Published online Apr 14, 2019. doi: 10.3748/wjg.v25.i14.1640
Peer-review started: February 17, 2019
First decision: February 26, 2019
Revised: March 9, 2019
Accepted: March 16, 2019
Article in press: March 16, 2019
Published online: April 14, 2019
Processing time: 57 Days and 20.3 Hours
The gravest prognostic factor in early gastric cancer is lymph-node metastasis, with an incidence of about 10% overall. About two-thirds of early gastric cancer patients can be diagnosed as node-negative prior to treatment based on clinic-pathological data. Thus, the tumor can be resected by endoscopic submucosal dissection. In the remaining third, surgical resection is necessary because of the possibility of nodal metastasis. Nevertheless, almost all patients can be cured by gastrectomy with D1+ lymph-node dissection. Laparoscopic or robotic gastrectomy has become widespread in East Asia because perioperative and oncological safety are similar to open surgery. However, after D1+ gastrectomy, functional symptoms may still result. Physicians must strive to minimize post-gastrectomy symptoms and optimize long-term quality of life after this operation. Depending on the location and size of the primary lesion, preservation of the pylorus or cardia should be considered. In addition, the extent of lymph-node dissection can be individualized, and significant gastric-volume preservation can be achieved if sentinel node biopsy is used to distinguish node-negative patients. Though the surgical treatment for early gastric cancer may be less radical than in the past, the operative method itself seems to be still in transition.
Core tip: The surgical treatment for early gastric cancer seems to be appropriately radical, because almost all patients can be cured by gastrectomy with lymph-node dissection up to D1+. However, after D1+ gastrectomy, multiple functional symptoms are caused by the loss of the stomach. Physicians must strive to reduce post-gastrectomy symptoms and optimize quality of life. About two-thirds of early gastric cancers are node-negative and can be resected by endoscopic submucosal dissection. The extent of lymph-node dissection can be individualized, and significant gastric preservation can be achieved, with sentinel-node biopsy. The operative method itself is still in transition.
- Citation: Kinami S, Nakamura N, Tomita Y, Miyata T, Fujita H, Ueda N, Kosaka T. Precision surgical approach with lymph-node dissection in early gastric cancer. World J Gastroenterol 2019; 25(14): 1640-1652
- URL: https://www.wjgnet.com/1007-9327/full/v25/i14/1640.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i14.1640
Gastric cancer is a public health concern worldwide, and especially in Asia[1,2]. Therefore, Japanese and South Korean physicians have focused on early detection of gastric cancer[3]. For decades, half of the gastric cancers detected in Japan and Korea have been early-stage cancer[4]. The outcome of surgical resection for early gastric cancer is excellent, and most clinicians recognize that early gastric cancer is curable. The focus of recent topical discussion of early gastric cancer is minimally invasive treatment. Many early gastric cancers have been resected endoscopically[5]. Less invasive approaches such as laparoscopic gastrectomy and robotic gastrectomy have also been widely carried out[6-8]. On the other hand, standard surgery for early gastric cancer is distal partial gastrectomy (DG) or total gastrectomy (TG), with lymph-node dissection[9], so, even with a laparoscopic approach, post-gastrectomy symptoms and functional effects cannot be ignored[10]. In this article, we reconsider surgical treatment options for early gastric cancer and discuss the most appropriate treatment.
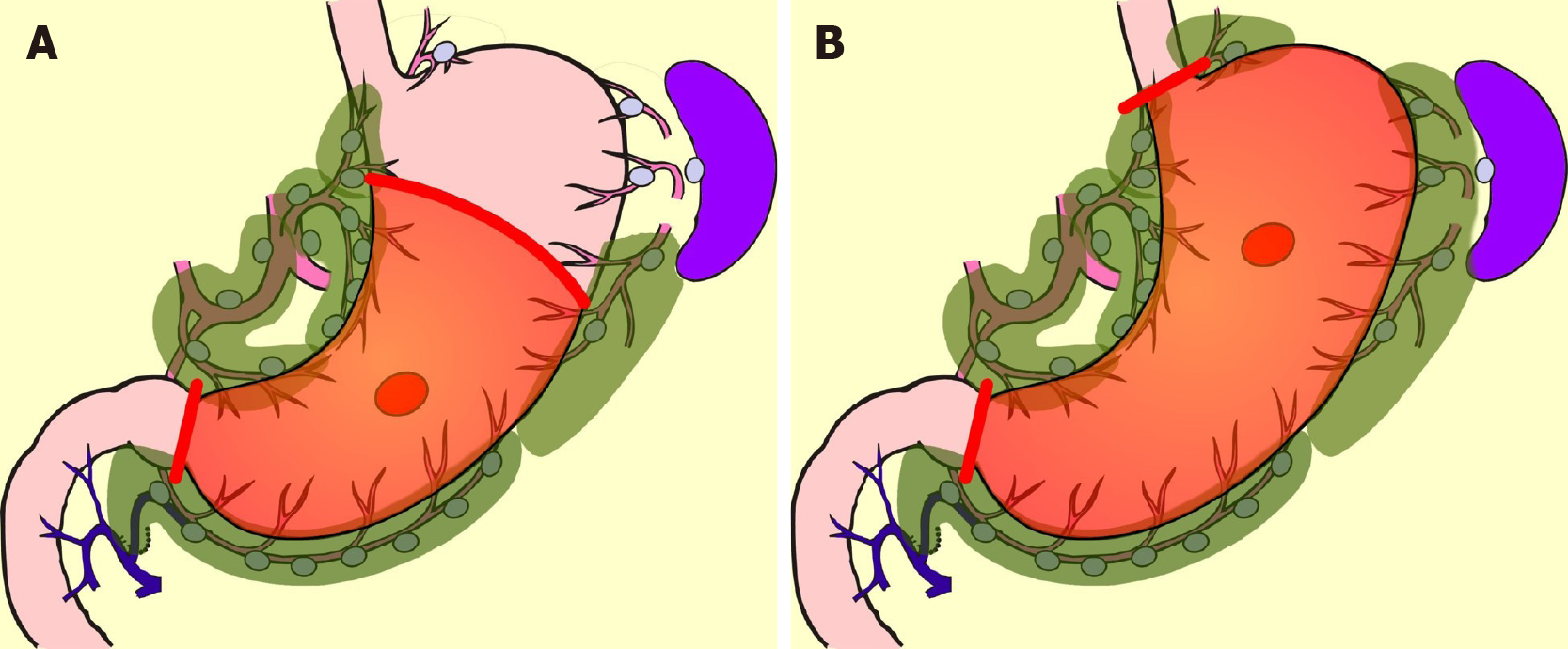
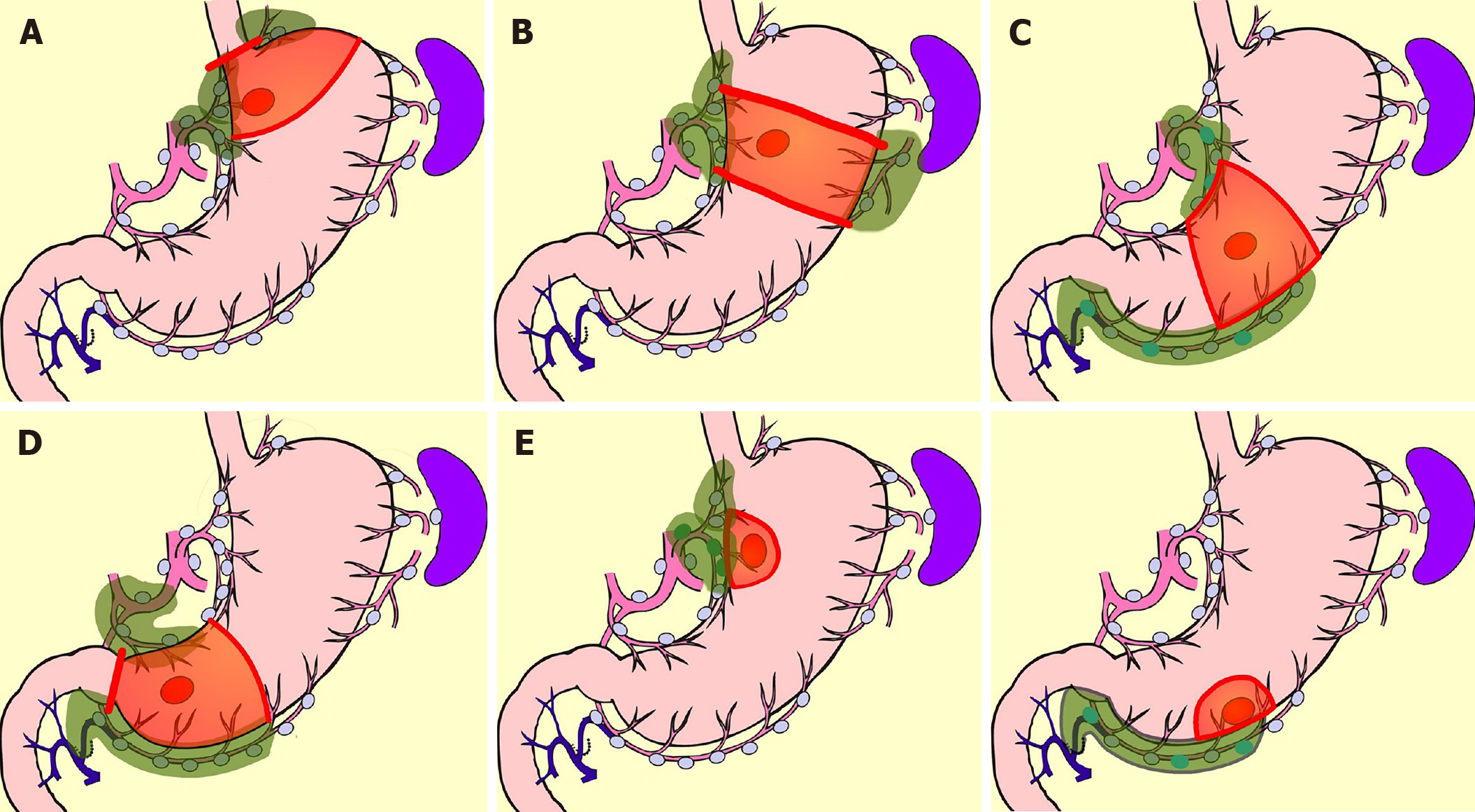
Early gastric cancer is defined as carcinoma in which depth of invasion is restricted to the mucosal layer or submucosa[11]. The presence or absence of lymph-node metastasis is irrelevant to the classification[11]. Most early gastric cancers are asymptomatic. Early gastric cancer is often detected with gastroscopy or barium meal during a health-screening checkup[12]. Advanced gastric cancer is frequently associated with hematogenous or peritoneal metastases, while in contrast, early gastric cancer has few such distant metastases. On the other hand, early gastric cancer is rarely associated with lymph-node metastasis. The frequency of lymph-node metastasis is 2%-3% in mucosal cancer and 15%-20% in submucosal cancer[13-21]. Numerous previous studies have examined the location of these lymph node metastases. In the classification of gastric carcinoma of the Japanese Gastric Cancer Association[22], the regional lymph nodes of the stomach are classified in detail and numbered. Currently, the extent of lymph-node metastasis has been evaluated in terms of the number of metastases, but in the past, regional nodes were grouped according to the location of the cancer, and the degree of lymph-node metastasis was evaluated based on which group of nodes the metastasis had reached. The precise data of nodal metastasis of early gastric cancer described in a representative literature are summarized in Table 1[23-26]. Most nodal metastasis in early gastric cancer was found to be limited to perigastric nodes and nodes number 7, 8a, and 9. Based on these results, the Japanese Gastric Cancer Association established D1+[27], the extent of lymph-node dissection for submucosal cancer (Figure 1). The disease-specific survival of D1+ gastrectomy for early gastric cancer is often given as 96%-98% in articles investigating the outcome of laparoscopic gastrectomy[28-30].
| Nodal location | Total cases | Perigastric nodes (%) | aLGA (%) | Suprapancreatic nodes (%) | SpH (%) | aPHA (%) | PAN (%) | ||||||||
| Station NO | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8a | 9 | 11p | 11d | 10 | 12a | 16 | |
| Kitamura et al[23] | 634 | 8.2 | 1.6 | 0.94 | 0.31 | 0.31 | 0.00 | 0.00 | 0.16 | 0.16 | |||||
| Tanaka et al[24] | 2368 | 0.97 | 0.08 | 4.6 | 3.2 | 0.51 | 2.4 | 1.4 | 0.63 | 0.72 | 0.42 | 0.00 | 0.00 | 0.00 | 0.00 |
| Nakajima et al[25] | 3630 | 0.90 | 0.11 | 5.9 | 3.9 | 0.47 | 3.4 | 1.1 | 1.1 | 1.1 | 0.36 | 0.03 | 0.08 | 0.06 | 0.25 |
| Yoshikawa et al[26]1 | 7151 | 19.01 | 2.01 | 1.81 | 0.131 | 0.131 | 0.001 | 0.001 | 0.001 | 0.001 | |||||
In summary, the characteristics of early gastric cancer are as follows: there are few hematogenous and peritoneal metastases; the determining prognostic factor is lymph node metastasis; the incidence of nodal metastasis is about 10% overall; and even with nodal metastases, almost all patients can be cured by gastrectomy with D1+ lymph-node dissection.
However, after D1+ gastrectomy, several functional symptoms are caused by the loss of the stomach. Reduction of gastric acid secretion impairs food digestive capacity. The amount of food intake decreases, nutritional status worsens, and body weight decreases. In addition, patients suffer from various postgastrectomy symptoms (PGS). They include reflux esophagitis, dumping syndrome, defecation abnormalities, anorexia, and abdominal pain. These symptoms are thought to compromise patients’ quality of life (QOL). In addition, several long-term aftereffects may occur, such as iron deficiency anemia, pernicious anemia, bone metabolic disorders, gastric stump cancer, cholelithiasis, and ileus[31-37]. Taking these disadvantages into consideration, if there is another therapeutic option, we would like to adopt it to avoid gastrectomy.
There are endoscopic treatments. The endoscopic treatments are alternatives to surgery using gastrofibroscopy. Gastroscopy was developed in Japan, and endoscopic treatments for gastric tumor were also invented and developed in Japan. The beginning of endoscopic treatment is endoscopic mucosal resection (EMR). EMR is a method of injecting physiological saline into the submucosal layer to lift the lesion, narrowing the mucosa with a snare, and removing the mucosa by applying a high frequency current[38]. During the same period, endoscopic treatments using lesion cauterization with laser, heating probe, argon plasma discharge, etc., had been attempted. These therapies have some advantages and serious disadvantages, and they have not attained therapeutic value sufficient to replace surgical treatment[39,40]. However, this situation has changed with the advent of endoscopic submucosal dissection (ESD) technique[40]. ESD is the method of dissecting and removing the specimen after incision of the mucosa around the entire circumference using a dedicated device. In ESD, the specimen is removed in one piece, which secures a safety margin, the treatment outcome is equal to prior surgical treatments, and it is possible for the patient to enjoy the same QOL as before the treatment. The greatest disadvantage of ESD was its technical difficulty, though this difficulty was decreased by the development of electrocautery equipment and advancements in the dedicated device. Nowadays, ESD is not just an alternative to surgery, it is becoming a complete replacement for certain types of surgery[40]. The indication for ESD is a case in which the clinic-pathological data available prior to treatment is presumed to be negative for nodal metastasis. The frequency of nodal metastasis is 2%-3% in mucosal cancer and 15%-20% in submucosal cancer. Therefore, ESD is indicated in most mucosal cancers. Gotoda et al[41] proposed an indication for ESD from a study of surgical cases of two Japanese high-volume centers. Since the probability of nodal metastasis was set not to exceed the mortality rate after surgical resection, these criteria includes cases with a very low, but not zero, percentage probability of nodal metastasis. Recently, the long-term prognosis of the cases who underwent ESD according to this indication has been reported, and it has been confirmed that it is equal to the prognosis of the surgical resection[42-44]. Current indications for ESD are shown in Tables 2 and 3. These are new recommendations, stated in Japanese gastric cancer treatment guidelines for 2018[9]. In the table of indications (Table 2), Zone A is a former absolute indication. Zone B is newly added absolute indication proven by prospective observational trial JCOG 0607. Zone C is the expanded indication. Although this area is considered to be sufficiently curable, caution is necessary because the proof of the prospective observational trial JCOG1009/1010 has not been completed yet. Zone D is labeled “relative indication”. This zone comprises an alternative treatment to surgery, reserved only for patients unable to endure surgical treatment. The guideline also defines the evaluation of curability after resection (Table 3). If the specimen is diagnosed as eCuraA or eCureB, the cancer is considered completely resected and no additional treatment is required. On the other hand, for eCuraC it is considered that the cancer has not been completely resected, and additional treatment is necessary. The lesion of eCuraC-1 needs additional local treatment, and in the case of cCuraC-2 surgical treatment with lymph-node dissection should be added.
| Depth of invasion (preoperative) | Clinical mucosal cancer | |||
| Intra-tumoral ulcer of ulcer scar | UL 0 | UL 1 | ||
| Tumor size (Long axis) | 2 cm | > 2 cm | 3 cm | > 3 cm |
| Differentiated | A | B | B | D |
| Undifferentiated | C | D | D | D |
| Updated evaluation of curability after endoscopic submucosal dissection | |
| eCuraA | En-bloc resection, predominantly differentiated adenocarcinoma, pathological mucosal cancer (pT1a), HM0, VM0, Ly0, V0 |
| UL0 (regardless of size) | |
| UL1, under 3 cm in diameter | |
| If size of undifferentiated component is > 2 cm, tumor is diagnosed as eCuraC-2 | |
| eCuraB | En-bloc resection, HM0, VM0, Ly0, V0 |
| UL0, under 2 cm in diameter, predominantly undifferentiated adenocarcinoma, pathological mucosal cancer (pT1a) | |
| UL1, under 3 cm in diameter, predominantly differentiated adenocarcinoma, pathological submucosal cancer within 500 µm (pT1b1) | |
| If there is an undifferentiated component in the submucosal layer, tumor is diagnosed as eCuraC-2 | |
| eCuraC-1 | Lesion meeting criteria of eCuraA or eCuraB except with positive lateral margin or non–en-bloc resection. |
| eCuraC-2 | The lesion meets none of eCuraA, eCuraB, or eCuraC-1 |
In cases where ESD is not indicated, surgical resection is necessary because of the possibility of nodal metastasis[20,41]. In East Asia, most surgical treatment for early gastric cancer is laparoscopic D1+ gastrectomy. Laparoscopic gastrectomy was introduced in Japan in 1991[45]. At the time, the quality of laparoscopic views was poor, and the instruments were inadequate to the task. Therefore, lymph-node dissection was extremely difficult. Laparoscopic surgeries for early gastric cancer at that time consisted of local resection (LR) without lymphadenectomy[46], and intra-gastric surgery dissecting the mucosa[47]. Though the pioneers also attempted laparoscopic DG, the extent of nodal dissection was confined to peri-gastric nodes[45]. The indication for these treatments were cases in which lymph-node metastasis was presumed to be absent based on clinico-pathological features[45-47]. These approaches gradually disappeared with the advent of ESD. Subsequently, the invention of ultrasonic activated devices and technological advancements by surgeons have enabled laparoscopic lymph-node dissection comparable to conventional open surgery. Therefore, laparoscopic DG or TG with D1+ or D2 came to be performed as daily practice, and gradually replaced conventional open surgery.
There are many articles comparing laparoscopic gastrectomy and conventional open gastrectomy for patients with early gastric cancer[48,49], and a meta-analysis of prospective trials has also been conducted[49]. Perioperative surgical safety and oncological safety are considered similar[48,49]. The advantages of laparoscopic gastrectomy over conventional open surgery are as follows: the smaller size of the incision; lower number of times analgesic is required; lesser amount of intraoperative hemorrhage; and fewer occurrences of wound dehiscence and respiratory complications. On the other hand, the drawbacks are high cost and longer operation time[49]. Although it is difficult to conclusively establish the minimal invasiveness of laparoscopic gastrectomy, it is suggested by the shorter time to first flatus and shorter hospital stays.
Because the size of the wound is clearly visible, laparoscopic surgery is an attractive option for hospitals. The high degree of difficulty of the laparoscopic gastrectomy is an attractive challenge for young surgeons. Thus, laparoscopic gastrectomy has become widespread in East Asia. However, the benefits of laparoscopic gastrectomy in the long term have not yet been sufficiently examined.
Various PGS occur after gastrectomy[34-37], and, these symptoms seem to worsen QOL[35]. The severity of these PGS and the deterioration of QOL are subjective measures, and objective evaluations are difficult. These items are patient-reported outcomes whose scientific validation must be performed using definitive questionnaires that have already been validated psychometrically. DAUGS[50] and PGSAS-45[34] have been reported as questionnaires specifically for post-gastrectomy patients. Of these, PGSAS-45 seems to be the de-facto standard to verify PGS and QOL, because it covers items that are considered important by gastrectomy specialists, it includes a short-form 8 (SF-8) for the assessment of generic QOL, and the standard values of Japanese patients are known based on data from more than 2500 cases[34].
Kinami et al[51] evaluated the superiority in PGS and QOL at least 1 year after surgery of patients who underwent laparoscopic gastrectomy, using data from the PGSAS study for additional analysis. The outcome measures included in the PGSAS-45 are classified into three domains: the symptom domain, the living status domain, and the QOL domain[34]. A few main outcome measures of PGSAS-45 were superior after laparoscopic, compared to conventional open, DG surgery. These measures were: The need of additional food; dissatisfaction with symptoms; and the mental component summary of SF-8. These items were of the living status or QOL domain, not the symptom domain. In contrast, for TG, there was no difference in the scores of main outcome measures between laparoscopic surgery and conventional open surgery. From this large-scale analysis, it was concluded that there is no advantage for laparoscopic TG from the viewpoint of the PGS[51]. Generally, the only difference between laparoscopic surgery and conventional open surgery is the length of the incision. Therefore, a large difference in PGS between laparoscopic and open surgery would not be expected.
Even if the advantages are small, such as the size of the incision, the number of times analgesic is used, and a slight improvement in QOL, it is reasonable to apply laparoscopic gastrectomy to the early gastric cancer, if the surgical and oncological safety are equivalent. However, if other approaches can be used to prevent PGS, palliation of PGS should be prioritized to benefit patients in the long term, rather than focusing on the small incision size afforded by laparoscopic surgery. Limited surgery is a method expected to palliate PGS. Limited surgical approaches consist of reduced resection area of the stomach and smaller extent of nodal dissection. These include pylorus-preserving gastrectomy (PPG), proximal gastrectomy (PG), and LR. In performing LR, the unconventional decision to omit lymph-node dissection is necessary. Therefore, LR is rarely done today, while ESD is routinely performed[52].
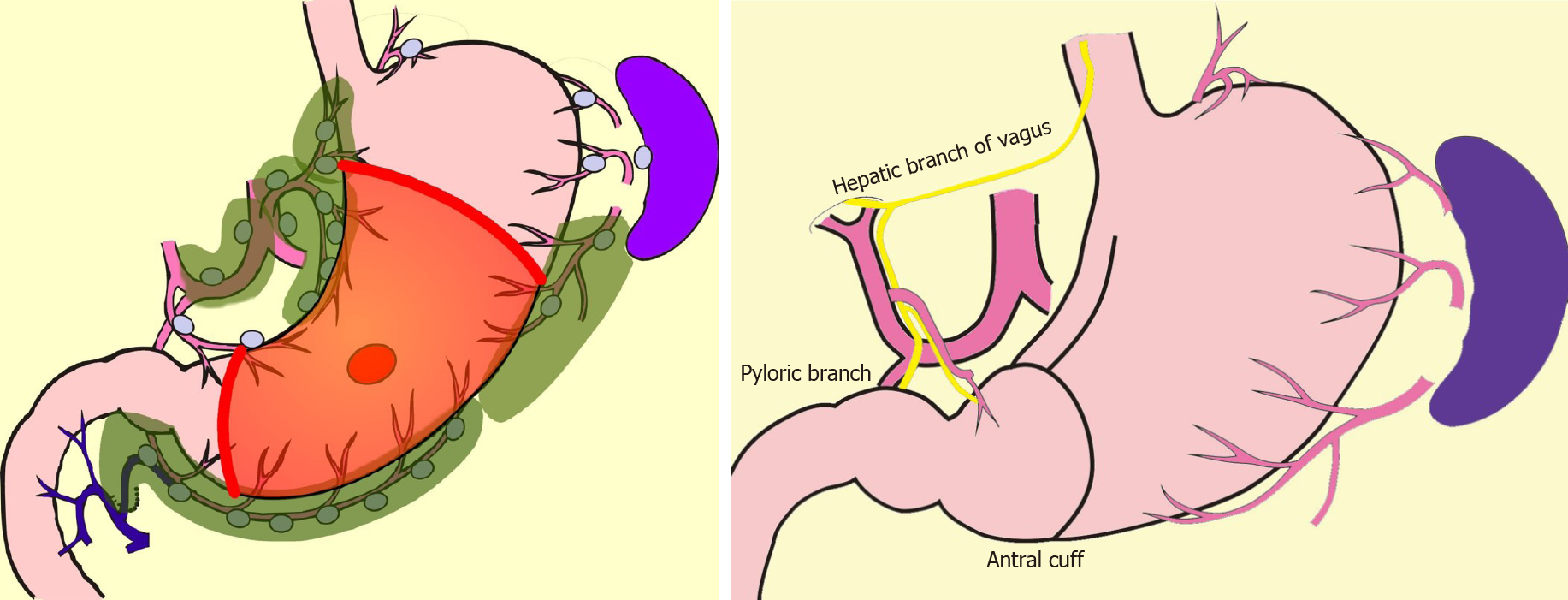
In comparison with DG, PPG is a pylorus-preserving procedure[53]. Generally, the right gastric artery and pyloric branch of the vagus nerve are preserved to secure an antral cuff of about 3 cm. It is expected to be effective for preventing dumping symptoms, regurgitation of duodenal juice into the stomach, and gallstone formation after gastrectomy[54,55]. This procedure is indicated in cases in which right gastric-artery lymph-node dissection can be omitted. Practically speaking, these patients will have tumors located at the middle part of the stomach with distal margins more than 4 cm from the pylorus[9]. Even if PPG is adopted, lymph-node dissection at nodes other than those located at the right gastric artery area is possible, and D1+ for PPG is included in the Japanese gastric cancer treatment guidelines[9] (Figure 2). The survival outcome of PPG is considered equal to that of DG[54,55]. In the PGSAS study, it was confirmed that, in comparison with Billroth I cases, diarrhea and dumping symptoms and the necessity of additional food was lower in PPG cases[56].
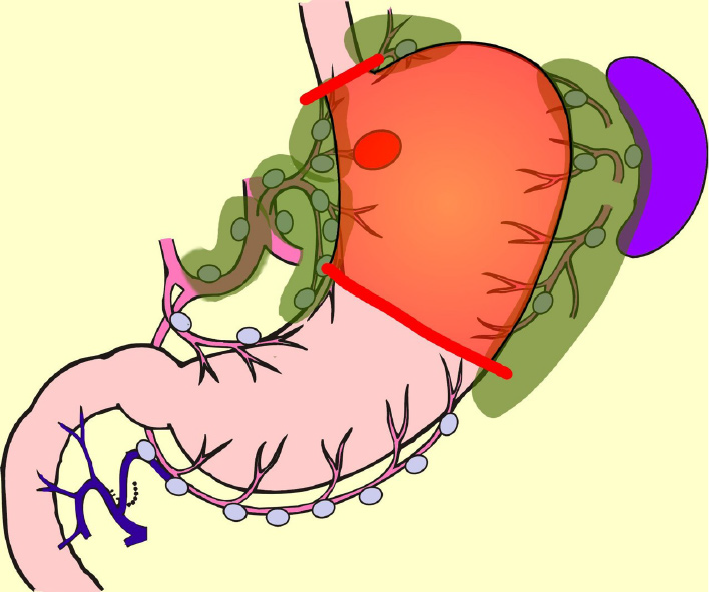
PG is an alternative to TG for early gastric cancer located in the stomach’s upper third. Compared with TG, PG preserves over one-half of the distal stomach and is considered to be superior in improving nutritional status and preventing anemia because of higher dietary intake and preserved secretion of gastric acid, Castle intrinsic factor, and gastrin. It is reported that if the cancer lesion is limited to the upper-third of the stomach, dissection of the lymph nodes along the right gastric artery and right gastroepiploic artery can be omitted without compromising radicality[57-60]. In the Japanese gastric cancer treatment guideline, D1+ for PG is set[9] (Figure 3). In the PGSAS study, PG prevented diarrhea and dumping symptoms, slightly diminished weight loss, and lowered the necessity of additional food than TG[36].
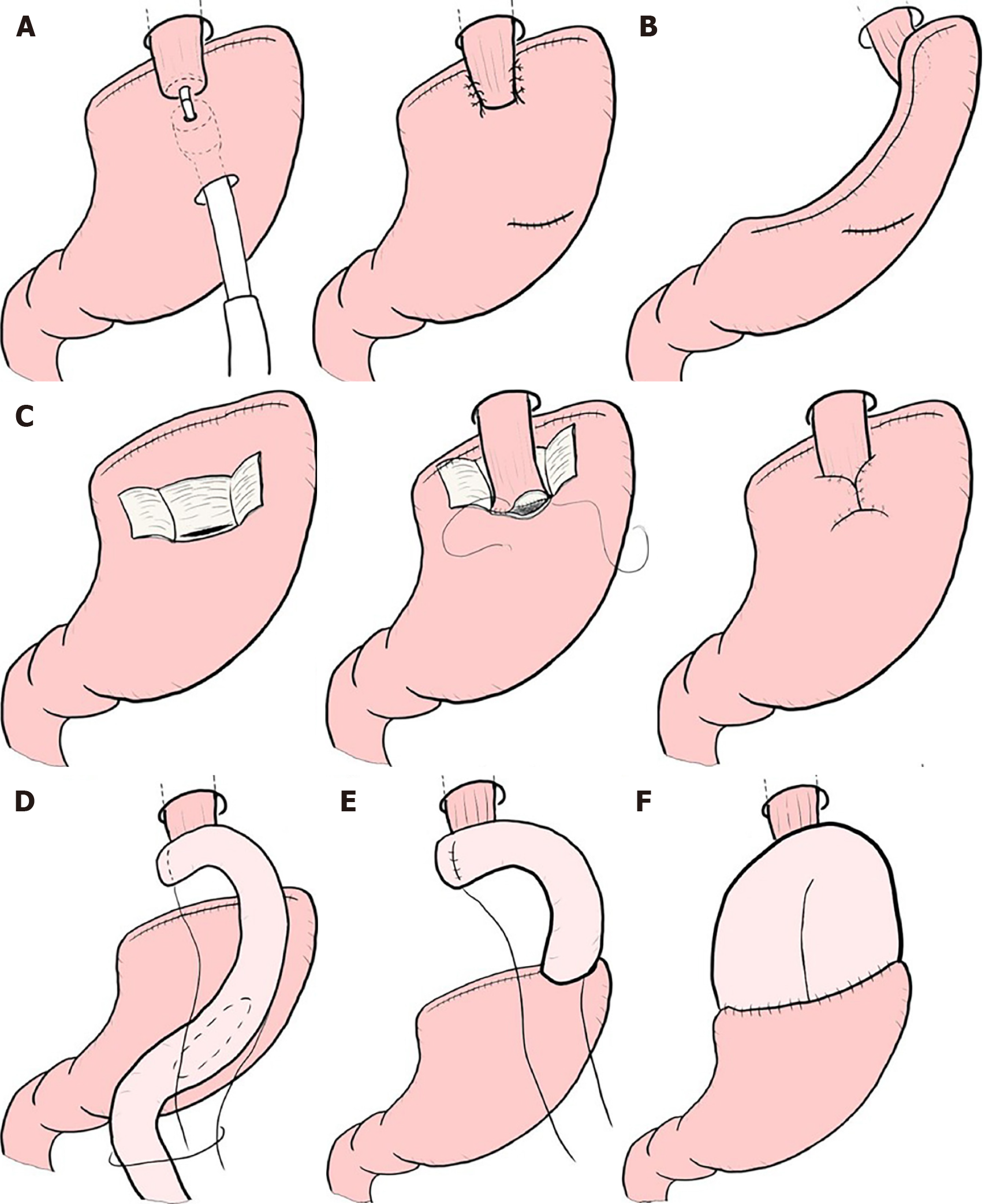
Though these limited surgeries are superior in terms of preserving some functions lost by gastrectomy, it is known that some patient dissatisfaction may result from PGS. PPG occasionally creates cases where the hospitalization period is extended due to either delayed gastric emptying or small-stomach symptoms[61]. There are also many cases in which reflux esophagitis or gastric stasis are generated after PG[62,63]. Due to these facts, such limited surgeries are sometimes considered difficult procedures. In a questionnaire survey conducted by gastric cancer specialists in Japan by the Japanese Society for Gastro-surgical Pathophysiology, only 30% of surgeons had adopted PPG and 70% of surgeons had adopted PG. Several attempts to reduce the aforementioned postoperative difficulties have been reported. In PPG, preservation of the infra-pyloric arteries and veins has been reported to be beneficial in preventing delayed gastric emptying[64-66]. The PGSAS study also concluded that size of proximal remnant stomach, gastro-gastric anastomoses with hand sewing, and adequate size of the antral cuff were helpful in reducing postoperative disability[67]. In PG, the reconstruction method is considered to be useful for the prevention of reflux esophagitis, and pyloroplasty and preservation of the hepatic and pyloric branches of vagus are considered to be useful for the reduction of gastric stasis. Reconstruction is particularly important, and various method to prevent reflux have been devised (Figure 4), but every method has its own advantages and disadvantages, and there is still no definitive operative method[68-76]. Methods to reduce postoperative damage of PG were also studied in the PGSAS study, and it has been concluded that a large distal remnant stomach and a pyloric bougie were effective[77].
Though PPG and PG are complicated procedures, reports of laparoscopic PPG and laparoscopic PG have increased recently[78-82]. However, the results of PPG and PG performed laparoscopically are equivalent to conventional open surgery in terms of safety and radicality, while preservation of function is not yet fully proven. Regarding function preservation, a retrospective study comparing laparoscopic PPG with laparoscopic DG was reported, again with fewer cases presenting with diarrhea and dumping symptoms[80]. However, reports comparing PPG or PG in conventional open surgery and laparoscopic surgery are not impressive[82]. Kinami et al[51] analyzed the data of the PGSAS study and found that laparoscopic PPG had better physical-component scores on SF-8 than open PPG, whereas there was no difference in the scores for main outcome measures between laparoscopic PG and open PG. From these results, it is concluded that the function preserved in PPG and PG in laparoscopic surgeries are equivalent to those in the conventional open surgeries. Thus, it appears that there is little long-term advantage in laparoscopic surgery.
As described above, studies of large cohorts using the PGSAS demonstrated that PPG and PG are superior in the occurrence rate of dumping symptoms and diarrhea, and the maintenance of eating habits. Nevertheless, comparing PPG and DG, or PG and TG, other functional outcomes were found to be similar[36,56]. Therefore, more extensive gastric preservation is required to prevent more PGS outbreaks and improve QOL. The extent of resection of the stomach is inseparable from that of lymph-node dissection. Therefore, in order to carry out better function-preserving procedures, it is necessary to boldly omit the lymph-node dissection in order to preserve blood circulation to the stomach.
What is the lymph-node metastasis rate of surgical cases for early gastric cancer today, when ESD has become standard? In the latest version of the Japanese gastric-cancer treatment guideline (5th edition, in Japanese), the results of research on equalization and actual conditions of gastric-cancer medical treatment in Japan as of fiscal 2013 is reported[9]. This study investigated the course of gastric-cancer treatment in 297 cancer hospitals in Japan. The total number of patients with gastric cancer was 44879, and the endoscopic treatment rate for pretreatment T1N0 gastric cancer was 64.1%. Therefore, at standard cancer treatment facilities in Japan, the number of primary surgical cases for T1N0 gastric cancer may be estimated to be approximately one-third. This is considering that 90% of early gastric cancers are negative for metastases. If all endoscopically treated cases are assumed to be node negative, 70% of surgical cases are calculated to be negative for metastasis. Therefore, only 30% of cases require D1+ (i.e., are those in which the possibility of lymph-node metastasis cannot be ruled out). D1+ may be unnecessary in 70% of cases.
Currently, however, it is difficult to reduce the extent of dissection below D1+. The frequency of metastasis in group 2 lymph nodes is low. There are two opinions: one is that there is no large benefit from the prophylactic dissection[16]; and the other is that there is a significant difference in the prognosis for D1 and D2[17]. The general view of specialists is that the extent of lymph-node dissection should not be indiscriminately reduced. In addition, there is no large difference in gastrectomy extent between D1+ and D1. Reduction of nodal dissection from D1+ to D1 is of little value in terms of PGS prevention. To prevent PGS, intraoperative diagnosis of node-negative patients should be required to establish the appropriateness of omitting peri-gastric nodal dissection, with its benefits of preservation of gastric blood flow and reduction in the resection area of stomach.
Currently, the best reliable method of intraoperative nodal diagnosis is the sentinel-node biopsy[83]. A sentinel node (SN) is defined as the node that receives lymphatic flow directly from a primary tumor. SN biopsy has been attempted most for gastric cancer among all gastrointestinal cancers[84-88]. In a multicenter prospective clinical trial in Japan, the SN concept was proved valid in early gastric cancer[85]. It is believed that the extent of lymph-node dissection can be reduced without compromising the radicality, if the node-negative patient is able to be diagnosed by SN biopsy[89]. Unfortunately, unlike breast cancer, in gastric cancer there is no room for additional nodal dissection after initial surgery, the prognosis of micro-metastasis is unknown, and a method for predicting non-SN metastases in patients with positive SN metastases has not been established. Rapid intraoperative diagnostic methods for nodal metastasis which can diagnose to the micro-metastasis level has not been established either. Therefore, it is considered premature to omit all lymphadenectomy by utilizing SN biopsy in gastric cancer. Lymphatic-basin dissection has been proposed as a realistic solution for omitting the nodal dissection[84-89]. This is a method for gastric SN biopsy, in which the lymphatic basin identified by dye mapping is removed en-bloc, and the SNs are identified ex-vivo after basin dissection, and is sent for intraoperative rapid diagnosis[88]. After SN biopsy, D2 gastrectomy is added if the patient is diagnosed as node-positive, but if the patient is diagnosed as node negative, additional dissection is omitted, the gastric feeding artery outside the basin is preserved, and the resection area of stomach is minimized. Thus, by adopting the SN biopsy, a large-area stomach-sparing function-preserving radical gastrectomy, as shown in Figure 5, can be performed. Kinami et al[88] reported that there was no recurrence in 174 cases in which nodal dissection outside the basin was omitted. Isozaki et al[90] reported that PGS in cases involving function-preserving procedures according to this protocol were clearly better than standard procedures.
A large-scale prospective study is currently ongoing in Korea to verify survival prognoses after gastric-cancer SN biopsy[91]. Additionally, a prospective trial to verify both prognosis and function-preservation effects of the function-preserving radical gastrectomy accompanied by basin dissection is on-going in Japan[89]. In addition, attempts to reproduce this function-preserving radical gastrectomy using laparoscopic surgery have also been reported[84,89,92]. If the two prospective studies described above show evidence supporting SN biopsy in gastric cancer, the surgery for early gastric cancer will eventually shift from the current laparoscopic D1+ gastrectomy to a laparoscopic, tailor-made, function-preserving radical gastrectomy, in which patients may be diagnosed as node-negative intraoperatively.
However, there are many problems to be solved in SN-directed, tailor-made function-preserving gastrectomy techniques. Solutions for the following issues are necessary: setting the proper range for lymphatic basin dissection; establishment of quick, convenient, universally-applicable intraoperative diagnosis method; and strategies for preventing dysfunction after surgery. Physicians should also pay attention to the risk of metachronous multiple gastric cancers when the remnant stomach area is large.
Early gastric cancer can be cured by surgical treatment, and physicians must be mindful of reduction of PGS and improvement of QOL over the long term after the operation. The authors intention in this article is to provide a roadmap for developing precision surgery for early gastric cancer from these three perspectives: Lymph-node dissection, function preservation, and a less invasive approach.
Lymph-node dissection: D1+ is an appropriate and standardized extent of dissection for early gastric cancer in view of radicality and safety. However, D1+ is not essential in all cases. The extent of lymph-node dissection can be individualized if SN biopsy is used to distinguish node-negative patients.
Function preserving: Depending on the location and size of the primary lesion, preservation of the pylorus or cardia should be considered. If the patient is diagnosed as node-negative by SN biopsy, significant, large-volume gastric preservation can be achieved.
Less invasive approach: There seems to be no problem with the use of laparoscopic surgery because the perioperative and oncological safety are similar to open surgery, but the physician should be aware that laparoscopic surgery is technically difficult. Prioritizing completion of laparoscopic surgery and forgoing conversion to limited or function-preserving open surgery is undesirable in terms of PGS reduction. However, if advances in surgical devices or technological innovations in robotic-assisted surgery are made, all operations will eventually be undertaken using a less invasive approach.
Though the surgical treatment for early gastric cancer is currently radical, the operative method itself seems to be still in a transitional stage. Evidence-based gastric-cancer SN biopsy and technological innovations in robotic-assisted surgery are sure to bring a new era of minimally invasive, significantly function-preserving surgery.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Demetrashvili Z, Norero E S-Editor: Yan JP L-Editor: A E-Editor: Song H
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55732] [Article Influence: 7961.7] [Reference Citation Analysis (132)] |
| 2. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13200] [Article Influence: 1466.7] [Reference Citation Analysis (3)] |
| 3. | Sumiyama K. Past and current trends in endoscopic diagnosis for early stage gastric cancer in Japan. Gastric Cancer. 2017;20:20-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 4. | Sobue T, Saika K. Epidemiology of stomach cancer (in Japanese with English abstract). Stomach Intest. 2018;53:522-524. |
| 5. | Nishizawa T, Yahagi N. Endoscopic mucosal resection and endoscopic submucosal dissection: Technique and new directions. Curr Opin Gastroenterol. 2017;33:315-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 6. | Wang S, Ling T, Zhao E, Cao H. The surgical treatment of gastric cancer in the era of minimally invasive surgery. Minerva Chir. 2017;72:334-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Tokunaga M, Kaito A, Sugita S, Watanabe M, Sunagawa H, Kinoshita T. Robotic gastrectomy for gastric cancer. Transl Gastroenterol Hepatol. 2017;2:57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Caruso S, Patriti A, Roviello F, De Franco L, Franceschini F, Coratti A, Ceccarelli G. Laparoscopic and robot-assisted gastrectomy for gastric cancer: Current considerations. World J Gastroenterol. 2016;22:5694-5717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 9. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (ver. 5). Tokyo: Kanehara-shuppan 2018; . |
| 10. | Davis JL, Ripley RT. Postgastrectomy Syndromes and Nutritional Considerations Following Gastric Surgery. Surg Clin North Am. 2017;97:277-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (2)] |
| 11. | Tasaka S. National survey of early gastric cancer (Japanese). Gastroenterol Endosc. 1962;4:4-14. |
| 12. | Hosokawa O, Shimizu M, Kaizaki Y, Miyanaga T, Asaumi Y, Ibe N, Hattori M, Dohden K, Hayashida Y, Hiranuma C. Current status of diagnosis for early gastric cancer (in Japanese with English abstract). Stomach Intest. 2009;44:455-464. |
| 13. | Takeda J, Hashimoto K, Machi J, Hirai Y, Yoshida C, Ohmori Y, Kakegawa T. Clinical and pathological evaluation of early gastric cancer and lymph node metastasis. Kurume Med J. 1987;34:183-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 14. | Sasako M, McCulloch P, Kinoshita T, Maruyama K. New method to evaluate the therapeutic value of lymph node dissection for gastric cancer. Br J Surg. 1995;82:346-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 334] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 15. | Isozaki H, Okajima K, Ichinona T, Fujii K, Nomura E, Izumi N, Ohyama T. Distant lymph node metastasis of early gastric cancer. Surg Today. 1997;27:600-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Yoshikawa T, Tsuburaya A, Kobayashi O, Sairenji M, Motohashi H, Noguchi Y. Is D2 lymph node dissection necessary for early gastric cancer? Ann Surg Oncol. 2002;9:401-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Miwa K, Miyazaki I, Sahara H, Fujimura T, Yonemura Y, Noguchi M, Falla R. Rationale for extensive lymphadenectomy in early gastric carcinoma. Br J Cancer. 1995;72:1518-1524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Seto Y, Shimoyama S, Kitayama J, Mafune K, Kaminishi M, Aikou T, Arai K, Ohta K, Nashimoto A, Honda I, Yamagishi H, Yamamura Y. Lymph node metastasis and preoperative diagnosis of depth of invasion in early gastric cancer. Gastric Cancer. 2001;4:34-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Kurihara N, Kubota T, Otani Y, Ohgami M, Kumai K, Sugiura H, Kitajima M. Lymph node metastasis of early gastric cancer with submucosal invasion. Br J Surg. 1998;85:835-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 88] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Gotoda T, Sasako M, Ono H, Katai H, Sano T, Shimoda T. Evaluation of the necessity for gastrectomy with lymph node dissection for patients with submucosal invasive gastric cancer. Br J Surg. 2001;88:444-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 133] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 21. | Kwee RM, Kwee TC. Predicting lymph node status in early gastric cancer. Gastric Cancer. 2008;11:134-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 129] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 22. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma 2017 (The 15th edition). Tokyo: Kanehara-shuppan 2018; . |
| 23. | Kitamura K, Yamaguchi T, Taniguchi H, Hagiwara A, Sawai K, Takahashi T. Analysis of lymph node metastasis in early gastric cancer: Rationale of limited surgery. J Surg Oncol. 1997;64:42-47. [PubMed] |
| 24. | Tanaka N, Katai H, Taniguchi H, Saka M, Morita S, Fukunaga T, Shimoda T. Surgical treatment for early gastric cancer (in Japanese with English abstract). Stomach Intest. 2009;44:700-706. |
| 25. | Nakajima H, Yamanaka K, Horii T, Nishina T, Ikeda T, Komeda M. A more comprehensive left ventricular repair for severely dilated cardiomyopathy. J Card Surg. 2006;21:62-64; discussion 65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 26. | Yoshikawa T, Tsuburaya A, Kobayashi O, Sairenji M, Motohashi H, Noguchi Y. Indications of limited surgery for gastric cancer with submucosal invasion--analysis of 715 cases with special reference to site of the tumor and level 2 lymph nodes. Hepatogastroenterology. 2003;50:1727-1730. [PubMed] |
| 27. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1723] [Cited by in RCA: 1895] [Article Influence: 135.4] [Reference Citation Analysis (0)] |
| 28. | Kikuchi S, Kuroda S, Nishizaki M, Kagawa T, Kanzaki H, Kawahara Y, Kagawa S, Tanaka T, Okada H, Fujiwara T. Management of early gastric cancer that meet the indication for radical lymph node dissection following endoscopic resection: A retrospective cohort analysis. BMC Surg. 2017;17:72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 29. | Kitano S, Shiraishi N, Uyama I, Sugihara K, Tanigawa N; Japanese Laparoscopic Surgery Study Group. A multicenter study on oncologic outcome of laparoscopic gastrectomy for early cancer in Japan. Ann Surg. 2007;245:68-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 518] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 30. | Lee JH, Yom CK, Han HS. Comparison of long-term outcomes of laparoscopy-assisted and open distal gastrectomy for early gastric cancer. Surg Endosc. 2009;23:1759-1763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 136] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 31. | Bolton JS, Conway WC 2nd. Postgastrectomy syndromes. Surg Clin North Am. 2011;91:1105-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 32. | Carvajal SH, Mulvihill SJ. Postgastrectomy syndromes: Dumping and diarrhea. Gastroenterol Clin North Am. 1994;23:261-279. [PubMed] |
| 33. | Harju E. Metabolic problems after gastric surgery. Int Surg. 1990;75:27-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 34. | Nakada K, Ikeda M, Takahashi M, Kinami S, Yoshida M, Uenosono Y, Kawashima Y, Oshio A, Suzukamo Y, Terashima M, Kodera Y. Characteristics and clinical relevance of postgastrectomy syndrome assessment scale (PGSAS)-45: Newly developed integrated questionnaires for assessment of living status and quality of life in postgastrectomy patients. Gastric Cancer. 2015;18:147-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 35. | Nakada K, Takahashi M, Ikeda M, Kinami S, Yoshida M, Uenosono Y, Kawashima Y, Nakao S, Oshio A, Suzukamo Y, Terashima M, Kodera Y. Factors affecting the quality of life of patients after gastrectomy as assessed using the newly developed PGSAS-45 scale: A nationwide multi-institutional study. World J Gastroenterol. 2016;22:8978-8990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 36. | Takiguchi N, Takahashi M, Ikeda M, Inagawa S, Ueda S, Nobuoka T, Ota M, Iwasaki Y, Uchida N, Kodera Y, Nakada K. Long-term quality-of-life comparison of total gastrectomy and proximal gastrectomy by postgastrectomy syndrome assessment scale (PGSAS-45): A nationwide multi-institutional study. Gastric Cancer. 2015;18:407-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 166] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 37. | Terashima M, Tanabe K, Yoshida M, Kawahira H, Inada T, Okabe H, Urushihara T, Kawashima Y, Fukushima N, Nakada K. Postgastrectomy Syndrome Assessment Scale (PGSAS)-45 and changes in body weight are useful tools for evaluation of reconstruction methods following distal gastrectomy. Ann Surg Oncol. 2014;21 Suppl 3:S370-S378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 38. | Tada M, Murakami A, Karita M, Yanai H, Okita K. Endoscopic resection of early gastric cancer. Endoscopy. 1993;25:445-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 277] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 39. | Ono H, Kondo H, Gotoda T, Shirao K, Yamaguchi H, Saito D, Hosokawa K, Shimoda T, Yoshida S. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. 2001;48:225-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1134] [Cited by in RCA: 1148] [Article Influence: 47.8] [Reference Citation Analysis (4)] |
| 40. | Gotoda T. Endoscopic resection of early gastric cancer. Gastric Cancer. 2007;10:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 495] [Article Influence: 27.5] [Reference Citation Analysis (1)] |
| 41. | Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, Kato Y. Incidence of lymph node metastasis from early gastric cancer: Estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1308] [Cited by in RCA: 1325] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 42. | Hirasawa T, Gotoda T, Miyata S, Kato Y, Shimoda T, Taniguchi H, Fujisaki J, Sano T, Yamaguchi T. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer. 2009;12:148-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 367] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 43. | Hatta W, Gotoda T, Oyama T, Kawata N, Takahashi A, Yoshifuku Y, Hoteya S, Nakamura K, Hirano M, Esaki M, Matsuda M, Ohnita K, Shimoda R, Yoshida M, Dohi O, Takada J, Tanaka K, Yamada S, Tsuji T, Ito H, Hayashi Y, Nakamura T, Shimosegawa T. Is radical surgery necessary in all patients who do not meet the curative criteria for endoscopic submucosal dissection in early gastric cancer? A multi-center retrospective study in Japan. J Gastroenterol. 2017;52:175-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 114] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 44. | Kawata N, Kakushima N, Takizawa K, Tanaka M, Makuuchi R, Tokunaga M, Tanizawa Y, Bando E, Kawamura T, Sugino T, Kusafuka K, Shimoda T, Nakajima T, Terashima M, Ono H. Risk factors for lymph node metastasis and long-term outcomes of patients with early gastric cancer after non-curative endoscopic submucosal dissection. Surg Endosc. 2017;31:1607-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 45. | Kitano S, Iso Y, Moriyama M, Sugimachi K. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc. 1994;4:146-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 46. | Ohgami M, Otani Y, Kumai K, Kubota T, Kim YI, Kitajima M. Curative laparoscopic surgery for early gastric cancer: Five years experience. World J Surg. 1999;23:187-192; discussion 192-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 161] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 47. | Ohashi S. Laparoscopic intraluminal (intragastric) surgery for early gastric cancer. A new concept in laparoscopic surgery. Surg Endosc. 1995;9:169-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 149] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 48. | Katai H, Sasako M, Fukuda H, Nakamura K, Hiki N, Saka M, Yamaue H, Yoshikawa T, Kojima K; JCOG Gastric Cancer Surgical Study Group. Safety and feasibility of laparoscopy-assisted distal gastrectomy with suprapancreatic nodal dissection for clinical stage I gastric cancer: A multicenter phase II trial (JCOG 0703). Gastric Cancer. 2010;13:238-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 259] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 49. | Zhang CD, Yamashita H, Zhang S, Seto Y. Reevaluation of laparoscopic versus open distal gastrectomy for early gastric cancer in Asia: A meta-analysis of randomized controlled trials. Int J Surg. 2018;56:31-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 50. | Nakamura M, Hosoya Y, Umeshita K, Yano M, Doki Y, Miyashiro I, Dannoue H, Mori M, Kishi K, Lefor AT. Postoperative quality of life: Development and validation of the "Dysfunction After Upper Gastrointestinal Surgery" scoring system. J Am Coll Surg. 2011;213:508-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 51. | Kinami S, Takahashi M, Urushihara T, Ikeda M, Yoshida M, Uenosono Y, Oshio A, Suzukamo Y, Terashima M, Kodera Y, Nakada K. Background factors influencing postgastrectomy syndromes after various types of gastrectomy. World J Clin Cases. 2018;6:1111-1120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 52. | Kinami S, Funaki H, Fujita H, Nakano Y, Ueda N, Kosaka T. Local resection of the stomach for gastric cancer. Surg Today. 2017;47:651-659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 53. | Maki T, Shiratori T, Hatafuku T, Sugawara K. Pylorus-preserving gastrectomy as an improved operation for gastric ulcer. Surgery. 1967;61:838-845. [PubMed] |
| 54. | Nunobe S, Sasako M, Saka M, Fukagawa T, Katai H, Sano T. Symptom evaluation of long-term postoperative outcomes after pylorus-preserving gastrectomy for early gastric cancer. Gastric Cancer. 2007;10:167-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 55. | Morita S, Katai H, Saka M, Fukagawa T, Sano T, Sasako M. Outcome of pylorus-preserving gastrectomy for early gastric cancer. Br J Surg. 2008;95:1131-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 56. | Fujita J, Takahashi M, Urushihara T, Tanabe K, Kodera Y, Yumiba T, Matsumoto H, Takagane A, Kunisaki C, Nakada K. Assessment of postoperative quality of life following pylorus-preserving gastrectomy and Billroth-I distal gastrectomy in gastric cancer patients: Results of the nationwide postgastrectomy syndrome assessment study. Gastric Cancer. 2016;19:302-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
| 57. | Katai H, Morita S, Saka M, Taniguchi H, Fukagawa T. Long-term outcome after proximal gastrectomy with jejunal interposition for suspected early cancer in the upper third of the stomach. Br J Surg. 2010;97:558-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 58. | Ikeguchi M, Kader A, Takaya S, Fukumoto Y, Osaki T, Saito H, Tatebe S, Wakatsuki T. Prognosis of patients with gastric cancer who underwent proximal gastrectomy. Int Surg. 2012;97:275-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 59. | Sugoor P, Shah S, Dusane R, Desouza A, Goel M, Shrikhande SV. Proximal gastrectomy versus total gastrectomy for proximal third gastric cancer: Total gastrectomy is not always necessary. Langenbecks Arch Surg. 2016;401:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 60. | Wen L, Chen XZ, Wu B, Chen XL, Wang L, Yang K, Zhang B, Chen ZX, Chen JP, Zhou ZG, Li CM, Hu JK. Total vs. proximal gastrectomy for proximal gastric cancer: A systematic review and meta-analysis. Hepatogastroenterology. 2012;59:633-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 61. | Michiura T, Nakane Y, Kanbara T, Nakai K, Inoue K, Yamamichi K, Kamiyama Y. Assessment of the preserved function of the remnant stomach in pylorus-preserving gastrectomy by gastric emptying scintigraphy. World J Surg. 2006;30:1277-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 62. | Yoo CH, Sohn BH, Han WK, Pae WK. Long-term results of proximal and total gastrectomy for adenocarcinoma of the upper third of the stomach. Cancer Res Treat. 2004;36:50-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 63. | Nakane Y, Michiura T, Inoue K, Sato M, Nakai K, Ioka M, Yamamichi K. Role of pyloroplasty after proximal gastrectomy for cancer. Hepatogastroenterology. 2004;51:1867-1871. [PubMed] |
| 64. | Nakabayashi T, Mochiki E, Garcia M, Haga N, Suzuki T, Asao T, Kuwano H. Pyloric motility after pylorus-preserving gastrectomy with or without the pyloric branch of the vagus nerve. World J Surg. 2002;26:577-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 65. | Nishizawa N, Hosoda K, Moriya H, Mieno H, Ema A, Ushiku H, Ishii S, Tanaka T, Washio M, Yokoi K, Harada H, Watanabe M, Yamashita K. Patients' preoperative background causes gastric stasis after laparoscopy-assisted pylorus-preserving gastrectomy. Asian J Endosc Surg. 2018;11:337-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 66. | Kiyokawa T, Hiki N, Nunobe S, Honda M, Ohashi M, Sano T. Preserving infrapyloric vein reduces postoperative gastric stasis after laparoscopic pylorus-preserving gastrectomy. Langenbecks Arch Surg. 2017;402:49-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 67. | Namikawa T, Hiki N, Kinami S, Okabe H, Urushihara T, Kawahira H, Fukushima N, Kodera Y, Yumiba T, Oshio A, Nakada K. Factors that minimize postgastrectomy symptoms following pylorus-preserving gastrectomy: Assessment using a newly developed scale (PGSAS-45). Gastric Cancer. 2015;18:397-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 68. | Sakuramoto S, Yamashita K, Kikuchi S, Futawatari N, Katada N, Moriya H, Hirai K, Watanabe M. Clinical experience of laparoscopy-assisted proximal gastrectomy with Toupet-like partial fundoplication in early gastric cancer for preventing reflux esophagitis. J Am Coll Surg. 2009;209:344-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 69. | Tokunaga M, Ohyama S, Hiki N, Hoshino E, Nunobe S, Fukunaga T, Seto Y, Yamaguchi T. Endoscopic evaluation of reflux esophagitis after proximal gastrectomy: Comparison between esophagogastric anastomosis and jejunal interposition. World J Surg. 2008;32:1473-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 70. | Nomura E, Lee SW, Kawai M, Yamazaki M, Nabeshima K, Nakamura K, Uchiyama K. Functional outcomes by reconstruction technique following laparoscopic proximal gastrectomy for gastric cancer: Double tract versus jejunal interposition. World J Surg Oncol. 2014;12:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 71. | Takagawa R, Kunisaki C, Kimura J, Makino H, Kosaka T, Ono HA, Akiyama H, Endo I. A pilot study comparing jejunal pouch and jejunal interposition reconstruction after proximal gastrectomy. Dig Surg. 2010;27:502-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 72. | Mochiki E, Fukuchi M, Ogata K, Ohno T, Ishida H, Kuwano H. Postoperative functional evaluation of gastric tube after laparoscopic proximal gastrectomy for gastric cancer. Anticancer Res. 2014;34:4293-4298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 73. | Hayami M, Hiki N, Nunobe S, Mine S, Ohashi M, Kumagai K, Ida S, Watanabe M, Sano T, Yamaguchi T. Clinical Outcomes and Evaluation of Laparoscopic Proximal Gastrectomy with Double-Flap Technique for Early Gastric Cancer in the Upper Third of the Stomach. Ann Surg Oncol. 2017;24:1635-1642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 109] [Article Influence: 13.6] [Reference Citation Analysis (1)] |
| 74. | Yamashita Y, Yamamoto A, Tamamori Y, Yoshii M, Nishiguchi Y. Side overlap esophagogastrostomy to prevent reflux after proximal gastrectomy. Gastric Cancer. 2017;20:728-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (1)] |
| 75. | Nakamura M, Yamaue H. Reconstruction after proximal gastrectomy for gastric cancer in the upper third of the stomach: A review of the literature published from 2000 to 2014. Surg Today. 2016;46:517-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 76. | Tomita R, Fujisaki S, Tanjoh K, Fukuzawa M. A novel operative technique on proximal gastrectomy reconstructed by interposition of a jejunal J pouch with preservation of the vagal nerve and lower esophageal sphincter. Hepatogastroenterology. 2001;48:1186-1191. [PubMed] |
| 77. | Inada T, Yoshida M, Ikeda M, Yumiba T, Matsumoto H, Takagane A, Kunisaki C, Fukushima R, Yabusaki H, Nakada K. Evaluation of QOL after proximal gastrectomy using a newly developed assessment scale (PGSAS-45). World J Surg. 2014;38:3152-3162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 78. | Jiang X, Hiki N, Nunobe S, Fukunaga T, Kumagai K, Nohara K, Katayama H, Ohyama S, Sano T, Yamaguchi T. Long-term outcome and survival with laparoscopy-assisted pylorus-preserving gastrectomy for early gastric cancer. Surg Endosc. 2011;25:1182-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 79. | Ueda Y, Shiroshita H, Etoh T, Inomata M, Shiraishi N. Laparoscopic proximal gastrectomy for early gastric cancer. Surg Today. 2017;47:538-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 80. | Hosoda K, Yamashita K, Sakuramoto S, Katada N, Moriya H, Mieno H, Watanabe M. Postoperative quality of life after laparoscopy-assisted pylorus-preserving gastrectomy compared With laparoscopy-assisted distal gastrectomy: A cross-sectional postal questionnaire survey. Am J Surg. 2017;213:763-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 81. | Ahn SH, Lee JH, Park DJ, Kim HH. Comparative study of clinical outcomes between laparoscopy-assisted proximal gastrectomy (LAPG) and laparoscopy-assisted total gastrectomy (LATG) for proximal gastric cancer. Gastric Cancer. 2013;16:282-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 82. | Kinoshita T, Gotohda N, Kato Y, Takahashi S, Konishi M, Kinoshita T. Laparoscopic proximal gastrectomy with jejunal interposition for gastric cancer in the proximal third of the stomach: A retrospective comparison with open surgery. Surg Endosc. 2013;27:146-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 83. | Morton DL, Wen DR, Wong JH, Economou JS, Cagle LA, Storm FK, Foshag LJ, Cochran AJ. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992;127:392-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3238] [Cited by in RCA: 2931] [Article Influence: 88.8] [Reference Citation Analysis (0)] |
| 84. | Kinami S, Kosaka T. Laparoscopic sentinel node navigation surgery for early gastric cancer. Transl Gastroenterol Hepatol. 2017;2:42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 85. | Kitagawa Y, Takeuchi H, Takagi Y, Natsugoe S, Terashima M, Murakami N, Fujimura T, Tsujimoto H, Hayashi H, Yoshimizu N, Takagane A, Mohri Y, Nabeshima K, Uenosono Y, Kinami S, Sakamoto J, Morita S, Aikou T, Miwa K, Kitajima M. Sentinel node mapping for gastric cancer: A prospective multicenter trial in Japan. J Clin Oncol. 2013;31:3704-3710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 233] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 86. | Miwa K. Sentinel node concept and its application for cancer surgery. Nihon Geka Gakkai Zasshi. 2000;101:307-310. [PubMed] |
| 87. | Miwa K, Kinami S, Taniguchi K, Fushida S, Fujimura T, Nonomura A. Mapping sentinel nodes in patients with early-stage gastric carcinoma. Br J Surg. 2003;90:178-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 179] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 88. | Kinami S, Fujimura T, Ojima E, Fushida S, Ojima T, Funaki H, Fujita H, Takamura H, Ninomiya I, Nishimura G, Kayahara M, Ohta T, Yoh Z. PTD classification: Proposal for a new classification of gastric cancer location based on physiological lymphatic flow. Int J Clin Oncol. 2008;13:320-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 89. | Takeuchi H, Goto O, Yahagi N, Kitagawa Y. Function-preserving gastrectomy based on the sentinel node concept in early gastric cancer. Gastric Cancer. 2017;20:53-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 90. | Isozaki H, Matsumoto S, Murakami S, Takama T, Sho T, Ishihara K, Sakai K, Takeda M, Nakada K, Fujiwara T. Diminished Gastric Resection Preserves Better Quality of Life in Patients with Early Gastric Cancer. Acta Med Okayama. 2016;70:119-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 91. | Park JY, Kim YW, Ryu KW, Nam BH, Lee YJ, Jeong SH, Park JH, Hur H, Han SU, Min JS, An JY, Hyung WJ, Cho GS, Jeong GA, Jeong O, Park YK, Jung MR, Yoon HM, Eom BW. Assessment of laparoscopic stomach preserving surgery with sentinel basin dissection versus standard gastrectomy with lymphadenectomy in early gastric cancer-A multicenter randomized phase III clinical trial (SENORITA trial) protocol. BMC Cancer. 2016;16:340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 92. | Kinami S, Oonishi T, Fujita J, Tomita Y, Funaki H, Fujita H, Nakano Y, Ueda N, Kosaka T. Optimal settings and accuracy of indocyanine green fluorescence imaging for sentinel node biopsy in early gastric cancer. Oncol Lett. 2016;11:4055-4062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |