Published online Nov 7, 2019. doi: 10.3748/wjg.v25.i41.6205
Peer-review started: August 8, 2019
First decision: September 18, 2019
Revised: September 26, 2019
Accepted: October 17, 2019
Article in press: October 17, 2019
Published online: November 7, 2019
Processing time: 90 Days and 16 Hours
Chronic biliary obstruction results in ischemia and hypoxia of hepatocytes, and leads to apoptosis. Apoptosis is very important in regulating the homeostasis of the hepatobiliary system. Endoplasmic reticulum (ER) stress is one of the signaling pathways that induce apoptosis. Moreover, the protein kinase RNA-like endoplasmic reticulum kinase (PERK)-induced apoptotic pathway is the main way; but its role in liver injury remains unclear. Yinchenhao decoction (YCHD) is a traditional Chinese medicine formula that alleviates liver injury and apoptosis, yet its mechanism is unknown. We undertook this study to investigate the effects of YCHD on the expression of ER stress proteins and hepatocyte apoptosis in rats with obstructive jaundice (OJ).
To investigate whether YCHD can attenuate OJ-induced liver injury and hepatocyte apoptosis by inhibiting the PERK-CCAAT/enhancer-binding protein homologous protein (CHOP)-growth arrest and DNA damage-inducible protein 34 (GADD34) pathway and B cell lymphoma/leukemia-2 related X protein (Bax)/B cell lymphoma/leukemia-2 (Bcl-2) ratio.
For in vivo experiments, 30 rats were divided into three groups: control group, OJ model group, and YCHD-treated group. Blood was collected to detect the indicators of liver function, and liver tissues were used for histological analysis. For in vitro experiments, 30 rats were divided into three groups: G1, G2, and G3. The rats in group G1 had their bile duct exposed without ligation, the rats in group G2 underwent total bile duct ligation, and the rats in group G3 were given a gavage of YCHD. According to the serum pharmacology, serum was extracted and centrifuged from the rat blood to cultivate the BRL-3A cells. Terminal deoxynucleotidyl transferase mediated dUTP nick end-labelling (TUNEL) assay was used to detect BRL-3A hepatocyte apoptosis. Alanine aminotransferase (ALT) and aspartate transaminase (AST) levels in the medium were detected. Western blot and quantitative real-time polymerase chain reaction (qRT-PCR) analyses were used to detect protein and gene expression levels of PERK, CHOP, GADD34, Bax, and Bcl-2 in the liver tissues and BRL-3A cells.
Biochemical assays and haematoxylin and eosin staining suggested severe liver function injury and liver tissue structure damage in the OJ model group. The TUNEL assay showed that massive BRL-3A rat hepatocyte apoptosis was induced by OJ. Elevated ALT and AST levels in the medium also demonstrated that hepatocytes could be destroyed by OJ. Western blot or qRT-PCR analyses showed that the protein and mRNA expression levels of PERK, CHOP, and GADD34 were significantly increased both in the rat liver tissue and BRL-3A rat hepatocytes by OJ. The Bax and Bcl-2 levels were increased, and the Bax/Bcl-2 ratio was also increased. When YCHD was used, the PERK, CHOP, GADD34, and Bax levels quickly decreased, while the Bcl-2 levels increased, and the Bax/Bcl-2 ratio decreased.
OJ-induced liver injury and hepatocyte apoptosis are associated with the activation of the PERK-CHOP-GADD34 pathway and increased Bax/Bcl-2 ratio. YCHD can attenuate these changes.
Core tip: Chronic biliary obstruction results in ischaemia and hypoxia of hepatocytes and leads to apoptosis. The protein kinase RNA-like endoplasmic reticulum kinase (PERK)-induced apoptotic pathway is the main and important way, however, its role in liver injury and hepatocyte apoptosis remains unclear. Yinchenhao decoction (YCHD) can alleviate liver injury and apoptosis, but whether YCHD can attenuates obstructive jaundice (OJ)-induced liver injury and hepatocyte apoptosis by inhibiting the PERK-induced pathway is still unknown. In this research study, we found that OJ induced liver injury and hepatocyte apoptosis by activating the PERK- CCAAT/enhancer-binding protein homologous protein (CHOP)-growth arrest and DNA damage-inducible protein 34 (GADD34) pathway and upregulating the B cell lymphoma/leukemia-2 related X protein (Bax)/ B cell lymphoma/leukemia-2 (Bcl-2) ratio. YCHD attenuated these changes by inhibiting the PERK-CHOP-GADD34 pathway and Bax/Bcl-2 ratio.
- Citation: Wu YL, Li ZL, Zhang XB, Liu H. Yinchenhao decoction attenuates obstructive jaundice-induced liver injury and hepatocyte apoptosis by suppressing protein kinase RNA-like endoplasmic reticulum kinase-induced pathway. World J Gastroenterol 2019; 25(41): 6205-6221
- URL: https://www.wjgnet.com/1007-9327/full/v25/i41/6205.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i41.6205
Obstructive jaundice (OJ) is defined by evaluated serum bilirubin levels and characterized by increased conjugated bilirubin levels without influencing unconjugated bilirubin levels[1]. Biliary obstruction can cause damage to all organs of the body, especially the liver. OJ affects tissues by releasing various inflammatory mediators that stimulate the inflammatory response and cytokine cascades. This can lead to reduced clearance of endotoxin, decreased immune function, vascular dysfunction, coagulation disorders, and even disseminated intravascular coagulation, impaired gastrointestinal mucosal barrier, damaged hepatic sinusoidal endothelium, and injured liver function[2,3], which eventually can cause hepatocyte ischaemia, hypoxia, and acidosis.
Apoptosis is a programmed mechanism of cell death caused by various physiological and pathological conditions characterized by DNA damage[4]. In 2014, Kosar et al[5] confirmed that serious cellular damage occurs in hepatocytes during OJ. If DNA damage in a cell cannot be resolved, apoptotic signaling mechanisms can be activated, forcing the cell to self-destruct[6]. There are three main apoptotic signaling pathways: The death receptor pathway, the mitochondrial pathway, and the endoplasmic reticulum (ER) stress pathway. The ER stress pathway, which is a newly characterized apoptotic signaling pathway, is considered a supplement of the mitochondrial pathway and is a research hotspot[7,8]. However, there are few studies on liver injury and hepatocyte apoptosis induced by OJ through activating the ER stress pathway.
The ER has three transmembrane receptors, inositol-requiring protein 1 (IRE1), protein kinase RNA-like endoplasmic reticulum kinase (PERK), and activating transcription factor 6 (ATF6), which collectively monitor the functions of the ER[9]. These three receptors represent three classical unfolded protein response (UPR) signaling pathways: The IRE1ɑ-XBP1 pathway, the PERK-eIF2ɑ pathway, and the ATF6 pathway[10,11]. Cellular ischaemia and hypoxia can induce ER stress and activate the UPR to restore normal physiological function to the ER[12]. It is known that the UPR is a pro-survival response by reducing gene transcription, attenuating protein translation, enhancing protein folding, and promoting ER-associated degradation of misfolded proteins to resist ER stress and restore ER homeostasis[13]. However, if the ER stress is too prolonged or too severe, the UPR fails to control ER stress, resulting in a change from pro-survival to pro-apoptotic signaling and, eventually, cell apoptosis[14].
However, these three signaling pathways do not directly induce apoptosis but activate apoptotic signaling molecules, including CCAAT/enhancer-binding protein homologous protein (CHOP), c-Jun NH2 terminal kinase (JNK), and caspases. There are three apoptotic pathways induced by the UPR: The IRE1ɑ-ASK-JNK pathway, the PERK-eIF2ɑ-CHOP signaling pathway, and the caspase-12 pathway[15]. The expression of CHOP has significant importance in inducing pro-survival to pro-apoptosis[11]. CHOP interacts with other transcription regulators, including growth arrest and DNA damage-inducible protein 34 (GADD34), the B cell lymphoma/leukemia-2 (Bcl-2) family, endoplasmic reticulum oxidoreductase 1, and Tribbles-related protein 3 pseudokinase, to induce apoptosis changes[16]. The Bcl-2 family is not only an important regulator of cell apoptosis, but it is also the most important regulator of the mitochondrial apoptosis pathway.
Yinchenhao decoction (YCHD) is used as a complementary and alternative treatment to ameliorate clinical symptoms, improve liver function, reduce liver inflammation and fibrosis, and improve patient quality of life with a high degree of safety, few side effects, and low toxicity[17]. Liu et al[18] compared five types of traditional Chinese medicine (TCM) and showed that YCHD exerted the most significant therapeutic effect in improving liver function in patients with liver cirrhosis. YCHD has been used for more than a thousand years to treat patients with viral hepatitis, cholestasis, primary biliary cirrhosis, and liver fibrosis[19]. Additionally, YCHD has been shown to inhibit hepatocyte apoptosis and promote the secretion and excretion of bile, which alleviates icterus[20]. YCHD consists of three herbal components: Artemisia capillaris Thunb (Herba Artemisiae Capillaris, Yin-Chen-Hao), Gardenia jasminoides Ellis (Fructus Gardeniae, Zhi-zi), and Rheum officinale Baill (Radix Rhei Officinalis, Da-huang) with a ratio of 3:2:1 in weight. Its major bioactive ingredients are geniposide, capillin, capilene, capillarisin, and rhein[17,21]. The YCHD components exert their effects on liver disease in a synergistic manner. For instance, capillarisin also acts as a choleretic[17]. Rhein has been shown to inhibit hepatic stellate cell activation and reverse liver fibrosis[22]. Many experiments have confirmed that the main YCHD components alleviate liver damage and inhibit apoptosis, but the main mechanism of YCHD has not been clarified.
The pharmacological evaluation of serum, which was first proposed by Iwama Hiroko in 1987[23], has become an important method to study the mechanisms of TCM. The core concept of serum pharmacology is to collect animal blood and to obtain serum after administering a TCM by gavage at defined times, followed by the addition of the serum to an in vitro tissue or cell system to study the pharmacodynamics and mechanism of TCM. This method prevents interference of the in vitro experiment from the physical and chemical properties of crude TCM and allows the study of the metabolized pharmacologically active products, following the process of digestion and absorption of the TCM and its biological transformation in the gastrointestinal tract. Collectively, these features allow us to evaluate the true pharmacological effects of TCMs[24]. Compared with the evaluation of TCMs directly added in in vitro studies, the results of in vitro studies evaluating the pharmacological effects in serum, derived from an animal model, may be more reliable and representative of the true in vivo effects of the TCM compound being investigated.
Therefore, the aim of this study was to determine the role of the PERK-induced ER stress pathways in liver injury and hepatocyte apoptosis, and the mechanism by which YCHD alleviates apoptosis and improves liver injury.
According to the current version of the “Synopsis of Prescriptions of the Golden Chamber”[25], the YCHD formula consists of 120 g of Artemisia capillaris Thunb (Herba Artemisiae Capillaris, Yin-Chen-Hao), 80 g of Gardenia jasminoides Ellis (Fructus Gardeniae, Zhi-zi), and 40 g of Rheum officinale Baill (Radix Rhei Officinalis, Da-huang), all of which were purchased from Tianjin Nankai Hospital. These herbals were mixed in water, decocted for 45 min and 30 min, successively, and then concentrated to 240 mL. As such, every 1 mL of liquid contains 1 g YCHD. The concentrated form of YCHD was stored at 4 °C.
Sixty male Wistar rats weighing 250-270 g were obtained from the Institute of the Environmental Medicine of the Chinese People’s Liberation Army Academy of Military Medical Sciences. The rats were housed under standard laboratory conditions, and provided with ad libitum food and water, with a 12-hour light/dark cycle, a mean humidity of 50 ± 5%, a mean temperature of 25 ± 2 °C, and external noise controlled to within 60 dB. The rats had one week of environmental adaptation.
Surgical common bile duct ligation is one of the most commonly used methods for the creation of an OJ animal model. In 2016, Aoki et al[26] showed that although total ligation of the bile duct resulted in a high mortality, this model also resulted in the conspicuous symptoms of jaundice and was suitable for short-term research studies. Tarcin et al[27] further showed that serum levels of alanine aminotransferase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), gamma-glutamyl transpeptidase (GGT), and total bilirubin (TBIL), and direct bilirubin (DBIL) rose and peaked at day 7 after bile duct ligation before they began to decline. Therefore, in the current experiment, total bile duct ligation was selected as the surgical method, and all rats were sacrificed on day 7.
Thirty rats were randomly selected and divided into three groups: A, control group (n = 10); B, OJ model group (n = 10); and C, YCHD-treated group (n = 10). The rats in group A had their bile duct exposed without ligation. The rats in groups B and C underwent total bile duct ligation. One hour after operation, the rats in group C were given a gavage of 1 mL of YCHD per 100 g body weight for 7 d. Blood samples of all rats were collected on day 7 from the abdominal aorta and were tested for biochemical parameters. Liver tissues were quickly dissected and rinsed in ice-cold saline, frozen in liquid nitrogen, and stored at -80 °C for histopathology, Western blot, and quantitative real-time polymerase chain reaction (qRT-PCR) analyses. Samples from the group C rats were collected one hour after gavage.
The remaining 30 rats were randomly divided into three groups on average: G1, G2, and G3. On the day of surgery, the rats in group G1 had their bile duct exposed without ligation, and the rats in group G2 underwent total bile duct ligation, and the rats in group G3 were just given a gavage of 1 mL of YCHD per 100 g body weight every day for 7 d. Blood samples of all rats were collected on day 7 from the abdominal aorta and centrifuged. Plasma was retained and stored at ﹣80 °C for cell study use.
The rat blood was collected and centrifuged at 300 g for 10 min at 20 °C, and the supernatants were collected. ALT, AST, TBIL, DBIL, GGT, and ALP levels were then measured using an automatic clinical analyser (ACA).
Liver tissues were fixed in 5% formaldehyde solution, embedded in paraffin, and sliced into 5-micron sections. Haematoxylin and eosin staining was performed to observe the histopathologic changes. Histological features were observed and captured with a light microscope.
BRL-3A rat hepatocytes were purchased from the Cell Bank of the Chinese Academy of Sciences and cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, United States) supplemented with 10% foetal bovine serum (Gibco, United States) and 1% penicillin-streptomycin solution (HyClone, United States) at 37 °C in a humidified atmosphere containing 5% CO2. The culture medium was replaced every 2 d. BRL-3A cells were divided into three groups: S, O, and M. In group S, BRL-3A cells were cultured in DMEM medium containing 10% serum from the G1 group with 1% penicillin-streptomycin solution. In group O, BRL-3A cells were cultured in DMEM medium containing 10% serum from the G2 group with 1% penicillin-streptomycin solution. In group M, BRL-3A cells were cultured in DMEM medium containing 10% serum from the G2 group and 10% serum from the G3 group with 1% penicillin-streptomycin solution. Cells were cultured for 6 h, 24 h, and 48 h. At each time point, cell culture medium was collected to detect the ALT and AST levels, and cultured BRL-3A cells were frozen in liquid nitrogen and stored at ﹣80 °C for TUNEL assay, Western blot, and qRT-PCR analyses.
ALT and AST levels in the cell culture medium were measured using an ACA.
BRL-3A cell apoptosis was detected by using an In-Situ Cell Death Detection Kit, Fluorescein (Roche-11684795910) (Sigma-Aldrich, United States) to perform TUNEL assay, according to the manufacturer’s protocol. Biotinylated nucleotides detected DNA fragments through terminal deoxynucleotidyl transferase. Biotinylated nucleotides were detected by TACS Blue Label (Trevigen, United States). Following TUNEL staining, the BRL-3A cells were counterstained with 4’-6-diamidino-2-phenylindole to detect the nuclei, as shown by blue staining. TUNEL-positive cells were defined as cells with green staining (wavelength, 488 nm).
qRT-PCR was performed to detect the mRNA expression levels of PERK, CHOP, GADD34, B cell lymphoma/leukemia-2 related X protein (Bax), and Bcl-2 both in rat liver tissues and BRL-3A rat hepatocytes. Total RNA was extracted from cells using TRIzol reagent (Invitrogen, United States) to detect the mRNA expression levels. cDNA was synthesized from RNA through reverse transcription (RT) by using the SuperScript III RT kit following the manufacturer’s instructions. The RT conditions for each cDNA amplification were 42 °C for 60 min and 85 °C for 10 min, and then the cDNA was stored in a -20 °C freezer. qRT-PCR was performed in triplicate with SYBR qPCR mix (Invitrogen, United States) in a total volume of 20 µL on the 7900 HT Fast Real-Time PCR System (Applied Biosystems, United States) using the following cycling parameters: 95 °C for 2 min and 40 cycles of 94 °C for 20 s, 60 °C for 20 s, and 72 °C for 30 s. The primer sequences used are shown in Tables 1 and 2. β-actin and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were used as the reference genes. Experiments were repeated at least three times for each group.
| Gene | Forward primer (5’-3’) | Reverse primer (5’-3’) |
| β-actin | GTTACCAGGGCTGCCTTCTC | GGGTTTCCCGTTGATGACC |
| PERK | GTTCATGGAGAATGGCGCTT | GACACTTTGCACACCAGGTT |
| CHOP | ACACCACCACACCTGAAAGC | TGGACACTGTCTCAAAGGCG |
| GADD34 | GTTTGCACGAGATCGAAGCC | CCCTGAAAGCAGGGGTAAGG |
| Bax | CCGAAATGTTTGCTGACG | AGCCGATCTCGAAGGAAGT |
| Bcl-2 | ACCTGAATGACCACCTAGAGC | TCCGACTGAAGAGCGAAC |
| Gene | Forward primer (5’-3’) | Reverse primer (5’-3’) |
| GADPH | GTTACCAGGGCTGCCTTCTC | GGGTTTCCCGTTGATGACC |
| PERK | GCTTGCTCCCACATCGGATA | CTAAGGACCTGCCGCGAG |
| CHOP | GATACTCGCTCTCCGCTCCA | CTTCCTTCTGGAACACTCTCTCC |
| GADD34 | TTACCTGGACAGAAGCCAGC | AGTGCACCTTTCTACCCTTCAG |
| Bax | TTGCTACAGGGTTTCATCCAGG | CACTCGCTCAGCTTCTTGGT |
| Bcl-2 | AGGATAACGGAGGCTGGGATG | CTCACTTGTGGCCCAGGTAT |
Western blot analysis was performed to detect the protein expression levels of PERK, CHOP, GADD34, Bax, and Bcl-2 both in rat liver tissues and BRL-3A rat hepatocytes. Total protein was obtained from cells using protein extraction solution and quantified using a BCA Protein Assay kit (ThermoFisher Scientific, United States). Proteins were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Following electrophoresis, proteins were transferred onto polyvinylidene difluoride membranes (Millipore, United States). After blocking with 5% blocking buffer, the membranes were incubated overnight at 4 °C with primary antibodies against β-actin (1:1000), GAPDH (1:1000), PERK (1:1000), CHOP (1:1000), GADD34 (1:1000), Bax (1:1000), and Bcl-2 (1:1000). After washing, the membranes were incubated with horseradish peroxidase-conjugated secondary antibody for 1 h. The immune complexes were detected by the enhanced chemiluminescence method, and Western blots were measured with the ChemiDoc MP Imaging System (Bio-Rad, United States). Each experiment was repeated at least three times for each group.
Data are presented as the mean ± standard deviation. Statistical differences among multiple groups were compared by one-way analysis of variance (ANOVA) using SPSS version 21.0. P < 0.05 was considered statistically significant.
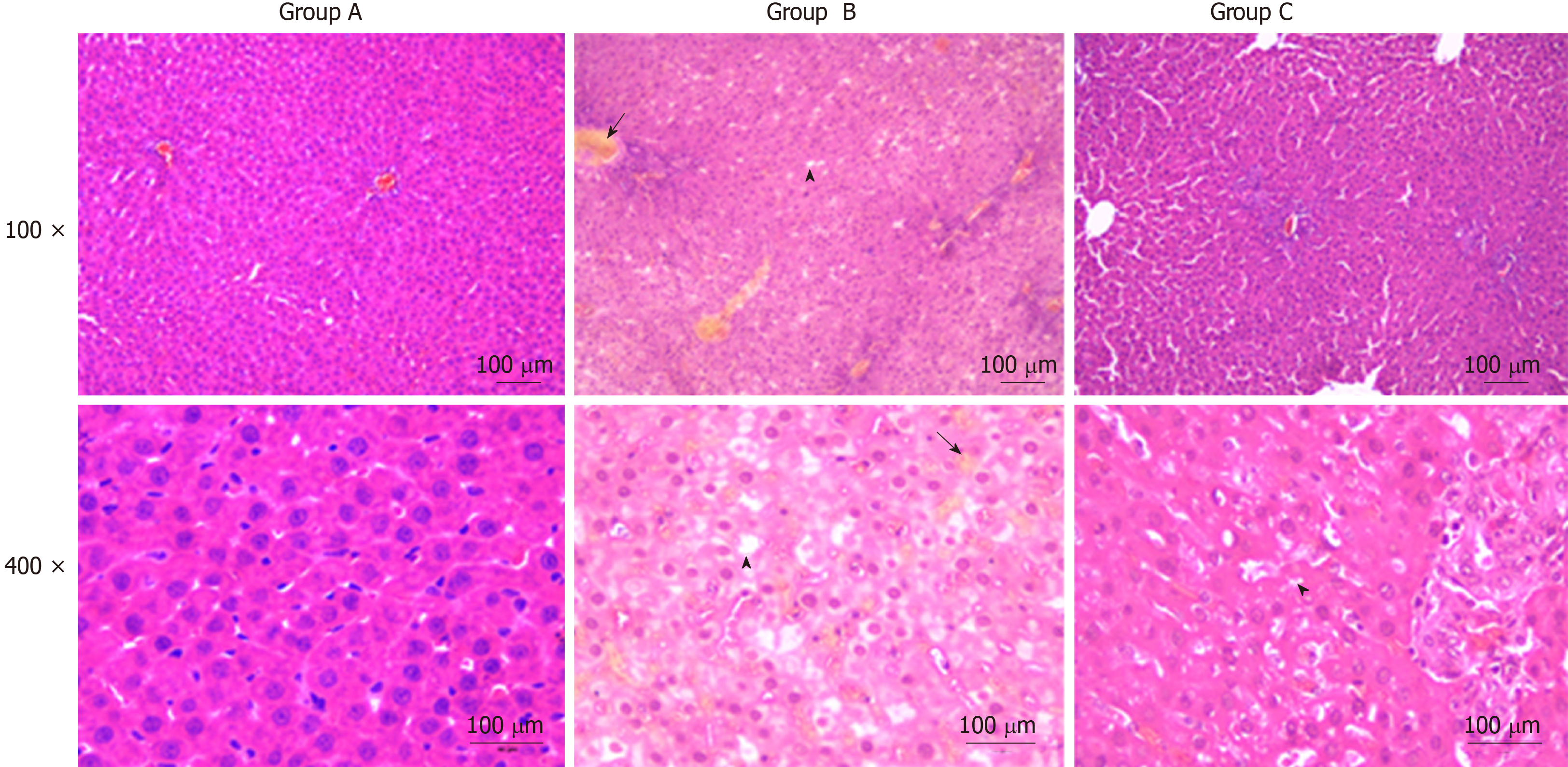
Liver histopathologic changes were detected to investigate the effects of YCHD on liver tissue injury. As shown in Figure 1, there were no abnormal liver tissue structure in the control group. The liver tissue in the OJ model group showed an unclear texture, the hepatic sinusoids were narrowed and congestive, and the hepatic lobule structures were disordered. Moreover, cholestasis, liver cell swelling, fatty degeneration and necrosis, cytoplasmic shrinkage, and hyperchromatic, inflammatory cell infiltration were observed. However, the histopathologic changes in the liver tissues were substantially ameliorated in the YCHD-treated group.
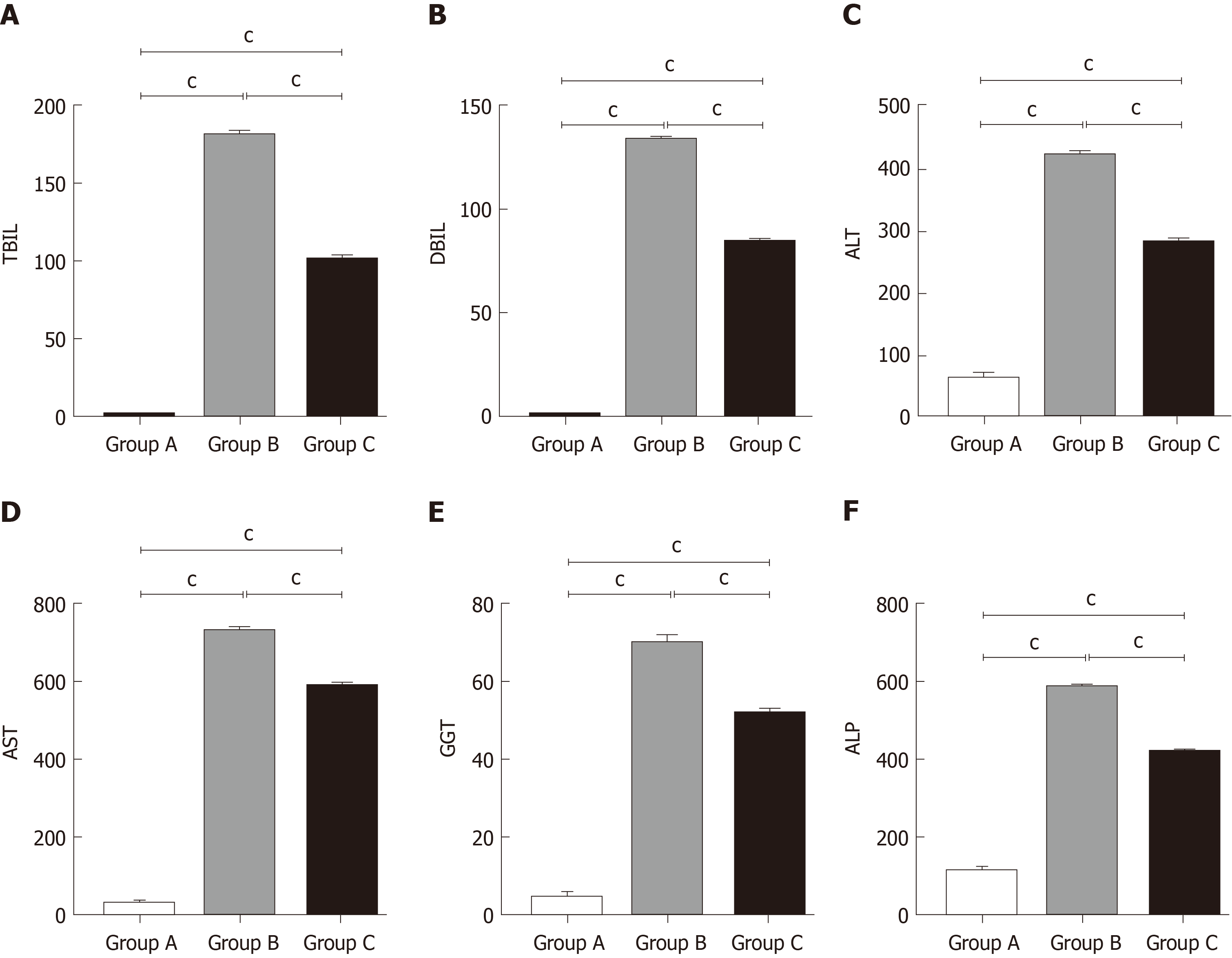
We detected liver function by measuring serum TBIL, DBIL, ALT, AST, GGT, and ALP levels. As shown in Figure 2, compared with the control group, these levels were remarkably increased in the OJ model group, but they decreased quickly after treatment with YCHD.
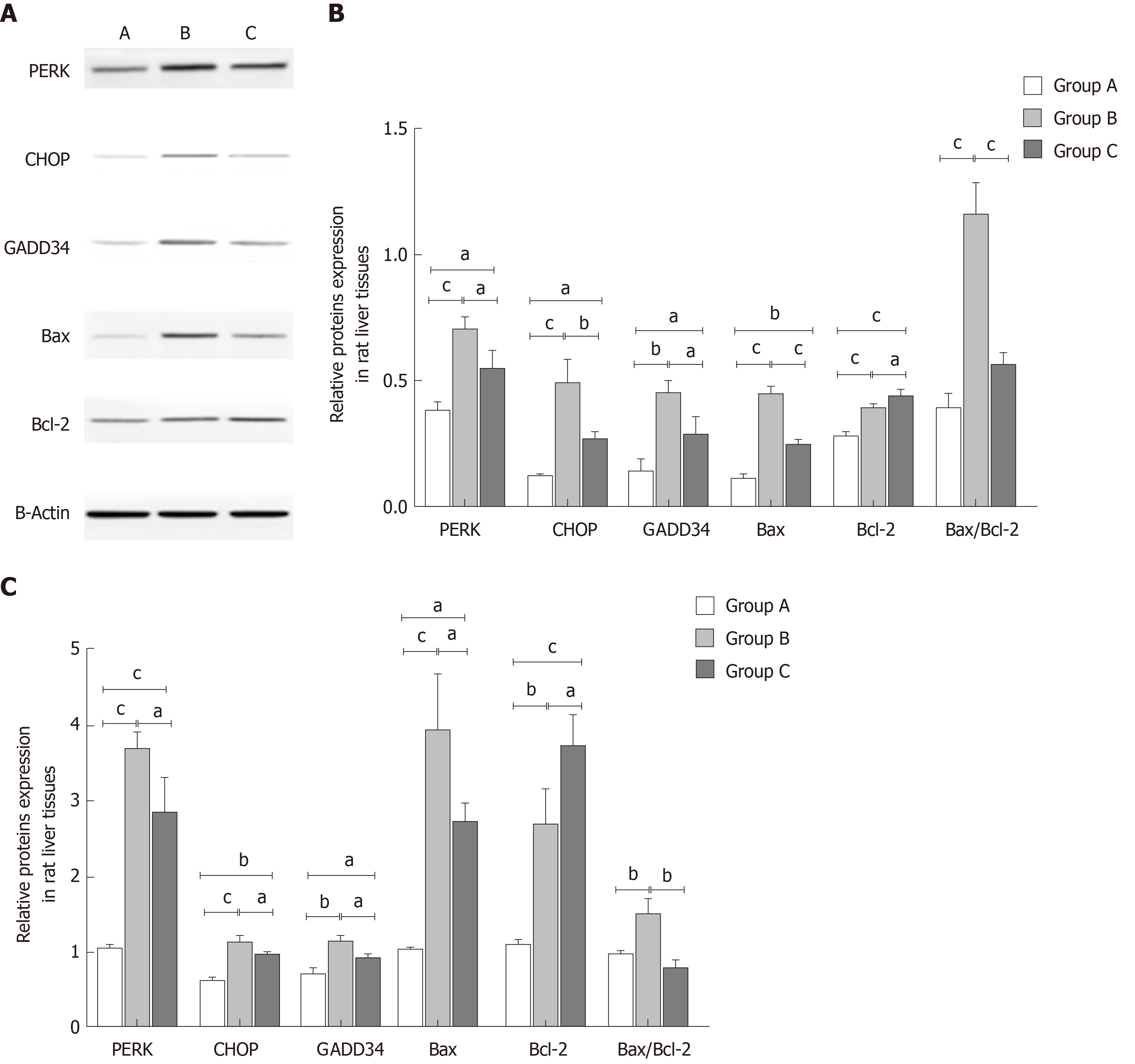
To investigate the mechanisms of YCHD in improving liver injury induced by OJ, both the protein and mRNA levels of PERK, CHOP, GADD34, Bax, and Bcl-2 in liver tissues were detected. As shown in Figure 3, compared with the control group, the protein and mRNA expression levels of PERK, CHOP, and GADD34 were upregulated. Additionally, the Bax and Bcl-2 levels were increased, and the Bax/Bcl-2 ratio was also increased. However, after treatment with YCHD, the PERK, CHOP, GADD34, and Bax levels were downregulated and the Bcl-2 levels were upregulated; however, the Bax/Bcl-2 ratio decreased.
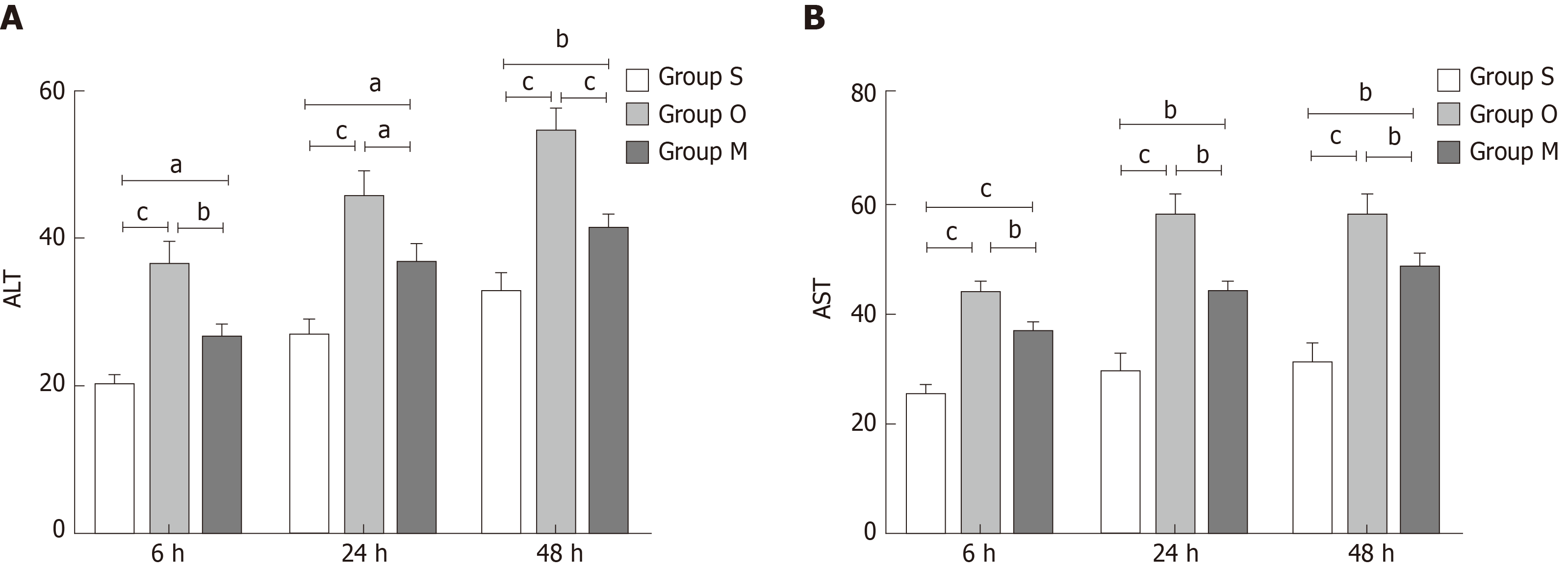
ALT and AST changes reflect hepatocyte injury. As shown in Figure 4, the ALT and AST levels were increased in group O compared with group S, but they decreased quickly after YCHD treatment.
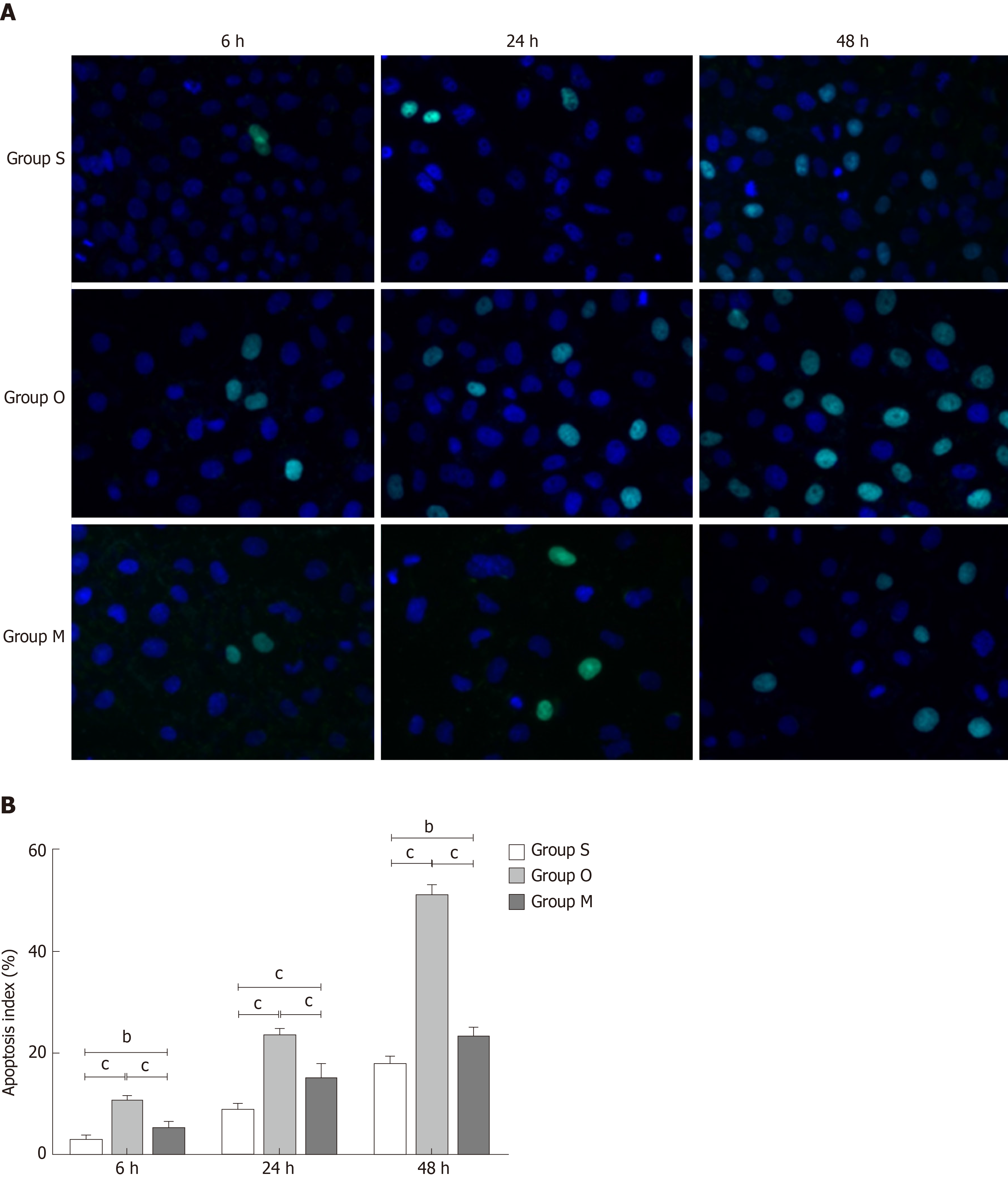
TUNEL assay was utilized to detect the cells apoptosis, and the results are shown in Figure 5A. Normal cells are shown in blue, and TUNEL positive cells are shown in green. Quantitative results, using the apoptosis index, are shown in Figure 5B. It is clear that the green staining and the apoptosis index increased significantly in group O; however, the green staining and apoptosis index decreased in group M.
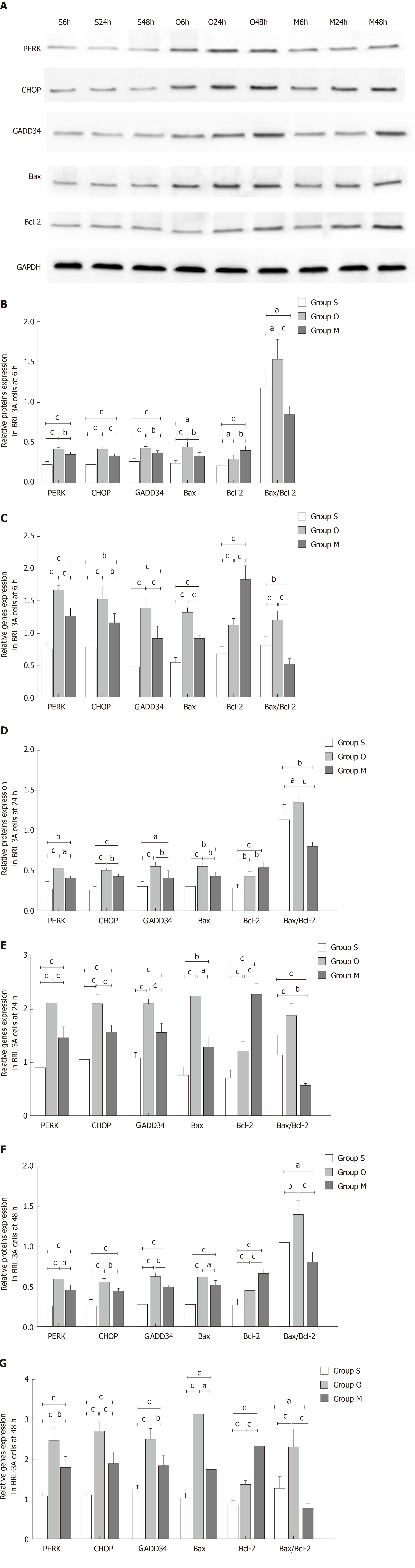
Next, we evaluated the roles of YCHD in inhibiting hepatocyte apoptosis induced by OJ by examining changes in the protein and mRNA expression levels of PERK, CHOP, GADD34, Bax, and Bcl-2 in BRL-3A rat hepatocytes. In BRL-3A rat hepatocytes that were cultured with OJ rat serum for 6 h, 24 h and 48 h, both the protein and mRNA levels of PERK, CHOP, and GADD34 were upregulated. Additionally, the Bax and Bcl-2 levels were increased, and the Bax/Bcl-2 ratio was also increased. In contrast, the BRL-3A rat hepatocytes cultured with OJ rat serum and rat serum containing YCHD for 6 h, 24 h and 48 h, the protein and mRNA expression levels of PERK, CHOP, GADD34, and Bax were downregulated, the Bcl-2 levels were upregulated, and the Bax/Bcl-2 ratio also decreased (Figure 6).
OJ has several causes, including biliary atresia, cholangiolithiasis, pancreatic tumours, cholangiocarcinoma, gallbladder carcinoma, and liver tumours, which can lead to bile decrease or cessation[28]. Chronic biliary obstruction can lead to a dramatic increase in biliary pressure, which results in increased portal vein and hepatic sinus pressure, causing further reduction in liver blood perfusion. Ischaemia and hypoxia in liver cells cause hepatocyte damage, resulting in the release of intracellular liver enzymes, including ALT and AST, which are released into the blood. In the present study, TBIL, DBIL, ALT, AST, GGT, and ALP levels were all obviously elevated in group B. Additionally, the rat liver structure in group B was seriously damaged. The above experiments demonstrated that OJ can seriously influence liver morphology and function.
The ER is essential for secretion and synthesis of membrane proteins, post-translational modification, protein folding, and oligomerization[29]. Therefore, it is necessary for the ER to maintain homeostasis to perform normal physiological functions. Ischaemia, hypoxia, acidosis, disorders of calcium metabolism, and oxidative stress result in unfolded or misfolded protein accumulation, which can cause ER stress. The ability of cells to deal with this disturbance is a critical factor in cell survival[30].
IRE1, PERK, and ATF6 activate a series of downstream signaling proteins that resist ER stress. Under normal physiological conditions, all three transducers are bound to an ER-resident chaperone, an immunoglobulin binding protein (Bip) also known as glucose regulating protein 78 (Grp78). When ER stress occurs, each transducer is activated and triggers downstream signaling cascades following Grp78/Bip dissociation to restore ER homeostasis[31,32]. However, if ER stress is prolonged or unresolved, cells become apoptotic. PERK is the key regulator of the response to ER stress[33]. PERK is a type I transmembrane protein with an ER luminal sensor and a cytoplasmic domain with Ser/Thr kinase activity. When dissociated from Grp78/Bip, PERK is activated by dimerization and autophosphorylation. Active PERK then phosphorylates a downstream factor, eukaryotic initiation factor 2α (eIF2α) at Ser51[34]. In the initiation of protein synthesis, translation of eIF2α combines with methionyl-tRNA to form a ternary complex, eIF2-GTP-tRNAmet, to recognize AUG start codons. Inactive eIF2-GDP is then converted to active eIF2-GTP that requires the guanine nucleotide exchange factor, eIF2B[32]. When ER stress occurs, phosphorylated eIF2α, which is activated by PERK, inhibits eIF2B translation of eIF2-GDP into eIF2-GTP, thereby reducing protein synthesis[35]. At the same time, specific genes, such as activating transcription factor 4 (ATF4), contain small non-coding upstream open reading frames in their 5’ untranslated region, which can bypass the eIF2α-dependent translation block, and express increasingly through phosphorylated eIF2α[36,37]. Fawcett et al showed that ATF4 could activate the downstream transcription factor, CHOP, in vivo[38]. CHOP is known as a key apoptosis factor, and the PERK-eIF2α-ATF4 pathway is most important for CHOP activation in the three arms of the UPR. GADD34 has a catalytic subunit, protein phosphatase 1, which can dephosphorylate eIF2α to restore the function of general Cap-dependent protein translation to resume protein synthesis and suppress ATF4 translation[39-41]. If the UPR fails in restoring ER homeostasis, increased protein synthesis leading to ATP depletion, oxidative stress, and cell death can occur (Han et al[42], 2013). The expression of GADD34 is related to cell apoptosis, and its overexpression can initiate or enhance the cell apoptosis[43].
CHOP can also induce apoptosis by regulating Bcl-2 family proteins. CHOP induces apoptosis by enhancing the expression of pro-apoptotic proteins and suppressing anti-apoptotic proteins. Bcl-2 and Bax are the most important and typical anti-apoptotic and pro-apoptotic proteins in the Bcl-2 family, respectively[44]. The Bax/Bcl-2 ratio plays a decisive role in the fate of cells. Bax and Bcl-2 regulate apoptosis by forming homodimers or heterodimers. Apoptotic signals activate Bax, which is transferred from the cytoplasm to the mitochondrial membrane. Bax is increased, and Bax/Bax homodimers promote cell apoptosis. When Bcl-2 is increased, Bcl-2/Bax heterodimers are formed, and the apoptosis trend is weakened; therefore, a function of Bcl-2 is to inhibit apoptosis[45,46].
In the present study, the TUNEL assay showed that apoptotic cells in group O were more frequent, and the levels of ALT and AST in cell culture medium were increased, which indicated that liver cells could be destroyed by OJ. The Western blot and qRT-PCR analyses showed that the protein and mRNA expression of PERK, CHOP, and GADD34 both in group B rat liver tissues and BRL-3A rat liver cells of group O were increased significantly. Additionally, the protein and mRNA levels of Bax and Bcl-2 increased, and the Bax/Bcl-2 ratio also went up, which indicated that the Bax increase dominated. These results indicated that OJ could injure liver tissues and promote hepatocyte apoptosis by activating the PERK-CHOP-GADD34 pathways and upregulating the Bax/Bcl-2 ratio.
YCHD has a recognized role in increasing bile secretion and excretion, enhancing bile flow, treating inflammation, and promoting diuresis[47-49]. YCHD is not only used for treating jaundice and liver disorders but also for inhibiting hepatic cell apoptosis[19-21,50,51]. In the present study, after the YCHD treatment, the liver tissue structure, liver function, and hepatocytes apoptosis indices were obviously improved. Additionally, the protein and mRNA expression levels of PERK, CHOP, GADD34, and Bax both in the rat liver tissues and BRL-3A cells were downregulated; however, the protein and mRNA levels of Bcl-2 increased, while the Bax/Bcl-2 ratio decreased, indicating that the Bcl-2 levels dominated. These results further supported that YCHD could suppress liver injury, improve liver function, and alleviate hepatocyte apoptosis by inhibiting the PERK-CHOP-GADD34 pathways and downregulating the Bax/Bcl-2 ratio.
In conclusion, OJ can induce liver damage and hepatocyte apoptosis by activating the PERK-CHOP-GADD34 pathways and upregulating the Bax/Bcl-2 ratio. Additionally, YCHD can improve liver function and reduce hepatocyte apoptosis by inhibiting the activation of the PERK-CHOP-GADD34 pathways and downregulating the Bax/Bcl-2 ratio.
Biliary obstruction can cause damage to all organs of the body, especially the liver. Chronic biliary obstruction results in ischaemia and hypoxia of hepatocytes and leads to apoptosis. Apoptosis is very important in regulating the homeostasis of the hepatobiliary system. Endoplasmic reticulum (ER) stress induced apoptosis is a hot research topic worldwide. However, there are few studies on liver injury and hepatocyte apoptosis induced by obstructive jaundice (OJ) through activating the ER stress pathway. Previous studies have confirmed that Yinchenhao decoction (YCHD) can effectively alleviate liver function injury and inhibit liver cell apoptosis, but the main mechanism of YCHD has not been clarified.
To provide scientific evidence for clinical application of YCHD in OJ treatment.
This study aimed to clarify the molecular mechanism of YCHD in alleviating OJ-induced liver injury and hepatocyte apoptosis.
In this study, the expression levels of protein kinase RNA-like endoplasmic reticulum-resident protein kinase (PERK), CCAAT/enhancer-binding protein homologous protein (CHOP), growth arrest- and DNA damage-inducible gene 34 (GADD34), B cell lymphoma/leukemia-2 gene (Bcl-2), and Bcl-2 related X protein (Bax) were detected in rats and BRL-3A rat hepatocytes, and the changes of PERK, CHOP, GADD34, Bax, and Bcl-2 were detected after the application of YCHD.
We found that OJ induced liver injury and hepatocyte apoptosis by activating the PERK-CHOP-GADD34 pathways and upregulating Bax/Bcl-2 ratio, and YCHD attenuated these changes by inhibiting the PERK-CHOP-GADD34 pathways and downregulating Bax/Bcl-2 ratio.
YCHD can attenuate OJ-induced liver injury and hepatocyte apoptosis by inhibiting the PERK-CHOP-GADD34 pathways and downregulating Bax/Bcl-2 ratio. These findings support the clinical use of YCHD for OJ treatment.
YCHD is a traditional Chinese medicine (TCM) formula that has thousands of years of history for OJ treatment. The combination of TCM and modern medicine can effectively treat OJ.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Exbrayat JM, Mizuguchi T, Slomiany BL S-Editor: Tang JZ L-Editor: Wang TQ E-Editor: Ma YJ
| 1. | Kurniawan J, Hasan I, Gani RA, Simadibrata M. Mortality-related Factors in Patients with Malignant Obstructive Jaundice. Acta Med Indones. 2016;48:282-288. [PubMed] |
| 2. | Qiu YD, Bai JL, Xu FG, Ding YT. Effect of preoperative biliary drainage on malignant obstructive jaundice: a meta-analysis. World J Gastroenterol. 2011;17:391-396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Sun C, Yan G, Li Z, Tzeng CM. A meta-analysis of the effect of preoperative biliary stenting on patients with obstructive jaundice. Medicine (Baltimore). 2014;93:e189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | Sun EW, Shi YF. Apoptosis: the quiet death silences the immune system. Pharmacol Ther. 2001;92:135-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 109] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Kosar NM, Tosun M, Polat C, Kahraman A, Arikan Y. Hepatocyte apoptotic index and p53 expression in obstructive jaundice rats. Bratisl Lek Listy. 2014;115:352-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Sherr CJ. Tumor surveillance via the ARF-p53 pathway. Genes Dev. 1998;12:2984-2991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 529] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 7. | Arora S, Tandon S. Mushroom Extracts Induce Human Colon Cancer Cell (COLO-205) Death by Triggering the Mitochondrial Apoptosis Pathway and Go/G1-Phase Cell Cycle Arrest. Arch Iran Med. 2015;18:284-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Fujiwara N, Inoue J, Kawano T, Tanimoto K, Kozaki K, Inazawa J. miR-634 Activates the Mitochondrial Apoptosis Pathway and Enhances Chemotherapy-Induced Cytotoxicity. Cancer Res. 2015;75:3890-3901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3842] [Cited by in RCA: 4523] [Article Influence: 347.9] [Reference Citation Analysis (0)] |
| 10. | Sano R, Reed JC. ER stress-induced cell death mechanisms. Biochim Biophys Acta. 2013;1833:3460-3470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1214] [Cited by in RCA: 1532] [Article Influence: 127.7] [Reference Citation Analysis (1)] |
| 11. | Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7:880-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1670] [Cited by in RCA: 1909] [Article Influence: 100.5] [Reference Citation Analysis (0)] |
| 12. | Sovolyova N, Healy S, Samali A, Logue SE. Stressed to death - mechanisms of ER stress-induced cell death. Biol Chem. 2014;395:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 163] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 13. | Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13:184-190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2167] [Cited by in RCA: 2095] [Article Influence: 149.6] [Reference Citation Analysis (0)] |
| 14. | Wang K. Molecular mechanisms of liver injury: apoptosis or necrosis. Exp Toxicol Pathol. 2014;66:351-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 15. | Cao Y, Hao Y, Li H, Liu Q, Gao F, Liu W, Duan H. Role of endoplasmic reticulum stress in apoptosis of differentiated mouse podocytes induced by high glucose. Int J Mol Med. 2014;33:809-816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 16. | Du K, Herzig S, Kulkarni RN, Montminy M. TRB3: a tribbles homolog that inhibits Akt/PKB activation by insulin in liver. Science. 2003;300:1574-1577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 668] [Cited by in RCA: 703] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 17. | Wu L, Zhou PQ, Xie JW, Zhu R, Zhou SC, Wang G, Wu ZX, Hao S. Effects of Yinchenhao decoction on self-regulation of renin-angiotensin system by targeting angiotensin converting enzyme 2 in bile duct-ligated rat liver. J Huazhong Univ Sci Technolog Med Sci. 2015;35:519-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Liu C, Sun M, Wang L, Wang G, Chen G, Liu C, Liu P. Effects of Yinchenhao Tang and related decoctions on DMN-induced cirrhosis/fibrosis in rats. Chin Med. 2008;3:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Wang X, Zhang A, Wang P, Sun H, Wu G, Sun W, Lv H, Jiao G, Xu H, Yuan Y, Liu L, Zou D, Wu Z, Han Y, Yan G, Dong W, Wu F, Dong T, Yu Y, Zhang S, Wu X, Tong X, Meng X. Metabolomics coupled with proteomics advancing drug discovery toward more agile development of targeted combination therapies. Mol Cell Proteomics. 2013;12:1226-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 20. | Zhang A, Sun H, Qiu S, Wang X. Advancing drug discovery and development from active constituents of yinchenhao tang, a famous traditional chinese medicine formula. Evid Based Complement Alternat Med. 2013;2013:257909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Arai M, Yokosuka O, Fukai K, Kanda T, Kojima H, Kawai S, Imazeki F, Hirasawa H, Saisho H. A case of severe acute hepatitis of unknown etiology treated with the Chinese herbal medicine Inchinko-to. Hepatol Res. 2004;28:161-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Guo MZ, Li XS, Xu HR, Mei ZC, Shen W, Ye XF. Rhein inhibits liver fibrosis induced by carbon tetrachloride in rats. Acta Pharmacol Sin. 2002;23:739-744. [PubMed] |
| 23. | Iwama H, Amagaya S, Ogihara Y. Effect of shosaikoto, a Japanese and Chinese traditional herbal medicinal mixture, on the mitogenic activity of lipopolysaccharide: a new pharmacological testing method. J Ethnopharmacol. 1987;21:45-53. [PubMed] [DOI] [Full Text] |
| 24. | Bochu W, Liancai Z, Qi C. Primary study on the application of Serum Pharmacology in Chinese traditional medicine. Colloids Surf B Biointerfaces. 2005;43:194-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Song SJ, Li ZL, Zhang XB. Effect of Yinchenhao decoction on expression of IRE1α protein in liver cells of rats with obstructive jaundice. Shijie Huaren Xiaohua Zazhi. 2016;24:2520-2524. |
| 26. | Aoki H, Aoki M, Yang J, Katsuta E, Mukhopadhyay P, Ramanathan R, Woelfel IA, Wang X, Spiegel S, Zhou H, Takabe K. Murine model of long-term obstructive jaundice. J Surg Res. 2016;206:118-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Tarcin O, Basaranoglu M, Tahan V, Tahan G, Sücüllü I, Yilmaz N, Sood G, Snyder N, Hilman G, Celikel C, Tözün N. Time course of collagen peak in bile duct-ligated rats. BMC Gastroenterol. 2011;11:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Unal Y, Tuncal S, Kosmaz K, Kucuk B, Kismet K, Cavusoglu T, Celepli P, Senes M, Yildiz S, Hucumenoglu S. The Effect of Calcium Dobesilate on Liver Damage in Experimental Obstructive Jaundice. J Invest Surg. 2019;32:238-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Ron D, Hubbard SR. How IRE1 reacts to ER stress. Cell. 2008;132:24-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 183] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 30. | Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4586] [Cited by in RCA: 5052] [Article Influence: 280.7] [Reference Citation Analysis (0)] |
| 31. | Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2:326-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1987] [Cited by in RCA: 2170] [Article Influence: 86.8] [Reference Citation Analysis (0)] |
| 32. | Shen Y, Meunier L, Hendershot LM. Identification and characterization of a novel endoplasmic reticulum (ER) DnaJ homologue, which stimulates ATPase activity of BiP in vitro and is induced by ER stress. J Biol Chem. 2002;277:15947-15956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 163] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 33. | Ron D, Harding HP. Protein-folding homeostasis in the endoplasmic reticulum and nutritional regulation. Cold Spring Harb Perspect Biol. 2012;4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 34. | Donnelly N, Gorman AM, Gupta S, Samali A. The eIF2α kinases: their structures and functions. Cell Mol Life Sci. 2013;70:3493-3511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 636] [Cited by in RCA: 655] [Article Influence: 54.6] [Reference Citation Analysis (0)] |
| 35. | Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2430] [Cited by in RCA: 2557] [Article Influence: 98.3] [Reference Citation Analysis (0)] |
| 36. | Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6:1099-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2332] [Cited by in RCA: 2504] [Article Influence: 100.2] [Reference Citation Analysis (0)] |
| 37. | Schröder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739-789. [PubMed] [DOI] [Full Text] |
| 38. | Fawcett TW, Martindale JL, Guyton KZ, Hai T, Holbrook NJ. Complexes containing activating transcription factor (ATF)/cAMP-responsive-element-binding protein (CREB) interact with the CCAAT/enhancer-binding protein (C/EBP)-ATF composite site to regulate Gadd153 expression during the stress response. Biochem J. 1999;339:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 251] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 39. | Novoa I, Zeng H, Harding HP, Ron D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. J Cell Biol. 2001;153:1011-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1112] [Cited by in RCA: 1096] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 40. | Connor JH, Weiser DC, Li S, Hallenbeck JM, Shenolikar S. Growth arrest and DNA damage-inducible protein GADD34 assembles a novel signaling complex containing protein phosphatase 1 and inhibitor 1. Mol Cell Biol. 2001;21:6841-6850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 217] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 41. | Ma Y, Hendershot LM. Delineation of a negative feedback regulatory loop that controls protein translation during endoplasmic reticulum stress. J Biol Chem. 2003;278:34864-34873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 338] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 42. | Han J, Back SH, Hur J, Lin YH, Gildersleeve R, Shan J, Yuan CL, Krokowski D, Wang S, Hatzoglou M, Kilberg MS, Sartor MA, Kaufman RJ. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat Cell Biol. 2013;15:481-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1032] [Cited by in RCA: 1323] [Article Influence: 110.3] [Reference Citation Analysis (0)] |
| 43. | Adler HT, Chinery R, Wu DY, Kussick SJ, Payne JM, Fornace AJ, Tkachuk DC. Leukemic HRX fusion proteins inhibit GADD34-induced apoptosis and associate with the GADD34 and hSNF5/INI1 proteins. Mol Cell Biol. 1999;19:7050-7060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 125] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 44. | Rossé T, Olivier R, Monney L, Rager M, Conus S, Fellay I, Jansen B, Borner C. Bcl-2 prolongs cell survival after Bax-induced release of cytochrome c. Nature. 1998;391:496-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 658] [Cited by in RCA: 668] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 45. | Brooks C, Dong Z. Regulation of mitochondrial morphological dynamics during apoptosis by Bcl-2 family proteins: a key in Bak? Cell Cycle. 2007;6:3043-3047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 46. | Lalier L, Cartron PF, Juin P, Nedelkina S, Manon S, Bechinger B, Vallette FM. Bax activation and mitochondrial insertion during apoptosis. Apoptosis. 2007;12:887-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 247] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 47. | Wang YH, Zhao CX, Chen BM, He M, Liu LQ, Li CY, Chen X. Reverse effect of Yinchenhao decoction in dimethyl nitrosamine-induced hepatic fibrosis in rats. Zhongguo Zhong Yao Za Zhi. 2014;39:1473-1478. [PubMed] |
| 48. | Lee TY, Chang HH, Wu MY, Lin HC. Yin-Chen-Hao-Tang ameliorates obstruction-induced hepatic apoptosis in rats. J Pharm Pharmacol. 2007;59:583-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 49. | Cai H, Song YH, Xia WJ, Jin MW. Aqueous extract of Yin-Chen-Hao decoction, a traditional Chinese prescription, exerts protective effects on concanavalin A-induced hepatitis in mice through inhibition of NF-kappaB. J Pharm Pharmacol. 2006;58:677-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 50. | Inao M, Mochida S, Matsui A, Eguchi Y, Yulutuz Y, Wang Y, Naiki K, Kakinuma T, Fujimori K, Nagoshi S, Fujiwara K. Japanese herbal medicine Inchin-ko-to as a therapeutic drug for liver fibrosis. J Hepatol. 2004;41:584-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 79] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 51. | Yamamoto M, Miura N, Ohtake N, Amagaya S, Ishige A, Sasaki H, Komatsu Y, Fukuda K, Ito T, Terasawa K. Genipin, a metabolite derived from the herbal medicine Inchin-ko-to, and suppression of Fas-induced lethal liver apoptosis in mice. Gastroenterology. 2000;118:380-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 100] [Article Influence: 4.0] [Reference Citation Analysis (0)] |