Published online Oct 21, 2019. doi: 10.3748/wjg.v25.i39.6025
Peer-review started: August 1, 2019
First decision: August 27, 2019
Revised: September 16, 2019
Accepted: September 27, 2019
Article in press: September 27, 2019
Published online: October 21, 2019
Processing time: 80 Days and 20.5 Hours
Allicin (2-propene-1-sulfinothioic acid S-2-propenyl ester, diallyl thiosulfinate) extracted from garlic, has proven activity against Helicobacter pylori (H. Pylori) infection. In recent years, clinical trials have explored its utility as an add-on therapy with variable outcomes reported.
To perform a systemic review of allicin as an add-on treatment for H. Pylori infection and assess its efficacy in randomized controlled trials (RCTs).
Electronic databases including MEDLINE, EMBASE, the Web of Science, the Cochrane Database, the China National Knowledge Infrastructure Database, Chinese VIP Information Databases, Chinese Medical Databases, and the Wan-Fang Database were searched for keywords including “allicin”, “Helicobacter pylori”, “randomized clinical trials”, and their synonyms. A meta-analysis was performed using the fixed-effects model for low heterogeneity and the random-effects model for high heterogeneity with sensitivity analysis. Bias was evaluated using Egger’s tests. Trial sequential analysis (TSA) was used to evaluate information size and treatment benefits. The Grading of Recommendations Assessment, Development and Evaluation (GRADE) was used to assess the level of quality, and studies were classed as “high quality”, “moderate quality”, “low quality”, and “very low quality”.
A total of eight RCTs consisting of 867 participants (435 from the allicin group and 432 from the control group) were included. Eradication rate in the allicin group (93.33%, 406/435) was significantly higher than that of the control group (83.56%, 361/432) [I2 = 0%, odds ratio (OR) = 2.75, 95% confidence interval (CI): 1.74-4.35, P < 0.001]. The healing rate of ulcers following H. pylori therapy in the allicin group (86.17%, 349/405) was significantly higher than that of the control group (75.87%, 305/402) [I2 = 0%, OR = 2.05, 95%CI: 1.39-3.03, P < 0.001]. The total remission rate of peptic ulcers across all allicin groups was 95.99%, which was significantly higher than that of controls [95.99% (359/374) vs 89.25% (332/372), I2 = 0, heterogeneity P = 0.84, OR = 3.13, 95%CI: 1.51-6.51, P =0.002]. No significant differences in side effects were observed. TSA suggested that the trials were of sufficient standard to draw reliable conclusions. The quality of outcomes including eradication rates and side effects was graded as “very low” due to downgrades for “risk of bias” and “indirectness”. Other outcomes such as ulcer healing rates and total ulcer remission rates were graded as "low" due to downgrades for “risk of bias”.
Allicin as an add-on therapy improves H. pylori eradication, healing of ulcers, and remission of symptoms. These results are suggested to be treated with caution due to limited quality.
Core tip: The present systematic review assessed the efficacy and safety of allicin as an add-on treatment to PPI triple therapy and bismuth containing quadruple therapy for Helicobacter pylori infection. As a result, allicin was confirmed to increase the eradication rate, healing rate of ulcers, and remission rate of digestive symptoms but not rate of side effects.
- Citation: Si XB, Zhang XM, Wang S, Lan Y, Zhang S, Huo LY. Allicin as add-on therapy for Helicobacter pylori infection: A systematic review and meta-analysis. World J Gastroenterol 2019; 25(39): 6025-6040
- URL: https://www.wjgnet.com/1007-9327/full/v25/i39/6025.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i39.6025
Helicobacter pylori (H. pylori) is a Gram-negative microaerophilic bacterium that colonizes the gastric mucosa[1]. Globally, ≥ 50% of individuals are infected, and the prevalence is higher in developing countries[2]. H. pylori is a major cause of gastritis and peptic ulcers, as well as atrophy, intestinal metaplasia, intraepithelial neoplasia, and mucosa-associated lymphoid tissue lymphoma (MALT)[1,2].
Proton pump inhibitors (PPIs) in combination with antibiotics have been used to treat H. pylori infection. PPI triple therapy (PTT), consisting of PPI and two antibiotics such as amoxicillin and clarithromycin, is the recommended front-line treatment[2] but its eradication rates have decreased to ~70% or lower[2]. Antibiotic resistance, particularly towards clarithromycin and metronidazole, is the major cause of this decline[3]. Bismuth containing quadruple therapy (BCQT) is now recommended as the main empirical therapy in regions with high clarithromycin and metronidazole resistance (> 15%)[1]. However, eradication rates using BCQT range from 70% to 94%, questioning its effectiveness as a therapeutic strategy[1,2,4]. Therefore, new strategies to treat H. pylori infection are needed.
Choosing new alternatives with high efficiency and less side effects is one of the possible desirable options in the treatment of H. pylori infection[5]. In recent years, a series of studies were performed to explore the anti-H. pylori activities and clinical application of various agents as alternative therapies, such as plants[6,7], probiotics[8], and gastric mucin[9]. However, most of them were done in vitro[5]. In vivo studies as well as clinical trials are needed.
Garlic (Allium sativum L.) is one of the most widely grown vegetable crops in Asia, and is a known medical plant worldwide. Garlic contains 33 sulfur compounds including allicin, alliin, ajoene, diallyl trisulfide (DATS) and others[10]. Previous in vitro studies have shown that garlic inhibits bacterial growth and colonization including H. pylori[11]. Several clinical trials using garlic oil and fresh oral garlic failed to show improvements in H. pylori infection[12,13].
Allicin (2-propene-1-sulfinothioic acid S-2-propenyl ester, diallyl thiosulfinate) is an active anti-H. pylori component of garlic[11]. Due to developments in pharmaceutical technology, commercial allicin tablets are available. Allicin in addition to PTT and BCQT has been trialed as an anti-H. pylori therapy, with variable results[14-23]. Based on these studies, we performed this meta-analysis to systemically review the efficacy and safety of allicin as an add-on therapy to PTT/BCQT for H. pylori infection.
Preplanned protocols were established with protocols.io (https:// http://www.protocols.io) under the title “Allicin as a Complementary Medicine of Triple/Quadruple Therapy for Helicobacter pylori; A Systemic review and Meta-analysis of Randomized Controlled Trials (protocol)”. (https://dx.doi.org/10.17504/protocols.io.4ybgxsn)
We performed a systemic literature search in MEDLINE, EMBASE, the Web of Science, the Cochrane Database, the China National Knowledge Infrastructure Database, Chinese VIP Information Databases, Chinese Medical Databases, and the Wan-Fang Database from inception to June 1, 2019. Academic journals, dissertations, and conference proceedings were included irrespective of gray literature status. The search terms included “Helicobacter pylori”, “allicin”, “randomized clinical trials”, and their synonyms. The search strategy is listed in Appendix 1, with PubMed as an example. Reference lists were searched for potentially relevant titles. Literature searches and analysis were preplanned prior to the systemic review.
The following criteria were used for literature selection: (1) The subjects enrolled were adults with H. pylori infection with/without H. pylori-related disease including gastritis and ulcers. The diagnosis of H. pylori infection was based on positive histology, rapid urease tests (RUT), or urease breath tests (UBT); (2) Subjects in the treatment group underwent interventions using allicin plus PTT or BCQT. The control group received PTT/BCQT alone; (3) PTT/BCQT regimens in both groups were identified; (4) The main outcome was the eradication rate. Secondary outcomes were side effects, the relief of digestive symptoms, and ulcer healing (healing rate and total effectiveness rate); and (5) The study design consisted of randomized controlled trials (RCTs).
Studies that met the following criteria were excluded: (1) Duplicate articles or evaluation of the same samples; (2) Articles published as reviews, meta-analysis, or protocols; (8) Studies recruiting children; and (9) In vitro studies.
All retrieved trials were independently screened by two reviewers (Xiao-Bei Si and Shuo Zhang). Titles and abstracts were screened for all relevant articles. Full texts were screened for further assessments according to the inclusion and exclusion criteria. Disagreements were resolved by discussion or through consultation with a second specialist.
Two reviewers (Xiao-Bei Si and Shuai Wang) independently extracted data from the included RCTs. Authors, publication year, sample size, interventions, eradication rate, and secondary outcomes (remission of digestive symptoms, healing rate of peptic ulcers, and side effects) were included. Eradication rate was defined as H. pylori negativity following eradication therapy[1]. Peptic ulcers were classed as healed, effective, or ineffective following endoscopic examination before and after eradication therapy. The healing of peptic ulcers was defined as the disappearance of ulcer lesions and surrounding inflammation. Effectiveness was defined as a reduction in ulcer lesions to ≤ 50% of the original size, whilst non-effectiveness was deemed as ≥ 50% of the lesion remaining. Healing rate was defined as the number of cured cases divided by the total number of cases. Total effectiveness rate was defined as the percentage of patients whose peptic ulcers were classed as healing and/or effectiveness [Total effectiveness rate = (total number – non-effectiveness number)/total number × 100%][24]. The remission of abdominal pain was defined as a disappearance of abdominal pain after treatment. Disappearing abdominal pain was defined as the time from the initiation of H. pylori treatment to the disappearance of abdominal pain. Side effect rate was defined as the percentage of patients with at least one side effect[24].
We evaluated the risk of bias of the included articles using the Cochrane handbook[25]. Methodological quality was assessed with regard to random sequence generation, allocation concealment, the blinding of participants and personnel, the blinding of outcome assessments, incomplete outcome data, selective reporting, and other bias. Risks of bias were categorized as “low”, “high”, or “unclear”.
Meta-analysis was performed using Comprehensive Meta-analysis software (version 2.2.064; Biostat Inc, Englewood, United States). Sensitivity analysis was performed depending on the heterogeneity across the included studies. Statistical heterogeneity was assessed by I2 statistics. The Chi-square test P < 0.10 or I2 values ≥ 50% indicated heterogeneity. Trials showing either clinical heterogeneity or statistical heterogeneity were combined according to the random-effects model. The fixed-effects model was otherwise used. Publication bias was analyzed using an Egger's test. Funnel plots were performed if >10 studies were included.
TSA was used to evaluate treatment benefits based on the sample sizes using TSA software (version 0.9.5.10 Beta; Copenhagen Trial Unit, Copenhagen, Denmark) with α = 5% and 1-β = 80%. The anticipated relative risk reduction was based on the pooled estimate of available trials. Boundaries were monitored to confirm the early termination of the trials when P-values were sufficiently small enough to confirm the anticipated effects. The chance of random errors increased due to insufficient comparisons and the repetitive testing of pooled data. When cumulative Z-curves crossed sequential monitoring boundaries, a sufficient level of evidence is obtained for the intervention. When Z-curves did not cross the boundaries, the conclusions for the intervention were not justified[26].
The quality of the meta-analysis was evaluated using The Grading of Recommendations Assessment, Development and Evaluation (GRADE). As per GRADE criteria, certainty was rated as downwards to include risk of bias, inconsistency, indirectness, imprecision, and publication bias. Certainty was increased through large effects, dose-response relationships, and the adjustment of all plausible residual confounding effects. Evidence was summarized into four categories: “High quality”, “moderate quality”, “low quality”, and “very low quality”[27].
We identified 211 records using our established search strategy. Of these, 82 were excluded as duplicated records, 114 were non-clinical trials, 14 were unrelated articles, and one trial was excluded due to allicin plus non-PTT/BCQT regimens. In total, 10 clinical trials were retrieved for further full text screening. One trial[14] was excluded as the participants underwent non-standard triple therapy of amoxicillin-bismuth-PPI. A single trial[23] was excluded as the triple therapies in the allicin and control groups differed. Thus, a total of eight RCTs with 867 participants (435 from the allicin group and 432 from the control group) were finally included. The sample population of each RCT ranged from 60 to 220. A flow chart of article screening and selection processes is shown in Figure 1. One study was performed in Turkey whilst the rest were performed in China. The characteristics of each included study are summarized in Table 1.
| Ref. | N | Participants | Diagnostic methods | Allicin group | Control group | Therapy duration | Therapies after eradication | Outcomes |
| Zhan et al, 2013[15] | 60 | Hp-infected patients with peptic ulcer | i OR ii | Am: 1000 mg b.i.d. F: 100 mg b.i.d E: 40 mg q.d. Al: 40 mg t.i.d. | Am: 1000 mg b.i.d. F: 100 mg b.i.d E: 40 mg q.d. | 7 d | E: 40 mg q.d. for another 3 weeks in both groups | a, b, c, d |
| i Hp histology; | ||||||||
| ii 14C-UBT or RUT in latest 7 days before endoscopy test | ||||||||
| Bai et al, 2008[16] | 198 | Hp-infected patients with peptic ulcer | i AND ii | Am: 1000 mg b.i.d. M: 400 mg b.i.d O: 20 mg b.i.d. Al: 40 mg t.i.d. | A: 1000 mg b.i.d. M: 400 mg b.i.d O: 20 mg b.i.d. | 7 d | O: 20 mg b.i.d. for another 3 weeks in both groups | a, b, c |
| i Histology or RUT; | ||||||||
| ii 14C-UBT | ||||||||
| Wang et al, 2006[17] | 61 | Hp-infected patients with peptic ulcer | i AND ii | F: 100 mg b.i.d C: 250 mg b.i.d O: 20 mg b.i.d. Al: 40 mg t.i.d. | F: 100 mg b.i.d C: 250 mg b.i.d O: 20 mg b.i.d. | 7 d | O: 20 mg b.i.d. for another 3 weeks in both groups | a, b, d |
| i Hp histology or RUT; | ||||||||
| ii 14C-UBT | ||||||||
| Li et al, 2014[18] | 86 | Hp-infected patients with peptic ulcer | 14C-UBT | Am: 1000 mg b.i.d. F: 100 mg b.i.d E: 40 mg q.d. Al: 40 mg t.i.d. | Am: 1000 mg b.i.d. F: 100mg b.i.d E: 40 mg q.d. | 7 d | E: 40 mg q.d. for another 4 weeks in both groups | a, b, c, d |
| Kochar et al, 2001[19] | 60 | Hp-infected patients with peptic ulcer | Hp histology | Am: 1000 mg b.i.d. C: 500 mg b.i.d La: 10 mg b.i.d. Al: 1.2 mg q.d. | Am: 1000 mg b.i.d. C: 500 mg b.i.d La: 10 mg b.i.d. | 14 d | None | a, d |
| Guan et al, 2017[20] | 90 | Hp-infected patients with peptic ulcer | 14C-UBT | Am: 1000 mg b.i.d. F: 100 mg b.i.d E: 40 mg q.d. Al: 40 mg t.i.d. | Am: 1000 mg b.i.d. F: 100 mg b.i.d E: 40 mg q.d. | 7 d | E: 40 mg q.d. for another 3 weeks in both groups | a, b, c, d |
| Zhao et al, 2015[21] | 92 | Hp-infected patients with peptic ulcer | 14C-UBT | T: 500 mg b.i.d. C: 500 mg b.i.d I: 5 mg b.i.d. B: 220 mg t.i.d. Al: 40 mg t.i.d. | T: 500 mg b.i.d. C: 500 mg b.i.d I: 5 mg b.i.d. B: 220 mg t.i.d. | 7 d | I: 5 mg b.i.d. for another 3 weeks in both groups | a, b, d, e |
| Chen et al, 2016[22] | 220 | Hp-infected patients with peptic ulcer | 14C-UBT | T: 500 mg b.i.d. C: 500 mg b.i.d I: 5 mg b.i.d. B: 220 mg t.i.d. Al: 40 mg t.i.d. | T: 500 mg b.i.d. C: 500 mg b.i.d I: 5 mg b.i.d. B: 220 mg t.i.d. | 7 d | Al: 40 mg t.i.d. and I: 5 mg b.i.d. for another 3 weeks in both groups | a, b, c, e |
We used the Cochrane handbook tool to assess the quality of the included studies. No studies reported methods of randomization or concealment, despite claims of “randomized trials”. No studies implemented blindness, and one study[22] reported incomplete outcome data. No studies reported the existence of reporting bias. Table 2 shows the results of quality assessments. No included studies registered published protocols. An Egger’s test showed no publication bias (Intercept: -1.21506, P = 0.25). Funnel plots were not performed due to the insufficient study number (n < 10).
| Ref. | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other bias |
| Zhan, 2013[15] | Unclear | Unclear | High risk of bias | Unclear | Low risk of bias | Unclear | Unclear |
| Bai, 2008[16] | Unclear | Unclear | High risk of bias | Unclear | Low risk of bias | Unclear | Unclear |
| Wang, 2006[17] | Unclear | Unclear | High risk of bias | Unclear | Low risk of bias | Unclear | Unclear |
| Li, 2014[18] | Unclear | Unclear | High risk of bias | Unclear | Low risk of bias | Unclear | Unclear |
| Kochar, 2001[19] | Unclear | Unclear | High risk of bias | Unclear | Low risk of bias | Unclear | Unclear |
| Guan, 2017[20] | Unclear | Unclear | High risk of bias | Unclear | Low risk of bias | Unclear | Unclear |
| Zhao, 2015[21] | Unclear | Unclear | High risk of bias | Unclear | Low risk of bias | Unclear | Unclear |
| Chen, 2016[22] | Unclear | Unclear | High risk of bias | Unclear | High risk of bias | Unclear | Unclear |
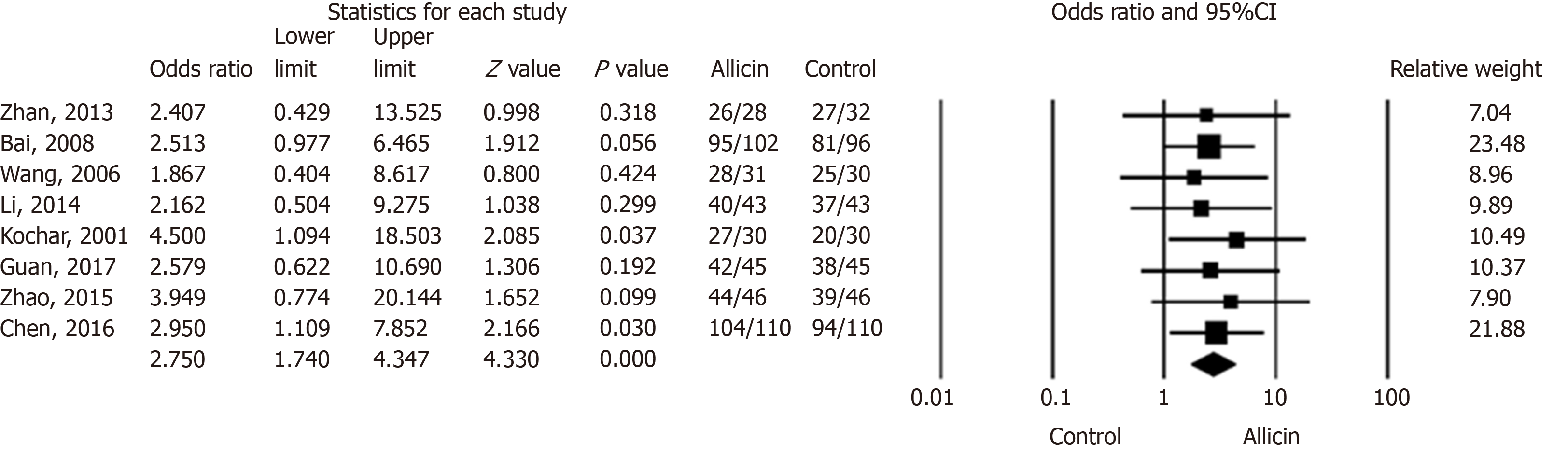
We compared the eradication rates of H. pylori between allicin and control groups. The eradication rates of the allicin group (93.33%, 406/435) were significantly higher than those of the control group (83.56%, 361/432) for intent-to-treat (ITT) analysis [I2 = 0%, heterogeneity P = 0.993, odds ratio (OR) = 2.75, 95% confidence interval (CI): 1.74-4.35, P < 0.001] (Figure 2) and per-protocol (PP) analysis [93.55% (406/434) vs 83.76% (361/431), I2 = 0%, heterogeneity P = 0.996, OR = 2.81, 95%CI: 1.77-4.47, P < 0.001][15-22].
Six studies[15-20] compared allicin plus PTT vs PTT alone. The eradication rates of the allicin group were significantly higher than those of the control group for both ITT analysis [92.47% (258/279) vs 82.61% (228/276), I2 = 0%, OR = 2.87, 95%CI: 1.65-4.99, P < 0.001] and PP analysis [92.81% (258/278) vs 82.91% (228/275), I2 = 0%, OR = 2.66, 95%CI: 1.53-4.64, P = 0.001]. A further two studies[21,22] compared allicin combined with PPI-bismuth-tinidazole-clarithromycin therapy vs PPI-bismuth-tinidazole-clarithromycin therapy. The eradication rates in the allicin and control groups were 94.87% (148/156) and 85.25% (133/156), respectively, which significantly differed for ITT/PP analyses [I2 = 0%, OR = 3.19, 95%CI: 1.38-7.38, P = 0.007]. Three studies[15,18,20] compared 7-day PPI-amoxicillin-furazolidone therapy with or without allicin and reported eradication rates of 92.46% (135/146) and 81.33% (122/150), respectively, for ITT/PP analyses [I2 = 0%, OR = 2.38, 95%CI: 0.99-5.71, P = 0.053] (Table 3).
| Comparison | Eradication rate (%) | Heterogeneity | OR | 95%CI | P value | ||
| Allicin group | Control | I2 (%) | P value | ||||
| Allicin + PTT vs PTT[15-20] (ITT) | 92.47 | 82.61 | 0 | 0.975 | 2.87 | 1.65-4.99 | < 0.001 |
| Allicin + PTT vs PTT[15-20] (PP) | 92.81 | 82.91 | 0 | 0.984 | 2.66 | 1.53-4.64 | 0.001 |
| Allicin-PPI-B-T-C vs PPI-B-T-C[21,22] (ITT/PP) | 94.87 | 85.25 | 0 | 0.764 | 3.19 | 1.38-7.38 | 0.007 |
| Allicin-PPI-Am-F vs PPI-Am-F[15,18,20] (ITT/PP) | 92.46 | 81.33 | 0 | 0.986 | 2.38 | 0.99-5.71 | 0.053 |
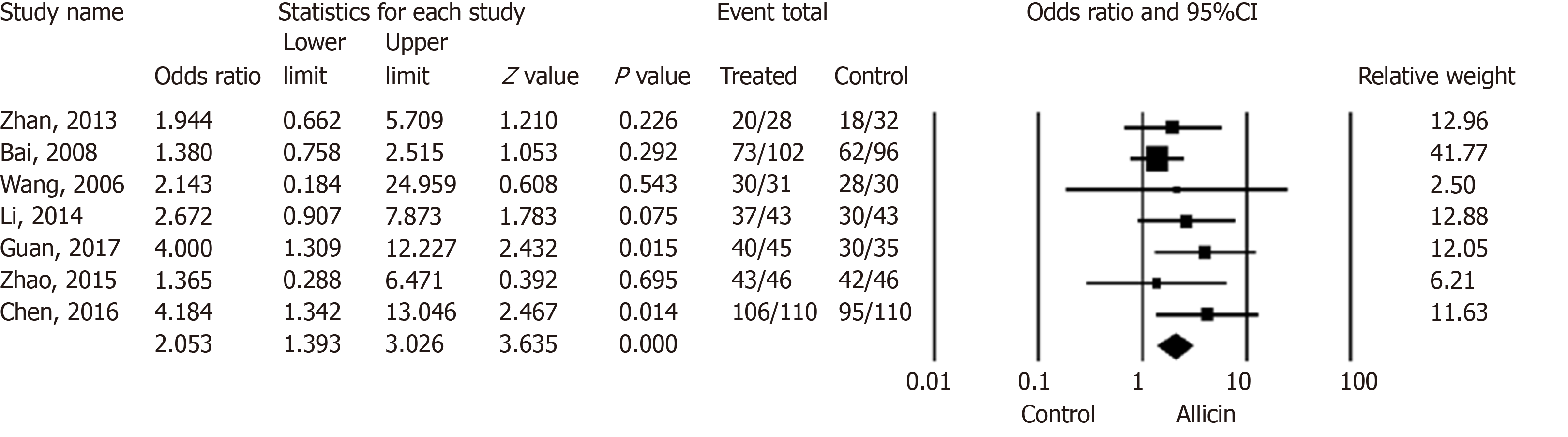
Patients with H. pylori related peptic ulcers across seven studies were included. The healing rates of ulcers after H. pylori eradication therapy in the allicin group were significantly higher those of the control group for ITT analysis [86.17% (349/405) vs 75.87% (305/402), I2 = 0%, heterogeneity P = 0.536, OR = 2.05, 95%CI: 1.39-3.03, P < 0.001] (Figure 3) and PP analysis [86.39% (349/404) vs 76.06% (305/401), I2 = 0%, heterogeneity P = 0.527, OR = 2.06, 95%CI: 1.40-3.05, P < 0.001][15-18,20-22].
Five studies [15-18,20] compared the healing rates following 7 days of allicin combined with PTT vs PTT alone. The allicin group showed significantly higher rates of healing rates compared to the control group for both ITT analysis [80.32% (200/249) vs 68.29% (168/246), I2 = 0%, OR = 2.14, 95%CI: 1.39-3.29, P = 0.001] and PP analysis [80.65% (200/248) vs 68.57% (168/245), I2 = 0%, OR = 1.93, 95%CI: 1.25-2.96, P = 0.003]. Upon comparison of allicin-PPI-bismuth-tinidazole-clarithromycin vs PPI-bismuth-tinidazole-clarithromycin, significantly higher healing rates in the allicin group were observed for ITT/PP analyses [95.51% (149/156) vs 87.82% (137/156), I2 = 22.924, OR = 2.83, 95%CI: 1.13-7.10, P = 0.026][21,22]. Allicin plus PPI-amoxicillin-furazolidone showed significantly higher healing rates than the control group for ITT/PP analyses [83.62% (97/116) vs 65.00% (78/120), I2 = 0%, OR = 1.90, 95%CI: 1.18-3.06][15,18,20] (Table 4).
| Comparison | Healing rate (%) | Heterogeneity | OR | 95%CI | P value | ||
| Allicin group | Control | I2 (%) | P value | ||||
| Allicin + PTT vs PTT[15-18,20] (ITT) | 80.32 | 68.29 | 0 | 0.527 | 2.14 | 1.39-3.29 | 0.001 |
| Allicin + PTT vs PTT[15-18,20] (PP) | 80.65 | 68.57 | 0 | 0.513 | 1.93 | 1.25-2.96 | 0.003 |
| Allicin-PPI-B-T-C vs PPI-B-T-C[21,22] (ITT/PP) | 95.51 | 87.82 | 22.924 | 0.255 | 2.83 | 1.13-7.10 | 0.026 |
| Allicin-PPI-Am-F vs PPI-Am-F[15,18,20] (ITT/PP) | 83.62 | 65.00 | 0 | 0.660 | 1.90 | 1.18-3.06 | 0.002 |
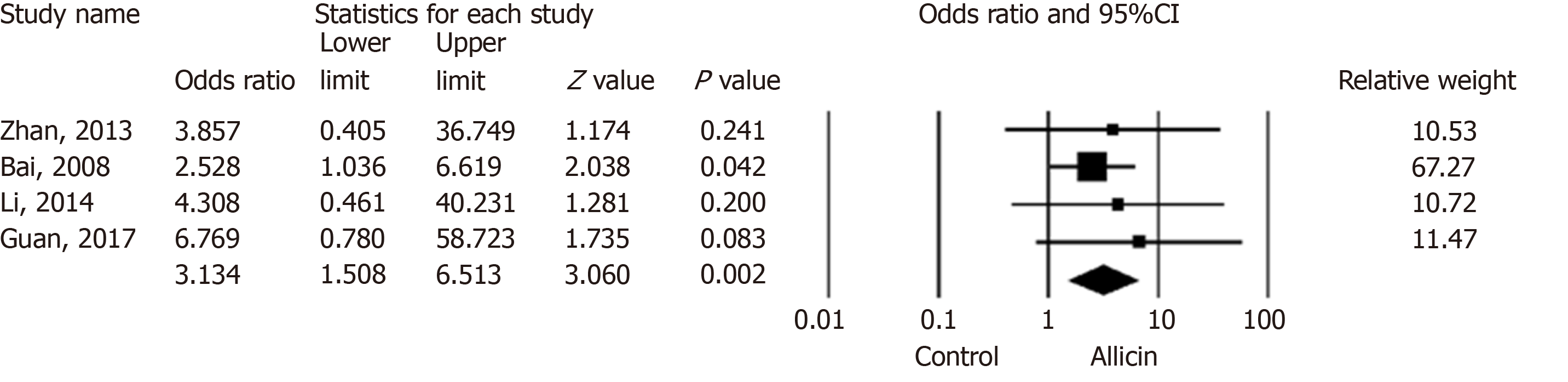
Six studies[15,16,18,20-22] reported peptic ulcer remission rates. The total remission across allicin groups was significantly higher than that of controls for ITT/PP analyses [95.99% (359/374) vs 89.25% (332/372), I2 = 0, heterogeneity P = 0.84, OR = 3.13, 95%CI: 1.51-6.51, P = 0.002] (Figure 4).
Four studies[15,16,18,20] compared allicin combined with PTT vs PTT alone for total remission rates for ITT/PP analyses [93.12% (203/218) vs 81.48% (176/216), I2 = 0, OR = 3.13, 95%CI: 1.51-6.51, P = 0.004]. Studies comparing BCQT plus allicin vs BCQT alone reported total remission rates of 100%[21,22]. Allicin plus PPI-amoxicillin-furazolidone showed higher total remission rates of 93.97% compared to 7-day PPI-amoxicillin-furazolidone therapy for ITT/PP analyses [93.97% (109/116) vs 80.83% (97/120), I2 = 0%, OR = 4.87, 95%CI: 1.36-17.50, P = 0.015][15,18,20] (Table 5).
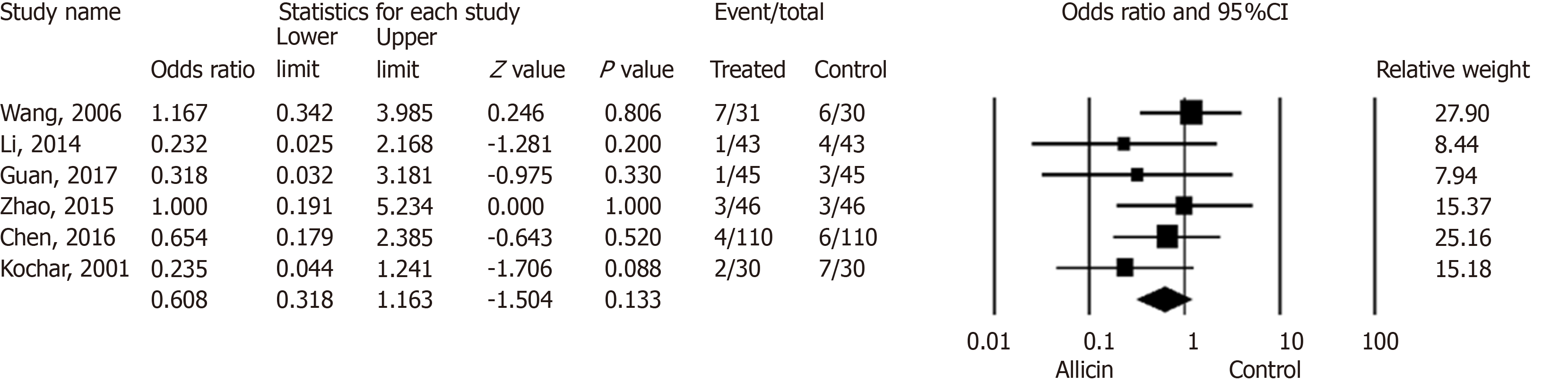
Six studies[17-22] reported side effects in the allicin group and control group without statistical significance for ITT analysis [5.90% (18/305) vs 9.53% (29/304), I2 = 0%, heterogeneity P = 0.591, OR = 0.61, 95%CI: 0.32-1.16, P = 0.133] (Figure 5) and PP analysis [5.92% (18/304) vs 9.57% (29/303), I2 = 0%, heterogeneity P = 0.593, OR = 0.61, 95%CI: 0.32-1.16, P = 0.132].
Four studies[17-20] compared allicin combined with PTT vs PTT alone for side effects. No significant differences were observed for ITT analysis [7.38% (11/149) vs 13.51% (20/148), OR = 0.52, 95%CI: 0.22-1.20, P = 0.125] and PP analysis [5.92% (18/304) vs 9.57% (29/303), OR = 0.61, 95%CI: 0.32-1.16, P = 0.132]. Two studies[18,20] compared allicin-PPI-amoxicillin-furazolidone therapy vs PPI-amoxicillin-furazolidone alone, which showed no significant differences between groups for ITT/PP analyses [2.27% (2/88) vs 7.95% (7/88), I2 = 0%, OR = 0.27, 95%CI: 0.054-1.34, P = 0.110]. No significant differences were observed for allicin-PPI-bismuth-tinidazole-clarithromycin vs PPI-bismuth-tinidazole-clarithromycin alone for ITT/PP analyses [4.49% (7/156) vs 5.77% (9/156), I2 = 0%, OR = 0.77, 95%CI: 0.28-2.13, P = 0.612][21,22] (Table 6).
| Comparison | Side effect rate (%) | Heterogeneity | OR | 95%CI | P value | ||
| Allicin group | Control | I2 (%) | P value | ||||
| Allicin + PTT vs PTT[17-20] (ITT) | 7.38 | 13.51 | 6.66 | 0.36 | 0.52 | 0.22-1.20 | 0.125 |
| Allicin + PTT vs PTT[17-20] (PP) | 5.92 | 9.57 | 0 | 0.59 | 0.61 | 0.32-1.16 | 0.132 |
| Allicin-PPI-B-T-C vs PPI-B-T-C[21,22] (ITT/PP) | 4.49 | 5.77 | 0 | 0.69 | 0.77 | 0.28-2.13 | 0.612 |
| Allicin-PPI-Am-F vs PPI-Am-F[18,20] (ITT/PP) | 2.27 | 7.95 | 0 | 0.85 | 0.27 | 0.054-1.34 | 0.110 |
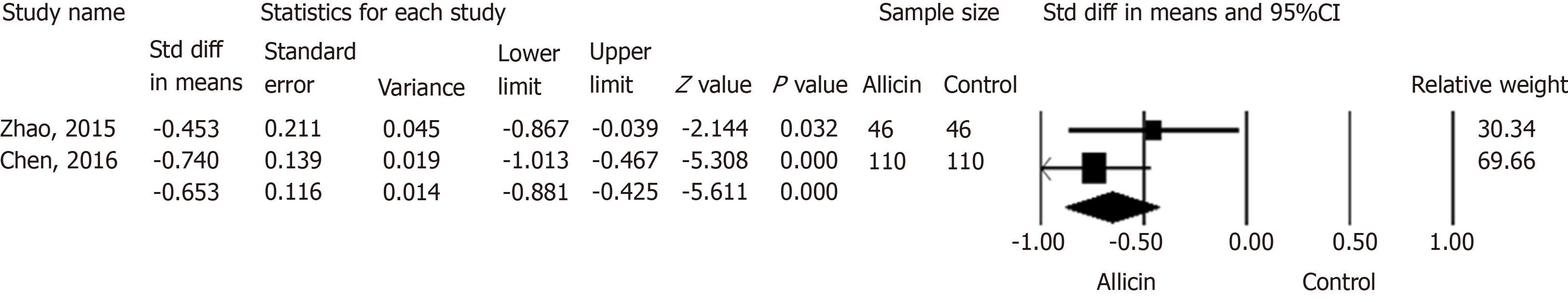
Two studies[21,22] reported the remission of abdominal pain in both groups. Chen et al[22] reported the subsidence of abdominal pain after 1.52 ± 0.5 d in the allicin group compared to 2.20 ± 1.2 d in control subjects. This significant difference was in contrast to that reported by Zhao et al[21] (average times of 1.55 ± 0.5 d and 1.80 ± 0.6 d in the allicin and control groups, respectively). A further meta-analysis showed more rapid cessation of abdominal pain in the allicin group [standard mean difference (SMD) = -0.653, 95%CI: -0.88--0.43, P < 0.001] (Figure 6).
From the sensitivity analysis, the individual removal of studies had no statistical significance and the pooled OR was unchanged.
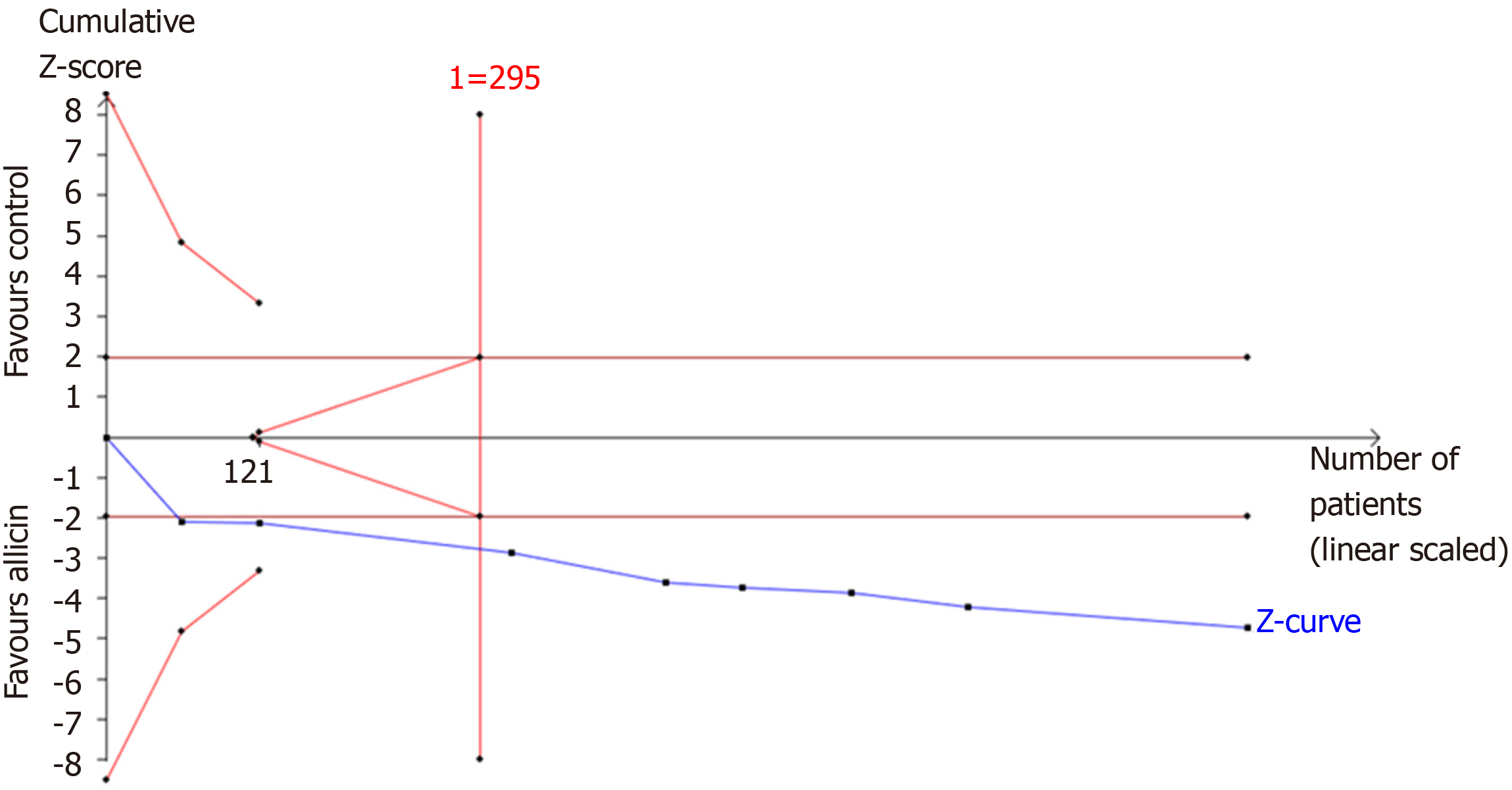
TSA of the eradication rates showed that the required information size (RIS) of 295 participants were required to calculate the eradication rates of our meta-synthesis, based on the following statistical indicators of I error probability (α = 5%): Type II error probability (β = 20%); relative risk reduction (RRR = -12.41%); and incidence in the control arm (Pc = 83.56%, derived from the meta-analysis data). Cumulative Z-curve crossed the trial sequential monitoring boundary, showing significant evidence of eradication rates. The cumulative values of the Z scores crossed conventional boundary values, trial sequential monitoring boundaries, and RIS line, suggesting that the trials were sufficient, and no alterations of the conclusions were likely (Figure 7).
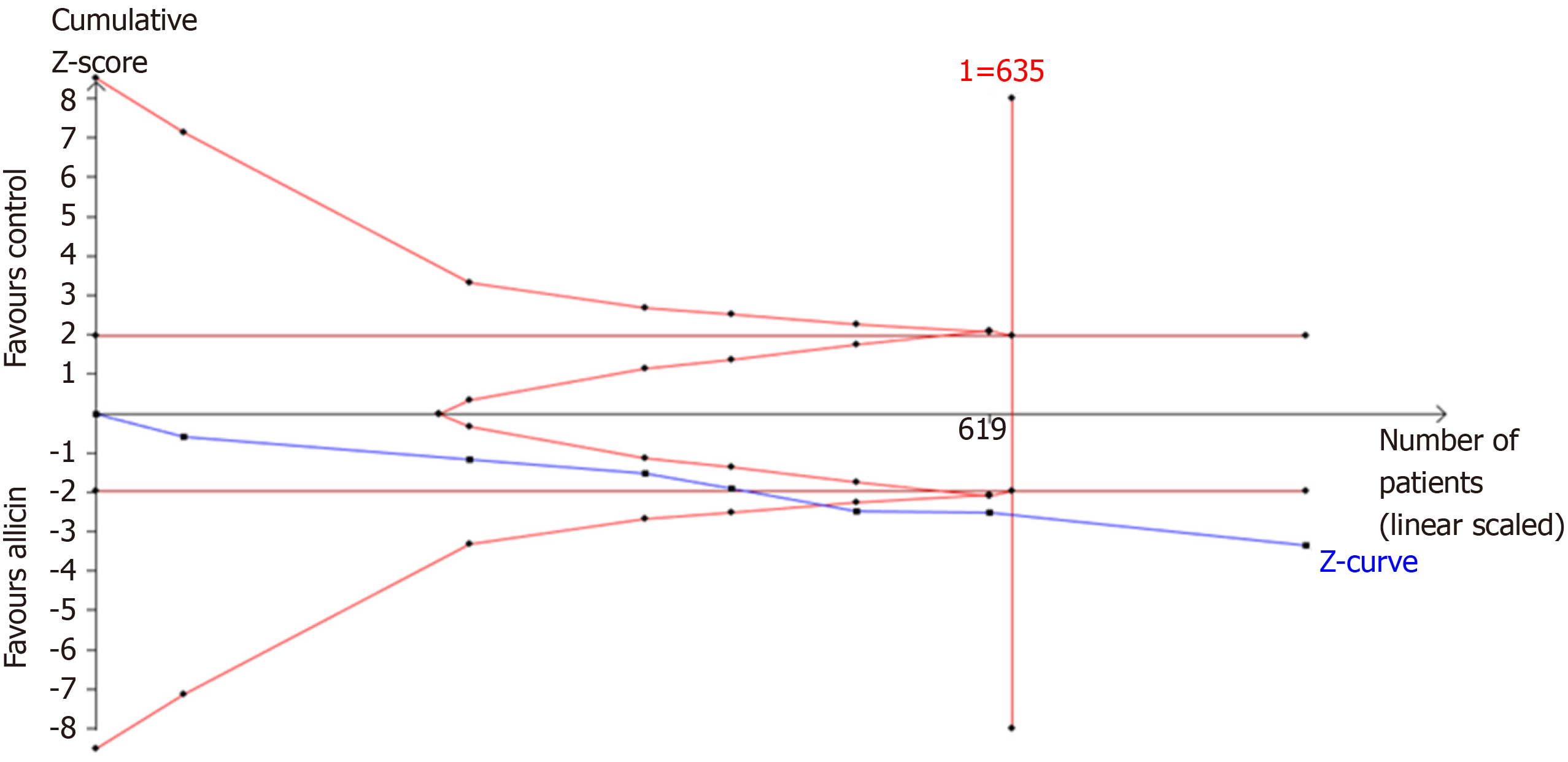
A further TSA of the healing rates showed that RIS of 635 participants was required to calculate the healing rates based on the following statistical indicators of I error probability (α = 5%): β = 20%; RRR = -10.49%; and Pc = 75.87%. Cumulative Z-curves crossed the trial sequential monitoring boundary, which showed sufficient evidence of statistically significant healing rates. Z-curves crossed the conventional boundary values, and trial sequential monitoring boundaries reached the RIS line, suggesting that the trials sufficiently drew reliable conclusions (Figure 8).
We downgraded by two levels for the “risk of bias” as the included studies did not state randomization methods, despite claiming to be randomized. In addition, the blinding of patients was not described in all included studies, which were therefore classed as non-blind. Studies were further downgraded due to “indirectness”. We assessed the efficacy of eradication rates as primary outcomes in patients with H. pylori infection. However, participants of the seven included studies (7/8, 87.50%) had H. pylori infection combined with peptic ulcers. These differences might lead to further bias. As a result, the overall certainty of the eradication rates was "very low" due to such downgrades. The outcomes of “healing rates of peptic ulcers” and “total remission rates of peptic ulcers” were “low” due to the “risk of bias” as all included studies did not state randomization and blinding methods despite claiming to be randomized. The outcome of “side effect rates” was graded as “very low” due to similar issues. GRADE evidence profiles are shown in Table 7.
| Participants (stu-dies) | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Overall quality of evidence |
| Eradication rate | ||||||
| 867(8 studies) | Very serious1 | No serious inconsistency | Serious2 | No serious imprecision | Undetected | Very low12 |
| Healing rate of ulcers | ||||||
| 807(7 studies) | Very serious1 | No serious inconsistency | No serious indirectness | No serious imprecision | Undetected | Low1 |
| Total remission rate of ulcers | ||||||
| 807(7 studies) | Very serious1 | No serious inconsistency | No serious indirectness | No serious imprecision | Undetected | Low1 |
| Side effect rate | ||||||
| 549(5 studies) | Very serious1 | No serious inconsistency | Serious3 | No serious imprecision | Undetected | Very low13 |
H. pylori infection is one of the pathogenic factors of gastritis, peptic ulcers, and MALT[1]. H. pylori eradication plays an important role in the treatment of digestive disease and reduces the lifetime risk of gastric cancer[1]. In recent years, antibiotic resistance, particularly metronidazole and clarithromycin, have threatened H. pylori therapy[2]. Nearly 15% of H. pylori isolates develop multiple drug resistance (resistance to three or more antibiotics)[3,28]. As a result, the eradication rates of PTT have decreased to 70%-85%[1,29]. Accordingly, clarithromycin-based triple therapy is not recommended in areas of high resistance[3]. Compared to PTT, BCQT is an accepted strategy to increase eradication rates[1], particularly in areas of clarithromycin and metronidazole resistance[1]. According to the meta-analysis performed by Venerito et al[30], the eradication rates of bismuth-based quadruple therapy range from 68.8% to 91.0% (intention-to-treat analysis) with a total eradication rate of 77.6%. Both therapies failed to effectively control H. pylori. Considering increased antibiotic resistance rates and treatment failure rates, new H. pylori treatment strategies need to be developed. Accordingly, finding alternative non-antibiotic approaches is one of new strategies for H. pylori treatment.
Garlic is anti-bacterial and has been used to treat infectious diseases. In 1998, Chung et al[31] first reported that garlic components can suppress H. pylori growth. Diallyl sulfide (DAS) or diallyl disulfide (DADS) was shown to elicit bactericidal effects on H. pylori cultures. During that period, commercial garlic preparations containing either garlic powder (GP) or garlic oil (GO) were assessed. GP is a preparation of sliced, dried, and pulverized garlic cloves to which water is added. GO is produced by heating crushed garlic cloves to 100 °C, collecting the vapor as a distillate, and diluting the final product in vegetable oil[32]. Although O'Gara et al demonstrated the anti-H. pylori effects of GO and GP in vitro, determined through minimal inhibitory concentrations ranging from 8 to 32 mg/mL and 250 to 500 mg/mL, respectively, subsequent clinical studies failed to confirm this activity. Aydin et al[12] reported a prospective cohort of 20 H. pylori infected individuals treated with GO (275 mg three times per day combined with omeprazole) in whom no significant effects on H. pylori eradication were observed. A non-randomized crossover trial by Graham et al[13] treated H. pylori infected individuals with fresh oral garlic, with no beneficial effects reported. In 2001, McNulty et al[33] performed a clinical trial with GO therapy in which the subjects received one 4 mg GO capsule with their meals four times per day for 14 d. No evidence of either H. pylori eradication or the improvement of symptoms was observed. These negative results failed to prove the inhibitory effects of fresh garlic and GO on H. pylori. These failures can, however, be rationalized. First, although the levels of allicin in commercial products are roughly equivalent to those of fresh crushed garlic, the conversion of allicin to other garlic sulfides can occur during the production process[12]. Second, it is accepted that the anti-H. pylori effects of garlic are dose dependent but no consensus on acceptable garlic doses exists. Whether effective doses were used in these trials remains unclear.
Allicin was first defined as an antimicrobial agent in 1944[34] and was subsequently shown to have anti-H. pylori effects[35], the mechanism(s) of which remain undefined[35]. H. pylori suppression may contribute to the anti-inflammatory effects of allicin, particularly the inhibition of IL-8 and TNF-α. H. pylori infection inhibits heat shock proteins (HSP) and promotes lipopolysaccharidase release[36]. In recent years, the artificial synthesis of allicin in commercial preparation (40 mg per tablet) improved allicin therapy. A series of clinical trials were performed in H. pylori infected individuals in whom allicin was administered as an add-on treatment to PTT/BCQT to treat H. pylori infection and H. pylori-related disease. Xue et al[37] performed a trial in which the eradication rate of allicin with ranitidine therapy were compared to that of ranitidine alone. Negative results were reported, suggesting that garlic products fail to eradicate H. pylori in the absence of antibiotics.
In this review, we analyzed trials that included allicin as an add-on treatment to PTT/BCQT for H. pylori infection. Allicin treated groups showed an eradication rate of ~93.33%. These results were graded as “good” (90%-95%) and highlight the benefits of allicin as an add-on treatment for H. pylori eradication[38]. Our meta-analysis also showed that allicin plus PTT/BCQT resulted in higher healing rates and total remission rates of peptic ulcers. Several mechanisms contribute to improved ulcer responses. First, inflammatory responses play a role in the development of peptic ulcers. Allicin inhibits the activation of NF-Kβ, which inhibits the production of TNF-α, leading to anti-inflammatory effects[39]. Second, garlic extracts have protective effects and alleviate oxidative stress in gastric tissue. Such process contributes to mucosal injury and ulcer development[40]. Third, peptic ulcers result from a disturbance of aggressive and defensive factors in the stomach. Garlic extracts may enhance NO synthesis through increasing the activity of constitutive nitric oxide synthase (cNOS) and promoting the maintenance of endothelial function[41-42].
In recent years, several options such as phytotherapy[6,7] or traditional Chinese medicine[43], probiotics[8], and nutraceutical agents[44] have been proposed as alternative treatments for H. pylori infection. In addition to allicin, several other add-on treatments to PTT/BCQT have been investigated as well, with clinical evidence published. Berberine, extracted from Coptis chinensis Franch, was used to treat H. pylori infection through its combination with PTT. The eradication rate of berberine plus PTT was 85.89 % according to previous meta-analysis[45]. Probiotics have been used as an add-on treatment for H. pylori infection. Gong et al[29] performed a meta-analysis to evaluate the efficacy of probiotics plus PTT with an eradication rate of 80.74% reported. A further meta-analysis evaluated the effects of probiotics plus BQCT with an eradication rate of 90.76% observed[8]. Yin et al[42] also performed a meta-analysis that evaluated the traditional Chinese medicine Jinghua Weikang capsules plus PTT with an eradication rate of 85.47%. These therapeutic regimens did not achieve the effectiveness of allicin plus PTT therapy for the treatment of H. pylori infection. However, the role of alternative treatment remains controversial. Previous studies mostly demonstrated that the agents as alternative treatment exhibit anti-inflammatory, immunomodulatory, and gastro-protective activities. Such activities contributed to improvement of peptic ulcer healing and remission of gastrointestinal tract symptoms. The anti-H. pylori activities were not well proved due to the lack of correlation between in vitro susceptibility and in vivo efficacy. What's more, no agent of alternative treatment was accepted to treat H. pylori infection as a monotherapy. In this regard, we speculate that anti-H. pylori activities of such agents, especially medical plants, exist while their active ingredients and effective dosages need to be further explored. The research and development process of allicin, i.e., identification of active ingredients followed by therapeutic dosage exploration, might be a typical case of clinical application of alternative medicine.
Two previous meta-analyses[46-47] showed comparable findings, but common insufficiencies limit the quality of their evidence. Both analyses[46-47] included studies of PPI-clarithromycin-bismuth ± allicin[14], whilst Hu et al[46] compared ranitidine plus allicin to allicin alone[37]. Both therapeutic regimens are not wildly accepted and may have caused an unneglected bias risk. The study by Hu et al[46] performed an adequate search strategy, but the H. pylori patients had duodenal ulcers, which narrowed the application of the evidence.
This is the first systematic review and meta-analysis of H. pylori treatment using allicin that was assessed using the GRADE system, although the quality of main outcome was graded as “very low” due to downgrades for the risk of bias and indirectness. What’s more, when considering this meta-analysis, potential limitations should be considered: (1) The included studies were of low quality, which limited the clinical evidence; (2) Although I2 statistics assessment showed no statistical heterogeneity, we considered existence of clinical heterogeneity of the included studies. First, seven included studies included the participants with H. pylori infection combined with H. pylori related ulcers while the rest one[19] included the participants with H. pylori infection alone. Second, the present review compared the efficacy of allicin plus PTT/BCQT vs PTT/BCQT alone. However, the PTT/BCQT regimens of included studies differed, especially components of antibiotics. Third, most of the included studies (7/8, 87.50%) were performed in China except for the study by Kochar et al[19]. Fourth, the eradication therapy period of the included studies ranged from 7 d to 14 d; and (3) Antibiotic resistance of the participants was not assessed, so the efficacy of allicin on antibiotic resistant H. pylori strains was not assessed.
In conclusion, this study provides evidence that allicin improves eradication rates, healing rates, the remission of peptic ulcers, and the remission of abdominal pain, but does not affect side effects when used as an add-on treatment for H. pylori infection and H. pylori related ulcers. However, the quality of this study was graded as “very low” for eradication and side effects rates and “low” for healing and total remission rates of peptic ulcers. These results should be treated with cautions due to limited quality of the included studies.
Allicin (2-propene-1-sulfinothioic acid S-2-propenyl ester, diallyl thiosulfinate), a compound of garlic, was proved to be active in inhibiting Helicobacter pylori (H. pylori) growth in vitro. However, several clinical trials using garlic oil and fresh oral garlic failed to show improvements in H. pylori infection. In recent years, due to developments in pharmaceutical technology, commercial allicin tablets are available, with a series of randomized clinical trials that explored allicin as an add-on therapy to PPI therapy or bismuth containing quadruple therapy to treat H. pylori infection.
Allicin as an add-on therapy to treat H. pylori infection has been trialed, with variable results. Whether allicin could be medicated as an anti-H. pylori drug is still inconclusive.
We performed a meta-analysis to evaluate the efficacy and safety of allicin as an add-on therapy, i.e., allicin plus PPI triple therapy or bismuth containing quadruple therapy for H. pylori infection.
Electronic databases including MEDLINE, EMBASE, Web of Science, etc. were searched. A meta-analysis was performed using the fixed-effects model for low heterogeneity and the random-effects model for high heterogeneity with sensitivity analysis. Bias was evaluated using Egger’s tests. Trial sequential analysis (TSA) was used to evaluate information size and treatment benefits. The Grading of Recommendations Assessment, Development and Evaluation (GRADE) was used to assess the level of quality.
A total of eight RCTs consisting of 867 participants were included. As a result, add-on therapy of allicin combined with PPI triple therapy (PTT) or bismuth containing quadruple therapy (BCQT) showed a significantly higher eradication rate (93.33% vs 83.56%, P < 0.001) and healing rates of ulcer (86.17% vs 75.87%, P < 0.001). In addition, the total remission rate of peptic ulcers across all allicin groups was significantly higher than that of controls (95.99% vs 89.25%, P = 0.002). Such outcomes were graded as “low” (ulcer healing rates and total ulcer remission rates) or “very low” (eradication rates and side effects rates) according to the GRADE assessment.
This study provides evidence that allicin improves eradication rates, healing rates, the remission of peptic ulcers, and the remission of abdominal pain, but does not affect side effects when used as an add-on treatment for H. pylori infection and H. pylori related ulcers. In other words, allicin plus PPI triple therapy or bismuth containing quadruple therapy may obtain better therapeutic effects.
The present review evaluated the efficacy and safety of allicin as an add-on therapy for H. pylori infection, with conclusion that allicin might improve healing rate and symptom remission of H. pylori related ulcers as well as H. pylori eradication rate. However, there are still many questions remaining unclear. On one hand, the exact mechanism of allicin as an anti-H. pylori drug is not clear up till now. On the other hand, further clinical evidence of high quality is still needed since the present evidence is of “low” or “very low” quality.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Slomiany BL, Vorobjova T S-Editor: Wang J L-Editor: Wang TQ E-Editor: Ma YJ
| 1. | Liu WZ, Xie Y, Lu H, Cheng H, Zeng ZR, Zhou LY, Chen Y, Wang JB, Du YQ, Lu NH; Chinese Society of Gastroenterology, Chinese Study Group on Helicobacter pylori and Peptic Ulcer. Fifth Chinese National Consensus Report on the management of Helicobacter pylori infection. Helicobacter. 2018;23:e12475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 331] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 2. | Hunt RH, Xiao SD, Megraud F, Leon-Barua R, Bazzoli F, van der Merwe S, Vaz Coelho LG, Fock M, Fedail S, Cohen H, Malfertheiner P, Vakil N, Hamid S, Goh KL, Wong BC, Krabshuis J, Le Mair A; World Gastroenterology Organization. Helicobacter pylori in developing countries. World Gastroenterology Organisation Global Guideline. J Gastrointestin Liver Dis. 2011;20:299-304. [PubMed] |
| 3. | Ranjbar R, Chehelgerdi M. Genotyping and antibiotic resistance properties of Helicobacter pylori strains isolated from human and animal gastric biopsies. Infect Drug Resist. 2018;11:2545-2554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Lee SW, Kim HJ, Kim JG. Treatment of Helicobacter pylori Infection in Korea: A Systematic Review and Meta-analysis. J Korean Med Sci. 2015;30:1001-1009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Ayala G, Escobedo-Hinojosa WI, de la Cruz-Herrera CF, Romero I. Exploring alternative treatments for Helicobacter pylori infection. World J Gastroenterol. 2014;20:1450-1469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 96] [Cited by in RCA: 95] [Article Influence: 8.6] [Reference Citation Analysis (1)] |
| 6. | Hajimahmoodi M, Shams-Ardakani M, Saniee P, Siavoshi F, Mehrabani M, Hosseinzadeh H, Foroumadi P, Safavi M, Khanavi M, Akbarzadeh T, Shafiee A, Foroumadi A. In vitro antibacterial activity of some Iranian medicinal plant extracts against Helicobacter pylori. Nat Prod Res. 2011;25:1059-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Ndip RN, Malange Tarkang AE, Mbullah SM, Luma HN, Malongue A, Ndip LM, Nyongbela K, Wirmum C, Efange SM. In vitro anti-Helicobacter pylori activity of extracts of selected medicinal plants from North West Cameroon. J Ethnopharmacol. 2007;114:452-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Si XB, Lan Y, Qiao L. A meta-analysis of randomized controlled trials of bismuth-containing quadruple therapy combined with probiotic supplement for eradication of Helicobacter pylori. Chin J Intern Med. 2017;56:752-759. [RCA] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 9. | Lee H, Kobayashi M, Wang P, Nakayama J, Seeberger PH, Fukuda M. Expression cloning of cholesterol alpha-glucosyltransferase, a unique enzyme that can be inhibited by natural antibiotic gastric mucin O-glycans, from Helicobacter pylori. Biochem Biophys Res Commun. 2006;349:1235-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Jeong JH, Jeong HR, Jo YN, Kim HJ, Shin JH, Heo HJ. Ameliorating effects of aged garlic extracts against Aβ-induced neurotoxicity and cognitive impairment. BMC Complement Altern Med. 2013;13:268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | O'Gara EA, Maslin DJ, Nevill AM, Hill DJ. The effect of simulated gastric environments on the anti-Helicobacter activity of garlic oil. J Appl Microbiol. 2008;104:1324-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Aydin A, Ersöz G, Tekesin O, Akçiçek E, Tuncyürek M. Garlic oil and Helicobacter pylori infection. Am J Gastroenterol. 2000;95:563-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Graham DY, Anderson SY, Lang T. Garlic or jalapeño peppers for treatment of Helicobacter pylori infection. Am J Gastroenterol. 1999;94:1200-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 45] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Zhu YG. The efficacy of allicin combined with Rabeprazole to treat Hp infection. China Med. 2008;94-95. [DOI] [Full Text] |
| 15. | Zhan YH, Peng NN. Allicin-Amoxicillin-Esomeprazole-Furazolidone therapy for Hp related duodenal ulcer. Shand Med J. 2013;63-64. [DOI] [Full Text] |
| 16. | Bai HG, Jiang LZ, Wang AG. Allicin containing quadruple therapy for Hp related duodenal ulcer. Chin Prec Med. 2008;47-48. [DOI] [Full Text] |
| 17. | Wang CE, Du ZH, Xiang H. Treatment of Helicobacter pylori-positive duodenal ulcer: A controlled trial. Med J West China. 2006;304-306. [DOI] [Full Text] |
| 18. | Li CM. The Clinical Observation of Quadruple Therapy in the Treatment of Duodenal Peptic Ulcer with Helicobacter Pylori Positive. Chin Foreign Med Res. 2014;20-21. [DOI] [Full Text] |
| 19. | Koçkar C, Oztürk M, Bavbek N. Helicobacter pylori eradication with beta carotene, ascorbic acid and allicin. Acta Medica (Hradec Kralove). 2001;44:97-100. [PubMed] |
| 20. | Guan AF. Quadruple Therapy for Hp related duodenal ulcer: a clinical trial. Linchuang Jianyan Zazhi (Electronic Edition). 2017;6:401. |
| 21. | Zhao SC, Chen W, Wang P. Clinical effect of allicin combined four-medicine on duodenal ulcer. Strait Pharm J. 2015;27:90-91. [DOI] [Full Text] |
| 22. | Chen W, Zhao SC, Wang P. Allicin Combined with ilaprazole for treating duodenal ulcer in 110 cases. Zhongguo Yaoye. 2016;25:114-116. |
| 23. | Jiao WL, Liu JY. Quadruple therapy for Hp infection with clarithromycin resistance. Zhongguo Wuzhenxue Zazhi. 2010;10:3834. |
| 24. | Pharmaceutical Bureau of the Ministry of Health of the People's Republic of China. Guiding Principles for Clinical Study of New Drugs (Traditional Chinese Medicine) in the Treatment of Peptic Ulcer. Zhonghua Zhongyiyao Zazhi. 1989;4:312-313. |
| 25. | Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18487] [Cited by in RCA: 24861] [Article Influence: 1775.8] [Reference Citation Analysis (3)] |
| 26. | Wetterslev J, Jakobsen JC, Gluud C. Trial Sequential Analysis in systematic reviews with meta-analysis. BMC Med Res Methodol. 2017;17:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 488] [Cited by in RCA: 847] [Article Influence: 105.9] [Reference Citation Analysis (0)] |
| 27. | Jensen EA, Foglia EE, Schmidt B. Evidence-Based Pharmacologic Therapies for Prevention of Bronchopulmonary Dysplasia: Application of the Grading of Recommendations Assessment, Development, and Evaluation Methodology. Clin Perinatol. 2015;42:755-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Vega AE, Cortiñas TI, Puig ON, Silva HJ. Molecular characterization and susceptibility testing of Helicobacter pylori strains isolated in western Argentina. Int J Infect Dis. 2010;14 Suppl 3:e85-e92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Gong Y, Li Y, Sun Q. Probiotics improve efficacy and tolerability of triple therapy to eradicate Helicobacter pylori: a meta-analysis of randomized controlled trials. Int J Clin Exp Med. 2015;8:6530-6543. [PubMed] |
| 30. | Venerito M, Krieger T, Ecker T, Leandro G, Malfertheiner P. Meta-analysis of bismuth quadruple therapy versus clarithromycin triple therapy for empiric primary treatment of Helicobacter pylori infection. Digestion. 2013;88:33-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 113] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 31. | Chung JG, Chen GW, Wu LT, Chang HL, Lin JG, Yeh CC, Wang TF. Effects of garlic compounds diallyl sulfide and diallyl disulfide on arylamine N-acetyltransferase activity in strains of Helicobacter pylori from peptic ulcer patients. Am J Chin Med. 1998;26:353-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Lawson LD, Hughes BG. Characterization of the formation of allicin and other thiosulfinates from garlic. Planta Med. 1992;58:345-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 115] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 33. | McNulty CA, Wilson MP, Havinga W, Johnston B, O'Gara EA, Maslin DJ. A pilot study to determine the effectiveness of garlic oil capsules in the treatment of dyspeptic patients with Helicobacter pylori. Helicobacter. 2001;6:249-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 34. | Cavallito CJ, Bailey JH. Allicin, the antibacterial principle of Allium sativum. I. Isolation, physical properties and antibacterial action. J Am Chem Soc. 1944;66:1950-1954. [RCA] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 386] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 35. | Cañizares P, Gracia I, Gómez LA, Martín de Argila C, Boixeda D, García A, de Rafael L. Allyl-thiosulfinates, the bacteriostatic compounds of garlic against Helicobacter pylori. Biotechnol Prog. 2004;20:397-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 36. | Zardast M, Namakin K, Esmaelian Kaho J, Hashemi SS. Assessment of antibacterial effect of garlic in patients infected with Helicobacter pylori using urease breath test. Avicenna J Phytomed. 2016;6:495-501. [PubMed] |
| 37. | Xue L. The efficacy of allicin combined with ranitidine to treat Hp infection. J Mod Med Heal. 2004;524. [DOI] [Full Text] |
| 38. | Graham DY, Lu H, Yamaoka Y. A report card to grade Helicobacter pylori therapy. Helicobacter. 2007;12:275-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 315] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 39. | Schäfer G, Kaschula CH. The immunomodulation and anti-inflammatory effects of garlic organosulfur compounds in cancer chemoprevention. Anticancer Agents Med Chem. 2014;14:233-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 114] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 40. | Colín-González AL, Santana RA, Silva-Islas CA, Chánez-Cárdenas ME, Santamaría A, Maldonado PD. The antioxidant mechanisms underlying the aged garlic extract- and S-allylcysteine-induced protection. Oxid Med Cell Longev. 2012;2012:907162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 174] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 41. | Kim-Park S, Ku DD. Garlic elicits a nitric oxide-dependent relaxation and inhibits hypoxic pulmonary vasoconstriction in rats. Clin Exp Pharmacol Physiol. 2000;27:780-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 42. | Ku DD, Abdel-Razek TT, Dai J, Kim-Park S, Fallon MB, Abrams GA. Garlic and its active metabolite allicin produce endothelium- and nitric oxide-dependent relaxation in rat pulmonary arteries. Clin Exp Pharmacol Physiol. 2002;29:84-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 43. | Yin RR, Chen HL, Wu X, Yang J, Wang Q. Efficacy and Safety of Jinghua Weikang Capsules Combined with Triple Therapy in the Treatment of Hp Ralated Chronic Gastritis or Peptic Ulcer: A Meta-analysis China Pharmacy. 2018;29:2256-2260. [DOI] [Full Text] |
| 44. | Manyi-Loh CE, Clarke AM, Ndip RN. Detection of phytoconstituents in column fractions of n-hexane extract of Goldcrest honey exhibiting anti-Helicobacter pylori activity. Arch Med Res. 2012;43:197-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 45. | Zhang D, Ke L, Ni Z, Chen Y, Zhang LH, Zhu SH, Li CJ, Shang L, Liang J, Shi YQ. Berberine containing quadruple therapy for initial Helicobacter pylori eradication: An open-label randomized phase IV trial. Medicine (Baltimore). 2017;96:e7697. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 46. | Hu X, Ma JJ, Dong WG. Meta-analysis for the effects of allicin on the treatment of duodenal peptic ulcer with Helicobacter pylori positive. Hainan Med J. 2017;2898-2902. [DOI] [Full Text] |
| 47. | Zhang MX, Yang J, Xu C. Allicin for Helicobacter pylori infection combined with duodenal ulcer: a meta-analysis. Linchuang Yixue. 2014;16:1135-1136. |