Published online Jul 7, 2019. doi: 10.3748/wjg.v25.i25.3196
Peer-review started: February 23, 2019
First decision: April 4, 2019
Revised: May 14, 2019
Accepted: May 31, 2019
Article in press: June 1, 2019
Published online: July 7, 2019
Processing time: 133 Days and 23.2 Hours
Colorectal cancer (CRC) is the third most common malignancy of the digestive tract and the fifth leading cause of cancer-related mortality in China. Sporamin, a Kunitz-type trypsin inhibitor isolated from sweet potato, is a potential anti-cancer agent with activities against a number of malignant tumor cells in vitro. The liver secretes a myriad of endocrine factors that may facilitate the growth and transformation of tumors in the development of CRC.
To investigate the effects of sporamin on liver morphology and biomarkers of xenografted CRC in the liver of athymic BALB/c mice.
Twenty-seven male BALB/c nude mice were randomly divided into control, vehicle, and sporamin groups. Mice in the latter two groups were intraperitoneally xenografted with LoVo colorectal carcinoma cells and intragastrically infused with saline or sporamin (0.5 g/kg body weight/d), respectively, for 3 wk. Hematoxylin and eosin (HE) staining of the sections was performed to observe morphological changes in hepatic tissue and real-time fluorescent quantitative PCR (qPCR) and enzyme-linked immunosorbent assay (ELISA) were used to measure the expression of β-catenin and vascular endothelial growth factor (VEGF) in the liver.
Sporamin significantly reduced the number and weight of tumor nodules formed in the abdominal cavity. Compared with the vehicle group, the mean tumor weight (± SD) in the sporamin group was significantly reduced (0.44 ± 0.10 g vs 0.26 ± 0.15 g) and the total number of tumors decreased from 93 to 55. HE staining showed that enlargement of the nucleus and synthesis of proteins within hepatocytes, as well as infiltration of inflammatory cells into the liver, were attenuated by sporamin. Immunohistochemical staining and ELISA showed that the concentrations of β-catenin and VEGF in the liver were significantly reduced by sporamin. Compared with the vehicle group, the expression of β-catenin measured in integrated optical density units per area was reduced in the sporamin group (47.29 ± 9.10 vs 26.14 ± 1.72; P = 0.003). Expression of VEGF was also reduced after sporamin intervention from 20.78 ± 2.06 in the vehicle group to 15.80 ± 1.09 in the sporamin group (P = 0.021). Compared with the vehicle group, the concentration of β-catenin decreased from 134.42 ± 22.04 pg/mL to 109.07 ± 9.65 pg/mL after sporamin intervention (P = 0.00002). qPCR indicated that compared to the vehicle group, relative mRNA expression of β-catenin and VEGF in the liver of mice in the sporamin-treated group was significantly reduced to 71% ± 1% (P = 0.000001) and 23% ± 7% (P = 0.00002), respectively, of the vehicle group levels.
Sporamin down-regulates the expression and secretion of β-catenin and VEGF in the liver, which subsequently inhibits the transcription of downstream genes involved in cancer progression and angiogenesis.
Core tip: Sporamin, a Kunitz-type trypsin inhibitor, restrains the growth of intraperitoneally xenografted LoVo [also known as colorectal cancer (CRC) cells] in athymic BALB/c mice. The mechanism determined by changes in morphology and tumor biomarkers in the liver involves sporamin-induced down-regulation of β-catenin secretion and vascular endothelial growth factor expression. This suppresses the formation of xenografted tumor nodules in vivo and subsequently inhibits the transcription of downstream genes involved in cancer progression and angiogenesis. The anti-cancer effects of sporamin against CRC are closely associated with its inhibitory effect on these tumor biomarkers. Further studies are warranted to elucidate the corresponding signal transduction events that mediate this process.
- Citation: Yang C, Zhang JJ, Zhang XP, Xiao R, Li PG. Sporamin suppresses growth of xenografted colorectal carcinoma in athymic BALB/c mice by inhibiting liver β-catenin and vascular endothelial growth factor expression. World J Gastroenterol 2019; 25(25): 3196-3206
- URL: https://www.wjgnet.com/1007-9327/full/v25/i25/3196.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i25.3196
Colorectal cancer (CRC) is the third most common malignancy of the digestive tract and the fifth leading cause of cancer-related mortality in China[1,2]. The age-standardized incidence rate of CRC in China has increased from 12.8 per 100000 in 2003 to 14.2 per 100000 in 2017, while the mortality rate has risen from 5.9 to 7.4 per 100000. China has lower rates of CRC incidence and mortality than most developed countries, but has a higher case-fatality ratio (14.0%) and mortality/incidence ratio (52.1%)[3].
Treatment for CRC generally consists of surgery, adjuvant radiation, and chemotherapy as well as immunotherapy. Due to the low survival rate of CRC patients, there is an urgent need for new agents to combat this malignancy[4]. Plants are a rich source of various phytochemicals that may exert anti-oxidative, proapoptotic, anti-proliferative, anti-metastatic, and anti-angiogenic effects, depending on tumor type[5-8]. Sporamin is a Kunitz-type trypsin inhibitor that is found in the dicotyledonous plant, sweet potato (Ipomoea batatas), which belongs to the Convolvulaceae family. The tuberous roots contain 0.49%-2.24% crude sporamin protein on a fresh-weight basis[9,10]. Previous studies have identified sporamin as a potential anti-cancer agent against a number of malignant tumor cells, including HT29, HCT116, and SW480 colorectal cancer cells[11], TCA8113 tongue carcinoma cells[12] as well as PANC-1 and BxPC-3 pancreatic cancer cells[13].
The liver plays a vital role in the development of digestive tract cancers. It secretes a myriad of endocrine factors that may facilitate the growth and transformation of tumors, including β-catenin and vascular endothelial growth factor (VEGF). It is also the main metastatic target of CRC[14-16]. Thus, substances that can reduce the levels of tumor biomarkers in the liver may have the potential to become promising anti-cancer agents in the future. However, the effects of sporamin on the expression and secretion of tumor biomarkers in the liver are currently unknown. Therefore, in the present study, LoVo colorectal carcinoma cells were intraperitoneally xenografted into athymic BALB/c nude mice and sporamin was given orally to the mice to observe its effect on the growth of tumors, with a focus on changes in the structure and function of the liver, especially the expression and secretion of β-catenin and VEGF.
Sporamin was extracted from sweet potatoes as previously reported[8]. Reagents used in these experiments were obtained from the following suppliers: SYBR Green and cDNA Reverse Transcription Kit, Thermo Scientific, Shanghai, China; TRIzol and diethyl pyrocarbonate, Invitrogen, Shanghai, China; anhydrous ethanol, chloroform, and isopropanol, Beijing Chemical Reagent Company, Beijing, China; Dulbecco's modified Eagle medium (DMEM), fetal bovine serum, and PBS buffer, Corning, NY, United States; penicillin-streptomycin mixture and trypsin, Keygen Biotech, Nanjing, China; VEGF and β-catenin ELISA kits, Mecenbio, Beijing, China; xylene, gradient ethanol, 1% hydrochloric acid ethanol, and chloral hydrate dry powder, Sinopharm Science and Technology, Beijing, China; distilled water, Experimental Platform of Capital Medical University, Beijing, China; neutral formalin fixative, Leagene, Beijing, China; 3% hydrogen peroxide, BSA, hematoxylin dyeing solution, neutral gum, DAB chromogenic reagent, and β-catenin polyclonal antibody, Solarbio, Shanghai, China; VEGF polyclonal antibody, Abcam, Beijing, China; HRP-conjugated goat anti-rabbit antibody, KPL, Wuhan, China; RNAlater RNA stabilization reagent, Sigma, St. Louis, MO, United States.
The colon cancer LoVo cell line was purchased from the Tumor Cell Bank of the Chinese Academy of Medical Sciences (Beijing, China). The cells were grown in DMEM high-glucose medium supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin and maintained at 37 °C in a humidified incubator with 5% CO2. The medium was changed every 48 h. Single cell suspensions containing 5 × 106 cells in 0.2 mL PBS were prepared during the logarithmic growth phase.
All experiments were approved by the local Ethics Committee for Animal Research Studies at Capital Medical University, Beijing, China (animal experiment ethics review number: AEEI-2016-018).
Twenty-seven male BALB/c nude mice aged 4-6 wk old and weighing 13-15 g were purchased from Vital River Laboratory Animal Technology Co. Ltd. (Beijing, China) and maintained under specific pathogen-free conditions with free access to drinking water throughout the experiments. Animals were housed in a restricted access room under a 12-h/12-h light/dark cycle with a controlled temperature of 18-29 °C, daily range of temperature ≤ 3 °C, relative humidity of 40%-70%, airflow velocity ≤ 0.18 m/s, and room air pressure gradient of 20-50 Pa.
After one week of acclimation, animals were randomly divided into three groups, with nine animals in each group, and we verified that there was no significant differences in body weight between the groups. The groups were as follows: (1) Control group; (2) Vehicle group; and (3) Sporamin group. In the latter two groups, 5 × 106 LoVo cells in 0.2 mL PBS were xenografted into the abdominal cavity of the mice. Mice in the control group were injected with an equal volume of PBS to mimic the operation in the other groups. Sporamin was dissolved in distilled water and given through intragastric infusion to the mice at a dose of 0.5 g/kg body weight/d. Mice in the control and vehicle groups were given the same amount of distilled water by intragastric infusion. The body weights of the mice were recorded every three days during the experiment. After three weeks of sporamin treatment, the mice were anesthetized with 10% chloral hydrate (0.1 mL/10 g body weight) and sacrificed by cervical dislocation. An autopsy of each mouse was carried out and the liver and the tumor nodes formed in the abdominal cavity were carefully counted, collected, and weighed. Then, the tissues samples were divided into three portions. Samples for pathological examinations were fixed in 10% neutral formalin and stored at room temperature. Samples for quantitative PCR (qPCR) were preserved in RNA stabilization reagent and stored at -20 °C. Samples for ELISA assays were directly stored at -80 °C in cryotubes.
Tissue samples fixed with formalin were subjected to paraffin embedding and sectioned at 5 μm for observation. Hematoxylin and eosin (HE) staining of the sections was performed to observe morphological changes in the tissues. After dewaxing and antigen retrieval, immunochemical assays were performed using specific polyclonal antibodies against VEGF and β-catenin. HRP-conjugated goat anti-rabbit antibody was then applied to the sections to label the primary antibodies. DAB chromogenic reagent and hematoxylin were then added and coverslips were added to sections after color development. The expression of VEGF and β-catenin in liver tissue was qualitatively analyzed with a high-magnification inverted fluorescence phase-contrast microscope (40×; DMIL, Leica, Germany) and photographs were taken. ImageJ was used for image analysis to compare the average optical density (AOD) of positive staining sites in each group.
Total RNA was extracted from the liver with TRIzol reagent. The mRNA was reverse-transcribed into cDNA and SYBR Green PCR Master Mix was used to determine the transcriptional expression of specific genes. The primer sequences were as follows: β-catenin forward, 5’-TCT GAG GAC AAG CCA CAG GA-3’ and reverse, 5’-GCA CCA ATG TCC AGT CCA AG-3’; VEGF forward, 5’-CTT CAG CTC GCT CCT CCA CT-3’ and reverse, 5’-CAG GCC TCT TCT TCC ACC AC-3’; β-actin forward, 5’-GTG CTA TGT TGC TCT AGA CTT CG-3’ and reverse, 5’-ATG CCA CAG GAT TCC ATA CC-3’. Amplification of the housekeeping gene beta-actin from the same samples was used as an internal control. Relative gene expression was calculated using the 2-ΔΔCt method[17] .
Liver samples were ground into homogenates and assayed according to the VEGF and β-catenin ELISA kit manufacturer’s instructions. The OD value of each sample well was measured with a microplate reader at a wavelength of 450 nm, and the concentrations of VEGF and β-catenin in the liver homogenates were calculated and presented in pg/mL. Reproducibility was evaluated in three independent experiments, and two standard curves were run on each plate.
Statistical analyses were performed using SPSS 21.0 software (IBM, Armonk, NY, United States). Image analysis was performed using ImageJ (https://imagej.nih.gov). Statistical charts were created using Prism5.0 (Graphpad, San Diego, CA, United States). All data are expressed as the mean ± standard deviation (SD). The differences between any two groups were tested by independent sample t-tests and comparisons of multiple groups were made by one-way ANOVA; Tukey’s test was used for multiple pairwise comparisons of means. A P-value < 0.05 was considered statistically significant.
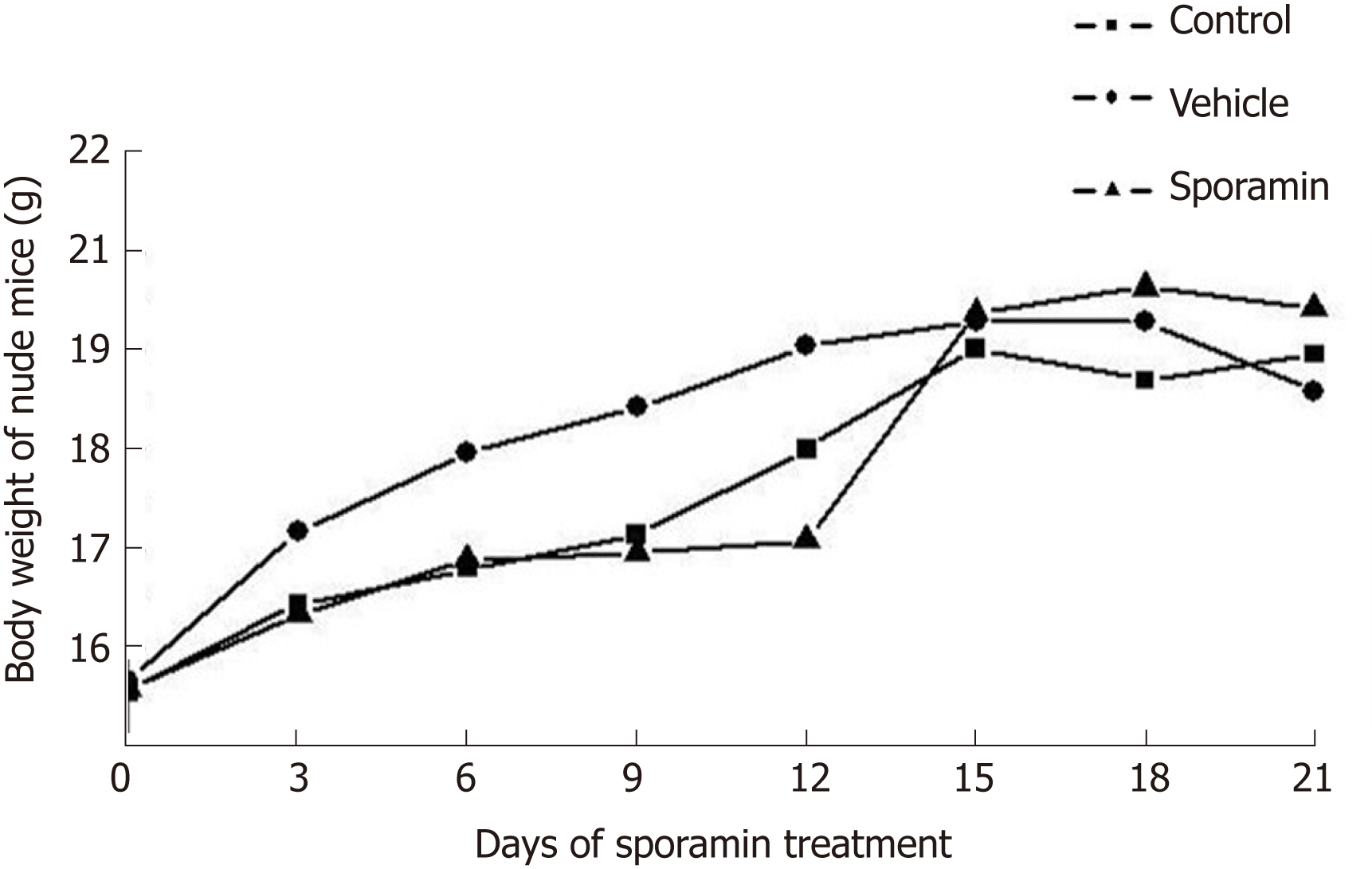
The body weight of the animals in all the three groups showed an upward trend as the experiment progressed. From day 9-15, body weight increased rapidly, but slowed from day 16-21. In comparison with the control and vehicle groups, it was noteworthy that the body weight of animals in the sporamin group was highest although there were no significant differences among these groups when the increase in body weight ceased on day 15 (P = 0.12; Figure 1). There were also no significant differences in liver weights or liver/body weight ratios among the three groups (Table 1).
| Group | Liver weight (g) | Liver/body weight |
| Control | 1.15 ± 0.17 | 0.06 ± 0.01 |
| Vehicle | 1.10 ± 0.24 | 0.06 ± 0.01 |
| Sporamin | 1.04 ± 0.24 | 0.06 ± 0.01 |
| F | 0.50 | 0.10 |
| P | 0.61 | 0.76 |
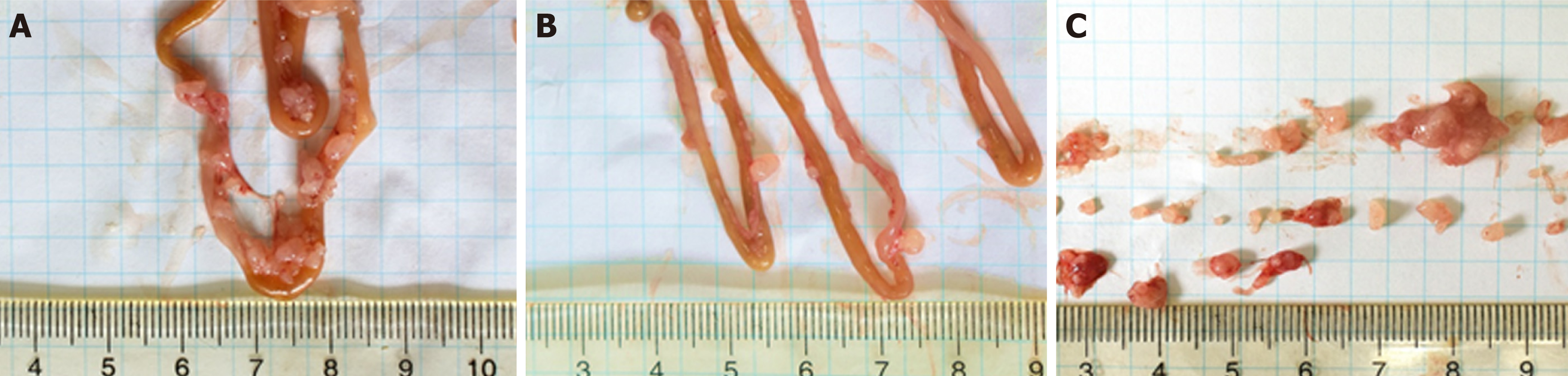
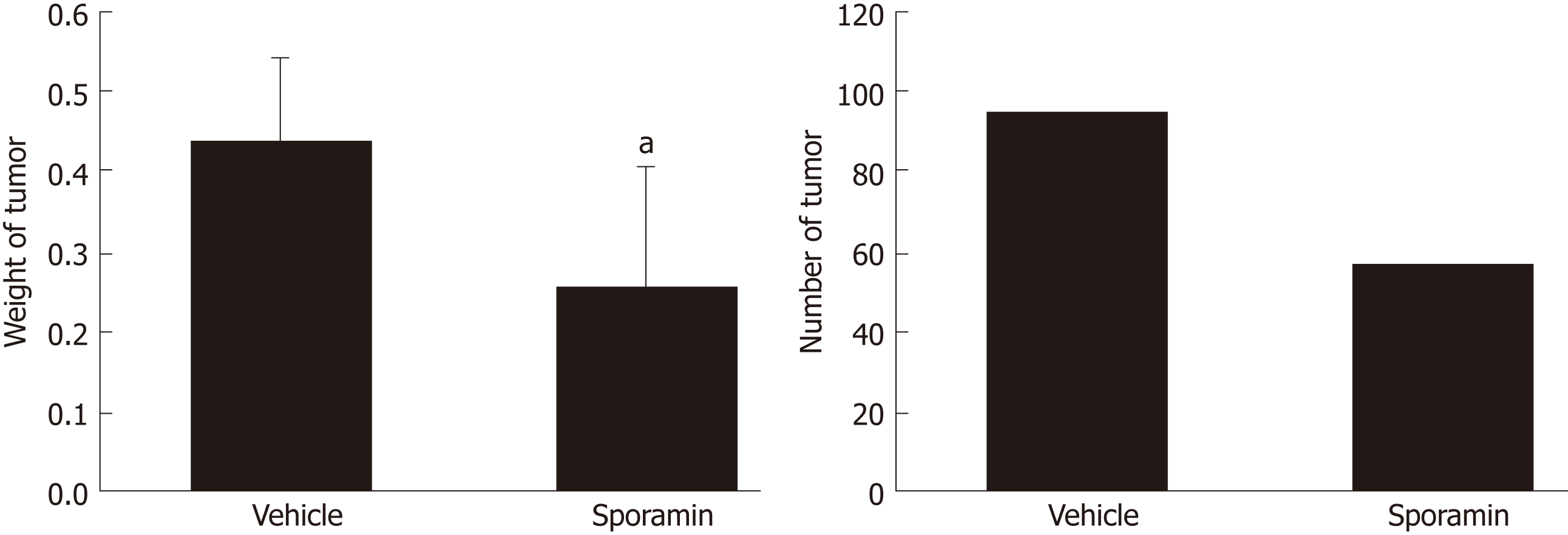
All of the nude mice that were inoculated with cancer cells developed tumor nodules in their abdominal cavities. Figure 2 shows that the tumors grew mainly on the mesentery instead of the intestine. They were rough, hard, and grayish white nodules differing in number and volume. Compared to the vehicle group, the total weight of the tumor nodules in the sporamin group was reduced by 51.15% (95% confidence interval: 0.16-0.31) and the total number of tumors was reduced from 93 to 55 (Figure 3). Compared with the vehicle group (0.44 ± 0.10 g), the mean tumor weight in the sporamin group (0.26 ± 0.15 g) was significantly reduced (P = 0.04).
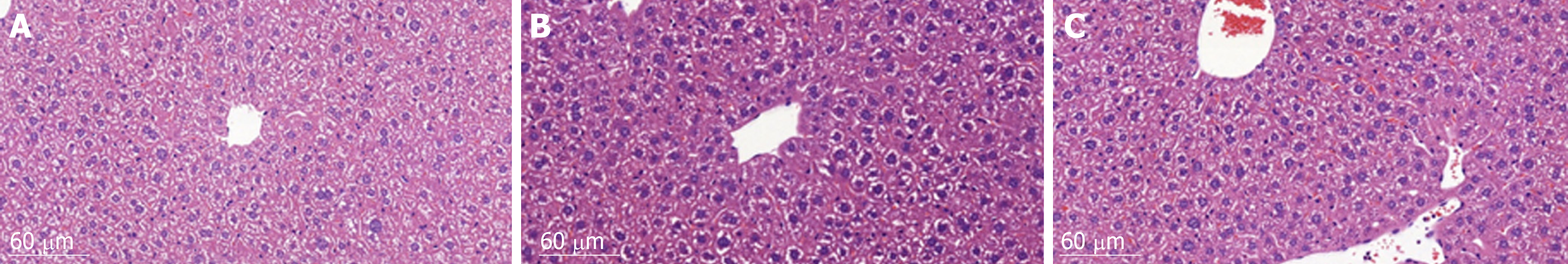
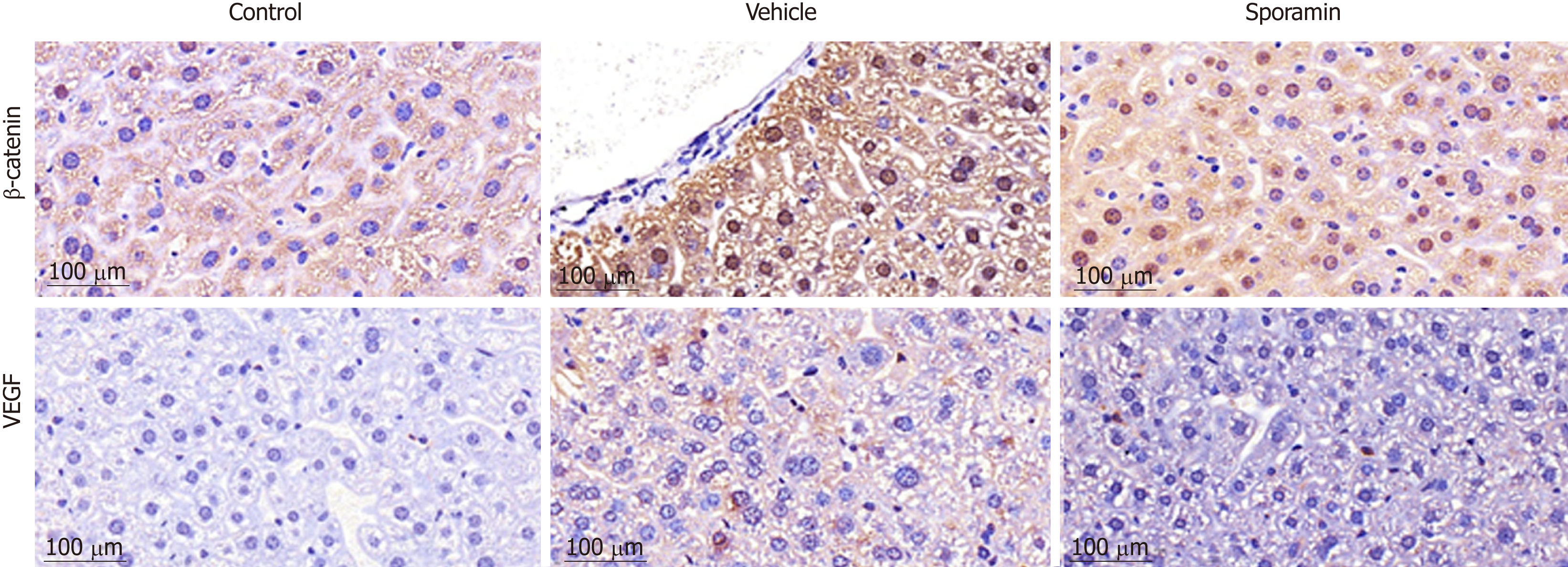
HE staining of liver histological sections showed that, compared to the control group, the size of hepatocytes in the vehicle group was enlarged, and nuclei were also enlarged and stained deeply blue. The cytoplasm displayed a basophilic change. Cell membranes and the borders between them became blurred. As a result, the hepatic sinusoid was compressed by the enlarged hepatocytes and the structure of the hepatic plate became irregular. There were also many lymphocytes which had infiltrated into the hepatic tissues, but no metastatic tumor cells were found. Compared to the vehicle group, the histomorphology of livers from animals in the sporamin group was similar to that of the control group with a relatively small cell size, clearer hepatic plate, and a reduced degree of blue staining of the cytoplasm, indicating that sporamin had restored the normal structure of the liver in these animals (Figure 4).
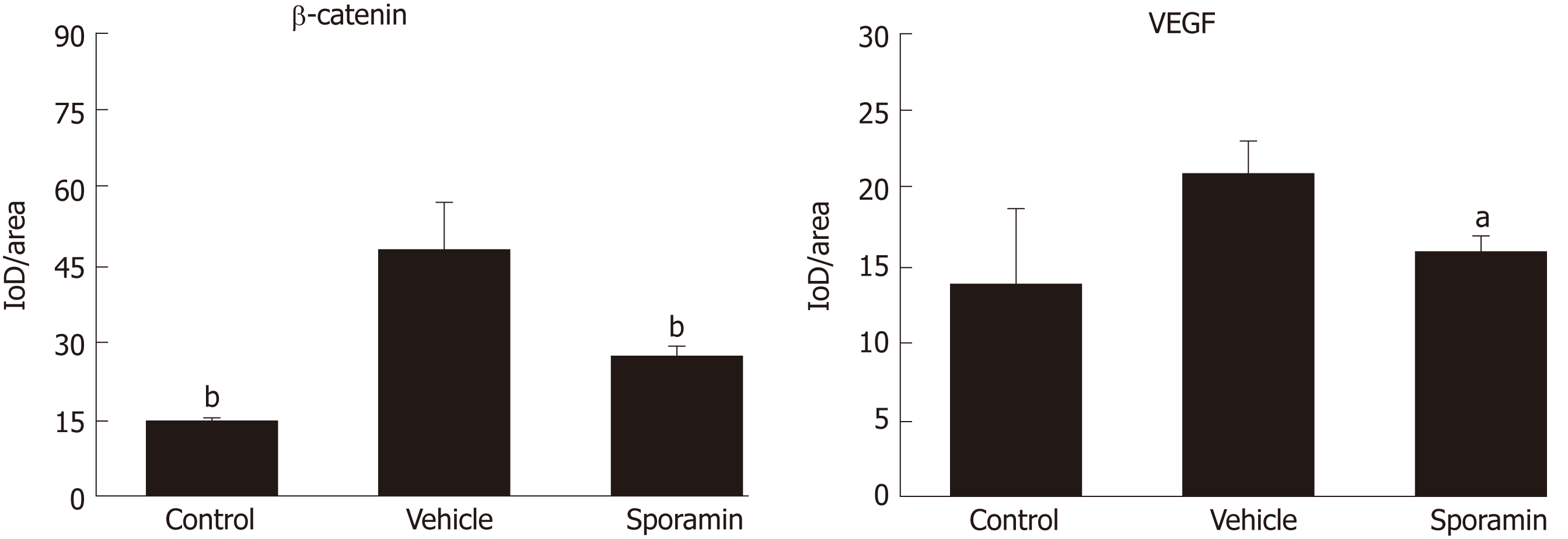
To investigate functional changes in the liver, antibodies against β-catenin and VEGF were applied to the liver sections to show the levels and intracellular locations of these tumor biomarkers. After antibody incubation, the nuclei of the liver cells were stained blue and biomarkers were stained brownish yellow or light yellow. As shown in Figure 5, high concentrations of β-catenin were found in the cytoplasm and also translocated to the nucleus, indicating that it had bound to its target genes and had initiated the transcription process. Quantitative analysis of the AOD of the positively stained areas in the liver tissue sections showed that, compared with the vehicle group (47.29 ± 9.10), the expression of β-catenin was significantly reduced by sporamin to 26.14 ± 1.72 (P = 0.003; Figure 6). In line with the increase in β-catenin expression and nuclear translocation in the vehicle group, the expression of the angiogenic factor VEGF, which is also a downstream target of β-catenin, was increased in the cytoplasm of hepatocytes in the vehicle group. Similarly, expression of the VEGF protein was also reduced after sporamin intervention from 20.78 ± 2.06 in the vehicle group to 15.80 ± 1.09 in the sporamin group (P = 0.021; Figure 6), sug-gesting that sporamin had an anti-angiogenic effect in these animals.
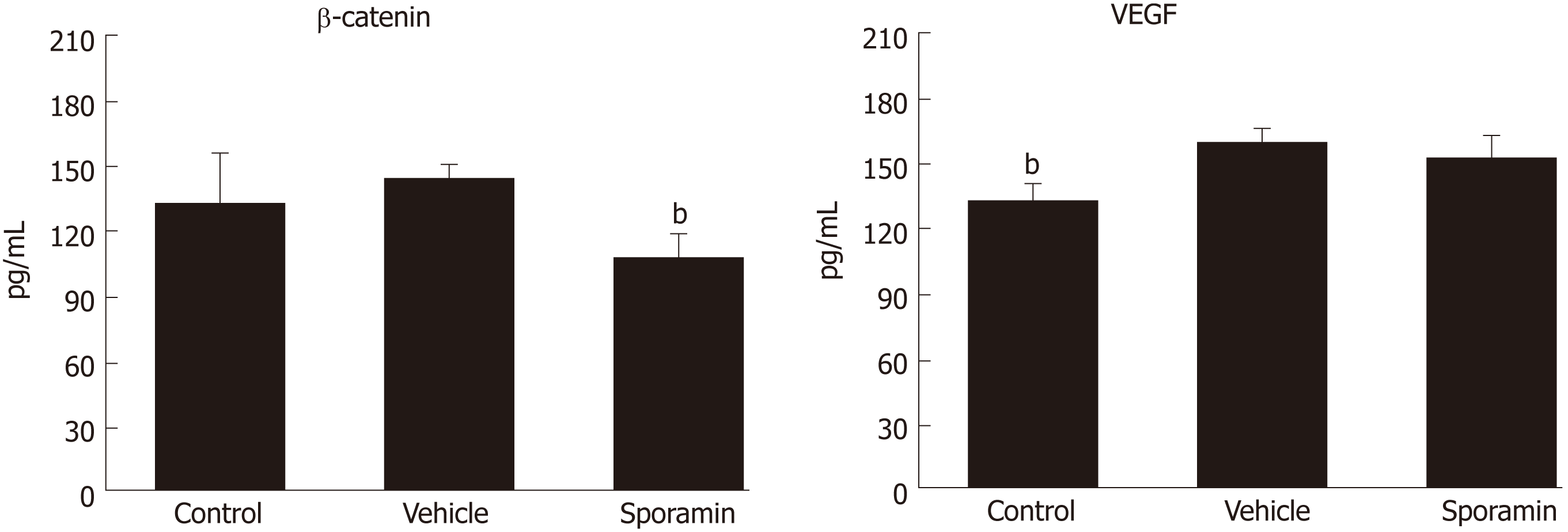
The concentrations of β-catenin and VEGF protein in liver tissue were assessed by ELISA. Figure 7 shows that the concentration of β-catenin increased, albeit non-significantly, from 134.42 ± 22.04 pg/mL in the control group to 143.33 ± 5.06 pg/mL in the vehicle group (P = 0.35). However, the concentration of VEGF in liver tissue was significantly increased from 132.05 ± 7.96 pg/mL in the control group to 158.73 ± 6.23 pg/mL in the vehicle group (P = 0.00007). Compared with the vehicle group, the concentration of β-catenin after sporamin intervention decreased to 109.07 ± 9.65 pg/mL (P = 0.00002). The concentration of VEGF also decreased to 150.90 ± 10.38 pg/mL but it was not significant (P = 0.14). These results are consistent with changes observed in the immunohistochemistry analysis.
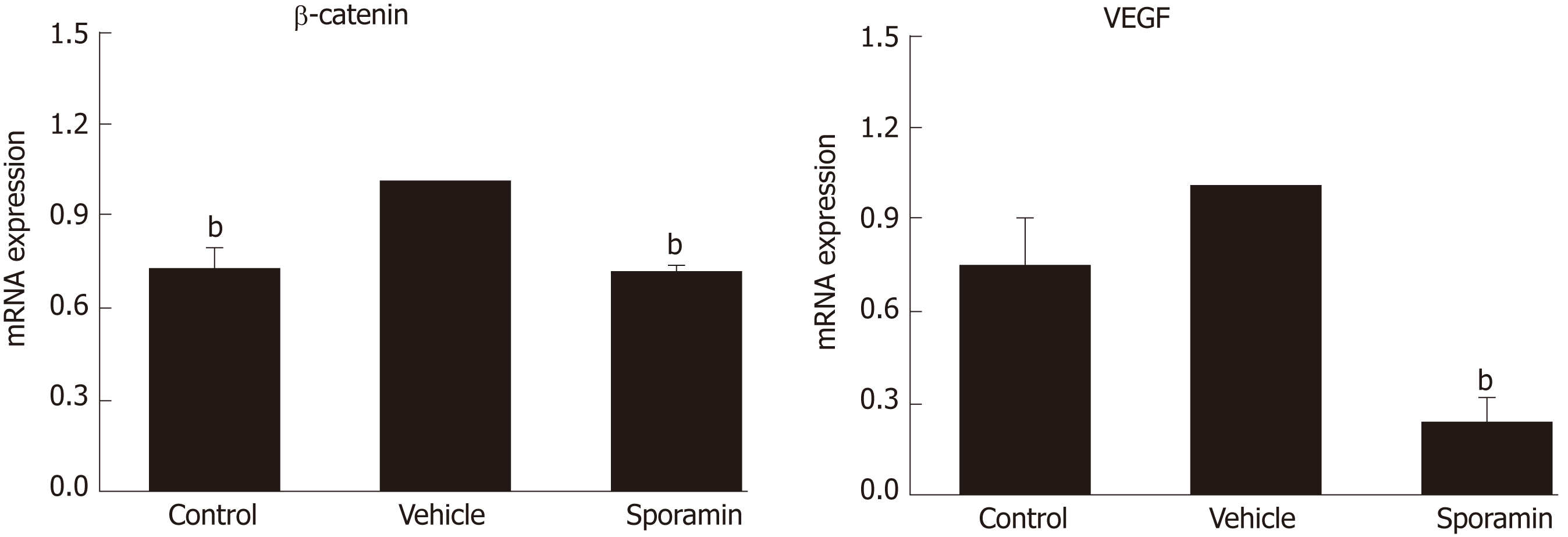
To further verify the effects of sporamin on the expression of β-catenin and VEGF at the transcriptional level, qPCR was conducted to detect the relative abundance of these mRNAs in the liver samples. Figure 8 shows that compared with the control group, relative expression of β-catenin mRNA in the vehicle group was significantly increased (P = 0.0004), but the increase was not significant for VEGF (P = 0.26). Compared with the vehicle group, the relative abundance of β-catenin and VEGF mRNA in the sporamin group was significantly reduced to 71% ± 1% (P = 0.000001) and 23% ± 7% (P = 0.00002) of the vehicle group levels, respectively.
CRC is one of the most common malignancies of the digestive tract in both developing and developed countries[18]. Metastasis and recurrence of the primary tumor are the main reasons for the high mortality rate of this disease. At present, chemotherapy still has severe side effects, which limits its use in many circumstances. In recent years, many studies have indicated that phytochemicals may be a promising source of new anti-cancer agents against CRC which will play an important role in chemoprevention of the disease (for review see[19]). In the current study, compared with the vehicle group, the number and total weight of tumor nodules formed in the abdominal cavities of the mice were significantly reduced by treatment with sporamin, a Kunitz type trypsin inhibitor obtained from the sweet potato. This is in line with previous studies which showed that sporamin can suppress the growth of a variety of cancer cell lines including human esophageal squamous cell carcinoma cells[20], human pancreatic cancer cells[13], and human tongue carcinoma cells[12], both in vitro and in vivo.
Considering that the liver is usually the first target organ of CRC metastasis, the present study mainly focused on changes in the structure and function of the liver in the tumor-bearing mice. The liver is the largest endocrine gland in the body which can secrete a great number of hormones, growth factors, and cytokines and plays a crucial role in the development and transformation of many malignant tumors. The liver is also the most common anatomical site for hematogenous metastases of CRC, which are one of the most difficult and challenging obstacles in the treatment of CRC [21]. In our study, although the weights of the body and the liver and the ratio of liver weight to body weight were not significantly different among the control, vehicle, and sporamin groups, we found that sporamin increased body weight and reduced liver weight compared with vehicle treatment, implying that sporamin was possibly beneficial for the general health status of the mice. This effect may be partially attributed to the nutritional effects of sporamin because it is a dietary protein with many biological activities[22].
In accordance with previous findings that colon cancer cells can induce the liver to synthesize and secrete a variety of hormones, growth factors, and cytokines, which facilitate the growth of the tumor and induce a systematic inflammatory status[23], our studies showed that after intraperitoneal tumor cell inoculation, the histomorphology and function of the liver were all significantly changed . In contrast, it was noteworthy that, compared with the vehicle group, sporamin demonstrated the ability to restore the normal structure and function of the liver. Hepatocyte enlargement, nuclear pyknosis, cell membrane blurring, and blue staining of the cytoplasm were all improved, suggesting that sporamin had attenuated protein synthesis within the cell and alleviated the hypertrophic status of the hepatocytes. Functionally, the accumulation of β-catenin in the cytoplasm as well as its translocation to the nucleus of the hepatocytes was both significantly attenuated by sporamin. As is well known, the activation of the Wnt/β-catenin signaling pathway plays a key role in the development of CRC[24]. As a transcriptional activator, the translocation of β-catenin from the cytoplasm to the nucleus initiates the transcription of a number of target genes[25] which participate in the malignant transformation of CRC[26]. Therefore, any agents that can reduce the level of β-catenin within the tumor cells and hepatocytes may exhibit a potent effect against CRC. For example, in ade-nomatous polyposis coli (APC) tumor suppressor gene-mutated APCmin/+ mice, the formation of colorectal adenomas is inhibited by suppressing the activation of the Wnt/β-catenin signaling pathway[27]. Therefore, our findings suggest that sporamin may partially exert its effect by inhibiting the synthesis and function of β-catenin in the liver.
VEGF is one of the most potent angiogenic factors that can be synthesized by tumor cells as well as hepatocytes after stimulation by various environmental factors such as hypoxia. VEGF is also a downstream target of the β-catenin pathway, and expression of VEGF is usually positively correlated with the expression of β-catenin in tumor cells and with the progression of the tumor[28]. Consequently, if the expression of β-catenin in tumor cells is reduced, the expression of VEGF usually decreases as well[29]. Our results are consistent with these previous findings and show that sporamin has an anti-angiogenic effect against CRC. As to the specific mechanisms by which sporamin inhibits the expression of β-catenin and VEGF as well as signaling events during this process, further investigation will be required.
In conclusion, our study suggests that sporamin can suppress the growth of xenografted colorectal tumor nodules in mice by restoring the normal structure of the liver and downregulating the expression and secretion of β-catenin and VEGF in the liver. These anti-cancer effects of sporamin against CRC are closely associated with its inhibitory effect on these tumor biomarkers. Further studies are warranted to elucidate the corresponding signal transduction events mediating this process.
Colorectal cancer (CRC) is the third most common malignancy of the digestive tract and the fifth leading cause of cancer-related mortality in China. Sporamin, a Kunitz-type trypsin inhibitor isolated from sweet potato, is a potential anti-cancer agent with activity against a number of malignant tumor cells in vitro. The liver secretes a myriad of endocrine factors that may facilitate the growth and transformation of tumors in the development of CRC.
Sporamin as a potential anti-cancer agent against a number of malignant tumor cells, including HT29, HCT116, and SW480 colorectal cancer cells, TCA8113 tongue carcinoma cells as well as PANC-1 and BxPC-3 pancreatic cancer cells. However, the effects of sporamin on the expression and secretion of tumor biomarkers in the liver are currently unknown. Therefore, in the present study, LoVo colorectal carcinoma cells were intraperitoneally xenografted into athymic BALB/c nude mice and sporamin was given orally to the mice to observe its effect on the growth of tumors, with a focus on changes in the structure and function of the liver, especially the expression and secretion of β-catenin and vascular endothelial growth factor (VEGF).
To investigate the effects of sporamin on liver morphology and biomarkers of xenografted CRC in the liver of BALB/c athymic mice.
Twenty-seven male BALB/c nude mice were randomly divided into control, vehicle, and sporamin groups. Mice in the latter two groups were intraperitoneally xenografted with LoVo colorectal carcinoma cells and intragastrically infused with saline or sporamin (0.5 g/kg body weight/d), respectively, for 3 weeks. Hematoxylin and eosin (HE) staining of the sections was performed to observe morphological changes in hepatic tissue and real-time fluorescent quantitative PCR and enzyme-linked immunosorbent assays (ELISA) were used to measure the expression of β-catenin and VEGF in the liver.
Sporamin significantly reduced the number and weight of tumor nodules formed in the abdominal cavity. Compared with the vehicle group, the mean tumor weight (± SD) in the sporamin group was significantly reduced (0.26 ± 0.15 g vs 0.44 ± 0.10 g) and the total number of tumors decreased from 93 to 55. HE staining showed that enlargement of the nucleus and synthesis of proteins within hepatocytes, as well as infiltration of inflammatory cells into the liver, were attenuated by sporamin. Immunohistochemical staining and ELISA showed that the concentrations of β-catenin and VEGF in the liver were significantly reduced by sporamin. Compared with the vehicle group, the expression of β-catenin measured in integrated optical density units per area was reduced in the sporamin group (47.29 ± 9.10 vs 26.14 ± 1.72; P = 0.003). Expression of VEGF was also reduced after sporamin intervention from 20.78 ± 2.06 in the vehicle group to 15.80 ± 1.09 in the sporamin group (P = 0.021). The secretion of VEGF and β-catenin in the liver was also assessed by ELISA, which showed that the concentration of VEGF in liver tissue increased significantly from 132.05 ± 7.96 pg/mL in the control group to 158.73 ± 6.23 pg/mL in the sporamin-treated group (P = 0.00007). Compared with the vehicle group, the concentration of β-catenin decreased from 134.42 ± 22.04 pg/mL to 109.07 ± 9.65 pg/mL after sporamin intervention (P = 0.00002). Quantitative PCR (qPCR) indicated that compared to the vehicle group, relative mRNA expression of β-catenin and VEGF in the liver of the sporamin-treated group was significantly reduced to 71% ± 1% (P = 0.000001) and 23% ± 7% (P = 0.00002), respectively, of the vehicle group levels.
Sporamin down-regulates the expression and secretion of β-catenin and VEGF in the liver, which subsequently inhibits the transcription of downstream genes involved in cancer progression and angiogenesis.
Our study suggests that sporamin can suppress the growth of xenografted colorectal tumor nodules in mice by restoring the normal structure of the liver and downregulating the expression and secretion of β-catenin and VEGF in the liver. These anti-cancer effects of sporamin against CRC are closely associated with its inhibitory effect on these tumor biomarkers. Further studies are warranted to elucidate the corresponding signal transduction events mediating this process.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Demir Y, Mohamed SY S-Editor: Ma RY L-Editor: Wang TQ E-Editor: Ma YJ
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12135] [Cited by in RCA: 12978] [Article Influence: 1442.0] [Reference Citation Analysis (2)] |
| 2. | Chen W. Cancer statistics: updated cancer burden in China. Chin J Cancer Res. 2015;27:1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 139] [Reference Citation Analysis (0)] |
| 3. | Zhu J, Tan Z, Hollis-Hansen K, Zhang Y, Yu C, Li Y. Epidemiological Trends in Colorectal Cancer in China: An Ecological Study. Dig Dis Sci. 2017;62:235-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 128] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 4. | Xie G, Raufman JP. Role of the Aryl Hydrocarbon Receptor in Colon Neoplasia. Cancers (Basel). 2015;7:1436-1446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Ho JW, Leung YK, Chan CP. Herbal medicine in the treatment of cancer. Curr Med Chem Anticancer Agents. 2002;2:209-214. [PubMed] |
| 6. | Scarpa ES, Ninfali P. Phytochemicals as Innovative Therapeutic Tools against Cancer Stem Cells. Int J Mol Sci. 2015;16:15727-15742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 7. | Shu L, Cheung KL, Khor TO, Chen C, Kong AN. Phytochemicals: cancer chemoprevention and suppression of tumor onset and metastasis. Cancer Metastasis Rev. 2010;29:483-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 168] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 8. | Crispin BJ, Seghi RR, Globe H. Effect of different metal ceramic alloys on the color of opaque and dentin porcelain. J Prosthet Dent. 1991;65:351-356. [PubMed] |
| 9. | Maeshima M, Sasaki T, Asahi T. Characterization of major proteins in sweet potato tuberous roots. Phytochemistry. 1985;166:515-523. [DOI] [Full Text] |
| 10. | Li PG, Mu TH. Recovery of sporamin from naturally fermented sweet potato starch slurry by foam fractionation. Int J Food Sci Tech. 2012;47:1889-1895. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Li PG, Mu TH, Deng L. Anticancer effects of sweet potato protein on human colorectal cancer cells. World J Gastroenterol. 2013;19:3300-3308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Yao J, Qian C. Sporamin induce apoptosis in human tongue carcinoma cells by down-regulating Akt/GSK-3 signaling. Fundam Clin Pharmacol. 2011;25:229-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Qian C, Chen X, Qi Y, Zhong S, Gao X, Zheng W, Mao Z, Yao J. Sporamin induces apoptosis and inhibits NF-κB activation in human pancreatic cancer cells. Tumour Biol. 2017;39:1010428317706917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Adam R, Lucidi V, Bismuth H. Hepatic colorectal metastases: methods of improving resectability. Surg Clin North Am. 2004;84:659-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Nguyen DX, Bos PD, Massagué J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1874] [Cited by in RCA: 1969] [Article Influence: 123.1] [Reference Citation Analysis (0)] |
| 16. | Rudmik LR, Magliocco AM. Molecular mechanisms of hepatic metastasis in colorectal cancer. J Surg Oncol. 2005;92:347-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117419] [Cited by in RCA: 133158] [Article Influence: 5548.3] [Reference Citation Analysis (1)] |
| 18. | Karsa LV, Lignini TA, Patnick J, Lambert R, Sauvaget C. The dimensions of the CRC problem. Best Pract Res Clin Gastroenterol. 2010;24:381-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 19. | Mahadevappa R, Kwok HF. Phytochemicals - A Novel and Prominent Source of Anti-cancer Drugs Against Colorectal Cancer. Comb Chem High Throughput Screen. 2017;20:376-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Qian CJ, Qi YX, Chen XY, Zeng JP, Yao J. Sporamin suppresses growth of human esophageal squamous cell carcinoma cells by inhibition of NFκB via an AKTindependent pathway. Mol Med Rep. 2017;16:9620-9626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Xu J, Qin X, Wang J, Zhang S, Zhong Y, Ren L, Wei Y, Zeng S, Wan D, Zheng S; Society of Surgery; Chinese Medical Association; Committee of Colorectal Cancer, Chinese Anti-cancer Association. Chinese guidelines for the diagnosis and comprehensive treatment of hepatic metastasis of colorectal cancer. J Cancer Res Clin Oncol. 2011;137:1379-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Senthilkumar R, Yeh KW. Multiple biological functions of sporamin related to stress tolerance in sweet potato (Ipomoea batatas Lam). Biotechnol Adv. 2012;30:1309-1317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Xu J, Ren L. [China Guideline for Diagnosis and Comprehensive Treatment of Colorectal Liver Metastases (Version 2018)]. Zhonghua Wei Chang Wai Ke Za Zhi. 2018;21:601-626. [PubMed] |
| 24. | Larriba MJ, González-Sancho JM, Barbáchano A, Niell N, Ferrer-Mayorga G, Muñoz A. Vitamin D Is a Multilevel Repressor of Wnt/b-Catenin Signaling in Cancer Cells. Cancers (Basel). 2013;5:1242-1260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 110] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 25. | Deep G, Panigrahi GK. Hypoxia-Induced Signaling Promotes Prostate Cancer Progression: Exosomes Role as Messenger of Hypoxic Response in Tumor Microenvironment. Crit Rev Oncog. 2015;20:419-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 26. | Ying Y, Zhu H, Liang Z, Ma X, Li S. GLP1 protects cardiomyocytes from palmitate-induced apoptosis via Akt/GSK3b/b-catenin pathway. J Mol Endocrinol. 2015;55:245-262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 27. | Gao C, Chen G, Kuan SF, Zhang DH, Schlaepfer DD, Hu J. FAK/PYK2 promotes the Wnt/β-catenin pathway and intestinal tumorigenesis by phosphorylating GSK3β. Elife. 2015;4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 28. | Dılek FH. Topak N, Tokyol Ç, Akbulut G, Dılek ON. β-Catenin and its relation to VEGF and cyclin D1 expression in pT3 rectosigmoid cancers. Turk J Gastroenterol. 2010;21:365-371. [PubMed] |
| 29. | Zhang K, Guo J, Ge Z, Zhang J. Nanosecond pulsed electric fields (nsPEFs) regulate phenotypes of chondrocytes through Wnt/β-catenin signaling pathway. Sci Rep. 2014;4:5836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |