Published online Oct 7, 2019. doi: 10.3748/wjg.v25.i37.5687
Peer-review started: June 3, 2019
First decision: July 21, 2019
Revised: August 30, 2019
Accepted: September 11, 2019
Article in press: September 11, 2019
Published online: October 7, 2019
Processing time: 121 Days and 7.2 Hours
Prospective study of 200 patients with hepatocellular carcinoma (HCC) that underwent liver transplant (LT) after drug-eluting beads transarterial chemoembolization (DEB-TACE) for downstaging versus bridging. Overall survival and tumor recurrence rates were calculated, eligibility for LT, time on the waiting list and radiological response were compared. After TACE, only patients within Milan Criteria (MC) were transplanted. More patients underwent LT in bridging group. Five-year post-transplant overall survival, recurrence-free survival has no difference between the groups. Complete response was observed more frequently in bridging group. Patients in DS group can achieve post-transplant survival and HCC recurrence-free probability, at five years, just like patients within MC in patients undergoing DEB-TACE.
To determine long-term outcomes of patients with HCC that underwent LT after DEB-TACE for downstaging vs bridging.
Prospective cohort study of 200 patients included from April 2011 through June 2014. Bridging group included patients within MC. Downstaging group (out of MC) was divided in 5 subgroups (G1 to G5). Total tumor diameter was ≤ 8 cm for G1, 2, 3, 4 (n = 42) and was > 8 cm for G5 (n = 22). Downstaging (n = 64) and bridging (n = 136) populations were not significantly different. Overall survival and tumor recurrence rates were calculated by the Kaplan-Meier method. Additionally, eligibility for LT, time on the waiting list until LT and radiological response were compared.
After TACE, only patients within MC were transplanted. More patients underwent LT in bridging group 65.9% (P = 0.001). Downstaging population presented: higher number of nodules 2.81 (P = 0.001); larger total tumor diameter 8.09 (P = 0.001); multifocal HCC 78% (P = 0.001); more post-transplantation recurrence 25% (P = 0.02). Patients with maximal tumor diameter up to 7.05 cm were more likely to receive LT (P = 0.005). Median time on the waiting list was significantly longer in downstaging group 10.6 mo (P = 0.028). Five-year post-transplant overall survival was 73.5% in downstaging and 72.3% bridging groups (P = 0.31), and recurrence-free survival was 62.1% in downstaging and 74.8% bridging groups (P = 0.93). Radiological response: complete response was observed more frequently in bridging group (P = 0.004).
Tumors initially exceeding the MC down-staged after DEB-TACE, can achieve post-transplant survival and HCC recurrence-free probability, at five years, just like patients within MC in patients undergoing DEB-TACE.
Core tip: The great finding of this work was that through a homogeneous technique of hepatic chemoembolization with drug eluting beads, it was possible to perform the procedures controlling the drug delivery and end point. In conclusion, as far as the degree of tumor necrosis as well as in relation to survival, there was no difference between the group within the Milan criteria (bridging) and the group outside the criteria (downstaging). Therefore, it is worth investing in the treatment of patients out of the Milan criteria so that they have a survival with the same expectations of the patients in criterion.
- Citation: Affonso BB, Galastri FL, da Motta Leal Filho JM, Nasser F, Falsarella PM, Cavalcante RN, de Almeida MD, Felga GEG, Valle LGM, Wolosker N. Long-term outcomes of hepatocellular carcinoma that underwent chemoembolization for bridging or downstaging. World J Gastroenterol 2019; 25(37): 5687-5701
- URL: https://www.wjgnet.com/1007-9327/full/v25/i37/5687.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i37.5687
Hepatocellular carcinoma (HCC) is the sixth leading cause of cancer and the third leading cause of cancer death worldwide. It is the number one oncologic cause of death in cirrhotic patients, with approximately one million deaths/year[1,2]. According to the Barcelona Clinic Liver Cancer (BCLC) staging classification, liver resection (LR), radiofrequency ablation (RFA), and liver transplantation (LT) are potentially curative treatments for HCC[3,4].
LT is a well-established modality for curative treatment of HCC because it removes the tumor, while excluding the cirrhotic environment, which could lead to the emergence of new malignant lesions[5]. Success rates of LT as a curative treatment are attributed to improved candidate selection using restrictive criteria based on number and tumor size, among which the most frequently used is the Milan criteria (MC)[6]. In centers that frequently perform LT, the 5-year post-transplant patient survival can achieve 75%-80%[7].
According to the BCLC, transarterial chemoembolization (TACE) is indicated as palliative treatment in patients with intermediate HCC (BCLC B). However, over the last several years, TACE has being indicated as downstaging (reduction in the size of tumor using locoregional therapies (LRT) in selected patients to meet acceptable criteria for LT)[7,8] and bridging (neo-adjuvant therapy attempt to avoid HCC growth while the patient is waiting for transplantation)[9]. Nevertheless, there is a lack of consistent data on radiological response, overall and recurrence-free survival after transplantation in this heterogeneous group of patients.
Drug-eluting beads (DEB-TACE) is a technology that has been developed to enhance tumor drug delivery and reduce systemic availability and toxicity. DEB-TACE loaded with doxorubicin is a safe and effective palliative treatment for HCC and offers clinical benefit to patients with more advanced disease[10]. Another benefit of this technology is that it allows for standardization of the chemoembolization technique, since it is possible to estimate the amount of drug delivered to each tumor. The purpose of this study was to compare the long-term outcomes of patients that underwent LT after DEB-TACE for downstaging versus bridging. Also, we aimed to investigate radiological tumor response after the first DEB-TACE session in both groups.
This study was a single-institution, prospective, cohort study, conducted at the Department of Interventional Radiology and approved by the research ethics committee (SGPP155711/CEP11/1704–CAAE0199.0.028.000-11). All patients signed an informed consent form and the study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
The present study included 200 consecutive patients with HCC, from April 1, 2011 to June 30, 2014, who underwent DEB-TACE at our institution using the outpatient treatment protocol previously described[11]. These patients were part of the liver transplant program and were divided into two groups: Bridging and downstaging (Figure 1). At that time, precise criteria for HCC downstaging related to the sum of the maximal tumor diameters were unclear; therefore, we included in the downstaging group all patients out of the MC[6], without vascular invasion based on cross-sectional magnetic resonance imaging (MRI) or computed tomography (CT) and without lymph node involvement by tumor or extra-hepatic tumor spread. Consistent with Yao et al[8], we classified the downstaging patients into 5 groups, as summarized in Table 1. It was identified a subgroup never before described, Group 4 which has a low tumor volume (less than 8 cm) but with 2 or 3 lesions above 5 cm. Patients who were within MC[6] or T2 of the United Network for Organs Sharing were classified as bridging group (Table 1) and were divided into 3 groups, Group 1 = one tumor; Group 2 = two tumors; and Group 3 = three tumors.
