Published online Aug 14, 2019. doi: 10.3748/wjg.v25.i30.4235
Peer-review started: January 22, 2019
First decision: February 13, 2019
Revised: July 3, 2019
Accepted: July 5, 2019
Article in press: July 5, 2019
Published online: August 14, 2019
Processing time: 204 Days and 15.5 Hours
There is a small and poorly studied population of patients with mild and limited Crohn’s disease (CD), who either spontaneously enter remission and can discontinue therapy, or be maintained on milder anti-inflammatory treatment.
To identify a group of children with mild CD who were not escalated to immunomodulators (azathioprine, mercaptopurine, or methotrexate) or biologics (infliximab or adalimumab) within the first two years after their Crohn’s diagnosis and outline the natural history and phenotypic features of these patients.
In a retrospective chart review of the inflammatory bowel disease database at Boston Children’s Hospital we reviewed all the mild CD patient’s clinic visits, laboratory studies, and procedures for the duration of time they were followed at the center. Patients were included if they had clear diagnosis of Crohn’s disease, and they were not escalated to immunosuppressive therapies for at least 2 years after the date of diagnosis. These mild CD patients were compared to controls diagnosed at a similar time, that were treated with immunomodulators or biologics. Data that was abstracted included: Age at diagnosis, sex, disease location utilizing the Paris classification, medical treatment, surgical treatment, endoscopic findings, histology, and hospitalizations. We also analyzed differences in the phenotypic features between those with mild CD and those with moderate to severe disease.
Out of 1205 patients with CD diagnosed between 1990 and 2013, we identified 29 patients that met the inclusion criteria, and they were matched with 58 controls. There were no significant differences between the disease behaviors at presentation, with approximately 90% of patients in each group having inflammatory disease. However, patients with mild disease were more likely to have disease limited to the colon (31% vs 12%, P = 0.03). In contrast, patients with moderate to severe disease (aka control group) were more likely to have ileocolonic disease (70% vs 45% in the mild group, P = 0.02). Of the 29 patients, only 8 required medication escalation to immunomodulators during the period of follow-up. The primary indication for escalation to immune suppressive therapies was corticosteroid dependence. We also found that patients treated without immunomodulators or biologics for mild CD continue to exhibit histologic intestinal inflammation. Of the 29 patients, three developed significant complications of ileal disease, though only one required surgical intervention during the period of follow-up.
We identified a cohort of children with mild CD, who were able to avoid the institution of immune suppressive therapies for several years, and generally had good outcomes during the period of follow-up. While a subset of these patients will eventually require either immunosuppression or surgery, the majority of them have a good quality of life despite having low-grade intestinal inflammation. Importantly, this subset of patients has managed to avoid the potential toxicities of immune suppression for several years. The majority of these patients have either colonic disease with minimal small bowel involvement or limited ileal disease.
Core tip: We demonstrate that a higher percentage of patients with mild Crohn’s have colonic disease compared to matched controls with moderate to severe disease. We show that patients with mild disease can be maintained for several years on milder treatments and the majority can avoid escalation to immunosuppressive therapies.
- Citation: Sharma Y, Bousvaros A, Liu E, Bender Stern J. Natural history of children with mild Crohn’s disease. World J Gastroenterol 2019; 25(30): 4235-4245
- URL: https://www.wjgnet.com/1007-9327/full/v25/i30/4235.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i30.4235
Over the last 15 years, immune suppressive agents (including immunomodulators such as thiopurines, and biologics such as infliximab and adalimumab) have become the treatment of choice in children and adults with moderate to severe Crohn’s disease (CD)[1,2]. However, there is a small and poorly studied population of patients with mild and limited Crohn’s, who either spontaneously enter remission and can discontinue therapy, or be maintained on milder anti-inflammatory treatment. These treatments include aminosalicylates, dietary manipulations, or antibiotics. Because clinical trials tend to focus on the moderate to severe population, definitive evidence from placebo-controlled trials in the milder population is lacking. Physicians are concerned about the risk of undertreating patients, and exposing them to complications of a subtle, progressive disease[2]. However, utilizing immuno-suppression on a group of patients with mild disease and a good prognosis may in fact be “overtreating”, exposing patients to the risk of such therapies without any benefit. The decision making is challenging, because the phenotypic features, natural history, and long-term outcomes of children with mild disease is unclear. In addition, there is no consensus definition on what constitutes “mild CD” .
In order to better outline the natural history of patients with mild CD, we identified a group of children with CD who were not escalated to immunomodulators or biologics within the first two years after their CD diagnosis. In this highly selected group of mild patients, we demonstrate that a higher percentage of patients have colonic disease compared to matched controls with moderate to severe disease. We also show that patients with mild disease can be maintained for several years on treatments such as aminosalicylates, antibiotics, or dietary changes, and the majority can avoid escalation to immunosuppressive therapies. However, subclinical inflammation and growth impairment may persist in this group of patients.
In order to identify patients with mild CD we conducted a retrospective review of our IBD database at Boston Children’s Hospital. At the time of review, the database contained 1205 patients with CD, diagnosed between the years of 1990 and 2013. Patients were included if they had clear diagnosis of CD, and they were not escalated to immunosuppressive therapies for at least 2 years after the date of diagnosis. No patients with ulcerative colitis or indeterminate colitis [aka inflammatory bowel disease unclassified (IBDU)] were included in this study. Exclusion criteria were usage of methotrexate, mercaptopurine, azathioprine, or biologic therapies (e.g., infliximab, adalimumab, or certolizumab) initiated within the first 2 years after diagnosis. We also excluded patients who developed surgical complications of CD requiring bowel resection within the first two years. Patients with perianal disease were not excluded.
The natural history of this mild group of CD patients was reviewed for the duration of time they were followed as patients at our center. We reviewed all clinic visits, laboratory studies, and procedures. Data that was abstracted included: age at diagnosis, sex, disease location utilizing the Paris classification, medical treatment, surgical treatment, endoscopic findings, histology, and hospitalizations[3]. We utilized a standardized case report form, and the abstracted data was entered into a REDCAP database.
To determine if there were differences in phenotypic features between the mild group and patients with moderate to severe disease, we identified a matched control group with moderate to severe disease. We matched each case with two “moderate to severe” controls (aka patients started on an immunomodulator or biologic within the first two years of diagnosis). Patients were matched by sex and age at diagnosis. Because treatment of CD has changed significantly in the last 20 years, we also matched patients by the year the patient was diagnosed (within 2 years). For example, a 12-year-old female diagnosed in 2000 would be matched with a similar patient diagnosed between 2000 and 2002, so as to avoid comparing a patient diagnosed in 2000 with a patient diagnosed in 2010.
Data were entered by using Redcap and converted to SAS software (Version 9.4; SAS Institute) for analysis. Descriptive statistics were used to summarize subject characteristics at disease diagnosis. For continuous variables, mean and standard deviation were derived and compared using independent T-tests; for categorical variables, frequencies in absolute numbers and percentages were assessed and compared using Chi-squared tests. In order to describe height data, we utilized Z scores, with a Z score reflecting the number of standard deviations above or below the mean for a specific data point or group of data points.
Cox proportional hazard models were used to identify risk factors of disease escalation among the mild group of patients with CD. Potential risk factors considered into the analysis include age at diagnosis, sex, Paris location, steroid use in the first 2 years and after 2 years following diagnosis of CD, maintained on antibiotics in the first 2 years following diagnosis of CD, and whether complications developed after diagnosis of CD. Kaplan-Meier curve was used to show time to escalation after 2 years of diagnosis.
All variables were analyzed in a univariate manner. All statistical tests were two-sided, with P value less than 0.05 considered significant.
Out of 1205 patients with CD diagnosed between 1990 and 2013, we identified 29 patients (15 male) that met the inclusion criteria and were matched with 58 controls. Table 1 summarizes the demographic characteristics between the cases and the control group. The mean age of diagnosis was 10 ± 4.2 years. All twenty-nine mild patients were treated with aminosalicylates and 5 patients had a combination of aminosalicylates and antibiotics. There were no significant differences between the disease behaviors at presentation, with approximately 90% of patients in each group having inflammatory disease (aka CD without the development of either perforating or fibrostenosing complications). However, patients with mild disease were more likely to have disease limited to the colon (31% vs 12%, P = 0.03). In contrast, patients with moderate to severe disease (aka control group) were more likely to have ileocolonic disease (70% vs 45% in the mild group, P = 0.02). At diagnosis, the mild group had a lower mean CRP and a higher albumin (CRP 1.54 mg/dL, Albumin 3.8 mg/dL), compared to the control group (CRP 5.9 mg/dL, Albumin 3.3 mg/dL). Hematocrit and ESR were similar for both groups (mild group mean HCT 35 %, ESR 32 mm/h; control group HCT 35 %, ESR 37 mm/h).
| Variables | Mild case (n = 29) | Moderate or severe case (n = 58) | P value |
| Age at diagnosis, yr | 10.0 (4.2) | 11.6 (3.3) | 0.06 |
| Male % | 15 (51.7) | 29 (52.7) | 0.93 |
| Paris Location at diagnosis | |||
| Lower 1/3 of ileum +/- base of cecum | 6 (20.7) | 7 (12.1) | 0.29 |
| Colon only | 9 (31.0) | 7 (12.1) | 0.03 |
| Ileum and colon | 13 (44.8) | 41 (70.7) | 0.02 |
| Upper GI proximal to ligament of treitz | 7 (24.1) | 25 (43.1) | 0.08 |
| Distal to ligament of treitz to proximal ileum | 1 (3.5) | 2 (3.5) | 1.00 |
| Disease behavior at diagnosis | |||
| Inflammatory (non-stricturing, non-penetrating) | 28 (96.6) | 51 (87.9) | 0.19 |
| Stricturing | 0 (0) | 3 (5.2) | 0.21 |
| Penetrating | 0 (0) | 1 (1.7) | 0.48 |
| Perianal | 3 (10.3) | 12 (20.7) | 0.23 |
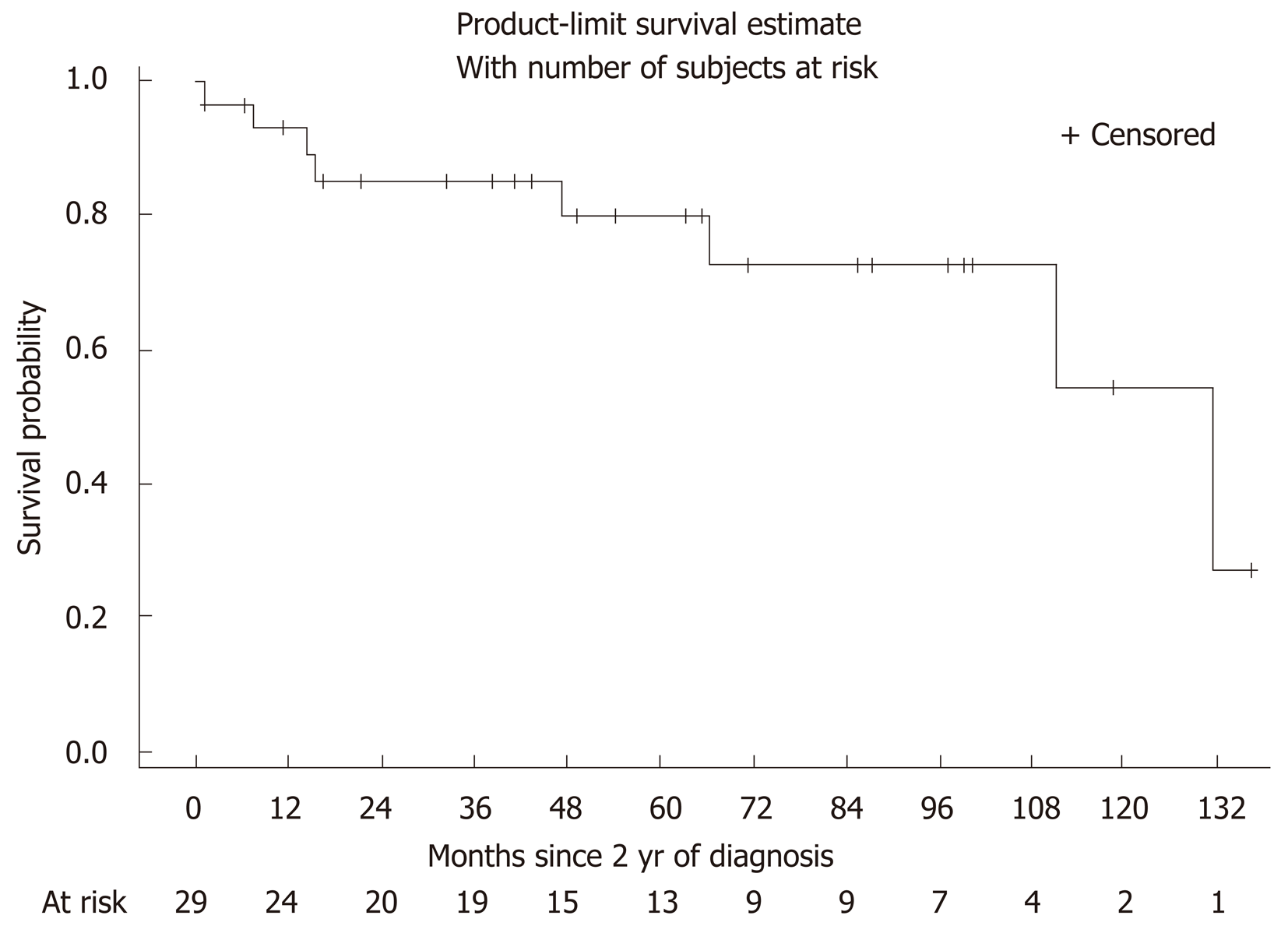
The mild CD group was followed for a mean of 4.5 years after diagnosis (range 1 month to 11 years). In order to look at long term outcomes via a survival curve, we did not include the first two years of therapy (as we defined our mild population by the absence of complications or drug escalation in those first two years). Figure 1 (Kaplan-Meier curve) illustrates the probability of avoiding escalation to immunomodulators or biologics during the period of follow-up. Of the 29 patients, 8 required medication escalation to immunomodulators during the period of follow-up (Table 2). The primary indication for escalation to immune suppressive therapies was corticosteroid dependence. Patients treated with corticosteroids during the follow-up period were more likely to undergo addition of immunomodulators (hazard ratio = 12.1, 95%CI: 1.4-102.7, P = 0.02) (Table 3). Only one of these 29 patients required bowel resection during the follow-up period (after 12 years), though 1 patient did require surgical intervention for perianal disease.
| n (%) | |
| Starting immunomodulator | 8 (27.6) |
| Starting biologic | 0 |
| Starting immunomodulator and biologic | 0 |
| Surgery | 0 |
| Continued on minimal therapy | 21 (72.4) |
| Univariate | P value | |
| Hazard ratio (95%CI) | ||
| Age at diagnosis | 1.24 (0.97-1.59) | 0.09 |
| Gender (female vs male) | 0.67 (0.15-3.00) | 0.60 |
| Paris location at diagnosis | ||
| Lower 1/3 of ileum +/- base of cecum | 1.29 (0.30-5.66) | 0.73 |
| Colon only | 0.96 (0.19-4.99) | 0.96 |
| Ileum and colon | 0.50 (0.10-2.53) | 0.40 |
| Upper GI proximal to Ligament of Treitz | 1.00 (0.19-5.17) | 1.00 |
| Distal to Ligament of Treitz to proximal ileum | 5.46 (0.61-49.27) | 0.13 |
| Any steroids in the first 2 yr | 1.26 (0.30-5.35) | 0.75 |
| Any steroids after the first 2 yr | 12.1 (1.4-102.7) | 0.02 |
| Complications | 5.6 (1.3-24.9) | 0.02 |
Of the 29 patients, 21 underwent follow-up colonoscopy at some point after their initial diagnosis. Fifteen of the 21 patients continued to demonstrate histologic inflammation consistent with active CD (including granulomata, cryptitis, and chronic active ileitis or colitis) (Table 4). One patient underwent two follow-up colonoscopies over an 8-year period. The first demonstrated active inflammation, while the most recent was completely normal.
| Year (age) of diagnosis | Duration of follow-up after initial 2 yr of treatment (mo) | Followup colonoscopy histology findings | Medications | Outcome/ complications |
| 2002 (10 yr) | 111 | 2007-normal colonoscopy | 5-ASA (Asacol), prednisone | Escalation to 6MP(age 20) Surgery for ileal stricture (age 22) |
| 2004 (12 yr) | 49 | 2009-Noncaseating granulomas in TI and throughout colon. | 5-ASA (Pentasa, Lialda) | Stayed on ASA, no escalation |
| 2001 (9 yr) | 32 | Not done | 5-ASA, prednisone | Stayed on ASA, no prednisone after induction, no escalation |
| 2002 (10 yr) | 131 | 2009- chronic active ileitis with ulceration | 5-ASA (Pentasa, Lialda) | 2015-escalation to mercaptopurine, then adalimumab |
| 2003 (10 yr) | 66 | 2010-severely active ileitis | 5-ASA (Pentasa), 3 courses of prednisone over 3 years | 2012-began mercaptopurine |
| 2005 (13 yr) | 15 | Not done | 5-ASA (Asacol) | Ileal phlegmon, treated with antibiotics and subsequent mercaptopurine |
| 2010 (16 yr) | 43 | 2014-nonnecrotizing granuloma throughout the colon | 5-ASA (Asacol) | Stayed on ASA, no escalation. |
| 2010 (16 yr) | 1 | Not done | 5-ASA (Asacol), hydrocortisone | Stayed on ASA, no steroid after initial induction |
| 2008 (14 yr) | 63 | 2010-mild active ileitis colitis in ascending and sigmoid colon | 5-ASA (Asacol), metronidazole | Stayed on ASA, no escalation |
| 2012 (17 yr) | 7 | 2014-nonnecrotizing granulomas in left colon | 5-ASA (Pentasa) | Escalation to mercaptopurine, then adalimumab |
| 2008 (14 yr) | 65 | 2012-normal ileum, chronic active colitis 2016-normal colonoscopy | 5-ASA (Pentasa), budesonide | No escalation |
| 2008 (13 yr) | 47 | 2014-mild active colitis, in ascending, granuloma in ileum | 5-ASA (Pentasa), metronidazole | 6MP initiated |
| 2007 (12 yr) | 71 | 2016-Inactive colitis at IC valve | 5-ASA (Asacol) | Ileal stricture in 2016, no surgery yet |
| 2008 (13 yr) | 21 | 2009-active colitis in cecum, inactive in remainder of colon | 5-ASA (Pentasa, Lialda) | Discontinued all therapy, never hospitalized |
| 2005 (10 yr) | 14 | None prior to escalation | 5-ASA (Pentasa), metronidazole | 6MP, then infliximab |
| 2010 (15 yr) | 6 | None | 5-ASA (Pentasa), prednisone induction | No escalation |
| 2005 (9 yr) | 85 | 2013-focal mild ileitis | 5-ASA (Pentasa), metronidazole | No escalation |
| 2000 (4 yr) | 1 | Not done | Sulfasalazine, flagyl | Begun infliximab for colonic and perianal disease |
| 2002 (12 yr) | 38 | 2012-normal colonoscopy | 5-ASA (mesalamine), prednisone | No escalation |
| 2006 (9 yr) | 87 | 2016-chronic active colitis, non- necrotizing granuloma in transverse colon | 5-ASA (Pentasa), two courses of steroids in first two years | No escalation |
| 2002 (4 yr) | 136 | 2010-mild active colitis | Sulfasalazine, budesonide | No escalation |
| 2001 (3 yr) | 100 | Not done | Sulfasalazine, budesonide | No escalation |
| 2003 (5 yr) | 118 | 2012-focal moderately active colitis | Sulfasalazine, prednisone | No escalation |
| 2009 (10 yr) | 41 | 2014-focal acute inflammation | 5ASA (Asacol), Budesonide | No escalation |
| 2005 (7 yr) | 99 | 2014-mildly active colitis | Pentasa | No escalation |
| 2007 (6 yr) | 97 | 2013- chronic inactive colitis | Pentasa, Sulfasalazine | No escalation |
| 2012 (10 yr) | 16 | 2013-mild active colitis with granulomata | Asacol, prednisone induction | No escalation |
| 2008 (3 yr) | 54 | Not done | Sulfasalazine, probiotics | No escalation |
| 2010 (3 yr) | 11 | 2012-chronic moderately active colitis | Sulfasalazine, prednisone induction | No escalation |
At diagnosis, the mean Z score for our aminosalicylate patient cohort on whom accurate height data was available (n = 27) was -0.07 (range -1.88 to 2.37). At the end of the period of follow- up, the mean Z score had declined slightly to -0.19 (range -2.83 to +1.75). The mean change in Z score from diagnosis to end of follow-up was -0.12, suggesting that the subclinical histologic inflammation could potentially contribute to impaired growth. Overall, 11 of 27 patients had a positive change in Z score from diagnosis to the end of follow-up, while 16 of 27 had a negative change.
Of the 29 total patients, three developed significant complications of ileal disease, though only one required surgical intervention during the period of follow-up. One patient, diagnosed at age 10, was escalated to mercaptopurine at age 20 for persistent ileal inflammation, and underwent limited ileal resection at age 22. The second presented with an ileal phlegmon that was managed medically and escalated to thiopurine therapy 39 months after diagnosis. The third patient presented with ileal narrowing and lower gastrointestinal bleeding and received both infliximab and thiopurine combination therapy with clinical improvement 63 months after diagnosis. No other patient developed serious complications of their IBD. The patients with complications during the follow-up period were also more likely to subsequently be treated with immunosuppression (hazard ratio = 5.6, 95%CI: 1.3-24.9, P = 0.02).
In this study, we have identified a cohort of children with mild Crohn’s disease, who were able to avoid the institution of immune suppressive therapies for several years, and generally had good outcomes during the period of follow-up. This cohort of children fall into two major categories: Those with limited ileal disease, which sometimes may progress to an ileal stricture over time, and those with predominantly colonic disease, who can have their symptoms controlled on aminosalicylate therapy, and who remained asymptomatic despite the ongoing presence of colonic inflammation. While a subset of these patients will eventually require either immunosuppression or surgery, the majority of them appear to have a good quality of life despite having low-grade intestinal inflammation, with the caveat that quality of life is not formally assessed with questionnaires during most clinical visits. Importantly, this subset of patients has managed to avoid the potential toxicities of immune suppression for several years. However, the persistent colonic inflammation may result in ongoing lack of catch-up growth, as our cohort had a mild falloff in mean Z score over the course of the study.
Most research on pediatric CD has focused on identifying and treating the population of patients with moderate to severe disease, who will benefit from early introduction of “top-down therapy” with immunomodulators (azathioprine, mercaptopurine, and methotrexate) and biologics (infliximab, adalimumab, and potentially ustekinumab and vedolizumab). Clinical, serologic, and genetic factors that are associated with an increased likelihood to develop complications such as stricturing or penetrating disease include deep fissuring colonic ulcers, the presence of antibodies to Saccharomyces cerevisiae (ASCA), and the presence of the NOD2 gene. A paper from the prospective RISK cohort utilizing propensity score matching, demonstrated that comparably ill patients have a better clinical outcome when treated with anti-TNF agents, than when treated with immunomodulators or amino-salicylates. However, it is unclear how effective immunomodulators and biologics are in preventing progression to surgery. A large prospective study from the pediatric inflammatory bowel disease registry demonstrated that early introduction of immunomodulators does not necessarily reduce the likelihood of surgery [4]. More recent data from RISK suggests that while early use of anti-TNF agents may reduce disease activity, anti-TNFs do not necessarily prevent the development of complications such as stricturing disease[5]. In contrast, there is almost no information on the phenotype and natural history of clinically mild CD. In a prospective cohort study in 2008, Dubinsky and colleagues demonstrated that the absence of serologic markers (ANCA, ASCA) correlated with a reduced likelihood of surgery in children with CD[6]. Siegel and colleagues have developed a web based tool that incorporates clinical characteristics, NOD 2 genetics, and serology, which will allow physicians and patients to predict the likelihood of complications in the future.
Our population was treated with a variety of agents, most notably sulfasalazine or mesalamine. Mild CD is frequently treated with nonimmune suppressive medications such as aminosalicylates[7]. Aminosalicylates have anti-inflammatory properties by inhibiting prostaglandin and leukotriene synthesis [8]. In addition, sulfasalazine may also have antimicrobial properties. The utilization of aminosalicylates in CD remains highly controversial. Those in favor of aminosalicylate use cite a meta-analysis of multiple trials by Hanauer et al[9], suggesting a modest reduction in the CD activity index. In contrast, those who feel aminosalicylates are of little or no value in the treatment of IBD state that the studies are highly conflicting, that the overall effect is clinically insignificant, and there is no evidence that aminosalicylates produce mucosal healing[10]. A recent meta-analysis of available clinical trials from the Cochrane group suggested that sulfasalazine “is modestly effective” at inducing remission in Crohn’s, though the efficacy is inferior to corticosteroids[11]. The same review suggested that high dose mesalamine is not superior to placebo in the treatment of Crohn’s. However, the clinical trial data is limited, and the group recommended additional studies be performed to more fully answer the question as to whether or not aminosalicylates are effective. Experts have suggested a limited role for aminosalicylates in the treatment of CD, though aminosalicylates probably has minimal efficacy when utilized as adjunctive treatment in patients receiving immunomodulators or biologics[12].
In spite of evidence suggesting limited if any efficacy in CD, aminosalicylate medications continue to be frequently prescribed by gastroenterologists[13]. A 2009 retrospective multicenter pediatric study conducted in the Netherlands analyzed disease behavior at diagnosis and its effect on prescribing behavior. They found that aminosalicylate monotherapy was more frequently prescribed for mild disease[14]. Additionally, a population-based cohort study was published in 2008 where they outlined the natural history of pediatric CD. They included all cases of pediatric CD in the EPIMAD registry diagnosed between 1988 and 2002 with a median follow-up time of 84 months. At diagnosis most patients had disease in the ileum or colon that was non-stricturing and non-penetrating in nature. Almost all children in this cohort received aminosalicylates at one point in time[15].
We have little knowledge about those with mild disease who spontaneously enter remission and can discontinue medical therapy all together. In a recently published Canadian study evaluating a population-based database with patients diagnosed from 1987 to 2002, about 50% of IBD patients were not on any medications 5 years after their diagnosis and yet still continued to do well. Long term medication non-use was defined as no IBD related medications for at least 1 year or longer. This was more commonly seen in CD as compared to UC[16]. In another study, one third of patients with CD at a tertiary care medical center were not on any IBD medications in a 2 year time span. Approximately 50% of those nonusers were in clinical remission per physician assessment. These non-users were older and had longer disease duration as compared to those on medications. There was no significant difference in disease location, disease behavior or the presence of perianal disease[17].
Other interventions which are utilized to treat mild CD include antibiotics and dietary interventions. Antibiotics such as ciprofloxacin and metronidazole are generally utilized short term, to treat “flares” of the disease characterized by abdominal pain or diarrhea[18]. A recent paper by Levine and colleagues suggested that the combination of azithromycin and metronidazole may be superior to the utilization of metronidazole alone at treating active Crohn’s[19]. There is little data however on the use of long-term antibiotics to prevent exacerbation of CD, and there are the potential risks of Clostridium difficile infection if recurrent courses of antibiotics are used, or neuropathy (particularly with metronidazole). While a subset of our patients did receive courses of antibiotic therapy, they were not utilized for long-term treatment.
Dietary therapy is also of interest to patients. While exclusive enteral nutrition is often utilized for induction of remission in patients with CD, data remains limited on utilization of diet as long-term maintenance therapy. Gupta and Baldassano presented outcomes of an enteral nutrition protocol in which patients took the majority of their calories as liquid diet, but also were able to take a subset of their calories as food. The majority of patients that utilize this protocol abandoned this intervention after 1 year, suggesting the challenges of complying with such a restrictive diet[20]. Other diets of interest to patients include the specific carbohydrate diet, which in at least one small study has been suggested to promote intestinal healing[21].
Our study has several limitations. Most importantly, the population is highly selected. Fewer than 5% of our patients met the inclusion criteria of being able to avoid escalation to immunomodulators or biologics within the first 2 years. It is unclear however whether disease severity warranted the introduction of more immunosuppression, or whether the practitioners treating these patients subscribed to a “top-down philosophy” of utilizing early immunosuppression. There is a possibility that patients that fit this mild phenotype might be treated with immunosuppression very early on. Though, the low frequency of such patients emphasizes that the vast majority of children with Crohn’s will in all likelihood require immunosuppression to treat their disease. In addition, though we could identify histologic inflammation in patients, we could not adequately retrospectively assess the extent of macroscopic (aka visible with the endoscope) inflammation, because the endoscopic reports did not contain formal endoscopic activity indices such as the SES-CD. Future prospective studies of mild CD that incorporate follow-up colonoscopy, should utilize respective endoscopic scoring systems.
Nevertheless, this study does suggest that there is a phenotype of mild CD, which may not necessarily require immunomodulators or biologics. Our case-control study suggests that the majority of these patients have either colonic disease with minimal small bowel involvement or limited ileal disease. Further characterization of this phenotype may require additional prospective studies, though possibly retrospective examinations of large cohorts (such as the risk cohort) may also help identify and better characterize this phenotype. In the future, we hope that additional genetic, microbiome, and serologic data may help us better predict a priori who constitutes this milder cohort of patients and whether they can be treated in a more conservative “step up” or “treat to target” manner, rather than all receiving immunomodulators or biologics early in the course of their disease. The current concept of “treat to target” encourages physicians to implement therapy, and then reassess the effects on the patient on clinical remission, laboratory remission and endoscopic remission 6-12 months after the therapy is initiated. Identification and better characterization of this mild subset of patients is very important, as most families would prefer to avoid immunosuppression if possible. However, the benefits of avoiding immune suppression must be weighed against the risks of potential future disease com-plications (including growth failure).
A great deal has been written on the treatment of moderate to severe Crohn’s disease. There is a subset of patients with milder disease, who may be able to avoid potent long-term immune suppression. However, there is little or no data on the natural history of patients who are not treated with immune suppressive agents. This is particularly relevant in children, who if treated with immune suppression agents, may be at risk for serious toxicities such as infection and lymphoma. However, the under treatment of Crohn’s disease could increase the risk of the development of complications. We therefore desired to study the natural history of patients who were not treated with immune suppressive agents.
Children with mild or limited Crohn’s disease are a poorly studied population. Phenotypic features, natural history, treatments used, and long-term outcomes of children with mild disease is unclear. We wanted to assess whether there are phenotypic differences such as disease location or disease behavior in mild vs moderate and/or severe Crohn’s disease at the time of diagnosis. We also wanted to define long-term outcome in the mild population. We wanted to see whether these patients develop complications or require surgery over time, and if so, what is the time to escalation in therapy and also, what are the predictors of escalation.
The objective of the study was to determine the prevalence of complications in patients who did not receive immunomodulators or biologics for at least 2 years after initial diagnosis. We identified a small subset of patients in our cohort who were treated this way. We then reviewed charts in order to determine the event-free survival, i.e., the time until treatment was escalated to immunomodulators, biologics, or surgery.
We conducted a retrospective chart review of the Inflammatory Bowel Disease Database at Boston Children’s Hospital. We went through the detailed clinic visits, laboratory studies and procedures for all the patients that met our inclusion criteria. And data was then filed into a Redcap database. Descriptive statistics were used to summarize subject characteristics at disease diagnosis. Chi-squared tests were utilized to assess categorical variables and independent T-tests were used to compare continuous variables. Z scores were used to describe height data.
We identified a subset of patients who were able to avoid immune suppression for prolonged periods of time, up to a decade. Interestingly, only a subset of these patients (approximately 30%) required escalation to more potent immunosuppression on clinical grounds. Patients that required steroid courses after the first 2 years and had more complications were more likely to require escalation in therapy. However, treatment without immunosuppressive drugs (such as aminosalicylates), while often resulting in clinical improvement, did not result in histologic healing in the vast majority of patients.
Not every child with new onset Crohn’s disease requires the early institution of immunosuppressive agents (such as methotrexate, mercaptopurine, or anti-TNF treatment) immediately after diagnosis. There is a small subset of children with mild Crohn’s disease, who can be maintained on aminosalicylate therapy alone, and not require drug escalation for several years. Currently, the phenotype that may have the best response to aminosalicylate therapy is mild colonic disease.
Mild Crohn’s patients may be able to be identified in the future through genetic or serologic predictive models. It’s important to stratify low risk patients and prevent overtreatment with immunomodulators and biologics. Future research should focus on establishing more genotypic and environmental data to better characterize this low risk population.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chiba T, Day AS S-Editor: Ma RY L-Editor: A E-Editor: Zhang YL
| 1. | Ruemmele FM, Veres G, Kolho KL, Griffiths A, Levine A, Escher JC, Amil Dias J, Barabino A, Braegger CP, Bronsky J, Buderus S, Martín-de-Carpi J, De Ridder L, Fagerberg UL, Hugot JP, Kierkus J, Kolacek S, Koletzko S, Lionetti P, Miele E, Navas López VM, Paerregaard A, Russell RK, Serban DE, Shaoul R, Van Rheenen P, Veereman G, Weiss B, Wilson D, Dignass A, Eliakim A, Winter H, Turner D; European Crohn's and Colitis Organisation; European Society of Pediatric Gastroenterology, Hepatology and Nutrition. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn's disease. J Crohns Colitis. 2014;8:1179-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 851] [Cited by in RCA: 843] [Article Influence: 76.6] [Reference Citation Analysis (0)] |
| 2. | Conrad MA, Rosh JR. Pediatric Inflammatory Bowel Disease. Pediatr Clin North Am. 2017;64:577-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 3. | Levine A, Griffiths A, Markowitz J, Wilson DC, Turner D, Russell RK, Fell J, Ruemmele FM, Walters T, Sherlock M, Dubinsky M, Hyams JS. Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflamm Bowel Dis. 2011;17:1314-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1216] [Cited by in RCA: 1144] [Article Influence: 81.7] [Reference Citation Analysis (0)] |
| 4. | Schaefer ME, Machan JT, Kawatu D, Langton CR, Markowitz J, Crandall W, Mack DR, Evans JS, Pfefferkorn MD, Griffiths AM, Otley AR, Bousvaros A, Kugathasan S, Rosh JR, Keljo DJ, Carvalho RS, Tomer G, Mamula P, Kay MH, Kerzner B, Oliva-Hemker M, Kappelman MD, Saeed SA, Hyams JS, Leleiko NS. Factors that determine risk for surgery in pediatric patients with Crohn's disease. Clin Gastroenterol Hepatol. 2010;8:789-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Kugathasan S, Denson LA, Walters TD, Kim MO, Marigorta UM, Schirmer M, Mondal K, Liu C, Griffiths A, Noe JD, Crandall WV, Snapper S, Rabizadeh S, Rosh JR, Shapiro JM, Guthery S, Mack DR, Kellermayer R, Kappelman MD, Steiner S, Moulton DE, Keljo D, Cohen S, Oliva-Hemker M, Heyman MB, Otley AR, Baker SS, Evans JS, Kirschner BS, Patel AS, Ziring D, Trapnell BC, Sylvester FA, Stephens MC, Baldassano RN, Markowitz JF, Cho J, Xavier RJ, Huttenhower C, Aronow BJ, Gibson G, Hyams JS, Dubinsky MC. Prediction of complicated disease course for children newly diagnosed with Crohn's disease: a multicentre inception cohort study. Lancet. 2017;389:1710-1718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 489] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 6. | Dubinsky MC, Kugathasan S, Mei L, Picornell Y, Nebel J, Wrobel I, Quiros A, Silber G, Wahbeh G, Katzir L, Vasiliauskas E, Bahar R, Otley A, Mack D, Evans J, Rosh J, Hemker MO, Leleiko N, Crandall W, Langton C, Landers C, Taylor KD, Targan SR, Rotter JI, Markowitz J, Hyams J; Western Regional Pediatric IBD Research Alliance; Pediatric IBD Collaborative Research Group; Wisconsin Pediatric IBD Alliance. Increased immune reactivity predicts aggressive complicating Crohn's disease in children. Clin Gastroenterol Hepatol. 2008;6:1105-1111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 188] [Cited by in RCA: 181] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 7. | Lahad A, Weiss B. Current therapy of pediatric Crohn's disease. World J Gastrointest Pathophysiol. 2015;6:33-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Desreumaux P, Ghosh S. Review article: mode of action and delivery of 5-aminosalicylic acid - new evidence. Aliment Pharmacol Ther. 2006;24 Suppl 1:2-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 129] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 9. | Hanauer SB, Strömberg U. Oral Pentasa in the treatment of active Crohn's disease: A meta-analysis of double-blind, placebo-controlled trials. Clin Gastroenterol Hepatol. 2004;2:379-388. [PubMed] |
| 10. | van Bodegraven AA, Mulder CJ. Indications for 5-aminosalicylate in inflammatory bowel disease: is the body of evidence complete? World J Gastroenterol. 2006;12:6115-6123. [PubMed] |
| 11. | Lim WC, Wang Y, MacDonald JK, Hanauer S. Aminosalicylates for induction of remission or response in Crohn's disease. Cochrane Database Syst Rev. 2016;7:CD008870. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 12. | Hanauer SB. Review article: aminosalicylates in inflammatory bowel disease. Aliment Pharmacol Ther. 2004;20 Suppl 4:60-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Gearry RB, Ajlouni Y, Nandurkar S, Iser JH, Gibson PR. 5-Aminosalicylic acid (mesalazine) use in Crohn's disease: a survey of the opinions and practice of Australian gastroenterologists. Inflamm Bowel Dis. 2007;13:1009-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Mesker T, van Rheenen PF, Norbruis OF, Uitentuis J, Waalkens HJ, Gonera G, van Overbeek LA, Butler J, Rings EH. Pediatric Crohn's disease activity at diagnosis, its influence on pediatrician's prescribing behavior, and clinical outcome 5 years later. Inflamm Bowel Dis. 2009;15:1670-1677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Vernier-Massouille G, Balde M, Salleron J, Turck D, Dupas JL, Mouterde O, Merle V, Salomez JL, Branche J, Marti R, Lerebours E, Cortot A, Gower-Rousseau C, Colombel JF. Natural history of pediatric Crohn's disease: a population-based cohort study. Gastroenterology. 2008;135:1106-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 467] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 16. | Melesse DY, Targownik LE, Singh H, Blanchard JF, Bernstein CN. Patterns and Predictors of Long-term Nonuse of Medical Therapy Among Persons with Inflammatory Bowel Disease. Inflamm Bowel Dis. 2015;21:1615-1622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Bhasin S, Singh H, Targownik LE, Israeli E, Bernstein CN. Rates and Reasons for Nonuse of Prescription Medication for Inflammatory Bowel Disease in a Referral Clinic. Inflamm Bowel Dis. 2016;22:919-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Norton C, Czuber-Dochan W, Artom M, Sweeney L, Hart A. Systematic review: interventions for abdominal pain management in inflammatory bowel disease. Aliment Pharmacol Ther. 2017;46:115-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 19. | Levine A, Turner D. Combined azithromycin and metronidazole therapy is effective in inducing remission in pediatric Crohn's disease. J Crohns Colitis. 2011;5:222-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Gupta K, Noble A, Kachelries KE, Albenberg L, Kelsen JR, Grossman AB, Baldassano RN. A novel enteral nutrition protocol for the treatment of pediatric Crohn's disease. Inflamm Bowel Dis. 2013;19:1374-1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Cohen SA, Gold BD, Oliva S, Lewis J, Stallworth A, Koch B, Eshee L, Mason D. Clinical and mucosal improvement with specific carbohydrate diet in pediatric Crohn disease. J Pediatr Gastroenterol Nutr. 2014;59:516-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 170] [Article Influence: 15.5] [Reference Citation Analysis (0)] |