Published online Feb 7, 2019. doi: 10.3748/wjg.v25.i5.622
Peer-review started: October 19, 2018
First decision: December 20, 2018
Revised: December 25, 2018
Accepted: January 14, 2019
Article in press: January 14, 2019
Published online: February 7, 2019
Processing time: 103 Days and 2.7 Hours
The Liver Imaging Reporting and Data System (LI-RADS), supported by the American College of Radiology (ACR), has been developed for standardizing the acquisition, interpretation, reporting, and data collection of liver imaging examinations in patients at risk for hepatocellular carcinoma (HCC). Diffusion-weighted imaging (DWI), which is described as an ancillary imaging feature of LI-RADS, can improve the diagnostic efficiency of LI-RADS v2017 with gadoxetic acid-enhanced magnetic resonance imaging (MRI) for HCC.
To determine whether the use of DWI can improve the diagnostic efficiency of LI-RADS v2017 with gadoxetic acid-enhanced magnetic resonance MRI for HCC.
In this institutional review board-approved study, 245 observations of high risk of HCC were retrospectively acquired from 203 patients who underwent gadoxetic acid-enhanced MRI from October 2013 to April 2018. Two readers independently measured the maximum diameter and recorded the presence of each lesion and assigned scores according to LI-RADS v2017. The test was used to determine the agreement between the two readers with or without DWI. In addition, the sensitivity (SE), specificity (SP), accuracy (AC), positive predictive value (PPV), and negative predictive value (NPV) of LI-RADS were calculated. Youden index values were used to compare the diagnostic performance of LI-RADS with or without DWI.
Almost perfect interobserver agreement was obtained for the categorization of observations with LI-RADS (kappa value: 0.813 without DWI and 0.882 with DWI). For LR-5, the diagnostic SE, SP, and AC values were 61.2%, 92.5%, and 71.4%, respectively, with or without DWI; for LR-4/5, they were 73.9%, 80%, and 75.9% without DWI and 87.9%, 80%, and 85.3% with DWI; for LR-4/5/M, they were 75.8%, 58.8%, and 70.2% without DWI and 87.9%, 58.8%, and 78.4% with DWI; for LR- 4/5/TIV, they were 75.8%, 75%, and 75.5% without DWI and 89.7%, 75%, and 84.9% with DWI. The Youden index values of the LI-RADS classification without or with DWI were as follows: LR-4/5: 0.539 vs 0.679; LR-4/5/M: 0.346 vs 0.467; and LR-4/5/TIV: 0.508 vs 0.647.
LI-RADS v2017 has been successfully applied with gadoxetate-enhanced MRI for patients at high risk for HCC. The addition of DWI significantly increases the diagnostic efficiency for HCC.
Core tip: The aim of this study was to determine whether the use of diffusion-weighted imaging (DWI) improves the diagnostic efficiency of the Liver Imaging Reporting and Data System (LI-RADS) v2017 with gadoxetic acid-enhanced magnetic resonance (MR) for hepatocellular carcinoma (HCC). A total of 245 observations in 203 patients were analyzed. The Youden index values of the LI-RADS classification without or with DWI were as follows: LR-4/5: 0.539 vs 0.679; LR-4/5/M: 0.346 vs 0.467; and LR-4/5/TIV: 0.508 vs 0.647. Using LI-RADS v2017 with gadoxetic acid-enhanced MR combined with DWI may result in a more accurate diagnosis of HCC.
- Citation: Zhang T, Huang ZX, Wei Y, Jiang HY, Chen J, Liu XJ, Cao LK, Duan T, He XP, Xia CC, Song B. Hepatocellular carcinoma: Can LI-RADS v2017 with gadoxetic-acid enhancement magnetic resonance and diffusion-weighted imaging improve diagnostic accuracy? World J Gastroenterol 2019; 25(5): 622-631
- URL: https://www.wjgnet.com/1007-9327/full/v25/i5/622.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i5.622
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the third leading cause of cancer-related deaths[1]. In high-risk patients, HCC can be diagnosed noninvasively by computed tomography (CT) and magnetic resonance imaging (MRI) without the need for further histopathological confirmation when the imaging features are characteristic[2-4].
The Liver Imaging Reporting and Data System (LI-RADS), supported by the American College of Radiology (ACR), has been developed for standardizing the acquisition, interpretation, reporting, and data collection of liver imaging examinations in patients at risk for HCC. Initially released in 2011, the system has been updated in 2013, 2014, 2017, and 2018 based on the evolution of published evidence, integration of new technology, and incorporation of user feedback[5-7]. Each liver observation is categorized according to its probability of HCC, from LR-1 to LR-5 (definitely benign, probably benign, intermediate, probably HCC, and definitely HCC)[5]. In LI-RADS v2017[8], if an observation is probably or definitely malignant but is not specific for HCC, LR-M is allocated. Moreover, a new diagnostic category, LR-NC and LR-TIV (previously LR-5V), has been added. Ancillary features can be applied to upgrade or downgrade the initially assigned LI-RADS category based on major features only[9].
MRI can be used for categorization of liver observations and diagnosis of HCC based on the major and ancillary features of LI-RADS[10-12]. Gadoxetic-acid disodium (Gd-EOB-DTPA), a hepatobiliary contrast agent, could provide information on tumor vasculature and hepatocyte function[13-15]. Diffusion-weighted imaging (DWI) can further quantitatively measure tissue proton diffusion and reflect tumor cellularity[16-18]. Thus, the combination of Gd-EOB-DTPA-enhanced MRI and DWI has the potential to improve the sensitivity (SE) and overall accuracy (AC) for diagnosing HCC.
The aim of this retrospective study was to evaluate the interrater reliability and diagnostic AC of LI-RADS v2017 with Gd-EOB-DTPA-enhanced MRI for HCC and to determine the incremental value of the ancillary feature “restricted diffusion” on DWI images.
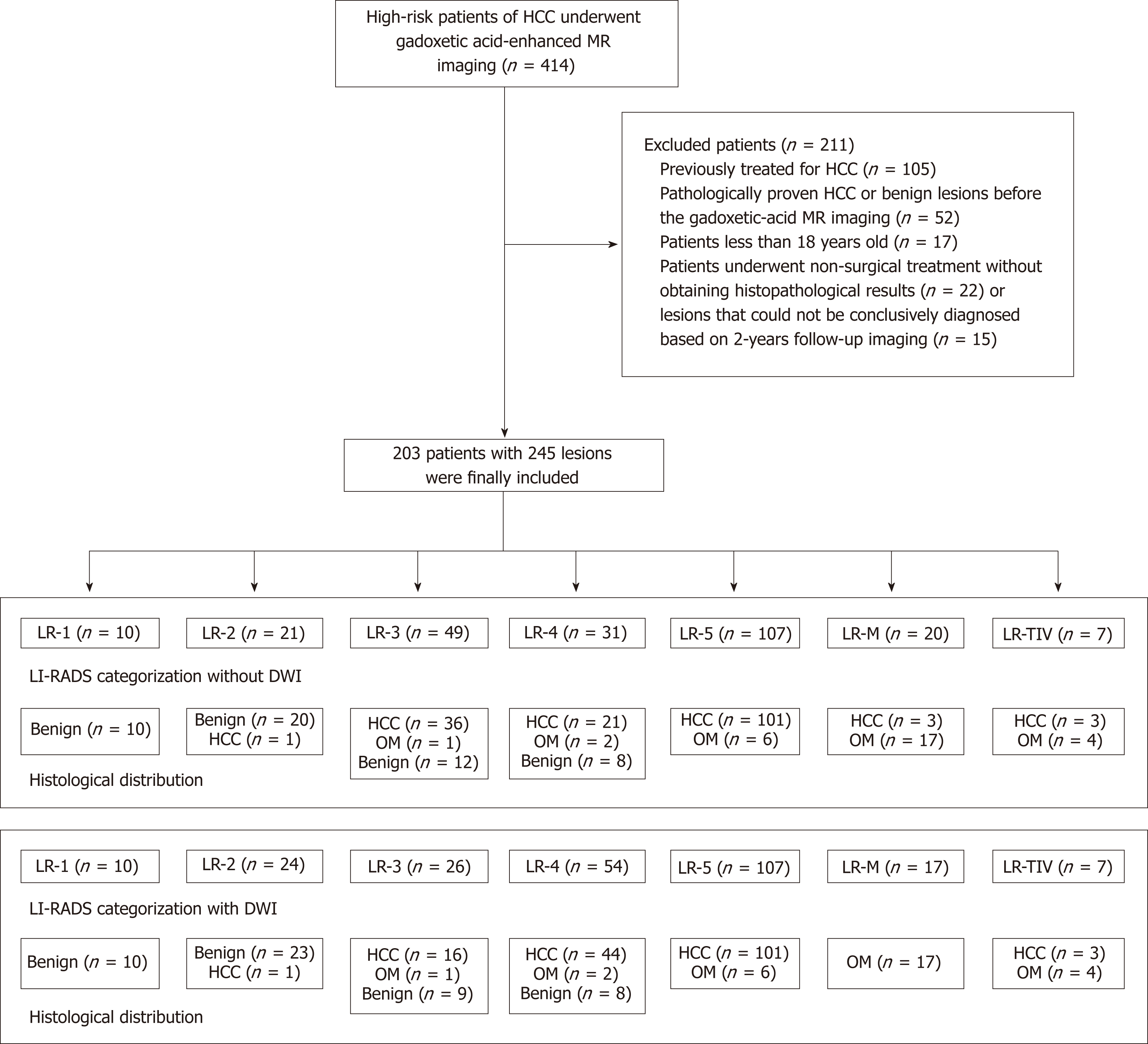
This retrospective cohort study was approved by our institutional review board, and the requirement for patient consent was waived. Between October 2013 and April 2018, a total of 414 consecutive patients who were at high risk [hepatitis B virus (HBV) infection, hepatitis C virus (HCV) infection, or hepatic cirrhosis] for HCC were enrolled. All included patients were confirmed by surgical pathology, needle biopsy, or more than two years of follow-up. Among these patients, 211 were excluded because of the exclusion criteria (Figure 1). The exclusion criteria were as follows: (1) previously treated for HCC (n =105); (2) pathologically proven HCC or benign lesions before gadoxetic acid-enhanced MRI (n = 52); (3) less than 18 years old (n = 17); and (4) underwent nonsurgical treatment without obtaining histopathological results (n = 22) or lesions that could not be conclusively diagnosed based on 2-year follow-up imaging (n = 15).
For all examinations, studies were carried out by using a 3.0 T MR system (MAGNETOM Skyra, Siemens Healthcare, Erlangen, Germany). An 18-channel phased-array torso coil was used for all measurements. Routine MRI sequences included in the standardized scanning protocol were a respiratory-triggered axial T2-weighted turbo spin echo (TSE) sequence with fat suppression; in and out of phase T1-weighted imaging acquired with a gradient recalled (GRE) dual echo sequence; and pre- and postcontrast T1-weighted three-dimensional VIBE sequences acquired with a GRE sequence in the arterial phase (20 s), portal venous phase (60 s), and delayed phase (180 s) after the injection of Gd-EOB-DTPA (Primovist, Bayer Pharma AG, Berlin, Germany) at a rate of 2 mL/s. The delay time for the hepatobiliary phase was 20 min. The detailed parameters of each acquisition sequence are shown in Table 1.
| Parameter | DWI | In/out of phase | T2-weighted imaging | VIBE |
| T1-weighted imaging | ||||
| Repetition time (ms) | 5600 | 81 | 2160 | 3.95 |
| Echo time (ms) | 68 | 1.4 | 100 | 1.92 |
| Field of view (mm2) | 380 × 289 | 400 × 325 | 433 × 433 | 400 × 296 |
| Scan matrix | 100 × 76 | 352 × 286 | 320 × 20288 | 352 × 256 |
| Slice thickness (mm) | 6 | 6 | 6 | 2 |
| Slice gap (mm) | 1 | 2.7 | 2.7 | 0 |
| Number of excitation | … | 1 | 2 | 1 |
Two radiologists (with more than ten years of experience in abdominal radiology) who were blinded to the clinical, laboratory, and pathology results reinterpreted the MR images. Each reader measured the maximum diameter and recorded the presence of each lesion and assigned scores according to LI-RADS v2017[11]. The scoring categories were as follows: LR-1 was definitely benign, LR-2 was probably benign, LR-3 was an intermediate probability of malignancy, LR-4 was probably HCC, and LR-5 was definitely HCC. Findings that were probably or definitely malignant but not HCC specific were categorized as LR-M and those with definite tumor in vein as LR-TIV. The final category results were compared with the pathology to assess diagnostic AC.
Categorical variables are reported as the number of cases and percentages. The kappa test was first used to determine the agreement between the two independent radiologists in each item. A kappa value of 0 indicates no agreement, kappa values of 0.01-0.20 represent slight agreement, 0.21-0.40 fair agreement, 0.41-0.60 moderate agreement, 0.61-0.80 good agreement, 0.81-0.99 almost perfect agreement, and 1 perfect agreement[19]. In addition, the SE, specificity (SP), AC, positive predictive value (PPV), and negative predictive value (NPV) were calculated for the diagnostic performance of LI-RADS. Youden index values were used to compare the diagnostic performance of LI-RADS with or without DWI. All statistical analyses were performed using SPSS 23.0 (SPSS Inc, Chicago, IL, United States).
During the study period, 414 consecutive patients were selected for potential inclusion. Of these patients, 203 (mean age: 50.31 ± 10.87 years; range: 26-77 years) with 245 hepatic lesions, including 157 (77.34%) men (50.06 ± 10.05 years old; range: 26-77 years old) and 46 (22.66%) women (51.17 ± 11.67 years old; range: 30-77 years old) who met the inclusion criteria, were ultimately included. In the study cohort, 19 patients had multiple HCCs. Of these patients, 195 had Child-Pugh A, and 8 had Child-Pugh B. In addition, 194 (95.57%) patients had HBV infection, 8 (3.94%) had HCV infection, and 1 (0.4%) had both HBV and HCV infections. The baseline characteristics of all patients are summarized in Table 2.
| Characteristic | Value |
| Clinical information | |
| Age (yr) | 50.31 ± 10.87 (range: 26-77) |
| Male/female | 157/46 |
| Etiology of liver disease | |
| Hepatitis B virus | 194 (95.57%) |
| Hepatitis C virus | 8 (3.94%) |
| Both hepatitis B and C virus | 1 (0.49%) |
| Tumor size (cm) | Median 5.3 (range: 1.1-12.8) |
| AFP level (ng/mL) | 102.3 ng/mL (range 1.2-15926.0) |
| Serum AST (≥ 35 IU/L) | 107 (52.7%) |
| Serum ALT (≥ 40 IU/L) | 82 (40.4%) |
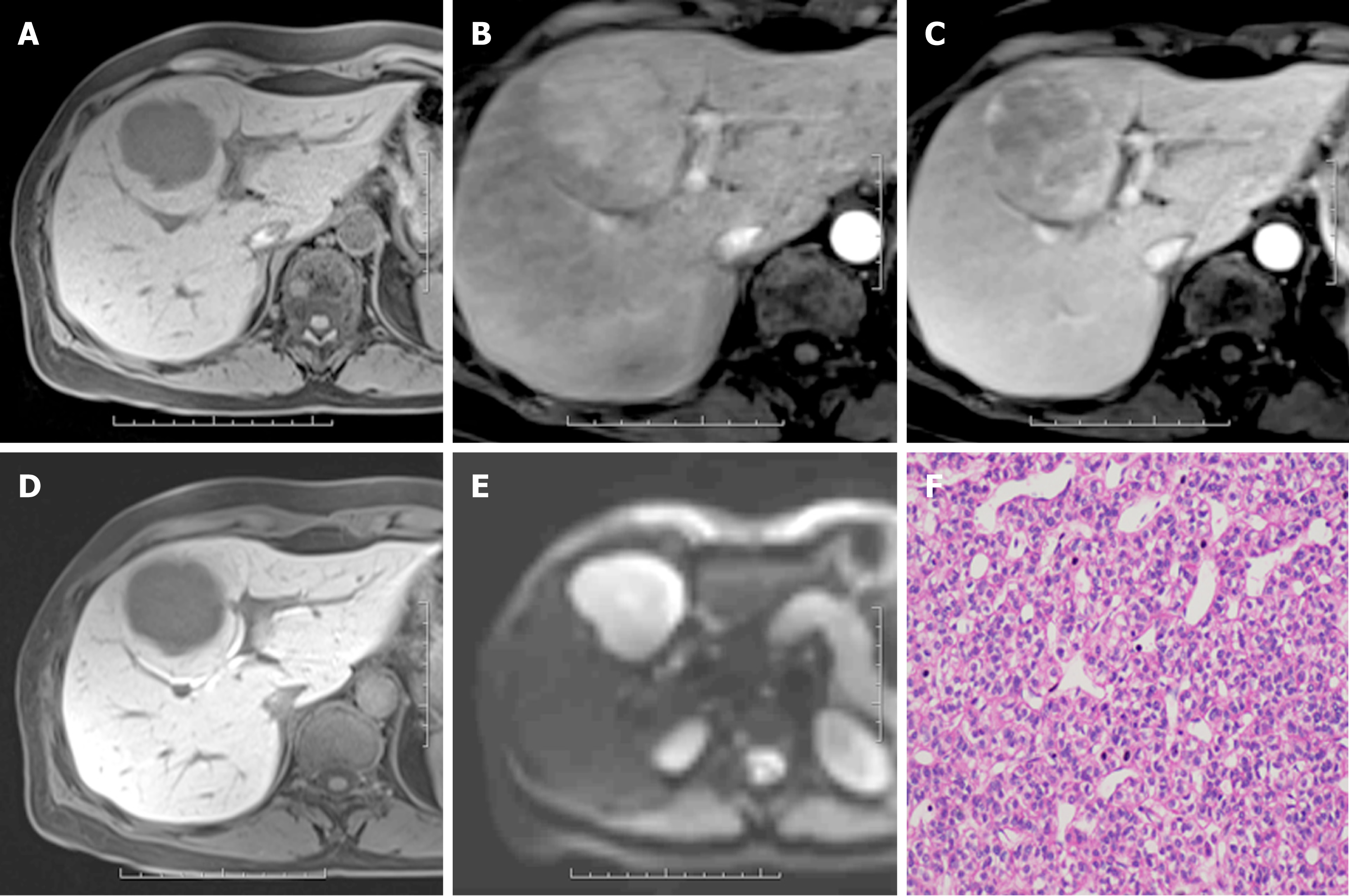
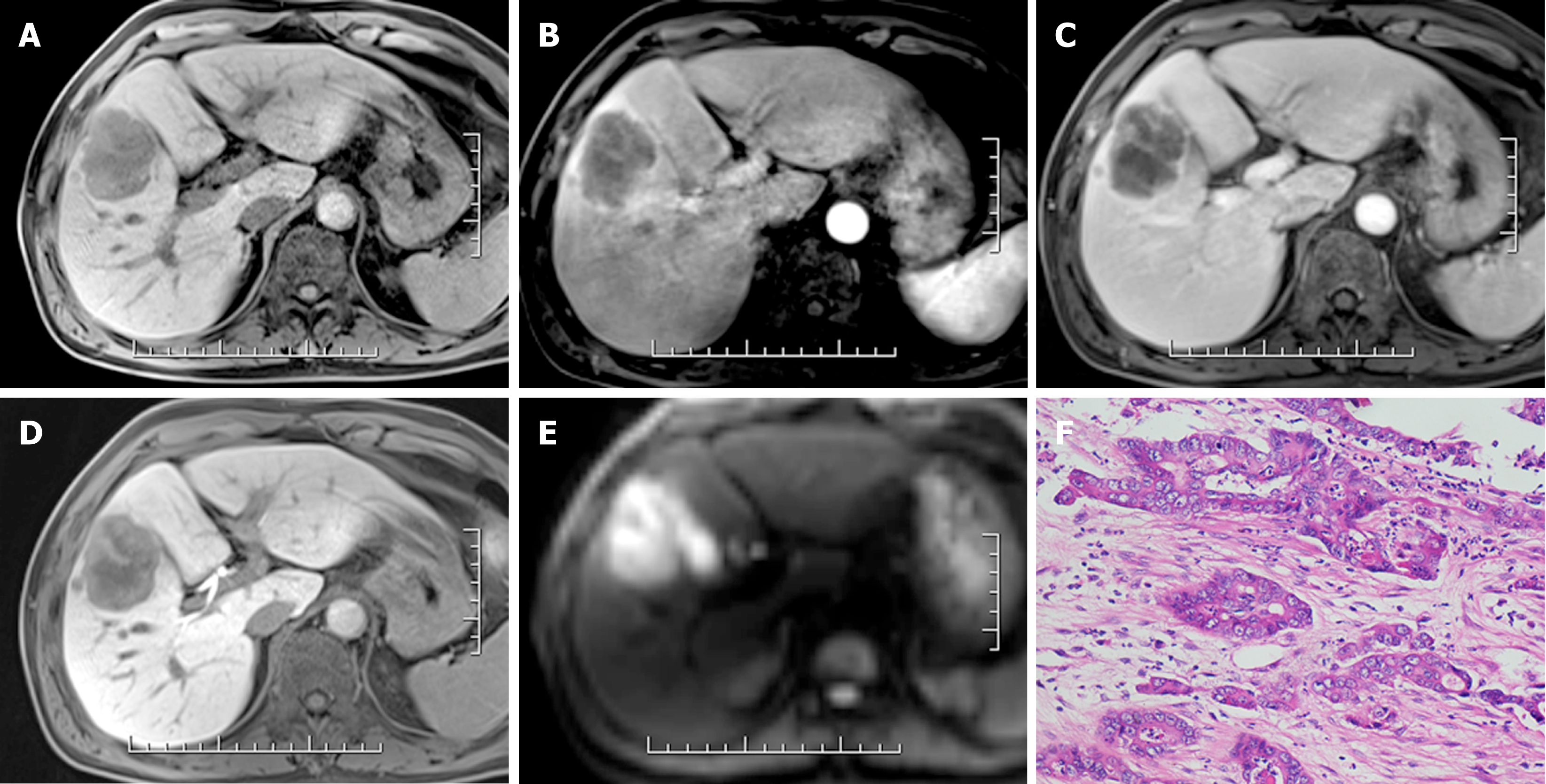
Of all 245 hepatic lesions, 195 (79.59%) were confirmed as malignant by histologic analysis, including 165 (67.35%) lesions diagnosed as HCC (Figure 2), 22 (8.98%) as intrahepatic cholangiocarcinoma (ICC) (Figure 3), 5 (2.04%) as combined HCC (cHCC), and 3 (1.22%) as sarcomatoid HCC (SHC). In addition, 50 (20.41%) lesions were diagnosed as benign by liver biopsy (n = 5) or two-year follow-up using CT or MRI (n = 45). The median diameter for hepatic lesions was 5.3 cm (range: 1.1-12.8 cm).
The agreement of the LI-RADS classification was almost perfect between the two observers [kappa = 0.813; 95% confidence interval (CI): 0.748-0.871]. When DWI images were jointly viewed for LI-RADS classification, the agreement between the two observers was markedly increased (kappa = 0.882; 95%CI: 0.834-0.928).
When MR images were reviewed without DWI, HCCs were diagnosed in zero of 10 (0%) LR-1 lesions, one (5%) of 21 LR-2, 36 (73.5%) of 49 LR-3, 21 (67.7%) of 31 LR-4, 101 (94.4%) of 107 LR-5, 3 (15%) of 20 LR-M, and 3 (42.9%) of 7 LR-TIV. However, when the MR and DWI images were jointly viewed for the LI-RADS classification, HCCs were diagnosed in zero of 10 (0%) LR-1 lesions, one (4%) of 24 LR-2, 16 (61.5%) of 26 LR-3, 44 (81.5%) of 54 LR-4, 101 (94.4%) of 107 LR-5, zero (0%) of 17 LR-M, and 3 (42.9%) of 7 LR-TIV. Regarding the diagnostic efficiency, when considering only lesions classified as LR-5, the diagnostic SE, SP, and AC values were 61.2%, 92.5%, and 71.4% without DWI and 61.2%, 92.5%, and 71.4% with DWI, respectively. For LR-4/5, the values were 73.9%, 80%, and 75.9% without DWI and 87.9%, 80%, and 85.3% with DWI, respectively. The Youden index value of this LI-RADS classification with DWI (0.679) was higher than that without DWI (0.539). For LR-4/5/M, the values were 75.8%, 58.8%, and 70.2% without DWI and 87.9%, 58.8%, and 78.4% with DWI, respectively. The Youden index value of this LI-RADS classification with DWI (0.467) was higher than that without DWI (0.346). For LR- 4/5/TIV, the values were 75.8%, 75%, and 75.5% without DWI and 89.7%, 75%, and 84.9% with DWI, respectively. The Youden index value of this LI-RADS classification with DWI (0.647) was higher than that without DWI (0.508). Detailed information about the diagnostic efficiency is summarized in Table 3.
| Group | DWI | Sensitivity (100%) | Specificity (100%) | Accuracy (100%) | PPV (100%) | NPV (100%) | Youden index |
| LR-5 | A-DWI | 61.2 (101/165) | 92.5 (74/80) | 71.4 (175/245) | 94.4 (101/107) | 53.6 (74/138) | 0.537 |
| P-DWI | 61.2 (101/165) | 92.5 (74/80) | 71.4 (175/245) | 94.4 (101/107) | 53.6 (74/138) | 0.537 | |
| LR-4/5 | A-DWI | 73.9 (122/165) | 80 (64/80) | 75.9 (186/245) | 88.4(122/138) | 59.8 (64/107) | 0.539 |
| P-DWI | 87.9 (145/165) | 80 (64/80) | 85.3 (209/245) | 90.1 (145/161) | 76.2 (64/84) | 0.679 | |
| LR-4/5/M | A-DWI | 75.8 (125/165) | 58.8 (47/80) | 70.2 (172/245) | 79.1 (125/158) | 54 (47/87) | 0.346 |
| P-DWI | 87.9 (145/165) | 58.8 (47/80) | 78.4 (192/245) | 81.5 (145/178) | 70.1 (47/67) | 0.467 | |
| LR-4/5/TIV | A-DWI | 75.8 (125/165) | 75 (60/80) | 75.5 (185/245) | 86.2 (125/145) | 60 (60/100) | 0.508 |
| P-DWI | 89.7 (148/165) | 75 (60/80) | 84.9 (208/245) | 88.1 (148/168) | 77.9 (60/77) | 0.647 |
The results of this study demonstrated that the use of LI-RADS v2017 on gadoxetic acid-enhanced MR can provide high diagnostic efficacy for HCC. Furthermore, when DWI and MR images were jointly viewed for LI-RADS classification, the diagnostic AC was significantly increased. Thus, using LI-RADS v2017 with gadoxetic acid-enhanced MR combined with DWI may result in a more accurate diagnosis of HCC.
Several studies have compared the interobserver agreement of liver nodule classification based on LI-RADS. Liu et al[20] showed that the interobserver agreement was 0.44, and another study showed that the observer consistency was 0.748[21]. The interobserver agreement in our study was higher than that in previous studies, which might be explained by the application of the hepatobiliary phase (HBP) on gadoxetic acid-enhanced MRI. As Gd-EOB-DTPA is a liver-specific contrast agent with a unique EOB group, it can be specifically taken up by normal hepatocytes (approximately 50% uptake rate), thereby producing an enhancing effect in liver cells after Gd-EOB-DTPA administration[22]. However, dysfunctional liver cells cannot take up special liver contrast agents. Therefore, this imaging modality could provide useful information to distinguish abnormal hepatocytes (including HCC) from normal hepatocytes. In addition, the interobserver agreement of LI-RADS categorization was increased when DWI and MR were jointly viewed for the classification. The combination of Gd-EOB-DTPA-enhanced MRI and the DWI sequence can significantly improve both the diagnostic AC and SP for chronic liver disease-associated HCC[23]. This finding might be explained by the ability of DWI to reflect the cellularity of tissue. Compared with normal tissue, tumor tissue with high cellularity could result in decreased extracellular space and limited water diffusion, represented by high signal intensity. In our study, a small number of lesions were classified as LR-3 without DWI; once DWI was added, these lesions were downgraded to LR-2 due to unrestricted diffusion. In addition, some lesions previously classified as LR-3 were upgraded to LR-4 due to restricted diffusion. Compared with the final pathological results, lesions degraded to LR-2 included atypical hemangioma and hepatic angiomyolipoma, and lesions upgraded to LR-4 were HCCs.
Our study shows that LI-RADS v2017 on gadoxetic acid-enhanced MR and DWI can improve the diagnostic efficiency for the evaluation of patients at risk for HCC. When considering only lesions classified as LR-5, the diagnostic efficiency of HCC did not change with or without DWI. In LI-RADS v2017, DWI is an ancillary feature that can be applied for category adjustment but cannot be used to upgrade to LR-5. In addition, observations classified as LR-5 in our study strongly suggested HCCs based on major features; accordingly, no observations were downgraded to LR-4. For LR-4/5, LR-4/5/TIV, and LR-4/5/M, all diagnostic efficiencies were improved when DWI was added (LR-4/5: 0.539 vs 0.679; LR-4/5/M: 0.346 vs 0.467; and LR-4/5/TIV: 0.508 vs 0.647). Although DWI is an ancillary feature, we could upgrade some LR-3 observations to LR-4, and many observations were confirmed as HCCs. Therefore, the diagnostic efficiency was improved. Our results were consistent with those of Kim et al[23] who reported similar diagnostic efficacy of LI-RADS on gadoxetate-enhanced MRI. However, compared with previous studies, the advantage of this study was the application of LI-RADS v2017, which offered more consummate categorization.
In our study, one of 21/24 (without and with DWI, respectively) category 2 lesions was ultimately diagnosed as HCC (well-differentiated). Some previous studies have also confirmed a few LR-2 lesions[20,24] as HCCs. For LR-3, 36 out of 49 lesions were HCCs without DWI; however, 23 out of the 49 LR-3 lesions mentioned above were reclassified as LR-2 (n = 3) with hypointensity and LR-4 (n = 20) with hyperintensity on DWI. Moreover, the final pathological outcomes confirmed the 20 reclassified LR-4 lesions as HCCs and the remaining 3 LR-2 lesions as benign. In addition, 3 LR-M lesions were reclassified as LR-4 due to DWI characteristics, and all of these lesions were confirmed as HCCs. Therefore, the application of DWI readjusted the categorization and enhanced the diagnostic efficiency for HCC. For LR-1, LR-5, and LR-TIV, the categorization was the same with or without DWI because the imaging signs for these LI-RADS classifications were sufficient to make accurate diagnoses. Some non-HCC malignancies in our study were categorized as LR-3, LR-4, or LR-5, demonstrating the difficulty of obtaining a perfectly specific diagnosis of HCC using LI-RADS v2017.
However, our study had several limitations. First, a selection bias may have been present due to the single-center, retrospective design. Thus, validating these results with studies in other centers with a prospective design is necessary. Second, a major feature, the growth threshold, was not investigated because of patients with doubtful liver malignancy who underwent liver surgery without a long-term follow-up. Thus, we lacked patients to meet the criterion. Third, definitely or probably benign observations were reported at the observers’ discretion; hence, the numbers of LR-1 and LR-2 observations were lower than the actual numbers of benign lesions, which may decrease the diagnostic AC for true negative patients. Fourth, the latest version of LI-RADS (v2018) was not applied to these patients. However, we believe that our data, a pool of categorization results by several readers during actual MRI interpretation, can better reflect clinical practice and may be broadly applied.
In conclusion, LI-RADS v2017 has been successfully applied with gadoxetate-enhanced MRI for patients at high risk for HCC. The addition of DWI significantly increases the diagnostic efficiency for HCC. However, these results need to be validated with studies in other centers in a prospective form.
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the third leading cause of cancer-related deaths. The Liver Imaging Reporting and Data System (LI-RADS), supported by the American College of Radiology (ACR), has been developed for standardizing the acquisition, interpretation, reporting, and data collection of liver imaging examinations in patients at risk for HCC. Ancillary features can be applied to upgrade or downgrade the initially assigned LI-RADS category based on major features only.
Magnetic resonance imaging (MRI) can be used for categorization of liver observations and diagnosis of HCC based on the major and ancillary features of LI-RADS. Gadoxetic-acid disodium (Gd-EOB-DTPA), a hepatobiliary contrast agent, could provide information of hepatocyte function. Diffusion-weighted imaging (DWI) can further quantitatively measure tissue diffusion and further reflect tumor cellularity. Thus, the combination of Gd-EOB-DTPA-enhanced MRI and DWI has the potential to improve the diagnostic accuracy (AC) for HCC.
In this study, we aimed to determine the usefulness of DWI in improving the diagnostic AC of LI-RADS v2017 classification. In addition, future research should focus on the comparison of LI-RADS v2017 and v2018.
In this institutional review board-approved study, a total of 414 consecutive patients at high risk for HCC were enrolled. Two radiologists who were blinded to the clinical, laboratory, and pathology results reinterpreted the MR images. Each reader measured the maximum diameter and recorded the presence of each lesion and assigned scores according to LI-RADS v2017. The ancillary feature “restricted diffusion” on DWI images could be used at radiologist discretion for category adjustment (upgrade or downgrade). The kappa test was used to determine the agreement between the two independent radiologists in each item. In addition, the sensitivity (SE), specificity (SP), AC, positive predictive value (PPV), and negative predictive value (NPV) were calculated for assessing the diagnostic performance of LI-RADS. Youden index values were used to compare the diagnostic performance of LI-RADS with or without DWI.
For LR-5, the diagnostic SE, SP, and AC values were 61.2%, 92.5%, and 71.4%, respectively, with or without DWI; for LR-4/5, they were 73.9%, 80%, and 75.9% without DWI and 87.9%, 80%, and 85.3% with DWI; for LR-4/5/M, they were 75.8%, 58.8%, and 70.2% without DWI and 87.9%, 58.8%, and 78.4% with DWI; for LR- 4/5/TIV, they were 75.8%, 75%, and 75.5% without DWI and 89.7%, 75%, and 84.9% with DWI. The Youden index values of the LI-RADS classification without or with DWI were as follows: LR-4/5: 0.539 vs 0.679; LR-4/5/M: 0.346 vs 0.467; and LR-4/5/TIV: 0.508 vs 0.647. The remaining problems that exist should be solved by using prospective, multi-center study to verify our results.
The ancillary feature “restricted diffusion” on DWI images could be used at radiologist discretion for category adjustment (upgrade or downgrade). The ability of DWI is to reflect the cellularity of tissue. Compared with normal tissue, tumor tissue with high cellularity could result in decreased extracellular space and limited water diffusion, represented by high signal intensity. In our study, a small number of lesions were classified as LR-3 without DWI; however, when DWI was added, these lesions were downgraded to LR-2 due to unrestricted diffusion. In addition, some lesions previously classified as LR-3 were upgraded to LR-4 due to restricted diffusion. Thus, our study also shows that the use of DWI can improve the diagnostic efficiency of LI-RADS v2017 with gadoxetic acid-enhanced MRI for HCC. We believe that our data, a pool of categorization results by several readers during actual MRI interpretation, can better be explained by clinical practice and may be broadly applied.
Our study shows that LI-RADS v2017 has been successfully applied with gadoxetate-enhanced MRI for patients at high risk for HCC. The addition of DWI significantly increased the diagnostic efficiency for HCC. For the future research, we intend to investigate interobserver or intraobserver variability through a multi-center study and apply the latest 2018 version of LI-RADS.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Boteon YL, Cerwenka H, Méndez-Sánchez N S- Editor: Ma RY L- Editor: Wang TQ E- Editor: Huang Y
| 1. | Komatsu N, Motosugi U, Maekawa S, Shindo K, Sakamoto M, Sato M, Tatsumi A, Miura M, Amemiya F, Nakayama Y, Inoue T, Fukasawa M, Uetake T, Ohtaka M, Sato T, Asahina Y, Kurosaki M, Izumi N, Ichikawa T, Araki T, Enomoto N. Hepatocellular carcinoma risk assessment using gadoxetic acid-enhanced hepatocyte phase magnetic resonance imaging. Hepatol Res. 2014;44:1339-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 2. | Reataza M, Imagawa DK. Advances in managing hepatocellular carcinoma. Front Med. 2014;8:175-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Roth CG, Mitchell DG. Hepatocellular carcinoma and other hepatic malignancies: MR imaging. Radiol Clin North Am. 2014;52:683-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Potretzke TA, Tan BR, Doyle MB, Brunt EM, Heiken JP, Fowler KJ. Imaging Features of Biphenotypic Primary Liver Carcinoma (Hepatocholangiocarcinoma) and the Potential to Mimic Hepatocellular Carcinoma: LI-RADS Analysis of CT and MRI Features in 61 Cases. AJR Am J Roentgenol. 2016;207:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 5. | Elsayes KM, Hooker JC, Agrons MM, Kielar AZ, Tang A, Fowler KJ, Chernyak V, Bashir MR, Kono Y, Do RK, Mitchell DG, Kamaya A, Hecht EM, Sirlin CB. 2017 Version of LI-RADS for CT and MR Imaging: An Update. Radiographics. 2017;37:1994-2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 179] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 6. | Chernyak V, Fowler KJ, Kamaya A, Kielar AZ, Elsayes KM, Bashir MR, Kono Y, Do RK, Mitchell DG, Singal AG, Tang A, Sirlin CB. Liver Imaging Reporting and Data System (LI-RADS) Version 2018: Imaging of Hepatocellular Carcinoma in At-Risk Patients. Radiology. 2018;289:816-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 772] [Article Influence: 110.3] [Reference Citation Analysis (0)] |
| 7. | Elsayes KM, Kielar AZ, Elmohr MM, Chernyak V, Masch WR, Furlan A, Marks RM, Cruite I, Fowler KJ, Tang A, Bashir MR, Hecht EM, Kamaya A, Jambhekar K, Kamath A, Arora S, Bijan B, Ash R, Kassam Z, Chaudhry H, McGahan JP, Yacoub JH, McInnes M, Fung AW, Shanbhogue K, Lee J, Deshmukh S, Horvat N, Mitchell DG, Do RKG, Surabhi VR, Szklaruk J, Sirlin CB. White paper of the Society of Abdominal Radiology hepatocellular carcinoma diagnosis disease-focused panel on LI-RADS v2018 for CT and MRI. Abdom Radiol (NY). 2018;43:2625-2642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 8. | Schima W, Heiken J. LI-RADS v2017 for liver nodules: how we read and report. Cancer Imaging. 2018;18:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Chernyak V, Tang A, Flusberg M, Papadatos D, Bijan B, Kono Y, Santillan C. LI-RADS® ancillary features on CT and MRI. Abdom Radiol (NY). 2018;43:82-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 10. | Narsinh KH, Cui J, Papadatos D, Sirlin CB, Santillan CS. Hepatocarcinogenesis and LI-RADS. Abdom Radiol (NY). 2018;43:158-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Santillan C, Chernyak V, Sirlin C. LI-RADS categories: concepts, definitions, and criteria. Abdom Radiol (NY). 2018;43:101-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 12. | Kim Y, Furlan A, Borhani AA, Bae KT. Computer-aided diagnosis program for classifying the risk of hepatocellular carcinoma on MR images following liver imaging reporting and data system (LI-RADS). J Magn Reson Imaging. 2018;47:710-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Schwope RB, May LA, Reiter MJ, Lisanti CJ, Margolis DJ. Gadoxetic acid: pearls and pitfalls. Abdom Imaging. 2015;40:2012-2029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Lim S, Kim YK, Park HJ, Lee WJ, Choi D, Park MJ. Infiltrative hepatocellular carcinoma on gadoxetic acid-enhanced and diffusion-weighted MRI at 3.0T. J Magn Reson Imaging. 2014;39:1238-1245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Tsurusaki M, Sofue K, Isoda H, Okada M, Kitajima K, Murakami T. Comparison of gadoxetic acid-enhanced magnetic resonance imaging and contrast-enhanced computed tomography with histopathological examinations for the identification of hepatocellular carcinoma: a multicenter phase III study. J Gastroenterol. 2016;51:71-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Chavhan GB, Alsabban Z, Babyn PS. Diffusion-weighted imaging in pediatric body MR imaging: principles, technique, and emerging applications. Radiographics. 2014;34:E73-E88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 17. | Zhu SC, Liu YH, Wei Y, Li LL, Dou SW, Sun TY, Shi DP. Intravoxel incoherent motion diffusion-weighted magnetic resonance imaging for predicting histological grade of hepatocellular carcinoma: Comparison with conventional diffusion-weighted imaging. World J Gastroenterol. 2018;24:929-940. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 18. | Mannelli L, Nougaret S, Vargas HA, Do RK. Advances in diffusion-weighted imaging. Radiol Clin North Am. 2015;53:569-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | Zidan M, Thomas RL, Slovis TL. What you need to know about statistics, part II: reliability of diagnostic and screening tests. Pediatr Radiol. 2015;45:317-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Liu W, Qin J, Guo R, Xie S, Jiang H, Wang X, Kang Z, Wang J, Shan H. Accuracy of the diagnostic evaluation of hepatocellular carcinoma with LI-RADS. Acta Radiol. 2018;59:140-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 21. | Lee SE, An C, Hwang SH, Choi JY, Han K, Kim MJ. Extracellular contrast agent-enhanced MRI: 15-min delayed phase may improve the diagnostic performance for hepatocellular carcinoma in patients with chronic liver disease. Eur Radiol. 2018;28:1551-1559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Wu JW, Yu YC, Qu XL, Zhang Y, Gao H. Optimization of hepatobiliary phase delay time of Gd-EOB-DTPA-enhanced magnetic resonance imaging for identification of hepatocellular carcinoma in patients with cirrhosis of different degrees of severity. World J Gastroenterol. 2018;24:415-423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Li X, Li C, Wang R, Ren J, Yang J, Zhang Y. Combined Application of Gadoxetic Acid Disodium-Enhanced Magnetic Resonance Imaging (MRI) and Diffusion-Weighted Imaging (DWI) in the Diagnosis of Chronic Liver Disease-Induced Hepatocellular Carcinoma: A Meta-Analysis. PLoS One. 2015;10:e0144247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Kim YY, An C, Kim S, Kim MJ. Diagnostic accuracy of prospective application of the Liver Imaging Reporting and Data System (LI-RADS) in gadoxetate-enhanced MRI. Eur Radiol. 2018;28:2038-2046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |