Published online Oct 28, 2019. doi: 10.3748/wjg.v25.i40.6094
Peer-review started: June 17, 2019
First decision: July 21, 2019
Revised: August 9, 2019
Accepted: September 9, 2019
Article in press: September 9, 2019
Published online: October 28, 2019
Processing time: 134 Days and 23.6 Hours
Direct-acting antiviral agents (DAAs) are extremely effective in eradicating hepatitis C virus (HCV) in chronically infected patients. However, the protective role of the sustained virologic response (SVR) achieved by second- and third-generation DAAs against the onset of hepatocellular carcinoma (HCC) and mortality is less well established.
To examine the occurrence of HCC or death from any cause in a retrospective-prospective study of patients treated with DAAs.
Patients were enrolled from a tertiary academic hospital center for liver disease management that collects subject data mainly from northeastern Italy. The study was conducted in 380 patients (age: 60 ± 13 years, 224 males, 32% with cirrhosis) treated with DAAs with or without SVR (95/5%), with a median follow up of 58 wk (interquartile range: 38-117). The baseline anthropometric features, HCV viral load, severity of liver disease, presence of extra-hepatic complications, coinfection with HIV and/or HBV, alcohol consumption, previous interferon use, alpha-fetoprotein levels, and renal function were considered to be confounders.
The incidence rate of HCC in patients with and without SVR was 1.3 and 59 per 100 person-years, respectively (incidence rate ratio: 44, 95%CI: 15-136, P < 0.001). Considering the combined endpoint of HCC or death from any cause, the hazard ratio (HR) for the SVR patients was 0.070 (95%CI: 0.025-0.194, P < 0.001). Other independent predictors of HCC or death were low HCV viremia (HR: 0.808, P = 0.030), low platelet count (HR: 0.910, P = 0.041), and presence of mixed cryoglobulinemia (HR: 3.460, P = 0.044). Considering SVR in a multi-state model, the independent predictors of SVR achievement were absence of cirrhosis (HR: 0.521, P < 0.001) and high platelet count (HR: 1.019, P = 0.026). Mixed cryoglobulinemia predicted the combined endpoint in patients with and without SVR (HR: 5.982, P = 0.028 and HR: 5.633, P = 0.047, respectively).
DAA treatment is effective in inducing SVR and protecting against HCC or death. A residual risk of HCC persists in patients with advanced liver disease or with complications, such as mixed cryoglobulinemia or renal failure.
Core tip: The protective role of a sustained virologic response (SVR) achieved by direct-acting antiviral agents (DAAs) against the chronic consequences of hepatitis C virus (HCV) infection is not well established. We examined the occurrence of hepatocellular carcinoma (HCC) or death from any cause in a retrospective-prospective study at our tertiary academic hospital center for liver disease management. We confirmed that DAA treatment is very effective in inducing SVR in HCV-infected patients and in protecting patients against HCC development or death. However, a residual risk of HCC persists, particularly in patients with advanced liver disease or with extra-hepatic HCV-related complications.
- Citation: Colussi G, Donnini D, Brizzi RF, Maier S, Valenti L, Catena C, Cavarape A, Sechi LA, Soardo G. Sustained virologic response to direct-acting antiviral agents predicts better outcomes in hepatitis C virus-infected patients: A retrospective study. World J Gastroenterol 2019; 25(40): 6094-6106
- URL: https://www.wjgnet.com/1007-9327/full/v25/i40/6094.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i40.6094
Chronic hepatitis C virus (HCV) infection is highly prevalent in many areas of the world and, because of its serious associated hepatic and extrahepatic complications, it constitutes an important burden for the global health system[1]. Chronic HCV infection can cause both liver disease [cirrhosis and hepatocellular carcinoma (HCC)] and extrahepatic diseases (lymphomas, vasculitis, progressive renal failure) that could lead to patient death[2]. The classic treatment of HCV infection with interferon-based regimens has shown a low efficacy in eradicating HCV infection, particularly in patients with a high grade of liver fibrosis, and cannot be offered to patients with more severe liver disease or several comorbidities due to multiple severe adverse effects[3,4].
New interferon-free therapies based on combinations of direct-acting antiviral agents (DAAs) have progressively improved HCV eradication, granting a sustained virologic response (SVR) in more than 95% of patients with fewer adverse effects than those treated with interferon therapy[5]. However, HCV eradication following treatment with DAAs has not provided clear evidence of a beneficial effect in reducing HCC development in the short term or death in those without advanced liver disease[6]. In fact, some studies have suggested an increased prevalence of early HCC recurrence after treatment with DAAs in patients with a previously cured HCC[7,8].
In Italy, the prevalence of HCV infection is among the highest in Western Europe, reaching 2% of seropositivity and a chronic viremic rate close to 73%[1]. According to the European Medicines Agency guidelines[9], DAAs have been approved by the Italian Medicines Agency (AIFA) since 2014, and they have been made available for HCV-infected patients with advanced liver fibrosis or with significant HCV-related extrahepatic complications. Since 2017, the treatment has been extended to patients with less advanced liver disease.
In this study, we characterized chronic HCV-infected patients and, after treatment with second- and third-generation interferon-free DAA regimens, we followed the patients for a median of 58 weeks. The aim of this study was to retrospectively analyze the predictors of both SVR and new occurrence of HCC or death after interferon-free DAA treatment. For this purpose, we have used the classic survival analysis and a more sophisticated multi-state survival model where SVR achievement was introduced as a time-dependent variable.
Patients of any age, sex, and race included in the Registry of Drug Monitoring for DAA use were considered for inclusion in this retrospective study, and the enrollment period lasted from January 2015 to January 2019. The patients were enrolled from a tertiary academic hospital center for liver disease management that collects subject data mainly from northeastern Italy. Patients were included if they had documented HCV chronic viremia and criteria for using DAAs according to the recommendations of the AIFA (Table S1 of the supplemental material) and the European Association for the Study of the Liver (EASL)[10,11]. Patients were excluded from the analysis if, after inclusion, they did not start DAA therapy or did not complete DAA treatment for reasons other than adverse effects. Patients were also excluded if they had incomplete data or were lost to follow up. The names of the DAAs used are specified in Table S2 of the supplemental material.
Some patients in the study had been previously treated with interferon-based regimens without achieving SVR or with adverse effects that precluded the continuation of therapy. These patients were subsequently treated with interferon-free DAAs and were included in the study. The choice of DAAs was made according to the EASL guidelines and under the surveillance of two expert hepatologists. Second- and third-generation DAAs were used with or without ribavirin according to the HCV genotype and inclusion criteria. Treatment was continued for 12 wk, and SVR was defined as undetectable HCV RNA at 12 and 24 wk after the end of therapy. Those patients who did not achieve SVR after 12 wk or did not maintain SVR after 24 wk were considered “nonresponders” or “relapsers”, respectively, and were treated again with different genotype-specific DAAs for another 12 wk (rescue treatment). If the first or rescue treatment with DAAs achieved SVR at 12 and 24 wk, the patients were considered “responders”; otherwise, the patients remained “nonresponders”.
Liver fibrosis was assessed by either liver biopsy or transient elastography (FibroScan TM®, EchoSensTM, Paris, France), and the results were converted to a Metavir score[12]. Cirrhosis was determined by the presence of suggestive clinical signs (altered serum aminotransferases levels, platelet counts, coagulation parameters, gamma-glutamyl-aminotransferase levels, total bilirubin levels, and serum albumin levels) and/or the presence of signs of portal hypertension (ultrasound splenomegaly and endoscopic esophageal or gastric varices), and a Metavir score of F4 (or corresponding Ishak)[13], or a liver stiffness measured by transient elastography higher than 14.5 kPa. Patients with cirrhosis were ranked by the Child-Turcotte-Pugh and MELD scores[14].
All patients were evaluated before starting DAAs and every 3 mo for 1 year by clinical, biochemical, and ultrasound examinations. Thereafter, the follow up was established according to hepatic disease severity. Regular medical controls varied from every 1 to 6 mo per patient. At baseline, we collected general clinical characteristics, anthropometric variables, and hemochromocytometric and coagulation parameters and assessed liver and kidney functions, plasma glucose and alpha-fetoprotein levels and the presence of cryoglobulins. The body mass index was calculated as the body weight divided by the squared height in meters. The glomerular filtration rate was estimated by the Modification of Diet in Renal Disease study equation with 4 variables. The presence of diabetes was documented according to the intake of oral antidiabetic agents and/or the administration of insulin therapy or by fasting blood glucose higher than 125 mg/dL on two separate days. Alcohol consumption was considered when the intake was equal to or greater than 20 g/d of ethanol for both sexes.
The presence of HCC was suspected by ultrasound examination and/or elevated alpha-fetoprotein levels (> 400 ng/mL) and was confirmed by contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI). When doubts persisted, CT-guided liver biopsy was performed. Patients with HCC were staged according to the Barcelona Clinic Liver Cancer system and were treated, whenever appropriate, by surgical resection according to the Milan criteria[15,16]. Unresectable HCC was treated by liver transplantation, percutaneous lesion radiofrequency or ethanol ablation, or trans-arterial chemoembolization. Patients with HCV viremia and previous HCC that had been treated by surgical resection, liver transplantation, or radical local ablation were considered cured after radiological confirmation (complete radiological response). These patients were treated with DAAs after confirmation of the complete radiological response according to the Italian guidelines and were included in the study. During the follow up, the occurrence of de-novo (new) HCC or death from any cause was registered for all patients until the end of the study. The patient data and informed consent were collected according to Italian laws on observational retrospective studies and the Italian Data Protection Authority. The study was approved by the Institutional Review Board of the University of Udine.
HCV infection was suspected by the detection of serologic anti-HCV antibodies using a standard immunoassay and was confirmed by the measurement of viremia using quantitative RNA analysis (Abbott RealTime HCV, Abbott, Rungis, France). HIV and HBV coinfections were evaluated by standard methods in all HCV-positive patients. The genotype of HCV was established by NS5b gene sequencing[17]. Blood and coagulation variables, plasma aminotransferases, gamma-glutamyl-aminotransferase, alkaline phosphatase, total bilirubin, creatinine, and glucose levels were assessed by automated laboratory methods. The prothrombin time was normalized using standard plasma and was expressed as the international normalized ratio (INR). Plasma albumin was measured using a colorimetric method (ALB2; Roche cobas®) and an automated system (Roche/Hitachi Cobas c 701, Roche Diagnostics GmbH, Mannheim). Cryoglobulins were assessed at baseline according to Vermeersch et al[18]. Specifically, the blood was clotted for 1 h, and the serum was separated by centrifugation at 37 °C and refrigerated at 4 °C to allow cryoglobulin precipitation (cryocrit) over 7 d. The cryoglobulin protein concentration was measured by spectrophotometry, and the proteins were characterized after rewarming at 37 °C using an immunologic assay. Only type II or III mixed cryoglobulinemia (MC) was considered potentially linked to HCV infection[2]. We considered a cryocrit greater than 1% to be clinically significant. The plasma alpha-fetoprotein level was measured using an immunoenzymatic chemiluminescence method.
Contrast-enhanced CT and MRI examinations to assess upper abdomen organs were performed by standardized methods using a 64-row multidetector CT scanner (Discovery HD 750, GE Healthcare, Milwaukee, WI, United States) and a 3.0-T magnet using a 32-channel surface coil (Achieva, Philips Medical System, Best, the Netherlands), respectively. Liver biopsies were evaluated by an expert liver histopathologist.
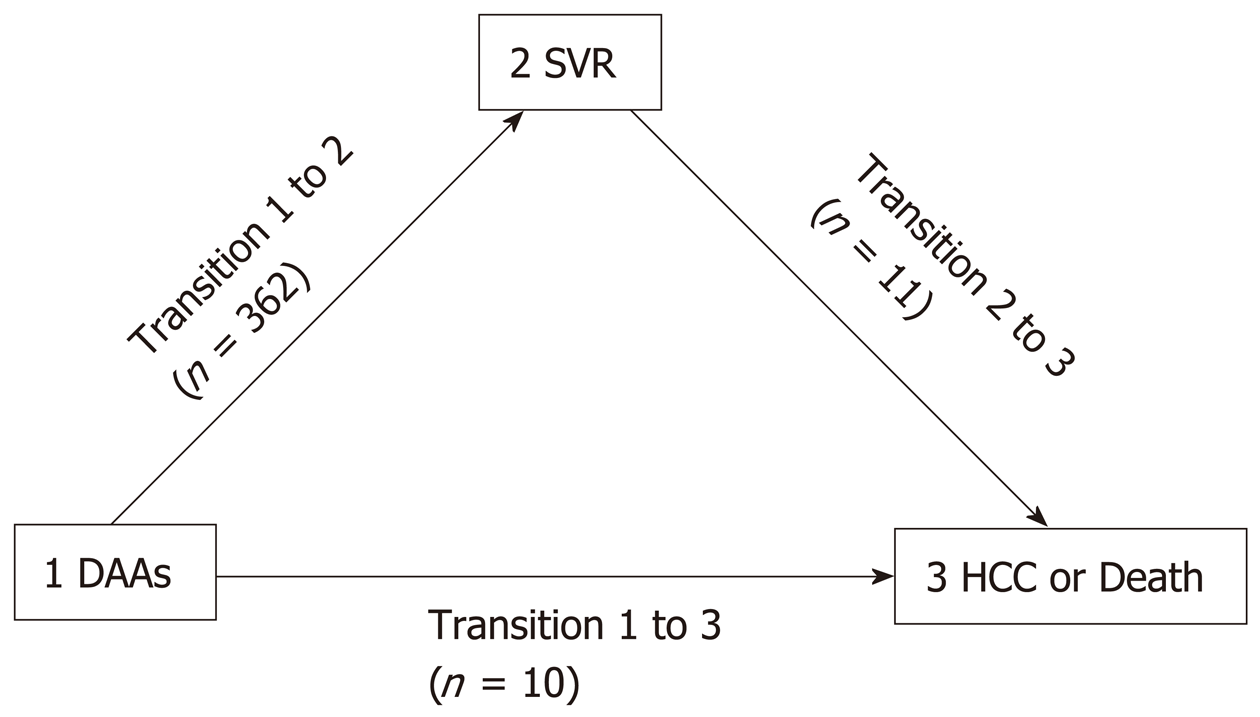
The data are presented as the means ± standard deviation or medians [interquartile range (IQR)] when appropriate. The normal distribution of variables was assessed by histogram analysis and the Shapiro-Wilks test for normality. Skewed variables were log transformed to achieve a normal distribution. Proportions were presented as counts (%). The mean difference was assessed by student’s t-test or by the nonparametric Wilcoxon-Mann-Whitney test when appropriate. Proportions were analyzed as contingency tables, and differences were assessed by Fisher’s exact test. Correlation analysis was performed by calculating the Pearson’s correlation coefficient. The variance in the continuous variables across factor levels was assessed by one-way analysis of variance (ANOVA), and Tukey’s method was used to correct for multiple comparisons. The incidence rate and incident rate ratio with 95%CIs were calculated as measures of incidence and for comparative purposes (analyzed by a chi-Squared test). Kaplan-Meier curves with a 95%CI and frequency tables were used to represent the event rates over time, and the nonparametric log-rank test was used to assess significant differences. Factors associated with the event rate were analyzed by Cox’s proportional hazards analysis in univariate and multivariate models, and the results were expressed as hazard ratios (HRs) at 95%CIs. To analyze the predictors of SVR achievement and the predictors of HCC or death occurrence with or without SVR achievement, multi-state analysis with 3 states (Figure 1) was conducted. In this model, SVR was considered as a time-dependent variable, and Cox’s proportional hazards were measured for each of the 3 state transitions using a clock-forward approach in a Markov chain model (Cox-Markov model)[19]. The multi-state Cox-Markov model is an extension of the classical survival analysis; with this model, we investigated the effect of different baseline cofactors for each disease state[20].
Multivariate model selection was assessed by the evaluation of the best model starting from a model that included age and sex and considered all other variables with univariate statistical or biological relevance by a stepwise forward approach based on the Akaike information criterion[21]. For statistical analysis, we used software R (The R Foundation for Statistical Computing, Vienna, Austria, version 3.5.2) using the “epiR” (version 0.9-99), “survival” (version 2.43-3), “mstate” (version 0.2.11), and “MASS” (version 7.3-51.1) packages[22].
The study cohort comprised 399 patients who were prescribed interferon-free DAAs. Two of the patients did not initiate DAAs because of alcohol abuse or decompensated cirrhosis, two patients did not complete DAA treatment because they did not comply with the therapy, and 15 patients were lost to follow up or had incomplete data. The remaining 380 patients were included in the analysis. Cirrhosis was diagnosed in 122 patients before starting DAA treatment. Eight patients had a previous diagnosis of HCC that was cured by orthotopic liver transplantation in six patients, and by surgical resection or percutaneous radiofrequency in the remaining two patients. We observed no significant interactions between DAAs and immunosuppressive agents that led us to modify the dose or drug type. The frequencies of the different interferon-free DAAs used and the genotypes of HCV are reported in Supplemental Tables S2 and S3, respectively. DAA treatment was effective in 362 patients (95%), and the overall incidence rate of HCC was 3.2 per 100 person-years. The incidence rate of HCC in patients who did not achieve SVR was 59 per 100 person-years, whereas it was 1.3 per 100 person-years in those with SVR (incidence rate ratio: 44, 95%CI: 15-136, P < 0.001). Five patients interrupted DAA treatment for adverse events, 1 in the group with SVR and 4 in the group without SVR (P < 0.001). The frequency of different adverse events reported during treatment is presented in Supplemental Table S4.
At baseline, the patients with SVR had a higher HCV viral load, platelet count, and plasma albumin levels than the patients without SVR, and the patients with SVR a lower occurrence of previous HCC, prevalence of cirrhosis, relapse of HCV infection after initial DAA treatment, and lower Metavir scores, INR, and plasma alpha-fetoprotein levels (Table 1) than the patients without SVR. Patients who developed HCC during the follow up after DAA therapy had, at baseline, a higher occurrence of previous HCC, relapse of HCV infection after initial DAA treatment, presence of plasma cryoglobulins, higher Metavir score, and prevalence of cirrhosis, INR, and plasma alpha-fetoprotein levels; these patients also had a lower HCV viral load, plasma albumin levels, and platelet count than patients without HCC (Table 1).
| Variable | All patients (n = 380) | No SVR (n = 18) | SVR (n = 362) | P value | No HCC (n = 363) | HCC (n = 17) | P value |
| General clinical characteristics | |||||||
| Age (yr) | 60 ± 13 | 62 ± 10 | 59 ± 13 | NS | 59 ± 13 | 61 ± 9 | NS |
| Male, n (%) | 224 (58.9) | 14 (77.8) | 210 (58.0) | NS | 213 (58.7) | 11 (64.7) | NS |
| BMI (kg/m2) | 24.9 ± 3.7 | 24.6 ± 2.7 | 24.9 ± 3.7 | NS | 24.9 ± 3.7 | 24.2 ± 3.2 | NS |
| Alcohol use, n (%) | 17 (4.5) | 2 (11.1) | 15 (4.1) | NS | 16 (4.4) | 1 (5.9) | NS |
| Diabetes, n (%) | 48 (12.6) | 4 (22.2) | 44 (12.1) | NS | 44 (12.1) | 4 (23.5) | NS |
| Plasma creatinine (mg/dL) | 0.86 ± 0.24 | 1.03 ± 0.42 | 0.86 ± 0.23 | NS | 0.86 ± 0.24 | 0.97 ± 0.30 | NS |
| eGFR (mL/min/1.73 m2) | 87 ± 25 | 91 ± 18 | 87 ± 25 | NS | 87 ± 25 | 86 ± 16 | NS |
| HCV infection-related variables | |||||||
| HCV viremia (n × 103) | 956 (203-2944) | 297 (47-1442) | 977 (219-3059) | 0.033 | 967 (219-3049) | 244 (36-1864) | NS |
| HIV coinfection, n (%) | 8 (2.1) | 0 | 8 (100) | - | 8 (100) | 0 | - |
| Previous use of interferon, n (%) | 137 (36.0) | 7 (38.9) | 130 (35.9) | NS | 129 (35.5) | 8 (47.1) | NS |
| Use of ribavirin association, n (%) | 89 (23.4) | 5 (27.8) | 84 (23.2) | NS | 84 (23.1) | 5 (29.4) | NS |
| SVR, n (%) | 362 (95.3) | - | 362 (100) | - | 355 (97.8) | 10 (58.8) | < 0.001 |
| Time to SVR (wk) | 12 (12-13) | - | 12 (12-13) | - | 12 (12-12) | 15 (13-23) | < 0.001 |
| HCV relapse, n (%) | 20 (5.3) | 12 (66.7) | 8 (2.2) | < 0.001 | 13 (3.6) | 7 (41.2) | < 0.001 |
| Mixed cryoglobulinemia, n (%) | 35 (9.2) | 4 (22.2) | 31 (8.6) | NS | 30 (8.3) | 5 (29.4) | 0.014 |
| Liver structure and function variables | |||||||
| Metavir score | 2.6 ± 1.2 | 3.7 ± 0.6 | 2.6 ± 1.2 | < 0.001 | 2.6 ± 1.2 | 3.9 ± 0.3 | < 0.001 |
| Cirrhosis, n (%) | 122 (32.1) | 14 (77.8) | 108 (29.8) | < 0.001 | 107 (29.5) | 15 (88.2) | < 0.001 |
| Plasma albumin (g/L) | 41.0 ± 5.4 | 37.6 ± 6.3 | 41.2 ± 5.3 | 0.027 | 41.4 ± 5.4 | 38.7 ± 5.0 | 0.047 |
| Plasma total bilirubin (g/dL) | 0.79 ± 0.41 | 0.92 ± 0.50 | 0.78 ± 0.41 | NS | 0.78 ± 0.41 | 0.94 ± 0.47 | NS |
| Plasma alpha-fetoprotein (ng/mL) | 4.10 (2.87-7.10) | 6.30 (5.20-8.70) | 4.10 (2.75-7.05) | 0.012 | 4.10 (2.70-6.80) | 8.00 (4.95-10.80) | 0.001 |
| Platelet count (n × 103/mL) | 167 ± 67 | 114 ± 67 | 170 ± 66 | 0.003 | 170 ± 66 | 98 ± 62 | < 0.001 |
| INR | 1.05 ± 0.12 | 1.14 ± 0.19 | 1.04 ± 0.11 | 0.038 | 1.05 ± 0.11 | 1.17 ± 0.17 | 0.013 |
| HCC-related variables | |||||||
| Previous HCC, n (%) | 8 (2.1) | 3 (16.7) | 5 (1.4) | 0.004 | 5 (1.4) | 3 (17.6) | 0.004 |
| HCC, n (%) | 17 (4.5) | 10 (55.5) | 7 (1.9) | < 0.001 | - | 17 (100) | - |
| Time to HCC (wk) | 26 (17-65) | 25 (12-57) | 40 (25-63) | NS | - | 26 (17-65) | - |
| Death, n (%) | 8 (2.1) | 3 (16.7) | 5 (1.4) | 0.004 | 4 (1.1) | 4 (23.5) | < 0.001 |
| Time to death (wk) | 65.5 (50.5-126.8) | 40 (28-79) | 76 (55-150) | NS | 65 (55-95) | 79 (34-127) | NS |
The baseline age, sex, BMI, alcohol intake, presence of diabetes, renal function, use of ribavirin in association with DAAs, previous use of interferon and plasma total bilirubin levels were not associated with the achievement of SVR or the development of HCC (Table 1). Although a different proportion between the type of inclusion criteria and presence of HCC was observed, no differences were seen between the inclusion criteria and SVR and between the HCV genotype and presence of SVR or HCC (Supplemental Table S3), whereas a difference in the proportion between the DAAs used and the presence of SVR or HCC was observed (P = 0.010 and P < 0.001, respectively; Supplemental Table S2). DAA treatment induced SVR similarly in patients who had previously used interferon and in those who had not, at rates of 94.8% and 95.5%, respectively. HCC developed in 5.8% or 3.7% of patients who had used interferon or not previously, respectively. The differences between these groups were not significant. Three of eight patients with a previously cured HCC showed recurrence of HCC, and all 3 of these patients did not achieve SVR after DAA treatment. The remaining 5 patients achieved SVR and did not develop HCC. The log of the HCV viral load was directly associated with the log of the platelet count (P = 0.014), and a significant difference was found between the log HCV viral load across Metavir scores (P = 0.014); specifically, the viral load in the Metavir F4 score was significantly lower than that in the F3 score (P = 0.032 after Tukey’s correction). An inverse relationship was found between the platelet count across Metavir scores (P < 0.001).
Patients with MC were more frequently of the female sex (95% vs 83%, P < 0.001), older (64 ± 10 vs 59 ± 13 years, P = 0.005), and with a worse renal function (eGFR 79 ± 24 vs 88 ± 25 mL/min/1.73 m2, P = 0.039) than patients without MC at baseline. No association was found between MC and the Metavir score, presence of cirrhosis, platelet count, albumin levels or INR. HIV coinfection was not associated with SVR or HCC. No HBV coinfection was detected in all included patients.
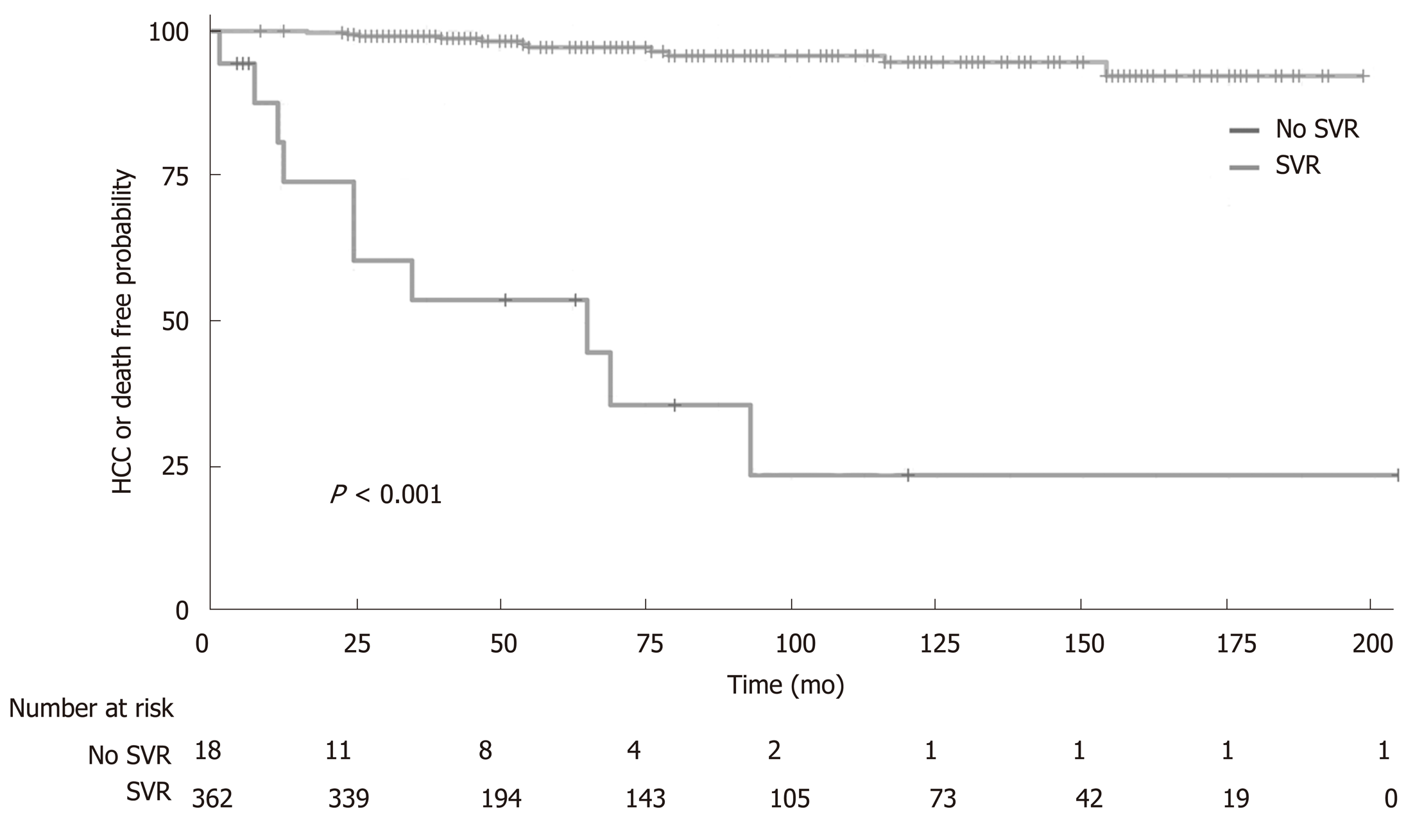
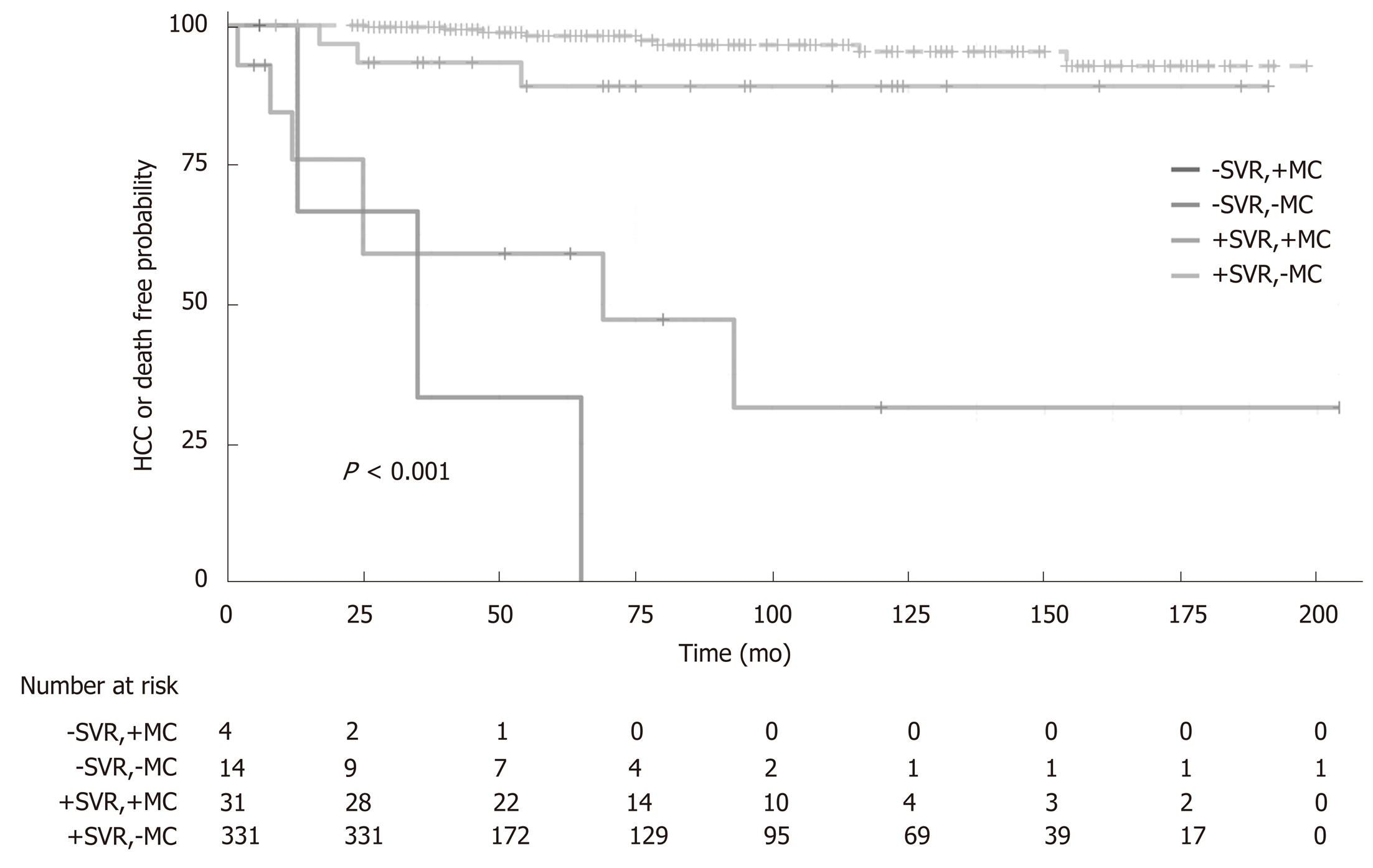
The effect of SVR on the combined endpoint of a new HCC occurrence or death from any cause during the follow up is represented by Kaplan-Meier curves in Figure 2. In a median follow up of 58 wk (IQR 38-117), the patients with SVR had a higher survival rate free from HCC than patients who did not achieve SVR (HR: 0.035, 95%CI: 0.015-0.084, P < 0.0001, Figure 2). In classical Cox’s multivariate proportional hazards analysis, the factors that best and independently predicted the combined endpoint were the log of HCV viremia (HR: 0.808, 95%CI: 0.666-0.980, P = 0.030), platelet count (HR: 0.910, 95%CI: 0.831-0.996, P = 0.041), presence of MC (HR: 3.460, 95%CI: 1.035-11.56, P = 0.044), and presence of SVR (HR: 0.070, 95%CI: 0.025-0.194, P < 0.001). The predictors were independent of age, sex, renal function, and presence of cirrhosis (Table 2). In Figure 3, we reported the independent effect of both the SVR and MC status on the combined endpoint of HCC or death from any cause. The effect of the presence of cirrhosis on the combined end-point by SVR achievement is represented in Figure S1 of the supplemental material.
| Univariate analysis | Multivariate analysis | |||
| Variable | HR (95%CI) | P value | HR (95%CI) | P value |
| Age (yr) | 1.003 (0.968-1.040) | 0.862 | 1.008 (0.958-1.060) | 0.760 |
| Male (no/yes) | 1.334 (0.538-3.308) | 0.534 | 2.492 (0.683-9.089) | 0.167 |
| HCV viremia (log n) | 0.811 (0.698-0.944) | 0.007 | 0.808 (0.666-0.980) | 0.030 |
| Cirrhosis (no/yes) | 9.259 (2.705-31.70) | < 0.001 | 3.392 (0.861-13.37) | 0.081 |
| Platelet count (n × 104/mL) | 0.806 (0.735-0.885) | < 0.001 | 0.910 (0.831-0.996) | 0.041 |
| eGFR (10 mL/min/1.73 m2) | 0.935 (0.780-1.122) | 0.473 | 0.774 (0.596-1.005) | 0.055 |
| Mixed cryoglobulinemia (no/yes) | 4.522 (1.822-11.23) | 0.001 | 3.460 (1.035-11.56) | 0.044 |
| SVR (no/yes) | 0.035 (0.015-0.084) | < 0.001 | 0.070 (0.025-0.194) | < 0.001 |
Since the baseline hazards did not satisfy the proportional hazards assumption in the multi-state Cox-Markov model, we used stratified hazards instead of proportional hazards to assess baseline hazards for each separate state transition, as shown in Figure 1 (Table 3). The analysis showed that the best and independent predictors of SVR achievement (transition 1→2) were the absence of cirrhosis (HR: 0.521, 95%CI: 0.404-0.672, P < 0.001) and higher platelet count (HR: 1.019, 95%CI: 1.002-1.036, P = 0.026); the predictor of HCC or death in patients who did not achieve SVR (transition 1→3) was the presence of MC (HR: 5.633, 95%CI: 1.022-31.05, P = 0.047); and the predictors of HCC or death of patients who achieved SVR (transition 2→3) were the presence of MC (HR: 5.982, 95%CI: 1.217-29.41, P = 0.028), lower platelet count (HR: 0.958, 95%CI: 0.939-0.979, P < 0.001), and lower eGFR (HR: 0.948, 95%CI: 0.915-0.985, P = 0.005).
| Variable | HR | 95%CI | P value |
| Transition 1→2 | |||
| Age (yr) | 0.997 | 0.989-1.005 | 0.493 |
| Male (no/yes) | 0.789 | 0.615-1.013 | 0.062 |
| HCV viremia (log n) | 0.983 | 0.940-1.029 | 0.470 |
| Cirrhosis (no/yes) | 0.521 | 0.404-0.672 | < 0.001 |
| Mixed cryoglobulinemia (no/yes) | 0.755 | 0.520-1.098 | 0.141 |
| Platelet count (n × 104/mL) | 1.019 | 1.002-1.036 | 0.026 |
| eGFR (10 mL/min/1.73 m2) | 1.003 | 0.998-1.008 | 0.235 |
| Transition 1→3 | |||
| Age (yr) | 0.975 | 0.912-1.043 | 0.466 |
| Male (no/yes) | 2.040 | 0.249-16.74 | 0.507 |
| HCV viremia (log n) | 0.771 | 0.589-1.009 | 0.058 |
| Cirrhosis (no/yes) | 5.704 | 0.620-52.51 | 0.124 |
| Mixed cryoglobulinemia (no/yes) | 5.633 | 1.022-31.05 | 0.047 |
| Platelet count (n × 104/mL) | 0.928 | 0.981-1.005 | 0.229 |
| eGFR (10 mL/min/1.73 m2) | 1.007 | 0.970-1.045 | 0.726 |
| Transition 2→3 | |||
| Age (yr) | 1.019 | 0.945-1.098 | 0.625 |
| Male (no/yes) | 3.613 | 0.692-18.88 | 0.128 |
| HCV viremia (log n) | 0.972 | 0.715-1.321 | 0.857 |
| Cirrhosis (no/yes) | 2.720 | 0.479-15.44 | 0.259 |
| Mixed cryoglobulinemia (no/yes) | 5.982 | 1.217-29.41 | 0.028 |
| Platelet count (n × 104/mL) | 0.958 | 0.935-0.982 | < 0.001 |
| eGFR (10 mL/min/1.73 m2) | 0.948 | 0.915-0.985 | 0.005 |
In this study, HCV-infected patients who achieved SVR after DAA treatment had a significantly higher survival rate free from new HCC occurrence and a lower rate of death from any cause than patients who did not achieve SVR. This association was strongly independent of confounders in a classical survival model where the presence of SVR was considered a baseline variable together with age, sex, HCV viremia, presence of cirrhosis, MC, platelet count, and renal function.
Although SVR achievement using interferon-based regimens is a predictor of HCC-free survival[23], similar evidence is less clear for DAAs. Different retrospective studies have reported that treatment with these agents did not reduce the incidence of HCC[7] but can even increase the risk of early HCC recurrence after treatment[7,8]. Conversely, other studies with larger samples have suggested a strong beneficial effect of SVR on both the occurrence and recurrence of HCC[24-27]. Our study demonstrated that the achievement of SVR is robustly associated with a better HCC-free survival than a lack of SVR and is independent of previous interferon-based regimen use. However, the lack of a control group precluded the possibility of testing whether DAA treatment per se can be a risk factor for HCC development, although the overall observed HCC incidence rate was similar to that in previous studies with different HCV treatments[6]. Notably, it should be considered that patients with fibrosis > F2 (Metavir) or cirrhosis that achieved SVR after DAA treatment should still need surveillance for their residual risk to develop HCC[28].
SVR is a condition more frequently achieved in HCV-infected patients with a lower grade of liver fibrosis and less advanced hepatic disease. Patients with cirrhosis and/or a decompensated liver disease are less prone to achieve SVR, and these patients are also those with the highest probability of developing HCC and dying due to several causes[29]. Additionally, in our study, the predictors of SVR achievement were a low Metavir score or the absence of cirrhosis and a high platelet count, indicating the expression of less advanced liver disease. In addition, in patients who achieved SVR, the signs of portal hypertension and impaired renal function, such as the markers of advanced liver disease, are independent predictors of HCC development and death from any cause. This is an important point, since the use of DAAs was only recently approved in Italy for HCV patients with less advanced hepatic disease. In those low-risk patients, the efficacy of HCV eradication on the prevention of HCC was indeed more pronounced. This extension of DAAs use is expected to significantly reduce the HCV epidemic and the global burden of HCV-related complications.
In our study, a low baseline viral load was a predictor of HCC development or death from any cause. This point has been explored by Duvoux et al[30] who evaluated the HCV viral load in 3 groups of patients with chronic HCV infection: (1) Without cirrhosis; (2) With compensated cirrhosis; and (3) With end-stage cirrhotic disease waiting for liver transplantation[30]. The HCV viral load was significantly lower in end-stage cirrhosis disease than in the other groups and, more interestingly, the viral load increased after liver transplantation. This inverse relationship occurred despite the HCV genotype. The authors concluded that HCV replication is lower in end-stage cirrhotic disease because of a reduced hepatocyte mass and/or an impaired cell-to-cell virus transmission probably due to extensive liver fibrosis[30]. These HCV chronic patients were also those with the lowest probability of achieving SVR and highest risk of developing HCC or dying from any cause. In our study, the inverse association between the HCV viral load and severity of liver disease has been suggested by the indirect relationship between the viral load and Metavir score, and the direct relationship between the viral load and platelet count. However, the role of HCV viremia as a predictive factor of HCC development or mortality in cirrhotic patients remains controversial[31].
Interestingly, we also observed that the baseline presence of an MC predicted the probability of developing HCC or dying from any cause independently of SVR achievement. Asymptomatic or symptomatic MC is associated with HCV viral infection by mechanisms that are not well understood. Generally, stimulation of the B-lymphocyte system by HCV facilitates the development of immortalized B-cell clones releasing IgM with rheumatoid factor activity and the formation of immunocomplexes between immunoglobulins and HCV molecular components that precipitate in plasma below 37 °C. These immunocomplexes activate complement, inducing vascular inflammation that leads to vascular inflammation and cryoglobulinemic syndrome[32]. It is not clear how the presence of cryoglobulinemia could influence SVR achievement and/or HCC development. The study of Passerini et al[33] showed no difference in SVR achievement at 12 wk after DAA treatment between HCV patients with and without serum cryoglobulins; however, a previous meta-analysis of 19 observational studies, with more than 2000 patients enrolled, demonstrated that the development of cirrhosis was 5 times as frequent in patients with MC as in those without (OR: 4.90, 95%CI: 3.32-7.15)[34]. Paradoxically, the long-term prospective study of Lauletta et al[35] showed that the presence of a cryoglobulinemic syndrome is an independent protective factor against the development of both cirrhosis and HCC, although no difference was found in the overall survival between patients with or without cryoglobulins in 15 years of observation[35]. Nevertheless, the prevalence of MC in our study was lower than that expected from previous studies (40%-60%)[33], likely because of our elevated cut-off point for MC diagnosis that could have led us to underestimate low-grade MC[18].
This study has several limitations that need to be highlighted. First, we included all patients who had been treated with DAAs at a single center, without selection criteria to increase the sample size and improve the statistical power of the study. However, the study population was heterogeneous, and several unknown confounders could have influenced the results. Thus, we have used statistical adjustments in inferential methods and performed multivariate analyses with significant and clinically important confounders, factoring in such heterogeneity as much as possible. Second, we had a low incidence of events and a low median observational time. This low incidence is because we included only HCV patients treated with second- and third-generation DAAs that were introduced in Italy only recently. This point is important because the low outcome rate together with the elevated SVR achievement rate could have limited the power of our multi-state Cox-Markov analysis to confirm a protective effect of SVR on HCC development and mortality. On the other hand, the strength of our study is that the use of a multi-state Cox-Markov model has permitted us to explore more deeply the relationships of the baseline variables with events in each different disease state, which is not possible with a classical survival Cox model. Third, the lack of a control group did not permit exploration of the hypothesis of DAAs as a potential risk factor for HCC development; however, this investigation was not among the aims of the study. Fourth, the follow up interval could be relatively short for HCC development, at least in a general population of HCV-infected patients. However, because the national guidelines limits DAA prescription to patients with advanced or complicated liver disease (Table S1 of supplemental material), we necessarily treated patients with a high risk of developing HCC in a shorter period.
In conclusion, we confirmed that DAA treatment is highly effective in eradicating HCV infection, and this eradication occurs more frequently in patients with less advanced liver disease. In addition, HCV-infected patients who achieved SVR after DAA treatment have a higher survival free from new HCC occurrence or death from any cause than patients who did not achieve SVR. In a classical survival model, this association was strongly independent of confounders.
Direct-acting antiviral agents (DAAs) are extremely effective in eradicating hepatitis C virus (HCV) in chronically infected patients. However, the protective role of a sustained virologic response (SVR) achieved by second- and third-generation DAAs against the onset of hepatocellular carcinoma (HCC) and mortality is less well established.
The main topic of this study was to establish the effectiveness of new generations of DAAs to induce SVR and to prevent HCC development or mortality in HCV-infected patients. These patients were characterized by an elevated risk for HCC because of their advanced or complicated liver disease. The strength of this study was the use of a multi-state Cox-Markov model. This model permitted us to explore more deeply the relationships of baseline variables with events in each different disease state. This last approach is not possible with a classical survival Cox model.
The aim of this study was to examine HCC occurrence or death from any cause in a retrospective-prospective study of patients treated with DAAs and to identify potential predictors of SVR achievement and HCC development.
The patients were enrolled from a tertiary academic hospital center for liver disease management that collects subject data mainly from northeastern Italy. The study was conducted using 380 patients (age: 60 ± 13 years, 224 males, 32% with cirrhosis) treated with DAAs with or without SVR (95/5%), with a median follow up of 58 wk (interquartile range: 38-117). The baseline anthropometric features, HCV viral load, severity of liver disease, presence of extra-hepatic complications, coinfection with HIV and/or HBV, alcohol consumption, previous interferon use, alpha-fetoprotein levels, and renal function were considered to be confounders. Survival analysis was conducted using a classical Cox model and a more advanced Cox-Markov multi-state model that considered SVR as a time-dependent variable that can influence the probability of the outcome.
The incidence rates of HCC in patients with and without SVR were 1.3 and 59 per 100 person-years, respectively (incidence rate ratio: 44, 95%CI: 15-136, P < 0.001). Considering the combined endpoint of HCC or death from any cause, the hazard ratio (HR) for SVR patients was 0.070 (95%CI: 0.025-0.194, P < 0.001). Other independent predictors of HCC or death were low HCV viremia (HR: 0.808, P = 0.030), low platelet count (HR: 0.910, P = 0.041), and presence of mixed cryoglobulinemia (HR: 3.460, P = 0.044). Considering SVR in a multi-state model, independent predictors of SVR achievement were the absence of cirrhosis (HR: 0.521, P < 0.001) and high platelet count (HR: 1.019, P = 0.026). Mixed cryoglobulinemia predicted the combined endpoint in patients with and without SVR (HR: 5.982, P = 0.028 and HR: 5.633, P = 0.047, respectively).
Treatment with DAAs is very effective in inducing SVR and protecting against HCC or death. A residual risk for HCC persists in patients with advanced liver disease or disease complicated by extra-hepatic manifestations, such as mixed cryoglobulinemia and renal failure.
New generations of DAAs should be recommended to treat all patients with HCV infection independently of the status of their liver disease or the presence of extra-hepatic manifestations. Further studies are needed to define a better strategy to treat HCV-infected patients with more advanced or complicated liver disease.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Carrier P, Grawish ME, Tuna N, Wang L, Yang SS, Zheng H S-Editor: Yan JP L-Editor: A E-Editor: Zhang YL
| 1. | Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014;61:S45-S57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1325] [Cited by in RCA: 1362] [Article Influence: 123.8] [Reference Citation Analysis (0)] |
| 2. | Cacoub P, Poynard T, Ghillani P, Charlotte F, Olivi M, Piette JC, Opolon P. Extrahepatic manifestations of chronic hepatitis C. MULTIVIRC Group. Multidepartment Virus C. Arthritis Rheum. 1999;42:2204-2212. [PubMed] [DOI] [Full Text] |
| 3. | Reddy KR, Wright TL, Pockros PJ, Shiffman M, Everson G, Reindollar R, Fried MW, Purdum PP, Jensen D, Smith C, Lee WM, Boyer TD, Lin A, Pedder S, DePamphilis J. Efficacy and safety of pegylated (40-kd) interferon alpha-2a compared with interferon alpha-2a in noncirrhotic patients with chronic hepatitis C. Hepatology. 2001;33:433-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 261] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 4. | Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4736] [Cited by in RCA: 4558] [Article Influence: 189.9] [Reference Citation Analysis (0)] |
| 5. | Andreone P, Colombo MG, Enejosa JV, Koksal I, Ferenci P, Maieron A, Müllhaupt B, Horsmans Y, Weiland O, Reesink HW, Rodrigues L, Hu YB, Podsadecki T, Bernstein B. ABT-450, ritonavir, ombitasvir, and dasabuvir achieves 97% and 100% sustained virologic response with or without ribavirin in treatment-experienced patients with HCV genotype 1b infection. Gastroenterology. 2014;147:359-365.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 282] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 6. | Waziry R, Hajarizadeh B, Grebely J, Amin J, Law M, Danta M, George J, Dore GJ. Hepatocellular carcinoma risk following direct-acting antiviral HCV therapy: A systematic review, meta-analyses, and meta-regression. J Hepatol. 2017;67:1204-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 380] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 7. | Conti F, Buonfiglioli F, Scuteri A, Crespi C, Bolondi L, Caraceni P, Foschi FG, Lenzi M, Mazzella G, Verucchi G, Andreone P, Brillanti S. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J Hepatol. 2016;65:727-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 698] [Article Influence: 77.6] [Reference Citation Analysis (0)] |
| 8. | Reig M, Mariño Z, Perelló C, Iñarrairaegui M, Ribeiro A, Lens S, Díaz A, Vilana R, Darnell A, Varela M, Sangro B, Calleja JL, Forns X, Bruix J. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J Hepatol. 2016;65:719-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 725] [Cited by in RCA: 803] [Article Influence: 89.2] [Reference Citation Analysis (0)] |
| 9. | European Medicines Agency. Assessment report-Procedure under Article 20 of Regulation (EC) No 726/2004 resulting from pharmacovigilance data [Internet]. 8 September 2016 [cited 2019 Apr 13]. Available from: https://www.ema.europa.eu/en/documents/referral/direct-acting-antivirals-hepatitis-c-article-20-procedure-prac-assessment-report_en.pdf. |
| 10. | European Association for Study of Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2014;60:392-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 635] [Cited by in RCA: 655] [Article Influence: 59.5] [Reference Citation Analysis (0)] |
| 11. | European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu.; European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C 2018. J Hepatol. 2018;69:461-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1281] [Cited by in RCA: 1209] [Article Influence: 172.7] [Reference Citation Analysis (0)] |
| 12. | Paranaguá-Vezozzo DC, Andrade A, Mazo DF, Nunes V, Guedes AL, Ragazzo TG, Moutinho R, Nacif LS, Ono SK, Alves VA, Carrilho FJ. Concordance of non-invasive mechanical and serum tests for liver fibrosis evaluation in chronic hepatitis C. World J Hepatol. 2017;9:436-442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Shiha G, Zalata K. Ishak versus METAVIR: Terminology, Convertibility and Correlation with Laboratory Changes in Chronic Hepatitis C. Liver Biopsy [Internet]. 2011 Sep 6 [cited 2019 Mar 30]. Available from: https://www.intechopen.com/books/liver-biopsy/ishak-versus-metavir-terminology-convertibility-and-correlation-with-laboratory-changes-in-chronic-h. |
| 14. | Butt AA, Ren Y, Lo Re V, Taddei TH, Kaplan DE. Comparing Child-Pugh, MELD, and FIB-4 to Predict Clinical Outcomes in Hepatitis C Virus-Infected Persons: Results From ERCHIVES. Clin Infect Dis. 2017;65:64-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 15. | Pons F, Varela M, Llovet JM. Staging systems in hepatocellular carcinoma. HPB (Oxford). 2005;7:35-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 206] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 16. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6029] [Article Influence: 861.3] [Reference Citation Analysis (3)] |
| 17. | Laperche S, Saune K, Dény P, Duverlie G, Alain S, Chaix ML, Gaudy C, Lunel F, Pawlotsky JM, Payan C, Pozzetto B, Tamalet C, Thibault V, Vallet S, Bouchardeau F, Izopet J, Lefrère JJ. Unique NS5b hepatitis C virus gene sequence consensus database is essential for standardization of genotype determinations in multicenter epidemiological studies. J Clin Microbiol. 2006;44:614-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Vermeersch P, Gijbels K, Mariën G, Lunn R, Egner W, White P, Bossuyt X. A critical appraisal of current practice in the detection, analysis, and reporting of cryoglobulins. Clin Chem. 2008;54:39-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med. 2007;26:2389-2430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1342] [Cited by in RCA: 1608] [Article Influence: 89.3] [Reference Citation Analysis (0)] |
| 20. | de Wreede LC, Fiocco M, Putter H. The mstate package for estimation and prediction in non- and semi-parametric multi-state and competing risks models. Comput Methods Programs Biomed. 2010;99:261-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 264] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 21. | Zhang Z. Variable selection with stepwise and best subset approaches. Ann Transl Med. 2016;4:136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 321] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 22. | R Core Team. R: A language and environment for statistical computing. [Internet]. Vienna, Austria: R Foundation for Statistical Computing, 2018. Available from: https://www.R-project.org/. |
| 23. | Singal AK, Singh A, Jaganmohan S, Guturu P, Mummadi R, Kuo YF, Sood GK. Antiviral therapy reduces risk of hepatocellular carcinoma in patients with hepatitis C virus-related cirrhosis. Clin Gastroenterol Hepatol. 2010;8:192-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 166] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 24. | Ioannou GN, Green PK, Berry K. HCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinoma. J Hepatol. 2017;pii:S0168-8278(17)32273-0. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 375] [Article Influence: 46.9] [Reference Citation Analysis (1)] |
| 25. | Lleo A, Aglitti A, Aghemo A, Maisonneuve P, Bruno S, Persico M; collaborators. Predictors of hepatocellular carcinoma in HCV cirrhotic patients treated with direct acting antivirals. Dig Liver Dis. 2019;51:310-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 26. | Romano A, Angeli P, Piovesan S, Noventa F, Anastassopoulos G, Chemello L, Cavalletto L, Gambato M, Russo FP, Burra P, Vincenzi V, Scotton PG, Panese S, Tempesta D, Bertin T, Carrara M, Carlotto A, Capra F, Carolo G, Scroccaro G, Alberti A. Newly diagnosed hepatocellular carcinoma in patients with advanced hepatitis C treated with DAAs: A prospective population study. J Hepatol. 2018;69:345-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 125] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 27. | Cabibbo G, Celsa C, Calvaruso V, Petta S, Cacciola I, Cannavò MR, Madonia S, Rossi M, Magro B, Rini F, Distefano M, Larocca L, Prestileo T, Malizia G, Bertino G, Benanti F, Licata A, Scalisi I, Mazzola G, Di Rosolini MA, Alaimo G, Averna A, Cartabellotta F, Alessi N, Guastella S, Russello M, Scifo G, Squadrito G, Raimondo G, Trevisani F, Craxì A, Di Marco V, Cammà C; Rete Sicilia Selezione Terapia – HCV (RESIST-HCV) and Italian Liver Cancer (ITA. LI.CA.) Group. Direct-acting antivirals after successful treatment of early hepatocellular carcinoma improve survival in HCV-cirrhotic patients. J Hepatol. 2019;71:265-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 154] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 28. | Su F, Ioannou GN. The impact of direct-acting antiviral therapy for hepatitis C on hepatocellular carcinoma risk. Curr Hepatol Rep. 2018;17:377-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Pham TT, Keast SL, Farmer KC, Thompson DM, Rathbun RC, Nesser NJ, Holderread BP, Skrepnek GH. Sustained Virologic Response and Costs Associated with Direct-Acting Antivirals for Chronic Hepatitis C Infection in Oklahoma Medicaid. J Manag Care Spec Pharm. 2018;24:664-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Duvoux C, Pawlotsky JM, Bastie A, Cherqui D, Soussy CJ, Dhumeaux D. Low HCV replication levels in end-stage hepatitis C virus-related liver disease. J Hepatol. 1999;31:593-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 31. | Noh R, Lee DH, Kwon BW, Kim YH, Kim SB, Song IH. Clinical Impact of Viral Load on the Development of Hepatocellular Carcinoma and Liver-Related Mortality in Patients with Hepatitis C Virus Infection. Gastroenterol Res Pract. 2016;2016:7476231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | Dammacco F, Lauletta G, Russi S, Leone P, Tucci M, Manno C, Monaco S, Ferrari S, Vacca A, Racanelli V. Clinical practice: hepatitis C virus infection, cryoglobulinemia and cryoglobulinemic vasculitis. Clin Exp Med. 2019;19:1-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 33. | Passerini M, Schiavini M, Magni CF, Landonio S, Niero F, Passerini S, Croci AL, Bolis M, Scalzi V, Gubertini G, Ricci ED, Galli M, Rizzardini G. Are direct-acting antivirals safe and effective in hepatitis C virus-cryoglobulinemia? virological, immunological, and clinical data from a real-life experience. Eur J Gastroenterol Hepatol. 2018;30:1208-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 34. | Kayali Z, Buckwold VE, Zimmerman B, Schmidt WN. Hepatitis C, cryoglobulinemia, and cirrhosis: a meta-analysis. Hepatology. 2002;36:978-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 109] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 35. | Lauletta G, Russi S, Conteduca V, Sansonno L, Dammacco F, Sansonno D. Impact of Cryoglobulinemic Syndrome on the Outcome of Chronic Hepatitis C Virus Infection: A 15-Year Prospective Study. Medicine (Baltimore). 2013;92:245-256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |