Published online Aug 21, 2019. doi: 10.3748/wjg.v25.i31.4360
Peer-review started: April 22, 2019
First decision: June 10, 2019
Revised: June 24, 2019
Accepted: July 19, 2019
Article in press: July 19, 2019
Published online: August 21, 2019
Processing time: 122 Days and 12.5 Hours
Hepatocellular carcinoma is one of the most frequent malignant tumors worldwide: Portal vein tumor thrombosis (PVTT) occurs in about 35%-50% of patients and represents a strong negative prognostic factor, due to the increased risk of tumor spread into the bloodstream, leading to a high recurrence risk. For this reason, it is a contraindication to liver transplantation and in several prognostic scores sorafenib represents its standard of care, due to its antiangiogenetic action, although it can grant only a poor prolongation of life expectancy. Recent scientific evidences lead to consider PVTT as a complex anatomical and clinical condition, including a wide range of patients with different prognosis and new treatment possibilities according to the degree of portal system involvement, tumor biological aggressiveness, complications caused by portal hypertension, patient’s clinical features and tolerance to antineoplastic treatments. The median survival has been reported to range between 2.7 and 4 mo in absence of therapy, but it can vary from 5 mo to 5 years, thus depicting an extremely variable scenario. For this reason, it is extremely important to focus on the most adequate strategy to be applied to each group of PVTT patients.
Core tip: Portal vein tumor thrombosis (PVTT) is a complex anatomical and clinical condition, including patients with different prognosis according to the degree of portal system involvement, tumor biological aggressiveness, complications caused by portal hypertension, patient’s clinical features and tolerance to antineoplastic treatments. For this reason, it is extremely important to focus on the most adequate treatment strategy for each group of PVTT patients.
- Citation: Cerrito L, Annicchiarico BE, Iezzi R, Gasbarrini A, Pompili M, Ponziani FR. Treatment of hepatocellular carcinoma in patients with portal vein tumor thrombosis: Beyond the known frontiers. World J Gastroenterol 2019; 25(31): 4360-4382
- URL: https://www.wjgnet.com/1007-9327/full/v25/i31/4360.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i31.4360
Hepatocellular carcinoma (HCC) is one of the most frequent malignant tumors worldwide accounting for 749000 new cases/year and about 745000 deaths/year, with an incidence that ranges from less than 5 per 100000 individuals in Northern Europe, United States and Japan to over 20 per 100000 in sub-Saharan Africa and Eastern Asia[1-3].
Portal vein tumor thrombosis (PVTT) occurs in about 35%-50% of patients[4] and involves the main trunk at the time of diagnosis in 15%-30% of cases[5]. However, PVTT prevalence is certainly underestimated, being incidentally found in about 62% of autoptic livers and in 14% of surgical specimens obtained from HCC patients[6]. In those with already known history of HCC, the probability of malignant vascular infiltration is as high as 30%, decreasing to 20% in case of contemporary diagnosis of both HCC and thrombosis[7].
The presence of PVTT is considered a strong negative prognostic factor, due to the increased risk of release into the bloodstream of cancer cells, leading to a high recurrence risk; for this reason, it is considered a contraindication to LT and is included in several HCC-prognostic scores [e.g., Barcelona-Clinic Liver Cancer (BCLC) staging system, French classification of HCC, Cancer of the Liver Italian Program (CLIP) classification, the Hong-Kong Liver Cancer (HKLC) staging system, Chinese University Prognostic Index (CUPI) score, and Japan Integrated Staging (JIS)][8-14]. In most of these classifications, sorafenib is the standard of care for patients with PVTT due to its antiangiogenetic action related to the inhibition of the vascular endothelial growth factor (VEGF). However, sorafenib can grant only a poor prolongation of life expectancy in these patients[15].
Recent scientific evidences lead to consider PVTT as a complex anatomical and clinical condition, including a wide range of patients with different prognosis and new treatment possibilities according to degree of the portal system involvement, tumor biological aggressiveness, patient’s clinical features and tolerance to antineoplastic treatments, severity of liver dysfunction, and complications caused by portal hypertension (e.g., variceal hemorrhage). The median survival has been reported to range between 2.7 and 4 mo in absence of therapy[16,17], but it can vary from 5 mo to 5 years according to liver function, tumor features and treatment applied, thus depicting an extremely variable scenario[4,8]. For this reason, it is extremely important to focus on the most adequate strategy to be applied to each group of PVTT patients.
Ultrasound (US) examination represents the first-line imaging technique for the detection of PVTT with sensitivity and specificity of 80%-100% and diagnostic accuracy of 88%-98%[18-20]. The thrombus appears as a hypo/isoechoic inhomogeneous material obstructing partially or completely the vessel, with an expansive aspect of the mass and difficult or absent detection of the infiltrated vascular walls. Colour and Power Doppler-US is able to detect pulsatile signs of arterial neovascularization within the endoluminal mass, while pulsed Doppler identifies high resistance index in intralesional arterial flow[18].
Contrast enhanced ultrasound (CEUS) represents the most sensitive and cheap method to depict neoplastic endovascular invasion, with a better diagnostic performance compared to computed tomography (CT)-scan (sensitivity 88%-100%, specificity 94%-96% in differential diagnosis between benign and neoplastic PVT)[21]; PVTT presents a HCC-like contrast behaviour, with a rapid wash-in during arterial phase and wash-out in the portal/late phase, while benign thrombus has no contrast enhancement in all the study phases[22].
CT-scan and magnetic resonance imaging (MRI) can distinguish between neoplastic and non-neoplastic portal thrombosis and add further information on thrombosis extension and presence of collateral vessels, with a sensitivity of 86% and 100% and a specificity of 100% and 90%, respectively. In particular, CT texture analysis and attenuation values grant the distinction between neoplastic and benign thrombosis on portal-phase CT imaging by analysing thrombus density, mean value of positive pixels and entropy[23-27].
Percutaneous US-guided fine-needle aspiration is another simple, safe and effective diagnostic method to distinguish non-neoplastic and neoplastic portal thrombosis when a definitive diagnosis is not attained by imaging methods[28].
Another promising tool to achieve differential diagnosis between non-malignant thrombosis and PVTT is 18F-Fluorodeoxyglucose positron emission tomography/CT (18F-FDG PET/CT), which seems to be able to identify metabolic abnormalities of the thrombus before the detection of the typical morphological and contrast enhanced signs detected on CT-scan. Hu et al[29] defined thrombus malignancy by visual analysis and a maximum standardized uptake value (SUVmax) > 3.35.
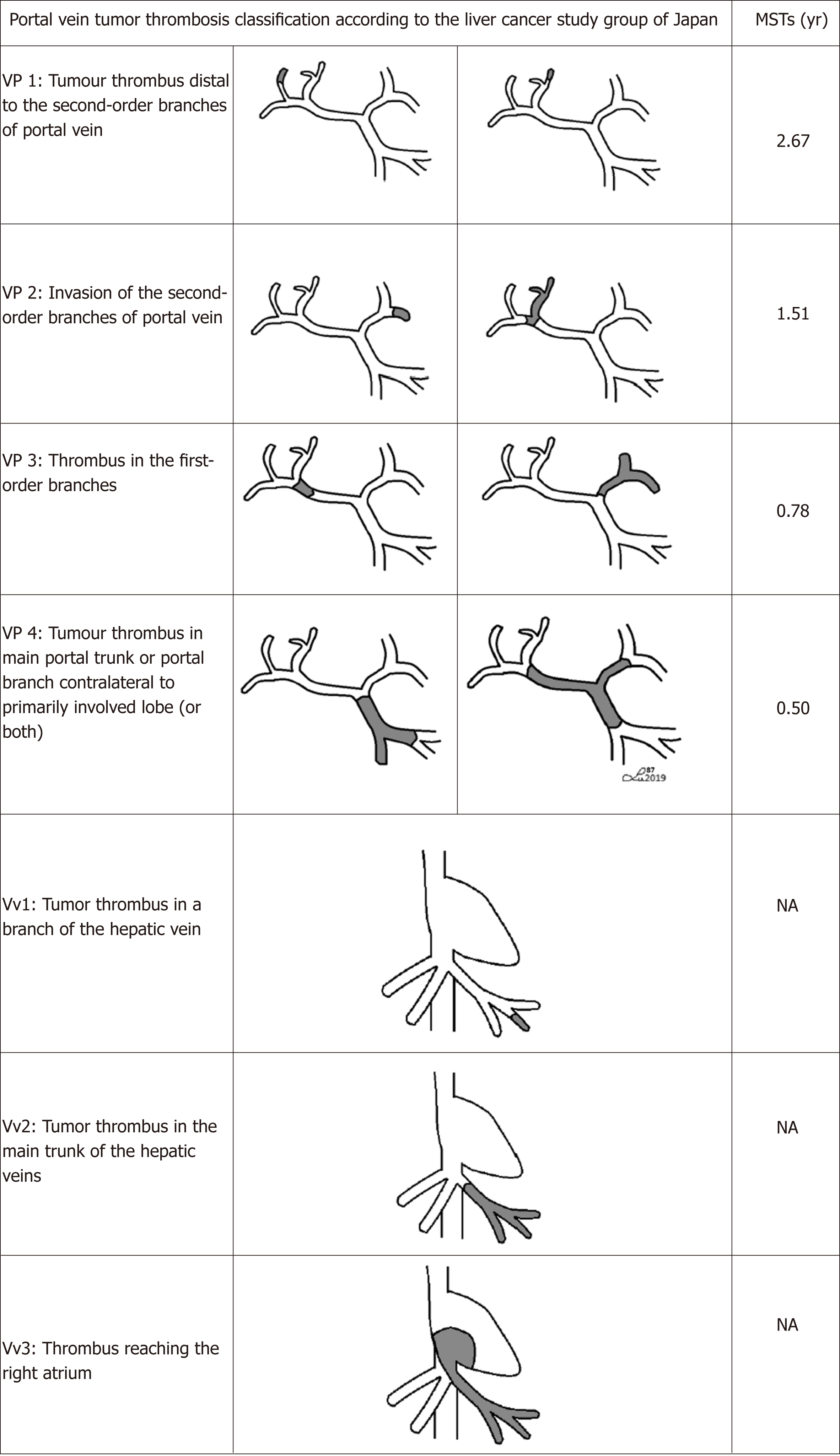
PVTT classification is crucial to define a proper therapeutic approach. There are many studies describing PVTT stages according to the classifications created in different medical centers. In 2010 Ikai et al[30,31] on behalf of the Liver Cancer Study Group of Japan (LCSGJ), distinguished four grades of PVTT and three grades of hepatic vein tumor involvement [Vp1: Presence of a tumor thrombus distal to the second-order branches of portal vein (but not involving them directly); Vp2: Invasion of the second-order branches of portal vein; Vp3: Presence of the thrombus in the first-order branches; Vp4: Tumor thrombus in the main trunk of the portal vein and/or a portal vein branch contralateral to the primarily involved lobe; Vv1: Tumor thrombus in a branch of the hepatic vein; Vv2: Tumor thrombus in the main trunk of the hepatic veins; Vv3: Thrombus reaching the right atrium] (Figure 1).
Another classification proposed by Cheng et al describes the portion of portal vein involved, identifying 4 categories: Type I0 indicating microscopic portal invasion, Type I combining Vp1 and Vp2, Type II corresponding to Vp3, Type III and IV indicating thrombus in the main trunk and involvement of superior mesenteric vein, respectively[32,33].
Finally, a simplified classification, divides patients with HCC and PVTT in group A (tumor thrombus in the main portal trunk or in both the left and right portal veins) and group B (involvement of either the left or the right portal vein)[34].
According to the Barcellona Clinic Liver Cancer (BCLC) classification, the recommended treatment for HCC with PVTT is sorafenib. However, many other treatment strategies have been attempted, with variable results. They include non-surgical procedures, such as systemic chemotherapy, radiation therapy, transarterial chemoembolization (TACE), microwave coagulation therapy (MCT), percutaneous ethanol injection (PEI), radiofrequency ablation (RFA), and surgical options such as resection and liver transplantation (LT).
Sorafenib is a multi-tyrosine-kinase inhibitor targeting Raf/Mek/Erk-pathways, vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) receptors, widely used as standard-of-care in the treatment of advanced HCC[35]. Its efficacy has been investigated by two phase-III-trials: In the Sorafenib Hepatocellular Carcinoma Assessment Randomized Protocol (SHARP, studying Western population), the median overall survival (OS) of patients with HCC and macrovascular invasion (n = 108 patients) was 8.1 mo vs 4.9 mo in the placebo group (n = 123 patients), while time to progression (TTP) was 4.1 mo vs 2.7 mo, respectively; these results were confirmed by the Asia-Pacific Trial, but, as sorafenib was only able to slightly prolong OS compared to placebo in advanced HCC (6.5 mo vs 4.2 mo, its effect in presence of PVTT was even smaller (5.6 mo vs 4.1 mo, TTP 2.7 mo vs 1.3 mo) in this study[35-38]. Yau et al[39] noticed a similar OS in both patients with and without PVTT treated with sorafenib. Subsequent studies in patients with PVTT classified as Vp2-3-4 did not reveal satisfying performances. Jeong et al[40] analysed the effects of sorafenib monotherapy in Vp3-4 PVTT, finding a 10% response rate and a 40% disease control rate (OS 3.1 mo; TTP 2.1 mo); the overall incidence of drug-related adverse events (AEs) was 90% and the most common AEs (grade 1-2) were dermatological, gastrointestinal and constitutional.
As regards the combination of systemic treatments with other techniques, Giorgio et al[41] demonstrated a better outcome for PVTT treated with both sorafenib and RFA compared to sorafenib alone (1-2-3 year survival rates were 60%, 35% and 26% vs 37%, 0% and 0%, respectively); AEs were almost superimposable in the two groups [abdominal pain, hand foot skin reaction (HFSR), asthenia, diarrhoea, weight loss]. A recent multicenter phase III trial by Park et al[42] demonstrated that, in advanced HCC, sorafenib combined with cTACE faced to sorafenib-alone does not achieve an increase in OS (12.8 mo vs 10.8 mo; HR = 0.91; 90%CI: 0.69-1.21; P = 0.290) but improved TTP (5.3 mo vs 3.5 mo; HR = 0.67; 90%CI: 0.53-0.85; P = 0.003), PFS (5.2 mo vs 3.6 mo; HR = 0.73; 90%CI: 0.59-0.91; P = 0.01) and tumor response rate (60.6% vs 47.3%, P = 0.005). Surprisingly, patients with Vp3-4 PVTT or invasion of other vessels seemed to have a survival benefit from sorafenib plus TACE, even though this trend was not statistically significant (HR = 0.52; 95%CI: 0.27–1.02). It is also relevant to point out that a high incidence of serious AEs was observed in the combination treatment group faced to sorafenib-alone (33.3% vs 19.8%, P = 0.006, respectively), including elevation of alanine aminotransferase (ALT), hyperbilirubinemia, ascites, HFSR, thrombocytopenia and anorexia.
It should be noted that data on the efficacy of other antiangiogenetic drugs in patients with PVTT are lacking. Indeed, in the registrative study of lenvatinib, which has been shown to be non-inferior to sorafenib for the treatment of patients with advanced HCC, patients with main portal trunk invasion were excluded[43].
Traditional chemotherapy is not usually included in HCC treatment algorithms because of its toxicity, which may be even more serious in patients with cirrhosis and liver function impairment.
Okada et al[44] conducted a phase II study in which different systemic chemotherapy regimens [Tegafur, Doxorubicin, Tegafur plus Uracil, Etoposide, Mitoxantrone, Interferon-gamma, Cisplatin, 5-fluorouracil (5-FU)] were administered to 71 patients with unresectable HCC, whose response varied from 0% to 20%; median survival time was 5.6 mo, 1-year and 2-years survival rates were 23% and 5%. Among them, 22 patients had Vp4 PVTT, with a reported median survival time of 3.9 mo. Itamoto et al[45] assessed hepatic arterial infusion of 5-FU and cisplatin in 7 patients with unresectable HCC and Vp3-4 PVTT: Response rate was 33%; a reduction in size or disappearance of thrombosis after chemotherapy was observed in 43% patients; mean and median survival times were 8 and 7.5 mo, respectively. No serious AEs occurred, whereas nausea and vomiting were the most common mild AEs requiring medical management. Yamasaki et al[46] obtained good results in a randomized study on HCC with PVTT by the addition of leucovorin to the protocol previously experimented by Itamoto[45], with a 56% response rate vs 20% (P = 0.022); indeed, for leucovorin arm, 1- and 2-year survival rates were 66.7% and 44.4% compared to 10% and 0% for the control group (P = 0.033).
Ando et al[47] examined 9 patients with advanced HCC and Vp4-PVTT for the efficacy of low-dose cisplatin and 5-FU arterial infusion by subcutaneously implanted injection-port: overall response rate was 44.4%, 3-year survival rate was 40%, median survival time 10.2 mo. Only tolerable AEs occurred (nausea, loss of appetite).
Interesting data also come from small studies using chemotherapy schemes including interferon (IFN)-alpha. Urabe et al[48] treated 16 patients with HCC and Vp3-4 PVTT with a combined scheme of methotrexate, 5-FU, cisplatin and IFN-alpha 2b; the reported response rate was 46.7%, median survival was 7 mo, and the 2-year survival rate was 57.1%. Transient severe hematologic AEs were registered and more than half of patients presented grade 2 nausea or vomiting. Similar results and safety data were described by Kaneko et al[49] using the same therapeutic regimen (overall response rate 45%, median survival 11 mo, 2-year survival 15%).
Some other studies showed encouraging results for the combination of subcutaneous IFN-alpha and intra-arterial 5-FU in the treatment of HCC with Vp3-4 PVTT: Response rates ranged from 43.6% to 63% and mean survival rates from 6.9 to 11.8 mo[50-52].
Hepatic arterial infusion chemotherapy (HAIC) has been also investigated in the treatment of PVTT, because of its property to carry anticancer drug directly at the tumor site, thus limiting systemic AEs. Song et al[53] showed that HAIC was more effective than sorafenib in the treatment of Vp2-4 PVTT (OS = 7.1 mo vs 5.5 mo, P = 0.011; TTP 3.3 mo vs 2.1 mo, P = 0.034); the most common AE in the sorafenib group was HFSR (45%, mainly grade 1-2), while in the HAIC group all patients had at least grade 1 AEs, and grade 3-4 AEs were more frequent, with 98% anemia, 84% thrombocytopenia, 74% bilirubin elevation and 72% ALT elevation (68% HAIC group vs 27% sorafenib group).
Ikeda et al[54] designed a phase II trial that proved a fair efficacy of intra-arterial cisplatin infusion in Vp3-4 PVTT, with a response rate of 28%, a mean survival time of 7.1 mo and grade 3 AEs (blood cell decrease, transient transaminases elevation) not requiring treatments. However, other similar experiences did not confirm such encouraging results[55-56].
Another interesting perspective is offered by metronomic chemotherapy protocol (MET) that should act by preventing tumor cell proliferation and angiogenesis and stimulating the immune system.
Yang et al[57] compared MET with cisplatin and 5-FU to sorafenib in the treatment of advanced HCC with PVTT, finding better outcomes as regards median survival time (158 d vs 117 d) and OS rate in the first group (P = 0.029). Main AEs in the MET group were leukopenia (48%), hyperbilirubinemia (30%), thrombocytopenia (22%), serum ALT elevation (17%); in the sorafenib group, mainly skin (28% HFSR) and gastrointestinal tract toxicity (40%), hyperbilirubinemia (36%) and increase in ALT serum levels (17%) were reported. The difference between grade 3-4 toxicity rates in both groups was not statistically significant. An Italian experience[58] proved that MET with capecitabine was effective in improving patients’ survival after sorafenib discontinuation compared to best supportive care. However, in this study, patients in the MET group showed a lower prevalence of PVTT, which represented itself a negative prognostic factor.
TACE is considered the preferential palliative therapy for multinodular HCC for patients with well-preserved liver function. TACE usefulness in PVTT, especially in type III/IV, remains uncertain, because of the limited effect on survival compared to systemic treatments and the potential risk of ischemia related post-TACE liver function failure; this risk appears increased if the collateral blood circulation surrounding the obstructed portal vein is insufficient[59-61]. The main studies concerning TACE in PVTT patients are summarized in Table 1.
| First author, year | Patients (patients) | Treatment | PVTT Class (Vp) | Median survival time(mo) | 1-yr survival rate | 2-yr survival rate | 3-yr survival rate | 5-yr survival rate |
| Okazaki M, 1991 | 163 | TACE | Vp 2 (48) Vp 3 (56) Vp 4 (59) | 4.3 mo 4 mo 3.8 mo | - | - | - | - |
| Chung JW, 1995 | 83 | TACE | Vp 3,4 (83) | 6 mo | 30% | 18% | 9% | - |
| Georgiades CS, 2005 | 32 | TACE | Vp 3,4 (32) | 9.5 mo | 25% | - | - | - |
| Luo J, 2011 | 84 | TACE | Vp 1,2 (40) Vp 3 (44) | 10.2 mo 5.3 mo | 30.9% 3.8% | 9.2% 0% | - | - |
| Niu ZJ, 2012 | 115 | TACE | Vp 1 (12) Vp 2 (52) Vp 3 (42) Vp 4 (9) | 19 11 7.1 4 | 27.8% | 6% | - | - |
| Peng ZW, 2012 | 402 | TACE | Vp 1 (54) Vp 2 (136) Vp 3 (166) Vp 4 (46) | - | 41.1% 37.9% 36.1% 30.4% | - | 8.9% 6% 4.2% 4.3% | 3.6% 0% 0% 0% |
| Ajit Y, 2014 | TACE | 6.2 mo | - | 22% | - | - | ||
| Liu L, 2014 | 188 | TACE | Vp 1,2 (98) Vp 3 (90) | 6 | 38% | 17% | 3% | - |
| Liu PH, 2014 | 181 | TACE | Vp 1,2 (181) | - | 60% | - | 42% | 33% |
| Chern MC, 2014 | 50 | TACE | Vp 1,2,3,4 | 6.2 mo (range, 1.7–50.9 mo) | 22% | 10% | 8% | - |
| Tawada A, 2014 | 81 | TACE | Vp 1,2,3,4 | NA | 45% | 23% | 20% | - |
| Ye JZ, 2014 | 338 | TACE (86 patients) | Vp 1,2,3,4 | 7.0 mo | 17.5% | 0% | 0% | - |
| Tan X, 2015 | 116 | TACE (64 patients) TACE+PVE (52 patients) | Type I, II, III (according to Shi et al) | 27.7 mo | 60.9% 80.7% | 41% 59% | 25% 36.5% | 0% 11.5% |
A metanalysis by Xue et al compared TACE and conservative treatment in 1601 patients with PVTT: 6-mo and 1-year survival were better in TACE group than in supportive therapy group, with good results for both Child-Pugh A and B patients[62]. These results were confirmed in another metanalysis by Leng et al[63]l including 600 patients.
A review by Silva et al[63] including 13 studies with 1933 patients with PVTT treated with TACE, showed a 1-, 3-, 5-year survival of 29%, 4% and 1%, respectively. Main trunk PVTT demonstrated the worst outcome, although the Modified Response Evaluation Criteria in Solid Tumors (mRECIST) response rates were similar in cases with main portal vein or portal vein branches thrombosis (14% vs 16%, P = 0.238).
Compared to conservative treatment, TACE allows to achieve a mean survival time of 19 mo vs 4 mo in type I PVTT (Vp1-2), 11.0 mo vs 1.43 mo in type II PVTT (Vp3), and 7.1/4.0 mo vs 1.3/1.0 mo in type III/IV(Vp4), respectively (P < 0.01)[65]. Chung et al reported a median OS of 5.6 mo vs 2.2. mo (P < 0.001) in a study in which TACE arm was compared faced to supportive care arm. The best survival results were achieved in patients with Vp4. The AEs rate was 28.9%, with gastrointestinal hemorrhage more frequent in the TACE group[66]. Another small study evidenced satisfying survival rates at 3-, 6- and 12-mo (82%, 71%, 47% respectively), with a median survival rate of 10 months and better OS in Child-Pugh A compared to Child B patients (15 mo vs 5 mo, respectively)[67]. Tawada et al[68] retrospectively analyzed the AEs in a group of patients with PVTT treated with TACE: The main grade 3 AEs were ALT and AST (Aspartate Aminotransferase) elevation (54.5% and 69.7%, respectively) whereas the occurrence of thrombocytopenia, hyponatremia, hyperbilirubinemia, leukopenia and anemia was rare. In this study, treatment response was evaluated by CT at one or two months after TACE, and was considered separately as “parenchymal-response” and “PVTT-response”. Mean survival times were 11.1 mo vs 5.5 mo for parenchymal response positive patients compared to negative ones, and 14.0 mo vs 5.8 mo for PVTT response positive patients compared to negative ones.
TACE efficacy in the treatment of PVTT seems to be related to the degree of hepatic arterial supply to the thrombus. Indeed, patients showing a good accumulation of lipiodol after TACE presented a better response to TACE, with an OS of 10 mo vs 2.7 mo (P < 0.001)[69].
When TACE is compared to sorafenib in BCLC-C HCC, the median OS seems to be similar (9.2 mo vs 7.4 mo; P = 0.377) but the incidence of AEs appears to be higher in the TACE group (30% vs 17%)[70].
The combination of sorafenib and TACE is reasonable considering their complementary effects: On one side, TACE determines hypoxia that could enhance the release of angiogenic factors; on the other side, sorafenib has an antiangiogenic activity (anti-VEGF-2 and 3, anti- PDGF Receptor) and suppresses tumour proliferation[71]. A metanalysis by Liu et al[72], examining 17 studies on the combination of sorafenib and TACE in unresectable HCC compared to TACE alone, showed benefits in TTP but not in OS (TTP: 7.1-9.0 mo; OS: 12-27 mo). AEs were mainly grade 1-2 (alopecia, fatigue, nausea, diarrhoea, HFSR, haematological events, hepatotoxicity) and manageable with sorafenib dose-reduction. Heavy limitations of this analysis were represented by the wide heterogeneity of accessible data, and lack of OS and TTP in non-comparative studies that were, however, included. Furthermore, the phase II trial START performed in Asia and assessing the effectiveness of the combination of TACE with Sorafenib showed promising results in PVTT patients in terms of 3-year OS (86.1%) with acceptable rates of AEs[73].
A retrospective study by Kim et al[74] demonstrated better OS and longer TTP for patients treated with the combination of TACE and RFA than with TACE or sorafenib alone.
Zhang et al[75] observed an improved outcome applying percutaneous transhepatic portal vein stenting (PTPVS) and TACE, with or without 3-dimensional conformal radiotherapy (3-DCRT): Median OS was 16.5 mo in the radiotherapy (RT) group vs 4.8 in the non-RT one. This study also demonstrates better results of PTPVS-TACE with 3-DCRT especially in avoiding stent re-occlusion due to tumour thrombus regrowth.
On the whole, TACE alone improves survival of selected patients with unresectable HCC, but its effectiveness in improving OS and TTP of PVTT patients is not definitively proven[76]. The association of TACE to other treatments could provide an additional benefit by improving local control of HCC growth but further data are needed in order to demonstrate a positive influence on OS.
HCC is a radio-resistant carcinoma, but, in selected patients with PVTT, RT was demonstrated to be effective with a dose-related response[77,78]. However, RT can lead to severe liver damage[79]. Indeed, concerns exist upon a “veno-occlusive-disease-mimicking” radiation-induced liver disease (RILDS) characterized by jaundice, severe hypertransaminasemia, hepatomegaly and ascites appearing 2-3 mo after RT. This threatening condition still remains without specific treatments and its prognosis is potentially negative[80]. However, a small volume of liver tissue could tolerate potentially tumoricidal high radiation doses. Indeed, Lawrence et al[81] demonstrated that the use of dose-volume histogram allows to concentrate a higher radiation dose on neoplastic tissue while sparing 2/3 of normal hepatic tissue, producing response rates 2-3 times higher faced to conventional therapy, thus obtaining a better local disease-control) and new technologies allow to achieve a highly selective target receiving high doses radiation and sparing normal tissue, thus avoiding RILDS[82].
Some attempts to make this technique more selective and tolerable were made through the use of 3-DCRT which allows to apply radiation selectively on both tumor mass and PVTT and to avoid irradiation on the surrounding hepatic and non-hepatic tissues[83].
Tang et al[84] compared RT to surgery in a retrospective study on 371 patients with resectable HCC and PVTT obtaining better outcomes for the RT arm: Median OS was 12.3 mo vs 10 mo, respectively; 1-, 2-, and 3-year OS was 51.6%, 28.4%, 19.9% for the RT group and 40.1%, 17.0%, 13.6% for the surgery group (P = 0.029). Other studies on Vp3-4 PVTT showed a survival rate of 5.3-7 mo, with response rates ranging between 32.6% and 83%[78,85-88].
A study by Nakazawa et al[89] comparing RT to placebo on high grade PVTT (Vp3-4, Vv2-3), showed a 48% overall objective response rate (complete plus partial response): 56% for Vp, 50% for Vv, 20% for both Vp and Vv). Median survival time in patients receiving RT was 10 mo vs 3.6 mo in the control group; furthermore, 1- and 2-year cumulative survival rates were significantly higher (38% and 20.7% vs 8.3% and 2.7%). No grade 3-4 AEs occurred; the most common AEs were fatigue, anorexia, leukopenia and hypertransaminasemia of grade 1 or 2.
A recent study performed using the propensity score matching highlighted the superiority of RT vs sorafenib in patients with Vp3-4 PVTT (OS 10.9 mo vs 4.8 mo; P = 0.025); complete regression was achieved in 3.6% patients, partial response in 50.2% and stable disease in 25.6% in the RT arm. Serious AEs were more frequent in the sorafenib group (hypertransaminasemia, anorexia, nausea, HFSR), while only one case of grade 3 leukopenia occurred in patients undergoing RT[90].
Positive results have also been reported on proton-beam therapy (PBT) in HCC with PVTT, because of the possibility to locally concentrate the radiation delivery. In a study by Sugahara et al[91] the median OS was 22 mo, with 91% 2-year local control rate and 48% 2-year survival rate; only a few patients presented grade 3 AEs, mainly consisting in leukocytopenia and thrombocytopenia. In another study 12 patients with Vp3-4 PVTT were studied after PBT: 2 of them obtained a complete response without recurrence 4.3 and 6.4 years after treatment. The remaining 10 showed partial response 1-3 mo after PBT (objective response rate 100%), with appearance of new HCC outside the irradiated volume after further 0.1-2.4 years from PBT. PFS and OS rates were 67%/24% and 88%/58% at 2 and 5 years, respectively. No grade 3 AEs were observed[92].
Several RT techniques (3-DCRT, intensity-modulated RT, stereotactic body RT (SBRT), and RT with proton beams and carbon ion beams) showed promising outcomes in HCC patients with and without PVTT: PBT, unlike RT with X-rays, allow to precisely deliver high-dose radiations to the target volume, sparing the normal liver tissue and the surrounding organs[93,94]. Furthermore, a metanalysis by Qi et al[95] demonstrated a higher rate of survival for PBT compared to CRT, with lower AEs.
Gamma knife radiosurgery (GKR) is a type of external radiation treatment applied especially on brain lesions, focusing with gamma beams radiations precisely on the target, thus avoiding damage to surrounding healthy brain tissue. It consists in a single application whereas RT requires multiple sessions to attenuate the effects of radiations on healthy tissues. In a few Chinese cancer centers, GKR has been applied on HCC-PVTT for more than a decade. A retrospective study by Lu et al[96] identified a remarkably increased median OS of HCC-PVTT patients receiving combined TACE and GKR faced to TACE alone. A subsequent retrospective study by Lu et al[97] investigated the efficacy and safety of GKR monotherapy on PVTT-HCC, highlighting an OS advantage in both patients with branch or main PVTT (6.1 mo for GKR group vs 3.0 mo for supportive therapy)[35,36]. Grade 1-2 AEs were predominant and easily managed. Despite the need of further future validation, GKR was reported to be effective on HCC with PVTT, without differences related to its extension[97].
The combination of RT with other treatment modalities represents also an interesting approach. For example, TACE determines the necrosis of the tumor thrombus by reducing the arterial supply and stimulates G0 cells to enter into the proliferating phase, allowing the increase of RT-related antitumor effect. The first study in patients with Vp2 PVTT was made by Chen et al[98] on 10 patients who received TACE combined with RT: 6 patients died after a period ranging from 3 to 10 mo (because of advanced HCC, liver failure, HCC-rupture) while the others survived more than 6 mo. Further studies also reached satisfying response rates[76,78,99-103].
Similarly, conventional chemotherapy could cause a significant enhancement in RT effect: Katamura et al[104] compared patients treated with intra-arterial 5-FU and IFN-alpha alone or combined with 3D-CRT in the treatment of PVTT (patients received 3D-CRT concomitantly with the first course of intra-arterial 5-FU/IFN): In the RT group an improvement in PVTT and a reduced incidence of portal hypertension-related AEs were noticed, the objective response rate of PVTT was higher in RT group (P = 0.012), but the median survival time was similar to non-RT group (7.5 mo vs 7.9 mo). Comparing the RT and non-RT groups, a complete PVTT resolution was achieved in 19% vs 6%, PR in 56% vs 19%, SD in 25% vs 44%, PD in 0% vs 31%, respectively.
Another study claimed the superiority of surgery plus neoadjuvant RT over surgery alone in preventing tumor recurrence (1-year OS 69.0% vs 35.6%, 2-year OS 20.4% vs 0%, 6-mo RR 49.0% vs 88.7%, 1-year RR 77.0% vs 97.7%)[83].
Finally, Nakagawa et al[105] demonstrated the relevant role of RT followed by percutaneous ethanol injection (PEI), RFA or TACE in HCC with Vp2-4 PVTT, with a 50% response rate and 1-, 3- and 5-year survival rate of 45.1%, 15.2% and 5.1% respectively. Table 2 summarizes the most important papers analyzing the effectiveness of RT alone or associated with other treatments in PVTT.
| First author, year | Patients (patients) | Treatment | PVTT Class (Vp) | Response rate | Survival rate(yr) | Mean survival time(mo) |
| Tazawa, 2001 | 24 | RT (50 Gy)+TACE | Vp3,4 | 50% | NA | 9.7 mo |
| Yamada, 2001 | 8 | RT (30 Gy) | Vp3 | 37.5% | NA | NA |
| Ishikura, 2002 | 20 | TACE+RT (50 Gy) | Vp3 | 50% | 25% (1-yr) | 5.3 mo |
| Yamada K, 2003 | 19 | TACE and 3D-RT (30Gy) | Vp3 | 57.9% | 40.6%(1-yr), 10.2% (2-yr) | 7 mo |
| Nakagawa, 2005 | 52 | 3D-CRT (39-60 Gy) | Vp2,3,4 | NA | 5.1% (5-yr) | NA |
| Kim DY, 2005 | 59 | 3D-CRT (39-70.2 Gy) | Vp3,4 | 45.8% | 20.7% (2-yr) | 10.7 mo |
| Hata, 2005 | 12 | Proton-beam therapy (50-72 Gy) | Vp3,4 | 100% | 88% (2-yr) 58% (5-yr) | 27 mo |
| Lin CS, 2006 | 43 | 3D-CRT (21patients) Conventional Rtp (22 patients) | Vp3,4 | 83% 75% | NA NA | 6.7 mo 6.0 mo |
| Hsu WC, 2006 | 53 | 3D-CRT+thalidomide | Vp3,4 | 50% | 84.8% (6mo), 60.0% (1-yr), 44.6% (2-yr) | 24.0 mo |
| Nakazawa, 2007 | 32 | RT | Vp3-4, Vv2-3 | 48% | 38.0% (1-yr), 20.7% (2-yr) | 10.0 mo |
| Toya, 2007 | 38 | 3D-CRT (23.4-59.5 Gy) | Vp NA | 44.7% | 39.4% (1-yr) | 9.6 mo (OS) |
| Shirai, 2009 | 26 | 3D-CRT using SPECT | Vp3,4 | 30.7% | 30% (2-yr) | 10.3 mo |
| Kang, 2013 | 101 | RT+TACE TACE+RT | Vp NA | 70.3% | A) 58.8% (1-yr), 29.4% (2-yr) B) 54.1% (1-yr), 27.0% (2-yr) | 17 mo 15 mo |
| Nakazawa, 2014 | 97 | 3D-CRT (30-56 Gy) | Vp3,4 | 45% | NA | 10.9 mo |
| Lee, 2014 | 46 | 3D-CRT (35-60 Gy) | Vp3,4 | 32.6% | 66.8% (1-yr) | NA |
Transarterial radioembolization (TARE) acts by delivering local beta radiation within HCC through the selective release of iodine-131-labeled lipiodol (131I), iodine (125I) or yttrium (90Y) in lobar, segmental, or subsegmental hepatic arteries supplying the tumor[106,107]. In contrast to TACE, for which PVTT is a technical issue and an absolute contraindication in case of Vp3-4 stage due to the increased risk of liver ischemic necrosis and treatment-related death, TARE can be performed in patients with PVTT without major concerns, thanks to the minimal embolic effect of 90Y-glass microspheres and consequent lower risk of liver ischemia[108].
In a small study on 32 patients, Shae et al[109] compared the effects of TARE and TACE in a subgroup of patients with major vascular invasion, obtaining a median survival of 12 mo vs 9 mo (3-year survival rate 20.3% vs 9.7%).
TARE has also been shown to be more tolerable than TACE or Sorafenib: The most frequent AE, observed in 20-55% of patients, is post-embolization syndrome (fever, nausea, abdominal pain, malaise), which does not require medical intervention in most cases[110]. However, in a retrospective, multicentre, therapy registry, doxorubicin drug eluting beads (DEBDOX) resulted to be safer in the treatment of unresectable HCC with PVTT, achieving better disease control (67% vs 20%; P = 0.0014) and median survival time than TARE[111]. Furthermore, although TARE was reported to be superior to sorafenib in prolonging survival of PVTT patients in some retrospective studies[112-115], two phase III trials (SorAfenib versus Radioembolization in Advanced Hepatocellular carcinoma – SARAH – and Selective Internal Radiation Therapy Versus Sorafenib – SIRveNIB)[116,117] failed to demonstrate a significant superiority of TARE compared to sorafenib. In particular, in the SARAH study the presence of PVTT seems to favor sorafenib in term of OS.
An interesting point could be the selection of a subgroup of PVTT patients in whom the benefit of TARE could be better. In a recent paper, Spreafico et al[118] developed a prognostic classification showing that patients with at least one measurable HCC lesion, PVTT non-occluding the main portal vein, absence of extra-hepatic metastasis, Child-Pugh < B7, and good performance status achieved the best therapeutic results.
Percutaneous treatments have been proposed for HCC complicated by PVTT, but particular caution must be paid because of the potential risk of damaging portal and biliary structures. However, some preliminary attempts have been done, with discrete results.
In a study planned in 2005 when sorafenib was not available and including 35 patients with main trunk PVTT and a single HCC nodule (size 3.7-5 cm) who underwent RFA of both the HCC lesion and PVTT, the 3-year survival rate was significantly higher when compared to no treatment (77% vs 0%)[41], with a mean survival time of 28.3 ± 3.8 mo vs 6.8 ± 0.5 mo (P < 0.001). A complete tumor necrosis and main portal trunk recanalization was achieved in 26 patients (74%). In another study[119], combined treatment with RFA and TACE on HCC complicated by PVTT was reported to achieve a mean survival time of 29.5 months, with a 1-, 3-, 5-year survival rate of 63%, 40% and 23%. Slightly inferior rates were obtained when using laser as ablation technique[120].
Cryotherapy has also been considered as a complementary treatment to sorafenib in patients with branch and main-trunk PVTT, with a significant increase in OS (12.5 mo vs 8.6 mo respectively) and good safety profiles[121].
The main limitation of ablation techniques is the extension and the location of PVTT within the portal system. Indeed, severe side effects such as neoplastic embolization, irreversible damages of vascular and biliary structures, gallbladder, gastric and duodenal walls have been occasionally reported after PVTT ablation using percutaneous ethanol injection, RFA or laser[41,120,122]. Electrochemotherapy (ECT) is an innovative method based on local application of short and intense electric pulses that temporarily permeabilize cellular membranes, permitting the delivery of therapeutic molecules. It has been used in a small prospective case series of HCC patients with Child-Pugh A-B liver cirrhosis and Vp3-4 PVTT: Post-treatment CEUS examination and biopsy of the portal thrombus showed in all cases a complete necrosis of tumor cells without involution signs in perivascular tissues and portal endothelium. During the subsequent 12-mo follow-up, no local recurrence was detected. The main concern on this promising technique is the occurrence of thrombosis of main portal trunk and fatal late gastrointestinal haemorrhage (17% of treated patients)[123].
Since 1980s, surgical resection has been adopted for the treatment of HCC with PVTT of the first-order branches[124]. In 1990s, Kumada et al[125] reported a surgical resection of a tumor thrombus extended in the main portal trunk, describing five possible surgical approaches: (1) Hepatolobectomy, consisting in the en-bloc resection of both HCC (located either in the left or the right lobe) and PVTT (confined to the first portal branch); (2) Thrombectomy with a balloon catheter that is introduced in the clamped portal vein by an incision and used to extract the thrombus by suction or curettage; (3) Portal vein bypass, consisting in the en-bloc resection of both the involved portal branches and the thrombus when its extraction is technically complicated, followed by the creation of a bypass-graft between portal trunk and umbilical vein; (4) Portal vein resection and anastomosis, performed in the presence of PVTT of the contralateral first branch, with an en-bloc resection of both the thrombus and the portal trunk, and the subsequent anastomosis between portal trunk and the residue of portal vein; and (5) Thrombectomy, with the extraction of the thrombus through an incision in portal wall at the bifurcation of right or left vein, while a biologic pump redirects portal and vena cava blood flow to the axillary vein; if PVTT removal is difficult, portal vein should be resected and subsequently reconstructed.
Nowadays, surgical approach to PVTT is based on three main methods: Hepatectomy, tumor thrombectomy, and en-bloc-resection of portal vein and thrombus with subsequent portal reconstruction. The first one represents an excellent solution in case of PVTT in the first portal branches in patients with satisfying clinical condition, as it grants a radical tumor and PVTT removal. The second one has the potential advantage of avoiding tumor residuals, but it is technically more difficult to perform, especially in case of extended thrombosis. The latter, applied to PVTT involving the bifurcation with or without the main and/or contralateral portal veins, presents high intraoperative mortality risk, but a more favorable result faced to thrombectomy that is technically less difficult, but with a higher risk of tumor residual or metastatization to the distal branches[126,127]. A study by Chok et al[128] compared these three surgical methods (hepatectomy, tumor thrombectomy, and en-bloc-resection of portal vein and thrombus), highlighting that median disease-free survival was 4.21, 1.51 and 3.78 mo, respectively. This implies that adjuvant treatments would be necessary to prevent the high incidence of recurrence but, unfortunately, they are still unavailable.
It should be noted that no prospective study on surgical treatment for PVTT is available to date. Some studies underlined the achievement of the same survival and disease-free survival outcomes for en-bloc resection of the involved liver segment and bifurcation with or without the main portal trunk compared to thrombectomy with peeling-off technique[129-131]. Conversely, a propensity score analysis by Zhang et al[132] demonstrated the superiority of en-bloc resection compared to thrombectomy with peeling-off resection in type I-II PVTT (mean survival time, MST 18.2 mo vs 10.9 mo, 1-, 3-, 5-year OS 68.9%, 34.3%, 30.8% vs 49.1%, 22.1%, 15.8%; recurrence rate 9.7% vs 23.9%, P = 0.005, respectively).
Back-flow thrombectomy (BFT) has been employed in order to expand therapeutic possibilities for Vp4 PVTT, which in Japan is surgically approached only in 8.8% cases, minimizing the risk of migration of floating thrombus and allowing further adjuvant treatments[133].
Beyond its pathological meaning and the debates on the most proper therapeutic approach, PVTT itself has a fundamental role in defining the possible evolution of HCC. In patients with HBV-related HCC macrovascular invasion, tumor differentiation and size are all important prognostic factors[134]. As regards surgical resection of large HCC (more than 10 cm), PVTT is an independent predictor of early recurrence-related mortality and a major negative prognostic factor[135]. Since PVTT invading portal trunk is considered to be a sign of advanced HCC, the purpose of surgical approach is not to improve survival, but to help preventing the consequences of excessively high portal pressure (e.g., variceal hemorrhage). However, even in patients with PVTT of the portal trunk, a slight improvement of survival has been reported even when compared to treatment with sorafenib[130,136-138]. A review of 24 studies including 4389 patients with PVTT undergoing surgical resection, evidenced a 1-year OS of 50% and 5-year OS of 18%[139,140]. Peng et al[141] showed a 1-, 3- and 5-years survival rate for type I and II PVTT of 42%, 14.1%, 11.1% for surgery and 37.8%, 7.3%, 0.5% for TACE, respectively; however, the size of the surgery group was about half of the TACE group (201 vs 402), and the subgroups analysis presented statistically significant results only in type I and II PVTT (P < 0.001 and 0.002), but not in type III and IV (P = 0.541 and 0.371); R0 resection could be achieved in 83% patients (88/106) with HCC and PVTT but no vascular wall invasion, as already indicated by Shi et al[142] who suggested thrombectomy as a proper treatment option if the tumor thrombus does not infiltrate portal venous wall. Further studies confirmed the superiority of surgery over TACE, especially in type I and II PVTT[143,144].
Liver resection and thrombectomy have a better 5-year OS if the PVTT is confined to the first or second branch of the main portal vein rather than to portal trunk[30,145-147]. A review analyzing 23 articles and 2412 patients noticed a substantial difference in 5-year post-resection survival related to PVTT stage: Vp1-2 45% (range: 25%-54%), Vp3 19% (range: 0%-38%), Vp4 14.5% (range: 0%-26.4%)[148]. Wang et al[149] suggested that, beyond type I and II PVTT (according to Chang’s classification), surgery could be effective also in selected cases of type III PVTT. Indeed, surgery allows an en-bloc removal of neoplastic tissue in type I and II but in type III it is mandatory to clamp portal vein, perform a thrombectomy and subsequent washing of the vascular lumen with saline solution, in order to remove possible neoplastic residuals.
At present, neither precise consensus nor indications have been established for each Vp-category: According to LCSGJ, Vp1-3 are candidate to resection, but Vp4 has a weak indication; taking into account Cheng’s classification, type I-III could be treated by surgery, while type IV represents a contraindication. A study by Sakamoto et al[125] proposed for Vp4 PVTT an en-bloc resection with reconstruction of the portal vein or thrombectomy. Another Japanese group[150] underlined that only Vp3 may have benefits from surgery, while Vp4 should be treated with alternative therapies.
Yamamoto et al[151,152] claimed that hepatectomy provides comparable survival rates for both Vp3-4 and Vp1-2 but in this series Vp3-4 group is really small faced to the Vp1-2 group. They also suggested that stage II-III patients [according to American Joint Committee on Cancer (AJCC)] with high serum alpha-fetoprotein levels should undergo neoadjuvant/adjuvant therapies.
A retrospective multicenter analysis of liver resection associated to thrombectomy performed in Italy did not report a significant difference of 5-year survival among Vp1, Vp2 and Vp3 patients[153]. Roayaie et al[154] reported a MST of 13.1 mo and 14% 5-year survival in patients with PVTT treated with resection, whereas in case of involvement of hepatic veins and vena cava MSTs felt to 4.7 mo. In case of HCC < 7 cm and AFP < 30 ng/mL, MST was 20 and 29 mo respectively. Prognostic elements such as tumor size, extension of vascular invasion and AFP resulted to be fundamental to define the outcome and should be considered when deciding if resection is possible. The main studies concerning the clinical outcome of patients with HCC and PVTT are reported in Table 3.
| First author, year | Patients (patients) | PVTT Class (Vp) | Mortality | 5-yr survival rate | Median survival time (mo) |
| Yamaoka, 1992 | 29 | 3-4 | 11% | 11.6% (3-yr) | NA |
| Ikai, 1998 | 26 | 3 4 | NA | 11% 4% | NA |
| Ohkubo, 2000 | 47 | 2-3-4 | 0% | 23.9% | 14 mo |
| Wu, 2000 | 97 15 | 1-2-3 4 | 3.1% 0% | 28.5% 26.4% | NA NA |
| Poon, 2003 | 20 | 3-4, Vv2 | 5.7% | 13.3% | 6.0 mo |
| Ikai et al, 2004 (21,711 patients treated from 1989 to 1999) | 17,867 1609 672 679 | 0 1 2 3-4 | NA | 56.5% 34.4% 27.0% 17.3% | NA |
| Pawlik, 2005 | 102 | 3, Vv 2-3 | 5.9% | 10% | 11 mo |
| Ikai, 2006 | 78 | 3-4 | 3.8% | 10.9% | 8.9 mo |
| Chen, 2007 | 286 152 | 1-3 4 | NA 2.6% | 18.1% 0% | 10.1 mo |
| Le Treut, 2006 | 26 | 3-4, Vv2-3 | 11.5% | 13% | 9.0 mo |
| Zhou, 2006 | 381 | 2-3-4 | NA | 12% | NA |
| Shi, 2011 | 144 189 86 22 | 1 2 3 4 | NA | 26.7% (3-yr) 16.9% (3-yr) 3.7% (3-yr) 0% (3-yr) | NA |
| Inoue et al. 2009 | 20 | 4 | 0% | 39.0% | NA |
| Ban, 2009 | 45 | 3 4 | 0% 0% | 41.8% 20.9 | 20.0 mo |
| Kondo, 2009 | 5 | 4 | 0% | 0% | 8.0 mo |
| Ikai et al 2010 (25,066 patients treated from 1994 to 2005) | 20,195 1978 820 1,021 | 0 1 2 3-4 | NA | 59.0% 39.1% 23.3% 18.3% | NA |
| Shi, 2010 | 169 78 | 3 4 | 0.6% 0% | 17.7% (3-yr) 3.6% (3-yr) | 15.0 mo 10.0 mo |
| Wang, 2013 | 25 | Vv3 | 0% | 13.5% | NA |
| Chok, 2013 | 88 | 1-2-3-4 | 3.3% | 11.2-14.3% | NA |
| Pesi, 2015 | 15 5 21 8 3 | 1 2 3 Vv2 Vv3 | NA | 53.3% 30.1% 20.0% NA NA | NA |
| Xu, 2015 | 16 40 | A B | 100% NA | 0% 5.2% | NA |
| Kokudo, 2016 | 1772 1475 1942 1285 | 1 2 3 4 | NA | NA | 2.67 yr 1.51 yr 0.78 yr 0.50 yr |
| Kudo, 2016 | 1908 714 852 | 1 2 3-4 | NA | 48.2% 29.2% 25.05 | NA |
A metanalysis by Hyun et al[155], examining 5986 patients from 18 studies, showed that primary hepatectomy is superior to TACE in BCLC-B/C stage HCC, with significant OS benefit (HR = 0.59; 95%CI: 0.51-0.67; P < 0.00001); as per BCLC-B, also in BCLC-C group, 1-, 3-, 5-years survival rates were higher for primary hepatectomy than TACE (62%, 42%, 20% vs 45%, 16%, 8%, respectively). Despite these intriguing results, the long-term effectiveness of surgery in advanced HCC is still to be proven, since no comparison was made between surgery versus standard systemic treatment with sorafenib in this group of patients. Zhang et al[156] proposed the preoperative Eastern Hepatobiliary Surgery Hospital (EHBH) PVTT scoring system predicting accurately the prognosis after R0 LR of HCC patients with type I-II PVTT: This method could facilitate the decision between conventional systemic treatment and surgery in patients with advanced HCC because of its accuracy in selecting patients who could be advantaged by LR.
Associated Liver Partition and Portal vein ligation for Staged hepatectomy (ALPPS) is an innovative surgical technique, first developed in 2012, for the management of liver metastases in patients with potentially small residual liver volume, that reduces the time of liver hypertrophy from 4-6 wk (in case of standard portal embolization) to 7-10 d. It consists of in situ splitting of the liver along the main portal scissura or the right side of the falciform ligament, associated with right portal vein ligation[157]: Vennarecci et al[158] demonstrated its efficacy in two cases of HCCs with major vascular invasion in which the classical approach of radiological or surgical portal-vein occlusion and subsequent resection after 4-6 wk could not be performed; these patients were still alive 7 and 12 mo after surgery.
A combination strategy involving surgery has been reported to be more beneficial than surgery alone[159]. However, the available data are still fragmentary and involve a limited number of cases. Then, further prospective randomized clinical trials are needed to achieve solid conclusions.
TACE following resection has been associated to a reduction in disease-recurrence and a prolongation of OS. This effect is obtained especially in patients with high-recurrence-risk such as those with large tumors and vascular invasion[5,160-164].
Neoadjuvant RT can achieve tumor downstaging allowing surgery and improving disease-free and OS without significant radio-mediated liver-damage[83]. In particular, PVTT seems to have a better radio-sensitivity than HCC itself (response rate 27% vs 13% respectively): This allows to reduce the duration of the intervention, the bleeding-risk and the possibility of micrometastatization by small thrombotic fragments during surgery[160]. Kamiyama et al[165] described the possible role of RT as neoadjuvant treatment before surgery in patients with Vp3-4 PVTT, helping to improve prognosis through the inactivation of thrombus tumor cells and consequent prevention of dissemination, with a mean survival time of 19.6 mo. Some studies also proved TARE with yttrium-90 to be effective in downstaging unresectable HCC with PVTT, increasing patients’ survival[166].
Sorafenib has been tested as adjuvant therapy to prevent recurrence after surgical intervention. However, the initial promising results on animal models[167], were not confirmed in humans. An increased overall survival was reported in animal models and in a small series of 12 patients with HCC in the BCLC-C stage treated with sorafenib after resection faced to the 24 ones treated with surgery alone[168].
Tang et al[169] reported that combination of surgery plus chemotherapy is effective for HCC with PVTT, increasing survival rates faced to other therapeutic strategies (chemotherapy alone, surgery alone, conservative treatment). Remarkable data were also obtained comparing hepatectomy plus thrombectomy to hepatectomy plus thrombectomy plus chemotherapy (1-year and 3-years survival rates of 47% vs 70% and of 22% vs 20%, respectively)[6]. Nagano et al[170] also showed a 100% overall 1-year survival using subcutaneous IFN-alpha and hepatic arterial infusion of 5-FU after palliative resection of advanced HCC with PVTT.
However, none of these studies clearly demonstrates that chemotherapy increases PVTT outcome after surgery.
According to international guidelines, HCC with PVTT still remains a contraindication for LT because of the elevated risk of tumor recurrence, but also because of technical difficulties and post-operative mortality[59,171-174].
However, based upon the positive experiences with living-donor LT after successful downstaging, the role of PVTT as an absolute contraindication to LT deserves a reconsideration. A recent study reported on the use a pre-waiting list LT protocol of intensive downstaging treatment in a living donor LT program based on concurrent chemoradiotherapy (CCRT) followed by HAIC[175]. Patients achieving successful downstaging underwent LT and median patient and graft survival was 33 months. Some important issues emerged from this study: Technical limitations due to anatomic modifications determined by downstaging treatments, the prognostic value of biomarkers (e.g., AFP, PIVKA-II – protein induced by vitamin K absence or antagonist-II), the high recurrence rate (3/8 patients).
An interesting study by Levi Sandri et al[176] showed encouraging results of TARE for PVTT-HCC downstaging prior to LT in four patients; DFS was 39.1 mo and in all cases complete thrombosis regression was achieved, demonstrating the potential role of TARE in tumour downstaging for LT candidates. These data underline the importance of TARE as a useful downstaging tool for PVTT-HCC opening a possible scenario for LT.
External radiation therapy has also been considered for PVTT as a bridging or downstaging therapy[177]. A small retrospective series of 10 patients receiving hypofractionated image-guided RT (HIGRT) followed by LT, presented pathologic complete response in 27% of cases; in the others, the lesions were decreased or stable in size[178]. Five years after LT all patients were alive and no recurrence was observed. In another study on 38 patients treated with HIGRT and LT, complete plus partial pathologic response was achieved in 68%[179].
HCC patients with PVTT treated with resection, TACE and LT present an increased survival rate compared to those receiving conservative treatment (30% vs 12% at 1 year and 10% vs 4% at 3 years)[6].
All these experiences contribute to endorse downstaging strategies as an essential preliminary condition to allow PVTT-HCC to undergo LT without an excessively consistent risk of recurrence.
Patients with HCC and PVTT still remain a subgroup with poor survival, despite the efforts to expand treatment indications outside the BCLC algorithm. The most evident survival advantages can be attributed to surgery and several centers are moving towards an extension of BCLC indications in these patients[136]. Recently released guidelines by European Association for the Study of the Liver (EASL) and American Association for the Study of Liver Diseases (AASLD) reflect this new tendency and the favourable results reported by both Eastern and Western surgical groups for patients with PVTT of the first/secondary branches (Vp1/2), support the differentiation of treatment algorithms according to PVTT extension[59,180].
The importance of the scientific debate on PVTT treatment is summarized by Wang et al[181] who focus on the exiguous survival advantage offered by palliative therapies (such as sorafenib) confronted with their high health-costs. A revision of western countries guidelines is required to fully recognize hepatic resection as a valid alternative for selected cases of advanced HCC. The reason why this strategy is not yet applied probably resides in the low incidence of HCC in Western countries compared with Eastern ones and the coexistence in developed countries of several comorbidities, such as cirrhosis, resulting in the risk of poor OS after resection, thus leading either to conservative surgical strategies or to merely palliative therapies. Despite these concerns, a study by Kokudo et al[182] reported a less than 4% postoperative 90-day mortality for hepatic resection in PVTT-HCC. For this reason, notwithstanding obvious technical difficulties of surgery in complex patients (e.g., cirrhotic, cardiopathic ones), it should be given a larger space as a therapeutic option in patients with first-order branch PVTT and normal liver function, because of its safety and efficacy. For those with PVTT in main trunk or contralateral branches, TARE and sorafenib (alone or combined) represent the most suitable solution.
There is still a deep gap of knowledge in the field of HCC associated with PVTT as only preliminary and insufficient data are available on the effectiveness of combining neoadjuvant treatment with resection and no prospective study comparing surgery with other treatments has been reported. In the meanwhile, the involvement of a multidisciplinary tumor board is mandatory in order to provide the best therapeutic decision for complex, selected cases, considering risks and benefits in the light of residual liver function which remains a decisive factor in the orientation of therapeutic choices.
Although sorafenib represents at the present time the only recommended approach in this category of patients, it is important to remind that, for selected cases, alternative therapeutic approaches with a good safety and effectiveness profile such as surgery and TARE are available, and that the sequential and personalized combination of different treatment modalities will probably lead in the future to a deep modification of BCLC treatment pathways.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li LQ, Montasser I S-Editor: Ma RY L-Editor: A E-Editor: Ma YJ
| 1. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20511] [Article Influence: 2051.1] [Reference Citation Analysis (20)] |
| 2. | Bosetti C, Levi F, Boffetta P, Lucchini F, Negri E, La Vecchia C. Trends in mortality from hepatocellular carcinoma in Europe, 1980-2004. Hepatology. 2008;48:137-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 181] [Article Influence: 10.6] [Reference Citation Analysis (1)] |
| 3. | Colombo M, de Franchis R, Del Ninno E, Sangiovanni A, De Fazio C, Tommasini M, Donato MF, Piva A, Di Carlo V, Dioguardi N. Hepatocellular carcinoma in Italian patients with cirrhosis. N Engl J Med. 1991;325:675-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 536] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 4. | Manzano-Robleda Mdel C, Barranco-Fragoso B, Uribe M, Méndez-Sánchez N. Portal vein thrombosis: what is new? Ann Hepatol. 2015;14:20-27. [PubMed] |
| 5. | Minagawa M, Makuuchi M, Takayama T, Ohtomo K. Selection criteria for hepatectomy in patients with hepatocellular carcinoma and portal vein tumor thrombus. Ann Surg. 2001;233:379-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 144] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 6. | Zhou Q, Wang Y, Zhou X, Peng B, Yang J, Liang L, Li J. Prognostic analysis for treatment modalities in hepatocellular carcinomas with portal vein tumor thrombi. Asian Pac J Cancer Prev. 2011;12:2847-2850. [PubMed] |
| 7. | Piscaglia F, Gianstefani A, Ravaioli M, Golfieri R, Cappelli A, Giampalma E, Sagrini E, Imbriaco G, Pinna AD, Bolondi L; Bologna Liver Transplant Group. Criteria for diagnosing benign portal vein thrombosis in the assessment of patients with cirrhosis and hepatocellular carcinoma for liver transplantation. Liver Transpl. 2010;16:658-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 8. | Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2645] [Cited by in RCA: 2873] [Article Influence: 110.5] [Reference Citation Analysis (1)] |
| 9. | Chevret S, Trinchet JC, Mathieu D, Rached AA, Beaugrand M, Chastang C. A new prognostic classification for predicting survival in patients with hepatocellular carcinoma. Groupe d'Etude et de Traitement du Carcinome Hépatocellulaire. J Hepatol. 1999;31:133-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 360] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 10. | Farinati F, Vitale A, Spolverato G, Pawlik TM, Huo TL, Lee YH, Frigo AC, Giacomin A, Giannini EG, Ciccarese F, Piscaglia F, Rapaccini GL, Di Marco M, Caturelli E, Zoli M, Borzio F, Cabibbo G, Felder M, Sacco R, Morisco F, Biasini E, Foschi FG, Gasbarrini A, Svegliati Baroni G, Virdone R, Masotto A, Trevisani F, Cillo U; ITA. LI.CA study group. Development and Validation of a New Prognostic System for Patients with Hepatocellular Carcinoma. PLoS Med. 2016;13:e1002006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 113] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 11. | A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28:751-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 977] [Cited by in RCA: 963] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 12. | Sohn JH, Duran R, Zhao Y, Fleckenstein F, Chapiro J, Sahu S, Schernthaner RE, Qian T, Lee H, Zhao L, Hamilton J, Frangakis C, Lin M, Salem R, Geschwind JF. Validation of the Hong Kong Liver Cancer Staging System in Determining Prognosis of the North American Patients Following Intra-arterial Therapy. Clin Gastroenterol Hepatol. 2017;15:746-755.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Leung TW, Tang AM, Zee B, Lau WY, Lai PB, Leung KL, Lau JT, Yu SC, Johnson PJ. Construction of the Chinese University Prognostic Index for hepatocellular carcinoma and comparison with the TNM staging system, the Okuda staging system, and the Cancer of the Liver Italian Program staging system: a study based on 926 patients. Cancer. 2002;94:1760-1769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 452] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 14. | Kitai S, Kudo M, Minami Y, Haji S, Osaki Y, Oka H, Seki T, Kasugai H, Sasaki Y, Matsunaga T. Validation of a new prognostic staging system for hepatocellular carcinoma: a comparison of the biomarker-combined Japan Integrated Staging Score, the conventional Japan Integrated Staging Score and the BALAD Score. Oncology. 2008;75 Suppl 1:83-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2800] [Cited by in RCA: 4097] [Article Influence: 585.3] [Reference Citation Analysis (6)] |
| 16. | Minagawa M, Makuuchi M. Treatment of hepatocellular carcinoma accompanied by portal vein tumor thrombus. World J Gastroenterol. 2006;12:7561-7567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 209] [Cited by in RCA: 231] [Article Influence: 12.2] [Reference Citation Analysis (1)] |
| 17. | Llovet JM, Bustamante J, Castells A, Vilana R, Ayuso Mdel C, Sala M, Brú C, Rodés J, Bruix J. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29:62-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 839] [Cited by in RCA: 907] [Article Influence: 34.9] [Reference Citation Analysis (1)] |
| 18. | Margini C, Berzigotti A. Portal vein thrombosis: The role of imaging in the clinical setting. Dig Liver Dis. 2017;49:113-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 19. | Tessler FN, Gehring BJ, Gomes AS, Perrella RR, Ragavendra N, Busuttil RW, Grant EG. Diagnosis of portal vein thrombosis: value of color Doppler imaging. AJR Am J Roentgenol. 1991;157:293-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 122] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Berzigotti A, Piscaglia F; EFSUMB Education and Professional Standards Committee. Ultrasound in portal hypertension--part 2--and EFSUMB recommendations for the performance and reporting of ultrasound examinations in portal hypertension. Ultraschall Med. 2012;33:8-32; quiz 30-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Tarantino L, Francica G, Sordelli I, Esposito F, Giorgio A, Sorrentino P, de Stefano G, Di Sarno A, Ferraioli G, Sperlongano P. Diagnosis of benign and malignant portal vein thrombosis in cirrhotic patients with hepatocellular carcinoma: color Doppler US, contrast-enhanced US, and fine-needle biopsy. Abdom Imaging. 2006;31:537-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 116] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 22. | Rossi S, Rosa L, Ravetta V, Cascina A, Quaretti P, Azzaretti A, Scagnelli P, Tinelli C, Dionigi P, Calliada F. Contrast-enhanced versus conventional and color Doppler sonography for the detection of thrombosis of the portal and hepatic venous systems. AJR Am J Roentgenol. 2006;186:763-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 90] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 23. | Tublin ME, Dodd GD, Baron RL. Benign and malignant portal vein thrombosis: differentiation by CT characteristics. AJR Am J Roentgenol. 1997;168:719-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 182] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 24. | Catalano OA, Choy G, Zhu A, Hahn PF, Sahani DV. Differentiation of malignant thrombus from bland thrombus of the portal vein in patients with hepatocellular carcinoma: application of diffusion-weighted MR imaging. Radiology. 2010;254:154-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 151] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 25. | Sandrasegaran K, Tahir B, Nutakki K, Akisik FM, Bodanapally U, Tann M, Chalasani N. Usefulness of conventional MRI sequences and diffusion-weighted imaging in differentiating malignant from benign portal vein thrombus in cirrhotic patients. AJR Am J Roentgenol. 2013;201:1211-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 26. | Canellas R, Mehrkhani F, Patino M, Kambadakone A, Sahani D. Characterization of Portal Vein Thrombosis (Neoplastic Versus Bland) on CT Images Using Software-Based Texture Analysis and Thrombus Density (Hounsfield Units). AJR Am J Roentgenol. 2016;207:W81-W87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 27. | Li C, Hu J, Zhou D, Zhao J, Ma K, Yin X, Wang J. Differentiation of bland from neoplastic thrombus of the portal vein in patients with hepatocellular carcinoma: application of susceptibility-weighted MR imaging. BMC Cancer. 2014;14:590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Rammohan A, Jeswanth S, Sukumar R, Anand L, Kumar PS, Srinivasan UP, Ravi R, Ravichandran P. Percutaneous ultrasound-guided fine-needle aspiration of portal vein thrombi as a diagnostic and staging technique for hepatocellular carcinoma. Abdom Imaging. 2013;38:1057-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Hu S, Zhang J, Cheng C, Liu Q, Sun G, Zuo C. The role of 18F-FDG PET/CT in differentiating malignant from benign portal vein thrombosis. Abdom Imaging. 2014;39:1221-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 30. | Ikai I, Arii S, Okazaki M, Okita K, Omata M, Kojiro M, Takayasu K, Nakanuma Y, Makuuchi M, Matsuyama Y, Monden M, Kudo M. Report of the 17th Nationwide Follow-up Survey of Primary Liver Cancer in Japan. Hepatol Res. 2007;37:676-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 324] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 31. | Ikai I, Itai Y, Okita K, Omata M, Kojiro M, Kobayashi K, Nakanuma Y, Futagawa S, Makuuchi M, Yamaoka Y. Report of the 15th follow-up survey of primary liver cancer. Hepatol Res. 2004;28:21-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 126] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 32. | Shuqun C, Mengchao W, Han C, Feng S, Jiahe Y, Guanghui D, Wenming C, Peijun W, Yuxiang Z. Tumor thrombus types influence the prognosis of hepatocellular carcinoma with the tumor thrombi in the portal vein. Hepatogastroenterology. 2007;54:499-502. [PubMed] |
| 33. | Shi J, Lai EC, Li N, Guo WX, Xue J, Lau WY, Wu MC, Cheng SQ. A new classification for hepatocellular carcinoma with portal vein tumor thrombus. J Hepatobiliary Pancreat Sci. 2011;18:74-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 147] [Article Influence: 10.5] [Reference Citation Analysis (1)] |
| 34. | Xu JF, Liu XY, Wang S, Wen HX. Surgical treatment for hepatocellular carcinoma with portal vein tumor thrombus: a novel classification. World J Surg Oncol. 2015;13:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 35. | Bruix J, Raoul JL, Sherman M, Mazzaferro V, Bolondi L, Craxi A, Galle PR, Santoro A, Beaugrand M, Sangiovanni A, Porta C, Gerken G, Marrero JA, Nadel A, Shan M, Moscovici M, Voliotis D, Llovet JM. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol. 2012;57:821-829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 664] [Cited by in RCA: 653] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 36. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10261] [Article Influence: 603.6] [Reference Citation Analysis (2)] |
| 37. | Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3854] [Cited by in RCA: 4648] [Article Influence: 273.4] [Reference Citation Analysis (0)] |
| 38. | Cheng AL, Guan Z, Chen Z, Tsao CJ, Qin S, Kim JS, Yang TS, Tak WY, Pan H, Yu S, Xu J, Fang F, Zou J, Lentini G, Voliotis D, Kang YK. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma according to baseline status: subset analyses of the phase III Sorafenib Asia-Pacific trial. Eur J Cancer. 2012;48:1452-1465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 225] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 39. | Yau T, Chan P, Ng KK, Chok SH, Cheung TT, Fan ST, Poon RT. Phase 2 open-label study of single-agent sorafenib in treating advanced hepatocellular carcinoma in a hepatitis B-endemic Asian population: presence of lung metastasis predicts poor response. Cancer. 2009;115:428-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 126] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 40. | Jeong SW, Jang JY, Shim KY, Lee SH, Kim SG, Cha SW, Kim YS, Cho YD, Kim HS, Kim BS, Kim KH, Kim JH. Practical effect of sorafenib monotherapy on advanced hepatocellular carcinoma and portal vein tumor thrombosis. Gut Liver. 2013;7:696-703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 41. | Giorgio A, Merola MG, Montesarchio L, Merola F, Santoro B, Coppola C, Gatti P, Amendola F, DI Sarno A, Calvanese A, Matteucci P, Giorgio V. Sorafenib Combined with Radio-frequency Ablation Compared with Sorafenib Alone in Treatment of Hepatocellular Carcinoma Invading Portal Vein: A Western Randomized Controlled Trial. Anticancer Res. 2016;36:6179-6183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 42. | Park JW, Kim YJ, Kim DY, Bae SH, Paik SW, Lee YJ, Kim HY, Lee HC, Han SY, Cheong JY, Kwon OS, Yeon JE, Kim BH, Hwang J. Sorafenib with or without concurrent transarterial chemoembolization in patients with advanced hepatocellular carcinoma: The phase III STAH trial. J Hepatol. 2019;70:684-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 150] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 43. | Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3128] [Cited by in RCA: 3818] [Article Influence: 545.4] [Reference Citation Analysis (1)] |
| 44. | Okada S, Okazaki N, Nose H, Yoshimori M, Aoki K. Prognostic factors in patients with hepatocellular carcinoma receiving systemic chemotherapy. Hepatology. 1992;16:112-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 45. | Itamoto T, Nakahara H, Tashiro H, Haruta N, Asahara T, Naito A, Ito K. Hepatic arterial infusion of 5-fluorouracil and cisplatin for unresectable or recurrent hepatocellular carcinoma with tumor thrombus of the portal vein. J Surg Oncol. 2002;80:143-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 46. | Yamasaki T, Kurokawa F, Shirahashi H, Kusano N, Hironaka K, Masuhara M, Okita K. Novel arterial infusion chemotherapy using cisplatin, 5-fluorouracil, and leucovorin for patients with advanced hepatocellular carcinoma. Hepatol Res. 2002;23:7-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 47. | Ando E, Yamashita F, Tanaka M, Tanikawa K. A novel chemotherapy for advanced hepatocellular carcinoma with tumor thrombosis of the main trunk of the portal vein. Cancer. 1997;79:1890-1896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 48. | Urabe T, Kaneko S, Matsushita E, Unoura M, Kobayashi K. Clinical pilot study of intrahepatic arterial chemotherapy with methotrexate, 5-fluorouracil, cisplatin and subcutaneous interferon-alpha-2b for patients with locally advanced hepatocellular carcinoma. Oncology. 1998;55:39-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 60] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 49. | Kaneko S, Urabe T, Kobayashi K. Combination chemotherapy for advanced hepatocellular carcinoma complicated by major portal vein thrombosis. Oncology. 2002;62 Suppl 1:69-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 50. | Sakon M, Nagano H, Dono K, Nakamori S, Umeshita K, Yamada A, Kawata S, Imai Y, Iijima S, Monden M. Combined intraarterial 5-fluorouracil and subcutaneous interferon-alpha therapy for advanced hepatocellular carcinoma with tumor thrombi in the major portal branches. Cancer. 2002;94:435-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 143] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 51. | Obi S, Yoshida H, Toune R, Unuma T, Kanda M, Sato S, Tateishi R, Teratani T, Shiina S, Omata M. Combination therapy of intraarterial 5-fluorouracil and systemic interferon-alpha for advanced hepatocellular carcinoma with portal venous invasion. Cancer. 2006;106:1990-1997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 203] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 52. | Ota H, Nagano H, Sakon M, Eguchi H, Kondo M, Yamamoto T, Nakamura M, Damdinsuren B, Wada H, Marubashi S, Miyamoto A, Dono K, Umeshita K, Nakamori S, Wakasa K, Monden M. Treatment of hepatocellular carcinoma with major portal vein thrombosis by combined therapy with subcutaneous interferon-alpha and intra-arterial 5-fluorouracil; role of type 1 interferon receptor expression. Br J Cancer. 2005;93:557-564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 116] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 53. | Song DS, Song MJ, Bae SH, Chung WJ, Jang JY, Kim YS, Lee SH, Park JY, Yim HJ, Cho SB, Park SY, Yang JM. A comparative study between sorafenib and hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis. J Gastroenterol. 2015;50:445-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 115] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 54. | Ikeda M, Okusaka T, Furuse J, Mitsunaga S, Ueno H, Yamaura H, Inaba Y, Takeuchi Y, Satake M, Arai Y. A multi-institutional phase II trial of hepatic arterial infusion chemotherapy with cisplatin for advanced hepatocellular carcinoma with portal vein tumor thrombosis. Cancer Chemother Pharmacol. 2013;72:463-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 55. | Kudo M, Takeuchi Y, Ueshima K, Yokosuka O, Obi S, Izumi N, Aikata H, Nagano H, Hatano E, Sasaki Y, Hino K, Kumada K, Yamamoto K, Imai Y, Iwadou S, Ogawa C, Okusaka T, Arai Y, Kanai F, Akazawa K, and SILIUS Study Group. Prospective randomized controlled phase III trial comparing the efficacy of sorafenib versus sorafenib in combination with low-dose cisplatin/fluorouracil hepatic arterial infusion chemotherapy in patients with advanced hepatocellular carcinoma. J Hepatol. 2016;64:S183–S212. [DOI] [Full Text] |
| 56. | Ikeda M, Shimizu S, Sato T, Morimoto M, Kojima Y, Inaba Y, Hagihara A, Kudo M, Nakamori S, Kaneko S, Sugimoto R, Tahara T, Ohmura T, Yasui K, Sato K, Ishii H, Furuse J, Okusaka T. Sorafenib plus hepatic arterial infusion chemotherapy with cisplatin versus sorafenib for advanced hepatocellular carcinoma: randomized phase II trial. Ann Oncol. 2016;27:2090-2096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 125] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 57. | Yang H, Woo HY, Lee SK, Han JW, Jang B, Nam HC, Lee HL, Lee SW, Song DS, Song MJ, Oh JS, Chun HJ, Jang JW, Lozada A, Bae SH, Choi JY, Yoon SK. A comparative study of sorafenib and metronomic chemotherapy for Barcelona Clinic Liver Cancer-stage C hepatocellular carcinoma with poor liver function. Clin Mol Hepatol. 2017;23:128-137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 58. | Trevisani F, Brandi G, Garuti F, Barbera MA, Tortora R, Casadei Gardini A, Granito A, Tovoli F, De Lorenzo S, Inghilesi AL, Foschi FG, Bernardi M, Marra F, Sacco R, Di Costanzo GG. Metronomic capecitabine as second-line treatment for hepatocellular carcinoma after sorafenib discontinuation. J Cancer Res Clin Oncol. 2018;144:403-414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 59. | European Association for the Study of the Liver; European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6048] [Article Influence: 864.0] [Reference Citation Analysis (3)] |
| 60. | Liu L, Zhang C, Zhao Y, Qi X, Chen H, Bai W, He C, Guo W, Yin Z, Fan D, Han G. Transarterial chemoembolization for the treatment of advanced hepatocellular carcinoma with portal vein tumor thrombosis: prognostic factors in a single-center study of 188 patients. Biomed Res Int. 2014;2014:194278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 61. | Yin J, Bo WT, Sun J, Xiang X, Lang JY, Zhong JH, Li LQ. New Evidence and Perspectives on the Management of Hepatocellular Carcinoma with Portal Vein Tumor Thrombus. J Clin Transl Hepatol. 2017;5:169-176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 62. | Xue TC, Xie XY, Zhang L, Yin X, Zhang BH, Ren ZG. Transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombus: a meta-analysis. BMC Gastroenterol. 2013;13:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 133] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 63. | Leng JJ, Xu YZ, Dong JH. Efficacy of transarterial chemoembolization for hepatocellular carcinoma with portal vein thrombosis: a meta-analysis. ANZ J Surg. 2016;86:816-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 64. | Silva JP, Berger NG, Tsai S, Christians KK, Clarke CN, Mogal H, White S, Rilling W, Gamblin TC. Transarterial chemoembolization in hepatocellular carcinoma with portal vein tumor thrombosis: a systematic review and meta-analysis. HPB (Oxford). 2017;19:659-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 65. | Niu ZJ, Ma YL, Kang P, Ou SQ, Meng ZB, Li ZK, Qi F, Zhao C. Transarterial chemoembolization compared with conservative treatment for advanced hepatocellular carcinoma with portal vein tumor thrombus: using a new classification. Med Oncol. 2012;29:2992-2997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 66. | Chung GE, Lee JH, Kim HY, Hwang SY, Kim JS, Chung JW, Yoon JH, Lee HS, Kim YJ. Transarterial chemoembolization can be safely performed in patients with hepatocellular carcinoma invading the main portal vein and may improve the overall survival. Radiology. 2011;258:627-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 250] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 67. | Ajit Y, Sudarsan H, Saumya G, Abhishek A, Navneet R, Piyush R, Anil A, Arun G. Transarterial chemoembolization in unresectable hepatocellular carcinoma with portal vein thrombosis: a perspective on survival. Oman Med J. 2014;29:430-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 68. | Tawada A, Chiba T, Ooka Y, Kanogawa N, Motoyama T, Saito T, Ogasawara S, Suzuki E, Maruyama H, Kanai F, Yoshikawa M, Yokosuka O. Efficacy of transarterial chemoembolization targeting portal vein tumor thrombus in patients with hepatocellular carcinoma. Anticancer Res. 2014;34:4231-4237. [PubMed] |
| 69. | Sun J, Shi J, Huang B, Cheng F, Guo W, Lau WY, Cheng S. The degree of hepatic arterial blood supply of portal vein tumor thrombus in patients with hepatocellular carcinoma and its impact on overall survival after transarterial chemoembolization. Oncotarget. 2017;8:79816-79824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 70. | Yu SJ, Kim YJ. Effective treatment strategies other than sorafenib for the patients with advanced hepatocellular carcinoma invading portal vein. World J Hepatol. 2015;7:1553-1561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 71. | Han K, Kim JH, Ko GY, Gwon DI, Sung KB. Treatment of hepatocellular carcinoma with portal venous tumor thrombosis: A comprehensive review. World J Gastroenterol. 2016;22:407-416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 72. | Liu L, Chen H, Wang M, Zhao Y, Cai G, Qi X, Han G. Combination therapy of sorafenib and TACE for unresectable HCC: a systematic review and meta-analysis. PLoS One. 2014;9:e91124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 73. | Chao Y, Chung YH, Han G, Yoon JH, Yang J, Wang J, Shao GL, Kim BI, Lee TY. The combination of transcatheter arterial chemoembolization and sorafenib is well tolerated and effective in Asian patients with hepatocellular carcinoma: final results of the START trial. Int J Cancer. 2015;136:1458-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 74. | Kim GA, Shim JH, Yoon SM, Jung J, Kim JH, Ryu MH, Ryoo BY, Kang YK, Lee D, Kim KM, Lim YS, Lee HC, Chung YH, Lee YS. Comparison of chemoembolization with and without radiation therapy and sorafenib for advanced hepatocellular carcinoma with portal vein tumor thrombosis: a propensity score analysis. J Vasc Interv Radiol. 2015;26:320-9.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 75. | Zhang XB, Wang JH, Yan ZP, Qian S, Du SS, Zeng ZC. Hepatocellular carcinoma with main portal vein tumor thrombus: treatment with 3-dimensional conformal radiotherapy after portal vein stenting and transarterial chemoembolization. Cancer. 2009;115:1245-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 76. | Ishikura S, Ogino T, Furuse J, Satake M, Baba S, Kawashima M, Nihei K, Ito Y, Maru Y, Ikeda H. Radiotherapy after transcatheter arterial chemoembolization for patients with hepatocellular carcinoma and portal vein tumor thrombus. Am J Clin Oncol. 2002;25:189-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 91] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 77. | Huang YJ, Hsu HC, Wang CY, Wang CJ, Chen HC, Huang EY, Fang FM, Lu SN. The treatment responses in cases of radiation therapy to portal vein thrombosis in advanced hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2009;73:1155-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 78. | Kim DY, Park W, Lim DH, Lee JH, Yoo BC, Paik SW, Kho KC, Kim TH, Ahn YC, Huh SJ. Three-dimensional conformal radiotherapy for portal vein thrombosis of hepatocellular carcinoma. Cancer. 2005;103:2419-2426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 152] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 79. | Fujino H, Kimura T, Aikata H, Miyaki D, Kawaoka T, Kan H, Fukuhara T, Kobayashi T, Naeshiro N, Honda Y, Tsuge M, Hiramatsu A, Imamura M, Kawakami Y, Hyogo H, Takahashi S, Yoshimatsu R, Yamagami T, Kenjo M, Nagata Y, Awai K, Chayama K. Role of 3-D conformal radiotherapy for major portal vein tumor thrombosis combined with hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma. Hepatol Res. 2015;45:607-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 80. | Yu JI, Park HC, Lim DH, Park WY. Predictive factors for Child-Pugh score elevation in hepatocellular carcinoma patients treated with conformal radiation therapy: dose-volume histogram analysis. Tumori. 2013;99:164-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 81. | Lawrence TS, Ten Haken RK, Kessler ML, Robertson JM, Lyman JT, Lavigne ML, Brown MB, DuRoss DJ, Andrews JC, Ensminger WD. The use of 3-D dose volume analysis to predict radiation hepatitis. Int J Radiat Oncol Biol Phys. 1992;23:781-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 232] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 82. | Dawson LA, Ten Haken RK. Partial volume tolerance of the liver to radiation. Semin Radiat Oncol. 2005;15:279-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 196] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 83. | Li N, Feng S, Xue J, Wei XB, Shi J, Guo WX, Lau WY, Wu MC, Cheng SQ, Meng Y. Hepatocellular carcinoma with main portal vein tumor thrombus: a comparative study comparing hepatectomy with or without neoadjuvant radiotherapy. HPB (Oxford). 2016;18:549-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 84. | Tang QH, Li AJ, Yang GM, Lai EC, Zhou WP, Jiang ZH, Lau WY, Wu MC. Surgical resection versus conformal radiotherapy combined with TACE for resectable hepatocellular carcinoma with portal vein tumor thrombus: a comparative study. World J Surg. 2013;37:1362-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 85. | Lin CS, Jen YM, Chiu SY, Hwang JM, Chao HL, Lin HY, Shum WY. Treatment of portal vein tumor thrombosis of hepatoma patients with either stereotactic radiotherapy or three-dimensional conformal radiotherapy. Jpn J Clin Oncol. 2006;36:212-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 86. | Hsu WC, Chan SC, Ting LL, Chung NN, Wang PM, Ying KS, Shin JS, Chao CJ, Lin GD. Results of three-dimensional conformal radiotherapy and thalidomide for advanced hepatocellular carcinoma. Jpn J Clin Oncol. 2006;36:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 87. | Lee JH, Kim DH, Ki YK, Nam JH, Heo J, Woo HY, Kim DW, Kim WT. Three-dimensional conformal radiotherapy for portal vein tumor thrombosis alone in advanced hepatocellular carcinoma. Radiat Oncol J. 2014;32:170-178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 88. | Toya R, Murakami R, Baba Y, Nishimura R, Morishita S, Ikeda O, Kawanaka K, Beppu T, Sugiyama S, Sakamoto T, Yamashita Y, Oya N. Conformal radiation therapy for portal vein tumor thrombosis of hepatocellular carcinoma. Radiother Oncol. 2007;84:266-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 89] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 89. | Nakazawa T, Adachi S, Kitano M, Isobe Y, Kokubu S, Hidaka H, Ono K, Okuwaki Y, Watanabe M, Shibuya A, Saigenji K. Potential prognostic benefits of radiotherapy as an initial treatment for patients with unresectable advanced hepatocellular carcinoma with invasion to intrahepatic large vessels. Oncology. 2007;73:90-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 90. | Nakazawa T, Hidaka H, Shibuya A, Okuwaki Y, Tanaka Y, Takada J, Minamino T, Watanabe M, Kokubu S, Koizumi W. Overall survival in response to sorafenib versus radiotherapy in unresectable hepatocellular carcinoma with major portal vein tumor thrombosis: propensity score analysis. BMC Gastroenterol. 2014;14:84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 98] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 91. | Sugahara S, Nakayama H, Fukuda K, Mizumoto M, Tokita M, Abei M, Shoda J, Matsuzaki Y, Thono E, Tsuboi K, Tokuuye K. Proton-beam therapy for hepatocellular carcinoma associated with portal vein tumor thrombosis. Strahlenther Onkol. 2009;185:782-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 92. | Hata M, Tokuuye K, Sugahara S, Kagei K, Igaki H, Hashimoto T, Ohara K, Matsuzaki Y, Tanaka N, Akine Y. Proton beam therapy for hepatocellular carcinoma with portal vein tumor thrombus. Cancer. 2005;104:794-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 85] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 93. | Kim TH, Park JW, Kim BH, Kim H, Moon SH, Kim SS, Woo SM, Koh YH, Lee WJ, Kim DY, Kim CM. Does Risk-Adapted Proton Beam Therapy Have a Role as a Complementary or Alternative Therapeutic Option for Hepatocellular Carcinoma? Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 94. | Kim JY, Lim YK, Kim TH, Cho KH, Choi SH, Jeong H, Kim DW, Park JH, Shin DH, Lee SB, Kim SS, Kim JY, Kim DY, Park JW. Normal liver sparing by proton beam therapy for hepatocellular carcinoma: Comparison with helical intensity modulated radiotherapy and volumetric modulated arc therapy. Acta Oncol. 2015;54:1827-1832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 95. | Qi WX, Fu S, Zhang Q, Guo XM. Charged particle therapy versus photon therapy for patients with hepatocellular carcinoma: a systematic review and meta-analysis. Radiother Oncol. 2015;114:289-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 98] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 96. | Lu XJ, Dong J, Ji LJ, Luo JH, Cao HM, Xiao LX, Zhou J, Ling CQ. Safety and efficacy of TACE and gamma knife on hepatocellular carcinoma with portal vein invasion. Gut. 2016;65:715-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 97. | Lu XJ, Dong J, Ji LJ, Xiao LX, Ling CQ, Zhou J. Tolerability and efficacy of gamma knife radiosurgery on hepatocellular carcinoma with portal vein tumor thrombosis. Oncotarget. 2016;7:3614-3622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 98. | Chen SC, Lian SL, Chang WY. The effect of external radiotherapy in treatment of portal vein invasion in hepatocellular carcinoma. Cancer Chemother Pharmacol. 1994;33 Suppl:S124-S127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 99. | Tazawa J, Maeda M, Sakai Y, Yamane M, Ohbayashi H, Kakinuma S, Miyasaka Y, Nagayama K, Enomoto N, Sato C. Radiation therapy in combination with transcatheter arterial chemoembolization for hepatocellular carcinoma with extensive portal vein involvement. J Gastroenterol Hepatol. 2001;16:660-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 79] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 100. | Yamada K, Soejima T, Sugimoto K, Mayahara H, Izaki K, Sasaki R, Maruta T, Matsumoto S, Hirota S, Sugimura K. Pilot study of local radiotherapy for portal vein tumor thrombus in patients with unresectable hepatocellular carcinoma. Jpn J Clin Oncol. 2001;31:147-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 101. | Yu W, Gu K, Yu Z, Yuan D, He M, Ma N, Lai S, Zhao J, Ren Z, Zhang X, Shao C, Jiang GL. Sorafenib potentiates irradiation effect in hepatocellular carcinoma in vitro and in vivo. Cancer Lett. 2013;329:109-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 102. | Chen SW, Lin LC, Kuo YC, Liang JA, Kuo CC, Chiou JF. Phase 2 study of combined sorafenib and radiation therapy in patients with advanced hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2014;88:1041-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 103. | Yoon SM, Lim YS, Won HJ, Kim JH, Kim KM, Lee HC, Chung YH, Lee YS, Lee SG, Park JH, Suh DJ. Radiotherapy plus transarterial chemoembolization for hepatocellular carcinoma invading the portal vein: long-term patient outcomes. Int J Radiat Oncol Biol Phys. 2012;82:2004-2011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 180] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 104. | Katamura Y, Aikata H, Takaki S, Azakami T, Kawaoka T, Waki K, Hiramatsu A, Kawakami Y, Takahashi S, Kenjo M, Toyota N, Ito K, Chayama K. Intra-arterial 5-fluorouracil/interferon combination therapy for advanced hepatocellular carcinoma with or without three-dimensional conformal radiotherapy for portal vein tumor thrombosis. J Gastroenterol. 2009;44:492-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 105. | Nakagawa K, Yamashita H, Shiraishi K, Nakamura N, Tago M, Igaki H, Hosoi Y, Shiina S, Omata M, Makuuchi M, Ohtomo K. Radiation therapy for portal venous invasion by hepatocellular carcinoma. World J Gastroenterol. 2005;11:7237-7241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 106. | Lau WY, Sangro B, Chen PJ, Cheng SQ, Chow P, Lee RC, Leung T, Han KH, Poon RT. Treatment for hepatocellular carcinoma with portal vein tumor thrombosis: the emerging role for radioembolization using yttrium-90. Oncology. 2013;84:311-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 126] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 107. | Mazzaferro V, Sposito C, Bhoori S, Romito R, Chiesa C, Morosi C, Maccauro M, Marchianò A, Bongini M, Lanocita R, Civelli E, Bombardieri E, Camerini T, Spreafico C. Yttrium-90 radioembolization for intermediate-advanced hepatocellular carcinoma: a phase 2 study. Hepatology. 2013;57:1826-1837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 398] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 108. | Memon K, Kulik L, Lewandowski RJ, Mulcahy MF, Benson AB, Ganger D, Riaz A, Gupta R, Vouche M, Gates VL, Miller FH, Omary RA, Salem R. Radioembolization for hepatocellular carcinoma with portal vein thrombosis: impact of liver function on systemic treatment options at disease progression. J Hepatol. 2013;58:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 109. | She WH, Cheung TT, Yau TC, Chan AC, Chok KS, Chu FS, Liu RK, Poon RT, Chan SC, Fan ST, Lo CM. Survival analysis of transarterial radioembolization with yttrium-90 for hepatocellular carcinoma patients with HBV infection. Hepatobiliary Surg Nutr. 2014;3:185-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 110. | Salem R, Lewandowski RJ, Mulcahy MF, Riaz A, Ryu RK, Ibrahim S, Atassi B, Baker T, Gates V, Miller FH, Sato KT, Wang E, Gupta R, Benson AB, Newman SB, Omary RA, Abecassis M, Kulik L. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010;138:52-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 738] [Cited by in RCA: 781] [Article Influence: 52.1] [Reference Citation Analysis (0)] |
| 111. | Akinwande O, Kim D, Edwards J, Brown R, Philips P, Scoggins C, Martin RC 2nd. Is radioembolization ((90)Y) better than doxorubicin drug eluting beads (DEBDOX) for hepatocellular carcinoma with portal vein thrombosis? A retrospective analysis. Surg Oncol. 2015;24:270-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 112. | Edeline J, Crouzet L, Campillo-Gimenez B, Rolland Y, Pracht M, Guillygomarc'h A, Boudjema K, Lenoir L, Adhoute X, Rohou T, Boucher E, Clément B, Blanc JF, Garin E. Selective internal radiation therapy compared with sorafenib for hepatocellular carcinoma with portal vein thrombosis. Eur J Nucl Med Mol Imaging. 2016;43:635-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 113. | de la Torre MA, Buades-Mateu J, de la Rosa PA, Lué A, Bustamante FJ, Serrano MT, Testillano M, Lorente S, Arenas JI, Gil C, Iñarrairaegui M, Sangro B. A comparison of survival in patients with hepatocellular carcinoma and portal vein invasion treated by radioembolization or sorafenib. Liver Int. 2016;36:1206-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 114. | Gramenzi A, Golfieri R, Mosconi C, Cappelli A, Granito A, Cucchetti A, Marinelli S, Pettinato C, Erroi V, Fiumana S, Bolondi L, Bernardi M, Trevisani F; BLOG (Bologna Liver Oncology Group). Yttrium-90 radioembolization vs sorafenib for intermediate-locally advanced hepatocellular carcinoma: a cohort study with propensity score analysis. Liver Int. 2015;35:1036-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 115. | Jia Z, Jiang G, Tian F, Zhu C, Qin X. A systematic review on the safety and effectiveness of yttrium-90 radioembolization for hepatocellular carcinoma with portal vein tumor thrombosis. Saudi J Gastroenterol. 2016;22:353-359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 116. | Vilgrain V, Pereira H, Assenat E, Guiu B, Ilonca AD, Pageaux GP, Sibert A, Bouattour M, Lebtahi R, Allaham W, Barraud H, Laurent V, Mathias E, Bronowicki JP, Tasu JP, Perdrisot R, Silvain C, Gerolami R, Mundler O, Seitz JF, Vidal V, Aubé C, Oberti F, Couturier O, Brenot-Rossi I, Raoul JL, Sarran A, Costentin C, Itti E, Luciani A, Adam R, Lewin M, Samuel D, Ronot M, Dinut A, Castera L, Chatellier G; SARAH Trial Group. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2017;18:1624-1636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 620] [Cited by in RCA: 594] [Article Influence: 74.3] [Reference Citation Analysis (0)] |
| 117. | Chow PHW, Gandhi M; Asia-Pacific Hepatocellular Carcinoma Trials Group: Phase III multi-centre open-label randomized controlled trial of selective internal radiation therapy (SIRT) versus sorafenib in locally advanced hepatocellular carcinoma: The SIRveNIB study. J Clin Oncol. 2017;35:4002-4002. [DOI] [Full Text] |
| 118. | Spreafico C, Sposito C, Vaiani M, Cascella T, Bhoori S, Morosi C, Lanocita R, Romito R, Chiesa C, Maccauro M, Marchianò A, Mazzaferro V. Development of a prognostic score to predict response to Yttrium-90 radioembolization for hepatocellular carcinoma with portal vein invasion. J Hepatol. 2018;68:724-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 115] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 119. | Zheng JS, Long J, Sun B, Lu NN, Fang D, Zhao LY, Du N. Transcatheter arterial chemoembolization combined with radiofrequency ablation can improve survival of patients with hepatocellular carcinoma with portal vein tumour thrombosis: extending the indication for ablation? Clin Radiol. 2014;69:e253-e263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 120. | Lu ZH, Shen F, Yan ZL, Li J, Yang JH, Zong M, Shi LH, Wu MC. Treatment of portal vein tumor thrombus of hepatocellular carcinoma with percutaneous laser ablation. J Cancer Res Clin Oncol. 2009;135:783-789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 121. | Yang Y, Lu Y, Wang C, Bai W, Qu J, Chen Y, Chang X, An L, Zhou L, Zeng Z, Lou M, Lv J. Cryotherapy is associated with improved clinical outcomes of sorafenib for the treatment of advanced hepatocellular carcinoma. Exp Ther Med. 2012;3:171-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 122. | Livraghi T, Grigioni W, Mazziotti A, Sangalli G, Vettori C. Percutaneous alcohol injection of portal thrombosis in hepatocellular carcinoma: a new possible treatment. Tumori. 1990;76:394-397. [PubMed] |
| 123. | Tarantino L, Busto G, Nasto A, Fristachi R, Cacace L, Talamo M, Accardo C, Bortone S, Gallo P, Tarantino P, Nasto RA, Di Minno MN, Ambrosino P. Percutaneous electrochemotherapy in the treatment of portal vein tumor thrombosis at hepatic hilum in patients with hepatocellular carcinoma in cirrhosis: A feasibility study. World J Gastroenterol. 2017;23:906-918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
| 124. | Lin TY, Lee CS, Chen KM, Chen CC. Role of surgery in the treatment of primary carcinoma of the liver: a 31-year experience. Br J Surg. 1987;74:839-842. [PubMed] |
| 125. | Yamaoka Y, Kumada K, Ino K, Takayasu T, Shimahara Y, Mori K, Tanaka A, Morimoto T, Taki Y, Washida M. Liver resection for hepatocellular carcinoma (HCC) with direct removal of tumor thrombi in the main portal vein. World J Surg. 1992;16:1172-6; discussion 1177. [PubMed] |
| 126. | Sakamoto K, Nagano H. Surgical treatment for advanced hepatocellular carcinoma with portal vein tumor thrombus. Hepatol Res. 2017;47:957-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 127. | Tanaka A, Morimoto T, Yamaoka Y. Implications of surgical treatment for advanced hepatocellular carcinoma with tumor thrombi in the portal vein. Hepatogastroenterology. 1996;43:637-643. [PubMed] |
| 128. | Chok KS, Cheung TT, Chan SC, Poon RT, Fan ST, Lo CM. Surgical outcomes in hepatocellular carcinoma patients with portal vein tumor thrombosis. World J Surg. 2014;38:490-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 129. | Wu CC, Hsieh SR, Chen JT, Ho WL, Lin MC, Yeh DC, Liu TJ, P'eng FK. An appraisal of liver and portal vein resection for hepatocellular carcinoma with tumor thrombi extending to portal bifurcation. Arch Surg. 2000;135:1273-1279. [PubMed] |
| 130. | Chan SL, Chong CC, Chan AW, Poon DM, Chok KS. Management of hepatocellular carcinoma with portal vein tumor thrombosis: Review and update at 2016. World J Gastroenterol. 2016;22:7289-7300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 133] [Cited by in RCA: 141] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 131. | Inoue Y, Hasegawa K, Ishizawa T, Aoki T, Sano K, Beck Y, Imamura H, Sugawara Y, Kokudo N, Makuuchi M. Is there any difference in survival according to the portal tumor thrombectomy method in patients with hepatocellular carcinoma? Surgery. 2009;145:9-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 132. | Zhang YF, Le Y, Wei W, Zou RH, Wang JH, OuYang HY, Xiao CZ, Zhong XP, Shi M, Guo RP. Optimal surgical strategy for hepatocellular carcinoma with portal vein tumor thrombus: a propensity score analysis. Oncotarget. 2016;7:38845-38856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 133. | Fukumoto T, Kido M, Takebe A, Tanaka M, Kinoshita H, Kuramitsu K, Komatsu S, Tsugawa D, Goto T, Asari S, Toyama H, Ajiki T, Ku Y. New macroscopic classification and back-flow thrombectomy for advanced hepatocellular carcinoma with portal vein tumor thrombus invading the contralateral second portal branch. Surg Today. 2017;47:1094-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 134. | Zhou L, Rui JA, Wang SB, Chen SG, Qu Q. Risk factors of microvascular invasion, portal vein tumor thrombosis and poor post-resectional survival in HBV-related hepatocellular carcinoma. Hepatogastroenterology. 2014;61:1696-1703. [PubMed] |
| 135. | Lim C, Compagnon P, Sebagh M, Salloum C, Calderaro J, Luciani A, Pascal G, Laurent A, Levesque E, Maggi U, Feray C, Cherqui D, Castaing D, Azoulay D. Hepatectomy for hepatocellular carcinoma larger than 10 cm: preoperative risk stratification to prevent futile surgery. HPB (Oxford). 2015;17:611-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 136. | Torzilli G, Belghiti J, Kokudo N, Takayama T, Capussotti L, Nuzzo G, Vauthey JN, Choti MA, De Santibanes E, Donadon M, Morenghi E, Makuuchi M. A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers: is it adherent to the EASL/AASLD recommendations?: an observational study of the HCC East-West study group. Ann Surg. 2013;257:929-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 417] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 137. | Wang JH, Wang CC, Hung CH, Chen CL, Lu SN. Survival comparison between surgical resection and radiofrequency ablation for patients in BCLC very early/early stage hepatocellular carcinoma. J Hepatol. 2012;56:412-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 272] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 138. | Kumada K, Ozawa K, Okamoto R, Takayasu T, Yamaguchi M, Yamamoto Y, Higashiyama H, Morikawa S, Sasaki H, Shimahara Y. Hepatic resection for advanced hepatocellular carcinoma with removal of portal vein tumor thrombi. Surgery. 1990;108:821-827. [PubMed] |
| 139. | Zhong JH, Torzilli G, Xing H, Li C, Han J, Liang L, Zhang H, Dai SY, Li LQ, Shen F, Yang T. Controversies and evidence of hepatic resection for hepatocellular carcinoma. BBA Clin. 2016;6:125-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 140. | Zhong JH, Rodríguez AC, Ke Y, Wang YY, Wang L, Li LQ. Hepatic resection as a safe and effective treatment for hepatocellular carcinoma involving a single large tumor, multiple tumors, or macrovascular invasion. Medicine (Baltimore). 2015;94:e396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 107] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 141. | Peng ZW, Guo RP, Zhang YJ, Lin XJ, Chen MS, Lau WY. Hepatic resection versus transcatheter arterial chemoembolization for the treatment of hepatocellular carcinoma with portal vein tumor thrombus. Cancer. 2012;118:4725-4736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 142. | Shi J, Lai EC, Li N, Guo WX, Xue J, Lau WY, Wu MC, Cheng SQ. Surgical treatment of hepatocellular carcinoma with portal vein tumor thrombus. Ann Surg Oncol. 2010;17:2073-2080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 222] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 143. | Liu PH, Lee YH, Hsia CY, Hsu CY, Huang YH, Chiou YY, Lin HC, Huo TI. Surgical resection versus transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombosis: a propensity score analysis. Ann Surg Oncol. 2014;21:1825-1833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 144. | Zheng N, Wei X, Zhang D, Chai W, Che M, Wang J, Du B. Hepatic resection or transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombus. Medicine (Baltimore). 2016;95:e3959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 145. | Chen XP, Qiu FZ, Wu ZD, Zhang ZW, Huang ZY, Chen YF, Zhang BX, He SQ, Zhang WG. Effects of location and extension of portal vein tumor thrombus on long-term outcomes of surgical treatment for hepatocellular carcinoma. Ann Surg Oncol. 2006;13:940-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 110] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 146. | Kudo M, Izumi N, Ichida T, Ku Y, Kokudo N, Sakamoto M, Takayama T, Nakashima O, Matsui O, Matsuyama Y. Report of the 19th follow-up survey of primary liver cancer in Japan. Hepatol Res. 2016;46:372-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 119] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 147. | Ban D, Shimada K, Yamamoto Y, Nara S, Esaki M, Sakamoto Y, Kosuge T. Efficacy of a hepatectomy and a tumor thrombectomy for hepatocellular carcinoma with tumor thrombus extending to the main portal vein. J Gastrointest Surg. 2009;13:1921-1928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 148. | Glantzounis GK, Paliouras A, Stylianidi MC, Milionis H, Tzimas P, Roukos D, Pentheroudakis G, Felekouras E. The role of liver resection in the management of intermediate and advanced stage hepatocellular carcinoma. A systematic review. Eur J Surg Oncol. 2018;44:195-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 149. | Wang K, Guo WX, Chen MS, Mao YL, Sun BC, Shi J, Zhang YJ, Meng Y, Yang YF, Cong WM, Wu MC, Lau WY, Cheng SQ. Multimodality Treatment for Hepatocellular Carcinoma With Portal Vein Tumor Thrombus: A Large-Scale, Multicenter, Propensity Mathching Score Analysis. Medicine (Baltimore). 2016;95:e3015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 150. | Higaki T, Yamazaki S, Moriguchi M, Nakayama H, Kurokawa T, Takayama T. Indication for surgical resection in patients with hepatocellular carcinoma with major vascular invasion. Biosci Trends. 2017;11:581-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 151. | Yamamoto Y, Ikoma H, Morimura R, Shoda K, Konishi H, Murayama Y, Komatsu S, Shiozaki A, Kuriu Y, Kubota T, Nakanishi M, Ichikawa D, Fujiwara H, Okamoto K, Sakakura C, Ochiai T, Otsuji E. Post-hepatectomy survival in advanced hepatocellular carcinoma with portal vein tumor thrombosis. World J Gastroenterol. 2015;21:246-253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 152. | Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5537] [Cited by in RCA: 6460] [Article Influence: 430.7] [Reference Citation Analysis (0)] |
| 153. | Pesi B, Ferrero A, Grazi GL, Cescon M, Russolillo N, Leo F, Boni L, Pinna AD, Capussotti L, Batignani G. Liver resection with thrombectomy as a treatment of hepatocellular carcinoma with major vascular invasion: results from a retrospective multicentric study. Am J Surg. 2015;210:35-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 154. | Roayaie S, Jibara G, Taouli B, Schwartz M. Resection of hepatocellular carcinoma with macroscopic vascular invasion. Ann Surg Oncol. 2013;20:3754-3760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 155. | Hyun MH, Lee YS, Kim JH, Lee CU, Jung YK, Seo YS, Yim HJ, Yeon JE, Byun KS. Hepatic resection compared to chemoembolization in intermediate- to advanced-stage hepatocellular carcinoma: A meta-analysis of high-quality studies. Hepatology. 2018;68:977-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 159] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 156. | Zhang XP, Gao YZ, Chen ZH, Chen MS, Li LQ, Wen TF, Xu L, Wang K, Chai ZT, Guo WX, Shi J, Xie D, Wu MC, Yee Lau W, Cheng SQ. An Eastern Hepatobiliary Surgery Hospital/Portal Vein Tumor Thrombus Scoring System as an Aid to Decision Making on Hepatectomy for Hepatocellular Carcinoma Patients With Portal Vein Tumor Thrombus: A Multicenter Study. Hepatology. 2019;69:2076-2090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 157. | Schnitzbauer AA, Lang SA, Goessmann H, Nadalin S, Baumgart J, Farkas SA, Fichtner-Feigl S, Lorf T, Goralcyk A, Hörbelt R, Kroemer A, Loss M, Rümmele P, Scherer MN, Padberg W, Königsrainer A, Lang H, Obed A, Schlitt HJ. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg. 2012;255:405-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 837] [Cited by in RCA: 932] [Article Influence: 71.7] [Reference Citation Analysis (0)] |
| 158. | Vennarecci G, Laurenzi A, Santoro R, Colasanti M, Lepiane P, Ettorre GM. The ALPPS procedure: a surgical option for hepatocellular carcinoma with major vascular invasion. World J Surg. 2014;38:1498-1503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 159. | Kamiyama T, Kakisaka T, Orimo T, Wakayama K. Hepatectomy for hepatocellular carcinoma with portal vein tumor thrombus. World J Hepatol. 2017;9:1296-1304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 160. | Ye JZ, Wang YY, Bai T, Chen J, Xiang BD, Wu FX, Li LQ. Surgical resection for hepatocellular carcinoma with portal vein tumor thrombus in the Asia-Pacific region beyond the Barcelona Clinic Liver Cancer treatment algorithms: a review and update. Oncotarget. 2017;8:93258-93278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 161. | Peng BG, He Q, Li JP, Zhou F. Adjuvant transcatheter arterial chemoembolization improves efficacy of hepatectomy for patients with hepatocellular carcinoma and portal vein tumor thrombus. Am J Surg. 2009;198:313-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 162. | Li Q, Wang J, Sun Y, Cui YL, Juzi JT, Li HX, Qian BY, Hao XS. Efficacy of postoperative transarterial chemoembolization and portal vein chemotherapy for patients with hepatocellular carcinoma complicated by portal vein tumor thrombosis--a randomized study. World J Surg. 2006;30:2004-2011; discussion 2012-2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 163. | Fukuda S, Okuda K, Imamura M, Imamura I, Eriguchi N, Aoyagi S. Surgical resection combined with chemotherapy for advanced hepatocellular carcinoma with tumor thrombus: report of 19 cases. Surgery. 2002;131:300-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 164. | Lau WY, Ho SK, Yu SC, Lai EC, Liew CT, Leung TW. Salvage surgery following downstaging of unresectable hepatocellular carcinoma. Ann Surg. 2004;240:299-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 112] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 165. | Kamiyama T, Nakanishi K, Yokoo H, Tahara M, Nakagawa T, Kamachi H, Taguchi H, Shirato H, Matsushita M, Todo S. Efficacy of preoperative radiotherapy to portal vein tumor thrombus in the main trunk or first branch in patients with hepatocellular carcinoma. Int J Clin Oncol. 2007;12:363-368. [PubMed] |
| 166. | Pracht M, Edeline J, Lenoir L, Latournerie M, Mesbah H, Audrain O, Rolland Y, Clément B, Raoul JL, Garin E, Boucher E. Lobar hepatocellular carcinoma with ipsilateral portal vein tumor thrombosis treated with yttrium-90 glass microsphere radioembolization: preliminary results. Int J Hepatol. 2013;2013:827649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 167. | Feng YX, Wang T, Deng YZ, Yang P, Li JJ, Guan DX, Yao F, Zhu YQ, Qin Y, Wang H, Li N, Wu MC, Wang HY, Wang XF, Cheng SQ, Xie D. Sorafenib suppresses postsurgical recurrence and metastasis of hepatocellular carcinoma in an orthotopic mouse model. Hepatology. 2011;53:483-492. [PubMed] [DOI] [Full Text] |
| 168. | Li J, Hou Y, Cai XB, Liu B. Sorafenib after resection improves the outcome of BCLC stage C hepatocellular carcinoma. World J Gastroenterol. 2016;22:4034-4040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 169. | Tang ZY, Zhou BH, Wang W, Du G, Liu ZY, Li J, Zhang SZ, Fu ZH. Curative Analysis of Several Therapeutic Methods for Primary Hepatocellular Carcinoma with Portal Vein Tumor Thrombus. Hepatogastroenterology. 2015;62:703-709. [PubMed] |
| 170. | Nagano H, Kobayashi S, Marubashi S, Wada H, Eguchi H, Tanemura M, Tomimaru Y, Umeshita K, Doki Y, Mori M. Combined IFN-α and 5-FU treatment as a postoperative adjuvant following surgery for hepatocellular carcinoma with portal venous tumor thrombus. Exp Ther Med. 2013;5:3-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 171. | Choi HJ, Kim DG, Na GH, Hong TH, You YK. Extended criteria for living donor liver transplantation in patients with advanced hepatocellular carcinoma. Transplant Proc. 2012;44:399-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 172. | Freeman RB. Transplantation for hepatocellular carcinoma: The Milan criteria and beyond. Liver Transpl. 2006;12:S8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 173. | Kaihara S, Kiuchi T, Ueda M, Oike F, Fujimoto Y, Ogawa K, Kozaki K, Tanaka K. Living-donor liver transplantation for hepatocellular carcinoma. Transplantation. 2003;75:S37-S40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 121] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 174. | Sapisochin G, Goldaracena N, Laurence JM, Dib M, Barbas A, Ghanekar A, Cleary SP, Lilly L, Cattral MS, Marquez M, Selzner M, Renner E, Selzner N, McGilvray ID, Greig PD, Grant DR. The extended Toronto criteria for liver transplantation in patients with hepatocellular carcinoma: A prospective validation study. Hepatology. 2016;64:2077-2088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 276] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 175. | Han DH, Joo DJ, Kim MS, Choi GH, Choi JS, Park YN, Seong J, Han KH, Kim SI. Living Donor Liver Transplantation for Advanced Hepatocellular Carcinoma with Portal Vein Tumor Thrombosis after Concurrent Chemoradiation Therapy. Yonsei Med J. 2016;57:1276-1281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 176. | Levi Sandri GB, Ettorre GM, Colasanti M, De Werra E, Mascianà G, Ferraro D, Tortorelli G, Sciuto R, Lucatelli P, Pizzi G, Visco-Comandini U, Vennarecci G. Hepatocellular carcinoma with macrovascular invasion treated with yttrium-90 radioembolization prior to transplantation. Hepatobiliary Surg Nutr. 2017;6:44-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 177. | Chino F, Stephens SJ, Choi SS, Marin D, Kim CY, Morse MA, Godfrey DJ, Czito BG, Willett CG, Palta M. The role of external beam radiotherapy in the treatment of hepatocellular cancer. Cancer. 2018;124:3476-3489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 178. | Mannina EM, Cardenes HR, Lasley FD, Goodman B, Zook J, Althouse S, Cox JA, Saxena R, Tector J, Maluccio M. Role of Stereotactic Body Radiation Therapy Before Orthotopic Liver Transplantation: Retrospective Evaluation of Pathologic Response and Outcomes. Int J Radiat Oncol Biol Phys. 2017;97:931-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 179. | O'Connor JK, Trotter J, Davis GL, Dempster J, Klintmalm GB, Goldstein RM. Long-term outcomes of stereotactic body radiation therapy in the treatment of hepatocellular cancer as a bridge to transplantation. Liver Transpl. 2012;18:949-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 128] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 180. | Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2107] [Cited by in RCA: 3023] [Article Influence: 431.9] [Reference Citation Analysis (3)] |
| 181. | Wang HL, Cucchetti A, Zhong JH, Ye XP, Gu JH, Ma L, Peng NF, Li LQ. Should hepatic resection be recommended to patients with hepatocellular carcinoma and portal vein invasion? J Hepatol. 2016;65:1057-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 182. | Kokudo T, Hasegawa K, Matsuyama Y, Takayama T, Izumi N, Kadoya M, Kudo M, Ku Y, Sakamoto M, Nakashima O, Kaneko S, Kokudo N; Liver Cancer Study Group of Japan. Survival benefit of liver resection for hepatocellular carcinoma associated with portal vein invasion. J Hepatol. 2016;65:938-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 365] [Article Influence: 40.6] [Reference Citation Analysis (0)] |