Published online Oct 7, 2019. doi: 10.3748/wjg.v25.i37.5711
Peer-review started: April 15, 2019
First decision: June 16, 2019
Revised: July 10, 2019
Accepted: August 7, 2019
Article in press: August 7, 2019
Published online: October 7, 2019
Processing time: 169 Days and 13.5 Hours
Laparoscopy has been widely used in general surgical procedures, but total laparoscopic pancreaticoduodenectomy (TLPD) is still a complex and challenging surgery that is only performed in a small number of patients at a few large academic medical centers. Although the safety and feasibility of TLPD have been established, few studies have compared it with open pancreaticoduodenectomy (OPD) with regard to perioperative and oncological outcomes. Therefore, we carried out a meta-analysis to evaluate whether TLPD is superior to OPD.
To compare the treatment outcomes of TLPD and OPD in order to assess the safety and feasibility of TLPD.
We conducted a systematic search of studies comparing TLPD with OPD that were published in the PubMed, EMBASE, and Cochrane Library databases through December 31, 2018. The studies comparing TLPD and OPD with at least one of the outcomes we were interested in and with more than 10 cases in each group were included in this analysis. The Newcastle-Ottawa scale was used to assess the quality of the nonrandomized controlled trials and the Jadad scale was used to assess the randomized controlled trials. Intraoperative data, postoperative complications, and oncologic outcomes were evaluated. The meta-analysis was performed using Review Manager Software version 5.3. Random or fixed-effects meta-analyses were undertaken to measure the pooled estimates.
A total of 4790 articles were initially identified for our study. After screening, 4762 articles were excluded and 28 studies representing 39771 patients (3543 undergoing TLPD and 36228 undergoing OPD) were eventually included. Patients who underwent TLPD had less intraoperative blood loss [weighted mean difference (WMD) = -260.08 mL, 95% confidence interval (CI): (-336.02, -184.14) mL, P < 0.00001], a lower blood transfusion rate [odds ratio (OR) = 0.51, 95%CI: 0.36-0.72, P = 0.0001], a lower perioperative overall morbidity (OR = 0.82, 95%CI: 0.73-0.92, P = 0.0008), a lower wound infection rate (OR = 0.48, 95%CI: 0.34-0.67, P < 0.0001), a lower pneumonia rate (OR = 0.72, 95%CI: 0.60-0.85, P = 0.0002), a shorter duration of intensive care unit (ICU) stay [WMD = -0.28 d, 95%CI (-2.88, -1.29) d, P < 0.00001] and a shorter length of hospital stay [WMD = -3.05 d, 95%CI (-3.93, -2.17), P < 0.00001], a lower rate of discharge to a new facility (OR = 0.55, 95%CI: 0.39-0.78, P = 0.0008), and a lower 30-d readmission rate (OR = 0.81, 95%CI: 0.68-0.95, P = 0.10) than those who underwent OPD. In addition, the TLPD group had a higher R0 rate (OR = 1.28, 95%CI: 1.13-1.44, P = 0.0001) and more lymph nodes harvested (WMD = 1.32, 95%CI: 0.57-2.06, P = 0.0005) than the OPD group. However, the patients who underwent TLPD experienced a significantly longer operative time (WMD = 77.92 min, 95%CI: 40.89-114.95, P < 0.0001) and had a smaller tumor size than those who underwent OPD [WMD = -0.32 cm, 95%CI: (-0.58, -0.07) cm, P = 0.01]. There were no significant differences between the two groups in the major morbidity, postoperative pancreatic fistula, delayed gastric emptying, postpancreatectomy hemorrhage, bile leak, gastroenteric anastomosis fistula, intra-abdominal abscess, bowel obstruction, fluid collection, reoperation, ICU admission, or 30-d and 90-d mortality rates. For malignant tumors, the 1-, 2-, 3-, 4- and 5-year overall survival rates were not significantly different between the two groups.
This meta-analysis indicates that TLPD is safe and feasible, and may be a desirable alternative to OPD, although a longer operative time is needed and only smaller tumors can be treated.
Core tip: This is a meta-analysis with the largest number of cases so far comparing total laparoscopic pancreaticoduodenectomy (TLPD) and open pancreaticoduodenectomy (OPD). In recent years, the reports or comparisions between TLPD and OPD are increasing, but most of them have a very small number of cases included, and the quality and reliability are limited. In this meta-analysis, we reviewed the published literature on this topic until now with the largest number of cases, thus the conclusion is much more reliable. In addition, our study analyzed the effects of laparoscopic skills not only on intraoperative parameters and postoperative complications, but also on oncological outcomes to ensure its safety and feasibility.
- Citation: Zhang H, Lan X, Peng B, Li B. Is total laparoscopic pancreaticoduodenectomy superior to open procedure? A meta-analysis. World J Gastroenterol 2019; 25(37): 5711-5731
- URL: https://www.wjgnet.com/1007-9327/full/v25/i37/5711.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i37.5711
Laparoscopic techniques have been widely applied in general surgical procedures and have been proved to be beneficial for some selected patients in terms of postoperative recovery and a shorter hospital stay[1,2]. However, since the first laparoscopic pancreaticoduodenectomy was introduced by Gagner et al[3] in 1994, total laparoscopic pancreaticoduodenectomy (TLPD) is still not universally performed and remains a formidable challenging and highly specialized procedure owing to the retroperitoneal surgical location, difficult dissection near the great vessels, critical intracorporeal anastomoses, and its high postoperative morbidity or mortality rates[4,5]. In recent years, with the innovations in laparoscopic techniques and instruments, and the accumulation of surgical experience[6], TLPD has been gradually performed at some major medical centers in properly selected patients and has gained popularity among general surgeons[7,8]. Even so, controversies regarding its perioperative and oncological safety still exist. Although a large number of studies have been performed to evaluate the feasibility and safety of TLPD recently[9-11], all these studies are retrospective analyses including a small number of cases, and there has been no large case-control studies or randomized controlled trials (RCTs).
The purpose of our study was to critically evaluate whether TLPD is superior to the open procedure. For this reason, we carried out a meta-analysis of TLPD vs open pancreaticoduodenectomy (OPD) to compare the intraoperative outcomes, postoperative complications, postoperative recovery, oncological safety, and overall survival (OS).
Our study complied with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines[12], and protocol can be accessed at the International Prospective Register of Systematic Reviews (registration number: CRD42019126173). Manuscripts comparing intraoperative, postoperative, and oncological outcomes and OS of patients with malignancies treated by TLPD or OPD were identified in medical databases.
We conducted a comprehensive literature search of studies published in the PubMed, EMBASE and Cochrane Library databases until December 31, 2018. Only full-text literature published in the English language was included and considered. The studies we identified were restricted to research performed in humans. The prespecified search terms were divided into two categories: The “laparoscopic” terms (“laparoscope” OR “laparoscopic” OR “laparoscopy” OR “minimally invasive” OR “laparoscopy-assisted”) and the “pancreaticoduodenectomy” terms (“pancreatoduo-denectomy” OR “pancreaticoduodenectomy” OR “pancreaticoduodenectomies” OR “pancreatoduodenectomies” OR “pancreatoduodenal resection” OR “duodenopancreatectomy” OR “duodenopancreatectomies” OR “Whipple” OR “pancreatic resection”). References from the articles we acquired were also searched manually to identify additional literature.
TLPD was defined as the resection of the pancreatic head, bile duct, and duodenum and reconstruction of the digestive tract performed completely intracorporeally by laparoscopy. Hybrid procedures, laparoscopic-assisted procedures, or robotic-assisted procedures were not included in this study.
Inclusion criteria: All published nonrandomized and randomized studies comparing TLPD and OPD with at least one of the outcomes we were interested in were included in this analysis. Moreover, the literature we incorporated into our study included full texts with a total number of TLPDs greater than 10. In addition, if several studies were from the same institution, the most recently published study or the one with the largest sample was selected for our analysis.
Exclusion criteria: Studies unrelated to our topic, those that did not include the outcomes we were interested in, those involving patients who underwent other types of pancreaticoduodenectomy (not TLPD or OPD), and those in which less than 10 TLPDs were performed were excluded from our analysis. In addition, other types of articles, such as case reports, reviews, meta-analyses, abstracts, and letters, were also excluded.
The Newcastle-Ottawa scale (NOS)[13] was used to assess the quality of the nonrandomized controlled trials we included in this meta-analysis. This scale includes three items: the selection of patients, the assessment of outcomes, and the comparability of the groups. The range of the NOS score is 0-9 stars and studies receiving more than six stars are regarded as high quality studies. The RCTs included in our study were assessed with the Jadad scale[14] which has a maximum possible score of 5. The RCTs with a score of more than two were regarded as high quality studies. Two authors (Hua Zhang and Xiang Lan) independently assessed the quality of the studies we included, a consensus was reached with the help of another author (Bing Peng) to resolve any disagreements between the two authors.
Two authors (Hua Zhang and Xiang Lan) independently extracted the data from the manuscripts included in the analysis. If they had any disagreements, another author (Bing Peng) was asked for help to resolve the disagreements until a consensus was reached. Data on the following were extracted: first author, publication year, country of the author, type of study, characteristics of the study population, NOS score, intraoperative outcomes (operative time, estimated blood loss, and transfusion rate), postoperative events (overall morbidity, severe complications rate, postoperative pancreatic fistula (POPF) rate, bile leak, delayed gastric emptying (DGE), postpancreatectomy hemorrhage (PPH), postoperative intra-peritoneal abscess, wound infection, intensive care unit (ICU) stay, length of hospital stay (LOS), readmission rate, reoperative rate, and perioperative mortality), oncological outcomes (tumor size, number of lymph nodes harvested, and R0 resection), and prognostic variables (1-, 2-, 3-, 4-, and 5-year OS rates). The definitions and classification of postoperative complications complied with the International Study Group of Pancreatic Surgery and the Clavien–Dindo classification[15-18].
The study was conducted according to the recommendations of the Cochrane Collaboration[19]. All statistical analyses in this study were performed with the software Review Manager version 5.3. I2 values were preferred for the quantification of statistical inconsistency, which was defined as the percentage of variation between studies due to heterogeneity, with values greater than 50% deemed to indicate significant heterogeneity. Continuous variables in this analysis were evaluated using the inverse variance statistical method, and the weighted mean difference (WMD) was also calculated. Dichotomous variables in this study were analyzed by the Mantel-Haenszel statistical method using the odds ratio (OR) as the summary statistic. Both statistical methods are reported with 95% confidence intervals (CIs). A random-effects model was used to present the results of heterogeneous data in the presence of low or moderate statistical inconsistency (I2 ≥ 50%), and a fix-effects model was used in the presence of high statistical inconsistency (I2 < 50%). Forest plots were constructed, and a P-value < 0.05 and a 95%CI that did not include the value 1 were considered statistically significant. Funnel plots were also constructed to detect and evaluate the publication bias.
A total of 4790 potential studies were initially identified from the medical databases initially. After excluding duplicates, 3791 articles remained. Subsequently, we reviewed the titles to identify literature that was not relevant to our topic, and these articles were excluded from our study. We scanned the abstracts or full texts of the remaining literature for our study, and eventually, 28 eligible articles[4,9-11,20-43] (including 2 RCTs, 20 retrospective studies and 6 matched case-control studies) met the inclusion criteria and were selected. The selection strategy is presented as a flowchart in Figure 1. The 28 selected articles with a total of 39771 patients (3543 undergoing TLPD and 36228 undergoing OPD) were from seven countries worldwide (2 United Kingdom, 16 United States, 2 France, 2 Korea, 3 China, 2 India, and 1 Spain). Each of the included studies included more than one variable we were interested in and compared the two groups. The NOS and the Jadad scales were used to assess the quality of the included studies and the quality scores ranged from 7-9 in retrospective studies and 3 in RCTs. The characteristics of the included studies are summarized in Table 1. Additionally, patients who underwent conversion to other types of surgeries in each study were included in the TLPD group.
| First author | Year | Country | Study design | Sample size | Conver-sion n (%) | ISGPF | ISGPS | Clavien-Dindo | Quali-ty Scor-es | |
| TLPD | OPD | |||||||||
| Zimmerman | 2018 | USA | Retrospective analysis | 280 | 6336 | 78 (27.86) | NR | NR | NR | 7a |
| Tran | 2016 | USA | Retrospective analysis | 681 | 14893 | NR | NR | NR | NR | 7a |
| Tee | 2015 | USA | Retrospective analysis | 113 | 225 | NR | YES | YES | YES | 8a |
| Tan | 2015 | China | Retrospective analysis | 30 | 30 | NR | YES | YES | NR | 9a |
| Stauffer | 2017 | USA | Retrospective analysis | 58 | 193 | NR | YES | YES | YES | 8a |
| Song | 2015 | Korea | Matched case-control study | 97 | 198 | NR | YES | YES | YES | 8a |
| Sharpe | 2015 | USA | Retrospective analysis | 384 | 4037 | NR | NR | NR | NR | 7a |
| Senthilna-than | 2015 | India | Retrospective analysis | 45 | 118 | NR | NR | NR | NR | 8a |
| Poves | 2018 | Spain | RCT | 32 | 29 | 8 (25.00) | YES | YES | YES | 4b |
| Palanivelu | 2017 | India | RCT | 32 | 32 | 1 (3.13) | YES | YES | YES | 3b |
| Meng | 2018 | China | Retrospective analysis | 58 | 58 | NR | YES | YES | YES | 9a |
| Lee | 2018 | Korea | Matched case-control study | 31 | 31 | NR | NR | NR | NR | 9a |
| Khaled | 2018 | UK | Matched case-control study | 15 | 15 | 1 (6.67) | YES | YES | YES | 9a |
| Kantor | 2017 | USA | Retrospective analysis | 828 | 7385 | NR | NR | NR | NR | 7a |
| Hakeem | 2014 | UK | Matched case-control study | 12 | 12 | NR | NR | NR | YES | 9a |
| Gerber | 2017 | USA | Retrospective analysis | 52 | 50 | NR | NR | NR | NR | 8a |
| Dokmak | 2015 | France | Matched case-control study | 46 | 46 | 3 (6.52) | YES | YES | YES | 9a |
| Delitto | 2016 | USA | Retrospective analysis | 52 | 50 | NR | YES | YES | YES | 8a |
| Croome | 2014 | USA | Retrospective analysis | 108 | 214 | 7 (6.48) | YES | YES | YES | 8a |
| Conrad | 2017 | USA | Retrospective analysis | 40 | 25 | 9 (22.50) | YES | YES | YES | 8a |
| Chopinet | 2018 | France | Retrospective analysis | 65 | 290 | NR | YES | YES | YES | 8a |
| Chen | 2018 | China | Retrospective analysis | 47 | 55 | NR | YES | YES | YES | 8a |
| Chapman | 2018 | USA | Retrospective analysis | 22 | 25 | 0 (0) | YES | YES | YES | 8a |
| Chapman | 2018 | USA | Retrospective analysis | 248 | 1520 | 74 (29.84) | NR | NR | NR | 7a |
| Asbun | 2012 | USA | Retrospective analysis | 53 | 215 | 9 (15.00) | YES | YES | NR | 8a |
| Zureikat | 2011 | USA | Matched case-control study | 14 | 14 | 2 (14.29) | YES | NR | YES | 9a |
| Mesleh | 2013 | USA | Retrospective analysis | 75 | 48 | 10 (13.33) | YES | YES | YES | 8a |
| Speicher | 2014 | USA | Retrospective analysis | 25 | 84 | NR | YES | NR | NR | 8a |
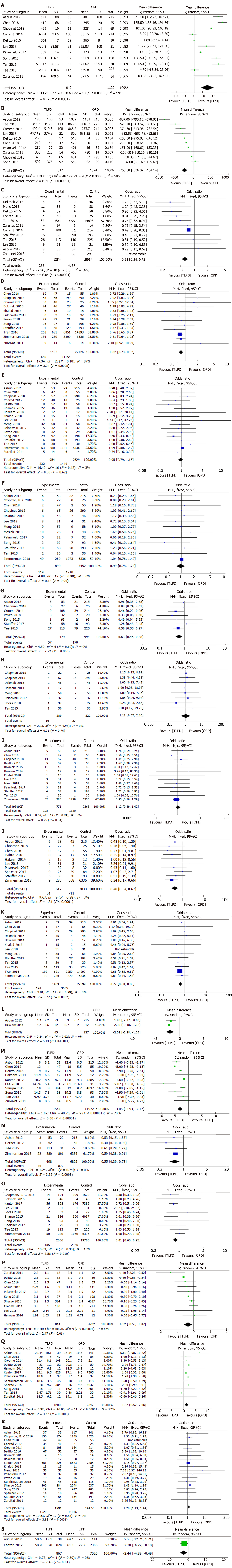
The results of the meta-analysis with regard to the variables we were interested in, for instance, the intraoperative parameters, postoperative complications, oncological outcomes, and OS rate, are reported in detail and summarized in Tables 2-4. Forest plots of the comparisons we interested are shown in Figure 2.
| Outcome of interest | No. of studies | Sample size | Heterogeneity | Model | Overall effect size | 95%CI of overall effect | P-value | ||
| TLPD | OPD | P | I2 | ||||||
| Operative time | 11 | 642 | 1129 | < 0.00001 | 99% | R | WMD = 77.92 | 40.89, 114.95 | < 0.0001 |
| EBL | 10 | 612 | 1324 | < 0.00001 | 98% | R | WMD = -260.08 | -336.02, -184.14 | < 0.00001 |
| Transfusion rate | 12 | 1254 | 15964 | 0.003 | 60% | R | OR = 0.51 | 0.36, 0.72 | 0.0001 |
| Overall morbidity | 12 | 1407 | 22126 | 0.10 | 37% | F | OR = 0.82 | 0.73, 0.92 | 0.0008 |
| Major morbidity | 16 | 886 | 1704 | 0.05 | 41% | F | OR = 0.88 | 0.70, 1.10 | 0.25 |
| POPF | 17 | 954 | 7629 | 0.42 | 3% | F | OR = 0.95 | 0.79, 1.15 | 0.62 |
| POPF (grade B/C) | 18 | 942 | 1821 | 0.47 | 0 | F | OR = 0.96 | 0.76, 1.22 | 0.75 |
| DGE | 13 | 890 | 7452 | 0.98 | 0 | F | OR = 0.99 | 0.78, 1.24 | 0.90 |
| DGE (grade B/C) | 7 | 479 | 994 | 0.60 | 0 | F | OR = 0.63 | 0.45, 0.88 | 0.006 |
| PPH | 13 | 771 | 7363 | 0.74 | 0 | F | OR = 1.12 | 0.89, 1.42 | 0.34 |
| PPH (grade B/C) | 7 | 454 | 966 | 0.91 | 0 | F | OR = 1.02 | 0.65, 1.60 | 0.95 |
| Bile leak | 8 | 289 | 522 | 0.96 | 0 | F | OR = 1.11 | 0.57, 2.16 | 0.76 |
| Gastroente-ric anastomosis fistula | 4 | 201 | 423 | 0.77 | 0 | F | OR = 0.62 | 0.16, 2.40 | 0.49 |
| Wound infection | 10 | 612 | 7033 | 0.38 | 7% | F | OR = 0.48 | 0.34, 0.67 | < 0.0001 |
| Intra-abdominal abscess | 6 | 455 | 6676 | 0.32 | 14% | F | OR = 0.97 | 0.71, 1.31 | 0.82 |
| Bowel obstruction | 2 | 73 | 73 | 0.33 | 0 | F | OR = 1.00 | 0.14, 7.31 | 1.00 |
| Fluid collection | 3 | 142 | 367 | 0.20 | 37% | F | OR = 1.50 | 0.90, 2.48 | 0.12 |
| Pneumonia | 13 | 1489 | 22399 | 0.99 | 0 | F | OR = 0.72 | 0.60, 0.85 | 0.0002 |
| Cardiac event | 6 | 1200 | 21877 | 0.75 | 0 | F | OR = 1.04 | 0.82, 1.32 | 0.75 |
| Reoperation | 14 | 881 | 7616 | 0.40 | 5% | F | OR = 1.10 | 0.83, 1.47 | 0.51 |
| ICU admission | 2 | 171 | 283 | 0.40 | 0 | F | OR = 0.90 | 0.53, 1.54 | 0.71 |
| ICU stay | 2 | 65 | 227 | 0.62 | 0 | F | WMD = -2.08 | -2.88, -1.29 | < 0.00001 |
| Diet Start Time | 3 | 175 | 284 | <0.0001 | 90% | R | WMD = -1.75 | -3.38, -0.12 | 0.04 |
| LOS | 10 | 1544 | 11922 | < 0.00001 | 78% | R | WMD = -3.05 | -3.93, -2.17 | < 0.00001 |
| Discharge to a new facility | 4 | 498 | 6826 | 0.74 | 0 | F | OR = 0.55 | 0.39, 0.78 | 0.0008 |
| 30-d readmission | 10 | 2006 | 19786 | 0.30 | 15% | F | OR = 0.81 | 0.68, 0.95 | 0.010 |
| 90-d readmission | 4 | 136 | 279 | 0.70 | 0 | F | OR = 1.07 | 0.62, 1.84 | 0.81 |
| 30-d mortality | 18 | 2870 | 35337 | 0.70 | 0 | F | OR = 1.00 | 0.81, 1.24 | 1.00 |
| 90-d mortality | 11 | 1273 | 9159 | 0.77 | 0 | F | OR = 0.77 | 0.58, 1.01 | 0.06 |
| Outcome of interest | No. of studies | Sample size | Heterogeneity | Model | Overall effect size | 95%CI of overall effect | P-value | ||
| TLPD | OPD | P | I2 | ||||||
| Tumor size | 10 | 814 | 4782 | < 0.00001 | 85% | R | WMD = -0.32 | -0.58, -0.07 | 0.01 |
| RLNs | 12 | 1600 | 12347 | < 0.00001 | 77% | R | WMD = 1.32 | 0.57, 2.06 | 0.0005 |
| R0 rate | 19 | 1991 | 14477 | 0.88 | 0 | F | OR = 1.28 | 1.13, 1.44 | 0.0001 |
| Time to adjuvant chemotherapy | 2 | 867 | 7526 | 0.39 | 0 | F | WMD = -2.44 | -4.39, -0.49 | 0.01 |
| RFS of all malignant tumor patients | |||||||||
| 1-yr RFS | 2 | 52 | 37 | NA | NA | F | OR = 0.44 | 0.16, 1.23 | 0.12 |
| 3-yr RFS | 2 | 52 | 37 | 0.22 | 35% | F | OR = 0.53 | 0.19, 1.47 | 0.22 |
| 5-yr RFS | 2 | 52 | 37 | 0.31 | 4% | F | OR = 0.40 | 0.12, 1.33 | 0.14 |
| OS of all malignant tumor patients | |||||||||
| 1-yr OS | 5 | 412 | 2206 | 0.007 | 72% | R | OR = 0.62 | 0.33, 1.19 | 0.15 |
| 2-yr OS | 4 | 400 | 2194 | 0.03 | 66% | R | OR = 0.61 | 0.32, 1.17 | 0.14 |
| 3-yr OS | 5 | 412 | 2206 | 0.08 | 51% | R | OR = 0.80 | 0.40, 1.62 | 0.54 |
| 4-yr OS | 4 | 400 | 2194 | 0.60 | 0 | F | OR = 0.73 | 0.41, 1.30 | 0.28 |
| 5-yr OS | 6 | 520 | 2420 | 0.52 | 0 | F | OR = 0.78 | 0.38, 1.59 | 0.49 |
| OS of PDAC patients | |||||||||
| 1-yr OS | 3 | 349 | 1947 | 0.28 | 22% | F | OR = 0.93 | 0.74, 1.18 | 0.57 |
| 2-yr OS | 3 | 349 | 1947 | 0.21 | 37% | F | OR = 0.93 | 0.70, 1.24 | 0.63 |
| 3-yr OS | 3 | 349 | 1947 | 0.60 | 0 | F | OR = 0.86 | 0.56, 1.34 | 0.52 |
| 4-yr OS | 3 | 349 | 1947 | 0.95 | 0 | F | OR = 0.97 | 0.49, 1.92 | 0.94 |
| 5-yr OS | 3 | 349 | 1947 | 0.27 | 17% | F | OR = 0.65 | 0.19, 2.23 | 0.49 |
| OS of periampullary adenocarcinoma patients | |||||||||
| 1-yr OS | 3 | 349 | 1947 | 0.28 | 22% | F | OR = 0.93 | 0.74, 1.18 | 0.57 |
| 2-yr OS | 3 | 349 | 1947 | 0.21 | 37% | F | OR = 0.93 | 0.70, 1.24 | 0.63 |
| 3-yr OS | 3 | 349 | 1947 | 0.60 | 0 | F | OR = 0.86 | 0.56, 1.34 | 0.52 |
| 4-yr OS | 3 | 349 | 1947 | 0.95 | 0 | F | OR = 0.97 | 0.49, 1.92 | 0.94 |
| 5-yr OS | 3 | 349 | 1947 | 0.27 | 17% | F | OR = 0.65 | 0.19, 2.23 | 0.49 |
| 5-yr OS | 6 | 520 | 2420 | 0.52 | 0 | F | OR = 0.78 | 0.38, 1.59 | 0.49 |
| Outcomes of interest | No. of Studies | Sample size | Heterogeneity | Model | Overall effect size | 95%CI of overall effect | P-value | ||
| TLPD | OPD | P | I2 | ||||||
| Operative time | 10 | 577 | 1064 | < 0.00001 | 98% | R | WMD = 74.66 | 43.71, 105.62 | < 0.00001 |
| EBL | 9 | 547 | 1034 | < 0.00001 | 95% | R | WMD = -280.46 | -347.73, -213.19 | < 0.00001 |
| Transfusion rate | 10 | 573 | 1071 | 0.15 | 32% | F | OR = 0.45 | 0.35, 0.58 | < 0.00001 |
| Overall morbidity | 9 | 381 | 607 | 0.31 | 15% | F | OR = 0.88 | 0.65, 1.18 | 0.38 |
| Major morbidity | 15 | 829 | 1414 | 0.49 | 0 | F | OR = 0.75 | 0.59, 0.96 | 0.02 |
| POPF | 15 | 617 | 1003 | 0.61 | 0 | F | OR = 0.82 | 0.64, 1.05 | 0.11 |
| POPF (grade B/C) | 17 | 877 | 1531 | 0.89 | 0 | F | OR = 0.86 | 0.66, 1.11 | 0.24 |
| DGE | 10 | 523 | 801 | 0.93 | 0 | F | OR = 0.93 | 0.64, 1.34 | 0.69 |
| DGE (grade B/C) | 7 | 479 | 994 | 0.60 | 0 | F | OR = 0.63 | 0.45, 0.88 | 0.006 |
| PPH | 11 | 434 | 737 | 0.84 | 0 | F | OR = 1.48 | 0.93, 2.36 | 0.10 |
| PPH (grade B/C) | 7 | 454 | 966 | 0.91 | 0 | F | OR = 1.02 | 0.65, 1.60 | 0.95 |
| Bile leak | 7 | 232 | 232 | 0.93 | 0 | F | OR = 1.00 | 0.45, 2.25 | 0.99 |
| Gastroente-ric anastomosis fistula | 3 | 136 | 133 | 0.54 | 0 | F | OR = 0.49 | 0.09, 2.73 | 0.42 |
| Wound infection | 9 | 332 | 697 | 0.46 | 0 | F | OR = 0.57 | 0.39, 0.84 | 0.005 |
| Intra-abdominal abscess | 5 | 175 | 340 | 0.23 | 29% | F | OR = 0.74 | 0.39, 1.38 | 0.34 |
| Bowel obstruction | 2 | 73 | 73 | 0.33 | 0 | F | OR = 1.00 | 0.14, 7.31 | 1.00 |
| Fluid collection | 2 | 77 | 77 | 0.96 | 0 | F | OR = 0.63 | 0.21, 1.89 | 0.41 |
| Pneumonia | 10 | 463 | 880 | 0.99 | 0 | F | OR = 0.79 | 0.54, 1.15 | 0.21 |
| Cardiac event | 4 | 239 | 648 | 0.48 | 0 | F | OR = 1.11 | 0.75, 1.62 | 0.61 |
| Reoperation | 12 | 544 | 990 | 0.46 | 0 | F | OR = 0.76 | 0.48, 1.21 | 0.25 |
| ICU admission | 2 | 171 | 283 | 0.40 | 0 | F | OR = 0.90 | 0.53, 1.54 | 0.71 |
| ICU stay | 2 | 65 | 227 | 0.62 | 0 | F | WMD = -2.08 | -2.88, -1.29 | < 0.00001 |
| Diet start time | 3 | 175 | 284 | <0.0001 | 90% | R | WMD = -1.75 | -3.38, -0.12 | 0.04 |
| LOS | 8 | 332 | 500 | 0.004 | 67% | R | WMD = -3.69 | -4.90, -2.48 | < 0.00001 |
| Discharge to a new facility | 3 | 218 | 490 | 0.60 | 0 | F | OR = 0.50 | 0.28, 0.87 | 0.01 |
| 30-d readmission | 6 | 340 | 508 | 0.77 | 0 | F | OR = 1.04 | 0.68, 1.59 | 0.86 |
| 90-d readmission | 4 | 136 | 279 | 0.70 | 0 | F | OR = 1.07 | 0.62, 1.84 | 0.81 |
| 30-d mortality | 12 | 527 | 1271 | 0.76 | 0 | F | OR = 1.26 | 0.59, 2.68 | 0.55 |
| 90-d mortality | 9 | 312 | 661 | 0.96 | 0 | F | OR = 0.60 | 0.31, 1.17 | 0.14 |
| Time to adjuvant chemothe-rapy | 1 | 39 | 141 | NA | NA | F | WMD = -5.50 | -12.71, 1.71 | 0.14 |
| Tumor size | 9 | 430 | 745 | 0.0002 | 73% | R | WMD = -0.36 | -0.63, -0.09 | 0.008 |
| RLNs | 10 | 388 | 925 | < 0.00001 | 79% | R | WMD = 1.28 | 0.29, 2.27 | 0.01 |
| R0 rate | 16 | 605 | 1535 | 0.87 | 0 | F | OR = 1.44 | 1.07, 1.94 | 0.02 |
| OS of PDAC | |||||||||
| 1-yr OS | 2 | 65 | 427 | 0.49 | 0 | F | OR = 0.65 | 0.37, 1.13 | 0.12 |
| 2-yr OS | 2 | 65 | 427 | 0.74 | 0 | F | OR = 0.54 | 0.27, 1.07 | 0.08 |
| 3-yr OS | 2 | 65 | 427 | 0.37 | 0 | F | OR = 0.98 | 0.46, 2.10 | 0.96 |
| 4-yr OS | 2 | 65 | 427 | 0.82 | 0 | F | OR = 1.04 | 0.43, 2.52 | 0.93 |
| 5-yr OS | 2 | 65 | 427 | 0.27 | 17% | F | OR = 0.65 | 0.19, 2.23 | 0.49 |
| OS of periampullary adenocarcinoma patients | |||||||||
| 1-yr OS | 2 | 23 | 234 | 0.004 | 88% | R | OR = 0.85 | 0.01, 107.31 | 0.95 |
| 3-yr OS | 2 | 23 | 234 | 0.01 | 84% | R | OR = 0.90 | 0.01, 86.00 | 0.96 |
| 5-yr OS | 2 | 23 | 234 | 0.26 | 20% | F | OR = 1.57 | 0.43, 5.73 | 0.49 |
The intraoperative parameters we were interested in included the operative time, estimated blood loss (EBL), and blood transfusion rate.
Eleven[10,20,23-26,32,35,37,40,41] of 28 studies reported data regarding the operative time, however, two studies[25,26] showed no significant differences between the two groups. The remaining studies demonstrated a longer operative time in the TLPD group than in the OPD group. Our study showed that the application of the laparoscopic technique may be associated with a significantly longer operative time (WMD = 77.92 min, 95%CI: 40.89-114.95 min, P < 0.0001) (Figure 2A).
The EBL was presented in ten studies[10,20,23-26,32,35,37,41] including 1936 cases (612 undergoing TLPD and 1324 undergoing OPD); except for the studies by Zureikat et al[10] and Song et al[37], which showed no significant difference, the others indicated that the TLPD was associated with less blood loss. Our study also revealed that there was a significant reduction in blood loss in the TLPD group (WMD = -260.08 mL, 95%CI: (-336.02, -184.14) mL, P < 0.00001) (Figure 2B).
The blood transfusion rate was reported in 12 studies[10,11,20,24-27,32,33,39,41,42] including 22034 patients (1577 undergoing TLPD and 20457 undergoing OPD); five of twelve studies (Conrad et al[11], Delitto et al[26], Dokmak et al[27], Meng et al[33] and Zureikat et al[10]) reported no significant difference between the two groups in terms of the transfusion rate, but the other studies showed a significantly lower rate in the TLPD group. The pooled analysis of all included studies showed a statistically significant decrease in transfusion rate in the TLPD group (OR = 0.51, 95%CI: 0.36-0.72, P = 0.0001) (Figure 2C).
The variables we were interested in and compared for postoperative complications were the overall morbidity, major morbidity (Clavien-Dindo grade III–V), POPF, bile leak, DGE, PPH, gastroenteric anastomosis fistula, wound infection, intra-abdominal abscess, bowel obstruction, fluid collection, pneumonia, cardiac event, reoperation rate, ICU admission, ICU stay, diet start time, LOS, rate of discharge to a new facility, readmission rate, and mortality. The results are presented in Table 2.
The overall morbidity was reported in 12 articles[10,11,23,24,27,31,35-37,39,42,43] including 23533 patients. Only Tran et al[42] found a higher morbidity in the OPD group. The remaining 11 studies did not show any significant difference between the two groups. Our meta-analysis of all the satisfactory studies indicated that the rate of overall postoperative complications in the TLPD group was significantly lower than that in the OPD group (OR = 0.82, 95%CI: 0.73-0.92, P = 0.0008) (Figure 2D). Sixteen studies[10,11,20,23-27,29,33-37,39,41] reported the major complication rate (Clavien-Dindo classification ≥ III), and most of them reported the same conclusion that the incidence was comparable in the two groups, but two of the studies obtained a completely different result; Poves et al[36] found that the rate was higher in the OPD group, whereas Chopinet et al[24] obtained a higher rate in the TLPD group. The pooled analysis revealed that the incidence between the two groups did not have any statistically significant differences (OR = 0.88, 95%CI: 0.70-1.10, P = 0.25).
POPF is a common complication after PD. Seventeen[10,11,20,23,24,26,27,29,31-33,35-37,39,40,43] studies demonstrated the occurrence of POPF, and most of the studies did not find any differences between the two groups. The remaining three studies arrived at a different result. Two[26,37] of the three studies showed that TLPD could significantly reduce the incidence of POPF, but Chopinet et al[24] found that TLPD was associated with an increased POPF rate. Our study also revealed that TLPD did not significantly decrease or increase the incidence of POPF (OR = 0.95, 95%CI: 0.79-1.15, P = 0.62) (Figure 2E). In terms of clinically significant pancreatic fistula [grade B/C according to the definition of the International Study Group of Pancreatic Fistula (ISGPF)], 18 studies[10,20,22-27,31,33-41] reported an incidence rate. The analysis of all the included cases indicated that the use of total laparoscopic techniques did not significantly affect the incidence of the clinically significant pancreatic fistula (OR = 0.96, 95%CI: 0.76-1.22, P = 0.75), which is similar to the results of the included studies.
In our study, 13[20,21,23,24,27,32-35,37,39,40,43] of the 28 studies reported the rate of DGE, and the results of all included studies were comparable between the two groups. Our meta-analysis also revealed that there was no statistically significant difference between the two groups in terms of DGE (OR = 0.99, 95%CI: 0.78-1.24, P = 0.90) (Figure 2F). However, for grades B and C DGE, the incidence was significantly decreased in the TLPD group (OR = 0.63, 95%CI: 0.45-0.88, P = 0.006), although only one[25] of the seven included studies[20,22,25,36,37,39,41] obtained the same result. In addition, we also found that the TLPD group had a shorter time to resume an oral diet [WMD = -1.75 d, 95%CI: (-3.38, -0.12), P =0.004] (Figure 2G).
Bile leak is another common postoperative complication that is mainly associated with the cholangiojejunostomy skills. With advancements in anastomosis, the incidence of bile leak has decreased. In our study, eight studies[22,24,27,29,33,35,36,40] including 43 patients (16 undergoing TLPD and 27 undergoing OPD) developed bile leak, and the meta-analysis revealed that the incidence in the TLPD group was comparable to that in the OPD group (OR = 1.11, 95%CI: 0.57-2.16, P = 0.76) (Figure 2H).
A total of 1425 patients in 13 studies[20,23,24,26,27,29,31-33,35,39,40,43] developed PPH. Although Chopinet et al[24] and Dokmak et al[27] found that the application of TLPD increased the incidence of PPH, the overall incidence was 13.62% in the TLPD group and 17.93% in the OPD group. Our pooled analysis of all the included studies did not demonstrate any significant differences between the two groups (OR = 1.12, 95%CI: 0.89-1.42, P = 0.34) (Figure 2I). Similarly, this analysis of included articles[20,25,33,35,36,39,41] did not show any statistically significant differences in terms of severe PPH (grade B/C) (OR = 1.02, 95%CI: 0.65-1.60, P = 0.95).
A total of 762 patients in ten studies[20,22,23,26,29,32,35,38,39,43] developed wound infections in our study, and the overall incidence was 8.33% in the TLPD group and 10.11% in the OPD group. The results of our analysis showed that the frequency of wound infections in the TLPD group was significantly lower than that in the OPD group (OR = 0.48, 95%CI: 0.34-0.67, P < 0.0001) (Figure 2J).
Pneumonia is also a common complication that occurs after abdominal surgery, especially in elderly patients. In our study, only Tran et al[42] demonstrated a lower incidence of pneumonia in the TLPD group, while the others[20,23,24,27,29,31-33,39-41,43] did not show any significant difference between the two groups. Our analysis of the involved studies revealed a lower incidence of pneumonia in the TLPD group (OR = 0.72, 95%CI: 0.60-0.85, P = 0.0002) (Figure 2K).
The ICU admission rate was reported in two studies[33,41] involving 71 patients (25 TLPDs and 46 OPDs), and the rate did not show any significant difference between the two groups (OR = 0.90, 95%CI: 0.53-1.54, P = 0.71). However, in terms of the duration of ICU stay, we observed that the TLPD group had a significantly shorter ICU stay than the OPD group [WMD = -0.28d, 95%CI: (-2.88, -1.29) d, P < 0.00001] (Figure 2L) through analysis of these two studies[20,29].
Our meta-analysis of the included studies[9,10,20,23,26,29,30,32,37,40] showed that the LOS was significantly shorter in the TLPD group [WMD = -3.05 d, 95%CI: (-3.93, -2.17) d, P < 0.00001] (Figure 2M), and it also revealed that patients who underwent TLPD were less frequently discharged to a new facility[20,28,41,43] (OR = 0.55, 95%CI: 0.39-0.78, P = 0.0008) (Figure 2N).
The 30- and 90-d mortality rates after surgery were reported in 18[9,10,21,23-26,29-33,37,38,40-43], 11[11,20-22,27,29,30,35,36,38,39] studies, and none of these studies revealed any statistically significant differences between the two groups (OR = 1.00, 95%CI: 0.81-1.24, P = 1.00; OR = 0.77, 95%CI: 0.58-1.01, P = 0.06).
A total of 14 articles[10,20,24,27,31-36,38,39,41,43] reported the reoperation rate; the overall reoperation rate was 7.95% in the TLPD group and 6.43% in the OPD group. The pooled analysis did not show any significant differences (OR = 1.10, 95%CI: 0.83-1.47, P = 0.51).
The 30-d readmission rate was observed in ten studies[9,21,27,30,32,36-38,41,43], and the readmission rate in the TLPD group was significantly lower than that in the OPD group (9.22% vs 11.95%) (OR = 0.81, 95%CI: 0.68-0.95, P = 0.010) (Figure 2O). However, for the 90-d readmission rate[22,35,36,39], there was no significant differences between the two groups (OR = 1.07, 95%CI: 0.62-1.84, P = 0.81).
For other outcomes with low incidences, such as gastroenteric anastomosis fistula[24,27,33,36] (OR = 0.62, 95%CI: 0.16-2.40, P = 0.49), intra-abdominal abscess[20,22,29,33,40,43] (OR = 0.97, 95%CI: 0.71-1.31, P = 0.82), bowel obstruction[31,33] (OR = 1.00, 95%CI: 0.14-7.31, P = 1.00), fluid collection[24,27,32] (OR = 1.50, 95%CI: 0.90-2.48, P = 0.12), and cardiac events[20,31,39,41-43] (OR = 1.04, 95%CI: 0.82-1.32, P = 0.75), we did not find any significant differences between the two groups.
The oncological outcomes we focused on were the tumor size, the number of lymph nodes harvested, the R0 resection rate, and the OS rate. The results are presented in Table 3. The meta-analysis of tumor size reported in the articles[9,10,20,23,25,26,29,32,35,37] demonstrated that the tumor size in the TLPD group was usually smaller than that in the OPD group, and the differences between the two groups were statistically significant [WMD = -0.32 cm, 95%CI: (-0.58, -0.07) cm, P = 0.01] (Figure 2P).
Twelve studies[4,9,10,20,23,25,26,29,30,35,37,40] reported the number of lymph nodes harvested, and our analysis of all the included studies demonstrated that the patients in the TLPD group had significantly more lymph nodes harvested than those in the OPD group (WMD = 1.32, 95%CI: 0.57-2.06, P = 0.0005) (Figure 2Q).
Regarding R0 resection in patients with malignant tumors, 19 articles[4,9-11,20,21,23,25-27,29-31,33,35-39] were included, and only Sharpe et al[9] and Delitto et al[26] showed significant differences. The analysis of the included studies showed that the TLPD group had a significantly higher R0 resection rate than the OPD group (OR = 1.28, 95%CI: 1.13-1.44, P = 0.0001) (Figure 2R).
In our study, we also found that the TLPD group had a shorter mean time to start their adjuvant therapies than the OPD group[20,30] [WMD = -2.44 d, 95%CI: (-4.39, -0.49) d, P = 0.01] (Figure 2S). The OS was another factor we were interested in when treating patients with malignant tumors. In our study, the 1-year[11,21,29,37,39] (OR = 0.62, 95%CI: 0.33-1.19, P = 0.15), 2-year[11,21,37,39] (OR = 0.61, 95%CI: 0.32-1.17, P = 0.14), 3-year[11,21,29,37,39] (OR = 0.80, 95%CI: 0.40-1.62, P = 0.54), 4-year[11,21,37,39] (OR = 0.73, 95%CI: 0.41-1.30, P = 0.28), and 5-year[11,21,25,29,37,39] (OR = 0.78, 95%CI: 0.38-1.59, P = 0.49) OS rates of the included studies were not significantly longer in the TLPD group. In addition, for patients with malignant tumors with the same origin as pancreatic ductal adenocarcinoma or periampullary adenocarcinoma, the application of TLPD did not affect the OS.
Considering the high heterogeneity between the two groups, we performed a sensitivity analysis to evaluate the quality of the results we obtained. A total of 22 studies[4,10,11,20,22,23,25-29,31-41] were enrolled in the sensitivity analysis and six[9,21,24,30,42,43] were excluded because their patients were from more than one institution. The results are presented in Table 4. The results of the items we were interested in were mostly consistent with those of the former analysis except for three, namely, the overall morbidity, pneumonia, and 30-d readmission rates, which were significantly lower in the TLPD group than in the OPD group in the former analysis, but became comparable (OR = 0.88, 95%CI: 0.65-1.18, P = 0.38; OR = 0.79, 95%CI: 0.54-1.15, P = 0.21; OR = 1.04, 95%CI: 0.68-1.59, P = 0.86). The incidence of major morbidity was comparable between the two groups in the prior analysis, but in the subsequent analysis, it was significantly lower in the TLPD group than in the OPD group (OR = 0.75, 95%CI: 0.59-0.96, P = 0.02).
Laparoscopic techniques are minimally invasive procedures that have been applied in a wide variety of general surgical procedures, including some pancreatic operations[30], and the techniques have proven to be more advantageous in terms of shortened LOS, reduced operative blood loss, decreased incidence of postoperative complications, and enhanced postoperative recovery[1,44-47]. However, with regard to pancreaticoduodenectomy, this procedure has only been performed at some major medical centers. In the last decade, with the continuous advancements in instrumentation and innovations in procedures[48], TLPD has been increasingly accepted and performed by general surgeons worldwide, but this challenging procedure is still in its early stages, and whether TLPD is superior to or comparable to OPD has remained unknown until now. To our knowledge, several systematic reviews or meta-analyses comparing minimally invasive pancreaticoduodenectomy (MIPD) and OPD have been published[49,50] , but none of them have compared TLPD and OPD specifically; hence, we performed this meta-analysis with the largest available dataset from the published literature.
This meta-analysis, based on 2 RCTs and 26 retrospective comparative studies of TLPD and OPD, preliminarily confirmed the feasibility and potential advantages of TLPD; among 39771 patients, including 3543 in the TLPD group and the remaining 36228 in the OPD group, there were no significant differences between the two groups in terms of the major morbidity, mortality, reoperation, and 90-d readmission rates, TLPD was associated with less EBL, lower blood transfusion rate, lower incidence of overall postoperative complications, shorter length of ICU stay and LOS, more harvested lymph nodes, and higher R0 resection rate than OPD. However, TLPD was associated with a longer operative time and a smaller tumor size.
The longer duration of surgery is the main disadvantage of TLPD and is always one of the reasons why some surgeons doubt its feasibility. This may be attributed to the complexity and difficulty of this laparoscopic procedure. Our analysis of the included studies also demonstrated that the operative time was significantly longer in the TLPD group than in the OPD group (P < 0.0001), as other studies have reported[20,27,34,51]. However, Dokmak[27,49,52] indicated that the operative time may decrease with the learning curve. Some studies in other fields have reported that longer operative time is harmful to postoperative recovery[53], but this result was not clear with this procedure. Additionally, the tumor size in our analysis was significantly smaller in the TLPD group (P = 0.01). The larger the tumor size is, the much closer the tumor can be to the great vessels, and thus the more difficult the tumor is to dissect; therefore, as most of surgeons had just started to perform this procedure and had limited experience, they preferred to select patients with smaller tumors to reduce the degree of difficulty; as a consequence, the results need more studies to further evaluate.
We found that the EBL and intraoperative blood transfusion rate were significantly decreased in the TLPD group (P < 0.00001, P = 0.0001) in our meta-analysis, which may be associated with the suitable selection of patients, the utilization of some instruments for hemostasis, and the improved or magnified visualization offered by the laparoscopic techniques, which enhances the view of the structures surrounding the operative region and guarantees a precise resection along the appropriate plane[8,27].
Pancreaticoduodenectomy is always associated with high postoperative morbidity, and this is another main factor that prevents some surgeons from performing TLPD. In our study, we found that the TLPD group had a higher overall morbidity, but the major morbidity did not show any significant differences between the two groups (P = 0.25). Considering that a variety of factors may have influenced the results, such as preoperative comorbidities and selection bias, additional studies are needed to verify the outcomes.
POPF and DGE are considered to be the two most common and severe complications of PD, especially POPF, which is a life-threatening complication, and the occurence of these complications may affect postoperative recovery and mortality[54]. Our pooled analysis indicated there was no difference in the incidence of the overall occurrence of POPF (P = 0.62) or clinically significant pancreatic fistula (P = 0.75) in the TLPD group. Similarly, our meta-analysis did not show any significant differences in DGE without heterogeneity, however, for severe DGE (Grade B/C), TLPD was associated with a significantly lower incidence (P = 0.006). Although the definitions of POPF in all the included studies complied with the ISGPF definition, and the application of a laparoscopic technique could enhance the precise resection to decrease its incidence in the TLPD group, some other factors may also have influenced its incidence, such as the pancreatic texture, diameter of pancreatic duct[55,56], and reconstruction techniques for the alimentary tract, which were not documented in the included literature. Consequently, our study preliminarily confirmed that TLPD is not inferior to OPD in terms of decreasing the incidences of POPF and DGE, despite some drawbacks.
In addition, we observed that the wound infection rate in the TLPD group was significantly lower than that in the OPD group (P < 0.0001), which may be attributed to the smaller incision. However, considering that other factors, such as the diagnostic criteria for wound infection and the application of antibiotics during the perioperative period, were not recorded in the literature, the results may be still controversial and require more high-quality and adequately designed trials to evaluate.
Shortening the LOS is a major advantage of MIPD. Our meta-analysis showed that TLPD was associated with a shorter LOS and ICU stay (P < 0.00001, P < 0.00001), which was also confirmed in the sensitivity analysis (P < 0.00001, P < 0.00001). The shortened length of stay indirectly demonstrates the quick recovery expected after TLPD, illustrates the decreased cost of the whole treatment process, and shows the shortened time to start adjuvant chemotherapy for patients with malignant tumors[20,22,25]. Additionally, our pooled analysis also indicated that the reoperation rate, readmission rate, and mortality rate in the TLPD group were comparable to those in the OPD group, which may prove the safety and feasibility of TLPD from another point of view.
In patients undergoing PD for malignant tumors, oncological safety is the main priority. In our pooled analysis, we found that the R0 resection rate in the TLPD group was significantly higher than that in the OPD group, which was also observed in the sensitivity analysis. With respect to the number of lymph nodes retrieved, our meta-analysis and sensitivity analysis of the included studies indicated that the TLPD could obtain significantly more lymph nodes (P = 0.0005, P = 0.01). Therefore, we suggest that TLPD is comparable to OPD or may be better than OPD in terms of oncological outcomes. Furthermore, the OS in our study showed no significant difference in our pooled analysis and sensitivity analysis. However, considering that whether the patients received adjuvant chemotherapy, which is associated with long-term oncological outcomes, was not documented in the literature, the results we obtained are not reliable, and more highly qualified long-term studies should be carried out to answer this question.
The strengths of our study lie in the following aspects. One is that our meta-analysis presents a detailed comparison between TLPD and OPD, and, to our knowledge, this is the first meta-analysis of these two specific surgical procedures. Furthermore, the large sample size is another strength, because we included all the available data from published studies, thus increasing the reliability of our study. Additionally, data extraction was performed independently by two authors to reduce errors and ensure the authenticity of our results. Nevertheless, there are some limitations in our meta-analysis that must be taken into consideration.
First, most of the studies we included were retrospective, and only two were RCTs, thus, the quality of the studies was restricted and some potential bias associated with the outcomes may have affected the results we calculated. Therefore, further cohort studies, case-control studies, or RCTs with large numbers of patients are essential to confirm the results.
Second, although most of the characteristics of the patients included in the comparative studies were documented, some others, such as the comorbidities, preoperative treatments, such as endoscopic nasobiliary drainage and percutaneous transhepatic cholangiodrainage, discharge criteria, and treatment with chemotherapy after surgery, were not recorded or adjusted for in the studies, which may have influenced the outcomes and OS. Furthermore, some studies we included had a small number of patients, which may indicate that the surgeons were in the initial stages or still in the learning curve and had limited skills and experiences. These factors may also have affected the perioperative and postoperative outcomes. Therefore, further studies with well-matched patients and well-adjusted confounders are needed.
Third, the comparison of the cost and the OS rate was reported in few studies with limited cases, and more high-quality studies are needed to prove the authenticity in the future. Therefore, we suggest that TLPD is comparable to OPD or may be better than OPD in terms of reducing blood loss, decreasing the blood transfusion and wound infection rates, shortening the length of ICU stay and LOS, increasing the number of lymph nodes harvested and the R0 resection rate, and improving the oncological outcomes, despite having a longer operative time and being used for smaller tumors. However, considering the bias in our study and the complexity of the procedure, we suggested that this procedure be performed at high-volume medical centers by teams who are experienced with pancreatic surgeries and laparoscopic techniques. In addition, we recommend that patients with small lesions distant from the major vessels undergo TLPD during the initial period. To eliminate the influence of bias, further studies with well-matched patients or RCTs should be performed to evaluate the efficacy and advantages of TLPD.
Total laparoscopic pancreaticoduodenectomy (TLPD) has been performed and grew in popularity among the general surgeons in some major medical centers worldwide. Studies about its safety and feasibility have been reported, but considering the research characteristics and study size, controversies regarding its perioperative and oncological safey still exist.
We hope to offer higher quality and more relible evidence in the selection of clinical treatment options for patients with pancreatic head or periampullary lesions.
To help identify which operation method is suitable and beneficial for patients with pancreatic head or periampullary lesions.
A systematic search was conducted in PubMed, EMBASE and Cochrane Library databases for studies concerning TLPD and open pancreaticoduodenectomy (OPD) for patients with pancreatic head or periampullary lesions. We followed the Preferred Reporting Items for Systematic Reviews and the PRISMA agreement, and and protocol can be accessed at the International Prospective Register of Systematic Reviews (registration number: CRD42019126173). The meta-analysis was performed using Review Manager Software version 5.3, and the quality was assessed using the Newcastle-Ottawa scale for the nonrandomized controlled trials and the Jadad scale for the randomized controlled trials.
Twenty-eight studies were eligible and selected in our analysis, including 3543 patients in the TLPD group and 36228 patients in the OPD group. Estimated blood loss (P < 0.00001) was less, intraoperative blood transfusion (P < 0.00001) and wound infection rate (P = 0.005) were lower, intensive care unit stay (P < 0.00001), length of hispital stay (P < 0.00001), and diet start time (P = 0.04) were shorter, R0 resection rate was higher (P = 0.02), and more lymph nodes was harvested (P = 0.01) in the TLPD group, although the operative time was longer (P < 0.00001) and the tumor size was smaller (P = 0.008). The overall morbidity, reoperation rate, and mortality showed no significant difference between the TLPD group and the OPD group. Moreover, the overall survival and recurrence-free survival afterTLPD were similar to those after OPD.
The current meta-analysis showed that TLPD may be an ideal alternative option for patients with pancreatic head or periampullary lesions and it can be beneficial for patiets.
The results of the current meta-analysis may offer surgeons more reliable evidence in choosing the surgery options for patients with pancreatic head or periampullary lesions.
The authors thank Professor Yuan Fang for the review of statistical analysis.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Afzal M, Gumbs A, Isaji S, Suzuki S, Tsoulfas G S-Editor: Yan JP L-Editor: Wang TQ E-Editor: Wu YXJ
| 1. | Huscher CG, Mingoli A, Sgarzini G, Sansonetti A, Di Paola M, Recher A, Ponzano C. Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: Five-year results of a randomized prospective trial. Ann Surg. 2005;241:232-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 650] [Cited by in RCA: 655] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 2. | Qiu JG, Wu H, Jiang H, Huang JW, Pankaj P, Xu YL, Wang JZ, Zeng Y. Laparoscopic fenestration vs open fenestration in patients with congenital hepatic cysts: A meta-analysis. World J Gastroenterol. 2011;17:3359-3365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Gagner M, Pomp A. Laparoscopic pylorus-preserving pancreatoduodenectomy. Surg Endosc. 1994;8:408-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 659] [Article Influence: 21.3] [Reference Citation Analysis (1)] |
| 4. | Senthilnathan P, Chinnusamy P, Ramanujam A, Sivakumar SG, Natesan AV, Chandramaliteeswaran C, Palanivelu PR, Ramakrishnan P, Subbiah R. Comparison of Pathological Radicality between Open and Laparoscopic Pancreaticoduodenectomy in a Tertiary Centre. Indian J Surg Oncol. 2015;6:20-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Drymousis P, Raptis DA, Spalding D, Fernandez-Cruz L, Menon D, Breitenstein S, Davidson B, Frilling A. Laparoscopic versus open pancreas resection for pancreatic neuroendocrine tumours: A systematic review and meta-analysis. HPB (Oxford). 2014;16:397-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 6. | Nigri G, Petrucciani N, La Torre M, Magistri P, Valabrega S, Aurello P, Ramacciato G. Duodenopancreatectomy: Open or minimally invasive approach? Surgeon. 2014;12:227-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Cho A, Yamamoto H, Nagata M, Takiguchi N, Shimada H, Kainuma O, Souda H, Gunji H, Miyazaki A, Ikeda A, Tohma T, Matsumoto I. Comparison of laparoscopy-assisted and open pylorus-preserving pancreaticoduodenectomy for periampullary disease. Am J Surg. 2009;198:445-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 8. | Qin H, Qiu J, Zhao Y, Pan G, Zeng Y. Does minimally-invasive pancreaticoduodenectomy have advantages over its open method? A meta-analysis of retrospective studies. PLoS One. 2014;9:e104274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Sharpe SM, Talamonti MS, Wang CE, Prinz RA, Roggin KK, Bentrem DJ, Winchester DJ, Marsh RD, Stocker SJ, Baker MS. Early National Experience with Laparoscopic Pancreaticoduodenectomy for Ductal Adenocarcinoma: A Comparison of Laparoscopic Pancreaticoduodenectomy and Open Pancreaticoduodenectomy from the National Cancer Data Base. J Am Coll Surg. 2015;221:175-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 152] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 10. | Zureikat AH, Breaux JA, Steel JL, Hughes SJ. Can laparoscopic pancreaticoduodenectomy be safely implemented? J Gastrointest Surg. 2011;15:1151-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 105] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 11. | Conrad C, Basso V, Passot G, Zorzi D, Li L, Chen HC, Fuks D, Gayet B. Comparable long-term oncologic outcomes of laparoscopic versus open pancreaticoduodenectomy for adenocarcinoma: A propensity score weighting analysis. Surg Endosc. 2017;31:3970-3978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 12. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ. 2009;339:b2700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13930] [Cited by in RCA: 13358] [Article Influence: 834.9] [Reference Citation Analysis (0)] |
| 13. | Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8858] [Cited by in RCA: 12669] [Article Influence: 844.6] [Reference Citation Analysis (0)] |
| 14. | Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials. 1996;17:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12275] [Cited by in RCA: 12889] [Article Influence: 444.4] [Reference Citation Analysis (1)] |
| 15. | Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M; International Study Group on Pancreatic Fistula Definition. Postoperative pancreatic fistula: An international study group (ISGPF) definition. Surgery. 2005;138:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3282] [Cited by in RCA: 3514] [Article Influence: 175.7] [Reference Citation Analysis (34)] |
| 16. | Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, Neoptolemos JP, Padbury RT, Sarr MG, Yeo CJ, Büchler MW. Postpancreatectomy hemorrhage (PPH): An International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery. 2007;142:20-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1411] [Cited by in RCA: 1951] [Article Influence: 108.4] [Reference Citation Analysis (0)] |
| 17. | Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, Neoptolemos JP, Padbury RT, Sarr MG, Traverso LW, Yeo CJ, Büchler MW. Delayed gastric emptying (DGE) after pancreatic surgery: A suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 2007;142:761-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1771] [Cited by in RCA: 2332] [Article Influence: 129.6] [Reference Citation Analysis (0)] |
| 18. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 24854] [Article Influence: 1183.5] [Reference Citation Analysis (0)] |
| 19. | Clarke M, Horton R. Bringing it all together: Lancet-Cochrane collaborate on systematic reviews. Lancet. 2001;357:1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 205] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 20. | Asbun HJ, Stauffer JA. Laparoscopic vs open pancreaticoduodenectomy: Overall outcomes and severity of complications using the Accordion Severity Grading System. J Am Coll Surg. 2012;215:810-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 295] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 21. | Chapman BC, Gajdos C, Hosokawa P, Henderson W, Paniccia A, Overbey DM, Gleisner A, Schulick RD, McCarter MD, Edil BH. Comparison of laparoscopic to open pancreaticoduodenectomy in elderly patients with pancreatic adenocarcinoma. Surg Endosc. 2018;32:2239-2248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 22. | Chapman BC, Gleisner A, Ibrahim-Zada I, Overbey DM, Paniccia A, Meguid C, Brauer B, Gajdos C, McCarter MD, Schulick RD, Edil BH. Laparoscopic pancreaticoduodenectomy: Changing the management of ampullary neoplasms. Surg Endosc. 2018;32:915-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Chen XM, Sun DL, Zhang Y. Laparoscopic versus open pancreaticoduodenectomy combined with uncinated process approach: A comparative study evaluating perioperative outcomes (Retrospective cohort study). Int J Surg. 2018;51:170-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 24. | Chopinet S, Fuks D, Rinaudo M, Massol J, Gregoire E, Lamer C, Belgaumkar A, Hardwigsen J, Le Treut YP, Gayet B. Postoperative Bleeding After Laparoscopic Pancreaticoduodenectomy: The Achilles' Heel? World J Surg. 2018;42:1138-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Croome KP, Farnell MB, Que FG, Reid-Lombardo KM, Truty MJ, Nagorney DM, Kendrick ML. Total laparoscopic pancreaticoduodenectomy for pancreatic ductal adenocarcinoma: Oncologic advantages over open approaches? Ann Surg. 2014;260:633-8; discussion 638-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 350] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 26. | Delitto D, Luckhurst CM, Black BS, Beck JL, George TJ, Sarosi GA, Thomas RM, Trevino JG, Behrns KE, Hughes SJ. Oncologic and Perioperative Outcomes Following Selective Application of Laparoscopic Pancreaticoduodenectomy for Periampullary Malignancies. J Gastrointest Surg. 2016;20:1343-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 27. | Dokmak S, Ftériche FS, Aussilhou B, Bensafta Y, Lévy P, Ruszniewski P, Belghiti J, Sauvanet A. Laparoscopic pancreaticoduodenectomy should not be routine for resection of periampullary tumors. J Am Coll Surg. 2015;220:831-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 150] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 28. | Gerber MH, Delitto D, Crippen CJ, George TJ, Behrns KE, Trevino JG, Cioffi JL, Hughes SJ. Analysis of the Cost Effectiveness of Laparoscopic Pancreatoduodenectomy. J Gastrointest Surg. 2017;21:1404-1410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Hakeem AR, Verbeke CS, Cairns A, Aldouri A, Smith AM, Menon KV. A matched-pair analysis of laparoscopic versus open pancreaticoduodenectomy: Oncological outcomes using Leeds Pathology Protocol. Hepatobiliary Pancreat Dis Int. 2014;13:435-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 30. | Kantor O, Talamonti MS, Sharpe S, Lutfi W, Winchester DJ, Roggin KK, Bentrem DJ, Prinz RA, Baker MS. Laparoscopic pancreaticoduodenectomy for adenocarcinoma provides short-term oncologic outcomes and long-term overall survival rates similar to those for open pancreaticoduodenectomy. Am J Surg. 2017;213:512-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 31. | Khaled YS, Fatania K, Barrie J, De Liguori N, Deshpande R, O'Reilly DA, Ammori BJ. Matched Case-Control Comparative Study of Laparoscopic Versus Open Pancreaticoduodenectomy for Malignant Lesions. Surg Laparosc Endosc Percutan Tech. 2018;28:47-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 32. | Lee CS, Kim EY, You YK, Hong TH. Perioperative outcomes of laparoscopic pancreaticoduodenectomy for benign and borderline malignant periampullary disease compared to open pancreaticoduodenectomy. Langenbecks Arch Surg. 2018;403:591-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Meng LW, Cai YQ, Li YB, Cai H, Peng B. Comparison of Laparoscopic and Open Pancreaticoduodenectomy for the Treatment of Nonpancreatic Periampullary Adenocarcinomas. Surg Laparosc Endosc Percutan Tech. 2018;28:56-61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 34. | Mesleh MG, Stauffer JA, Bowers SP, Asbun HJ. Cost analysis of open and laparoscopic pancreaticoduodenectomy: A single institution comparison. Surg Endosc. 2013;27:4518-4523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 35. | Palanivelu C, Senthilnathan P, Sabnis SC, Babu NS, Srivatsan Gurumurthy S, Anand Vijai N, Nalankilli VP, Praveen Raj P, Parthasarathy R, Rajapandian S. Randomized clinical trial of laparoscopic versus open pancreatoduodenectomy for periampullary tumours. Br J Surg. 2017;104:1443-1450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 294] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 36. | Poves I, Burdío F, Morató O, Iglesias M, Radosevic A, Ilzarbe L, Visa L, Grande L. Comparison of Perioperative Outcomes Between Laparoscopic and Open Approach for Pancreatoduodenectomy: The PADULAP Randomized Controlled Trial. Ann Surg. 2018;268:731-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 265] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 37. | Song KB, Kim SC, Hwang DW, Lee JH, Lee DJ, Lee JW, Park KM, Lee YJ. Matched Case-Control Analysis Comparing Laparoscopic and Open Pylorus-preserving Pancreaticoduodenectomy in Patients With Periampullary Tumors. Ann Surg. 2015;262:146-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 162] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 38. | Speicher PJ, Nussbaum DP, White RR, Zani S, Mosca PJ, Blazer DG, Clary BM, Pappas TN, Tyler DS, Perez A. Defining the learning curve for team-based laparoscopic pancreaticoduodenectomy. Ann Surg Oncol. 2014;21:4014-4019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 144] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 39. | Stauffer JA, Coppola A, Villacreses D, Mody K, Johnson E, Li Z, Asbun HJ. Laparoscopic versus open pancreaticoduodenectomy for pancreatic adenocarcinoma: Long-term results at a single institution. Surg Endosc. 2017;31:2233-2241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 122] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 40. | Tan CL, Zhang H, Peng B, Li KZ. Outcome and costs of laparoscopic pancreaticoduodenectomy during the initial learning curve vs laparotomy. World J Gastroenterol. 2015;21:5311-5319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 49] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 41. | Tee MC, Croome KP, Shubert CR, Farnell MB, Truty MJ, Que FG, Reid-Lombardo KM, Smoot RL, Nagorney DM, Kendrick ML. Laparoscopic pancreatoduodenectomy does not completely mitigate increased perioperative risks in elderly patients. HPB (Oxford). 2015;17:909-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 42. | Tran TB, Dua MM, Worhunsky DJ, Poultsides GA, Norton JA, Visser BC. The First Decade of Laparoscopic Pancreaticoduodenectomy in the United States: Costs and Outcomes Using the Nationwide Inpatient Sample. Surg Endosc. 2016;30:1778-1783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 43. | Zimmerman AM, Roye DG, Charpentier KP. A comparison of outcomes between open, laparoscopic and robotic pancreaticoduodenectomy. HPB (Oxford). 2018;20:364-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 44. | Clinical Outcomes of Surgical Therapy Study Group. Nelson H, Sargent DJ, Wieand HS, Fleshman J, Anvari M, Stryker SJ, Beart RW Jr, Hellinger M, Flanagan R Jr, Peters W, Ota D. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004;350:2050-2059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2606] [Cited by in RCA: 2518] [Article Influence: 119.9] [Reference Citation Analysis (0)] |
| 45. | Kim HH, Hyung WJ, Cho GS, Kim MC, Han SU, Kim W, Ryu SW, Lee HJ, Song KY. Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: An interim report--a phase III multicenter, prospective, randomized Trial (KLASS Trial). Ann Surg. 2010;251:417-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 562] [Cited by in RCA: 624] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 46. | Correa-Gallego C, Dinkelspiel HE, Sulimanoff I, Fisher S, Viñuela EF, Kingham TP, Fong Y, DeMatteo RP, D'Angelica MI, Jarnagin WR, Allen PJ. Minimally-invasive vs open pancreaticoduodenectomy: Systematic review and meta-analysis. J Am Coll Surg. 2014;218:129-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 132] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 47. | Pędziwiatr M, Małczak P, Pisarska M, Major P, Wysocki M, Stefura T, Budzyński A. Minimally invasive versus open pancreatoduodenectomy-systematic review and meta-analysis. Langenbecks Arch Surg. 2017;402:841-851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 48. | Satyadas T, Kanhere HA, Lauder C, Maddern GJ. Evolution in technique of laparoscopic pancreaticoduodenectomy: A decade long experience from a tertiary center. J Hepatobiliary Pancreat Sci. 2010;17:367-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 49. | de Rooij T, Klompmaker S, Abu Hilal M, Kendrick ML, Busch OR, Besselink MG. Laparoscopic pancreatic surgery for benign and malignant disease. Nat Rev Gastroenterol Hepatol. 2016;13:227-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 101] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 50. | de Rooij T, Lu MZ, Steen MW, Gerhards MF, Dijkgraaf MG, Busch OR, Lips DJ, Festen S, Besselink MG; Dutch Pancreatic Cancer Group. Minimally Invasive Versus Open Pancreatoduodenectomy: Systematic Review and Meta-analysis of Comparative Cohort and Registry Studies. Ann Surg. 2016;264:257-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 149] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 51. | Chalikonda S, Aguilar-Saavedra JR, Walsh RM. Laparoscopic robotic-assisted pancreaticoduodenectomy: A case-matched comparison with open resection. Surg Endosc. 2012;26:2397-2402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 191] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 52. | Wellner UF, Küsters S, Sick O, Busch C, Bausch D, Bronsert P, Hopt UT, Karcz KW, Keck T. Hybrid laparoscopic versus open pylorus-preserving pancreatoduodenectomy: Retrospective matched case comparison in 80 patients. Langenbecks Arch Surg. 2014;399:849-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 53. | Bailey MB, Davenport DL, Vargas HD, Evers BM, McKenzie SP. Longer operative time: Deterioration of clinical outcomes of laparoscopic colectomy versus open colectomy. Dis Colon Rectum. 2014;57:616-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 54. | Zhao Z, Yin Z, Hang Z, Ji G, Feng Q, Zhao Q. A systemic review and an updated meta-analysis: Minimally invasive vs open pancreaticoduodenectomy. Sci Rep. 2017;7:2220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 55. | Gouma DJ, van Geenen RC, van Gulik TM, de Haan RJ, de Wit LT, Busch OR, Obertop H. Rates of complications and death after pancreaticoduodenectomy: Risk factors and the impact of hospital volume. Ann Surg. 2000;232:786-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 650] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 56. | Tranchart H, Gaujoux S, Rebours V, Vullierme MP, Dokmak S, Levy P, Couvelard A, Belghiti J, Sauvanet A. Preoperative CT scan helps to predict the occurrence of severe pancreatic fistula after pancreaticoduodenectomy. Ann Surg. 2012;256:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 127] [Article Influence: 9.8] [Reference Citation Analysis (0)] |