| Downstaging and bridging protocol | |
| Inclusion Criteria | |
| Bridging | |
| Patients who were within MC or UNOS T2 | |
| Downstaging subgroups (HCC exceeding MC) | |
| Group 1 = 1 lesion > 5 and ≤ 8 cm | |
| Group 2 = 2 or 3 lesions at least one > 3 and ≤ 5 cm with the sum of the maximal tumor diameters ≤ 8 cm | |
| Group 3 = 4 or 5 lesions each ≤ 3 cm with the sum of the maximal tumor diameters ≤ 8 cm | |
| Group 4 = 2 or 3 lesions at least one > 5cm with the sum of the maximal tumor diameters ≤ 8 cm | |
| Group 5 = total tumor diameter > 8 cm | |
| Absence of vascular invasion based on cross-sectional MRI or CT | |
| Criteria for successful downstaging | |
| Residual tumor(s) within MC for deceased donor liver transplant | |
| In patients with 4 or 5 tumors, successful downstaging requires complete necrosis (based on cross-sectional MRI or CT) of at least 1 to 2 tumor(s), respectively, so that there will be no more than 3 lesions with viable tumor each ≤ 3 cm to meet MC | |
| Criteria for downstaging failure and exclusion from liver transplant | |
| Progression of tumor(s) to beyond inclusion criteria for downstaging and bridging based on tumor size and number | |
| Vascular invasion based on cross-sectional MRI or CT | |
| Lymph node involvement by tumor or extra-hepatic spread of tumor | |
All patients underwent multiphasic abdomen CT (Aquilion One 320, Toshiba, Tokyo, Japan, Aquillon 64, Toshiba, Tokyo, Japan-Aquilion Vision 640 Toshiba, Tokyo, Japan) or MRI (GE 2 HDXT-1.5T, General Electric, Boston, Massachusetts, United States and Siemens Espree-1.5T, Siemens AG, Berlin, Germany). The overwhelming majority of the imaging examinations were performed with MRI. All images were acquired using the following parameters: 120kV voltage; tube current (sure exposure 3D SD 10.00, Max 500 Min 100 mAs, reconstruction slice thickness: 1 or 3 mm - depending on the acquired sequence). Patients submitted to abdominal CT received an intravenous bolus injection of 1.7 mL of contrast/kg body weight of the non-ionic iodinated contrast agent Henetix® 350mg I/mL, Guerbet-Rio de Janeiro, Brazil (350 mg I/mL Iobitridol). Patients who underwent abdominal MRI received intravenous bolus injection of 0.2 mL/kg patient weight of the paramagnetic contrast agent Magnevistam®, Bayer-Leverkusen, Germany (469 mg/mL Dimeglumine Gadopentetate), both with injection rate of 3 to 4 mL/s. HCC diagnosis for a lesion ≥ 1 cm was based on either CT or MRI demonstrating arterial phase enhancement and washout during the delayed images, according to the American Association for the Study of Liver Diseases guidelines[12,13]. Hepatic nodules < 1 cm were not counted as HCC. Percutaneous biopsy was not routinely performed.
Criteria for downstaging failure and exclusion from liver transplant are summarized in Table 1. There was no time limit or DEB-TACE session limit for completing downstaging. Eligibility for LT and time on the waiting list until LT were compared between the downstaging and bridging groups.
DEB-TACE protocol was previously described by Nasser et al[11] and Cavalcante et al[14]. Briefly, DEB-TACE procedures were performed under local anesthesia with lidocaine 2%, sedation and analgesia, with venous administration of midazolam and fentanyl.
Using a unilateral femoral artery approach, diagnostic angiograms of the superior mesenteric, celiac trunk, and common hepatic artery were performed with the purpose of outlining the hepatic artery anatomy, delineate the tumor, identify its feeding vessels, and evaluate portal vein patency. In each DEB-TACE session, feeding vessels were catheterized with a 2.8F microcatheter (Progreat, Terumo, Japan), and embolization of the tumors was performed with injection of iodinated contrast medium mixed with one vial of DC-BEAD 100-300μm (Biocompatibles, United Kingdom) or HepaSphere 50-100 μm (Merit Medical Systems, United States) loaded with 50 mg of Doxorubicin. If the endpoint was not achieved after the injection of loaded beads, additional bland beads (Beadblock, Biocompatibles, United Kingdom or Contour, Boston, United States) were injected until the endpoint (complete stasis) was reached. For patients with more than one tumor, DEB-TACE began by the largest nodule to reach the smallest tumor, regardless of how many sessions were required[11]. Vascular lake phenomenon was defined as a localized pooling of contrast media within the tumor, which persists in the venous phase of angiography, resembling extravasation[14].
Tumor response was assessed through imaging studies (contrast-enhanced MRI or multiphasic abdomen CT) and performed 30-45 d after DEB-TACE, according to the modified response evaluation criteria in solid tumors (mRECIST) guidelines[15], as follows: (1) Target lesion response: response of the treated nodules was evaluated by comparing the baseline sum of diameters of target lesions before DEB-TACE with the sum of diameters of viable target lesions after DEB-TACE in each patient; (2) Complete response (CR) was defined as disappearance of any intra-tumoral enhancement in all target lesions; Partial response (PR) was defined as at least a 30% decrease in the sum of diameters of viable target lesions; Stable disease (SD) was defined as any case that does not qualify for either PR or progressive disease; Disease progression was defined as an increase of at least 20% in the sum of the diameters of viable target lesions; (3) Objective response (OR) rate was defined as the sum of CR and PR.
Five-year post-transplant overall survival and recurrence-free survival were evaluated and compared between the two groups.
Statistical analysis was performed with SPSS version 15.0 (IBM, Armonk, NY, United States). Quantitative characteristics were described by group (bridging and downstaging) before and after transplantation using summary measures (mean, standard deviation, median, minimum and maximum) and compared with Student's t-tests or Mann-Whitney tests. Qualitative characteristics were described by group (bridging and downstaging) before and after transplantation. Associations were tested with chi-square tests or exact tests (Fisher's exact test or likelihood ratio test). Overall survival and recurrence-free survival were estimated using bivariate Cox regression and multivariate Cox regression to verify the influence of significant characteristics on survival. Overall survival and recurrence-free survival were evaluated, by group, using the Kaplan-Meier method. Receiver operating characteristic (ROC) curve was generated to identify maximal tumor diameter most associated with liver transplant in the downstaging group. P value of 0.05 or less was considered significant.
A total of 200 patients were enrolled during the inclusion period: 64 in downstaging group and 136 in bridging group. Three patients could not perform DEB-TACE and were excluded. Two patients were excluded as a result of hepatic artery dissection during the procedure. One patient was excluded because she presented with respiratory failure after sedation and the procedure was interrupted before embolization (Figure 1).
The groups did not significantly differ in terms of age, gender, etiology of liver disease, Child score, or baseline alpha-fetoprotein levels. At presentation, the downstaging population presented a greater number of nodules, median of 2.81 nodules vs 1.47 (P = 0.001); increased total tumor diameter 8.09 vs 3.73 (P = 0.001); increased multifocal HCC 78% vs 34.6% of samples (P = 0.001); and increased vascular lake phenomenon 34.3% vs 12.5% (P = 0.001) (Table 2).
| Variable | Group | Total (n = 200) | P value | |
| Downstaging (n = 64) | Bridging (n = 136) | |||
| Gender (Male), n (%) | 53 (82.8) | 112 (82.4) | 165 (82.5) | 0.936 |
| Age (yr) (mean ± SD, range) | 57.6 ± 6.9 (41-71) | 58.2 ± 8.8 (27-77) | 58.0 ± 8.2 (27-77) | 0.6261 |
| Etiology, n (%) | 0.0692 | |||
| Hepatitis B | 7 (10.9) | 5 (3.7) | 12 (6.0) | |
| Hepatitis C | 43 (67.2) | 78 (57.4) | 121 (60.5) | |
| Hepatitis B/C | 1 (1.6) | 6 (4.4) | 7 (3.5) | |
| Alcohol | 6 (9.4) | 26 (19.1) | 32 (16.0) | |
| Alcohol/Hepatitis | 2 (3.1) | 2 (1.5) | 4 (2.0) | |
| Other | 5 (7.8) | 19 (14.0) | 24 (12.0) | |
| Child-Pugh score, n (%) | 0.2442 | |||
| A (5-6) | 24 (41.4) | 72 (53.7) | 96 (50.0) | |
| B (7-9) | 30 (51.7) | 52 (38.8) | 82 (42.7) | |
| C (10-15) | 4 (6.9) | 10 (7.5) | 14 (7.3) | |
| MELD, mean ± SD | 13 (7-28) | 11 (6-23) | 12 (6-28) | 0.0553 |
| AFP (ng/dL) (mean ± SD, range) | 12.3 (0.9-22.411) | 10.1 (0.6-12.784) | 11.0 (0.6-22.411) | 0.3353 |
| Coagulopathy, n (%) | 0.095 | |||
| No | 41 (64.1) | 70 (51.5) | 111 (55.5) | |
| Yes | 23 (35.9) | 66 (48.5) | 89 (44.5) | |
| Thrombocytopenia, n (%) | 0.297 | |||
| No | 27 (42.2) | 47 (34.6) | 74 (37.0) | |
| Yes | 37 (57.8) | 89 (65.4) | 126 (63.0) | |
| HCC multifocal, n (%) | < 0.001 | |||
| Single tumor | 14 (21.9) | 89 (65.4) | 103 (51.5) | |
| Multinodular | 50 (78.1) | 47 (34.6) | 97 (48.5) | |
| Number of nodules (mean ± SD, range) | 2 (1-9) | 1 (1-3) | 1 (1-9) | < 0.0013 |
| Maximal tumor diameter (mean ± SD, range) | 8.09 ± 2.75 (4.6-17.2) | 3.75 ± 1.20 (1.4-7.5) | 5.14 ± 2.74 (1.4-17.2) | < 0.0011 |
| Pseudocapsule, n (%) | 0.118 | |||
| No | 9 (17.0) | 30 (28.3) | 39 (24.5) | |
| Yes | 44 (83) | 76 (71.7) | 120 (75.5) | |
| Vascular lake phenomenon, n (%) | < 0.001 | |||
| No | 42 (65.6) | 119 (87.5) | 161 (80.5) | |
| Yes | 22 (34.4) | 17 (12.5) | 39 (19.5) | |
Several variables that would increase the chance of the individual undergoing transplant were evaluated to identify LT predictors. Patients with coagulopathy (RNI > 1.2) and thrombocytopenia (platelet count < 150.000/mm3)[16,17] were more likely to be transplanted (Table 3). After TACE, only patients within MC were transplanted. More patients underwent LT in the bridging group than in downstaging (65.9% vs 33.9%, P = 0.001) (Tables 3 and 4). Among the downstaging patients, G4 demonstrated the best eligibility for orthotopic liver transplantation (OLT) (60%) and did not have any cases of HCC recurrence (Table 4).
| Variable | Liver transplant | P value | |
| No | Yes | ||
| Gender (Male), n (%) | 70 (42.9) | 93 (57.1) | 0.451 |
| Age (yr), (mean, ± SD, range) | 58.1 ± 8.7 (27-77) | 57.9 ± 7.8 (32-73) | 0.8861 |
| Child-Pugh score, n (%) | 0.644 | ||
| A (5-6) | 43 (44.8) | 53 (55.2) | |
| B (7-9) | 33 (40.7) | 48 (59.3) | |
| C (10-15) | 7 (53.8) | 6 (46.2) | |
| MELD, mean ± SD, range | 11 (6-28) | 12 (7-23) | 0.0552 |
| AFP (ng/dL), (mean, ± SD, range) | 12.75 (0.6-22,411) | 8.8 (1.1-8,216) | 0.3352 |
| Coagulopathy, n (%) | 0.001 | ||
| No | 60 (55.0) | 49 (45.0) | |
| Yes | 27 (30.7) | 61 (69.3) | |
| Trombocytopenia, n (%) | 0.002 | ||
| No | 42 (58.3) | 30 (41.7) | |
| Yes | 45 (36.0) | 80 (64.0) | |
| Group, n (%) | < 0.001 | ||
| Downstaging | 41 (66.1) | 21 (33.9) | |
| Bridging | 46 (34.1) | 89 (65.9) | |
| Vascular lake phenomenon, n (%) | 0.776 | ||
| No | 71 (44.7) | 88 (55.3) | |
| Yes | 16 (42.1) | 22 (57.9) | |
| Total | 87 (44.2) | 110 (55.8) | |
| All patients before LT | Eligibility for LT, n (%) | Patients who received LT | |||||
| HCC | Number of patients | Total tumor diameter mean ± SD (cm), range | Number of sessions of DEB-TACE until LT (mean) | Deaths < 30 d (n) | Deaths > 30 d (n) | Recurrence (n, %) | |
| Bridging | 135 | 89 (65.9) | 8 | 14 | 5 (5.81) | ||
| Group 1 | 90 | 3.22 ± 0.90 (2-5) | 55 (61.1) | 1.74 | 5 | 8 | 1 |
| Group 2 | 27 | 4.16 ± 0.64 (3.2-5.7) | 19 (70.3) | 1.63 | 3 | 2 | 1 |
| Group 3 | 18 | 5.70 ± 0.81 (4.7-7.5) | 15 (83.3) | 1.6 | 0 | 4 | 3 |
| Downstaging | 62 | 21 (33.9) | 0 | 3 | 5 (25) | ||
| Group 1 | 11 | 6.52 ± 0.70 (5.7-8) | 3 (27.3) | 1.33 | 0 | 0 | 0 |
| Group 2 | 19 | 6.53 ± 0.95 (4.6-7.9) | 11 (57.9) | 1.54 | 0 | 2 | 3 |
| Group 3 | 5 | 6.56 ± 0.56 (5.9-7.2) | 2 (40) | 2.5 | 0 | 1 | 1 |
| Group 4 | 5 | 7.2 ± 0.34 (6.9-7.7) | 3 (60) | 3 | 0 | 0 | 0 |
| Group 5 | 22 | 10.92 ± 2.92 (8.2-17.2) | 2 (9.1) | 4 | 0 | 0 | 1 |
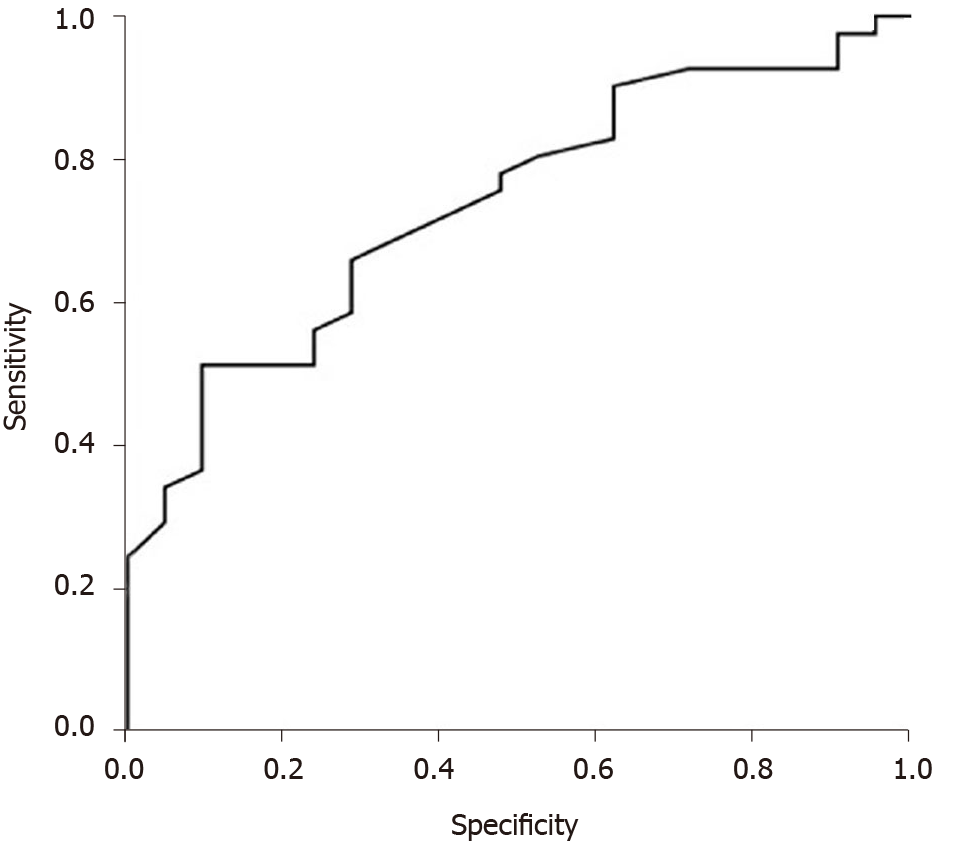
Median time on the waiting list for LT (interval between the first DEB-TACE to LT) in the downstaging group was significantly longer 10.6 months (range, 1.7 to 20.1 mo) than in the bridging group 6.6 mo (range, 0.6 to 30.5 mo) (P = 0.028) (Table 5). ROC curve analysis revealed that patients with maximal tumor diameter up to 7.05 cm were more likely to receive LT during DEB-TACE (P = 0.005) than patients with maximal tumor diameter more than 7.05, with sensitivity of 65.9% and specificity of 71.4% (Figure 2).
| Variable | Group | Total | P value | |
| Downstaging | Bridging | |||
| Overall survival (yr), (mean ± SD, range) | n = 20 | n = 87 | n = 107 | > 0.05 |
| 3.15 ± 1.33 (0.52-5.07) | 2.97 ± 1.66 (0-5.77) | 3.00 ± 1.60 (0-5.77) | ||
| Recurrence-free survival, (mean ± SD, range) | n = 20 | n = 86 | n = 106 | > 0.05 |
| 3.02 ± 1.37 (0.34-5.07) | 2.93 ± 1.69 (0-5.77) | 2.95 ± 1.63 (0-5.77) | ||
| Time of the waiting list until OLT (mo), (median, range) | n = 21 | n = 89 | n = 110 | < 0.051 |
| 10.6 (1.70-20.1) | 6.6 (0.60-30.47) | 7.0 (0.60-30.47) | ||
CR was observed more frequently in bridging than in downstaging group (P = 0.004). However, since PR occurred more often in the downstaging group, there was no statistically significant difference in OR between groups (P = 0.105) (Table 6). Six patients from the bridging group were submitted to LT after the first DEB-TACE procedure, thereby occurring before the imaging study (less than 30 d after DEB-TACE).
Overall survival and recurrence-free survival were estimated using bivariate Cox regression and multivariate Cox regression to verify the influence of baseline characteristics (age, gender, etiology, CHILD score, MELD, alpha-fetoprotein, number of nodules, maximal tumor diameter) on survival. There was no influence of baseline characteristics (P > 0.05), and/or between groups (bridging versus downstaging) on overall survival (P = 0.662) (Table 7) or recurrence-free survival (P = 0.874) (Table 8).
| Variável | HR (not adjusted) | 95%CI | P value | HR (adjusted) | 95%CI | P value | ||
| Inferior | Superior | Inferior | Superior | |||||
| Age (yr) | 1.02 | 0.97 | 1.07 | 0.469 | 1.01 | 0.96 | 1.06 | > 0.05 |
| Gender (male) | 0.46 | 0.19 | 1.09 | 0.078 | 0.51 | 0.16 | 1.63 | > 0.05 |
| Etiology | ||||||||
| Hepatitis B | 1.00 | 1.00 | ||||||
| Hepatitis C | 1.92 | 0.25 | 14.61 | 0.529 | 1.31 | 0.14 | 12.29 | > 0.05 |
| Hepatitis B/C | 1.50 | 0.09 | 23.93 | 0.776 | 1.14 | 0.06 | 22.88 | > 0.05 |
| Alcohol | 2.92 | 0.34 | 25.16 | 0.330 | 2.58 | 0.24 | 27.33 | > 0.05 |
| Alcohol/Hepatitis | 4.96 | 0.31 | 79.60 | 0.258 | 0.00 | 0.00 | > 0.05 | |
| Other | 1.73 | 0.18 | 16.66 | 0.636 | 1.60 | 0.14 | 18.40 | > 0.05 |
| Child-Pugh score | ||||||||
| A | 1.00 | 1.00 | ||||||
| B | 0.97 | 0.43 | 2.17 | 0.940 | 0.84 | 0.31 | 2.25 | > 0.05 |
| C | 0.00 | 0.00 | 0.983 | NA | > 0.05 | |||
| MELD | 0.99 | 0.86 | 1.14 | 0.875 | 1.07 | 0.90 | 1.27 | > 0.05 |
| Alpha-fetoprotein | ||||||||
| < 10 | 1.00 | 1.00 | ||||||
| 10-100 | 1.47 | 0.62 | 3.49 | 0.383 | 1.37 | 0.47 | 3.97 | > 0.05 |
| 100-1000 | 2.02 | 0.65 | 6.26 | 0.225 | 1.94 | 0.54 | 6.97 | > 0.05 |
| > 1000 | NA | 0.983 | NA | > 0.05 | ||||
| Number of nodules | 1.08 | 0.68 | 1.70 | 0.747 | 1.05 | 0.47 | 2.37 | > 0.05 |
| Maximal tumor diameter (cm) | 0.96 | 0.76 | 1.21 | 0.733 | 1.10 | 0.66 | 1.86 | > 0.05 |
| Group (Bridging) | 1.83 | 0.55 | 6.13 | 0.325 | 1.53 | 0.23 | 10.18 | > 0.05 |
| Variável | HR (not adjusted) | 95%CI | P value | HR (adjusted) | 95%CI | P value | ||
| Inferior | Superior | Inferior | Superior | |||||
| Age (yr) | 1.03 | 0.98 | 1.09 | 0.285 | 1.01 | 0.96 | 1.08 | > 0.05 |
| Gender (male) | 0.53 | 0.21 | 1.32 | 0.174 | 0.69 | 0.20 | 2.41 | > 0.05 |
| Etiology | ||||||||
| Hepatitis B | 1.00 | 1.00 | ||||||
| Hepatitis C | 2.00 | 0.26 | 15.21 | 0.504 | 1.46 | 0.16 | 13.70 | > 0.05 |
| Hepatitis B/C | 1.54 | 0.10 | 24.58 | 0.761 | 1.49 | 0.07 | 30.71 | > 0.05 |
| Alcohol | 2.82 | 0.33 | 24.30 | 0.345 | 2.53 | 0.23 | 27.36 | > 0.05 |
| Alcohol/Hepatitis | 10.15 | 0.91 | 112.71 | 0.059 | 6.07 | 0.26 | 142.70 | > 0.05 |
| Other | 1.78 | 0.19 | 17.20 | 0.617 | 1.90 | 0.16 | 22.79 | > 0.05 |
| Child-Pugh score | ||||||||
| A | 1.00 | 1.00 | ||||||
| B | 1.21 | 0.55 | 2.64 | 0.642 | 1.01 | 0.36 | 2.81 | > 0.05 |
| C | NA | 0.982 | # | > 0.05 | ||||
| MELD | 1.01 | 0.88 | 1.15 | 0.917 | 1.04 | 0.87 | 1.25 | > 0.05 |
| Alpha-fetoprotein | ||||||||
| < 10 | 1.00 | 1,00 | ||||||
| 10-100 | 1.36 | 0.58 | 3.17 | 0.483 | 1.43 | 0.50 | 4.12 | > 0.05 |
| 100-1000 | 2.15 | 0.70 | 6.61 | 0.182 | 2.38 | 0.65 | 8.70 | > 0.05 |
| > 1000 | NA | 0.982 | NA | > 0.05 | ||||
| Number of nodules | 1.28 | 0.83 | 1.98 | 0.261 | 1.34 | 0.61 | 2.94 | > 0.05 |
| Maximal tumor diameter (cm) | 1.06 | 0.85 | 1.31 | 0.619 | 1.04 | 0.63 | 1.72 | > 0.05 |
| Group (Bridging) | 1.04 | 0.39 | 2.76 | 0.935 | 1.15 | 0.20 | 6.78 | > 0.05 |
In an intragroup analysis, there was no statistical difference between overall survival (P = 0.955) and recurrence-free survival (P = 0.955) observed in subgroups 1, 2, and 3 of the bridging group. However, in the downstaging group, it seems that subgroup 3 had worse overall survival (P = 0.04) and worse recurrence-free survival (P = 0.027), compared to the other subgroups (Table 4). Post-transplantation recurrence occurred more frequently in the downstaging group 25% (5/20) than in the bridging group 5.81% (5/86) (P = 0.020); however, these events did not significantly affect recurrence-free survival (P = 0.874).
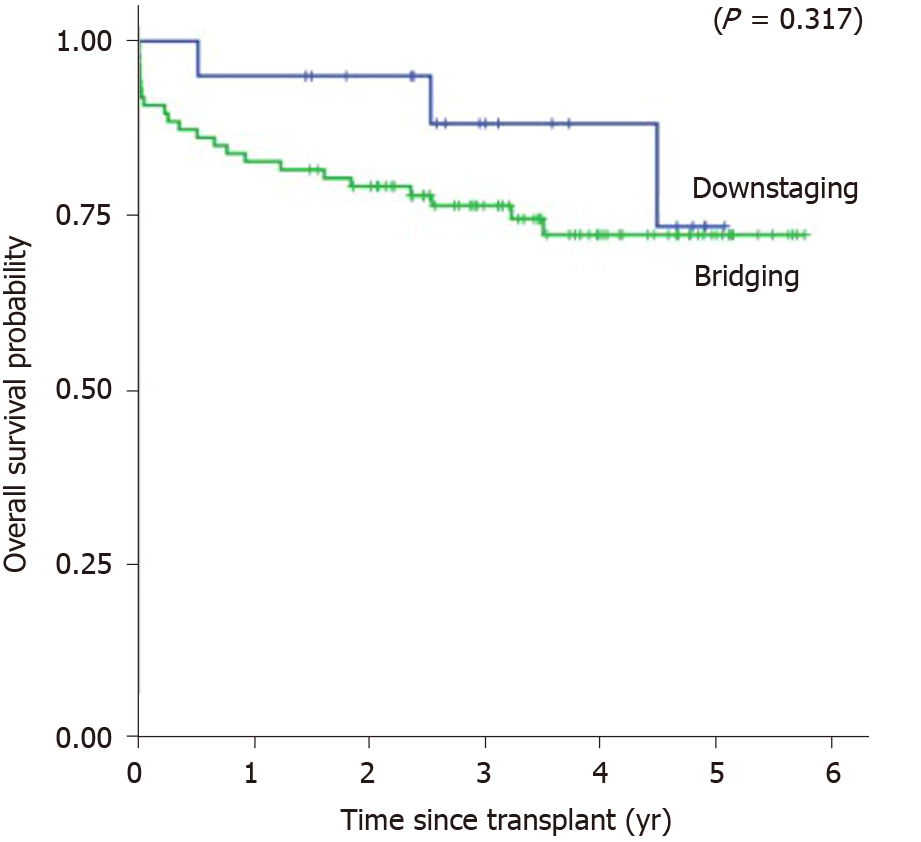
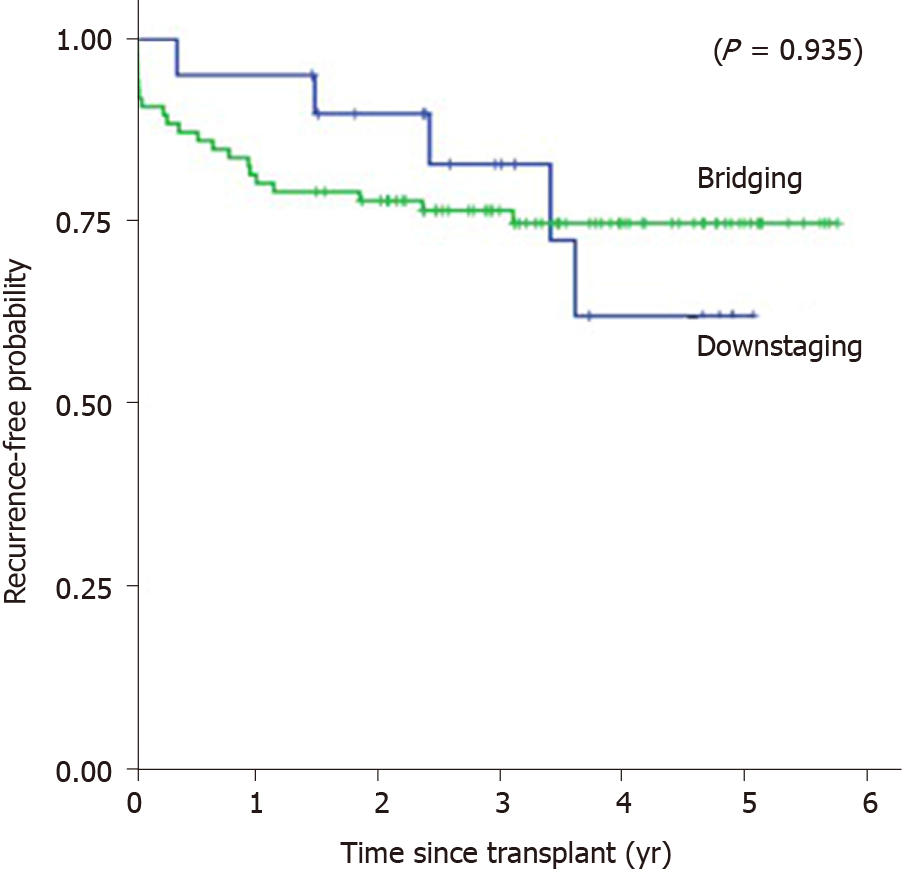
Kaplan-Meier’s 1, 3, and 5-year post-transplant overall survival probability were 95%, 88.2%, 73.5% in the downstaging group, and 82.8%, 76.5%, 72.3% in the bridging group (P = 0.317), respectively (Figure 3). Median overall survival was 1150 d or 3.15 years (SD = 1.33, range from 0.52 to 5.07) in the downstaging group, and 1083 d or 2.97 years (SD = 1.66, range from 0 to 5.77) in bridging group. Kaplan-Meier’s 1, 3, and 5-year post-transplant recurrence-free survival probability were 95%, 82.8%, 62.1% in the downstaging group, and 80.2%, 76.5%, 74.8% in the bridging group (P = 0.935), respectively (Figure 4). Recurrence-free survival was 1104 d or 3.02 years (SD = 1.37, range from 0.34 to 5.07) in downstaging group, and 1070 days or 2.93 years (SD = 1.69, range from 0 to 5.77) in bridging group (Table 6).
The best approach to patients with HCC beyond MC is controversial given the scarcity of available organs for transplantation and the impreciseness of identifying patients who are most likely to benefit from LT. In 2010, downstaging of HCC has been identified as a priority for research in the field of LT[18]. Since that time, we have not found in the literature many published data evaluating this controversial subject. The majority of papers is limited by small sample size, short duration of follow-up, and absence of a comparison group[7-9,19-24]. Most studies have used MC as the endpoint for downstaging[9,19,22-24]. There appears to persist a doubt if patients beyond MC should undergo LT after successful downstaging. There is a lack of information in the literature regarding long-term overall and recurrence-free survival in these patients[25].
The current understanding regarding neo-adjuvant treatment for HCC is that it would be most appropriate for: Controlling HCC progression for expected long waiting times (bridging), identifying patients with different probabilities of cancer progression (selection criterion), and reducing tumors sizes to meet acceptable criteria for LT (downstaging)[26]. However, there is no strong evidence that neo-adjuvant treatments should be applied if the expected waiting time for LT is shorter than 6 mo[25-27].
The present study included patients that have used neo-adjuvant therapy, DEB-TACE loaded with Doxorubicin, for HCC patients undergoing bridging and downstaging. Patients fulfilling the MC were immediately included on the waiting list for LT, whereas patients beyond MC were listed only after they met MC, regardless of how many DEB-TACE sessions. Few studies followed the same protocol: none exclusively using DEB-TACE and the majority using many different kinds of LRT[19,20,22]. Graziadei et al[19], used conventional TACE (cTACE) for both groups: patients fulfilling the MC were started immediately after listing for OLT and patients beyond MC were included on the waiting list after showing response to the first TACE. Ravaioli et al[20], used many kinds of LRT, such as LR, percutaneous ethanol injection (PEI), RFA, and cTACE for both groups. Additionally, patients fulfilling the MC were listed immediately for LT and patients beyond MC were listed after completing the pre-established downstaging protocol. De Luna et al., employed transcatheter arterial chemoinfusion for both groups. Patients fulfilling the MC were listed immediately for OLT and patients beyond MC were included on the waiting list after reaching MC (downstaged)[22].
Few studies have used LRT in patients beyond MC in order to achieve downstaging[7-9,21,23,24]. Some studies compared their results with other LRT, such as transarterial radioembolization[9]; some studies did not compare between groups[8,9,21,23,24]; and other studies used patients within the MC on the waiting list for LT as a comparative group[7]. Among these, the most significant study, highlighted by the sample and methodology, was published recently by Yao et al[7]. In a prospective cohort, the authors reported the outcomes of 118 patients (largest study group) exceeding the MC that underwent LRT (cTACE, RFA, and PEI) in a downstaging well-established protocol with the intent for LT and compared to 488 patients within the MC on presentation[7].
In the present study, at the presentation, the two populations did not have significantly different baseline demographic characteristics. Vascular lake phenomenon was much more frequent in downstaging population 34.3% (P = 0.001), perhaps since vascular lake phenomenon occurs more frequently in tumor of size ≥ 3.0 cm[14].
More patients underwent LT in the bridging group (66%) compared to the downstaging group (34%, P = 0.001). In the literature, eligibility for LT reported for the bridging group ranges from 68% to 85.4%[19,20,22]. Yao et al[7], reported 68% eligibility for LT in the MC group, similarly seen in our study. In a recent study, a dropout rate of 2.58% due to tumor progression was observed in patients who received bridging LRT, while the rate among patients who did not receive LTR was 8.18% (P = 0.01)[28]. Among downstaging patients, neo-adjuvant success rates regarding eligibility for LT widely vary from 11% to more than 70%[29]. This large range reflects the heterogeneity of the criteria used to include patients in a downstaging protocol, differences in the LTR protocol itself, and several different criteria used worldwide on when to include a patient on a transplant list.
In the downstaging protocol, it was included patients with maximal tumor diameter ≤ 8 cm (G1, G2, G3, and G4) and > 8 cm (G5). In this way, eligibility for LT was 34%. However, if we closely look at eligibility for LT in subgroups with maximal tumor diameter up to 8 cm, eligibility for LT would be 47.5%, closer to Yao et al[7]’s reported eligibility of 54%. Maximal tumor diameter appears to influence success of HCC downstaging due to eligibility for LT, so much so that Yao et al[7], limit maximal tumor diameter to 8 cm in their downstaging protocol.
In the present study, G1, G2, and G3 used the same criteria described by Yao et al[7]. Nevertheless, we found a new group of patients with maximal tumor diameter ≤ 8 cm (G4 = 2 or 3 lesions at least one > 5 cm with the sum of the maximal tumor diameters ≤ 8 cm), not previously described. Among patients in the downstaging group, we sought to verify if there was a cutoff limit that related to maximal tumor diameter, and a better chance for the patient to be submitted to LT. We found that patients in the downstaging group with maximal tumor diameter up to 7.05 cm had a greater chance of LT (P = 0.005). Contrary to this finding, G4, with a mean maximal tumor diameter of 7.2 cm, had the best eligibility for LT (60%). On the other hand, corroborating Yao et al[7]’s impression, G5, where the maximal tumor diameter was 10.9 cm on average, had the worst eligibility for LT (2 patients of 22; 9.1%). Other variables that were implicated with a greater chance of LT were coagulopathy, thrombocytopenia, and belonging to the bridging group.
Studies provided a cautionary note with an anticipated higher recurrence rate post LT: the further the tumor burden is beyond the MC (the Metro ticket concept)[25,30]. In our study, post LT recurrence occurred more frequently in the downstaging group 25% (5/20) compared to the bridging group 5.81% (5/86) (P = 0.02); however, this finding did not interfere with recurrence-free survival (P = 0.874). Group 4 did not have a case of HCC recurrence. In the downstaging group, HCC recurrence rate was higher than Yao et al[7]’s rate (7.5%), but similar to Ravaioli et al[20]’s rate (18%).
Our results suggest that patients, with tumors initially exceeding the MC down-staged after DEB-TACE, can achieve post-transplant survival and HCC recurrence-free probability, at five years, just like patients within MC. And, that most patients who have a total tumor diameter less than 7.0 cm will require up to 2 sessions of DEB-TACE to reach downstaging and/or to be transplanted. Patients with total tumor diameter greater than 7.0 cm will require 2.5 or more sessions. As far as we know, this study is the only one in the literature that compares long-term results between two cohorts (downstaging and bridging) that used DEB-TACE with doxorubicin exclusively as LRT[25]. No baseline characteristic, including AFP levels, served as a predictor of poor overall or recurrence-free survival after LT.
There is no evidence that one type of LRT is clearly superior to another, but TACE, especially cTACE, is the most frequently one used[26,31]. In HCC, a temporary overproduction of vascular endothelial growth factor (VEGF) is caused by a single session of TACE. And an increase in serum VEGF is related to tumor growth via neoangiogenesis, metastatic seeding, and cancer cell migration and survival[32]. Lipiodol emulsion, used in cTACE, causes unstable ischemia with reperfusion injury in the targeted tissue[32]. On the other hand, the beads used in DEB-TACE lead to irreversible permanent embolization and ischemia[33]. Schicho et al[32], compared cTACE with DEB-TACE, finding that VEGF plasma levels were significantly higher in cTACE patients as soon as 24 h after treatment (P = 0.01) and remained at high levels 28 d after cTACE (P = 0.03), compared to DEB-TACE patients. Thus, perhaps DEB-TACE should be preferentially used when TACE is the LRT option that is considered. We found one paper in the literature that uses DEB-TACE with doxorubicin for downstaging, but the study did not include a comparative group[24].
Response to TACE as a selection criterion is promptly identifiable in clinical practice and may reflect biological properties, such as tumor aggressiveness[34]. There is an excellent correlation between pathologic degree of tumor necrosis post LRT found in MRI using mRECIST criteria and explant pathology[35]. In the present study, we verified that there was an association between degree of response in both groups after first DEB-TACE (P = 0.004). The bridging group presented with more CR events and less PR events than the downstaging group. However, the OR rate was not statistically significant different between the two groups (P = 0.105).
We recognize that the subgroup analysis is strongly limited by the low number of patients included in each subgroup but the absence of difference between groups in the final outcome shows the equivalence of results by the technique employed. Successful downstaging of HCC to within MC with DEB-TACE loaded with doxorubicin was associated with a lower rate of LT and four times more HCC recurrence than the bridging group. Despite this finding, both groups had comparable post-transplant survival and recurrence-free probabilities. OR did not differ between groups.
Transarterial chemoembolization (TACE) is the most common neoadjuvant therapy modality in the pre-transplant setting and drug-eluting beads TACE (DEB-TACE) is therapy with fewer adverse effects.
Bridging and downstaging patients for liver transplantation (LT) outcomes comparation still have not been fully elucidate.
To determine long-term outcomes of patients with hepatocellular carcinoma (HCC) that underwent LT after DEB-TACE for downstaging vs bridging.
Compare the overall survival, tumor recurrence, rate of LT, waiting time on list and radiological response for hepatocellular carcinoma after DEB-TACE in downstaging and bridging patients. This was a single-center, observational prospective study with controlled and uniform chemoembolization technique.
After TACE, only patients within Milan Criteria (MC) were transplanted. More patients underwent LT in bridging group 65.9% (P = 0.001). Downstaging population presented: higher number of nodules 2.81 (P = 0.001); larger total tumor diameter 8.09 (P = 0.001); multifocal HCC 78% (P = 0.001); more post-transplantation recurrence 25% (P = 0.02). Patients with maximal tumor diameter up to 7.05cm were more likely to receive LT (P = 0.005). Median time on the waiting list was significantly longer in downstaging group 10.6 mo (P = 0.028). Five-year post-transplant overall survival was 73.5% in downstaging and 72.3% bridging groups (P = 0.31), and recurrence-free survival was 62.1% in downstaging and 74.8% bridging groups (P = 0.93). Radiological response: Complete response was observed more frequently in bridging group (P = 0.004). There were no difference between the groups in five-years post-transplant overall survival and recurrence-free survival.
Tumors initially exceeding the MC down-staged after DEB-TACE, can achieve post-transplant survival and HCC recurrence-free probability, at five years, just like patients within MC in patients undergoing DEB-TACE.
It is worth investing in patients in the downstaging group to meet their liver transplant criteria because of the results within 5 years after transplantation.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Allam N, Chiu KW, Cholongitas E, Makisalo H S-Editor: Yan JP L-Editor: A E-Editor: Li X
| 1. | Rossi L, Zoratto F, Papa A, Iodice F, Minozzi M, Frati L, Tomao S. Current approach in the treatment of hepatocellular carcinoma. World J Gastrointest Oncol. 2010;2:348-359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 65] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 2. | Raoul JL, Sangro B, Forner A, Mazzaferro V, Piscaglia F, Bolondi L, Lencioni R. Evolving strategies for the management of intermediate-stage hepatocellular carcinoma: available evidence and expert opinion on the use of transarterial chemoembolization. Cancer Treat Rev. 2011;37:212-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 409] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 3. | Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2645] [Cited by in RCA: 2876] [Article Influence: 110.6] [Reference Citation Analysis (1)] |
| 4. | Lei JY, Yan LN, Wang WT. Transplantation vs resection for hepatocellular carcinoma with compensated liver function after downstaging therapy. World J Gastroenterol. 2013;19:4400-4408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Majno P, Lencioni R, Mornex F, Girard N, Poon RT, Cherqui D. Is the treatment of hepatocellular carcinoma on the waiting list necessary? Liver Transpl. 2011;17 Suppl 2:S98-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5110] [Cited by in RCA: 5313] [Article Influence: 183.2] [Reference Citation Analysis (0)] |
| 7. | Yao FY, Mehta N, Flemming J, Dodge J, Hameed B, Fix O, Hirose R, Fidelman N, Kerlan RK, Roberts JP. Downstaging of hepatocellular cancer before liver transplant: long-term outcome compared to tumors within Milan criteria. Hepatology. 2015;61:1968-1977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 370] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 8. | Yao FY, Kerlan RK, Hirose R, Davern TJ, Bass NM, Feng S, Peters M, Terrault N, Freise CE, Ascher NL, Roberts JP. Excellent outcome following down-staging of hepatocellular carcinoma prior to liver transplantation: an intention-to-treat analysis. Hepatology. 2008;48:819-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 409] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 9. | Lewandowski RJ, Kulik LM, Riaz A, Senthilnathan S, Mulcahy MF, Ryu RK, Ibrahim SM, Sato KT, Baker T, Miller FH, Omary R, Abecassis M, Salem R. A comparative analysis of transarterial downstaging for hepatocellular carcinoma: chemoembolization versus radioembolization. Am J Transplant. 2009;9:1920-1928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 437] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 10. | Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, Pitton M, Sergent G, Pfammatter T, Terraz S, Benhamou Y, Avajon Y, Gruenberger T, Pomoni M, Langenberger H, Schuchmann M, Dumortier J, Mueller C, Chevallier P, Lencioni R; PRECISION V Investigators. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33:41-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1063] [Cited by in RCA: 1208] [Article Influence: 75.5] [Reference Citation Analysis (0)] |
| 11. | Nasser F, Cavalcante RN, Galastri FL, de Rezende MB, Felga GG, Travassos FB, De Fina B, Affonso BB. Safety and feasibility of same-day discharge of patients with hepatocellular carcinoma treated with transarterial chemoembolization with drug-eluting beads in a liver transplantation program. J Vasc Interv Radiol. 2014;25:1012-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Bruix J, Sherman M; Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4333] [Cited by in RCA: 4508] [Article Influence: 225.4] [Reference Citation Analysis (0)] |
| 13. | McEvoy SH, McCarthy CJ, Lavelle LP, Moran DE, Cantwell CP, Skehan SJ, Gibney RG, Malone DE. Hepatocellular carcinoma: illustrated guide to systematic radiologic diagnosis and staging according to guidelines of the American Association for the Study of Liver Diseases. Radiographics. 2013;33:1653-1668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 14. | Cavalcante RN, Nasser F, Motta-Leal-Filho JM, Affonso BB, Galastri FL, De Fina B, Garcia RG, Wolosker N. Occurrence of Vascular Lake Phenomenon as a Predictor of Improved Tumor Response in HCC Patients That Underwent DEB-TACE. Cardiovasc Intervent Radiol. 2017;40:1044-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3353] [Cited by in RCA: 3303] [Article Influence: 220.2] [Reference Citation Analysis (36)] |
| 16. | Peltan ID, Vande Vusse LK, Maier RV, Watkins TR. An International Normalized Ratio-Based Definition of Acute Traumatic Coagulopathy Is Associated With Mortality, Venous Thromboembolism, and Multiple Organ Failure After Injury. Crit Care Med. 2015;43:1429-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 17. | Finger PT. Book review: the eye: basic sciences in practice. N Engl J Med. 1996;334:1550-1553. [DOI] [Full Text] |
| 18. | Thomas MB, Jaffe D, Choti MM, Belghiti J, Curley S, Fong Y, Gores G, Kerlan R, Merle P, O'Neil B, Poon R, Schwartz L, Tepper J, Yao F, Haller D, Mooney M, Venook A. Hepatocellular carcinoma: consensus recommendations of the National Cancer Institute Clinical Trials Planning Meeting. J Clin Oncol. 2010;28:3994-4005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 323] [Article Influence: 21.5] [Reference Citation Analysis (1)] |
| 19. | Graziadei IW, Sandmueller H, Waldenberger P, Koenigsrainer A, Nachbaur K, Jaschke W, Margreiter R, Vogel W. Chemoembolization followed by liver transplantation for hepatocellular carcinoma impedes tumor progression while on the waiting list and leads to excellent outcome. Liver Transpl. 2003;9:557-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 336] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 20. | Ravaioli M, Grazi GL, Piscaglia F, Trevisani F, Cescon M, Ercolani G, Vivarelli M, Golfieri R, D'Errico Grigioni A, Panzini I, Morelli C, Bernardi M, Bolondi L, Pinna AD. Liver transplantation for hepatocellular carcinoma: results of down-staging in patients initially outside the Milan selection criteria. Am J Transplant. 2008;8:2547-2557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 299] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 21. | Chapman WC, Majella Doyle MB, Stuart JE, Vachharajani N, Crippin JS, Anderson CD, Lowell JA, Shenoy S, Darcy MD, Brown DB. Outcomes of neoadjuvant transarterial chemoembolization to downstage hepatocellular carcinoma before liver transplantation. Ann Surg. 2008;248:617-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 234] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 22. | De Luna W, Sze DY, Ahmed A, Ha BY, Ayoub W, Keeffe EB, Cooper A, Esquivel C, Nguyen MH. Transarterial chemoinfusion for hepatocellular carcinoma as downstaging therapy and a bridge toward liver transplantation. Am J Transplant. 2009;9:1158-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 23. | Barakat O, Wood RP, Ozaki CF, Ankoma-Sey V, Galati J, Skolkin M, Toombs B, Round M, Moore W, Mieles L. Morphological features of advanced hepatocellular carcinoma as a predictor of downstaging and liver transplantation: an intention-to-treat analysis. Liver Transpl. 2010;16:289-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 24. | Yu CY, Ou HY, Weng CC, Huang TL, Chen TY, Leung-Chit L, Hsu HW, Chen CL, Cheng YF. Drug-Eluting Bead Transarterial Chemoembolization as Bridge Therapy for Hepatocellular Carcinoma Before Living-Donor Liver Transplantation. Transplant Proc. 2016;48:1045-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Kulik L, Heimbach JK, Zaiem F, Almasri J, Prokop LJ, Wang Z, Murad MH, Mohammed K. Therapies for patients with hepatocellular carcinoma awaiting liver transplantation: A systematic review and meta-analysis. Hepatology. 2018;67:381-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 222] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 26. | Toso C, Mentha G, Kneteman NM, Majno P. The place of downstaging for hepatocellular carcinoma. J Hepatol. 2010;52:930-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 27. | Cescon M, Cucchetti A, Ravaioli M, Pinna AD. Hepatocellular carcinoma locoregional therapies for patients in the waiting list. Impact on transplantability and recurrence rate. J Hepatol. 2013;58:609-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 117] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 28. | Xing M, Sakaria S, Dhanasekaran R, Parekh S, Spivey J, Knechtle SJ, Zhang D, Kim HS. Bridging Locoregional Therapy Prolongs Survival in Patients Listed for Liver Transplant with Hepatocellular Carcinoma. Cardiovasc Intervent Radiol. 2017;40:410-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Bryce K, Tsochatzis EA. Downstaging for hepatocellular cancer: harm or benefit? Transl Gastroenterol Hepatol. 2017;2:106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL, Adam R, Neuhaus P, Salizzoni M, Bruix J, Forner A, De Carlis L, Cillo U, Burroughs AK, Troisi R, Rossi M, Gerunda GE, Lerut J, Belghiti J, Boin I, Gugenheim J, Rochling F, Van Hoek B, Majno P; Metroticket Investigator Study Group. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1574] [Article Influence: 92.6] [Reference Citation Analysis (1)] |
| 31. | Yao FY, Breitenstein S, Broelsch CE, Dufour JF, Sherman M. Does a patient qualify for liver transplantation after the down-staging of hepatocellular carcinoma? Liver Transpl. 2011;17 Suppl 2:S109-S116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 32. | Schicho A, Hellerbrand C, Krüger K, Beyer LP, Wohlgemuth W, Niessen C, Hohenstein E, Stroszczynski C, Pereira PL, Wiggermann P. Impact of Different Embolic Agents for Transarterial Chemoembolization (TACE) Procedures on Systemic Vascular Endothelial Growth Factor (VEGF) Levels. J Clin Transl Hepatol. 2016;4:288-292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 33. | Facciorusso A, Di Maso M, Muscatiello N. Drug-eluting beads versus conventional chemoembolization for the treatment of unresectable hepatocellular carcinoma: A meta-analysis. Dig Liver Dis. 2016;48:571-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 174] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 34. | Otto G, Schuchmann M, Hoppe-Lotichius M, Heise M, Weinmann A, Hansen T, Pitton MP. How to decide about liver transplantation in patients with hepatocellular carcinoma: size and number of lesions or response to TACE? J Hepatol. 2013;59:279-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 35. | Gordic S, Corcuera-Solano I, Stueck A, Besa C, Argiriadi P, Guniganti P, King M, Kihira S, Babb J, Thung S, Taouli B. Evaluation of HCC response to locoregional therapy: Validation of MRI-based response criteria versus explant pathology. J Hepatol. 2017;67:1213-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |