Published online Mar 14, 2020. doi: 10.3748/wjg.v26.i10.1042
Peer-review started: November 13, 2019
First decision: December 4, 2019
Revised: December 20, 2019
Accepted: February 9, 2020
Article in press: February 9, 2020
Published online: March 14, 2020
Processing time: 122 Days and 4.6 Hours
Postoperative adjuvant transcatheter arterial chemoembolization (PA-TACE) has improved overall survival (OS) in patients with hepatocellular carcinoma (HCC). However, the prognostic and predictive factors remain unclear.
To assess the prognostic factors and the predictors of PA-TACE benefit for OS in patients with resected HCC.
Univariate and multivariate analyses were performed to identify the potential prognostic factors for OS. In order to assess the predictive factors of PA-TACE benefit, the interaction variables between treatments for each subgroup were evaluated using the Cox proportional hazards regression model.
A total of 378 patients (PA-TACE vs surgery alone, 189:189) from three centers were included after a propensity-score 1:1 matching analysis. Compared to the group receiving surgery alone, PA-TACE prolonged the OS rate in patients with resected HCC (P < 0.001). The Barcelona Clinic Liver Cancer system and ferritin-to-hemoglobin ratio (FHR) were used as the prognostic factors for OS in both groups. Age (P = 0.023) and microscopic vascular invasion (MVI) (P = 0.002) were also identified in the PA-TACE group, while gender (P = 0.027), hepatitis B virus (P = 0.034) and albumin-bilirubin grade (P = 0.027) were also selected in the surgery alone group. In addition, PA-TACE resulted in longer OS than surgery alone across subgroups [all hazard ratios (PA-TACE-to-surgery alone) < 1]. Notably, a significantly prolonged OS following PA-TACE was observed in patients with high FHR (P = 0.038) and without MVI (P = 0.048).
FHR and Barcelona Clinic Liver Cancer stages were regarded as prognostic factors for OS. Moreover, high FHR and the absence of MVI were important predictive factors, which can be used to assist clinicians in selecting which patients could achieve a better OS with PA-TACE.
Core tip: We have identified the prognostic and predictive factors that can assist clinicians in selecting hepatocellular carcinoma patients who could achieve a better overall survival with postoperative adjuvant transcatheter arterial chemoembolization (PA-TACE). Our study demonstrated that PA-TACE showed a better outcome with longer overall survival in each variable compared to surgery alone. Both Barcelona Clinic Liver Cancer staging and the ferritin-to-hemoglobin ratio demonstrated significance as prognostic factors, whereas high ferritin-to-hemoglobin ratio and the absence of microscopic vascular invasion were predictive factors. The potential prognostic factors identified in this study could prove to be helpful for the future design of clinical trials regarding PA-TACE.
- Citation: Chen MY, Juengpanich S, Hu JH, Topatana W, Cao JS, Tong CH, Lin J, Cai XJ. Prognostic factors and predictors of postoperative adjuvant transcatheter arterial chemoembolization benefit in patients with resected hepatocellular carcinoma. World J Gastroenterol 2020; 26(10): 1042-1055
- URL: https://www.wjgnet.com/1007-9327/full/v26/i10/1042.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i10.1042
Liver cancer is the fourth leading cause of cancer-related mortality and is currently the main cause of liver-related death worldwide[1,2]. Hepatocellular carcinoma (HCC) is the most common type of liver cancer, and causes nearly one million deaths every year[2-4]. Overall, the 5-year survival rate of patients with HCC is less than 20%. Surgery is recommended as one of the most effective radical treatments for patients with early-stage HCC[5,6], whereas systemic therapy such as sorafenib is recommended as the first-line drug for the treatment of patients with advanced HCC[7]. Even though transcatheter arterial chemoembolization (TACE) is not the first choice for early-stage HCC, it is regarded as one of the primary treatment options for HCC patients with intermediate and advanced stages. In addition, TACE is widely recommended in preoperative and postoperative therapy, due to satisfactory results in eliminating tiny invisible tumor spots with few complications[8]. Recent studies have demonstrated that postoperative adjuvant TACE (PA-TACE) can improve the outcome of HCC associated with hepatic vein invasion and prolong overall survival (OS) in patients with multinodular HCC. It is recommended that patients should undergo PA-TACE when the liver function has recovered at 1-3 months after the initial operation[9]. However, it is still unclear whether PA-TACE can prolong OS in patients with HCC after R0 hepatectomy.
The prognostic factors and predictors of PA-TACE benefit in a patient after surgery remains limited. Although analyses of potential predictive factors for PA-TACE benefit have been studied using data from an observational study on HCC patients with microscopic vascular invasion (MVI), the number of patients and the lack of variables prevented accurate differentiation between prognostic and predictive factors[10]. A prognostic factor is a clinical, laboratory or molecular variable which is correlated with the natural course of the disease regardless of treatment, whereas a predictive factor is a variable used to identify a subgroup of patients most likely to benefit from a specific treatment[11,12]. Moreover, elucidation of predictive factors related to treatment response is key to designing and analyzing future clinical trials, when PA-TACE is regarded as a comparator[13].
Therefore, we aim to compare OS following PA-TACE and surgery alone, and to assess prognostic factors for OS and predictive factors of PA-TACE benefit in patients with HCC after R0 hepatectomy.
Patients with resectable HCC were identified and selected from three centers between January 2010 and January 2014. The inclusion criteria were as follows: (1) Patients underwent surgery with/without PA-TACE; (2) Pathology-confirmed HCC; and (3) Preoperative diagnosis of a single intrahepatic mass. The exclusion criteria were as follows: (1) Tumor size larger than 5 cm; (2) Positive margin; (3) Combined with other tumors; (4) Concurrently treated with conventional medicine; (5) Barcelona Clinic Liver Cancer (BCLC) stage higher than stage A; (6) OS less than 3 mo; and (7) Lack of necessary preoperative laboratory test results. Briefly, patients with Child-Pugh A and BCLC (0-A stage) enrolled in the study had undergone R0 hepatectomy for HCC with/without PA-TACE and had not received any other previous treatment. Details of the enrolled patients are summarized in Figure 1.
An informed consent was obtained from each patient before surgery. The medical ethics committee at the three medical centers approved this study. To protect patient confidentiality, during this retrospective clinical study, only preoperative laboratory test results without identifiable information were used. This study complied with the provisions in the Helsinki Declaration, the regulations of each Medical Institutional Review Board and local law.
According to our medical center regulations, following the selection of suitable patients for PA-TACE, all patients were suggested to undergo PA-TACE when the patient had recovered liver function at 1 month after the initial operation. The patients were followed up once every 3 mo. At each follow-up visit, tumor markers, abdominal ultrasound, and liver function assessments were performed. Every 6 mo or when recurrence was suspected, enhanced computed tomography scanning or magnetic resonance imaging was performed.
A standardized data form was created to collect all relevant information on the patients’ demographic and clinical characteristics, including age, gender, body mass index, BCLC stage, hepatitis B virus (HBV), hepatitis C virus (HCV), cirrhosis, family history, MVI, alpha-fetoprotein (AFP), albumin-bilirubin (ALBI) grade, neutrophil-to-lymphocyte ratio (NLR), ferritin, ferritin-to-hemoglobin ratio (FHR), C-reactive protein, treatment details, follow-up, etc. Continuous variables (age, AFP, NLR, ferritin, FHR, C-reactive protein) were transformed into categorical variables based on recognized cutoff values or median.
OS was the primary focus of this study. In order to avoid migration from retrospective analysis, a propensity-score 1:1 matching analysis was performed by R 3.6.0 (http://www.r-project.org) with the MatchIt package. The OS in the two treatment groups (PA-TACE vs surgery alone) was estimated and compared using Kaplan-Meier curves and the log-rank test, respectively. Univariate and multivariate analyses were then performed to identify potential prognostic factors for OS in each group. Finally, variables with significance in the univariate analyses (P < 0.05), were included in the multivariate analyses by the back-ward step using the Cox proportional hazards regression model. The number of events and 5-year survival rates were summarized based on Kaplan-Meier curves for OS across subgroups. In order to evaluate the treatment effect among subgroups, the potential interaction between treatment and each subgroup was assessed by hazard ratios (HRs) using a stratified Cox proportional hazard model. A P value for interaction based on HRs was used to estimate the strength of predictive value[14]. Variables with a P value less than 0.05 were regarded as significant.
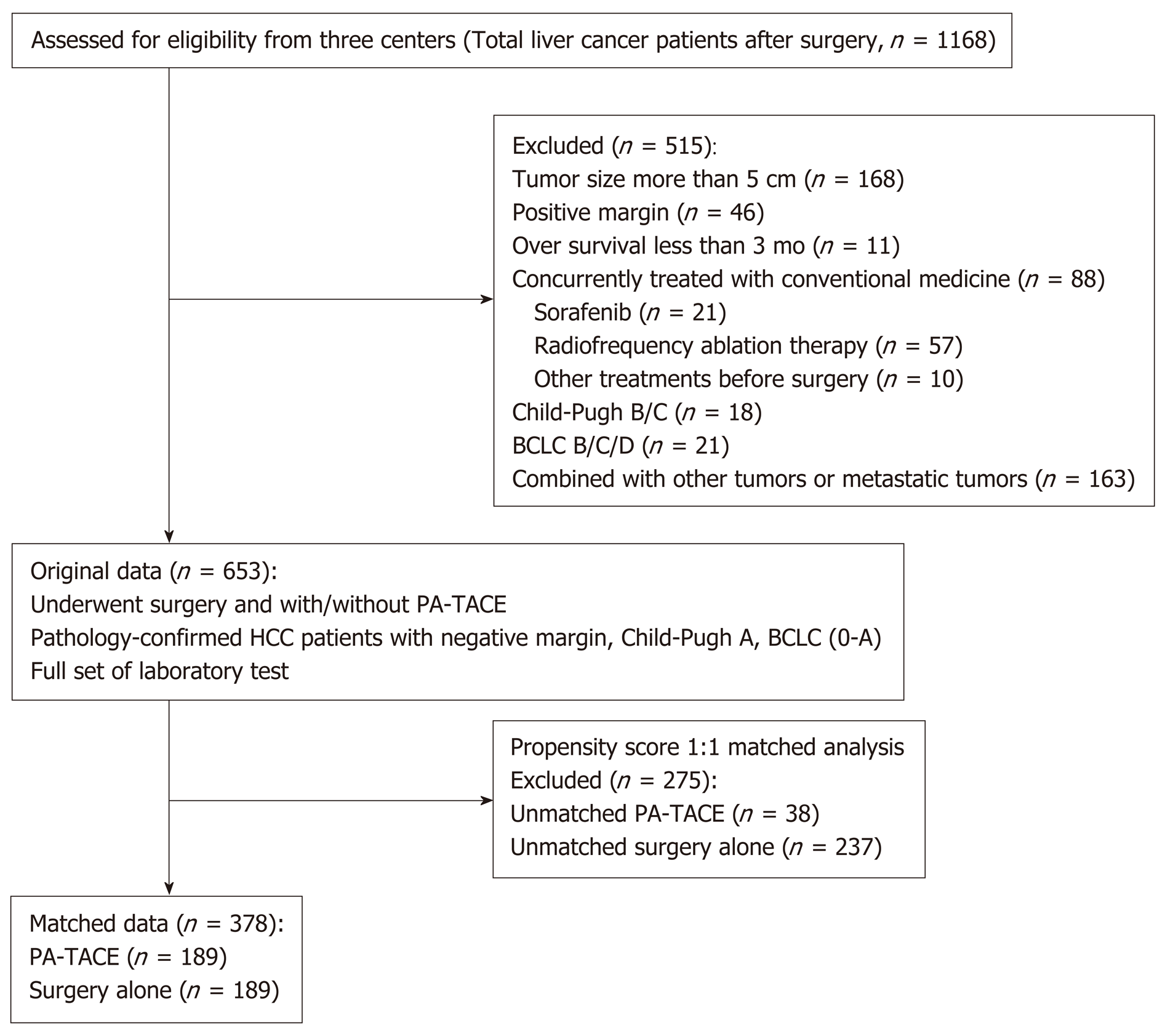
A total of 653 patients were selected from three centers as original data, and 378 patients (PA-TACE vs surgery alone, 189:189) were identified for exploratory analysis after a propensity-score 1:1 matching analysis. No significant difference was observed in each variable in the matched data (Table 1). All Kaplan-Meier curves for OS between the PA-TACE and surgery alone group showed significance before and after propensity score matching (Figure 2). In the PA-TACE group with matched data, the median age was 55 years, ranging from 26 to 86 years, and 170 patients (89.9%) were male. Almost half of the patient population in this study had a body mass index of less than 23. Most patients in this study were at BCLC stage A (79.9%), with HBV infection (88.4%) and with or without HCV infection (96.8%). 56.1% of the patients presented with cirrhosis, 14.8% presented with MVI, and 12.2% had a family history of HCC. Moreover, elevations in laboratory values, including high AFP (> 200 ng/mL), high NLR [> median (2.2)], high ferritin (> 400 ng/mL), high FHR [> median (23)], were present in almost half of the patients in this study. Overall, similar results were observed in the surgery alone group (Table 1).
| Original data | Matched data | |||||
| Characteristic | PA-TACE | Surgery alone | P value | PA-TACE | Surgery alone | P value |
| Age (yr) | < 0.001 | 0.889 | ||||
| < 65 | 196 (73.0) | 281 (66.0) | 158 (83.6) | 159 (84.1) | ||
| ≥ 65 | 31 (13.7) | 145 (34.0) | 31 (16.4) | 30 (15.9) | ||
| Gender | 0.245 | 0.866 | ||||
| Male | 197 (86.8) | 355 (83.3) | 170 (89.9) | 169 (89.4) | ||
| Female | 30 (13.2) | 71 (16.7) | 19 (10.1) | 20 (10.6) | ||
| BMI | 0.074 | 0.905 | ||||
| < 23 | 97 (42.7) | 188 (44.1) | 84 (44.4) | 81 (42.9) | ||
| 23-25 | 76 (33.5) | 110 (25.8) | 57 (30.2) | 61 (32.3) | ||
| ≥ 25 | 54 (23.8) | 128 (30.0) | 48 (25.4) | 47 (24.9) | ||
| BCLC stage | 0.509 | 0.614 | ||||
| 0 | 43 (18.9) | 90 (21.1) | 38 (20.1) | 42 (22.2) | ||
| A | 184 (81.1) | 336 (78.9) | 151 (79.9) | 147 (77.8) | ||
| HBV | < 0.001 | 0.362 | ||||
| Negative | 25 (11.0) | 101 (23.7) | 22 (11.6) | 28 (14.8) | ||
| Positive | 202 (89.0) | 325 (76.3) | 167 (88.4) | 161 (85.2) | ||
| HCV | 0.282 | 0.351 | ||||
| Negative | 218 (96.0) | 402 (94.4) | 183 (96.8) | 182 (96.3) | ||
| Positive | 3 (1.4) | 3 (0.7) | 0 (0) | 2 (1.1) | ||
| Unknown | 6 (2.6) | 21 (4.9) | 6 (3.2) | 5 (2.6) | ||
| Cirrhosis | 0.067 | 0.678 | ||||
| No | 114 (50.2) | 182 (42.7) | 83 (43.9) | 79 (41.8) | ||
| Yes | 113 (49.8) | 224 (57.3) | 106 (56.1) | 110 (58.2) | ||
| Family history | 0.605 | 0.876 | ||||
| No | 200 (88.1) | 381 (89.4) | 166 (87.8) | 165 (87.3) | ||
| Yes | 27 (11.9) | 45 (10.6) | 23 (12.2) | 24 (12.7) | ||
| MVI | 0.006 | 0.884 | ||||
| No | 187 (82.4) | 383 (89.9) | 161 (85.2) | 162 (85.7) | ||
| Yes | 40 (17.6) | 43 (10.1) | 28 (14.8) | 27 (14.3) | ||
| AFP (ng/mL) | < 0.001 | 1.000 | ||||
| ≤ 200 | 96 (42.3) | 252 (59.2) | 96 (50.8) | 96 (50.8) | ||
| > 200 | 131 (57.7) | 174 (40.8) | 93 (49.2) | 93 (49.2) | ||
| ALBI grade | 0.001 | 0.678 | ||||
| 1 | 129 (56.8) | 223 (52.3) | 104 (55.0) | 108 (57.1) | ||
| 2 | 92 (40.5) | 203 (47.7) | 85 (45.0) | 81 (42.9) | ||
| 3 | 6 (2.6) | 0 (0) | - | - | ||
| NLR | 0.986 | 0.918 | ||||
| ≤ Median | 116 (51.1) | 218 (51.2) | 86 (45.5) | 85 (45.0) | ||
| > Median | 111 (48.9) | 208 (48.8) | 103 (54.5) | 104 (55.0) | ||
| Ferritin (ng/mL) | 0.196 | 0.914 | ||||
| ≤ 400 | 147 (64.8) | 297 (69.7) | 125 (66.1) | 124 (65.6) | ||
| > 400 | 80 (35.2) | 129 (30.3) | 64 (33.9) | 65 (34.4) | ||
| FHR | 0.095 | 0.918 | ||||
| ≤ Median | 114 (50.2) | 243 (57.0) | 98 (51.9) | 97 (51.3) | ||
| > Median | 113 (49.8) | 183 (43.0) | 91 (48.1) | 92 (48.7) | ||
| CRP (mg/L) | 0.089 | 0.341 | ||||
| ≤ 5 | 176 (77.5) | 304 (71.4) | 138 (73.0) | 146 (77.2) | ||
| > 5 | 51 (22.5) | 122 (28.6) | 51 (27.0) | 43 (22.8) | ||
| Times | - | - | ||||
| 1 | 166 (73.1) | - | 136 (71.9) | - | ||
| 2 | 55 (24.2) | - | 47 (24.9) | - | ||
| > 3 | 6 (2.7) | - | 6 (3.2) | - | ||
Univariate and multivariate analyses were performed to identify potential prognostic factors for OS using the Cox proportional hazards regression model. Factors with a P-value of less than 0.05 were selected after univariate analyses. Four factors, including age (P = 0.043), BCLC stage (P = 0.015), MVI (P = 0.005), and FHR (P = 0.001), were identified in the PA-TACE group, and, five factors, including gender (P = 0.001), BCLC stage (P = 0.045), HBV (P = 0.038), ALBI grade (P < 0.001) and FHR (P < 0.001), were identified in the surgery alone group (Table 2).
| PA-TACE | Surgery alone | |||||
| Characteristic | Hazard ratio | 95%CI | P value | Hazard ratio | 95%CI | P value |
| Age (yr) | 0.043 | 0.166 | ||||
| < 65 | Reference | Reference | ||||
| ≥ 65 | 0.300 | 0.093-0.963 | 0.043 | 1.490 | 0.848-2.619 | 0.166 |
| Gender | 0.578 | 0.001 | ||||
| Male | Reference | Reference | ||||
| Female | 0.785 | 0.334-1.844 | 0.578 | 0.382 | 0.217-0674 | 0.001 |
| BMI | 0.187 | 0.121 | ||||
| < 23 | Reference | Reference | ||||
| 23-25 | 0.497 | 0.233-1.060 | 0.070 | 0.859 | 0.525-1.407 | 0.546 |
| ≥ 25 | 0.912 | 0.476-1.747 | 0.781 | 0.517 | 0.275-0.971 | 0.040 |
| BCLC stage | 0.015 | 0.045 | ||||
| 0 | Reference | Reference | ||||
| A | 4.287 | 1.333-13.785 | 0.015 | 1.839 | 1.014-3.403 | 0.045 |
| HBV | 0.693 | 0.038 | ||||
| Negative | Reference | Reference | ||||
| Positive | 1.205 | 0.478-3.038 | 0.693 | 2.414 | 1.049-5.553 | 0.038 |
| HCV | 0.367 | 0.645 | ||||
| Negative | Reference | Reference | ||||
| Positive | - | - | 0 | 0.000-NA | 0.963 | |
| Unknown | 0.047 | 0-36.104 | 0.367 | 0.390 | 0.054-2.804 | 0.350 |
| Cirrhosis | 0.166 | 0.129 | ||||
| No | Reference | Reference | ||||
| Yes | 1.516 | 0.841-2.730 | 0.166 | 1.440 | 0.899-2.307 | 0.129 |
| Family history | 0.936 | 0.543 | ||||
| No | Reference | Reference | ||||
| Yes | 0.966 | 0.411-2.269 | 0.936 | 0.806 | 0.402-1.615 | 0.543 |
| MVI | 0.005 | 0.439 | ||||
| No | Reference | Reference | ||||
| Yes | 2.557 | 1.332-4.910 | 0.005 | 1.275 | 0.689-2.359 | 0.439 |
| AFP (ng/mL) | 0.429 | 0.416 | ||||
| ≤ 200 | Reference | Reference | ||||
| > 200 | 1.255 | 0.715-2.203 | 0.429 | 1.203 | 0.771-1.878 | 0.416 |
| ALBI grade | 0.085 | < 0.001 | ||||
| 1 | Reference | Reference | ||||
| 2 | 1.637 | 0.934-2.871 | 0.085 | 2.303 | 1.466-3.620 | < 0.001 |
| 3 | - | - | - | - | - | - |
| NLR | 0.962 | 0.578 | ||||
| ≤ Median | Reference | Reference | ||||
| > Median | 0.986 | 0.563-1729 | 0.962 | 0.881 | 0.564-1.376 | 0.578 |
| Ferritin (ng/mL) | 0.056 | 0.323 | ||||
| ≤ 400 | Reference | Reference | ||||
| > 400 | 1.731 | 0.986-3.040 | 0.056 | 1.447 | 0.695-3.015 | 0.323 |
| FHR | 0.001 | < 0.001 | ||||
| ≤ Median | Reference | Reference | ||||
| > Median | 2.830 | 1.541-5.198 | 0.001 | 4.084 | 2.452-6.802 | < 0.001 |
| CRP (mg/L) | 0.670 | 0.875 | ||||
| ≤ 5 | Reference | Reference | ||||
| > 5 | 0.864 | 0.441-1.691 | 0.670 | 1.043 | 0.616-1.767 | 0.875 |
| Times | 0.193 | - | ||||
| 1 | Reference | - | - | |||
| 2 | 0.661 | 0.319-1.370 | 0.266 | - | - | |
| 3 | 2.171 | 0.668-7.059 | 0.197 | - | - | |
According to multivariate analyses, FHR (all P < 0.001) and BCLC stage (PA-TACE vs surgery alone, P = 0.021 and P = 0.036, respectively) were regarded as prognostic factors for OS in both groups. In addition, five prognostic factors, including age (P = 0.023) and MVI (P = 0.002) for PA-TACE, and gender (P = 0.027), HBV (P = 0.034), and ALBI grade (P = 0.027) for surgery alone (Table 3) were identified.
| PA-TACE | Surgery Alone | |||||
| Characteristic | Hazard ratio | 95%CI | P value | Hazard ratio | 95%CI | P value |
| Age (yr) | 0.023 | - | ||||
| < 65 | Reference | - | - | - | ||
| ≥ 65 | 0.257 | 0.080-0.826 | 0.023 | - | - | - |
| Gender | - | 0.027 | ||||
| Male | - | - | - | Reference | ||
| Female | - | - | - | 0.523 | 0.294-0.930 | 0.027 |
| BCLC stage | 0.021 | 0.036 | ||||
| 0 | Reference | Reference | ||||
| A | 3.972 | 1.23-12.825 | 0.021 | 1.963 | 1.044-3.689 | 0.036 |
| HBV | - | 0.034 | ||||
| Negative | - | - | - | Reference | ||
| Positive | - | - | - | 1.982 | 1.013-4.736 | 0.034 |
| MVI | 0.002 | - | ||||
| No | Reference | - | - | - | ||
| Yes | 2.850 | 1.475-5.506 | 0.002 | - | - | - |
| ALBI grade | - | 0.027 | ||||
| 1 | - | - | - | Reference | ||
| 2 | - | - | - | 1.732 | 1.064-2.821 | 0.027 |
| 3 | - | - | - | - | - | - |
| FHR | < 0.001 | < 0.001 | ||||
| ≤ Median | Reference | Reference | ||||
| > Median | 3.338 | 1.810-6.157 | < 0.001 | 1.963 | 1.044-3.689 | < 0.001 |
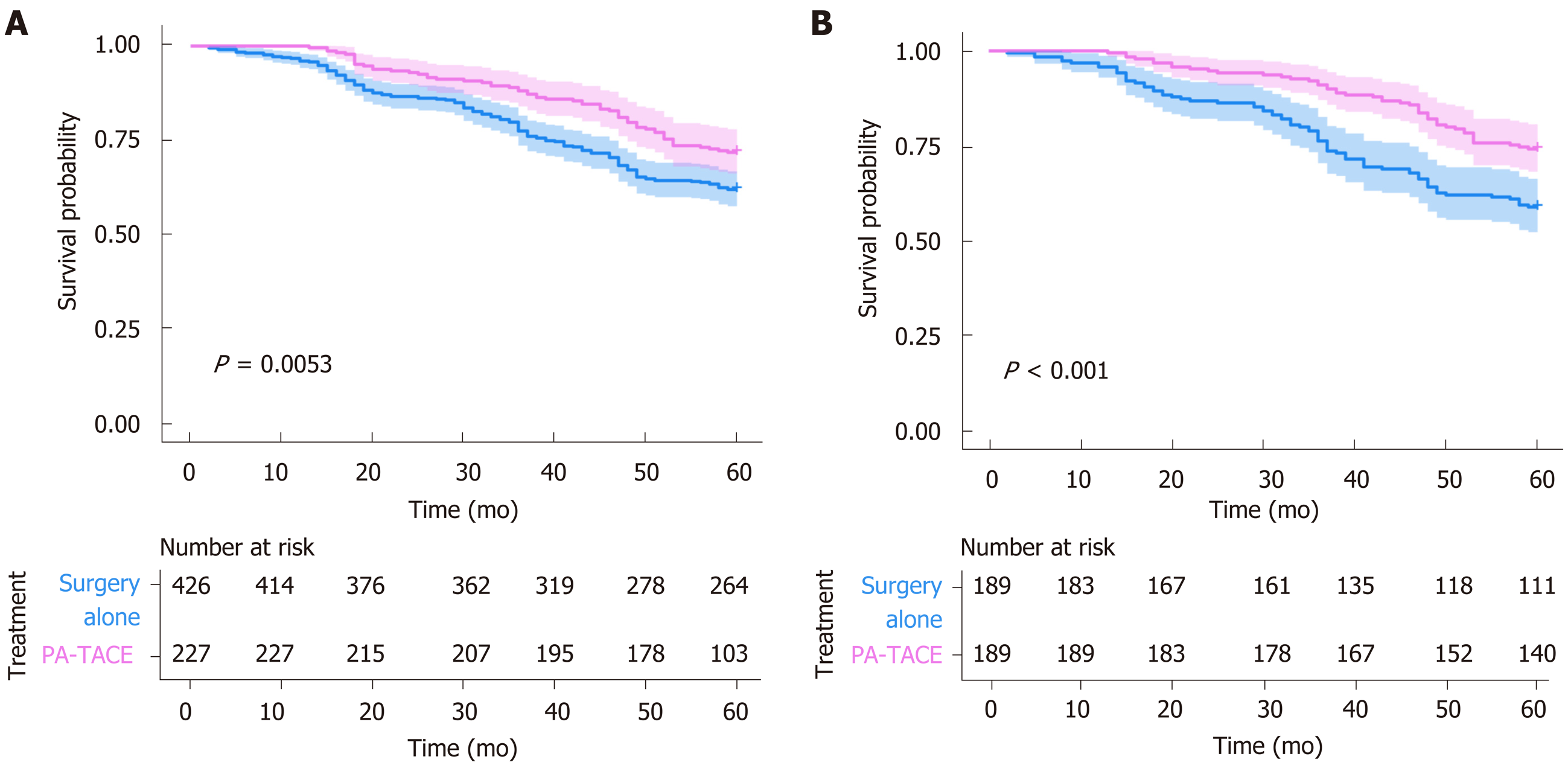
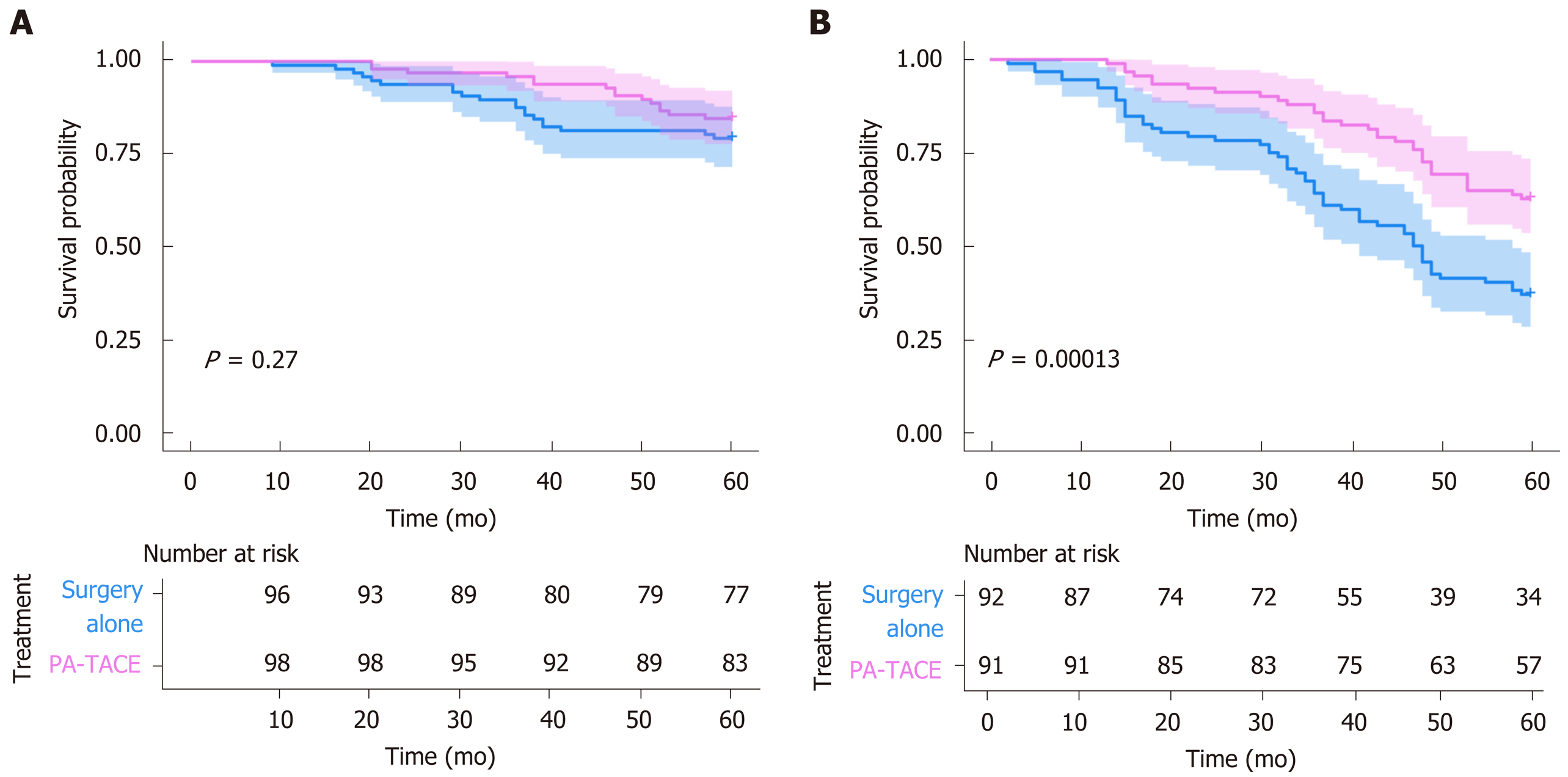
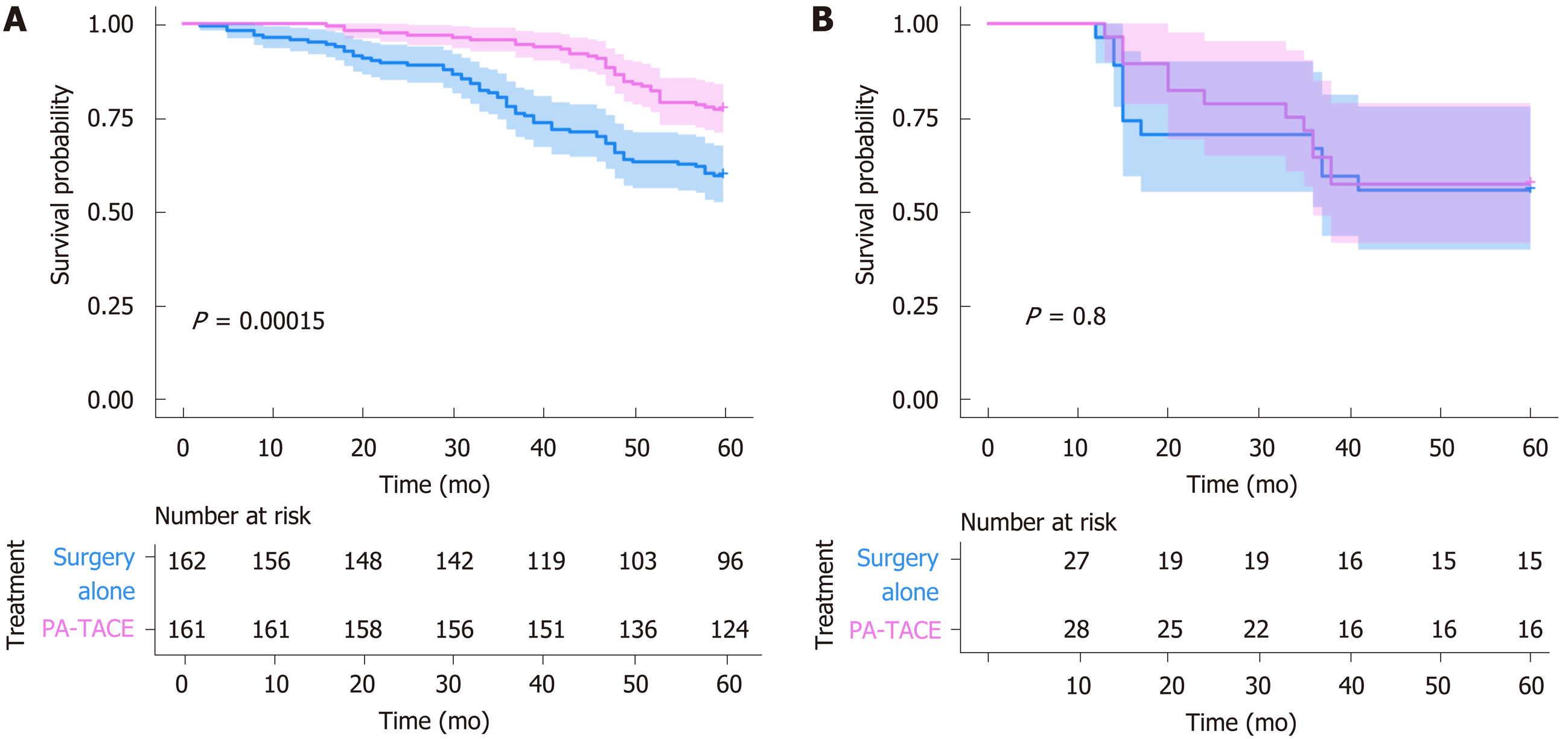
Compared to surgery alone, PA-TACE showed a statistically significant benefit across subgroups [all HRs (PA-TACE-to-surgery alone) < 1] (Table 4). In addition, according to treatment response to PA-TACE and surgery alone, PA-TACE had a better outcome compared to surgery alone, with longer OS in each variable. Notably, significantly prolonged OS following PA-TACE (P < 0.05) was observed in patients with high FHR (Figure 3) and the absence of MVI (Figure 4).
| Events, n (%) | 5-yr survival rate, % | ||||||
| Characteristic | PA-TACE | Surgery alone | PA-TACE | Surgery alone | HR(P/S) | 95%CI | P value for interaction |
| Age (yr) | 0.059 | ||||||
| < 65 | 46 (29.3) | 63 (39.6) | 70.9 | 60.4 | 0.653 | 0.347-0.956 | |
| ≥ 65 | 3 (9.7) | 15 (50.0) | 90.3 | 50.0 | 0.164 | 0.040-0.584 | |
| Gender | 0.131 | ||||||
| Male | 43 (25.3) | 63 (37.3) | 74.7 | 62.7 | 0.598 | 0.406-0.882 | |
| Female | 4 (21.1) | 7 (35.0) | 78.9 | 65.0 | 0.270 | 0.104-0.703 | |
| BMI | 0.261 | ||||||
| < 23 | 26 (31.0) | 38 (46.9) | 69.0 | 53.1 | 0.530 | 0.321-0.873 | |
| 23-25 | 9 (15.8) | 27 (44.3) | 84.2 | 55.7 | 0.315 | 0.148-0.671 | |
| ≥ 25 | 14 (29.2) | 13 (27.7) | 70.8 | 72.3 | 0.973 | 0.457-2.070 | |
| BCLC stage | 0.236 | ||||||
| 0 | 3 (7.9) | 12 (28.6) | 92.1 | 71.4 | 0.256 | 0.072-0.909 | |
| A | 46 (30.5) | 66 (44.9) | 69.5 | 55.1 | 0.570 | 0.391-0.830 | |
| HBV | 0.274 | ||||||
| Negative | 5 (22.7) | 6 (21.4) | 77.3 | 78.6 | 0.988 | 0.302-3.247 | |
| Positive | 44 (26.3) | 72 (44.7) | 73.7 | 55.3 | 0.493 | 0.339-0.717 | |
| HCV | 0.527 | ||||||
| Negative | 49 (26.8) | 77 (42.3) | 73.2 | 57.7 | 0.560 | 0.376-0.770 | |
| Positive | 0 (0) | 0 (0) | 100 | 100 | - | - | |
| Unknown | 0 (0) | 1 (20.0) | 100 | 80.0 | 0.013 | 0.000-1357 | |
| Cirrhosis | 0.943 | ||||||
| No | 17 (20.5) | 26 (32.9) | 79.5 | 67.1 | 0.550 | 0.298-1.013 | |
| Yes | 32 (30.2) | 52 (47.3) | 69.8 | 52.7 | 0.535 | 0.345-0.832 | |
| Family History | 0.793 | ||||||
| No | 43 (25.9) | 69 (41.8) | 74.1 | 58.2 | 0.528 | 0.360-0.772 | |
| Yes | 6 (26.1) | 9 (37.5) | 73.9 | 62.5 | 0.612 | 0.218-1.721 | |
| MVI | 0.048 | ||||||
| No | 37 (23.0) | 66 (40.7) | 77.0 | 59.3 | 0.469 | 0.313-0.701 | |
| Yes | 12 (42.9) | 12 (44.4) | 57.1 | 55.6 | 0.911 | 0.605-1.708 | |
| AFP | 0.984 | ||||||
| ≤ 200 ng/mL | 22 (22.9) | 36 (37.5) | 77.1 | 62.5 | 0.529 | 0.311-0.898 | |
| > 200 ng/mL | 27 (29.0) | 42 (45.2) | 71.0 | 54.8 | 0.533 | 0.328-0.865 | |
| ALBI grade | 0.333 | ||||||
| 1 | 23 (22.1) | 32 (29.6) | 77.9 | 70.4 | 0.647 | 0.378-1.105 | |
| 2 | 26 (30.6) | 46 (56.8) | 69.4 | 43.2 | 0.453 | 0.280-0.734 | |
| 3 | - | - | - | - | - | - | |
| NLR | 0.791 | ||||||
| ≤ Median | 23 (26.7) | 36 (42.4) | 73.3 | 57.6 | 0.510 | 0.302-0.860 | |
| > Median | 26 (25.2) | 42 (40.3) | 74.8 | 59.6 | 0.562 | 0.344-0.917 | |
| Ferritin | 0.289 | ||||||
| ≤ 400 ng/mL | 27 (21.6) | 33 (26.6) | 78.4 | 73.4 | 0.691 | 0.354-1.349 | |
| > 400 ng/mL | 22 (34.4) | 45 (69.2) | 65.6 | 30.8 | 0.450 | 0.294-0.688 | |
| FHR | 0.038 | ||||||
| ≤ Median | 15 (15.3) | 20 (20.6) | 84.7 | 79.4 | 0.748 | 0.450-1.244 | |
| > Median | 34 (37.4) | 58 (63.0) | 62.6 | 37.0 | 0.348 | 0.209-0.582 | |
| CRP | 0.853 | ||||||
| ≤ 5 mg/L | 38 (27.5) | 60 (41.1) | 72.5 | 58.9 | 0.556 | 0.370-0.835 | |
| > 5 mg/L | 11 (21.6) | 18 (41.9) | 78.4 | 58.1 | 0.513 | 0.242-1.088 | |
The improved outcome and prolonged OS of PA-TACE have been demonstrated in previous studies. However, it is still unclear whether the combination of PA-TACE and surgery can improve the prognosis of patients with resectable HCC. In this retrospective, multi-center study, we analyzed the potential prognostic factors and predictive factors of PA-TACE benefit in patients with resectable HCC. We utilized propensity-score 1:1 matching before exploratory analysis, which was beneficial in reducing bias due to the confounding variables and accurately distinguished the prognostic factors from the predictive factors. In this study, FHR was not only considered an independent prognostic factor (P < 0.001) in both groups, but it was also one of the most significant predictive factors of PA-TACE benefit (P = 0.038) in patients with resectable HCC. Most importantly, we reduced selective bias by (1) choosing patients with HCC who were identified and selected from three different centers; (2) using a standardized data form which was created to collect all the relevant information regarding these patients; and (3) using propensity score matching, which was carried out to avoid migration from the retrospective case studies.
There are various clinical elements that affect the prognosis of patients who have undergone PA-TACE. In this study, various factors were considered in the prediction of prognosis including ferritin, FHR, BCLC stage, MVI, HBC, and HCV, etc. The upregulation of ferritin is usually observed in patients with HCC[15,16] and is accompanied by a poor OS[17-19]. Previous studies have shown that serum ferritin reflects the iron load of the body and originates from hepatocytes and macrophages[20]. Rapid development would require a large amount of iron for the proliferation and progression of cancer cells, leading to the association of high iron levels in malignant cancers[21]. The higher the serum ferritin level is, the more impact it has on the OS. High serum ferritin level results in multiple system malfunctions which worsen the patient’s condition and overall health[22,23]. Moreover, cancer can lead to low hemoglobin levels due to multifactorial issues, such as immune, nutritional and metabolic components[21,24]. Therefore, serum ferritin is regarded as one of the biomarkers of HCC.
FHR is a unique factor that indicates multiple pathophysiological processes related to the relationship between the tumor and the host[25]. It is calculated by dividing serum ferritin, which is increased due to tumor progression, and hemoglobin, which reflects the current overall condition of the patient. The increase in FHR is associated with advanced tumor progression, leading to a higher likelihood of PA-TACE in patients with advanced HCC. Our study indicated that high FHR was associated with tumor progression and poor OS. According to these results, patients with higher FHR had poorer OS compared to those with lower FHR in both groups (P < 0.001). Significantly prolonged OS following PA-TACE was observed in patients with high FHR (P = 0.038). Normal liver cells primarily depend on the blood supply provided by the hepatic portal vein, while HCC cells primarily receive most of their blood supply from the hepatic artery[26]. Therefore, PA-TACE treatment may be able to decrease the number of residual tumor cells and improve overall long-term survival. In addition, a high FHR level is a predictive factor that is used to assist clinicians in managing and selecting patients who could receive maximal benefit from PA-TACE after hepatectomy. Therefore, FHR is an important marker for the prediction of prognosis after treatment in HCC patients.
The BCLC staging system is commonly used to manage patients with HCC. The treatment plan for patients with HCC is usually derived from BCLC guidelines[27]. It is indicated that patients with preserved liver function and a tumor diameter of less than 5 cm will benefit most from PA-TACE. In our study, the BCLC stage was considered another prognostic factor in both groups. Patients with BCLC stage 0 had a longer OS than those with BCLC stage A. However, patients had similar benefits from PA-TACE regardless of their BCLC stage (P = 0.236). All HRs showed significance in BCLC stage 0 and A (HR = 0.256, 95%CI: 0.072-0.909 and HR = 0.570, 95%CI: 0.391-0.830, respectively). Recent studies have shown that deterioration in liver function is known to be a risk factor for hepatectomy. The prognosis of patients is determined by how advanced the liver disease is[28]. Hence, the BCLC stage can be regarded as a prognostic factor but not a predictive factor.
Previous studies have suggested that HCC with a large tumor diameter and vascular invasion are better for predicting prognosis. MVI has been indicated as a strong predictor of tumor recurrence and OS in patients receiving surgery alone[29]. Residual tumor cells that are supplied by the malignant MVI are usually left behind after surgical resection, resulting in the prognosis of patients without MVI being better than those with MVI. Due to the correlation between MVI and poor OS, MVI is considered one of the most powerful prognostic factors[10]. According to our results, patients with MVI who received surgery alone showed poor OS. However, patients without MVI had a prolonged OS following PA-TACE (P = 0.048). PA-TACE should be able to eliminate some of the MVI and residual tumor cells, improving the OS of patients who have undergone PA-TACE. Therefore, MVI may be a predictive factor of PA-TACE treatment benefit and a prognostic factor for patients receiving surgery alone.
Various studies have been conducted on the elements that impact the long-term prognosis of patients that have undergone PA-TACE. Overall, most prognostic factors used in our study were consistent with those in previous studies. Both studies by Kim et al[30] and Cai et al[31] reported the same results, which showed that ALBI was one of the most important prognostic factors for predicting OS in patients who had undergone PA-TACE after hepatectomy. Studies have shown that HBV reactivation is an obstacle for hepatic cell regeneration, which results in reduced OS in patients with HBV infection. However, PA-TACE can also eliminate HBV within tumor cells and lower the viral load, thus slightly increasing OS in HCC patients with HBV infection.
There are some limitations in this study. The major limitation is the retrospective nature of the data acquired. Although a propensity score matching analysis was performed, potential bias could not be completely eliminated. Moreover, several clinical variables in the data could not be measured, for example, PA-TACE drugs and dosages may have varied across the three different centers. Hence, a prospective study is required to confirm PA-TACE treatment efficacy and its impact on OS. In addition, even though this study was based on multiple medical centers, most patients with HCC also had HBV infection and less than 10% of patients with HCV infection were included. Therefore, future studies should pay more attention to HCV, and multicenter randomized controlled trials are required to verify these results. This study has provided many paths for future research. Tumors evolve spontaneously, and a reliable way of predicting OS and response to PA-TACE is essential for physicians to create a treatment plan that will improve the patient’s prognosis. By using the potential prognostic factors identified in this study, artificial intelligence could be used to assess the clinical outcome of patients with HCC.
In conclusion, PA-TACE resulted in a better outcome with longer OS in each variable compared to surgery alone. Prolonged OS following PA-TACE (P < 0.05) was observed in patients with high FHR and the absence of MVI. This study demonstrated that FHR and BCLC stages were the most important prognostic factors for OS. Moreover, high FHR [> median (23)] and absence of MVI were predictive factors of PA-TACE benefit, and can be used to assist clinicians in selecting which patients would achieve a better OS with PA-TACE.
Transcatheter arterial chemoembolization (TACE) is one of the primary treatment options for hepatocellular carcinoma (HCC) patients with intermediate and advanced stages, and is widely recommended in preoperative and postoperative therapy, due to satisfactory results in eliminating tiny invisible tumor spots with few complications. Recent studies have demonstrated that postoperative adjuvant TACE (PA-TACE) can improve the outcome of HCC associated with hepatic vein invasion and prolong overall survival (OS) in patients with multinodular HCC. However, it is still unclear whether PA-TACE can prolong OS in patients with HCC after R0 hepatectomy.
Although analyses of potential predictive factors for PA-TACE benefit have been carried out using data from an observational study on HCC patients with microscopic vascular invasion (MVI), the number of patients and the lack of variables prevented accurate differentiation between prognostic and predictive factors. Therefore, we aimed to compare OS following PA-TACE and surgery alone, and to assess prognostic factors for OS and predictive factors of PA-TACE benefit in patients with HCC after R0 hepatectomy.
The main objective of this study was to identify the prognostic and predictive factors that can assist clinicians in selecting HCC patients who would achieve a better OS with PA-TACE. We conducted a retrospective, multi-center study to assess the prognostic factors for HCC and the predictive factors of PA-TACE. The potential prognostic factors identified in this study could lead to the utilization of artificial intelligence to assess the clinical outcome of HCC patients.
A total of 653 patients were selected from three centers as original data, and 378 patients (PA-TACE vs surgery alone, 189:189) were identified for exploratory analysis after a propensity-score 1:1 matching analysis. Univariate and multivariate analyses were performed to identify the potential prognostic factors for OS. In order to assess the predictive factors of PA-TACE benefit, the interaction variables between treatments for each subgroup were evaluated using the Cox proportional hazards regression model.
Compared to the group receiving surgery alone, PA-TACE prolonged the OS rate in patients with resected HCC (P < 0.001). The Barcelona Clinic Liver Cancer system and ferritin-to-hemoglobin ratio (FHR) were used as the prognostic factors for OS in both groups. PA-TACE showed longer OS than surgery alone across subgroups [all hazard ratios (PA-TACE-to-surgery alone) < 1]. Notably, the significantly prolonged OS following PA-TACE was observed in patients with high FHR (P = 0.038) and without MVI (P = 0.048).
Previous studies have demonstrated improved outcomes following PA-TACE, but it is still unclear whether the combination of PA-TACE and surgery improves the prognosis of patients with resectable HCC. This study demonstrated that FHR and Barcelona Clinic Liver Cancer stages were the most important prognostic factors for OS. Moreover, high FHR [> median (23)] and absence of MVI were predictive factors of PA-TACE benefit, and can be used to assist clinicians in selecting which patients would achieve a better OS with PA-TACE. This study has provided many paths for future research. Tumors evolve spontaneously, and a reliable way of predicting OS and response to PA-TACE is essential for physicians to create a treatment plan that will improve the patient’s prognosis. By using the potential prognostic factors identified in this study, artificial intelligence could be used to assess the clinical outcome of patients with HCC. However, due to the limitations in our study, a prospective study is needed in the future to confirm PA-TACE treatment efficacy and its impact on OS.
Due to the limitations in this study, several lessons can be learned. Although a propensity score matching analysis was performed, potential bias could not be completely eliminated. Moreover, several clinical variables in the data could not be measured. Hence, a prospective study is needed to confirm PA-TACE treatment efficacy and its impact on OS. This study has provided many paths for future research. Tumors evolve spontaneously, and a reliable way of predicting OS and response to PA-TACE is essential for physicians to create a treatment plan that will improve the patient’s prognosis. By using the potential prognostic factors identified in this study, artificial intelligence could be used to assess the clinical outcome of patients with HCC.
We are grateful to Cai Y for providing language help, and all our colleagues for assistance in this study.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Boscá L, Bramhall SR, Que J S-Editor: Yan JP L-Editor: Webster JR E-Editor: Zhang YL
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55806] [Article Influence: 7972.3] [Reference Citation Analysis (132)] |
| 2. | Miller KD, Goding Sauer A, Ortiz AP, Fedewa SA, Pinheiro PS, Tortolero-Luna G, Martinez-Tyson D, Jemal A, Siegel RL. Cancer Statistics for Hispanics/Latinos, 2018. CA Cancer J Clin. 2018;68:425-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 318] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 3. | DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for African Americans, 2019. CA Cancer J Clin. 2019;69:211-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 531] [Article Influence: 88.5] [Reference Citation Analysis (0)] |
| 4. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13300] [Cited by in RCA: 15469] [Article Influence: 2578.2] [Reference Citation Analysis (2)] |
| 5. | Cai X. Laparoscopic liver resection: the current status and the future. Hepatobiliary Surg Nutr. 2018;7:98-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 6. | Juengpanich S, Shi L, Iranmanesh Y, Chen J, Cheng Z, Khoo AK, Pan L, Wang Y, Cai X. The role of natural killer cells in hepatocellular carcinoma development and treatment: A narrative review. Transl Oncol. 2019;12:1092-1107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | Chen M, Hu J, Cao J, Cai X. Comprehensive Consideration before the Decision-Making of the Systemic Treatment in Patients with Advanced Hepatocellular Carcinoma. Liver cancer. 2019;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Zhang Z, Liu Q, He J, Yang J, Yang G, Wu M. The effect of preoperative transcatheter hepatic arterial chemoembolization on disease-free survival after hepatectomy for hepatocellular carcinoma. Cancer. 2000;89:2606-2612. [PubMed] |
| 9. | Wang H, Du PC, Wu MC, Cong WM. Postoperative adjuvant transarterial chemoembolization for multinodular hepatocellular carcinoma within the Barcelona Clinic Liver Cancer early stage and microvascular invasion. Hepatobiliary Surg Nutr. 2018;7:418-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 10. | Sun JJ, Wang K, Zhang CZ, Guo WX, Shi J, Cong WM, Wu MC, Lau WY, Cheng SQ. Postoperative Adjuvant Transcatheter Arterial Chemoembolization After R0 Hepatectomy Improves Outcomes of Patients Who have Hepatocellular Carcinoma with Microvascular Invasion. Ann Surg Oncol. 2016;23:1344-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 132] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 11. | Adolfsson J, Steineck G. Prognostic and treatment-predictive factors-is there a difference? Prostate Cancer Prostatic Dis. 2000;3:265-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Bruix J, Cheng AL, Meinhardt G, Nakajima K, De Sanctis Y, Llovet J. Corrigendum to "Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: Analysis of two phase III studies" [J hepatol 67 (2017) 999-1008]. J Hepatol. 2018;69:990-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Chen M, Cao J, Zhang B, Pan L, Cai X. A Nomogram for Prediction of Overall Survival in Patients with Node-negative Gallbladder Cancer. J Cancer. 2019;10:3246-3252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ. 2003;326:219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2009] [Cited by in RCA: 2386] [Article Influence: 108.5] [Reference Citation Analysis (0)] |
| 15. | Jung M, Mertens C, Tomat E, Brüne B. Iron as a Central Player and Promising Target in Cancer Progression. Int J Mol Sci. 2019;20:pii: E273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 191] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 16. | Wang Y, Yu L, Ding J, Chen Y. Iron Metabolism in Cancer. Int J Mol Sci. 2018;20:pii: E95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 197] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 17. | Jiao Y, Wilkinson J 4th, Di X, Wang W, Hatcher H, Kock ND, D'Agostino R Jr, Knovich MA, Torti FM, Torti SV. Curcumin, a cancer chemopreventive and chemotherapeutic agent, is a biologically active iron chelator. Blood. 2009;113:462-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 186] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 18. | Manz DH, Blanchette NL, Paul BT, Torti FM, Torti SV. Iron and cancer: recent insights. Ann N Y Acad Sci. 2016;1368:149-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 349] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 19. | Torti SV, Manz DH, Paul BT, Blanchette-Farra N, Torti FM. Iron and Cancer. Annu Rev Nutr. 2018;38:97-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 307] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 20. | Cohen LA, Gutierrez L, Weiss A, Leichtmann-Bardoogo Y, Zhang DL, Crooks DR, Sougrat R, Morgenstern A, Galy B, Hentze MW, Lazaro FJ, Rouault TA, Meyron-Holtz EG. Serum ferritin is derived primarily from macrophages through a nonclassical secretory pathway. Blood. 2010;116:1574-1584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 331] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 21. | Macciò A, Madeddu C, Gramignano G, Mulas C, Tanca L, Cherchi MC, Floris C, Omoto I, Barracca A, Ganz T. The role of inflammation, iron, and nutritional status in cancer-related anemia: results of a large, prospective, observational study. Haematologica. 2015;100:124-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 152] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 22. | Gao S, Zhao D, Qi Y, Wang M, Zhao F, Sun J, Liu J. The association between serum ferritin levels and the risk of new-onset type 2 diabetes mellitus: A 10-year follow-up of the Chinese Multi-Provincial Cohort Study. Diabetes Res Clin Pract. 2017;130:154-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Gye HJ, Kim JM, Yoo C, Shim SH, Won YS, Sung KC, Lee MY, Park KH. Relationship between high serum ferritin level and glaucoma in a South Korean population: the Kangbuk Samsung health study. Br J Ophthalmol. 2016;100:1703-1707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Lee CL, Tsai CH, Yeh DC, Lin CS, Li YF, Tzeng HE. Hemoglobin level trajectories in the early treatment period are related with survival outcomes in patients with breast cancer. Oncotarget. 2017;8:1569-1579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Lee S, Jeon H, Shim B. Prognostic Value of Ferritin-to-Hemoglobin Ratio in Patients with Advanced Non-Small-Cell Lung Cancer. J Cancer. 2019;10:1717-1725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Portolani N, Coniglio A, Ghidoni S, Giovanelli M, Benetti A, Tiberio GA, Giulini SM. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann Surg. 2006;243:229-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 706] [Cited by in RCA: 728] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 27. | Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2107] [Cited by in RCA: 3023] [Article Influence: 431.9] [Reference Citation Analysis (3)] |
| 28. | Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2121] [Cited by in RCA: 3237] [Article Influence: 462.4] [Reference Citation Analysis (1)] |
| 29. | Hu H, Han XK, Long XR, Fan J, Yan ZP, Wang JH, Liu R. Prognostic nomogram for post-surgical treatment with adjuvant TACE in hepatitis B virus-related hepatocellular carcinoma. Oncotarget. 2016;7:58302-58314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Kim JH, Sinn DH, Lee JH, Hyun D, Cho SK, Shin SW, Chang Y, Kim YJ, Yoon JH, Kang W, Gwak GY, Paik YH, Lee JH, Koh KC, Paik SW, Choi MS. Novel Albumin-Bilirubin Grade-Based Risk Prediction Model for Patients with Hepatocellular Carcinoma Undergoing Chemoembolization. Dig Dis Sci. 2018;63:1062-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Cai XR, Chen ZH, Liu MM, Lin JX, Zhang XP, Chen J, Lin Q, Ma XK, Wen JY, Xie SD, Wu XY, Dong M. Modified CLIP score with the albumin-bilirubin grade retains prognostic value in HBV-related hepatocellular carcinoma patients treated with trans-catheter arterial chemoembolization therapy. J Cancer. 2018;9:2380-2388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |