Published online Jun 21, 2019. doi: 10.3748/wjg.v25.i23.2924
Peer-review started: November 22, 2018
First decision: December 12, 2018
Revised: March 12, 2019
Accepted: March 29, 2019
Article in press: March 30, 2019
Published online: June 21, 2019
Processing time: 212 Days and 3.3 Hours
The digestive tract is the maximal immunizing tissue in the body, and mucosal integrity and functional status of the gut is very important to maintain a healthy organism. Severe infection is one of the most common causes of gastrointestinal dysfunction, and the pathogenesis is closely related to endotoxemia and intestinal barrier injury. Bifidobacterium is one of the main probiotics in the human body that is involved in digestion, absorption, metabolism, nutrition, and immunity. Bifidobacterium plays an important role in maintaining the intestinal mucosal barrier integrity. This study investigated the protective mechanism of Bifidobacterium during ileal injury in rats.
To investigate the effects of Bifidobacterium on cytokine-induced neutrophil chemoattractant (CINC) and insulin-like growth factor 1 (IGF-1) in the ileum of rats with endotoxin injury.
Preweaning rats were randomly divided into three groups: Control (group C), model (group E) and treatment (group T). Group E was intraperitoneally injected with lipopolysaccharide (LPS) to create an animal model of intestinal injury. Group T was intragastrically administered Bifidobacterium suspension 7 d before LPS. Group C was intraperitoneally injected with normal saline. The rats were killed at 2, 6 or 12 h after LPS or physiological saline injection to collect ileal tissue samples. The expression of ileal CINC mRNA was evaluated by reverse transcription-polymerase chain reaction (RT-PCR), and expression of ileal IGF-1 protein and mRNA was detected by immunohistochemistry and RT-PCR, respectively.
The ileum of rats in Group C did not express CINC mRNA, ileums from Group E expressed high levels, which was then significantly decreased in Group T (F = 23.947, P < 0.05). There was no significant difference in CINC mRNA expression at different times (F = 0.665, P > 0.05). There was a high level of IGF-1 brown granules in ileal crypts and epithelial cells in Group C, sparse staining in Group E, and dark, dense brown staining in Group T. There was a significant difference between Groups C and E and Groups E and T (P < 0.05). There was no significant difference in IGF-1 protein expression at different times (F = 1.269, P > 0.05). IGF-1 mRNA expression was significantly different among the three groups (P < 0.05), though not at different times (F = 0.086, P > 0.05).
Expression of CINC mRNA increased in the ileum of preweaning rats with endotoxin injury, and exogenous administration of Bifidobacterium reduced CINC mRNA expression. IGF-1 protein and mRNA expression decreased in the ileum of preweaning rats with endotoxin injury, and exogenous administration of Bifidobacterium prevented the decrease in IGF-1 expression. Bifidobacterium may increase IGF-1 expression and enhance intestinal immune barrier function in rats with endotoxin injury.
Core tip: The purpose of this article is to investigate the effects of Bifidobacterium on cytokine-induced neutrophil chemoattractant and insulin-like growth factor 1 (IGF-1) in the ileum of rats with endotoxin injury. It was found that Bifidobacterium may increase IGF-1 expression and enhance intestinal immune barrier function in rats with endotoxin injury.
- Citation: Wang W, Sun M, Zheng YL, Sun LY, Qu SQ. Effects of Bifidobacterium infantis on cytokine-induced neutrophil chemoattractant and insulin-like growth factor-1 in the ileum of rats with endotoxin injury. World J Gastroenterol 2019; 25(23): 2924-2934
- URL: https://www.wjgnet.com/1007-9327/full/v25/i23/2924.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i23.2924
Severe infection is one of the most common causes of gastrointestinal dysfunction, and the pathogenesis is closely related to endotoxemia and intestinal barrier injury. Bifidobacterium is one of the main probiotics in the human body involved in digestion, absorption, metabolism, nutrition, and immunity to infection. In particular, Bifidobacterium plays an important role in maintaining intestinal mucosal barrier integrity[1]. Insulin-like growth factor 1 (IGF-1) is a polypeptide that is primarily secreted from the liver, but it is also expressed in other organs such as the intestine, making it part of the intestinal immune barrier[2]. This study investigated the protective mechanism of Bifidobacterium through ileal injury in preweaning rats.
Bifidobacterium infantis (KLDS2.0002) suspension was purchased from the Key Laboratory of Dairy Science of Northeast Agricultural University. Escherichia coli (O55: B5) lipopolysaccharide (LPS) was purchased from Sigma. Immunohistochemical IGF-1 rabbit anti-rat IgG (primary antibody) was purchased from Wuhan Boshide Bioengineering Co. Ltd. (Wuhan, China), and horseradish-peroxidase-labeled IgG (secondary antibody) and concentrated DAB kits were purchased from Beijing Zhongshan Jinqiao Biotechnology Co. Ltd (Beijing, China). The primers for IGF-1, cytokine-induced neutrophil chemoattractant (CINC) and β-actin were designed and synthesized by Shanghai Shengneng Bocai Biological Technology Co. Ltd. A reverse transcription-polymerase chain reaction (RT-PCR) kit was purchased from Promega (Beijing, China).
Instruments and equipment were obtained as follows: ultramicrotome (LKB, Sweden); GIS gel image processing system (Tanon, Shanghai, China); TC-XP gene amplification instrument (Hangzhou Bioer Technology Co. Ltd., Hangzhou, China); GE-100 gel electrophoresis system (Hangzhou Bioer Technology Co. Ltd., Hangzhou, China); Eclipse E800 Camera system (Nikon, Japan); CMIAS2001 Beihang Motic Image Analysis system (Beijing Mike Audi Image Technology Co. Ltd., Beijing, China); HFsafe 1200 biosafety cabinet (LiShen Scientific Instrument Co. Ltd., Shanghai, China); AE200s electronic analytical balance (Mettler Toledo Instruments Shanghai Co. Ltd., Shanghai, China); MDF-U53V 80 °C low temperature freezer (SANSY, Japan); Neofuge 23R desktop high speed refrigerated centrifuge (Shanghai, China); microsample feeder (Eppendorf AG, Germany); and XW-80A vortex mixer (Shanghai, China).
Healthy 18-day-old preweaning Wistar rats (31.16 ± 6.38 g) were provided by the Laboratory Animal Center of the Second Affiliated Hospital of Harbin Medical University. The rats were randomly divided into three groups: Control (group C), model (group E) and Bifidobacterium treatment (group T). The animals were sacrificed 2, 6 and 12 h after intraperitoneal injection of endotoxin (LPS) or normal saline, and ileal samples were collected. Each group had eight preweaning rats for each time point. Animals that died during the experiment were not included.
TPY medium: 10 g casein peptone, 2.5 g yeast extract powder, 5 g glucose, 5 g soy peptone, 1.0 g Tween 80, 0.5 g L-cysteine hydrochloric acid, 5 mL salt-mixture solution, and 995 mL distilled water, adjusted to pH 7.2, and sterilized at 121 °C for 20 min. Salt-mixture solution: 20.0 g K2HPO4, 5.0 g MgCl2∙6H2O, 2.5 g ZnSO4∙7H2O, 1.5 g CaCl2 and 0.5 g FeC13, added to 100 mL distilled water.
Activation of freeze-dried strains: Before activation, cryopreserved strains were placed at room temperature for several hours. The ampoule was wiped with 70% alcohol in a sterile room, opened, and 0.2 mL TPY medium was added. The strains taken by sterilized platinum earrings were inoculated into TPY medium and onto agar plates. After anaerobic incubation for 48-72 h at 37 °C, three generations were continuously activated to enhance the viability of the strains.
Morphological observation: Platinum earrings were used to remove bacteria from a single colony or liquid culture and then smeared onto a microscope slide for Gram staining. The bacterial morphology was observed under the microscope. Generation of strains: After microscopic examination, strains with good morphology and no bacterial colonies in the solid medium were selected, and lines were drawn on TPY solid medium. At the same time, TPY liquid medium was added to bacteria to culture anaerobically at 37 °C for 48-72 h.
Measurement of viable bacteria: The 0.5 mL suspension was gradiently diluted 10 times with sterilized normal saline. The 0.2 mL suspension of the appropriate dilution gradient was extracted and placed on the TPY agar plate two or three times for each dilution gradient, and the suspension was uniformly spread out using a sterilized rod. After ventilation in the corridor of the anaerobic incubator, the suspension was transferred to an incubator for anaerobic cultivation at 37 °C for 48 h. Finally, bacterial colonies were counted after the strains were grown.
Rats in groups E and T were intraperitoneally injected with 5 mg/kg LPS (5 mg/mL dissolved in normal saline). In group T, 0.5 mL Bifidobacterium suspension (2.0 × 109 CFU/mL) was administered intragastrically twice daily 1 wk before LPS, until the end of the experiment. In group C, 1 mL/kg normal saline was intraperitoneally injected. The preweaning rats in each group were returned to their cage after treatment and continued to receive rat’s milk.
After the animals were sacrificed, the abdomen was aseptically dissected, and the contents of the intestinal cavity were washed with ice-cold physiological saline. A 0.5–1 cm length of ileum 2–3 cm away from the ileocecal junction was removed and fixed in 4% paraformaldehyde dissolved in 0.1 M PBS. The tissue was embedded in paraffin and sectioned for immunohistochemical study. A 4-5 cm length of ileum 3-4 cm away from the ileocecal junction was removed and placed in an RNase-free Eppendorf tube and stored at – 80 °C prior to total RNA extraction and RT-PCR.
The SP method (peroxidase-labeled streptomycin, and streptavidin/peroxidase) was used for immunohistochemistry. Paraffin sections were dewaxed and washed twice with 0.01 M PBS at pH 7.4 for 5 min. The sections were incubated in recently prepared 3% H2O2 (in 80% methanol) at room temperature for 5-10 min to eliminate endogenous peroxidase activity and washed three times in PBS for 5 min. Antigen retrieval was performed with 1 mM EDTA (pH 9.0) under high temperature and high pressure for 10 min. When returning to room temperature, sections were washed with distilled water, then washed three times with PBS for 5 min, covered with 10% normal goat serum (PBS diluted) and incubated at room temperature for 20 min to reduce non-specific staining. Excess liquid was discarded. A total of 50 μL rabbit anti-rat IGF-1 antibody (1:100) was incubated at 4 °C overnight or 37 °C for 1 hour, followed by three washes in PBS for 5 min each. Then, 50 μL biotin-labeled secondary antibody was incubated at 37 °C for 10-30 min, and washed three times with PBS for 5 min. Finally, 50 μL horseradish-peroxidase-labeled streptavidin was incubated at room temperature for 20 min and washed three times in PBS for 5 min. The sections were stained with newly prepared diaminobenzidine color developing agent at room temperature for 5-10 min, with the degree of dying being monitored under microscope, then washed with distilled water for 5 min. The sections were stained again with hematoxylin for 2 min, and washed with distilled water for 5 min. They were differentiated by 0.1% hydrochloric acid alcohol, and washed in flowing water for 30 min. The stained sections were dehydrated in graded series of ethanol, cleared, and sealed with gum. For the negative controls, PBS was used in place of the rabbit anti-rat IGF-1 primary antibody. The other steps were the same as above, excluding non-specific staining.
Homogeneous brown staining was considered positive. For immunohistochemical analysis, five to eight stained sections were selected for each time point. Five non-overlapping views were randomly selected under light microscopy (× 400), and the optical density of each view was measured by Nikon Eclipse E800 Image Acquisition System and Beihang Motic Pathological Image Analysis System.
For total RNA extraction, ileal tissues preserved at –80 °C were ground in liquid nitrogen under aseptic conditions, then poured into an Eppendorf tube. TRIzol Reagent (50-200 mg tissue/mL TRIzol) was added to the ileal tissues for 5 min at room temperature to facilitate dissociation of nucleic acid–protein complexes. Protein and DNA were removed by addition of 0.2 mL chloroform, and the supernatant was extracted by rapid vibration and centrifugation at 12000 rpm at 4 °C for 15 min.
RNA was precipitated by addition of 0.5 mL isopropanol. After mixing and incubating at room temperature for 10 min, the samples were centrifuged at 12000 rpm at 4 °C for 15 min. The supernatant was discarded, and the precipitate was resuspended in 1 mL 75% ethanol (prepared in 0.1% DEPC water) and centrifuged at 7500 rpm at 4 °C for 5 min. The supernatant was discarded and dried for 5-10 min. The RNA was dissolved in RNase-free DEPC-treated water to a volume of 20 mL, mixed, and stored at – 20 °C.
For reverse transcription into cDNA, 2 μL RNA was added to the total reaction of 20 μL. The reverse transcription reaction solution was prepared as follows: 2 μL 10 × buffer, 4 μL MgCl2 (25 mM), 2 μL dNTP (10 mM), 0.5 μL RNase, 0.7 μL AMV (22 U/μL), 1 μL oligo-dT 15 (50 μM), 2 μL total RNA and 7.8 μL enzyme-free water. The reverse transcription reaction was carried out at 42 °C for 15 min, 95 °C for 15 min, and 0-5 °C for 5 min. The cDNA concentration was determined by ultraviolet spectrophotometry.
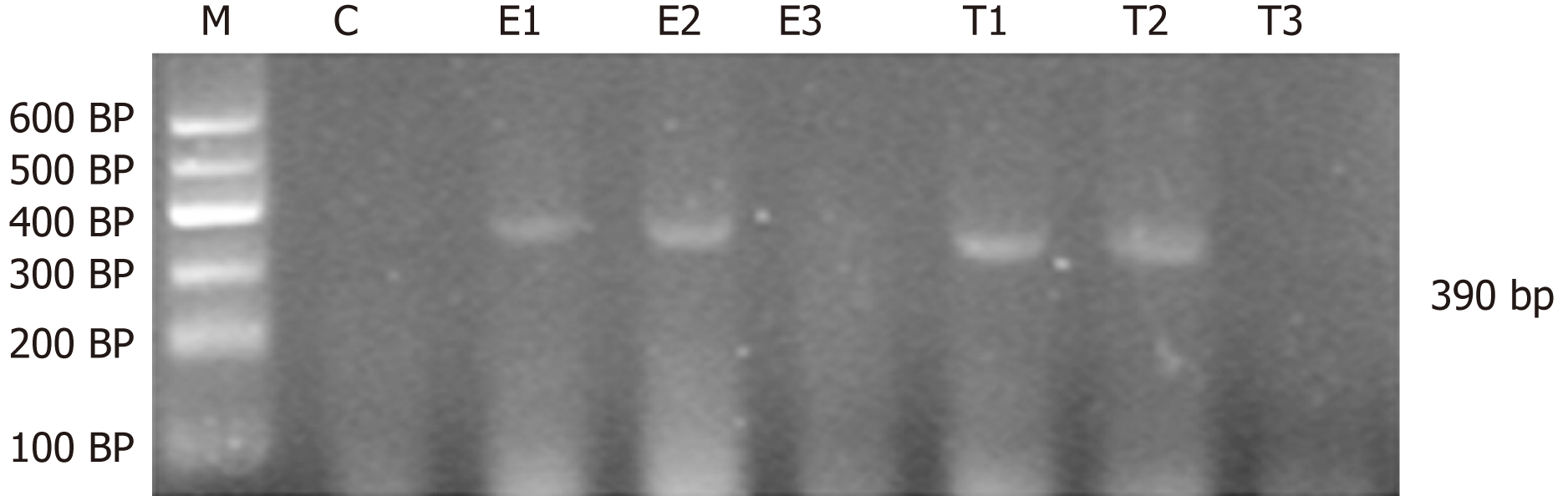
Amplification of CINC, IGF-1 and β-actin was as follows: CINC, primer sequence: 5′-TGA GCT GCG CAG TCA GTG CCT GCA-3′, antisense primer sequence: 5′-ACA CCC TTT AGC ATC TTT TGG ACA-3′. The length of product was 390 bp. IGF-1, primer sequence: 5′-GCT GCC ACT TGG ATC GCT ATT C-3′; antisense primer sequence: 5′-CGT CCC GGG TCG TTT ACA CA-3′. The length of product was 300 bp. β-actin, primer sequence: 5′-CAT CTG CTG GAA GGT GGA CA-3′; antisense primer sequence: 5′-GAG AGG GAA ATC GTG CGT GAC-3′. The length of product was 452 bp.
The total reaction system volume was 25 μL, with 2 μg cDNA (the volume of cDNA was calculated according to the cDNA concentration of each sample); each reaction system was brought to 25 μL with enzyme-free water. Other specific components and volumes were as follows: 1.0 μL MgCl2 (50 mM), 2.5 μL 10 × buffer, 0.5 μL dNTPs (10 mM), and 0.5 μL Taq polymerase (5 U/μL). The amplification conditions for CINC were: 94 °C for 5 min; 35 cycles of 94 °C for 30 s, 55 °C for 30 s and 72 °C for 30 s; then 72 °C for 7 min. The amplification conditions for IGF-1 were: 94 °C for 5 min; 35 cycles of 94 °C for 30 s, 55 °C for 30 s and 72 °C for 30 s; then 72 °C for 7 min. The amplification conditions for β-actin were: 94 °C for 5 min; 30 cycles of 94 °C for 30 s, 56 °C for 40 s and 72 °C for 22 s; then 72 °C for 7 min.
For semi-quantitative analysis of PCR products, PCR reaction products (3-5 μL) were mixed with ethidium bromide and subjected to 2% agarose gel electrophoresis. The PCR products were quantified by ImageJ analysis, and β-actin was the internal reference for RNA detection. The relative expression of genes = optical density of tested genes/intrinsic optical density.
Statistical analyses were performed using SPSS version 21.0 software. The results were expressed as mean ± SD. The variance analysis of factorial design was used for statistical analysis. P < 0.05 was considered statistically significant.
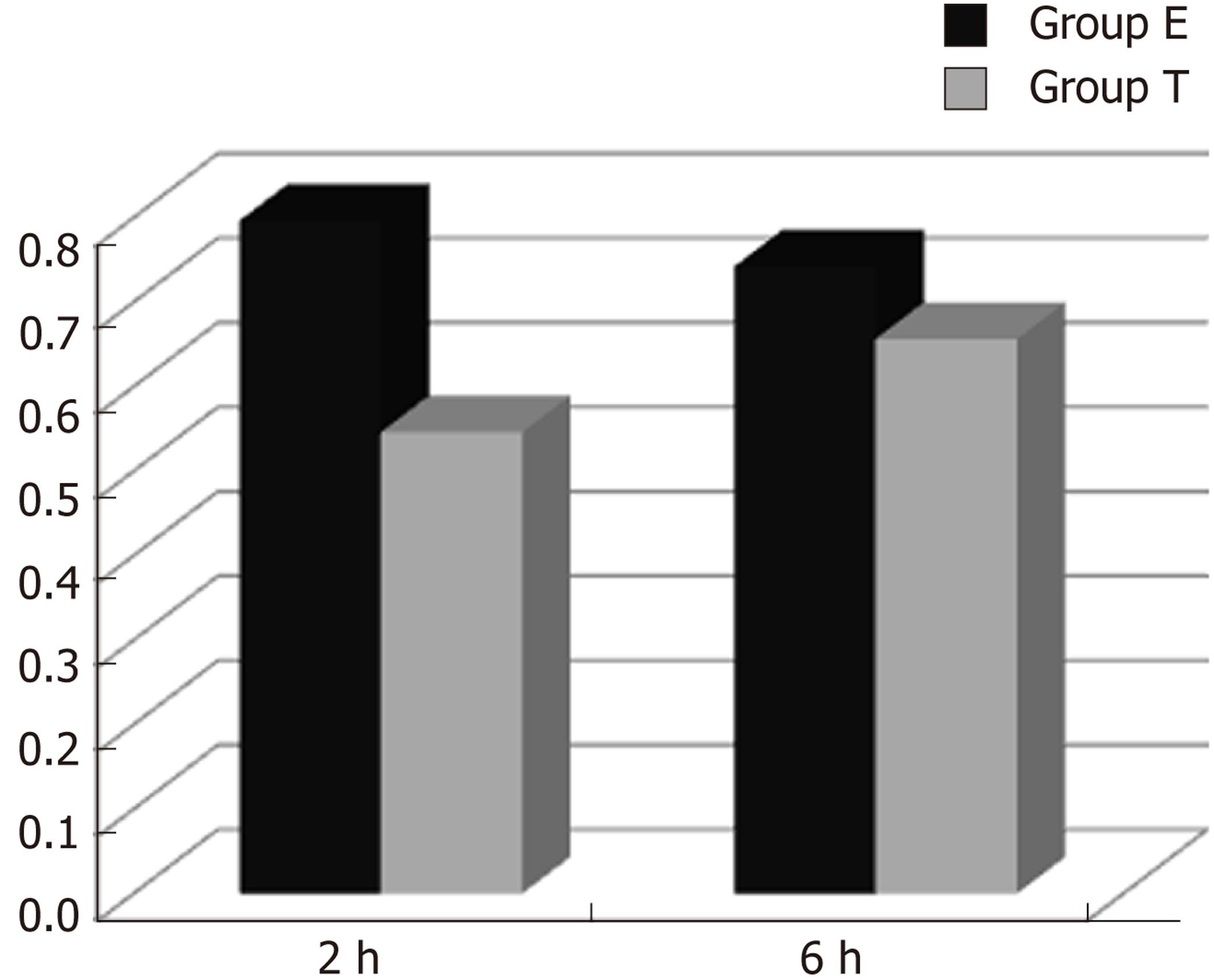
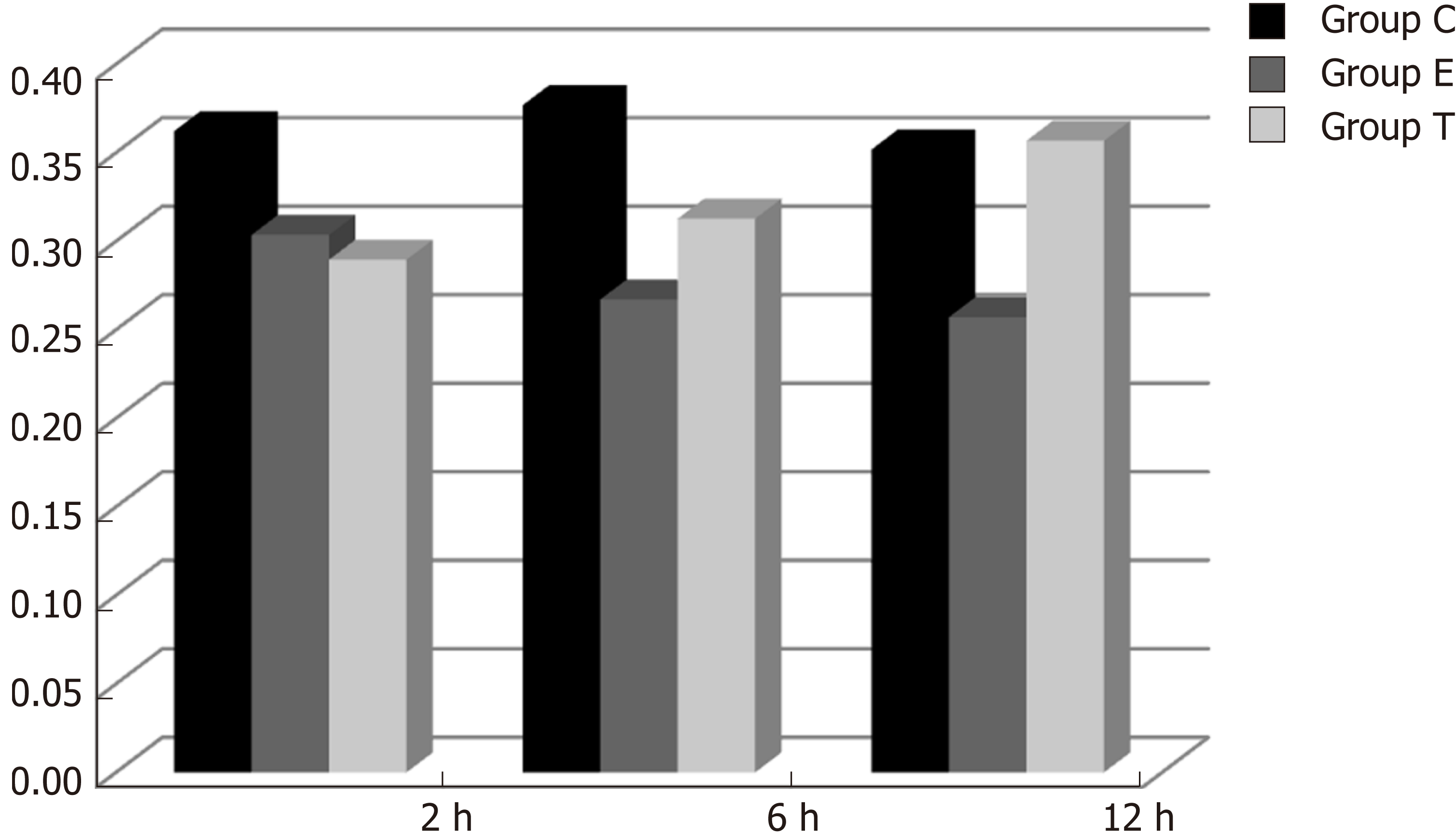
CINC mRNA was not expressed in group C at any time examined. There was a significant difference between groups T and E (F = 23.947, P < 0.05). There was no significant difference in expression within a group at different time points (F = 0.665, P > 0.05) (Figures 1 and 2).
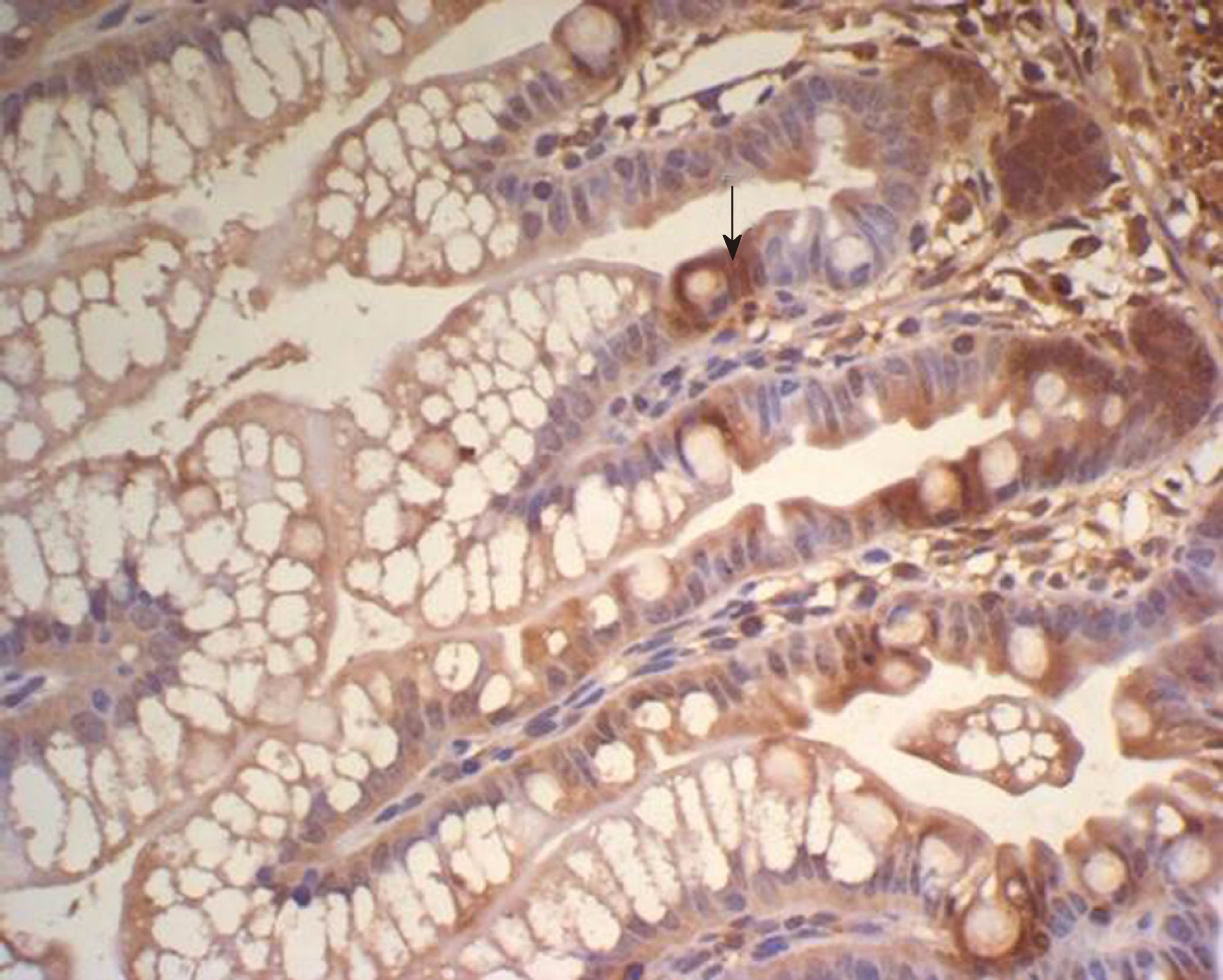
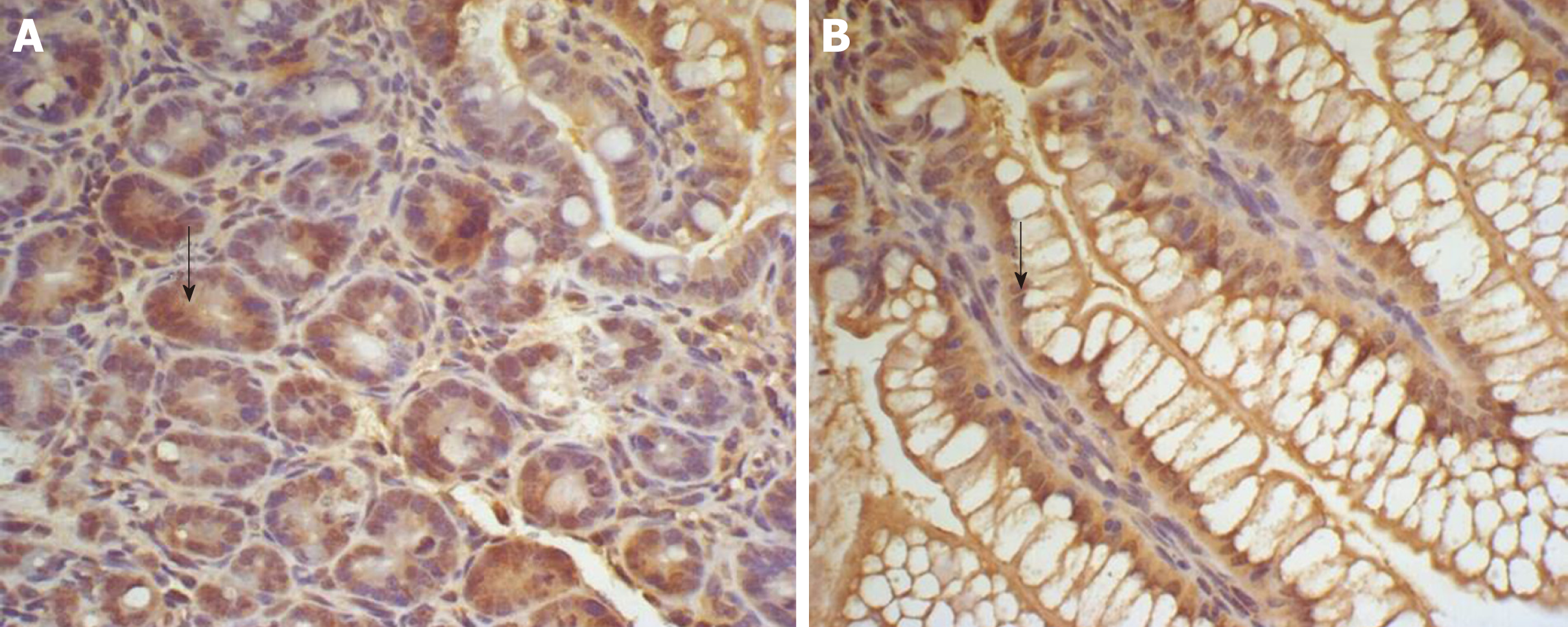
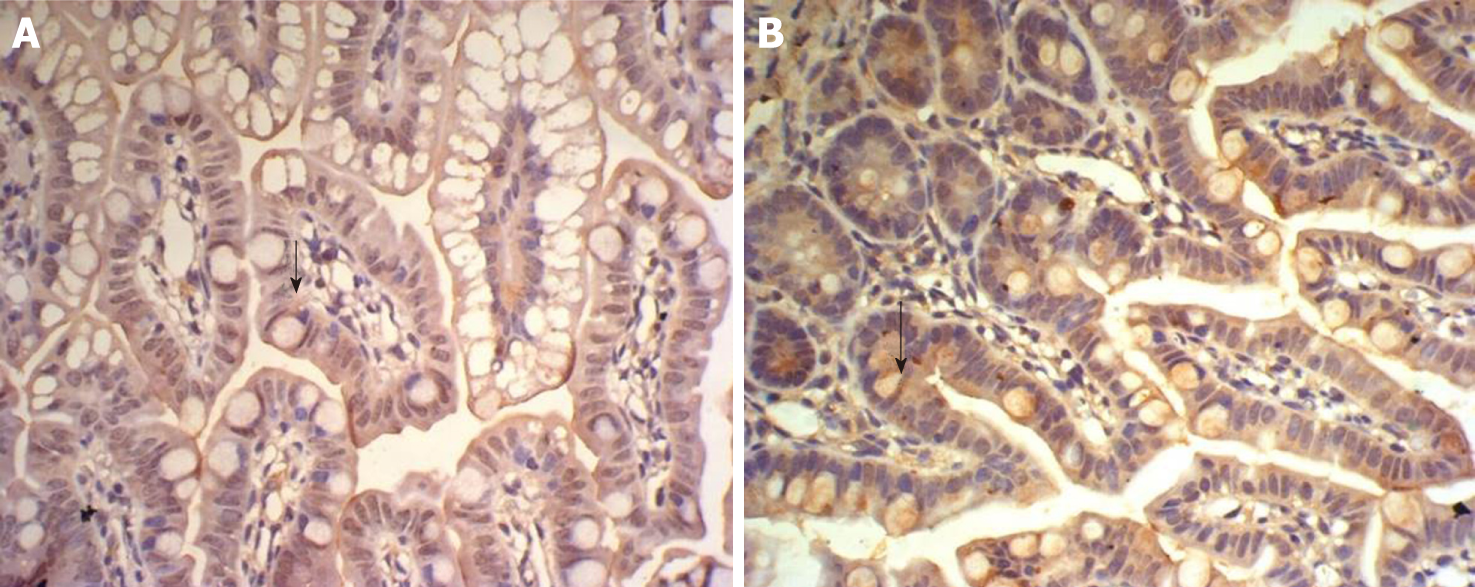
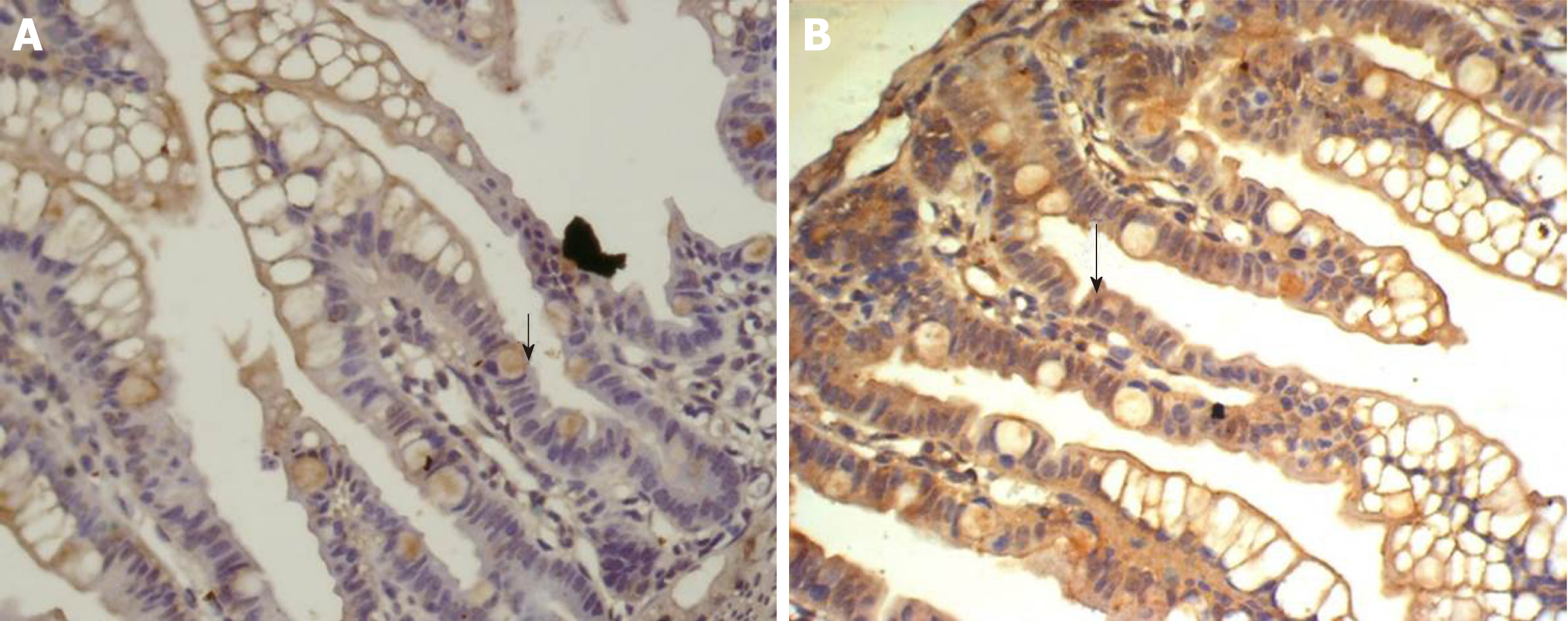
There was some IGF-1 expression in group C at every time point examined. There were more brown granules in the intestinal crypts and epithelial cells compared to the other groups. The brown staining became faint and sparse in group E. The brown staining became dark and dense in group T. There was no significant difference in staining at different time points (F = 1.269, P > 0.05), although there were significant differences among groups (F = 32.463, P < 0.05). Further analysis showed significant differences between groups C and E and groups E and T (P < 0.05) (Figures 3-7).
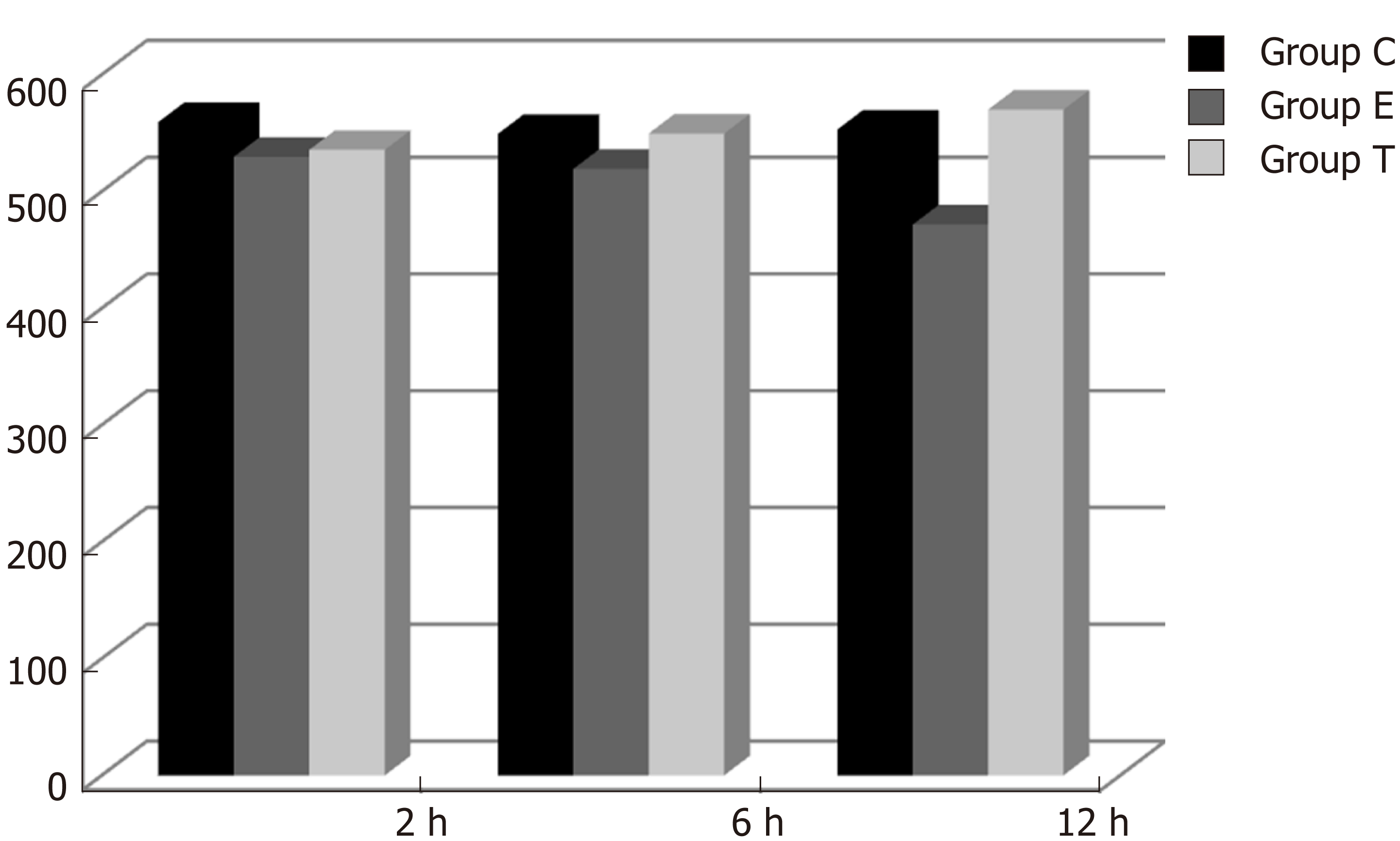
IGF-1 mRNA was highly expressed in group C. There was no significant difference in expression at different times (F = 0.086, P > 0.05), although there were significant differences between groups (F = 46.670, P < 0.05). Further analysis revealed that there was a significant difference between groups C and E, groups C and T, and groups E and T (P < 0.05) (Figure 8).
Gastrointestinal dysfunction plays an important role in the development of multiple organ dysfunction syndrome (MODS). The intestine is one of the target organs injured in MODS, and it plays an important role in initiating MODS, in which intestinal mucosal barrier damage is a key factor. Bacterial or endotoxin translocation is closely related to excessive growth of opportunistic pathogens in the intestine, weakened intestinal immunity, and intestinal mucosal damage[3]. The intestinal flora is a key component of the mechanical, immune and biological barriers of the intestinal mucosa, as it plays a key role in preventing bacterial or endotoxin translocation.
Bifidobacterium is one of the main components of the intestinal mucosal barrier and is one of the main probiotics in the human body. It is involved in digestion, absorption, nutrition, metabolism, anti-infective immunity, and especially in maintaining the integrity of the intestinal mucosal barrier. The digestive tract is the largest bacterial habitat in the body. The micro-ecological stability of the intestine can be destroyed by fasting, antacids or antibiotics in critically ill patients, which can disrupt the stability of the intestinal micro-ecology. Consequently, intestinal flora imbalance is the primary cause of bacterial translocation and intestinal infection. It has been reported that Bifidobacterium has an effect on rotavirus enteritis[4] and can prevent necrotizing enterocolitis in premature rats[5,6], where it has been shown to have a therapeutic effect on necrotizing enterocolitis[7,8]. Bifidobacterium also improves immunity and the inflammatory response in weaning rats with colitis[9], and there has been an increased research focus on the effects of Bifidobacterium on metabolic syndrome[10-15]. In recent years, Bifidobacterium has been used to prevent cardiac damage[16], and its effect on the immune state in early life has been studied[17]. However, the intestinal protective mechanism of Bifidobacterium has not been entirely elucidated.
In inflammatory reactions, neutrophils in the blood must be activated after entering tissue spaces through capillary walls, where they then exert their biological effects. Neutrophil chemotaxis is mediated by CINC, a murine chemotactic cytokine similar to human interleukin-8. CINC-1 expression is increased in rat models of ulcerative colitis[18], intestinal and lung ischemia/reperfusion models and endotoxin injury models[19,20]. CINC-1 expression in the lung tissue of rats is increased by endotoxin[20,21] and chronic intermittent hypoxia[22].
In the present study, there was no significant CINC mRNA expression in the ileum of group C rats at any time point examined. CINC mRNA expression was high at 2 and 6 h in group E, which meant that the model of intestinal injury was successful. CINC mRNA expression in the ileum of rats in group T was significantly decreased compared to group E (P < 0.05), which indicated that Bifidobacterium relieved ileal inflammation and protected the rat intestine.
IGF-1 is a multifunctional cellular regulatory factor that is mainly secreted by the liver. Thus, the liver is the main source of circulating IGF-1, but IGF-1 is also ex-pressed in other tissues including the intestine, condylar cartilage cells in rats[23], T lymphocytes in mice dendritic epithelia[24], brain tissue of rats[25,26], placenta of pregnant mice[27-29], and colonic smooth muscle cells in diabetic rats[27]. IGF-1 plays a role in cell growth[30-32], differentiation[33,34] and metabolism[35]. One recent study focused on continuous IGF-1 expression in the intestine in a rat model of short bowel syndrome[36]. Other studies have shown that exogenous IGF-1 improved intestinal barrier function in rats with cirrhosis[37] or acute necrotizing pancreatitis[38], where survival rates have been significantly improved by minimal invasive interventions[39-43]. How IGF-1 is expressed during intestinal infection and how Bifidobacterium affects that expression have not been reported.
In this study, IGF-1 protein and mRNA expression in the ileum decreased after intraperitoneal injection of endotoxin, as reported previously[44]. Intragastric administration of Bifidobacterium increased IGF-1 protein and mRNA expression, indicating that IGF-1 plays an important role in the recovery of ileal mucosal damage. Bifidobacterium may enhance the immunological barrier function of the intestine by inhibiting local inflammatory responses and increasing IGF-1 expression.
Severe infection is one of the most common causes of gastrointestinal dysfunction, and its pathogenesis is closely related to endotoxemia and intestinal barrier injury.
Bifidobacterium plays an important role in maintaining the integrity of the intestinal mucosal barrier.
This study investigated the protective mechanism of Bifidobacterium during ileal injury in rats.
Using endotoxin injured rat models, ileal cytokine-induced neutrophil chemoattractant (CINC) mRNA expression was evaluated by reverse transcription-polymerase chain reaction (RT-PCR), and expression of ileal insulin-like growth factor 1 (IGF-1) protein and mRNA was detected by immunohistochemistry and RT-PCR, respectively.
There was a significant difference in CINC mRNA expression between the different groups (P < 0.05). There was a significant difference in IGF-1 brown granule expression among the different groups (P < 0.05), and expression of IGF-1 mRNA significantly differed among the three groups (P < 0.05)
Bifidobacterium may increase IGF-1 expression and enhance intestinal immune barrier function in rats with endotoxin injury.
This study can provide a new therapeutic tool and theoretical support for gastrointestinal dys-function.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Higuchi K, Jung DH, Pourshafie MR S-Editor: Yan JP L-Editor: Filipodia E-Editor: Ma YJ
| 1. | Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda NN, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke JM, Topping DL, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K, Ohno H. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2951] [Cited by in RCA: 3750] [Article Influence: 312.5] [Reference Citation Analysis (0)] |
| 2. | Zhang R, Shi Y, Guan X. Regulatory role of the GHSOCS2-IGF-1 axis in the pathogenesis of intestinal mucosal barrier dysfunction in ulcerative colitis. Shijie Huaren Xiaohua Zazhi. 2010;18:2442-2447. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (1)] |
| 3. | Zhu R, Ma XC. Clinical Value of Ultrasonography in Diagnosis of Pulmonary Embolism in Critically Ill Patients. J Transl Int Med. 2017;5:200-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Rigo-Adrover M, Saldaña-Ruíz S, van Limpt K, Knipping K, Garssen J, Knol J, Franch A, Castell M, Pérez-Cano FJ. A combination of scGOS/lcFOS with Bifidobacterium breve M-16V protects suckling rats from rotavirus gastroenteritis. Eur J Nutr. 2017;56:1657-1670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Satoh T, Izumi H, Iwabuchi N, Odamaki T, Namba K, Abe F, Xiao JZ. Bifidobacterium breve prevents necrotising enterocolitis by suppressing inflammatory responses in a preterm rat model. Benef Microbes. 2016;7:75-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Wu SF, Chiu HY, Chen AC, Lin HY, Lin HC, Caplan M. Efficacy of different probiotic combinations on death and necrotizing enterocolitis in a premature rat model. J Pediatr Gastroenterol Nutr. 2013;57:23-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Tang J, Zou W, Li MX, Lv H, Huang LG, Yuan WM. Regulation of bifidobacterium on Wnt/β-Catenin signal pathway of intestinal tissue in newborn rats with necrotizing enterocolitis. Zhonghua Shiyong Linchuang Erke Zazhi. 2016;31:302-305. [DOI] [Full Text] |
| 8. | Su H, Lv H, Zou W, Li MX, Huang LG, Li J, Yuan WM. Protective Effects of Biifdobacterium on intestinal tissue of newborn rats with necrotizing enterocolitis and its regulation. Zhonghua Weichankexue Zazhi. 2015;18:290-295. [DOI] [Full Text] |
| 9. | Izumi H, Minegishi M, Sato Y, Shimizu T, Sekine K, Takase M. Bifidobacterium breve alters immune function and ameliorates DSS-induced inflammation in weanling rats. Pediatr Res. 2015;78:407-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Zhu G, Ma F, Wang G, Wang Y, Zhao J, Zhang H, Chen W. Bifidobacteria attenuate the development of metabolic disorders, with inter- and intra-species differences. Food Funct. 2018;9:3509-3522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 11. | Plaza-Díaz J, Plaza-Díaz J, Robles-Sánchez C, Abadía-Molina F, Sáez-Lara MJ, Vilchez-Padial LM, Gil Á, Gómez-Llorente C, Fontana L. Gene expression profiling in the intestinal mucosa of obese rats administered probiotic bacteria. Sci Data. 2017;4:170186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Plaza-Díaz J, Robles-Sánchez C, Abadía-Molina F, Morón-Calvente V, Sáez-Lara MJ, Ruiz-Bravo A, Jiménez-Valera M, Gil Á, Gómez-Llorente C, Fontana L. Adamdec1, Ednrb and Ptgs1/Cox1, inflammation genes upregulated in the intestinal mucosa of obese rats, are downregulated by three probiotic strains. Sci Rep. 2017;7:1939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Kim SJ, Park SH, Sin HS, Jang SH, Lee SW, Kim SY, Kwon B, Yu KY, Kim SY, Yang DK. Hypocholesterolemic Effects of Probiotic Mixture on Diet-Induced Hypercholesterolemic Rats. Nutrients. 2017;9:pii: E293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 14. | Plaza-Diaz J, Gomez-Llorente C, Abadia-Molina F, Saez-Lara MJ, Campaña-Martin L, Muñoz-Quezada S, Romero F, Gil A, Fontana L. Effects of Lactobacillus paracasei CNCM I-4034, Bifidobacterium breve CNCM I-4035 and Lactobacillus rhamnosus CNCM I-4036 on hepatic steatosis in Zucker rats. PLoS One. 2014;9:e98401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 15. | Bordoni A, Amaretti A, Leonardi A, Boschetti E, Danesi F, Matteuzzi D, Roncaglia L, Raimondi S, Rossi M. Cholesterol-lowering probiotics: In vitro selection and in vivo testing of bifidobacteria. Appl Microbiol Biotechnol. 2013;97:8273-8281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 16. | Sadeghzadeh J, Vakili A, Sameni HR, Shadnoush M, Bandegi AR, Zahedi Khorasani M. The Effect of Oral Consumption of Probiotics in Prevention of Heart Injury in a Rat Myocardial Infarction Model: A Histopathological, Hemodynamic and Biochemical Evaluation. Iran Biomed J. 2017;21:174-181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Rigo-Adrover MD, Franch À, Castell M, Pérez-Cano FJ. Preclinical Immunomodulation by the Probiotic Bifidobacterium breve M-16V in Early Life. PLoS One. 2016;11:e0166082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Algieri F, Zorrilla P, Rodriguez-Nogales A, Garrido-Mesa N, Bañuelos O, González-Tejero MR, Casares-Porcel M, Molero-Mesa J, Zarzuelo A, Utrilla MP, Rodriguez-Cabezas ME, Galvez J. Intestinal anti-inflammatory activity of hydroalcoholic extracts of Phlomis purpurea L. and Phlomis lychnitis L. in the trinitrobenzenesulphonic acid model of rat colitis. J Ethnopharmacol. 2013;146:750-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Chen CB, Tu WF, Xi WB. Effects of sufentanil pretreatment on long injury induced by ischemia-reperfusion hind limbs in Rats. Linchuang Mazuixue Zazhi. 2015;31:63-66. |
| 20. | Dong S, Zhong Y, Yang K, Xiong X, Mao B. Intervention effect and dose-dependent response of tanreqing injection on airway inflammation in lipopolysaccharide-induced rats. J Tradit Chin Med. 2013;33:505-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Sun YH, Cui Y, Sun XJ, Li XQ. Effects of sevoflurane on neutrophil chemokine-1 and intercellular adhesion molecule-1 in endotoxin-induced acute lung injury in rats. Guangdong Yixue. 2014;35. [DOI] [Full Text] |
| 22. | Han Q, Li G, Mak JC, Zhang Y, Ip MS, Zhang N. [The role of heme oxygenase-1 in the protection of chronic intermittent hypoxia-induced lung injury in vivo]. Zhonghua Jie He He Hu Xi Za Zhi. 2015;38:516-519. [PubMed] |
| 23. | Jiang LT, Wei L, Xie YY, Zhou Q. Proliferation and Differentiation of Mandibular Condylar Chondrocytes Require IGF-1 Signal Pathway Related-proteins. Kouqiang Hemian Waike Zazhi. 2013;23:171-177. |
| 24. | Deng X, Chen F, Liu J, Zhou Z, Jia C. [Expression of coxsackie-adenovirus receptor in keratinocytes of mouse skin after heat stimulation and the effect of coxsackie-adenovirus receptor on dendritic epidermal T lymphocytes]. Zhonghua Shao Shang Za Zhi. 2014;30:40-45. [PubMed] |
| 25. | Zhang Y, Shi B, Huang KX. Study on the protective effect of Mongolian treasure pill on cerebral tissue of neonatal rats with hypoxic brain injury. Zhongguo Fuyou Baojian. 2014;29:2596-2598. |
| 26. | Yan QF, Chen YC, Huang SP, Heng XP, Chen Y, Li L, Wang SM, Kang WQ, Chen CY, Ye MJ, Zheng HL. Dangua recipe on intervening IGF-1 in encephalon of mice with Apo E-/-Diabetes Mellitus. Jilin Zhongyiyao Zazhi. 2015;35:404-406. |
| 27. | Zhang YD, Liang JW, Wang J, Liang B. Pregnant rats intake different levels of glucose to influence the placental IGFs expression. Zhonghua Linchuang Yishi Zazhi. 2015;9:470-474. |
| 28. | Wu MM, Yang F, Qu Y, Mu DZ. [Effects of maternal folate deficiency on the methylation of insulin-like growth factor system in the offspring rats]. Zhongguo Dang Dai Er Ke Za Zhi. 2017;19:470-474. [PubMed] |
| 29. | Wang Y, Xu XY, Tang YR, Yang WW, Yuan YF, Ning YJ, Yu YJ, Lin L. Effect of endogenous insulin-like growth factor and stem cell factor on diabetic colonic dysmotility. World J Gastroenterol. 2013;19:3324-3331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Wang DH, Qi HX, Luo KJ, Chen PY. Effects of puerarin subcutaneous injection on body and bone metabolism in pregnant rats on low-protein diet during pregnancy. Shangdong Yiyao. 2017;57:46-48. |
| 31. | Wu WY, Wang JY, Xu XJ. Body mass catch-up growth in premature infants and infants less than gestational age and its correlation with igf-1. Shangdong Yiyao. 2015;1:69-71. |
| 32. | Han H, Li Y. Effect of rat nerve growth factor on nerve conduction velocity and serum IGF-1in patients with diabetic peripheral neuropathy. Zhongguo Shiyong Yikan. 2014;41:85-87. |
| 33. | Jiang ZM, Lu DX, Li HX. Therapeutic effect of mouse nerve growth factor on acute cerebral infarction. Shandong Yiyao. 2015;34:106-107. |
| 34. | Yan QY, Li Z, Hu WT, Fang Y, Wang SN, Qiu ZD, Zhang SM. Roles of Insulin-like Growth Factor-1 in the Induction of Murine Induced Pluripotent Stem Cells. Shengjing Sunshang Yu Gongneng Chongjian. 2013;8:157-159. |
| 35. | Dong X, Chang G, Ji XF, Tao DB, Wang YX. The relationship between serum insulin-like growth factor I levels and ischemic stroke risk. PLoS One. 2014;9:e94845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 36. | Sangild PT, Ney DM, Sigalet DL, Vegge A, Burrin D. Animal models of gastrointestinal and liver diseases. Animal models of infant short bowel syndrome: Translational relevance and challenges. Am J Physiol Gastrointest Liver Physiol. 2014;307:G1147-G1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 37. | Zhao TY, Su LP, Ma CY, Zhai XH, Duan ZJ, Zhu Y, Zhao G, Li CY, Wang LX, Yang D. IGF-1 decreases portal vein endotoxin via regulating intestinal tight junctions and plays a role in attenuating portal hypertension of cirrhotic rats. BMC Gastroenterol. 2015;15:77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 38. | Cui PL, Lv D, Wang YB, Yang ZX, Xu YQ. Effects of glutamine on expression of IGF-1 and intestinal mucosal barrier in rats with acute necrotic pancreatitis. Shandong Yiyao. 2014;54:14-17. |
| 39. | Adler DG. Single-operator experience with a 20-mm diameter lumen apposing metal stent to treat patients with large pancreatic fluid collections from pancreatic necrosis. Endosc Ultrasound. 2018;7:422-423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 40. | Poincloux L, Chabrot P, Mulliez A, Genes J, Boyer L, Abergel A. Interventional endoscopic ultrasound: A new promising way for intrahepatic portosystemic shunt with portal pressure gradient. Endosc Ultrasound. 2017;6:394-401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 41. | Adler DG, Shah J, Nieto J, Binmoeller K, Bhat Y, Taylor LJ, Siddiqui AA. Placement of lumen-apposing metal stents to drain pseudocysts and walled-off pancreatic necrosis can be safely performed on an outpatient basis: A multicenter study. Endosc Ultrasound. 2019;8:36-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 42. | Huang JY. Role of EUS-guided liver biopsy in benign parenchymal disease (with video). Endosc Ultrasound. 2018;7:236-239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 43. | Rana SS, Gupta R, Kang M, Sharma V, Sharma R, Gorsi U, Bhasin DK. Percutaneous catheter drainage followed by endoscopic transluminal drainage/necrosectomy for treatment of infected pancreatic necrosis in early phase of illness. Endosc Ultrasound. 2018;7:41-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 44. | He Y, Yuan X, Zhou G, Feng A. Activation of IGF-1/IGFBP-3 signaling by berberine improves intestinal mucosal barrier of rats with acute endotoxemia. Fitoterapia. 2018;124:200-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |