Published online Apr 14, 2019. doi: 10.3748/wjg.v25.i14.1753
Peer-review started: February 6, 2019
First decision: February 21, 2019
Revised: February 27, 2019
Accepted: March 11, 2019
Article in press: March 12, 2019
Published online: April 14, 2019
Processing time: 72 Days and 23.5 Hours
We recently reported on a hereditary enteropathy associated with a gene encoding a prostaglandin transporter and referred to as chronic enteropathy associated with SLCO2A1 gene (CEAS). Crohn’s disease (CD) is a major differential diagnosis of CEAS, because these diseases share some clinical features. Therefore, there is a need to develop a convenient screening test to distinguish CEAS from CD.
To examine whether prostaglandin E major urinary metabolites (PGE-MUM) can serve as a biomarker to distinguish CEAS from CD.
This was a transactional study of 20 patients with CEAS and 98 patients with CD. CEAS was diagnosed by the confirmation of homozygous or compound heterozygous mutation of SLCO2A1. We measured the concentration of PGE-MUM in spot urine by radioimmunoassay, and the concentration was compared between the two groups of patients. We also determined the optimal cut-off value of PGE-MUM to distinguish CEAS from CD by receiver operating characteristic (ROC) curve analysis.
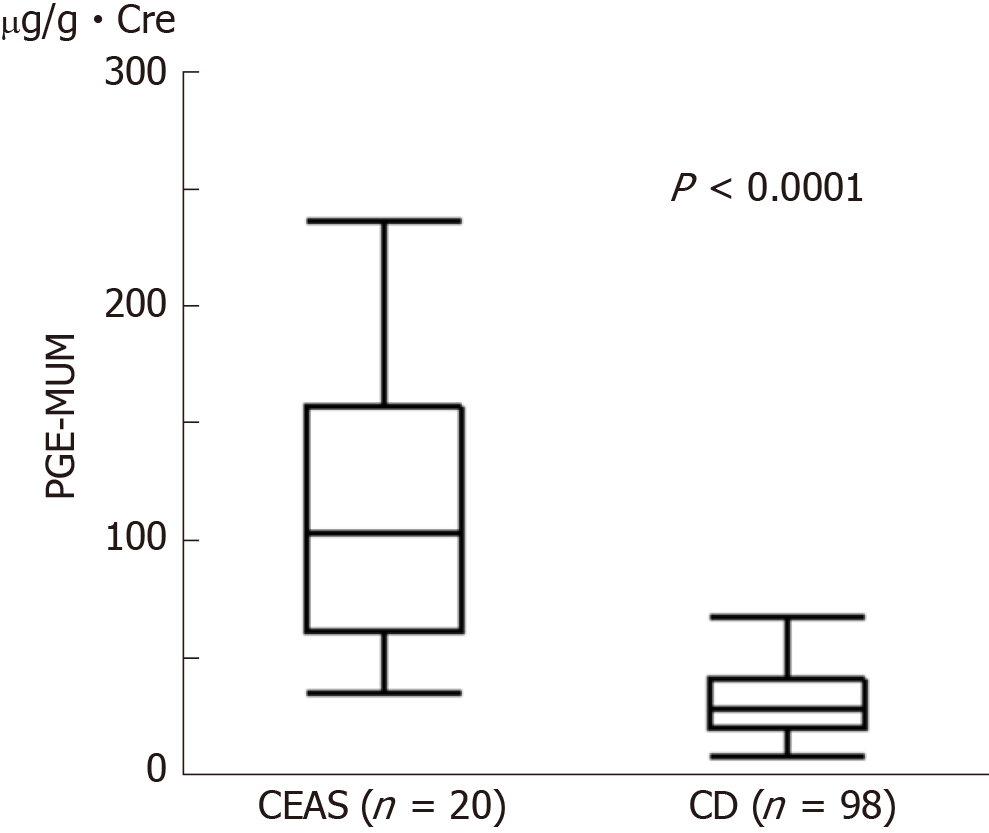
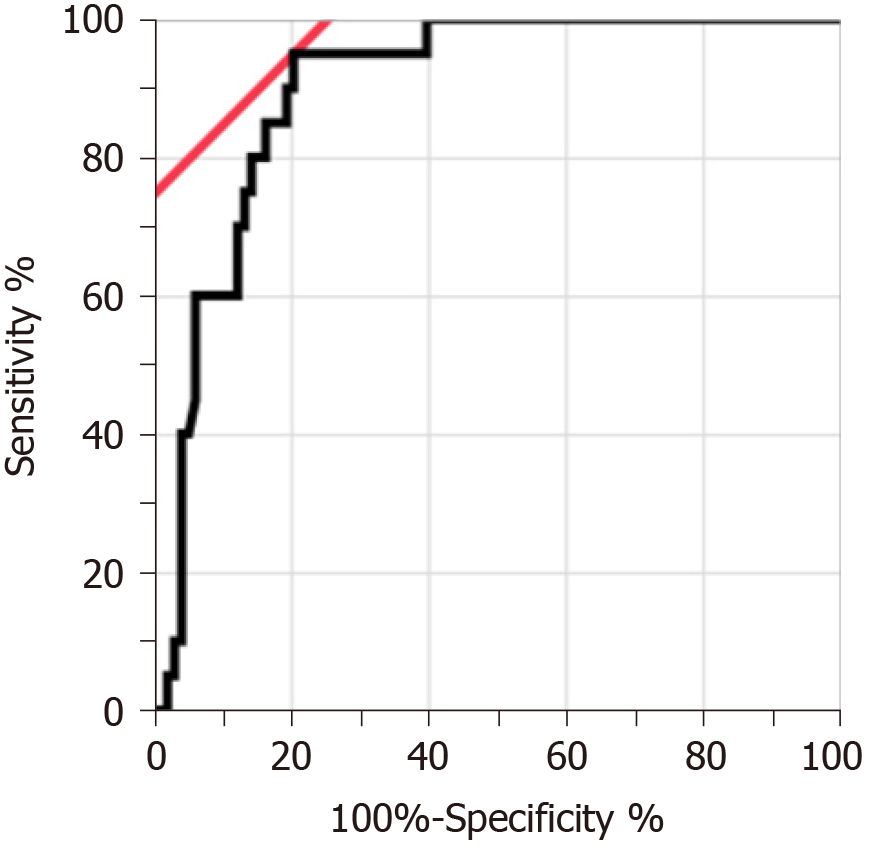
Twenty Japanese patients with CEAS and 98 patients with CD were enrolled. PGE-MUM concentration in patients with CEAS was significantly higher than that in patients with CD (median 102.7 vs 27.9 μg/g × Cre, P < 0.0001). One log unit increase in PGE-MUM contributed to 7.3 increase in the likelihood for the diagnosis of CEAS [95% confidence interval (CI) 3.2-16.7]. A logistic regression analysis revealed that the association was significant even after adjusting confounding factors (adjusted odds ratio 29.6, 95%CI 4.7-185.7). ROC curve analysis revealed the optimal PGE-MUM cut-off value for the distinction of CEAS from CD to be 48.9 μg/g × Cre with 95.0% sensitivity and 79.6% specificity.
PGE-MUM measurement is a convenient, non-invasive and useful test for the distinction of CEAS from CD.
Core tip: Chronic enteropathy associated with SLCO2A1 gene (CEAS) is a rare hereditary enteropathy associated with a gene encoding a prostaglandin transporter. It is sometimes difficult to distinguish CEAS from Crohn’s disease (CD), because these diseases share some clinical features. We report the usefulness of prostaglandin E-major urinary metabolites (PGE-MUM) to differentiate CEAS from CD. PGE-MUM concentration was significantly higher in CEAS than in CD, and optimal cut-off value for the distinction of CEAS from CD to be 48.9 μg/g × Cre with sensitivity of 95.0% and specificity of 79.6%. In clinical practice, PGE-MUM measurement might be useful as a screening test for CEAS.
- Citation: Matsuno Y, Umeno J, Esaki M, Hirakawa Y, Fuyuno Y, Okamoto Y, Hirano A, Yasukawa S, Hirai F, Matsui T, Hosomi S, Watanabe K, Hosoe N, Ogata H, Hisamatsu T, Yanai S, Kochi S, Kurahara K, Yao T, Torisu T, Kitazono T, Matsumoto T. Measurement of prostaglandin metabolites is useful in diagnosis of small bowel ulcerations. World J Gastroenterol 2019; 25(14): 1753-1763
- URL: https://www.wjgnet.com/1007-9327/full/v25/i14/1753.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i14.1753
The use of capsule endoscopy and balloon-assisted endoscopy has enabled the precise observation of small bowel mucosal lesions in gastrointestinal disease pathology, such as Crohn’s disease (CD), intestinal tuberculosis, and nonsteroidal anti-inflammatory drug (NSAID)-induced enteropathy[1-5]. While characteristic endoscopic findings of small bowel inflammation have been reported, it is sometimes difficult to correctly diagnose patients by endoscopic findings alone in cases lacking characteristic features of the disease.
We previously reported an independent disease entity termed chronic nonspecific multiple ulcers of the small intestine (CNSU) characterized by multiple small intestinal ulcers of nonspecific histology[6-9]. In addition, we recently found that CNSU is caused by recessive inheritance of the SLCO2A1 gene and proposed a new nomenclature for this disease: Chronic enteropathy associated with SLCO2A1 gene (CEAS)[10]. Therefore, it is now possible to diagnose CEAS based on a combination of clinical features and genetic analysis. However, it is sometimes difficult to differentiate CEAS from CD because these diseases share clinical features such as persistent anemia and hypoproteinemia, susceptible age in puberty, and a predominance of ileal involvement. The differentiation between CEAS and CD is clinically essential to avoid ineffective therapies because patients with CEAS do not respond to treatment with corticosteroids or biologics[7,9]. Moreover, since the patients with CEAS usually show low inflammatory markers in blood test[11], it is a particularly important problem to distinguish CEAS from CD with low disease activity. In clinical practice, however, it is unrealistic to perform genetic screening for all patients suspected as CD or CEAS.
The SLCO2A1 gene encodes a prostaglandin transporter that mediates the cellular uptake of prostaglandins. SLCO2A1 is also the causative gene of primary hypertrophic osteoarthropathy (PHO)[12-14]. An increase in urinary levels of prostaglandin E2 (PGE2) and prostaglandin E metabolites in SLCO2A1-deficient PHO individuals has been confirmed[14]. PGE2 is known to have the protective effect on the mucosal injury, however it is difficult to directly measure PGE2 level in local tissues or in the blood because of its extremely rapid metabolism[15]. Prostaglandin E-major urinary metabolite (PGE-MUM) is known to be stable and we have also previously demonstrated an increase in the urinary levels of prostaglandin E metabolites in CEAS patients[10]. Therefore, this study investigated whether PGE-MUM are useful for differentiating CEAS from CD.
Among the participants of our previous studies[10,16,17] and the patients diagnosed as CEAS or CD at Kyushu University hospital, we enrolled 20 Japanese patients with CEAS and 98 patients with CD for the present investigation. A diagnosis of CEAS was confirmed based on published clinical criteria[18,19] with genetic analysis (Supplementary Table 1). The diagnosis of CD was based on the Japanese diagnostic criteria for CD[20]. No patients were administrated prostaglandin analogues or NSAIDs at the time of urine sample collection. All urine and blood samples were collected after obtaining written informed consent. This study was approved by the institutional review board at each collecting site in accordance with the Declaration of Helsinki Principles.
| CEAS (n = 20) | CD (n = 98) | P value1 | |
| Age, yr | 56 (41-63) | 44 (38-54) | 0.098 |
| Gender | |||
| Male | 5 (25) | 66 (67) | 0.0008 |
| Female | 15 (75) | 32 (33) | |
| Smoking | |||
| Never smoker | 16 (80) | 68 (69) | 0.42 |
| Current smoker | 1 (5) | 13 (13) | |
| Former smoker | 3 (15) | 17 (18) | |
| Pulmonary disease | 1 (5) | 5 (5) | 1 |
| History of surgery | 13 (65) | 59 (60) | 0.8 |
| CRP, g/dL | 0.30 (0.11-0.53) | 0.13 (0.03-0.58) | 0.26 |
| Disease location | |||
| L1, ileal | 34 (35) | ||
| L2, colonic | 14 (14) | ||
| L3, ileocolonic | 50 (51) | ||
| Disease duration, yr | 33 (19-42) | 20 (13-29) | 0.0055 |
Clinical information including age, gender, history of surgery, concomitant drugs, serum C reactive protein (CRP) levels, disease location, and disease duration was collected from each participating institution. Because the PGE2 metabolite concentration was reported to increase in cases of thiazide-induced hyponatremia[21] and laxative sennoside administration[22], we collected information about the use of these medications. Because the PGE-MUM concentration was reported to be influenced by age, gender, smoking habit, and pulmonary inflammatory conditions including chronic fibrosing interstitial pneumonia[15,23], we also collected information about smoking habit and pulmonary disease. Age was determined at the time of urine sample collection. Smoking habit was defined as never, current (> 6 mo on a daily basis), and former (cessation of smoking > 6 mo). Pulmonary diseases included chronic respiratory disease, diffuse lung disease, and any other lung or airway disease. History of surgery was defined as a history of intestinal resection at the time of study enrolment. The site of involvement was determined by radiographic and/or endoscopic findings, and these patients were categorized according to the Montreal classification[24]. Disease duration was defined as the period from the onset of symptoms until study enrolment.
We used genomic DNA samples extracted from peripheral blood in our previous studies[10,16,17,25]. In advance, we checked and confirmed that none of the patients with CD had homozygous or compound heterozygous SLCO2A1 mutations with regards to the major six sites in our previous report[10], including c.421G>T, c.664G>A, c.940+1G>A, c.1372G>T, c.1461+1G>C, and c.1807C>T. The diagnosis of CEAS was confirmed with 90.2% sensitivity by genotyping these 6 mutations on the basis of our previous investigation.
All PGE-MUM concentrations were measured at SRL Inc. (Tokyo, Japan) by using a bicyclic PGE-MUM radioimmunoassay kit (Institute of Isotopes Co., Ltd. Budapest, Hungary). PGE-MUM, 7α-hydroxy-5, 11-diketotetranorprosta-1, 16-dioic acid derived from PGE1 and PGE2 can be transformed to bicyclic PGE-MUM by alkali treatment. Each urine sample was collected and submitted individually according to the manufacturer’s protocol described by Fujiwara et al[22]. The urine samples were frozen and stored at -20 °C until the assay. PGE-MUM concentrations were corrected by the urinary creatinine concentration.
Categorical variables were expressed as the number (%) and continuous variables were expressed as the median (interquartile range). Comparison of the variables between CEAS and CD were performed using the chi-squared test, Fisher’s exact probability test, or the Mann–Whitney U-test, where appropriate. The associations between clinical factors and the PGE-MUM concentration were estimated using univariable linear regression analyses. Because of the skewed distribution of the PGE-MUM concentration, values were log-transformed and used for regression analysis.
For the analysis of the clinical significance of PGE-MUM concentration to differentiate CEAS from CD, the logistic regression model was used to estimate the odds ratios (ORs) with 95% confidence intervals (CIs). The diagnostic accuracy of PGE-MUM concentration was evaluated using the area under the curve (AUC) with the receiver operating characteristic (ROC). The optimal cut-off value of PGE-MUM concentration for the diagnosis of CEAS was determined based on the maximum Youden Index (sensitivity + specificity - 1). In addition, sensitivity and specificity were compared using several cut-off values to confirm the diagnostic accuracy of this cut-off value. Statistical analyses were performed using JMP version 13.0 (SAS Institute, Cary, NC, United States). A P value of 0.05 or smaller was considered statistically significant.
The enrolled subjects consisted of 20 patients with CEAS (CEAS group) and 98 patients with CD (CD group). The clinical characteristics of the patients are shown in Table 1. In the CEAS group, female patients were more frequent compared with the CD group. The disease duration was significantly longer in the CEAS group than in the CD group (median 33 vs 20 years, P = 0.0055). There was no significant difference in smoking status or medical history of pulmonary disease. Because most patients with CD were in clinical remission at the time of urine sample collection, the CRP levels remained low in the CD group.
Overall, 118 urinary samples were obtained from CEAS and CD patients. The PGE-MUM concentration ranged from 7.7 to 402.0 (median 34.1) μg/g × Cre. Table 2 shows the result of univariable linear regression analysis. A positive history of pulmonary disease and history of surgery were significantly associated with a higher PGE-MUM concentration (P < 0.0001 and P = 0.029, respectively). Serum CRP value was marginally associated with PGE-MUM concentration (P = 0.078). Factors with a P value < 0.1 were included in the multivariate analysis considering the possibility of confounding factors.
| Univariable linear regression (P value) | |
| Age | P = 0.47 (0.067) |
| Gender (1, female; 0, male) | P = 0.26 (0.10) |
| Smoking (1, Current or former smoker; 0, Never smoker) | P = 0.17 (-0.13) |
| Pulmonary disease | P < 0.0001 (0.36) |
| History of surgery | P = 0.029 (0.20) |
| CRP | P = 0.078 (0.16) |
| Disease duration | P = 0.0040 (0.26) |
The PGE-MUM concentration was significantly higher in the CEAS group than in the CD group [median interquartile range (IQR): 102.7 (62.0-155.0) μg/g × Cre vs 27.9 (19.6-40.0) μg/g × Cre, P < 0.0001, Figure 1]. Crude OR of 1 log unit increase in the PGE-MUM concentration for the distinction between CEAS and CD was 7.3 (95%CI 3.2-16.7, Table 3). This prediction model showed good differentiating ability between CEAS and CD, with an AUC of 0.90 (95%CI 0.82-0.95). This association was significant even after adjusting for possible confounding factors including age, gender, medical history of pulmonary disease, history of surgery, disease duration, and serum CRP value (model 3). The multivariate-adjusted OR was 29.6 (95%CI 4.7-185.7), with an AUC of 0.95 (95%CI 0.86-0.98).
| Model 1 | Model 2 | Model 3 | |
| Odds ratio of 1 log unit change | 7.3 | 6 | 29.6 |
| (95%CI) | (3.2-16.7) | (2.6-13.9) | (4.7-185.7) |
| AUC (95%CI) | 0.90 (0.82-0.94) | 0.93 (0.87-0.96) | 0.95 (0.86-0.98) |
The PGE-MUM concentration in the colonic CD group (L2) was significantly lower than in the other groups (median: 16.5 μg/g × Cre vs 29.5 μg/g × Cre, P = 0.036), and the PGE-MUM concentration of the ileal CD group (L1) was significantly higher than in the colonic CD group (median: 32.9 μg/g × Cre vs 16.5 μg/g × Cre, P = 0.017). When we confined the subjects to those with ileal CD, the PGE-MUM concentration in the CEAS group was still significantly higher than that in the ileal CD group (median 102.7 μg/g × Cre vs 32.9 μg/g × Cre, P < 0.0001).
Figure 2 shows the ROC curve of the PGE-MUM concentration to differentiate CEAS from CD. Based on the ROC analysis, the optimal cut-off value was identified as 48.9 μg/g × Cre, which differentiated CEAS from CD with 95.0% sensitivity and 79.6% specificity (Supplementary Table 2). The AUC was 0.90 (95%CI, 0.82-0.95).
Among the CD group, six patients had particularly high PGE-MUM concentrations (> 100 μg/g × Cre). Although we confirmed that they did not harbor the six major mutations in the SLCO2A1 gene, we screened all 14 coding exons and intron-exon boundaries of the SLCO2A1 gene using Sanger sequencing to exclude a possible misdiagnosis of CEAS. No homozygous or compound heterozygous SLCO2A1 mutations were found in the six CD patients.
CEAS was initially reported as a novel disease entity referred to as "C" in the Japanese literature in the 1960s[26]. We recently reported it is an autosomal recessive inherited disease[8] caused by loss-of-function mutations in the SLCO2A1 gene[10]. Small bowel lesions in CEAS are characterized by multiple shallow ulcers with a circular or eccentric oblique configuration[18]. In contrast, CD typically develops longitudinal ulcers and cobblestoning, and ulcerations are predominantly located on the mesenteric side. However, both CEAS and CD involve the ileum with occasional ileal stenoses, and the clinical features of CEAS and CD are similar; therefore, it is not easy to differentiate CEAS from CD based on clinical manifestations alone[11]. Although immunohistochemical staining for SLCO2A1 protein in biopsy specimens from the GI tract was reported to be useful to distinguish CEAS from CD[27,28], a more convenient and non-invasive screening test is required.
PGE-MUM, a major urinary metabolite derived from PGE2, is clinically used to assess the severity of inflammatory conditions such as interstitial pneumonia[23], cystic fibrosis[29], and ulcerative colitis[30,31]. Similarly, urinary PGE2 metabolite concentrations in patients with CD are higher than those in patients with colorectal neoplasms and healthy controls[32]. Indeed, PGE-MUM concentrations in patients with CD (median 27.9, IQR 19.6-40.0 μg/g × Cre) in the present study were higher than those in Japanese healthy controls (median 13.1, IQR 10.3-17.2 μg/g × Cre) as reported in previous studies[15,23]. Nevertheless, the PGE-MUM concentration, a surrogate marker of physiological PGE2 levels[30], in patients with CEAS was significantly higher than in patients with CD. This difference was still significant even after the adjustment for confounding factors. In addition, we demonstrated that the provisional cut-off value (48.9 μg/g × Cre) of PGE-MUM had a differentiating ability with 95.0% sensitivity and 79.6% specificity (AUC 0.90). Therefore, PGE-MUM is useful to distinguish CEAS from CD.
Although prostaglandins are mediators of active inflammation, many experimental studies have indicated that they play pivotal roles in maintaining mucosal homeostasis[33] and mucosal repair[34] of the intestine. PGE2 is mainly derived from arachidonic acid by cyclooxygenase and PGE synthase and PGE2 acts through four different receptor subtypes (EP1, EP2, EP3 and EP4), which partly determines the various physiological effects of PGE2 including the regulation of acid secretion, bicarbonate secretion, mucus production, and mucosal blood flow[35]. In particular, dimethyl PGE2 (dmPGE2), a stable derivative of PGE2, was reported to exert a potent inhibitory effect against indomethacin-induced intestinal injuries in a rat model via activation of EP3 and EP4 receptors[36]. It was also shown that the deleterious effect of indomethacin on the healing of intestinal ulcers was induced by an EP4 antagonist and suppressed by the co-administration of PGE2 as well as an EP4 agonist[34]. These results indicate that the protective effect of PGE2 on mucosal injury of the intestine is caused by EP3 and EP4 activation.
PHO, which develops skin and bone disorders caused by impaired prostaglandin metabolism, is classified into two types: PHOAR2 caused by the SLCO2A1 gene[12], and PHOAR1 caused by the HPGD gene[37]. Prostaglandin-signaling is terminated by the cellular uptake of prostaglandins via the prostaglandin transporter, followed by intracellular oxidation by 15-ketoprostaglandin dehydrogenase (15-PGDH) encoded by the HPGD gene[38]. Thus, cellular uptake of prostaglandins is the first critical step in inactivating prostaglandins. The SLCO2A1 gene mediates the degradation of prostaglandins via their intracellular uptake[39,40]. The HPGD gene is also related to the degradation of prostaglandins by encoding intracellular oxidation enzymes of 15-PGDH. In both conditions, significantly elevated plasma PGE2 levels have been observed[13,37]. Because PGE2 promotes the proliferation of osteoblasts in bone tissue via the expression of vascular endothelial growth factor[41,42], various clinical manifestations of PHO such as digital clubbing, periostosis, acroosteolysis, painful joint enlargement, and thickened skin, are suggested to be the consequence of an overabundance of PGE2. Although PGE2 is protective against gastrointestinal mucosal injuries[35,42], multiple ileal ulcers occur in patients with CEAS, in whom systemic levels of PGE2 are elevated. Considering our previous result that the prostaglandin transporter was expressed on the cellular membrane of vascular endothelial cells[10], it can be presumed that impaired prostaglandin use in the intestinal mucosa caused by mutation of the SLCO2A1 gene is a major factor in the pathogenesis of intestinal ulcers in CEAS.
Cryptogenic multifocal ulcerous stenosing enteritis (CMUSE) is another disease entity that causes gastrointestinal ulcerations resembling CEAS[43,44]. Recently, recessive mutations in the PLA2G4A gene, encoding cytoplasmic phospholipase A2-α (cPLA2α), which hydrolyses cellular membrane phospholipids into arachidonic acids, have been identified as a cause of CMUSE[44,45]. Because the loss-of-function of cPLA2α decreases systemic levels of PGE2 (approximated by PGE-MUM) and thromboxane A2[45], multiple small bowel ulcers, as well as the dysfunction of platelet aggregation are observed in patients with CMUSE[46]. Although precise analyses of the morphologic features of gastrointestinal lesions in CMUSE have not been reported yet, it seems likely that CEAS and CMUSE share morphologic features regarding the gastrointestinal lesions. In this sense, the measurement of PGE-MUM concentrations can be assumed to be useful to differentiate CEAS from CMUSE, although genetic testing is mandatory to confirm the diagnosis.
This study had several limitations. First, the sample size of the patients with CEAS was relatively small because CEAS is a rare disease. Therefore, we could not validate the cut-off value for the distinction between the two diseases. Second, because most of our CD patients were in clinical remission, the cut-off value may not be appropriate for the distinction of CEAS from active CD. Indeed, some patients with quiescent CD had markedly high PGE-MUM concentrations. Third, we could not completely rule out the possibility that the patients with CD had recessive mutations in the SLCO2A1 gene. Although we checked the major six mutations of the SLCO2A1 gene in patients with CD, 9.8% of the mutations might be overlooked based on our previous result[10].
In conclusion, the measurement of PGE-MUM might be a convenient, non-invasive and useful test to differentiate CEAS from CD, although genetic analysis is mandatory for the confirmation of CEAS. Further studies are necessary to clarify the pathogenesis of CEAS to explore therapeutic targets of this disease.
Chronic enteropathy associated with SLCO2A1 gene (CEAS) is a rare small intestinal disease, but distinct clinical condition from Crohn’s disease (CD). To date, we can make a correct diagnosis of CEAS by combination of clinical features and genetic analysis. However, CEAS is sometimes misdiagnosed as CD, because these diseases have some clinical features in common.
A more convenient and non-invasive screening test will help to diagnose CEAS correctly.
To investigate the usefulness of prostaglandin E-major urinary metabolites (PGE-MUM) to distinguish CEAS from CD.
Participants were diagnosed CEAS and CD by clinical diagnostic criteria and genetic analysis. All participants’ PGE-MUM concentrations were measured by radioimmunoassay. We analyzed differentiating ability of PGE-MUM measurement between CEAS and CD.
Twenty patients with CEAS and 98 patients with CD were enrolled. It was found that the PGE-MUM concentrations of patients with CEAS were significantly higher than those of patients with CD and this correlation was still statistically significant after adjusting the possible confounding factors. Additionally, the present study showed the provisional cut-off value (48.9 μg/g × Cre) of PGE-MUM had a differentiating ability with 95.0% sensitivity and 79.6% specificity (area under the curve 0.90).
The measurement of PGE-MUM can serve as a useful biomarker to differentiate CEAS from CD, although genetic analysis is mandatory for the diagnosis of CEAS.
Our study showed the usefulness of PGE-MUM to differentiate CEAS from CD. In the present research, the sample size of the patients with CEAS were relatively small, the cut-off value for the distinction of CEAS from CD should be validated with large sample size of patients with CEAS in the future research.
We appreciate the patients for participating in this study. We also appreciate Ms. Risa Tsuneyoshi for technical assistance and Drs. Tomohiko Moriyama, Shin Fujioka, Naoya Kubokura, Yoichiro Nuki, Ema Washio, and Shinichi Kawano for their assistance with clinical characterization.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Caboclo JF, Slomiany BL, Tsoulfas G S-Editor: Yan JP L-Editor: A E-Editor: Song H
| 1. | Iddan G, Meron G, Glukhovsky A, Swain P. Wireless capsule endoscopy. Nature. 2000;405:417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1994] [Cited by in RCA: 1386] [Article Influence: 55.4] [Reference Citation Analysis (1)] |
| 2. | Yamamoto H, Sekine Y, Sato Y, Higashizawa T, Miyata T, Iino S, Ido K, Sugano K. Total enteroscopy with a nonsurgical steerable double-balloon method. Gastrointest Endosc. 2001;53:216-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 896] [Cited by in RCA: 861] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 3. | Matsumoto T, Moriyama T, Esaki M, Nakamura S, Iida M. Performance of antegrade double-balloon enteroscopy: Comparison with push enteroscopy. Gastrointest Endosc. 2005;62:392-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Matsumoto T, Kudo T, Esaki M, Yano T, Yamamoto H, Sakamoto C, Goto H, Nakase H, Tanaka S, Matsui T, Sugano K, Iida M. Prevalence of non-steroidal anti-inflammatory drug-induced enteropathy determined by double-balloon endoscopy: A Japanese multicenter study. Scand J Gastroenterol. 2008;43:490-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 91] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 5. | Esaki M, Matsumoto T, Watanabe K, Arakawa T, Naito Y, Matsuura M, Nakase H, Hibi T, Matsumoto T, Nouda S, Higuchi K, Ohmiya N, Goto H, Kurokawa S, Motoya S, Watanabe M. Use of capsule endoscopy in patients with Crohn's disease in Japan: A multicenter survey. J Gastroenterol Hepatol. 2014;29:96-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Matsumoto T, Iida M, Matsui T, Yao T, Watanabe H, Yao T, Okabe H. Non-specific multiple ulcers of the small intestine unrelated to non-steroidal anti-inflammatory drugs. J Clin Pathol. 2004;57:1145-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Matsumoto T, Iida M, Matsui T, Yao T. Chronic nonspecific multiple ulcers of the small intestine: A proposal of the entity from Japanese gastroenterologists to Western enteroscopists. Gastrointest Endosc. 2007;66:S99-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Matsumoto T, Kubokura N, Matsui T, Iida M, Yao T. Chronic nonspecific multiple ulcer of the small intestine segregates in offspring from consanguinity. J Crohns Colitis. 2011;5:559-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Esaki M, Umeno J, Kitazono T, Matsumoto T. Clinicopathologic features of chronic nonspecific multiple ulcers of the small intestine. Clin J Gastroenterol. 2015;8:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Umeno J, Hisamatsu T, Esaki M, Hirano A, Kubokura N, Asano K, Kochi S, Yanai S, Fuyuno Y, Shimamura K, Hosoe N, Ogata H, Watanabe T, Aoyagi K, Ooi H, Watanabe K, Yasukawa S, Hirai F, Matsui T, Iida M, Yao T, Hibi T, Kosaki K, Kanai T, Kitazono T, Matsumoto T. A Hereditary Enteropathy Caused by Mutations in the SLCO2A1 Gene, Encoding a Prostaglandin Transporter. PLoS Genet. 2015;11:e1005581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 103] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 11. | Umeno J, Esaki M, Hirano A, Fuyuno Y, Ohmiya N, Yasukawa S, Hirai F, Kochi S, Kurahara K, Yanai S, Uchida K, Hosomi S, Watanabe K, Hosoe N, Ogata H, Hisamatsu T, Nagayama M, Yamamoto H, Abukawa D, Kakuta F, Onodera K, Matsui T, Hibi T, Yao T, Kitazono T, Matsumoto T; CEAS study group. Clinical features of chronic enteropathy associated with SLCO2A1 gene: A new entity clinically distinct from Crohn's disease. J Gastroenterol. 2018;53:907-915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 12. | Seifert W, Kühnisch J, Tüysüz B, Specker C, Brouwers A, Horn D. Mutations in the prostaglandin transporter encoding gene SLCO2A1 cause primary hypertrophic osteoarthropathy and isolated digital clubbing. Hum Mutat. 2012;33:660-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Zhang Z, Xia W, He J, Zhang Z, Ke Y, Yue H, Wang C, Zhang H, Gu J, Hu W, Fu W, Hu Y, Li M, Liu Y. Exome sequencing identifies SLCO2A1 mutations as a cause of primary hypertrophic osteoarthropathy. Am J Hum Genet. 2012;90:125-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 145] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 14. | Zhang Z, He JW, Fu WZ, Zhang CQ, Zhang ZL. Mutations in the SLCO2A1 gene and primary hypertrophic osteoarthropathy: A clinical and biochemical characterization. J Clin Endocrinol Metab. 2013;98:E923-E933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Okayasu I, Ohnishi H, Sarandi I, Shojima J, Komatsu J, Oritsu M, Sasabe M, Nanami KO, Matsuura M, Azumi J, Ito S, Fujiwara M. Significant increase of prostaglandin E-major urinary metabolite in male smokers: A screening study of age and gender differences using a simple radioimmunoassay. J Clin Lab Anal. 2014;28:32-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Hirano A, Yamazaki K, Umeno J, Ashikawa K, Aoki M, Matsumoto T, Nakamura S, Ninomiya T, Matsui T, Hirai F, Kawaguchi T, Takazoe M, Tanaka H, Motoya S, Kiyohara Y, Kitazono T, Nakamura Y, Kamatani N, Kubo M. Association study of 71 European Crohn's disease susceptibility loci in a Japanese population. Inflamm Bowel Dis. 2013;19:526-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | Yamazaki K, Umeno J, Takahashi A, Hirano A, Johnson TA, Kumasaka N, Morizono T, Hosono N, Kawaguchi T, Takazoe M, Yamada T, Suzuki Y, Tanaka H, Motoya S, Hosokawa M, Arimura Y, Shinomura Y, Matsui T, Matsumoto T, Iida M, Tsunoda T, Nakamura Y, Kamatani N, Kubo M. A genome-wide association study identifies 2 susceptibility Loci for Crohn's disease in a Japanese population. Gastroenterology. 2013;144:781-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 18. | Matsumoto T, Nakamura S, Esaki M, Yada S, Koga H, Yao T, Iida M. Endoscopic features of chronic nonspecific multiple ulcers of the small intestine: Comparison with nonsteroidal anti-inflammatory drug-induced enteropathy. Dig Dis Sci. 2006;51:1357-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Hosoe N, Ohmiya N, Hirai F, Umeno J, Esaki M, Yamagami H, Onodera K, Bamba S, Imaeda H, Yanai S, Hisamatsu T, Ogata H, Matsumoto T; CEAS Atlas Group. Chronic Enteropathy Associated With SLCO2A1 Gene [CEAS]-Characterisation of an Enteric Disorder to be Considered in the Differential Diagnosis of Crohn's Disease. J Crohns Colitis. 2017;11:1277-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 20. | Hisabe T, Hirai F, Matsui T, Watanabe M. Evaluation of diagnostic criteria for Crohn's disease in Japan. J Gastroenterol. 2014;49:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Ware JS, Wain LV, Channavajjhala SK, Jackson VE, Edwards E, Lu R, Siew K, Jia W, Shrine N, Kinnear S, Jalland M, Henry AP, Clayton J, O'Shaughnessy KM, Tobin MD, Schuster VL, Cook S, Hall IP, Glover M. Phenotypic and pharmacogenetic evaluation of patients with thiazide-induced hyponatremia. J Clin Invest. 2017;127:3367-3374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 22. | Fujiwara M, Okayasu I, Oritsu M, Komatsu J, Yoshitsugu M, Katoh Y, Bandoh T, Toyoshima H, Kase Y, Sugihara K, Kanno J, Hayashi Y. Significant increase in prostaglandin E-main urinary metabolite by laxative administration: Comparison with ulcerative colitis. Digestion. 2000;61:201-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Horikiri T, Hara H, Saito N, Araya J, Takasaka N, Utsumi H, Yanagisawa H, Hashimoto M, Yoshii Y, Wakui H, Minagawa S, Ishikawa T, Shimizu K, Numata T, Arihiro S, Kaneko Y, Nakayama K, Matsuura T, Matsuura M, Fujiwara M, Okayasu I, Ito S, Kuwano K. Increased levels of prostaglandin E-major urinary metabolite (PGE-MUM) in chronic fibrosing interstitial pneumonia. Respir Med. 2017;122:43-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: Controversies, consensus, and implications. Gut. 2006;55:749-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1970] [Cited by in RCA: 2354] [Article Influence: 123.9] [Reference Citation Analysis (2)] |
| 25. | Fuyuno Y, Yamazaki K, Takahashi A, Esaki M, Kawaguchi T, Takazoe M, Matsumoto T, Matsui T, Tanaka H, Motoya S, Suzuki Y, Kiyohara Y, Kitazono T, Kubo M. Genetic characteristics of inflammatory bowel disease in a Japanese population. J Gastroenterol. 2016;51:672-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Okabe H, Sakimura M. “Hi Tokui-sei Tahatsu-sei Shouchou Kaiyou-shou”. Stomach Intest. 1968;3:1539-1549. |
| 27. | Yamaguchi S, Yanai S, Nakamura S, Kawasaki K, Eizuka M, Uesugi N, Sugai T, Umeno J, Esaki M, Matsumoto T. Immunohistochemical differentiation between chronic enteropathy associated with SLCO2A1 gene and other inflammatory bowel diseases. Intest Res. 2018;16:393-399. |
| 28. | Yanai S, Yamaguchi S, Nakamura S, Kawasaki K, Toya Y, Yamada N, Eizuka M, Uesugi N, Umeno J, Esaki M, Okimoto E, Ishihara S, Sugai T, Matsumoto T. Distinction of Chronic Enteropathy associated with SLCO2A1 Gene from Crohn’s Disease. Gut Liver. 2018;13:62-66. |
| 29. | Jabr S, Gartner S, Milne GL, Roca-Ferrer J, Casas J, Moreno A, Gelpí E, Picado C. Quantification of major urinary metabolites of PGE2 and PGD2 in cystic fibrosis: Correlation with disease severity. Prostaglandins Leukot Essent Fatty Acids. 2013;89:121-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Arai Y, Arihiro S, Matsuura T, Kato T, Matsuoka M, Saruta M, Mitsunaga M, Matsuura M, Fujiwara M, Okayasu I, Ito S, Tajiri H. Prostaglandin E-major urinary metabolite as a reliable surrogate marker for mucosal inflammation in ulcerative colitis. Inflamm Bowel Dis. 2014;20:1208-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 31. | Arai Y, Matsuura T, Matsuura M, Fujiwara M, Okayasu I, Ito S, Arihiro S. Prostaglandin E-Major Urinary Metabolite as a Biomarker for Inflammation in Ulcerative Colitis: Prostaglandins Revisited. Digestion. 2016;93:32-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 32. | Johnson JC, Schmidt CR, Shrubsole MJ, Billheimer DD, Joshi PR, Morrow JD, Heslin MJ, Washington MK, Ness RM, Zheng W, Schwartz DA, Coffey RJ, Beauchamp RD, Merchant NB. Urine PGE-M: A metabolite of prostaglandin E2 as a potential biomarker of advanced colorectal neoplasia. Clin Gastroenterol Hepatol. 2006;4:1358-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 33. | Otani T, Yamaguchi K, Scherl E, Du B, Tai HH, Greifer M, Petrovic L, Daikoku T, Dey SK, Subbaramaiah K, Dannenberg AJ. Levels of NAD(+)-dependent 15-hydroxyprostaglandin dehydrogenase are reduced in inflammatory bowel disease: Evidence for involvement of TNF-alpha. Am J Physiol Gastrointest Liver Physiol. 2006;290:G361-G368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 34. | Hatazawa R, Ohno R, Tanigami M, Tanaka A, Takeuchi K. Roles of endogenous prostaglandins and cyclooxygenase isozymes in healing of indomethacin-induced small intestinal lesions in rats. J Pharmacol Exp Ther. 2006;318:691-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 35. | Takeuchi K, Kato S, Amagase K. Prostaglandin EP receptors involved in modulating gastrointestinal mucosal integrity. J Pharmacol Sci. 2010;114:248-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 36. | Kunikata T, Tanaka A, Miyazawa T, Kato S, Takeuchi K. 16,16-Dimethyl prostaglandin E2 inhibits indomethacin-induced small intestinal lesions through EP3 and EP4 receptors. Dig Dis Sci. 2002;47:894-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 75] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 37. | Uppal S, Diggle CP, Carr IM, Fishwick CW, Ahmed M, Ibrahim GH, Helliwell PS, Latos-Bieleńska A, Phillips SE, Markham AF, Bennett CP, Bonthron DT. Mutations in 15-hydroxyprostaglandin dehydrogenase cause primary hypertrophic osteoarthropathy. Nat Genet. 2008;40:789-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 204] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 38. | Nakanishi T, Tamai I. Roles of Organic Anion Transporting Polypeptide 2A1 (OATP2A1/SLCO2A1) in Regulating the Pathophysiological Actions of Prostaglandins. AAPS J. 2017;20:13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 39. | Kanai N, Lu R, Satriano JA, Bao Y, Wolkoff AW, Schuster VL. Identification and characterization of a prostaglandin transporter. Science. 1995;268:866-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 298] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 40. | Nomura T, Lu R, Pucci ML, Schuster VL. The two-step model of prostaglandin signal termination: In vitro reconstitution with the prostaglandin transporter and prostaglandin 15 dehydrogenase. Mol Pharmacol. 2004;65:973-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 116] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 41. | Harada S, Nagy JA, Sullivan KA, Thomas KA, Endo N, Rodan GA, Rodan SB. Induction of vascular endothelial growth factor expression by prostaglandin E2 and E1 in osteoblasts. J Clin Invest. 1994;93:2490-2496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 277] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 42. | Rao R, Redha R, Macias-Perez I, Su Y, Hao C, Zent R, Breyer MD, Pozzi A. Prostaglandin E2-EP4 receptor promotes endothelial cell migration via ERK activation and angiogenesis in vivo. J Biol Chem. 2007;282:16959-16968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 110] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 43. | Shoesmith JH, Tate GT, Wright CJ. Multiple Strictures Of The Jejunum. Gut. 1964;5:132-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 44. | Perlemuter G, Guillevin L, Legman P, Weiss L, Couturier D, Chaussade S. Cryptogenetic multifocal ulcerous stenosing enteritis: An atypical type of vasculitis or a disease mimicking vasculitis. Gut. 2001;48:333-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 71] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 45. | Adler DH, Cogan JD, Phillips JA, Schnetz-Boutaud N, Milne GL, Iverson T, Stein JA, Brenner DA, Morrow JD, Boutaud O, Oates JA. Inherited human cPLA(2alpha) deficiency is associated with impaired eicosanoid biosynthesis, small intestinal ulceration, and platelet dysfunction. J Clin Invest. 2008;118:2121-2131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 46. | Brooke MA, Longhurst HJ, Plagnol V, Kirkby NS, Mitchell JA, Rüschendorf F, Warner TD, Kelsell DP, MacDonald TT. Cryptogenic multifocal ulcerating stenosing enteritis associated with homozygous deletion mutations in cytosolic phospholipase A2-α. Gut. 2014;63:96-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |