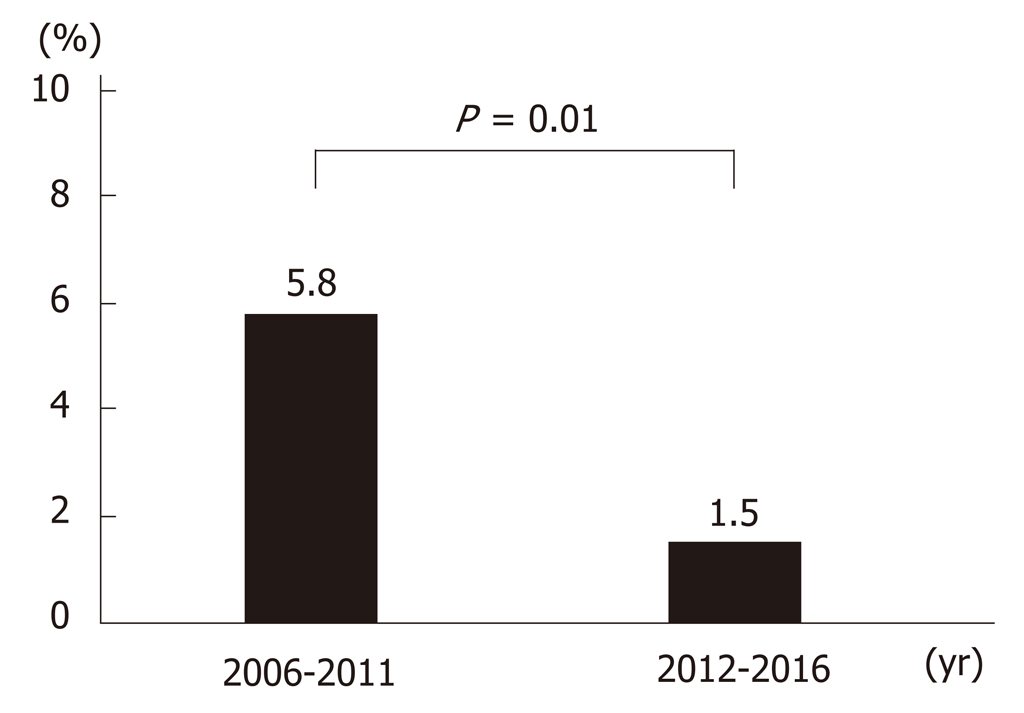Published online Nov 14, 2019. doi: 10.3748/wjg.v25.i42.6342
Peer-review started: May 27, 2019
First decision: July 21, 2019
Revised: September 11, 2019
Accepted: November 1, 2019
Article in press: November 1, 2019
Published online: November 14, 2019
Processing time: 172 Days and 21.8 Hours
The two main causes of gastric ulcer bleeding are Helicobacter pylori (H. pylori) infection and ulcerogenic medicines, although the number of cases caused by each may vary with age. In Japan, the rate of H. pylori infection has fallen over the last decade and the number of prescriptions for non-steroidal anti-inflammatory drugs (NSAIDs) and antithrombotic drugs is increasing as the population ages. Methods of treatment for gastric ulcer bleeding have advanced with the advent of hemostatic forceps and potassium-competitive acid blocker (P-CAB). Thus, causes and treatments for gastric ulcer bleeding have changed over the last decade.
To examine the trends of gastric ulcer bleeding over 10 years in the metropolitan area of Japan.
This is a single-center retrospective study. A total of 564 patients were enrolled from inpatients admitted to our hospital with gastric ulcer bleeding between 2006 and 2016. Age, medication history, H. pylori infection, method of treatment, rate of rebleeding, and the length of hospitalization were analyzed. Factors associated with gastric ulcer bleeding were evaluated using Fisher’s exact test, Pearson’s Chi-squared test or Student’s t-test as appropriate. The Jonckheere-Terpstra test was used to evaluate trends. A per-protocol analysis was used to examine the rate of H. pylori infection.
There was a significant increase in the mean age over time (P < 0.01). The rate of H. pylori infection tended to decrease over the study period (P = 0.10), whereas the proportion of patients taking antithrombotic agents or NSAIDs tended to increase (P = 0.07). Over time, the use of NSAIDs and antithrombotic drugs increased with age. By contrast, the rate of H. pylori infection during the study period fell with age. H. pylori-induced ulcers accounted for the majority of cases in younger patients (< 70 years old); however, the rate decreased with age (P < 0.01). The method of treatment trend has changed significantly over time. The main method of endoscopic hemostasis has changed from clipping and injection to forceps coagulation (P < 0.01), and frequently prescribed medicines have changed from proton pump inhibitor to P-CAB (P < 0.01). The rate of rebleeding during the latter half of the study was significantly lower than that in the first half.
These trends, gastric ulcers caused by ulcerogenic drugs were increasing with age and H. pylori-induced ulcers were more common in younger patients, were observed.
Core tip: The two main causes of gastric ulcer bleeding are Helicobacter pylori (H. pylori) infection and ulcerogenic medicines, although the number of cases caused by each may vary with age. The aim of this study was to examine changes of gastric ulcer bleeding over 10 years. The mean age increased significantly. The rate of H. pylori infection tended to decrease, whereas the proportion of patients taking antithrombotic agents or non-steroidal anti-inflammatory drugs tended to increase. H. pylori-induced ulcers accounted for the majority in younger patients and the proportion of gastric ulcers caused by ulcerogenic drugs was increasing with age.
- Citation: Kubosawa Y, Mori H, Kinoshita S, Nakazato Y, Fujimoto A, Kikuchi M, Nishizawa T, Suzuki M, Suzuki H. Changes of gastric ulcer bleeding in the metropolitan area of Japan. World J Gastroenterol 2019; 25(42): 6342-6353
- URL: https://www.wjgnet.com/1007-9327/full/v25/i42/6342.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i42.6342
The two main causes of gastric ulcer are Helicobacter pylori (H. pylori) infection and non-steroidal anti-inflammatory drugs (NSAIDs)/antiplatelet drugs (mainly low-dose aspirin)[1]. Some reports have shown that the use of both anticoagulants and antiplatelet drugs increases the risk of gastrointestinal bleeding more than antiplatelet drugs alone[2,3]. The rate of H. pylori infection in Japan has fallen over the last decade, from 74.7% (1970s) to 53.0% (1990s) and 35.1% (2010s), mainly due to extensive implementation of H. pylori eradication therapy and improved hygiene[4].
A national census showed that the number of elderly (> 65 years old) and late-stage elderly (> 75 years old) people has increased rapidly over the last 10 years[5]. According to the Japan National Database, most NSAIDs, antiplatelet drugs, and anticoagulants are prescribed to persons over 65 years old[6]. Therefore, the number of prescriptions for NSAIDs and antithrombotic drugs is increasing as the population ages.
Endoscopic methods for treating gastric ulcer bleeding have advanced during the last 10 years[7]. In the past, injection and clipping were the major methods used for endoscopic hemostasis[8,9]. Endoscopic submucosal dissection for early gastric cancer is performed widely in Japan[10]. Accordingly, the use of hemostatic forceps with soft coagulation is now used to stem gastric ulcer bleeding[11]. In addition, a first-in-class potassium-competitive acid blocker (P-CAB), which provides more rapid, stronger, and continuous gastric acid suppression than conventional proton pump inhibitors (PPIs), was introduced to the market in 2015. Thus, the causes of and treatments for gastric ulcer bleeding have changed over the last decade. In this study, we examined the trends in the causes and treatments of gastric ulcer bleeding in the metropolitan area of Japan, and examined the relationship between factors that cause gastric ulcer bleeding.
Data from patients admitted to National Hospital Organization Tokyo Medical Center with a gastric ulcer between 2006 and 2016 were examined retrospectively. Patients were selected from the inpatient database. Gastric cancer patients were excluded. All patients underwent upper gastrointestinal endoscopy during hospitalization. Collected data included age, medication history (NSAIDs, antithrombotics, and antacids), H. pylori infection, method of treatment (endoscopic procedure or medicine), rate of rebleeding, and length of hospital stay. Rebleeding was defined as the need for further endoscopic treatment after the first treatment. H. pylori infection was diagnosed if one of the following tests was positive: The serum H. pylori antibody test, the H. pylori stool antigen test, the urea breath test, the rapid urease test, or histological examination of biopsy tissue.
The main aim was to examine whether there was a change in the trend of causes, treatments, and prognosis of gastric ulcer bleeding over 10 years. The secondary aim was to examine the relationship between factors that cause gastric ulcer bleeding, and whether this was related to age or time. To evaluate risk factors associated with age and time, the study was divided into two time periods: 2006–2011 and 2012–2016. The study protocol was approved by the Committee for Medical Ethics of the National Hospital Organization Tokyo Medical Center (Approval ID No. R18-043; June 4, 2018) and was conducted in accordance with the Declaration of Helsinki (1975) and its later amendment (1983). Written informed consent was not required, and informed consent included an opt-out clause approved by the Medical Ethics Committee. The study was observational and reported in accordance with the Strengthening Reporting of Observational Studies in Epidemiology guidelines[12].
All data are expressed as the mean ± standard deviation. Factors associated with gastric ulcer bleeding were evaluated using Fisher’s exact test, Pearson’s Chi-squared test or Student’s t-test as appropriate. The Jonckheere-Terpstra test was used to evaluate trends. A per-protocol analysis was used to examine the rate of H. pylori infection. A P value < 0.05 was considered statistically significant, and a P value < 0.10 was considered marginally significant. All statistical analyses were performed using SPSS 25 for Windows (SPSS Inc., Chicago, IL, United States).
A total of 586 patients were selected; however, 22 patients found to have gastric cancer by pathology were excluded. The remaining 564 patients (343 men and 221 women; mean age, 70.2 ± 14.3 years) were examined (Table 1). Of these, 455 were tested for H. pylori infection and 74.7% were positive. The mean age of H. pylori-positive patients was lower than that of H. pylori-negative patients (66.6 ± 13.6 vs 72.3 ± 14.3, respectively; P < 0.01). Overall, 38.8% patients took anticoagulants, antiplatelets, or NSAIDs; the mean age of these patients was higher than that of patients who did not take any of these medicines (77.1 ± 10.3 vs 65.8 ± 14.7, respectively; P < 0.01). Significantly more H. pylori-negative patients than H. pylori-positive patients took anticoagulants, antiplatelets, or NSAIDs (48.7% vs 30.6%, respectively; P < 0.01). The rate of H. pylori infection in patients who took anticoagulants, antiplatelets, or NSAIDs was significantly lower than that in patients who did not take any antithrombotic drugs or NSAIDs (47.5% vs 68.4%; P < 0.01). With respect to the number of ulcers, a single ulcer was more common in H. pylori-positive patients than in H. pylori-negative patients (75.8% vs 64.3%; P = 0.02). A single ulcer was also more common in patients who did not take any medicines than in those who did (73.6% vs 61.7%; P = 0.02). Significantly more H. pylori-negative than H. pylori-positive patients were taking antacids prior to admission (24.4% vs 10.3%, respectively; P < 0.01).
| All patients | H. pylori-positive | H. pylori-negative | P value | Anticoagulants/antiplatelet drug/NSAIDs | No drugs | P value | |
| Total | 564 | 340 | 115 | 219 | 345 | ||
| Male/female | 343/221 | 223/117 | 66/49 | 0.111 | 127/92 | 216/129 | 0.271 |
| Age (mean ± SD) | 70.2 ± 14.3 | 66.6 ± 13.6 | 72.3 ± 14.3 | < 0.012 | 77.1 ± 10.3 | 65.8 ± 14.7 | < 0.012 |
| H. pylori status | |||||||
| Positive | 340 (60.3) | - | - | 104 (47.5) | 236 (68.4) | < 0.011 | |
| Negative | 115 (20.4) | - | - | 56 (25.6) | 59 (17.1) | ||
| Unknown | 109 (19.3) | - | - | 59 (26.9) | 50 (14.5) | ||
| Toxic agents | |||||||
| Anticoagulants/antiplatelets/NSAIDs | 219 (38.8) | 104 (30.6) | 56 (48.7) | < 0.011 | 219 (100) | 0 | |
| Anticoagulants | 43 (7.6) | 19 (5.6) | 12 (10.4) | 43 (19.6) | 0 | ||
| Antiplatelets | 130 (23.0) | 60 (17.6) | 33 (28.7) | 130 (59.4) | 0 | ||
| NSAIDs | 174 (30.9) | 85 (25.0) | 39 (33.9) | 174 (79.5) | 0 | ||
| Two or more drugs | 76 (13.5) | 50 (14.7) | 26 (22.6) | (50.7) | 0 | ||
| None | 345 (61.2) | 236 (69.4) | 59 (51.3) | 0 | 345 (100) | ||
| Single or multiple | |||||||
| Single | 400 (70.9) | 251 (73.8) | 71 (61.7) | 0.021 | 138 (63.0) | 254 (73.6) | 0.011 |
| Multiple | 108 (29.1) | 89 (26.2) | 44 (38.3) | 81 (37.0) | 91 (26.4) | ||
| Location (single ulcer) | |||||||
| U | 127 (22.5) | 80 (23.5) | 15 (13.0) | < 0.013 | 47 (21.5) | 76 (22.0) | 0.173 |
| M | 216 (38.3) | 151 (44.4) | 26 (22.6) | 66 (30.1) | 143 (41.4) | ||
| L | 57 (10.1) | 18 (5.3) | 30 (26.1) | 25 (11.4) | 32 (9.3) | ||
| Forrest Classification | 0.103 | < 0.013 | |||||
| Ia | 25 (4.4) | 14 (4.1) | 5 (4.3) | 11 (5.0) | 14 (4.1) | ||
| Ib | 44 (7.8) | 18 (5.3) | 11 (9.6) | 24 (11.0) | 20 (5.8) | ||
| IIa | 280 (49.6) | 188 (55.3) | 46 (40.0) | 91 (41.6) | 189 (54.8) | ||
| IIb | 32 (5.7) | 16 (4.7) | 7 (6.1) | 21 (9.6) | 11 (3.2) | ||
| IIc | 73 (12.9) | 42 (12.4) | 17 (14.8) | 28 (12.8) | 45 (13.0) | ||
| III | 110 (19.5) | 62 (18.2) | 29 (25.2) | 44 (20.1) | 66 (19.1) | ||
| Previous antacids | 86 (15.3) | 35 (10.3) | 28(24.4) | < 0.011 | 41 (18.7) | 46 (13.3) | 0.631 |
| PPIs | 37 (6.6) | 7 (2.1) | 17(14.8) | 18 (8.2) | 19 (5.5) | ||
| H2 blockers | 49 (8.7) | 28 (8.2) | 11 (9.6) | 23 (10.5) | 26 (7.5) | ||
| P-CAB | 1 (0.2) | 028 (8.2) | 011 (9.6) | 023 (10.5) | 1 (0.3) | ||
| None | 477 (84.6) | 305 (89.7) | 87 (75.7) | 178 (81.3) | 299 (86.7) |
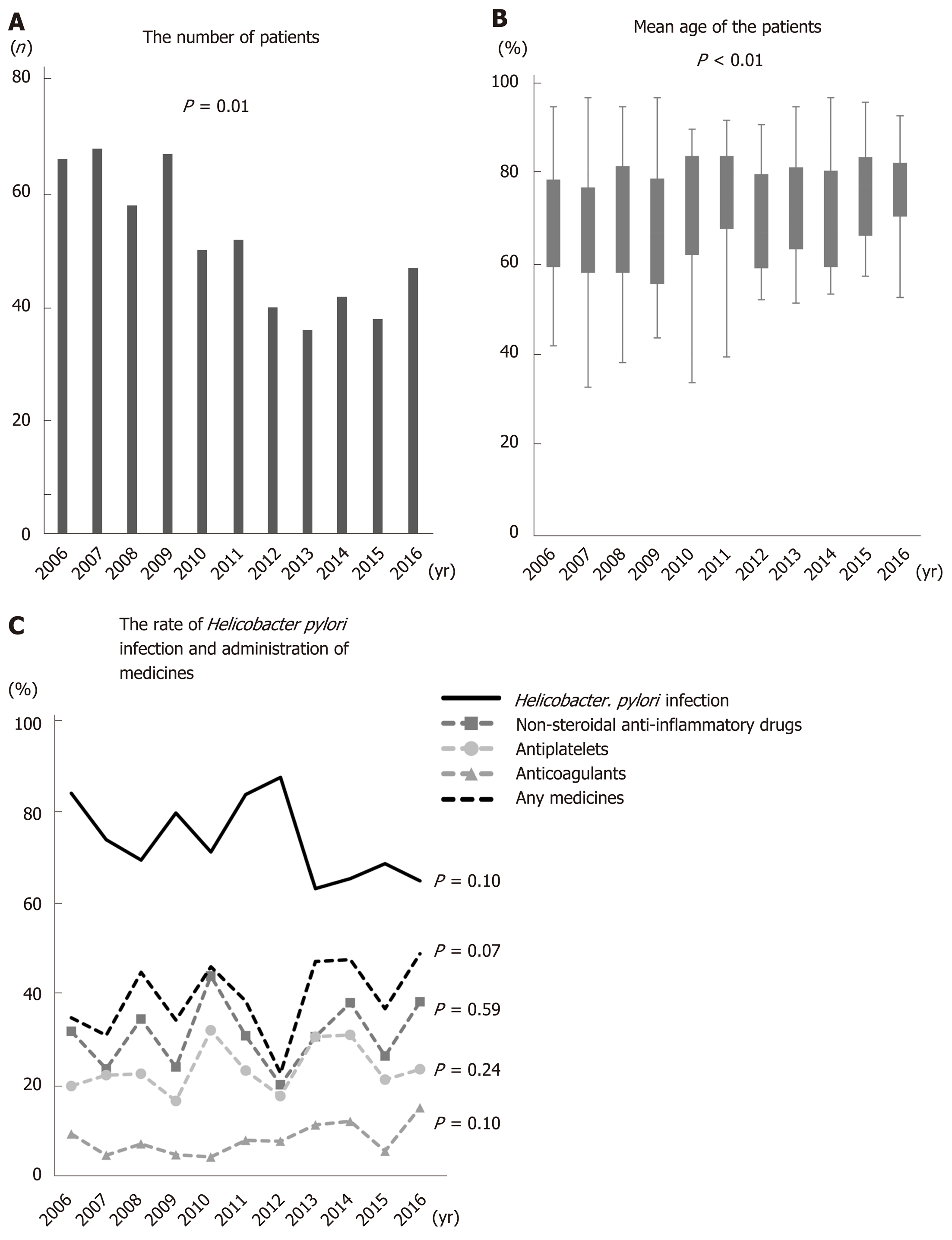
Figure 1A shows the number of patients in each year. The number of patients decreased significantly as time passed. The mean age of gastric ulcer patients increased significantly from 2006 to 2016 (the mean age in 2006, 2007, 2008, 2009, 2010, 2011, 2012, 2013, 2014, 2015, and 2016 was 68.6, 67.5, 69.4, 66.5, 71.7, 72.5, 67.6, 71.8, 70.1, 75.0, and 75.6, respectively; P < 0.01) (Figure 1B). By contrast, the rate of H. pylori infection decreased with age. The percentage of patients taking any antithrombotics or NSAIDs tended to rise over time (P = 0.07), although the numbers of patients taking each individual drug did not show a significant trend over time (NSAIDs: P = 0.59, antiplatelets: P = 0.24, anticoagulants: P = 0.10). Over the study period, NSAIDs were the most common drug taken (Figure 1C).
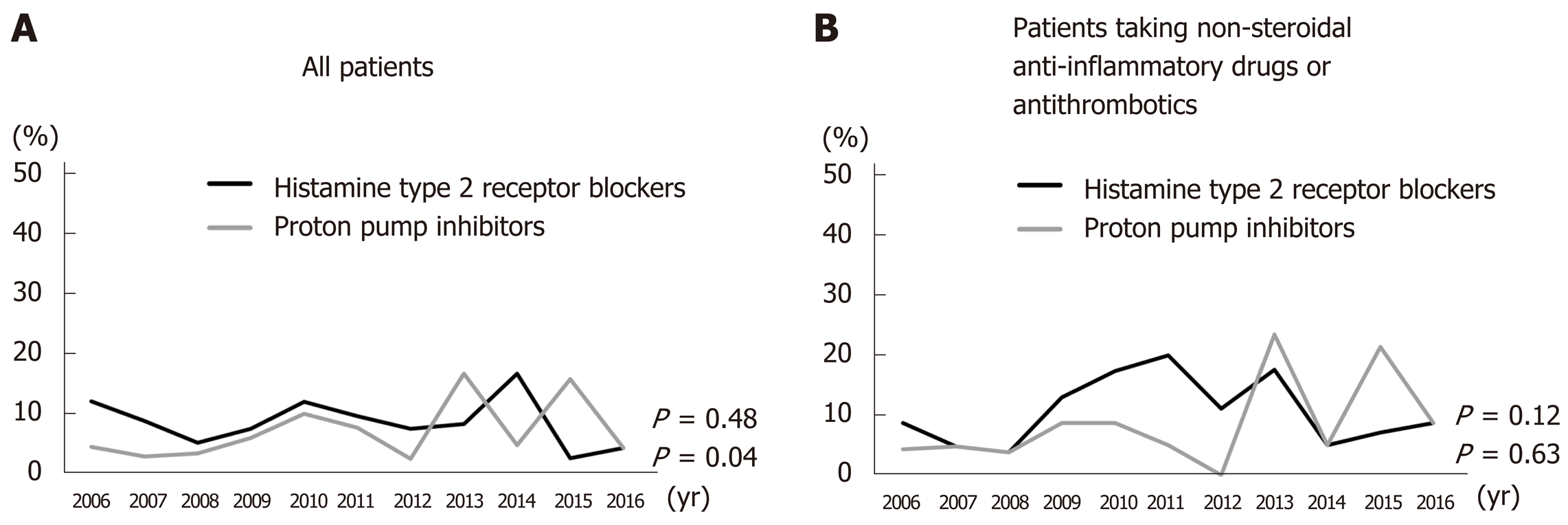
PPI administration prior to treatment increased significantly from 2006 to 2016 (P = 0.04); however, there was no significant increase in the administration of histamine type 2 receptor blockers (H2 blockers) (P = 0.48) (Figure 2A). There was no significant trend over time in the numbers of patients taking H2 blockers or PPIs (P = 0.63 and 0.12, respectively) concurrent with NSAIDs, antiplatelets, and/or anticoagulants (Figure 2B).
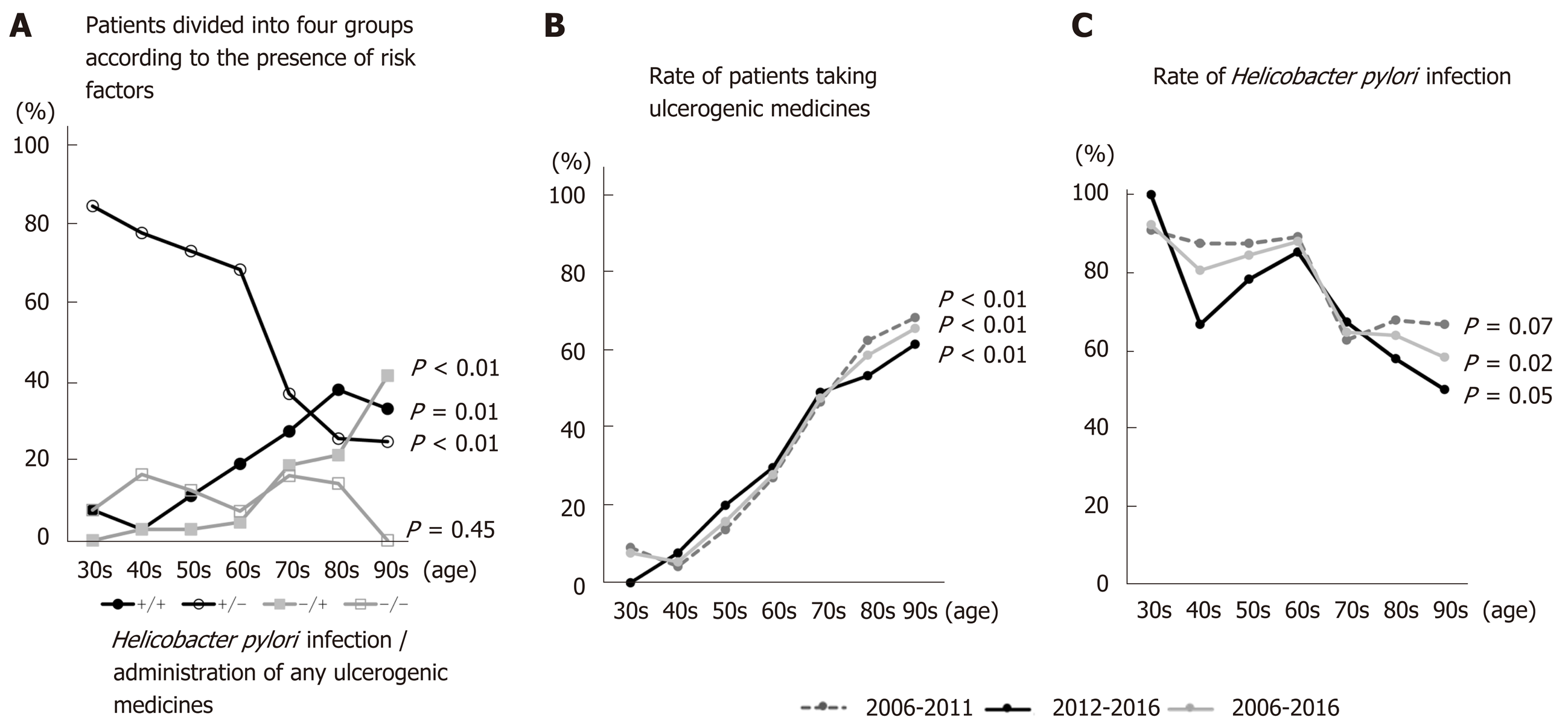
Enrolled patients were divided into four groups according to H. pylori infection and administration of NSAIDs and/or antithrombotics: H. pylori (+) and medicine (+), H. pylori (+) and medicine (-), H. pylori (-) and medicine (+), and H. pylori (-) and medicine (-). The numbers in the H. pylori (+) and medicine (-) group fell significantly as ages increased from the 30s to the 90s (P < 0.01) (Figure 3A). By contrast, the numbers in the H. pylori (+) and medicine (+) and H. pylori (-) and medicine (+) groups increased as age increased from the 30s to the 90s (P = 0.01 and P < 0.01, respectively) (Figure 3A). There were no significant changes in the H. pylori (-) and medicine (-) group, the so-called idiopathic ulcer patients, with age (P = 0.45) (Figure 3A). As patients aged from their 30s to their 70s, the H. pylori (+) and medicine (-) group accounted for the largest proportion; however, this was replaced by the H. pylori (+) and medicine (+) group when patients entered their 80s (Figure 3A). Figure 3B and C shows the rate of H. pylori infection in patients taking NSAIDs, antiplatelets, or anticoagulants during 2006–2011, 2012-2016, and 2006–2016. The rate of H. pylori infection tended to fall with age throughout the study period (P = 0.07, P = 0.05, and P = 0.02 for 2006–2011, 2012–2016, and 2006–2016, respectively). However, the number of patients taking NSAIDs and/or antithrombotics increased with age (P < 0.01, P < 0.01, and P < 0.01 for 2006–2011, 2012–2016, and 2006–2016, respectively).
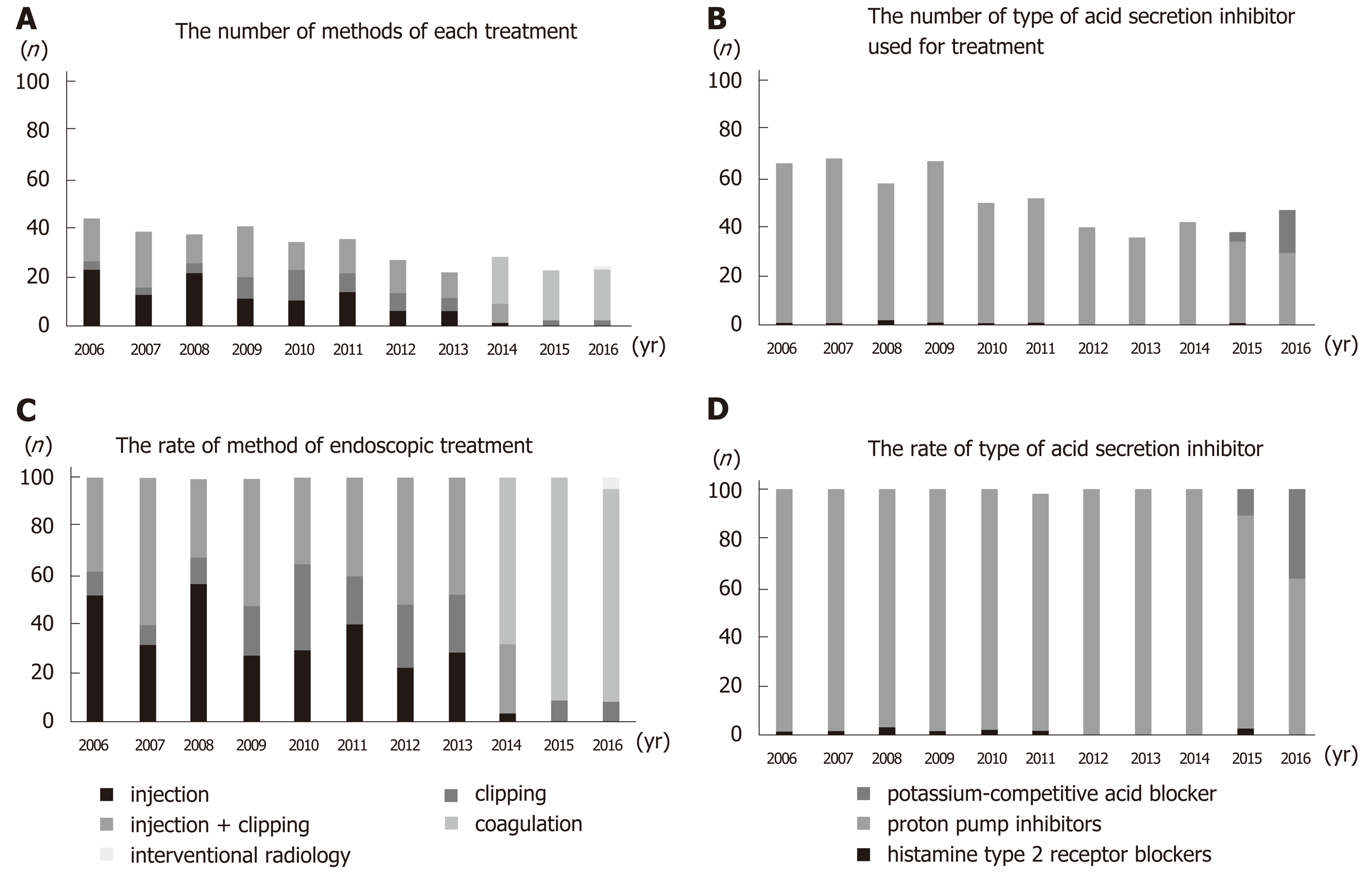
Up until 2013, the main treatment for gastric ulcer bleeding was endoscopic hemostasis, injection (epinephrine or absolute alcohol), and clipping. From 2014, this was replaced by forceps coagulation (P < 0.01) (Figure 4A and 4C). Up until 2014, the most common medical treatment for gastric ulcers was PPIs (96%–100%). However, vonoprazan became available in 2015, and P-CAB administration increased significantly thereafter (P < 0.01) (Figure 4B and 4D). Length of hospitalization fell over time (mean 14.9, 12.9, 14.3, 11.6, 12.4, 14.5, 11.6, 16.6, 13.2, 11.1, and 10.7 d in 2006, 2007, 2008, 2009, 2010, 2011, 2012, 2013, 2014, 2015, and 2016, respectively; P = 0.12). The rate of rebleeding did not show a significant trend over time (3.0%, 2.9%, 8.6%, 7.5%, 0.0%, 13.5%, 0.0%, 2.8%, 2.4%, 2.6%, and 0.0% in 2006, 2007, 2008, 2009, 2010, 2011, 2012, 2013, 2014, 2015, and 2016, respectively; P = 0.12); however, the rate during the latter half (2012–2016) of the study was significantly lower than that in the first half (2006–2011) (1.5% vs 5.8%; P = 0.01) (Figure 5).
Ulcers classified as Forrest Ia, Ib and IIa are considered as high risk of rebleeding, and thereby are recommended to undergo endoscopic hemostasis. In this study, the rebleeding rate of Forrest classification did not show a significant trend (8.0%, 6.8%, 5.7%, 9.4%, 0%, and 0% in Forrest classification Ia, Ib, IIa, IIb, IIc, and III, respectively; P = 0.13). As we described above, the rebleeding rate during the latter half (2012–2016) of the study was significantly lower than that in the first half, however, the total rate of Forrest Ia, Ib and IIa patients was not significantly different in the first half and the latter half (63.7% vs 58.6%, P = 0.24).
The results presented herein show that the mean age of bleeding gastric ulcer patients increased significantly over time (2006-2016), and that the rate of H. pylori infection decreased over time (Figure 1). The incidence of ulcer caused by H. pylori infection in the absence of NSAIDs and antithrombotics decreased as patients aged from their 30s to their 90s (Figure 3A). However, the numbers of H. pylori (+) and medicine (+) and H. pylori (-) and medicine (+) patients increased as they aged from their 30s to their 90s (Figure 3A). There was no clear change in the rates of H. pylori infection or administration of NSAIDs, antiplatelets, and/or anticoagulants between the first half (2006-2011) and the latter half (2012-2016) of the study (Figure 3B and C). Thus, the increasing age of gastric ulcer patients reflects the aging population in general; the aging population also accounts for the decreasing rate of H. pylori infection and the increasing rate of ulcerogenic drug administration.
In 2013, the Japanese Ministry of Health, Labour, and Welfare approved insurance coverage for H. pylori eradication therapy for all patients with H. pylori-associated chronic gastritis to prevent gastric carcinogenesis[13-15]. However, this expansion in H. pylori eradication therapy did not have a significant impact on the rate of gastric ulcer in the present study because we found no clear change in the rate of H. pylori infection between 2006-2011 and 2012-2016. Jyotheeswaran et al[16] reported that, in the United States, white gastric ulcer patients showed lower prevalence of H. pylori than non-white patients (53% vs 78%, respectively; P < 0.01). This is in agreement with findings from other studies showing that the prevalence of H. pylori in whites is lower than that in non-whites[17,18]. These findings suggest that expansion of H. pylori eradication in Japan may alter the causes of gastric ulcer in the future. Therefore, future trends have attracted public interest.
In Japan, the use of NSAIDs/aspirin in elderly patients is increasing[6]. Thus, non-H. pylori-induced gastric ulcers are relatively more common[19]. In this study, we found that the use of PPIs or H2 blockers by patients taking anticoagulants/an-tiplatelets/NSAIDs was about 18.7%, which was low. Previous reports have suggested that administration of antacids prevents gastrointestinal toxicity induced by NSAIDs and low-dose aspirin[20,21]. However, some patients still had gastric ulcer bleeding even when taking antacids. This may be because H2 blockers are ineffective in preventing ulcers[22] and PPIs do not work as effectively in patients with the CYP2C19 polymorphism[23]. Recently, vonoprazan has shown superior efficacy to lansoprazole for preventing low-dose aspirin-associated ulcers and recurrence of NSAID-associated peptic ulcers[24-26]. Therefore, more widespread use of vonoprazan for primary and secondary prevention of drug-induced ulcers should reduce the incidence.
The proportion of patients with idiopathic gastric ulcer was 13.0% (59/455, Table 1); this did not change with age (P = 0.45; Figure 3A). In Asia in the 1990s, idiopathic ulcers accounted for a small percentage of all ulcers; however, several studies in the 2000s reported that the proportion of idiopathic ulcers had reached 10%-30%, indicating that the incidence of idiopathic ulcers in Asia is rising[27]. Idiopathic gastric ulcers are more common in older people, are more resistant to acid suppression therapy, and are associated with a greater risk of bleeding and recurrence and greater overall mortality than ‘traditional’ H. pylori-positive gastric ulcers[28-30]. Although recurrence can occur if an idiopathic ulcer is not treated, there is no consensus on whether maintenance therapy with acid-suppressive agents prevents recurrence effectively[27]. A large prospective cohort study from Hong Kong showed that neither H2 blockers nor PPIs prevented relapse of idiopathic ulcer hemorrhage[31]. Consequently, due to a lack of an effective treatment, the incidence of idiopathic ulcer will continue to increase.
The rate of H. pylori (+) and medicine (-) ulcers (i.e., H. pylori-induced ulcers) was highest in patients aged < 70. Thus, H. pylori eradication treatment is expected to prevent secondary ulcers in younger cases. H. pylori testing and treatment strategies remain important for primary and secondary prevention of H. pylori-induced gastric ulcers[32,33].
The rate of rebleeding in the latter half (2012-2016) of the study was significantly lower than that in the first half (2006-2011) (Figure 5). From 2014, the most common endoscopic hemostasis method was forceps coagulation (Figure 4A and C). Forceps coagulation is as safe and effective as clipping[11]. Arima et al[34] reported that forceps coagulation achieved hemostasis more quickly than clipping. Antacids also play an important role in the treatment of gastric ulcers. PPIs are superior to H2 blockers with respect to reducing the number of rebleeding episodes and the need for surgery[35,36]. Vonoprazan was introduced in 2015, and its use in this study increased dramatically up until 2016 (Figure 4B and D). Vonoprazan is as effective as lansoprazole with respect to healing acute peptic ulcers[24,37,38]; however, there is little evidence to suggest that it is better at preventing rebleeding. Maintaining a high intragastric pH is important to achieve hemostasis and prevent rebleeding. Vonoprazan has a greater acid-inhibitory effect than other PPIs from the first day of administration. It also has a longer duration of action. The pH 4 holding time ratios of vonoprazan are significantly longer than those of other PPIs[26,39]. Thus, forceps coagulation and vonoprazan may reduce the rate of rebleeding or improve ulcer healing.
Forrest classification is useful in deciding adaptation of endoscopic hemostasis. Endoscopic hemostasis can prevent rebleeding significantly in Forrest Ia, Ib and IIa[40]. It suggest Forrest Ia, Ib and IIa ulcers have higher risk of rebleeding than those of Forrest IIb, IIc and III. In this study, the rate of Forrest Ia, Ib and IIa patients was not significantly different in the first half and the latter half (63.7% vs 58.6%, P = 0.24). This result showed that the decreased rate of rebleeding in the latter half was not caused by the changes of the distribution in Forrest classification. However, this result supports the hypothesis that the decreased rate of rebleeding in the latter half may reflect the changes of the treatments. Further studies are warranted to definitively confirm that endoscopic forceps coagulation and vonoprazan are more effective than conventional PPIs and endoscopic clipping and injection (epinephrine or absolute alcohol) for preventing of rebleeding.
This study has several limitations. It was a single center retrospective study; the H. pylori infection status of some patients was unknown, and the methods used to diagnose H. pylori infection were not standardized. Moreover, information of the previous ulcer bleeding was not available.
In conclusion, the mean age of patients with gastric ulcer is increasing, which probably reflects Japan’s current aging population. The incidence of gastric ulcers caused by ulcerogenic drugs is also increasing with age; however, H. pylori-induced ulcers are more common in younger patients (< 70 years old).
The two main causes of gastric ulcer bleeding are Helicobacter pylori (H. pylori) infection and ulcerogenic medicines, although the number of cases caused by each may vary with age. In Japan, the rate of H. pylori infection has fallen over the last decade and the number of prescriptions for non-steroidal anti-inflammatory drugs (NSAIDs) and antithrombotic drugs is increasing as the population ages. In Japan, the aging population is advancing rapidly. Methods of treatment for gastric ulcer bleeding have advanced with the advent of hemostatic forceps and potassium-competitive acid blocker (P-CAB).
The causes and treatments for gastric ulcer bleeding have changed over the last decade. However at present, gastric ulcer bleeding is common disease. Hence, we examined whether overtime there was a change in the trend of causes and treatment that may possibly reveal an effective treatment for decreasing gastric ulcer bleeding in the future.
The main aim was to examine whether there was a change in the trend of causes, treatments, and prognosis of gastric ulcer bleeding over 10 years. The secondary aim was to examine the relationship between factors that cause gastric ulcer bleeding, and whether this was related to age or time.
Data from patients admitted to the National Hospital Organization Tokyo Medical Center with a gastric ulcer between 2006 and 2016 were examined retrospectively. Patients were selected from the database of inpatients. We excluded gastric cancer patients. Collected data included age, medication history, H. pylori infection, method of treatment, rate of rebleeding, and length of hospitalization. Factors associated with gastric ulcer bleeding were evaluated using Fisher’s exact test, Pearson’s Chi-squared test or Student’s t-test as appropriate. The Jonckheere-Terpstra test was used to evaluate trends. A per-protocol analysis was used to examine the rate of H. pylori infection.
There was a significant increase in the mean age over time (P < 0.01). The rate of H. pylori infection tended to decrease over the study period (P = 0.10), whereas the proportion of patients taking antithrombotic agents or NSAIDs tended to increase (P = 0.07). Over time, the use of NSAIDs and antithrombotic drugs increased with age. By contrast, the rate of H. pylori infection during the study period fell with age. H. pylori-induced ulcers accounted for the majority of cases in younger patients (< 70 years old); however, the rate decreased with age (P < 0.01). The method of treatment trend has changed significantly over time. The main method of endoscopic hemostasis has changed from clipping and injection to forceps coagulation (P < 0.01), and frequently prescribed medicines have changed from proton pump inhibitor to P-CAB (P < 0.01). The rate of rebleeding during the latter half of the study was significantly lower than that in the first half. The rate of Forrest Ia, Ib and IIa patients was not significantly different in the first half and the latter half (P = 0.24).
The mean age of gastric ulcer patients increased significantly in this decade. The rate of H. pylori infection decreased and the percentage of patients taking any antithrombotics or NSAIDs tended to rise over time. Gastric ulcers caused by ulcerogenic drugs are increasing with age and H. pylori-induced ulcers are more common in younger patients. H. pylori induced ulcer is more common in younger patients. H. pylori induced ulcer would decrease and the main cause of gastric ulcer would be ulcerogenic medicines in the future. Expansion of antacid administration for preventing injury from ulcerogenic drugs may decrease gastric ulcer bleeding. Expansion of eradication therapy of H. pylori and administration of antacid for preventing injury from ulcerogenic drugs may decrease gastric ulcer bleeding.
The mean age of gastric ulcer bleeding patients was increasing. There was the trends, that the gastric ulcers caused by ulcerogenic drugs were increasing with age and H. pylori-induced ulcers were more common in younger patients. Advancement in treatment, both endoscopic hemostasis and medicine, may contribute to reduction of rebleeding. This study suggests forceps coagulation and P-CAB are superior methods of treatment for gastric ulcer bleeding. Further research is needed to confirm this hypothesis. Equally important is to clarify and understand the cause and effective treatment for idiopathic ulcer. To confirm the superiority of forceps coagulation and P-CAB for the prevention of gastric ulcer bleeding, randomized prospective study is warranted. In addition, a large multi-center study may be useful in clarifying the characteristics of idiopathic ulcer.
For the English proofreading and editing, the authors would like to thank John Surya.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Berkane S, Link A, Slomiany BL S-Editor: Tang JZ L-Editor: A E-Editor: Zhang YL
| 1. | Sung JJ, Kuipers EJ, El-Serag HB. Systematic review: the global incidence and prevalence of peptic ulcer disease. Aliment Pharmacol Ther. 2009;29:938-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 270] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 2. | Hallas J, Dall M, Andries A, Andersen BS, Aalykke C, Hansen JM, Andersen M, Lassen AT. Use of single and combined antithrombotic therapy and risk of serious upper gastrointestinal bleeding: population based case-control study. BMJ. 2006;333:726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 270] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 3. | Ibáñez L, Vidal X, Vendrell L, Moretti U, Laporte JR; Spanish-Italian Collaborative Group for the Epidemiology of Gastrointestinal Bleeding. Upper gastrointestinal bleeding associated with antiplatelet drugs. Aliment Pharmacol Ther. 2006;23:235-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 95] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Kamada T, Haruma K, Ito M, Inoue K, Manabe N, Matsumoto H, Kusunoki H, Hata J, Yoshihara M, Sumii K, Akiyama T, Tanaka S, Shiotani A, Graham DY. Time Trends in Helicobacter pylori Infection and Atrophic Gastritis Over 40 Years in Japan. Helicobacter. 2015;20:192-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 5. | Heisei 22 nenndo kokusei chosa. Statistics Bureau, Ministry of Internal Affairs and Communications. 2011. Available from: https://www.stat.go.jp/english/data/zensho/pdf/outline2.pdf. |
| 6. | Japanese Ministry of Health, Labor and Welfare. The second time NDB open data in 2015. |
| 7. | Toka B, Eminler AT, Karacaer C, Uslan MI, Koksal AS, Parlak E. Comparison of monopolar hemostatic forceps with soft coagulation versus hemoclip for peptic ulcer bleeding: a randomized trial (with video). Gastrointest Endosc. 2019;89:792-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | Panés J, Viver J, Forné M, Garcia-Olivares E, Marco C, Garau J. Controlled trial of endoscopic sclerosis in bleeding peptic ulcers. Lancet. 1987;2:1292-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 100] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Raju GS, Gajula L. Endoclips for GI endoscopy. Gastrointest Endosc. 2004;59:267-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 122] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 10. | Kakushima N, Fujishiro M. Endoscopic submucosal dissection for gastrointestinal neoplasms. World J Gastroenterol. 2008;14:2962-2967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 114] [Cited by in RCA: 127] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 11. | Nagata S, Kimura S, Ogoshi H, Hidaka T. Endoscopic hemostasis of gastric ulcer bleeding by hemostatic forceps coagulation. Dig Endosc. 2010;22 Suppl 1:S22-S25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335:806-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3438] [Cited by in RCA: 6169] [Article Influence: 342.7] [Reference Citation Analysis (0)] |
| 13. | Shiota S, Murakawi K, Suzuki R, Fujioka T, Yamaoka Y. Helicobacter pylori infection in Japan. Expert Rev Gastroenterol Hepatol. 2013;7:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 14. | Ito M, Takata S, Tatsugami M, Wada Y, Imagawa S, Matsumoto Y, Takamura A, Kitamura S, Matsuo T, Tanaka S, Haruma K, Chayama K. Clinical prevention of gastric cancer by Helicobacter pylori eradication therapy: a systematic review. J Gastroenterol. 2009;44:365-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Fukase K, Kato M, Kikuchi S, Inoue K, Uemura N, Okamoto S, Terao S, Amagai K, Hayashi S, Asaka M; Japan Gast Study Group. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet. 2008;372:392-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 876] [Cited by in RCA: 934] [Article Influence: 54.9] [Reference Citation Analysis (0)] |
| 16. | Jyotheeswaran S, Shah AN, Jin HO, Potter GD, Ona FV, Chey WY. Prevalence of Helicobacter pylori in peptic ulcer patients in greater Rochester, NY: is empirical triple therapy justified? Am J Gastroenterol. 1998;93:574-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 96] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Suzuki H, Mori H. World trends for H. pylori eradication therapy and gastric cancer prevention strategy by H. pylori test-and-treat. J Gastroenterol. 2018;53:354-361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 102] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 18. | O'Neill CM, Weitz IC, O'Connell C, Liebman HA. Ethnic and racial difference in Helicobacter pylori infection in patients with immune thrombocytopenia treated at a major urban medical center. Platelets. 2019;30:413-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Musumba C, Jorgensen A, Sutton L, Van Eker D, Moorcroft J, Hopkins M, Pritchard DM, Pirmohamed M. The relative contribution of NSAIDs and Helicobacter pylori to the aetiology of endoscopically-diagnosed peptic ulcer disease: observations from a tertiary referral hospital in the UK between 2005 and 2010. Aliment Pharmacol Ther. 2012;36:48-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Hooper L, Brown TJ, Elliott R, Payne K, Roberts C, Symmons D. The effectiveness of five strategies for the prevention of gastrointestinal toxicity induced by non-steroidal anti-inflammatory drugs: systematic review. BMJ. 2004;329:948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 157] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 21. | Taha AS, McCloskey C, Prasad R, Bezlyak V. Famotidine for the prevention of peptic ulcers and oesophagitis in patients taking low-dose aspirin (FAMOUS): a phase III, randomised, double-blind, placebo-controlled trial. Lancet. 2009;374:119-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 162] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 22. | Mo C, Sun G, Wang YZ, Lu ML, Yang YS. PPI versus Histamine H2 Receptor Antagonists for Prevention of Upper Gastrointestinal Injury Associated with Low-Dose Aspirin: Systematic Review and Meta-analysis. PLoS One. 2015;10:e0131558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | El Rouby N, Lima JJ, Johnson JA. Proton pump inhibitors: from CYP2C19 pharmacogenetics to precision medicine. Expert Opin Drug Metab Toxicol. 2018;14:447-460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 161] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 24. | Kawai T, Oda K, Funao N, Nishimura A, Matsumoto Y, Mizokami Y, Ashida K, Sugano K. Vonoprazan prevents low-dose aspirin-associated ulcer recurrence: randomised phase 3 study. Gut. 2018;67:1033-1041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (1)] |
| 25. | Mizokami Y, Oda K, Funao N, Nishimura A, Soen S, Kawai T, Ashida K, Sugano K. Vonoprazan prevents ulcer recurrence during long-term NSAID therapy: randomised, lansoprazole-controlled non-inferiority and single-blind extension study. Gut. 2018;67:1042-1051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 26. | Mori H, Suzuki H. Role of Acid Suppression in Acid-related Diseases: Proton Pump Inhibitor and Potassium-competitive Acid Blocker. J Neurogastroenterol Motil. 2019;25:6-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 27. | Iijima K, Kanno T, Koike T, Shimosegawa T. Helicobacter pylori-negative, non-steroidal anti-inflammatory drug: negative idiopathic ulcers in Asia. World J Gastroenterol. 2014;20:706-713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Chow DK, Sung JJ. Non-NSAID non-H. pylori ulcer disease. Best Pract Res Clin Gastroenterol. 2009;23:3-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Quan C, Talley NJ. Management of peptic ulcer disease not related to Helicobacter pylori or NSAIDs. Am J Gastroenterol. 2002;97:2950-2961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 30. | Chung CS, Chiang TH, Lee YC. A systematic approach for the diagnosis and treatment of idiopathic peptic ulcers. Korean J Intern Med. 2015;30:559-570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 31. | Wong GL, Au KW, Lo AO, Tse YK, Ching JY, To KF, Chan FK. Gastroprotective therapy does not improve outcomes of patients with Helicobacter pylori-negative idiopathic bleeding ulcers. Clin Gastroenterol Hepatol. 2012;10:1124-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Chen Q, Liang X, Long X, Yu L, Liu W, Lu H. Cost-effectiveness analysis of screen-and-treat strategy in asymptomatic Chinese for preventing Helicobacter pylori-associated diseases. Helicobacter. 2019;24:e12563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 33. | Pohl H, Finlayson SR, Sonnenberg A, Robertson DJ. Helicobacter pylori-associated ulcer bleeding: should we test for eradication after treatment? Aliment Pharmacol Ther. 2005;22:529-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 34. | Arima S, Sakata Y, Ogata S, Tominaga N, Tsuruoka N, Mannen K, Shiraishi R, Shimoda R, Tsunada S, Sakata H, Iwakiri R, Fujimoto K. Evaluation of hemostasis with soft coagulation using endoscopic hemostatic forceps in comparison with metallic hemoclips for bleeding gastric ulcers: a prospective, randomized trial. J Gastroenterol. 2010;45:501-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Lanas A, Artal A, Blás JM, Arroyo MT, Lopez-Zaborras J, Sáinz R. Effect of parenteral omeprazole and ranitidine on gastric pH and the outcome of bleeding peptic ulcer. J Clin Gastroenterol. 1995;21:103-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 72] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 36. | van Rensburg C, Barkun AN, Racz I, Fedorak R, Bornman PC, Beglinger C, Balanzó J, Devière J, Kupcinskas L, Luehmann R, Doerfler H, Schäfer-Preuss S. Clinical trial: intravenous pantoprazole vs. ranitidine for the prevention of peptic ulcer rebleeding: a multicentre, multinational, randomized trial. Aliment Pharmacol Ther. 2009;29:497-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Miwa H, Uedo N, Watari J, Mori Y, Sakurai Y, Takanami Y, Nishimura A, Tatsumi T, Sakaki N. Randomised clinical trial: efficacy and safety of vonoprazan vs. lansoprazole in patients with gastric or duodenal ulcers - results from two phase 3, non-inferiority randomised controlled trials. Aliment Pharmacol Ther. 2017;45:240-252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 38. | Jenkins H, Sakurai Y, Nishimura A, Okamoto H, Hibberd M, Jenkins R, Yoneyama T, Ashida K, Ogama Y, Warrington S. Randomised clinical trial: safety, tolerability, pharmacokinetics and pharmacodynamics of repeated doses of TAK-438 (vonoprazan), a novel potassium-competitive acid blocker, in healthy male subjects. Aliment Pharmacol Ther. 2015;41:636-648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 231] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 39. | Sakurai Y, Mori Y, Okamoto H, Nishimura A, Komura E, Araki T, Shiramoto M. Acid-inhibitory effects of vonoprazan 20 mg compared with esomeprazole 20 mg or rabeprazole 10 mg in healthy adult male subjects--a randomised open-label cross-over study. Aliment Pharmacol Ther. 2015;42:719-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 274] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 40. | Sacks HS, Chalmers TC, Blum AL, Berrier J, Pagano D. Endoscopic hemostasis. An effective therapy for bleeding peptic ulcers. JAMA. 1990;264:494-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 92] [Article Influence: 2.6] [Reference Citation Analysis (0)] |