Published online Jun 14, 2019. doi: 10.3748/wjg.v25.i22.2706
Peer-review started: March 13, 2019
First decision: March 27, 2019
Revised: April 2, 2019
Accepted: April 19, 2019
Article in press: April 20, 2019
Published online: June 14, 2019
Processing time: 94 Days and 15.5 Hours
Proton pump inhibitors (PPIs) are common medications within the practice of gastroenterology. These drugs, which act through the irreversible inhibition of the hydrogen/potassium pump (H+/K+-ATPase pump) in the gastric parietal cells, are used in the treatment of several acid-related disorders. PPIs are generally well tolerated but, through the long-term reduction of gastric acid secretion, can increase the risk of an imbalance in gut microbiota composition (i.e., dysbiosis). The gut microbiota is a complex ecosystem in which microbes coexist and interact with the human host. Indeed, the resident gut bacteria are needed for multiple vital functions, such as nutrient and drug metabolism, the production of energy, defense against pathogens, the modulation of the immune system and support of the integrity of the gut mucosal barrier. The bacteria are collected in communities that vary in density and composition within each segment of the gastrointestinal (GI) tract. Therefore, every change in the gut ecosystem has been connected to an increased susceptibility or exacerbation of various GI disorders. The aim of this review is to summarize the recently available data on PPI-related microbiota alterations in each segment of the GI tract and to analyze the possible involvement of PPIs in the pathogenesis of several specific GI diseases.
Core tip: The gut microbiota plays a fundamental role in the maintenance of human health. However, several drugs, including proton pump inhibitors, can cause dysbiosis, which in turn is responsible for different extra-intestinal and intestinal diseases. An up-to-date review of the literature was conducted to identify changes in gut microbiota composition related to chronic proton pump inhibitor therapy and to highlight the possible pathogenic involvement of dysbiosis in gastrointestinal disorders.
- Citation: Bruno G, Zaccari P, Rocco G, Scalese G, Panetta C, Porowska B, Pontone S, Severi C. Proton pump inhibitors and dysbiosis: Current knowledge and aspects to be clarified. World J Gastroenterol 2019; 25(22): 2706-2719
- URL: https://www.wjgnet.com/1007-9327/full/v25/i22/2706.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i22.2706
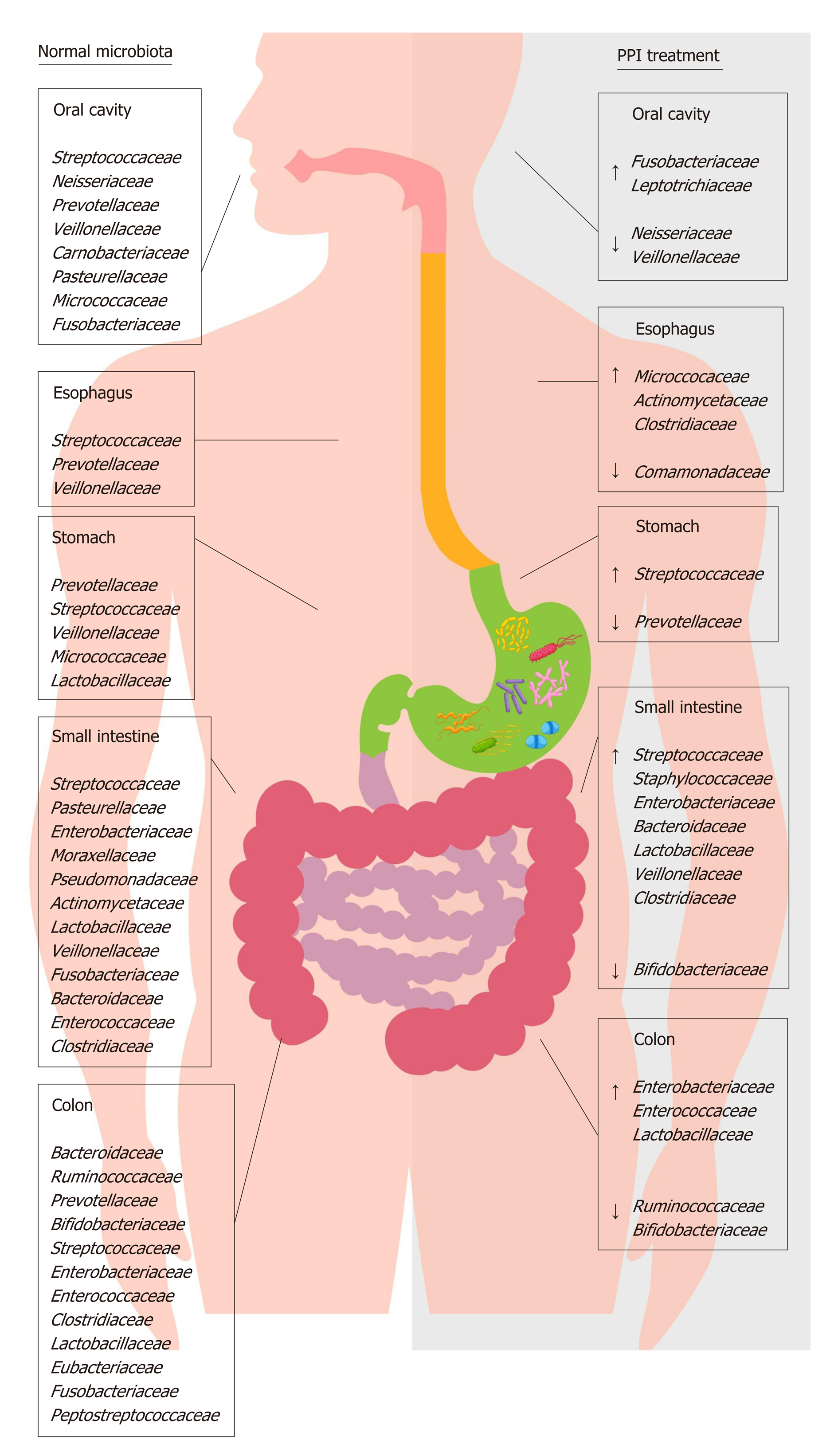
Proton pump inhibitors (PPIs) are acid-suppressive agents and are among the most widely used and over-used drugs in the world[1]. PPIs are the first-choice treatment for acid-related disorders such as bleeding, peptic ulcers, gastroesophageal reflux, erosive esophagitis (ERD) and certain dyspepsia subtypes[2-5]. The use of PPIs is generally well tolerated, although their long-term use exposes patients to an increased risk of developing extra-intestinal disorders[6-8], likely due to PPI-driven gastric hypo-chlorhydria, which can cause also significant changes in gut microbiota composition[9-11] (Figure 1). The gut microbiota has a key role in metabolic, nutritional, physiological, defensive and immunological processes in the human body, and its composition is closely connected to individuals’ health and the diseases they experience[12,13]. Changes in this microbial equilibrium that is, dysbiosis can promote and influence the course of many intestinal and extra-intestinal diseases[14-16]. The impact of PPIs on gut microbiota composition is currently a popular topic, and over the years, several interesting manuscripts have been published that expand the knowledge in this field[17-20].
The aim of the current review is to summarize the more recent evidence on the effect of PPIs on the gut microbiota, focusing on various areas within the gastrointestinal (GI) tract, and to discuss the possible role of the associated dysbiosis in the pathogenesis of several GI disorders. In doing so, this study seeks to better understand how PPIs could alter, via gut microbiota imbalance, human homeostasis.
Despite the continuous introduction of different bacteria from humans’ external environment, the oral microbiota remains less variable compared to other areas of the GI tract. It is primarily composed of Firmicutes and Bacteroidetes, with Actino-bacteria, Proteobacteria and Fusobacteria also present[21]. In terms of genera, the most present are Streptococcus, Neisseria, Prevotella, Gemella, Granulicatella and Veillonella[22,23]. The oral microbiota helps to enrich and shape the bacterial communities of the gut through the continuous inflow of food and saliva[24-26]. It has been reported that inflammatory diseases such as gingivitis and periodontitis, which cause a shift in the composition of the oral microbial community, can promote the production of toxic and carcinogenic metabolites, cytolytic enzymes and oral pathogen-derived lipopolysaccharide (LPS) that are able to colonize extra-oral sites due to transient bacteremia[27-29]. The spread of such toxic compounds has been reported to contribute to the development of many GI diseases, including irritable bowel syndrome (IBS), inflammatory bowel diseases (IBD) and cancer[30-33].
Currently, little data has been presented about the relationship between PPI use and oral microbiota composition. One study showed that, in healthy volunteers, a four-week esomeprazole administration of PPI caused an increase of Fusobacterium and Leptotrichia in the periodontal pocket, associated with a decrease of Neisseria and Veillonella in saliva and a parallel increase of Streptococcus in fecal samples; this suggests that PPIs may cause both oral and gut microbiota alterations[34]. Based on these data, the oral cavity could represent a potential source of microbiota information related to oral and non-oral disorders; it could also be an important indicator of dysbiosis in other areas of the GI tract.
The esophagus has a distinct microbiota, with a relatively stable environmental bacterial composition; it does not simply contain a transient microbial population originating from swallowing (i.e., from the oral cavity) or reflux (i.e., from the stomach). The distal tract is mostly colonized by Firmicutes, followed by Bacteroidetes, Actinobacteria, Proteobacteria and Fusobacteria, with the most represented genera being Streptococcus, followed by Prevotella and Veillonella[35]. On the basis of the differences in genera proportion, two types of microbiota have been identified in the esophagus: Type I, present in healthy subjects, is characterized by a predominance of Gram-positive taxa (especially Streptococcus), whereas type II, associated with ERD and Barrett esophagus (BE), is constituted by a predominance of Gram-negative taxa, including Veillonella, Prevotella, Haemophilus, Neisseria, Rothia, Granulicatella, Campylobacter, Porphyromonas, Fusobacterium and Actinomyces, with a relative decrease in the abundance of Streptococcus[36,37]. It is likely that this switch in favor of Gram-negative bacteria could cause an LPS-mediated activation of innate immunity, inducing a dangerous cycle of dysbiosis-inflammation-dysbiosis and mucosal damage[38].
Since the early 1980s, a dysbiosis-mediated inflammatory response and the increased production of pro-carcinogenic bacterial compounds have been thought to contribute to carcinogenesis[39], a hypothesis recently re-confirmed and studied[40]. Both ERD and BE are considered precursor conditions to esophageal adenocarcinoma (EAC)[41]. In both these conditions, a significant enrichment of Campylobacter concisus has been reported[42,43]. This bacterium could have a role in EAC, promoting the metaplastic processes in the early stages of cancer through an increase in the interleukin (IL)-18 expression and downregulation of transforming growth factor beta 1, nuclear factor kappa B (NF-κB) and signal transducer and activator of transcription 3 signaling involved in the EAC cascade[42-44]. Moreover, the presence of Fusobacterium nucleatum (F. nucleatum) has been described in esophageal cancers and has been associated with a poor prognosis, suggesting its potential role as a prognostic biomarker[45]. Finally, esophageal samples of BE with high-grade dysplasia and EAC show a decreased microbiota diversity and a relative abundance of Lactobacillales that, through their capability to acidify the microenvironment and to produce harmful substances such as hydrogen peroxide, might contribute to the development of these diseases[46].
Currently, studies related to the effects of PPIs on the esophageal microbiota and their ability to reverse the microbial switch that occurs in ERD and BE are scarce. PPI treatment can alter esophageal microbiota, causing an increase in the abundance of Firmicutes and a decrease in the abundance of Bacteroidetes and Proteobacteria[47]. This evidence, obtained through both aspirates and biopsies, suggests that some bacterial families can colonize an esophagus exposed to lesser acidic refluxes, even if their role needs to be ascertained. A recent epidemiological study revealed that, in the absence of other risk factors, the long-term use of PPIs is associated with an increased risk of EAC[48]. The authors hypothesized that PPI therapy itself could predispose patients to EAC, likely through the colonization of non-gastric microbes capable of producing nitrosamines, which are known to possess carcinogenic potential for both EAC and esophageal squamous carcinoma. This concept stands in contrast with the actual guidelines that recommend PPI use in patients with non-dysplastic BE[49] because their long-term use significantly decreases the risk of the progression to high-grade dysplasia and EAC[50-52]. It has been hypothesized that the reduction of gastric acid reflux in the esophagus induced by PPIs avoids the death of acid-sensitive bacteria that have beneficial effects in the maintenance of a type I microbiota[53].
The gastric microbiota is composed mainly of Firmicutes, Bacteroidetes, Proteo-bacteria and Actinobacteria, with the most abundant genera being Streptococcus, followed by Veillonella, Prevotella, Fusobacterium and Rothia[54]. The impact of PPIs on gastric pH and the gastric microbiota has been the starting point for research in this field, but only recently have data demonstrated the importance of the consequences of long-term PPI use. PPIs have unfavorable effects on gastric functions and host defensive mechanisms, causing delayed gastric emptying, decreased gastric mucus viscosity, increased bacterial load and increased bacterial translocation[55-57]. Streptococcaceae are the most abundant family observed during PPI therapy, followed by Prevotellaceae, Campylobacteraceae and Leptotrichiaceae[58]. The primary abundance of Streptococcaceae was also demonstrated in dyspeptic patients during PPI treatment, suggesting that this ecological switch in favor of Streptococcaceae could be an independent indicator of gastric dysbiosis due to these drugs[59].
It is important to keep in mind that hypochlorhydria promotes a reduction in microbial diversity and the growth of microbes that have genotoxic potential, with an increase in the nitrate/nitrite reductase bacterial functions involved in cancer development[60]. Moreover, high gastric pH values can give rise to a different bacterial balance characterized by a significant increase in oral bacteria, such as Pepto-streptococcus stomatis, Streptococcus anginosus, Parvimonas micra, Slackia exigua and Dialister pneumosintes. Through the induction of different metabolic pathways, such bacteria could have a role in gastric cancer (GC) progression[61]. Therefore, to better understand the power of promoting the survival and spread of potentially genotoxic bacteria in the stomach and other GI regions, it will be crucial to define the effects of PPIs in gastric microbiota composition. However, the role of PPIs in GC development is under debate, with some studies and meta-analyses reporting an increased risk of developing GC in long-term PPI users[62,63] up to 2.4 times greater, even after Helicobacter pylori (H. pylori) eradication, according to a recent study[64] and other meta-analyses not confirming such a risk[65,66].
Gastric dysbiosis occurs as a result of H. pylori-related gastritis. H. pylori pro-inflammatory activity affects the luminal microenvironment and modifies the gastric microbiota. During the infection, the gastric microbiota is predominantly constituted of Proteobacteria, followed by Firmicutes, Bacteroidetes and Actinobacteria[54]. It is noteworthy that, depending on the site of H. pylori colonization, the dysbiosis can be associated with either an increase or decrease in acid secretion, which further influences gastric microbiota composition. H. pylori infection can lead to antrum-predominant gastritis, in which the oxyntic mucosa is not inflamed but a gastrin-driven increase in acid output occurs, along with the possible development of duodenal ulcer[67,68]. In addition, when the infection does spread to the oxyntic mucosa, it causes pangastritis, which is associated with hypochloridria, and is responsible for the development of chronic atrophic gastritis, intestinal metaplasia and, finally, dysplasia and GC[69,70]. Several studies have shown that the bacterial migration from the antrum to gastric body and fundus occurs more frequently during long-term PPIs use[71]. Therefore, it is recommended to eradicate H. pylori infection in all patients who require long-term PPI therapy to stop the pro-inflammatory stimulus and still reduce the risk of GC[72,73].
The density and composition of the bacterial population in the small intestinal tracts (i.e., the duodenum, jejunum and ileum) are influenced by several factors, including transit time, the presence of chemical factors, oxygen levels and the presence of antimicrobial substances that modulate bacterial growth[74]. Regarding the duodenal and jejunum, the predominant phyla are Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria and Fusobacteria. Facultative anaerobes such as Streptococcus, Haemophilus, Escherichia, Actinomyces and obligate anaerobes such as Veillonella, Prevotella and Fusobacterium are the most abundant genera[75-77]. Despite several sampling techniques having been used, the human ileal microbiota remains poorly characterized[78]. Previously, evidence based on biopsies collected by retrograde colonoscopy showed that the major phylogenetic groups are similar between the distal ileum and rectum[79,80]. In contrast, a recent study on samples collected surgically revealed profound differences between ileal and colonic microbiota, suggesting that the microbiota of the distal ileum appears to be constituted mainly by facultative anaerobic species within the Bacilli class (e.g., Streptococcaceae, Lactobacillacae, Aerococcaceae, and Carnobacteriaceae) and not by strict anaerobic species from the Clostridia class. However, it is likely that these results are not completely repre-sentative of normal ileal flora due to several influencing factors, such as the high age of the patients studied, comorbidity and antibiotic use[78].
Chronic treatment with PPIs strongly impacts small intestine microbiota and, in particular, causes small intestinal bacterial overgrowth (SIBO), likely due to the loss of the gastric acid defensive barrier[81-83]. SIBO is a condition defined by the presence of more than 105 bacteria per ml of upper gut aspirate and characterized mostly by weight loss, diarrhea, bloating and malabsorption[84]. In jejunal samples of SIBO patients, an overgrowth of microaerophilic microorganisms such as Streptococcus, Staphylococcus, Escherichia, and Klebsiella and anaerobic bacteria such as Bacteroides, Lactobacillus, Veillonella and Clostridium was found[85]. Likely, the increased production of toxic agents such as ammonia, D-lactate, endogenous bacterial peptidoglycans, serum endotoxin and bacterial compounds stimulates the secretion of proin-flammatory cytokines, causing symptoms to develop and the malabsorption of fat and lipophilic vitamins by the deconjugation of bile acids to occur[86,87].
PPI-induced dysbiosis may represent a risk factor for hepatic encephalopathy (HE) and spontaneous bacterial peritonitis (SBP) in cirrhotic patients[88-90]. In such patients, the development of SIBO is prompted by intestinal dysmotility and the alteration of mucosal barrier integrity, facilitating the spread of pathogens and bacterial metabolism products, such as nitrogenous substances and toxins. This spread occurs through the circulatory and lymphatic systems and results in a plausibly increased risk of SBP, HE and more generally life-threatening infections[91,92].
It is noteworthy that some of the microbial changes caused by PPIs are the same as the alterations already present in patients with cirrhosis and especially in patients with decompensated cirrhosis including the relative increase of potentially pathogenic bacteria such as Staphylococcaeae, Enterobacteriaceae and Enterococcaceae[93]. This dysbiosis has also been shown to be related to the occurrence of HE and SBP, implying a poor prognosis and disease progression. For this reason, the use of minimally absorbed antibiotics, such as rifaximin, and prebiotics, such as lactulose, represents the cornerstone of treatment for HE[94]. Therefore, in patients with liver diseases, regardless of the severity of the underlying hepatopathy, PPIs may increase the risk of complications and should be administered only in the presence of a specific therapeutic indication.
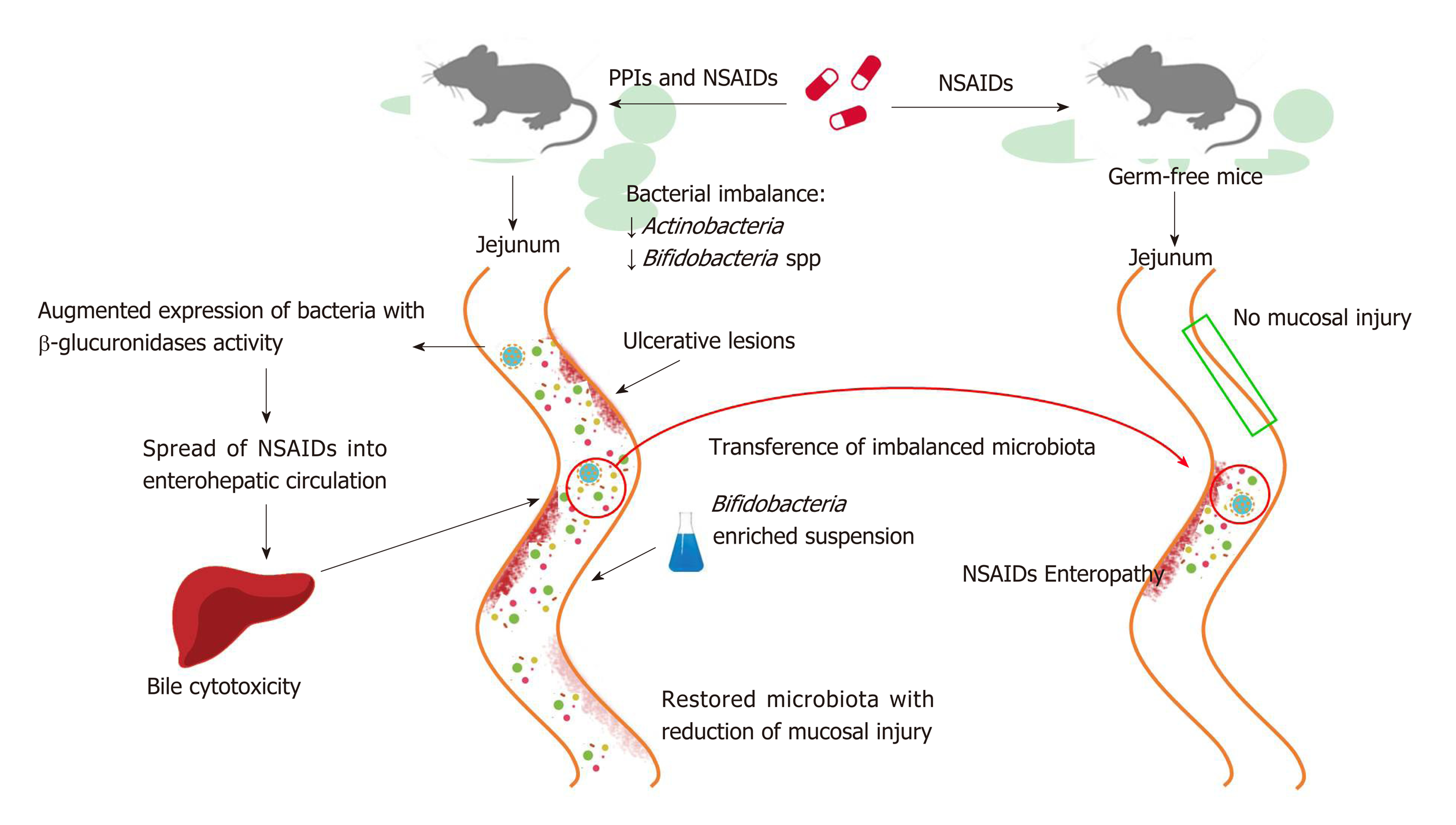
PPIs have also been reported to exacerbate the mucosal damage caused by non-steroidal anti-inflammatory drugs (NSAIDs) in the distal portion of the small bowel to the ligament of Treitz[95,96], which stands in contrast to the protective effects of PPIs on NSAIDs-induced upper GI mucosal injury[97]. Even if the exact mechanism by which it occurs is not clear, bacterial imbalance can play an important role and, as such, has been investigated in a number of studies conducted in murine models (Figure 2). In rats, a PPI-driven significant reduction of Actinobacteria and Bifidobacteria spp. in the jejunum was shown to exacerbate NSAID-induced enteropathy[98]. Moreover, PPIs augmented the expression of bacteria with beta-glucuronidases activity, and this microbial imbalance could promote the spread of NSAIDs into enterohepatic circulation, increasing bile cytotoxicity and subsequently causing ulcerative lesions[99,100]. The role of dysbiosis in mucosal injuries was further supported by the beneficial effects of the co-administration of a Bifidobacteria-enriched commensal bacteria suspension, which was able to reduce mucosal damage[98]. Moreover, germ-free mice are less susceptible to intestinal lesions induced by NSAIDs and it has been documented that NSAID-induced enteropathy is transferable via microbiota[98,101,102]. Based on these observations, confirmation of the role of dysbiosis in humans is needed.
The colon harbors the largest number of microbes per unit volume of the whole GI tract[103]. On fecal and biopsy samples, the four predominant phyla are Firmicutes and Bacteroidetes, followed by Actinobacteria and Proteobacteria[104,105], with a discrete inter-individual variability, especially regarding bacterial species and strains. The most represented bacterial clusters, called enterotypes, are constituted by a variation in the levels of one of these three genera: Bacteroides (enterotype 1), Prevotella (enterotype 2) and Ruminococcus (enterotype 3)[106,107]. PPIs by reducing gastric acid secretion can produce profound changes in the colonic microbiota, mainly characterized by a decrease in the abundance of commensal bacteria, which is associated with a reduction in microbial diversity and an increase of oral bacteria in the stool[108,109]. Therefore, it is reasonable to assume that PPI-driven dysbiosis significantly impacts host health.
PPIs can influence the onset of enteric infections, resulting in an increased risk of Clostridium difficile infection (CDI), as well as Salmonella, Campylobacter and diarrheagenic Escherichia coli (E. coli)[110-113]. Even if not fully clarified, it has been hypothesized that, in CDI, a reduction in alpha diversity and a decrease in the abundance of bacteria of the Ruminococcoceae associated with an increase in the Enterobacteriaceae, Enterococcoceae and Lactobacillaceae families observed during long-term PPI treatment could facilitate the onset of infection[109]. This likely occurs because the increase of Proteobacteria members promotes the induction and maintenance of a pro-inflammatory environment[114,115].
It is likely that PPI use predisposes patients to the development of IBS[116]. As previously stated, the long-term PPI use facilitates the induction of enteric infection, and, through secondary changes in microbiota composition, these drugs may influence gut–brain axis functions, prompting IBS onset[117,118]. That dysbiosis plays a role in IBS, and especially the strong association between gut infection and IBS, has been supported by several studies[119,120]. Indeed, it has been found that 10%-30% of patients who develop post-infectious IBS following an infectious gastroenteritis can be considered to be in a post-inflammatory condition, exacerbated by acute stress[121-124]. Moreover, the role of gut microbiota composition in the pathogenesis of IBS is sustained by the success of certain probiotics in IBS symptom amelioration[125,126]. The IBS dysbiosis mostly consists of a reduction in alpha diversity and an imbalance between microbial groups, represented by a scarce amount Bifidobacteria and Lactobacilli members and an increase in potentially pathogenic Enterobacteriaceae, such as E. coli[127,128]; this is similar to what has been observed in patients who have undergone chronic PPI therapy[109].
Regarding IBDs, various observations have led researchers to postulate that chronic PPI administration may have a negative effect on such conditions[129,130]. At present, the data on microbial imbalance during IBD have not been not fully elucidated. Some studies have documented a reduced abundance of Firmicutes and Bacteroidetes, while others have reported an increase[131,132]. Overall, an increase in Proteobacteria has almost always been described[133,134]. It is thought that during IBD, a reduction in protective bacteria occurs in parallel with an increase in pro-inflammatory bacteria. Both in Crohn’s disease (CD) and ulcerative colitis (UC), higher concentrations of E. coli have been observed, and particularly of a variant called adherent-invasive E. coli, which is able to colonize the ileal mucosa and is responsible for the early inflam-matory state[135,136]. Specifically, a reduction in anti-inflammatory bacteria, such as Faecalibacterium prausnitzii, Bifidobacterium adolescentis, and Dialister invisus, has been observed in CD sample analyses associated with unknown species of Clostridium (especially clusters IV and XIVa)[137]. In UC, a decrease in Akkermansia muciniphila, Roseburia and Faecalibacterium prausnitzii, along with an increase in Fusobacterium species, has been documented[138,139]. In the context of dysbiosis, PPIs may lead to short-term flare ups in the course of IBDs. This is likely because IBD patients are particularly susceptible to the development of bacterial superinfections, especially those caused by Clostridium difficile, Campylobacter, Salmonella, Shigella and Entamoeba histolytica, which represent harmful stimuli that can induce a relapse of the disease in a microenvironment that is already altered[140,141]. Moreover, the expansion of Proteobacteria could facilitate a mucosal immune response in genetically predisposed individuals, leading to the development and continuation of chronic intestinal inflammation[133,142,143].
Finally, our review focused on colorectal cancer (CRC). Nowadays, it is well known that the only microorganism that has a primary and direct role in the development of GI tumors is H. pylori. However, the intricate and overall action that the imbalance of gut microbiota can play in conditioning the colon microenvironment and favoring oncogenesis is emerging, with great interest on the part of researchers[144-146]. As previously stated, PPI therapy facilitates the presence of oral bacteria in the stool[108,109]. In this context, the role of F. nucleatum should be carefully analyzed. F. nucleatum is a commensal anaerobic bacterium of the oral cavity associated with periodontal disorders, and it has been found in large quantities in CRC[147]. Its pro-inflammatory activity in the intestinal mucosa has been well described, and its presence could be related to patient outcome[148,149]. This microorganism has two adhesion proteins, FapA and FadA, the latter of which mediates the invasion of the bacterium into the intestinal epithelium. The consequence of this invasion is the promotion of NF-κB signaling and the expression of several cytokines, such as IL-6, IL-8, IL-10, IL-18 and TNF-α. The net result of these changes is the creation of a pro-inflammatory milieu for tumor growth, favored by the FapA-mediated suppression of T cells’ cytotoxic activity[147,150]. Therefore, studies have specified that identifying the presence of this bacterium in PPI users and especially those with concomitant oral disorders is a necessity.
Last, chronic hypergastrinemia, typically present during PPI use, can promote the growth of malignant colonic epithelial cells, facilitating the deleterious sequencing adenoma-carcinoma[151-153]. Nevertheless, it is fundamental to highlight that many studies over the years have not found a direct correlation between PPI use in clinical practice and an increased risk of CRC[154-156].
In an era in which gut microbiota science enjoys much attention[157,158], it seems crucial to define which types of drugs have an impact on gut microbiota composition. The evidence indicates that PPIs which are widely used in gastroenterology clinical practice likely through their acid-antisecretory effects, are able to modify the host microbiota in each segment of the GI tract and can contribute to dysbiosis development; this dysbiosis can, in turn, facilitate the onset of certain GI disorders. Moreover, the gastric hypochlorhydria caused by PPIs favors the survival and migration of oral bacteria in lower areas of the GI tract, with a possible establishment of a pro-inflammatory microenvironment. Further prospective studies are necessary to define how the microbial changes due to PPIs impact human health. Moreover, therapeutic strategies, such as probiotic supplementation, could be a useful approach to prevent dysbiosis during PPI treatment; however, the validity of this observation remains to be seen. Currently, the use of PPIs is recommended only when strictly necessary due to their possible ability to induce dysbiosis.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bang CS, Dinç T, Filik L, Li Y, Ulaşoğlu C S-Editor: Yan JP L-Editor: A E-Editor: Zhang YL
| 1. | Scarpignato C, Pelosini I, Di Mario F. Acid suppression therapy: Where do we go from here? Dig Dis. 2006;24:11-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 76] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 2. | Bardou M, Toubouti Y, Benhaberou-Brun D, Rahme E, Barkun AN. Meta-analysis: Proton-pump inhibition in high-risk patients with acute peptic ulcer bleeding. Aliment Pharmacol Ther. 2005;21:677-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 83] [Article Influence: 4.2] [Reference Citation Analysis (1)] |
| 3. | DeVault KR, Castell DO; American College of Gastroenterology. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. Am J Gastroenterol. 2005;100:190-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 579] [Cited by in RCA: 526] [Article Influence: 26.3] [Reference Citation Analysis (2)] |
| 4. | Sharma VK, Leontiadis GI, Howden CW. Meta-analysis of randomized controlled trials comparing standard clinical doses of omeprazole and lansoprazole in erosive oesophagitis. Aliment Pharmacol Ther. 2001;15:227-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 5. | Suzuki H, Okada S, Hibi T. Proton-pump inhibitors for the treatment of functional dyspepsia. Therap Adv Gastroenterol. 2011;4:219-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 6. | Islam MM, Poly TN, Walther BA, Dubey NK, Anggraini Ningrum DN, Shabbir SA, Jack Li YC. Adverse outcomes of long-term use of proton pump inhibitors: A systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2018;30:1395-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 7. | de la Coba Ortiz C, Argüelles Arias F, Martín de Argila de Prados C, Júdez Gutiérrez J, Linares Rodríguez A, Ortega Alonso A, Rodríguez de Santiago E, Rodríguez-Téllez M, Vera Mendoza MI, Aguilera Castro L, Álvarez Sánchez Á, Andrade Bellido RJ, Bao Pérez F, Castro Fernández M, Giganto Tomé F. Proton-pump inhibitors adverse effects: A review of the evidence and position statement by the Sociedad Española de Patología Digestiva. Rev Esp Enferm Dig. 2016;108:207-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 8. | Eom CS, Jeon CY, Lim JW, Cho EG, Park SM, Lee KS. Use of acid-suppressive drugs and risk of pneumonia: A systematic review and meta-analysis. CMAJ. 2011;183:310-319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 305] [Cited by in RCA: 277] [Article Influence: 19.8] [Reference Citation Analysis (1)] |
| 9. | Tsuda A, Suda W, Morita H, Takanashi K, Takagi A, Koga Y, Hattori M. Influence of Proton-Pump Inhibitors on the Luminal Microbiota in the Gastrointestinal Tract. Clin Transl Gastroenterol. 2015;6:e89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 133] [Article Influence: 13.3] [Reference Citation Analysis (1)] |
| 10. | Freedberg DE, Toussaint NC, Chen SP, Ratner AJ, Whittier S, Wang TC, Wang HH, Abrams JA. Proton Pump Inhibitors Alter Specific Taxa in the Human Gastrointestinal Microbiome: A Crossover Trial. Gastroenterology. 2015;149:883-5.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 238] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 11. | Takagi T, Naito Y, Inoue R, Kashiwagi S, Uchiyama K, Mizushima K, Tsuchiya S, Okayama T, Dohi O, Yoshida N, Kamada K, Ishikawa T, Handa O, Konishi H, Okuda K, Tsujimoto Y, Ohnogi H, Itoh Y. The influence of long-term use of proton pump inhibitors on the gut microbiota: An age-sex-matched case-control study. J Clin Biochem Nutr. 2018;62:100-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 12. | Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Nageshwar Reddy D. Role of the normal gut microbiota. World J Gastroenterol. 2015;21:8787-8803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1421] [Cited by in RCA: 1837] [Article Influence: 183.7] [Reference Citation Analysis (58)] |
| 13. | Gerritsen J, Smidt H, Rijkers GT, de Vos WM. Intestinal microbiota in human health and disease: The impact of probiotics. Genes Nutr. 2011;6:209-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 492] [Cited by in RCA: 466] [Article Influence: 33.3] [Reference Citation Analysis (1)] |
| 14. | Belizário JE, Faintuch J, Garay-Malpartida M. Gut Microbiome Dysbiosis and Immunometabolism: New Frontiers for Treatment of Metabolic Diseases. Mediators Inflamm. 2018;2018:2037838. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 185] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 15. | DeGruttola AK, Low D, Mizoguchi A, Mizoguchi E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm Bowel Dis. 2016;22:1137-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 657] [Article Influence: 73.0] [Reference Citation Analysis (0)] |
| 16. | Carding S, Verbeke K, Vipond DT, Corfe BM, Owen LJ. Dysbiosis of the gut microbiota in disease. Microb Ecol Health Dis. 2015;26:26191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 514] [Cited by in RCA: 800] [Article Influence: 80.0] [Reference Citation Analysis (0)] |
| 17. | Freedberg DE, Lebwohl B, Abrams JA. The impact of proton pump inhibitors on the human gastrointestinal microbiome. Clin Lab Med. 2014;34:771-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 123] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 18. | Singh A, Cresci GA, Kirby DF. Proton Pump Inhibitors: Risks and Rewards and Emerging Consequences to the Gut Microbiome. Nutr Clin Pract. 2018;33:614-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 19. | Minalyan A, Gabrielyan L, Scott D, Jacobs J, Pisegna JR. The Gastric and Intestinal Microbiome: Role of Proton Pump Inhibitors. Curr Gastroenterol Rep. 2017;19:42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 20. | Imhann F, Vich Vila A, Bonder MJ, Lopez Manosalva AG, Koonen DPY, Fu J, Wijmenga C, Zhernakova A, Weersma RK. The influence of proton pump inhibitors and other commonly used medication on the gut microbiota. Gut Microbes. 2017;8:351-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 132] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 21. | Zaura E, Nicu EA, Krom BP, Keijser BJ. Acquiring and maintaining a normal oral microbiome: Current perspective. Front Cell Infect Microbiol. 2014;4:85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 164] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 22. | Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005;43:5721-5732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1867] [Cited by in RCA: 2021] [Article Influence: 101.1] [Reference Citation Analysis (0)] |
| 23. | Arweiler NB, Netuschil L. The Oral Microbiota. Adv Exp Med Biol. 2016;902:45-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 157] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 24. | Andersson AF, Lindberg M, Jakobsson H, Bäckhed F, Nyrén P, Engstrand L. Comparative analysis of human gut microbiota by barcoded pyrosequencing. PLoS One. 2008;3:e2836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 743] [Cited by in RCA: 741] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 25. | Segata N, Haake SK, Mannon P, Lemon KP, Waldron L, Gevers D, Huttenhower C, Izard J. Composition of the adult digestive tract bacterial microbiome based on seven mouth surfaces, tonsils, throat and stool samples. Genome Biol. 2012;13:R42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 607] [Cited by in RCA: 763] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 26. | Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner AC, Yu WH, Lakshmanan A, Wade WG. The human oral microbiome. J Bacteriol. 2010;192:5002-5017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2580] [Cited by in RCA: 2247] [Article Influence: 149.8] [Reference Citation Analysis (0)] |
| 27. | Zhang Y, Wang X, Li H, Ni C, Du Z, Yan F. Human oral microbiota and its modulation for oral health. Biomed Pharmacother. 2018;99:883-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 310] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 28. | Han YW, Wang X. Mobile microbiome: Oral bacteria in extra-oral infections and inflammation. J Dent Res. 2013;92:485-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 353] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 29. | Li X, Kolltveit KM, Tronstad L, Olsen I. Systemic diseases caused by oral infection. Clin Microbiol Rev. 2000;13:547-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 509] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 30. | Fourie NH, Wang D, Abey SK, Sherwin LB, Joseph PV, Rahim-Williams B, Ferguson EG, Henderson WA. The microbiome of the oral mucosa in irritable bowel syndrome. Gut Microbes. 2016;7:286-301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 31. | Lucas López R, Grande Burgos MJ, Gálvez A, Pérez Pulido R. The human gastrointestinal tract and oral microbiota in inflammatory bowel disease: A state of the science review. APMIS. 2017;125:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 32. | Ahn J, Chen CY, Hayes RB. Oral microbiome and oral and gastrointestinal cancer risk. Cancer Causes Control. 2012;23:399-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 179] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 33. | Karpiński TM. Role of Oral Microbiota in Cancer Development. Microorganisms. 2019;7:pii: E20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 271] [Cited by in RCA: 243] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 34. | Mishiro T, Oka K, Kuroki Y, Takahashi M, Tatsumi K, Saitoh T, Tobita H, Ishimura N, Sato S, Ishihara S, Sekine J, Wada K, Kinoshita Y. Oral microbiome alterations of healthy volunteers with proton pump inhibitor. J Gastroenterol Hepatol. 2018;33:1059-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 35. | Pei Z, Bini EJ, Yang L, Zhou M, Francois F, Blaser MJ. Bacterial biota in the human distal esophagus. Proc Natl Acad Sci USA. 2004;101:4250-4255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 338] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 36. | Abrams JA. The microbiome as a potential biomarker for oesophageal adenocarcinoma. Lancet Gastroenterol Hepatol. 2017;2:4-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 37. | Yang L, Francois F, Pei Z. Molecular pathways: Pathogenesis and clinical implications of microbiome alteration in esophagitis and Barrett esophagus. Clin Cancer Res. 2012;18:2138-2144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 135] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 38. | Di Pilato V, Freschi G, Ringressi MN, Pallecchi L, Rossolini GM, Bechi P. The esophageal microbiota in health and disease. Ann N Y Acad Sci. 2016;1381:21-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 39. | Lau WF, Wong J, Lam KH, Ong GB. Oesophageal microbial flora in carcinoma of the oesophagus. Aust N Z J Surg. 1981;51:52-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 40. | Baba Y, Iwatsuki M, Yoshida N, Watanabe M, Baba H. Review of the gut microbiome and esophageal cancer: Pathogenesis and potential clinical implications. Ann Gastroenterol Surg. 2017;1:99-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 91] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 41. | Cook MB, Corley DA, Murray LJ, Liao LM, Kamangar F, Ye W, Gammon MD, Risch HA, Casson AG, Freedman ND, Chow WH, Wu AH, Bernstein L, Nyrén O, Pandeya N, Whiteman DC, Vaughan TL. Gastroesophageal reflux in relation to adenocarcinomas of the esophagus: A pooled analysis from the Barrett's and Esophageal Adenocarcinoma Consortium (BEACON). PLoS One. 2014;9:e103508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 114] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 42. | Macfarlane S, Furrie E, Macfarlane GT, Dillon JF. Microbial colonization of the upper gastrointestinal tract in patients with Barrett's esophagus. Clin Infect Dis. 2007;45:29-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 121] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 43. | Blackett KL, Siddhi SS, Cleary S, Steed H, Miller MH, Macfarlane S, Macfarlane GT, Dillon JF. Oesophageal bacterial biofilm changes in gastro-oesophageal reflux disease, Barrett's and oesophageal carcinoma: Association or causality? Aliment Pharmacol Ther. 2013;37:1084-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 157] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 44. | Kaakoush NO, Castaño-Rodríguez N, Man SM, Mitchell HM. Is Campylobacter to esophageal adenocarcinoma as Helicobacter is to gastric adenocarcinoma? Trends Microbiol. 2015;23:455-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (1)] |
| 45. | Yamamura K, Baba Y, Nakagawa S, Mima K, Miyake K, Nakamura K, Sawayama H, Kinoshita K, Ishimoto T, Iwatsuki M, Sakamoto Y, Yamashita Y, Yoshida N, Watanabe M, Baba H. Human Microbiome Fusobacterium Nucleatum in Esophageal Cancer Tissue Is Associated with Prognosis. Clin Cancer Res. 2016;22:5574-5581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 323] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 46. | Elliott DRF, Walker AW, O'Donovan M, Parkhill J, Fitzgerald RC. A non-endoscopic device to sample the oesophageal microbiota: A case-control study. Lancet Gastroenterol Hepatol. 2017;2:32-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 121] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 47. | Amir I, Konikoff FM, Oppenheim M, Gophna U, Half EE. Gastric microbiota is altered in oesophagitis and Barrett's oesophagus and further modified by proton pump inhibitors. Environ Microbiol. 2014;16:2905-2914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 144] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 48. | Brusselaers N, Engstrand L, Lagergren J. Maintenance proton pump inhibition therapy and risk of oesophageal cancer. Cancer Epidemiol. 2018;53:172-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 49. | Shaheen NJ, Falk GW, Iyer PG, Gerson LB; American College of Gastroenterology. ACG Clinical Guideline: Diagnosis and Management of Barrett's Esophagus. Am J Gastroenterol. 2016;111:30-50; quiz 51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 990] [Cited by in RCA: 1058] [Article Influence: 117.6] [Reference Citation Analysis (0)] |
| 50. | de Jonge PJ, Steyerberg EW, Kuipers EJ, Honkoop P, Wolters LM, Kerkhof M, van Dekken H, Siersema PD. Risk factors for the development of esophageal adenocarcinoma in Barrett's esophagus. Am J Gastroenterol. 2006;101:1421-1429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 76] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 51. | Nguyen DM, El-Serag HB, Henderson L, Stein D, Bhattacharyya A, Sampliner RE. Medication usage and the risk of neoplasia in patients with Barrett's esophagus. Clin Gastroenterol Hepatol. 2009;7:1299-1304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 120] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 52. | Singh S, Garg SK, Singh PP, Iyer PG, El-Serag HB. Acid-suppressive medications and risk of oesophageal adenocarcinoma in patients with Barrett's oesophagus: A systematic review and meta-analysis. Gut. 2014;63:1229-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 199] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 53. | Neto AG, Whitaker A, Pei Z. Microbiome and potential targets for chemoprevention of esophageal adenocarcinoma. Semin Oncol. 2016;43:86-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 54. | Bruno G, Rocco G, Zaccari P, Porowska B, Mascellino MT, Severi C. Helicobacter pylori Infection and Gastric Dysbiosis: Can Probiotics Administration Be Useful to Treat This Condition? Can J Infect Dis Med Microbiol. 2018;2018:6237239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 55. | Scarpignato C, Gatta L, Zullo A, Blandizzi C; SIF-AIGO-FIMMG Group; Italian Society of Pharmacology, the Italian Association of Hospital Gastroenterologists, and the Italian Federation of General Practitioners. Effective and safe proton pump inhibitor therapy in acid-related diseases - A position paper addressing benefits and potential harms of acid suppression. BMC Med. 2016;14:179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 275] [Cited by in RCA: 294] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 56. | Huang JQ, Hunt RH. Pharmacological and pharmacodynamic essentials of H(2)-receptor antagonists and proton pump inhibitors for the practising physician. Best Pract Res Clin Gastroenterol. 2001;15:355-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 78] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 57. | Wandall JH. Effects of omeprazole on neutrophil chemotaxis, super oxide production, degranulation, and translocation of cytochrome b-245. Gut. 1992;33:617-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 144] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 58. | Parsons BN, Ijaz UZ, D'Amore R, Burkitt MD, Eccles R, Lenzi L, Duckworth CA, Moore AR, Tiszlavicz L, Varro A, Hall N, Pritchard DM. Comparison of the human gastric microbiota in hypochlorhydric states arising as a result of Helicobacter pylori-induced atrophic gastritis, autoimmune atrophic gastritis and proton pump inhibitor use. PLoS Pathog. 2017;13:e1006653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 149] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 59. | Paroni Sterbini F, Palladini A, Masucci L, Cannistraci CV, Pastorino R, Ianiro G, Bugli F, Martini C, Ricciardi W, Gasbarrini A, Sanguinetti M, Cammarota G, Posteraro B. Effects of Proton Pump Inhibitors on the Gastric Mucosa-Associated Microbiota in Dyspeptic Patients. Appl Environ Microbiol. 2016;82:6633-6644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 60. | Ferreira RM, Pereira-Marques J, Pinto-Ribeiro I, Costa JL, Carneiro F, Machado JC, Figueiredo C. Gastric microbial community profiling reveals a dysbiotic cancer-associated microbiota. Gut. 2018;67:226-236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 341] [Cited by in RCA: 476] [Article Influence: 68.0] [Reference Citation Analysis (1)] |
| 61. | Coker OO, Dai Z, Nie Y, Zhao G, Cao L, Nakatsu G, Wu WK, Wong SH, Chen Z, Sung JJY, Yu J. Mucosal microbiome dysbiosis in gastric carcinogenesis. Gut. 2018;67:1024-1032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 304] [Cited by in RCA: 476] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 62. | Ahn JS, Eom CS, Jeon CY, Park SM. Acid suppressive drugs and gastric cancer: A meta-analysis of observational studies. World J Gastroenterol. 2013;19:2560-2568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 79] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 63. | Brusselaers N, Wahlin K, Engstrand L, Lagergren J. Maintenance therapy with proton pump inhibitors and risk of gastric cancer: A nationwide population-based cohort study in Sweden. BMJ Open. 2017;7:e017739. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 150] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 64. | Cheung KS, Chan EW, Wong AYS, Chen L, Wong ICK, Leung WK. Long-term proton pump inhibitors and risk of gastric cancer development after treatment for Helicobacter pylori: A population-based study. Gut. 2018;67:28-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 339] [Article Influence: 48.4] [Reference Citation Analysis (1)] |
| 65. | Song H, Zhu J, Lu D. Long-term proton pump inhibitor (PPI) use and the development of gastric pre-malignant lesions. Cochrane Database Syst Rev. 2014;CD010623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 66. | Eslami L, Nasseri-Moghaddam S. Meta-analyses: Does long-term PPI use increase the risk of gastric premalignant lesions? Arch Iran Med. 2013;16:449-458. [PubMed] |
| 67. | Kusters JG, van Vliet AH, Kuipers EJ. Pathogenesis of Helicobacter pylori infection. Clin Microbiol Rev. 2006;19:449-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1446] [Cited by in RCA: 1529] [Article Influence: 80.5] [Reference Citation Analysis (1)] |
| 68. | Graham DY. History of Helicobacter pylori, duodenal ulcer, gastric ulcer and gastric cancer. World J Gastroenterol. 2014;20:5191-5204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 202] [Cited by in RCA: 229] [Article Influence: 20.8] [Reference Citation Analysis (3)] |
| 69. | Sung J, Kim N, Lee J, Hwang YJ, Kim HW, Chung JW, Kim JW, Lee DH. Associations among Gastric Juice pH, Atrophic Gastritis, Intestinal Metaplasia and Helicobacter pylori Infection. Gut Liver. 2018;12:158-164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 70. | Correa P. Helicobacter pylori and gastric carcinogenesis. Am J Surg Pathol. 1995;19 Suppl 1:S37-S43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 151] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 71. | Malfertheiner P, Kandulski A, Venerito M. Proton-pump inhibitors: Understanding the complications and risks. Nat Rev Gastroenterol Hepatol. 2017;14:697-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 189] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 72. | Malfertheiner P, Megraud F, O'Morain CA, Gisbert JP, Kuipers EJ, Axon AT, Bazzoli F, Gasbarrini A, Atherton J, Graham DY, Hunt R, Moayyedi P, Rokkas T, Rugge M, Selgrad M, Suerbaum S, Sugano K, El-Omar EM; European Helicobacter and Microbiota Study Group and Consensus panel. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 2017;66:6-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2220] [Cited by in RCA: 1983] [Article Influence: 247.9] [Reference Citation Analysis (1)] |
| 73. | Ford AC, Forman D, Hunt RH, Yuan Y, Moayyedi P. Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: Systematic review and meta-analysis of randomised controlled trials. BMJ. 2014;348:g3174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 456] [Cited by in RCA: 442] [Article Influence: 40.2] [Reference Citation Analysis (1)] |
| 74. | Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J. 2017;474:1823-1836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1710] [Cited by in RCA: 2056] [Article Influence: 257.0] [Reference Citation Analysis (7)] |
| 75. | Sundin OH, Mendoza-Ladd A, Zeng M, Diaz-Arévalo D, Morales E, Fagan BM, Ordoñez J, Velez P, Antony N, McCallum RW. The human jejunum has an endogenous microbiota that differs from those in the oral cavity and colon. BMC Microbiol. 2017;17:160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 76. | Wacklin P, Kaukinen K, Tuovinen E, Collin P, Lindfors K, Partanen J, Mäki M, Mättö J. The duodenal microbiota composition of adult celiac disease patients is associated with the clinical manifestation of the disease. Inflamm Bowel Dis. 2013;19:934-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 144] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 77. | Zhong L, Shanahan ER, Raj A, Koloski NA, Fletcher L, Morrison M, Walker MM, Talley NJ, Holtmann G. Dyspepsia and the microbiome: Time to focus on the small intestine. Gut. 2017;66:1168-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 141] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 78. | Villmones HC, Haug ES, Ulvestad E, Grude N, Stenstad T, Halland A, Kommedal Ø. Species Level Description of the Human Ileal Bacterial Microbiota. Sci Rep. 2018;8:4736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 79. | Wang M, Ahrné S, Jeppsson B, Molin G. Comparison of bacterial diversity along the human intestinal tract by direct cloning and sequencing of 16S rRNA genes. FEMS Microbiol Ecol. 2005;54:219-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 239] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 80. | Dave M, Johnson LA, Walk ST, Young VB, Stidham RW, Chaudhary MN, Funnell J, Higgins PD. A randomised trial of sheathed versus standard forceps for obtaining uncontaminated biopsy specimens of microbiota from the terminal ileum. Gut. 2011;60:1043-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 81. | Fujimori S. What are the effects of proton pump inhibitors on the small intestine? World J Gastroenterol. 2015;21:6817-6819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 82. | Spiegel BM, Chey WD, Chang L. Bacterial overgrowth and irritable bowel syndrome: Unifying hypothesis or a spurious consequence of proton pump inhibitors? Am J Gastroenterol. 2008;103:2972-2976. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 83. | Lo WK, Chan WW. Proton pump inhibitor use and the risk of small intestinal bacterial overgrowth: A meta-analysis. Clin Gastroenterol Hepatol. 2013;11:483-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 245] [Article Influence: 20.4] [Reference Citation Analysis (1)] |
| 84. | Liang S, Xu L, Zhang D, Wu Z. Effect of probiotics on small intestinal bacterial overgrowth in patients with gastric and colorectal cancer. Turk J Gastroenterol. 2016;27:227-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 85. | Bouhnik Y, Alain S, Attar A, Flourié B, Raskine L, Sanson-Le Pors MJ, Rambaud JC. Bacterial populations contaminating the upper gut in patients with small intestinal bacterial overgrowth syndrome. Scand J Gastroenterol. 2008;43 Suppl 9:1030-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 120] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 86. | Bures J, Cyrany J, Kohoutova D, Förstl M, Rejchrt S, Kvetina J, Vorisek V, Kopacova M. Small intestinal bacterial overgrowth syndrome. World J Gastroenterol. 2010;16:2978-2990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 383] [Cited by in RCA: 364] [Article Influence: 24.3] [Reference Citation Analysis (4)] |
| 87. | Fan X, Sellin JH. Review article: Small intestinal bacterial overgrowth, bile acid malabsorption and gluten intolerance as possible causes of chronic watery diarrhoea. Aliment Pharmacol Ther. 2009;29:1069-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 88. | Nardelli S, Gioia S, Ridola L, Farcomeni A, Merli M, Riggio O. Proton Pump Inhibitors Are Associated With Minimal and Overt Hepatic Encephalopathy and Increased Mortality in Patients With Cirrhosis. Hepatology. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 89. | Zhu J, Qi X, Yu H, Yoshida EM, Mendez-Sanchez N, Zhang X, Wang R, Deng H, Li J, Han D, Guo X. Association of proton pump inhibitors with the risk of hepatic encephalopathy during hospitalization for liver cirrhosis. United European Gastroenterol J. 2018;6:1179-1187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 90. | Dam G, Vilstrup H, Watson H, Jepsen P. Proton pump inhibitors as a risk factor for hepatic encephalopathy and spontaneous bacterial peritonitis in patients with cirrhosis with ascites. Hepatology. 2016;64:1265-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 143] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 91. | Sturgeon JP, Shawcross DL. Recent insights into the pathogenesis of hepatic encephalopathy and treatments. Expert Rev Gastroenterol Hepatol. 2014;8:83-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 92. | Gupta A, Dhiman RK, Kumari S, Rana S, Agarwal R, Duseja A, Chawla Y. Role of small intestinal bacterial overgrowth and delayed gastrointestinal transit time in cirrhotic patients with minimal hepatic encephalopathy. J Hepatol. 2010;53:849-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 152] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 93. | Bajaj JS, Heuman DM, Hylemon PB, Sanyal AJ, White MB, Monteith P, Noble NA, Unser AB, Daita K, Fisher AR, Sikaroodi M, Gillevet PM. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol. 2014;60:940-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 836] [Article Influence: 76.0] [Reference Citation Analysis (0)] |
| 94. | Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, Weissenborn K, Wong P. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1583] [Cited by in RCA: 1408] [Article Influence: 128.0] [Reference Citation Analysis (1)] |
| 95. | Marlicz W, Loniewski I, Grimes DS, Quigley EM. Nonsteroidal anti-inflammatory drugs, proton pump inhibitors, and gastrointestinal injury: Contrasting interactions in the stomach and small intestine. Mayo Clin Proc. 2014;89:1699-1709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 96. | Gwee KA, Goh V, Lima G, Setia S. Coprescribing proton-pump inhibitors with nonsteroidal anti-inflammatory drugs: Risks versus benefits. J Pain Res. 2018;11:361-374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 97. | Scheiman JM. The use of proton pump inhibitors in treating and preventing NSAID-induced mucosal damage. Arthritis Res Ther. 2013;15 Suppl 3:S5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 98. | Wallace JL, Syer S, Denou E, de Palma G, Vong L, McKnight W, Jury J, Bolla M, Bercik P, Collins SM, Verdu E, Ongini E. Proton pump inhibitors exacerbate NSAID-induced small intestinal injury by inducing dysbiosis. Gastroenterology. 2011;141:1314-1322, 1322.e1-1322.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 342] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 99. | Blackler RW, Motta JP, Manko A, Workentine M, Bercik P, Surette MG, Wallace JL. Hydrogen sulphide protects against NSAID-enteropathy through modulation of bile and the microbiota. Br J Pharmacol. 2015;172:992-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 100. | Syer SD, Blackler RW, Martin R, de Palma G, Rossi L, Verdu E, Bercik P, Surette MG, Aucouturier A, Langella P, Wallace JL. NSAID enteropathy and bacteria: A complicated relationship. J Gastroenterol. 2015;50:387-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 101. | Uejima M, Kinouchi T, Kataoka K, Hiraoka I, Ohnishi Y. Role of intestinal bacteria in ileal ulcer formation in rats treated with a nonsteroidal antiinflammatory drug. Microbiol Immunol. 1996;40:553-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 82] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 102. | Robert A, Asano T. Resistance of germfree rats to indomethacin-induced intestinal lesions. Prostaglandins. 1977;14:333-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 231] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 103. | Sender R, Fuchs S, Milo R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016;14:e1002533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2328] [Cited by in RCA: 2919] [Article Influence: 324.3] [Reference Citation Analysis (0)] |
| 104. | Khanna S, Tosh PK. A clinician's primer on the role of the microbiome in human health and disease. Mayo Clin Proc. 2014;89:107-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 139] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 105. | Watt E, Gemmell MR, Berry S, Glaire M, Farquharson F, Louis P, Murray GI, El-Omar E, Hold GL. Extending colonic mucosal microbiome analysis-assessment of colonic lavage as a proxy for endoscopic colonic biopsies. Microbiome. 2016;4:61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 106. | Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto JM, Bertalan M, Borruel N, Casellas F, Fernandez L, Gautier L, Hansen T, Hattori M, Hayashi T, Kleerebezem M, Kurokawa K, Leclerc M, Levenez F, Manichanh C, Nielsen HB, Nielsen T, Pons N, Poulain J, Qin J, Sicheritz-Ponten T, Tims S, Torrents D, Ugarte E, Zoetendal EG, Wang J, Guarner F, Pedersen O, de Vos WM, Brunak S, Doré J; MetaHIT Consortium, Antolín M, Artiguenave F, Blottiere HM, Almeida M, Brechot C, Cara C, Chervaux C, Cultrone A, Delorme C, Denariaz G, Dervyn R, Foerstner KU, Friss C, van de Guchte M, Guedon E, Haimet F, Huber W, van Hylckama-Vlieg J, Jamet A, Juste C, Kaci G, Knol J, Lakhdari O, Layec S, Le Roux K, Maguin E, Mérieux A, Melo Minardi R, M'rini C, Muller J, Oozeer R, Parkhill J, Renault P, Rescigno M, Sanchez N, Sunagawa S, Torrejon A, Turner K, Vandemeulebrouck G, Varela E, Winogradsky Y, Zeller G, Weissenbach J, Ehrlich SD, Bork P. Enterotypes of the human gut microbiome. Nature. 2011;473:174-180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5822] [Cited by in RCA: 5032] [Article Influence: 359.4] [Reference Citation Analysis (2)] |
| 107. | Shanahan F. The colonic microbiota and colonic disease. Curr Gastroenterol Rep. 2012;14:446-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 108. | Jackson MA, Goodrich JK, Maxan ME, Freedberg DE, Abrams JA, Poole AC, Sutter JL, Welter D, Ley RE, Bell JT, Spector TD, Steves CJ. Proton pump inhibitors alter the composition of the gut microbiota. Gut. 2016;65:749-756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 500] [Cited by in RCA: 626] [Article Influence: 69.6] [Reference Citation Analysis (0)] |
| 109. | Imhann F, Bonder MJ, Vich Vila A, Fu J, Mujagic Z, Vork L, Tigchelaar EF, Jankipersadsing SA, Cenit MC, Harmsen HJ, Dijkstra G, Franke L, Xavier RJ, Jonkers D, Wijmenga C, Weersma RK, Zhernakova A. Proton pump inhibitors affect the gut microbiome. Gut. 2016;65:740-748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 780] [Cited by in RCA: 933] [Article Influence: 103.7] [Reference Citation Analysis (0)] |
| 110. | Janarthanan S, Ditah I, Adler DG, Ehrinpreis MN. Clostridium difficile-associated diarrhea and proton pump inhibitor therapy: A meta-analysis. Am J Gastroenterol. 2012;107:1001-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 366] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 111. | Trifan A, Stanciu C, Girleanu I, Stoica OC, Singeap AM, Maxim R, Chiriac SA, Ciobica A, Boiculese L. Proton pump inhibitors therapy and risk of Clostridium difficile infection: Systematic review and meta-analysis. World J Gastroenterol. 2017;23:6500-6515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 202] [Cited by in RCA: 188] [Article Influence: 23.5] [Reference Citation Analysis (5)] |
| 112. | Leonard J, Marshall JK, Moayyedi P. Systematic review of the risk of enteric infection in patients taking acid suppression. Am J Gastroenterol. 2007;102:2047-56; quiz 2057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 423] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 113. | Bavishi C, Dupont HL. Systematic review: The use of proton pump inhibitors and increased susceptibility to enteric infection. Aliment Pharmacol Ther. 2011;34:1269-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 325] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 114. | Rizzatti G, Lopetuso LR, Gibiino G, Binda C, Gasbarrini A. Proteobacteria: A Common Factor in Human Diseases. Biomed Res Int. 2017;2017:9351507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 388] [Cited by in RCA: 790] [Article Influence: 98.8] [Reference Citation Analysis (0)] |
| 115. | Hakansson A, Molin G. Gut microbiota and inflammation. Nutrients. 2011;3:637-682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 272] [Cited by in RCA: 321] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 116. | Schmulson MJ, Frati-Munari AC. Bowel symptoms in patients that receive proton pump inhibitors. Results of a multicenter survey in Mexico. Rev Gastroenterol Mex. 2019;84:44-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 117. | Distrutti E, Monaldi L, Ricci P, Fiorucci S. Gut microbiota role in irritable bowel syndrome: New therapeutic strategies. World J Gastroenterol. 2016;22:2219-2241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 210] [Cited by in RCA: 206] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 118. | Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. 2015;28:203-209. [PubMed] |
| 119. | Thabane M, Kottachchi DT, Marshall JK. Systematic review and meta-analysis: The incidence and prognosis of post-infectious irritable bowel syndrome. Aliment Pharmacol Ther. 2007;26:535-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 311] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 120. | Marshall JK, Thabane M, Garg AX, Clark WF, Salvadori M, Collins SM; Walkerton Health Study Investigators. Incidence and epidemiology of irritable bowel syndrome after a large waterborne outbreak of bacterial dysentery. Gastroenterology. 2006;131:445-50; quiz 660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 228] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 121. | DuPont AW. Postinfectious irritable bowel syndrome. Clin Infect Dis. 2008;46:594-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 122. | Kanazawa M, Fukudo S. Relationship between infectious gastroenteritis and irritable bowel syndrome. Clin J Gastroenterol. 2014;7:14-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 123. | Thompson JR. Is irritable bowel syndrome an infectious disease? World J Gastroenterol. 2016;22:1331-1334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 124. | Kiank C, Taché Y, Larauche M. Stress-related modulation of inflammation in experimental models of bowel disease and post-infectious irritable bowel syndrome: Role of corticotropin-releasing factor receptors. Brain Behav Immun. 2010;24:41-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 125. | Moayyedi P, Ford AC, Talley NJ, Cremonini F, Foxx-Orenstein AE, Brandt LJ, Quigley EM. The efficacy of probiotics in the treatment of irritable bowel syndrome: A systematic review. Gut. 2010;59:325-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 452] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 126. | Aragon G, Graham DB, Borum M, Doman DB. Probiotic therapy for irritable bowel syndrome. Gastroenterol Hepatol (N Y). 2010;6:39-44. [PubMed] |
| 127. | Si JM, Yu YC, Fan YJ, Chen SJ. Intestinal microecology and quality of life in irritable bowel syndrome patients. World J Gastroenterol. 2004;10:1802-1805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 157] [Cited by in RCA: 152] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 128. | El-Salhy M. Recent developments in the pathophysiology of irritable bowel syndrome. World J Gastroenterol. 2015;21:7621-7636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 72] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 129. | Shah R, Richardson P, Yu H, Kramer J, Hou JK. Gastric Acid Suppression Is Associated with an Increased Risk of Adverse Outcomes in Inflammatory Bowel Disease. Digestion. 2017;95:188-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 130. | Juillerat P, Schneeweiss S, Cook EF, Ananthakrishnan AN, Mogun H, Korzenik JR. Drugs that inhibit gastric acid secretion may alter the course of inflammatory bowel disease. Aliment Pharmacol Ther. 2012;36:239-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 131. | Ahmed I, Roy BC, Khan SA, Septer S, Umar S. Microbiome, Metabolome and Inflammatory Bowel Disease. Microorganisms. 2016;4:pii: E20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 138] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 132. | Marchesi JR, Adams DH, Fava F, Hermes GD, Hirschfield GM, Hold G, Quraishi MN, Kinross J, Smidt H, Tuohy KM, Thomas LV, Zoetendal EG, Hart A. The gut microbiota and host health: A new clinical frontier. Gut. 2016;65:330-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1341] [Cited by in RCA: 1559] [Article Influence: 173.2] [Reference Citation Analysis (0)] |
| 133. | Mukhopadhya I, Hansen R, El-Omar EM, Hold GL. IBD-what role do Proteobacteria play? Nat Rev Gastroenterol Hepatol. 2012;9:219-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 574] [Article Influence: 44.2] [Reference Citation Analysis (1)] |
| 134. | Matsuoka K, Kanai T. The gut microbiota and inflammatory bowel disease. Semin Immunopathol. 2015;37:47-55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 407] [Cited by in RCA: 572] [Article Influence: 52.0] [Reference Citation Analysis (1)] |
| 135. | Darfeuille-Michaud A, Boudeau J, Bulois P, Neut C, Glasser AL, Barnich N, Bringer MA, Swidsinski A, Beaugerie L, Colombel JF. High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn's disease. Gastroenterology. 2004;127:412-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1075] [Cited by in RCA: 1123] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 136. | Sokol H, Lepage P, Seksik P, Doré J, Marteau P. Temperature gradient gel electrophoresis of fecal 16S rRNA reveals active Escherichia coli in the microbiota of patients with ulcerative colitis. J Clin Microbiol. 2006;44:3172-3177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 116] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 137. | Joossens M, Huys G, Cnockaert M, De Preter V, Verbeke K, Rutgeerts P, Vandamme P, Vermeire S. Dysbiosis of the faecal microbiota in patients with Crohn's disease and their unaffected relatives. Gut. 2011;60:631-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 692] [Cited by in RCA: 794] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 138. | Shen ZH, Zhu CX, Quan YS, Yang ZY, Wu S, Luo WW, Tan B, Wang XY. Relationship between intestinal microbiota and ulcerative colitis: Mechanisms and clinical application of probiotics and fecal microbiota transplantation. World J Gastroenterol. 2018;24:5-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 307] [Cited by in RCA: 459] [Article Influence: 65.6] [Reference Citation Analysis (6)] |
| 139. | Machiels K, Joossens M, Sabino J, De Preter V, Arijs I, Eeckhaut V, Ballet V, Claes K, Van Immerseel F, Verbeke K, Ferrante M, Verhaegen J, Rutgeerts P, Vermeire S. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut. 2014;63:1275-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1036] [Cited by in RCA: 1332] [Article Influence: 121.1] [Reference Citation Analysis (3)] |
| 140. | Mylonaki M, Langmead L, Pantes A, Johnson F, Rampton DS. Enteric infection in relapse of inflammatory bowel disease: Importance of microbiological examination of stool. Eur J Gastroenterol Hepatol. 2004;16:775-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 165] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 141. | Singh S, Graff LA, Bernstein CN. Do NSAIDs, antibiotics, infections, or stress trigger flares in IBD? Am J Gastroenterol. 2009;104:1298-313; quiz 1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 124] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 142. | Sartor RB. Pathogenesis and immune mechanisms of chronic inflammatory bowel diseases. Am J Gastroenterol. 1997;92:5S-11S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 143. | Cho JH. The genetics and immunopathogenesis of inflammatory bowel disease. Nat Rev Immunol. 2008;8:458-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 666] [Cited by in RCA: 688] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 144. | Jobin C. Colorectal cancer: Looking for answers in the microbiota. Cancer Discov. 2013;3:384-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 145. | Ahn J, Sinha R, Pei Z, Dominianni C, Wu J, Shi J, Goedert JJ, Hayes RB, Yang L. Human gut microbiome and risk for colorectal cancer. J Natl Cancer Inst. 2013;105:1907-1911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 566] [Cited by in RCA: 710] [Article Influence: 59.2] [Reference Citation Analysis (1)] |
| 146. | Raskov H, Burcharth J, Pommergaard HC. Linking Gut Microbiota to Colorectal Cancer. J Cancer. 2017;8:3378-3395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 101] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 147. | Shang FM, Liu HL. Fusobacterium nucleatum and colorectal cancer: A review. World J Gastrointest Oncol. 2018;10:71-81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 192] [Cited by in RCA: 195] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 148. | Flanagan L, Schmid J, Ebert M, Soucek P, Kunicka T, Liska V, Bruha J, Neary P, Dezeeuw N, Tommasino M, Jenab M, Prehn JH, Hughes DJ. Fusobacterium nucleatum associates with stages of colorectal neoplasia development, colorectal cancer and disease outcome. Eur J Clin Microbiol Infect Dis. 2014;33:1381-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 384] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 149. | Tilg H, Adolph TE, Gerner RR, Moschen AR. The Intestinal Microbiota in Colorectal Cancer. Cancer Cell. 2018;33:954-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 548] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 150. | Gao R, Gao Z, Huang L, Qin H. Gut microbiota and colorectal cancer. Eur J Clin Microbiol Infect Dis. 2017;36:757-769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 144] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 151. | Joshi SN, Gardner JD. Gastrin and colon cancer: A unifying hypothesis. Dig Dis. 1996;14:334-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 152. | Thorburn CM, Friedman GD, Dickinson CJ, Vogelman JH, Orentreich N, Parsonnet J. Gastrin and colorectal cancer: A prospective study. Gastroenterology. 1998;115:275-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 207] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 153. | Georgopoulos SD, Polymeros D, Triantafyllou K, Spiliadi C, Mentis A, Karamanolis DG, Ladas SD. Hypergastrinemia is associated with increased risk of distal colon adenomas. Digestion. 2006;74:42-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 154. | Yang YX, Hennessy S, Propert K, Hwang WT, Sedarat A, Lewis JD. Chronic proton pump inhibitor therapy and the risk of colorectal cancer. Gastroenterology. 2007;133:748-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 155. | van Soest EM, van Rossum LG, Dieleman JP, van Oijen MG, Siersema PD, Sturkenboom MC, Kuipers EJ. Proton pump inhibitors and the risk of colorectal cancer. Am J Gastroenterol. 2008;103:966-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 156. | Robertson DJ, Larsson H, Friis S, Pedersen L, Baron JA, Sørensen HT. Proton pump inhibitor use and risk of colorectal cancer: A population-based, case-control study. Gastroenterology. 2007;133:755-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 157. | Guarner F. Decade in review-gut microbiota: The gut microbiota era marches on. Nat Rev Gastroenterol Hepatol. 2014;11:647-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 158. | O’Grady J, O'Connor EM, Shanahan F. Review article: Dietary fibre in the era of microbiome science. Aliment Pharmacol Ther. 2019;49:506-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 101] [Article Influence: 16.8] [Reference Citation Analysis (0)] |