Published online Oct 28, 2019. doi: 10.3748/wjg.v25.i40.6063
Peer-review started: July 16, 2019
First decision: August 18, 2019
Revised: September 3, 2019
Accepted: September 27, 2019
Article in press: September 28, 2019
Published online: October 28, 2019
Processing time: 107 Days and 7.9 Hours
Studies have shown that insulin-like growth factor 2 mRNA-binding protein 1 (IGF2BP1) plays critical roles in the genesis and development of human cancers.
To investigate the clinical significance and role of IGF2BP1 in pancreatic cancer.
Expression levels of IGF2BP1 and microRNA-494 (miR-494) were mined based on Gene Expression Omnibus datasets and validated in both clinical samples and cell lines by quantitative real-time polymerase chain reaction and Western blot. The relationship between IGF2BP1 expression and clinicopathological factors of pancreatic cancer patients was analyzed. The effect and mechanism of IGF2BP1 on pancreatic cancer cell proliferation were investigated in vitro and in vivo. Analyses were performed to explore underlying mechanisms of IGF2BP1 upregulation in pancreatic cancer and assays were carried out to verify the post-transcriptional regulation of IGF2BP1 by miR-494.
We found that IGF2BP1 was upregulated and associated with a poor prognosis in pancreatic cancer patients. We showed that downregulation of IGF2BP1 inhibited pancreatic cancer cell growth in vitro and in vivo via the AKT signaling pathway. Mechanistically, we showed that the frequent upregulation of IGF2BP1 was attributed to the downregulation of miR-494 expression in pancreatic cancer. Furthermore, we discovered that reexpression of miR-494 could partially abrogate the oncogenic role of IGF2BP1.
Our results revealed that upregulated IGF2BP1 promotes the proliferation of pancreatic cancer cells via the AKT signaling pathway and confirmed that the activation of IGF2BP1 is partly due to the silencing of miR-494.
Core tip: Insulin-like growth factor 2 mRNA-binding protein 1 (IGF2BP1) exerts vital roles in the development of various cancers; however, the expression, functional role, and regulatory mechanisms of IGF2BP1 in pancreatic cancer remain unclear. In this study, the expression levels of IGF2BP1 and miR-494 were mined based on Gene Expression Omnibus datasets and validated in both clinical samples and cell lines. The relationship between IGF2BP1 expression and clinicopathological factors of pancreatic cancer patients was analyzed. The effect of IGF2BP1 on pancreatic cancer cell proliferation and underlying regulatory mechanism were investigated. Our study suggests that IGF2BP1 may be a new therapeutic target for pancreatic cancer.
- Citation: Wan BS, Cheng M, Zhang L. Insulin-like growth factor 2 mRNA-binding protein 1 promotes cell proliferation via activation of AKT and is directly targeted by microRNA-494 in pancreatic cancer. World J Gastroenterol 2019; 25(40): 6063-6076
- URL: https://www.wjgnet.com/1007-9327/full/v25/i40/6063.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i40.6063
Pancreatic ductal adenocarcinoma (PDAC) is one of the most common causes of cancer-related deaths worldwide[1]. Currently, the treatment options for patients with inoperable pancreatic cancer are very limited, and the clinical outcomes remain unsatisfactory[2,3]. Although some understanding of the molecular pathogenesis of pancreatic cancer development and progression was obtained[4-6], new therapy targets are necessary to improve the survival rate of pancreatic cancer patients. Insulin-like growth factor 2 mRNA-binding protein 1 (IGF2BP1), also known as IMP-1 or CRD-BP, belongs to a conserved family of oncofetal RNA-binding proteins, which include IGF2BP2 and IGF2BP3[7,8]. IGF2BP1 controls the translation and stability of its target RNAs by posttranscriptional regulation[9]. Increasing evidence indicates that the IGF2BP family plays critical roles in the genesis and development of human cancers. IGF2BP2 expression predicts poor outcomes in gallbladder cancer patients and high tumor growth rates in xenograft models[10]. IGF2BP1 reduces the metastatic potential of breast cancer in a mouse model[11], regulates the expression and activity of GLI1 in the development of basal cell carcinoma[12], and acts as an adaptor protein that recruits the CCR4-NOT complex and thereby initiates the degradation of the long noncoding RNA (lncRNA) highly upregulated in liver cancer (HULC) in hepatocarcinoma[13]. Currently, very little is known regarding IGF2BP1 expression in PDAC and its role in genesis or progression of this malignancy.
MicroRNAs (miRNAs) play important roles in cellular differentiation and proliferation in embryo development and cancer progression. MiRNAs bind to the 3’ untranslated region (3'-UTR) of the target mRNAs, which results in mRNA cleavage or translational repression [14,15]. This miRNA-mediated gene silencing, which has been shown in numerous studies, plays critical roles in pancreatic cancer development and progression. MiR-381 targeted the chemokine receptor-4 mRNA 3’-UTR to aggravate PDAC carcinogenesis[16], and miR-1181 inhibited cell invasion and proliferation via STAT3 in pancreatic cancer[17].
In this study, we demonstrated that IGF2BP1 was overexpressed in PDAC when compared to its expression in matched normal control tissues. IGF2BP1 promoted PDAC cell proliferation both in vitro and in vivo through the AKT signaling pathway. We also determined that dysregulation of miR-494 contributed to the upregulation of IGF2BP1. Thus, our results provide a new molecular mechanism of oncogenesis and suggest a potential therapeutic target for pancreatic cancer.
Surgical specimens of pancreatic tumors and adjacent nontumor tissues were collected between January 2015 and December 2015 from 30 patients with histologically confirmed pancreatic adenocarcinomas from Henan Cancer Hospital (Zhengzhou, China). None of these patients received preoperative chemotherapy or radiotherapy. Normal pancreatic tissues from three patients with benign pancreatic diseases were also collected and histologically classified. This study was approved by the Human Research Ethics Committee of Zhengzhou University.
Human pancreatic cancer cell lines (Capan-2, Mia PaCa-2, Panc-1, and Panc 0327) were purchased from the American Typical Culture Center (Manassas, VA, United States), and the immortalized human pancreatic ductal epithelial (HPDE) cell line was purchased from the Cell Repository of the Chinese Academy of Sciences (Shanghai, China). Cells were cultured in RPMI 1640 (Capan-2, Panc 0327, and HPDE) or DMEM (Mia PaCa-2 and Panc-1) supplemented with 10% fetal bovine serum (Gibco, Carlsbad, CA, United States) in a humidified atmosphere with 5% CO2 at 37 °C. All cells in our study were authenticated using short tandem repeat DNA profiling within 2 mo. All mature miRNA mimics, inhibitors, primers, and siRNAs were purchased from RiboBio company (Guangzhou, China). Cell transfection was conducted using Lipofectamine 2000 (Invitrogen, Carlsbad, United States) according to the manufacturer’s instructions.
Formalin-fixed and paraffin-embedded tissue sections were deparaffinized in xylene and rehydrated in a graded series of alcohol solutions, followed by antigen retrieval and blockage with 3% bovine serum albumin for 30 min. Tissue sections were incubated with primary antibodies at optimal concentrations overnight at 4 °C. Then, the biotinylated sections were incubated with the secondary antibody (Boster, Wuhan, China) for 1 h at room temperature. Finally, the sections were stained with a diaminobenzidine (DAB) kit (Boster, Wuhan, China) and counterstained with hematoxylin (Boster, Wuhan, China). Staining was independently assessed by two experienced pathologists at the The Affiliated Cancer Hospital of Zhengzhou University. Images were obtained using a microscope (Olympus, Tokyo, Japan). IGF2BP1 staining intensity was classified as 0 (negative), 1 (weak), 2 (moderate), or 3 (strong). The staining proportion was quantified as 0 (negative), 1 (0.01%–50%), and 2 (51%–100%). The staining score of each sample was calculated as the proportional score × intensity score. Patients were grouped as low IGF2BP1 expression when the staining score was ≤ 2, and as high IGF2BP1 expression when the score was ≥ 3.
The lentiviral vectors for human IGF2BP1 overexpression (Lv-IGF2BP1) and knockdown (Lv-sh-IGF2BP1) and control empty vectors were constructed and synthesized by GeneChem Corporation (Shanghai, China). Lentiviral infection was performed according to the manufacturer’s protocol. The primary antibodies included IGF2BP1 (ab124930, Abcam), pan-AKT (C67E7, CST), p-AKT (D9E, CST), and GAPDH (Boster, Wuhan, China).
Total RNA isolation was performed using a TRIzol kit (Invitrogen, Carlsbad, CA, United States) according to the manufacturer’s instructions. For cDNA synthesis, equal amounts of RNA were transcribed, and random primers (Takara Bio, Inc., Dalian, China) were used for reverse transcription according to the manufacturer’s instructions. RT-qPCR assays of mRNA expression levels were performed using a SYBR Green PCR Kit (RR420A; Takara, Dalian, China) on an ABI Prism 7500 (Applied Biosystems, Foster City, CA, United States) according to the manufacturer’s instructions. The housekeeping genes GAPDH and U6 were used as reference genes. The primers used were: GAPDH forward, 5’-AGAAGGCTGGGGCTCATTTG-3’ and reverse: 5’-TGAGAGCTGTCCATTGGTAG AG-3’; IGF2BP1 forward, 5’-CAAAGGAGCCGGAAAATTCAAAT-3’ and reverse, 5’-CGTCTCACTCTC GGTGTTCA-3’. The relative gene expression was quantified and analyzed by the 2−ΔΔCt method.
Tissue and cell proteins were extracted using RIPA buffer (Beyotime Biotechnology, Shanghai, China) containing protease and phosphatase inhibitors. After protein concentration determination and denaturation, the samples were subjected to sodium dodecyl-polyacrylamide gel electrophoresis, and then transferred onto polyvinylidene fluoride (PVDF) membranes (Millipore, Bedford, MA, United States) as described[9]. After incubation with relevant primary and secondary antibodies (Boster, Wuhan, China), the blots were visualized by enhanced chemiluminescence (Boster, Wuhan, China).
One thousand pancreatic cancer cells were cultured in 96-well plates in triplicate and were maintained in complete medium. After transfection, cell viability was measured by the CCK-8 assay (Dojindo Laboratories, Kumamoto, Japan) according to the manufacturer’s instructions.
After transfection, cell apoptosis was detected using a FACSCalibur flow cytometer (BD Biosciences, MA, United States) after staining using the Annexin V/PI apoptosis kit (MultiSciences, Hangzhou, China) according to the manufacturer’s instructions. For cell cycle analysis, transfected pancreatic cancer cells were harvested, washed with cold PBS, fixed in 70% ethanol at -20 °C for 24 h, and stained with a cell cycle kit (MultiSciences, Hangzhou, China).
Six-week-old female BALB/c nude mice were purchased from HFK Bioscience (Beijing, China). All mice were maintained under specific pathogen-free conditions in the Central Animal Laboratory of Zhengzhou University. For subcutaneous xenograft model creation, 5 × 106 cells suspended in 100 μL of PBS were injected into the flank of mice. Tumor volume and mouse weight were calculated every week. Tumor volume was calculated using the formula length × width2/2. Animal studies were conducted under the principles and procedures outlined in the NIH Guide for the Care and Use of Laboratory Animals and approved by the Ethics Committee of Zhengzhou University.
The 3’-UTR and the 3’-UTR mutant of human IGF2BP1 were constructed and synthesized by GeneChem RiboBio Corporation (Guang zhou, China). For the reporter assays, pancreatic cancer cells were cotransfected with wild-type reporter plasmid and miR-494 mimics. After transfection for 48 h, luciferase activity was measured using the Dual-Glo Luciferase Reporter System according to the manufacturer’s instructions.
GSE15471, GSE32678, and GSE62165 datasets were downloaded from GEO. Gene expression profiling was analyzed with R software (version 3.3.3) using the limma package to calculate the differentially expressed mRNAs and miRNAs. GSEA was conducted using GSEA 3.0 software (Broad Institute, Cambridge, MA, United States) to identify the biologically associated gene sets.
All experiments were carried out in three biological replicates at least. Statistical analyses were performed using SPSS 17.0 software (IBM Corp., Armonk, NY, United States). The log-rank test was used to analyze the effect of expression on patients’ overall survival (OS). ANOVA and student’s t-test were used to compare differences between groups. P < 0.05 was considered statistically significant.
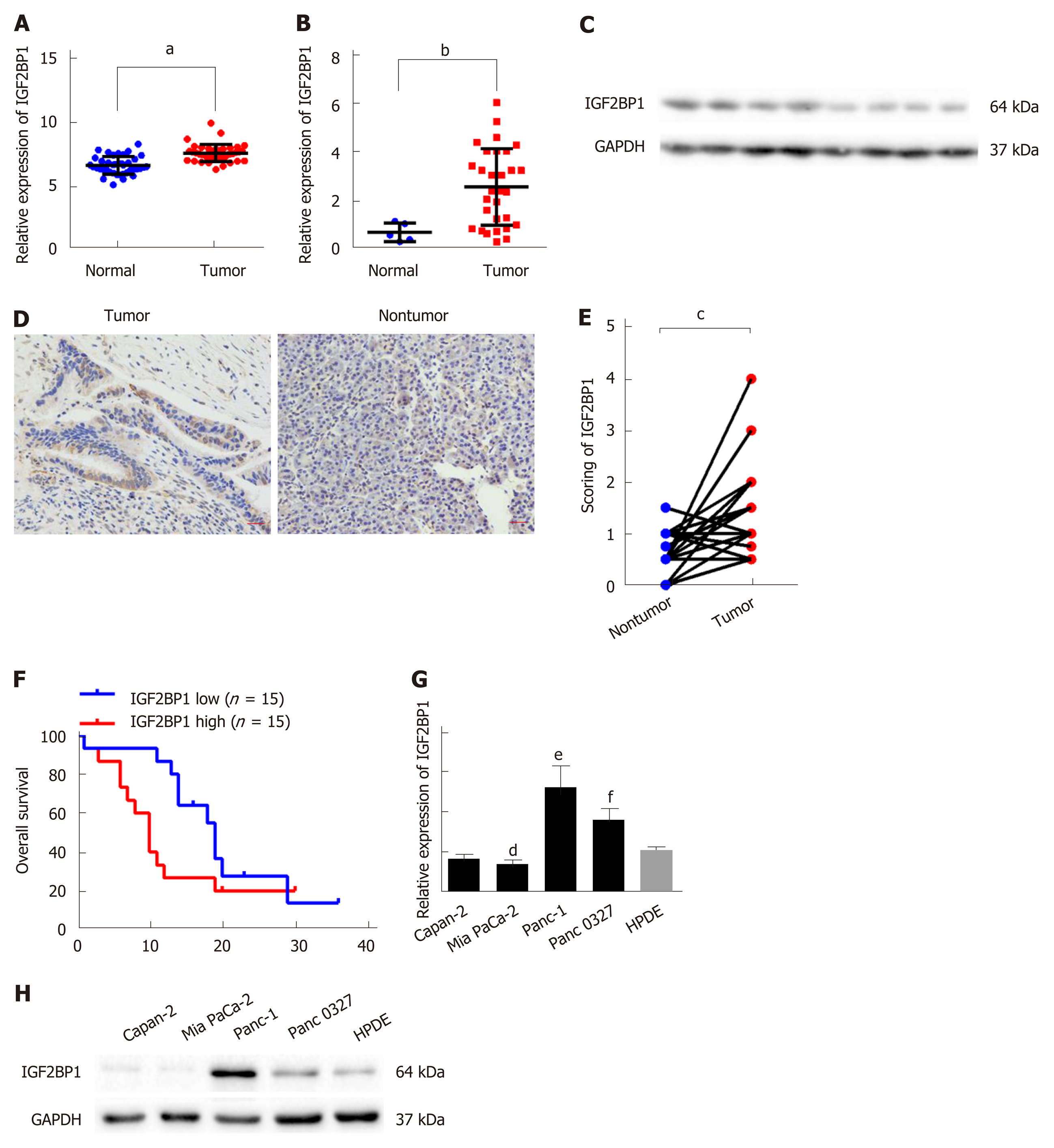
To study the expression of IGF2BP1 in pancreatic cancer, we analyzed the mRNA expression levels of IGF2BP1 in the GEO dataset GSE15471. The results indicated that the mRNA expression level of IGF2BP1 in the pancreatic cancer samples was much higher than that in the normal tissue samples (Figure 1A). Next, we confirmed elevated IGF2BP1 mRNA and protein levels in pancreatic cancer samples by RT-qPCR and immunoblotting (Figure 1B and C and Supplementary Figure 1). To investigate the clinical implication of IGF2BP1 during the development of pancreatic cancer, we performed IHC staining in 30 pairs of pancreatic cancer and matched nontumor specimens (Figure 1D; Table 1). Quantitative analysis of the IHC data revealed that IGF2BP1 was upregulated in cancer tissues compared with adjacent nontumor tissues (Figure 1E). Clinicopathological correlation analysis showed that overexpression of IGF2BP1 was correlated with tumor size (Table 1). Kaplan-Meier survival analysis showed that patients with tumoral overexpression of IGF2BP1 demonstrated a shorter OS time than patients with low expression (Figure 1F; 10 mo vs 19 mo; log-rank test, P < 0.05). The mRNA and protein levels of IGF2BP1 were also examined in several pancreatic cancer cell lines. We found that IGF2BP1 mRNA and protein levels were aberrantly upregulated in human pancreatic cancer cell lines compared to the HPDE cell line (Figure 1G and H). Collectively, we conclude that IGF2BP1 is overexpressed in pancreatic cancer and predicts a poor prognosis.
| Factor | n | High | Low | P value |
| Sex | 15 | 15 | 0.475 | |
| Female | 13 | 7 | 6 | |
| Male | 17 | 8 | 9 | |
| Age, yr | 0.368 | |||
| < 50 | 20 | 14 | 6 | |
| ≥ 50 | 10 | 6 | 4 | |
| Tumor size | < 0.01 | |||
| ≥ 2.5 cm | 13 | 9 | 4 | |
| < 2.5 cm | 17 | 5 | 12 |
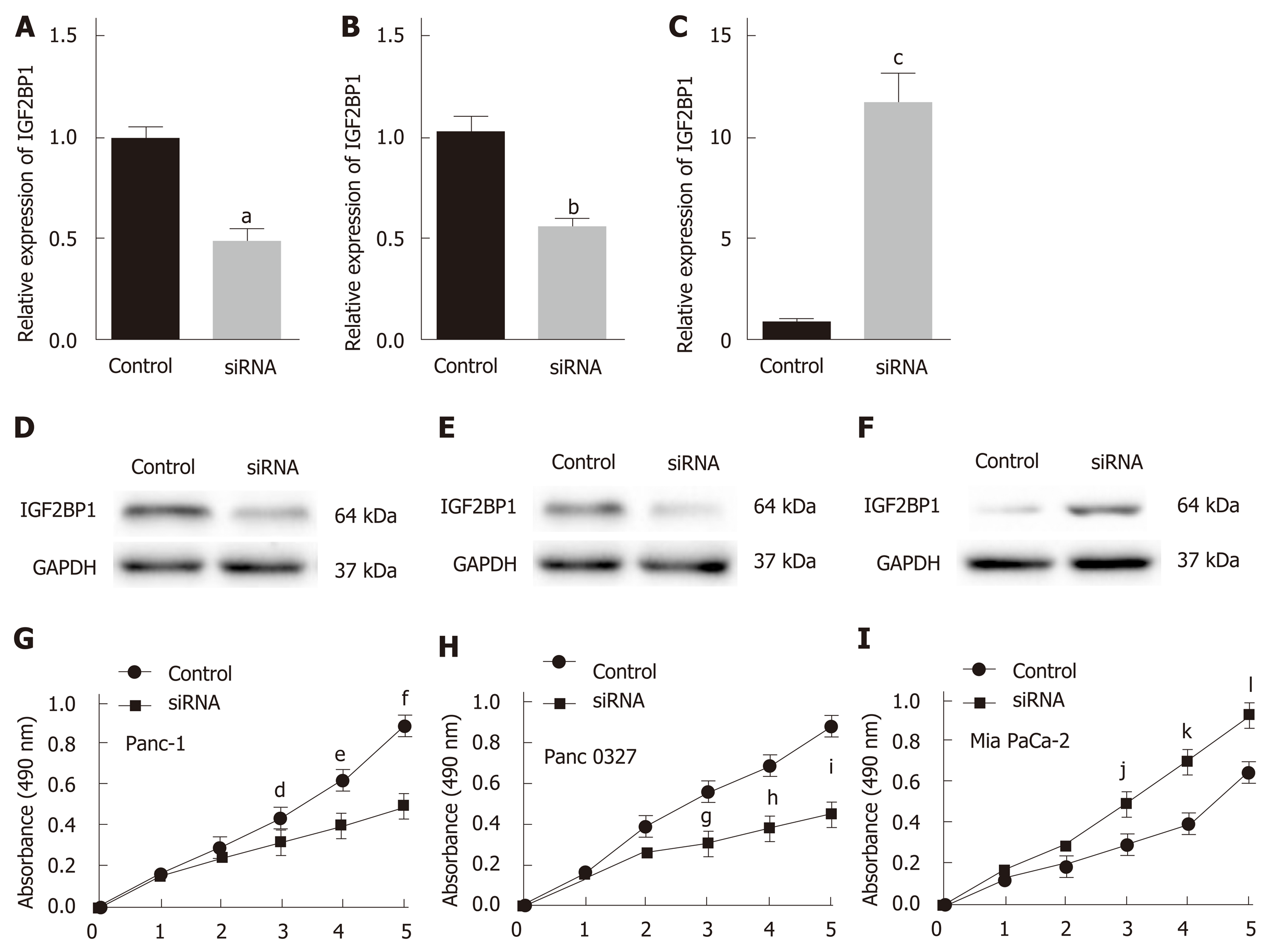
To investigate the biological function of IGF2BP1 in pancreatic cancer, we knocked down IGF2BP1 expression in two IGF2BP1-high-expressing (Panc-1 and Panc 0327) cell lines by siRNA transfection and ectopic expression in the IGF2BP1-low-expressing Mia PaCa-2 cell line. The transfection efficiency was measured by RT-qPCR and Western blot (Figure 2A-F). CCK-8 assays revealed that knockdown of IGF2BP1 markedly inhibited the growth of both Panc-1 and Panc 0327 cells (Figure 2G and H), whereas overexpression of IGF2BP1 significantly accelerated the growth of Mia PaCa-2 cells (Figure 2I). These results suggest that IGF2BP1 promotes pancreatic cancer cell proliferation in vitro.
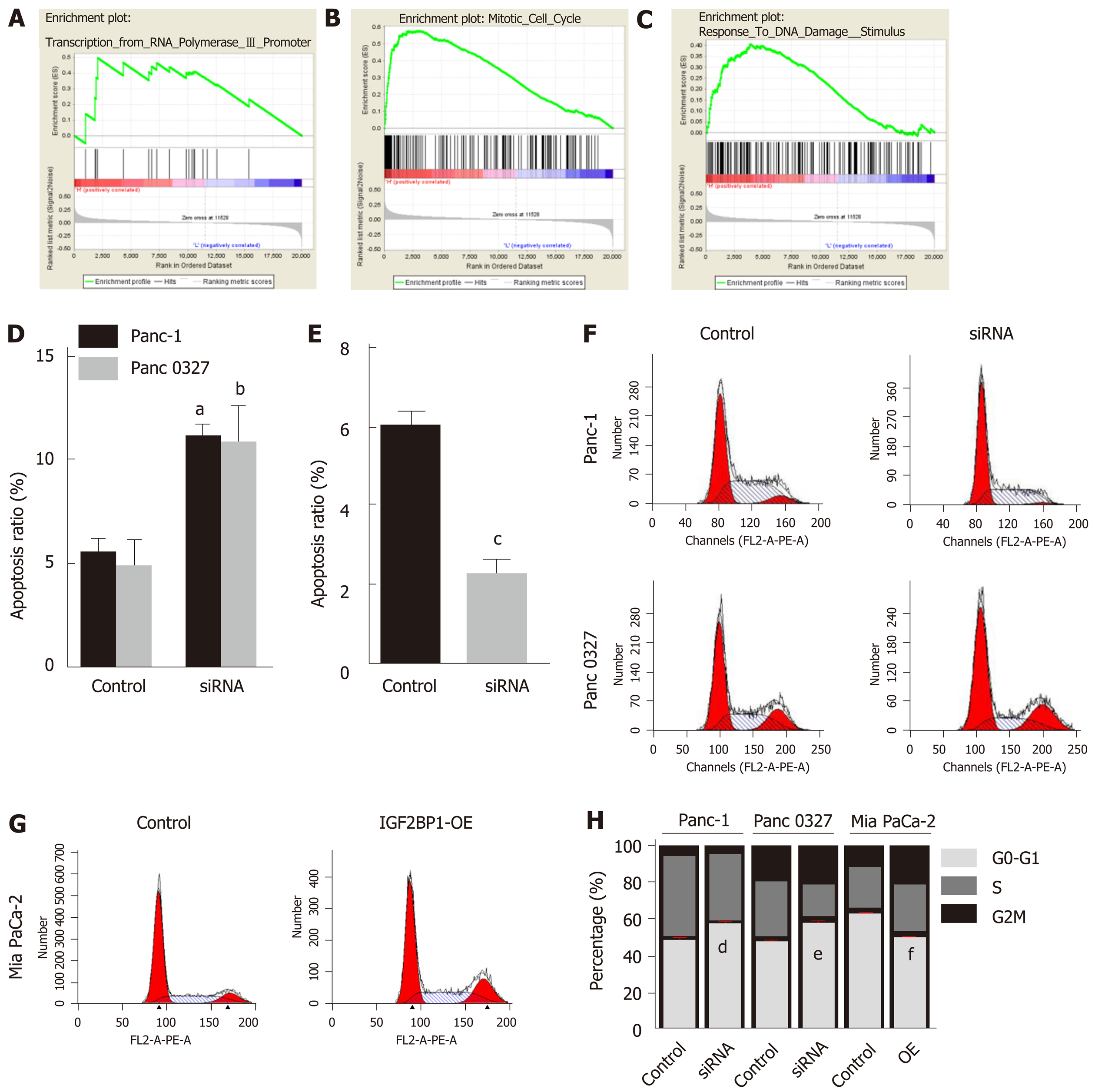
To understand the potential mechanism by which IGF2BP1 promotes pancreatic cancer cell proliferation, we conducted GSEA based on a published pancreatic cancer dataset GSE62165. IGF2BP1 upregulation was found to be positively correlated with the transcription of RNA polymerase promoter III, the mitotic cell cycle, and the response to DNA damage stimulus (Figure 3A-C). We further examined whether the inhibition of cell proliferation was due to a perturbation of the cell cycle or increased apoptosis by flow cytometry. Depletion of IGF2BP1 induced the apoptosis of Panc-1 and Panc 0327 cells (Figure 3D and Supplementary Figure 2). Overexpression of IGF2BP1 decreased the apoptosis of Mia PaCa-2 cells (Figure 3E and Supplementary Figure 3). Depletion of IGF2BP1 increased the proportion of Panc-1 and Panc 0327 cells in the G1 phase and reduced the proportions in the S and G2 phases (Figure 3F and 3H). Additionally, overexpression of IGF2BP1 promoted the transition of Mia PaCa-2 cells in G1 to enter the S and G2 phases compared with control cells (Figure 3G and H). Overall, the present results demonstrated that IGF2BP1 promotes pancreatic cancer cell growth by inducing apoptosis and accelerating the G1 to S phase transition.
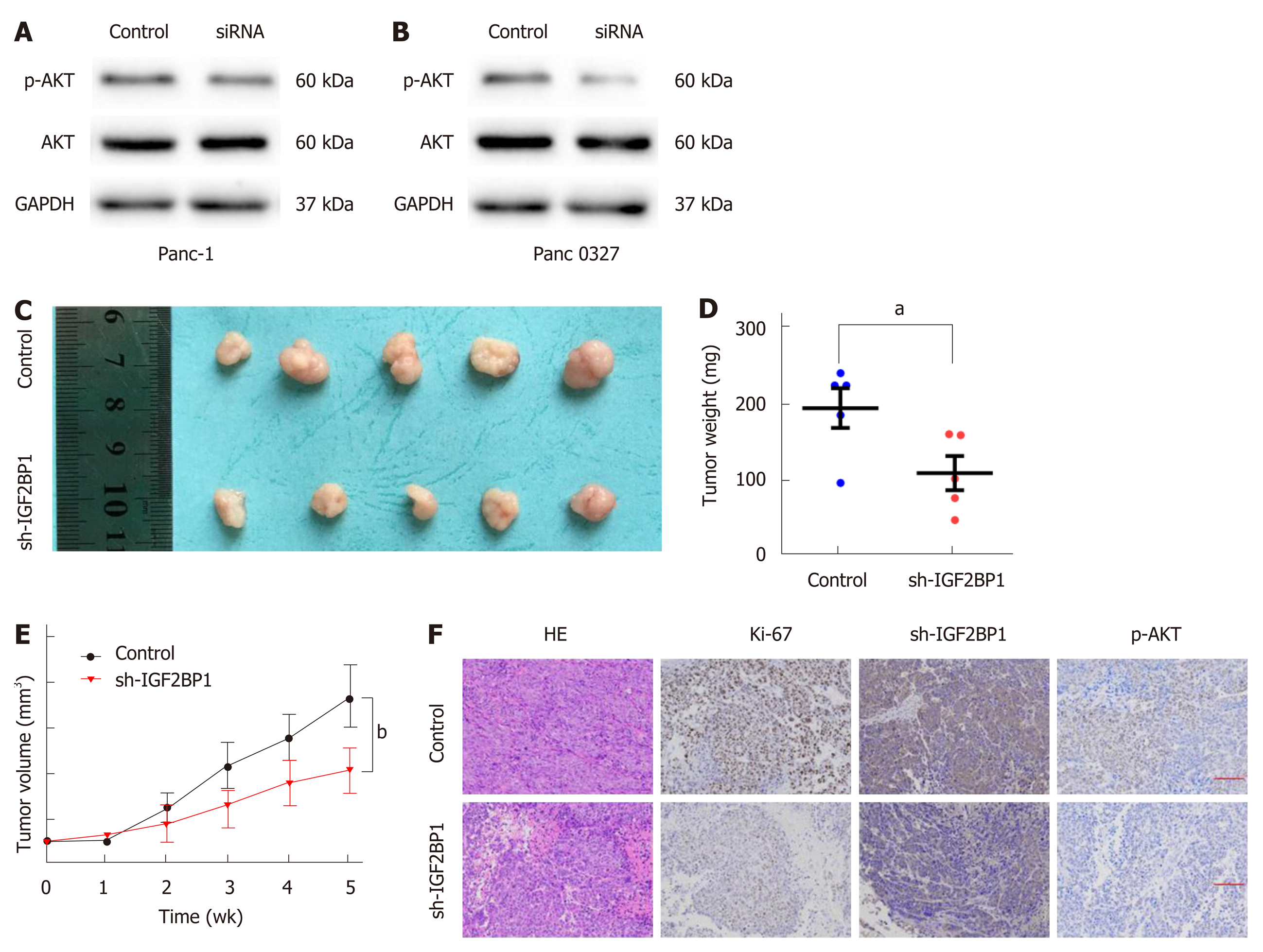
It has been reported that AKT activation is necessary for cancer progression. To determine whether IGF2BP1 activates AKT in pancreatic cancer, Western blot was performed. As presented in Figure 4A and B, silencing of IGF2BP1 in Panc-1 and Panc 0327 cells resulted in notably decreased phosphorylation of AKT. To verify the above findings in vivo, a subcutaneous xenograft model was established in nude mice (Figure 4C). Mice of the IGF2BP1 knockdown group showed a slower increase in tumor volume and less weight loss compared with the control group (Figure 4D and E). In addition, Ki-67 and p-AKT staining was also reduced in the IGF2BP1 knockdown group (Figure 4F). Taking these results together, our study demonstrated that IGF2BP1 promotes pancreatic cancer growth by activating the AKT signaling pathway.
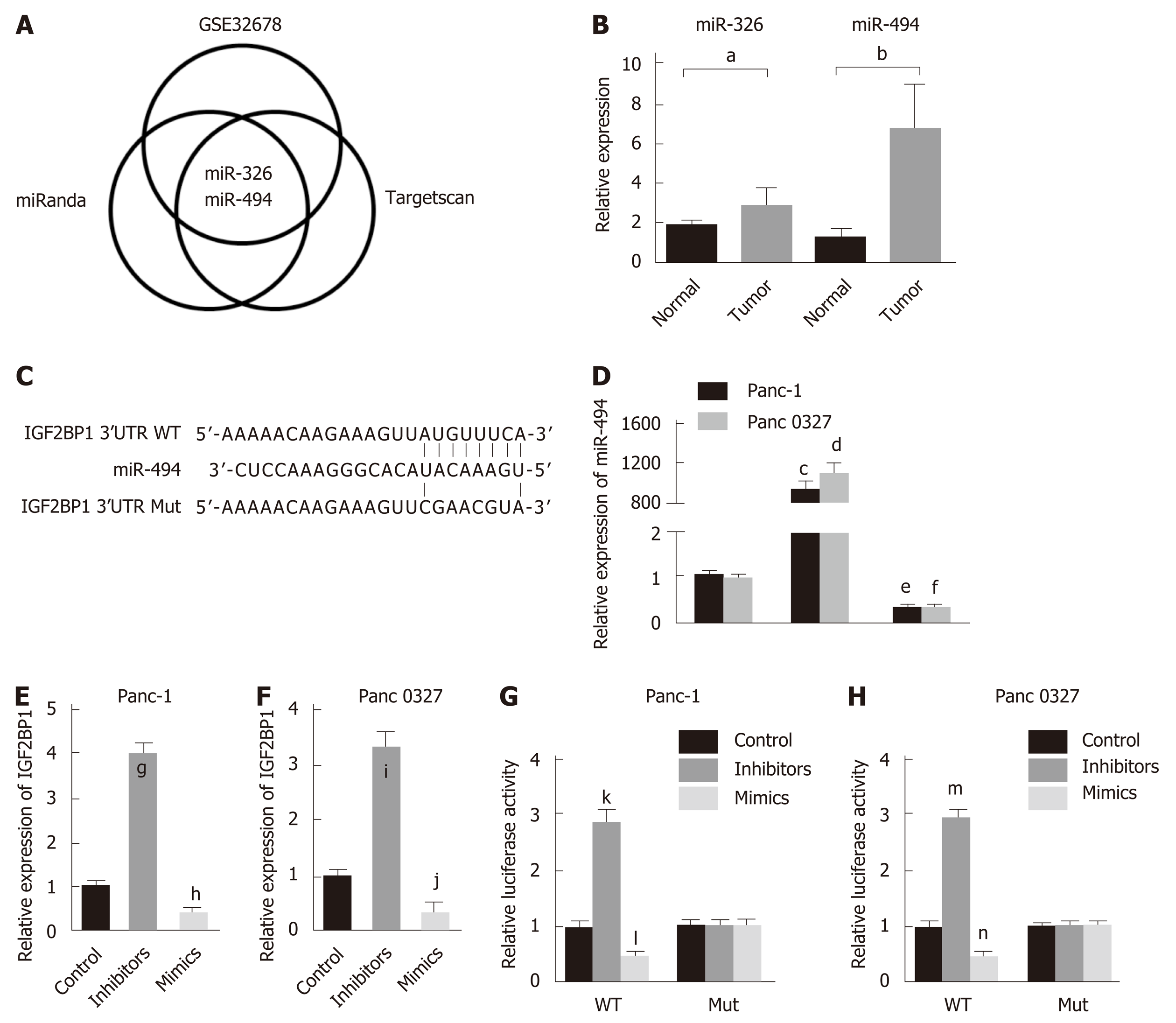
Whether IGF2BP1 is regulated at the posttranscriptional level in pancreatic cancer remains unknown. To identify the potential miRNAs targeting IGF2BP1, two online bioinformatics tools, microRNA.org and TargetScan, were used, and the prediction results were comprehensively analyzed. After searching the GEO dataset GSE32678, we found that only two miRNAs, miR-494 and miR-326, were downregulated (Figure 5A). The more frequent low expression of miR-494 was further validated by RT-qPCR in our cohort of pancreatic cancer patients (Figure 5B, n = 30). Thus, we speculated that miR-494 exhibited the greatest potential to regulate IGF2BP1, and the predicted binding site is shown in Figure 5C. To verify whether IGF2BP1 is a direct target of miR-494, wild-type (WT) and mutated (Mut) IGF2BP1 3’-UTR-coupled luciferase reporters were designed. Panc-1 and Panc 0327 cells were transfected with miR-494 mimics, inhibitors, or control sequences (Figure 5D). IGF2BP1 mRNA was markedly downregulated by the miR-494 mimics and upregulated by the miR-494 inhibitors (Figure 5E and F). In both cell lines, the luciferase activity was notably suppressed by the miR-494 mimics and enhanced by the miR-494 inhibitors in the WT group, with no significant change observed in the Mut group (Figure 5G and H). Therefore, our results showed that IGF2BP1 is a direct target of miR-494 in pancreatic cancer.
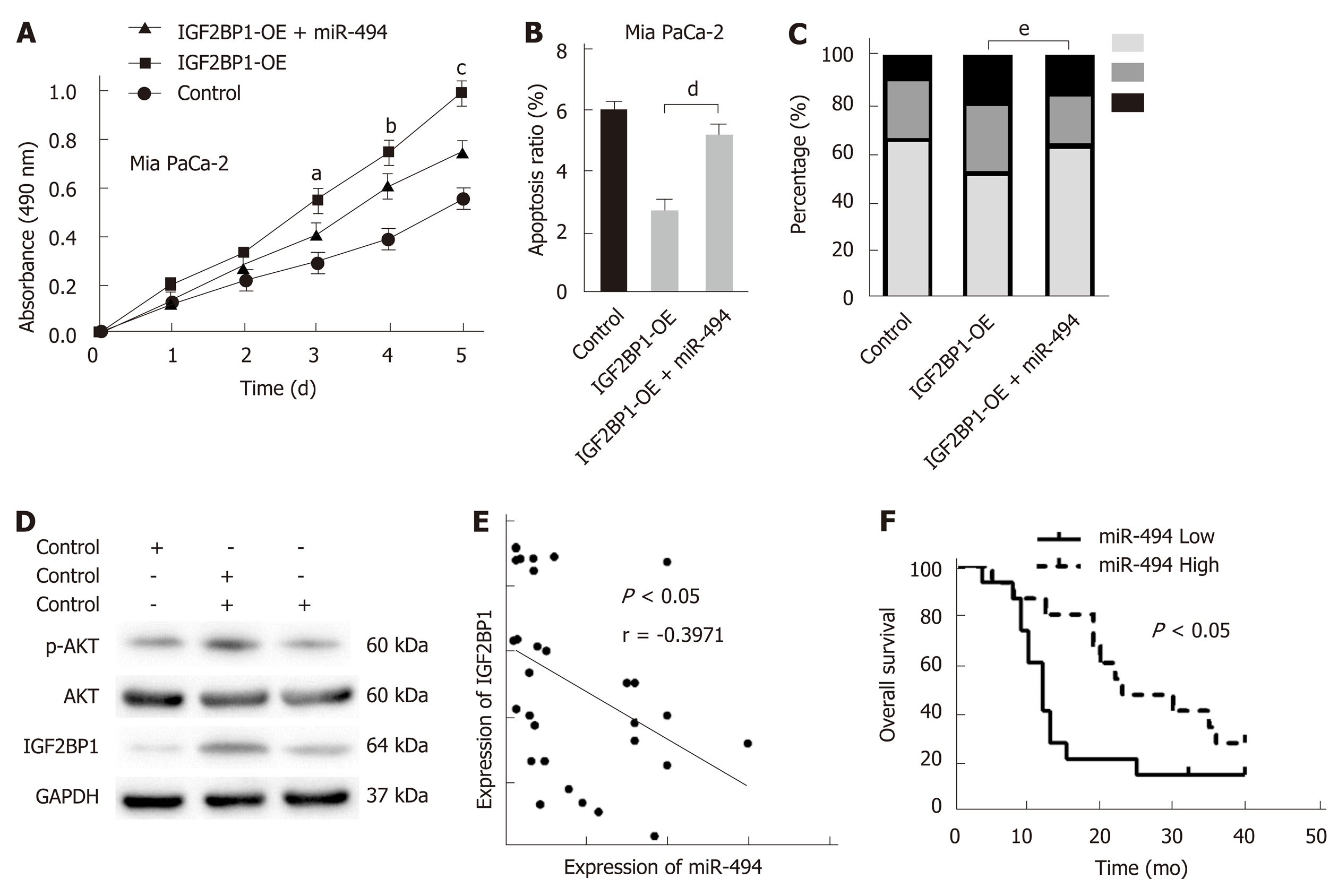
To confirm whether IGF2BP1 is a functional target of miR-494, rescue experiments were performed in Mia PaCa-2 cells. The growth promotion caused by IGF2BP1 reexpression was partly reversed by the miR-494 mimics as demonstrated by the CCK-8 assays (Figure 6A). Cell apoptosis analysis revealed that reexpression of miR-494 induced apoptosis of Mia PaCa-2 cells, which was preserved by the regulation of IGF2BP1 (Figure 6B). Cell cycle analysis revealed that Mia PaCa-2 cells accumulated in the G1 phase due to upregulated IGF2BP1, and the cells partially transitioned into the S/G2 phase following cotransfection with the miR-494 mimics (Figure 6C). Furthermore, cotransfection of miR-494 reduced phosphorylation levels of AKT (Ser473), which was mediated by IGF2BP1 overexpression (Figure 6D). Furthermore, a negative correlation between IGF2BP1 and miR-494 expression was observed in our clinical samples (Figure 6E, n = 30). Kaplan–Meier curves demonstrated that pancreatic cancer patients with high miR-494 expression (n = 15) had a better OS than those with low miR-494 expression (n = 15) (Figure 6F; 12 mo vs 23 mo, log-rank test, P < 0.05). These findings suggested that miR-494 modulates the function of IGF2BP1, and could influence pancreatic cancer cell survival by regulating IGF2BP1.
In this study, upregulation of IGF2BP1 was observed in the GEO samples and validated in an expanded cohort. We identified IGF2BP1 as a prognostic indicator and a vital factor in the development of pancreatic cancer via the AKT signaling pathway. Additionally, the upregulation of IGF2BP1 was due to miR-494 dysregulation. Therefore, IGF2BP1 might be a novel therapeutic target for the treatment of pancreatic cancer in the future.
IGF2BP1 is a well-known oncofetal protein linked to several human cancers. High IGF2BP1 expression is a poor prognostic marker in basal cell carcinoma with an activated Hedgehog signaling pathway[12]. IGF2BP1 leads to an increased lncRNA HULC half-life and higher steady-state expression to promote the carcinogenesis of hepatomas[13]. Here, we demonstrated that stable knockdown of IGF2BP1 drastically inhibited pancreatic cancer cell proliferation and reduced tumor formation in nude mice, whereas reexpression of IGF2BP1 increased pancreatic cancer cell tumorigenesis. Accordingly, upregulation of IGF2BP1 in human pancreatic cancer was observed and associated with a more aggressive clinicopathological phenotype and poorer prognosis. These findings together suggest that IGF2BP1 is an oncogene in pancreatic cancer.
Previous studies have shown that dysregulation of miRNAs profoundly influences the expression of IGF2BP1. For example, miRNA-196b inhibits cell proliferation and induces apoptosis in HepG2 cells by targeting IGF2BP1[18]. MiR-506 inhibits cell proliferation and invasion by targeting IGF2BP1 in glioblastoma[19]. MiR-625 functions as an anti-metastatic miRNA and plays an important role in hepatocellular carcinoma progression by modulating the IGF2BP1/PTEN pathway[20]. MiRNA-873 suppresses glioblastoma tumorigenesis and metastasis by inhibiting the expression of IGF2BP1[21]. Based on these studies, we considered the possible involvement of miRNA in regulating IGF2BP1 expression. We identified downregulated miR-494 as a negative regulator of IGF2BP1. MiR-494 interacted with the IGF2BP1 3’-UTR in a seed sequence-dependent manner and repressed IGF2BP1 expression. MiR-494 is a frequently downregulated miRNA in human pancreatic cancer and is negatively associated with IGF2BP1 expression in human pancreatic cancer, implying that loss of miR-494 stabilizes IGF2BP1 and thereby contributes to its upregulation.
MiR-494 has been found to be a tumor-suppressive miRNA that is downregulated in various types of solid tumors. It inhibits cellular proliferation, migration, and invasion by targeting SIRT1 and is a potential prognostic marker in epithelial ovarian cancer[22]. However, there are also reports about the tumor promotor function of miR-494 in cancers. MiR-494 promotes cervical cancer proliferation through the regulation of PTEN[23], and is an independent prognostic marker and promotes cell migration and invasion of colorectal cancer cells by directly targeting PTEN[24]. In the present study, we found that miR-494 was downregulated in pancreatic cancer and was associated with the prognosis of pancreatic cancer patients. Next, bioinformatics analysis found a complementary sequence between the 3’-UTR of IGF2BP1 and miR-494. Then, we used RT-qPCR and a dual-luciferase reporter assay to confirm that IGF2BP1 is a target of miR-494. These findings indicate that miR-494 is downregulated and exerts a tumor suppressive role in pancreatic cancer.
In this work, we demonstrated that IGF2BP1 is an oncogene which is important for pancreatic cancer tumor growth. Frequent upregulation of IGF2BP1 results from a posttranscriptional activating mechanism.
Pancreatic cancer is one of the most common causes of cancer-related deaths worldwide. Current treatment options for patients with pancreatic cancer are very limited, and the clinical outcomes remain unsatisfactory. New therapy targets are necessary to improve the survival rates of pancreatic cancer patients. Insulin-like growth factor 2 mRNA-binding protein 1 (IGF2BP1) plays critical roles in the genesis and development of human cancers. Little is known regarding IGF2BP1 expression and its role in the carcinogenesis or progression of pancreatic ductal adenocarcinoma (PDAC).
Our findings will provide a new therapeutic target for pancreatic cancer.
To study the expression, function, and regulatory mechanisms of IGF2BP1 in pancreatic cancer.
We detected the expression levels of IGF2BP1 and miR-494 in Gene Expression Omnibus datasets and validated in clinical samples by quantitative real-time polymerase chain reaction and Western blot. The relationship between IGF2BP1 expression and clinicopathological factors of pancreatic cancer patients was analyzed. The effect and mechanism of IGF2BP1 on pancreatic cancer cell proliferation were investigated in vitro and in vivo. Analyses were performed to explore underlying mechanisms of IGF2BP1 upregulation in pancreatic cancer and assays were carried out to verify the post- transcriptional regulation of IGF2BP1 by miR-494.
In the present study, we found that IGF2BP1 was upregulated and associated with a poor prognosis of pancreatic cancer patients. We showed that knockdown of IGF2BP1 reduced pancreatic cancer cell proliferation in vitro and in vivo via the AKT signaling pathway. Meanwhile, the frequent upregulation of IGF2BP1 was attributed to the downregulation of miR-494 expression in pancreatic cancer.
IGF2BP1 is upregulated and promotes the proliferation of pancreatic cancer via the AKT signaling pathway. Upregulation of IGF2BP1 is partly due to the silencing of miR-494.
This study provides insight into the role of IGF2BP1 in promoting pancreatic cancer development by activating AKT. IGF2BP1 might be a new therapeutic target for pancreatic cancer.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Barreto SG, Tanase C S-Editor: Tang JZ L-Editor: Wang TQ E-Editor: Zhang YL
| 1. | Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3134] [Cited by in RCA: 3538] [Article Influence: 393.1] [Reference Citation Analysis (0)] |
| 2. | Tamtaji OR, Mirhosseini N, Reiter RJ, Behnamfar M, Asemi Z. Melatonin and pancreatic cancer: Current knowledge and future perspectives. J Cell Physiol. 2019;234:5372-5378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 3. | McGuigan A, Kelly P, Turkington RC, Jones C, Coleman HG, McCain RS. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 2018;24:4846-4861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1338] [Cited by in RCA: 1258] [Article Influence: 179.7] [Reference Citation Analysis (39)] |
| 4. | Tanase CP, Neagu AI, Necula LG, Mambet C, Enciu AM, Calenic B, Cruceru ML, Albulescu R. Cancer stem cells: involvement in pancreatic cancer pathogenesis and perspectives on cancer therapeutics. World J Gastroenterol. 2014;20:10790-10801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Tanase CP, Neagu M, Albulescu R, Hinescu ME. Advances in pancreatic cancer detection. Adv Clin Chem. 2010;51:145-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Pistol-Tanase C, Raducan E, Dima SO, Albulescu L, Alina I, Marius P, Cruceru LM, Codorean E, Neagu TM, Popescu I. Assessment of soluble angiogenic markers in pancreatic cancer. Biomark Med. 2008;2:447-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Degrauwe N, Suvà ML, Janiszewska M, Riggi N, Stamenkovic I. IMPs: an RNA-binding protein family that provides a link between stem cell maintenance in normal development and cancer. Genes Dev. 2016;30:2459-2474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 231] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 8. | Bell JL, Wächter K, Mühleck B, Pazaitis N, Köhn M, Lederer M, Hüttelmaier S. Insulin-like growth factor 2 mRNA-binding proteins (IGF2BPs): post-transcriptional drivers of cancer progression? Cell Mol Life Sci. 2013;70:2657-2675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 366] [Cited by in RCA: 576] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 9. | Gutschner T, Hämmerle M, Pazaitis N, Bley N, Fiskin E, Uckelmann H, Heim A, Groβ M, Hofmann N, Geffers R, Skawran B, Longerich T, Breuhahn K, Schirmacher P, Mühleck B, Hüttelmaier S, Diederichs S. Insulin-like growth factor 2 mRNA-binding protein 1 (IGF2BP1) is an important protumorigenic factor in hepatocellular carcinoma. Hepatology. 2014;59:1900-1911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 149] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 10. | Kessler SM, Lederer E, Laggai S, Golob-Schwarzl N, Hosseini K, Petzold J, Schweiger C, Reihs R, Keil M, Hoffmann J, Mayr C, Kiesslich T, Pichler M, Kim KS, Rhee H, Park YN, Lax S, Obrist P, Kiemer AK, Haybaeck J. IMP2/IGF2BP2 expression, but not IMP1 and IMP3, predicts poor outcome in patients and high tumor growth rate in xenograft models of gallbladder cancer. Oncotarget. 2017;8:89736-89745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Nwokafor CU, Sellers RS, Singer RH. IMP1, an mRNA binding protein that reduces the metastatic potential of breast cancer in a mouse model. Oncotarget. 2016;7:72662-72671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Noubissi FK, Kim T, Kawahara TN, Aughenbaugh WD, Berg E, Longley BJ, Athar M, Spiegelman VS. Role of CRD-BP in the growth of human basal cell carcinoma cells. J Invest Dermatol. 2014;134:1718-1724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Hämmerle M, Gutschner T, Uckelmann H, Ozgur S, Fiskin E, Gross M, Skawran B, Geffers R, Longerich T, Breuhahn K, Schirmacher P, Stoecklin G, Diederichs S. Posttranscriptional destabilization of the liver-specific long noncoding RNA HULC by the IGF2 mRNA-binding protein 1 (IGF2BP1). Hepatology. 2013;58:1703-1712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 202] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 14. | Zhou Y, Huang T, Siu HL, Wong CC, Dong Y, Wu F, Zhang B, Wu WK, Cheng AS, Yu J, To KF, Kang W. IGF2BP3 functions as a potential oncogene and is a crucial target of miR-34a in gastric carcinogenesis. Mol Cancer. 2017;16:77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 110] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 15. | Tang H, Zhu J, Du W, Liu S, Zeng Y, Ding Z, Zhang Y, Wang X, Liu Z, Huang J. CPNE1 is a target of miR-335-5p and plays an important role in the pathogenesis of non-small cell lung cancer. J Exp Clin Cancer Res. 2018;37:131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 16. | Gao S, Cai Y, Zhang H, Hu F, Hou L, Xu Q. Long noncoding RNA DLEU1 aggravates pancreatic ductal adenocarcinoma carcinogenesis via the miR-381/CXCR4 axis. J Cell Physiol. 2019;234:6746-6757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 17. | Wang J, Guo XJ, Ding YM, Jiang JX. miR-1181 inhibits invasion and proliferation via STAT3 in pancreatic cancer. World J Gastroenterol. 2017;23:1594-1601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Rebucci M, Sermeus A, Leonard E, Delaive E, Dieu M, Fransolet M, Arnould T, Michiels C. miRNA-196b inhibits cell proliferation and induces apoptosis in HepG2 cells by targeting IGF2BP1. Mol Cancer. 2015;14:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 19. | Luo Y, Sun R, Zhang J, Sun T, Liu X, Yang B. miR-506 inhibits the proliferation and invasion by targeting IGF2BP1 in glioblastoma. Am J Transl Res. 2015;7:2007-2014. [PubMed] |
| 20. | Zhou X, Zhang CZ, Lu SX, Chen GG, Li LZ, Liu LL, Yi C, Fu J, Hu W, Wen JM, Yun JP. miR-625 suppresses tumour migration and invasion by targeting IGF2BP1 in hepatocellular carcinoma. Oncogene. 2015;34:965-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 21. | Wang RJ, Li JW, Bao BH, Wu HC, Du ZH, Su JL, Zhang MH, Liang HQ. MicroRNA-873 (miRNA-873) inhibits glioblastoma tumorigenesis and metastasis by suppressing the expression of IGF2BP1. J Biol Chem. 2015;290:8938-8948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 22. | Yang A, Wang X, Yu C, Jin Z, Wei L, Cao J, Wang Q, Zhang M, Zhang L, Zhang L, Hao C. microRNA-494 is a potential prognostic marker and inhibits cellular proliferation, migration and invasion by targeting SIRT1 in epithelial ovarian cancer. Oncol Lett. 2017;14:3177-3184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Yang YK, Xi WY, Xi RX, Li JY, Li Q, Gao YE. MicroRNA-494 promotes cervical cancer proliferation through the regulation of PTEN. Oncol Rep. 2015;33:2393-2401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 24. | Sun HB, Chen X, Ji H, Wu T, Lu HW, Zhang Y, Li H, Li YM. miR494 is an independent prognostic factor and promotes cell migration and invasion in colorectal cancer by directly targeting PTEN. Int J Oncol. 2014;45:2486-2494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |