59:1627-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 126] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 2. | Rodríguez-Roisin R, Krowka MJ. Hepatopulmonary syndrome--a liver-induced lung vascular disorder. N Engl J Med. 2008;358:2378-2387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 422] [Article Influence: 24.8] [Reference Citation Analysis (1)] |
| 3. | Schenk P, Fuhrmann V, Madl C, Funk G, Lehr S, Kandel O, Müller C. Hepatopulmonary syndrome: prevalence and predictive value of various cut offs for arterial oxygenation and their clinical consequences. Gut. 2002;51:853-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 209] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 4. | Krowka MJ, Wiesner RH, Heimbach JK. Pulmonary contraindications, indications and MELD exceptions for liver transplantation: a contemporary view and look forward. J Hepatol. 2013;59:367-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 5. | Raevens S, Geerts A, Van Steenkiste C, Verhelst X, Van Vlierberghe H, Colle I. Hepatopulmonary syndrome and portopulmonary hypertension: recent knowledge in pathogenesis and overview of clinical assessment. Liver Int. 2015;35:1646-1660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 6. | Abrams GA, Jaffe CC, Hoffer PB, Binder HJ, Fallon MB. Diagnostic utility of contrast echocardiography and lung perfusion scan in patients with hepatopulmonary syndrome. Gastroenterology. 1995;109:1283-1288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 244] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 7. | Abrams GA, Nanda NC, Dubovsky EV, Krowka MJ, Fallon MB. Use of macroaggregated albumin lung perfusion scan to diagnose hepatopulmonary syndrome: a new approach. Gastroenterology. 1998;114:305-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 150] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 8. | Krowka MJ, Wiseman GA, Burnett OL, Spivey JR, Therneau T, Porayko MK, Wiesner RH. Hepatopulmonary syndrome: a prospective study of relationships between severity of liver disease, PaO(2) response to 100% oxygen, and brain uptake after (99m)Tc MAA lung scanning. Chest. 2000;118:615-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 165] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 9. | Arguedas MR, Abrams GA, Krowka MJ, Fallon MB. Prospective evaluation of outcomes and predictors of mortality in patients with hepatopulmonary syndrome undergoing liver transplantation. Hepatology. 2003;37:192-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 238] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 10. | Surasi DS, Manapragada P, Bhambhvani P. Lung perfusion imaging in hepatopulmonary syndrome using (99m)Tc macroaggregated albumin. J Nucl Cardiol. 2015;22:586-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Vedrinne JM, Duperret S, Bizollon T, Magnin C, Motin J, Trepo C, Ducerf C. Comparison of transesophageal and transthoracic contrast echocardiography for detection of an intrapulmonary shunt in liver disease. Chest. 1997;111:1236-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 92] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Fuhrmann V, Madl C, Mueller C, Holzinger U, Kitzberger R, Funk GC, Schenk P. Hepatopulmonary syndrome in patients with hypoxic hepatitis. Gastroenterology. 2006;131:69-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 13. | Gates GF, Orme HW, Dore EK. Cardiac shunt assessment in children with macroaggregated albumin technetium-99m. Radiology. 1974;112:649-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 23] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | MacDonald A, Burrell S. Infrequently performed studies in nuclear medicine: Part 1. J Nucl Med Technol. 2008;36:132-43; quiz 145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Wade OL, Bishop JM. Cardiac output and regional blood flow. Oxford: Blackwell Scientific Publications, 1962: 86-89. |
| 16. | Guevara M, Bru C, Ginès P, Fernández-Esparrach G, Sort P, Bataller R, Jiménez W, Arroyo V, Rodés. Increased cerebrovascular resistance in cirrhotic patients with ascites. Hepatology. 1998;28:39-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 96] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Kalambokis G, Tsianos EV. Pitfalls in the assessment of intrapulmonary shunt using lung perfusion scintigraphy in patients with cirrhosis. Liver Int. 2011;31:138-9; author reply 139-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 18. | Zhao H, Tsauo J, Zhang X, Ma H, Weng N, Wang L, Li X. Pulmonary transit time derived from pulmonary angiography for the diagnosis of hepatopulmonary syndrome. Liver Int. 2018;38:1974-1981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Rodríguez-Roisin R, Krowka MJ, Hervé P, Fallon MB; ERS Task Force Pulmonary-Hepatic Vascular Disorders (PHD) Scientific Committee. Pulmonary-Hepatic vascular Disorders (PHD). Eur Respir J. 2004;24:861-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 507] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 20. | Kalambokis G, Baltayiannis G, Tsiouris S, Pappas K, Kokkinou P, Fotopoulos A, Tsianos EV. Scintigraphic evaluation of intrapulmonary shunt in normoxemic cirrhotic patients and effects of terlipressin. Hepatol Res. 2010;40:1015-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Faraci FM. Effects of endothelin and vasopressin on cerebral blood vessels. Am J Physiol. 1989;257:H799-H803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Wolfe JD, Tashkin DP, Holly FE, Brachman MB, Genovesi MG. Hypoxemia of cirrhosis: detection of abnormal small pulmonary vascular channels by a quantitative radionuclide method. Am J Med. 1977;63:746-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 113] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Zhao H, Tsauo J, Ma HY, Li X. The role of macroaggregated albumin lung perfusion scan in hepatopulmonary syndrome: are we ready to draw conclusions? Liver Int. 2015;35:1918-1919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (1)] |