Published online Nov 28, 2019. doi: 10.3748/wjg.v25.i44.6551
Peer-review started: September 6, 2019
First decision: October 14, 2019
Revised: November 8, 2019
Accepted: November 13, 2019
Article in press: November 13, 2019
Published online: November 28, 2019
Processing time: 83 Days and 6.7 Hours
Regimens involving direct-acting antiviral agents (DAAs) are recommended for the treatment of infection with hepatitis C virus (HCV) genotypes 1, 2 and 3. But real-world data is still not enough, especially in Asia.
To investigate the efficacy and safety of DAA-based regimens in a real-life setting in China.
This study included 366 patients infected with HCV genotypes 1, 2 and 3, with or without cirrhosis, who were observed between May 2015 and December 2018. They were treated with ledipasvir and sofosbuvir (SOF) (genotype 1) with or without ribavirin (RBV), SOF and RBV (genotype 2), or SOF and daclatasvir (genotype 3), with or without RBV, for 12 or more wk. The participants’ sustained virological responses (SVR) at post-treatment week 12 (SVR12) was the primary endpoint. The occurrence of adverse events and drug-drug interactions were recorded.
In the 366 patients, genotype 1 (59.0%) was the most common genotype, followed by genotypes 2 (34.4%) and 3 (6.6%). Liver cirrhosis was diagnosed in 154 (42.1%) patients. Fifty (13.7%) patients were treatment-experienced. Intention-to-treat analysis revealed that SVR12 was 86.3% (316/366). For modified intention-to-treat analysis, SVR12 was achieved in 96.6% of overall patients (316/327), 96.3% in patients with genotype 1, 97.5% in those with genotype 2, and 95.0% in those with genotype 3. Most of the treatment failures were due to lack of follow-up (3 cases had non-responses, 1 had virological breakthrough, 11 relapsed and 36 did not participate in the follow-up). There was no significant difference in SVR between different genotypes and liver statuses (P < 0.05). Patients with lower alanine aminotransferase levels at baseline who achieved an end of treatment response were more likely to achieve SVR12 (P < 0.05). High SVR was observed regardless of age, gender, liver status, alpha-fetoprotein, HCV RNA levels or history of antiviral therapy (P > 0.05 for all). The cumulative hepatocellular carcinoma occurrence and recurrence rate after using the DAAs was 0.9%. Most of the adverse events were mild. We found two cases of special adverse events. One case involved facial and bilateral lower extremity edema, and the other case showed an interesting change in lipid levels while on medication. No severe adverse events were noted.
The DAA-based regimens tested in this study have excellent effectiveness and safety in all patients infected with HCV genotypes 1, 2 and 3, including those with cirrhosis.
Core tip: Direct-acting antiviral agent (DAA)-based regimens are currently the preferred treatment for hepatitis C. However, there is not enough data reporting the results of real-world research, especially in countries such as China where DAAs have only been approved for using in recent years. We found that there was no significant difference in sustained virological responses (SVR) between patients with different genotypes and liver statuses. Patients with lower alanine aminotransferase levels at baseline who achieved end of treatment response were more likely to achieve SVR at post-treatment week 12. Also, we found two cases of special adverse events. One case involved facial and bilateral lower extremity edema, which was due to drug-drug interactions, and the other case showed an interesting change in lipid levels while the patient was on medication.
- Citation: Yang Y, Wu FP, Wang WJ, Shi JJ, Li YP, Zhang X, Dang SS. Real life efficacy and safety of direct-acting antiviral therapy for treatment of patients infected with hepatitis C virus genotypes 1, 2 and 3 in northwest China. World J Gastroenterol 2019; 25(44): 6551-6560
- URL: https://www.wjgnet.com/1007-9327/full/v25/i44/6551.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i44.6551
Hepatitis C virus (HCV) has infected approximately 3% of the world's population[1-3], and 70% to 80% of patients have chronic hepatitis. The leading cause of liver cirrhosis and hepatocellular carcinoma (HCC) is hepatitis. No effective vaccine to prevent hepatitis C is currently available[4-6]. Thus, the treatment of hepatitis C is particularly important. There are several HCV genotypes, including 1a, 1b, 1c, 2a, 2b, 2c, 3a, 3b, 4, 5 and 6, which correspond to the number of HCV gene sequences, of which genotypes 1, 2 and 3 are the most common types[7-10]. Clinical care has advanced for patients with HCV-related liver disease in recent years. But, in some countries, such as China, the standard care has been a combination therapy using pegylated interferon and ribavirin (RBV). However, this treatment has shown a low rate of effectiveness. Due to severe adverse reactions and harsh indications, many patients cannot tolerate this treatment. Since the advent of direct-acting antiviral drugs (DAAs) in 2011, the landscape of HCV treatment has changed remarkably. These oral combinations make it possible to achieve exceptional cure rates with better tolerability, minimal side effects and a shorter duration of treatment.
Among the NS5B polymerase nucleoside inhibitors, sofosbuvir (SOF) is a potent pan-genotypic inhibitor. Many clinical trials based on SOF have shown high rates of sustained virological response (SVR). A phase 3, randomized, open-label study involving patients infected with HCV genotype 1 with or without cirrhosis achieved SVR rates between 94% and 99% with SOF and ledipasvir[11]. Among patients infected with HCV genotype 2, the VALENCE trial reported that therapy with SOF-RBV for 12 wk resulted in a high rate of SVR (93%)[12]. One year later, another phase III trial, ASTRAL-3, reported a higher rate of SVR (99%) using a regimen of SOF-velpatasvir (VEL)[13]. In patients infected with genotype 3, the ALLY-3 trial showed that patients treated with a regimen of daclatasvir and SOF achieved SVR rates of 63% and 96%, with and without cirrhosis, respectively[14].
Although high rates of SVR have been shown, results derived from controlled settings may not be representative to the real world. Also, data gathered from different races is insufficient. Therefore, evaluations of drugs in real-world settings are critical, especially in patients such as those from northwest China, where clinical trial data is limited.
Our study was a single center, prospective, observational study. Prospective patients were identified by a search of the clinical records at the Department of Infectious Diseases of the Second Affiliated Hospital of Xi'an Jiaotong University, in order to evaluate the treatment outcomes in a real-world cohort of patients infected with HCV genotypes 1, 2 and 3 who were evaluated between May 2015 and December 2018.
All the patients who had been diagnosed as having a chronic HCV infection (positive anti-HCV antibody for greater than six months with detectable HCV RNA), with or without cirrhosis, including those who were treatment naïve or had previously been exposed to interferon or direct-acting antiviral therapy, were included in our study. Patients who had co-infections with hepatitis B virus (HBV) were also included. Patients meeting the following criteria were excluded: Human immunodeficiency virus co-infection, pregnant and lactating female patients, male patients with fertility willingness, patients under the age of 18, those with chronic kidney disease, and those with advanced liver disease (Child-Pugh C). All the patients clearly understood the potential benefits and risks and were willing to receive antiviral treatment. All the patients voluntarily joined this study after giving informed consent.
The treatment regimens used are in accordance with the European Association of Study of the Liver guidelines (2015) and are summarized in Table 1. Before the treatment, the patients were informed about the potential drug-drug interactions (DDIs) and were advised to consult pharmacists before starting any new treatment.
| Patients | Genotype 1 | Genotype 2 | Genotype 3 | |
| Chronic hepatitis | Treatment naïve | SOF + LDV for 12 wk | SOF + RBV for 12 wk | SOF + DCV for 12 wk |
| Treatment experienced | SOF + RBV for 16-20 wk | SOF + DCV + RBV for 12 wk or 24 wk without RBV | ||
| Cirrhosis, compensated or decompensated | SOF + LDV + RBV for 12 wk or 24 wk without RBV | SOF + RBV for 16-20 wk | SOF + DCV + RBV for 24 wk | |
Each patient’s detailed medical history was recorded. Clinical examinations were performed on all patients. Baseline laboratory values were collected in all patients within 3 mo before starting treatment. The following data were recorded at the time of enrollment: Age, gender, fibrosis stage, history of treatment, anti-HCV antibody (ELISCAN HCV; RFCL Limited, Dehradun, India), genotype (Abbott Molecular Inc., Des Plaines, IL, United States), and any comorbidities, such as diabetes, HBV co-infection, hypertension and fatty liver disease. The baseline clinical chemistry results are shown in Table 2. HCV RNA was analyzed using COBAS TaqMan HCV Test 2.0 (Roche Diagnostics Corporation, Indianapolis, IN, United States). FibroScan transabdominal ultrasound and abdominal computerized tomography were also performed on the patients. Liver stiffness value > 12.5 kPa (FibroScan®, Echosens, France), clinical manifestations such as esophageal varices and ascites or distinct sonographic signs of portal hypertension indicated cirrhosis.
| Total (n = 366) | Genotype 1 (n = 216) | Genotype 2 (n = 126) | Genotype 3 (n = 24) | |
| Age (yr) | 52.2 ± 12.0 | 51.7 ± 12.8 | 53.6 ± 10.0 | 49.8 ± 14.1 |
| Gender (males, %) | 174 (47.5) | 101 (46.8) | 62 (49.2) | 11 (45.8) |
| TBIL (µmol/L) | 19.63 ± 10.60 | 19.62 ± 10.77 | 19.52 ± 10.72 | 20.07 ± 8.47 |
| ALT (U/L) | 53.48 ± 45.18 | 56.95 ± 47.79 | 51.55 ± 40.35 | 54.33 ± 36.91 |
| AST (U/L) | 51.89 ± 39.12 | 50.79 ± 41.96 | 48.9 ± 33.74 | 53.5 ± 33.31 |
| Albumin (g/L) | 36.6 ± 8.6 | 38.9 ± 8.6 | 41.2 ± 8.5 | 36.6 ± 8.6 |
| TCHO (mmol/L) | 3.85 ± 0.60 | 3.81 ± 0.58 | 3.85 ± 0.66 | 4.14 ± 0.41 |
| Hemoglobin (mg/dL) | 120.3 ± 23.6 | 120.4 ± 23.3 | 121.2 ± 23.2 | 114.8 ± 27.9 |
| Platelets (109/L) | 147.7 ± 75.8 | 144.5 ± 74.8 | 155.7 ± 77.8 | 134.3 ± 73.6 |
| AFP (ng/mL) | 4.69 ± 5.79 | 5.01 ± 7.33 | 4.30 ± 2.02 | 3.81 ± 2.27 |
| Treatment experienced, n (%) | 50 (13.7) | 26 (12.0) | 20 (15.9) | 4 (16.7) |
| Cirrhosis, n (%) | 154 (42.1) | 96 (43.5) | 46 (36.5) | 12 (50) |
| HBV/HCV co-infection, n (%) | 25 (6.8) | 12 (5.6) | 9 (7.1) | 4 (16.7) |
| Diabetes mellitus, n (%) | 17 (4.6) | 12 (5.6) | 4 (3.2) | 1 (4.2) |
| Hypertension, n (%) | 24 (6.6) | 16 (7.4) | 8 (6.3) | 0 (0) |
| Fatty liver disease, n (%) | 52 (14.2) | 31 (14.4) | 16 (12.7) | 5 (20.8) |
This study was approved by the Institutional Review Board of Xi’an Jiaotong University.
HCV RNA was monitored at 4 wk [rapid virological response (RVR)], the end of therapy [end of treatment response (ETR)] and 12 wk after the treatment (SVR12). Viral relapse was defined as detectable HCV RNA after treatment. Non-response was defined as failure to achieve a 1 log 10 reduction in HCV RNA after 12 wk of treatment. Viral breakthrough was defined as detectable HCV RNA after a period of initial response while still on therapy.
All the patients were assessed for adverse reactions, including severe fatigue, depression, insomnia, skin reactions, and dyspnea. Adverse hematological reactions included neutropenia and anemia. If hypocytosis occurred, routine bloodwork would be examined more frequently. When hemoglobin was < 100 g/L or ≥ 85 g/L with no significant cardiovascular disease, the following steps were taken: During the subsequent 4 wk of treatment, if hemoglobin decreased by ≥ 20 g/L, RBV was reduced to 600 mg/d (200 mg in the morning and 400 mg at night), and if hemoglobin decreased to 85 g/L or remained below 120 g/L after 4 wk of RBV reduction, RBV was discontinued.
The statistical methods of this study were reviewed by Lei-Lei Pei from Institute of Public Health Xi’an Jiaotong University.
For continuous variables, the outcome is expressed as the mean ± standard deviation or as median and range. It was compared using the Kruskal-Wallis H test or the Mann-Whitney U test. For categorical data, the outcome is presented as percentage, and the differences were tested using the χ2 test. Logistic regression was used to analyze which variables had a statistical impact on SVR. SVR12 was evaluated by an intention-to-treat (ITT) analysis, which was based on the initial treatment assignment, including all patients who received at least one dose of the medicine. SVR12 was also analyzed by a modified ITT (mITT) analysis. The significance level was set at P < 2009;0.05. All analyses were performed using SPSS 25.0 software.
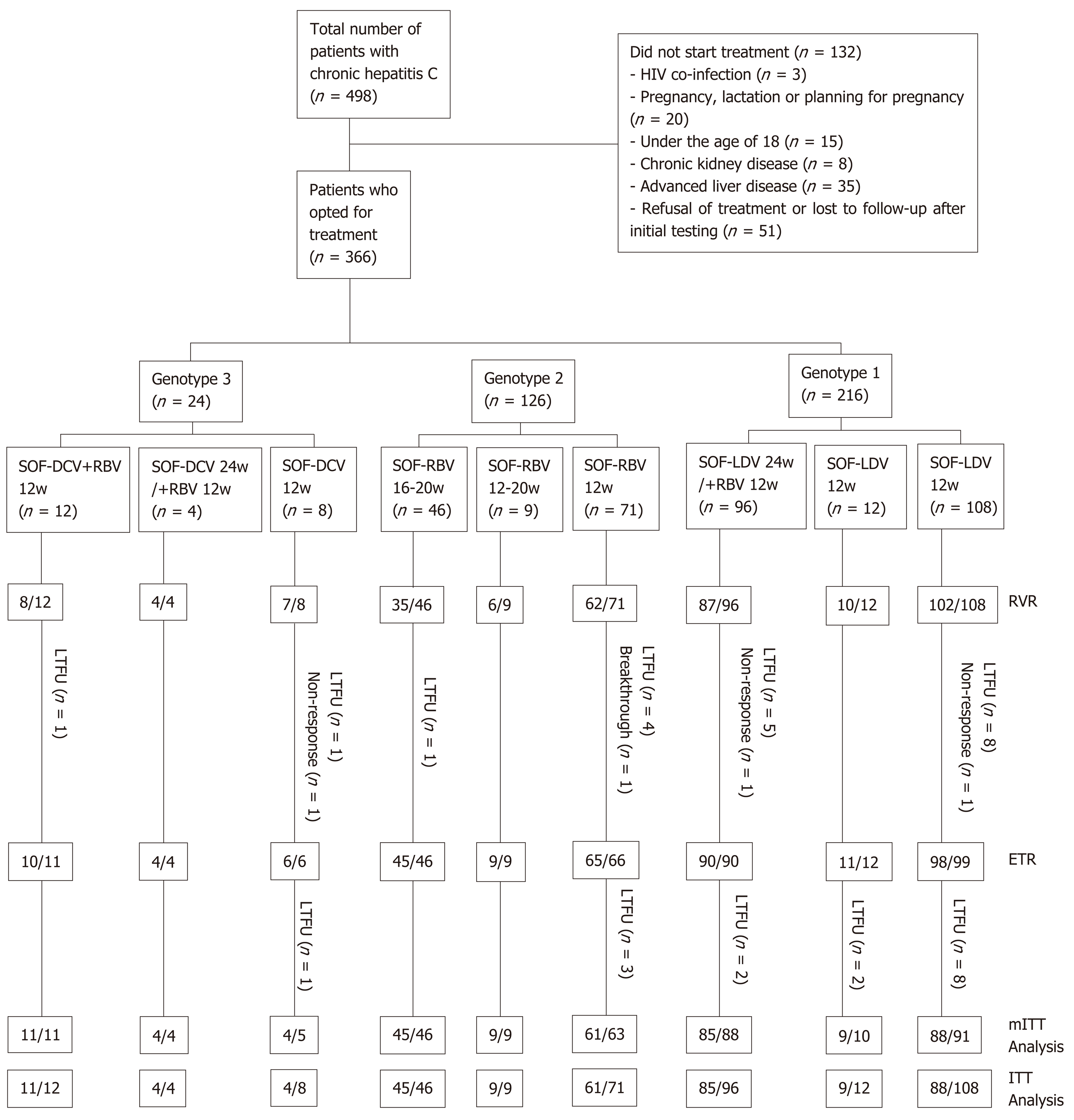
Between May 2015 and December 2018, a total of 498 patients were diagnosed with HCV infection, and 366 of them commenced treatment with DAAs (Figure 1). There were 216 patients with genotype 1 (1a, 10.2%; 1b, 68.5%; subtype not specified, 21.3%), 108 with genotype 2 (2a, 68.3%; 2b, 5.6%; subtype not specified, 26.2%) and 24 with genotype 3 (3a, 25.0%; 3b, 12.5%; subtype not specified, 62.5%). Of the patients, 12 (3.3%) were found to have controlled co-existing HCC. Table 2 shows the baseline characteristics of the patients. The mean age of the patients was 52.2 ± 12.0 years, and 192 (47.5%) were male. Fifty (13.7%) patients had been treated with interferon (IFN) or DAAs before. Some of the patients (154, 42.1%) had cirrhosis. Most of the cirrhotic patients (123, 79.9%) had a Child-Pugh score of A, while the rest of them had a Child-Pugh score of B. A total of 25 (6.8%) of the patients had HBV co-infection, 17 (4.6%) had diabetes mellitus, 24 (6.6) had hypertension, and 52 (14.2%) had fatty liver disease.
The baseline clinical, biochemical, hematological and virologic characteristics of the patients based on the severity of their liver disease are shown in Table 3. Patients with cirrhosis (including compensated and decompensated cirrhosis) were older. They had higher levels of total bilirubin, alanine transaminase (ALT), aspartate ami-notransferase (AST), alpha-fetoprotein (AFP), liver stiffness measurement (LSM) score, fibrosis 4 score, and AST-to-platelet ratio index (APRI). Patients with cirrhosis had lower levels of albumin, hemoglobin, platelets, and HCV RNA compared to patients without (P < 0.05 for all).
| Non-cirrhosis (n = 212) | Cirrhosis (compensated and decompensated) (n = 154) | P value | |
| Age (yr) | 48.5 ± 12.6 | 57.3 ± 8.9 | < 0.001 |
| Gender (males, %) | 115 (54.2) | 77 (50.0) | 0.423 |
| TBil (µmol/L) | 15.04 ± 8.78 | 25.95 ± 9.59 | < 0.001 |
| ALT (U/L) | 41.74 ± 43.00 | 64.88 ± 44.81 | 0.029 |
| AST (U/L) | 31.47 ± 26.67 | 68.12 ± 43.44 | 0.034 |
| Albumin (g/L) | 44.5 ± 4.5 | 33.0 ± 8.6 | < 0.001 |
| TCHO (mmol/L) | 3.86 ± 0.55 | 3.82 ± 0.66 | 0.349 |
| Hemoglobin (mg/dL) | 135.3 ± 10.6 | 99.5 ± 20.4 | 0.002 |
| Platelets (109/L) | 195.3 ± 59.8 | 86.1 ± 32.2 | < 0.001 |
| AFP (ng/mL) | 4.04 ± 1.79 | 5.58 ± 8.61 | 0.045 |
| Log10 HCV RNA (IU/mL) | 6.6 ± 0.8 | 6.3 ± 1.3 | 0.040 |
| LSM (kPa) | 5.71 ± 1.7 | 10.26 ± 5.1 | < 0.001 |
| Fibrosis 4 score | 1.43 ± 1.0 | 7.49 ± 6.0 | < 0.001 |
| APRI | 1.06 ± 0.9 | 2.3 ± 1.5 | < 0.001 |
In our study, RVR was achieved in 321 (87.7%) of 366 patients, and ETR was achieved in 338 (98.5%) of 343 patients. ITT analysis showed that 316 (86.3%) patients reached SVR12, accounting for 182 (84.3%) of 216 patients with genotype 1, 115 (91.3%) of 126 with genotype 2, and 19 (79.2%) of 24 with genotype 3 (P = 0.375). SVR (mITT analysis) was 96.3% in patients with genotype 1, 97.5% in those with genotype 2, and 95.0% in those with genotype 3. No statistically significant difference in SVR was found among patients with different genotypes (P = 0.759). In patients without cirrhosis (96.2%), SVR12 rates were similar to those of patients with cirrhosis (97.2%, P = 0.971). Of all the patients, a total of 51 (13.6%) failed to achieve SVR, most of which (n = 36) were due to loss to follow-up. In addition, 3 patients had non-responses, 1 had a viral breakthrough, and 11 relapsed. Patients with genotype 3 had more treatment failures (non-response in 1, and 1 had a relapse). After treatment, the patients’ liver stiffness as determined by Fibroscan decreased by 0.64 ± 1.1, and patients with cirrhosis had an even more decrease (P = 0.023).
In the correlation analysis between the baseline characteristics and SVR (Table 4), we found that patients with lower ALT levels (P = 0.034) were more likely to achieve SVR12. However, there were no significant difference in age, gender, bilirubin, AST, albumin, cholesterol, hemoglobin, platelets, AFP, liver status, or HCV RNA levels (P > 0.05 for all). Additionally, patients who achieved ETR were more likely to achieve SVR12.
| Non-SVR12 (n = 11) | SVR12 (n = 315) | P value | |
| Age (yr) | 54.1 ± 13.5 | 51.8 ±11.9 | 0.098 |
| Gender (males, %) | 4 (36.4) | 162 (51.4)3 | 0.080 |
| TBil (µmol/L) | 20.16 ± 10.13 | 19.58 ± 10.69 | 0.184 |
| ALT (U/L) | 62.45 ± 53.85 | 52.28 ± 46.14 | 0.034 |
| AST (U/L) | 42.73 ± 30.30 | 47.29 ± 39.74 | 0.516 |
| Albumin (g/L) | 37.7 ± 10.0 | 39.6 ± 8.6 | 0.214 |
| TCHO (mmol/L) | 3.93 ± 0.59 | 3.85 ± 0.60 | 0.879 |
| Hemoglobin (mg/dL) | 119.1 ± 15.0 | 119.5 ± 23.8 | 0.497 |
| Platelets (109/L) | 165.6 ± 68.4 | 144.9 ± 76.0 | 0.167 |
| AFP (ng/mL) | 5.03 ± 4.49 | 4.77 ± 6.15 | 0.407 |
| Log10 HCV RNA (IU/mL) | 6.5 ± 0.6 | 6.5 ± 1.0 | 0.450 |
| Cirrhosis, n (%) | 5 (45.5) | 138 (43.8) | 0.621 |
| Treatment experienced, n (%) | 1 (9.1) | 49 (15.6) | 0.052 |
A total of 114 (31.1%) patients reported that they had at least one adverse event, but no serious adverse reactions (SARs) occurred. Most of the adverse events were mild or moderate, such as fatigue (66, 18.0%), rash (25, 6.8%), anemia (16, 4.4%), and myocardial enzymes abnormality (7, 1.9%), which were mainly due to the use of RBV. The patients with cirrhosis tended to have more adverse reactions (P = 0.027). Twelve wk after the end of treatment, one patient had developed HCC, and two others had a recurrence of HCC. The cumulative HCC occurrence and recurrence rate after DAA treatment was 0.9%. The patient with newly discovered HCC also had cirrhosis and experienced treatment failure. These two cases of recurrence were in patients with cirrhosis and SVR.
We found two cases of special adverse events. In one case, a patient had had benign prostatic hyperplasia in addition to hepatitis C. His HCV viral load was 5.8 log IU/mL, and his genotype was 3b. After 24 h of co-administration with tamsulosin hydrochloride, edema occurred in his face and the patient also had bilateral lower extremity edema. The edema disappeared after he discontinued tamsulosin hydrochloride. We checked medicine specifications of tamsulosin hydrochloride and the DAA regimens but none of them mentioned edema in adverse reactions. So, we believed that his edema was due to DDIs between tamsulosin hydrochloride and DAAs. In the other case, we observed an interesting change in the serum lipids. A middle-aged man on stable methadone maintenance therapy took VEL/SOF for antiviral therapy. Before the new treatment started, his total cholesterol and triglyceride levels were basically normal. After starting antiviral treatment, his triglyceride level rose rapidly, and peaked 8 wk later. His cholesterol increased slowly and then decreased slowly. But 24 h after withdrawing from VEL/SOF, his triglyceride and total cholesterol levels were basically normal. There was no severe adverse reaction during his antiviral therapy. The viral load was still negative at the end of the treatment.
Chronic HCV infection is a global health problem. It can be associated with many several hepatic and extrahepatic disorders. Of patients with chronic infection, many develop cirrhosis and HCC. Before 2011, the standard antiviral therapy was pegylated interferon-alpha and RBV for 24–48 wk[15,16]. After a few years, unremitting research has led to the discovery of several DAAs that target several molecular targets. DAA-based regimens can greatly increase the efficacy and shorten the duration of HCV therapy. Since about 2015, DAAs have had access to the Chinese market, and Chinese patients with hepatitis C have been treated with legal DAA regimens. Since this is such a short amount of time, the real-world data on the use of DAA drugs in China has not been sufficient.
Our study is a large, prospective, real-world study evaluating the efficacy and safety of DAA-based regimens in patients infected with genotype 1, 2, and 3 observed from 2015 to 2018. In this study, a total of 498 patients were diagnosed with HCV infections, but only 366 (73.5%) of them received treatments. Most of the patients had genotypes 1 and 2, which basically reflects the prevalence of genotypes among HCV sufferers in China[17]. We noticed that in our study, patients with genotype 3 were younger than patients with genotypes 1 and 2, and patients with genotype 3 had a higher proportion of HBV/HCV co-infections, though this difference was not statistically significant. We discovered that genotype 3 was highly prevalent among intravenous drug users, who were younger and more likely to be associated with other blood-borne diseases. Also, we found that the patients with cirrhosis were older. Those patients had higher levels of total bilirubin, ALT, AST and AFP, LSM scores, fibrosis 4 scores and APRIs. They also had lower levels of albumin, hemoglobin, platelets and HCV RNA compared to the patients without cirrhosis, which is consistent with our clinical experiences.
Overall, the patients in our study achieved high rates of SVR12 (96.6%) on the mITT analysis, which are roughly identical to those observed in the phase III trials. The SVR rates were consistently high in all patients regardless of the regimens used. No statistically significant difference in SVR was found among patients with different genotypes and with different liver statuses. In addition, patients with cirrhosis (97.2%) responded similarly to those without cirrhosis (96.2%) 12 wk after the end of the treatment. Analysis of the changes of liver stiffness before and after treatment showed that the decrease of liver stiffness was significantly correlated with liver status. In patients with cirrhosis, LSM decreased even more compared to patients with chronic infections. However, it should be noted that we divided our patients into cirrhotic (compensated cirrhosis and decompensated cirrhosis) and non-cirrhotic groups (chronic hepatitis and liver fibrosis), then we made the comparison, which may lead to bias.
Interestingly, by analyzing the treatment failures, we found that the patients with genotype 3 were more likely to relapse. Although the total number is small (24 patients), the proportion of patients experiencing a relapse or no response was much higher than that of patients with the other genotypes. Other researchers have also found that genotype 3 patients had poorer clinical outcomes than patients with the other genotypes[18]. In our study, 12 patients had controlled co-existing HCC at baseline. After DAA antiviral therapy, one patient developed HCC, and two had HCC recurrence. The incidence of HCC was lower than that in the untreated patients[19-21]. The patient with newly discovered HCC had cirrhosis and experienced treatment failure. These two cases of recurrence both had cirrhosis and SVR. These findings indicate that patients with cirrhosis and experience treatment failure are more likely to develop HCC. This is the same result as those obtained in other studies[22-24]. Therefore, when patients also have cirrhosis, especially those patients with treatment failure, we should pay more attention to their tumor markers and conduct regular imageological liver examinations.
Also, we found that the level of ALT at baseline was the only characteristic associated with SVR. Patients who had lower levels of ALT at baseline and achieved ETR were more likely to achieve SVR, which is consistent with other research[25]. We also found that SVR rates were independent of either liver status or baseline HCV viral load. Furthermore, whether patients had received antiviral therapy before had no influence on SVR. Hence, based on these findings, and regardless of liver status, HCV viral load and prior treatment experience, the appropriate DAA-based regimens and an adequate course of treatment can achieve satisfactory curative effects.
The safety of our study was quite satisfactory. Although 31.1% of patients experienced one or more adverse events, no SARs were observed. Also, most of the adverse events were mild or moderate, of which fatigue was the most common adverse event. Patients with liver cirrhosis or who were given RBV in their regimens should receive more attention because they are more likely to have adverse reactions. In our study, patients with complications such as hypertension were told to try to avoid taking drugs that have potential DDIs with DAAs. However, we observed two cases of special adverse events. One was facial and bilateral lower extremity edema, which was due to DDIs and the other was an interesting change in lipid levels while on DAA medication. No similar cases have been reported yet. These adverse events indicate that even now, real-world data on the use of DAA drugs is still needed and meaningful.
This study had a few limitations. It was a single center study and the number of genotype 3 patients was quite small. Some patients used Indian generics for antiviral therapy. The drug availabilities may also lead to bias. However, our study provided data from the real-life experience of using DAAs in northwest China, and confirmed their efficacy and safety, thus providing physicians with the data needed for potential positive treatment outcomes. The long-term efficacy and safety of DAAs need to be studied, but the short-term results are encouraging.
Regimens involving direct-acting antiviral agents (DAAs) are recommended for the treatment of infection with hepatitis C virus (HCV) genotypes 1, 2 and 3. But real-world data is still not enough, especially in Asia.
Although high rates of sustained virological responses (SVR) have been shown in trials, results derived from controlled settings may not be representative to the real world. Drugs may have many unexpected adverse events in clinical applications. Also, data gathered from different races is insufficient. Therefore, evaluations of drugs in real-world settings are critical, especially in patients such as those from northwest China, where clinical trial data is limited.
Our study aimed to investigate the efficacy and safety of DAA-based regimens in a Chinese real-life setting in China and find out whether there are any differences in different genotypes and liver statuses.
This study included 366 patients infected with HCV genotypes 1, 2 and 3, with or without cirrhosis, who were observed between May 2015 and December 2018. They were treated with ledipasvir and sofosbuvir (SOF) (genotype 1) with or without ribavirin (RBV), SOF and RBV (genotype 2), or SOF and daclatasvir (genotype 3), with or without RBV, for 12 or more wk. The participants’ SVR at post-treatment week 12 (SVR12) was the primary endpoint. The occurrence of adverse events and drug-drug interactions were recorded.
In the 366 patients, genotype 1 (59.0%) was the most common genotype, followed by genotypes 2 (34.4%) and 3 (6.6%). Liver cirrhosis was diagnosed in 154 (42.1%) patients. Fifty (13.7%) patients were treatment-experienced. Intention-to-treat analysis revealed that SVR12 was 86.3% (316/366). For modified intention-to-treat analysis, SVR12 was achieved in 96.6% of overall patients (316/327), 96.3% in patients with genotype 1, 97.5% in those with genotype 2, and 95.0% in those with genotype 3. Most of the treatment failures were due to lack of follow-up (3 cases had non-responses, 1 had virological breakthrough, 11 relapsed and 36 did not participate in the follow-up). There was no significant difference in SVR between different genotypes and liver statuses (P < 0.05). Patients with lower alanine aminotransferase levels at baseline who achieved an end of treatment response were more likely to achieve SVR12 (P < 0.05). High SVR was observed regardless of age, gender, liver status, alpha-fetoprotein, HCV RNA levels or history of antiviral therapy (P > 0.05 for all). The cumulative hepatocellular carcinoma occurrence and recurrence rate after using the DAAs was 0.9%. Most of the adverse events were mild. We found two cases of special adverse events. One case involved facial and bilateral lower extremity edema, and the other case showed an interesting change in lipid levels while on medication. No severe adverse events were noted.
The DAA-based regimens tested in this study have excellent effectiveness and safety in all patients infected with HCV genotypes 1, 2 and 3, including those with cirrhosis. In clinical applications, there are indeed unexpected adverse events.
The antiviral effect of DAAs is quite satisfying. However, in the clinic, many adverse events may occur due to combination with other medications and individual variation. Strict monitoring is required for clinical use of DAAs. Besides, the long-term efficacy and safety of DAAs need to be studied.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Johansen S, Kanda T, Koshy A, Rukavina M S-Editor: Tang JZ L-Editor: Wang TQ E-Editor: Zhang YL
| 1. | Spearman CW, Dusheiko GM, Hellard M, Sonderup M. Hepatitis C. Lancet. 2019;394:1451-1466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 284] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 2. | Stepanova M, Younossi ZM. Economic Burden of Hepatitis C Infection. Clin Liver Dis. 2017;21:579-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 3. | Younossi ZM. Hepatitis C Infection: A Systemic Disease. Clin Liver Dis. 2017;21:449-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Ghasemi F, Rostami S, Meshkat Z. Progress in the development of vaccines for hepatitis C virus infection. World J Gastroenterol. 2015;21:11984-12002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Hesamizadeh K, Sharafi H, Rezaee-Zavareh MS, Behnava B, Alavian SM. Next Steps Toward Eradication of Hepatitis C in the Era of Direct Acting Antivirals. Hepat Mon. 2016;16:e37089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 6. | Bukh J. The history of hepatitis C virus (HCV): Basic research reveals unique features in phylogeny, evolution and the viral life cycle with new perspectives for epidemic control. J Hepatol. 2016;65:S2-S21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 178] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 7. | Petruzziello A, Marigliano S, Loquercio G, Cozzolino A, Cacciapuoti C. Global epidemiology of hepatitis C virus infection: An up-date of the distribution and circulation of hepatitis C virus genotypes. World J Gastroenterol. 2016;22:7824-7840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 514] [Cited by in RCA: 568] [Article Influence: 63.1] [Reference Citation Analysis (7)] |
| 8. | D'Ambrosio R, Degasperi E, Colombo M, Aghemo A. Direct-acting antivirals: the endgame for hepatitis C? Curr Opin Virol. 2017;24:31-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 9. | Manns MP, Buti M, Gane E, Pawlotsky JM, Razavi H, Terrault N, Younossi Z. Hepatitis C virus infection. Nat Rev Dis Primers. 2017;3:17006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 342] [Article Influence: 42.8] [Reference Citation Analysis (1)] |
| 10. | Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2:161-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1493] [Cited by in RCA: 1472] [Article Influence: 184.0] [Reference Citation Analysis (0)] |
| 11. | Afdhal N, Reddy KR, Nelson DR, Lawitz E, Gordon SC, Schiff E, Nahass R, Ghalib R, Gitlin N, Herring R, Lalezari J, Younes ZH, Pockros PJ, Di Bisceglie AM, Arora S, Subramanian GM, Zhu Y, Dvory-Sobol H, Yang JC, Pang PS, Symonds WT, McHutchison JG, Muir AJ, Sulkowski M, Kwo P; ION-2 Investigators. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370:1483-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1065] [Cited by in RCA: 1064] [Article Influence: 96.7] [Reference Citation Analysis (0)] |
| 12. | Zeuzem S, Dusheiko GM, Salupere R, Mangia A, Flisiak R, Hyland RH, Illeperuma A, Svarovskaia E, Brainard DM, Symonds WT, Subramanian GM, McHutchison JG, Weiland O, Reesink HW, Ferenci P, Hézode C, Esteban R; VALENCE Investigators. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N Engl J Med. 2014;370:1993-2001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 660] [Cited by in RCA: 638] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 13. | Foster GR, Afdhal N, Roberts SK, Bräu N, Gane EJ, Pianko S, Lawitz E, Thompson A, Shiffman ML, Cooper C, Towner WJ, Conway B, Ruane P, Bourlière M, Asselah T, Berg T, Zeuzem S, Rosenberg W, Agarwal K, Stedman CA, Mo H, Dvory-Sobol H, Han L, Wang J, McNally J, Osinusi A, Brainard DM, McHutchison JG, Mazzotta F, Tran TT, Gordon SC, Patel K, Reau N, Mangia A, Sulkowski M; ASTRAL-2 Investigators; ASTRAL-3 Investigators. Sofosbuvir and Velpatasvir for HCV Genotype 2 and 3 Infection. N Engl J Med. 2015;373:2608-2617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 649] [Article Influence: 64.9] [Reference Citation Analysis (0)] |
| 14. | Nelson DR, Cooper JN, Lalezari JP, Lawitz E, Pockros PJ, Gitlin N, Freilich BF, Younes ZH, Harlan W, Ghalib R, Oguchi G, Thuluvath PJ, Ortiz-Lasanta G, Rabinovitz M, Bernstein D, Bennett M, Hawkins T, Ravendhran N, Sheikh AM, Varunok P, Kowdley KV, Hennicken D, McPhee F, Rana K, Hughes EA; ALLY-3 Study Team. All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology. 2015;61:1127-1135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 504] [Cited by in RCA: 511] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 15. | Carter W, Connelly S, Struble K. Reinventing HCV Treatment: Past and Future Perspectives. J Clin Pharmacol. 2017;57:287-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | González-Grande R, Jiménez-Pérez M, González Arjona C, Mostazo Torres J. New approaches in the treatment of hepatitis C. World J Gastroenterol. 2016;22:1421-1432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 115] [Cited by in RCA: 99] [Article Influence: 11.0] [Reference Citation Analysis (4)] |
| 17. | Duan Z, Jia JD, Hou J, Lou L, Tobias H, Xu XY, Wei L, Zhuang H, Pan CQ. Current challenges and the management of chronic hepatitis C in mainland China. J Clin Gastroenterol. 2014;48:679-686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Huang JF, Huang CF, Yeh ML, Dai CY, Yu ML, Chuang WL. Updates in the management and treatment of HCV genotype 3, what are the remaining challenges? Expert Rev Anti Infect Ther. 2018;16:907-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Dimitroulis D, Damaskos C, Valsami S, Davakis S, Garmpis N, Spartalis E, Athanasiou A, Moris D, Sakellariou S, Kykalos S, Tsourouflis G, Garmpi A, Delladetsima I, Kontzoglou K, Kouraklis G. From diagnosis to treatment of hepatocellular carcinoma: An epidemic problem for both developed and developing world. World J Gastroenterol. 2017;23:5282-5294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 228] [Cited by in RCA: 229] [Article Influence: 28.6] [Reference Citation Analysis (4)] |
| 20. | Meringer H, Shibolet O, Deutsch L. Hepatocellular carcinoma in the post-hepatitis C virus era: Should we change the paradigm? World J Gastroenterol. 2019;25:3929-3940. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Ferrarese A, Germani G, Gambato M, Russo FP, Senzolo M, Zanetto A, Shalaby S, Cillo U, Zanus G, Angeli P, Burra P. Hepatitis C virus related cirrhosis decreased as indication to liver transplantation since the introduction of direct-acting antivirals: A single-center study. World J Gastroenterol. 2018;24:4403-4411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Ioannou GN, Green PK, Berry K. HCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinoma. J Hepatol. 2017;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 376] [Article Influence: 47.0] [Reference Citation Analysis (1)] |
| 23. | ANRS collaborative study group on hepatocellular carcinoma (ANRS CO22 HEPATHER, CO12 CirVir and CO23 CUPILT cohorts). Lack of evidence of an effect of direct-acting antivirals on the recurrence of hepatocellular carcinoma: Data from three ANRS cohorts. J Hepatol. 2016;65:734-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 330] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 24. | Conti F, Buonfiglioli F, Scuteri A, Crespi C, Bolondi L, Caraceni P, Foschi FG, Lenzi M, Mazzella G, Verucchi G, Andreone P, Brillanti S. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J Hepatol. 2016;65:727-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 699] [Article Influence: 77.7] [Reference Citation Analysis (0)] |
| 25. | Kim NJ, Locke CJ, Park H, Magee C, Bacchetti P, Khalili M. Race and Hepatitis C Care Continuum in an Underserved Birth Cohort. J Gen Intern Med. 2019;34:2005-2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |