Published online Sep 21, 2019. doi: 10.3748/wjg.v25.i35.5356
Peer-review started: May 4, 2019
First decision: May 30, 2019
Revised: August 8, 2019
Accepted: August 24, 2019
Article in press: August 24, 2019
Published online: September 21, 2019
Processing time: 143 Days and 10.3 Hours
Immunosuppression has undoubtedly raised the overall positive outcomes in the post-operative management of solid organ transplantation. However, long-term exposure to immunosuppression is associated with critical systemic morbidities. De novo malignancies following orthotopic liver transplants (OLTs) are a serious threat in pediatric and adult transplant individuals. Data from different experiences were reported and compared to assess the connection between immunosuppression and de novo malignancies in liver transplant patients.
To study the role of immunosuppression on the incidence of de novo malignancies in liver transplant recipients.
A systematic literature examination about de novo malignancies and immunosuppression weaning in adult and pediatric OLT recipients was described in the present review. Worldwide data were collected from highly qualified institutions performing OLTs. Patient follow-up, immunosuppression discontinuation and incidence of de novo malignancies were reported. Likewise, the review assesses the differences in adult and pediatric recipients by describing the adopted immunosuppression regimens and the different type of diagnosed solid and blood malignancy.
Emerging evidence suggests that the liver is an immunologically privileged organ able to support immunosuppression discontinuation in carefully selected recipients. Malignancies are often detected in liver transplant patients undergoing daily immunosuppression regimens. Post-transplant lymphoproliferative diseases and skin tumors are the most detected de novo malignancies in the pediatric and adult OLT population, respectively. To date, immunosuppression withdrawal has been achieved in up to 40% and 60% of well-selected adult and pediatric recipients, respectively. In both populations, a clear benefit of immunosuppression weaning protocols on de novo malignancies is difficult to ascertain because data have not been specified in most of the clinical experiences.
The selected populations of tolerant pediatric and adult liver transplant recipients greatly benefit from immunosuppression weaning. There is still no strong clinical evidence on the usefulness of immunosuppression withdrawal in OLT recipients on malignancies. An interesting focus is represented by the complete reconstitution of the immunological pathways that could help in decreasing the incidence of de novo malignancies and may also help in treating liver transplant patients suffering from cancer.
Core tip: A systematic literature examination about de novo malignancies and immunosuppression weaning both in adult and pediatric orthotopic liver transplant recipients was described in the present review. Even though conclusive evidence on immunosuppression withdrawal in orthotopic liver transplant recipients with regard to malignancies are lacking, we can argue that the reconstitution of the immunological pathway could decrease the incidence of de novo malignancies and may also help in treating liver transplant patients suffering from cancers.
- Citation: Manzia TM, Angelico R, Gazia C, Lenci I, Milana M, Ademoyero OT, Pedini D, Toti L, Spada M, Tisone G, Baiocchi L. De novo malignancies after liver transplantation: The effect of immunosuppression-personal data and review of literature. World J Gastroenterol 2019; 25(35): 5356-5375
- URL: https://www.wjgnet.com/1007-9327/full/v25/i35/5356.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i35.5356
It has been shown that progress in surgical techniques and enhanced standards in patient selection, standard of care, peri-operative management, survival rates and quality of life after orthotopic liver transplant (OLT) has remarkably improved over the last three decades. This has led to OLT being the treatment of choice for end-stage acute and chronic liver failure. However, the life-long immunosuppression (IS) regimens following transplantation still burden OLT recipients. In fact, major risks include infections, oncogenic viruses and renal, cardiovascular and metabolic complications along with a worrisome time-dependent susceptibility to de novo malignancies (DNMs)[1]. The incidence of DNMs among transplant patients is two to four times higher than in the healthy population[2]. These numbers increase to greater than 19 times in the pediatric counterpart[3], and DNM-related mortality is becoming the most prevalent cause of death amongst transplant subjects[4-6]. Beyond the therapeutic strategies for DNMs after OLT, IS drug minimization or withdrawal has been proposed.
Several studies have demonstrated the tolerogenic potential of the liver[7,8]. Because of its unique anatomy, several cell types in the liver have the capacity to act as antigen-presenting cells. In fact, dendritic cells, Kupffer cells and hepatocytes are capable of presenting antigens that activate CD8+ T cells[7]. These mechanisms are believed to play a role in allowing IS discontinuation and a permanent IS-free state (IFS) in up to 30%-40% of adult OLT recipients and in up to 60% of the pediatric population[9,10]. The present review aimed to detect the role of IS and its minimization or withdrawal in OLT adult and pediatric recipients on DNMs.
The primary goal of the current review was to assess the incidence and the characteristics of the diagnosed DNMs after an OLT in adult and pediatric populations in comparison with the non-transplanted immunocompetent population. The secondary goals were to determine: the incidence and outcome of those recipients that were successfully weaned off IS; and to address whether the maintenance of an IFS decreases the incidence of DNMs in LT recipients.
A literature review was conducted in February 2019 through MEDLINE databases (via PubMed) and Google Scholar to find studies pertaining to OLTs, DNMs, IS regimens and the clinical operational tolerance (COT) threshold. Articles published in languages other than English were excluded. All texts were full text accessible. Multiple keywords were used: “de novo tumor”, “adult”, “pediatric”, “liver transplantation”, “malignancy”, “review” and “operational tolerance”. The combination of words was used to maximize the results and achieve the highest possibility of articles related to the field of the present review. A flow chart of the article selection is provided in Figure 1.
Studies published in journals describing DNMs and risk factors for their development were searched for both adult and pediatric OLT recipients including experiences from article bibliographies. Records on post-transplant lymphoproliferative diseases (PTLDs), skin, head, neck, breast, lung, prostate, kidney, colorectal and other DNMs were collected and discussed from systematic reviews, randomized clinical trials, observational studies and case-control studies. IS regimens included calcineurin inhibitors (CNIs), corticosteroids, azathioprine, mammalian target of rapamycin inhibitors (mTORi) and antibody-mediated induction therapies. No time limits were applied to provide the closest results to the effective impact of DNMs on OLT patients. Non-English articles and cohorts of patients who underwent allografts other than liver were excluded from this review.
Information extracted from each selected article was first author name, year of publication, number of patients, follow-up period, characteristics of the detected malignancies, number of tolerant patients and study outcomes.
Recurrence and DNMs are the most frequent cause of mortality in adult OLT recipients[11] with an incidence up to 26%[12]. Conversely to cardiovascular complications, mortality from DNMs is increasing fast[13]: OLT recipients experience the highest onset rate of lymphomas (57%), and both PTLDs and non-PTLD tumors appear to develop after a shorter time in OLT recipients than other solid organ transplant patients[14]. Moreover, liver-localized PTLDs may originate from the donor and their treatment effect is very different. According to the donor/host origin of PTLDs, the prognostic significance might significantly change: Donor originated PTLDs might have different clinical and pathological features compared with the case of host originated PTLDs[15].
The probability of developing non-skin malignancies is higher in patients who underwent OLT for primary sclerosing cholangitis (PSC) (22% at 10 years) or alcoholic liver disease (ALD) (18% at 10 years)[16]. In particular, alcohol abuse[17] correlates with a three-fold increased risk of developing DNMs, and similar results are encountered in smokers of long duration due to the induced DNA damage[18,19] (Table 1). Overall, skin cancers are commonly diagnosed DNMs along with PTLDs after an OLT. The incidence of other malignancies is subject to a large variability due to the majority of epidemiological data coming from registry databases or single-center retrospective studies.
| Number and % incidence of de novo malignancy types in all orthotopic liver transplant recipients | ||||||||||||||
| Author | Yr | Number of patients | Number of DNM | PTLD | Skin and Kaposi | Head and Neck | Lung | Renal | Colon | Prostate | Breast | Gynecological | Others | |
| Jonas et al[72] | 1997 | 458 | 33 | 7 (1.5) | 8 (1.7) | 2 (0.4) | 3 (0.7) | 0 | 0 | 0 | 3 (0.7) | 7 (1.5) | 3 (0.6) | |
| Jain et al[134] | 1998 | 1000 | 57 | NA | 24 (2.4) | 7 (0.7) | 8 (0.8) | 2 (0.2) | 4 (0.4) | 3 (0.3) | 3 (0.3) | 0 | 6 (0.6) | |
| Kelly et al[135] | 1998 | 888 | 31 | NA | 8 (0.9) | 6 (0.7) | 1 (0.1) | 0 | 3 (0.3) | 0 | 2 (0.2) | 1 (0.1) | 10 (1.1) | |
| Jimenez et al[136] | 2002 | 505 | 62 | 13 (2.6) | 16 (3.2) | 10 (2.0) | 6 (1.2) | NA | NA | NA | NA | NA | 17 (3.3) | |
| Saigal et al[73] | 2002 | 1140 | 30 | NA | 14 (1.2) | 3 (0.3) | 0 | 3 (0.3) | 1 (0.1) | 0 | 2 (0.2) | 1 (0.1) | 6 (0.5) | |
| Sanchez et al[137] | 2002 | 1421 | 125 | 35 (2.5) | 42 (3.0) | 4 (0.3) | 11 (0.8) | 3 (0.2) | 9 (0.6) | 0 | 7 (0.5) | 2 (0.1) | 13 (0.8) | |
| Benlloch et al[17] | 2004 | 772 | 41 | 10 (1.3) | NA | 9 (1.2) | 8 (1.0) | 3 (0.4) | 2 (0.3) | 1 (0.1) | 2 (0.3) | 2 (0.3) | 4 (0.5) | |
| Oo et al[138] | 2005 | 1778 | 141 | 18 (1.0) | 51 (2.9) | NA | 14 (0.8) | NA | 18 (1.0) | NA | 11 (0.6) | 1 (0.06) | 28 (1.6) | |
| Yao et al[12] | 2006 | 1043 | 53 | 9 (0.9) | 17 (0.6) | 3 (0.3) | 5 (0.5) | 2 (0.2) | 6 (0.6) | 0 | 4 (0.4) | 2 (0.2) | 5 (0.5) | |
| Aberg et al[81] | 2008 | 540 | 39 | 9 (1.7) | 11 (2.0) | 2 (0.3) | NA | 2 (0.3) | 2 (0.3) | 2 (0.3) | 1 (0.1) | NA | 10 (1.8) | |
| Jiang et al[20] | 2008 | 2034 | 113 | 44 (2.1) | NA | 3 (0.1) | 10 (0.5) | 4 (0.2) | 14 (0.7) | 5 (0.2) | 5 (0.2) | NA | 24 (1.2) | |
| Baccarani et al[139] | 2010 | 417 | 43 | 9 (2.1) | 8 (1.9) | 8 (1.9) | 4 (0.9) | 0 | 2 (0.5) | 0 | 1 (0.2) | 3 (0.7) | 8 (1.9) | |
| Engels et al[5] | 2011 | 37888 | 1563 | 365 (1.0) | NA | NA | 300 (0.8) | 67 (1.8) | NA | NA | NA | NA | 831 (2.2) | |
| Chatrath et al[140] | 2013 | 534 | 80 | 16 (3.0) | 24 (4.5) | 9 (1.7) | 13 (2.4) | 1 (0.2) | 1 (0.2) | 1 (0.2) | NA | 1 (0.2) | 14 (2.6) | |
| Schrem et al[141] | 2013 | 2000 | 120 | 23 (1.1) | NA | 11 (0.5) | 14 (0.7) | 7 (0.3) | 13 (0.6) | 5 (0.2) | 8 (0.4) | 10 (0.5) | 29 (1.4) | |
| Krynitz et al[32] | 2013 | 1221 | 150 | 27 (2.2) | 58 (4.7) | 4 (0.3) | 6 (0.5) | 2 (0.1) | 6 (0.5) | 4 (0.3) | 7 (0.6) | 10 (0.8) | 26 (2.1) | |
| Mouchli et al[62] | 2017 | 373 | 64 | 22 (5.9) | 5 (1.3) | NA | NA | 11 (2.9) | 11 (2.9) | 7 (1.9) | 5 (1.3) | 3 (0.8) | 0 | |
| Egeli et al[142] | 2017 | 429 | 9 | NA | 1 (0.2) | 2 (0.4) | 5 (1.1) | NA | NA | NA | NA | NA | 1 (0.2) | |
| Taborelli et al[143] | 2018 | 2832 | 266 | 37 (1.3) | 72 (2.5) | 34 (1.2) | 28 (1.0) | 4 (0.2) | 21 (0.7) | 2 (0.1) | 4 (0.2) | 3 (0.1) | 65 (2.1) | |
Major de novo malignancy incidence in adult OLT recipients
PTLDs: PTLDs are the second most diagnosed DNMs after an OLT accounting for around 35% of non-skin malignancies[20]. Most PTLDs are due to Epstein-Barr virus (EBV). Even if a clear cut-off range of EBV-DNA levels has not been well recognized, virus detection may be sufficient to reveal early PTLDs[2,21-24]. Although the mortality still remains high (up to 85% and 69% after 1 and 5 years, respectively), PTLDs are decreasing due to the PTLD type, prognosis and efficacy of the available treatments[23,25,26].
Certain types of IS regimens including anti-thymocyte globulin, cyclosporine (CsA) or muromonab-CD3 are more likely to determine the onset of PTLDs[27,28]. The survival rate was significantly better in patients undergoing tacrolimus regimens compared to CsA (81.2% vs 50% after 5 years from the PTLD diagnosis)[29]. Multidisciplinary approaches that include IS weaning, interferon, surgery, radiotherapy and chemotherapy were attempted to reduce the incidence or recurrence from PTLDs[30].
Non-PTLDs: The most represented malignancies in adult OLT recipients are skin cancers[31-33] despite their lower recurrence after other SOTs[33-35]. Non-melanoma skin cancer are the most represented, and OLT recipients express a much higher risk when compared to the healthy population[36]. The vast majority of non-melanoma skin cancer is represented by squamous cell carcinomas and basal cell carcinomas[18,35]. However, a recent report from Rademacher et al[37] described an inverted trend with a decline in the incidence of skin cancer in the OLT population. This suggests that the characteristics of the analyzed cohort and a more deliberate use of sun blockers, avoidance of direct UV radiation and the type of IS adopted may play a role[38,39].
Human papilloma virus infections, aging, pallor of skin, previous cutaneous malignancies, blue or hazelnut eyes, CD4 lymphocytopenia and history of actinic keratosis are associated to skin tumors after an OLT[18,35,40,41]. In addition, PSC (considered as an indication for transplant[42]), male phenotype, Caucasian ethnicity and monoclonal induction therapy[40] represent relevant assets. A longstanding clinical experience proved CsA to be the strongest predictor; in fact CsA-treated patients developed a skin malignancy in a shorter time than patients treated with tacrolimus, making CsA an independent and specific risk factor for skin cancer[43].
A strong link between IS and DNM development is also found in the Kaposi sarcoma (KS), a multifocal angioproliferative muco-cutaneous tumor[27] that affects immunodeficient patients infected with human herpesvirus-8. However, in contrast with other DNMs[34,44,45], the KS incidence among the OLT population is constantly dropping. KS affects OLT patients around 500-fold more than the general population[27,44,46,47]. Thus, a tailored IS administration and a meticulous use of chemotherapy are crucial to avoid the outset of KS. Of note, a low blood viral concentration often limits the human herpesvirus-8 detection in most affected patients[34,48]. Typical KS diagnosis might also be missed by an inexperienced pathologist[49]. Even though there are ongoing trials on novel treatments for KS, evidence suggests that switching the IS regimen from CsA/tacrolimus to mTORi represents the best option to reduce the growth of KS[44,49,50].
Head and neck cancer are less common, but they are still the most serious DNMs in the OLT population. Although no screening exam is approved to diagnose these malignancies[2,51], specific follow-up guidelines by the European Association for the Study of Liver highly recommended that smokers and former alcoholic OLT patients are screened[52]. A recent study by Piselli et al[53] on 2770 OLT recipients confirmed that these subjects are more prone to develop head and neck cancer especially in those with a previous history of smoking and alcoholic liver disease. The 5-year survival rate has been reported around 35% with a standardized incidence ratio (SIR) that increased to 11.2% in OLT subjects with alcoholic liver disease.
Tobacco seems to be involved in the development of pharyngeal and tongue cancer, whereas alcohol plays a predominant role in the onset of oropharyngeal and upper aerodigestive squamous tumors in OLT individuals[54,55]. Hence, regular screenings should be performed on ears, nose and throat especially if there is a prior history of smoking.
Lung cancer accounts about 26% of the total deaths related to post OLT DNMs[56]. In fact OLT recipients showed between two- and three-fold higher incidence than the general population[34]. Better outcomes in OLT subjects with no history of smoking were observed. Nevertheless, the survival rate in both OLT individuals and in the healthy population after being diagnosed with lung cancer was similar. Therefore, the major gamechanger is mainly represented by cigarette smoking[57].
OLT recipients have a high prevalence of colorectal malignancies usually diagnosed between the 1st and 4th year after OLT; the risk rises to 5.6% considering the PSC recipients[58]. Although the information about patients suffering from both PSC and inflammatory bowel disease are still scarce, the higher risk in developing colorectal malignancies has been well recognized[59,60] and a special surveillance in these patients is currently strongly recommended[61]. Moreover, after 5 years the risk goes up to 15%, and a closer follow up must be mandatory in order to early detect any tumour development[58,62,63]. Despite being identified at earlier stages, the prognosis of colorectal metastasis in OLT recipients is still worse than the general population mostly due to the IS regimens that reduce the immune cell activity[64,65].
OLT recipients did not show an overall increased risk of prostate cancer when compared to the general population[2,34,66,67]. Non-prostate genitourinary neoplasms are usually more lethal and develop earlier in OLT recipients. Renal malignancies after OLT have a SIR of 3.3, and annual ultrasound screenings after OLT are strongly encouraged[27,34].
Young OLT females under CsA-based IS are more likely to develop breast fibroadenomas compared to males[68,69]. In fact, CsA seems to: enhance the fibroblast activity; influence the hypothalamic-pituitary axis and interfere with the prolactin receptors on lymphocytes[34,70]. Furthermore, the capability of CsA to regulate the expression of pyruvate kinase M2 in different breast cancer cell lines is giving new insights about its role in cancer therapy[71]. A switch to tacrolimus is high advisable because the mass dimension seems to decrease in dimension after conversion[68,69]. Non-breast gynecological tumors are often more represented in the OLT patients than in the healthy population[27,72,73]. This might be explained by a pre-OLT stricter screening program towards breast cancer diagnosis that should also be more enforced in gynecological malignancies[27].
DNMs account for 5%-16% of non-hepatic related deaths after pediatric OLT[74] and together with cardiovascular complications are becoming the major cause of late death after transplantation. In children, the risk of developing DNMs is 19-fold higher than adults, and tumors are more aggressive and less responsive to treatments[6]. Therefore, the early detection and prompt therapeutic management of DNMs in pediatric recipients is essential to achieve satisfactory results. As in adults, the major risk factors for DNMs after pediatric OLT include IS regimens as well as viral infections such as EBV, cytomegalovirus, human papilloma virus and human herpesvirus-8[75]. Due to the paucity of data in the pediatric population, data on DNMs after OLT in children are reported mainly in registry transplant studies including other solid organ (kidney, lung, heart)[76,77]. Therefore, records on the incidence and types of DNMs after pediatric OLT are limited to single case series and mainly related to PTLDs.
PTLDs: PTLDs are the most frequent DNMs after pediatric OLT with an incidence of 5%-20%. In 90%-95% of cases, PTLDs are related to EBV and cytomegalovirus infections[78]. The risk of developing PTLDs from EBV primary infections increases to 7-fold compared to the reactivation of a pre-existing infection[79,80]. Worldwide, different series confirmed that EBV-related PTLDs were the most common DNM, ranging between 35% and 80% of all neoplasms either in liver and in kidney pediatric transplant recipients[76].
The subtypes of PTLDs might vary from benign polymorphic conditions to aggressive monomorphic states such as lymphomas. From a large registry analysis of DNMs after pediatric OLTs, non-Hodgkin’s lymphoma is the most frequent (71%) type of PTLD, out of which nodal diffuse large-B cell lymphoma and Burkitt’s lymphoma are the most detected, while Hodgkin’s lymphoma and leukemia account for 8% and 4%, respectively[3]. Non-Hodgkin’s lymphoma occurs mainly in younger patients: Estimated SIR is 123 (95% confidence interval 3.12-686) for children aged < 17 years old, 55.7 (95% confidence interval 6.74-201) for recipients aged between 17 and 39 years old and 9.42 (95% confidence interval 3.06-22.0) for patients ≥ 40 years old[81]. Several series suggest that donor-derived PTLD might be more likely to relapse in transplanted organs when compared with recipient-derived PTLD. In addition, donor-derived PTLD seems to appear earlier in the post-transplant period and present a more positive 5-year prognosis than the ones arising from recipients[81].
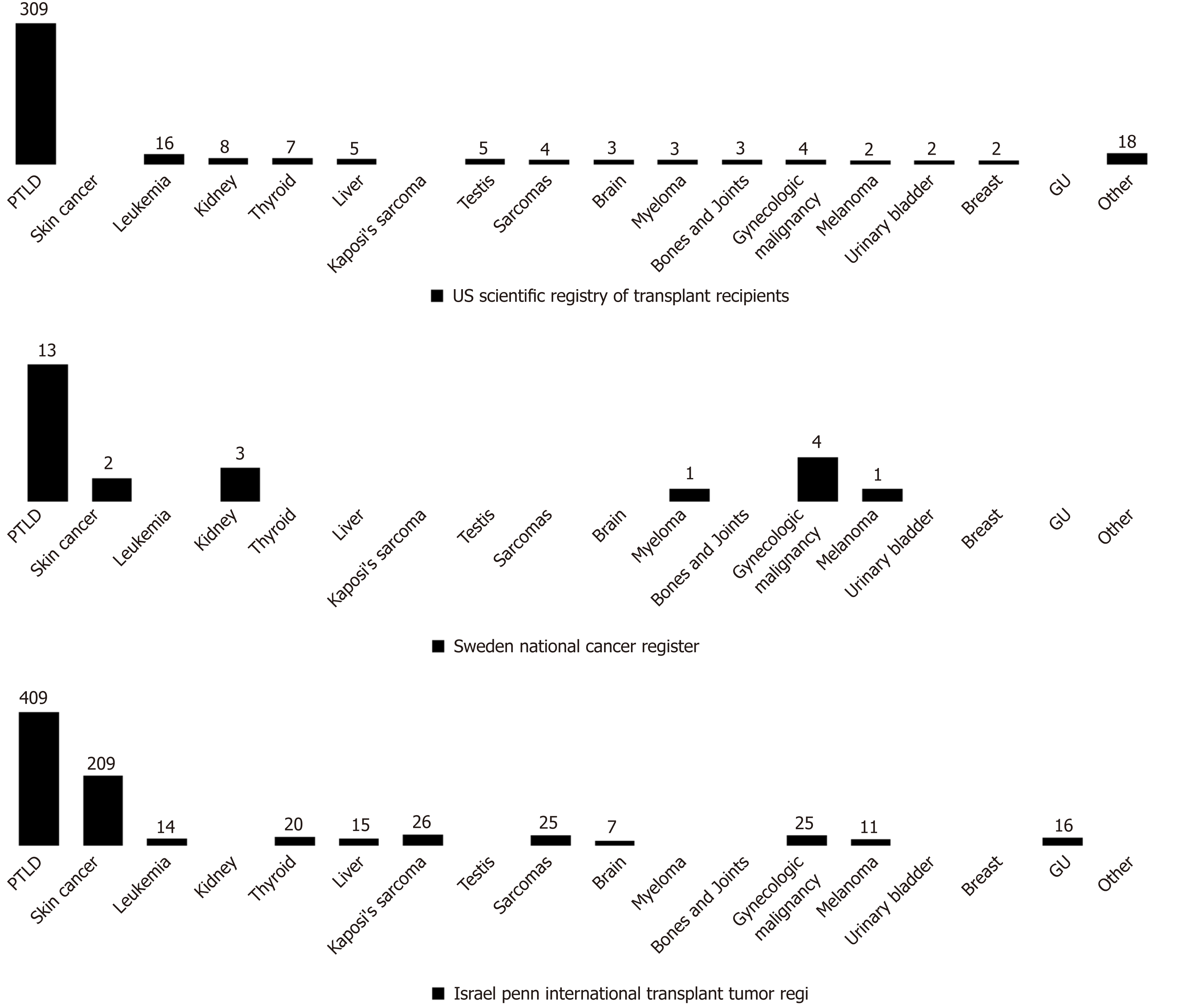
Non-PTLD: Non-PTLD neoplasms are rare in pediatric OLT recipients, so that, the incidence of non-PTLD malignancy is unclear due to paucity of data (Figure 2). Non-melanoma skin cancer is the most common non-PTLD DNMs represented mainly by squamous cell carcinomas[78]. Cases of melanomas have also been reported with a higher incidence than in adults. Other non-PTLDs include gynecologic neoplasms, KS, papillary thyroid tumors, sarcomas, brain tumors, renal cell carcinoma, liver tumors, testis neoplasms and bladder cancer.
The incidence of PTLD versus non-PTLD malignancies differs among age groups. Data from the Israel Penn International Transplant Tumor Registry[78] reported that children with post-transplant non-PTLD DNMs are older than recipients developing PTLD malignancies (13.2 vs 7.9 years of age, P < 0.0001). Moreover, from the time of transplantation, non-PTLD tumors are diagnosed within 99.2 months (P < 0.0001) while PTLD malignancies are detected within 60.2 months (P < 0.0001), and the latest to onset are usually vulvar and perineal cancer (113 mo)[78].
The Consensus on Managing Modifiable Risk in Transplantation group extensively described the main risk factors for graft loss in kidney and OLT recipients and provided useful recommendations to extend the long-term graft survival and to decrease the chances of DNMs onset[82]. IS drugs activate different pathways in the immune system and need to be carefully selected[83]. The primary disease needs to be considered in order to prescribe the most appropriate IS treatment. Of interest, mTORi might play a slight protective role reducing the incidence of DNMs especially within the 1st year of the transplant[84-86]. Similar data were described for mycophenolate mofetil[87]. The use of mTORi, mycophenolate mofetil, and tacrolimus represents the first choice when cancer develops in transplant recipients. There are no reports of such use of mTORi in the pediatric population. On the other hand, CNIs seem to have a cancer-promoting influence that might be related to their blood level concentration. Antilymphocyte medications also influence the onset of DNMs in long-lasting IS, while corticosteroids do not directly affect the risk of developing DNMs unless they are associated with chronic IS[88,89]. The association of multiple agents in lifelong IS regimens might be responsible for a substantially higher risk of DNMs. For these reasons, the discontinuation of IS (especially carcinogenic IS) should always be considered in transplant patients[88]. The primary aim is to achieve a COT status defined as a condition of non-reactivity of the immune system with a good graft function and no rejection in the absence of IS[90,91].
On the other hand, the non-compliance to IS considerably reduced the mid- and long-term survival of transplanted organs. It is estimated that about 10% of deaths or graft loss in adult OLT individuals were due to a poor compliance to the IS regimen[92,93]. Therefore, patients unintentionally or surreptitiously do not comply with IS regimens[94-96] due to the most disparate reasons are more likely to lose the graft. The cost and necessity of IS along with the prescribed dosage and the size of daily pills represents irresponsible behaviors that might compromise the patient compliance. Physicians should always be alerted for the possibility of these situations. For these reasons, it is important to establish a positive connection between the recipient and the healthcare provider[82,97].
Therefore, the spectrum of DNMs can also be reduced with a deeper understanding of the reasons for negligent conduct. Earlier studies demonstrated that patients already benefited from reminders of the importance of IS medication combined with counseling and psychological interventions[82,95,96]. Likewise, OLT individuals who do not regularly take daily medications face higher risks of graft rejection and elevated chances of developing DNMs. Consequently, the IS withdrawal must be physician-driven and always under close clinical surveillance.
The “Holy Grail” of transplantation is the achievement of an IFS. As mentioned above, long-lasting IS exposes patients to multiple adverse effects such as infections, tumors and target organ damage. The paramount importance of COT in LT can be achieved in selected recipients starting from a cautious IS minimization and constantly monitoring the liver function tests (LFTs)[9,98]. Unfortunately, as shown in most series only 30% of well-selected LT recipients can be safely weaned from IS[9,98-101] (Table 2).
| First authors | Yr/Trial start | Number of patients | Complete IS weaning, % | Median follow-up mo from IS withdrawal | Rejection rate, for acute, % | Weaned patients due to DNM diagnosis |
| Ramos et al[110] | 1995 | 39 | 41 | 15 | 38.4 | None |
| Devlin et al[144] | 1998 | 18 | 27.8 | > 36 | 44.4 | None |
| Eason et al[145] | 2005 | 18 | 5.5 | 9 | 61.1 | None |
| Girlanda et al[146] | 2005 | 18 | 11 | 84 | 5.5 | None |
| Tisone et al[109] | 2006 | 34 | 23.4 | 45.5 ± 5.8 | 21.0 | None |
| Assy et al[147] | 2007 | 26 | 42 | 6 | 58.0 | None |
| Pons et al[148] | 2008 | 12 | 38.0 | 10-30 | 58.0 | None |
| Tryphonopoulos et al[149] | 2010 | 23 | 22.0 | 87 ± 3.0 | 5.0 | None |
| Manzia et al[106] | 2013 | 28 | 21.4 | 113 ± 20.0 | 21.0 | None |
| De la Garza et al[150] | 2013 | 24 | 62.5 | 14.0 | 37.5 | None |
| Benitez et al[151] | 2014 | 102 | 40.2 | 48.9 | 59.8 | None |
| Bohne et al[152] | 2014 | 34 | 50 | 12 | 44.1 | None |
| Todo et al[153] | 2016 | 10 | 70 | NA | 30.0 | None |
| Manzia et al[107] | 2018 | 75 | 42.6 | 78.5 | 0 | None |
| Shaked[154] | 2005 (clinical trial) | 275 | 20.3 | NA | 5.5 | None |
| Markman[155] | 2016 (ongoing trial) | 60 | NA | NA | NA | NA |
| Markman et al[156] | 2019 (ongoing trial) | NA | NA | NA | NA | NA |
The molecular mechanisms responsible for graft acceptance still need to be fully understood, but the liver seems less likely to cause rejection in their hosts than other organs. Multiple theories were hypothesized: (1) The production of higher levels of major histocompatibility complex might affect the recipient immune response[102]; (2) An OLT donor leukocytes migrating in the recipient blood stream could influence the graft tolerance because their irradiation causes organ rejection[103]; and (3) Donor hematopoietic stem cells might determine a chimeric effect in the recipient[104]. Moreover, the huge amount of blood that is constantly flowing in the liver exposes it to plenty of bacteria and antigens that could enhance a COT status[90].
New insights on human leukocyte antigen donor-specific antibody/antibodies (DSA) are emerging in OLT recipients. A recent study described how the IS management and IS withdrawal protocols might affect the onset of de novo DSA (dnDSA) after OLT especially during the transition to IS monotherapy in the 1st year after the OLT[105]. Interestingly, a higher dnDSA prevalence was found in patients undergoing IS minimization (51.7%) and IS-free patients (66.7%). These findings suggest that monitoring dnDSA is high advisable and the IS minimization or withdrawal should be taken in consideration after at least 1 year from OLT in order to prevent negative consequences on the graft.
The Tor Vergata experience: In the last decade, our Liver Unit from Tor Vergata Institute described multiple trials attempting IS minimization and IS withdrawal after OLTs[9,10,90,106-109]. The first purpose was to minimize the uptake of IS drugs in the first years post-OLT. Afterwards, patients with stable LFTs, no rejection or autoimmune disease who underwent IS minimization were discontinued from IS. Initially, LFTs are monitored every week and then monthly within the 1st year during the IS withdrawal process[90]. IS was resumed in patients who had double the normal LFT levels during follow-up or when a liver biopsy showed features of acute rejection[90].
From April 1998 to December 2014, in the HPB and Transplant Unit, 299 OLT were performed. Of these, 65 (21.7%) patients with a mean follow-up of 81 months were considered for weaning protocol while 234 (78.2%, mean follow-up of 125.6 months) were under CNIs or mTORi and mycophenolate mofetil IS regimens. In unpublished series, data on DNMs were compared in order to address the differences in DNM incidence during a median follow-up of 4 years (Table 3).
| Patients under standard IS,n = 234Median age: 53.6 ± 7.1 yr | Tolerant patients,n = 22Median age: 52.3 ± 6.0 yr | Non-tolerant patients, n = 43Median age: 51.5 ± 9.6 yr | |
| Number of patients | 234 | 22 | 43 |
| Median follow-up time from OLT to IS weaning, mo | - | 112.9 | 59.8 |
| Median follow-up time from weaning start to IS withdrawal, mo | - | 6.0 | 4.9 |
| Median follow-up time with no IS, mo | - | 92.3 | 2.3 |
| Median follow-up time after IS resumption, mo | - | - | 149.1 |
| Patients who developed DNMs, % | 13.7 | 0 | 6.4 |
| Median time from OLT to DNM development, mo | 44.5 | - | 113.0 |
| Incidence and type of DNMs | (n = 32) Lung (7) Head and neck (5) Colon (4) Oral cavity (4) PTLD (4) Genito-urinary (3) Esophagus (2) Liver (1) Mesothelioma (1) KS (1) | None | Bladder (1) Larynx (1) Lung (1) |
Among the 65 recruited patients enrolled in local IS withdrawal protocol[106,108,109], 22 (33.8%) were successfully weaned from IS (tolerant; Tol), while 43 (66.2%) were non-tolerant (Non-Tol) and needed IS resumption after an observed upsurge of the LFTs or biopsy-proven acute rejection. In the Tol group, none experienced DNMs versus two (4.6%) in the Non-Tol group and thirty-two (13%) in the standard immunosuppressed recipients (Table 4). LT recipients under daily IS showed an increased relative risk of 4.45 of developing DNMs versus Tol and Non-Tol recipients and a SIR of 1.5 when compared to the general population.
| First author | Yr | Type of study | Number of patients | DNM-Patients indicated to withdraw IS | Complete IS weaning, % | Time interval: OLT to withdrawal, mo/yr | Rejection rate, for acute, % |
| Ramos et al[110] | 1995 | Prospective | 20 (12-20 yr at entry) (59 total patients) | 2 | 27.10% | > 5 yr | 20.3% |
| Mazariegos et al[157] | 1997 | Historical cohort (self-weaned) and prospective cases | 31 (≤ 20 yr) (100 total patients) | 12 | Pediatric cohort: 29% | > 5 yr | 10% |
| Takatsuki et al[111] | 2001 | Prospective | 63 | NA | 38.1% | ≥ 2 yr | 25.4% |
| Oike et al[112] | 2002 | Prospective | 115 | NA | 42.6% | ≥ 2 yr | 20% |
| Koshiba et al[113] | 2007 | Retrospective | 581 | NA | 15% | ≥ 2 yr | 1.5% |
| Ohe et al[114] | 2012 | Historical cohort | 190 | NA | 44.2% | ≥ 2 yr | 26.3% |
| Hurwitz et al[116] | 2004 | Retrospective | 38 | 19 (PTLD) | 21% (n = 4 PTLD; n = 4 EBV) | Mean time to PTLD onset: 1.8 ± 2.3 yr; Mean time to EBV infection onset: 1.1 ± 1.1 yr | 55.2% |
| Lee et al[117] | 2009 | Prospective | 5 | 1 (PTLD) | 100% | 1.2-2 yr | 0% |
| Feng et al[118,158] (WISP-R trial) | 2012 | Prospective | 20 | NA | 60% | ≥ 4 yr | 35% |
| Feng et al[159] (iWITH trial, partial results, 2016) | 2012 | Prospective | 88 | NA | 60% | ≥ 4 yr | 40% |
| Waki et al[120] | 2013 | Retrospective | 52 | NA | 42.5% | > 2 yr | 57.5% |
| Lin et al[121] | 2015 | Prospective | 16 | NA | 40% | > 2 yr | 40% |
Role of immunosuppression minimization and withdrawal in pediatric OLT recipients: Because chronic IS significantly affects the long-term outcomes of pediatric OLT recipients, children were the primary OLT population who experienced IS minimization and withdrawal protocols (Table 4).
Ramos et al[110] reported the first clinical trial of IS weaning where 20 pediatric patients underwent drug discontinuation for long-term IS complications (in two cases for de novo squamous cell carcinomas) reaching COT in 16 patients (27.1%). Takatsuki et al[111] reported the result of a prospective trial where a COT status was reached in 24 (38%) out of 63 children after ≥ 2 years from the OLT, and this promising COT rate remained similar in the subsequent trials from the same study group[112-114]. All tolerant patients had normal LFTs after 1-year follow-up, and no rejection episodes were reported. However, almost 6% of selected COT patients showed signs of allograft fibrosis at histological finding, driving the introduction of a protocol liver biopsy for patients undergoing IS withdrawal[115].
Hurwitz et al[116] described the only report focusing on the effects of IS withdrawal on DNMs after pediatric OLTs. Thirty-eight pediatric OLT recipients affected by PTLDs (n = 19) or severe EBV infection (n = 19) after a mean time of 1.8 ± 2.3 years and 1.1 ± 1.1 years from OLT, respectively, attempted IS withdrawal in combination with antiviral drugs with or without chemotherapy. A complete IS withdrawal was achieved in eight (21%) patients for 4.2 ± 1.7 years with an overall 84% survival rate. Episodes of rejection that did occur after stopping IS were successfully treated with standard therapy with no graft loss. Although the results are tempered by the intrinsic limitations of retrospective studies, the authors state that the mortality risk from cancer well outweighs the risk of graft loss due to acute rejection from IS withdrawal. Also, Lee et al[117] reported in his COT series another case of a successful IS weaning in a child with a de novo PTLD with a 3-year follow-up.
Feng et al[118] published the results from a pilot prospective multi-centric trial aiming to withdraw IS in order to reduce drug-related complications: Out of 20 pediatric OLT recipients attempting COT, 12 (60%) children successfully discontinued (over a period at least of 36 wk) IS, while 8 patients experienced rejection resolved by IS resumption. Recently, the authors reported that after a 5-year follow-up all COT recipients have normal LFTs and no histological inflammation or fibrosis, despite some patients were found with DSA and modest increases in sinusoidal C4d staining[119]. These promising results suggested that in selected pediatric OLT recipients, COT was feasible; yet selection criteria (such as clinical and biomarkers criteria) are needed to identify the children who could successfully attempt IS withdrawal. High rates (40%-42%) of successful COT were also reported by other series[120,121]. Likewise, Waki et al[120] demonstrated that Non-tol patients were associated with post-transplant human leukocyte antigen antibodies. This could represent a future screening criterion to select children who could discontinue IS regimen.
The outset of DNMs in LT recipients seems to be connected to the IS regimen. In fact, IS drugs downregulate different pathways both of the adaptive and the innate immune response leading to a higher risk of tumor relapse after OLT. Hepatocellular carcinoma represents one of the indications for OLT. Due to the nature of the transplant indication itself, it would be beneficial to quickly tailor or withdraw IS because these recipients face a higher risk of recurrent hepatocellular carcinoma[90,122]. Thus, immediately after OLT, CNIs should be discontinued to minimize this threat as they seem more likely to trigger DNMs[123,124]. Conversely, mTORi seems to reduce the impact of DNMs at least within 5 years post-OLT[125].
The IS non-adherence must be always avoided due to its dangerous effects often underestimated in the overall graft longevity[126]. Nowadays, COT can be achieved in almost 30% of adult OLT individuals after a meticulous selection, but it is hard to accomplish for other solid organ transplant subjects because COT is organ dependent[127]. Strict criteria from the studies cited in Table 3 include IS regimens and IS drug blood levels, stable allograft function, no history of rejection or autoimmune diseases and a similar human leukocyte antigen match between donors and recipients. All these conditions need to be met in order to attempt COT. The accomplishment of a complete IFS in pediatric OLT recipients proved to be suitable in carefully designated patients albeit the heterogeneous considered cohorts. In fact, up to 60% of the total recipients were successfully withdrawn from IS while preserving a normal graft function.
Histological findings are as important as biochemical assessments in the definition of COT, even if not all studies reported liver biopsies features after weaning off IS. OLT recipients with normal LFTs might hide relevant graft inflammation or fibrosis that offset the risk of organ injury. In addition, modern studies stressed the relevance of histological features when outlining future trials. Considerations on graft fibrosis, independent from IS maintenance or withdrawal, need further investigations to fully understand the etiopathogenetic pathways involved. To the best of our knowledge, no clinical experience has been reported so far on IS withdrawal due to DNMs occurrence. Therefore, we can only speculate that the reconstitution of the immunological pathways can counteract the tumor growing.
The main drawback of the present review is that most COT studies explored have been fitted in order to address the possibility to achieve COT status and not in those who experienced DNMs. In fact, the majority of studies on IS withdrawal is referred to patients who demonstrated a stable clinical pathway with normal LFTs and no rejection post-OLT. An international registry including all adult and pediatric IS weaning experiences might represent an interesting approach to both gain knowledge about the entity of DNMs in OLT subjects and the final outcomes after IS withdrawal in such patients.
The minimization of IS dosages would provide multiple beneficial aspects that include: (1) Releasing from all IS burdens; (2) Remarkable savings in IS drugs[107]; and (3) Increased quality of life after the reduction of daily medications, which can positively influence compliance and graft outcomes in long-term treatments[9,128]. COT immunological biomarkers are constantly researched because their clinical predictor role would represent a game changer in the transplantation field. The blood stream represents the most used source of non-invasive liver tolerance biomarkers due to its potential never-ending amount[129,130]. Unfortunately, the lack of consistent assays and validated biomarkers that might predict graft failure currently represent an arduous issue. Patients are in desperate need of alternative treatments to lifelong IS, and until reliable biomarkers are available the gold standard for rejection diagnosis is still represented by liver biopsies[131].
In the last few decades, there have been multiple efforts to reach an IFS in OLT recipients. These attempts might lead to ethical concerns as they shift to a potential unsafe option, which could raise future complications. Patients demand the best long-term quality of life after such a tough experience of an organ transplantation. Researchers methodically commit to fulfill this urgency, and physicians struggle to prevent the recurrence of physical and psychological complications that mainly result from the IS itself or from the primary disease recurrence.
A COT status perfectly frames the overarching goal of transplantation, which aims to provide the best quality of life for transplant recipients who would not be burdened by the IS threats while providing economic benefits. From these perspectives an IFS remains the most enticing path to follow and considered worth it in spite of all the challenges to overcome. Likewise, the relatively recent field of regenerative medicine is constantly gaining ground through new outstanding findings. Specifically, the astonishing capabilities of the extracellular matrix capable of closely emulating the ideal milieu of native organs enhancing cell growth, migration and proliferation is promising to offer innovative hints for future research[132,133].
We are still far away from a translational side of these results, but the immense potential of regenerative medicine surely represents a hope for future therapies and IS avoidance. More than 60 years ago the transplantation era began after the first successful transplantation was performed among identical twins, and the first case of COT was described. Since that moment, tolerance continues to be a grueling problem albeit remarkable steps were taken over the past decades. In fact, when experienced hands were called to action, undeniable evidence proved that a stable IFS is achievable in carefully selected OLT recipients. Clues that COT is no longer intangible is becoming clearer, and the concept that considered IS weaning protocols as detrimental procedures should be now considered out-of-date. However, an in-depth knowledge is certainly required as many immunological pathways responsible for COT still remain arcane, and crucial challenges about tolerance need to be addressed with further investigations.
Immunosuppression (IS) has undoubtedly raised the overall positive outcomes in the post-operative management of solid organ transplantation. However, long-term exposure to IS is associated with critical systemic morbidities. De novo malignancies (DNMs) following orthotopic liver transplants (OLTs) are a serious threat in pediatric and adult transplant individuals. Data from different experiences were reported and compared to assess the connection between IS and DNMs in liver transplant patients.
DNMs represent a major threat in OLT children and adults. Multiple experiences were described to analyze the connection between IS and DNMs in liver transplant patients. Different pathways seem to be involved in the incidence of DNMs, but molecular mechanisms are still unknown. Giving an answer to this concern might lead to a solution for the complications related to the long-term use of IS.
To study the role of IS on the incidence of DNMs in liver transplant recipients.
A systematic literature examination of DNMs and IS weaning in adult and pediatric OLT recipients was described in the present review. Data from worldwide clinical trials was collected from highly qualified institutions performing OLTs. Patient follow-up, IS discontinuation and incidence of DNMs were reported. Likewise, the review assesses the differences in adult and pediatric recipients by describing the adopted IS regimens and the type of diagnosed solid and blood malignancy.
Emerging evidence suggests that the liver is an immunologically privileged organ able to support IS discontinuation in carefully selected recipients. Malignancies are often detected in liver transplant patients undergoing daily IS regimens. Post-transplant lymphoproliferative diseases and skin tumors are the most detected DNMs in pediatric and adult OLT patients, respectively. To date, IS withdrawal has been achieved in 40% and 60% of well-selected adult and pediatric recipients, respectively. In both populations, a clear benefit of IS weaning protocols on DNMs is difficult to ascertain because data have not been specified in most of the clinical experiences.
The selected populations of tolerant pediatric and adult liver transplant recipients greatly benefit from IS weaning. There is still no strong clinical evidence on the usefulness of IS withdrawal in OLT recipients on malignancies. An interesting focus is represented by the complete reconstitution of the immunological pathways that could help in decreasing the incidence of DNMs and may also help in treating liver transplanted patients suffering from cancer.
Most of the current studies on IS withdrawal describe patients with a stable clinical pathway with normal liver function test levels and no history of rejection post-OLT. In the future, an international registry including all IS weaning experiences in OLT patients would offer a promising database to explore the connections between DNMs and the final outcomes after IS withdrawal in such patients. Seriate graft biopsies should always be considered in future studies to take into account the risk of graft fibrosis. Fibrosis is independent from IS maintenance or withdrawal, and further investigations are strongly suggested to fully understand the etiopathogenetic pathways involved. The minimization of IS dosages may decrease all IS complications and induce remarkable savings in IS drugs. Moreover, the recipient’s quality of life after the reduction of daily medications could significantly boost their compliance and graft outcomes in the long-term. IS withdrawal is still arduous to realize. However, it is possible, and it is supported by the described cases of clinical operational tolerance in OLT individuals. In-depth investigations are needed to study the possibilities of achieving a complete IS-free state and clinical operational tolerance in OLT patients affected by DNMs because few studies explore this possibility. Regenerative medicine and clinical operational tolerance biomarkers are new promising frontiers that could provide novel insights about tolerance mechanisms in order to replace liver biopsies as the currently recognized gold standard method for rejection diagnosis.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B, B, B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Alexopoulou A, Bhatti ABH, Ding JX, Ho CM, Mikulic D, Ramsay MA, Sipahi AM, Zheng H S-Editor: Yan JP L-Editor: Filipodia E-Editor: Ma YJ
| 1. | Asfar S, Metrakos P, Fryer J, Verran D, Ghent C, Grant D, Bloch M, Burns P, Wall W. An analysis of late deaths after liver transplantation. Transplantation. 1996;61:1377-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 77] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Herrero JI. Screening of de novo tumors after liver transplantation. J Gastroenterol Hepatol. 2012;27:1011-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Yanik EL, Smith JM, Shiels MS, Clarke CA, Lynch CF, Kahn AR, Koch L, Pawlish KS, Engels EA. Cancer Risk After Pediatric Solid Organ Transplantation. Pediatrics. 2017;139:pii: e20163893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 4. | Adami J, Gäbel H, Lindelöf B, Ekström K, Rydh B, Glimelius B, Ekbom A, Adami HO, Granath F. Cancer risk following organ transplantation: A nationwide cohort study in Sweden. Br J Cancer. 2003;89:1221-1227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 529] [Cited by in RCA: 515] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 5. | Engels EA, Pfeiffer RM, Fraumeni JF, Kasiske BL, Israni AK, Snyder JJ, Wolfe RA, Goodrich NP, Bayakly AR, Clarke CA, Copeland G, Finch JL, Fleissner ML, Goodman MT, Kahn A, Koch L, Lynch CF, Madeleine MM, Pawlish K, Rao C, Williams MA, Castenson D, Curry M, Parsons R, Fant G, Lin M. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011;306:1891-1901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1114] [Cited by in RCA: 1093] [Article Influence: 78.1] [Reference Citation Analysis (0)] |
| 6. | Zhou J, Hu Z, Zhang Q, Li Z, Xiang J, Yan S, Wu J, Zhang M, Zheng S. Spectrum of De Novo Cancers and Predictors in Liver Transplantation: Analysis of the Scientific Registry of Transplant Recipients Database. PLoS One. 2016;11:e0155179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Moris D, Lu L, Qian S. Mechanisms of liver-induced tolerance. Curr Opin Organ Transplant. 2017;22:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Levitsky J, Feng S. Tolerance in clinical liver transplantation. Hum Immunol. 2018;79:283-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 9. | Manzia TM, Angelico R, Ciano P, Mugweru J, Owusu K, Sforza D, Toti L, Tisone G. Impact of immunosuppression minimization and withdrawal in long-term hepatitis C virus liver transplant recipients. World J Gastroenterol. 2014;20:12217-12225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Manzia TM, Angelico R, Toti L, Lai Q, Ciano P, Angelico M, Tisone G. Hepatitis C virus recurrence and immunosuppression-free state after liver transplantation. Expert Rev Clin Immunol. 2012;8:635-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Fung JJ, Jain A, Kwak EJ, Kusne S, Dvorchik I, Eghtesad B. De novo malignancies after liver transplantation: A major cause of late death. Liver Transpl. 2001;7:S109-S118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 149] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 12. | Yao FY, Gautam M, Palese C, Rebres R, Terrault N, Roberts JP, Peters MG. De novo malignancies following liver transplantation: A case-control study with long-term follow-up. Clin Transplant. 2006;20:617-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Pruthi J, Medkiff KA, Esrason KT, Donovan JA, Yoshida EM, Erb SR, Steinbrecher UP, Fong TL. Analysis of causes of death in liver transplant recipients who survived more than 3 years. Liver Transpl. 2001;7:811-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 203] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 14. | Penn I. Posttransplantation de novo tumors in liver allograft recipients. Liver Transpl Surg. 1996;2:52-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 89] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Nuckols JD, Baron PW, Stenzel TT, Olatidoye BA, Tuttle-Newhall JE, Clavien PA, Howell DN. The pathology of liver-localized post-transplant lymphoproliferative disease: A report of three cases and a review of the literature. Am J Surg Pathol. 2000;24:733-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Watt KD, Pedersen RA, Kremers WK, Heimbach JK, Sanchez W, Gores GJ. Long-term probability of and mortality from de novo malignancy after liver transplantation. Gastroenterology. 2009;137:2010-2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 191] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 17. | Benlloch S, Berenguer M, Prieto M, Moreno R, San Juan F, Rayón M, Mir J, Segura A, Berenguer J. De novo internal neoplasms after liver transplantation: Increased risk and aggressive behavior in recent years? Am J Transplant. 2004;4:596-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 100] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 18. | Herrero JI, España A, Quiroga J, Sangro B, Pardo F, Alvárez-Cienfuegos J, Prieto J. Nonmelanoma skin cancer after liver transplantation. Study of risk factors. Liver Transpl. 2005;11:1100-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 63] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Das R, Kundu S, Laskar S, Choudhury Y, Ghosh SK. Assessment of DNA repair susceptibility genes identified by whole exome sequencing in head and neck cancer. DNA Repair (Amst). 2018;66-67:50-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Jiang Y, Villeneuve PJ, Fenton SS, Schaubel DE, Lilly L, Mao Y. Liver transplantation and subsequent risk of cancer: Findings from a Canadian cohort study. Liver Transpl. 2008;14:1588-1597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Bakker NA, van Imhoff GW, Verschuuren EA, van Son WJ. Presentation and early detection of post-transplant lymphoproliferative disorder after solid organ transplantation. Transpl Int. 2007;20:207-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 97] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 22. | Dierickx D, Tousseyn T, De Wolf-Peeters C, Pirenne J, Verhoef G. Management of posttransplant lymphoproliferative disorders following solid organ transplant: An update. Leuk Lymphoma. 2011;52:950-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Mizuno S, Hayasaki A, Ito T, Fujii T, Iizawa Y, Kato H, Murata Y, Tanemura A, Kuriyama N, Azumi Y, Kishiwada M, Usui M, Sakurai H, Isaji S. De Novo Malignancy Following Adult-to-Adult Living Donor Liver Transplantation Focusing on Posttransplantation Lymphoproliferative Disorder. Transplant Proc. 2018;50:2699-2704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Shroff R, Rees L. The post-transplant lymphoproliferative disorder-a literature review. Pediatr Nephrol. 2004;19:369-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 116] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 25. | Jain A, Nalesnik M, Reyes J, Pokharna R, Mazariegos G, Green M, Eghtesad B, Marsh W, Cacciarelli T, Fontes P, Abu-Elmagd K, Sindhi R, Demetris J, Fung J. Posttransplant lymphoproliferative disorders in liver transplantation: A 20-year experience. Ann Surg. 2002;236:429-36; discussion 436-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 170] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 26. | Kremers WK, Devarbhavi HC, Wiesner RH, Krom RA, Macon WR, Habermann TM. Post-transplant lymphoproliferative disorders following liver transplantation: Incidence, risk factors and survival. Am J Transplant. 2006;6:1017-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 95] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 27. | Burra P, Rodriguez-Castro KI. Neoplastic disease after liver transplantation: Focus on de novo neoplasms. World J Gastroenterol. 2015;21:8753-8768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 28. | Dantal J, Soulillou JP. Immunosuppressive drugs and the risk of cancer after organ transplantation. N Engl J Med. 2005;352:1371-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 185] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 29. | Newell KA, Alonso EM, Whitington PF, Bruce DS, Millis JM, Piper JB, Woodle ES, Kelly SM, Koeppen H, Hart J, Rubin CM, Thistlethwaite JR. Posttransplant lymphoproliferative disease in pediatric liver transplantation. Interplay between primary Epstein-Barr virus infection and immunosuppression. Transplantation. 1996;62:370-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 196] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 30. | Eshraghian A, Imanieh MH, Dehghani SM, Nikeghbalian S, Shamsaeefar A, Barshans F, Kazemi K, Geramizadeh B, Malek-Hosseini SA. Post-transplant lymphoproliferative disorder after liver transplantation: Incidence, long-term survival and impact of serum tacrolimus level. World J Gastroenterol. 2017;23:1224-1232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 31. | Ducroux E, Boillot O, Ocampo MA, Decullier E, Roux A, Dumortier J, Kanitakis J, Jullien D, Euvrard S. Skin cancers after liver transplantation: Retrospective single-center study on 371 recipients. Transplantation. 2014;98:335-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | Krynitz B, Edgren G, Lindelöf B, Baecklund E, Brattström C, Wilczek H, Smedby KE. Risk of skin cancer and other malignancies in kidney, liver, heart and lung transplant recipients 1970 to 2008--a Swedish population-based study. Int J Cancer. 2013;132:1429-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 261] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 33. | Tran M, Sander M, Ravani P, Mydlarski PR. Incidence of melanoma in organ transplant recipients in Alberta, Canada. Clin Transplant. 2016;30:1271-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Burra P, Shalaby S, Zanetto A. Long-term care of transplant recipients: De novo neoplasms after liver transplantation. Curr Opin Organ Transplant. 2018;23:187-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 35. | Herrero JI, España A, D'Avola D, Pardo F, Iñarrairaegui M, Rotellar F, Sangro B, Quiroga J. Subsequent nonmelanoma skin cancer after liver transplantation. Transplant Proc. 2012;44:1568-1570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Euvrard S, Kanitakis J, Claudy A. Skin cancers after organ transplantation. N Engl J Med. 2003;348:1681-1691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1186] [Cited by in RCA: 1107] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 37. | Rademacher S, Seehofer D, Eurich D, Schoening W, Neuhaus R, Oellinger R, Denecke T, Pascher A, Schott E, Sinn M, Neuhaus P, Pratschke J. The 28-year incidence of de novo malignancies after liver transplantation: A single-center analysis of risk factors and mortality in 1616 patients. Liver Transpl. 2017;23:1404-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 38. | Jiyad Z, Olsen CM, Burke MT, Isbel NM, Green AC. Azathioprine and Risk of Skin Cancer in Organ Transplant Recipients: Systematic Review and Meta-Analysis. Am J Transplant. 2016;16:3490-3503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 136] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 39. | Santana AL, Felsen D, Carucci JA. Interleukin-22 and Cyclosporine in Aggressive Cutaneous Squamous Cell Carcinoma. Dermatol Clin. 2017;35:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 40. | Berg D, Otley CC. Skin cancer in organ transplant recipients: Epidemiology, pathogenesis, and management. J Am Acad Dermatol. 2002;47:1-17; quiz 18-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 524] [Cited by in RCA: 477] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 41. | Carroll RP, Ramsay HM, Fryer AA, Hawley CM, Nicol DL, Harden PN. Incidence and prediction of nonmelanoma skin cancer post-renal transplantation: A prospective study in Queensland, Australia. Am J Kidney Dis. 2003;41:676-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 93] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 42. | Otley CC, Cherikh WS, Salasche SJ, McBride MA, Christenson LJ, Kauffman HM. Skin cancer in organ transplant recipients: Effect of pretransplant end-organ disease. J Am Acad Dermatol. 2005;53:783-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 43. | Mithoefer AB, Supran S, Freeman RB. Risk factors associated with the development of skin cancer after liver transplantation. Liver Transpl. 2002;8:939-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 68] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 44. | Piselli P, Busnach G, Citterio F, Frigerio M, Arbustini E, Burra P, Pinna AD, Bresadola V, Ettorre GM, Baccarani U, Buda A, Lauro A, Zanus G, Cimaglia C, Spagnoletti G, Lenardon A, Agozzino M, Gambato M, Zanfi C, Miglioresi L, Di Gioia P, Mei L, Ippolito G, Serraino D; Immunosuppression and Cancer Study Group. Risk of Kaposi sarcoma after solid-organ transplantation: Multicenter study in 4,767 recipients in Italy, 1970-2006. Transplant Proc. 2009;41:1227-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 45. | Piselli P, Taborelli M, Cimaglia C, Serraino D; Italian Transplant & Cancer Cohort Study. Decreased incidence of Kaposi sarcoma after kidney transplant in Italy and role of mTOR-inhibitors: 1997-2016. Int J Cancer. 2019;145:597-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 46. | Berber I, Altaca G, Aydin C, Dural A, Kara VM, Yigit B, Turkmen A, Titiz MI. Kaposi's sarcoma in renal transplant patients: Predisposing factors and prognosis. Transplant Proc. 2005;37:967-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 47. | Euvrard S, Kanitakis J. Skin cancers after liver transplantation: What to do? J Hepatol. 2006;44:27-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 48. | Haq IU, Dalla Pria A, Papanastasopoulos P, Stegmann K, Bradshaw D, Nelson M, Bower M. The clinical application of plasma Kaposi sarcoma herpesvirus viral load as a tumour biomarker: Results from 704 patients. HIV Med. 2016;17:56-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 49. | Schneider JW, Dittmer DP. Diagnosis and Treatment of Kaposi Sarcoma. Am J Clin Dermatol. 2017;18:529-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 50. | Stallone G, Schena A, Infante B, Di Paolo S, Loverre A, Maggio G, Ranieri E, Gesualdo L, Schena FP, Grandaliano G. Sirolimus for Kaposi's sarcoma in renal-transplant recipients. N Engl J Med. 2005;352:1317-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 742] [Cited by in RCA: 677] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 51. | Jain A, Patil VP, Fung J. Incidence of de novo cancer and lymphoproliferative disorders after liver transplantation in relation to age and duration of follow-up. Liver Transpl. 2008;14:1406-1411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 52. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Liver transplantation. J Hepatol. 2016;64:433-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 543] [Cited by in RCA: 710] [Article Influence: 78.9] [Reference Citation Analysis (0)] |
| 53. | Piselli P, Burra P, Lauro A, Baccarani U, Ettorre GM, Vizzini GB, Rendina M, Rossi M, Tisone G, Zamboni F, Bortoluzzi I, Pinna AD, Risaliti A, Galatioto L, Vennarecci G, Di Leo A, Nudo F, Sforza D, Fantola G, Cimaglia C, Verdirosi D, Virdone S, Serraino D; Italian Transplant and Cancer Cohort Study. Head and neck and esophageal cancers after liver transplant: Results from a multicenter cohort study. Italy, 1997-2010. Transpl Int. 2015;28:841-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 54. | Bellamy CO, DiMartini AM, Ruppert K, Jain A, Dodson F, Torbenson M, Starzl TE, Fung JJ, Demetris AJ. Liver transplantation for alcoholic cirrhosis: Long term follow-up and impact of disease recurrence. Transplantation. 2001;72:619-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 112] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 55. | Jain A, DiMartini A, Kashyap R, Youk A, Rohal S, Fung J. Long-term follow-up after liver transplantation for alcoholic liver disease under tacrolimus. Transplantation. 2000;70:1335-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 89] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 56. | Daniel KE, Eickhoff J, Lucey MR. Why do patients die after a liver transplantation? Clin Transplant. 2017;31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 57. | Kocher F, Finkenstedt A, Fiegl M, Graziadei I, Gamerith G, Oberaigner W, Vogel W, Hilbe W. Liver Transplantation-Associated Lung Cancer: Comparison of Clinical Parameters and Outcomes. Clin Lung Cancer. 2015;16:e75-e81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 58. | Vera A, Gunson BK, Ussatoff V, Nightingale P, Candinas D, Radley S, Mayer A, Buckels JA, McMaster P, Neuberger J, Mirza DF. Colorectal cancer in patients with inflammatory bowel disease after liver transplantation for primary sclerosing cholangitis. Transplantation. 2003;75:1983-1988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 109] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 59. | Brentnall TA, Haggitt RC, Rabinovitch PS, Kimmey MB, Bronner MP, Levine DS, Kowdley KV, Stevens AC, Crispin DA, Emond M, Rubin CE. Risk and natural history of colonic neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis. Gastroenterology. 1996;110:331-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 206] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 60. | Higashi H, Yanaga K, Marsh JW, Tzakis A, Kakizoe S, Starzl TE. Development of colon cancer after liver transplantation for primary sclerosing cholangitis associated with ulcerative colitis. Hepatology. 1990;11:477-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 90] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 61. | Lindor KD, Kowdley KV, Harrison ME; American College of Gastroenterology. ACG Clinical Guideline: Primary Sclerosing Cholangitis. Am J Gastroenterol. 2015;110:646-59; quiz 660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 337] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 62. | Mouchli MA, Singh S, Loftus EV, Boardman L, Talwalkar J, Rosen CB, Heimbach JK, Wiesner RH, Hasan B, Poterucha JJ, Kymberly WD. Risk Factors and Outcomes of De Novo Cancers (Excluding Nonmelanoma Skin Cancer) After Liver Transplantation for Primary Sclerosing Cholangitis. Transplantation. 2017;101:1859-1866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 63. | Singh S, Loftus EV, Talwalkar JA. Inflammatory bowel disease after liver transplantation for primary sclerosing cholangitis. Am J Gastroenterol. 2013;108:1417-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 64. | Buell JF, Papaconstantinou HT, Skalow B, Hanaway MJ, Alloway RR, Woodle ES. De novo colorectal cancer: Five-year survival is markedly lower in transplant recipients compared with the general population. Transplant Proc. 2005;37:960-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 65. | Johnson EE, Leverson GE, Pirsch JD, Heise CP. A 30-year analysis of colorectal adenocarcinoma in transplant recipients and proposal for altered screening. J Gastrointest Surg. 2007;11:272-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 66. | Carenco C, Faure S, Herrero A, Assenat E, Duny Y, Danan G, Bismuth M, Chanques G, Ursic-Bedoya J, Jaber S, Larrey D, Navarro F, Pageaux GP. Incidence of solid organ cancers after liver transplantation: Comparison with regional cancer incidence rates and risk factors. Liver Int. 2015;35:1748-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 67. | Nordin A, Åberg F, Pukkala E, Pedersen CR, Storm HH, Rasmussen A, Bennet W, Olausson M, Wilczek H, Ericzon BG, Tretli S, Line PD, Karlsen TH, Boberg KM, Isoniemi H. Decreasing incidence of cancer after liver transplantation-A Nordic population-based study over 3 decades. Am J Transplant. 2018;18:952-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 68. | Tanaka N, Ueno T, Takama Y, Yamanaka H, Tazuke Y, Bessho K, Okuyama H. Fibroadenoma in adolescent females after living donor liver transplantation. Pediatr Transplant. 2017;21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 69. | Iaria G, Pisani F, De Luca L, Sforza D, Manuelli M, Perrone L, Bellini I, Angelico R, Tisone G. Prospective study of switch from cyclosporine to tacrolimus for fibroadenomas of the breast in kidney transplantation. Transplant Proc. 2010;42:1169-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 70. | Foxwell BM, Woerly G, Husi H, Mackie A, Quesniaux VF, Hiestand PC, Wenger RM, Ryffel B. Identification of several cyclosporine binding proteins in lymphoid and non-lymphoid cells in vivo. Biochim Biophys Acta. 1992;1138:115-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 71. | Jiang K, He B, Lai L, Chen Q, Liu Y, Guo Q, Wang Q. Cyclosporine A inhibits breast cancer cell growth by downregulating the expression of pyruvate kinase subtype M2. Int J Mol Med. 2012;30:302-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 72. | Jonas S, Rayes N, Neumann U, Neuhaus R, Bechstein WO, Guckelberger O, Tullius SG, Serke S, Neuhaus P. De novo malignancies after liver transplantation using tacrolimus-based protocols or cyclosporine-based quadruple immunosuppression with an interleukin-2 receptor antibody or antithymocyte globulin. Cancer. 1997;80:1141-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 73. | Saigal S, Norris S, Muiesan P, Rela M, Heaton N, O'Grady J. Evidence of differential risk for posttransplantation malignancy based on pretransplantation cause in patients undergoing liver transplantation. Liver Transpl. 2002;8:482-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 115] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 74. | Soltys KA, Mazariegos GV, Squires RH, Sindhi RK, Anand R; SPLIT Research Group. Late graft loss or death in pediatric liver transplantation: An analysis of the SPLIT database. Am J Transplant. 2007;7:2165-2171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 114] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 75. | Simard JF, Baecklund E, Kinch A, Brattström C, Ingvar A, Molin D, Adami J, Fernberg P, Wilczek H, Ekbom A, Smedby KE. Pediatric organ transplantation and risk of premalignant and malignant tumors in Sweden. Am J Transplant. 2011;11:146-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 76. | Debray D, Baudouin V, Lacaille F, Charbit M, Rivet C, Harambat J, Iserin F, Di Filippo S, Guyot C; Pediatric Transplantation Working Group of the French Speaking Society of Transplantation. De novo malignancy after solid organ transplantation in children. Transplant Proc. 2009;41:674-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 77. | Karakoyun M, Önen Ş, Baran M, Çakır M, Ömür Ecevit Ç, Kılıç M, Kantar M, Aksoylar S, Özgenç F, Aydoğdu S. Post-transplant malignancies in pediatric liver transplant recipients: Experience of two centers in Turkey. Turk J Gastroenterol. 2018;29:89-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 78. | Buell JF, Gross TG, Thomas MJ, Neff G, Muthiah C, Alloway R, Ryckman FC, Tiao GM, Woodle ES. Malignancy in pediatric transplant recipients. Semin Pediatr Surg. 2006;15:179-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 79. | Aucejo F, Rofaiel G, Miller C. Who is at risk for post-transplant lymphoproliferative disorders (PTLD) after liver transplantation? J Hepatol. 2006;44:19-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 80. | Mynarek M, Schober T, Behrends U, Maecker-Kolhoff B. Posttransplant lymphoproliferative disease after pediatric solid organ transplantation. Clin Dev Immunol. 2013;2013:814973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 81. | Aberg F, Pukkala E, Höckerstedt K, Sankila R, Isoniemi H. Risk of malignant neoplasms after liver transplantation: A population-based study. Liver Transpl. 2008;14:1428-1436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 129] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 82. | Neuberger JM, Bechstein WO, Kuypers DR, Burra P, Citterio F, De Geest S, Duvoux C, Jardine AG, Kamar N, Krämer BK, Metselaar HJ, Nevens F, Pirenne J, Rodríguez-Perálvarez ML, Samuel D, Schneeberger S, Serón D, Trunečka P, Tisone G, van Gelder T. Practical Recommendations for Long-term Management of Modifiable Risks in Kidney and Liver Transplant Recipients: A Guidance Report and Clinical Checklist by the Consensus on Managing Modifiable Risk in Transplantation (COMMIT) Group. Transplantation. 2017;101:S1-S56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 221] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 83. | Rodríguez-Perálvarez M, De la Mata M, Burroughs AK. Liver transplantation: Immunosuppression and oncology. Curr Opin Organ Transplant. 2014;19:253-260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 84. | Alberú J, Pascoe MD, Campistol JM, Schena FP, Rial Mdel C, Polinsky M, Neylan JF, Korth-Bradley J, Goldberg-Alberts R, Maller ES; Sirolimus CONVERT Trial Study Group. Lower malignancy rates in renal allograft recipients converted to sirolimus-based, calcineurin inhibitor-free immunotherapy: 24-month results from the CONVERT trial. Transplantation. 2011;92:303-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 168] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 85. | Kauffman HM, Cherikh WS, Cheng Y, Hanto DW, Kahan BD. Maintenance immunosuppression with target-of-rapamycin inhibitors is associated with a reduced incidence of de novo malignancies. Transplantation. 2005;80:883-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 463] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 86. | Thimonier E, Guillaud O, Walter T, Decullier E, Vallin M, Boillot O, Dumortier J. Conversion to everolimus dramatically improves the prognosis of de novo malignancies after liver transplantation for alcoholic liver disease. Clin Transplant. 2014;28:1339-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 87. | Kaltenborn A, Schrem H. Mycophenolate mofetil in liver transplantation: A review. Ann Transplant. 2013;18:685-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 88. | Bhat M, Mara K, Dierkhising R, Watt KDS. Immunosuppression, Race, and Donor-Related Risk Factors Affect De novo Cancer Incidence Across Solid Organ Transplant Recipients. Mayo Clin Proc. 2018;93:1236-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 89. | Zhu H, Sun Q, Tan C, Xu M, Dai Z, Wang Z, Fan J, Zhou J. Tacrolimus promotes hepatocellular carcinoma and enhances CXCR4/SDF‑1α expression in vivo. Mol Med Rep. 2014;10:585-592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 90. | Angelico R, Parente A, Manzia TM. Using a weaning immunosuppression protocol in liver transplantation recipients with hepatocellular carcinoma: A compromise between the risk of recurrence and the risk of rejection? Transl Gastroenterol Hepatol. 2017;2:74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 91. | Lerut J, Sanchez-Fueyo A. An appraisal of tolerance in liver transplantation. Am J Transplant. 2006;6:1774-1780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 160] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 92. | De Geest S, Burkhalter H, Bogert L, Berben L, Glass TR, Denhaerynck K; Psychosocial Interest Group; Swiss Transplant Cohort Study. Describing the evolution of medication nonadherence from pretransplant until 3 years post-transplant and determining pretransplant medication nonadherence as risk factor for post-transplant nonadherence to immunosuppressives: The Swiss Transplant Cohort Study. Transpl Int. 2014;27:657-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 93. | O'Carroll RE, McGregor LM, Swanson V, Masterton G, Hayes PC. Adherence to medication after liver transplantation in Scotland: A pilot study. Liver Transpl. 2006;12:1862-1868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 71] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 94. | Griva K, Davenport A, Harrison M, Newman SP. Non-adherence to immunosuppressive medications in kidney transplantation: Intent vs. forgetfulness and clinical markers of medication intake. Ann Behav Med. 2012;44:85-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 95. | De Bleser L, Dobbels F, Berben L, Vanhaecke J, Verleden G, Nevens F, De Geest S. The spectrum of nonadherence with medication in heart, liver, and lung transplant patients assessed in various ways. Transpl Int. 2011;24:882-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 96. | Prendergast MB, Gaston RS. Optimizing medication adherence: An ongoing opportunity to improve outcomes after kidney transplantation. Clin J Am Soc Nephrol. 2010;5:1305-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 97. | Tielen M, van Exel J, Laging M, Beck DK, Khemai R, van Gelder T, Betjes MG, Weimar W, Massey EK. Attitudes to medication after kidney transplantation and their association with medication adherence and graft survival: A 2-year follow-up study. J Transplant. 2014;2014:675301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 98. | Orlando G, Soker S, Wood K. Operational tolerance after liver transplantation. J Hepatol. 2009;50:1247-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 127] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 99. | Orlando G. Finding the right time for weaning off immunosuppression in solid organ transplant recipients. Expert Rev Clin Immunol. 2010;6:879-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 100. | Sánchez-Fueyo A. Identification of tolerant recipients following liver transplantation. Int Immunopharmacol. 2010;10:1501-1504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 101. | Sánchez-Fueyo A. Hot-topic debate on tolerance: Immunosuppression withdrawal. Liver Transpl. 2011;17 Suppl 3:S69-S73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 102. | Sumimoto R, Kamada N. Specific suppression of allograft rejection by soluble class I antigen and complexes with monoclonal antibody. Transplantation. 1990;50:678-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 111] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 103. | Sriwatanawongsa V, Davies HS, Calne RY. The essential roles of parenchymal tissues and passenger leukocytes in the tolerance induced by liver grafting in rats. Nat Med. 1995;1:428-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 101] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 104. | Qian S, Demetris AJ, Murase N, Rao AS, Fung JJ, Starzl TE. Murine liver allograft transplantation: Tolerance and donor cell chimerism. Hepatology. 1994;19:916-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 346] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 105. | Jucaud V, Shaked A, DesMarais M, Sayre P, Feng S, Levitsky J, Everly MJ. Prevalence and Impact of De Novo Donor-Specific Antibodies During a Multicenter Immunosuppression Withdrawal Trial in Adult Liver Transplant Recipients. Hepatology. 2019;69:1273-1286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 106. | Manzia TM, Angelico R, Baiocchi L, Toti L, Ciano P, Palmieri G, Angelico M, Orlando G, Tisone G. The Tor Vergata weaning of immunosuppression protocols in stable hepatitis C virus liver transplant patients: The 10-year follow-up. Transpl Int. 2013;26:259-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 107. | Manzia TM, Angelico R, Toti L, Angelico C, Quaranta C, Parente A, Blasi F, Iesari S, Sforza D, Baiocchi L, Lerut J, Tisone G. Longterm Survival and Cost-Effectiveness of Immunosuppression Withdrawal After Liver Transplantation. Liver Transpl. 2018;24:1199-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 108. | Orlando G, Manzia T, Baiocchi L, Sanchez-Fueyo A, Angelico M, Tisone G. The Tor Vergata weaning off immunosuppression protocol in stable HCV liver transplant patients: The updated follow up at 78 months. Transpl Immunol. 2008;20:43-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 70] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 109. | Tisone G, Orlando G, Cardillo A, Palmieri G, Manzia TM, Baiocchi L, Lionetti R, Anselmo A, Toti L, Angelico M. Complete weaning off immunosuppression in HCV liver transplant recipients is feasible and favourably impacts on the progression of disease recurrence. J Hepatol. 2006;44:702-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 125] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 110. | Ramos HC, Reyes J, Abu-Elmagd K, Zeevi A, Reinsmoen N, Tzakis A, Demetris AJ, Fung JJ, Flynn B, McMichael J. Weaning of immunosuppression in long-term liver transplant recipients. Transplantation. 1995;59:212-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 111. | Takatsuki M, Uemoto S, Inomata Y, Egawa H, Kiuchi T, Fujita S, Hayashi M, Kanematsu T, Tanaka K. Weaning of immunosuppression in living donor liver transplant recipients. Transplantation. 2001;72:449-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 214] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 112. | Oike F, Yokoi A, Nishimura E, Ogura Y, Fujimoto Y, Kasahara M, Kaihara S, Kiuchi T, Egawa H, Uemoto S, Tanaka K. Complete withdrawal of immunosuppression in living donor liver transplantation. Transplant Proc. 2002;34:1521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 113. | Koshiba T, Li Y, Takemura M, Wu Y, Sakaguchi S, Minato N, Wood KJ, Haga H, Ueda M, Uemoto S. Clinical, immunological, and pathological aspects of operational tolerance after pediatric living-donor liver transplantation. Transpl Immunol. 2007;17:94-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 144] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 114. | Ohe H, Waki K, Yoshitomi M, Morimoto T, Nafady-Hego H, Satoda N, Li Y, Zhao X, Sakaguchi S, Uemoto S, Bishop GA, Koshiba T. Factors affecting operational tolerance after pediatric living-donor liver transplantation: Impact of early post-transplant events and HLA match. Transpl Int. 2012;25:97-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 115. | Yoshitomi M, Koshiba T, Haga H, Li Y, Zhao X, Cheng D, Miyagawa A, Sakashita H, Tsuruyama T, Ohe H, Ueda M, Okamoto S, Egawa H, Wood K, Sakaguchi S, Manabe T, Tanaka K, Uemoto S. Requirement of protocol biopsy before and after complete cessation of immunosuppression after liver transplantation. Transplantation. 2009;87:606-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 116. | Hurwitz M, Desai DM, Cox KL, Berquist WE, Esquivel CO, Millan MT. Complete immunosuppressive withdrawal as a uniform approach to post-transplant lymphoproliferative disease in pediatric liver transplantation. Pediatr Transplant. 2004;8:267-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 117. | Lee JH, Lee SK, Lee HJ, Seo JM, Joh JW, Kim SJ, Kwon CH, Choe YH. Withdrawal of immunosuppression in pediatric liver transplant recipients in Korea. Yonsei Med J. 2009;50:784-788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 118. | Feng S, Ekong UD, Lobritto SJ, Demetris AJ, Roberts JP, Rosenthal P, Alonso EM, Philogene MC, Ikle D, Poole KM, Bridges ND, Turka LA, Tchao NK. Complete immunosuppression withdrawal and subsequent allograft function among pediatric recipients of parental living donor liver transplants. JAMA. 2012;307:283-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 263] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 119. | Feng S, Demetris AJ, Spain KM, Kanaparthi S, Burrell BE, Ekong UD, Alonso EM, Rosenthal P, Turka LA, Ikle D, Tchao NK. Five-year histological and serological follow-up of operationally tolerant pediatric liver transplant recipients enrolled in WISP-R. Hepatology. 2017;65:647-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 120. | Waki K, Sugawara Y, Mizuta K, Taniguchi M, Ozawa M, Hirata M, Nozawa M, Kaneko J, Takahashi K, Kadowaki T, Terasaki PI, Kokudo N. Predicting operational tolerance in pediatric living-donor liver transplantation by absence of HLA antibodies. Transplantation. 2013;95:177-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 121. | Lin NC, Wang HK, Yeh YC, Liu CP, Loong CC, Tsai HL, Chen CY, Chin T, Liu C. Minimization or withdrawal of immunosuppressants in pediatric liver transplant recipients. J Pediatr Surg. 2015;50:2128-2133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 122. | Santopaolo F, Lenci I, Milana M, Manzia TM, Baiocchi L. Liver transplantation for hepatocellular carcinoma: Where do we stand? World J Gastroenterol. 2019;25:2591-2602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 83] [Cited by in RCA: 78] [Article Influence: 13.0] [Reference Citation Analysis (3)] |
| 123. | Cholongitas E, Mamou C, Rodríguez-Castro KI, Burra P. Mammalian target of rapamycin inhibitors are associated with lower rates of hepatocellular carcinoma recurrence after liver transplantation: A systematic review. Transpl Int. 2014;27:1039-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 134] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 124. | Rodríguez-Perálvarez M, Tsochatzis E, Naveas MC, Pieri G, García-Caparrós C, O'Beirne J, Poyato-González A, Ferrín-Sánchez G, Montero-Álvarez JL, Patch D, Thorburn D, Briceño J, De la Mata M, Burroughs AK. Reduced exposure to calcineurin inhibitors early after liver transplantation prevents recurrence of hepatocellular carcinoma. J Hepatol. 2013;59:1193-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 188] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 125. | Geissler EK, Schnitzbauer AA, Zülke C, Lamby PE, Proneth A, Duvoux C, Burra P, Jauch KW, Rentsch M, Ganten TM, Schmidt J, Settmacher U, Heise M, Rossi G, Cillo U, Kneteman N, Adam R, van Hoek B, Bachellier P, Wolf P, Rostaing L, Bechstein WO, Rizell M, Powell J, Hidalgo E, Gugenheim J, Wolters H, Brockmann J, Roy A, Mutzbauer I, Schlitt A, Beckebaum S, Graeb C, Nadalin S, Valente U, Turrión VS, Jamieson N, Scholz T, Colledan M, Fändrich F, Becker T, Söderdahl G, Chazouillères O, Mäkisalo H, Pageaux GP, Steininger R, Soliman T, de Jong KP, Pirenne J, Margreiter R, Pratschke J, Pinna AD, Hauss J, Schreiber S, Strasser S, Klempnauer J, Troisi RI, Bhoori S, Lerut J, Bilbao I, Klein CG, Königsrainer A, Mirza DF, Otto G, Mazzaferro V, Neuhaus P, Schlitt HJ. Sirolimus Use in Liver Transplant Recipients With Hepatocellular Carcinoma: A Randomized, Multicenter, Open-Label Phase 3 Trial. Transplantation. 2016;100:116-125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 245] [Cited by in RCA: 349] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 126. | Adams DH, Sanchez-Fueyo A, Samuel D. From immunosuppression to tolerance. J Hepatol. 2015;62:S170-S185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 122] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 127. | Orlando G, Hematti P, Stratta RJ, Burke GW, Di Cocco P, Pisani F, Soker S, Wood K. Clinical operational tolerance after renal transplantation: Current status and future challenges. Ann Surg. 2010;252:915-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 128. | Karam VH, Gasquet I, Delvart V, Hiesse C, Dorent R, Danet C, Samuel D, Charpentier B, Gandjbakhch I, Bismuth H, Castaing D. Quality of life in adult survivors beyond 10 years after liver, kidney, and heart transplantation. Transplantation. 2003;76:1699-1704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 122] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 129. | Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4510] [Cited by in RCA: 4127] [Article Influence: 172.0] [Reference Citation Analysis (0)] |
| 130. | Londoño MC, Danger R, Giral M, Soulillou JP, Sánchez-Fueyo A, Brouard S. A need for biomarkers of operational tolerance in liver and kidney transplantation. Am J Transplant. 2012;12:1370-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 131. | Feng S, Bucuvalas J. Tolerance after liver transplantation: Where are we? Liver Transpl. 2017;23:1601-1614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 132. | Park KM, Hussein KH, Hong SH, Ahn C, Yang SR, Park SM, Kweon OK, Kim BM, Woo HM. Decellularized Liver Extracellular Matrix as Promising Tools for Transplantable Bioengineered Liver Promotes Hepatic Lineage Commitments of Induced Pluripotent Stem Cells. Tissue Eng Part A. 2016;22:449-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 90] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 133. | Edgar L, Altamimi A, García Sánchez M, Tamburrinia R, Asthana A, Gazia C, Orlando G. Utility of extracellular matrix powders in tissue engineering. Organogenesis. 2018;14:172-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 134. | Jain AB, Yee LD, Nalesnik MA, Youk A, Marsh G, Reyes J, Zak M, Rakela J, Irish W, Fung JJ. Comparative incidence of de novo nonlymphoid malignancies after liver transplantation under tacrolimus using surveillance epidemiologic end result data. Transplantation. 1998;66:1193-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 152] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 135. | Kelly DM, Emre S, Guy SR, Miller CM, Schwartz ME, Sheiner PA. Liver transplant recipients are not at increased risk for nonlymphoid solid organ tumors. Cancer. 1998;83:1237-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 136. | Jiménez C, Rodríguez D, Marqués E, Loinaz C, Alonso O, Hernández-Vallejo G, Marín L, Rodríguez F, García I, Moreno E. De novo tumors after orthotopic liver transplantation. Transplant Proc. 2002;34:297-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 137. | Sanchez EQ, Marubashi S, Jung G, Levy MF, Goldstein RM, Molmenti EP, Fasola CG, Gonwa TA, Jennings LW, Brooks BK, Klintmalm GB. De novo tumors after liver transplantation: A single-institution experience. Liver Transpl. 2002;8:285-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 100] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 138. | Oo YH, Gunson BK, Lancashire RJ, Cheng KK, Neuberger JM. Incidence of cancers following orthotopic liver transplantation in a single center: Comparison with national cancer incidence rates for England and Wales. Transplantation. 2005;80:759-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 68] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 139. | Baccarani U, Piselli P, Serraino D, Adani GL, Lorenzin D, Gambato M, Buda A, Zanus G, Vitale A, De Paoli A, Cimaglia C, Bresadola V, Toniutto P, Risaliti A, Cillo U, Bresadola F, Burra P. Comparison of de novo tumours after liver transplantation with incidence rates from Italian cancer registries. Dig Liver Dis. 2010;42:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 140. | Chatrath H, Berman K, Vuppalanchi R, Slaven J, Kwo P, Tector AJ, Chalasani N, Ghabril M. De novo malignancy post-liver transplantation: A single center, population controlled study. Clin Transplant. 2013;27:582-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 141. | Schrem H, Kurok M, Kaltenborn A, Vogel A, Walter U, Zachau L, Manns MP, Klempnauer J, Kleine M. Incidence and long-term risk of de novo malignancies after liver transplantation with implications for prevention and detection. Liver Transpl. 2013;19:1252-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 142. | Egeli T, Unek T, Ozbilgin M, Agalar C, Derici S, Akarsu M, Unek IT, Aysin M, Bacakoglu A, Astarcıoglu I. De Novo Malignancies After Liver Transplantation: A Single Institution Experience. Exp Clin Transplant. 2019;17:74-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 143. | Taborelli M, Piselli P, Ettorre GM, Lauro A, Galatioto L, Baccarani U, Rendina M, Shalaby S, Petrara R, Nudo F, Toti L, Sforza D, Fantola G, Cimaglia C, Agresta A, Vennarecci G, Pinna AD, Gruttadauria S, Risaliti A, Di Leo A, Burra P, Rossi M, Tisone G, Zamboni F, Serraino D; Italian Transplant & Cancer Cohort Study. Risk of virus and non-virus related malignancies following immunosuppression in a cohort of liver transplant recipients. Italy, 1985-2014. Int J Cancer. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 144. | Devlin J, Doherty D, Thomson L, Wong T, Donaldson P, Portmann B, Williams R. Defining the outcome of immunosuppression withdrawal after liver transplantation. Hepatology. 1998;27:926-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 173] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 145. | Eason JD, Cohen AJ, Nair S, Alcantera T, Loss GE. Tolerance: Is it worth the risk? Transplantation. 2005;79:1157-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 83] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 146. | Girlanda R, Rela M, Williams R, O'Grady JG, Heaton ND. Long-term outcome of immunosuppression withdrawal after liver transplantation. Transplant Proc. 2005;37:1708-1709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 66] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 147. | Assy N, Adams PC, Myers P, Simon V, Minuk GY, Wall W, Ghent CN. Randomized controlled trial of total immunosuppression withdrawal in liver transplant recipients: Role of ursodeoxycholic acid. Transplantation. 2007;83:1571-1576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 148. | Pons JA, Revilla-Nuin B, Baroja-Mazo A, Ramírez P, Martínez-Alarcón L, Sánchez-Bueno F, Robles R, Rios A, Aparicio P, Parrilla P. FoxP3 in peripheral blood is associated with operational tolerance in liver transplant patients during immunosuppression withdrawal. Transplantation. 2008;86:1370-1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 144] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 149. | Tryphonopoulos P, Ruiz P, Weppler D, Nishida S, Levi DM, Moon J, Tekin A, Velez M, Neuman DR, Island E, Selvaggi G, Tzakis AG. Long-term follow-up of 23 operational tolerant liver transplant recipients. Transplantation. 2010;90:1556-1561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 150. | de la Garza RG, Sarobe P, Merino J, Lasarte JJ, D'Avola D, Belsue V, Delgado JA, Silva L, Iñarrairaegui M, Sangro B, Sola JJ, Pardo F, Quiroga J, Herrero JI. Trial of complete weaning from immunosuppression for liver transplant recipients: Factors predictive of tolerance. Liver Transpl. 2013;19:937-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 151. | Benítez C, Londoño MC, Miquel R, Manzia TM, Abraldes JG, Lozano JJ, Martínez-Llordella M, López M, Angelico R, Bohne F, Sese P, Daoud F, Larcier P, Roelen DL, Claas F, Whitehouse G, Lerut J, Pirenne J, Rimola A, Tisone G, Sánchez-Fueyo A. Prospective multicenter clinical trial of immunosuppressive drug withdrawal in stable adult liver transplant recipients. Hepatology. 2013;58:1824-1835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 250] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 152. | Bohne F, Londoño MC, Benítez C, Miquel R, Martínez-Llordella M, Russo C, Ortiz C, Bonaccorsi-Riani E, Brander C, Bauer T, Protzer U, Jaeckel E, Taubert R, Forns X, Navasa M, Berenguer M, Rimola A, Lozano JJ, Sánchez-Fueyo A. HCV-induced immune responses influence the development of operational tolerance after liver transplantation in humans. Sci Transl Med. 2014;6:242ra81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 153. | Todo S, Yamashita K, Goto R, Zaitsu M, Nagatsu A, Oura T, Watanabe M, Aoyagi T, Suzuki T, Shimamura T, Kamiyama T, Sato N, Sugita J, Hatanaka K, Bashuda H, Habu S, Demetris AJ, Okumura K. A pilot study of operational tolerance with a regulatory T-cell-based cell therapy in living donor liver transplantation. Hepatology. 2016;64:632-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 329] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 154. | Shaked A. Gradual Withdrawal of Immune System Suppressing Drugs in Patients Receiving a Liver Transplant. [accessed]. 2019;ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine Available from: https://ClinicalTrials.gov/show/NCT00135694 ClinicalTrials.gov Identifier: NCT00135694. |
| 155. | Markman JF. Evaluation of Donor Specific Immune Senescence and Exhaustion as Biomarkers of Tolerance Post Liver Transplantation. [accessed]. 2019;ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine Available from: https://ClinicalTrials.gov/show/NCT02533180 ClinicalTrials.gov Identifier: NCT02533180. |
| 156. | Markman JF, Feng S. Liver Transplantation With Tregs at MGH. [accessed]. 2019;ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine Available from: https://ClinicalTrials.gov/show/NCT03577431 ClinicalTrials.gov Identifier: NCT03577431. |
| 157. | Mazariegos GV, Reyes J, Marino IR, Demetris AJ, Flynn B, Irish W, McMichael J, Fung JJ, Starzl TE. Weaning of immunosuppression in liver transplant recipients. Transplantation. 1997;63:243-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 290] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 158. | Feng S. Withdrawal of Immunosuppression in Pediatric Liver Transplant Recipients. [accessed]. 2019;ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://ClinicalTrials.gov/show/NCT00320606 ClinicalTrials.gov Identifier: NCT00320606. |
| 159. | Feng S, Bucuvalas J, Demetris A, Spain K, Kanaparthi S, Magee J, Mazariegos G. The iWITH Investigators Primary Outcome of iWITH: A Multi-Center Clinical Trial of Complete Immunosuppression Withdrawal (ISW) in Stable Pediatric Liver Transplant (LT) Recipients. Am J Transplant. 2016;16:2016. |