Published online Apr 14, 2019. doi: 10.3748/wjg.v25.i14.1741
Peer-review started: January 14, 2019
First decision: February 13, 2019
Revised: March 13, 2019
Accepted: March 15, 2019
Article in press: March 16, 2019
Published online: April 14, 2019
Processing time: 91 Days and 16.2 Hours
Patients with hypothalamic-pituitary disease have the feature of central obesity, insulin resistance, and dyslipidemia, and there is increased prevalence of liver dysfunction consistent with non-alcoholic fatty liver disease (NAFLD) in this population. The causes of hypopituitarism in the reported studies varied and combined pituitary hormone deficiency including central diabetes insipidus is much common in this population. This retrospective cross-sectional study was performed to analyze the clinical characteristics and related factors with NAFLD and cirrhosis in Chinese adult hypopituitary/panhypopituitary patients.
To analyze the clinical characteristics of and related risk factors for NAFLD in Chinese adult hypopituitary patients.
Adult Chinese patients with hypopituitarism and/or panhypopituitarism were enrolled at the Pituitary Center of Peking Union Medical College Hospital between August 2012 and April 2018. According to abdominal ultrasonography, these patients were divided into an NAFLD (-) group and an NAFLD (+) group, and the latter was further divided into an NAFLD group and a cirrhotic group. The data, such as patient characteristics, diagnosis, and treatment, were extracted from medical records, and statistical analysis was performed.
A total of 36 male and 14 female adult Chinese patients with hypopituitarism were included in this retrospective study; 43 (87.0%) of these patients exhibited growth hormone (GH) deficiency, and 39 (78.3%) had diabetes insipidus. A total of 27 (54.0%) patients were diagnosed with NAFLD, while seven patients were cirrhotic. No significant differences were noted in serum GH or insulin-like growth factor 1 among patients with cirrhosis, subjects with NAFLD, and those without NAFLD. However, plasma osmolality and serum sodium concentration of the cirrhotic patients were 314.9 mOsm/kgH2O and 151.0 mmol/L, respectively, which were significantly higher than those of the NAFLD patients (P = 0.036 and 0.042, respectively). Overweight/obesity and insulin resistance were common metabolic disorders in this population. The body mass index (BMI) and homeostasis model assessment of insulin resistance parameters of the cirrhotic patients were 27.7 kg/m2 and 9.57, respectively, which were significantly higher than those of the patients without NAFLD (P = 0.011 and 0.044, respectively). A correlation analysis was performed, and fasting insulin concentration was positively associated with plasma osmolality in patients with NAFLD, after adjusting for gender, age, and BMI (r = 0.540, P = 0.046), but no correlation was noted in patients without NAFLD.
NAFLD is common in patients with hypopituitarism. Plasma osmolality and serum sodium levels of hypopituitary patients with cirrhosis are higher than those of subjects with NAFLD, and fasting insulin concentration is positively associated with plasma osmolality in patients with NAFLD, which suggests that hyperosmolality might be a contributor to the worsening of NAFLD in hypopituitary patients.
Core tip: Fifty-four percent of hypopituitary patients in this retrospective study were diagnosed with non-alcoholic fatty liver disease (NAFLD), which was significantly higher than that of the general adult population in China. Growth hormone (GH) deficiency has been considered an important contributing factor to NAFLD in those patients. However, no significant differences were noted in GH or insulin-like growth factor 1 among patients with cirrhosis, subjects with NAFLD, and those without NAFLD in this study. Interestingly, we found that plasma osmolality and serum sodium concentration of the cirrhotic patients were significantly higher than those of the NAFLD patients. In addition, fasting insulin concentration was positively associated with plasma osmolality in patients with NAFLD. Additional studies are required to confirm that hyperosmolality may be a significant contributor to the worsening of NAFLD in hypopituitary patients.
- Citation: Yuan XX, Zhu HJ, Pan H, Chen S, Liu ZY, Li Y, Wang LJ, Lu L, Yang HB, Gong FY. Clinical characteristics of non-alcoholic fatty liver disease in Chinese adult hypopituitary patients. World J Gastroenterol 2019; 25(14): 1741-1752
- URL: https://www.wjgnet.com/1007-9327/full/v25/i14/1741.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i14.1741
Primary non-alcoholic fatty liver disease (NAFLD) is caused by an excess of fat in the liver (steatosis) that is not the result of excessive alcohol consumption or other secondary causes[1,2]. The disease can be presented in the form of hepatic steatosis, inflammatory non-alcoholic steatohepatitis (NASH), fibrosis and/or cirrhosis[1,2]. Approximately 2%-3% of NAFLD patients develop NASH, which carries a 20% potential risk of evolving further and causing cirrhosis[1,3]. Furthermore, NAFLD patients are five times more likely to develop hepatocellular carcinoma in the absence of cirrhosis compared with hepatitis C patients[4,5]. However, the pathogenesis of NAFLD and its progression are a complex process, and the “multiple-hit” hypothesis proposed by Buzzetti et al[6] in 2016 suggested that simple steatosis and NASH not only exhibited different risks of progression but might also reflect different disease entities in terms of pathogenesis. Multiple insults, including insulin resistance, obesity, and gut microbiota, contribute to the development of steatosis and liver inflammation[6].
Patients with hypothalamic-pituitary disease present with central obesity, insulin resistance, and dyslipidemia, whereas liver dysfunction and NAFLD are common symptoms in this subpopulation[7-10]. Hong et al[8] reported that the frequency of fatty liver on abdominal ultrasonography in male subjects with hypopituitarism was significantly higher compared with that of control subjects (70.6% vs 32.5%). Growth hormone (GH) deficiency (GHD) has been considered an important contributing factor to these metabolic changes in hypopituitary patients[11-13]. However, the causes of hypopituitarism in the reported studies varied, and combined pituitary hormone deficiency, including central diabetes insipidus, was more common in this subpopulation. To the best of our knowledge, the imbalance of central diabetes insipidus and concomitant serum electrolyte concentration has not been investigated with regard to the pathophysiology of NAFLD in patients with hypopituitarism. The aim of this retrospective cross-sectional study was to compare Chinese adult hypopituitary patients with NAFLD and those without NAFLD in terms of their clinical manifestations, endocrine function, metabolic parameters, and serum electrolyte parameters and to analyze the associated factors with NAFLD and cirrhosis.
The present study is a retrospective cross-sectional observational study. The study protocol was approved by the Ethics Committee of the Peking Union Medical College Hospital (PUMCH; No. JS1233).
A total of 36 male and 14 female adult Chinese patients with hypopituitarism and/or panhypopituitarism were consecutively enrolled at the Pituitary Center of PUMCH between August 2012 and April 2018. According to abdominal ultrasonography, these patients were divided into an NAFLD (-) group and an NAFLD (+) group, and the latter was further divided into an NAFLD group and a cirrhotic group according to the histological and/or clinical diagnostic criteria of cirrhosis.
Hypopituitarism/panhypopituitarism was diagnosed clinically based on clinical manifestations, basal evaluation of pituitary function, and provocative tests. Hypopituitarism/panhypopituitarism was confirmed as follows: GHD was diagnosed as a peak serum GH level lower than 3 μg/L in both insulin tolerance tests (ITTs) and L-arginine tests[14]. Hypogonadotropic hypogonadism (HH) was defined by a reduction in the levels of luteinizing hormone (LH) and follicle stimulating hormone (FSH) for premenopausal women with a low serum estradiol concentration, and for adult men with a morning serum total testosterone concentration lower than 10.4 nmol/L[15]. HH was diagnosed via gonadotropin releasing hormone analogue (triptorelin) stimulation tests as follows: LH60min was lower than 4 IU/L (indispensable tests for delayed or absent puberty)[16]. Secondary hypocortisolism was diagnosed based on a peak serum cortisol response lower than 18 μg/dL (497.5 nmol/L) in ITTs[14]. Secondary hypothyroidism was diagnosed from a serum free thyroxine (FT4) level below the laboratory reference range in conjunction with a low, normal, or mildly elevated thyroid stimulating hormone (TSH) level in the setting of pituitary disease[17]. Central diabetes insipidus was considered when serum osmolality was higher than 295 mOsm/kg, while the corresponding urine osmolality was lower than 300 mOsm/kg in fluid deprivation tests, and a subsequent response to arginine vasopressin (AVP) was observed[18].
Diagnosis of NAFLD was confirmed by fatty infiltration of the liver on abdominal ultrasonography in association with or without abnormal liver enzymes. The patients were diagnosed as cirrhotic by the development of clinically evident complications of liver diseases (such as esophageal varices, ascites, jaundice, hepatic encephalopathy, portal hypertension, hypersplenism, and hepatocellular carcinoma) or by liver biopsy.
The exclusion criteria were as follows: (1) patients with isolated pituitary hormone deficiencies; (2) patients with primary and secondary autoimmune hypophysitis treated with glucocorticoids; (3) patients with hypopituitarism secondary to functional pituitary adenomas, including somatotrophin, lactotrophin, thyrotrophin, corticotrophin, gonadotrophin, and plurihormonal and double adenomas; (4) patients with positive hepatitis B or C serology or with evidence of inherited, autoimmune, cholestatic, drug-induced, or metabolic liver disease using standard clinical, laboratory, imaging, and histologic criteria; (5) patients with weekly alcohol intake of more than 21 units for men or 14 units for women; (6) patients with liver dysfunction of a specific etiology other than hypopituitarism and NAFLD; and (7) children and adolescents.
The data were extracted from medical records, which included patient characteristics, such as age, gender, race, anthropometric parameters including height, weight, body mass index (BMI), waist circumference (WC), and blood pressure, diagnosis of the cause of hypopituitarism/panhypopituitarism, laboratory results, abdominal ultrasonography results, complications and comorbidities, treatments, and hormone replacements. The time of diagnosis of hypopituitarism/panhypopituitarism was obtained from the date of symptoms and signs if applicable, or the time of clinical diagnosis. The time of diagnosis of NAFLD was considered to be the date of abdominal ultrasonography that was indicative of primary NAFLD. Hormone replacement therapy refers to regular and dose titration replacement for at least 3 months. Biochemical and hormone levels were measured within three days after admission. Homeostasis model assessment of insulin resistance (HOMA-IR) was obtained according to the formula: (fasting plasma glucose × fasting serum insulin)/22.5.
The statistical package for the social science (SPSS, version 22.0; SPSS Inc., Chicago, IL, United States) was used for data and statistical analyses. Continuous data conforming to a normal distribution are expressed as the mean ± standard deviation, while continuous data of partial distribution are represented by the median values [interquartile range (IQR)]. Frequency data are expressed as the number (proportion) of patients with a condition. The Student’s t-test and Wilcoxon Signed-Rank test were used for continuous variables, and the chi-square or Fisher’s exact test was used for categorical variables. A P-value less than 0.05 was considered significant.
A total of 36 male and 14 female patients with hypopituitarism and/or panhypopituitarism were included in the present study. Their mean age was 26.9 years (range from 18.0 to 41.5 years). The frequency of fatty liver on abdominal ultrasonography was 54.0%. A total of two patients were diagnosed as cirrhotic by liver biopsy, while five patients were diagnosed as cirrhotic by the development of clinically evident complications of liver disease as indicated in the Materials and Methods section. Three cirrhotic patients were decompensated, one of whom had upper gastrointestinal bleeding, another two had hypersplenism, ascites, esophageal and gastric varices, and hepatopulmonary syndrome. The clinical characteristics of hypopituitary patients with NAFLD and without NAFLD are shown in Table 1, and a comparative analysis was completed. No significant differences were noted in the gender ratio among patients with cirrhosis, those with NAFLD, and those without NAFLD. Cirrhotic patients were younger than NAFLD patients (P = 0.009), with no significant difference in the course of hypopituitarism. The course of hypopituitarism of cirrhotic patients was significantly longer than that of patients without NAFLD (median course 13.0 years and 3.0 years, respectively, P = 0.023), although there was no significant difference in the age between the two groups.
| NAFLD (-) (n = 23) | NAFLD (+) | ||
| NAFLD (n = 20) | Cirrhosis (n = 7) | ||
| Male n (%) | 17 (73.9) | 13 (65.0) | 6 (85.7) |
| Age (yr) | 22.8 (19.6-30.4) | 30.8 (24.5-34.6) | 21.0 (19.0-25.2) |
| Duration (yr) | 3.0 (1.0-13.0)a | 8.5 (1.1-19.0) | 13.0 (11.0-18.0) |
| BMI (kg/m2) | 22.2 (19.3-25.3)a | 27.9 (25.4-31.7) | 27.7 (24.2-32.1) |
| WC (cm) | 73.0 (81.5-96.3)a | 102.0 (92.5-109.8) | 110.5 (91.5-128.8) |
| SBP (mmHg) | 108.7 ± 15.3 | 122.0 ± 13.4 | 108.4 ± 15.5 |
| DBP (mmHg) | 70.0 ± 12.5 | 77.1 ± 11.1 | 64.4 ± 8.1 |
| GH (ng/mL) | 0.10 (0.05-0.30) | 0.10 (0.08-0.10) | 0.10 (0.05-0.25) |
| IGF1 (ng/mL) | 66.0 (28.0-113.0) | 107.0 (43.0-141.0) | 92.5 (25.0-205.3) |
| FT3 (pg/mL) | 2.61 (2.24-3.15) | 2.43 (2.25-3.08) | 2.53 (2.15-2.72) |
| FT4 (ng/dL) | 0.86 (0.66-1.01) | 0.88 (0.70-1.11) | 0.97 (0.46-1.14) |
| TSH (μIU/mL) | 1.907 (0.151-2.968) | 2.098 (0.806-2.557) | 3.083 (0.091-4.945) |
| GLU (mmol/L) | 5.0 (4.6-5.3) | 4.9 (4.5-5.3) | 5.3 (4.7-8.9) |
| HOMA-IR | 2.60 (1.60-3.71)a | 3.92 (2.62-6.02) | 9.57 (2.23-10.94) |
| HbA1C (%) | 5.2 (4.7-5.5) | 5.5 (5.3-5.9) | 6.7 (4.7-8.8) |
| TC (mmol/L) | 4.98 (4.18-5.74) | 5.07 (4.70-7.03) | 5.31 (4.55-6.16) |
| TG (mmol/L) | 1.29 (0.98-1.78) | 1.89 (1.06-2.48) | 2.15 (1.16-2.89) |
| HDL-C (mmol/L) | 1.17 (0.97-1.44) | 0.99 (0.89-1.49) | 0.93 (0.70-1.41) |
| LDL-C (mmol/L) | 2.91 ± 0.97 | 3.29 ± 1.08 | 3.34 ± 0.72 |
| UA (μmol/L) | 418.91 ± 88.25 | 457.42 ± 111.01 | 493.14 ± 212.83 |
| hsCRP (mg/L) | 2.07 (1.14-5.24) | 3.43 (2.27-6.68) | 2.28 (1.50-13.71) |
| Posm (mOsm/kgH2O) | 305.2 (298.8-314.6) | 299.3 (293.7-305.5) b | 314.9 (296.0-336.1) |
| Na (mmol/L) | 146.0 (143.0-150.0) | 143.0 (140.0-146.5) b | 151.0 (141.0-162.0) |
| Cl (mmol/L) | 109.0 (108.0-114.0) | 106.0 (104.3-110.8) b | 118.0 (108.0-130.0) |
| K (mmol/L) | 4.20 ± 0.30 | 4.10 ± 0.30 | 4.10 ± 0.20 |
| Cr (μmol/L) | 66.8 ± 15.2 | 70.2 ± 16.9 | 64.4 ± 17.0 |
The underlying etiology of hypopituitarism is illustrated in Table 2. The profile of the causes of hypopituitarism in the cirrhotic group was similar to those of the NAFLD (-) and the NAFLD groups (P = 0.431 and 0.466, respectively). Intracranial germ cell tumors were the most common cause of hypopituitarism in patients without NAFLD (43.5%), while congenital hypopituitarism was the most common cause in NAFLD and cirrhotic patients (45.0% and 42.9%, respectively), followed by craniopharyngioma and other causes.
| NAFLD (-) (n = 23) | NAFLD (+) | |||
| NAFLD (n = 20) | Cirrhosis (n = 7) | |||
| Etiology | Congenital hypopituitarism | 7 (30.4) | 9 (45.0) | 3 (42.9) |
| Intracranial germ cell tumor | 10 (43.5) | 4 (20.0) | 3 (42.9) | |
| Craniopharyngioma | 1 (4.3) | 2 (10.0) | 1 (14.3) | |
| Others | 5 (21.7) | 5 (25.0) | 0 (0.0) | |
| Distribution of pituitary deficiencies | Gonadotrophins | 23 (100) | 20 (100) | 7 (100.0) |
| Growth hormone | 20 (87.0) | 19 (95.0) | 7 (100.0) | |
| Thyroid | 18 (78.3) | 12 (60.0) | 7 (100.0) | |
| Adrenal | 14 (60.9) | 13 (65.0) | 6 (85.7) | |
| Diabetes insipidus | 18 (78.3) | 13 (65.0) | 5 (71.4) | |
| Number of pituitary axis deficiencies | 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 2 | 2 (8.7) | 3 (15.0) | 0 (0.0) | |
| 3 | 6 (26.1) | 4 (20.0) | 0 (0.0) | |
| 4 | 4 (17.4) | 6 (30.0) | 3 (42.9) | |
| 5 | 11 (47.8) | 7 (35.0) | 4 (57.1) | |
The distribution of deficiency in anterior pituitary hormones is shown in Table 2, and all patients exhibited gonadotropin deficiency. The subjects who were on hormone replacement were evaluated clinically and biochemically at regular intervals, according to standard clinical practice, to ensure the physiological replacement of the deficient hormones. Although four (57.1%) cirrhotic patients were panhypopituitary, no significant differences were noted with regard to the distribution of deficiency in anterior pituitary hormones and to the proportion of patients who received physiological hormone replacement. No patient received GH therapy in the present study. No significant difference was noted in serum GH or insulin-like growth factor 1 (IGF1). Serum thyroid hormone levels were similar in cirrhotic, NAFLD, and NAFLD (-) patients, as shown in Table 1. In addition, there was no difference in the proportion of patients with hypothalamic dysfunction in cirrhotic, NAFLD, and NAFLD (-) patients.
No patients were taking medications, such as diuretics, that could affect plasma osmolality and serum sodium for the treatment of cirrhotic conditions. A total of 36 (72%) patients also had diabetes insipidus; however, 21 of them (58.3%) did not receive treatment with desmopressin. No significant differences were noted in the proportion of patients with diabetes insipidus or in the proportion of patients who received desmopressin treatment, between patients with and without NAFLD. In addition, no significant differences were observed between the NAFLD and the cirrhotic groups, as shown in Table 2. However, plasma osmolality of patients with cirrhosis was significantly higher than that of NAFLD patients (median plasma osmolality = 314.9 mOsm/kgH2O and 299.3 mOsm/kgH2O, respectively, P = 0.036). Similar differences were observed in the concentrations of serum sodium and chlorine, as shown in Table 1, but no significant differences were noted between patients with cirrhosis and those without NAFLD.
The comparison of the clinical characteristics demonstrated that no significant differences were noted in systolic blood pressure (SBP) or diastolic blood pressure (DBP) between patients with and without NAFLD. SBP and DBP of cirrhotic patients were lower than those of the NAFLD patients, although no statistically significant differences were observed (P adjusted for age = 0.116 and 0.050, respectively). The median BMI and WC in cirrhotic patients were 27.7 kg/m2 and 110.5 cm, respectively. These values were significantly higher than those of patients without NAFLD (22.2 kg/m2 and 73.0 cm, P = 0.011 and 0.040, respectively) but similar to those of the NAFLD patients (BMI = 27.9 kg/m2 and WC = 102.0 cm, P = 0.912 and 0.396, respectively). The HOMA-IR of cirrhotic patients was significantly higher than that of patients without NAFLD (P = 0.045), although no significant difference was noted between the cirrhotic patients and NAFLD patients. A correlation analysis was performed, and it showed that fasting insulin concentration was positively associated with plasma osmolality in patients with NAFLD after adjusting for gender, age, and BMI (r = 0.540, P = 0.046), but no correlation was noted in total hypopituitary patients or in patients without NAFLD. There was no correlation between HOMA-IR and plasma osmolality. In contrast to previous studies[7-9], no significant difference was noted in the levels of fasting plasma glucose, glycated hemoglobin A1C (HbA1C), total cholesterol (TC), low density lipoprotein cholesterol (LDL-C), high density lipoprotein cholesterol (HDL-C), or triglycerides (TG), as shown in Table 1.
Serum aminotransferase and bilirubin levels were considered elevated if they were above the normal laboratory reference range. No significant differences were noted among cirrhotic, NAFLD, and NAFLD (-) patients with regard to tserum aminotransferase and bilirubin levels, as shown in Table 3. The median levels of γ-glutamyltransferase (GGT) in cirrhotic patients were higher than those in patients without NAFLD (52.0 U/L and 23.0 U/L, respectively, P = 0.046), although no significant difference was noted between NAFLD patients and cirrhotic patients (P = 0.862). The comparison of the tests for hepatic synthetic function, including serum albumin, prothrombin time (PT), and international normalized ratio (INR), demonstrated that the average PT and INR in cirrhotic patients were significantly higher than those in the NAFLD patients and NAFLD (-) patients (as shown in Table 3), although no significant difference was noted between patients with and without NAFLD. The cirrhotic patients frequently exhibited a number of hematological abnormalities, including varying degrees of cytopenia. Thrombocytopenia was the most common type of hematological abnormality, while leukopenia and anemia developed later in the disease course. As shown in Table 3, white blood counts and blood platelet counts were lower in cirrhotic patients than in NAFLD and NAFLD (-) patients, although no significant differences were noted between patients with and without NAFLD.
| Index | NAFLD (-) (n = 23) | NAFLD (+) | ||
| NAFLD (n = 20) | Cirrhosis (n = 7) | |||
| Liver enzymes | ALT (U/L) | 26.0 (20.0-41.0) | 35.0 (27.3-81.8) | 23.0 (19.0-59.0) |
| ALB (g/L) | 46.8 ± 3.5a | 47.7 ± 3.0a | 49.6 ± 4.6 | |
| TBil (μmol/L) | 8.7 (6.9-14.4)b | 12.3 (8.0-17.1)a | 13.2 (9.9-15.9) | |
| DBil (μmol/L) | 2.6 (2.2-4.4) | 3.4 (2.6-5.5) | 4.4 (3.2-5.1) | |
| GGT (U/L) | 23.0 (16.0-33.0)a | 52.0 (29.0-77.0) | 52.0 (23.0-141.0) | |
| ALP (U/L) | 77.0 (60.5-122.5) | 82.0 (69.0-92.0) | 71.0 (62.0-117.0) | |
| AST (U/L) | 28.0 (22.5-38.5) | 36.0 (26.0-55.0) | 26.0 (20.0-59.0) | |
| TBA (μmol/L) | 2.90 (1.35-3.25) | 3.60 (2.30-7.00) | 3.60 (1.30-9.10) | |
| LD (U/L) | 233.9 ± 54.8 | 227.6 ± 41.4 | 215.3 ± 60.8 | |
| Routine blood tests | WBC (×109/L) | 6.53 ± 2.61a | 6.32 ± 1.71a | 4.02 ± 1.14 |
| NEUT# (×109/L) | 3.65 ± 1.90b | 3.44 ± 1.24a | 1.75 ± 0.30 | |
| PLT (×109/L) | 250.5 ± 81.1c | 273.0 ± 90.1c | 91.1 ± 53.3 | |
| Coagulation function tests | PT (s) | 12.11 ± 1.06c | 11.87 ± 1.01c | 15.43 ± 2.24 |
| PT% (%) | 87.3 ± 8.8c | 94.3 ± 14.9c | 62.6 ± 12.1 | |
| INR | 1.05 ± 0.08c | 1.01 ± 0.10c | 1.33 ± 0.17 | |
| FgB (g/L) | 2.97 ± 0.78b | 3.09 ± 0.81b | 1.89 ± 0.59 | |
| APTT (s) | 31.35 (25.70-35.15) | 28.45 (25.30-38.33) | 33.50 (30.48-41.58) | |
| TT (s) | 18.34 ± 0.97b | 17.82 ± 0.94c | 19.75 ± 1.08 | |
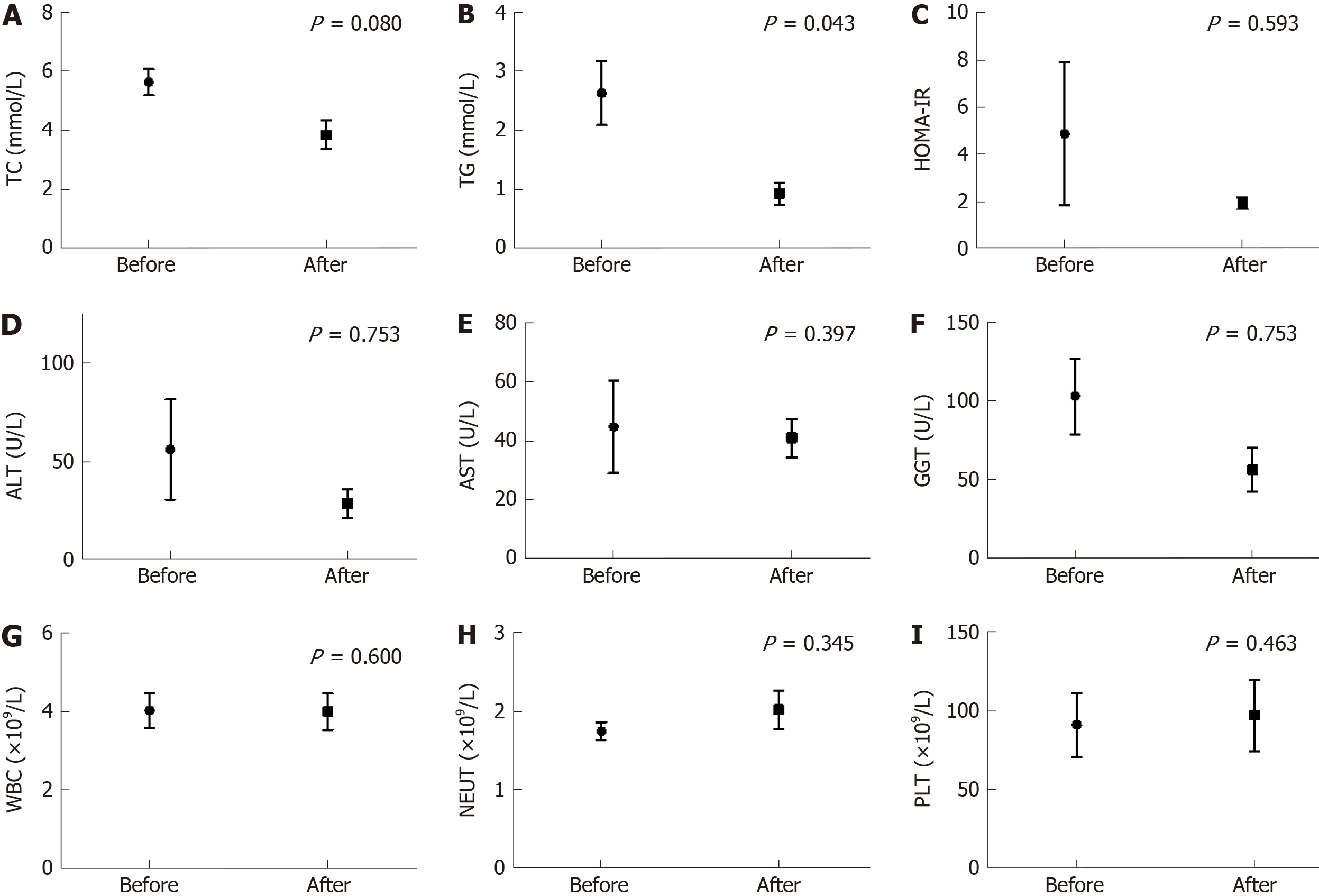
The biochemical indicators of the seven cirrhotic patients were retested following regular hormone replacement, as shown in Figure 1. Liver function was generally stable, which was reflected by serum aminotransferase levels, white blood count, and blood platelet count. TG was significantly lower than that before hormone replacement (P = 0.043). The fasting serum insulin and HOMA-IR levels were decreased following regular hormone replacement, although no significant differences were obtained (P = 0.593 and 0.593, respectively). Serum sodium was decreased from 152.9 mmol/L to 143.6 mmol/L after hormone replacement (P = 0.046); however, no significant difference was noted in plasma osmolality (319.6 mOsm/kgH2O and 300.1 mOsm/kgH2O, P = 0.080).
NAFLD is the most common cause of chronic liver dysfunction and a major global health problem over the past decades, whereas hypopituitarism/panhypopituitarism is a rare cause of NAFLD, which is easily misdiagnosed by endocrinologists and gastroenterologists. The data reported in the present study showed that the frequency of fatty liver in hypopituitary patients based on abdominal ultrasonography was 54.0%, which is significantly higher than that of the general adult population in China (approximately 15%), reported in 2013[19]. The incidence of cirrhosis in the present study population (patients with hypopituitarism and NAFLD) was 28%, which was significantly higher than that in the general NAFLD population but similar to that reported in a longitudinal cohort study (29%)[10,20].
Disturbances in endocrine hypothalamic-pituitary axes have been associated with NAFLD, including GHD, hypothyroidism, and hypogonadism[21]. GHD has been considered an important contributing factor to the metabolic change encountered in hypopituitary patients. The majority of the patients in the present study had GHD, and the average IGF1 level was considerably low in this patient group. Comparative analysis demonstrated no significant differences in serum growth hormone or IGF1 levels between hypopituitary patients with NAFLD and those without. Moreover, no significant differences were noted in the distribution of anterior pituitary deficiencies or hormone replacement in current hypopituitary patients with and without NAFLD. The causes of hypopituitarism varied, and combined pituitary hormone deficiency, including central diabetes insipidus, was more common in this population. Therefore, we speculated that this condition would be the result of multihormonal deficiency and that additional aggravating factors would be responsible for the pathophysiology of NAFLD in hypopituitary patients.
Congenital hypopituitarism, intracranial germ cell tumor, and craniopharyngioma were common causes of hypopituitarism in the present study. The latter two disorders usually affect the secretion of AVP. Combined deficits in AVP secretion and thirst sensation can result in life-threatening hyperosmolality and hypernatremia. In the current study, 72% of patients with hypopituitarism presented with central diabetes insipidus. Thus, hypernatremia manifested in these hypopituitary patients. Plasma osmolality levels of cirrhotic patients were significantly higher than those of NAFLD patients, and similar differences were noted with regard to serum sodium and serum chlorine levels. Homeostasis of electrolytes and the normal concentration of plasma osmolality are essential for the maintenance of normal cellular function. Cell volume changes are triggered by osmotic substances, thereby activating signals that contribute to the control and regulation of metabolism and gene expression. Following the induction of hyperosmolality, cells shrink and may undergo apoptosis[22-24]. Moreover, some components of insulin signaling, such as the enzyme protein kinase B, are sensitive to hyperosmolarity[22-24]. Hyperosmolality can inhibit insulin-induced glucose uptake, glycogen synthesis, and lipogenesis in 3T3-L1 adipocytes[25,26]. Therefore, the dehydration of hepatocytes, which is triggered by hyperosmolarity, can also induce insulin and cytokine resistance and promote the progression of NAFLD[27,28].
The pathogenesis of NAFLD has not been fully elucidated, and multiple etiologies have been proposed. The most prevalent hypothesis includes insulin resistance as the key mechanism leading to hepatic steatosis and possibly to steatohepatitis and liver fibrosis[6,29]. The data reported in the present study indicated that the metabolic changes accompanied by hypopituitarism/panhypopituitarism notably caused an increase in the weight and obesity parameters, insulin resistance, and hyperlipidemia, which is consistent with the findings reported previously[7-10]. HOMA-IR in patients with hypopituitarism and cirrhosis was significantly higher than that in patients without NAFLD. Moreover, fasting insulin concentration was positively associated with plasma osmolality in patients with NAFLD, although no correlation was noted between HOMA-IR and plasma osmolality. However, peripheral IR, pancreatic β-cell dysfunction, and even diabetes mellitus may occur in cirrhotic patients[30,31]. Therefore, the effect of the hyperosmolar state on the progression of NAFLD in hypopituitary patients requires further studies.
The sensitivity of ultrasound has been reported to range from 53% to 100% and its specificity from 77% to 98%[32]. Higher diagnostic sensitivities and specificities are achieved during the evaluation of moderate to severe hepatic steatosis cases, whereas lower values are noted during all grades of hepatic steatosis[32]. The accurate diagnosis of NASH and cirrhosis can be conducted by liver biopsy. Adams et al[10] reported that in ten patients with hypothalamic and pituitary dysfunction whose NAFLD symptoms were confirmed by liver biopsy, six were cirrhotic (29% of the total cohort), two exhibited NASH with fibrosis (10.5% of the total cohort), and two presented with simple steatosis. In the present study, two of our patients underwent liver biopsy, which indicated the lack of differential diagnosis between simple steatosis and NASH from NAFLD. Consequently, the incidence of NASH and fibrosis in our cohort was not clear. Only two of the seven cirrhotic patients received regular hormone replacement, while three of them received this therapy until they developed symptoms of decompensated cirrhosis, such as upper gastrointestinal bleeding and hypersplenism. Comparative analysis of our patients indicated that the variables of hepatic synthetic function, white blood count, and blood platelet count of cirrhotic patients were significantly lower than those of patients with hypopituitarism without NAFLD. However, no significant differences were noted in the level of serum aminotransferase or bilirubin. Following a period of regular hormone replacement, the biochemical indicators of the seven cirrhotic patients exhibited no further deterioration and were reflected by serum aminotransferase levels, white blood count, and blood platelet count, although it was unknown whether the pathological indicators of liver cirrhosis had improved. Therefore, routine liver blood tests could not be used to assess the progression of liver fibrosis and cirrhosis in NAFLD subjects, and liver biopsy was performed if necessary[1,2].
In conclusion, our data demonstrated a high prevalence of NAFLD and cirrhosis in patients with hypopituitarism. Notably, hypopituitary patients with cirrhosis exhibited significantly higher BMI and HOMA-IR compared with those without NAFLD. In addition, fasting insulin concentration was positively associated with plasma osmolality in patients with NAFLD, following adjustment for gender, age, and BMI. We reported for the first time that plasma osmolality and serum sodium levels of hypopituitary patients with cirrhosis were significantly higher than those of hypopituitary patients with NAFLD. Additional studies are required to confirm that hyperosmolality may be a significant contributor to the deterioration of NAFLD in hypopituitary patients. Routine liver blood tests could not be used to assess the progression of liver fibrosis and cirrhosis in subjects with NAFLD.
The present study was limited by insufficient sample size, as hypopituitarism is a rare condition. We did not use corrections for multiple comparisons because the findings from this analysis were general associations rather than affirmative findings. Thus, prospective, multicenter, cohort studies and animal experiments are required in the future to facilitate further understanding of the NAFLD pathophysiology in hypopituitary patients.
Non-alcoholic fatty liver disease (NAFLD) is a major global health problem with a substantial rise in prevalence over the last decades. Patients with hypothalamic-pituitary disease have the features of central obesity, insulin resistance, and dyslipidemia, and there is an increased prevalence of liver dysfunction consistent with NAFLD in this population. Growth hormone deficiency (GHD) has been considered as an important contributing factor to these metabolic changes in hypopituitary patients. However, the causes of hypopituitarism in the reported studies varied, and combined pituitary hormone deficiency, including central diabetes insipidus, was more common in this population. This retrospective cross-sectional study was performed to analyze clinical characteristics of NAFLD in Chinese adult hypopituitary/panhypopituitary patients, and to explore the risk factors that lead to rapid progression to cirrhosis.
The research motivation of the present study was to identify possible risk factors related to NAFLD in patients with hypopituitarism by summarizing the characteristics of NAFLD in this patient population, in order to prevent and delay the occurrence and progression of NAFLD in patients with hypopituitarism in future.
The main objectives of the present study were to analyze clinical characteristics of NAFLD in Chinese adult hypopituitary/panhypopituitary patients, and to explore the risk factors that lead to rapid progression to cirrhosis. Additional studies are required to research the mechanism of rapid progression of NAFLD to cirrhosis in hypopituitary patients.
The present study is a retrospective cross-sectional observational study. A total of 50 adult Chinese patients with hypopituitarism and/or panhypopituitarism were enrolled. The data were extracted from the medical records, including patients’ characteristics, diagnosis and treatment, biochemical and hormonal tests, and abdominal ultrasound. And then statistical analysis was performed.
Fifty-four percent of hypopituitary patients in this study were diagnosed with NAFLD, and seven patients were cirrhotic, which was significantly higher than that of the general population. Body mass index (BMI) and homeostasis model assessment of insulin resistance (HOMA-IR) of the cirrhotic patients were significantly higher than those of the patients without NAFLD. Moreover, plasma osmolality and serum sodium concentration of the cirrhotic patients were significantly higher than those of the NAFLD patients, and fasting insulin concentration was positively associated with plasma osmolality in patients with NAFLD, following adjustment for gender, age, and BMI.
The present study demonstrated a high prevalence of NAFLD and cirrhosis in patients with hypopituitarism. Hypopituitary patients with cirrhosis exhibited significantly higher BMI and HOMA-IR compared with those without NAFLD. In addition, fasting insulin concentration was positively associated with plasma osmolality in patients with NAFLD, following adjustment for gender, age, and BMI. Moreover, we report for the first time that plasma osmolality and serum sodium levels of hypopituitary patients with cirrhosis were significantly higher than those of hypopituitary patients with NAFLD. Additional studies are required to confirm that hyperosmolality may be a significant contributor to the rapid progression of NAFLD in hypopituitary patients.
There is a high prevalence of NAFLD and cirrhosis in patients with hypopituitarism, but the factors that lead to rapid progression to cirrhosis are not clear. Further studies are needed to determine whether hyperosmolality contributes to the deterioration of NAFLD in hypopituitary patients.
We are grateful to Yue-Lun Zhang for his recommendations on the statistical analysis.
Date sharing statement: No additional data are available.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sitkin S, Gatselis NK S-Editor: Ma RY L-Editor: Wang TQ E-Editor: Song H
| 2. | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3544] [Cited by in RCA: 4911] [Article Influence: 701.6] [Reference Citation Analysis (8)] |
| 3. | Reccia I, Kumar J, Akladios C, Virdis F, Pai M, Habib N, Spalding D. Non-alcoholic fatty liver disease: A sign of systemic disease. Metabolism. 2017;72:94-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 135] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 4. | Mittal S, El-Serag HB, Sada YH, Kanwal F, Duan Z, Temple S, May SB, Kramer JR, Richardson PA, Davila JA. Hepatocellular Carcinoma in the Absence of Cirrhosis in United States Veterans is Associated With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2016;14:124-131.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 486] [Article Influence: 54.0] [Reference Citation Analysis (1)] |
| 5. | Singal AG, El-Serag HB. Hepatocellular Carcinoma From Epidemiology to Prevention: Translating Knowledge into Practice. Clin Gastroenterol Hepatol. 2015;13:2140-2151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 406] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 6. | Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism. 2016;65:1038-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1490] [Cited by in RCA: 2102] [Article Influence: 233.6] [Reference Citation Analysis (1)] |
| 7. | Gardner CJ, Irwin AJ, Daousi C, McFarlane IA, Joseph F, Bell JD, Thomas EL, Adams VL, Kemp GJ, Cuthbertson DJ. Hepatic steatosis, GH deficiency and the effects of GH replacement: a Liverpool magnetic resonance spectroscopy study. Eur J Endocrinol. 2012;166:993-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Hong JW, Kim JY, Kim YE, Lee EJ. Metabolic parameters and nonalcoholic fatty liver disease in hypopituitary men. Horm Metab Res. 2011;43:48-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Irie M, Itoh Y, Miyashita Y, Tsushima T, Shirai K. Complications in adults with growth hormone deficiency--a survey study in Japan. Endocr J. 2004;51:479-485. [PubMed] |
| 10. | Adams LA, Feldstein A, Lindor KD, Angulo P. Nonalcoholic fatty liver disease among patients with hypothalamic and pituitary dysfunction. Hepatology. 2004;39:909-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 186] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 11. | Matsumoto R, Fukuoka H, Iguchi G, Nishizawa H, Bando H, Suda K, Takahashi M, Takahashi Y. Long-term effects of growth hormone replacement therapy on liver function in adult patients with growth hormone deficiency. Growth Horm IGF Res. 2014;24:174-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | Nakajima K, Hashimoto E, Kaneda H, Tokushige K, Shiratori K, Hizuka N, Takano K. Pediatric nonalcoholic steatohepatitis associated with hypopituitarism. J Gastroenterol. 2005;40:312-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Jonas MM, Krawczuk LE, Kim HB, Lillehei C, Perez-Atayde A. Rapid recurrence of nonalcoholic fatty liver disease after transplantation in a child with hypopituitarism and hepatopulmonary syndrome. Liver Transpl. 2005;11:108-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Ursula Kaiser KKYH, Melmed S, Polonsky KS, Larsen PR, Kronenberg HM. Pituitary Physiology and Diagnostic Evaluation. Melmed S, Polonsky KS, Larsen PR, Kronenberg HM. Williams Textbook Of Endocrinology, 13th ed. Canada: Patricia Tannian 2015; 182-214. |
| 15. | Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, Montori VM; Task Force, Endocrine Society. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536-2559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1450] [Cited by in RCA: 1339] [Article Influence: 89.3] [Reference Citation Analysis (0)] |
| 16. | Mao JF, Xu HL, Duan J, Chen RR, Li L, Li B, Nie M, Min L, Zhang HB, Wu XY. Reversal of idiopathic hypogonadotropic hypogonadism: a cohort study in Chinese patients. Asian J Androl. 2015;17:497-502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Sherlock M, Reulen RC, Alonso AA, Ayuk J, Clayton RN, Sheppard MC, Hawkins MM, Bates AS, Stewart PM. ACTH deficiency, higher doses of hydrocortisone replacement, and radiotherapy are independent predictors of mortality in patients with acromegaly. J Clin Endocrinol Metab. 2009;94:4216-4223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 140] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 18. | Alan G, Robinson JGV. Posterior Pituitary. In: Melmed S, Polonsky KS, Larsen PR, Kronenberg HM, editor. Williams Textbook of Endocrinology. 13th ed. Canada: Patricia Tannian 2015; 300-313. |
| 19. | Fan JG. Epidemiology of alcoholic and nonalcoholic fatty liver disease in China. J Gastroenterol Hepatol. 2013;28 Suppl 1:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 213] [Article Influence: 17.8] [Reference Citation Analysis (2)] |
| 20. | Kabbany MN, Conjeevaram Selvakumar PK, Watt K, Lopez R, Akras Z, Zein N, Carey W, Alkhouri N. Prevalence of Nonalcoholic Steatohepatitis-Associated Cirrhosis in the United States: An Analysis of National Health and Nutrition Examination Survey Data. Am J Gastroenterol. 2017;112:581-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 177] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 21. | Hoffmann A, Müller HL. RETRACTION Novel perspectives on hypothalamic-pituitary dysfunction as a risk factor for non-alcoholic fatty liver disease. Minerva Endocrinol. 2017;42:132-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Schliess F, Häussinger D. Osmosensing and signaling in the regulation of liver function. Contrib Nephrol. 2006;152:198-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Reinehr R, Becker S, Höngen A, Haüssinger D. The Src family kinase Yes triggers hyperosmotic activation of the epidermal growth factor receptor and CD95. J Biol Chem. 2004;279:23977-23987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Reinehr R, Schliess F, Häussinger D. Hyperosmolarity and CD95L trigger CD95/EGF receptor association and tyrosine phosphorylation of CD95 as prerequisites for CD95 membrane trafficking and DISC formation. FASEB J. 2003;17:731-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 87] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 25. | Meier R, Thelen M, Hemmings BA. Inactivation and dephosphorylation of protein kinase Balpha (PKBalpha) promoted by hyperosmotic stress. EMBO J. 1998;17:7294-7303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 141] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 26. | Chen D, Elmendorf JS, Olson AL, Li X, Earp HS, Pessin JE. Osmotic shock stimulates GLUT4 translocation in 3T3L1 adipocytes by a novel tyrosine kinase pathway. J Biol Chem. 1997;272:27401-27410. [PubMed] |
| 27. | Randhawa VK, Thong FS, Lim DY, Li D, Garg RR, Rudge R, Galli T, Rudich A, Klip A. Insulin and hypertonicity recruit GLUT4 to the plasma membrane of muscle cells by using N-ethylmaleimide-sensitive factor-dependent SNARE mechanisms but different v-SNAREs: role of TI-VAMP. Mol Biol Cell. 2004;15:5565-5573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Gual P, Gonzalez T, Grémeaux T, Barres R, Le Marchand-Brustel Y, Tanti JF. Hyperosmotic stress inhibits insulin receptor substrate-1 function by distinct mechanisms in 3T3-L1 adipocytes. J Biol Chem. 2003;278:26550-26557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Ercin CN, Dogru T, Genc H, Celebi G, Aslan F, Gurel H, Kara M, Sertoglu E, Tapan S, Bagci S, Rizzo M, Sonmez A. Insulin Resistance but Not Visceral Adiposity Index Is Associated with Liver Fibrosis in Nondiabetic Subjects with Nonalcoholic Fatty Liver Disease. Metab Syndr Relat Disord. 2015;13:319-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Kumar R. Hepatogenous Diabetes: An Underestimated Problem of Liver Cirrhosis. Indian J Endocrinol Metab. 2018;22:552-559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 31. | Orsi E, Grancini V, Menini S, Aghemo A, Pugliese G. Hepatogenous diabetes: Is it time to separate it from type 2 diabetes? Liver Int. 2017;37:950-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (3)] |
| 32. | Esterson YB, Grimaldi GM. Radiologic Imaging in Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Clin Liver Dis. 2018;22:93-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |