Published online Mar 14, 2020. doi: 10.3748/wjg.v26.i10.1029
Peer-review started: November 21, 2019
First decision: January 7, 2020
Revised: February 15, 2020
Accepted: February 21, 2020
Article in press: February 21, 2020
Published online: March 14, 2020
Processing time: 114 Days and 6.1 Hours
Nonalcoholic fatty liver disease (NAFLD) is a global metabolism-associated liver disease. Mesencephalic astrocyte-derived neurotrophic factor (MANF) is a newly discovered secreted protein that is involved in metabolic homeostasis. However, much remains to be discovered about its function in hepatic lipid metabolism; thus, we assessed whether MANF could regulate hepatic metabolism.
To establish in vivo and in vitro NAFLD models to explore the role of MANF in hepatic lipid metabolism.
HepG2 cells treated with free fatty acids (FFAs) and ob/ob mice were used as NAFLD models. Liver tissues collected from wild type and ob/ob mice were used to detect MANF expression. Cells were treated with FFAs for different durations. Moreover, we used lentiviral constructs to establish overexpression and knockdown cell models in order to interfere with MANF expression levels and observe whether MANF influences hepatic steatosis. Western blot analysis and quantitative real-time PCR were used to detect protein and gene expression, and oil red O staining was used to visualize intracellular lipid droplets.
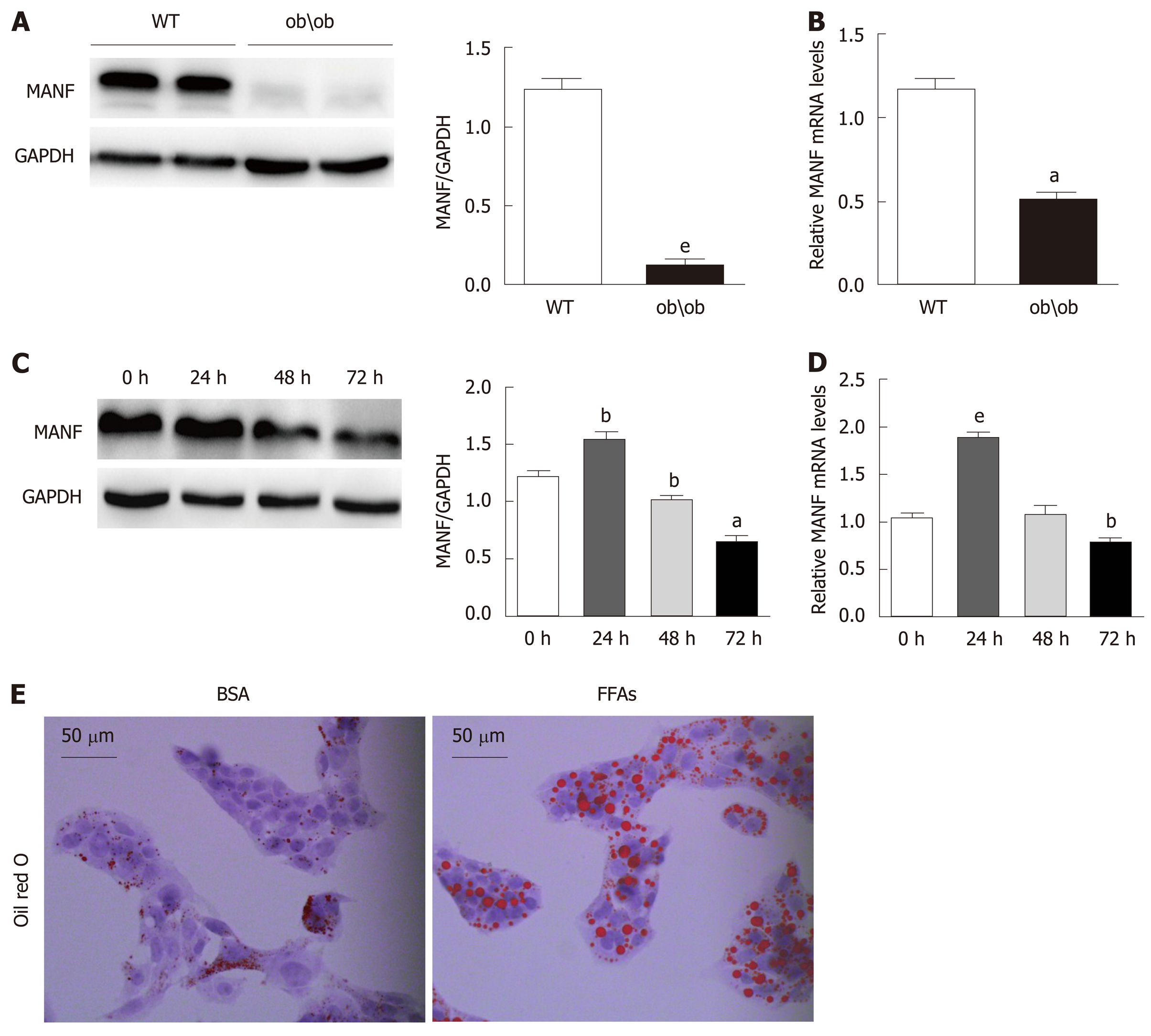
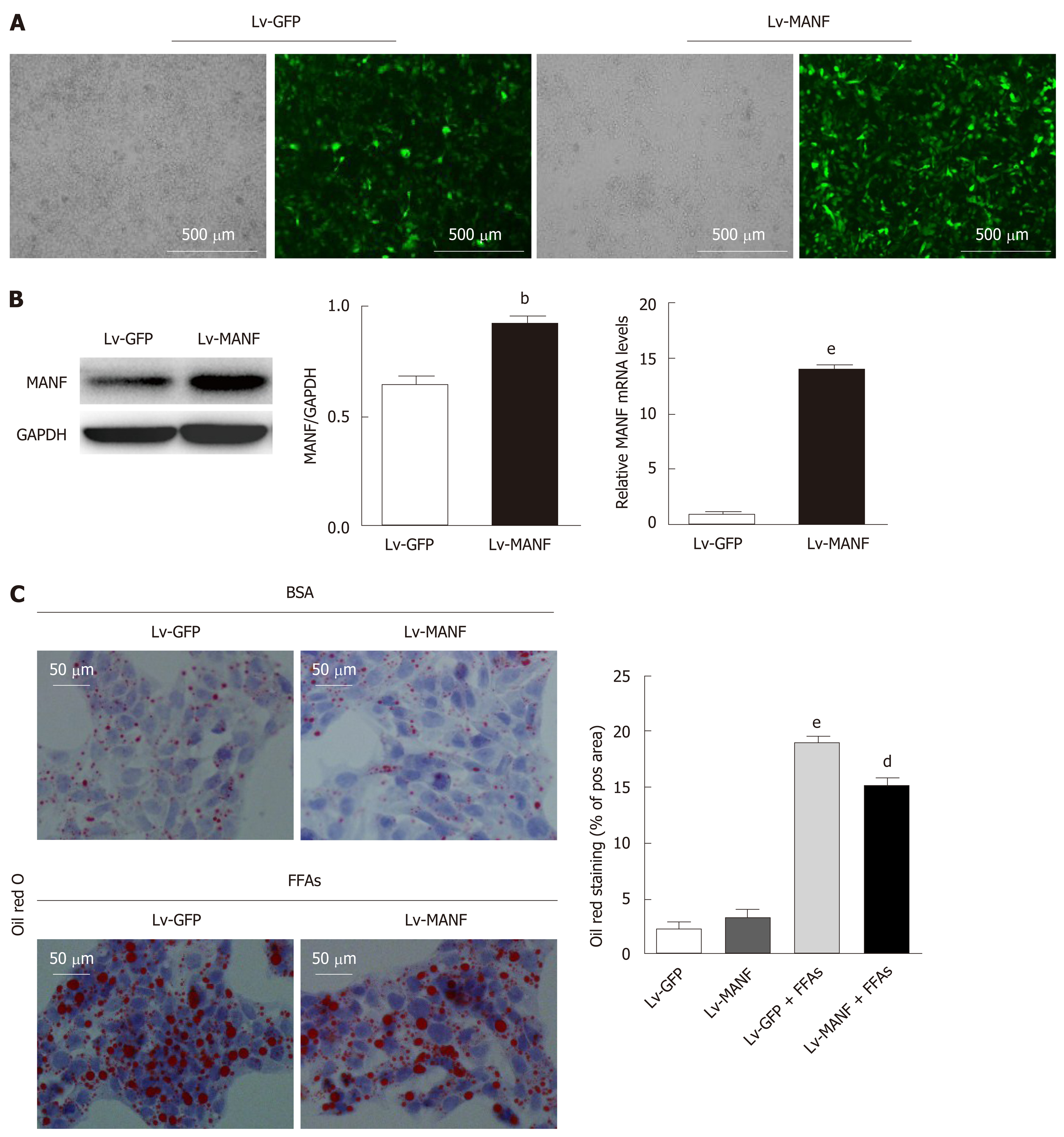
Hepatic MANF protein and mRNA expression in wild type mice were 10-fold and 2-fold higher, respectively, than those in ob/ob mice. The MANF protein was temporarily increased by 1.3-fold after stimulation with FFAs for 24 h and gradually decreased to 0.66-fold that of the control at the 72 h time point in HepG2 cells. MANF deficiency upregulated the expression of genes involved in fatty acid synthesis, cholesterol synthesis, and fatty acid uptake and aggravated HepG2 cell steatosis, while MANF overexpression inhibited fatty acid synthesis and uptake and cholesterol synthesis, and rescued HepG2 cells from FFAs-induced steatosis. Furthermore, a significant decrease in triglyceride levels was observed in the MANF overexpression group compared with the control group (0.4288 ± 0.0081 mmol/g vs 0.3746 ± 0.0121 mmol/g, P < 0.05) upon FFAs treatment. There was also a 17% decrease in intracellular total cholesterol levels between the MANF overexpression group and the control group (0.1301 ± 0.0059 mmol/g vs 0.1088 ± 0.0009 mmol/g, P < 0.05) upon FFAs treatment. Moreover, MANF suppressed lipid deposition in HepG2 cells.
Our findings indicate that MANF improves the phenotype of liver cell steatosis and may be a potential therapeutic target in hepatic steatosis processes.
Core tip: We first uncovered an important function of mesencephalic astrocyte-derived neurotrophic factor (MANF) in the pathogenesis of nonalcoholic fatty liver disease. We found that MANF exerts a significant effect on hepatic fatty metabolism. This study suggests for the first time that MANF expression was increased at an early stage and gradually decreased afterward under high free fatty acids stimulation in HepG2 cells. Moreover, the results from gain-and-loss functional experiments showed that loss of MANF accelerated lipogenesis and aggravated HepG2 cell steatosis, while MANF overexpression inhibited lipogenesis and rescued HepG2 cell steatosis from free fatty acids treatment.
- Citation: He M, Wang C, Long XH, Peng JJ, Liu DF, Yang GY, Jensen MD, Zhang LL. Mesencephalic astrocyte-derived neurotrophic factor ameliorates steatosis in HepG2 cells by regulating hepatic lipid metabolism. World J Gastroenterol 2020; 26(10): 1029-1041
- URL: https://www.wjgnet.com/1007-9327/full/v26/i10/1029.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i10.1029
Nonalcoholic fatty liver disease (NAFLD) is a clinicopathological condition characterized by the intracellular accumulation of lipids in hepatocytes followed by steatosis and inflammation in the liver. This multifactorial disease affects approximately 25% of the average population and 70%-90% of overweight/obese patients[1]. The pathogenic mechanisms of NAFLD are known to include lipid accumulation, inflammation, and oxidative stress. However, the precise mechanisms behind the pathogenesis of NAFLD are largely unknown. Given the high prevalence and severe prognosis of NAFLD, understanding the underlying mechanisms has become a priority.
Mesencephalic astrocyte-derived neurotrophic factor (MANF) is a newly discovered secreted protein that was originally isolated from a rat mesencephalic type-1 astrocyte cell line[2]. The structure of MANF includes two important domains. The N-terminal domain is homologous with saposin-like proteins, which are a large family of small, cysteine-rich proteins that interact with lipids and membranes[3]. The C-terminal domain is homologous with SAF-A/B, Acinus, and PIAS proteins and may have reductase or disulfide isomerase activity[4]. MANF is widely expressed in neuronal and nonneuronal tissues, particularly secretory tissues such as the liver and pancreas[5]. Studies have shown that MANF could exert protective functions in multiple diseases, such as intracerebral hemorrhage and Parkinson's disease[6,7]. Both in vivo and in vitro studies indicated that the cytoprotective effect of MANF is not limited to the nervous system, and it can also protect pancreatic beta cells from inflammatory stress-induced cell death[8] and improve pancreatic β cell proliferation and survival, while MANF knockout mice display a severe diabetic phenotype[9]. Furthermore, human studies have shown that circulating MANF levels are increased in patients with type 1 diabetes, newly diagnosed prediabetes, and type 2 diabetes, suggesting a potential role for MANF in metabolic diseases[9-12].
More interestingly, a recent study showed that MANF plays an increasingly significant role in obesity and participates in regulating food intake and body weight[13], protecting the liver against aging and metabolic stress-related damage, and that nonalcoholic steatohepatitis patients showed decreased levels of circulating MANF[14]. However, much remains to be explored about the role of MANF in hepatic lipid homeostasis. Regarding the potential role of MANF in metabolic diseases and its protective effect against metabolic stress, we proposed that MANF may play a potential protective role in NAFLD and have beneficial effects on lipid stress-induced liver cell steatosis.
All animal experiments were approved by the Animal Care and Use Ethics Committee of Chongqing Medical University (approved protocol No. 2019-48). The animal protocol was designed to minimize pain or discomfort to the animals. Adult male mice [C57BL/6J, 8-week-old, 18 ± 3 g, wild-type (WT) mice, n = 4] and 8-week-old male ob/ob mice (n = 4) were maintained in an environment with a 12 h light/dark cycle at 21-25 °C with free access to water and food. Genetically obese mice (ob/ob, Model Animal Research Center of Nanjing University, Nanjing, Jiangsu, China) fed a normal control chow diet were used for the fatty liver model, and WT mice (Chongqing Medical University, Chongqing, China) fed a normal control chow diet served as controls. After 12 wk of feeding, mice were euthanized with 10 g/L pentobarbital sodium, and liver tissues were collected and immediately stored at -80 °C or fixed in 40 g/L paraformaldehyde for further analyses.
Human HepG2 cells were obtained from Procell Life Science and Technology (Wuhan, Hubei, China). The cells were incubated in Dulbecco's Modified Eagle’s Medium (HyClone, Logan, United States) with 100 g/L fetal bovine serum (ExCell Bio, Shanghai, China) and 10 g/L penicillin-streptomycin solution (Beyotime, Shanghai, China). All cells were placed in a clean humidified incubator containing 50 mL/L CO2 at 37 °C. For the MANF knockdown experiment, HepG2 cells were transfected with lentivirus (Lv) expressing short hairpin RNA-targeted MANF, and the lentiviral vector alone was used as the control. For the MANF overexpression experiment, HepG2 cells were transfected with lentivirus expressing MANF, and lentiviral vector alone was used as a control. All lentiviruses were designed, synthesized, and packed by HanBio (Hanbio Biotechnology Co. Ltd., Shanghai, China). To construct a nonalcoholic fatty liver model in vitro, HepG2 cells were cultured in medium with 0.5 mmol/L FFAs (oleate:palmitate = 2:1, Sigma-Aldrich, St. Louis, MO, United States) containing 10 g/L low fatty acid bovine serum albumin (BSA, Sigma) as previously reported[15], and cells treated with BSA served as controls.
Total protein was extracted from mouse liver tissues or HepG2 cells with RIPA lysis buffer (Beyotime) containing phosphatase inhibitor cocktail A (Beyotime) and protease inhibitor (Roche, Basel, Switzerland), and the protein concentration was measured with a BCA Protein Assay kit (Beyotime). Protein samples (30 µg) were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (120 mL/L gel) and transferred to a 0.22 μm polyvinylidene difluoride membrane (Merck Millipore, Darmstadt, Germany). Then, the membrane was blocked with 50 g/L BSA for 2 h at room temperature, followed by incubation with primary antibody at 4 °C for 12-16 h and then secondary antibody for 1-2 h at room temperature. The protein expression level was detected using the chemiluminescence method, and the images were captured by a computer. GAPDH served as a control. The antibodies included anti-MANF (Abcam, Cambridge, MA, United States, ab67271, 1:1000), anti-GAPDH (Proteintech Group, Wuhan, Hubei, China, 60004-1-Ig, 1:5000) and HRP-coupled goat anti-rabbit or goat anti-mouse IgG secondary antibody (Zhongshan Co., Beijing, China, ZB-2301, ZB-2305, 1:5000).
Total RNA was extracted from mouse liver tissues and cells with TRIzol Reagent (Ambion, Carlsbad, CA, United States) according to the manufacturer’s instructions. RNA concentration and quality were measured with a Nanodrop2000 (Thermo Scientific). RNA was then reverse transcribed into cDNA using a PrimeScriptRT reagent kit (Takara Bio, Japan) according to the manufacturer’s protocol. Quantitative PCR was performed using a TB GreenTM Premix Ex TaqTM II kit (Takara) and an ABI StepOnePlus Real-Time PCR System (Life Technologies, CA, United States). The expression levels were normalized to the level of β-actin mRNA in the same samples. The data were analyzed by the 2-ΔΔCt method. The primers used are listed in Table 1.
| Gene | Species | Primers |
| MANF | Homo sapiens | F: 5’-CGGTTGTGCTACTATATCGGGG-3’ |
| R: 5’-GGCCAGAGGCTTTGATACCT-3’ | ||
| ACCα | Homo sapiens | F: 5’-ATGTCTGGCTTGCACCTAGTA-3’ |
| R: 5’-CCCCAAAGCGAGTAACAAATTCT-3’ | ||
| FAS | Homo sapiens | F: 5’-AGATTGTGTGATGAAGGACATGG-3’ |
| R: 5’-TGTTGCTGGTGAGTGTGCATT-3’ | ||
| SREBP-1C | Homo sapiens | F: 5’-CGGAACCATCTTGGCAACAGT-3’ |
| R: 5’-CGCTTCTCAATGGCGTTGT-3’ | ||
| HMGCR | Homo sapiens | F: 5’-TGATTGACCTTTCCAGAGCAAG-3’ |
| R: 5’-CTAAAATTGCCATTCCACGAGC-3’ | ||
| CD36 | Homo sapiens | F: 5’-GGCTGTGACCGGAACTGTG-3’ |
| R: 5’-AGGTCTCCAACTGGCATTAGAA-3’ | ||
| FABP-1 | Homo sapiens | F: 5’-GTGTCGGAAATCGTGCAGAAT-3’ |
| R: 5’-GACTTTCTCCCCTGTCATTGTC-3’ | ||
| β-actin | Homo sapiens | F: 5’-GCCGACAGGATGCAGAAGG-3’ |
| R: 5’-TGGAAGGTGGACAGCGAGG-3’ | ||
| MANF | Mus musculus | F: 5’-TCTGGGACGATTTTACCAGGA-3’ |
| R: 5’-TCTTGCTTCACGGCAAAACTTTA-3’ | ||
| β-actin | Mus musculus | F: 5’-TCACTGTCCACCTTCCAGCAGATG-3’ |
| R: 5’-CTCAGTAACAGTCCGCCTAGAAGC-3’ |
At the end of treatment, HepG2 cells on 6-well plates were washed with phosphate-buffered saline (PBS, HyClone), fixed with 40 g/L paraformaldehyde fixing solution (Boster Biological Technology Co., Ltd, Wuhan, Hubei, China) for 30 min at room temperature, washed with PBS, and dipped in 600 mL/L isopropanol for 1 min. Subsequently, the cells were stained with 5 g/L oil red O solution (Sigma) in 600 mL/L isopropanol for 15 min and then washed with distilled water three times. Finally, cells were counterstained with hematoxylin staining solution (Zhongshan Co.) for 5 s, washed with distilled water, and mounted with glycerol jelly mounting medium (Beyotime). The cells were then visualized under a light microscope (Olympus, Tokyo, Japan), and images were captured. Staining was quantified using ImageJ (National Institute of Health, Bethesda, MD, United States) and analyzed as previously described[16,17].
Intracellular triglyceride (TG) and total cholesterol (TC) contents were quantified with a triglyceride assay kit and a total cholesterol assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China) according to the manufacturer’s instructions.
Statistical analyses were performed using SPSS statistical software (Version 21.0, SPSS Inc., Chicago, IL, United States). Data distributions were assessed using the Shapiro-Wilk test. Differences in normally distributed data were analyzed by independent-samples t-tests (two groups) or one-way ANOVA (three or more groups) followed by Tukey’s test for data that were homoscedastic or Tamhane’s T2 analysis for data that were heteroscedastic. For all statistical tests, P < 0.05 was considered indicative of statistical significance. All the data are presented as the mean ± SE, and the graphics were plotted by GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA, United States).
To explore the expression of MANF in fatty liver, we detected its expression in liver samples from ob/ob mice and WT mice (animals analyzed: 4/4). Hepatic MANF protein and mRNA expression in WT mice were 10-fold (P < 0.01) and 2-fold (P < 0.05) higher, respectively, than those in ob/ob mice (Figure 1A and B). Interestingly, the MANF protein expression was firstly raised to 1.3 times (P < 0.01) upon FFAs treatment for 24 h and subsequently reduced by 47% (P < 0.05) at the 72 h (Figure 1C). The MANF mRNA level was temporarily increased by 2 times (P < 0.001) with FFAs stimulation for 24 h and gradually reduced by 25% (P < 0.01) at the 72 h time point in HepG2 cells (Figure 1D). Furthermore, to determine the expression of MANF under lipid stress in vitro, HepG2 cells were treated with BSA or 0.5 mmol/L FFAs, and oil red O staining showed that intracellular lipid droplets were significantly increased in HepG2 cells under FFAs stimulation (Figure 1E).
To further explore the function of MANF in lipid-induced stress, we used lentiviral vectors to overexpress MANF and screened the stably transfected cell line (Figure 2A). The mRNA and protein expression were assessed by quantitative real-time PCR and Western blot, respectively (Figure 2B). As shown in Figure 2C, without FFAs treatment, MANF overexpression did not impact the lipid droplet area, while upon FFAs treatment, MANF overexpression significantly diminished the lipid droplet content in HepG2 cells (P < 0.01).
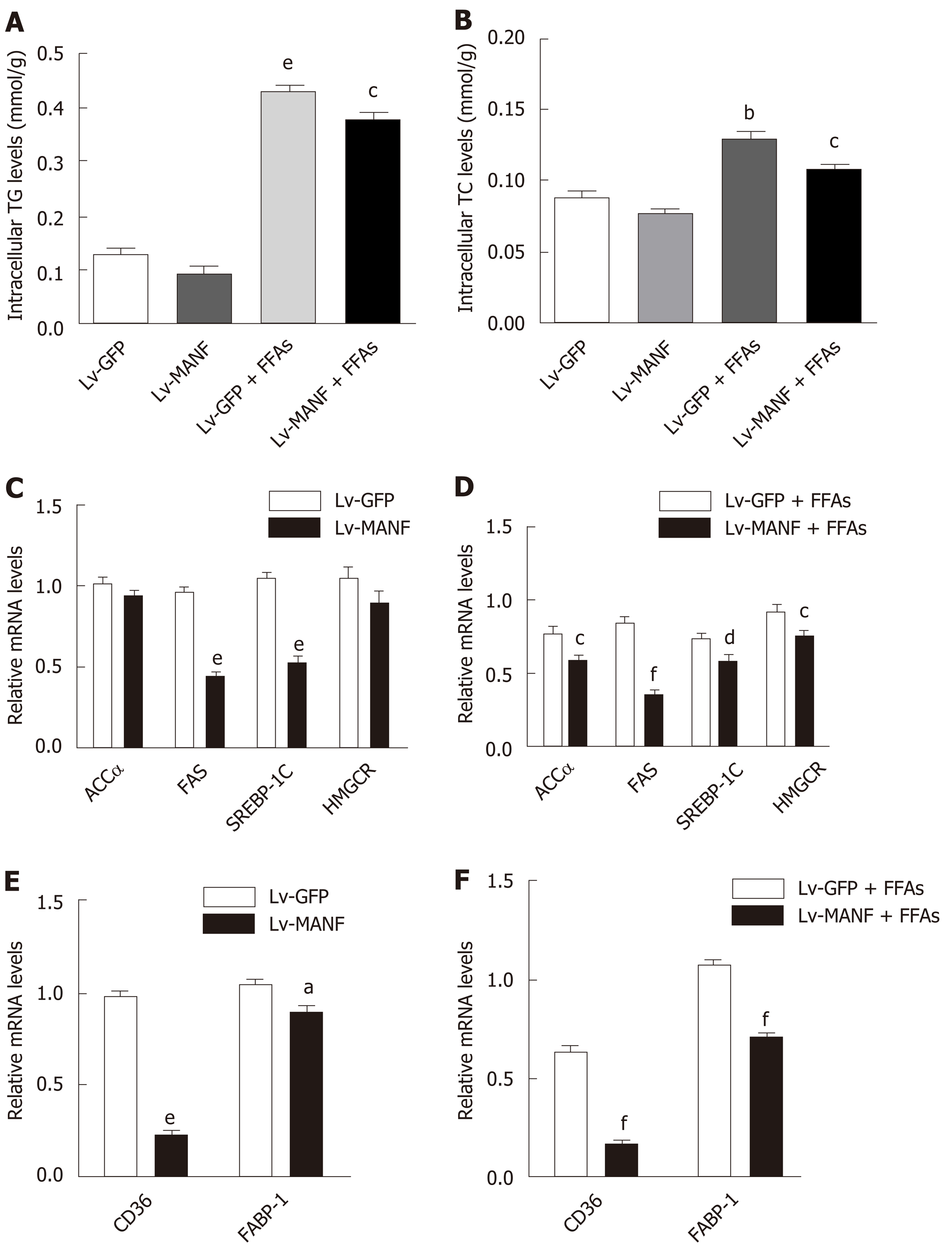
Next, we determined the intracellular content of TG and TC in HepG2 cells. The results showed a significant decrease in TG levels (0.3746 ± 0.0121 mmol/g) in the MANF over-expression group compared with those in the control group (0.4288 ± 0.0081 mmol/g) upon FFAs treatment (P < 0.05 Figure 3A). Furthermore, there was also a 17% decrease in intracellular TC levels (Figure 3B) in the MANF overexpression group (0.1088 ± 0.0009 mmol/g) compared with those in the control group (0.1301 ± 0.0059 mmol/g) after FFAs treatment (P < 0.05). Thus, to investigate the mechanisms of MANF responsible for suppressing lipogenesis, we detected the mRNA levels of lipid metabolism genes related to fatty acid synthesis (acetyl-CoA carboxylase α [ACCα], fatty acid synthase [FAS], sterol regulatory element binding transcription factor 1C [SREBP-1C]), cholesterol synthesis (3-hydroxy3-methylglutaryl [HMG] coenzyme A [CoA] reductase [HMGCR]), and fatty acid uptake (fatty acid translocase/CD36, fatty acid binding protein-1 [FABP-1]) in HepG2 cells.
The results showed that the expression of ACCα (P < 0.05) and SREBP-1C (P < 0.01) was lowered by 25% in the MANF overexpression group compared with that in the control group under FFAs treatment. Furthermore, there was a 57% reduction in FAS expression (P < 0.001) and a 18% decrease in HMGCR expression (P < 0.05) in the MANF overexpression group following FFAs stimulation (Figure 3C and D). After overexpression of MANF, the expression levels of CD36 and FABP-1 decreased by 71% and 33%, respectively (all P < 0.001, Figure 3E and F).
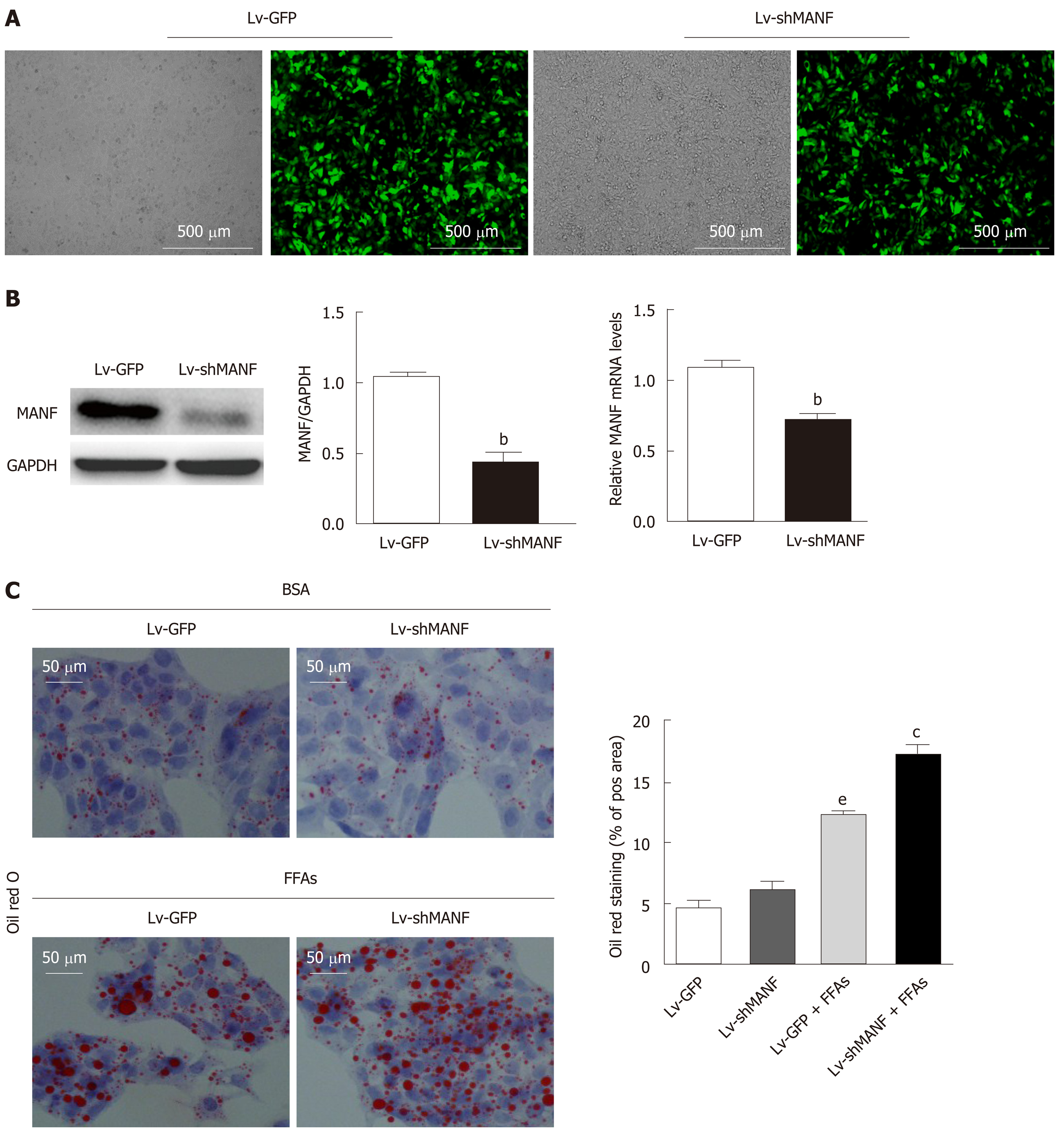
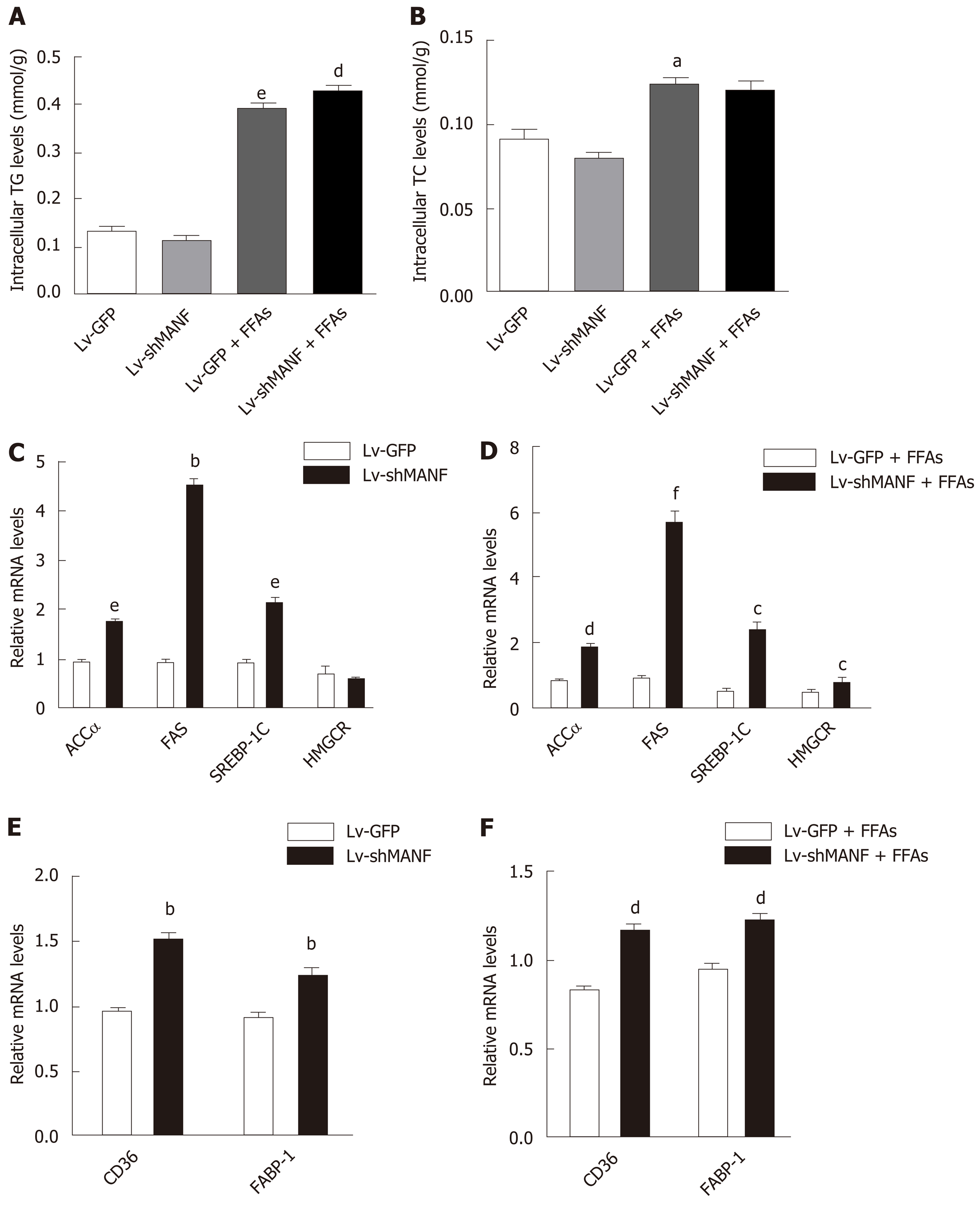
We then constructed a stable MANF-downregulated HepG2 cell line and explored the role of MANF deficiency in hepatic lipid accumulation (Figure 4A). The interference efficiency was also determined by quantitative real-time PCR and Western blot (Figure 4B). Oil red O staining showed no significant differences in lipid droplets between the MANF knockdown group and the control group in the absence of lipid stress. Additionally, under FFAs stimulation, the lipid droplet area in the MANF knockdown group was 37% greater than that in the control group (P < 0.05, Figure 4C).
Without FFAs treatment, MANF deficiency has little impact on intracellular TG levels. Interestingly, the intracellular TG levels were significantly increased in the MANF short hairpin RNA group (0.4294 ± 0.0072 mmol/g) compared with the control group (0.3946 ± 0.0041 mmol/g) under stimulation with FFAs (P < 0.01, Figure 5A). However, the intracellular TC levels were not significantly different between the MANF short hairpin RNA group and the control group with or without FFAs treatment (Figure 5B). In addition, the expression of genes related to fatty acid synthesis, cholesterol synthesis (Figure 5C and D), and fatty acid uptake (Figure 5E and F) was examined. The results showed that the expression of ACCα, FAS, SREBP-1C, and HMGCR in the MANF knockdown group was 2-fold (P < 0.01), 5.7-fold (P < 0.001), 4.8-fold (P < 0.05), and 1.6-fold (P < 0.05) higher, respectively, than that in the control group after FFAs treatment. The mRNA levels of CD36 and FABP-1 in the MANF knockdown group were 1.3-fold higher than those in the control group (P < 0.01).
Our study uncovered an important function of MANF in the pathogenesis of NAFLD. In the present study, both in vivo and in vitro studies provided basic pathological insights into a potential correlation between MANF and hepatic steatosis, which was functionally validated by up- and down-regulated MANF expression in vitro. Notably, MANF expression was increased in the early stage and gradually decreased under high FFAs stimulation. MANF deficiency increased lipogenesis and aggravated HepG2 cell steatosis, while MANF overexpression inhibited lipogenesis and rescued HepG2 cells from FFAs-induced steatosis. Our results demonstrated that MANF is a potential therapeutic target in hepatic steatosis processes.
Liver tissues from ob/ob mice showed significantly decreased mRNA and protein expression of MANF. This finding is in accordance with a previous study showing that plasma MANF levels were significantly reduced in nonalcoholic steatohepatitis patients and HFD-induced mice[14]. Our in vitro results suggested that MANF expression was increased in HepG2 cells during the first 24 h of stimulation by FFAs overload and then gradually decreased at the 48 h and 72 h time points. We speculated that the changes in MANF expression are due to diverse phases of the disease. A compensatory increase in MANF may protect against the progression of NAFLD in the early stage, similar to increased insulin secretion in the early stage of type 2 diabetes, but as the disease progresses, MANF expression is decreased, which in turn exacerbates the disease. However, further studies are needed to explore the differences in MANF expression in various stages of NAFLD.
Moreover, our clinical studies (data not shown) showed that plasma MANF levels were negatively associated with blood glucose and TG levels and positively associated with high-density lipoprotein cholesterol levels, which further indicated that MANF may play a protective role in glucolipid metabolism. Moreover, a high circulating TG level is the key feature of insulin resistance and dyslipidemia, and TG levels are significantly different between patients with or without NAFLD[18]. The accumulation of lipids, especially in the form of TGs, in the liver has been well accepted to set the stage for the progression of fatty liver disease and seems to be the hallmark of NAFLD[19]. Most importantly, the lipid accumulation and steatosis of HepG2 cells were worsened by MANF deficiency and attenuated by upregulating MANF, especially under stimulation with FFAs. NAFLD is frequently initiated and progresses when the susceptible genotype interacts with environmental factors such as sedentary lifestyle and overnutrition[20,21]. The present results suggest that MANF might be a new genetic background contributor to NAFLD and deserves further research.
To further investigate the mechanism by which MANF regulates hepatic lipid metabolism, the expression of genes involved in fatty acid synthesis, cholesterol synthesis, and fatty acid uptake was detected. ACCα, the rate-controlling enzyme in de novo lipogenesis, plays a crucial role in fatty acid metabolism[22]. In addition, its inhibition could reverse hepatic lipid accumulation[23]. FAS catalyzes the synthesis of palmitate from acetyl-CoA and malonyl-CoA, while SREBP-1C, a membrane-bound transcription factor, positively regulates the above lipogenic enzymes, including ACCα and FAS[24-28]. The SREBP protein is synthesized as a precursor that is attached to the nuclear membrane and endoplasmic reticulum. SREBP-1c is a major isoform related to the fatty acid metabolism in the liver and SREBP-1c overexpression raised the hepatic TG accumulation. We have found that overexpression of MANF could downregulate this gene while MANF knockdown could increase SREBP-1c expression, which implies that MANF could affect SREBP-1c expression and thus have a potential role in the regulation of lipogenesis. Our results demonstrated that the downregulation of MANF could enhance these genes and result in the accumulation of lipids, while the upregulation of MANF could significantly downregulate these genes and attenuate HepG2 cell steatosis, showing improved accumulation of lipids, especially TGs. Moreover, HMGCR, a key enzyme responsible for cholesterol synthesis[29], was reduced by MANF overexpression, indicating the advantageous effect of MANF in excessive cholesterol deposition. We detected genes involved in fatty acid uptake in addition to those involved in fatty acid synthesis. Hepatic fatty acid uptake mainly depends on the FABP family and the scavenger receptor CD36. CD36 expression is much lower in normal hepatocytes but is greatly induced by high-fat diets, leading to TG accumulation and hepatic cell steatosis[30-35], while disruption of its expression could attenuate NAFLD[36]. FABP-1 is a liver-specific FABP, and moderate inhibition of FABP-1 function could ameliorate lipid accumulation in the liver[37]. Most interestingly, modulating MANF expression could definitely affect fatty acid uptake by regulating CD36, and overexpression of MANF could inhibit FABP-1 expression, while MANF deficiency can increase FABP-1 expression. These results definitely showed that regulating MANF may offer an attractive therapeutic strategy for NAFLD.
There are a few limitations in this study. First, NAFLD encompasses simple steatosis to nonalcoholic steatohepatitis as well as further progression to cirrhosis and liver carcinoma, and we did not detect MANF expression in various stages of NAFLD. However, we verified the tendency for the first time in an in vitro study, and the gain and loss of function of the gene gave definite causal implications for MANF in the development of liver cell steatosis. Second, gain- and loss-of-function experiments involving MANF in an animal model of NAFLD still need to be performed. Nevertheless, the results from our experiments are sufficient to demonstrate a novel function of MANF in the pathogenesis of hepatic cell steatosis.
In conclusion, our study revealed a novel role of MANF in regulating hepatic lipid metabolism and steatosis in an in vitro model of NAFLD. This study is the first to indicate that hepatic MANF expression was induced upon FFAs overload and gradually decreased thereafter. MANF deficiency could significantly inhibit genes involved in lipogenesis and lipid uptake, potentially leading to attenuation of liver cell steatosis. Moreover, rescuing hepatic MANF may be a promising therapeutic target for the treatment of NAFLD, and additional intracellular mechanisms remain to be explored to fully understand the mechanism.
Nonalcoholic fatty liver disease (NAFLD) is a global metabolism-associated liver disease. Hepatic steatosis, inflammation, and insulin resistance are the primary pathologic changes of NAFLD. Although NAFLD has become a serious threat to human public health, its pathogenic mechanisms are largely unknown.
Mesencephalic astrocyte-derived neurotrophic factor (MANF) is a newly discovered conserved neurotrophic factor. Recent studies found that MANF is involved in diabetes, obesity, and metabolic homeostasis. However, much remains to be discovered about its function in hepatic lipid metabolism; thus, we detected whether MANF could regulate hepatic metabolism.
This study aimed to detect MANF expression in both in vivo and in vitro NAFLD models and explore the role of MANF in hepatic lipid metabolism.
HepG2 cells treated with free fatty acids and ob/ob mice were used as NAFLD models. Liver tissues collected from WT and ob/ob mice were used to detect MANF expression. Cells were treated with free fatty acids for different durations. Moreover, we established overexpression and knockdown cell models with lentiviruses to interfere with MANF expression levels in order to observe whether MANF influences hepatic steatosis.
Hepatic MANF expression was obviously decreased in ob/ob mice compared with wild type mice. The MANF level was elevated temporarily and gradually decreased in HepG2 cells as the duration of treatment with high free fatty acids increased. MANF deficiency accelerated lipogenesis and aggravated HepG2 cell steatosis, while MANF overexpression inhibited lipogenesis and rescued HepG2 cells from free fatty acids-induced steatosis.
MANF alleviated lipid deposition and suppressed lipogenes, showing a potential protective role in NAFLD.
MANF may be a potential therapeutic target in hepatic steatosis processes.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fatkhudinov T, Aureliano M, Hosomi R S-Editor: Zhou JJ L-Editor: Wang TQ E-Editor: Zhang YL
| 1. | Sahini N, Borlak J. Recent insights into the molecular pathophysiology of lipid droplet formation in hepatocytes. Prog Lipid Res. 2014;54:86-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 2. | Petrova P, Raibekas A, Pevsner J, Vigo N, Anafi M, Moore MK, Peaire AE, Shridhar V, Smith DI, Kelly J, Durocher Y, Commissiong JW. MANF: a new mesencephalic, astrocyte-derived neurotrophic factor with selectivity for dopaminergic neurons. J Mol Neurosci. 2003;20:173-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Parkash V, Lindholm P, Peränen J, Kalkkinen N, Oksanen E, Saarma M, Leppänen VM, Goldman A. The structure of the conserved neurotrophic factors MANF and CDNF explains why they are bifunctional. Protein Eng Des Sel. 2009;22:233-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 4. | Lindahl M, Saarma M, Lindholm P. Unconventional neurotrophic factors CDNF and MANF: Structure, physiological functions and therapeutic potential. Neurobiol Dis. 2017;97:90-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 136] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 5. | Lindholm P, Peränen J, Andressoo JO, Kalkkinen N, Kokaia Z, Lindvall O, Timmusk T, Saarma M. MANF is widely expressed in mammalian tissues and differently regulated after ischemic and epileptic insults in rodent brain. Mol Cell Neurosci. 2008;39:356-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 155] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 6. | Xu W, Gao L, Li T, Zheng J, Shao A, Zhang J. Mesencephalic Astrocyte-Derived Neurotrophic Factor (MANF) Protects Against Neuronal Apoptosis via Activation of Akt/MDM2/p53 Signaling Pathway in a Rat Model of Intracerebral Hemorrhage. Front Mol Neurosci. 2018;11:176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 7. | Zhang Z, Shen Y, Luo H, Zhang F, Peng D, Jing L, Wu Y, Xia X, Song Y, Li W, Jin L. MANF protects dopamine neurons and locomotion defects from a human α-synuclein induced Parkinson's disease model in C. elegans by regulating ER stress and autophagy pathways. Exp Neurol. 2018;308:59-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 8. | Hakonen E, Chandra V, Fogarty CL, Yu NY, Ustinov J, Katayama S, Galli E, Danilova T, Lindholm P, Vartiainen A, Einarsdottir E, Krjutškov K, Kere J, Saarma M, Lindahl M, Otonkoski T. MANF protects human pancreatic beta cells against stress-induced cell death. Diabetologia. 2018;61:2202-2214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 9. | Lindahl M, Danilova T, Palm E, Lindholm P, Võikar V, Hakonen E, Ustinov J, Andressoo JO, Harvey BK, Otonkoski T, Rossi J, Saarma M. MANF is indispensable for the proliferation and survival of pancreatic β cells. Cell Rep. 2014;7:366-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 161] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 10. | Wu T, Zhang F, Yang Q, Zhang Y, Liu Q, Jiang W, Cao H, Li D, Xie S, Tong N, He J. Circulating mesencephalic astrocyte-derived neurotrophic factor is increased in newly diagnosed prediabetic and diabetic patients, and is associated with insulin resistance. Endocr J. 2017;64:403-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 11. | Galli E, Härkönen T, Sainio MT, Ustav M, Toots U, Urtti A, Yliperttula M, Lindahl M, Knip M, Saarma M, Lindholm P. Increased circulating concentrations of mesencephalic astrocyte-derived neurotrophic factor in children with type 1 diabetes. Sci Rep. 2016;6:29058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 12. | Yavarna T, Al-Dewik N, Al-Mureikhi M, Ali R, Al-Mesaifri F, Mahmoud L, Shahbeck N, Lakhani S, AlMulla M, Nawaz Z, Vitazka P, Alkuraya FS, Ben-Omran T. High diagnostic yield of clinical exome sequencing in Middle Eastern patients with Mendelian disorders. Hum Genet. 2015;134:967-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 153] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 13. | Yang S, Yang H, Chang R, Yin P, Yang Y, Yang W, Huang S, Gaertig MA, Li S, Li XJ. MANF regulates hypothalamic control of food intake and body weight. Nat Commun. 2017;8:579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 14. | Sousa-Victor P, Neves J, Cedron-Craft W, Ventura PB, Liao CY, Riley RR, Soifer I, van Bruggen N, Kolumam GA, Villeda SA, Lamba DA, Jasper H. MANF regulates metabolic and immune homeostasis in ageing and protects against liver damage. Nat Metab. 2019;1:276-290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 15. | Feldstein AE, Werneburg NW, Canbay A, Guicciardi ME, Bronk SF, Rydzewski R, Burgart LJ, Gores GJ. Free fatty acids promote hepatic lipotoxicity by stimulating TNF-alpha expression via a lysosomal pathway. Hepatology. 2004;40:185-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 586] [Cited by in RCA: 614] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 16. | Wen J, Lin T, Wu W, Yang Y, Luo C, Zhou C, Wan J, Liu S, Wang D, Wang P, Li J. Tiaopi huxin recipe improved endothelial dysfunction and attenuated atherosclerosis by decreasing the expression of caveolin-1 in ApoE-deficient mice. J Cell Physiol. 2019;234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Zhang L, Yang M, Ren H, Hu H, Boden G, Li L, Yang G. GLP-1 analogue prevents NAFLD in ApoE KO mice with diet and Acrp30 knockdown by inhibiting c-JNK. Liver Int. 2013;33:794-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 18. | Trojak A, Waluś-Miarka M, Woźniakiewicz E, Małecki MT, Idzior-Waluś B. Nonalcoholic fatty liver disease is associated with low HDL cholesterol and coronary angioplasty in patients with type 2 diabetes. Med Sci Monit. 2013;19:1167-1172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Day CP, James OF. Steatohepatitis: a tale of two "hits"? Gastroenterology. 1998;114:842-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2953] [Cited by in RCA: 3130] [Article Influence: 115.9] [Reference Citation Analysis (36)] |
| 20. | Anstee QM, Day CP. The genetics of NAFLD. Nat Rev Gastroenterol Hepatol. 2013;10:645-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 265] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 21. | Anstee QM, Seth D, Day CP. Genetic Factors That Affect Risk of Alcoholic and Nonalcoholic Fatty Liver Disease. Gastroenterology. 2016;150:1728-1744.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 194] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 22. | Brownsey RW, Zhande R, Boone AN. Isoforms of acetyl-CoA carboxylase: structures, regulatory properties and metabolic functions. Biochem Soc Trans. 1997;25:1232-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Goedeke L, Bates J, Vatner DF, Perry RJ, Wang T, Ramirez R, Li L, Ellis MW, Zhang D, Wong KE, Beysen C, Cline GW, Ray AS, Shulman GI. Acetyl-CoA Carboxylase Inhibition Reverses NAFLD and Hepatic Insulin Resistance but Promotes Hypertriglyceridemia in Rodents. Hepatology. 2018;68:2197-2211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 191] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 24. | Shimano H. Sterol regulatory element-binding proteins (SREBPs): transcriptional regulators of lipid synthetic genes. Prog Lipid Res. 2001;40:439-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 573] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 25. | Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109:1125-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 1749] [Article Influence: 76.0] [Reference Citation Analysis (0)] |
| 26. | Nakamuta M, Kohjima M, Morizono S, Kotoh K, Yoshimoto T, Miyagi I, Enjoji M. Evaluation of fatty acid metabolism-related gene expression in nonalcoholic fatty liver disease. Int J Mol Med. 2005;16:631-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 27. | Kohjima M, Enjoji M, Higuchi N, Kato M, Kotoh K, Yoshimoto T, Fujino T, Yada M, Yada R, Harada N, Takayanagi R, Nakamuta M. Re-evaluation of fatty acid metabolism-related gene expression in nonalcoholic fatty liver disease. Int J Mol Med. 2007;20:351-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 28. | Horton JD, Bashmakov Y, Shimomura I, Shimano H. Regulation of sterol regulatory element binding proteins in livers of fasted and refed mice. Proc Natl Acad Sci USA. 1998;95:5987-5992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 519] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 29. | Ma S, Sun W, Gao L, Liu S. Therapeutic targets of hypercholesterolemia: HMGCR and LDLR. Diabetes Metab Syndr Obes. 2019;12:1543-1553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 30. | Bechmann LP, Gieseler RK, Sowa JP, Kahraman A, Erhard J, Wedemeyer I, Emons B, Jochum C, Feldkamp T, Gerken G, Canbay A. Apoptosis is associated with CD36/fatty acid translocase upregulation in non-alcoholic steatohepatitis. Liver Int. 2010;30:850-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 31. | Miquilena-Colina ME, Lima-Cabello E, Sánchez-Campos S, García-Mediavilla MV, Fernández-Bermejo M, Lozano-Rodríguez T, Vargas-Castrillón J, Buqué X, Ochoa B, Aspichueta P, González-Gallego J, García-Monzón C. Hepatic fatty acid translocase CD36 upregulation is associated with insulin resistance, hyperinsulinaemia and increased steatosis in non-alcoholic steatohepatitis and chronic hepatitis C. Gut. 2011;60:1394-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 329] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 32. | Greco D, Kotronen A, Westerbacka J, Puig O, Arkkila P, Kiviluoto T, Laitinen S, Kolak M, Fisher RM, Hamsten A, Auvinen P, Yki-Järvinen H. Gene expression in human NAFLD. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1281-G1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 326] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 33. | Su X, Abumrad NA. Cellular fatty acid uptake: a pathway under construction. Trends Endocrinol Metab. 2009;20:72-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 330] [Cited by in RCA: 313] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 34. | Koonen DP, Jacobs RL, Febbraio M, Young ME, Soltys CL, Ong H, Vance DE, Dyck JR. Increased hepatic CD36 expression contributes to dyslipidemia associated with diet-induced obesity. Diabetes. 2007;56:2863-2871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 380] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 35. | Zhou J, Febbraio M, Wada T, Zhai Y, Kuruba R, He J, Lee JH, Khadem S, Ren S, Li S, Silverstein RL, Xie W. Hepatic fatty acid transporter Cd36 is a common target of LXR, PXR, and PPARgamma in promoting steatosis. Gastroenterology. 2008;134:556-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 479] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 36. | Wilson CG, Tran JL, Erion DM, Vera NB, Febbraio M, Weiss EJ. Hepatocyte-Specific Disruption of CD36 Attenuates Fatty Liver and Improves Insulin Sensitivity in HFD-Fed Mice. Endocrinology. 2016;157:570-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 345] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 37. | Mukai T, Egawa M, Takeuchi T, Yamashita H, Kusudo T. Silencing of FABP1 ameliorates hepatic steatosis, inflammation, and oxidative stress in mice with nonalcoholic fatty liver disease. FEBS Open Bio. 2017;7:1009-1016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |