Published online Apr 14, 2019. doi: 10.3748/wjg.v25.i14.1684
Peer-review started: January 31, 2019
First decision: February 26, 2019
Revised: March 5, 2019
Accepted: March 16, 2019
Article in press: March 16, 2019
Published online: April 14, 2019
Processing time: 73 Days and 4.4 Hours
Recently, more and more studies have demonstrated the pivotal role of programmed death 1/programmed death ligand 1 (PD-1/PD-L1) pathway in the immune evasion of tumors from the host immune system. However, the role of PD-1/PD-L1 pathway in gastric neuroendocrine carcinomas (G-NECs) remains unknown.
To investigate the expression of PD-1/PD-L1 and role of PD-1/PD-L1 pathway in G-NECs, which occur rarely but are highly malignant and clinically defiant.
We investigated the expression of PD-L1 on tumor cells and PD-1+, CD8+, and FOXP3+ T cell infiltration by immunohistochemistry in 43 resected G-NEC tissue specimens. The copy number alterations of PD-L1 were assessed by qRT-PCR.
Most of the G-NECs tumor cells exhibited a near-uniform expression pattern of PD-L1, while some showed a tumor-stromal interface enhanced pattern. Of the 43 G-NECs, 21 (48.8%) were classified as a high PD-L1 expression group, and the high expression of PD-L1 was associated with poor overall survival (OS). The high expression of PD-L1 was correlated with abundant PD-1+ tumor infiltrating lymphocytes (TILs) instead of CD8+ TILs and FOXP3+ regulatory T cells (Tregs). Our analysis also suggested that the infiltration of CD8+ TILs tended to be a favorable factor for OS, although the difference did not reach the statistical significance (P = 0.065). Meanwhile, PD-L1 was significantly overexpressed in cases with copy number gain as compared with those without.
Our data demonstrated for the first time that high expression of PD-L1 in G-NECs is associated with a poor prognosis, while the high expression may be due to the copy number variation of PD-L1 gene or stimulation of TILs. These results provide a basis for the immunotherapy targeting PD-1/PD-L1 pathway in G-NECs.
Core tip: This study for the first time demonstrated that programmed death ligand 1 (PD-L1) can be expressed by gastric neuroendocrine carcinoma (G-NEC) cancer cells and the high PD-L1 expression was associated with a poor prognosis. And the high expression of PD-L1 may be due to the copy number variation of PD-L1 gene or stimulation of tumor infiltrating lymphocytes. These findings provide important implications for the potential use of antibody therapies targeting the PD-1/PD-L1 signaling pathway in G-NECs.
- Citation: Yang MW, Fu XL, Jiang YS, Chen XJ, Tao LY, Yang JY, Huo YM, Liu W, Zhang JF, Liu PF, Liu Q, Hua R, Zhang ZG, Sun YW, Liu DJ. Clinical significance of programmed death 1/programmed death ligand 1 pathway in gastric neuroendocrine carcinomas. World J Gastroenterol 2019; 25(14): 1684-1696
- URL: https://www.wjgnet.com/1007-9327/full/v25/i14/1684.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i14.1684
Neuroendocrine neoplasms (NENs), which used to be called neuroendocrine tumors, are benign or high-grade malignant. Meanwhile, they are pathologically and clinically heterogeneous rare tumors. In the past several years, the incidence of NENs has gradually increased over time[1,2], from 1.09/100000 in 1973 to 5.25/100000 in 2004 in the United States[3]. However, there was no improvement in outcomes because of the limited awareness of this disease[4,5]. As the most malignant subgroup, neuroendocrine carcinomas (NECs) are characterized by high-grade cytological atypia, apparent pleomorphism, extensive necrosis, and prominent mitotic activity[6]. As one of the most common type of NECs, gastric NECs (G-NECs) are poorly differentiated and high-grade malignant and might be either small-cell carcinomas or large-cell NECs in histology. Since the limited knowledge of the epidemiological and clinical characters for G-NECs, this disease deserves more attention.
Programmed death 1 (PD-1), first discovered in 1992 when searching genes responsible for programmed cell death by Ishida et al[7], is an immunoinhibitory receptor expressed by activated T cells, B cells, myeloid cells, and other antigen-presenting cells. Programmed death ligand 1 (PD-L1), a member of the B7 gene family, originally called B7H1 by Dong et al[8], was reported in 2000 by Freeman et al[9]. It is an immunomodulatory glycoprotein, which cannot be detected in normal liver parenchyma, breast, colon, kidney, uterus, muscle, pancreas, or gastric tissue[10,11], but could be selectively expressed in various malignancies, such as esophageal cancer[12], gastric cancer[11], pancreatic cancer[13], colorectal cancer[14], breast cancer[15], thymoma[16], and thymic cancer[17].
The host immune functions play an important role in inhibiting the development of malignant tumors. And the induction of anti-tumor immune responses needs the host immune system to identify the tumor antigen efficiently and to activate various T cells. Binding of PD-1 and PD-L1 inhibits phosphatidylinositol 3-kinase/Akt, suppresses T-cell IL2 production, and then reduces T-cell proliferation and survival[18]. Therefore, some malignant tumors can escape from immune-surveillance mechanisms by the expression of PD-L1[18]. Then, the blockade of the PD-1/PD-L1 pathway has become an attractive target in cancer therapy. The success of immunotherapy in various malignancies such as advanced melanoma, non–small cell lung cancer, and renal cell carcinoma have suggested that immunotherapy can be a promising alternative by blocking PD-1/PD-L1 signaling[19,20]. Until now, no study has evaluated the role of PD-1/PD-L1 in G-NECs.
CD8+ tumor infiltrating lymphocytes (TILs) are the key effector in antitumor immune response, and its presence has been reported to be a favorable prognostic factor in some malignancies, such as colorectal cancer[21], esophageal cancer[22,23], and breast cancer[24]. Meanwhile, it has been reported that the upregulation of PD-L1 on tumour cells may be driven by the stimulation of CD8+ T cells in melanoma[25,26]. FOXP3, as a forkhead-family transcription factor, controls regulatory T cell (Treg) development and function[27]. Tregs are a subset of T lymphocytes possessing the immunoregulatory capacity to suppress the proliferation and cytokine secretion of effector T lymphocytes. And previous studies have reported that the infiltration of FOXP3+ Tregs is correlated with the upregulation of PD-L1 in gastric cancer[28], breast cancer[29], and colorectal cancer[14].
In this study, we first detected PD-L1 expression in 43 G-NECs and analyzed the relationship between PD-L1 expression and patients’ prognosis. Then, we investigated PD-1+ T cells, CD8+ T cells, and FOXP3+ Treg cells in NECs and their associations with clinicopathological parameters and PD-L1 expression. PD-L1 gene copy number alterations and its relationship to PD-L1 expression were also examined.
This study examined 43 formalin-fixed, paraffin-embedded (FFPE) tissue samples from patients with G-NECs treated at Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, from 2007 to 2014. We diagnosed, graded, and staged G-NECs by the 2010 WHO classification[30]. Cases with mixed tumors were excluded. TNM staging was done according to the 7th Edition of the AJCC Cancer TNM Classification[31]. None of these patients had received tumor-related treatment before. For all cases enrolled in this study, information on age, gender, tumor size, tumor location, T classification, lymph node metastasis, liver metastasis, pathological stage, pathology, treatment, and outcome was reviewed.
The overall survival (OS) time was calculated from the date of surgery to death, or August 30, 2016, the ultimate follow-up deadline. Research Ethics Committee of Ren Ji Hospital approved this study and all participants signed informed consent forms.
Deparaffinage and rehydration of G-NEC tissues were first performed with xylene and graded alcohol, respectively. Activity of endogenous peroxidase was quenched with 3% hydrogen peroxide at room temperature for 15 min. Antigen retrieval was achieved at high temperature and high pressure. Blocking was done with 10% bull serum albumin for 30 min, then slides were incubated with antibodies against CD8 (1:200, 17335-1-AP, ProteinTech, United States), FOXP3 (1:200, 22228-1-AP, ProteinTech), PD-1 (1:25, ab140950, Abcam, United Kindom), and PD-L1 (1:500, ab205921, Abcam) at 4 ˚C overnight. The next day, the slides were incubated with a corresponding peroxidase-labeled secondary antibody for 30 min at room temperature. Finally, positive staining was visualized with diaminobenzidine tetrahydrochloride (Maixin Biotech, China) and counterstained with hematoxylin.
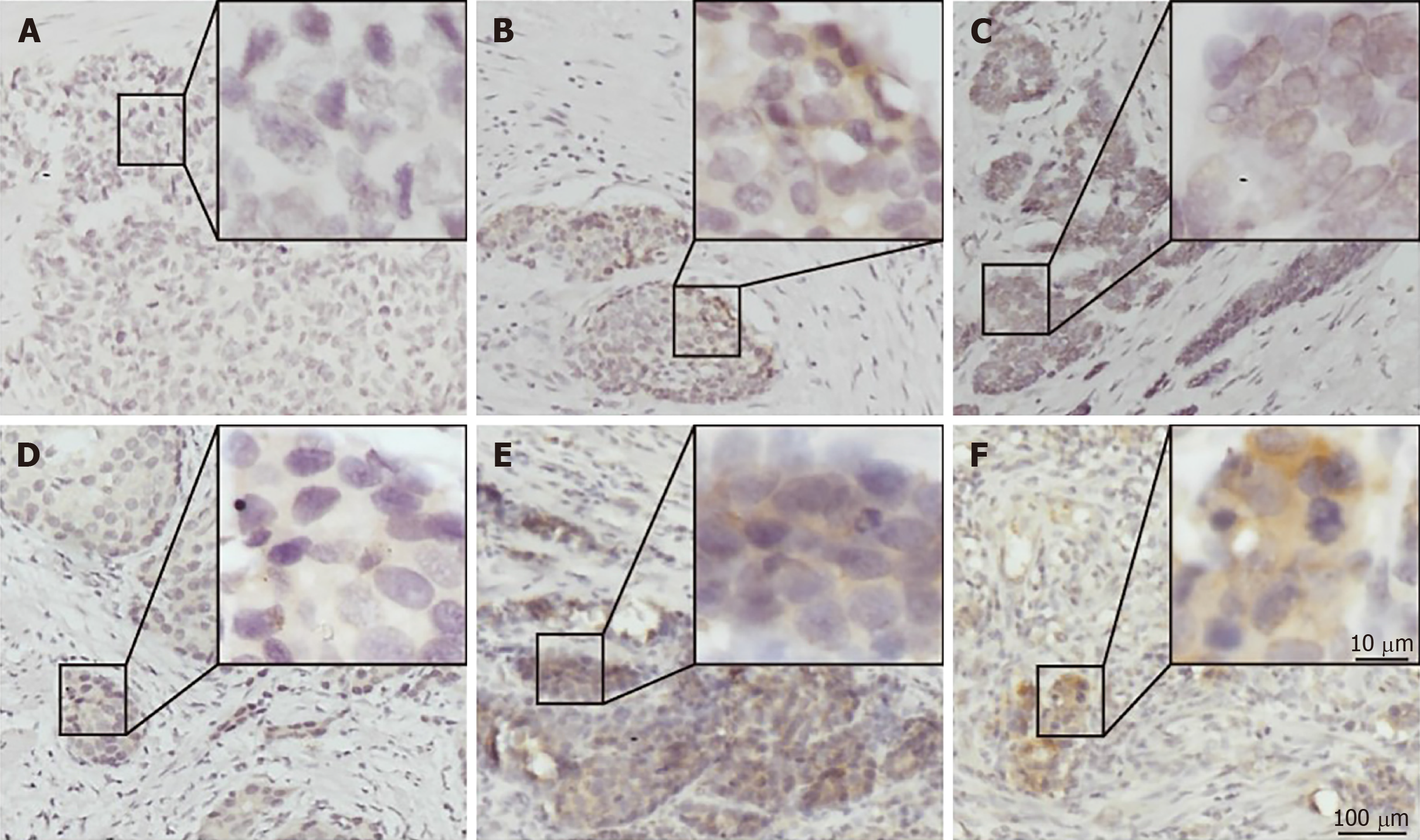
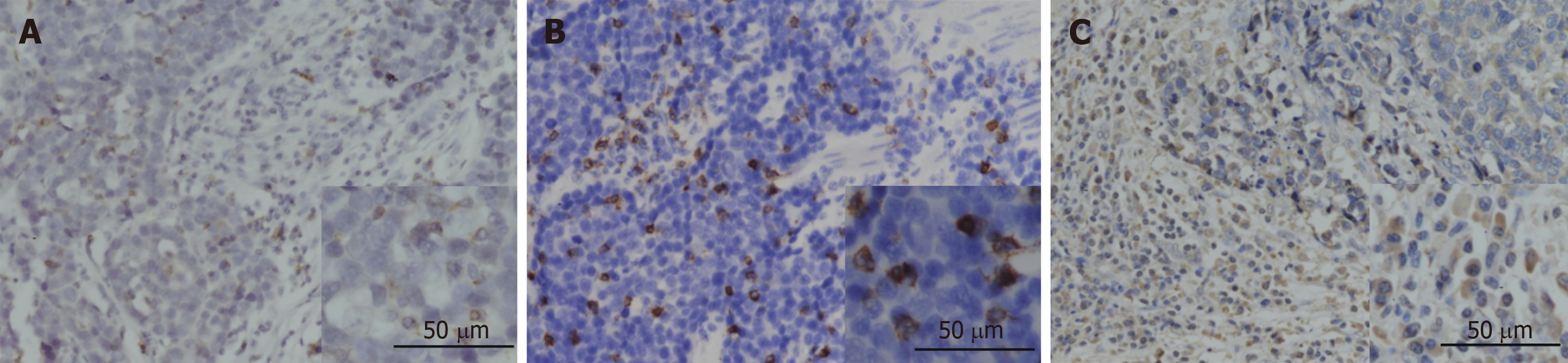
The final score of PD-L1 expression was assessed according to the percent of positive cell score × staining intensity score as follows: 0, 0-5%; 1, 6%-35%; 2, 36%-70%; 3, 71%-100%; 0, no staining; 1, weak staining; 2, moderate staining; 3, strong staining. The tissues with a final score < 4 were defined as PD-L1 low expression and those with a final score ≥ 4 were classified as PD-L1 high expression. The densities of PD-1+, CD8+, and FOXP3+ cells were measured in four high power fields from each tumor by an experienced pathologist and the average density was calculated. Low infiltration of PD-1+, CD8+, and FOXP3+ cells was defined as less than the median value.
Tissue DNA was extracted from FFPE tissue using QIAamp DNA FFPE Tissue Kit (QIAGEN, 56404, Germany). Copy number assay of PD-L1 was performed by qRT-PCR, CD274 TaqMan Copy Number Assays (Hs03704252_cn, Applied Biosystems, United States) was used to evaluate PD-L1 copy number while TaqMan Copy Number Reference Assay RNase P (Applied Biosystems 4403328, United States) was used to normalize the results. TaqMan Genotyping Master Mix (Applied Biosystems 4381656, United States) was used to perform the qRT-PCR on a 7500 real-time PCR system (Applied Biosystems, United States). Results were analyzed with CopyCaller Software (Applied Biosystems, United States).
Data are presented as the mean ± SD. Statistical analyses and graphical representations were performed using SPSS 22.0 software (IBM Corp., Armonk, NY, United States) and GraphPad Prism 6 (San Diego, CA, United States) software, respectively. χ2 test and Fisher’s exact test were used to evaluate the correlation of PD-L1, PD-1, FOXP3, and CD8 with clinic-pathologic parameters in patients with G-NECs. Survival curves were evaluated using the Kaplan-Meier method and differences were analyzed by the log-rank test. Identification of factors that had a significant influence on survival was performed by univariate and multivariate Cox regression analyses. Comparison between two groups was performed by the student’s t-test or Mann–Whitney U test. P-values (two-sided) < 0.05 were considered statistically significant.
A total of 43 patients diagnosed with G-NECs were enrolled in this study and the clinicopathologic features are described in detail in Table 1. The cases included 35 males (81.4%) and 8 females (18.6%) with a median age of 62 years (range, 33-82 years). Nearly half of the tumors were located in cardia, while the others were distributed evenly in the corpus and the antrum of the stomach, with two cases located in the residual stomach anastomosis. The majority of patients were diagnosed with stage III disease (81.4%), two cases with stage II (4.65%), and six cases with stage IV (13.95%). For the patients with liver metastases, one of six received resection of both primary and metastatic tumors, while the others received palliative resection of primary tumor. None of the patients received neoadjuvant therapy prior to the surgical resection. The median OS was 31 mo (range, 1-90 mo).
| Clinicopathological feature | n (%) |
| Age (yr) | |
| < 60/≥ 60 | 15 (34.88)/28 (65.12) |
| mean ± SD | 62.26 ± 10.46 |
| Gender | |
| Male/female | 35 (81.40)/8 (18.60) |
| Tumor location | |
| Cardia | 21 (48.84) |
| Corpus | 10 (23.26) |
| Antrum | 10 (23.26) |
| Residual stomach anastomosis | 2 (4.65) |
| Tumor size (max diameter, cm) | |
| ≤ 5/> 5 | 20 (46.51)/23 (53.49) |
| mean ± SD | 5.47 ± 3.18 |
| T classification | |
| T1/T2/T3/T4 | 0 (0)/4 (9.30)/0 (0)/39 (90.70) |
| Lymph node metastasis | |
| N0/N1 | 12 (27.91)/31 (72.09) |
| Liver metastasis | |
| Absent/present | 37 (86.05)/6 (13.95) |
| Pathological stage | |
| I/II/III/IV | 0 (0)/2 (4.65)/35 (81.40)/6 (13.95) |
| Pathology | |
| Small cell carcinomas | 39 (90.70) |
| Large cell carcinomas | 4 (9.30) |
| Operation | |
| Curative resection | 38 (88.37) |
| Palliative resection | 5 (11.63) |
| Neoadjuvant therapy | |
| No/present | 100 (100)/0 (0) |
| Follow-up | |
| Median OS (mo) | 31.0 |
PD-L1 was mainly expressed on both the membrane and cytoplasm of tumor cells (Figure 1C and F). Most of the G-NEC tissues demonstrated a near-uniform expression pattern of PD-L1 (Figure 1E and F), while five specimens showed a tumor-stromal interface enhanced pattern (Figure 1B). The expression score of PD-L1 was evaluated based on the product of ratio of positive cells (range, 0-3) and staining intensity (range, 0-3), as shown in Figure 1. We predefined the expression score less than 4 as low expression group while the others were high expression group, and 21 (48.8%) of 43 cases were classified as high PD-L1 expression.
The expression of PD-1 was almost on the surface of immune cells in the stroma among tumor nests (Figure 2A), and the expression of CD8 and FOXP3 was mainly on both membrane and cytoplasm of immune cells in the stroma among tumor nests (Figure 2B and C). We defined the expression of PD-1, CD8, and FOXP3 by the number of positive immune cells. The median value of PD-1+ cells in a high power field was 12, and the median values of CD8+ and FOXP3+ cells were 22 and 102.
Table 2 shows the correlation between immune markers and clinicopathologic characteristics. We found that the FOXP3+ Treg cell infiltration was significantly associated with age (P = 0.019), gender (P = 0.015), and tumor location (P = 0.018). It demonstrated that female patients, patients with elder age, and patients with G-NECs at the corpus were more likely to have a high infiltration of Treg cells. Importantly, we also found that patients with high infiltration of PD-1 positive immune cells tended to have higher expression of PD-L1 (P = 0.009). The infiltration of CD8+ cells was not associated with any clinical parameters or other immune markers. And all the five specimens with a tumor-stromal interface enhanced pattern of PD-L1 had high CD8+ T cell infiltration.
| Low CD8 n (%) | High CD8 n (%) | P-value | Low FOXP3 n (%) | High FOXP3 n (%) | P-value | Low PD-1 n (%) | High PD-1 n (%) | P-value | Low PD-L1 n (%) | High PD-L1 n (%) | P-value | ||
| Age (yr) | |||||||||||||
| < 60 | 7 | 8 | 0.835 | 11 | 4 | 0.019 | 9 | 6 | 0.284 | 7 | 8 | 0.666 | |
| ≥ 60 | 14 | 14 | 10 | 18 | 12 | 16 | 15 | 13 | |||||
| Gender | |||||||||||||
| Male | 17 | 18 | 0.942 | 14 | 21 | 0.015 | 16 | 19 | 0.391 | 19 | 16 | 0.391 | |
| Female | 4 | 4 | 7 | 1 | 5 | 3 | 3 | 5 | |||||
| Tumor location | |||||||||||||
| Cardia | 9 | 12 | 0.492 | 11 | 10 | 0.018 | 10 | 11 | 0.999 | 11 | 10 | 0.489 | |
| Corpus | 7 | 3 | 1 | 9 | 5 | 5 | 5 | 5 | |||||
| Antrum | 4 | 6 | 7 | 3 | 5 | 5 | 4 | 6 | |||||
| Residual stomach anastomosis | 1 | 1 | 2 | 0 | 1 | 1 | 2 | 0 | |||||
| Tumor size | |||||||||||||
| ≤ 5 cm | 12 | 8 | 0.172 | 11 | 9 | 0.451 | 11 | 9 | 0.451 | 10 | 10 | 0.887 | |
| > 5 cm | 9 | 14 | 10 | 13 | 10 | 13 | 12 | 11 | |||||
| T classification | |||||||||||||
| T1/2 | 3 | 1 | 0.272 | 2 | 2 | 0.961 | 2 | 2 | 0.961 | 1 | 3 | 0.272 | |
| T3/4 | 18 | 21 | 19 | 20 | 19 | 20 | 21 | 18 | |||||
| Lymph node metastasis | |||||||||||||
| ≤ 7 | 5 | 7 | 0.558 | 3 | 9 | 0.052 | 5 | 7 | 0.558 | 6 | 6 | 0.924 | |
| > 7 | 16 | 15 | 18 | 13 | 16 | 15 | 16 | 15 | |||||
| Liver metastasis | |||||||||||||
| Absent | 17 | 20 | 0.346 | 18 | 19 | 0.951 | 16 | 21 | 0.068 | 19 | 18 | 0.951 | |
| Present | 4 | 2 | 3 | 3 | 5 | 1 | 3 | 3 | |||||
| CD8 | |||||||||||||
| High | |||||||||||||
| Low | |||||||||||||
| FOXP3 | |||||||||||||
| Low | 9 | 12 | 0.443 | ||||||||||
| Hight | 12 | 10 | |||||||||||
| PD-1 | |||||||||||||
| Low | 13 | 8 | 0.094 | 12 | 9 | 0.287 | |||||||
| High | 8 | 14 | 9 | 13 | |||||||||
| PD-L1 | |||||||||||||
| Low | 9 | 13 | 0.287 | 12 | 10 | 0.443 | 15 | 7 | 0.009 | ||||
| High | 12 | 9 | 9 | 12 | 6 | 15 | |||||||
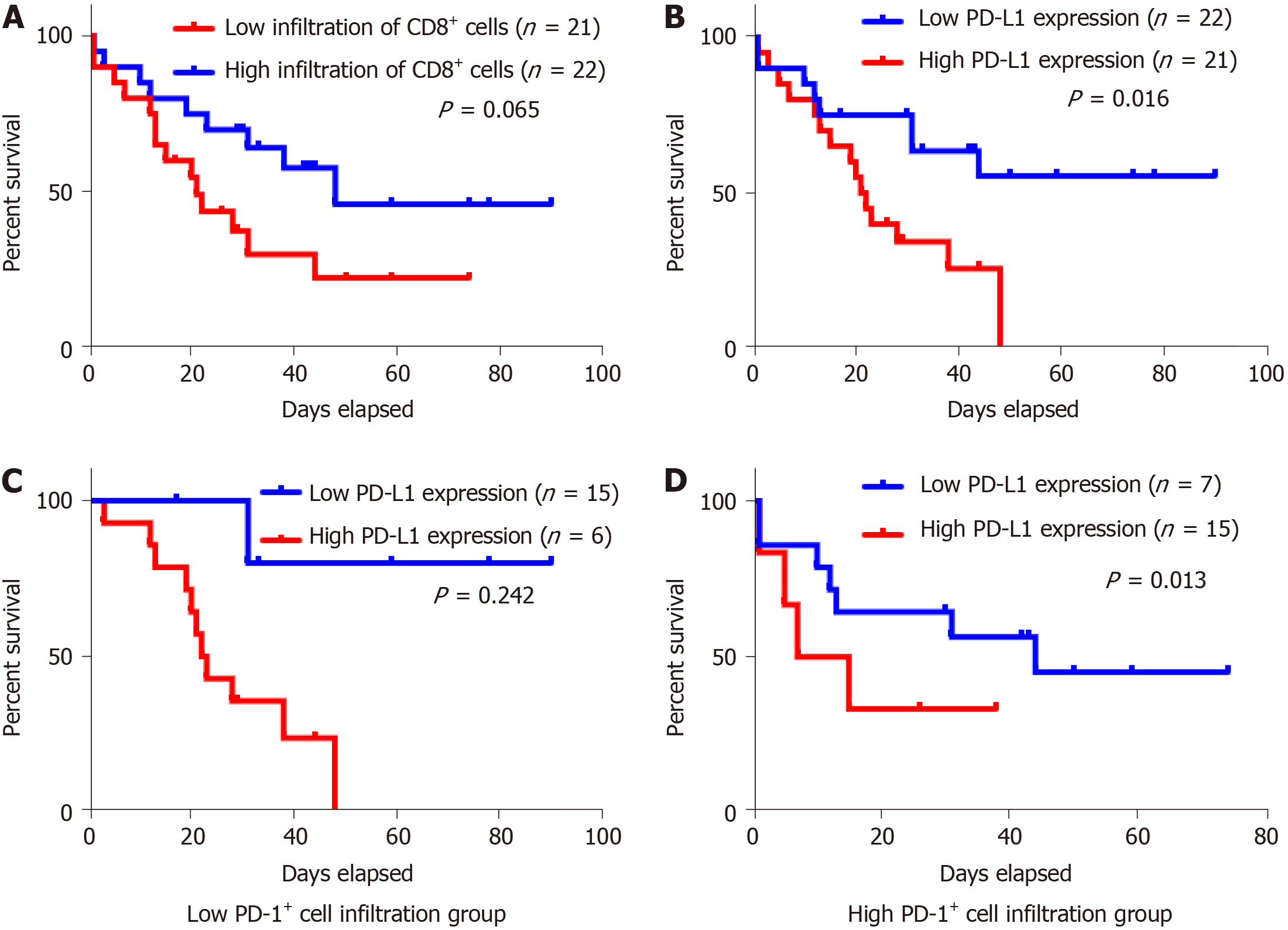
For Kaplan-Meier analysis, the OS of patients with high expression of PD-L1 was significantly shorter compared with patients with low PD-L1 expression (P = 0.016). We also found that patients with high CD8+ T cell infiltration may have a better clinical prognosis, however, due to the limited patient cohort, the P-value did not reach the statistical significance (P = 0.065). Univariate and multivariate analyses were performed to assess the impact of potential prognostic factors on OS. As shown in Table 3, corpus tumor [hazard ratio (HR) = 3.034, P = 0.032], liver metastasis (HR = 3.515, P = 0.016), and PD-L1 expression (HR = 2.846, P = 0.021) were significantly associated with OS in univariate analysis, while multivariate analysis demonstrated that only liver metastasis (HR = 4.045, P = 0.031) and PD-L1 expression (HR = 3.646, P = 0.009) were independent prognostic factors. Based on the theory that PD-L1 played a role in immune evasion by suppressing the function of PD-1 positive immune cells, we divided the patients into two groups depending on the infiltration of PD-1 positive cells and performed the Kaplan-Meier analysis to explore the influence of PD-L1 expression on patients’ survival. The results suggested that in the low PD-1+ cell infiltration group, patients’ survival was not associated with PD-L1 expression statistically (Figure 3C, P = 0.242); while in the high PD-1+ cells infiltration group, patients with high expression of PD-L1 had a significantly shorter survival (Figure 3D, P = 0.013).
| Prognostic parameter | Univariate | Multivariate | ||||
| HR | 95%CI | P-value | HR | 95%CI | P-value | |
| Age (yr) | ||||||
| < 60 | 1.0 (Reference) | |||||
| ≥ 60 | 2.718 | 0.921-8.023 | 0.07 | |||
| Gender | ||||||
| Male | 1.0 (Reference) | |||||
| Female | 0.915 | 0.308-2.718 | 0.873 | |||
| Tumor location | ||||||
| Cardia | 1.0 (Reference) | |||||
| Corpus | 3.034 | 1.100-8.374 | 0.032 | 2.878 | 0.971-8.529 | 0.057 |
| Antrum | 2.331 | 0.817-6.648 | 0.114 | 2.51 | 0.285-22.125 | 0.407 |
| Residual stomach anastomosis | 1.401 | 0.176-11.159 | 0.75 | 1.373 | 0.399-4.718 | 0.615 |
| Tumor size | ||||||
| ≤ 5 cm | 1.0 (Reference) | |||||
| > 5 cm | 1.605 | 0.693-3.717 | 0.269 | |||
| T classification | ||||||
| T1/2 | 1.0 (Reference) | |||||
| T3/4 | 0.76 | 0.407-1.420 | 0.39 | |||
| Lymph node metastasis | ||||||
| Absent | 1.0 (Reference) | |||||
| Present | 1.976 | 0.731-5.342 | 0.179 | |||
| Liver metastasis | ||||||
| Absent | 1.0 (Reference) | |||||
| Present | 3.515 | 1.269-9.731 | 0.016 | 4.045 | 1.140-14.351 | 0.031 |
| CD8 | ||||||
| Low | 1.0 (Reference) | |||||
| High | 0.46 | 0.197-1.074 | 0.073 | |||
| FOXP3 | ||||||
| Low | 1.0 (Reference) | |||||
| High | 1.375 | 0.593-3.186 | 0.458 | |||
| PD-1 | ||||||
| Low | 1.0 (Reference) | |||||
| High | 0.954 | 0.420-2.167 | 0.91 | |||
| PD-L1 | ||||||
| Low | 1.0 (Reference) | |||||
| High | 2.846 | 1.170-6.922 | 0.021 | 3.646 | 1.372-9.688 | 0.009 |
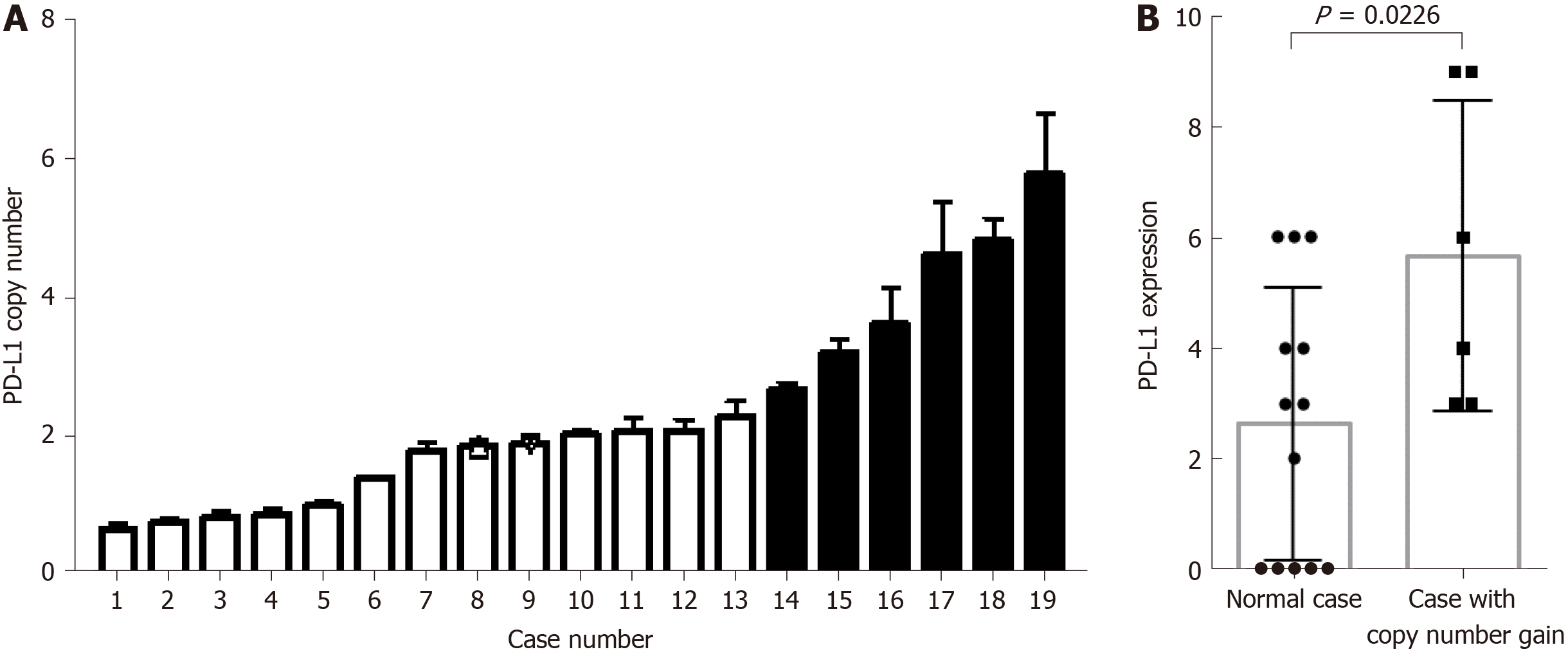
The G-NEC tissues demonstrated broad PD-L1 expression. Given the near-uniform expression pattern, we considered that genetic alteration may contribute to the PD-L1 expression. To address this issue, we analyzed the copy number variation in the tissue of G-NEC patients. In a cohort of 19 G-NEC patients, we found that 6/19 demonstrated a copy number gain of PD-L1 compared to normal human tissue. Furthermore, we found that the copy number of PD-L1 was positively correlated with the expression of PD-L1 at the protein level (Figure 4). However, we found no correlation between copy number gain and OS of patients (data not shown).
In this study, our data showed that more than half of G-NECs were diagnosed at an advanced stage (regional lymph node metastasis 72.09%, and liver metastasis 13.95%). And the median OS was only 31 mo. Meanwhile, as a rare group of poorly differentiated tumors with high-grade malignancy, novel therapeutic approaches are lack because of its rarity, complexity, and heterogeneity. Now, cancer immunotherapy is considered to be a major treatment backbone in the following decade[32]. Overexpression of PD-L1 and PD-1 in tumor and tumor stromal cells has been reported in various types of cancers, and antibodies targeting either PD-1 or PD-L1 have produced significant anti-tumor activity in several malignancies, such as non–small-cell lung cancer, melanoma, renal-cell cancer, bladder cancer, and head and neck cancer[20,33,34]. Therefore, there is an urgent need to define the significance of PD-1/PD-L1 pathway in G-NECs, with the potential to provide a new promising therapeutic approach.
In a recent study, Kim et al[35] evaluated the expression of PD-L1 in 32 metastatic GEP-NENs including one gastric NEN. Meanwhile, Cavalcanti et al[36] detected the expression of PD-L1 in 57 GEP-NENs including ten gastric NENs. They found that the expression of PD-L1 was associated with higher WHO tumor grade (grade 3) and it can be used as a predictive and prognostic marker for survival. So far, these are the only reports about PD-1/PD-L1 in GEP-NENs, and there is no research about PD-1/PD-L1 in G-NECs.
In our study, we first investigated the expression of PD-L1 in 43 G-NECs and the relationship between PD-L1 expression and clinical data. We found that 21 (48.8%) of 43 patients can be classified as high PD-L1 expression, and these people had a significant worse prognosis. This result is consistent with many previous studies focusing on gastric cancer[11], esophageal cancer[12], pancreatic cancer[13], and breast cancer[29]. And we also found that the expression of PD-L1 was an independent prognostic factor in G-NECs independent of tumor size and N classification.
PD-1, the ligand of PD-L1, expressed by activated T cells, B cells, myeloid cells, and other antigen-presenting cells, was also investigated in this research. According to previous studies, PD-1 expression in TILs is associated with PD-L1 expression in tumor cells[17,37], and increased number of PD-1+ TILs was also reported to be a significant predictor of poor survival[17,38,39]. In this study, we confirmed the positive association between PD-1+ TIL number and tumor cell PD-L1 expression. Then, the subgroup analysis indicated that PD-L1 had a role in predicting the prognosis only in PD-1+ group. These results supported that PD-L1 expressed by tumor cells interacts with PD-1+ TILs to suppress antitumor activity. However, in this study, we found no significant correlation between PD-1+ TILs and survival.
In our study, PD-L1 copy number analysis was performed to investigate the mechanisms underlying PD-L1 overexpression in G-NECs. We found that a large number of patients demonstrated a copy number alteration and PD-L1 expression was significantly higher in cases with PD-L1 copy number gain, implying that PD-L1 gene alteration is a mechanism of PD-L1 overexpression, similar to a study in thymic cancer[17]. Meanwhile, we noticed that survival analyses based on PD-L1 expression were inconsistent. Some recent research indicated that PD-L1 expression was associated with favorable OS in several malignancies, such as colorectal cancer, thymic cancer, breast cancer, and non-small cell lung cancer[14,17,40,41]. And in these studies, the authors detected a significant positive correlation between PD-L1 expression and increased TILs, especially CD8+ TILs, the key effector in antitumor immune response. Moreover, in melanoma, it was revaled that the up-regulation of immunosuppressive molecules PD-L1 and FOXP3+ Tregs is driven by CD8+ T cells[25], which means that the upregulated expression of PD-L1, PD-1, and FOXP3 might be a negative feedback mechanism following CD8+ T cell infiltration, to against activation of host antitumor immunity. Although the discrepancy between PD-L1 expression and prognosis can be attributed to a difference in experiment method and tumor heterogeneity, we determined CD8+ TILs and FOXP3+ Tregs in G-NECs.
As a glycoprotein, CD8 plays an important role in cell-mediated immunity by binding to the major histocompatibility complex class I molecule together with the T-cell receptor to stimulate the cytotoxic effect of TILs on cancer cells[42,43]. And it has been reported as a favorable prognostic factor in many malignancies[21-24]. In accordance with the previous research, we found that patients with high CD8+ T cell infiltration may have a better clinical prognosis, although it did not reach the statistical significance(P = 0.065). We found no association between PD-L1 expression and CD8+ TILs, however, we noticed that five specimens with high CD8+ T cell infiltration exhibited a tumor-stromal interface enhanced expression pattern of PD-L1. And this might support the correlation between tumor cell PD-L1 expression and CD8+ T cell infiltration in the stroma. And there was also no correlation between FOXP3+ Tregs, CD8+ TILs infiltration, and PD-L1 expression.
Finally, as a retrospective study, there are selection bias and some other limitations in our results. The small sample size of this research might result in some bias in the multivariable prognosis analysis, so larger sample studies are needed. In this research, we used IHC to investigate the expression of PD-L1, and our data might be limited by the lack of standardization of IHC, for example specificity and reproducibility of antibodies, definition of optimal positivity cut-off, and interpretative subjectivity.
In conclusion, this study for the first time demonstrated that the high PD-L1 expression by tumor cells was associated with a poor prognosis in G-NECs, especially in the PD-1+ subgroup. Although we did not find a significant correlation between CD8+ TILs and PD-L1 expression, we partly demonstrated the role of CD8+ TILs as a favorable prognostic biomarker for G-NEC patients. Due to the coinstantaneous impact of copy number variation and TILs’ stimulation on the expression of PD-L1, the mechanism of high expression of PD-L1 in G-NECs remains complicated. Most importantly, our findings provide important implications for the potential use of antibody therapies targeting the PD-1/PD-L1 signaling pathway in G-NECs.
Recently, more and more studies have demonstrated the pivotal role of programmed death 1/programmed death ligand 1 (PD-1/PD-L1) pathway in the immune evasion of tumors from the host immune system. However, the role of PD-1/PD-L1 pathway in gastric neuroendocrine carcinomas (G-NECs) remains unknown.
G-NECs are highly malignant, clinically defiant, and lack of effective treatment. Recent research has proved the role of treatment targeting PD-1/PD-L1 pathway in several other advanced cancers. This study for the first time demonstrated that PD-L1 can be expressed by G-NEC cancer cells and the high PD-L1 expression was associated with a poor prognosis. These findings provide important implications for the potential use of antibody therapies targeting the PD-1/PD-L1 signaling pathway in G-NECs.
We performed this research to investigate the expression of PD-1/PD-L1 and role of PD-1/PD-L1 pathway in G-NECs.
We investigated the expression of PD-L1 on tumor cells and PD-1+, CD8+, and FOXP3+ T cell infiltration by immunohistochemistry in 43 resected G-NECs tissue specimens, while the copy number alterations of PD-L1 were assessed by qRT-PCR. Statistical analyses and graphical representations were performed using SPSS 22.0 software and GraphPad Prism 6 software, respectively. χ2 test and Fisher’s exact test were used to evaluate the correlation of PD-L1, PD-1, FOXP3, and CD8 with clinicopathologic parameters in patients with G-NECs. Survival curves were evaluated using the Kaplan-Meier method and differences were analyzed by the log-rank test. Identification of factors that had a significant influence on survival was performed by univariate and multivariate Cox regression analyses. Comparison between two groups was performed by the Student’s t-test or Mann–Whitney U test.
We found that most of the G-NECs tumor cells exhibited a near-uniform expression pattern of PD-L1, while some showed a tumor-stromal interface enhanced pattern. Of the 43 G-NECs, 21 (48.8%) were classified as a high PD-L1 expression group, and the high expression of PD-L1 was associated with poor overall survival (OS). The high expression of PD-L1 was correlated with abundant PD-1+ tumor infiltrating lymphocytes (TILs) instead of CD8+ TILs and FOXP3+ regulatory T cells (Tregs). Our analysis also suggested that the infiltration of CD8+ TILs tended to be a favorable factor for OS, although the difference did not reach the statistical significance (P = 0.065). Meanwhile, PD-L1 was significantly overexpressed in cases with copy number gain as compared with those without. However, as a retrospective study, the small sample size of this research might result in some bias in the multivariable prognosis analysis, so larger sample studies are needed.
Our data demonstrated for the first time that the high expression of PD-L1 in G-NECs was associated with a poor prognosis, while the high expression may be due to the copy number variation of PD-L1 gene or stimulation of TILs. These results provide a basis for the immunotherapy targeting PD-1/PD-L1 pathway in G-NECs.
By this study, we found that PD-1/PD-L1 pathway is involved in G-NECs. In the following, in vitro cell experiments and in vivo animal experiments are needed.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Bramhall S, Cerwenka H, Okubo Y S-Editor: Yan JP L-Editor: Wang TQ E-Editor: Song H
| 1. | Sorbye H, Welin S, Langer SW, Vestermark LW, Holt N, Osterlund P, Dueland S, Hofsli E, Guren MG, Ohrling K, Birkemeyer E, Thiis-Evensen E, Biagini M, Gronbaek H, Soveri LM, Olsen IH, Federspiel B, Assmus J, Janson ET, Knigge U. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): The NORDIC NEC study. Ann Oncol. 2013;24:152-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 708] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 2. | Gastrointestinal Pathology Study Group of Korean Society of Pathologists; Cho MY, Kim JM, Sohn JH, Kim MJ, Kim KM, Kim WH, Kim H, Kook MC, Park DY, Lee JH, Chang H, Jung ES, Kim HK, Jin SY, Choi JH, Gu MJ, Kim S, Kang MS, Cho CH, Park MI, Kang YK, Kim YW, Yoon SO, Bae HI, Joo M, Moon WS, Kang DY, Chang SJ. Current Trends of the Incidence and Pathological Diagnosis of Gastroenteropancreatic Neuroendocrine Tumors (GEP-NETs) in Korea 2000-2009: Multicenter Study. Cancer Res Treat. 2012;44:157-165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 171] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 3. | Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A, Evans DB. One hundred years after "carcinoid": Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063-3072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3022] [Cited by in RCA: 3239] [Article Influence: 190.5] [Reference Citation Analysis (0)] |
| 4. | Lepage C, Rachet B, Coleman MP. Survival from malignant digestive endocrine tumors in England and Wales: A population-based study. Gastroenterology. 2007;132:899-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 5. | Modlin IM, Oberg K, Chung DC, Jensen RT, de Herder WW, Thakker RV, Caplin M, Delle Fave G, Kaltsas GA, Krenning EP, Moss SF, Nilsson O, Rindi G, Salazar R, Ruszniewski P, Sundin A. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008;9:61-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1268] [Cited by in RCA: 1180] [Article Influence: 69.4] [Reference Citation Analysis (0)] |
| 6. | Hijioka S, Hosoda W, Mizuno N, Hara K, Imaoka H, Bhatia V, Mekky MA, Tajika M, Tanaka T, Ishihara M, Yogi T, Tsutumi H, Fujiyoshi T, Sato T, Hieda N, Yoshida T, Okuno N, Shimizu Y, Yatabe Y, Niwa Y, Yamao K. Does the WHO 2010 classification of pancreatic neuroendocrine neoplasms accurately characterize pancreatic neuroendocrine carcinomas? J Gastroenterol. 2015;50:564-572. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 7. | Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887-3895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1899] [Cited by in RCA: 2196] [Article Influence: 66.5] [Reference Citation Analysis (0)] |
| 8. | Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1806] [Cited by in RCA: 1961] [Article Influence: 75.4] [Reference Citation Analysis (0)] |
| 9. | Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027-1034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3572] [Cited by in RCA: 4036] [Article Influence: 161.4] [Reference Citation Analysis (0)] |
| 10. | Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat Med. 2002;8:793-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2838] [Cited by in RCA: 3538] [Article Influence: 153.8] [Reference Citation Analysis (0)] |
| 11. | Wu C, Zhu Y, Jiang J, Zhao J, Zhang XG, Xu N. Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance. Acta Histochem. 2006;108:19-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 400] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 12. | Ohigashi Y, Sho M, Yamada Y, Tsurui Y, Hamada K, Ikeda N, Mizuno T, Yoriki R, Kashizuka H, Yane K, Tsushima F, Otsuki N, Yagita H, Azuma M, Nakajima Y. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res. 2005;11:2947-2953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 566] [Cited by in RCA: 638] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 13. | Nomi T, Sho M, Akahori T, Hamada K, Kubo A, Kanehiro H, Nakamura S, Enomoto K, Yagita H, Azuma M, Nakajima Y. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res. 2007;13:2151-2157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 645] [Cited by in RCA: 699] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 14. | Droeser RA, Hirt C, Viehl CT, Frey DM, Nebiker C, Huber X, Zlobec I, Eppenberger-Castori S, Tzankov A, Rosso R, Zuber M, Muraro MG, Amicarella F, Cremonesi E, Heberer M, Iezzi G, Lugli A, Terracciano L, Sconocchia G, Oertli D, Spagnoli GC, Tornillo L. Clinical impact of programmed cell death ligand 1 expression in colorectal cancer. Eur J Cancer. 2013;49:2233-2242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 354] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 15. | Baptista MZ, Sarian LO, Derchain SF, Pinto GA, Vassallo J. Prognostic significance of PD-L1 and PD-L2 in breast cancer. Hum Pathol. 2016;47:78-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 181] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 16. | Yokoyama S, Miyoshi H, Nishi T, Hashiguchi T, Mitsuoka M, Takamori S, Akagi Y, Kakuma T, Ohshima K. Clinicopathologic and Prognostic Implications of Programmed Death Ligand 1 Expression in Thymoma. Ann Thorac Surg. 2016;101:1361-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 17. | Yokoyama S, Miyoshi H, Nakashima K, Shimono J, Hashiguchi T, Mitsuoka M, Takamori S, Akagi Y, Ohshima K. Prognostic Value of Programmed Death Ligand 1 and Programmed Death 1 Expression in Thymic Carcinoma. Clin Cancer Res. 2016;22:4727-4734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 18. | Pedoeem A, Azoulay-Alfaguter I, Strazza M, Silverman GJ, Mor A. Programmed death-1 pathway in cancer and autoimmunity. Clin Immunol. 2014;153:145-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 207] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 19. | Hassel JC. Ipilimumab plus nivolumab for advanced melanoma. Lancet Oncol. 2016;17:1471-1472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Philips GK, Atkins M. Therapeutic uses of anti-PD-1 and anti-PD-L1 antibodies. Int Immunol. 2015;27:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 264] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 21. | Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, Nagura H, Ohtani H. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998;58:3491-3494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Schumacher K, Haensch W, Röefzaad C, Schlag PM. Prognostic significance of activated CD8(+) T cell infiltrations within esophageal carcinomas. Cancer Res. 2001;61:3932-3936. [PubMed] |
| 23. | Cho Y, Miyamoto M, Kato K, Fukunaga A, Shichinohe T, Kawarada Y, Hida Y, Oshikiri T, Kurokawa T, Suzuoki M, Nakakubo Y, Hiraoka K, Murakami S, Shinohara T, Itoh T, Okushiba S, Kondo S, Katoh H. CD4+ and CD8+ T cells cooperate to improve prognosis of patients with esophageal squamous cell carcinoma. Cancer Res. 2003;63:1555-1559. [PubMed] |
| 24. | Huang Y, Ma C, Zhang Q, Ye J, Wang F, Zhang Y, Hunborg P, Varvares MA, Hoft DF, Hsueh EC, Peng G. CD4+ and CD8+ T cells have opposing roles in breast cancer progression and outcome. Oncotarget. 2015;6:17462-17478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 159] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 25. | Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, Gajewski TF. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med. 2013;5:200ra116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1113] [Cited by in RCA: 1403] [Article Influence: 127.5] [Reference Citation Analysis (0)] |
| 26. | Taube JM, Young GD, McMiller TL, Chen S, Salas JT, Pritchard TS, Xu H, Meeker AK, Fan J, Cheadle C, Berger AE, Pardoll DM, Topalian SL. Differential Expression of Immune-Regulatory Genes Associated with PD-L1 Display in Melanoma: Implications for PD-1 Pathway Blockade. Clin Cancer Res. 2015;21:3969-3976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 203] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 27. | Kasprowicz DJ, Smallwood PS, Tyznik AJ, Ziegler SF. Scurfin (FoxP3) controls T-dependent immune responses in vivo through regulation of CD4+ T cell effector function. J Immunol. 2003;171:1216-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 79] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 28. | Hou J, Yu Z, Xiang R, Li C, Wang L, Chen S, Li Q, Chen M, Wang L. Correlation between infiltration of FOXP3+ regulatory T cells and expression of B7-H1 in the tumor tissues of gastric cancer. Exp Mol Pathol. 2014;96:284-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 118] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 29. | Li Z, Dong P, Ren M, Song Y, Qian X, Yang Y, Li S, Zhang X, Liu F. PD-L1 Expression Is Associated with Tumor FOXP3(+) Regulatory T-Cell Infiltration of Breast Cancer and Poor Prognosis of Patient. J Cancer. 2016;7:784-793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 130] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 30. | Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumours of the digestive system. In: WHO classification of tumours. 4th ed. Lyon: IARC 2010; 417. |
| 31. | Edge SB, Compton CC. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5537] [Cited by in RCA: 6452] [Article Influence: 430.1] [Reference Citation Analysis (0)] |
| 32. | Teng MW, Ngiow SF, Ribas A, Smyth MJ. Classifying Cancers Based on T-cell Infiltration and PD-L1. Cancer Res. 2015;75:2139-2145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 879] [Cited by in RCA: 1162] [Article Influence: 116.2] [Reference Citation Analysis (0)] |
| 33. | Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443-2454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8900] [Cited by in RCA: 9883] [Article Influence: 760.2] [Reference Citation Analysis (0)] |
| 34. | Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, Brahmer JR, Lawrence DP, Atkins MB, Powderly JD, Leming PD, Lipson EJ, Puzanov I, Smith DC, Taube JM, Wigginton JM, Kollia GD, Gupta A, Pardoll DM, Sosman JA, Hodi FS. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1801] [Cited by in RCA: 1827] [Article Influence: 166.1] [Reference Citation Analysis (0)] |
| 35. | Kim ST, Ha SY, Lee S, Ahn S, Lee J, Park SH, Park JO, Lim HY, Kang WK, Kim KM, Park YS. The Impact of PD-L1 Expression in Patients with Metastatic GEP-NETs. J Cancer. 2016;7:484-489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 107] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 36. | Cavalcanti E, Armentano R, Valentini AM, Chieppa M, Caruso ML. Role of PD-L1 expression as a biomarker for GEP neuroendocrine neoplasm grading. Cell Death Dis. 2017;8:e3004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 89] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 37. | Fan Y, Ma K, Wang C, Ning J, Hu Y, Dong D, Dong X, Geng Q, Li E, Wu Y. Prognostic value of PD-L1 and PD-1 expression in pulmonary neuroendocrine tumors. Onco Targets Ther. 2016;9:6075-6082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 38. | Muenst S, Soysal SD, Gao F, Obermann EC, Oertli D, Gillanders WE. The presence of programmed death 1 (PD-1)-positive tumor-infiltrating lymphocytes is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2013;139:667-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 281] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 39. | Thompson RH, Dong H, Lohse CM, Leibovich BC, Blute ML, Cheville JC, Kwon ED. PD-1 is expressed by tumor-infiltrating immune cells and is associated with poor outcome for patients with renal cell carcinoma. Clin Cancer Res. 2007;13:1757-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 441] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 40. | Schalper KA, Velcheti V, Carvajal D, Wimberly H, Brown J, Pusztai L, Rimm DL. In situ tumor PD-L1 mRNA expression is associated with increased TILs and better outcome in breast carcinomas. Clin Cancer Res. 2014;20:2773-2782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 380] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 41. | Schmidt LH, Kümmel A, Görlich D, Mohr M, Bröckling S, Mikesch JH, Grünewald I, Marra A, Schultheis AM, Wardelmann E, Müller-Tidow C, Spieker T, Schliemann C, Berdel WE, Wiewrodt R, Hartmann W. PD-1 and PD-L1 Expression in NSCLC Indicate a Favorable Prognosis in Defined Subgroups. PLoS One. 2015;10:e0136023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 198] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 42. | Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: Impact on clinical outcome. Nat Rev Cancer. 2012;12:298-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3108] [Cited by in RCA: 3599] [Article Influence: 276.8] [Reference Citation Analysis (0)] |
| 43. | Diana A, Wang LM, D'Costa Z, Allen P, Azad A, Silva MA, Soonawalla Z, Liu S, McKenna WG, Muschel RJ, Fokas E. Prognostic value, localization and correlation of PD-1/PD-L1, CD8 and FOXP3 with the desmoplastic stroma in pancreatic ductal adenocarcinoma. Oncotarget. 2016;7:40992-41004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |