Published online Apr 28, 2019. doi: 10.3748/wjg.v25.i16.1928
Peer-review started: March 19, 2019
First decision: March 27, 2019
Revised: April 3, 2019
Accepted: April 10, 2019
Article in press: April 10, 2019
Published online: April 28, 2019
Processing time: 37 Days and 12.2 Hours
Upper gastrointestinal (UGI) tract involvement of inflammatory bowel disease (IBD) is commonly seen in pediatric patients. Upper endoscopy is included in the routine workup of children with suspected IBD to enhance the diagnosis and management of these patients. Currently, childhood IBD is classified into ulcerative colitis (UC), atypical UC, Crohn’s disease (CD) and IBD unclassified. Histologic confirmation of UGI tract involvement, in particular the presence of epithelioid (non-caseating) granulomas, is helpful in confirming the diagnosis of IBD and its classification. Herein, we reviewed selected IBD-associated UGI tract manifestations in children. Lymphocytic esophagitis, seen predominantly in CD, is histologically characterized by increased intraepithelial lymphocytes (> 20 in one high-power field) in a background of mucosal injury with absence of granulocytes. Focally enhanced gastritis is a form of gastric inflammation in pediatric IBD marked by a focal lymphohistiocytic pit inflammation with or without granulocytes and plasma cells in a relatively normal background gastric mucosa. Duodenal inflammation seen in children with IBD includes cryptitis, villous flattening, increased intraepithelial lymphocytes, and lamina propria eosinophilia. Finally, epithelioid granulomas not associated with ruptured gland/crypt are a diagnostic feature of CD. The clinicopathologic correlation and differential diagnosis of each microscopic finding are discussed. Clinicians and pathologists should be cognizant of the utility and limitations of these histologic features.
Core tip: Upper gastrointestinal tract inflammation is frequently observed in pediatric patients with inflammatory bowel disease. Distinct inflammatory patterns such as lymphocytic esophagitis and focally enhanced gastritis are helpful in rendering the diagnosis of inflammatory bowel disease in an otherwise non-specific case. Epithelioid granulomas are the only specific microscopic finding to distinguish Crohn disease from ulcerative colitis. Meanwhile, duodenal inflammation demonstrates various non-specific histologic findings in the setting of pediatric inflammatory bowel disease. This review highlights the diagnostic criteria and differential diagnosis of each pathologic finding.
- Citation: Abuquteish D, Putra J. Upper gastrointestinal tract involvement of pediatric inflammatory bowel disease: A pathological review. World J Gastroenterol 2019; 25(16): 1928-1935
- URL: https://www.wjgnet.com/1007-9327/full/v25/i16/1928.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i16.1928
Inflammatory bowel disease (IBD) is a complex and multifactorial disorder of the gastrointestinal tract characterized by relapsing inflammation with a peak age of onset between 15 and 30 years of age[1]. The incidence of pediatric IBD varies based on the geographical region with the highest incidence seen in Europe (0.2-23 per 100000) and North America (1.1-15.2 per 100000)[2]. Benchimol et al[3] reported increasing prevalence of childhood-onset IBD in Canada over time. The incidence, however, is not statistically changed with an exception of the rapid increase in the youngest age group (6 mo to 5 years of age)[3]. A population-based epidemiological study from Wisconsin[4], United States, reported the annual incidence of IBD 9.5 per 100000 children with 19% of cases occurred in the first decade of life. The incidence of Crohn’s disease (CD), ulcerative colitis (UC) and indeterminate colitis in the study was 6.6, 2.4, and 0.5 per 100000 children, respectively[4].
Phenotype classification of IBD is necessary for accurate management and prognostication. The pediatric IBD Porto Group of the European Society of Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN)[5] classifies pediatric IBD into five subgroups: UC, atypical UC, colonic CD, small bowel CD, and IBD unclassified (IBDU). The classification is based on the endoscopic and pathologic findings.
Despite many similarities between IBDs in children and adults, the pediatric-onset IBD often presents with atypical features, making the classification of IBD in children challenging. In addition, upper gastrointestinal (UGI) tract involvement is more commonly identified in pediatric patients compared to adults[6]. Previously considered to be a specific finding of CD, UGI tract involvement is no longer used to distinguish CD from UC as it may be seen in both entities[7]. Herein, we review the UGI tract manifestations of pediatric IBD including lymphocytic esophagitis (LE), focally enhanced gastritis (FEG), duodenal inflammation, and epithelioid granulomas with an emphasis on their pathologic characteristics and differential diagnosis. The pathologic characteristics of these entities are summarized in Table 1. Monogenic IBD, a completely separate entity characterized by its early onset and association with underlying genetic defects, is not discussed in this review.
| Entity | Prevalence (%) | Histologic diagnostic criteria | Differential diagnosis | |
| CD | UC | |||
| Lymphocytic esophagitis | 12-28[15,20] | 7[15,20] | > 20 IELs/HPF; No significant granulocytes; Mucosal injury (edema; dyskeratosis). | Candidiasis, lichen planus esophagitis, lichenoid esophagitis. |
| Focally enhanced gastritis | 54-55[31,32] | 21-30[31,32] | Focal pit injury (lymphohistiocytes ± plasma cells or granulocytes); Relatively normal background mucosa. | Lymphoid aggregate, H. pylori-associated gastritis. |
| Duodenitis | 33-48[13,35,36,37] | 0-29[13,35,36,37] | Cryptitis; Villous blunting; Increased IELs (> 20 IELs/100 enterocytes); Lamina propria eosinophilia | Celiac disease, H. pylori infection, nonsteroidal anti-inflammatory medications, bacterial overgrowth, autoimmune diseases |
| Epithelioid granulomas | 2.7 (esophagus); 20.1 (stomach); 3.8 (duodenum)[40] | 0 | Collection of histiocytes; Non-caseating; Surrounded by lymphocytes; Not associated with ruptured gland/crypt | Chronic granulomatous disease, common variable immunodeficiency, and infection |
UGI tract inflammation is noted in approximately half of the children with IBD during the initial assessment[6,8]. Castellaneta et al[8] reported that the most frequently involved sites were the stomach (67%) followed by the esophagus (54%) and duodenum (22%). The clinical manifestations include epigastric and abdominal pain, nausea, vomiting, and weight loss[8]. However, a subset of patients with UGI tract inflammation were asymptomatic at initial presentation[8,9,10]. Therefore, ESPGHAN Revised Porto Criteria recommended that esophagogastroduodenoscopy (EGD) should be performed in all children at the initial evaluation of IBD with two or more biopsies obtained from each site irrespective of the UGI tract manifestations and endoscopic appearances[7,11].
Routine EGD allows a comprehensive evaluation of the extent of disease involvement and is potentially helpful to classify the disease in a subset of patients with otherwise nonspecific pancolitis[12]. The European registries (EUROKIDS and the Hungarian Paediatric IBD Registry) reported that 35%-67% of CD patients demonstrated macroscopic abnormalities of the UGI tract at EGD and 9%-24% of them showed characteristic endoscopic findings of CD (aphthous lesions, ulcerations, cobblestone appearance, and stenosis)[13,14].
Histologically, various inflammatory patterns of the UGI tract may be seen in childhood IBD. The esophageal involvement of these patients consists of active esophagitis, chronic esophagitis (including LE), and reflux esophagitis[8,9,15]. The inflammatory patterns seen in the stomach include chronic active and inactive gastritis (including Helicobacter pylori-associated gastritis), chronic atrophic gastritis, and FEG[10,16,17]. The duodenal inflammation is characterized by crypt inflammation, villous blunting, and increased intraepithelial lymphocytes[18]. Whereas the aforementioned findings can be seen in all IBD subgroups, epithelioid (non-caseating) granulomas are a specific histologic feature to render the diagnosis CD[5].
Initially described by Rubio et al[19] in 2006, LE has been associated with IBD in pediatric patients, particularly CD[15]. The prevalence of LE in children with CD ranges from 12.2 to 28%[15,20]. However, LE should not be used as a distinguishing feature between CD and UC, as a small proportion (7%) of children with UC also demonstrate LE[15]. Clinically, children with LE do not demonstrate symptoms that distinguish them from non-LE patients[15]. Moreover, the endoscopic findings are not specific; the esophageal mucosa might be unremarkable, edematous, or with erosions, similar to patients with reflux esophagitis[15,20].
LE is a diagnosis made on the basis of histologic findings. However, there is no widely accepted diagnostic criteria. The initial description[19] defined LE as having greater than 20 intraepithelial lymphocytes (IELs) per high-power field (HPF) with rare granulocytes, while the articles of LE in pediatric patients[15,20] defined LE as esophageal mucosa with greater than 50 IELs per HPF and less than 1 granulocyte per 50 IELs. Patil et al[21] argued that there is currently not enough evidence to support formal counting of lymphocytes to diagnose LE in daily practice. The skepticism is primarily due to the lack of data on the normal range of IELs in the esophageal mucosa. Putra et al[22] evaluated esophageal mucosal biopsies of 17 asymptomatic volunteers (age: 34 ± 9 years) and reported that the cut-offs for IEL (mean + 2SD) at gastroesophageal junction, distal esophagus, and mid esophagus were 62, 46, and 41 IELs per HPF, respectively. Meanwhile, there is no available data on the normal esophageal lymphocyte count in healthy children.
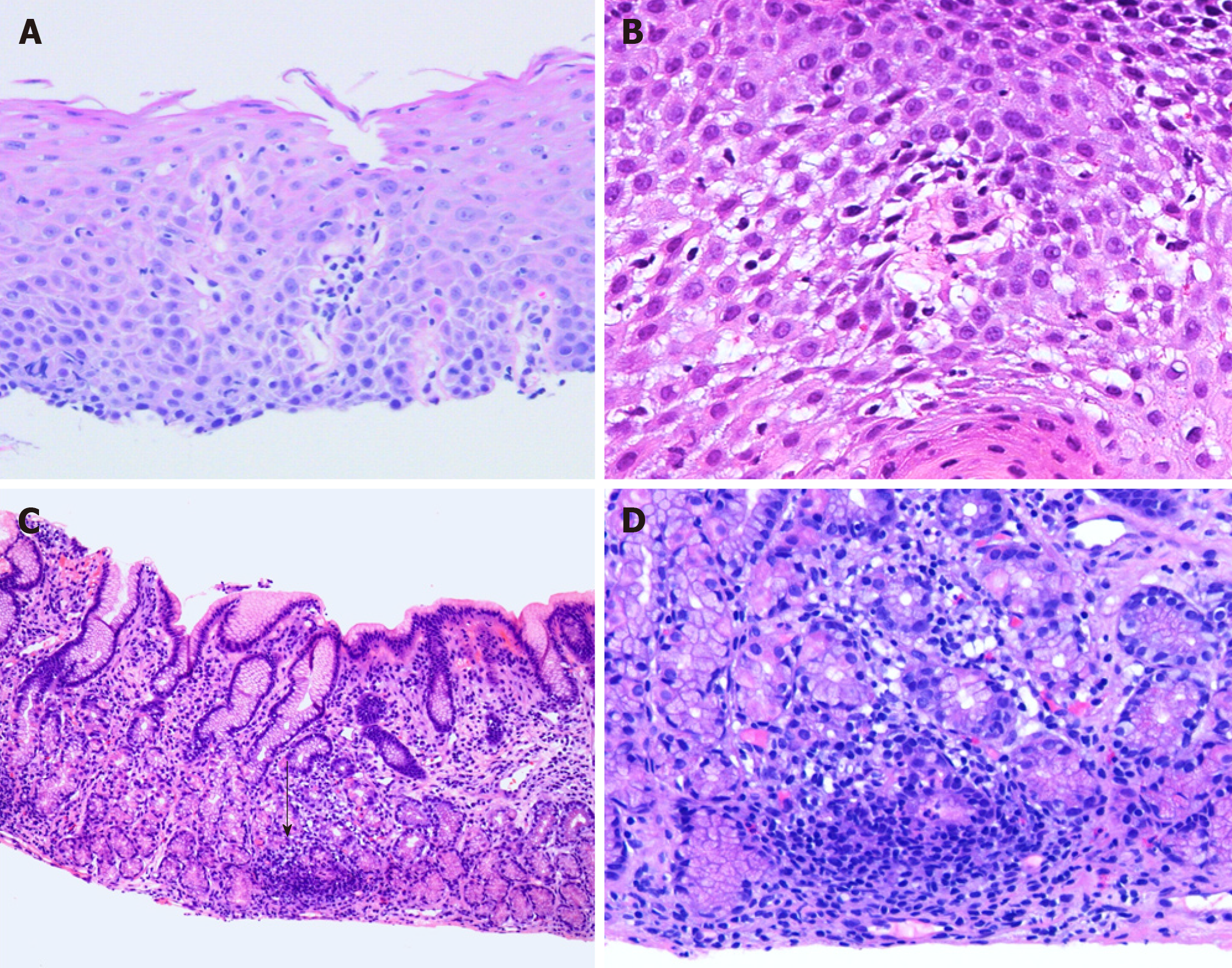
In practice, the authors use the diagnostic criteria established by Rubio et al[19] (greater than 20 IELs per HPF with no significant granulocytes; Figure 1A and B). Mucosal injury, in the form of edema or dyskeratosis, also often accompanies intraepithelial lymphocytosis[21]. IELs are more prominent in patients with CD and they usually demonstrate peripapillary distribution[20].
It is important to note that LE is not specific for childhood IBD and this association is not observed in adult patients[23]. LE is also seen in patients with esophageal candidiasis, motility disorders, gastroesophageal reflux disease, and immune-mediated disorders, among others[19,21-27]. Therefore, in the pathology report of LE patients with no clinical history of IBD, a note stating that LE is a nonspecific histologic finding and listing the clinical correlates is advised[21].
In addition to the aforementioned conditions, LE also shows overlap histologic findings with lichen planus esophagitis and lichenoid esophagitis[28]. Microscopically, lichen planus esophagitis is characterized by atrophic epithelial changes, a prominent band-like infiltrate in the epithelium and lamina propria, and scattered degenerated keratinocytes with direct immunofluorescence studies showing globular IgM deposits at the dermo-epidermal junction and complement staining in apoptotic keratinocytes[28,29]. Lichenoid esophagitis is a term reserved for biopsies showing histologic findings similar to lichen planus esophagitis with negative direct immunofluorescence studies; this histologic pattern has been associated with viral hepatitis and human immunodeficiency virus (HIV) infection[28].
FEG was introduced by Oberhuber et al[30] as a frequent type of gastritis in CD patients. In children, FEG is identified in both CD and UC patients with a reported prevalence of 54%-55% and 21%-30%[31,32], respectively, therefore it does not reliably distinguish between the two entities. The reported sensitivity and specificity of FEG for IBD to be 36%-41% and 94%-97%, respectively[32,33]. Children with FEG are 15.4 times more likely to have an IBD[32]. Although not entirely specific, the association between FEG and IBD is also observed in adult patients[30].
Clinically, there is no significant difference in the UGI tract symptoms between IBD children with and without FEG[10]. Abnormal endoscopic findings are more commonly identified in patients with FEG (49% to 68.7%)[10,31]; these abnormalities include aphthoid ulcers and submucosal bleeding[10].
FEG predominantly involves the antrum and is histologically defined as a focal pit (involving at least one foveolum/gastric gland) inflammation consisting of lymphocytes and histiocytes resulting in epithelial injury (Figure 1C and D)[30]. The lesion may contain plasma cells, eosinophils, and neutrophils; while, the background mucosa should be relatively normal[30,31,33]. In the setting of increased lymphoplasmacytic infiltrate in the lamina propria, the authors prefer to use the term “chronic active/inactive gastritis”.
Multifocal involvement is common and the total number of glands involved is higher in UC patients (6.4 ± 5.1 glands) compared to those with CD patients (4.0 ± 3.0 glands)[31]. CD patients with FEG are more likely than those without FEG to have active ileitis and granulomas elsewhere in gastrointestinal tract. Meanwhile, there is no correlation reported between FEG and other gastrointestinal findings in UC patients[31].
Histologically, FEG should be distinguished from lymphoid aggregates in the gastric biopsies. Multiple levels along with absence or presence of epithelial injury may resolve this issue. In addition, the presence of neutrophils should prompt a search for Helicobacter pylori organisms.
Alper et al[34] reported that duodenitis is identified in 12.7% of children undergoing endoscopy with celiac disease and IBD as the most common etiologies. The prevalence of duodenitis in childhood CD and UC ranges from 33%-48% and 0%-29%, respectively[13,35-37]. More than half of children with biopsy-proven duodenitis demonstrate unremarkable duodenal mucosa on endoscopy[34]. Macroscopic abnormalities associated with duodenitis include edema, erythema, erosions, scalloping, and ulcers[34].
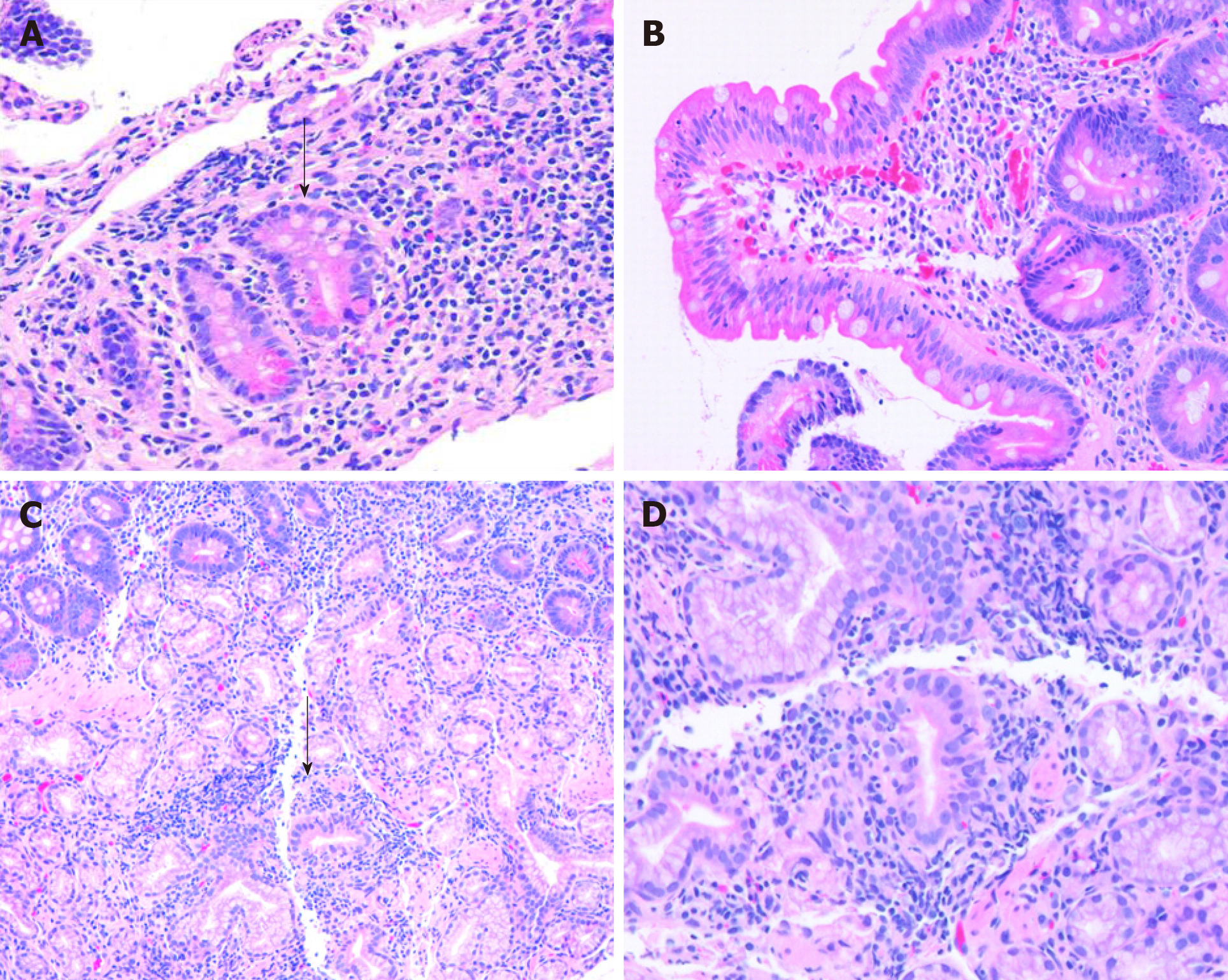
Histologically, there is no specific histologic feature that reliably distinguish IBD from non-IBD associated duodenitis with the exception of CD-associated granulomas. Hardee et al[18] reported that cryptitis is significantly more frequently seen in children with IBD compared to patients with celiac disease and other underlying etiologies (Figure 2). Other histologic abnormalities noted in the duodenal mucosa of children with IBD include increased IELs [greater than 20 IELs per 100 enterocytes on hematoxylin and eosin (H and E) sections], villous blunting, and lamina propria eosinophilia (greater than 20 eosinophils per HPF)[18].
In the absence of IBD, other diagnostic considerations for duodenal abnormalities such as celiac disease, Helicobacter pylori infection, nonsteroidal anti-inflammatory medications, bacterial overgrowth, and certain autoimmune diseases should be considered[34,38].
The presence of villous blunting and duodenal lymphocytosis in untreated celiac disease is more frequent and prominent compared to children with IBD. However, the presence of partial villous blunting or mild increased IELs should raise the possibility of early celiac disease. Alper et al[39] reported that although false-positive anti-tissue transglutaminase antibodies (tTG) could occur in children with IBD, the prevalence of celiac disease in these patients is similar to general population.
De Matos et al[40] reported that epithelioid granulomas were identified in 61% of children with CD who underwent EGD and colonoscopy at diagnosis. A significant proportion of these patients (13.4%) showed granulomas only in the UGI tract biopsies, emphasizing the importance of EGD in this patient population[40]. The frequency of granulomas in the esophagus, stomach, and duodenum of untreated CD patients was 2.7%, 20.1%, and 3.8%, respectively[40]. Meanwhile, a higher proportion of children showed granulomas in the terminal ileum (34.8%) and large intestine (35.3%)[40].
Histologically, CD-associated granulomas are characterized by collections of at least 5 epithelioid histiocytes with or without multinucleated giant cells, without necrotizing component (caseation), and away from ruptured gland/crypt[40,41]. The histologic differential diagnosis for granulomas includes chronic granulomatous disease, common variable immune deficiency, and an infectious process[42]. Therefore, clinical correlation to exclude other diagnostic considerations is crucial.
The presence of granulomas has been reported to represent a more severe disease phenotype. CD patients with granulomas prior to treatment are associated with perianal disease, gastritis, hypoalbuminemia, subsequent biologic treatment and hospitalization[40,43].
UGI tract manifestation of IBD is more frequently observed in children compared to adults. Pathologic findings of LE, FEG, duodenal inflammation, and epithelioid granulomas would support the diagnosis of childhood IBD in the appropriate clinical setting. With the exception of CD-associated granulomas, the histologic findings of these entities are non-specific; thus, cannot reliably classify childhood IBD. Clinicians should be mindful of the utility and limitations of these histologic findings, and pathologists should be aware of the differential diagnosis and clinical correlates of each entity.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Canada
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ciccone MM, Day AS, Gheita TA, Serban ED S-Editor: Ma RY L-Editor: A E-Editor: Song H
| 1. | Markowitz J. Crohn’s disease and ulcerative colitis. In: Wylie R, Hyams JS. Pediatric gastrointestinal disease. Philadelphia: WB Saunders 1999; 320-340. |
| 2. | Sýkora J, Pomahačová R, Kreslová M, Cvalínová D, Štych P, Schwarz J. Current global trends in the incidence of pediatric-onset inflammatory bowel disease. World J Gastroenterol. 2018;24:2741-2763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 271] [Cited by in RCA: 262] [Article Influence: 37.4] [Reference Citation Analysis (6)] |
| 3. | Benchimol EI, Bernstein CN, Bitton A, Carroll MW, Singh H, Otley AR, Vutcovici M, El-Matary W, Nguyen GC, Griffiths AM, Mack DR, Jacobson K, Mojaverian N, Tanyingoh D, Cui Y, Nugent ZJ, Coulombe J, Targownik LE, Jones JL, Leddin D, Murthy SK, Kaplan GG. Trends in Epidemiology of Pediatric Inflammatory Bowel Disease in Canada: Distributed Network Analysis of Multiple Population-Based Provincial Health Administrative Databases. Am J Gastroenterol. 2017;112:1120-1134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 190] [Cited by in RCA: 240] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 4. | Adamiak T, Walkiewicz-Jedrzejczak D, Fish D, Brown C, Tung J, Khan K, Faubion W, Park R, Heikenen J, Yaffee M, Rivera-Bennett MT, Wiedkamp M, Stephens M, Noel R, Nugent M, Nebel J, Simpson P, Kappelman MD, Kugathasan S. Incidence, clinical characteristics, and natural history of pediatric IBD in Wisconsin: a population-based epidemiological study. Inflamm Bowel Dis. 2013;19:1218-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 5. | Birimberg-Schwartz L, Zucker DM, Akriv A, Cucchiara S, Cameron FL, Wilson DC, Lazowska I, Yianni L, Paul SP, Romano C, Kolacek S, Buderus S, Pærregaard A, Russell RK, Escher JC, Turner D, Pediatric IBD Porto group of ESPGHAN. Development and Validation of Diagnostic Criteria for IBD Subtypes Including IBD-unclassified in Children: a Multicentre Study From the Pediatric IBD Porto Group of ESPGHAN. J Crohns Colitis. 2017;11:1078-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Van Limbergen J, Russell RK, Drummond HE, Aldhous MC, Round NK, Nimmo ER, Smith L, Gillett PM, McGrogan P, Weaver LT, Bisset WM, Mahdi G, Arnott ID, Satsangi J, Wilson DC. Definition of phenotypic characteristics of childhood-onset inflammatory bowel disease. Gastroenterology. 2008;135:1114-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 759] [Cited by in RCA: 701] [Article Influence: 41.2] [Reference Citation Analysis (1)] |
| 7. | Levine A, Koletzko S, Turner D, Escher JC, Cucchiara S, de Ridder L, Kolho KL, Veres G, Russell RK, Paerregaard A, Buderus S, Greer ML, Dias JA, Veereman-Wauters G, Lionetti P, Sladek M, Martin de Carpi J, Staiano A, Ruemmele FM, Wilson DC; European Society of Pediatric Gastroenterology, Hepatology, and Nutrition. ESPGHAN revised porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J Pediatr Gastroenterol Nutr. 2014;58:795-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 822] [Cited by in RCA: 992] [Article Influence: 90.2] [Reference Citation Analysis (0)] |
| 8. | Castellaneta SP, Afzal NA, Greenberg M, Deere H, Davies S, Murch SH, Walker-Smith JA, Thomson M, Srivistrava A. Diagnostic role of upper gastrointestinal endoscopy in pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2004;39:257-261. [PubMed] |
| 9. | Hummel TZ, ten Kate FJ, Reitsma JB, Benninga MA, Kindermann A. Additional value of upper GI tract endoscopy in the diagnostic assessment of childhood IBD. J Pediatr Gastroenterol Nutr. 2012;54:753-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Putra J, Ornvold K. Focally enhanced gastritis in children with inflammatory bowel disease: a clinicopathological correlation. Pathology. 2017;49:808-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Oliva S, Thomson M, de Ridder L, Martín-de-Carpi J, Van Biervliet S, Braegger C, Dias JA, Kolacek S, Miele E, Buderus S, Bronsky J, Winter H, Navas-López VM, Assa A, Chong SKF, Afzal NA, Smets F, Shaoul R, Hussey S, Turner D, Cucchiara S. Endoscopy in Pediatric Inflammatory Bowel Disease: A Position Paper on Behalf of the Porto IBD Group of the European Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2018;67:414-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (1)] |
| 12. | Turner D, Griffiths AM. Esophageal, gastric, and duodenal manifestations of IBD and the role of upper endoscopy in IBD diagnosis. Curr Gastroenterol Rep. 2009;11:234-237. [PubMed] |
| 13. | Kovacs M, Muller KE, Arato A, Lakatos PL, Kovacs JB, Varkonyi A, Solyom E, Polgar M, Nemes E, Guthy I, Tokodi I, Toth G, Horvath A, Tarnok A, Tomsits E, Csoszánszky N, Balogh M, Vass N, Bodi P, Dezsofi A, Gardos L, Micskey E, Papp M, Szucs D, Cseh A, Molnar K, Szabo D, Veres G; Hungarian IBD Registry Group (HUPIR). Diagnostic yield of upper endoscopy in paediatric patients with Crohn's disease and ulcerative colitis. Subanalysis of the HUPIR registry. J Crohns Colitis. 2012;6:86-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | de Bie CI, Buderus S, Sandhu BK, de Ridder L, Paerregaard A, Veres G, Dias JA, Escher JC, EUROKIDS Porto IBD Working Group of ESPGHAN. Diagnostic workup of paediatric patients with inflammatory bowel disease in Europe: results of a 5-year audit of the EUROKIDS registry. J Pediatr Gastroenterol Nutr. 2012;54:374-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 15. | Ebach DR, Vanderheyden AD, Ellison JM, Jensen CS. Lymphocytic esophagitis: a possible manifestation of pediatric upper gastrointestinal Crohn's disease. Inflamm Bowel Dis. 2011;17:45-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 16. | Basturk A, Artan R, Yılmaz A, Gelen MT. Gastritis Associated with Initially Pediatric Crohn's Disease and Ulcerative Colitis. Pediatr Gastroenterol Hepatol Nutr. 2018;21:163-169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Papamichael K, Konstantopoulos P, Mantzaris GJ. Helicobacter pylori infection and inflammatory bowel disease: is there a link? World J Gastroenterol. 2014;20:6374-6385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 63] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (1)] |
| 18. | Hardee S, Alper A, Pashankar DS, Morotti RA. Histopathology of duodenal mucosal lesions in pediatric patients with inflammatory bowel disease: statistical analysis to identify distinctive features. Pediatr Dev Pathol. 2014;17:450-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Rubio CA, Sjödahl K, Lagergren J. Lymphocytic esophagitis: a histologic subset of chronic esophagitis. Am J Clin Pathol. 2006;125:432-437. [PubMed] |
| 20. | Sutton LM, Heintz DD, Patel AS, Weinberg AG. Lymphocytic esophagitis in children. Inflamm Bowel Dis. 2014;20:1324-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 21. | Patil DT, Hammer S, Langer R, Yantiss RK. Lymphocytic esophagitis: an update on histologic diagnosis, endoscopic findings, and natural history. Ann N Y Acad Sci. 2018;1434:185-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Putra J, Muller KE, Hussain ZH, Parker S, Gabbard S, Brickley EB, Lacy BE, Rothstein R, Lisovsky M. Lymphocytic Esophagitis in Nonachalasia Primary Esophageal Motility Disorders: Improved Criteria, Prevalence, Strength of Association, and Natural History. Am J Surg Pathol. 2016;40:1679-1685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | Lisovsky M, Westerhoff M, Zhang X. Lymphocytic esophagitis: a histologic pattern with emerging clinical ramifications. Ann N Y Acad Sci. 2016;1381:133-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Abuquteish D, Siddiqui I, Putra J. Lymphocytic Esophagitis: Inflammatory Pattern of Candida Esophagitis in a Patient With Ulcerative Colitis. Int J Surg Pathol. 2019;1066896918824023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 25. | Xue Y, Suriawinata A, Liu X, Li Z, Gabbard S, Rothstein R, Lacy B, Lisovsky M. Lymphocytic Esophagitis With CD4 T-cell-predominant Intraepithelial Lymphocytes and Primary Esophageal Motility Abnormalities: A Potential Novel Clinicopathologic Entity. Am J Surg Pathol. 2015;39:1558-1567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 26. | Pasricha S, Gupta A, Reed CC, Speck O, Woosley JT, Dellon ES. Lymphocytic Esophagitis: An Emerging Clinicopathologic Disease Associated with Dysphagia. Dig Dis Sci. 2016;61:2935-2941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Haque S, Genta RM. Lymphocytic oesophagitis: clinicopathological aspects of an emerging condition. Gut. 2012;61:1108-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 28. | Salaria SN, Abu Alfa AK, Cruise MW, Wood LD, Montgomery EA. Lichenoid esophagitis: clinicopathologic overlap with established esophageal lichen planus. Am J Surg Pathol. 2013;37:1889-1894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 29. | Abraham SC, Ravich WJ, Anhalt GJ, Yardley JH, Wu TT. Esophageal lichen planus: case report and review of the literature. Am J Surg Pathol. 2000;24:1678-1682. [PubMed] |
| 30. | Oberhuber G, Püspök A, Oesterreicher C, Novacek G, Zauner C, Burghuber M, Vogelsang H, Pötzi R, Stolte M, Wrba F. Focally enhanced gastritis: a frequent type of gastritis in patients with Crohn's disease. Gastroenterology. 1997;112:698-706. [PubMed] |
| 31. | Ushiku T, Moran CJ, Lauwers GY. Focally enhanced gastritis in newly diagnosed pediatric inflammatory bowel disease. Am J Surg Pathol. 2013;37:1882-1888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 32. | Roka K, Roma E, Stefanaki K, Panayotou I, Kopsidas G, Chouliaras G. The value of focally enhanced gastritis in the diagnosis of pediatric inflammatory bowel diseases. J Crohns Colitis. 2013;7:797-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 33. | McHugh JB, Gopal P, Greenson JK. The clinical significance of focally enhanced gastritis in children. Am J Surg Pathol. 2013;37:295-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 34. | Alper A, Hardee S, Rojas-Velasquez D, Escalera S, Morotti RA, Pashankar DS. Prevalence and Clinical, Endoscopic, and Pathological Features of Duodenitis in Children. J Pediatr Gastroenterol Nutr. 2016;62:314-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 35. | Tobin JM, Sinha B, Ramani P, Saleh AR, Murphy MS. Upper gastrointestinal mucosal disease in pediatric Crohn disease and ulcerative colitis: a blinded, controlled study. J Pediatr Gastroenterol Nutr. 2001;32:443-448. [PubMed] |
| 36. | Sonnenberg A, Melton SD, Genta RM. Frequent occurrence of gastritis and duodenitis in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:39-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 37. | Genta RM, Sonnenberg A. Non-Helicobacter pylori gastritis is common among paediatric patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2012;35:1310-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 38. | Hammer ST, Greenson JK. The clinical significance of duodenal lymphocytosis with normal villus architecture. Arch Pathol Lab Med. 2013;137:1216-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 39. | Alper A, Rojas-Velasquez D, Pashankar DS. Prevalence of Anti-tissue Transglutaminase Antibodies and Celiac Disease in Children With Inflammatory Bowel Disease. J Pediatr Gastroenterol Nutr. 2018;66:934-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 40. | De Matos V, Russo PA, Cohen AB, Mamula P, Baldassano RN, Piccoli DA. Frequency and clinical correlations of granulomas in children with Crohn disease. J Pediatr Gastroenterol Nutr. 2008;46:392-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 41. | Pierik M, De Hertogh G, Vermeire S, Van Assche G, Van Eyken P, Joossens S, Claessens G, Vlietinck R, Rutgeerts P, Geboes K. Epithelioid granulomas, pattern recognition receptors, and phenotypes of Crohn's disease. Gut. 2005;54:223-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 42. | Jevon GP, Madhur R. Endoscopic and histologic findings in pediatric inflammatory bowel disease. Gastroenterol Hepatol (NY). 2010;6:174-180. [PubMed] |
| 43. | Rothschild B, Rinawi F, Herman Y, Nir O, Shamir R, Assa A. Prognostic significance of granulomas in children with Crohn's disease. Scand J Gastroenterol. 2017;52:716-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |