Published online Oct 7, 2019. doi: 10.3748/wjg.v25.i37.5604
Peer-review started: July 24, 2019
First decision: August 18, 2019
Revised: September 8, 2019
Accepted: September 11, 2019
Article in press: September 11, 2019
Published online: October 7, 2019
Processing time: 67 Days and 17.4 Hours
Esophageal squamous cell carcinoma (ESCC) is one of the main causes of human death. It is usually already in middle or advanced stage when diagnosed due to its hidden symptoms in early stage. Therefore, patients have already lost the best surgical timing when diagnosed. Radiotherapy and chemotherapy are standard treatment methods for ESCC clinically, but the efficacy and prognosis of patients from them are still unsatisfactory. Therefore, it is of great clinical significance to seek for biomarkers that can predict the radiotherapy and chemotherapy response and prognosis of ESCC patients.
To explore the clinical value of plasma miR-21 and miR-93 in ESCC.
A total of 128 ESCC patients admitted to the First Affiliated Hospital of Zhenzhou University were enrolled as a study group and treated with concurrent radiotherapy and chemotherapy, and other 45 healthy people during the same period were enrolled as a control group. The expression of plasma miR-21 and miR-93 was determined using quantitative real-time polymerase chain reaction, and the correlation of expression of plasma miR-21 and miR-93 with clinical pathological parameters about the patients was analyzed. The receiver operating characteristic (ROC) curve was adopted to assess the diagnostic value of plasma miR-21 and miR-93 for clinical pathological features of ESCC patients, the Logistic regression analysis adopted to analyze the risk factors for radiotherapy and chemotherapy efficacy in ESCC patients, and the Cox regression analysis to identify the prognostic factors for ESCC patients.
The study group showed significantly higher relative expression of plasma miR-21 and miR-93 than the control group (P < 0.01). The area under the ROC curve (AUC) of plasma miR-21 for diagnosing T stage, N stage, M stage, and pathological differentiation of ESCC was 0.819, 0.758, 0.824, and 0.725, respectively, and that of plasma miR-93 for diagnosing T stage, N stage, and M stage of ESCC was 0.827, 0.815, and 0.814, respectively. The AUC of combined plasma miR-21 and miR-93 for predicting radiotherapy and chemotherapy efficacy before radiotherapy and chemotherapy was 0.894, and the AUCs of them for predicting the 3-year overall survival (OS) were 0.861 and 0.807, respectively. T stage (P < 0.05), M stage (P < 0.05), miR-21(P < 0.01), and miR-93 (P < 0.05) were independent risk factors for radiotherapy and chemotherapy efficacy, and T stage (P < 0.01), N stage (P < 0.05), M stage (P < 0.01), miR-21 (P < 0.01), and miR-93 (P < 0.01) were independent prognostic factors for ESCC patients.
MiR-21 and miR-93 can be adopted as effective biomarkers for predicting radiotherapy and chemotherapy efficacy in ESCC and the 3-year OS of ESCC patients.
Core tip: In order to observe the roles of miR-21 and miR-93 in predicting radiotherapy and chemotherapy efficacy and prognosis in esophageal squamous cell carcinoma (ESCC), quantitative real-time polymerase chain reaction was adopted to determine the expression of plasma miR-21 and miR-93 in ESCC patients. Multivariate Logistic regression analysis revealed that patients with high T stage and M stage and high expression of miR-21 (> 5.80) and miR-93 (> 4.71) suffered an increased risk of ineffective radiotherapy and chemotherapy, and multivariate Cox regression analysis revealed that patients with high T stage, N stage, and M stage, and high expression of miR-21 (> 5.60), and miR-93 (> 3.87) suffered an increased risk of death in 3 years.
- Citation: Wang WT, Guo CQ, Cui GH, Zhao S. Correlation of plasma miR-21 and miR-93 with radiotherapy and chemotherapy efficacy and prognosis in patients with esophageal squamous cell carcinoma. World J Gastroenterol 2019; 25(37): 5604-5618
- URL: https://www.wjgnet.com/1007-9327/full/v25/i37/5604.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i37.5604
Esophageal carcinoma, a main cause of cancer death, is the eighth most common malignant tumor in human beings, with more than 480 thousand new patients and about 400 thousand deaths for it each year[1]. Esophageal carcinoma is classified into esophageal adenocarcinoma and esophageal squamous cell carcinoma (ESCC) based on its histological type, with 80% of cases being ESCC[2]. Surgery, neoadjuvant chemoradiotherapy, and combined treatment are main therapeutic methods for ESCC in clinic practice, and treatment decision-making for ESCC is made mainly according to TNM staging[3]. Due to the lack of typical clinical manifestations in the early stage, esophageal carcinoma is usually already in advanced stage when diagnosed, and most patients have already suffered distant spread and metastasis, so patients still show a very poor prognosis and non-optimistic survival rate even after treatment of surgery and radiotherapy[4]. Concurrent radiotherapy and chemotherapy are an important therapeutic method for ESCC, but the prognosis of patients after this treatment is quite different due to heterogeneity of the response of different patients to radiotherapy and chemotherapy[5]. The survival rate of patients with esophageal carcinoma has significantly improved with the continuous development of therapeutic regimens, but their 5-year overall survival (OS) is still less than 20%[6]. Therefore, it is of great significance to seek for biomarkers that can predict the radiotherapy and chemotherapy response and prognosis of ESCC patients in clinical practice.
MicroRNAs (miRNAs), a group of small single-strand non-coding RNAs, can inhibit mRNA translation or induce mRNA degradation by specifically binding to the 3' untranslated region sequence of mRNA target, thus regulating post-transcriptional gene expression[7]. MiRNAs participate in the progression, proliferation, and metastasis of cancer cells in almost all human cancers including ESCC[8]. MiRNAs are expressed in peripheral blood and tissues. Previous studies have confirmed that miRNAs may become potential biomarkers for diagnosis, treatment, or prognosis of malignant tumors in clinical practice[9,10]. It has been proved that miR-21 is highly expressed in many solid tumors, such as ESCC, oral squamous cell carcinoma, and gastric cancer, so it is considered as a new target for cancer diagnosis or treatment[11]. Previous studies pointed out that miR-21 could regulate the invasion, migration, and epithelial-mesenchymal transition of esophageal carcinoma cells[12,13]. A study by Ansari et al found that ESCC tissues showed significantly higher expression of miR-93 than nonneoplastic tissues[14]. Thus, miR-21 and miR-93 may play roles as tumor-promoting genes in ESCC. However, there are few studies on the roles of plasma miR-21 and miR-93 in predicting radiotherapy and chemotherapy efficacy and prognosis.
This study determined the expression of plasma miR-21 and miR-93 in ESCC patients, and explored their roles in predicting radiotherapy and chemotherapy response and prognosis.
A total of 128 ESCC patients admitted to our hospital were enrolled as a study group and treated with concurrent radiotherapy and chemotherapy, and other 45 healthy people during the same period were enrolled as a control group. The study group consisted of 95 males and 33 females between 53 and 79 years old with a mean age of 65.3 ± 8.6 years. The control group consisted of 30 males and 15 females between 49 and 78 years old with a mean age of 63.8 ± 8.3 years. This study, in line with the Declaration of Helsinki, has been approved by the Ethics Committee of the First Affiliated Hospital of Zhenzhou University. The patients and their family members have been informed through telephone or letter, on which they signed an informed consent form.
Patients meeting the following criteria were included: Patients confirmed with ESCC based on cytology or histology[15]; patients meeting the 8th edition of staging criteria for esophageal carcinoma released by the American Joint Committee on Cancer and Union for International Cancer Control in 2017[16]; patients without any radical radiotherapy or chemotherapy experience, without contraindication for radiotherapy or chemotherapy, with expected survival time > 3 mo, and with complete clinical data and follow-up data. The following patients were excluded: Patients combined with liver cirrhosis, cardiorespiratory dysfunction, hematologic disease, acute infection, autoimmune diseases, other malignant tumors, or acute and chronic infectious diseases; patients with perforation or hemorrhage indication; and those who cannot tolerate radiotherapy and chemotherapy, rejected the therapeutic regimen of this study, cannot accept periodic follow-up, or withdrew from the study halfway. The inclusion criteria were applicable to the study group, and all subjects in the control group were healthy people.
The ESCC patients were all treated with three-dimensional conformal radiotherapy[17]. The preventive exposure area and the area of primary tumor and lymph metastases were given 50 Gy/25f (5-6 wk) and 60 Gy/30f (6-7 wk), respectively. Concurrent chemotherapy was performed to the patients for 2 cycles as follows[18]: The patients were given 80 mg/m2 cisplatin (Qilu Pharmaceutical Co., Ltd., China, H37021358) through intravenous drip on the 1st day and the 29th day of radiotherapy, and 500 mg/m2 fluorouracil (Shanghai Xudong Haipu Pharmaceutical co., Ltd, China, H31020593) through intravenous drip from the 1st day to 5th day, and from the 29th day to 33rd d of radiotherapy.
The patients were subject to chest computed tomography and barium swallow-based esophageal radiography 3 mo after treatment, and assessed in accordance with the Response Evaluation Criteria in Solid Tumors (RECIST, revision 1.1) as follows[19]: Complete remission (CR) referred to the result that the lesion disappeared completely for 4 wk or more; partial response (PR) referred to the result that the lesion length was reduced by 30% or more for 4 wk or more; progressive disease (PD) referred to the result that a new lesion occurred and the lesion length was increased by 20% or more; and stable disease (SD) referred to the result that the lesion length was shortened or increased by a percentage between that of PR and that of PD. In terms of radiotherapy and chemotherapy efficacy, CR and PR were regarded as effective, and SD and PD were regarded as ineffective.
Elbow venous blood (5 mL) was drawn from all subjects before and after treatment into a vacuum test tube with EDTA-K2, and centrifuged at 820 g for 10 min. The upper plasma (2 mL) was transferred to an Eppendorf (EP) tube and centrifuged at 16000 g for 10 min to precipitate cell debris. Then, the supernatant was saved to a new EP tube at ﹣80 °C for later analysis. The total RNA of the supernatant was extracted according to the instructions of mirVanaTM miRNA Isolation Kit (Thermo Fisher Scientific-China, AM1561), and the concentration of RNA was determined using a NanoDrop 1000 ultraviolet spectrophotometer (NanoDrop Technologies, Inc. Wilmington, DE, United States). RNA was reversely transcribed into cDNA using the TaqMan MicroRNA Kit (Thermo Fisher Scientific-China, 4366596), and cDNA was taken as a template to carry out polymerase chain reaction (PCR) amplification. U6 was adopted as an internal reference gene. The upstream and downstream primers of miR-21 are 5'-GTTAGCTTATCAGACTGA-3' and 5'-GTGCAGGGTCCGAGGTAT-3', respectively; those of miR-93 are 5'-AGTCTCTGGCTGACTACATCACAG-3' and 5'-CTACTCACAAAACAGGAGTGGAATC-3’, respectively, and those of U6 are 5'-CTCGCTTCGGCAGCACA-3’ and 5'-AACGCTTCACGAATTTGCGT-3’, respectively. The primer sequences were all designed by Shanghai Sangon Biotech Co., Ltd., China. Quantitative determination was performed to miR-21 and miR-93 by real-time PCR on an Applied Biosystems ViiATM 7 system using the miRNA RT-qPCR Detection Kit (Applied Biosystems, Foster City, CA, United States). PCR amplification cycle conditions were: 90 °C for 5 min and 40 cycles of 90 °C for 5 s, 60 °C for 30 s, and 72 °C for 5 s. All samples were repeatedly determined three times, and the results are expressed by 2--ΔCT.
Patients were followed by telephone, visits, and other methods, once every 3 mo, to track the final outcome of patients after treatment. OS refers to the time from the beginning of radiotherapy and chemotherapy to death or the last follow-up.
SPSS20.0 (IBM Corp, Armonk, NY, United States) was used for statistical analyses, and Graphpad Prism6 (Graphpad Software, San Diego, CA, United States) was adopted for drawing figures about data in this study. Measurement data are expressed as the mean ± standard deviation (SD). Group comparison about measurement data was performed by the independent-samples t test, and comparison before and after treatment was performed by the paired t test. Enumeration data are expressed as the number of cases/percentage [n (%)], and comparison within groups about enumeration data was performed by the chi-square test. The Youden index of receiver operating characteristic (ROC) curves was adopted to assess the diagnostic value of miR-21 and miR-93 for clinicopathological features of ESCC patients. Logistic regression equation and ROC curves were adopted to judge the predictive value of miR-21 combined with miR-94 for assessing radiotherapy and chemotherapy efficacy in ESCC patients, and Logistic regression analysis was adopted to analyze the risk factors for radiotherapy and chemotherapy efficacy in ESCC patients. Kaplan-Meier method was applied to calculate OS, the Log-rank test applied to assess the difference in survival time between groups, and univariate and multivariate Cox regression analyses to identify the prognostic factors for ESCC patients. P < 0.05 indicated a significant difference.
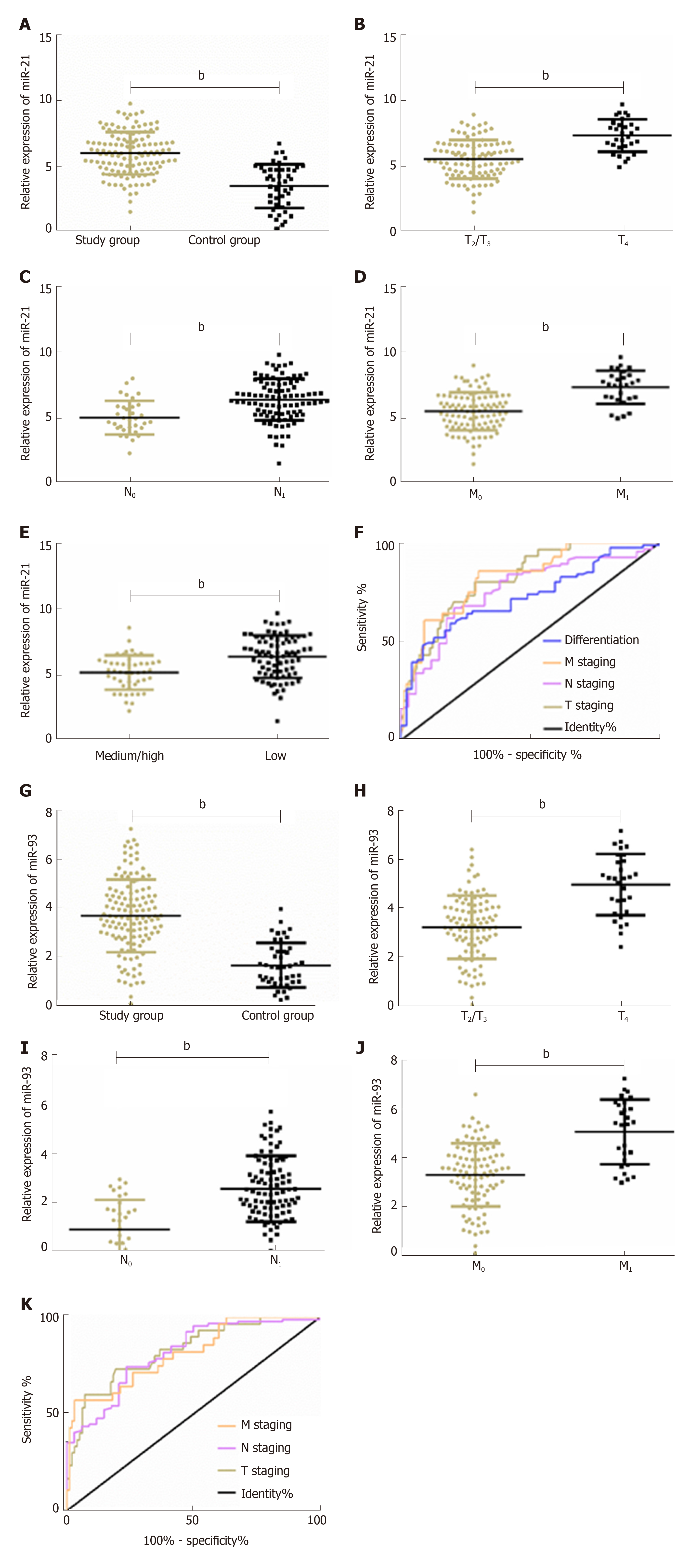
The study group showed significantly higher relative expression of plasma miR-21 and miR-93 than the control group (both P < 0.01). Plasma miR-21 and miR-93 were closely related to T stage, N stage, and M stage (P < 0.01), and plasma miR-21 was closely related to pathological differentiation (P < 0.01). ROC curve analysis showed that the area under the ROC curve (AUC) of miR-21 for diagnosing T stage, N stage, M stage, and pathological differentiation of ESCC was 0.819, 0.758, 0.824, and 0.725, respectively, and that of plasma miR-93 for diagnosing T stage, N stage, and M stage of ESCC was 0.827, 0.815, and 0.814, respectively. More details are shown in Figure 1 and Tables 1 and 2.
| Pathological feature | AUC | 95%CI | SE | Cut-off | Sensitivity (%) | Specificity (%) |
| MiR-21 | ||||||
| T stage | 0.819 | 0.740-0.899 | 0.041 | 6.52 | 80.00 | 71.43 |
| N stage | 0.758 | 0.666-0.851 | 0.047 | 5.90 | 67.02 | 79.41 |
| M stage | 0.824 | 0.740-0.908 | 0.043 | 6.34 | 85.71 | 70.00 |
| Pathological differentiation | 0.725 | 0.637-0.812 | 0.044 | 6.20 | 80.85 | 59.26 |
| MiR-93 | ||||||
| T stage | 0.827 | 0.742-0.911 | 0.043 | 4.37 | 73.33 | 80.61 |
| N stage | 0.815 | 0.733-0.896 | 0.041 | 3.34 | 74.47 | 76.47 |
| M stage | 0.814 | 0.722-0.906 | 0.047 | 5.36 | 57.14 | 97.00 |
| Category | n | miR-21 | t/F | P value | miR-93 | t/F | P-value |
| Gender | 0.867 | 0.387 | 0.337 | 0.737 | |||
| Male | 95 | 6.06 ± 1.61 | 3.63 ± 1.75 | ||||
| Female | 33 | 5.77 ± 1.78 | 3.74 ± 1.14 | ||||
| Age (yr) | 0.590 | 0.556 | 0.704 | 0.483 | |||
| ≤ 65 | 54 | 5.89 ± 1.61 | 3.57 ± 1.67 | ||||
| > 65 | 74 | 6.06±1.61 | 3.76 ± 1.38 | ||||
| Smoking history | 1.108 | 0.270 | 1.013 | 0.313 | |||
| Yes | 52 | 5.80 ± 1.47 | 3.53 ± 1.70 | ||||
| No | 76 | 6.12 ± 1.69 | 3.80 ± 1.31 | ||||
| History of alcoholism | 1.694 | 0.093 | 1.286 | 0.201 | |||
| Yes | 60 | 5.73 ± 1.54 | 3.49 ± 1.46 | ||||
| No | 68 | 6.21 ± 1.65 | 3.83 ± 1.52 | ||||
| T stage | 6.078 | < 0.001b | 6.489 | < 0.001d | |||
| T2/T3 | 98 | 5.57 ± 1.47 | 3.27 ± 1.31 | ||||
| T4 | 30 | 7.37 ± 1.24 | 5.03 ± 1.27 | ||||
| N stage | 4.503 | < 0.001b | 6.363 | < 0.001d | |||
| N0 | 34 | 5.00 ± 1.28 | 2.46 ± 1.21 | ||||
| N1 | 94 | 6.35 ± 1.57 | 4.12 ± 1.34 | ||||
| M stage | 6.111 | < 0.001b | 6.315 | < 0.001d | |||
| M0 | 100 | 5.58 ± 1.45 | 3.30 ± 1.30 | ||||
| M1 | 28 | 7.44 ± 1.28 | 5.06 ± 1.34 | ||||
| Tumor site | 0.219 | 0.804 | 0.404 | 0.669 | |||
| Upper 1/3 of thoracic part | 44 | 6.10 ± 1.46 | 3.52 ± 1.45 | ||||
| Middle 1/3 of thoracic part | 59 | 5.89 ± 1.69 | 3.78 ± 1.37 | ||||
| Lower 1/3 of thoracic part | 25 | 6.02 ± 1.70 | 3.75 ± 1.88 | ||||
| Pathological differentiation | 4.379 | < 0.001b | 1.371 | 0.172 | |||
| Medium/high differentiation | 47 | 5.16 ± 1.38 | 3.09 ± 1.42 | ||||
| Low differentiation | 81 | 6.43 ± 1.61 | 3.92 ± 1.50 | ||||
| Tumor length (cm) | 0.684 | 0.495 | 1.219 | 0.225 | |||
| ≤ 5 | 30 | 5.81 ± 1.55 | 3.39 ± 1.54 | ||||
| > 5 | 98 | 6.04 ± 1.63 | 3.77 ± 1.48 |
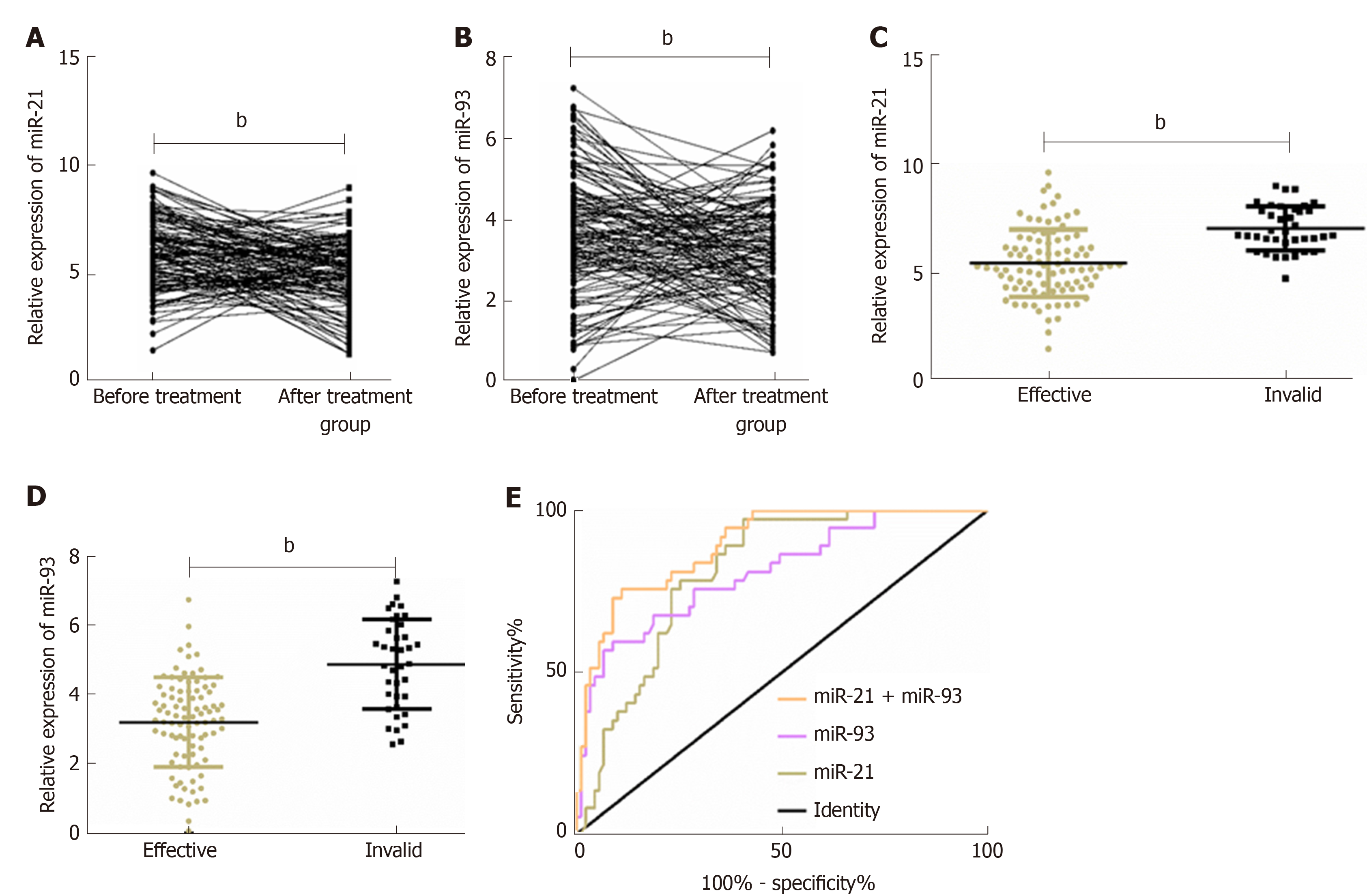
The relative expression of plasma miR-21 and miR-93 in ESCC patients after radiotherapy and chemotherapy was significantly lower than that before treatment (P < 0.01). After radiotherapy and chemotherapy, 91 out of the 128 patients got CR or PR, and other 37 patients got SD or PD. The relative expression of plasma miR-21 and miR-93 in patients with an effective response to radiotherapy and chemotherapy was significantly lower than that of patients with an ineffective response to radiotherapy and chemotherapy (P < 0.01). ROC curves of plasma miR-21 and miR-93 for assessing radiotherapy and chemotherapy efficacy in ESCC patients were drawn, and it was showed that the AUC of miR-21 for assessing radiotherapy and chemotherapy efficacy in ESCC patients was 0.811, and the optimal critical value was 5.80; the AUC of miR-93 for it was 0.810, and the optimal critical value was 4.71. Binary Logistic regression analysis was performed further. A Logistic regression model was obtained by taking miR-21 and miR-93 as independent variables: Logit (P) = -10.851 + 0.854 miR-21 + 1.091 miR-93. The AUC of combined miR-21 and miR-93 for assessing radiotherapy and chemotherapy efficacy was 0.894. More details are shown in Table 3 and Figure 2.
| Index | AUC | 95%CI | SE | Cut-off | Sensitivity (%) | Specificity (%) |
| MiR-21 | 0.811 | 0.738-0.884 | 0.037 | 5.80 | 97.30 | 59.34 |
| MiR-93 | 0.810 | 0.726-0.894 | 0.043 | 4.71 | 59.46 | 91.21 |
| MiR-21 + MiR-93 | 0.894 | 0.838-0.950 | 0.028 | 0.41 | 75.68 | 89.01 |
The patients with an effective response to radiotherapy and chemotherapy were compared with those with an ineffective response in clinical data and relevant indexes, and the optimal critical values of miR-21 and miR-93 for assessing radiotherapy and chemotherapy efficacy in ESCC patients were 5.80 and 4.71, respectively. Patients with an effective response to radiotherapy and chemotherapy and those with an ineffective response showed no significant difference in gender, age, history of alcoholism, tumor site, or tumor length, but showed significant differences in smoking history, T stage, N stage, M stage, pathological differentiation, miR-21, and miR-93 (P < 0.05). Multivariate Logistic regression analysis was adopted for factors with significant differences, which revealed that T stage (P < 0.05), M stage (P < 0.05), miR-21 (P < 0.01), and miR-93 (P < 0.05) were independent risk factors for radiotherapy and chemotherapy efficacy, and patients with high T stage, M stage, and high expression of miR-21 (> 5.80) and miR-93 (> 4.71) suffered an increased risk of ineffective radiotherapy and chemotherapy. More details are shown in Tables 4-6.
| Category | n | Effective (n = 91) | Ineffective (n = 37) | χ2 | P-value |
| Gender | 0.058 | 0.810 | |||
| Male | 95 | 67 (73.63) | 28 (75.68) | ||
| Female | 33 | 24 (26.37) | 9 (24.32) | ||
| Age (Yr) | 0.404 | 0.525 | |||
| ≤ 65 | 54 | 40 (43.96) | 14 (37.84) | ||
| > 65 | 74 | 51 (56.04) | 23 (62.16) | ||
| Smoking history | 5.733 | 0.017a | |||
| No | 52 | 43 (47.25) | 9 (24.32) | ||
| Yes | 76 | 48 (52.75) | 28 (75.68) | ||
| History of alcoholism | 0.839 | 0.360 | |||
| No | 60 | 45 (49.45) | 15 (40.54) | ||
| Yes | 68 | 46 (50.55) | 22 (59.46) | ||
| T stage | 11.380 | < 0.001b | |||
| T2/T3 | 98 | 77 (84.62) | 21 (56.76) | ||
| T4 | 30 | 14 (15.38) | 16 (43.24) | ||
| N stage | 4.543 | 0.033a | |||
| N0 | 34 | 29 (31.87) | 5 (13.51) | ||
| N1 | 94 | 62 (68.13) | 32 (86.49) | ||
| M stage | 13.900 | < 0.001b | |||
| M0 | 100 | 79 (86.81) | 21 (56.76) | ||
| M1 | 28 | 12 (13.19) | 16 (43.24) | ||
| Tumor site | 0.283 | 0.868 | |||
| Upper 1/3 of thoracic part | 44 | 30 (32.97) | 14 (37.84) | ||
| Middle 1/3 of thoracic part | 59 | 43 (47.25) | 16 (43.24) | ||
| Lower 1/3 of thoracic part | 25 | 18 (19.78) | 7 (18.92) | ||
| Pathological differentiation | 5.105 | 0.024a | |||
| Moderate/high differentiation | 47 | 39 (42.86) | 8 (21.62) | ||
| Low differentiation | 81 | 52 (57.14) | 29 (78.38) | ||
| Tumor length (cm) | 0.592 | 0.442 | |||
| ≤ 5 | 30 | 23 (25.27) | 7 (18.92) | ||
| > 5 | 98 | 68 (74.73) | 30 (81.08) | ||
| MiR-21 | 14.870 | < 0.001b | |||
| ≤ 5.80 | 72 | 61 (67.03) | 11 (29.73) | ||
| > 5.80 | 56 | 30 (32.97) | 26 (70.27) | ||
| MiR-93 | 12.590 | < 0.001b | |||
| ≤ 4.71 | 99 | 78 (85.71) | 21 (56.76) | ||
| > 4.71 | 29 | 13 (14.29) | 16 (43.24) |
| Factor | Variable | Assignment | Factor |
| Smoking history | X1 | No = 1, Yes = 2 | Smoking history |
| T stage | X2 | T2/T3 = 1, T4 = 2 | T stage |
| N stage | X3 | N0 = 1, N1 = 2 | N stage |
| M stage | X4 | M0 = 1, M1 = 2 | M stage |
| Pathological differentiation | X5 | Moderate/high differentiation = 1, low differentiation = 2 | Pathological differentiation |
| MiR-21 | X6 | ≤ 5.80 = 1, > 5.80 = 2 | MiR-21 |
| MiR-93 | X7 | ≤ 4.71 = 1, > 4.71 = 2 | MiR-93 |
| Variable | B | SE | Wald | P-value | OR | 95%CI |
| Smoking history | 0.103 | 0.478 | 0.046 | 0.830 | 1.108 | 0.434-2.829 |
| T stage | 1.093 | 0.506 | 4.676 | 0.031a | 2.984 | 1.108-8.037 |
| N stage | 0.954 | 0.713 | 1.792 | 0.181 | 2.597 | 0.642-10.502 |
| M stage | 1.047 | 0.454 | 5.312 | 0.021a | 2.848 | 1.170-6.935 |
| Pathological differentiation | 0.644 | 0.600 | 1.155 | 0.283 | 1.905 | 0.588-6.168 |
| MiR-21 | 1.901 | 0.602 | 9.961 | 0.002b | 6.692 | 2.055-21.79 |
| MiR-93 | 1.026 | 0.456 | 5.064 | 0.024a | 2.791 | 1.142-6.823 |
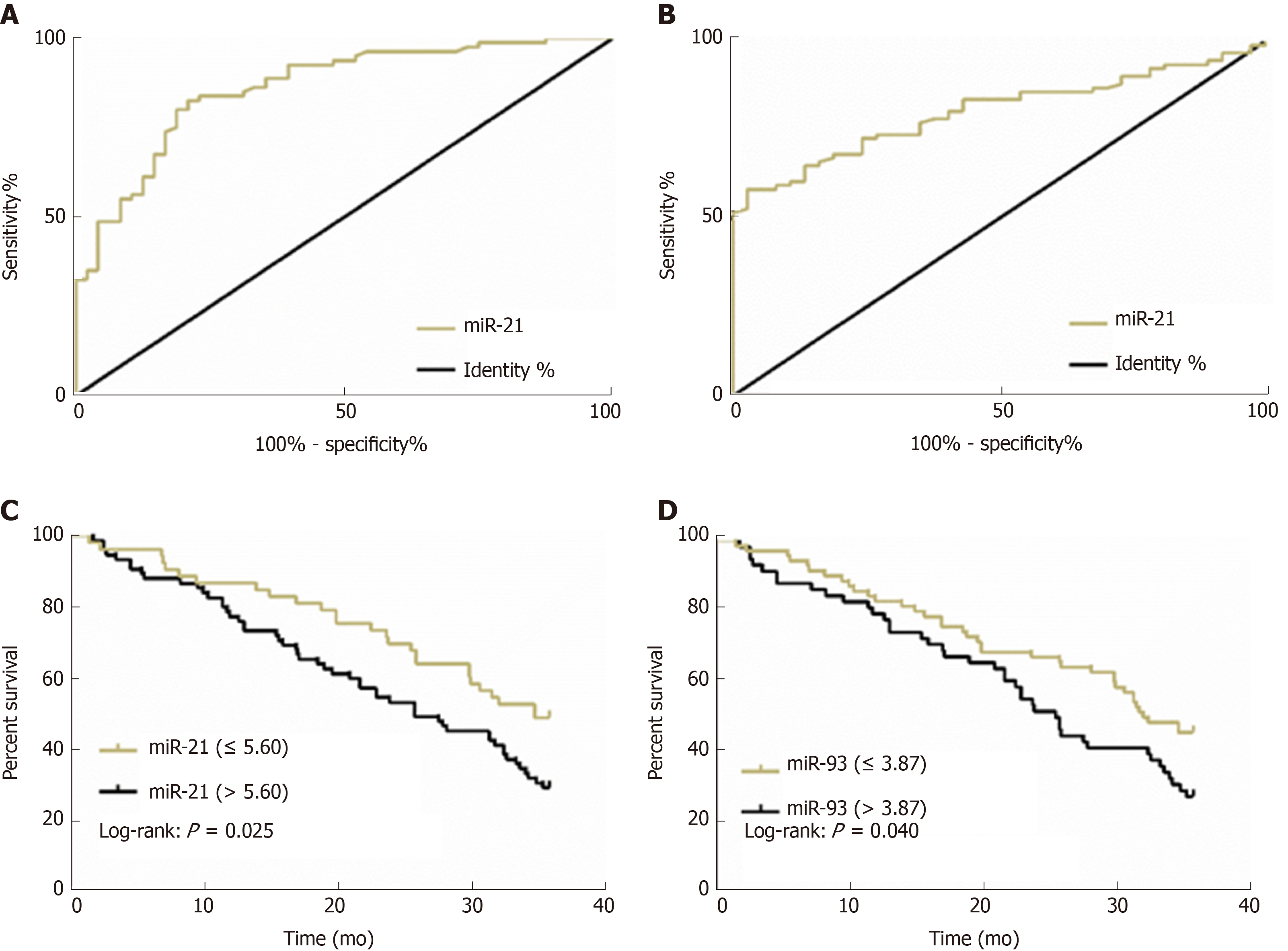
The 128 ESCC patients were followed successfully for 3 years, and their 3-year OS was 37.50% (48/128). According to the 3-year OS of ESCC patients, ROC curves of miR-21and miR-93 for assessing 3-year OS were drawn. The AUCs of miR-21 and miR-93 for assessing 3-year OS were 0.861 and 0.807, respectively, and their optimal critical values were 5.60 and 3.87, respectively. When the optimal critical values of miR-21 (5.60) and miR-93 (3.87) for assessing 3-year OS were taken as cut points, ESCC patients with plasma miR-21 ≤ 5.60 and miR-93 ≤ 3.87 showed a significant difference in 3-year OS (P < 0.05) compared with those with plasma miR-21 > 5.60 and miR-93 > 3.87. Multivariate Cox regression analysis revealed that T stage (P < 0.01), N stage (P < 0.05), M stage (P < 0.01), miR-21(P < 0.01), and miR-93 (P < 0.01) were all independent prognostic factors for ESCC patients, and patients with high T stage, N stage, and M stage, and high expression of miR-21 (> 5.60) and miR-93 (> 3.87) suffered an increased risk of death in 3 years. More details are shown in Figure 3 and Tables 7 and 8.
| Index | AUC | 95%CI | SE | Cut-off | Sensitivity (%) | Specificity (%) |
| MiR-21 | 0.861 | 0.797-0.926 | 0.033 | 5.60 | 82.50 | 79.17 |
| MiR-93 | 0.807 | 0.733-0.880 | 0.037 | 3.87 | 62.50 | 92.50 |
| Index | Univariate | Multivariate | ||
| HR (95%CI) | P-value | HR (95%CI) | P-value | |
| Gender (male vs female) | 1.178 (0.508-2.686) | 0.713 | ||
| Age (≤ 65 yr vs > 65 yr) | 1.167 (0.439-3.158) | 0.770 | ||
| Smoking history (no vs yes) | 0.631 (0.259-1.483) | 0.288 | ||
| History of alcoholism (no vs yes) | 0.802 (0.447-1.459) | 0.468 | ||
| T stage (T2/T3 vs T4) | 3.183 (1.762-5.783) | < 0.001b | 3.168 (1.537-6.537) | 0.003b |
| N stage (N0 vs N1) | 1.318 (1.067-1.638) | 0.015a | 1.307 (1.057-1.736) | 0.017a |
| M stage (M0 vs M1) | 2.438 (1.934-3.062) | < 0.001b | 1.735 (1.402-2.281) | <0.001b |
| Tumor site (Upper 1/3 vs middle 1/3 vs lower 1/3 of thoracic part) | 1.289 (0.557-2.825) | 0.548 | ||
| Pathological differentiation (moderate/high differentiation vs low differentiation) | 0.753 (0.567-1.008) | 0.048a | 0.802 (0.557-1.159) | 0.243 |
| Tumor length (≤ 5 cm vs > 5 cm) | 0.687 (0.283-1.675) | 0.394 | ||
| Radiotherapy and chemotherapy (effective vs ineffective) | 1.695 (1.237-2.248) | 0.001b | 1.335 (0.975-1.846) | 0.080 |
| MiR-21 (≤ 5.60 vs > 5.60) | 4.083 (2.158-7.763) | < 0.001b | 2.438 (1.276-4.71) | 0.007b |
| MiR-93 (≤ 3.87 vs > 3.87) | 4.485 (1.993-10.067) | < 0.001b | 2.198 (1.256-3.875) | 0.009b |
ESCC shows an increasing incidence in human beings. Its early symptoms are relatively hidden, so most patients are already in middle or advanced stage, and have lost the best chance of surgical resection[20,21]. Radiotherapy and chemotherapy are standard treatment methods for ESCC clinically, but the efficacy and OS of patients from them are still unsatisfactory[22]. Therefore, it is of great significance for treatment and prognosis of ESCC to explore the radiotherapy and chemotherapy efficacy and prognosis, and relevant biomarkers.
TNM staging is mostly performed based on postoperative pathologic biopsy, but for patients with an advanced disease, it is sometimes difficult to obtain cancer tissue specimens[23]. Blood miRNAs are easy to sample and determine, and their sampling causes no trauma, so they can be used as non-invasive biomarkers for diagnosis and prognosis of various tumors, with less trauma to patients than pathological biopsy[24,25]. In this study, the ESCC patients showed significantly increased relative expression of plasma miR-21 and miR-93. MiR-21 and miR-93 were closely related to T stage, N stage, and M stage, and miR-21 was closely related to pathological differentiation. ROC curves showed that plasma miR-93 and miR-21 had diagnostic value for these pathological parameters, indicating that miR-93 and miR-21 may become potential molecular markers in clinical diagnosis of ESCC. Clinically, assessments of TNM stage and pathological differentiation are mostly based on histology and imaging[26], so detection of miR-21 and miR-93 is expected to assist the diagnosis of pathological features of ESCC patients. Further study revealed that after radiotherapy and chemotherapy, the expressions of miR-21 and miR-93 significantly decreased, and the AUC of miR-21 combined with miR-93 for assessing radiotherapy and chemotherapy efficacy in ESCC patients was 0.894, indicating that the observed changes of miR-21 and miR-93 could be adopted to predict radiotherapy and chemotherapy efficacy. A study by Kurashige et al[27] revealed that ESCC patients showed highly expressed serum miR-21, and their expression of serum miR-21 was significantly decreased after chemotherapy, which is similar to our study. However, there are few studies on whether miR-21 and miR-93 are related to the radiotherapy and chemotherapy efficacy in ESCC patients. This study revealed that patients with high expression of miR-21 (> 5.80) and miR-93 (> 4.71) suffered an increased risk of ineffective radiotherapy and chemotherapy. Therefore, observation of the expression of miR-21 and miR-93 is helpful to judge the radiotherapy and chemotherapy efficacy in ESCC patients. Previous studies revealed that high expression of plasma miR-23a, related to lymphatic vessel invasion and deep invasion, was an independent risk factor for chemotherapy resistance in ESCC, and overexpression of miR-23a caused obvious drug resistance against 5-fluorouracil and cisplatin in vitro[28]. A study by Komatsu et al[29] pointed out that miR-21 was highly expressed in ESCC patients, and it could be used to predict the chemical resistance of patients. In addition, a study by Fu et al[30] showed that miR-93 could participate in the regulation of chemosensitivity to cisplatin in ovarian cancer through PTEN/Akt signal pathway regulators. According to the above studies and our study, miR-21 and miR-93 may play a key role in the development and chemotherapy resistance of ESCC, but the mechanism is still unclear. A study by Tanaka et al[31] revealed that expression of serum miR-21, significantly up-regulated in ESCC patients, was closely related to neoplasm stage, lymph node metastasis, and inflammatory response. In addition, a study by Li et al[32] confirmed that miR-93 was closely related to clinical stage, lymph node metastasis, and T stage of patients with head and neck squamous cell carcinoma; the patients with high miR-93 expression showed a significantly lower survival rate than those with low expression, and lymph node metastasis was related to a poor prognosis of patients with high miR-93 expression. Therefore, higher expression of miR-21 and miR-93 in ESCC patients may indicate a more serious disease due to high invasion and metastasis of cancer at this time, so patients with high expression of miR-21 and miR-93 suffered an increased risk of ineffective treatment. Finally, we followed the 128 ESCC patients for 3 years, and found that their 3-year OS was 37.50%, which is similar to a previous study[33]. The AUCs of miR-21 and miR-93 for assessing the 3-year OS of ESCC patients were 0.861 and 0.807, respectively, and Cox regression analysis revealed that patients with high T stage, N stage, M stage, and high expression of miR-21 (> 5.60) and miR-93 (> 3.87) suffered an increased risk of death in 3 years, which indicated that detection of miR-21 and miR-93 can be adopted for judging the prognosis of ESCC patients. A study by Komatsu et al[34] revealed that high expression of plasma miR-21 and miR-375 were independent prognostic factors for the 3-year survival rate of ESCC patients undergoing radical esophagectomy, which is similar to our study. Therefore, miR-21 and miR-93 are reliable biomarkers for treatment and prognosis evaluation of ESCC patients, but the regulatory mechanism needs further exploration.
This study has confirmed the roles of plasma miR-21 and miR-93 in radiotherapy and chemotherapy and prognosis of patients, but it still has some deficiencies. First, the correlation of miR-21 and miR-93 with toxic and side effects during chemoradiotherapy has not been observed. Second, the specific regulatory mechanisms of miR-21 and miR-93 in radiotherapy and chemotherapy of ESCC have not been discussed. Finally, the sample size included is small, which may cause selectivity bias, so the sample size needs to be expanded to further support this study.
To sum up, with up-regulated expression in plasma of ESCC patients, miR-21 and miR-93 can be adopted as effective biomarkers for assessing radiotherapy and chemotherapy efficacy and 3-year OS in ESCC patients.
Esophageal squamous cell carcinoma (ESCC), a common malignant tumor in human, is usually already in middle or advanced stage when diagnosed due to its hidden symptoms in early stage. Concurrent radiotherapy and chemotherapy are an important therapeutic method for ESCC in the middle or advanced stage, but the prognosis of patients after this treatment is quite different due to heterogeneity of the response of different patients to radiotherapy and chemotherapy. Under such a background, studying markers associated with radiotherapy and chemotherapy response and prognosis in ESCC patients will help to develop more targeted and individual therapies.
MicroRNAs (miRNAs) play an important role in human malignant tumors. MiR-21 and miR-93 may be helpful for ESCC therapy, so this study aimed to explore their clinical value in ESCC.
The study aimed to investigate the correlation of miR-21 and miR-93 with chemoradiotherapy and prognosis of ESCC patients, and their relationship may provide clues for optimal treatment of ESCC.
Quantitative real-time polymerase chain reaction was applied to determine the expressions- of plasma miR-21 and miR-93 in ESCC patients. The data were analyzed using a variety of statistical methods. For example, receiver operating characteristic curve was adopted to assess the diagnostic value of miR-21 and miR-93 for clinicopathological features of ESCC patients, the Logistic regression adopted to analyze the risk factors for radiotherapy and chemotherapy efficacy in ESCC patients, and the Cox regression to identify the prognostic factors for ESCC patients.
In this study, it was determined that the expression of miR-21 and miR-93 was significantly up-regulated in the plasma of ESCC patients, and they had diagnostic value for pathological characteristics of ESCC patients. In addition, miR-21 and miR-93 were independent risk factors for chemoradiotherapy efficacy, and independent prognostic factors for ESCC patients.
This study confirmed for the first time that plasma miR-21 and miR-93 have diagnostic value for pathological characteristics of ESCC patients. In addition, ESCC patients with high expressions of miR-21 and miR-93 suffer a high risk of failed radiotherapy and chemotherapy and death in 3 years. These results can provide a theoretical basis for evaluation, treatment, and prognosis of ESCC.
This study has proved the clinical value of plasma miR-21 and miR-93 in ESCC patients. However, in the future, it is required to perform an in vitro study to observe the drug resistance regulation mechanisms of miR-21 and miR-93 in radiotherapy and chemotherapy of ESCC cells to supplement this study. The mechanisms may be important for the treatment of ESCC patients. In addition, this study has only included 128 ESCC patients, so the sample size needs to be expanded further to verify the conclusions.
We would like to express our gratitude to the participants in the study.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mohamed SY, Fujiwara T S-Editor: Tang JZ L-Editor: Wang TQ E-Editor: Ma YJ
| 1. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21363] [Article Influence: 2136.3] [Reference Citation Analysis (3)] |
| 2. | Lin DC, Wang MR, Koeffler HP. Genomic and Epigenomic Aberrations in Esophageal Squamous Cell Carcinoma and Implications for Patients. Gastroenterology. 2018;154:374-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 185] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 3. | Wang J, Wu N, Zheng QF, Yan S, Lv C, Li SL, Yang Y. Evaluation of the 7th edition of the TNM classification in patients with resected esophageal squamous cell carcinoma. World J Gastroenterol. 2014;20:18397-18403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Wang C, Wang J, Chen Z, Gao Y, He J. Immunohistochemical prognostic markers of esophageal squamous cell carcinoma: a systematic review. Chin J Cancer. 2017;36:65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 5. | Hummel R, Sie C, Watson DI, Wang T, Ansar A, Michael MZ, Van der Hoek M, Haier J, Hussey DJ. MicroRNA signatures in chemotherapy resistant esophageal cancer cell lines. World J Gastroenterol. 2014;20:14904-14912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 60] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 6. | Choi S, Cui C, Luo Y, Kim SH, Ko JK, Huo X, Ma J, Fu LW, Souza RF, Korichneva I, Pan Z. Selective inhibitory effects of zinc on cell proliferation in esophageal squamous cell carcinoma through Orai1. FASEB J. 2018;32:404-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 7. | Zang WQ, Yang X, Wang T, Wang YY, Du YW, Chen XN, Li M, Zhao GQ. MiR-451 inhibits proliferation of esophageal carcinoma cell line EC9706 by targeting CDKN2D and MAP3K1. World J Gastroenterol. 2015;21:5867-5876. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Gao S, Zhao ZY, Zhang ZY, Zhang Y, Wu R. Prognostic Value of MicroRNAs in Esophageal Carcinoma: A Meta-Analysis. Clin Transl Gastroenterol. 2018;9:203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | He FC, Meng WW, Qu YH, Zhou MX, He J, Lv P, Ming L. Expression of circulating microRNA-20a and let-7a in esophageal squamous cell carcinoma. World J Gastroenterol. 2015;21:4660-4665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Zhou SL, Wang LD. Circulating microRNAs: novel biomarkers for esophageal cancer. World J Gastroenterol. 2010;16:2348-2354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Chen X, Cai S, Li B, Zhang X, Li W, Liang H, Cao X, Wang L, Wu Z. MicroRNA21 regulates the biological behavior of esophageal squamous cell carcinoma by targeting RASA1. Oncol Rep. 2019;41:1627-1637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Guraya S. Prognostic significance of circulating microRNA-21 expression in esophageal, pancreatic and colorectal cancers; a systematic review and meta-analysis. Int J Surg. 2018;60:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 13. | Li X, Chen D, Li M, Gao X, Shi G, Zhao H. The CADM2/Akt pathway is involved in the inhibitory effect of miR-21-5p downregulation on proliferation and apoptosis in esophageal squamous cell carcinoma cells. Chem Biol Interact. 2018;288:76-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Ansari MH, Irani S, Edalat H, Amin R, Mohammadi Roushandeh A. Deregulation of miR-93 and miR-143 in human esophageal cancer. Tumour Biol. 2016;37:3097-3103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Zhang D, Zheng Y, Wang Z, Huang Q, Cao X, Wang F, Liu S. Comparison of the 7th and proposed 8th editions of the AJCC/UICC TNM staging system for esophageal squamous cell carcinoma underwent radical surgery. Eur J Surg Oncol. 2017;43:1949-1955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Jain S, Dhingra S. Pathology of esophageal cancer and Barrett's esophagus. Ann Cardiothorac Surg. 2017;6:99-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 17. | Chen M, Liu P, Chen Y, Chen Z, Shen M, Liu X, Li X, Lin Y, Yang R, Ni W, Zhou X, Zhang L, Tian Y, Chen J. Primary tumor regression patterns in esophageal squamous cell cancer treated with definitive chemoradiotherapy and implications for surveillance schemes. Cancer Manag Res. 2019;11:3361-3369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Ordu AD, Nieder C, Geinitz H, Kup PG, Deymann LF, Scherer V, Combs SE, Fakhrian K. Radio(chemo)therapy for locally advanced squamous cell carcinoma of the esophagus: long-term outcome. Strahlenther Onkol. 2015;191:153-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Chen MQ, Lin QL, Chen YG, Guo JH, Xu BH, Tian Y. Neoadjuvant chemotherapy may not benefit esophageal squamous cell carcinoma patients treated with definitive chemoradiotherapy. J Chin Med Assoc. 2017;80:636-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Ma H, Hasim A, Mamtimin B, Kong B, Zhang HP, Sheyhidin I. Plasma free amino acid profiling of esophageal cancer using high-performance liquid chromatography spectroscopy. World J Gastroenterol. 2014;20:8653-8659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | He Y, Jin J, Wang L, Hu Y, Liang D, Yang H, Liu Y, Shan B. Evaluation of miR-21 and miR-375 as prognostic biomarkers in oesophageal cancer in high-risk areas in China. Clin Exp Metastasis. 2017;34:73-84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | Li F, Lv JH, Liang L, Wang JC, Li CR, Sun L, Li T. Downregulation of microRNA-21 inhibited radiation-resistance of esophageal squamous cell carcinoma. Cancer Cell Int. 2018;18:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Yu H, Duan B, Jiang L, Lin M, Sheng H, Huang J, Gao H. Serum miR-200c and clinical outcome of patients with advanced esophageal squamous cancer receiving platinum-based chemotherapy. Am J Transl Res. 2013;6:71-77. [PubMed] |
| 24. | Brase JC, Wuttig D, Kuner R, Sültmann H. Serum microRNAs as non-invasive biomarkers for cancer. Mol Cancer. 2010;9:306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 309] [Cited by in RCA: 352] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 25. | Wittmann J, Jäck HM. Serum microRNAs as powerful cancer biomarkers. Biochim Biophys Acta. 2010;1806:200-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 136] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 26. | Sun J, Song K, Feng X, Gao S. MicroRNA-367 is a potential diagnostic biomarker for patients with esophageal squamous cell carcinoma. Biochem Biophys Res Commun. 2016;473:363-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Kurashige J, Kamohara H, Watanabe M, Tanaka Y, Kinoshita K, Saito S, Hiyoshi Y, Iwatsuki M, Baba Y, Baba H. Serum microRNA-21 is a novel biomarker in patients with esophageal squamous cell carcinoma. J Surg Oncol. 2012;106:188-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 28. | Komatsu S, Ichikawa D, Kawaguchi T, Takeshita H, Miyamae M, Ohashi T, Okajima W, Imamura T, Kiuchi J, Arita T, Konishi H, Shiozaki A, Fujiwara H, Okamoto K, Otsuji E. Plasma microRNA profiles: identification of miR-23a as a novel biomarker for chemoresistance in esophageal squamous cell carcinoma. Oncotarget. 2016;7:62034-62048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 29. | Komatsu S, Ichikawa D, Kawaguchi T, Miyamae M, Okajima W, Ohashi T, Imamura T, Kiuchi J, Konishi H, Shiozaki A, Fujiwara H, Okamoto K, Otsuji E. Circulating miR-21 as an independent predictive biomarker for chemoresistance in esophageal squamous cell carcinoma. Am J Cancer Res. 2016;6:1511-1523. [PubMed] |
| 30. | Fu X, Tian J, Zhang L, Chen Y, Hao Q. Involvement of microRNA-93, a new regulator of PTEN/Akt signaling pathway, in regulation of chemotherapeutic drug cisplatin chemosensitivity in ovarian cancer cells. FEBS Lett. 2012;586:1279-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 158] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 31. | Tanaka Y, Kamohara H, Kinoshita K, Kurashige J, Ishimoto T, Iwatsuki M, Watanabe M, Baba H. Clinical impact of serum exosomal microRNA-21 as a clinical biomarker in human esophageal squamous cell carcinoma. Cancer. 2013;119:1159-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 358] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 32. | Li G, Ren S, Su Z, Liu C, Deng T, Huang D, Tian Y, Qiu Y, Liu Y. Increased expression of miR-93 is associated with poor prognosis in head and neck squamous cell carcinoma. Tumour Biol. 2015;36:3949-3956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 33. | Lyu X, Huang J, Mao Y, Liu Y, Feng Q, Shao K, Gao S, Jiang Y, Wang J, He J. Adjuvant chemotherapy after esophagectomy: is there a role in the treatment of the lymph node positive thoracic esophageal squamous cell carcinoma? J Surg Oncol. 2014;110:864-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 34. | Komatsu S, Ichikawa D, Takeshita H, Konishi H, Nagata H, Hirajima S, Kawaguchi T, Arita T, Shiozaki A, Fujiwara H, Okamoto K, Otsuji E. Prognostic impact of circulating miR-21 and miR-375 in plasma of patients with esophageal squamous cell carcinoma. Expert Opin Biol Ther. 2012;12 Suppl 1:S53-S59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |