Published online Apr 21, 2019. doi: 10.3748/wjg.v25.i15.1854
Peer-review started: February 21, 2019
First decision: March 5, 2019
Revised: March 10, 2019
Accepted: March 16, 2019
Article in press: March 16, 2019
Published online: April 21, 2019
Processing time: 56 Days and 17 Hours
Gastric cancer is one of the most common and deadly malignancies worldwide. Despite recent medical progress, the 5-year survival rate of gastric cancer is still unsatisfactory. 5-fluorouracil (5-Fu) is one of the first-line antineoplastic treatments for gastric cancer, as it can effectively induce cancer cell apoptosis. However, the effect of 5-Fu is limited due to drug resistance of the malignant tumor. Previous studies have reported that Sotetsuflavone from Cycas revoluta Thunb. can markedly suppress lung cancer cell proliferation by apoptosis, though its effect on gastric cancer remains unknown.
To investigate the inhibitory effect of Cycas revoluta Thunb. and to determine whether it can overcome gastric cancer cell drug resistance to 5-Fu.
Cell viability was examined to determine whether the natural extract of Cycas revoluta Thunb. induced gastric cancer cell death. The half-maximal effective concentration and the half-maximal lethal concentration were calculatede. Wound-healing and transwell assays were performed to examine gastric cancer cell motility. Clonogenic assays were performed to investigate the synergistic effects of Cycas revoluta Thunb. with 5-Fu, and apoptotic bodies were detected by Hoechst staining. Western blotting was performed to examine the expression of related proteins and to investigate the molecular mechanism of Cycas revoluta Thunb.-induced cancer cell apoptosis. The expressions of proteins, including mammalian target of rapamycin (mTOR) and p-AKT, were detected in different combinations of treatments for 48 h, then analyzed by ECL detection.
Gastric cancer cells were more sensitive to the natural extract of Cycas revoluta Thunb. compared to normal gastric epithelial cells, and the extract effectively inhibited gastric cancer cell migration and invasion. The extract improved the anti-cancer effect of 5-Fu by enhancing the chemosensitization of gastric cancer cells. Extract plus 5-Fu further reduced the expression of the drug-resistance-related proteins p-AKT and mTOR after 48 h compared to 5-Fu alone. Compared to 5-Fu treatment alone, mTOR and p-AKT expression was significantly reduced by about 50% and 75%, respectively. We also found that the natural extract of Cycas revoluta Thunb. further increased 5-Fu-induced gastric cancer cell apoptosis. Expression of apoptosis-related protein X-linked inhibitor of apoptosis protein and apoptosis inducing factor were significantly reduced and increased, respectively, in the 5-Fu-resistant gastric cancer line SGC-7901/R treated with extract plus 5-Fu, while the expression of survivin did not change.
The natural extract of Cycas revoluta Thunb. effectively inhibited gastric cancer cell growth and enhanced the anti-cancer effect of 5-Fu through the AKT-mTOR pathway.
Core tip: 5-fluorouracil (5-Fu) is an effective treatment for gastric cancer, which is one of the most common and deadly malignancies worldwide. However, the effect of 5-Fu is limited by the drug resistance of gastric cancer. Here, we report that natural extract of Cycas revoluta Thunb. effectively inhibits gastric cancer cell growth, migration and invasion. Furthermore, it can be used in combination with 5-Fu to enhance its anti-cancer effects through the AKT-mTOR pathway.
- Citation: Cui XL, Li KJ, Ren HX, Zhang YJ, Liu XD, Bu BG, Wang L. Extract of Cycas revoluta Thunb. enhances the inhibitory effect of 5-fluorouracil on gastric cancer cells through the AKT-mTOR pathway. World J Gastroenterol 2019; 25(15): 1854-1864
- URL: https://www.wjgnet.com/1007-9327/full/v25/i15/1854.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i15.1854
Gastric cancer remains the fourth most common malignancy diagnosed worldwide, especially in Eastern Asia, Eastern Europe and Central and South America[1-3]. It also is the third main cause of death related to malignancy, just behind lung and liver cancer[4]. In 2012, there were about 951,600 new patients diagnosed with gastric cancer, and over 700,000 deaths related to gastric cancer have been recorded[5].
With a broad spectrum of activity against malignant cells, 5-fluorouracil (5-Fu) is commonly employed against gastric, liver and colorectal cancers[6-8]. As a prevalent chemotherapeutic drug in clinical practice, 5-Fu can inhibit cancer cell proliferation and DNA replication, including gastric, breast and colorectal cancer cells, by inhibiting thymidylate synthase from synthesizing thymine, which ultimately induces apoptosis[9-11].
Apoptosis is an important molecular process for stable and orderly human growth. It is strictly controlled and its dysregulation is linked to many diseases, including cancer[12,13]. This complex process is regulated by a series of key proteins, such as X-linked inhibitor of apoptosis protein (XIAP), apoptosis inducing factor (AIF) and survivin. XIAP is a strong apoptotic regulator[14-18] and inhibits caspase-3, -7, and -9, which are all part of the mammalian apoptotic signaling pathway. AIF is released and promotes apoptosis by intrinsic signaling cascades[19,20] when mitochondria respond to apoptotic stimuli, such as the translocation of BH3 interacting domain death agonist (Bid)[21]. Survivin is a unique inhibitor of apoptosis (IAP), as it does not directly interact with caspases but with some adaptors or cofactors[22-26].
Although 5-Fu is widely used as an anticancer drug, it has some serious problems, such as low effective response rate and severe side effects. One of the most critical concerns is the increasing cases of drug resistant malignant tumor. Many 5-Fu drug-resistance-related proteins have been identified. For example, P-glycoprotein (P-gp) functions as a molecular ‘pump’ to expel chemotherapy drugs from the inside of the cell, and resistance to 5-Fu can be reversed when P-gp expression is reduced[27]. AKT is considered a key protein in the phosphiotidylinositol-3-kinase (PI3K)/Akt signaling pathway. It is activated at the plasma membrane by phosphorylation of Thr308 and Ser473 residues, and it can phosphorylate various downstream substrates related to drug sensitivity[28]. Mammalian target of rapamycin (mTOR), a serine/threonine kinase, is a main downstream effector of the PI3K/AKT signaling pathway[29]. It has also been reported that 5-Fu drug resistance may be mediated by the AKT-mTOR pathway[30,31].
Fortunately, drug resistance can be reduced when used in combination with other compounds. Previous studies have reported that chemosensitization of cancer cells to 5-Fu can be achieved by using dietary fats, particularly n-3 polyunsaturated fatty acids (PUFAs), puerarin, iRGD, and troxerutin[32-35].
Some Chinese medicines, including Cycas revoluta Thunb., have demonstrated chemosensitization effects, which provides some novel insights for anti-cancer treatments. According to ancient records, Cycas revoluta Thunb. is an evergreen palm woody plant[36] with useful medicinal value, such as reducing fever and alleviating congestion. A component of the extract, Sotetsuflavone, was identified to have strong anti-tumor activity against lung cancer cells[37,38]. Because it can effectively induce lung cancer cell apoptosis, we studied whether it could inhibit growth, migration and invasion of malignant gastric cancer cells. Furthermore, we evaluated its potential for chemosensitization in combination with 5-Fu and investigated its potential molecular mechanism.
The leaf of Cycas revoluta Thunb. was acquired from AnGuo herbal medicine market in HeBei Province of China. DMEM (12800017) and trypsin (25300054) were purchased from Life Technologies (Carlsbad, CA, United States). MTT (M2128) and 5-Fu (F6627) were purchased from Sigma (Saint Louis, MO, United States). Antibodies against p-gp, XIAP, p-Akt, AIF, mTOR, survivin, and GAPDH were purchased from Abcam (Shanghai, China).
The powder of the Cycas revoluta Thunb. leaf was extracted by reflux extraction with 80% ethanol. The extracts were collected and concentrated under reduced pressure until there was no irritating odor. The product was dissolved in water and filtered. The filtrate was then extracted with dichloromethane, concentrated under reduced pressure and dried.
The MGC-803, SGC-790, and HGC-27 cell lines were obtained from ATCC (Manassas, VA, United States). SNU-5 cells were obtained from the Cell Resource Center of Shanghai Institute of Life Sciences, Chinese Academy of Sciences. GES-1 cells were obtained from the Genetics department of Beijing Cancer Research Institute. The SGC-7901/R line was obtained from Shanghai Institute of Medicine, Chinese Academy of Sciences. Cell lines were cultured at 5% CO2 and 37˚C in DMEM medium containing fetal bovine serum (10%), penicillin (100 U/mL), and streptomycin (100 U/mL). Cells were used and analyzed at logarithmic growth phase.
Cells were grown in 96-well plates for cell viability tests. Gastric cell lines were treated with the extraction of Cycas revoluta Thunb. or 5-Fu after 24 h. The viability rate was measured by ATPlite assay (Perkin Elmer, Waltham, MA, United States)[39]. One thousand cells with different treatments were seeded into culture dish in clonogenic assays. The number of colonies was measured after 9 d.
MGC803 and HGC27 cells were cultured in six-well tissue culture plates and tested when the confluence reached 80%. Wounds were created by sterile pipette tips (10-μL), and loosely attached cells were washed out with phosphate-buffered saline. Light microscope was employed to photograph the progression of cell migration at different times, and the number of migrated cells was calculated in the scratched region.
Twenty-four-well Boyden chambers with 8-mm pore size filters (BD Falcon, Corning-Costar, New York, NY, United States) were used for this assay. Samples were suspended and seeded in the insert chamber with DMEM/F12 media and were incubated at 37˚C in 5% CO2 for 24 h to allow cells to migrate into the bottom, which contained DMEM/F12 media and 10% FBS. The number of cells that migrated was counted after staining with DAPI.
Total protein was extracted with NP40 lysis buffer. Sodium dodecyl sulfate polyacrylamide gel electrophoresis (commonly known as SDS-PAGE) and polyvinylidene difluoride membrane were used for separating and transferring samples. Membranes were blocked in tris buffered saline tween-20 (TBST) solution containing 5% nonfat dry milk for 1 h. Primary antibodies were added overnight at 4˚C and then rinsed three times for 10 min in TBST. Membranes were incubated in secondary antibodies for 1.5 h before being washed. The results were analyzed by ECL detection system.
After treatment with extract, samples were collected and washed with precooled phosphate-buffered saline (PBS). They were resuspended in 300 μL of binding buffer diluted in PBS. After incubation for 10 min, 5 μL Annexin V-FITC was added, followed by 5 μL PI for 5 min. Samples were then rinsed three times with precooled PBS and fixed in 4% paraformaldehyde for 30 min. After washing with PBS for three times, Hoechst was added to the plate dropwise and incubated at room temperature for 15 min. The results were observed under a fluorescence microscope and photographed after a final PBS wash.
The statistical methods used in this study were reviewed by Zhimin Shi from the College of Hebei University of Engineering. Experiments were repeated at least three times, and the data were processed by SPSS 20.0 statistical software. The standard deviation and Least Significant Difference were calculated by Student-Newman-Keuls test or Dunnett T3 test, in which aP < 0.05 and bP < 0.01.
To investigate tumor inhibition effects of different doses (0 μg/mL-350 μg/mL) of Cycas revoluta Thunb. extract, we performed cell viability assays. The results showed that Cycas revoluta Thunb. extract significantly inhibited gastric cancer cell viability after 24 h, especially at the low and medium doses (0 μg/mL-250 μg/mL) (Figure 1A). For treatments under 250 μg/mL extract, gastric cancer cell viability (MGC-803, SGC-790, HGC-27 and SUN-5) dramatically decreased with increasing concentrations of extract, while that of normal human gastric epithelial cells (GES-1a0 remained stable, which suggested that gastric cancer cells were more sensitive to Cycas revoluta Thunb. natural extract than normal gastric cells. We then analyzed the half-maximal effective concentration (EC50) and the half-maximal lethal concentration (LC50) of all cell lines (Figure 1B). The EC50 values of gastric cancer cells ranged from 176.44 μg/mL to 194.88 μg/mL and the LC50 values ranged from 135.23 μg/mL to 152.20 μg/mL. Compared to the EC50 (291.32 μg/mL) and LC50 (280.27 μg/mL) values for GES-1 cells, the Cycas revoluta Thunb. Extract was obviously more effective against gastric cancer cells. Additionally, high concentrations of extract (250 μg/mL-350 μg/mL), the viability rate of gastric cancer cells increased, which may be due to a screening effect for resistant cells or other adaptive mechanism.
To determine the effect of Cycas revoluta Thunb. natural extract on gastric cancer cell migration, we performed wound-healing assays. MGC-803 and HGC-27 gastric cancer cell lines were selected for the test, and cells were treated with a low dose of extract (60 μg/mL), which reduced cell viability by about 20%. Our results showed that Cycas revoluta Thunb. extract significantly reduced gastric cancer cell migration after 24 h of extract treatment, especially for MGC-803 cells, whose wound width was over twice that of control cells (Figure 2A). To further investigate its effect on gastric cancer cell invasion, we performed transwell invasion assays. Cycas revoluta Thunb. natural extract markedly reduced the invasion ability of both MGC-803 and HGC-27 cell lines (Figure 2B). Taken together, these results demonstrate that the Cycas revoluta Thunb. natural extract effectively inhibited malignant gastric cancer cell migration and invasion.
To determine whether Cycas revoluta Thunb. natural extract can be used in combination with other anti-cancer drugs, we chose 5-Fu, one of the most widely used chemotherapy drugs, for clonogenic assays.
By assessing colony formation ability, we found that although the inhibitory effect of 5-Fu was stronger than that of the extract, combining the two drugs enhanced the inhibitory effect of 5-Fu (Figure 3A).
To investigate whether the increased inhibitory effect was due to chemosensitization, we performed cell viability assays with a low dose of extract (60 μg/mL) and increasing doses of 5-Fu for 24 h. We found that the Cycas revoluta Thunb. natural extract significantly sensitized gastric cancer cells to 5-Fu (Figure 3B). The EC50 and LC50 values dramatically decreased in MGC-803 (1.6 times and 2.8 times) and HGC-27 cells (1.8 times and 3.5 times), suggesting that Cycas revoluta Thunb. natural extract had additive and synergistic effects with 5-Fu in inhibiting gastric cancer cells. To further confirm this result, we examined the expression of three key drug-resistance-related proteins including p-gp, p-AKT and mTOR by western blot. We found that all three proteins significantly decreased when 5-Fu was used in combination with Cycas revoluta Thunb. natural extract (Figure 3C), which suggested reduced drug-resistance of gastric cancer cells.
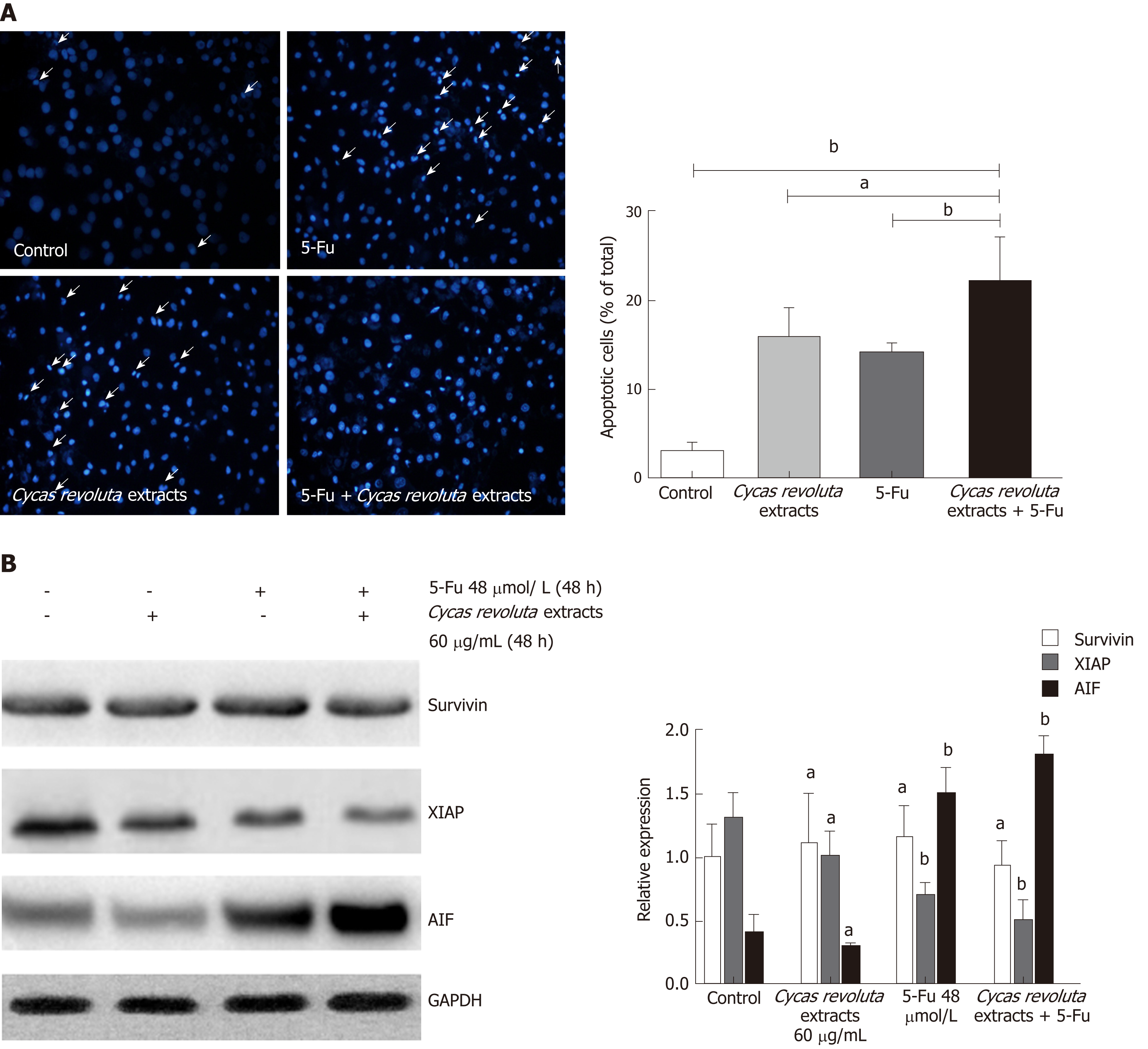
Because the Sotetsuflavone in Cycas revoluta Thunb. induced lung cancer cell apoptosis, we performed Hoechst 33258 staining in the 5-Fu-resistant gastric cancer line SGC-7901/R after treatment with the extract, 5-Fu or both. Both the extract and 5-Fu induced SGC-7901/R cell apoptosis, demonstrating that Cycas revoluta Thunb. extract can similarly induce gastric cancer cell apoptosis (Figure 4A). Moreover, the combination of 5-Fu and extract dramatically increased the extent of apoptosis (Figure 4A), suggesting that the Cycas revoluta Thunb. extract-induced chemosensitization of 5-Fu may be mediated via apoptosis . To confirm this hypothesis, we examined the expressional level of three important proteins involved in the apoptosis pathway. XIAP and AIF expression significantly decreased and increased, respectively, while the survivin expression remained stable (Figure 4B). This result demonstrated that the chemosensitive enhancement of 5-Fu and Cycas revoluta Thunb. extract may be due to further activation of apoptosis.
As one of the most common cancers, gastric cancer has been frequently diagnosed and has led to thousands of deaths worldwide. Around 500,000 people in China died from it just in 2015[3]. Although 5-Fu is often employed as chemotherapy against gastric cancer, its effect varies, likely due to the drug resistance of gastric tumors[10,11,27]. To overcome the problem of increasing drug resistance, 5-Fu is usually used with other compounds to enhance cancer cell sensitivity. According to previous studies, the traditional Chinese medicine Cycas revoluta Thunb. exhibited this potential synergistic effect[37,38]. Therefore, we investigated its inhibitory effect and the effect of chemosensitive enhancement with 5-Fu in gastric cancer.
In this study, we found that Cycas revoluta Thunb. extract effectively inhibited gastric cancer cell growth with little effect on normal gastric cells at low and medium doses (0 μg/mL-250 μg/mL). Combined with the significant decrease in EC50 and LC50 values for gastric cancer cells, we conclude that gastric cancer cells are more sensitive to Cycas revoluta Thunb. extract than normal gastric cells. However, the inhibitory effect on normal cells dramatically increased when the concentration of the extract was over 250 μg/mL. In contrast, the viability rate of cancer cells increased. This finding may be due to the strong screening effect of high concentrations of Cycas revoluta Thunb. natural extract, in which drug-resistant cancer cells rapidly proliferate, or employ other adaptive mechanisms. This result also suggests that the dose of Cycas revoluta Thunb. natural extract should be strictly controlled during practical application. Additionally, we demonstrated that Cycas revoluta Thunb. natural extract significantly decreased the migration and invasion ability of gastric cancer cells, further confirming its inhibitory effect on gastric cancer cells.
To determine whether Cycas revoluta Thunb. natural extract could be used with 5-Fu, we carried out clonogenic assays. The results showed that 5-Fu exhibited a stronger inhibitory effect than the extract, but combining the two drugs further inhibited cancer cell colony formation. By analyzing EC50 and LC50 values, we can conclude that cancer cell sensitivity to 5-Fu increased in the presence of Cycas revoluta Thunb. natural extract. This result was further confirmed by detecting the expression of the drug-resistance-related proteins p-gp, p-AKT and mTOR. p-AKT and mTOR expression were even further decreased in 5-Fu treatments when combined with Cycas revoluta Thunb. natural extract, suggesting that the changes in expression are highly related to Cycas revoluta Thunb-mediated enhancement of sensitivity of gastric cancer cells. mTOR is involved in AKT phosphorylation, which activates this enzyme. The activation of the AKT-mTOR pathway has been widely observed in various cancers, such as bladder cancer, breast cancer and non-small cell lung cancer[40-45]. This pathway plays an important role in regulating proliferation, survival, metastasis, and drug resistance of tumors, such as paclitaxel or endocrine therapy[44,45]. Some preclinical and clinical evidence has also suggested that NEAT1, BAG-1 and XPC are involved in the enhanced drug resistance of cancer cells mediated by the AKT-mTOR pathway[41,42,44,45], which may provide some clues for us to further explore the mechanism that the extract sensitizes gastric cancer cells to 5-Fu through the AKT-mTOR pathway.
Hoechst 33258 staining proved that using the two compounds together obviously increased the rate of cancer cell apoptosis, suggesting that Cycas revoluta Thunb. natural extract further induces apoptosis in 5-Fu treatments. This hypothesis was further confirmed by examining the expression of the apoptosis-related proteins XIAP, AIF and survivin. We observed that the activator AIF increased and inhibitor XIAP decreased, further explaining the increased apoptosis of gastric cancer cells. However, survivin expression remained stable, which suggests that this enhancement of apoptosis may not be mediated by survivin.
In conclusion, this study suggests that Cycas revoluta Thunb. natural extract can inhibit gastric cancer cell growth, migration and invasion. Moreover, it can be used to enhance the effect of 5-Fu through the AKT-mTOR pathway, which provides a promising strategy in chemotherapy against gastric cancer.
As one of the most frequent cancers, gastric cancer caused more than 700,000 deaths in just 2012 worldwide. Although 5-fluorouracil (5-Fu) is often employed as treatment against gastric cancer, its effect is severely affected by drug resistance of gastric cancer cells. Cycas revoluta Thunb. Extract has shown promise as a cancer treatment, though its effect on gastric cancer remains unknown.
To find new ways for chemical sensitization of cancer cells and improve the effect of 5-Fu during chemotherapy against malignancies.
To explore the anti-cancer effect of Cycas revoluta Thunb. in gastric cancer and investigate its chemical sensitization effect against gastric cancer cells during 5-Fu treatment.
The half-maximal effective concentration and the half-maximal lethal concentration of drugs were determined by cell viability test. The effect of Cycas revoluta Thunb. on gastric cancer cell migration was investigated by wound-healing and transwell assay. The synergistic effect between Cycas revoluta Thunb. and 5-Fu was confirmed by clonogenic assay and apoptosis detection. The expression of crucial proteins was measured by western blotting.
We found that the natural extract of Cycas revoluta Thunb. Preferentially killed gastric cancer cells compared to normal gastric cells. In addition, the extract significantly inhibited gastric cancer cell growth, migration and invasion. Cycas revoluta Thunb. can also improve the inhibitory effects of 5-Fu and effectively induce cell apoptosis. Western blotting analysis showed that P-glycoprotein, p-AKT and mammalian target of rapamycin (mTOR) expression markedly decreased, suggesting that AKT-mTOR pathway plays an important role in chemical sensitization induced by Cycas revoluta Thunb.
Our study demonstrated that the natural extract of Cycas revoluta Thunb. can significantly inhibit gastric cancer cell growth, migration and invasion. Furthermore, it can also improve the effect of 5-Fu and promote apoptosis during chemotherapy. Therefore, our study provides a new drug for improving the clinical effect of chemotherapy in gastric cancer. Our study also showed that Cycas revoluta Thunb. Enhanced the effects of 5-Fu through the AKT-mTOR pathway, offering a novel mechanism for the chemical sensitization effect of Cycas revoluta Thunb.
In the future, research may reveal the main component of Cycas revoluta Thunb. that enhances the sensitivity of cancer cells and further develop for its application in anti-cancer treatments. The identification of the molecular pathway related to AKT-mTOR may further explain the underlying mechanism.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kazuya S, Tanabe S S-Editor: Ma RY L-Editor: Filipodia E-Editor: Ma YJ
| 1. | den Hoed CM, Kuipers EJ. Gastric Cancer: How Can We Reduce the Incidence of this Disease? Curr Gastroenterol Rep. 2016;18:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 117] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 2. | Song Z, Wu Y, Yang J, Yang D, Fang X. Progress in the treatment of advanced gastric cancer. Tumour Biol. 2017;39:1010428317714626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 644] [Article Influence: 80.5] [Reference Citation Analysis (0)] |
| 3. | Yang C, Jiang H, Huang S, Hong H, Huang X, Wang X, Liao W, Wang X, Chen X, Jiang L. The prognostic role of pretreatment thrombocytosis in gastric cancer: A systematic review and meta-analysis. Medicine (Baltimore). 2018;97:e11763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20504] [Article Influence: 2050.4] [Reference Citation Analysis (20)] |
| 5. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21364] [Article Influence: 2136.4] [Reference Citation Analysis (3)] |
| 6. | Fan YL, Fan BY, Li Q, Di HX, Meng XY, Ling N. Preparation of 5-fluorouracil-loaded nanoparticles and study of interaction with gastric cancer cells. Asian Pac J Cancer Prev. 2014;15:7611-7615. [PubMed] |
| 7. | Lei Z, Tan IB, Das K, Deng N, Zouridis H, Pattison S, Chua C, Feng Z, Guan YK, Ooi CH, Ivanova T, Zhang S, Lee M, Wu J, Ngo A, Manesh S, Tan E, Teh BT, So JB, Goh LK, Boussioutas A, Lim TK, Flotow H, Tan P, Rozen SG. Identification of molecular subtypes of gastric cancer with different responses to PI3-kinase inhibitors and 5-fluorouracil. Gastroenterology. 2013;145:554-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 340] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 8. | Li X, Cai H, Zheng W, Tong M, Li H, Ao L, Li J, Hong G, Li M, Guan Q, Yang S, Yang D, Lin X, Guo Z. An individualized prognostic signature for gastric cancer patients treated with 5-Fluorouracil-based chemotherapy and distinct multi-omics characteristics of prognostic groups. Oncotarget. 2016;7:8743-8755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Wang T, Huang B, Guo R, Ma J, Peng C, Zu X, Tang H, Lei X. A let-7b binding site SNP in the 3'-UTR of the Bcl-xL gene enhances resistance to 5-fluorouracil and doxorubicin in breast cancer cells. Oncol Lett. 2015;9:1907-1911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Wu Y, Qi Y, Liu H, Wang X, Zhu H, Wang Z. AMPK activator AICAR promotes 5-FU-induced apoptosis in gastric cancer cells. Mol Cell Biochem. 2016;411:299-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Zhang JT, Zhou WL, He C, Liu T, Li CY, Wang L. 5-Fluorouracil induces apoptosis of colorectal cancer cells. Genet Mol Res. 2016;15:15017326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Emoto Y, Yoshizawa K, Kinoshita Y, Yuki M, Yuri T, Tsubura A. Green tea extract attenuates MNU-induced photoreceptor cell apoptosis via suppression of heme oxygenase-1. J Toxicol Pathol. 2016;29:61-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Kao SJ, Lee WJ, Chang JH, Chow JM, Chung CL, Hung WY, Chien MH. Suppression of reactive oxygen species-mediated ERK and JNK activation sensitizes dihydromyricetin-induced mitochondrial apoptosis in human non-small cell lung cancer. Environ Toxicol. 2017;32:1426-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 14. | Ding WB, Wang YX, Dong CW. microRNA15b induced SMCC7721 apoptosis via down-regulation of XIAP. Eur Rev Med Pharmacol Sci. 2017;21:542-548. [PubMed] |
| 15. | Gao C, Yu H, Yan C, Zhao W, Liu Y, Zhang D, Li J, Liu N. X-linked inhibitor of apoptosis inhibits apoptosis and preserves the blood-brain barrier after experimental subarachnoid hemorrhage. Sci Rep. 2017;7:44918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Li X, Chen W, Zeng W, Wan C, Duan S, Jiang S. microRNA-137 promotes apoptosis in ovarian cancer cells via the regulation of XIAP. Br J Cancer. 2017;116:66-76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 17. | Qin S, Yang C, Zhang B, Li X, Sun X, Li G, Zhang J, Xiao G, Gao X, Huang G, Wang P, Ren H. XIAP inhibits mature Smac-induced apoptosis by degrading it through ubiquitination in NSCLC. Int J Oncol. 2016;49:1289-1296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Yang WZ, Zhou H, Yan Y. XIAP underlies apoptosis resistance of renal cell carcinoma cells. Mol Med Rep. 2018;17:125-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Candé C, Cohen I, Daugas E, Ravagnan L, Larochette N, Zamzami N, Kroemer G. Apoptosis-inducing factor (AIF): a novel caspase-independent death effector released from mitochondria. Biochimie. 2002;84:215-222. [PubMed] |
| 20. | Miyake K, Bekisz J, Zhao T, Clark CR, Zoon KC. Apoptosis-inducing factor (AIF) is targeted in IFN-α2a-induced Bid-mediated apoptosis through Bak activation in ovarian cancer cells. Biochim Biophys Acta. 2012;1823:1378-1388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491-501. [PubMed] |
| 22. | Chantalat L, Skoufias DA, Kleman JP, Jung B, Dideberg O, Margolis RL. Crystal structure of human survivin reveals a bow tie-shaped dimer with two unusual alpha-helical extensions. Mol Cell. 2000;6:183-189. [PubMed] |
| 23. | Gu F, Li L, Yuan QF, Li C, Li ZH. Down-regulation of survivin enhances paclitaxel-induced Hela cell apoptosis. Eur Rev Med Pharmacol Sci. 2017;21:3504-3509. [PubMed] |
| 24. | Jaiswal PK, Goel A, Mittal RD. Survivin: A molecular biomarker in cancer. Indian J Med Res. 2015;141:389-397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 186] [Cited by in RCA: 240] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 25. | Khan Z, Khan AA, Yadav H, Prasad GBKS, Bisen PS. Survivin, a molecular target for therapeutic interventions in squamous cell carcinoma. Cell Mol Biol Lett. 2017;22:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 26. | Sokołowska J, Urbańska K. Survivin expression in correlation with apoptotic activity in canine lymphomas. Pol J Vet Sci. 2017;20:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 27. | Tang H, Zeng L, Wang J, Zhang X, Ruan Q, Wang J, Cui S, Yang D. Reversal of 5-fluorouracil resistance by EGCG is mediate by inactivation of TFAP2A/VEGF signaling pathway and down-regulation of MDR-1 and P-gp expression in gastric cancer. Oncotarget. 2017;8:82842-82853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 28. | Jin P, Wong CC, Mei S, He X, Qian Y, Sun L. MK-2206 co-treatment with 5-fluorouracil or doxorubicin enhances chemosensitivity and apoptosis in gastric cancer by attenuation of Akt phosphorylation. Onco Targets Ther. 2016;9:4387-4396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | He K, Zheng X, Li M, Zhang L, Yu J. mTOR inhibitors induce apoptosis in colon cancer cells via CHOP-dependent DR5 induction on 4E-BP1 dephosphorylation. Oncogene. 2016;35:148-157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 30. | Li Q, Mou LJ, Tao L, Chen W, Sun XT, Xia XF, Wu XY, Shi XL. Inhibition of mTOR suppresses human gallbladder carcinoma cell proliferation and enhances the cytotoxicity of 5-fluorouracil by downregulating MDR1 expression. Eur Rev Med Pharmacol Sci. 2016;20:1699-1706. [PubMed] |
| 31. | Sun Y, Jiang Y, Huang J, Chen H, Liao Y, Yang Z. CISD2 enhances the chemosensitivity of gastric cancer through the enhancement of 5-FU-induced apoptosis and the inhibition of autophagy by AKT/mTOR pathway. Cancer Med. 2017;6:2331-2346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 32. | Guo XF, Yang ZR, Wang J, Lei XF, Lv XG, Dong WG. Synergistic antitumor effect of puerarin combined with 5-fluorouracil on gastric carcinoma. Mol Med Rep. 2015;11:2562-2568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Rani I, Sharma B, Kumar S, Kaur S, Agnihotri N. Apoptosis mediated chemosensitization of tumor cells to 5-fluorouracil on supplementation of fish oil in experimental colon carcinoma. Tumour Biol. 2017;39:1010428317695019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 34. | Xu GY, Tang XJ. Troxerutin (TXN) potentiated 5-Fluorouracil (5-Fu) treatment of human gastric cancer through suppressing STAT3/NF-κB and Bcl-2 signaling pathways. Biomed Pharmacother. 2017;92:95-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 35. | Zhang L, Xing Y, Gao Q, Sun X, Zhang D, Cao G. Combination of NRP1-mediated iRGD with 5-fluorouracil suppresses proliferation, migration and invasion of gastric cancer cells. Biomed Pharmacother. 2017;93:1136-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 36. | Kowalska MT, Itzhak Y, Puett D. Presence of aromatase inhibitors in cycads. J Ethnopharmacol. 1995;47:113-116. [PubMed] |
| 37. | Wang S, Hu Y, Yan Y, Cheng Z, Liu T. Sotetsuflavone inhibits proliferation and induces apoptosis of A549 cells through ROS-mediated mitochondrial-dependent pathway. BMC Complement Altern Med. 2018;18:235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 38. | Wang S, Yan Y, Cheng Z, Hu Y, Liu T. Sotetsuflavone suppresses invasion and metastasis in non-small-cell lung cancer A549 cells by reversing EMT via the TNF-α/NF-κB and PI3K/AKT signaling pathway. Cell Death Discov. 2018;4:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 39. | Pulito C, Mori F, Sacconi A, Goeman F, Ferraiuolo M, Pasanisi P, Campagnoli C, Berrino F, Fanciulli M, Ford RJ, Levrero M, Pediconi N, Ciuffreda L, Milella M, Steinberg GR, Cioce M, Muti P, Strano S, Blandino G. Metformin-induced ablation of microRNA 21-5p releases Sestrin-1 and CAB39L antitumoral activities. Cell Discov. 2017;3:17022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 40. | Sathe A, Nawroth R. Targeting the PI3K/AKT/mTOR Pathway in Bladder Cancer. Methods Mol Biol. 2018;1655:335-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 120] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 41. | Sharma VR, Gupta GK, Sharma AK, Batra N, Sharma DK, Joshi A, Sharma AK. PI3K/Akt/mTOR Intracellular Pathway and Breast Cancer: Factors, Mechanism and Regulation. Curr Pharm Des. 2017;23:1633-1638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 42. | Ciruelos Gil EM. Targeting the PI3K/AKT/mTOR pathway in estrogen receptor-positive breast cancer. Cancer Treat Rev. 2014;40:862-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 246] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 43. | Ippen FM, Alvarez-Breckenridge CA, Kuter BM, Fink AL, Bihun IV, Lastrapes M, Penson T, Schmidt SP, Wojtkiewicz GR, Ning J, Subramanian M, Giobbie-Hurder A, Martinez-Lage M, Carter SL, Cahill DP, Wakimoto H, Brastianos PK. The dual PI3K/mTOR-pathway inhibitor GDC-0084 achieves antitumor activity in PIK3CA-mutant breast cancer brain metastases. Clin Cancer Res. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 44. | Li B, Gu W, Zhu X. NEAT1 mediates paclitaxel-resistance of non-small cell of lung cancer through activation of Akt/mTOR signaling pathway. J Drug Target. 2019;1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 45. | Guerrero-Zotano A, Mayer IA, Arteaga CL. PI3K/AKT/mTOR: role in breast cancer progression, drug resistance, and treatment. Cancer Metastasis Rev. 2016;35:515-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 301] [Article Influence: 37.6] [Reference Citation Analysis (0)] |