Published online Oct 7, 2019. doi: 10.3748/wjg.v25.i37.5578
Peer-review started: June 17, 2019
First decision: July 21, 2019
Revised: August 14, 2019
Accepted: August 19, 2019
Article in press: July 21, 2019
Published online: October 7, 2019
Processing time: 108 Days and 20.8 Hours
Helicobacter pylori (H. pylori) is a gram-negative bacterium that infects approximately 4.4 billion individuals worldwide. However, its prevalence varies among different geographic areas, and is influenced by several factors. The infection can be acquired by means of oral-oral or fecal-oral transmission, and the pathogen possesses various mechanisms that improve its capacity of mobility, adherence and manipulation of the gastric microenvironment, making possible the colonization of an organ with a highly acidic lumen. In addition, H. pylori presents a large variety of virulence factors that improve its pathogenicity, of which we highlight cytotoxin associated antigen A, vacuolating cytotoxin, duodenal ulcer promoting gene A protein, outer inflammatory protein and gamma-glutamyl transpeptidase. The host immune system, mainly by means of a Th1-polarized response, also plays a crucial role in the infection course. Although most H. pylori-positive individuals remain asymptomatic, the infection predisposes the development of various clinical conditions as peptic ulcers, gastric adenocarcinomas and mucosa-associated lymphoid tissue lymphomas. Invasive and non-invasive diagnostic methods, each of them with their related advantages and limitations, have been applied in H. pylori detection. Moreover, bacterial resistance to antimicrobial therapy is a major challenge in the treatment of this infection, and new therapy alternatives are being tested to improve H. pylori eradication. Last but not least, the development of effective vaccines against H. pylori infection have been the aim of several research studies.
Core tip:Helicobacter pylori (H. pylori) is a bacterium that infects more than half of the world’s population. The mechanisms of such infections are complex and deeply studied. In addition, the clinical outcomes are variable and depend on both pathogen and host characteristics. Moreover, the adequate clinical management by means of proper diagnosis and effective treatment is crucial for reaching success in bacterial eradication. This article aims to provide a broad overview of H. pylori infection, from pathogenesis to clinical management.
- Citation: de Brito BB, da Silva FAF, Soares AS, Pereira VA, Santos MLC, Sampaio MM, Neves PHM, de Melo FF. Pathogenesis and clinical management of Helicobacter pylori gastric infection. World J Gastroenterol 2019; 25(37): 5578-5589
- URL: https://www.wjgnet.com/1007-9327/full/v25/i37/5578.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i37.5578
Helicobacter pylori (H. pylori) is a gram-negative bacterium that inhabits the gastric environment of more than half of the world population[1]. Studies have demonstrated that the prevalence of H. pylori-positive status varies according to different factors such as age, geographical area, living condition and socioeconomic status[2]. Oral-oral transmission seems to be the main route of H. pylori transmission. This explains the common occurrence of the infection among members of the same family, such as parents and children. In this way, the sharing of utensils during feeding seems to be important for infection establishment[3]. Fecal-oral transmission is another form of infection that occurs through ingestion of contaminated water mainly due to unsatisfactory basic sanitation conditions[4]. Therefore, it is important to highlight that increasing socioeconomic status and the improvement of living conditions are factors that greatly influence the reduction in H. pylori infection prevalence[5].
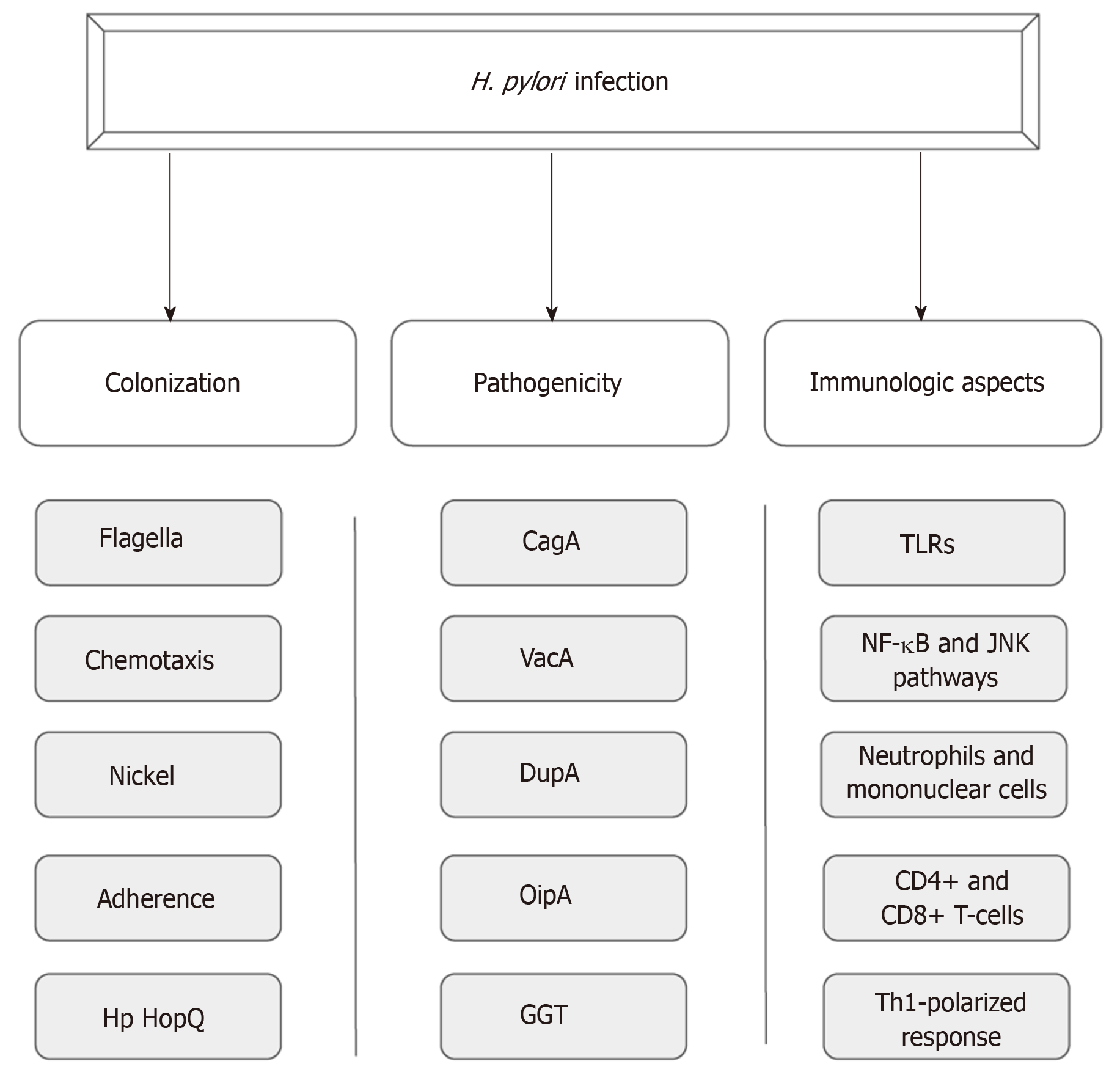
Until Warren and Marshall’s discovery of H. pylori infection in gastric mucosa, it was believed that the gastric environment was sterile because of its high acidity[6,7]. Aiming for successful colonization under such hostile conditions, the bacterium uses a wide range of mechanisms that provide improved mobility, robust adherence to epithelial cells and an enzymatic apparatus that allows the establishment of an appropriate microenvironment for infection perpetuation[8-10]. In addition, the potential of pathogenicity of this infection is provided by certain virulence factors such as cytotoxin associated antigen A (CagA), vacuolating cytotoxin (VacA), duodenal ulcer promoting gene A protein (DupA), outer inflammatory protein (OipA) and gamma-glutamyl transpeptidase (GGT)[11-15]. Moreover, the host immune system plays a crucial role in the course of the infection, likely by means of a Th1-polarized response against the pathogen (Figure 1)[16].
Although most H. pylori-positive individuals are asymptomatic, such infections predispose the development of diseases like peptic ulcers and gastric adenocarcinomas[17]. In this way, proper clinical management with a well-made diagnosis followed by effective treatment are important steps in the improvement of a patient’s clinical outcome[18]. A variety of invasive and non-invasive diagnostic methods have been used for H. pylori detection and, regarding treatment, bacterial resistance represents a major challenge in infection eradication[19,20]. In this sense, new therapy regimens as well as probiotic implementation have been tried in order to improve treatment results[21,22]. Moreover, the efforts of several researchers have been directed towards the development of vaccines against H. pylori infection.
H. pylori successful colonization of the hostile gastric environment requires special mechanisms. Firstly, after reaching the gastric environment, H. pylori uses its crucial flagellar motility for swimming in gastric content, what allows the bacterium to get in the gastric mucus layer[8]. Four to eight sheathed flagella compose the flagellar group situated on a single or on both poles of the bacterium[23-25]. H. pylori flagella can also provide different movements according to the media in which the bacterium is located. In liquid media, it presents a “swimming motility”, whereas in soft agar and on the surface of solid media, “spreading” and “swarming” movements can be observed, respectively[25]. Various studies have shown that several mutations in genes that encode specific flagellar proteins such as fliD, FlaA and FlaB impair the proper motility of H. pylori, which can reduce or even cease its capacity to colonize the gastric mucosal layer[26-28].
Besides flagella, H. pylori mobility also depends on chemotaxic action in response to different molecules, such as mucin, sodium bicarbonate, urea, sodium chloride and some specific amino acids[29,30]. At least ten H. pylori genes are related to reception, signal transduction, and processing of chemotactic stimuli[31]. Different H. pylori chemoreceptors have been described: T1pA, B, C, and D, CheA kinase and various coupling proteins. These proteins are all crucial for bacterium colonization, as demonstrated by various studies over recent years[32].
In addition, some transition metals are essential for living organisms, as they serve as cofactors for enzymatic reactions and some physiological processes, especially for enzymes that carry out the genetic material replication and transcription, attenuation of oxidative stress, and cellular energy production. In bacteria, these metals are crucial for survival and successful infection[33]. Nickel is an indispensable metal for H. pylori, since it is the cofactor for two important enzymes: urease and hydrogenase. These enzymes have a strong role in the infection process[10]. The activity of H. pylori urease contributes to the colonization of the microorganism, once this enzyme catalyzes the hydrolysis of urea to carbon dioxide and ammonia, which are buffer substances that attenuate the acidity of the stomach environment[34]. In turn, hydrogenase is part of a signaling cascade that induces an alternative airway, allowing H. pylori to use molecular hydrogen as a source of energy for its metabolism[35].
Adhesion molecules (Table 1) and surface receptors of gastric cells are also important in the interaction between bacteria and host[9,36]. One of the most well-characterized molecules is the blood group antigen binding adhesin A (BabA), which carries out specific binding to Lewis H-1 antigens[37,38]. Bacteria with high BabA expression are more virulent, and cause duodenal ulcer and gastric adenocarcinoma pathogenesis[39]. Recently, another bacterial-host interaction was identified through the adhesion of the outer membrane Hp HopQ. These adhesins bind to the CEACAMs (cell adhesion molecules related to the carcinoembryonic antigen) 1, 3, 5 and 6. That binding gives rise to cell signaling mediated by the HopQ-CEACAM interaction, which allows the translocation of CagA, the main virulence factor of H. pylori, thus increasing proinflammatory mediators in the host cell[40-42].
| Adhesin | Functions | Ref. |
| BabA | Specific binding to the b and H-1 Lewis antigens from the surface of the gastric epithelial cells | [105] |
| SabA | Binding to Lex, which is upregulated in gastric epithelial cells by H. pylori after initial colonization mediated by BabA. Also allows the adherence of the bacterium to laminin, an extracellular matrix protein | [106,107] |
| AlpA and AlpB | Mediation of adherence to gastric mucosal cells and promotion of inflammatory intracellular signaling cascades (might induce IL-8 and IL-6) | [108] |
| OipA | Adhesion to the gastric mucosa cells and promotion of proinflammatory environment (associated with IL-8 increase, mucosal damage and duodenal ulcer) | [109] |
| HopQ | Interaction with CEACAM family proteins of gastric mucosal cells, allowing CagA translocation. Might inhibits the activity of natural killer cells and T cells | [110] |
| HopZ | Interaction with undeterm ined receptors, promoting adhesion to gastric cells | [111] |
CagA is a bacterial protein that induces specific modifications in the morphology of epithelial cells while altering cell polarity, leading to a “hummingbird” phenotype. Changes in cytoskeleton associated with the development of gastric adenocarcinoma can also be triggered by this virulence factor[43]. The CagA gene is contained in a cag pathogenicity island, a region that also possesses the coding sequence of a type IV secretion system (T4SS)[11]. This bacterial structure is responsible for performing the translocation of CagA, as well as peptidoglycans, into host cells[44]. Within the host cell, CagA undergoes tyrosine phosphorylation at a Glu-Pro-Ile-Tyr-Ala (EPIYA) motif, a variable C-terminal CagA region that can be composed by different EPIYA segments (EPIYA-A, EPIYA-B, EPIYA-C and EPIYA-D)[45]. EPIYA-A and EPIYA-B segments have been found in most cagA-positive H. pylori strains, while EPIYA-C and EPIYA-D segments are related to Western and Eastern strains, respectively[46]. H. pylori strains containing EPIYA-D or at least two EPIYA-C segments in its cagA gene are associated with a higher risk of cancer development[47]. In addition, Queiroz et al[48] demonstrated in a Brazilian population that first-degree relatives of patients with gastric cancer tend to be infected by H. pylori strains containing two or more EPIYA-C segments. After phosphorylation, CagA activates SHP-2 (SH2-containing protein-tyrosine phosphatase), which promotes the cell changes mentioned above[49].
Other various virulence factors have been related to an increased H. pylori capacity to impair gastric homeostasis. Among them, VacA is a determinant protein for H. pylori pathogenicity, and its gene is present in almost all bacterial strains. VacA promotes the formation of acidic vacuoles in the cytoplasm of gastric epithelial cells. Consequently, the integrity of mitochondria, cytoplasmic membrane, and endomembranous structures is destabilized, leading cells to collapse[50]. Moreover, this protein might also promote the activation and suppression of the immune response, inducing immune tolerance and persistent H. pylori infection through its activities on T-cells and antigen-presenting cells[51]. The set of changes performed by this virulence factor adds to enhanced gastritis, as well as to ulcer and cancer development[12].
Another bacterial protein, DupA, seems to provide a higher acid resistance to the bacterium, and also might promote an increase in the production of IL-8 in the antral gastric mucosa. Enhanced IL-8 levels lead to mucosal inflammation and polymorphonuclear leukocyte infiltration, which contributes to the emergence of gastritis and duodenal ulcers[13]. Interestingly, the relation between dupA-positive H. pylori strains and duodenal ulcers has been observed in Asian countries, but not in the Western population[52]. Furthermore, our group demonstrated that the presence of functional dupA in H. pylori strains has been considered as a protective factor for gastric carcinoma development[53]. The gene products of dupA are homologues of the VirB4 ATPase, which is related to the mounting of the secretion apparatus, however, the probable association of dupA with H. pylori T4SS still needs to be better elucidated[13].
OipA, an outer membrane protein, contributes to both adhesion and increased inflammation by inducing enhanced IL-8 production[54,55]. The discovery of the relationship between OipA and the increased development of peptic ulcers and gastric cancer resulted in a larger number of studies on this H. pylori virulence factor[14]. The functional status of OipA has been described as an important factor in the outcome of the infection, since the expression of the oipA gene is regulated by a repair process called “slipped strand mispairing”, which depends on the quantity of CT dinucleotide repeats in the oipA 5’ region. Such a process determines whether oipA is nonfunctional or functional in a given bacterial strain, and the latter condition is related to increased gastric pathogenicity[56,57]. In addition, OipA might be related to changes in β-catenin signaling, cell proliferation and reduction of cell-cell junctions[58].
The enzyme GGT is a N-terminal nucleophile hydrolase also produced by H. pylori that catalyzes the conversion of glutamine into glutamate and ammonia, as well as the hydrolysis of glutathione into glutamate and cysteinylglycine[15]. Its activity leads to the production of reactive oxygen species (ROS), which, like ammonia, induce cell-cycle arrest, apoptosis and necrosis[59,60]. In addition, studies have demonstrated that this enzyme inhibits T cell proliferation and dendritic cell differentiation[61,62]. Higher GGT activity has been observed in peptic ulcer patients when compared to individuals with other gastroduodenal diseases[63].
Complex host immune responses, embracing innate and adaptive mechanisms, are induced by H. pylori infection[64,65]. Given the initial contact with the pathogen, various H. pylori antigens such as lipoteichoic acid, lipoproteins, lipopolysaccharide, HSP-60, NapA, DNA, and RNA bind to gastric cell receptors, including toll-like receptor (TLR) 1, TLR2, TLR4, TLR5, TLR6, and TLR10 located on epithelial cell membranes, and TLR9, found in intracellular vesicles[66,67]. Such interaction promotes, among other signaling pathways, NF-κB and c-jun N-terminal kinase activation, followed by proinflammatory cytokine release[68]. Besides receptor activation by pathogen-associated molecular patterns, injection of CagA through T4SS also leads to the production of cytokines, in another NF-κB-dependent process[69].
Subsequently, gastric mucosa is infiltrated by neutrophils and mononuclear cells, resulting in the production of nitric oxide and ROS[70]. Moreover, CD4+ and CD8+ T cells, components of adaptive immunity, are also recruited. A preferential activation of CD4+ cells to the detriment of CD8+ cells might occur, and a specific response is directed to the bacterium[71]. Regarding general cytokine profiles in H. pylori-positive patients, studies have suggested a Th1-polarized response, characterized by scarce IL-4 (a Th2 cytokine) and enhanced levels of gamma interferon, tumor necrosis factor, IL-1β, IL-6, IL-7, IL-8, IL-10, and IL-18[72,73]. With the exception of IL-10, which seems to play a role in limiting the inflammatory response, other increased cytokines might promote proinflammatory effects during H. pylori infection. Furthermore, we demonstrated that an increase in IL-17 is also associated with H. pylori infection, especially in adults[74]. In regard to immunoglobulin production, H. pylori-specific serum IgM antibodies can be detected in patient serum 4 wk after infection[75]. In chronic infection, serum IgA and IgG immunoglobulins are directed toward several bacterial antigens[76,77]. Such inflammation is asymptomatic in most H. pylori-positive patients, however it increases the risk of duodenal and gastric ulcer disease, as well as gastric malignancy development[78].
As a consequence of the mechanisms explained above, H. pylori-positive individuals are under increased risk of presenting various clinical manifestations[17]. The course of infection is variable and strongly dependent on host factors. Besides this, the pattern of gastric mucosal involvement is correlated with the risk of initiation and progression of different gastric disorders. Development of antral-predominant gastritis is associated with duodenal ulcers, while a corpus-predominant gastritis and multifocal atrophy tend to turn into gastric ulcers, gastric atrophy, intestinal metaplasia and gastric carcinoma[79]. Among gastrointestinal conditions, dyspepsia and peptic ulcer disease are frequently observed in clinical practice, and bacterial detection, when it is present, followed by infection eradication are crucial steps in the management of such disorders[80]. In addition, recent studies have associated H. pylori infection with a wide range of diseases. The infection was linked with the pathophysiology of neurological, dermatological, hematologic, cardiovascular, ocular, metabolic, hepatobiliary and allergic diseases[81].
Various diagnostic tests, with their specific advantages and disadvantages, are offered for H. pylori detection. Histology is the precursor method for H. pylori infection diagnosis, which, in such a technique, consists in the observation of typical bacteria associated with inflammatory reactions in the tissue slides. This method includes the use of several stains, such as Giemsa staining, and immunostaining to allow pathogen detection[19]. Another important H. pylori diagnostic method, the rapid urease test (RUT), detects an increase in reagent pH after the addition of a biopsy specimen containing H. pylori to the reagent. Such pH variation is caused by the conversion of the urea test reagent into ammonia. RUT is a relatively cheap, quick, easy, specific and widely available test[82]. Polymerase chain reaction (PCR) has also been applied for H. pylori detection. Al-Moayad et al[83] concluded that standardized PCR allows an accuracy superior to that observed in RUT, with improved detection in specimens with lower bacterial charge[84]. However, the necessity of endoscopy is an important limitation of the three methods mentioned above, and the advances in non-invasive diagnostic techniques have strengthened the idea of prioritizing the use of diagnostic alternatives for which endoscopy is dispensable.
The urea breath test (UBT) is now the main non-invasive method for such a diagnosis, gradually taking the place of RUT as the most suitable method for H. pylori detection. This test is based on the mechanism of bacterium degradation of 13C or 14C-labeled urea into CO2, which can be measured in the exhaled air using a mass or infrared spectrometer[85]. A Brazilian study[86] evaluated the use of a locally manufactured isotope in UBT, trying to reduce the importation costs of this substance, which is considered a limitation for the performance of this method in many countries. The assay concluded that the substrate manufactured in Brazil with reduced costs had similar performance when compared to the one imported from foreign countries. Such a reduction in the costs of this substrate can contribute to the dissemination of UBT use around the world.
A less expensive option for UBT, stool antigen tests (SATs), are good alternatives for H. pylori diagnosis. SATs can be made by means of enzyme immunoassay or immuno-chromatography[87]. In addition, a new promising non-invasive method, the urine test for the diagnosis of H. pylori infection, has been largely studied as an alternative. A meta-analysis from 2017[88], which included 23 studies, showed that testing for antibodies in urine samples might be a good diagnostic option. However, further studies are necessary to confirm the accuracy of this method. Finally, new strategies for serologic diagnosis of H. pylori infection have been developed through the discovery of specific serological markers. A recent study evaluated the accuracy of the “hook-associated protein 2 homologue”, FliD, as a marker of this infection. The use of the Flid ELISA method in the detection of H. pylori infection provided high specificity (99%) and sensibility (97%). Moreover, this method presents a simple technique at low cost[89].
There is not a universally accepted regimen for the treatment of H. pylori infection. However, all of them target the regressing symptomatology and healing of the mucosa damaged by the infection process[20]. Since the 1997 Maarstricht consensus, the standard triple therapy with proton pump inhibitors (PPI) in standard dose, clarithromycin (500 mg), and amoxicillin (1 g) twice daily for 7 d have been employed in most countries as a first-line regimen to eradicate H. pylori. The quadruple therapy, with addition of bismuth (120 mg) to the regimen, has also been used as a first-line regimen[90]. However, the increase in microbial resistance to clarithromycin, whose prevalence varies with time and geographic region, is leading to changes in the therapeutic regimen. The indiscriminate use of azithromycin and erythromycin in the treatment of respiratory infections and cross-resistance among macrolide antibiotics may be responsible for the increased microbial resistance to clarithromycin[91]. As a consequence, longer therapeutic regimens have been used for H. pylori eradication[92]. In areas with high clarithromycin resistance, the addition of metronidazole (500 mg) concomitantly with PPI, clarithromycin and amoxicillin twice daily for 5 d, characterizing a quadruple therapy, improves the efficacy of the treatment, with an intention-to-treat higher than 90%[21]. Moreover, in regions with clarithromycin resistance above 15%-20%, and quinolone resistance below 10%, clarithromycin could be substituted by levofloxacin (250/500 mg) in triple therapy. Such exchange increases the per-protocol and intention-to-treat eradication rates of the treatment[21,93]. In addition, the use of hybrid therapy has been suggested as an alternative to the standard approaches in some countries. This therapeutic scheme consists of administering PPI and amoxicillin for 14 d, and then adding both clarithromycin and nitroimidazole as a quadruple therapy for the final 7 d[94]. Finally, faced with such a situation, studies have proposed the use of tailored therapy as a possible new first-line treatment. Conducting tests for identifying the susceptibility of the bacterial strains to the different regimens appears to be a great alternative for bacterial eradication[95].
Probiotics are being used in the prevention and treatment of many gastrointestinal infections, so it is strongly believed that they might be useful for the treatment of H. pylori infection[20]. Research about the use of probiotics for this purpose are typically divided into treatments with and without antibiotics, and data available in the literature are still controversial[96]. Zagari et al[97] showed that probiotic supplementation did not improve either the efficacy or tolerability of the treatment, regardless of the species of microorganism used. On the other hand, some studies suggest that probiotics help in the restoration of the intestinal microbiota disturbed by antibiotics, leading to a decrease in side effects and, consequently, increased adherence to treatment, corroborating successful therapy[98]. However, no effect has been observed against H. pylori infection using treatment with probiotics alone. Other studies claim that the use of probiotics in combination with antimicrobial therapy has a potentiating effect by increasing eradication rates; however, the relationship with adverse effects is still uncertain[99]. The beneficial effects of probiotics on this infection may be associated with immunological and non-immunological mechanisms, such as substance production, gastric mucosal strengthening, and regulation of immune function[100]. As seen, the role of probiotics in this infection eradication is not well-established and consolidated, and the use of different species of microorganisms, doses and research methods contribute to such uncertainties.
The development of vaccines is a promising alternative that targets the prophylaxis and/or the treatment of the infection (Table 2)[101]. Recently, studies have focused on the development of reverse vaccines with the help of bioinformatics, and five antigenic epitopes have been prioritized as potential vaccine candidates: babA, sabA, fecA, vacA and omp16[20]. However, their development has been a major challenge in the H. pylori field, since many studies have not been successful in experimental models. In contrast, a randomized phase 3 study with children has been conducted in China, which was efficacious and safe in providing oral vaccines with recombinant B urease against H. pylori[102]. However, a more accurate evaluation of its long-term effect is required. In another study by Wang et al[103], intramuscular administration was compared with oral administration of the multi-epitope vaccine, evidencing a better protection rate by oral administration. The development of nanovaccines is also being explored, and presents a nice potential to become an excellent alternative in triggering an effective immunological response against H. pylori infection[90,104].
| Vaccine | Prophylactic | Therapeutic |
| EpiVax/Helicobacter pylori vaccine | Yes | Yes |
| Helicovaxor® | Yes | No |
| Imevax/IMX101 | Yes | No |
| Wuhu Kangwei Biological Technology | Yes | No |
Although the knowledge about the different H. pylori infection characteristics have been expanded since its discovery, much still needs to be done for a broader understanding of its underlying mechanisms. Furthermore, the new diagnostic methods should be better explored in order to reduce health expenditure and to provide less invasive diagnostic alternatives to patients. Finally, the growing resistance of H. pylori to antimicrobial therapy alerts to the necessity of developing satisfactory strategies for bacterial eradication, as well as vaccine implementation aiming at reducing infection prevalence.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Amiri M, Day AS, Gazouli M, Ierardi E, Talebi Bezmin Abadi A S-Editor: Yan JP L-Editor: Filipodia E-Editor: Ma YJ
| 1. | Alzahrani S, Lina TT, Gonzalez J, Pinchuk IV, Beswick EJ, Reyes VE. Effect of Helicobacter pylori on gastric epithelial cells. World J Gastroenterol. 2014;20:12767-12780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 105] [Cited by in RCA: 128] [Article Influence: 11.6] [Reference Citation Analysis (6)] |
| 2. | Peleteiro B, Bastos A, Ferro A, Lunet N. Prevalence of Helicobacter pylori infection worldwide: A systematic review of studies with national coverage. Dig Dis Sci. 2014;59:1698-1709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 228] [Article Influence: 20.7] [Reference Citation Analysis (7)] |
| 3. | Urita Y, Watanabe T, Kawagoe N, Takemoto I, Tanaka H, Kijima S, Kido H, Maeda T, Sugasawa Y, Miyazaki T, Honda Y, Nakanishi K, Shimada N, Nakajima H, Sugimoto M, Urita C. Role of infected grandmothers in transmission of Helicobacter pylori to children in a Japanese rural town. J Paediatr Child Health. 2013;49:394-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (3)] |
| 4. | Goh KL, Chan WK, Shiota S, Yamaoka Y. Epidemiology of Helicobacter pylori infection and public health implications. Helicobacter. 2011;16 Suppl 1:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 254] [Article Influence: 18.1] [Reference Citation Analysis (1)] |
| 5. | Laszewicz W, Iwańczak F, Iwańczak B; Task Force of the Polish Society of Gastroenterology; Task Force of the Polish Society of Gastroenterology. Seroprevalence of Helicobacter pylori infection in Polish children and adults depending on socioeconomic status and living conditions. Adv Med Sci. 2014;59:147-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 6. | Warren JR, Marshall B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;1:1273-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 454] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 7. | Wroblewski LE, Peek RM. Helicobacter pylori, Cancer, and the Gastric Microbiota. Adv Exp Med Biol. 2016;908:393-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 8. | Eaton KA, Morgan DR, Krakowka S. Motility as a factor in the colonisation of gnotobiotic piglets by Helicobacter pylori. J Med Microbiol. 1992;37:123-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 207] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
| 9. | Alm RA, Bina J, Andrews BM, Doig P, Hancock RE, Trust TJ. Comparative genomics of Helicobacter pylori: Analysis of the outer membrane protein families. Infect Immun. 2000;68:4155-4168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 246] [Article Influence: 9.8] [Reference Citation Analysis (1)] |
| 10. | Camilo V, Sugiyama T, Touati E. Pathogenesis of Helicobacter pylori infection. Helicobacter. 2017;22 Suppl 1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 110] [Article Influence: 13.8] [Reference Citation Analysis (3)] |
| 11. | Fischer W. Assembly and molecular mode of action of the Helicobacter pylori Cag type IV secretion apparatus. FEBS J. 2011;278:1203-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 12. | Boquet P, Ricci V. Intoxication strategy of Helicobacter pylori VacA toxin. Trends Microbiol. 2012;20:165-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 13. | Lu H, Hsu PI, Graham DY, Yamaoka Y. Duodenal ulcer promoting gene of Helicobacter pylori. Gastroenterology. 2005;128:833-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 223] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 14. | Yamaoka Y, Kwon DH, Graham DY. A M(r) 34,000 proinflammatory outer membrane protein (oipA) of Helicobacter pylori. Proc Natl Acad Sci U S A. 2000;97:7533-7538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 318] [Article Influence: 12.7] [Reference Citation Analysis (2)] |
| 15. | Shibayama K, Wachino J, Arakawa Y, Saidijam M, Rutherford NG, Henderson PJ. Metabolism of glutamine and glutathione via gamma-glutamyltranspeptidase and glutamate transport in Helicobacter pylori: Possible significance in the pathophysiology of the organism. Mol Microbiol. 2007;64:396-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 93] [Article Influence: 5.2] [Reference Citation Analysis (4)] |
| 16. | Bamford KB, Fan X, Crowe SE, Leary JF, Gourley WK, Luthra GK, Brooks EG, Graham DY, Reyes VE, Ernst PB. Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenterology. 1998;114:482-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 425] [Article Influence: 15.7] [Reference Citation Analysis (1)] |
| 17. | Malfertheiner P, Venerito M, Schulz C. Helicobacter pylori Infection: New Facts in Clinical Management. Curr Treat Options Gastroenterol. 2018;16:605-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Abadi AT, Kusters JG. Management of Helicobacter pylori infections. BMC Gastroenterol. 2016;16:94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Patel SK, Pratap CB, Jain AK, Gulati AK, Nath G. Diagnosis of Helicobacter pylori: What should be the gold standard? World J Gastroenterol. 2014;20:12847-12859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 146] [Cited by in RCA: 181] [Article Influence: 16.5] [Reference Citation Analysis (3)] |
| 20. | Safavi M, Sabourian R, Foroumadi A. Treatment of Helicobacter pylori infection: Current and future insights. World J Clin Cases. 2016;4:5-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 88] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (1)] |
| 21. | Federico A, Gravina AG, Miranda A, Loguercio C, Romano M. Eradication of Helicobacter pylori infection: Which regimen first? World J Gastroenterol. 2014;20:665-672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Ayala G, Escobedo-Hinojosa WI, de la Cruz-Herrera CF, Romero I. Exploring alternative treatments for Helicobacter pylori infection. World J Gastroenterol. 2014;20:1450-1469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 96] [Cited by in RCA: 92] [Article Influence: 8.4] [Reference Citation Analysis (1)] |
| 23. | Versalovic J, Carroll KC, Funke G, Jorgensen JH, Landry ML, Warnock DL. Manual of clinical microbiology. 10th ed. American Society for Microbiology. 2011;900-915. [DOI] [Full Text] |
| 24. | Ottemann KM, Lowenthal AC. Helicobacter pylori uses motility for initial colonization and to attain robust infection. Infect Immun. 2002;70:1984-1990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 234] [Article Influence: 10.2] [Reference Citation Analysis (3)] |
| 25. | Gu H. Role of Flagella in the Pathogenesis of Helicobacter pylori. Curr Microbiol. 2017;74:863-869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 124] [Article Influence: 15.5] [Reference Citation Analysis (1)] |
| 26. | Clyne M, Ocroinin T, Suerbaum S, Josenhans C, Drumm B. Adherence of isogenic flagellum-negative mutants of Helicobacter pylori and Helicobacter mustelae to human and ferret gastric epithelial cells. Infect Immun. 2000;68:4335-4339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (2)] |
| 27. | Eaton KA, Suerbaum S, Josenhans C, Krakowka S. Colonization of gnotobiotic piglets by Helicobacter pylori deficient in two flagellin genes. Infect Immun. 1996;64:2445-2448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 28. | Kim JS, Chang JH, Chung SI, Yum JS. Molecular cloning and characterization of the Helicobacter pylori fliD gene, an essential factor in flagellar structure and motility. J Bacteriol. 1999;181:6969-6976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 29. | Worku ML, Karim QN, Spencer J, Sidebotham RL. Chemotactic response of Helicobacter pylori to human plasma and bile. J Med Microbiol. 2004;53:807-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (6)] |
| 30. | Mizote T, Yoshiyama H, Nakazawa T. Urease-independent chemotactic responses of Helicobacter pylori to urea, urease inhibitors, and sodium bicarbonate. Infect Immun. 1997;65:1519-1521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (2)] |
| 31. | Alm RA, Ling LS, Moir DT, King BL, Brown ED, Doig PC, Smith DR, Noonan B, Guild BC, deJonge BL, Carmel G, Tummino PJ, Caruso A, Uria-Nickelsen M, Mills DM, Ives C, Gibson R, Merberg D, Mills SD, Jiang Q, Taylor DE, Vovis GF, Trust TJ. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature. 1999;397:176-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1387] [Cited by in RCA: 1358] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 32. | Aizawa SI, Harwood CS, Kadner RJ. Signaling components in bacterial locomotion and sensory reception. J Bacteriol. 2000;182:1459-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Becker KW, Skaar EP. Metal limitation and toxicity at the interface between host and pathogen. FEMS Microbiol Rev. 2014;38:1235-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 176] [Article Influence: 16.0] [Reference Citation Analysis (3)] |
| 34. | Eaton KA, Brooks CL, Morgan DR, Krakowka S. Essential role of urease in pathogenesis of gastritis induced by Helicobacter pylori in gnotobiotic piglets. Infect Immun. 1991;59:2470-2475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Olson JW, Maier RJ. Molecular hydrogen as an energy source for Helicobacter pylori. Science. 2002;298:1788-1790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 217] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 36. | Kalali B, Mejías-Luque R, Javaheri A, Gerhard M. H. pylori virulence factors: Influence on immune system and pathology. Mediators Inflamm. 2014;2014:426309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 37. | Oliveira AG, Santos A, Guerra JB, Rocha GA, Rocha AM, Oliveira CA, Cabral MM, Nogueira AM, Queiroz DM. babA2- and cagA-positive Helicobacter pylori strains are associated with duodenal ulcer and gastric carcinoma in Brazil. J Clin Microbiol. 2003;41:3964-3966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 38. | Björnham O, Bugaytsova J, Borén T, Schedin S. Dynamic force spectroscopy of the Helicobacter pylori BabA-Lewis b binding. Biophys Chem. 2009;143:102-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 39. | Mahdavi J, Sondén B, Hurtig M, Olfat FO, Forsberg L, Roche N, Angstrom J, Larsson T, Teneberg S, Karlsson KA, Altraja S, Wadström T, Kersulyte D, Berg DE, Dubois A, Petersson C, Magnusson KE, Norberg T, Lindh F, Lundskog BB, Arnqvist A, Hammarström L, Borén T. Helicobacter pylori SabA adhesin in persistent infection and chronic inflammation. Science. 2002;297:573-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 665] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 40. | Javaheri A, Kruse T, Moonens K, Mejías-Luque R, Debraekeleer A, Asche CI, Tegtmeyer N, Kalali B, Bach NC, Sieber SA, Hill DJ, Königer V, Hauck CR, Moskalenko R, Haas R, Busch DH, Klaile E, Slevogt H, Schmidt A, Backert S, Remaut H, Singer BB, Gerhard M. Helicobacter pylori adhesin HopQ engages in a virulence-enhancing interaction with human CEACAMs. Nat Microbiol. 2016;2:16189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 183] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 41. | Königer V, Holsten L, Harrison U, Busch B, Loell E, Zhao Q, Bonsor DA, Roth A, Kengmo-Tchoupa A, Smith SI, Mueller S, Sundberg EJ, Zimmermann W, Fischer W, Hauck CR, Haas R. Helicobacter pylori exploits human CEACAMs via HopQ for adherence and translocation of CagA. Nat Microbiol. 2016;2:16188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 131] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 42. | Zhao Q, Busch B, Jiménez-Soto LF, Ishikawa-Ankerhold H, Massberg S, Terradot L, Fischer W, Haas R. Integrin but not CEACAM receptors are dispensable for Helicobacter pylori CagA translocation. PLoS Pathog. 2018;14:e1007359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 43. | Tsutsumi R, Higashi H, Higuchi M, Okada M, Hatakeyama M. Attenuation of Helicobacter pylori CagA x SHP-2 signaling by interaction between CagA and C-terminal Src kinase. J Biol Chem. 2003;278:3664-3670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 232] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 44. | Suarez G, Romero-Gallo J, Sierra JC, Piazuelo MB, Krishna US, Gomez MA, Wilson KT, Peek RM. Genetic Manipulation of Helicobacter pylori Virulence Function by Host Carcinogenic Phenotypes. Cancer Res. 2017;77:2401-2412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 45. | Kanada R, Uchida T, Tsukamoto Y, Nguyen LT, Hijiya N, Matsuura K, Kodama M, Okimoto T, Murakami K, Fujioka T, Yanagisawa S, Moriyama M. Genotyping of the cagA gene of Helicobacter pylori on immunohistochemistry with East Asian CagA-specific antibody. Pathol Int. 2008;58:218-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 46. | Yamaoka Y, Osato MS, Sepulveda AR, Gutierrez O, Figura N, Kim JG, Kodama T, Kashima K, Graham DY. Molecular epidemiology of Helicobacter pylori: Separation of H. pylori from East Asian and non-Asian countries. Epidemiol Infect. 2000;124:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 90] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 47. | Basso D, Zambon CF, Letley DP, Stranges A, Marchet A, Rhead JL, Schiavon S, Guariso G, Ceroti M, Nitti D, Rugge M, Plebani M, Atherton JC. Clinical relevance of Helicobacter pylori cagA and vacA gene polymorphisms. Gastroenterology. 2008;135:91-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 294] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 48. | Queiroz DM, Silva CI, Goncalves MH, Braga-Neto MB, Fialho AB, Fialho AM, Rocha GA, Rocha AM, Batista SA, Guerrant RL, Lima AA, Braga LL. Higher frequency of cagA EPIYA-C phosphorylation sites in H. pylori strains from first-degree relatives of gastric cancer patients. BMC Gastroenterol. 2012;12:107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 49. | Higashi H, Tsutsumi R, Muto S, Sugiyama T, Azuma T, Asaka M, Hatakeyama M. SHP-2 tyrosine phosphatase as an intracellular target of Helicobacter pylori CagA protein. Science. 2002;295:683-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 755] [Cited by in RCA: 782] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 50. | Atherton JC, Peek RM, Tham KT, Cover TL, Blaser MJ. Clinical and pathological importance of heterogeneity in vacA, the vacuolating cytotoxin gene of Helicobacter pylori. Gastroenterology. 1997;112:92-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 406] [Article Influence: 14.5] [Reference Citation Analysis (1)] |
| 51. | Djekic A, Müller A. The Immunomodulator VacA Promotes Immune Tolerance and Persistent Helicobacter pylori Infection through Its Activities on T-Cells and Antigen-Presenting Cells. Toxins (Basel). 2016;8:pii: E187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 52. | Yamaoka Y. Roles of the plasticity regions of Helicobacter pylori in gastroduodenal pathogenesis. J Med Microbiol. 2008;57:545-553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 53. | Queiroz DM, Rocha GA, Rocha AM, Moura SB, Saraiva IE, Gomes LI, Soares TF, Melo FF, Cabral MM, Oliveira CA. dupA polymorphisms and risk of Helicobacter pylori-associated diseases. Int J Med Microbiol. 2011;301:225-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 54. | Farzi N, Yadegar A, Aghdaei HA, Yamaoka Y, Zali MR. Genetic diversity and functional analysis of oipA gene in association with other virulence factors among Helicobacter pylori isolates from Iranian patients with different gastric diseases. Infect Genet Evol. 2018;60:26-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 55. | Miftahussurur M, Yamaoka Y, Graham DY. Helicobacter pylori as an oncogenic pathogen, revisited. Expert Rev Mol Med. 2017;19:e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 56. | Liu J, He C, Chen M, Wang Z, Xing C, Yuan Y. Association of presence/absence and on/off patterns of Helicobacter pylori oipA gene with peptic ulcer disease and gastric cancer risks: A meta-analysis. BMC Infect Dis. 2013;13:555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 57. | Sallas ML, Dos Santos MP, Orcini WA, David ÉB, Peruquetti RL, Payão SLM, Rasmussen LT. Status (on/off) of oipA gene: Their associations with gastritis and gastric cancer and geographic origins. Arch Microbiol. 2019;201:93-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 58. | Franco AT, Johnston E, Krishna U, Yamaoka Y, Israel DA, Nagy TA, Wroblewski LE, Piazuelo MB, Correa P, Peek RM. Regulation of gastric carcinogenesis by Helicobacter pylori virulence factors. Cancer Res. 2008;68:379-387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 239] [Cited by in RCA: 234] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 59. | Shibayama K, Kamachi K, Nagata N, Yagi T, Nada T, Doi Y, Shibata N, Yokoyama K, Yamane K, Kato H, Iinuma Y, Arakawa Y. A novel apoptosis-inducing protein from Helicobacter pylori. Mol Microbiol. 2003;47:443-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 75] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 60. | Kim KM, Lee SG, Kim JM, Kim DS, Song JY, Kang HL, Lee WK, Cho MJ, Rhee KH, Youn HS, Baik SC. Helicobacter pylori gamma-glutamyltranspeptidase induces cell cycle arrest at the G1-S phase transition. J Microbiol. 2010;48:372-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 61. | Schmees C, Prinz C, Treptau T, Rad R, Hengst L, Voland P, Bauer S, Brenner L, Schmid RM, Gerhard M. Inhibition of T-cell proliferation by Helicobacter pylori gamma-glutamyl transpeptidase. Gastroenterology. 2007;132:1820-1833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 141] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 62. | Oertli M, Noben M, Engler DB, Semper RP, Reuter S, Maxeiner J, Gerhard M, Taube C, Müller A. Helicobacter pylori γ-glutamyl transpeptidase and vacuolating cytotoxin promote gastric persistence and immune tolerance. Proc Natl Acad Sci U S A. 2013;110:3047-3052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 186] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 63. | Gong M, Ling SS, Lui SY, Yeoh KG, Ho B. Helicobacter pylori gamma-glutamyl transpeptidase is a pathogenic factor in the development of peptic ulcer disease. Gastroenterology. 2010;139:564-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 64. | Yoshikawa T, Naito Y. The role of neutrophils and inflammation in gastric mucosal injury. Free Radic Res. 2000;33:785-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 64] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 65. | Crabtree JE, Mahony MJ, Taylor JD, Heatley RV, Littlewood JM, Tompkins DS. Immune responses to Helicobacter pylori in children with recurrent abdominal pain. J Clin Pathol. 1991;44:768-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 84] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 66. | Nagashima H, Iwatani S, Cruz M, Jiménez Abreu JA, Uchida T, Mahachai V, Vilaichone RK, Graham DY, Yamaoka Y. Toll-like Receptor 10 in Helicobacter pylori Infection. J Infect Dis. 2015;212:1666-1676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 67. | Smith SM. Role of Toll-like receptors in Helicobacter pylori infection and immunity. World J Gastrointest Pathophysiol. 2014;5:133-146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 85] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 68. | Smith MF, Mitchell A, Li G, Ding S, Fitzmaurice AM, Ryan K, Crowe S, Goldberg JB. Toll-like receptor (TLR) 2 and TLR5, but not TLR4, are required for Helicobacter pylori-induced NF-kappa B activation and chemokine expression by epithelial cells. J Biol Chem. 2003;278:32552-32560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 327] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 69. | Alandiyjany MN, Croxall NJ, Grove JI, Delahay RM. A role for the tfs3 ICE-encoded type IV secretion system in pro-inflammatory signalling by the Helicobacter pylori Ser/Thr kinase, CtkA. PLoS One. 2017;12:e0182144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 70. | Wilson KT, Ramanujam KS, Mobley HL, Musselman RF, James SP, Meltzer SJ. Helicobacter pylori stimulates inducible nitric oxide synthase expression and activity in a murine macrophage cell line. Gastroenterology. 1996;111:1524-1533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 134] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 71. | Lundgren A, Suri-Payer E, Enarsson K, Svennerholm AM, Lundin BS. Helicobacter pylori-specific CD4+ CD25high regulatory T cells suppress memory T-cell responses to H. pylori in infected individuals. Infect Immun. 2003;71:1755-1762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 246] [Article Influence: 11.2] [Reference Citation Analysis (1)] |
| 72. | Crabtree JE, Shallcross TM, Heatley RV, Wyatt JI. Mucosal tumour necrosis factor alpha and interleukin-6 in patients with Helicobacter pylori associated gastritis. Gut. 1991;32:1473-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 397] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 73. | Lindholm C, Quiding-Järbrink M, Lönroth H, Hamlet A, Svennerholm AM. Local cytokine response in Helicobacter pylori-infected subjects. Infect Immun. 1998;66:5964-5971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 74. | Freire de Melo F, Rocha AM, Rocha GA, Pedroso SH, de Assis Batista S, Fonseca de Castro LP, Carvalho SD, Bittencourt PF, de Oliveira CA, Corrêa-Oliveira R, Magalhães Queiroz DM. A regulatory instead of an IL-17 T response predominates in Helicobacter pylori-associated gastritis in children. Microbes Infect. 2012;14:341-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 75. | Nurgalieva ZZ, Conner ME, Opekun AR, Zheng CQ, Elliott SN, Ernst PB, Osato M, Estes MK, Graham DY. B-cell and T-cell immune responses to experimental Helicobacter pylori infection in humans. Infect Immun. 2005;73:2999-3006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (1)] |
| 76. | Mattsson A, Quiding-Järbrink M, Lönroth H, Hamlet A, Ahlstedt I, Svennerholm A. Antibody-secreting cells in the stomachs of symptomatic and asymptomatic Helicobacter pylori-infected subjects. Infect Immun. 1998;66:2705-2712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 77. | Perez-Perez GI, Dworkin BM, Chodos JE, Blaser MJ. Campylobacter pylori antibodies in humans. Ann Intern Med. 1988;109:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 310] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 78. | Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1848] [Cited by in RCA: 1907] [Article Influence: 82.9] [Reference Citation Analysis (3)] |
| 79. | Malfertheiner P, Megraud F, O'Morain CA, Atherton J, Axon AT, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T, El-Omar EM, Kuipers EJ; European Helicobacter Study Group. Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut. 2012;61:646-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1719] [Cited by in RCA: 1588] [Article Influence: 122.2] [Reference Citation Analysis (5)] |
| 80. | Liu L, Gao H, Wang H, Yu W, Zhu K, Zhang Y, Guo J. Comparison of Esophageal Function Tests to Investigate the Effect of Helicobacter Pylori Infection on Gastroesophageal Reflux Disease (GERD). Med Sci Monit. 2018;24:4791-4797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 81. | Gravina AG, Zagari RM, De Musis C, Romano L, Loguercio C, Romano M. Helicobacter pylori and extragastric diseases: A review. World J Gastroenterol. 2018;24:3204-3221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 244] [Cited by in RCA: 202] [Article Influence: 28.9] [Reference Citation Analysis (6)] |
| 82. | Wang YK, Kuo FC, Liu CJ, Wu MC, Shih HY, Wang SS, Wu JY, Kuo CH, Huang YK, Wu DC. Diagnosis of Helicobacter pylori infection: Current options and developments. World J Gastroenterol. 2015;21:11221-11235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 218] [Cited by in RCA: 264] [Article Influence: 26.4] [Reference Citation Analysis (8)] |
| 83. | Al-Moayad EE, Alghalibi SM, Al-Shamahy HA, Nasher AT, Al-Hebshi NN. Normalized real-time PCR for diagnosis of H. pylori infection. Qatar Med J. 2014;2014:123-129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 84. | Costamagna G, Zullo A, Bizzotto A, Spada C, Hassan C, Riccioni ME, Marmo C, Strangio G, Di Rienzo TA, Cammarota G, Gasbarrini A, Repici A. Real-time diagnosis of H. pylori infection during endoscopy: Accuracy of an innovative tool (EndoFaster). United European Gastroenterol J. 2016;4:339-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 85. | Cutler AF, Prasad VM. Long-term follow-up of Helicobacter pylori serology after successful eradication. Am J Gastroenterol. 1996;91:85-88. [PubMed] |
| 86. | Coelho LG, Sant'Ana CR, Oliveira RB, Cezar RCE, Araujo ACC, Silva RCTD, Trindade OR, Coelho MC, Ferrioli E, Bendassolli JA. Performance of the 13C-urea breath test for the diagnosis of H. pylori infection using a substrate synthesized in Brazil: A preliminary study. Clinics (Sao Paulo). 2018;73:e16553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 87. | Shimoyama T. Stool antigen tests for the management of Helicobacter pylori infection. World J Gastroenterol. 2013;19:8188-8191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 95] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 88. | Gong Y, Li Q, Yuan Y. Accuracy of testing for anti-Helicobacter pylori IgG in urine for H. pylori infection diagnosis: A systematic review and meta-analysis. BMJ Open. 2017;7:e013248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 89. | Khalifeh Gholi M, Kalali B, Formichella L, Göttner G, Shamsipour F, Zarnani AH, Hosseini M, Busch DH, Shirazi MH, Gerhard M. Helicobacter pylori FliD protein is a highly sensitive and specific marker for serologic diagnosis of H. pylori infection. Int J Med Microbiol. 2013;303:618-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 90. | Malfertheiner P, Mégraud F, O'Morain C, Bell D, Bianchi Porro G, Deltenre M, Forman D, Gasbarrini G, Jaup B, Misiewicz JJ, Pajares J, Quina M, Rauws E. Current European concepts in the management of Helicobacter pylori infection--the Maastricht Consensus Report. The European Helicobacter Pylori Study Group (EHPSG). Eur J Gastroenterol Hepatol. 1997;9:1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 160] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 91. | Wani FA, Bashir G, Khan MA, Zargar SA, Rasool Z, Qadri Q. Antibiotic resistance in Helicobacter pylori: A mutational analysis from a tertiary care hospital in Kashmir, India. Indian J Med Microbiol. 2018;36:265-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 92. | Hu Y, Zhu Y, Lu NH. Novel and Effective Therapeutic Regimens for Helicobacter pylori in an Era of Increasing Antibiotic Resistance. Front Cell Infect Microbiol. 2017;7:168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 107] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 93. | Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am J Gastroenterol. 2017;112:212-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 744] [Cited by in RCA: 1017] [Article Influence: 127.1] [Reference Citation Analysis (1)] |
| 94. | Hsu PI, Wu DC, Wu JY, Graham DY. Modified sequential Helicobacter pylori therapy: Proton pump inhibitor and amoxicillin for 14 days with clarithromycin and metronidazole added as a quadruple (hybrid) therapy for the final 7 days. Helicobacter. 2011;16:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 147] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 95. | Ierardi E, Giorgio F, Iannone A, Losurdo G, Principi M, Barone M, Pisani A, Di Leo A. Noninvasive molecular analysis of Helicobacter pylori: Is it time for tailored first-line therapy? World J Gastroenterol. 2017;23:2453-2458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 96. | Ierardi E, Giorgio F, Losurdo G, Di Leo A, Principi M. How antibiotic resistances could change Helicobacter pylori treatment: A matter of geography? World J Gastroenterol. 2013;19:8168-8180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 80] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 97. | Zagari RM, Romiti A, Ierardi E, Gravina AG, Panarese A, Grande G, Savarino E, Maconi G, Stasi E, Eusebi LH, Farinati F, Conigliaro R, Bazzoli F, Romano M. The "three-in-one" formulation of bismuth quadruple therapy for Helicobacter pylori eradication with or without probiotics supplementation: Efficacy and safety in daily clinical practice. Helicobacter. 2018;23:e12502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 98. | Zhu XY, Liu F. Probiotics as an adjuvant treatment in Helicobacter pylori eradication therapy. J Dig Dis. 2017;18:195-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 99. | Dang Y, Reinhardt JD, Zhou X, Zhang G. The effect of probiotics supplementation on Helicobacter pylori eradication rates and side effects during eradication therapy: A meta-analysis. PLoS One. 2014;9:e111030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 107] [Article Influence: 9.7] [Reference Citation Analysis (1)] |
| 100. | Lv Z, Wang B, Zhou X, Wang F, Xie Y, Zheng H, Lv N. Efficacy and safety of probiotics as adjuvant agents for Helicobacter pylori infection: A meta-analysis. Exp Ther Med. 2015;9:707-716. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 101. | Sutton P, Boag JM. Status of vaccine research and development for Helicobacter pylori. Vaccine. 2018;pii:S0264-410X(18)30017-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 102. | Zeng M, Mao XH, Li JX, Tong WD, Wang B, Zhang YJ, Guo G, Zhao ZJ, Li L, Wu DL, Lu DS, Tan ZM, Liang HY, Wu C, Li DH, Luo P, Zeng H, Zhang WJ, Zhang JY, Guo BT, Zhu FC, Zou QM. Efficacy, safety, and immunogenicity of an oral recombinant Helicobacter pylori vaccine in children in China: A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;386:1457-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 209] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 103. | Wang B, Pan X, Wang H, Zhou Y, Zhu J, Yang J, Li W. Immunological response of recombinant H. pylori multi-epitope vaccine with different vaccination strategies. Int J Clin Exp Pathol. 2014;7:6559-6566. [PubMed] |
| 104. | Milani M, Sharifi Y, Rahmati-Yamchi M, Somi MH, Akbarzadeh A. Immunology and vaccines and nanovaccines for Helicobacter pylori infection. Expert Rev Vaccines. 2015;14:833-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 105. | Rad R, Gerhard M, Lang R, Schöniger M, Rösch T, Schepp W, Becker I, Wagner H, Prinz C. The Helicobacter pylori blood group antigen-binding adhesin facilitates bacterial colonization and augments a nonspecific immune response. J Immunol. 2002;168:3033-3041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 121] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 106. | Sakamoto S, Watanabe T, Tokumaru T, Takagi H, Nakazato H, Lloyd KO. Expression of Lewisa, Lewisb, Lewisx, Lewisy, siayl-Lewisa, and sialyl-Lewisx blood group antigens in human gastric carcinoma and in normal gastric tissue. Cancer Res. 1989;49:745-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 107. | Walz A, Odenbreit S, Mahdavi J, Borén T, Ruhl S. Identification and characterization of binding properties of Helicobacter pylori by glycoconjugate arrays. Glycobiology. 2005;15:700-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 91] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 108. | Lu H, Wu JY, Beswick EJ, Ohno T, Odenbreit S, Haas R, Reyes VE, Kita M, Graham DY, Yamaoka Y. Functional and intracellular signaling differences associated with the Helicobacter pylori AlpAB adhesin from Western and East Asian strains. J Biol Chem. 2007;282:6242-6254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 109. | Yamaoka Y, Kikuchi S, el-Zimaity HM, Gutierrez O, Osato MS, Graham DY. Importance of Helicobacter pylori oipA in clinical presentation, gastric inflammation, and mucosal interleukin 8 production. Gastroenterology. 2002;123:414-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 229] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 110. | Gur C, Maalouf N, Gerhard M, Singer BB, Emgård J, Temper V, Neuman T, Mandelboim O, Bachrach G. The Helicobacter pylori HopQ outermembrane protein inhibits immune cell activities. Oncoimmunology. 2019;8:e1553487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (2)] |
| 111. | Acio-Pizzarello CR, Acio AA, Choi EJ, Bond K, Kim J, Kenan AC, Chen J, Forsyth MH. Determinants of the regulation of Helicobacter pylori adhesins include repeat sequences in both promoter and coding regions as well as the two-component system ArsRS. J Med Microbiol. 2017;66:798-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (1)] |